Abstract
The integration of phytochemistry into forensic science has emerged as a groundbreaking frontier, providing unprecedented insights into nature's secrets through the precise application of phytochemical fingerprinting of phytotoxins as a cutting-edge approach. This study explores the dynamic intersection of phytochemistry and forensic science, highlighting how the unique phytochemical profiles of toxic plants and their secondary metabolites, serve as distinctive markers for forensic investigations. By utilizing advanced techniques such as Ultra-High-Performance Liquid Chromatography (UHPLC) and High-Resolution Mass Spectrometry (HRMS), the detection and quantification of plant-derived are made more accurate in forensic contexts. Real-world case studies are presented to demonstrate the critical role of plant toxins in forensic outcomes and legal proceedings. The challenges, potential, and future prospects of integrating phytochemical fingerprinting of plant toxins into forensic science were discussed. This review aims to illuminate phytochemical fingerprinting of plant toxins as a promising tool to enhance the precision and depth of forensic analyses, offering new insights into the complex stories embedded in plant toxins.
Keywords: Forensic phytochemistry, Phytochemical fingerprinting, Plant toxins, Advanced chromatography
Introduction
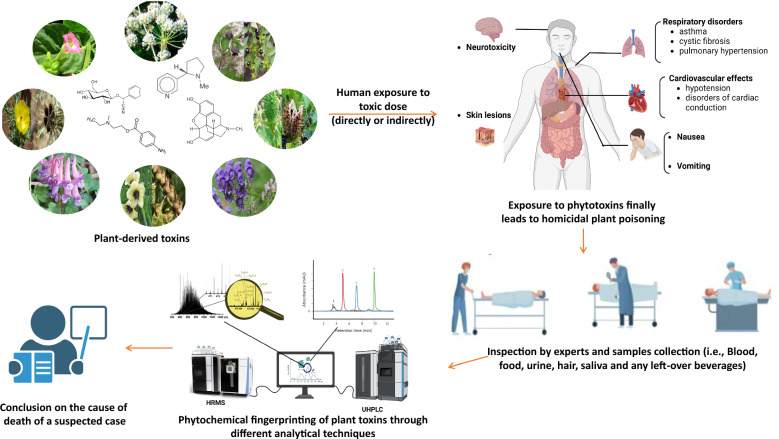
In the complex field of forensic science, a new discipline has emerged that intertwines botany and criminal investigation—forensic phytochemistry [1]. This growing field leverages plant-based evidence to illuminate mysteries that often evade traditional investigative techniques [2]. As we delve into the intersection of nature and crime, the importance of forensic phytochemistry becomes increasingly clear [3]. The exploration of phytochemical fingerprints emerges as a promising, cutting-edge method for unraveling the mysteries hidden within the toxic plants that populate our environment. Unlike traditional forensic analyses that primarily focus on human-derived evidence, forensic phytochemistry expands the investigative scope by incorporating the distinct chemical fingerprints imprinted on plant matter [4]. The field of forensic botany has been recognized since the mid-1900s, specifically when Arthur Koehler presented wood evidence in a court setting during the trial of the Charles Lindbergh baby kidnapping case [5].
The utilization of plants at both microscopic and macroscopic levels such as their organs (including leaves, stems, seeds, fruits, and roots), tissues (such as pollen grains, spores, fibers, and cork), and even their chemical components (including secondary metabolites, isotopes, DNA, and starch grains) as forensic evidence has been practiced since ancient times [6]. The Plantae kingdom encompasses approximately 400,000 species, with an additional 2,000 species documented annually, spans terrestrial and aquatic ecosystems and consist of multicellular, eukaryotic, and autotrophic organisms [7]. From leaves and pollen to seeds and extracts, plants carry a silent witness to their surroundings and interactions, becoming invaluable tools in the pursuit of justice [8–10]. Plant toxins are natural secondary metabolites produced by plants as a defense mechanism against herbivores, pathogens, and competing plants [11, 12]. While these toxins play an essential role in protecting plants, they can pose significant risks to humans when consumed or upon contact. Plant toxins vary widely in their chemical nature and effects on human health. Organic compounds, including alkaloids, terpenes, flavonoids, tannins, cardiac and cyanogenic glycosides, proanthocyanidins, phenylpropanoids, lignans, nitrogen-containing compounds, resins, oxalates, and certain proteins or amino acids, represent the main poisonous substances found in plants [12]. The release of these toxic biological agents is even considered as a bioterrorism [11]. Additionally, some poisonous plants are capable of accumulating inorganic compounds from the soil.
This study addresses the critical need for more refined and advanced methodologies in plant toxin analysis by exploring phytochemical fingerprinting. By gaining a comprehensive understanding of the unique chemical profiles of toxic plants, forensic scientists can better attribute toxins to specific botanical sources. From analyzing botanical fingerprints to examining real-world case studies, we navigate the intricate intersection of nature's secrets and forensic investigation, reshaping the landscape of criminal justice [13]. Unlocking the secrets encoded in phytochemical fingerprints not only enhances our understanding of toxic plant species but also contribute to more accurate and reliable forensic investigations. The potential implications are broad, with applications in criminal cases, environmental monitoring, and emergency response scenarios. This review utilizes state-of-the-art chromatographic and spectroscopic techniques, including Ultra-High-Performance Liquid Chromatography (UHPLC) and High-Resolution Mass Spectrometry (HRMS) to develop detailed phytochemical fingerprints. By integrating these advanced analytical methods, we aim to provide forensic scientists with a comprehensive and efficient tool for forensic scientists in the identification and analysis of plant toxins.
Literature screening and data extraction
Inclusion criteria
Scientific Credibility: Priority was given to papers published in peer-reviewed journals recognized in the fields of forensic science and botany.
Subject Relevance: Studies directly addressing phytotoxins and their applications in forensic science were selected.
Publication Date: Recent publications were prioritized to ensure up-to-date information; however, foundational classic references were also included.
Exclusion criteria
Low-Quality Studies: Papers lacking reliable data or presenting unsupported experimental results were excluded.
Irrelevance: Studies not directly related to the topic, such as those focusing on areas far removed from plant toxins or forensic science, were omitted.
Overly Analytical Focus: References addressing purely analytical topics without practical applications in forensic science were not included.
Non-Refereed Sources: Conference papers that were not peer-reviewed were excluded.
The selection procedure for the references used in this research is summarized as follows:
Assimilation
Keywords: forensic phytochemistry, phytochemical fingerprinting, plant toxins, advanced chromatography.
Sources: PubMed, Scopus, Google Scholar, Web of Science.
Publisher Websites: Elsevier, Wiley, Springer, Royal Society of Chemistry (RSC).
Article Types: Research papers, case reports, books, and reviews (Total: 234).
Screening:
Exclusions (n = 37): Duplicated publications (n = 12), studies not meeting selection criteria (n = 18), and articles unavailable for access (n = 7).
Suitability:
Final database (n = 197): Research papers (n = 86), case reports (n = 6), books (n = 36), reviews (n = 69).
Historical cases of plant poisoning
Forensic phytochemistry had made a significant impact beyond theoretical applications, demonstrated in real-world case studies that underscore its utility in criminal investigations [14, 15]. These cases vividly demonstrate how plant-based evidence can be a pivotal in solving crimes, offering a unique intersection between nature and forensic science. Throughout history, numerous cases of plant poisoning incidents have showcased the forensic importance of plant-related toxicology. Below are several notable historical incidents:
Case study 1: the poisonous garden
In a high-profile poisoning case, forensic phytochemistry played a central role when investigators examined the victim's surroundings [14]. Analysis of plant samples from the crime scene revealed presence of toxic compounds unique to a rare plant species. This finding not only identified the poisonous agent but also traced it to a specific geographical origin [16], ultimately helping to apprehend the perpetrator. Plant-derived toxins have been used by criminals in cases involving homicide, theft, and rape cases. Toxicity can manifest in different forms, including skin irritation from direct contact, or internal poisoning through ingestion, absorption through the dermal layer or inhalation.
Case study 2: Socrates’ death
The ancient Greek philosopher Socrates was sentenced to death in 399 BC by ingesting a cup of hemlock [17]. Hemlock (Conium maculatum) contains the potent neurotoxin coniine, which led to respiratory paralysis followed by Socrates' eventual death. This case is a well-known example of the intentional use of a plant toxin for execution.
Case study 3: the Borgia family poisonings
The notorious Borgia family, active during the Italian Renaissance, was rumored to have used various toxic plants for political assassinations [18]. Nightshade (Atropa belladonna) which contain the alkaloid atropine, was one of the substances suspected to be involved. These poisonings highlight the historical use of plant toxins in political intrigue.
Case study 4: locoweed poisoning in livestock
In North America, particularly in the western regions, locoweeds (Astragalus and Oxytropis genera) are known to cause poisoning in livestock [19]. Consumption of locoweeds by cattle and horses leads to a condition known as locoism, characterized by neurological symptoms. Forensic investigation into livestock deaths often focus on identifying the specific locoweed species responsible.
Case study 5: water Hemlock poisonings
Water hemlock (Cicuta species) is considered one of the most toxic plants in North America [20]. Several accidental ingestions have resulted in poisoning. Notable victims include explorers Meriwether Lewis and Christopher McCandless. Forensic examination in such cases involve identifying the plant and its toxic components.
Case study 6: Foxglove poisonings
Foxglove (Digitalis purpurea) contains cardiac glycosides, which can be fatal if ingested in large quantities [21]. Historical cases include both accidental ingestions and intentional poisonings. The digitalis compounds have medicinal uses, but improper administration can be dangerous. Forensic analysis helps detect the presence of foxglove toxins in the body.
Case study 7: Cassava cyanide poisoning
Cassava (Manihot esculenta) contains cyanogenic glycosides, which can release hydrogen cyanide when metabolized [22]. In regions where cassava is a dietary staple, cases of cyanide poisoning have occurred due to improper processing or consumption of inadequately prepared cassava products. Forensic investigation in these instances involve detecting cyanide in post-mortem samples.
These historical cases underscore the critical role of plant-related toxicology in forensic investigations. Identifying specific plant toxins, understanding their mechanisms of action, and distinguishing between accidental and intentional poisonings are essential aspects of forensic analysis. Advances in analytical techniques over time have improved the detection and quantification of plant toxins, enhancing to the field of forensic toxicology.
Toxic plants in forensic pathology
Toxic plants can play a significant role in forensic pathology, often influencing the determination of the cause of death [14]. These plants pose diverse challenges in forensic pathology, ranging from accidental poisonings to intentional acts of self-harm or homicide. Forensic pathologists, in collaboration with toxicologists and phytochemists, play a crucial role in identifying and understanding the implications of plant toxins during postmortem examinations. Comprehensive investigations and detailed documentation are essential to uncover the role of toxic plants in the cause of deaths and contribute to inform legal and public health outcomes. Below are several implications and considerations when dealing with toxic plants in forensic pathology:
Accidental poisoning
Ingestion of Toxic Plants Accidental ingestion of toxic plants, especially by children or individuals unfamiliar with their toxicity, can lead to poisoning [23]. Forensic pathologists must evaluate the possibility of accidental ingestion when determining the cause of death.
Suicidal poisoning
Plant Toxins as Suicide Means Some individuals may intentionally ingest toxic plants as a method of suicide [24]. The discovery of plant toxins in postmortem examinations raises questions about the intent behind the poisoning, requiring a detailed investigation.
Homicidal poisoning
Use of Plant Toxins in Homicides In certain cases, plant toxins may be used as a means of homicide [25]. Forensic pathologists need to identify patterns that suggest intentional poisoning, including the presence of specific plant toxins.
