Abstract
To investigate the effects of row ratio configurations on intercropping advantages and related rhizosphere microbial communities, a field experiment involving five treatments of different rows of broomcorn millet, i.e., P1M1 (1 row of broomcorn millet intercropped with 1 row of alfalfa), P2M3, P1M2, P1M3 and broomcorn millet alone (SP), was conducted on the Loess Plateau of China. We analyzed the yield, nutritional content of broomcorn millet, the soil nutrient availability and the diversity and community composition of AMF (arbuscular mycorrhizal fungi) and diazotrophs in the rhizosphere of broomcorn millet. The results showed that compared with monocultures, alfalfa-millet intercropping system under different row ratio configurations significantly increased the yield of broomcorn millet and the absorption of PTP and PTK (total phosphorus and potassium of broomcorn millet). In addition, the broomcorn millet-alfalfa intercropping system also improved soil nutrition, with the decrease of the row ratio of broomcorn millet, the changes of TN, NH4+-N and microbial biomass in the rhizosphere of broomcorn millet were consistent, which was opposite to NO3−-N. Moreover, co-occurrence network and PLS-PM (partial least squares path modelling) analysis showed alfalfa-broomcorn millet intercropping system changed the community diversity and composition of soil microorganisms, increased the improvement of soil nutrition (TN, NH4+-N and microbial biomass), and promoted the absorption of different nutrients by plants (N, P and K) mainly through the negative regulation of AMF and the synergistic effect of AMF on diazotrophs, and finally increased crop yield. This shows that broomcorn millet-alfalfa intercropping can increase plant nutrient content by adjusting soil nutrients and soil microbial activities, thereby increasing yield. Furthermore, we found that 1P2M was the best ratio of alfalfa-millet intercropping system, which may provide reliable suggestions and selection basis for future agricultural production practices.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-024-06011-6.
Keywords: Row ratio configuration, Intercropping system, Rhizosphere microbial community, Microorganism-plant interactions
Introduction
Cover crops, offer a valuable solution for enhancing resource utilization and productivity [1, 2], mainly because they increase plant diversity [3] and facilitate the synchronization of N release and plant N demands [2]. Nevertheless, the integration of cover crops into traditional crop rotations faces significant limitations due to the short summer growing season [4] and the lower temperatures and precipitation [5]. Recent studies have demonstrated the innovative practice of integrating cover crops into traditional forage cropping systems such as alfalfa and corn to effectively combine the benefits of dual-purpose cover crops, which contributes to improving soil health and livestock production [5, 6]. Notably, this innovative practice is fully coincident with an ancient agricultural conservation technique in which annual cereal is intercropped in alfalfa cropping systems to achieve both immediate economic and soil health benefits in China, especially in the first year of establishment [7]. Cuartero et al. found that a first-year melon / cowpea intercropping system improves soil nutrients and changes the soil microbial community [8], which also showed that the intercropping system had significant microbial effects in the short term. Intercropping system efficacy heavily relies on the row configurations of the intercropped crop [9], which may result from the competition and facilitation outcomes shaped interactions between legumes and cereals, involving altered distributions of root systems [10], soil nitrogen availability [11] and radiation interception [12]. However, sufficient studies on cover crop row ratio configurations to ensure cover crop intercropping success are scarce; filling this knowledge gap can contribute to a deeper understanding of the theory of intercropping cover crops, support the design of sustainable cropping systems and facilitate their application.
Intercropping systems, especially those combining alfalfa and cereals, are widely acknowledged as sustainable practices in northern China [13–15]. The advantages of intercropping may be attributed mainly to a combination of enhanced crop productivity and land sustainability [15] by optimizing resource utilization efficiency and enhancing biodiversity [13]. Although extensive research has focused on suitable row configurations in intercropping systems, their effects on nutrient advantages and related microbial communities are highly context dependent, presumably owing to plant species identity, agroclimatic situations and management practices [16, 17]. However, previous studies have focused primarily on legumes because of their N2 fixation capacity and economic significance and have overlooked the pivotal roles of cereals in efficient resource utilization and soil microbe interactions [18–20]. Given the differences in plant physiology between cereals and legumes, a comprehensive understanding of the responses of nutrient advantages and related rhizosphere microbial community to row ratio configurations from the perspective of cereals is imperative to improve the efficiency of intercropping.
Arbuscular mycorrhizal fungi (AMF) and diazotrophs (which express the nifH gene) significantly contribute to intercropping benefits [21] and sustain plant growth and soil function [22], though which are susceptible to environmental disturbances [23]. Additionally, common mycorrhizal networks (CMNs) play crucial roles in nutrient distribution among intercropped crop species and maintaining competitive balance [21]. Therefore, the “crop‒crop‒microorganism” theory has been proposed as a potential mechanism explaining microorganism‒plant interactions that impact high yield in intercropping systems [24]. Despite extensive research on the role of microbial communities in intercropping yield advantages [21], the direct response patterns of AMF and diazotrophic bacteria during cover crop‒alfalfa intercropping remain poorly understood. Understanding changes in soil microbial diversity and composition will deepen our understanding of plant‒microbial interactions in intercropping systems [25].
Broomcorn millet (Panicum miliaceum) plays a crucial role as a major grain crop in northern Shaanxi, China, as a result of its excellent nutritional quality, short growth cycle and drought tolerance [26]. The objective of this study was to gain a more comprehensive understanding of how row ratio configurations affect the variation in plant nutrient uptake, soil nutrient availability and related microbial communities in the rhizosphere of broomcorn millet in broomcorn millet‒alfalfa intercropping systems. We hypothesized that (1) different row ratio configurations significantly affect plant nutrient uptake and soil nutrient availability; (2) different row ratio configurations result in significant shifts in the diversity and composition of AMF and diazotrophic communities; and (3) there are links between row configurations and soil nutrient availability and the recruitment of beneficial microbes. This study are expected to highlight that suitable row ratio configurations were more effective at improving the relative efficiency of intercropping and the maximum benefits obtained.