Identification of plant toxins
Challenges in Identification Identifying plant toxins in postmortem samples can be challenging due to factors such as postmortem changes, toxin degradation, and the need for specialized analytical techniques [26].
Collaboration with Toxicologists Forensic pathologists often collaborate with forensic toxicologists who employ advanced analytical methods to identify and quantify plant toxins in biological samples.
Postmortem changes and decomposition
Effect on Toxin Detection Postmortem changes and decomposition processes can affect the detection of plant toxins [27]. Early postmortem examinations are preferred to minimize these challenges and obtain more accurate results.
Clinical presentation vs. Autopsy findings
Discrepancies in Symptoms In cases of plant poisoning, there may be discrepancies between the clinical symptoms reported before death and the autopsy findings [28]. This emphasizes the importance of comprehensive toxicological analyses to confirm the presence of plant toxins.
Histopathological examination
Organ Damage Plant toxins may cause specific histopathological changes in organs [29]. Histopathological examination of tissues can provide additional evidence about the effects of plant toxins on the body and assist in determining the cause of death.
Documentation and case history:
Detailed Case Documentation Forensic pathologists must meticulously document the circumstances surrounding the death, including any information related to the ingestion or exposure to toxic plants [30]. This documentation is crucial for conducting comprehensive forensic analyses.
Collaboration with phytochemists and public health concerns:
Collaboration with phytochemists becomes essential when dealing with plant-related poisonings. Botanists can help identify specific plant species and provide insights into the toxicity levels. The discovery of toxic plants in forensic pathology raises public health concerns, as identifying poisonous plants and assessing their potential risks can lead to preventive measures against accidental poisonings [31].
Legal implications
Forensic Testimony Forensic pathologists may be required to provide expert testimony in legal proceedings [32]. Clear communication of findings, including the presence of plant toxins and their role in the cause of death, is crucial for legal clarity.
Toxic principles of plant origin
The study of plant toxins in forensic science involves the identification, analysis, and interpretation of toxic substances derived from plants, particularly in the context of criminal investigations or legal proceedings [33, 34]. Phytotoxins (plant derived toxic principles) play a significant role in forensic cases related to poisoning, environmental crimes, and even biological warfare. It's important to note that the toxicity of plant compounds varies widely, and many plants that produce toxins are harmless when consumed in moderation or properly processed. Additionally, some plants with toxic components have been historically used in traditional medicine for therapeutic purposes. Plant toxins are systematically categorized into four distinct classes based on their lethal oral dose as ascertained through rat experimentation: class Ia: extremely hazardous (LD50 of 5 mg or less per kg of body weight); class Ib: highly hazardous (LD50 of 5 to 50 mg/kg of body weight); class II: moderately hazardous (LD50 of 50 to 500 mg/kg of body weight); and class III: slightly hazardous (LD50 of 500 mg and above per kg of body weight) [35, 36]. Consequently, proper identification and understanding of these toxins are crucial for ensuring safety and avoiding adverse effects. Forensic plant toxins can be classified into different categories based on their chemical nature and the effects they have on the human body.
Alkaloids
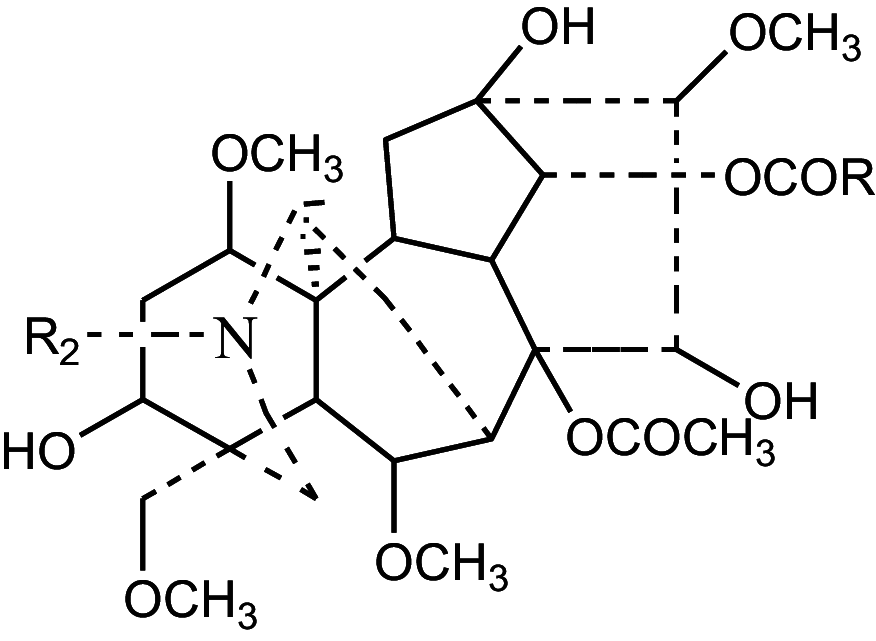
Alkaloids are naturally occurring organic compounds that contain basic nitrogen atoms with diverse structural forms. Some alkaloids have stimulant properties, while others are toxic to the nervous system, potentially leading to poisoning or even death at higher doses [37, 38]. Plant-derived toxic alkaloids of forensic significance belong to various classes, each characterized by unique structural features and toxicity profiles. Table. 1 provides an overview of the chemistry and occurrence of plant-derived toxic alkaloids that are employed as toxicological markers in forensic science.
Table 1.
The chemistry and occurrence of plant-derived toxic alkaloids of forensic significance
| Chemical nature | Toxicological marker | Natural source | Toxicity class* | References | ||
|---|---|---|---|---|---|---|
| Hazardous plant parts | Toxic plant species | Plant family | ||||
Pyridine alkaloids 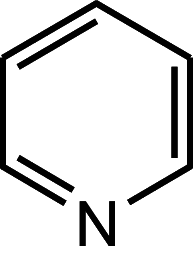
|
Nicotine Cotinine |
Leaves |
Tobacco plant Nicotiana tabacum |
Solanaceae | Ib | [37] |
Piperidine alkaloids 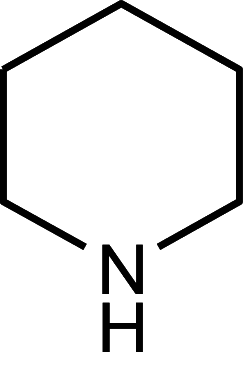
|
Anabasine Anatabine Nornicotine |
Leaves |
Tobacco plant Nicotiana glauca |
Solanaceae | Ib | [119] |
|
Coniine N-methyl-coniine Coniceine γ-coniceine Cicutoxin Conhydrine Pseudoconhydrine |
Leaves Stems Seeds |
Poison-hemlock Conium maculatum |
Apiaceae | Ia | [120] | |
|
Sedamine Sedinine |
All parts | Common stonecrop Sedum acre | Crassulaceae | II | [121] | |
Pyrrolizidine alkaloids 
|
Symphytine Echimidine Allantoin |
All parts Roots |
Comfrey Symphytum officinale |
Boraginaceae | II | [122] |
| Senecionine |
All parts especially Flowers |
Tansy ragwort Senecio jacobaea |
Asteraceae | II | [123] | |
| Lycopsamine | All parts |
Common bugloss Anchusa officinalis |
Boraginaceae | II | [124] | |
| Colchicine |
All parts, especially seeds, bulbs |
Autumn crocus Colchicum autumnale |
Liliaceae | Ia | [125] | |
|
Temuline Loline |
All parts |
Poison darnel Lolium temulentum |
Poaceae | Ib-II | [126] | |
Quinolizidine alkaloids 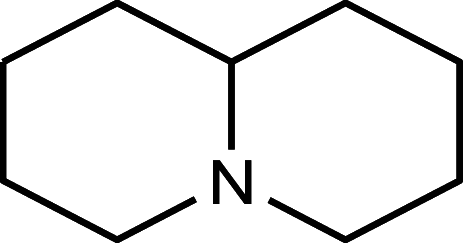
|
Nupharine Desoxynupharidine |
All parts |
yellow pond lily Nuphar lutea |
Nymphaeaceae | II | [127] |
|
Lupanine Sparteine |
Seeds |
Lupin Lupinus polyphyllus Broom Cytisus scoparius |
Fabaceae |
Ib-II II |
[128] | |
|
N-methylcytisine Cytisine |
All parts especially Seeds |
Spanish broom Spartium junceum |
Fabaceae | Ib | [129] | |
| Anagyrine | All parts |
Dyer's greenweed Genista tinctoria |
Fabaceae | [130] | ||
|
Benzophenanthridine alkaloids
|
Chelidonine Chelerythrine |
All parts, reddish latex |
Celandine Chelidonium majus |
Papaveraceae | Ib-II | [131] |
| Sanguinarine | Seeds |
Prickly-poppy Argemone mexicana |
Papaveraceae | Ia | [132] | |
|
Benzylisoquinoline alkaloids
|
Protopine | Rhizomes Roots |
Three-leaf corydalis Corydalis ternata |
Papaveraceae | II | [132] |
|
Isoquinoline alkaloids
|
Berberine |
All parts, especially latex |
Goldenseal Hydrastis canadensis |
Ranunculaceae | II | [132] |
|
Oregon grape Berberis aquifolium |
Berberidaceae | II | ||||
| Rhoeadine Protopin |
Common poppy Papaver rhoeas |
Papaveraceae | II | [133] | ||
|
Galanthamine Lycorine Tazettine |
Bulbs |
Spring Snowflake Leucojum vernum |
Amaryllidaceae | II | [134] | |
|
Glaucine Magnoflorine Protopine Sanguinarine |
All parts |
yellow hornpoppy Glaucium flavum |
Papaveraceae | II | [135] | |
|
Bulbocapnine Protoberberine Apomorphine |
All parts, especially tubers |
Hollowroot Corydalis cava |
Papaveraceae | II | [136, 137] | |
|
Opium Morphine Codeine Thebaine Noscapine |
Latex of seeds |
The poppy plant Papaver somniferum |
Papaveraceae | Ib | [38] | |
|
Steroidal alkaloids
|
Buxine Cyclobuxine | Leaves |
Common box Buxus sempervirens |
Buxaceae | Ib | [138] |
|
Protoveratrine A Protoveratrine B Germerine Cyclopamine |
Roots |
False hellebore Veratrum album |
Melanthiaceae | Ia | [139] | |
|
Imperialine Tuliposide A Tulipalin A |
Bulbs |
Crown imperial Fritillaria imperialis |
Liliaceae | II | [140] | |
|
Tropane alkaloids
|
Atropine |
Leaves Flowers Roots Seeds |
Deadly nightshade Atropa belladonna |
Solanaceae | Ia | [119, 141] |
| Hyoscyamine |
Black henbane Hyoscyamus niger |
|||||
| Scopolamine |
jimsonweed Datura stramonium |
|||||
| Cocaine | Leaves |
Coca Plant Erythroxylum coca |
Erythroxylaceae | Ia | [142] | |
|
Terpene alkaloids
|
Aconitine Mesaconitine Hypaconitine Jesaconitine Napelline |
Roots |
Monkshood Aconitum species |
Ranunculaceae | Ia | [143, 144] |
|
Lycoctonine Delcosine |
Seeds |
Forking larkspur Consolida regalis |
Ranunculaceae | Ia | [145] | |
|
Delphinine Nudicauline Staphisine Ajacine |
Seeds |
Candle larkspur Delphinium elatum |
Ranunculaceae | Ib | [146] | |
|
Indole alkaloids
|
Strychnine Brucine Vomicine Protostrychnine Strychnine-N-oxide |
Seeds | Strychnos nux-vomica | Loganiaceae | Ia | [147] |
|
Ergotamine Ergotoxine |
Seeds | Ipomoea coerulea | Convolvulaceae | Ib | [148] | |
| Vincamine | Aerial parts |
Lesser periwinkle Vinca minor |
Apocynaceae | III | [149] | |
|
Indolizidine alkaloids
|
Swainsonine | Seeds Leaves Flowers |
Locoweed Astragalus molissimus |
Fabaceae | II | [150] |
|
Steroidal glycoalkaloids
|
Soladulcidine, Solanine, Solasodine, Chaconine |
Green fruits Leaves |
Potato Solanum tuberosum Tomato S. lycopersicum Black nightshade S. nigrum |
Solanaceae | Ib-II | [151] |
|
Tryptamine alkaloids
|
Psilocybin Psilocin Baeocystine |
Fruiting body |
Magic mushroom Psilocybe azurescens |
Strophariaceae | II | [152] |
|
β-keto-amphetamine alkaloids
|
Cathine Cathinone Norephedrine |
Leaves |
Khat plant Catha edulis |
Celastraceae | Ib | [153] |
* Class Ia: extremely hazardous; Ib: highly hazardous; II: moderately hazardous; III: slightly hazardous as adapted from Wink, 2009 [121]
Glycosides
Glycosides are compounds that consist of a sugar molecule linked to another non-sugar moiety (aglycone) such as cyanogenic glycosides (found in cassava), which release hydrogen cyanide upon metabolism, posing a significant toxicity risk, cardiac glycosides (found in oleander), which affect heart function, and glucosinolates (found in cruciferous vegetables), which can disrupt thyroid function. Another class, saponins, are amphipathic glycosides found in beans, asparagus, and some medicinal plants. Large quantities of saponins can disrupt cell membranes and may have hemolytic effects [39]. Different classes of glycosides of forensic significance are listed in Table 2.