Materials and methods
Experimental region
The experiments were conducted in 2022 at the Jajiawa Experimental Station, Yanan Institute of Agricultural Science, Shaanxi Province, China (36°48′10′′N, 109°20′58′′E). The area is characterized by a mid-temperate continental semiarid monsoon climate, with an average annual temperature of 8.8 °C, an average annual precipitation of 581.1 mm, and a total annual sunshine duration of 2,489.5 h. The elevation of this region is 1007.6 m, and the total sunshine duration from April to October is 514 h. The basic physical and chemical properties of the soil in the experimental site were PH = 8.64, SOC(g/kg) = 5.08, TN(g/kg) = 0.18, TP(g/kg) = 0.51.
Experimental design and sample collection
The treatments included five row ratio configurations (P1M1 [1 row of broomcorn millet intercropped with 1 row of alfalfa], P1M2, P1M3, P2M3 and broomcorn millet alone [SP]). The plot size for each treatment was 5.0 × 8.0 m (width × length). The alfalfa variety was Zhongmu No.1(Medicago sativa), fall dormant plant with high yield and high quality ( Vitality Agricultural Science and Technology Co. Ltd., China). The broomcorn millet variety was Yumi No.1 ( Panicum miliaceum), which has the characteristics of high quality, high yield and strong disease resistant (College of Agronomy, Northwest A&F University, China). The experimental site was established on April 12, 2022; each treatment was replicated four times in a randomized block design, and each replicate plot was spaced 1 m apart. On April 23, 2022, alfalfa plants were sown at a rate of 15 kg·ha− 1 with a row spacing of 30 cm and a depth of 0.5–1.5 cm. Sowing of broomcorn millet were performed in the corresponding experimental plots in the middle of the corresponding alfalfa rows on May 23. The whole test plot was subjected to natural rainfall without artificial irrigation or fertilizer application. The weeding method was artificial weeding with no herbicide (Fig 1).
Fig. 1.
Schematic diagrams of experimental design. SP: Monoculture of broomcorn millet, P1M1: one row of alfalfa intercropped with one row of broomcorn millet, P1M2: two rows of alfalfa intercropped with one row of broomcorn millet, P1M3: three rows of alfalfa intercropped with one row of broomcorn millet, P2M3: three rows of alfalfa intercropped with two rows of broomcorn millet
Alfalfa and broomcorn millet were removed as forage grasses by being cut at the early flowering stage on July 15 and September 18 in 2022, after which the aboveground parts of the broomcorn millet and the soil samples of the broomcorn millet rhizosphere were collected. In accordance with the five-point sampling method, five soil samples from one plot were mixed into a composite sample. The plant roots around the sampling points were collected, and plants with similar growth characteristics were selected. The soil around the root system (0–5 mm from the root) was then gently brushed off and collected as rhizosphere soil samples. Each sample was immediately stored in liquid nitrogen until it was returned to the laboratory. Each soil sample was divided into three parts: one was stored at − 80 °C for DNA extraction and microbiological analysis; one was stored at 4 °C for soil microbial biomass carbon, nitrogen and phosphorus (MBC, MBN, and MBP); and one was air-dried for measurements of soil nutrition.
Sample chemical analysis
Air-dried samples and some fresh soil were then passed through 2 mm, 1 mm, 0.15 mm sieve, respectively. The pH was measured with a pH meter at a water: soil ratio of 1:2.5. The soil organic carbon (SOC) content and total carbon content of broomcorn millet (PTC) were determined using the potassium dichromate oxidation method [27]. Soil total nitrogen (TN) levels were measured using the Kjeldahl method; soil total phosphorus (TP) levels were measured by digestion with H2SO4 and HClO4 [22]; total nitrogen, phosphorus and potassium of broomcorn millet (PTN, PTP, PTK) were measured by digestion with H2SO4 and H2O2; and soil NH4+-N and NO3−-N were measured by extraction with 1.0 mol·L− 1 KCl [9]. All samples were analysed in a continuous flow analyser (AA3, SEAL, Germany). The MBC, MBN, MBP were measured using the chloroform fumigation leaching method. The natural abundance of carbon and nitrogen stable isotopes of 13C‰ and 15N‰ contents in soil samples were determined at the Analysis and Testing Center of the Institute of Agricultural Environment and Sustainable Development, Chinese Academy of Agricultural Sciences.
DNA extraction and library preparation
The soil samples were subjected to nucleic acid extraction using an OMEGA soil DNA kit (D5625–01) (Omega Bio-Tek, Norcross, USA) before the quality and quantity of the DNA were determined with a spectrophotometer (NanoDrop ND-2000, Wilmington, USA), after which the DNA was purified by 2% agarose gel electrophoresis. The 16S rRNA region of the fungus was amplified with the primers AMV4.5NF (5′-AAGCTCGTAGTTGAATTTCG-3′) and AMDGR (5′-CCCAACTATCCCTATTAATCAT-3′) [28]. The nifH gene was amplified with the primers PolyF (5′-TGCGAYCCSAARGCBGACTC-3′) and PolyR (5′-ATSGCCATCATYTCRCCGGA-3′) [29]. The obtained products were sent for paired-end sequencing on an Illumina MiSeq (2 × 250 bp) platform at Shanghai Paisano Biotechnology Co., Ltd. (Shanghai, China). QIIME2 2019.4 was used to demultiplex the raw sequence data [30]. The sequences were then quality filtered, denoised, and merged, and chimaeras were removed using the DADA2 plugin [31]. Nonsingleton amplicon sequence variants (ASVs/OTUs) were aligned with MAFFT [32].