Table 2.
The chemistry and occurrence of plant-derived toxic glycosides of forensic significance
| Chemical nature | Toxicological marker | Natural Source | Toxicity class* | References | ||
|---|---|---|---|---|---|---|
| Hazardous plant parts | Toxic plant species | Plant family | ||||
Cyanogenic Glycosides 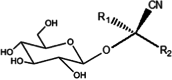
|
Aroin | Stems |
Arum cyrenaicum Arum italicum |
Araceae | Ia | [154] |
|
Amygdalin Prunasin |
Seeds |
Bitter almond Prunus dulcis |
Rosaceae | Ib-II | [155] | |
| Hydrocyanic acid | Roots |
Cassava Manihot esculenta Manihot utilissima |
Euphorbiaceae | Ib | [156] | |
| Linamarin Lotaustralin | [157] | |||||
| Dhurrin | grains |
Great Millet Sorghum bicolor |
Poaceae | Ib | [158] | |
|
Cardiac Glycosides (Steroidal Glycosides)
|
Cerberin | Seeds |
Suicide Tree Cerbera odollam |
Apocynaceae | Ia | [33, 159] |
|
Thevetin A, B Thevetoxin Neriifolin Peruviside Ruvoside |
Seeds |
Yellow Oleander Thevetia peruviana |
Apocynaceae | Ia | [160] | |
|
Calotoxin, Calotropins DI Calotropins DII |
Latex in Leaves Stem Bark Fruits |
Crown flower Calotropis gigantea |
Asclepiadaceae | Ia | [161] | |
|
Oleandrin Oleandrigenin |
Leaves Stems |
Oleander Nerium oleander |
Apocynaceae | Ia | [162] | |
|
Cardenolides (Digoxin-Digitoxin- Lanatoside) |
Leaves |
Foxglove Digitalis purpurea |
Plantaginaceae | Ia | [163] | |
| Convalatoxin | Leaves Flowers |
Lily of the valley Convallaria majalis |
Asparagaceae | Ib | [164] | |
| Adonitoxin | All parts |
Yellow Pheasant's Eye Adonis vernalis |
Ranunculaceae | Ib | [165] | |
| Proscillardin A | Bulbs |
Sea squill Scilla maritima |
Asparagaceae | Ib | [6] | |
| Hellebrim, Hellebrigenin | Rhizomes | Black hellebore Helleborus niger | Ranunculaceae | Ia | [166] | |
|
Alcoholic Glycosides
|
Salicin Salicilin |
Leaves Flowers Roots |
Primula spp. | Primulaceae | III | [167] |
|
Antraquinone Glycosides
|
Aloin A, B Antron Anthranol |
Latex in Leaves |
Aloe sp. | Liliaceae | II | [168] |
|
Hydroquinone Glycosides
|
Arbutin | All parts |
Leather bergenia Bergenia crassifolia |
Saxifragaceae | Ib | [169, 170] |
|
Saponin Glycosides
|
Tigonin | All parts | Digitalis lanata | Plantaginaceae | Ia | [171] |
| Gitonin | All parts | Digitalis purpurea | II | |||
| Triterpene saponins | Rhizomes Roots | Primula sp. | Primulaceae | II-III | [172] | |
| Saponariosides A, B | All parts | Saponaria officinalis | Caryophyllaceae | II | [173] | |
| Aescin (escin) | Seeds (Conkers) |
Horse chestnut Aesculus spp. |
Sapindaceae | II | [174] | |
| Taxine A,B,C Taxicin I, II | Leaves, Seeds Bark |
Yew plant Taxus spp. |
Taxaceae | Ia | [175] | |
|
Thioglycosides (Glucosinolates)
|
Gluco-tropeolin | Leaves |
Nasturtium Tropaeolum majus |
Tropaeolaceae | III | [176] |
| Sinigroside | Seeds |
Mustard Brassica sp. |
Brassicaceae | Ia | [177] | |
|
Triterpine glycosides
|
Passiflorine | Leaves |
Passion flower Passiflora edulis Passiflora incarnata |
Passifloraceae | II-III | [178] |
* Class Ia: extremely hazardous; Ib: highly hazardous; II: moderately hazardous; III: slightly hazardous as adapted from Wink, 2009 [121]
Proteins
Certain plant-derived proteins such as Toxalbumin and Mucunain, act as toxins by interfering with cellular functions or causing damage to organs [40, 41]. Lectins (e.g., ricin from castor beans and phytohemagglutinin from red kidney beans), can interfere with protein synthesis or cause agglutination of red blood cells, leading to toxicity [42]. Protease inhibitors (e.g., trypsin inhibitors from soybeans and chymotrypsin inhibitors from potatoes), can interfere with protein digestion, potentially causing nutrient deficiencies over time [43]. These proteins and other toxic compounds are detailed in Table 3. Additional classes of plant toxins including phenols, anthraquinones, Tannins, sesquiterpene, sesquiterpene lactones and cannabinoids are summarized in Table 4.
Table 3.
The chemistry and occurrence of plant-derived toxic proteins and amino acids of forensic significance
| Chemical nature | Toxicological marker | Natural Source | Toxicity class* | References | ||
|---|---|---|---|---|---|---|
| Hazardous plant parts | Toxic plant species |
Plant family | ||||
|
Proteins
|
Toxalbumin | Seeds |
Hondala Adenia palmata |
Passifloraceae | Ia | [41] |
| Mucunain | Seeds |
Velvet bean Mucuna prurita |
Fabaceae | Ib | [179] | |
Lectins 
|
Arbin | Seeds |
Rosary pea Abrus precatorius |
Fabaceae | Ia | [40] |
| Ricin | Seeds |
Castor bean Ricinus communis |
Euphorbiaceae | Ia | [42] | |
| Mistletoe | Leaves |
Mistletoe Viscum album |
Santalaceae | II | [180] | |
| Curcin | Seeds | Jatropha curcas | Euphorbiaceace | Ia | [181] | |
| Phytohemagglutinin | Seeds | Red kidney beans Phaseolus vulgaris | Fabaceae | II | [182] | |
| Crotine | All parts | Croton sp. | Euphorbiaceae | Ia | [183] | |
| Snow-drop lectine | Bulb |
Snowdrop Gallanthus nivalis |
Amaryllidaceae | II | [184] | |
Non-protein amino acid 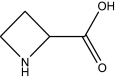
|
Azetidine 2-carboxylic acid | Roots | Convallaria majalis | Asparagaceae | Ib | [185] |
*Class Ia: extremely hazardous; Ib: highly hazardous; II: moderately hazardous as adapted from Wink, 2009 [121]
Table 4.
The chemistry and occurrence of other plant-derived toxins of forensic significance
| Chemical nature | Toxicological marker | Natural source | Toxicity class* | References | ||
|---|---|---|---|---|---|---|
| Hazardous plant parts | Toxic plant species | Plant family | ||||
|
Phenolic compounds
|
Hydroquinone | Seeds | Xanthium strumarium | Asteraceae | II | [186] |
| Salligenin | Roots | Primula sp. | Primulaceae | II | [187] | |
|
Benzopyran
|
Coumarins | Peel | Citrus sp. | Rutaceae | II | [188] |
| Chromones | Seeds | Angelica arhangelica | Apiaceae | II | [189, 190] | |
|
Flavonoids Flavan, Flavone Isoflavone Catechins |
All parts | Euphorbia sp. | Euphorbiaceae | II | [39, 128, 191] | |
|
Antraquinones
|
Hypericin | Flowers Leaves | Hypericum perforatum | Hypericaceae | II | [192] |
|
Sesquiterpene
|
Picrotoxin | Seeds |
Poison berry Anamirta cocculus |
Menispermaceae | Ib | [193] |
|
Sesquiterpene lactone
|
Parthenin |
Pollen Trichomes |
Parthenium Parthenium hysterophorus |
Asteraceae | II | [55] |
|
Carboxylic acid
|
Oxalic acid and its salt, Raphides |
Leaves Roots |
Dumb cane Dieffenbachia seguine |
Araceae | III | [194] |
|
Polyhydroxy alcohol
|
Tremetol | Leaves |
White snakeroot Ageratina altissima |
Asteraceae | Ib | [195] |
|
Polyketides
|
Cicutoxin | Roots |
Water hemlock Cicuta virosa |
Apiaceae | Ib | [196] |
|
Cannabinoids
|
Tetra-hydro Cannabinol |
Trichomes |
Marijuana Cannabis sativa |
Cannabaceae | Ib | [197] |
* Class Ib: highly hazardous; II: moderately hazardous; III: slightly hazardous as adapted from Wink, 2009 [121]
Phenols
Phenols are secondary metabolites produced by plants that serve as chemical defenses against herbivores, pathogens, and various environmental stressors. As plant toxins, phenols play a crucial role in deterring herbivores by affecting their digestion, growth, and overall health. These compounds can directly inhibit digestive enzymes, reducing nutrient absorption and making plant tissues less digestible [44]. This mechanism makes phenol-rich plants less palatable and nutritious for herbivores, which may deter them from repeated feeding.
Different types of phenols have distinct toxic effects. For example, tannins (a subset of phenols) can bind to dietary proteins, forming complexes that lower protein digestibility [45]. Some phenols can also interfere with cellular functions in herbivores, causing oxidative stress and cellular damage when ingested in large amounts [46, 47]. Moreover, phenols may cause bitter flavors or even adverse physiological responses, further protecting the plant. In essence, phenols function as natural toxins that allow plants to enhance their survival by reducing herbivory and protecting against disease, thereby contributing significantly to plant defense strategies.