Sequence analysis
The R package in QIIME2 was used for sequence analysis. Alpha diversity was calculated and presented in box plots. Beta diversity was calculated using Bray–Curtis metrics for analysis, which were visualized by nonmetric multidimensional scaling (NMDS) [33]. The R “VennDiagram” package was used to draw Venn diagrams, which can be used to visualize shared and unique ASVs/OTUs between samples/groups based on the occurrence of ASVs/OTUs in the samples/groups. The “classify _ samples _ ncv” function in the q2-sample classifier of machine learning methods in QIIME2 was used for random forest analysis and nested hierarchical cross-validation.
Statistical analysis
Significance analysis was performed with least significant difference (LSD) and Duncan’s post hoc multiple comparisons tests (P < 0.05). GraphPad Prism 9 was used to construct graphs for this section. The species correlation matrix was generated using the “psych” package in R and imported into Gephi 0.10.1 for visualization. The Mantel test and partial least squares path modelling (PLS‒PM) were used to determine the correlations. The “dply”, “plspm”, and “ggplot 2” packages in R3.6.2 were used to analyse and plot the data.
Results
Nutrition uptake in broomcorn millet
Compared with that of the monocultures, the uptake of PTP and PTK by broomcorn millet increased under intercropping and decreased with the decreasing row ratio of broomcorn millet; while intercropping minimally affected the PTN content and decreased the PTC content. For example, the PTP contents decreased in the following order: P1M1 > P2M3 > P1M2 > P1M3 > SP, and the PTP contents was significantly greater than that of SP by 41–163%; in particular, the PTP contents were significantly greater in the P1M1 and P2M3 treatments than in the monocultures (P < 0.05). Intercropping significantly decreased the yield of broomcorn millet compared to monoculture (P < 0.05), and yield decreased with the decrease of row ratio configurations in the first cutting, while the values in P2M3 was lowest in the second cutting (Table 1).
Table 1.
Nutrition and yield of broomcorn millet under different row ratio configurations
| Nutrition and yield | Row ratio configuration | |||||
|---|---|---|---|---|---|---|
| SP | P1M1 | P2M3 | P1M2 | P1M3 | ||
| Nutrition (g•kg− 1) | PTC | 32.82 ± 4.64 a | 28.13 ± 5.44 a | 26.22 ± 4.19 a | 25.78 ± 2.53 a | 29.83 ± 7.49 a |
| PTN | 7.71 ± 0.46 a | 7.93 ± 0.98 a | 7.70 ± 0.50 a | 7.87 ± 0.50 a | 7.43 ± 0.58 a | |
| PTP | 2.22 ± 0.28 c | 5.83 ± 0.57 a | 4.48 ± 0.56 ab | 4.19 ± 0.73 ab | 3.13 ± 0.73 bc | |
| PTK | 27.56 ± 1.47 a | 33.28 ± 1.68 a | 27.55 ± 2.66 a | 32.16 ± 2.31 a | 30.39 ± 1.40 a | |
|
Yield (t•hm− 2) |
1st cutting | 2.24 ± 0.16 a | 0.73 ± 0.03 b | 0.40 ± 0.08 c | 0.35 ± 0.06 c | 0.20 ± 0.02 c |
| 2nd cutting | 3.37 ± 0.54 a | 0.99 ± 0.17 b | 0.58 ± 0.03 b | 1.15 ± 0.13 ab | 0.74 ± 0.05 b | |
| Total year | 5.61 ± 0.69 a | 1.72 ± 0.16 b | 0.98 ± 0.08 b | 2.25 ± 0.75 b | 0.94 ± 0.07 b | |
Values represent the mean of four replicates. Different letters indicate significant differences between treatments (P < 0.05). Broomcorn millet were cut first on July 15 and second on September 18 in 2022
Rhizosphere soil chemical properties
Compared with those in monocultures, the contents of SOC and NO3−-N under intercropping decreased significantly with decreasing row ratio (P < 0.05), and the TN contents under P2M3 and P1M3 were significantly greater than those under the other treatments (Fig. 2A). The contents of MBC, MBN, MBP and NH4+-N in intercropping systems tended to increase with decreasing row ratio configurations.
Fig. 2.
Nutrition of the broomcorn millet and its rhizosphere soil samples (a), relationship between 13C‰, 15N‰, C%, N% and C/N (b). Note: 13C‰: soil stable carbon isotope; 15N‰: soil stable nitrogen isotope; C%: percentage of total carbon; N%: percentage of total nitrogen; C/N: soil carbon to nitrogen ratio. The results are means ± (standard error). Different letters in chart indicate significant difference (P < 0.05) in different row ratio configurations by the ANOVA and LSD multiple test
Linear regressions of soil 13C‰, C%, 15N‰, N% and C/N were performed under different row ratio configurations (Fig. 2B). There was a positive linear correlation between 13C‰ and C/N (R2 = 0.12) and a negative correlation between C% and C/N (R2 = 0.1457), but neither correlation reached significance. However, there was a significant negative linear correlation between the 15N‰ and C/N (P < 0.05, R2 = 0.2799). In addition, there was a highly significant negative linear correlation between the N% and C/N (P < 0.0001, R2 = 0.8844).
Diversity, composition and structure of AMF and diazotroph communities
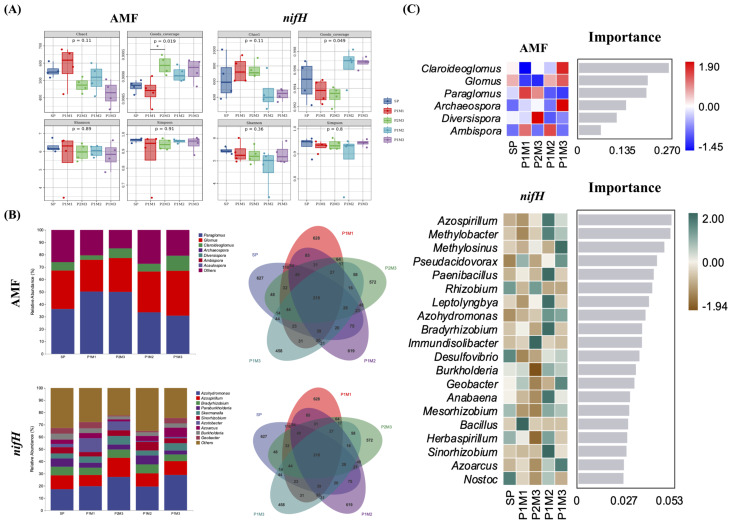
A total of 6,927 AMF-associated soil ASVs and 3,288 nifH-related OTUs were obtained from the twenty soil samples collected. The diversity indices of AMFs significantly differed across row ratio configurations. For example, Good’s coverage was ordered as P2M3 > P1M3 > P1M2 > P1M1. However, the alpha diversity analysis revealed no significant differences in the nifH population of broomcorn millet under different row ratio configurations (Fig. 3A), and the overall trend was similar.
Fig. 3.
The alpha diversity (a), community composition and Veen (b) and random forests analysis (c) of microbial communities of AMF and nifH in the rhizosphere of broomcorn millet under different row ratio configurations. All the data of alpha diversity is presented based on the box line plots of Chao1, Goods_coverage, Shannon, and Observed_species of ASVs/OTUs. The number under the diversity index label is the p-value of the Kruskal-Wallis test. The relative abundance of taxa and Veen show levels of genus in AMF and nifH. Random Forests Analysis at genus show in AMF and nifH
At the genus level, Paraglomus (30.88–50.37%) and Glomus (25.60–36.26%) were the dominant genera for AMF, whereas Azohydromonas (17.25–29.04%) and Azospirillum (9.00–15.80%) were the most abundant taxa for diazotrophs. In general, the proportion of dominant genera in the intercropping system was greater than that in the monocropping system, and as the row ratio decreased, the proportion of dominant genera first increased but then decreased, with the P2M3 treatment reaching the highest point. AMF shared 218 ASVs, whereas nifH shared 219 OTUs, and the number of unique OTUs associated with nifH decreased in the intercropping systems (Fig. 3B).
Claroideoglomus was the most important fungus in the AMF community, followed by Glomus and Paraglomus. In the diazotrophic community, Azospirillum and Mesorhizobium were the most important genera, followed by Methylosinus; more important genera were identified in the nifH community than that in AMF. NMDS analyses revealed that the community structures of the AMF and diazotrophs differed among the row ratio configurations (Fig. 4), among them, the community structure of AMF and nifH under P2M3 was significantly different from that of SP ( P < 0.05), and the inter-group difference analysis also confirmed this.
Fig. 4.
The non-metric multidimensional scaling (NMDS) (a) and analysis of inter-group differences (b) between microbial communities in the rhizosphere of broomcorn millet under different row ratio configurations
Co-occurrence patterns between AMF and diazotrophic bacterial communities
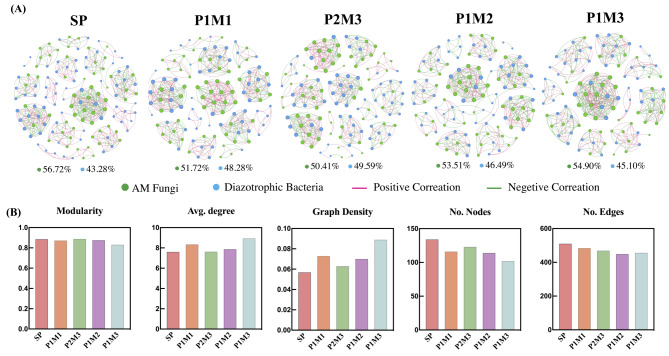
With decreasing row ratio, the node proportion of the AMF community first decreased but then increased, and the greatest number of nodes and the largest proportion of AMF were detected in monocultures, whereas the proportion of nodes among diazotrophic bacteria in monocultures was the smallest, reaching a maximum of 49.59% in P2M3 (Fig. 5A). In P1M3, modularity was the smallest, but the average degree and graph density were the greatest; the number of nodes and edges as well as the proportion of positively correlations also decreased significantly (Fig. 5B).
Fig. 5.
The co-occurrence networks patterns (a) and features (b) of AMF and diazotrophic bacteria communities in different row ratio configurations. Connections indicate strong (Spearman’s |r|>0.8) and significant (P < 0.01) correlations. The green node indicates AMF and the blue node denotes nifH. The node size represents the degree of ASV/OTUs. The red line indicates a positive interaction between two separate nodes and the green line denotes a negative interaction. Numbers represent the nodes proportion
Relationships among soil properties and soil microbial diversity
There were highly significant positive correlations between MBC, MBN and MBP and significant positive correlations between PTP and PTK and between PTK and NH4+-N. There was also a significant negative correlation between 15N‰ and TN. The correlations between nifH community diversity and NO3−-N and 15N‰ were statistically significant (P < 0.05) (Fig. 6A).
Fig. 6.
Network heat map (a), PLS-PM (b) and standardized effects from PLS-PM (c) between broomcorn millet and soil nutrition and diversity of AMF and diazotrophic bacteria under different row ratio configurations. The significant and nonsignificant relationships between different variables are represented by continuous and dashed arrows. Red arrows showed positive impacts while blue arrows showed negative impacts. The goodness-of-fit (GOF) statistics were examined and indicated that the model adequately fitted the data
The PLS‒PM results revealed that the row ratio configuration had a significant positive effect on soil nutrition (P < 0.01) and yield of broomcorn millet (P < 0.05), and soil nutrition (P < 0.05) and AMF diversity (P < 0.001) had significant positive and negative effects on yield of broomcorn millet. Moreover, the soil nutrition and AMF had significant negative and positive effects on nifH diversity respectively (P < 0.05) (Fig. 6B). In addition, the negative effect of AMF on yield was strongest, whereas the total effect was weakened due to indirect positive effects. (Fig. 6C).