Tannins
Tannins are phenolic compounds widely found in plants and play a key defensive role, acting as plant toxins against herbivores. Hydrolysable tannins and condensed tannins (proanthocyanidins) represent two distinct classifications within the realm of these compounds. The two categories exhibit notable differences in their nutritional properties and toxicological implications. Condensed tannins exert a more significant impact on digestibility reduction in comparison to hydrolysable tannins, whereas the latter may induce diverse toxicological responses due to hydrolysis occurring within the rumen [48]. Tannins reduce digestibility and can damage the digestive systems of animals that consume plants rich in these compounds by binding to dietary proteins and forming complex compounds that hinder their digestion and absorption. This binding inhibits digestive enzymes, such as pepsin, making tannin-rich plants less nutritious for ruminants [45, 49].
In this way, tannins serve as a natural plant toxin and a defensive strategy, deterring herbivores from fully benefiting from plant nutrients, and in some cases, causing toxicity or discouraging them from consuming tannin-rich plants.
Anthraquinones
Different anthraquinones such as Hypericin found primarily in Hypericum perforatum known for its phototoxic properties [50]. it can induce harmful effects when exposed to light, particularly ultraviolet (UV) light. When hypericin absorbs light, it can enter an excited state and generate reactive oxygen species (ROS). These highly reactive molecules can damage cellular components, including lipids, proteins, and DNA, leading to oxidative stress and cell death [51, 52].
Sesquiterpene
Sesquiterpenes such as Picrotoxin is derived from the seeds of the plant Anamirta cocculus. It is more widely recognized for its neurotoxic properties [53]. Picrotoxin is known to act as a non-competitive antagonist of the gamma-aminobutyric acid (GABA) receptor. GABA is a major inhibitory neurotransmitter in the central nervous system. By inhibiting GABA receptor function, picrotoxin disrupts normal neuronal signaling, leading to increased neuronal excitability and potential seizures [53, 54].
Sesquiterpene lactone
Such as Parthenin found primarily in Parthenium hysterophorus. It has gained attention not only for its phytotoxic and toxic effects on animals but also for its potential in vitro and in vivo genotoxicity [55]. Studies have shown that parthenin can induce DNA damage through the formation of reactive oxygen species (ROS), which can lead to oxidative stress and subsequent harm to DNA. Oxidative damage can result in single- and double-strand breaks, base modifications, and cross-linking of DNA strands [55]. Parthenin exposure has been associated with increased rates of chromosomal aberrations in cultured cells. These abnormalities can result from improper DNA repair mechanisms in response to damage, leading to further genomic instability [56, 57].
Cannabinoids
Cannabinoids are a diverse class of chemical compounds found primarily in the cannabis plant (Cannabis sativa), known for their psychoactive and therapeutic properties [58]. Among the various cannabinoids, tetrahydrocannabinol (THC) that binds to cannabinoid receptors in the brain and body, leading to a range of effects, including euphoria, altered perception, and relaxation [59]. Tetrahydrocannabinol (THC) exemplifies the dual nature of cannabinoids, as both a beneficial therapeutic agent and a potential plant toxin. Understanding its mechanisms of toxicity and the contexts in which these effects arise is essential for safe use, particularly in medical and recreational settings [60].
Phytochemical fingerprints: nature's unique identifier in forensic phytochemistry
Botanical fingerprints represent a groundbreaking concept in forensic phytochemistry, offering a distinctive and powerful means of identifying plant-based evidence. Analogous to human fingerprints, these botanical imprints capture unique chemical signatures of individual plant species, providing investigators with a powerful tool for tracing origins, movements, and connections involved in criminal investigations [2, 3].
Chemical diversity in plants
Plants produce a vast array of secondary metabolites, resulting in a wide range of chemical compounds [61, 62]. These include alkaloids, flavonoids, and terpenoids, which contribute to the distinct chemical profiles that characterize each plant species [8]. Forensic scientists utilize this chemical diversity to create phytochemical fingerprints, akin to the genetic code that differentiate each plant apart.
Geographical signatures
One of the remarkable aspects of phytochemical fingerprints is their ability to carry geographical information. Different regions yield distinct environmental conditions that influence the composition of plant secondary metabolites. By analyzing these chemical variations, investigators can deduce the geographic origin of plant samples. These insights can provide crucial information regarding crime scene locations or the movement of evidence [16, 63]. For example, chemical profiling of terpenoids and phytocannabinoids, can help to elucidate the geographic origin of confiscated marijuana [64]. In criminal investigations, these phytochemical fingerprints serve as a bridge linking crime scenes to suspects or connecting different crime scenes. A unique combination of plant compounds found at different locations can suggest a common origin or shared environmental factors, potentially tying together different aspects of an investigation.
Temporal dynamics
Phytochemical fingerprints are not static; they evolve over time and in response to environmental changes [65, 66]. This temporal aspect allows forensic experts to trace the age of plant materials, heling establish event timelines, corroborate alibis, or uncover long-buried evidence in historical cases.
Phytochemical fingerprinting techniques in plant toxin detection
Modern techniques used in phytochemical fingerprinting play a crucial role in the forensic detection and quantification of plant toxins. These methods provide accurate and sensitive measurements, helping forensic scientists to identify specific toxins, determine their concentrations, and establish connections between plant exposure and adverse health effects. Two of the most widely used techniques in plant toxin analysis are chromatography and mass spectrometry.
Chromatography
Chromatography is a separation technique that exploits the different affinities of compounds between a mobile phase (liquid or gas) and a stationary phase (solid or liquid) [67]. The separation is based on factors such as molecular size, polarity, or charge [68].
Gas Chromatography (GC): This technique is effective for analyzing volatile compounds [69], and plant toxins that can be vaporized without decomposition are often analyzed using GC.
Liquid Chromatography (LC): Suitable for a wide range of compounds, including non-volatile and polar substances [70]. High-Performance Liquid Chromatography (HPLC) is a common subtype used in plant toxin analysis.
Chromatography is commonly employed for separating and isolating plant toxins from complex matrices, such as biological samples (e.g., blood, urine) or plant materials.
Mass Spectrometry (MS)
Mass spectrometry is a technique that measures the mass-to-charge ratio of charged particles. It involves ionizing the sample and then analyzing the resulting ions based on their mass and charge [71, 72].
Gas Chromatography-Mass Spectrometry (GC–MS): Integrates the separation capabilities of GC with the sensitivity and specificity of MS [73]. It is commonly used for volatile plant toxins.
Liquid Chromatography-Mass Spectrometry (LC–MS): Combines the separation power of LC with the mass analysis of MS [74]. It is suitable for a broader range of compounds, including polar and non-volatile plant toxins.
Mass spectrometry is employed for the qualitative and quantitative analysis of plant toxins. It helps identify specific compounds by their mass spectra, providing valuable information about the molecular structure and aiding in the confirmation of toxin presence.
For instance, the aqueous extracts of ten plant samples; Tussilago farfara, Senecio vulgaris, Lithospermum officinale, Anchusa officinalis, Echium italicum, Heliotropium europaeum, Symphytum officinale, Eupatorium cannabinum, Borago officinalis and Petasites hybridus, collected from Orto botanicodellaScuola Medica Salernitana, located in Salerno, Italy, Salerno, Italy, were subjected to analysis by Ultra-high performance liquid chromatography-MS/MS (UPLC − MS/MS). This MS-based approach successfully identified 88 pyrrolizidine alkaloids (PAs) across a total of 282 samples, achieving an identification limit of 0.6 − 30 μg kg−1 [75].
Kalb et al., [76] employed MS-based methods to detect and quantify two protein toxins: ricin and RCA120. The developed method was successfully detected the two protein toxins in nine samples of castor bean plants. However, samples with a concentration of 0.414 ng/mL could not be detected. Thirty plant toxins belonged to five predominant groups of plant toxins, which include terpenoids, alkaloids, flavonoids, steroids and aromatic polyketides, were successfully detected using reversed phase liquid chromatography coupled with high-resolution mass spectrometry (LC − HRMS) with 40 times higher detection sensitivity than that of preceding techniques [77].
Nuclear magnetic resonance (NMR) spectroscopy
NMR spectroscopy measures the interactions between nuclear spins and an external magnetic field, providing information about the chemical structure and molecular dynamics of compounds [78]. In plant toxin analysis, NMR is used to confirm the chemical structure of identified compounds, complementing mass spectrometry by providing additional structural details [79]. NMR-based metabolic profiling techniques were employed to track changes in many plant metabolites for quality control and for detecting adulteration of products [80]. NMR spectroscopy was employed to describe the structures of large toxic plant proteins such as ricin from the castor bean plant [81]. The high-resolution 3D structure of viscotoxin A3, a phytotoxin found in from Viscum album L., has been determined in solution by 1H-NMR spectroscopy and deposited in the Protein Data Bank under the id. code 1ED0 [82].
Enzyme-linked immunosorbent assay (ELISA)
ELISA is an immunological technique that employs antibodies to detect the presence of specific antigens, including plant toxins [83]. It is often used as a rapid and cost-effective screening method for detecting the presence of plant toxins in samples. While it may not offer the same specificity of chromatography and mass spectrometry, ELISA serves as a valuable preliminary tool for large-scale analyses.
These analytical techniques, when used in combination, offer a comprehensive approach for plant toxin detection and quantification in forensic investigations. Advances in instrumentation, sensitivity, and data analysis methods continue to enhance the accuracy and reliability of these techniques.
Forensic investigation protocols
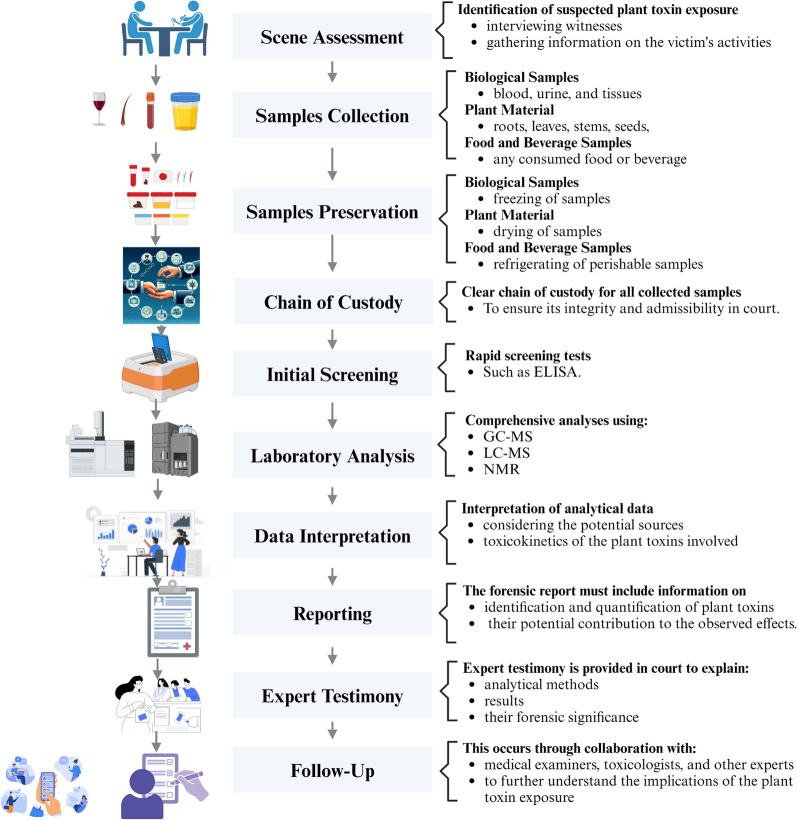
The forensic investigation of suspected plant toxin poisoning requires a systematic approach to ensure the proper collection, preservation, and analysis of relevant samples [84]. Below is a general protocol for conducting such forensic investigations and a flow chart highlighting these procedures is shown in Fig. 1:
Fig. 1.