Discussion
Soil nutrient changes in alfalfa-millet system driven by row configuration
The potential benefits of intercropping cereals with legumes on soil quality and fertility are well known [34], primarily by increasing biodiversity and land sustainability [15]. It has been reported that intercropped soybean [20], alfalfa [35] and faba bean improved the uptake of N, P and K in cereal, which are in accordance with our results that intercropping with alfalfa increased the uptake of P and K of broomcorn millet, mainly because of the stronger rhizosphere interactions, the increased decomposition of root residues and the regulation of soil bacterial community [36]. Notably, P uptake significantly decreased as the row ratio of broomcorn millet decreased in our study, which is closely associated with the changes of land equivalent ratio and the efficient use of the light environment [16].
However, our results indicated that intercropped broomcorn millet presented lower SOC contents in the soil than monocropped broomcorn millet did. One possible explanation is short growing season with insufficient C sequestration [17, 34]. In addition, these results might be related to the promotion of SOC decomposition, likely by the addition of fresh organic matter and the increased soil N availability [17]. Moreover, analyses of isotopic compositions revealed that soil 13C‰ was inconsistent with alterations in SOC accumulation, implying that intercropping time was also an important factor affecting soil C cycling [2, 37].
It’s known to all that intercropping significantly increased the soil available N (involved in N transfer through CMN) and the efficiency of N2 [38]. Our findings coincided with those of previous studies in which soil TN and NH4+-N under intercropping systems were higher than those in monoculture systems. However, our results demonstrated that NO3−-N was significantly lower in broomcorn millet intercropped with alfalfa than that in monocultures, possibly because of the consumption of soil nutrients by broomcorn millet [39], and sampling depth, time or crop preferences, could also have an impact on the measurement results, which caused the inconsistent trend of change between soil TN, NH4+-N and NO3–-N. Similarly, we found that the response of N nutrition in the rhizosphere of intercropped broomcorn millet was fully coincident with the lower soil 15N‰ and further revealed that increased N2 fixation contributed to increased N storage [40]. Our data are essential for understanding the cycles and balances of soil nutrients and potential management practices that optimize cover crop row ratio configurations in forage-based intercropping systems.
Overall patterns of AMF communities and diazotrophs in response to row ratio configurations
Annual cereal‒legume intercropping systems can increase soil mycorrhizal activity and native AMF propagules, especially the mycorrhizal colonization levels of cereal [41]. Our data indicated that the row ratio configuration led to changes in the soil AMF community composition in the intercropping system. The results of random forest analysis revealed that Paraglomus and Glomus were the dominant genera for root AMF, meaning that the different row ratio configurations shifted the AMF-dominant genera in favour of Glomus, which has the ability to protect soil organic carbon and is possible to better obtain carbon sources from the soil and retain them for plant nutrients [42]. The primary factors contributing to these findings are likely the implementation of crop cover intercropping [43] and interspecific competition among plants [44].
Our analysis revealed that the rhizosphere nifH community composition significantly differed across row ratio configurations, which is consistent with previous work [39, 45] and further suggests that intercropping with cover crops is critical for N cycling microbial populations [46]. In our study, the negative correlations among rhizosphere microorganisms in intercropped broomcorn were significantly greater than those observed in monocultures and provide evidence that cover crops contribute to increased rhizosphere microbes through competition for resources and niches [46, 47]. A more complex mycorrhizosphere may facilitate the transport of soil rhizodeposits, ultimately resulting in changes in root-related diazotrophic communities [23, 48]. Furthermore, the proportion of nifH nodes in the intercropping system was greater than that in the monoculture system, whereas that in the AMF system was the opposite, probably as a result of changes in plant intraspecific and interspecific competition [44], fully corroborating the findings of a previous study showing that diazotrophic and AMF communities exhibited different community assemblies and ecological networks [23].
Our study indicated that the degrees of node and edge variation had significant effects on co-occurrence networks, thus implying that row ratio configurations play important roles in shaping communities and regulating multiple soil ecosystem services [2]. Based on the results of this study, we found that the rhizosphere soil nutrients, microbial diversity and community structure of broomcorn millet under P1M2 row ratio configuration were the most different from others, and the total yield was the highest. Therefore, P1M2 was selected as the recommended row ratio configuration of alfalfa-broomcorn millet intercropping systems. More work is needed to clarify the extent to which competition or cooperation occurs in the soil microbial community in long-term experiment.
The correlation between AMF, diazotrophs and nutrition under different row ratio configurations
Soil microbial biomass is involved in the cycling of nutrients in soils and is susceptible to management practices and environmental conditions [40]. This study revealed greater levels of microbial biomass in the rhizospheres of intercropped broomcorn millet than in those of monocultured broomcorn millet, which coincides with the results of other studies [49, 50], and soil nutrient availability and microbial biomass indicators exhibited similar trends along with decreases in the row ratio of broomcorn millet, which meant that row ratio configurations altered the plant‒soil-microbial interaction, subsequently influencing intercropping efficiency [1, 51]. However, Sun et al. (2018) [52] suggested that the differences in the distribution of roots and nutritional requirements of alfalfa after the first year of establishment are due to resource competition between the intercropped crops [51]. Further detailed investigations are needed to elucidate the long-term responses of microbial community to row ratio configurations in intercropping systems.
Compared with monocultures, intercropping increases plant diversity, resulting in different root exudates, which in turn affects microbial biomass and its diversity, thus affecting the absorption of nutrients by crops and finally change the yield [16]. Moreover, the row ratio influences the availability of soil nutrients indirectly affecting the soil microbial composition and co-occurrence patterns [53]. Soil nutrition in intercropping systems has the potential to directly regulate the structure of microbial communities, either by mediating microbial richness or by modulating metabolic activities in soils [54]. Our data further suggest that row ratio configuration strengthened the role of AMF by reducing soil N availability, which subsequently influenced the function of diazotrophs [23]. Further work incorporating multi-omics analyses could help reveal the contributions of AMF and diazotrophs to plant‒soil-microbial interactions in the broomcorn millet rhizosphere.
Conclusions
The results of this study showed that compared with monocultures, alfalfa-millet intercropping system under different row ratios significantly increased the yield of broomcorn millet and the absorption of P and K elements. In addition, the broomcorn millet-alfalfa intercropping system also improved soil nutrition, with the decrease of the row ratio of broomcorn millet, the changes of TN, NH4+-N and microbial biomass in the rhizosphere of broomcorn millet were consistent, which was opposite to NO3−-N. Moreover, alfalfa-broomcorn millet intercropping system regulated the community structure and composition of soil microorganisms related to nitrogen cycle, increased the improvement of soil nutrition. This shows that alfalfa-broomcorn millet intercropping can increase plant nutrient content by adjusting soil nutrients and soil microbial activities, thereby increasing yield. Furthermore, we found that 1P2M was the best ratio of alfalfa-millet intercropping system, which may provide reliable suggestions and selection basis for future agricultural production practices.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was financially supported by the National Key R&D Program of China (2022YFD1300803), the National Natural Science Foundation of China (32071878).
Abbreviations
- P1M1
1 row of broomcorn millet intercropped with 1 row of alfalfa
- SP
Broomcorn millet alone
- AMF
Arbuscular Mycorrhizal Fungi
- PTC, PTP, PTK, PTN
Total Carbon, Phosphorus, Potassium, Nitrogen Content of Broomcorn Millet
- SOC
Soil Organic Carbon
- TP, TN
Soil Total Phosphorus, Nitrogen
- MBC, MBN, MBP
Microbial Biomass Carbon, Phosphorus, Nitrogen
- NH4+-N
Ammonium Nitrogen
- NO3−-N
Nitrate Nitrogen
- 13C‰
Carbon Isotope Content in Soil
- 15N‰
Nitrogen Isotope Content in Soil
- CMNs
Common Mycorrhizal Networks
- ANOVA
Analysis Of Variance
- DNA
Deoxyribonucleic Acid
- PCR
Polymerase Chain Reaction
- PCoA
The Principal Coordinates Analysis
- NMDS
Nonmetric Multidimensional Scaling
- PLS‒PM
Partial Least Squares Path Modelling
- NCBI
National Center for Biotechnology Information
- SRA
Sequence Read Archive
Author contributions
The idea and guidance of the study came from S.H. Material preparation, data collection, and analysis were done by S.L, while A.Y, R.L and M.W were involved in helping with the chemical property determination process. The first draft of the manuscript was written by S.L and S.H, whereas M.L and P.Y all commented on earlier versions of the manuscript. All authors read and approved the final manuscript.
Funding
National Key R&D Program of China (2022YFD1300803); National Natural Science Foundation of China (32071878).
Data availability
The 16S rRNA and ITS gene sequences of bacteria and fungi used this manuscript were submitted to the NCBI SRA databas (https://www.ncbi.nlm.nih.gov/, accessed on 27 February 2024), and the accession numbers are PRJNA1081141 and PRJNA1081170.
Declarations
Ethics approval and consent to participate
All methods were carried out in compliance with local and national regulations. Clinical trial number: not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dzvene AR, Tesfuhuney WA, Walker S, Ceronio G. Management of cover crop intercropping for live mulch on plant productivity and growth resources: a review. Air Soil Water Res. 2023;16:1–12. [Google Scholar]
- 2.Chinta YD, Araki H. Responses of bulk and rhizosphere soil microbiomes to different cover crop inputs and their connection and contribution to soil fertility and plant growth. Pedobiologia. 2023;101:150907. [Google Scholar]
- 3.Bukovsky-Reyes S, Isaac ME, Blesh J. Effects of intercropping and soil properties on root functional traits of cover crops. Agr Ecosyst Environ. 2019;285:106641. [Google Scholar]
- 4.Dobbratz M, Baker JM, Grossman J, Wells MS, Ginakes P. Rotary zone tillage improves corn establishment in a kura clover living mulch. Soil till Res. 2019;189:229–35. [Google Scholar]
- 5.Brooker AP, Renner KA, Basso B. Interseeding cover crops in corn: establishment, biomass, and competitiveness in on-farm trials. Agron J. 2020;112:3733–43. [Google Scholar]
- 6.Ogunleye AM, Opoku A, Solomon JKQ, Payne W. Cool-season cover crop effects on forage productivity and short-term soil health in a semi-arid environment. Renew Agr Food Syst. 2023;38:e26. [Google Scholar]
- 7.Chen BS. Cultivation of forage grasses and forage crops. China Agricultural Science and Technology; 2001.