Forensic investigation protocols: From samples collection till conclusion about the reasons of suspected death
Scene assessment
Identification of suspected plant toxin exposure involves interviewing witnesses, gathering information on the victim's recent activities, diet, and exposures and finally searching for the presence of plants or plant-based products in the environment that could be linked to poisoning.
Sample collection
Biological Samples: Collection of biological samples such as blood, urine, and tissues (particularly liver and kidney) from the deceased or affected individuals, both ante-mortem and post-mortem specimens for comparison.
Plant Material: Collection of samples of any suspected plants or plant-based products including roots, leaves, stems, seeds, or other parts that may contain toxins.
Food and Beverage Samples: Collection of any food or beverage consumed that are suspected to be contaminated with plant toxins, ensuring that they are preserved in clean, sealed containers.
Sample preservation
Biological Samples: Freeze samples intended for chemical analysis to prevent decomposition/degradation and store them in appropriate containers to avoid cross-contamination.
Plant Material: Dry plant samples for long-term preservation and store them in a cool, dry place away from direct sunlight.
Food and Beverage Samples: Refrigerate perishable samples and freeze or dry samples intended for long-term storage.
Chain of custody
Establishment and maintain a clear chain of custody for all collected samples. Document the handling, storage, and transfer of each sample to ensure its integrity and admissibility in court.
Initial screening
Use rapid screening tests, such as ELISA, to quickly detect the presence of plant toxins in samples. While these tests provide initial indications, they may lack the specificity of more advanced analytical methods.
Laboratory analysis: chromatography and mass spectrometry
Conduct a comprehensive analyses using GC–MS or LC–MS to identify and quantify specific plant toxins in biological and plant materials. Confirm the chemical structure of identified compounds using NMR spectroscopy, then compare the obtained results with control samples to ensure accuracy and reliability.
Data interpretation
Interpret the analytical data in the context of the case, considering the potential sources of exposure and toxicokinetic of the involved plant toxins.
Reporting
Prepare a detailed forensic report summarizing the findings, methods used, and conclusions. This forensic report should include information on the identification and quantification of plant toxins and their potential contribution to the observed effects.
Expert testimony
If required, provide expert testimony in court to explain the analytical methods, results, and their forensic significance.
Follow-up
Collaborate with medical examiners, toxicologists, and other experts to further investigate the implications of the plant toxin exposure. Also, monitor any potential public health concerns and implement preventive measures if necessary.
A comprehensive, well-documented forensic investigation including thorough sample collection, preservation, and analysis is essential for building a strong case in incidents involving suspected plant toxin poisoning. Collaboration among various experts and adherence to established protocols ensure the reliability of the findings in legal proceedings.
Challenges in identifying plant toxins in forensic phytochemistry
Identifying plant toxins during postmortem examinations is a complex process presents several challenges due to the dynamic nature of postmortem changes, including degradation and metabolic alterations [26, 27]. Thus, it requires careful consideration of these factors for accurate toxicological assessments in cases involving suspected plant toxin poisoning. Forensic experts must navigate these challenges, select appropriate analytical methods, and collaborate across disciplines to provide accurate and reliable results. Here are some key challenges:
Rapid postmortem changes
Autolysis and Putrefaction After death, enzymatic autolysis and microbial putrefaction can rapidly degrade tissues and substances present in the body [85], affecting the stability and detectability of plant toxins.
Metabolism
Postmortem Metabolism Metabolic processes can continue for some time after death, potentially altering the chemical structure of plant toxins [86]. Metabolites formed postmortem might differ from those produced in the living organism.
Biotransformation by microorganisms
Microorganisms present in the body can further metabolize plant toxins, complicating the identification process [87, 88].
Sampling challenges
Tissue Availability The availability of intact tissues for analysis may be limited due to autolysis and putrefaction, making it challenging to obtain representative samples for toxicological analysis [89]. The distribution of plant toxins may also vary within tissues, requiring sampling from different body compartments for a comprehensive analysis.
Toxin degradation
Chemical Decomposition Plant toxins may undergo chemical decomposition over time, especially when exposed to environmental factors such as temperature, humidity, and microbial activity [90]. This can lead to the loss of the parent compound or the formation of degradation products.
Volatility Some plant toxins are volatile and susceptible to evaporation, especially in cases where samples are not properly handled or stored.
Postmortem interval (PMI) impact
Time-Dependent Changes The duration between death and the postmortem examination (postmortem interval) can significantly affect tissue integrity and the detectability of plant toxins. Earlier postmortem analysis generally yields more reliable results than delayed examinations [91].
Forensic context
Distinguishing Antemortem and Postmortem Exposure Differentiating between antemortem exposure and postmortem contamination or generation of toxins can be challenging. Forensic toxicologists must carefully consider the circumstances surrounding the death and consider any potential sources of postmortem exposure [92].
Geographic and environmental factors
The geographic distribution of certain plants and environmental conditions significantly influence the prevalence of plant toxins, adding a geographical context to forensic investigations of suspected plant poisoning [93]. Incorporating geographic and ecological considerations into forensic investigations is essential for understanding the factors that affect plant toxicity. By considering the unique regional environmental conditions and plant compositions, forensic experts can better interpret the circumstances surrounding suspected plant poisoning cases. This multidisciplinary approach enhances the accuracy and relevance of forensic analyses in diverse geographic settings.
Endemic plants and toxins
Regional Variability Certain plants and their toxins are endemic to specific geographic regions. The prevalence of these plants may vary based on factors such as climate, soil type, and altitude [94]. Forensic investigators must be aware of local endemic plants and their toxins, as regional knowledge can aid in identifying potential sources of poisoning.
Climate and soil conditions
Temperature and Precipitation Climate factors, including temperature and precipitation, influence the types of plants that can thrive in a given area. Certain toxins may be produced or accumulated more abundantly in specific climates [95]. Climate change may shift plant distributions, affecting both the prevalence and toxicity of certain species, which may require forensic investigations to adapt to these changing environmental changes.
Soil Composition Soil conditions, such as pH and nutrient levels, play a role in plant growth and toxin production [96]. Certain toxins may be more prevalent in plants growing in specific soil types.
Altitude and elevation
Altitudinal Gradients Elevation affects plant distribution and, consequently, the prevalence of plant toxins [97]. Some plants may produce higher concentrations of toxins at different altitudes. Investigators should consider these variations in altitude when assessing potential plant poisoning cases.
Biotic interactions
Ecological Relationships Biotic interactions among plants, such as competition and mutualism, can affect toxin production [98]. Competitive interactions may lead to increased toxin levels, while mutualistic relationships may result in toxin production as a defense mechanism. The introduction of invasive plant species can alter local ecosystems and impact toxin prevalence, potentially introducing new toxins or altering the concentrations of existing ones.
Seasonal variation
Plant Phenology The timing of plant growth and reproduction stages, known as phenology, can influence toxin levels [99]. Certain toxins may be more concentrated in specific plant parts or seasons. Seasonal changes can affect the risk of toxin exposure, especially for individuals who rely on foraging or harvesting plants for food or medicinal purposes.
Strategies for improving detection and analysis of plant toxins in forensic science
To enhance the detection and analysis of plant toxins, several advanced techniques and strategies can be employed to increase accuracy, resolution, and speed in forensic investigations (Fig. 2).
Fig. 2.
Forensic analysis workflow for plant toxicological impact assessment and toxin identification. Phytochemical fingerprinting of plant-derived Toxins of Forensic significance through Ultra-High-Performance Liquid Chromatography (UHPLC) and High-Resolution Mass Spectrometry (HRMS)
High-resolution sophisticated chromatographic techniques
Ultra-High-Performance Liquid Chromatography (UHPLC) Utilizing UHPLC for improved separation and analysis of plant toxins [100]. This technology offers higher resolution, sensitivity, and faster analysis times compared to traditional chromatographic methods. It offers several advantages over traditional High-Performance Liquid Chromatography (HPLC), making it a preferred choice for many analytical applications. Here are some key advantages of UHPLC:
Increased Resolution UHPLC uses smaller particle sizes in the stationary phase, resulting in a higher efficiency and improved resolution, this allows for better separation of complex mixtures and the detection of closely eluting peaks [101].
Higher Speed and Throughput The use of smaller particles and increased surface area of interaction in UHPLC leads to faster analyte separation. This results in significantly reduced analysis times, leading to higher sample throughput. Shorter columns and higher flow rates contribute to quicker analyses [102].
Enhanced Sensitivity UHPLC systems typically operate at higher pressures, which allows for the use of smaller particle sizes without sacrificing efficiency. This increased pressure enhances sensitivity and allows for the detection of lower concentration analytes [103].
Improved Peak Shapes UHPLC produces narrower and sharper peaks compared to HPLC [104]. This is attributed to the smaller particle sizes, reduced band broadening, and increased efficiency, resulting in better peak shapes and improved accuracy in peak quantification.
Reduced Solvent Consumption The higher efficiency of UHPLC allows for the use of smaller elution volumes and reduced solvent consumption per analysis, making it both cost-effective and environmentally friendly [105].
Compatibility with Mass Spectrometry (MS) UHPLC is well-suited for coupling with mass spectrometry detection due to its higher efficiency and sensitivity. This combination enhances the capabilities for compound identification and quantification in complex samples. [106].
Increased Productivity and Sample Throughput The faster analysis times, improved resolution, and higher sensitivity contribute to increased overall productivity and sample throughput. Laboratories can analyze more samples in a given time frame, improving efficiency [107].
Versatility in Column Lengths UHPLC systems offer flexibility in choosing column lengths, allowing analysts to select columns tailored to the specific requirements of their separations [108]. This adaptability enhances the versatility of UHPLC for different applications.
Improved Spectral Quality UHPLC provides high spectral quality due to the narrow peaks it generates, which is beneficial for impurity profiling and compound identification [101].
Compatibility with Existing HPLC Methods UHPLC instruments are designed to be compatible with existing HPLC methods, making it easier for laboratories to transition to UHPLC without significant changes to their existing workflows [109].
Mass spectrometry improvements
High-Resolution Mass Spectrometry (HRMS) Integration of HRMS for accurate identification and quantification of plant toxins [110], provides superior resolution and can distinguish between closely related compounds, reducing the likelihood of false positives. HRMS offers several advantages compared to conventional mass spectrometry techniques.
High Mass Accuracy HRMS provides high mass accuracy, allowing for precise determination of the mass-to-charge ratio (m/z) of ions, making it essential for identifying complex compounds [111].
Increased Resolution HRMS systems have higher resolving power, enabling the separation and identification of closely spaced peaks in a mass spectrum [112]. This enhanced resolution is valuable for analyzing complex mixtures and resolving isobaric compounds. The high resolution and mass accuracy of HRMS facilitate detailed structural elucidation of compounds. This is valuable in the characterization of unknown or novel compounds in research and discovery applications.
Accurate Elemental Composition Determination HRMS allows for accurate determination of the elemental composition of ions based on their accurate mass [113]. This is particularly useful in determining molecular formulas and reducing the likelihood of false identifications.
Discrimination of Isotopes HRMS can distinguish between isotopes with similar masses, providing additional information about the elemental composition of compounds [112]. This capability is especially valuable in isotopic labeling studies and metabolic profiling.
Broad Analytical Range HRMS instruments can cover a wide mass range, making them versatile for the analysis of various compounds, from small molecules to large biomolecules [114]. This broad analytical range enhances the applicability of HRMS in different fields.
Flexibility in Acquisition Modes HRMS systems offer various acquisition modes, such as full-scan, targeted, and data-dependent acquisition [115]. This flexibility allows researchers to adapt the analysis to different experimental needs, maximizing the information obtained from a single experiment.
Reduced Interference HRMS can reduce the likelihood of interference from co-eluting compounds due to its ability to resolve ions with similar masses [116]. This is particularly advantageous in complex samples where accurate identification of individual components is challenging.
Compatibility with Chromatographic Techniques HRMS can be coupled with various chromatographic techniques, such as liquid chromatography (LC) and gas chromatography (GC), allowing for the separation of complex mixtures before mass spectrometric analysis [117]. This enhances the specificity and sensitivity of the analysis.
Advancements in Ionization Techniques HRMS benefits from advancements in ionization techniques, such as electrospray ionization (ESI) and matrix-assisted laser desorption/ionization (MALDI), expanding its capabilities in different sample types and applications [110, 118].
Adopting these advancements in technology and methodologies can significantly improve the capabilities of forensic science in detecting and analyzing plant toxins. The integration of cutting-edge tools and collaborative approaches holds the potential to enhance the accuracy, speed, and reliability of forensic investigations involving plant-related poisonings.
Conclusion and prospective directions
Plant-derived toxins as natural secondary metabolites, pose significant risks to human and animal health. The concept of phytochemical fingerprinting of plant toxins has been transformed forensic investigations by enabling precise identification and analysis of plant toxins. This innovative approach can be achieved through advanced chromatographic and spectroscopic techniques such as UHPLC and HRMS. These innovations will provide forensic scientists with even greater precision in identifying and characterizing plant compounds, strengthening the discriminatory power of botanical evidence. By analyzing the distinct profiles of plant toxins, forensic scientists can provide critical insights into crime scenes, identifying or eliminating the involvement of specific plants or toxins. As a result, forensic phytochemistry enhances the overall accuracy and reliability of criminal investigations, adding a new dimension to forensic science. The future of forensic phytochemistry holds promising avenues that can further revolutionize investigative processes and enhance the reliability of botanical evidence. Forensic phytochemistry is likely to extend its applications to environmental forensics. The analysis of plant evidence may contribute to investigations related to environmental crimes, such as illegal logging or pollution, providing a unique perspective on ecological impact assessments. Moreover, the future promises the development of portable and field-deployable analytical devices. These tools will enable real-time, on-site analyses by forensic investigators, accelerating the collection and interpretation of evidence, and supporting timely decision-making in the field. As forensic phytochemistry evolves, its role in both criminal and environmental investigations will undoubtedly expand, strengthening the pursuit of justice across a broader range of forensic contexts.
Author contributions
M.G.A., A.S.A.E., and N.Z. conceptualizing, writing and editing the manuscript.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Availability of data and materials
All the data are available in the manuscript.
Declarations
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The authors declare that they have no competing of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Coyle HM. Forensic botany: principles and applications to criminal casework. Boca Raton: CRC Press; 2004. [Google Scholar]
- 2.Hall DW, Byrd J. Forensic botany: a practical guide. Boca Raton: John Wiley & Sons; 2012. [Google Scholar]
- 3.Bock JH, Norris DO. Forensic plant science. Amsterdam: Academic Press; 2015. [Google Scholar]
- 4.Coyle HM, Lee C-L, Lin W-Y, Lee HC, Palmbach TM. Forensic botany: using plant evidence to aid in forensic death investigation. Croat Med J. 2005;46(4):606. [PubMed] [Google Scholar]
- 5.Coyle HM, Ladd C, Palmbach T, Lee HC. The green revolution: botanical contributions to forensics and drug enforcement. Croat Med J. 2001;42:340–5. [PubMed] [Google Scholar]
- 6.Bozorgi M, Amin GR, Ostad SN, Samadi N, Nazem E, Shekarchi M. Toxicological, chemical and antibacterial evaluation of squill vinegar, a useful product in Persian traditional medicine. Res J Pharmacogn. 2017;4:33–9. [Google Scholar]
- 7.Sharrock S, Oldfield S, Wilson O. Plant conservation report 2014: a review of progress towards the global strategy for plant conservation 2011–2020. 2014.
- 8.Oliveira M, Azevedo L, Ballard D, Branicki W, Amorim A. Using plants in forensics: state-of-the-art and prospects. Plant Sci. 2023;336: 111860. [DOI] [PubMed] [Google Scholar]
- 9.Oliveira M, Lackner M, Amorim A, Araujo R. Feasibility of mitochondrial single nucleotide polymorphisms to detect and identify Aspergillus fumigatus in clinical samples. Diagn Microbiol Infect Dis. 2014;80:53–8. [DOI] [PubMed] [Google Scholar]
- 10.Qu D, Qiao D-F, Chen X-C, Feng C-Q, Luo Q-Z, Tan X-H. Fatal poisoning by accidental ingestion of the “heartbreak grass” (Gelsemium elegans) verified by toxicological and medico-legal analyses. For Sci Int. 2021;321: 110745. [DOI] [PubMed] [Google Scholar]
- 11.Coyle HM, Lee HC, Palmbach TM. Forensic botany plants as evidence in criminal cases and as agents of bioterrorism. In: Coyle HM, editor. Forensic DNA applications. 2nd ed. Boca Raton: CRC Press; 2023. [Google Scholar]
- 12.Bernhoft A, Siem H, Bjertness E, Meltzer M, Flaten T, Holmsen E. Bioactive compounds in plants–benefits and risks for man and animals. Nor Acad Sci Lett Oslo. 2010;4:13–4. [Google Scholar]
- 13.Nugroho D, Chanthai S, Oh W-C, Benchawattananon R. Fluorophores-rich natural powder from selected medicinal plants for detection latent fingerprints and cyanide. Sci Prog. 2023;106:00368504231156217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dubey NK, Dwivedy AK, Chaudhari AK, Das S. Common toxic plants and their forensic significance. In: Dubey NK, editor. Natural products and drug discovery. Amsterdam: Elsevier; 2018. p. 349–74. [Google Scholar]
- 15.Anywar G. Historical Use of Toxic Plants. In: Anywar G, editor. Poisonous plants and phytochemicals in drug discovery. Ltd: John Wiley & Sons; 2020. p. 1–17. [Google Scholar]
- 16.Raje SC, Bhagat DS, Nimbalkar RK, Shejul SK, Bumbrah GS, Sankhla MS. Contributions and current trends of forensic botany in crime scene investigation. Forensic Sci J. 2022;21:1–2. [Google Scholar]
- 17.Hotti H, Rischer H. The killer of socrates: coniine and related alkaloids in the plant kingdom. Molecules. 2017;22:1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Retief FP, Cilliers L. Poisoning during the Renaissance: The Medicis and the Borgias. 2000.
- 19.Stegelmeier BL, James LF, Panter KE, Ralphs MH, Gardner DR, Molyneux RJ, et al. The pathogenesis and toxicokinetics of locoweed (Astragalus and Oxytropis spp.) poisoning in livestock. J Nat Toxins. 1999;8:35–45. [PubMed] [Google Scholar]
- 20.Dodson C, Dunmire WW. Mountain wildflowers of the southern rockies: revealing their natural history. London: UNM Press; 2007. [Google Scholar]
- 21.Norn S, Kruse PR. Hjerteglykosider: fra oldtiden over Witherings digitalis til endogen glykosider [Cardiac glycosides: from ancient history through Withering’s foxglove to endogeneous cardiac glycosides]. Dan Med Arbog. 2004;2004:119–32. [PubMed] [Google Scholar]
- 22.Siritunga D, Sayre R. Engineering cyanogen synthesis and turnover in cassava (Manihot esculenta). Plant Mol Biol. 2004;56:661–9. [DOI] [PubMed] [Google Scholar]
- 23.Plants T. Ingestion of toxic plants by herbivores. J Range Manag. 45: 36–45.
- 24.Strzelecki A, Pichon N, Gaulier JM, Amiel JB, Champy P, Clavel M. Acute toxic herbal intake in a suicide attempt and fatal refractory Ventricular Arrhythmia. Basic Clin Pharmacol Toxicol. 2010;107:698–9. [DOI] [PubMed] [Google Scholar]
- 25.Sinha M, Sahu M, Sallawad SS, Ahirwar B, Isukapatla AR. Characteristics and Identifying features of various natural toxins and poisonous plants used as weapons in forensic cases. Res J Pharm Technol. 2017;10:4237–41. [Google Scholar]
- 26.Shedge R, Krishan K, Warrier V, Kanchan T. Postmortem changes. In: Shedge R, Krishan K, Warrier V, Kanchan T, editors. Postmortem changes. Treasure Island: StatPearls Publishing; 2019. [Google Scholar]
- 27.Almulhim AM, Menezes RG. Evaluation of postmortem changes. Treasure Island: StatPearls Publishing; 2020. [PubMed] [Google Scholar]
- 28.Nelson L, Shih R, Balick M. Handbook of poisonous and injurious plants. Boston: Springer, US; 2007. [Google Scholar]
- 29.Kuete V. Physical, hematological, and histopathological signs of toxicity induced by African medicinal plants. In: Kuete V, editor. Toxicological survey of African medicinal plants. Amsterdam: Elsevier; 2014. p. 635–57. [Google Scholar]
- 30.Dolinak D, Matshes E, Lew EO. Forensic pathology: principles and practice. Amsterdam: Elsevier; 2005. [Google Scholar]
- 31.Matsuyama S, Nishi K. Genus identification of toxic plant by real-time PCR. Int J Legal Med. 2011;125:211–7. [DOI] [PubMed] [Google Scholar]
- 32.Weedn VW. Bases of forensic pathology expert testimony with emphasis on Iowa v Tyler. Acad Forensic Pathol. 2021;11:185–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaillard Y, Pepin G. Poisoning by plant material: review of human cases and analytical determination of main toxins by high-performance liquid chromatography–(tandem) mass spectrometry. J Chromatogr B Biomed Sci App. 1999;733:181–229. [DOI] [PubMed] [Google Scholar]
- 34.Patil A, Paikrao HM, Patil S. The chemistry and biology of the plant poisons and their forensic significance. In: Patil A, editor. Studies in natural products chemistry. Amsterdam: Elsevier; 2023. p. 255–321. [Google Scholar]
- 35.Tahir F, Ali E, Hassan SA, Bhat ZF, Walayat N, Nawaz A, et al. Cyanogenic glucosides in plant-based foods: occurrence, detection methods, and detoxification strategies—a comprehensive review. Microchem J. 2024;199: 110065. [Google Scholar]
- 36.Wink M, Van Wyk B-E. Mind-altering and poisonous plants of the world. New York: Timber Press; 2008. [Google Scholar]
- 37.Benowitz NL. Nicotine safety and toxicity. Oxford: Oxford University Press; 1998. [Google Scholar]