- 8.Cuartero J, Pascual JA, Vivo JM, Özbolat O, Sánchez-Navarro V, Egea-Cortines M, Zornoza R, Mena MM, Garcia E, Ros M. A first-year melon/cowpea intercropping system improves soil nutrients and changes the soil microbial community. Agr Ecosyst Environ. 2022;328:107856. [Google Scholar]
- 9.Wu Y, Chen WJ, Li Q, Guo ZQ, Li YZ, Zhao ZW, Zhai JY, Liu GB, Xue S. Ecoenzymatic stoichiometry and nutrient limitation under a natural secondary succession of vegetation on the Loess Plateau, China. Land Degrad Dev. 2021;32:399–409. [Google Scholar]
- 10.Ren YY, Wang XL, Zhang SQ, Palta JA, Chen YL. Influence of spatial arrangement in maize-soybean intercropping on root growth and water use efficiency. Plant Soil. 2017;415:131–44. [Google Scholar]
- 11.Chen N, Li XY, Simunek J, Shi HB, Zhang YH, Hu Q. Quantifying inter-species nitrogen competition in the tomato-corn intercropping system with different spatial arrangements. Agr Syst. 2022;201:103461. [Google Scholar]
- 12.Bitew Y, Derebe B, Worku A, Chakelie G. Response of maize and common bean to spatial and temporal differentiation in maize-common bean intercropping. PLoS ONE. 2021;16:e0257203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun BR, Peng Y, Yang HY, Li ZJ, Gao YZ, Wang C, Yan YL, Liu YM. Alfalfa (Medicago sativa L.)/maize (Zea mays L.) intercropping provides a feasible way to improve yield and economic incomes in farming and pastoral areas of northeast China. PLoS ONE. 2014;9(10):e110556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su KQ, Mu L, Zhou T, Kamran M, Yang HM. Intercropped alfalfa and spring wheat reduces soil alkali-salinity in the arid area of northwestern China. Plant Soil. 2022;499:275–92. [Google Scholar]
- 15.Zhang G, Zhang C, Yang Z, Dong S. Root distribution and N acquisition in an alfalfa and corn intercropping system. J Agr Sci. 2013;5:128. [Google Scholar]
- 16.Fan Y, Wang Z, Liao D, Raza MA, Wang B, Zhang J, Yang F. Uptake and utilization of nitrogen, phosphorus and potassium as related to yield advantage in maize-soybean intercropping under different row configurations. Sci Rep. 2020;10(1):9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang W, Li MY, Zhou R, Zhu SG, Tao HY, Khan A, Uzamurera AG, Wang BZ, Jin JM, Ma Y, Li WB, Tao XP, Xiong YC. Effects of interspecific interactions on soil carbon emission and efficiency in the semiarid intercropping systems. Soil till Res. 2023;234:105857. [Google Scholar]
- 18.Chen GD, Zhai YL, Zhou JG, Li YF, Lin J, Wan SM, Wu QZ. Optimizing maize belt width enhances productivity in wheat/maize intercropping systems. Sustainability-Basel. 2022;14:16137. [Google Scholar]
- 19.Wu K, Jiang C, Zhou S, Yang H. Optimizing arrangement and density in maize and alfalfa intercropping and the reduced incidence of the invasive fall armyworm (Spodoptera frugiperda) in southern China. Field Crops Res. 2022;287:108637. [Google Scholar]
- 20.Cheiere T, Lorin M, Corre-Hellou G. Choosing the right associated crop species in soybean-based intercropping systems: using a functional approach to understand crop growth dynamics. Field Crops Res. 2023;298:108964. [Google Scholar]
- 21.Wang GH, Sheng LC, Zhao D, Sheng JD, Wang XR, Liao H. Allocation of nitrogen and carbon is regulated by nodulation and mycorrhizal networks in soybean/maize intercropping system. Front Plant Sci. 2016;7:226662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang M, Wu Y, Zhao QF, Jiang YK, Liu GB, Sun W, Ma LH, Xue S. Secondary vegetation succession on the Loess Plateau altered the interaction between arbuscular mycorrhizal fungi and nitrogen-fixing bacteria. For Ecol Manag. 2023;530:120744. [Google Scholar]
- 23.Wang JC, Wang J, He JZ, Zhu YG, Qiao NH, Ge Y. Arbuscular mycorrhizal fungi and plant diversity drive restoration of nitrogen-cycling microbial communities. Mol Ecol. 2021;30:4133–46. [DOI] [PubMed] [Google Scholar]
- 24.Chamkhi I, Cheto S, Geistlinger J, Zeroual Y, Kouisni L, Bargaz A, Ghoulam C. Legume-based intercropping systems promote beneficial rhizobacterial community and crop yield under stressing conditions. Ind Crop Prod. 2022;183:114958. [Google Scholar]
- 25.Pan JW, Wu HL, Xiang WH, Ouyang S, Chen L, Zeng YL, Deng XW, Zhao ZH, Zeng WX, Kuzyakov Y. Soil microbial richness and community composition are primarily mediated by functional trait diversity of fine roots in subtropical forests. Plant Soil. 2023;497:485–501. [Google Scholar]
- 26.Wei SN, Jeong EC, Li YF, Kim HJ, Ahmadi F, Kim JG. Evaluation of forage production, feed value, and ensilability of proso millet (L). J Anim Sci Technol. 2022;64:38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liao HK, Zheng CL, Li J, Long J. Dynamics of soil microbial recovery from cropland to orchard along a 20-year chronosequence in a degraded karst ecosystem. Sci Total Environ. 2018;639:1051–9. [DOI] [PubMed] [Google Scholar]
- 28.Sato K, Suyama Y, Saito M, Sugawara K. A new primer for discrimination of arbuscular mycorrhizal fungi with polymerase chain reaction-denature gradient gel electrophoresis. Grassl Sci. 2005;51:179–81. [Google Scholar]
- 29.Poly F, Monrozier LJ, Bally R. Improvement in the RFLP procedure for studying the diversity of nifH genes in communities of nitrogen fixers in soil. Res Microbiol. 2001;152:95–103. [DOI] [PubMed] [Google Scholar]
- 30.Bolyen E, Rideout JR, Dillon MR, Bokulich NA, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019;37:1091–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13:581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katoh K, Misawa K, Kuma K, Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2022;30:3059–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu M, Li S, Bai J, Wang K, Qu Y, Long M, Yang P, Hu T, He S. Arbuscular mycorrhizal fungi and diazotrophic diversity and community composition responses to soybean genotypes from different maturity groups. Agron. 2023;13(7):1713. [Google Scholar]