- 38.Li J, Zhang F, Gu Y, Ye Y, Li L, Liu M, et al. Forensic aspects about fatal morphine intoxication of an unusual body packer: case report and literature review. Forensic Sci Int Rep. 2021;3: 100207. [Google Scholar]
- 39.Refahy LA-G. Study on flavonoids and triterpenoids content of some Euphorbiaceae plants. J Life Sci. 2011;5:100–7. [Google Scholar]
- 40.Dickers KJ, Bradberry SM, Rice P, Griffiths GD, Vale JA. Abrin poisoning. Toxicol Rev. 2003;22:137–42. [DOI] [PubMed] [Google Scholar]
- 41.Holstege CP. Criminal poisoning: clinical and forensic perspectives. New York: Jones & Bartlett Publishers; 2010. [Google Scholar]
- 42.Sandvig K, Olsnes S, Pihl A. Kinetics of binding of the toxic lectins abrin and ricin to surface receptors of human cells. J Biol Chem. 1976;251:3977–84. [PubMed] [Google Scholar]
- 43.Kim J-Y, Park S-C, Hwang I, Cheong H, Nah J-W, Hahm K-S, et al. Protease inhibitors from plants with antimicrobial activity. Int J Mol Sci. 2009;10:2860–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Downs JW, Wills BK. Phenol toxicity. 2019. [PubMed]
- 45.Maugeri A, Lombardo GE, Cirmi S, Süntar I, Barreca D, Laganà G, et al. Pharmacology and toxicology of tannins. Arch Toxicol. 2022;96:1257–77. [DOI] [PubMed] [Google Scholar]
- 46.Murray AR, Kisin E, Castranova V, Kommineni C, Gunther MR, Shvedova AA. Phenol-induced in vivo oxidative stress in skin: evidence for enhanced free radical generation, thiol oxidation, and antioxidant depletion. Chem Res Toxicol. 2007;20:1769–77. [DOI] [PubMed] [Google Scholar]
- 47.Toschi A, Piva A, Grilli E. Phenol-rich botanicals modulate oxidative stress and epithelial integrity in intestinal epithelial cells. Animals. 2022;12:2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar R, Vaithiyanathan S. Occurrence, nutritional significance and effect on animal productivity of tannins in tree leaves. Anim Feed Sci Technol. 1990;30:21–38. [Google Scholar]
- 49.Kumar R, Horigome T. Fractionation, characterization and protein-precipitating capacity of the condensed tannins from Robinia pseudoacacia L. leaves. J Agric Food Chem. 1986;34:487–9. [Google Scholar]
- 50.Theodossiou TA, Hothersall JS, De Witte PA, Pantos A, Agostinis P. The multifaceted photocytotoxic profile of hypericin. Mol Pharm. 2009;6:1775–89. [DOI] [PubMed] [Google Scholar]
- 51.Shukla V, Asthana S, Gupta P, Dwivedi PD, Tripathi A, Das M. toxicity of naturally occurring anthraquinones. In: Shukla V, editor. advances in molecular toxicology. Amsteradam: Elsevier; 2017. p. 1–50. [Google Scholar]
- 52.Abegg VF, Panajatovic MV, Mancuso RV, Allard JA, Duthaler U, Odermatt A, et al. Mechanisms of hepatocellular toxicity associated with the components of St. John’s Wort extract hypericin and hyperforin in HepG2 and HepaRG cells. Toxicol Lett. 2024;393:1–13. [DOI] [PubMed] [Google Scholar]
- 53.Olsen RW. Picrotoxin-like channel blockers of GABAA receptors. Proc Natl Acad Sci. 2006;103:6081–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pressly B, Vasylieva N, Barnych B, Singh V, Singh L, Bruun DA, et al. Comparison of the toxicokinetics of the convulsants picrotoxinin and tetramethylenedisulfotetramine (TETS) in mice. Arch Toxicol. 2020;94:1995–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramos A, Rivero R, Visozo A, Piloto J, García A. Parthenin, a sesquiterpene lactone of Parthenium hysterophorus L. is a high toxicity clastogen. Mutat Res Toxicol Environ Mutagen. 2002;514:19–27. [DOI] [PubMed] [Google Scholar]
- 56.Batish DR, Singh HP, Kohli RK, Kaur S, Saxena DB, Yadav S. Assessment of phytotoxicity of parthenin. Z Für Naturforschung C. 2007;62:367–72. [DOI] [PubMed] [Google Scholar]
- 57.Hussain A, Khan AA, Ali M, Zamani GY, Iqbal Z, Ullah Q, et al. In-vitro and in-vivo assessment of toxic effects of Parthenium hysterophorus leaves extract. J Chil Chem Soc. 2022;67:5484–9. [Google Scholar]
- 58.Śmiarowska M, Białecka M, Machoy-Mokrzyńska A. Cannabis and cannabinoids: pharmacology and therapeutic potential. Neurol Neurochir Pol. 2022;56:4–13. [DOI] [PubMed] [Google Scholar]
- 59.Alves P, Amaral C, Teixeira N, Correia-da-Silva G. Cannabis sativa: Much more beyond Δ9-tetrahydrocannabinol. Pharmacol Res. 2020;157: 104822. [DOI] [PubMed] [Google Scholar]
- 60.Sarne Y, Mechoulam R. Cannabinoids: between neuroprotection and neurotoxicity. Curr Drug Targets CNS Neurol Disord. 2005;4:677–84. [DOI] [PubMed] [Google Scholar]
- 61.Mohamed NZ, Shaban L, Safan S, El-Sayed ASA. Physiological and metabolic traits of Taxol biosynthesis of endophytic fungi inhabiting plants: plant-microbial crosstalk, and epigenetic regulators. Microbiol Res. 2023;272: 127385. [DOI] [PubMed] [Google Scholar]
- 62.Mohamed NZ, Shaaban L, Safan S, El-Sayed ASA. Phytochemical and metabolic profiling of the different Podocarpus species in Egypt: Potential antimicrobial and antiproliferative activities. Heliyon. 2023;9:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.ElSohly MA, Stanford DF, Murphy TP. Chemical fingerprinting of Cannabis as a means of source identification. In: Stanford DF, editor. Marijuana and the cannabinoids. Totowa: Humana Press; 2007. p. 51–66. [Google Scholar]
- 64.Rivera D, Mozayani A, Obi E, Drake JM. The structural complexities of Cannabis sativa L. and profiling techniques for geographic source determination. J Forensic Res. 2019;10:1000443. [Google Scholar]
- 65.Weyermann C, Roux C, Champod C. Initial results on the composition of fingerprints and its evolution as a function of time by GC/MS analysis. J Forensic Sci. 2011;56:102–8. [DOI] [PubMed] [Google Scholar]
- 66.Yang L, Wen K-S, Ruan X, Zhao Y-X, Wei F, Wang Q. Response of plant secondary metabolites to environmental factors. Molecules. 2018;23:762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smith I. Chromatography. Amsterdam: Elsevier; 2013. [Google Scholar]
- 68.Coskun O. Separation techniques: chromatography. North Clin Istanb. 2016;3:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mukadam M, Shaikh MFS, Anam MS, Ahmed K, Mandlekar MBF. Gas chromatography of volatile oil. Int J Pharm Res Appl. 2021;6:399–406. [Google Scholar]
- 70.Fanali S, Haddad PR, Poole C, Riekkola M-L. Liquid chromatography: applications. Amsterdam: Elsevier; 2017. [Google Scholar]
- 71.Duckworth HE, Barber RC, Venkatasubramanian VS. Mass spectroscopy. 2nd ed. Cambridge: Cambridge University Press; 1986. [Google Scholar]
- 72.Ahuja V, Singh A, Paul D, Dasgupta D, Urajová P, Ghosh S, et al. Recent Advances in the detection of food toxins using mass spectrometry. Chem Res Toxicol. 2023;36:1834–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Al-Rubaye AF, Hameed IH, Kadhim MJ. A review: uses of gas chromatography-mass spectrometry (GC-MS) technique for analysis of bioactive natural compounds of some plants. Int J Toxicol Pharmacol Res. 2017;9:81–5. [Google Scholar]
- 74.Pitt JJ. Principles and applications of liquid chromatography-mass spectrometry in clinical biochemistry. Clin Biochem Rev. 2009;30:19–34. [PMC free article] [PubMed] [Google Scholar]
- 75.Rizzo S, Celano R, Piccinelli AL, Serio S, Russo M, Rastrelli L. An analytical platform for the screening and identification of pyrrolizidine alkaloids in food matrices with high risk of contamination. Food Chem. 2023;406: 135058. [DOI] [PubMed] [Google Scholar]
- 76.Kalb SR, Schieltz DM, Becher F, Astot C, Fredriksson S-Å, Barr JR. Recommended mass spectrometry-based strategies to identify ricin-containing samples. Toxins. 2015;7:4881–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liang X, Christensen JH, Nielsen NJ. Enhancing the power of liquid chromatography–mass spectrometry for chemical fingerprinting of phytotoxins in the environment. J Chromatogr A. 2021;1642: 462027. [DOI] [PubMed] [Google Scholar]
- 78.Bovey FA, Mirau PA, Gutowsky HS. Nuclear magnetic resonance spectroscopy. Amsterdam: Elsevier; 1988. [Google Scholar]
- 79.Alkefai NH, Mir SR, Amin S, Ahamad J, Munjal K, Gauttam VK. NMR in analysis of food toxins. In: Alkefai NH, editor. Analysis of naturally occurring food toxins of plant origin. Boca Raton: CRC Press; 2022. [Google Scholar]
- 80.Holmes E, Tang H, Wang Y, Seger C. The assessment of plant metabolite profiles by NMR-based methodologies. Planta Med. 2006;72:771–85. [DOI] [PubMed] [Google Scholar]
- 81.Rosengren KJ, Daly NL, Craik DJ. NMR of peptide toxins. In: Rosengren KJ, editor. Annual reports on NMR spectroscopy. Amsterdam: Academic Press; 2009. p. 89–147. [Google Scholar]
- 82.Romagnoli S, Ugolini R, Fogolari F, Schaller G, Urech K, Giannattasio M, et al. NMR structural determination of viscotoxin A3 from Viscum album L. Biochem J. 2000;350(Pt 2):569. [PMC free article] [PubMed] [Google Scholar]
- 83.Sakamoto S, Putalun W, Vimolmangkang S, Phoolcharoen W, Shoyama Y, Tanaka H, et al. Enzyme-linked immunosorbent assay for the quantitative/qualitative analysis of plant secondary metabolites. J Nat Med. 2018;72:32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moawed A, Naguib DM. Medicolegal responsibility in acute poisoning cases: highlight on the related Egyptian laws. Egypt Soc Clin Toxicol J. 2023;11:1–9. [Google Scholar]
- 85.Forbes SL, Perrault KA, Comstock JL. Microscopic post-mortem changes: the chemistry of decomposition. In: Forbes SL, editor. Taphonomy of human remains: forensic analysis of the dead and the depositional environment. Hoboken: John Wiley & Sons, Ltd; 2017. [Google Scholar]
- 86.Szeremeta M, Pietrowska K, Niemcunowicz-Janica A, Kretowski A, Ciborowski M. Applications of metabolomics in forensic toxicology and forensic medicine. Int J Mol Sci. 2021;22:3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Skopp G. Preanalytic aspects in postmortem toxicology. Forensic Sci Int. 2004;142:75–100. [DOI] [PubMed] [Google Scholar]
- 88.der Rogowska-van Molen MA, Berasategui-Lopez A, Coolen S, Jansen RS, Welte CU. Microbial degradation of plant toxins. Environ Microbiol. 2023;25:2988–3010. [DOI] [PubMed] [Google Scholar]
- 89.Uekusa K, Hayashida M, Ohno Y. Forensic toxicological analyses of drugs in tissues in formalin solutions and in fixatives. Forensic Sci Int. 2015;249:165–72. [DOI] [PubMed] [Google Scholar]
- 90.Kot-Wasik A, Dabrowska D, Namiesnik J. The Importance of degradation in the fate of selected organic compounds in the environment. Part I. General considerations. Pol J Environ Stud. 2004;13:15. [Google Scholar]