- 34.Aguilera-Huertas J, Parras-Alcántara L, González-Rosado M, Lozano-García B. Intercropping in rainfed Mediterranean olive groves contributes to improving soil quality and soil organic carbon storage. Agr Ecosyst Environ. 2024;361:108826. [Google Scholar]
- 35.Nasar J, Shao Z, Arshad A, Jones FG, Liu S, Li C, Gao Q. The effect of maize–alfalfa intercropping on the physiological characteristics, nitrogen uptake and yield of maize. Plant Biol. 2020;22(6):1140–9. [DOI] [PubMed] [Google Scholar]
- 36.Sun L, Dong X, Wang Y, Maker G, Agarwal M, Ding Z. Tea-soybean intercropping improves tea quality and nutrition uptake by inducing changes of rhizosphere bacterial communities. Microorganisms. 2022;10(11):2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bielenberg H, Clark JD, Sanyal D, Wolthuizen J, Karki D, Rahal A, Bly A. Cover crop composition in long-term no-till soils in semi-arid environments do not influence soil health measurements after one year. Soil Sc Soc Am J. 2023;87:528–40. [Google Scholar]
- 38.Silva LS, Laroca JVD, Coelho AP, Gonsalves EC, Gomes RP, Pacheco LP, et al. Does grass-legume intercropping change soil quality and grain yield in integrated crop-livestock systems? Appl Soil Ecol. 2022;170:104257. [Google Scholar]
- 39.Zheng W, Gong QL, Zhao ZY, Liu J, Zhai BN, Wang ZH, Li ZY. Changes in the soil bacterial community structure and enzyme activities after intercrop mulch with cover crop for eight years in an orchard. Eur J Soil Biol. 2018;86:34–41. [Google Scholar]
- 40.Cong WF, Hoffland E, Li L, Six J, Sun JH, Bao XG, Zhang FS, van der Werf W. Intercropping enhances soil carbon and nitrogen. Global Change Biol. 2015;21:1715–26. [DOI] [PubMed] [Google Scholar]
- 41.Koskey G, Avio L, Turrini A, Sbrana C, Bàrberi P. Durum wheat-lentil relay intercropping enhances soil mycorrhizal activity but does not alter structure of arbuscular mycorrhizal fungal community within roots. Agr Ecosyst Environ. 2023;357:108696. [Google Scholar]
- 42.Qiu YP, Guo LJ, Xu XY, Zhang L, Zhang KC, Chen MF, Zhao YX, Burkey KO, Shew HD, Zobel RW, Zhang Y, Hu SJ. Warming and elevated ozone induce tradeoffs between fine roots and mycorrhizal fungi and stimulate organic carbon decomposition. Sci Adv. 2021;7(28):eabe9256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.García-González I, Martínez‐García LB, Barel JM, Martens H, Snoek LB, Hontoria C, De Deyn GB. Cover crop identity determines root fungal community and arbuscular mycorrhiza colonization in following main crops. Eur J Soil Sci. 2023;74(6):e13427. [Google Scholar]
- 44.Newberger DR, Minas IS, Manter DK, Vivanco JM. Shifts of the soil microbiome composition induced by plant-plant interactions under increasing cover crop densities and diversities. Sci Rep. 2023;13(1):17150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giusti B, Hogue R, Jeanne T, Lucotte M. Impacts of winter wheat and cover crops on soil microbial diversity in a corn-soybean no-till cropping system in Quebec (Canada). Agrosys Geosci Env. 2023;6(1):e20349. [Google Scholar]
- 46.Erhunmwunse AS, Mackowiak CL, Blount ARS, Dubeux JJr, Ogram A, Liao HL. Short-term perennial peanut integration into bahiagrass system influence on soil microbial-mediated nitrogen cycling activities and microbial co-occurrence networks. Eur J Soil Biol. 2023;119:103566. [Google Scholar]
- 47.Xiao D, He XY, Xu ZH, Bai SH, Zhang W, Hu PL, et al. Strong cooperations among diazotroph and arbuscular mycorrhizal fungi taxa promote free-living nitrogen fixation at soil-rock mixing layer. Geoderma. 2023;437:116600.
- 48.Veresoglou SD, Verbruggen E, Makarova O, Mansour I, Sen R, Rillig MC. Arbuscular mycorrhizal fungi alter the community structure of ammonia oxidizers at high fertility via competition for soil NH4+. Microb Ecol. 2019;78:147–58. [DOI] [PubMed] [Google Scholar]
- 49.Nyawade SO, Karanja NN, Gachene CKK, Gitari HI, Schulte-Geldermann E, Parker ML. Short-term dynamics of soil organic matter fractions and microbial activity in smallholder potato-legume intercropping systems. Appl Soil Ecol. 2019;142:123–35. [Google Scholar]
- 50.Chen XD, Wang Y, Wang JH, Condron LM, Guo B, Liu JL, Qiu GY, Li H. Impact of ryegrass cover crop inclusion on soil phosphorus and pqqc-and phod-harboring bacterial communities. Soil till Res. 2023;234:105823. [Google Scholar]
- 51.Yu L, Tang Y, Wang Z, Gou Y, Wang J. Nitrogen-cycling genes and rhizosphere microbial community with reduced nitrogen application in maize/soybean strip intercropping. Nutr Cycl Agrosyst. 2019;113(1):35–49. [Google Scholar]
- 52.Sun T, Li ZZ, Wu Q, Sheng TT, Du MY. Effects of alfalfa intercropping on crop yield, water use efficiency, and overall economic benefit in the Corn Belt of Northeast China. Field Crops Res. 2018;216:109–19. [Google Scholar]
- 53.Qiu LP, Zhang Q, Zhu HS, Reich PB, Banerjee S, van der Heijden MGA, et al. Erosion reduces soil microbial diversity, network complexity and multifunctionality. ISME J. 2021;15:2474–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang XY, Jia J, Chen LT, Chu HY, He JS, Zhang YJ, Feng XJ. Aridity and NPP constrain contribution of microbial necromass to soil organic carbon in the Qinghai-Tibet alpine grasslands. Soil Biol Biochem. 2021;156:108213. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The 16S rRNA and ITS gene sequences of bacteria and fungi used this manuscript were submitted to the NCBI SRA databas (https://www.ncbi.nlm.nih.gov/, accessed on 27 February 2024), and the accession numbers are PRJNA1081141 and PRJNA1081170.