- 91.Mohammed AT, Abdelfattah-Hassan A, Abdo S, Ali MIM, Wagih E. Estimation of the time since death based on the post-mortem histopathological changes in a rat brain: an observational study. J Adv Vet Res. 2023;13:526–30. [Google Scholar]
- 92.Yu K, Wu H, Shen C, Li H, Wei X, Liu R, et al. Identification of antemortem and postmortem fractures in a complex environment by FTIR spectroscopy based on a rabbit tibial fracture self-control model. Int J Legal Med. 2021;135:2385–94. [DOI] [PubMed] [Google Scholar]
- 93.Ma Y, Fei X, Li J, Liu Y, Wei A. Effects of location, climate, soil conditions and plant species on levels of potentially toxic elements in Chinese Prickly Ash pericarps from the main cultivation regions in China. Chemosphere. 2020;244: 125501. [DOI] [PubMed] [Google Scholar]
- 94.Günthardt BF, Hollender J, Hungerbühler K, Scheringer M, Bucheli TD. Comprehensive toxic plants–phytotoxins database and its application in assessing aquatic micropollution potential. J Agric Food Chem. 2018;66:7577–88. [DOI] [PubMed] [Google Scholar]
- 95.van der Fels-Klerx HJ, Olesen JE, Naustvoll L-J, Friocourt Y, Mengelers MJB, Christensen JH. Climate change impacts on natural toxins in food production systems, exemplified by deoxynivalenol in wheat and diarrhetic shellfish toxins. Food Addit Contam Part A. 2012;29:1647–59. [DOI] [PubMed] [Google Scholar]
- 96.Moraghan JT, Mascagni HJ Jr. Environmental and soil factors affecting micronutrient deficiencies and toxicities. In: Moraghan JT, editor. micronutrients in agriculture. Hoboken: John Wiley & Sons, Ltd; 1991. [Google Scholar]
- 97.Anic V, Hinojosa LF, Díaz-Forester J, Bustamante E, de la Fuente LM, Casale JF, et al. influence of soil chemical variables and altitude on the distribution of high-alpine plants: the case of the Andes of central Chile. Arct Antarct Alp Res. 2010;42:152–63. [Google Scholar]
- 98.Agrawal AA, Petschenka G, Bingham RA, Weber MG, Rasmann S. Toxic cardenolides: chemical ecology and coevolution of specialized plant–herbivore interactions. New Phytol. 2012;194:28–45. [DOI] [PubMed] [Google Scholar]
- 99.Jarzomski CM, Stamp NE, Bowers MD. Effects of plant phenology, nutrients and herbivory on growth and defensive chemistry of plantain. Plantago lanceolata Oikos. 2000;88:371–9. [Google Scholar]
- 100.Vaclavik L, Krynitsky AJ, Rader JI. Targeted analysis of multiple pharmaceuticals, plant toxins and other secondary metabolites in herbal dietary supplements by ultra-high performance liquid chromatography–quadrupole-orbital ion trap mass spectrometry. Anal Chim Acta. 2014;810:45–60. [DOI] [PubMed] [Google Scholar]
- 101.Ahmed F, Eticha T, Hymete A, Ashenef A, Ahmed F, Eticha T, et al. Principles and applications of ultra-high-performance liquid chromatography. In: Ahmed F, editor., et al., High performance liquid chromatography—recent advances and applications. New York: IntechOpen; 2023. [Google Scholar]
- 102.Tanna N, Plumb RS, Molloy BJ, Rainville PD, Wilson ID. Enhanced chromatographic efficiency obtained with vacuum jacketed columns facilitates the rapid UHPLC/MS/MS-based analysis of fasiglifam in rat plasma. Talanta. 2023;254: 124089. [DOI] [PubMed] [Google Scholar]
- 103.Fekete S, Schappler J, Veuthey J-L, Guillarme D. Current and future trends in UHPLC. TrAC Trends Anal Chem. 2014;63:2–13. [Google Scholar]
- 104.Wu H, Guo J, Chen S, Liu X, Zhou Y, Zhang X, et al. Recent developments in qualitative and quantitative analysis of phytochemical constituents and their metabolites using liquid chromatography–mass spectrometry. J Pharm Biomed Anal. 2013;72:267–91. [DOI] [PubMed] [Google Scholar]
- 105.Cielecka-Piontek J, Zalewski P, Jelińska A, Garbacki P. UHPLC: the greening face of liquid chromatography. Chromatographia. 2013;76:1429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nováková L, Svoboda P, Pavlík J. Ultra-high performance liquid chromatography. In: Nováková L, editor. Liquid chromatography. 2nd ed. Boston: Elsevier; 2017. p. 719–69. [Google Scholar]
- 107.Kaplitz AS, Kresge GA, Selover B, Horvat L, Franklin EG, Godinho JM, et al. High-throughput and ultrafast liquid chromatography. Anal Chem. 2020;92:67–84. [DOI] [PubMed] [Google Scholar]
- 108.D’Atri V, Fekete S, Clarke A, Veuthey J-L, Guillarme D. Recent advances in chromatography for pharmaceutical analysis. Anal Chem. 2019;91:210–39. [DOI] [PubMed] [Google Scholar]
- 109.Fabel S. Method Transfer between HPLC and UHPLC. In: Fabel S, editor. Chromatographic methods development. New Delhi: Jenny Stanford Publishing; 2019. [Google Scholar]
- 110.Alvarez-Rivera G, Ballesteros-Vivas D, Parada-Alfonso F, Ibañez E, Cifuentes A. Recent applications of high-resolution mass spectrometry for the characterization of plant natural products. TrAC Trends Anal Chem. 2019;112:87–101. [Google Scholar]
- 111.Arrebola-Liébanas FJ, Romero-González R, Garrido FA. RMS: fundamentals and basic concepts. In: Arrebola-Liébanas FJ, editor. Applications in high resolution mass spectrometry. Boston: Elsevier; 2017. p. 1–14. [Google Scholar]
- 112.Geer Wallace MA, McCord JP. High-resolution mass spectrometry. In: Geer Wallace MA, editor. Breathborne biomarkers and the human volatilome. 2nd ed. Boston: Elsevier; 2020. p. 253–70. [Google Scholar]
- 113.Rathahao-Paris E, Alves S, Junot C, Tabet J-C. High resolution mass spectrometry for structural identification of metabolites in metabolomics. Metabolomics. 2015;12:10. [Google Scholar]
- 114.Junot C, Fenaille F, Colsch B, Bécher F. High resolution mass spectrometry based techniques at the crossroads of metabolic pathways. Mass Spectrom Rev. 2014;33:471–500. [DOI] [PubMed] [Google Scholar]
- 115.You Y, Proctor RM, Guo K, Li X, Xue E, Guan F, et al. Use of high resolution/accurate mass full scan/data-dependent acquisition for targeted/non-targeted screening in equine doping control. Anal Methods. 2021;13:1565–75. [DOI] [PubMed] [Google Scholar]
- 116.Celma A, Ahrens L, Gago-Ferrero P, Hernández F, López F, Lundqvist J, et al. The relevant role of ion mobility separation in LC-HRMS based screening strategies for contaminants of emerging concern in the aquatic environment. Chemosphere. 2021;280: 130799. [DOI] [PubMed] [Google Scholar]
- 117.López-Ruiz R, Maldonado-Reina AJ, Marín-Sáez J, Romero-González R, Martínez-Vidal JL, Garrido FA. Unravelling plant protection product analysis: use of chromatography techniques (GC and LC) and high resolution mass spectrometry. Trends Environ Anal Chem. 2023;37: e00191. [Google Scholar]
- 118.Lai Y-H, Wang Y-S. Advances in high-resolution mass spectrometry techniques for analysis of high mass-to-charge ions. Mass Spectrom Rev. 2023;42:2426–45. [DOI] [PubMed] [Google Scholar]
- 119.Steenkamp PA, van Heerden FR, van Wyk B-E. Accidental fatal poisoning by Nicotiana glauca: identification of anabasine by high performance liquid chromatography/photodiode array/mass spectrometry. Forensic Sci Int. 2002;127:208–17. [DOI] [PubMed] [Google Scholar]
- 120.Vetter J. Poison hemlock (Conium maculatum L.). Food Chem Toxicol. 2004;42:1373–82. [DOI] [PubMed] [Google Scholar]
- 121.Wink M. Mode of action and toxicology of plant toxins and poisonous plants. Mitt Julius Kühn-Inst. 2009;421:93–112. [Google Scholar]
- 122.Salehi B, Sharopov F, Boyunegmez Tumer T, Ozleyen A, Rodríguez-Pérez C, Ezzat MS, et al. Symphytum species: a comprehensive review on chemical composition, food applications and phytopharmacology. Molecules. 2019;24:2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Berendonk C, Cerff D, Hünting K, Wiedenfeld H, Becerra J, Kuschak M. Pyrrolizidine alkaloid level in Senecio jacobaea and Senecio erraticus-the effect of plant organ and forage conservation. Proc 23rd Gen Meet Eur Grassld Fed Grassl Chang World Kiel Ger. 2010;15:669–71. [Google Scholar]
- 124.Broch-Due AI, Aasen AJ. Alkaloids of Anchusa officinalis L. identification of pyrrolizidine alkaloid lycopsamine. Acta Chem Scand Ser B. 1980;34:75–7. [Google Scholar]
- 125.Razinger G, Kozelj G, Gorjup V, Grenc D, Brvar M. Accidental poisoning with autumn crocus (Colchicum autumnale): a case series. Clin Toxicol. 2021;59:493–9. [DOI] [PubMed] [Google Scholar]
- 126.Dannhardt G, Steindl L. Alkaloids of Lolium temulentum: isolation, identification and pharmacological activity. Planta Med. 2007;51:212–4. [DOI] [PubMed] [Google Scholar]
- 127.Wagner HK, Wolff PM. New natural products and plant drugs with pharmacological, biological or therapeutical activity: proceedings of the first international congress on medicinal plant research, section A, held at the University of Munich, Germany, September 6–10, 1976. Springer Science & Business Media; 2012.
- 128.Gavilan J, Mennickent D, Ramirez-Molina O, Triviño S, Perez C, Silva-Grecchi T, et al. 17 Oxo sparteine and lupanine, obtained from Cytisus scoparius, exert a neuroprotection against soluble oligomers of amyloid-β Toxicity by nicotinic acetylcholine receptors. J Alzheimers Dis. 2019;67:343–56. [DOI] [PubMed] [Google Scholar]
- 129.Petruczynik A, Wróblewski K, Misiurek J, Plech T, Szalast K, Wojtanowski K, et al. Determination of cytisine and N-methylcytisine from selected plant extracts by high-performance liquid chromatography and comparison of their cytotoxic activity. Toxins. 2020;12:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cely-Veloza W, Kato MJ, Coy-Barrera E. Quinolizidine-type alkaloids: chemodiversity, occurrence, and bioactivity. ACS Omega. 2023;8:27862–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vavrečková C, Gawlik I, Müller K. Benzophenanthridine alkaloids of Chelidonium majus; II. potent inhibitory action against the growth of human keratinocytes. Planta Med. 2007;62:491–4. [DOI] [PubMed] [Google Scholar]
- 132.Dalvi RR. Sanguinarine: its potential, as a liver toxic alkaloid present in the seeds of Argemone mexicana. Experientia. 1985;41:77–8. [DOI] [PubMed] [Google Scholar]
- 133.Günaydın YK, Dündar ZD, Çekmen B, Akıllı NB, Köylü R, Cander B. Intoxication due to Papaver rhoeas (Corn Poppy): five case reports. Case Rep Med. 2015;2015: 321360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sun S, Wei Y, Cao Y, Deng B. Simultaneous electrochemiluminescence determination of galanthamine, homolycorine, lycorenine, and tazettine in Lycoris radiata by capillary electrophoresis with ultrasonic-assisted extraction. J Chromatogr B. 2017;1055–1056:15–9. [DOI] [PubMed] [Google Scholar]
- 135.Zamfirache IIB. Contributions to the chemical study of some Papaveraceae species capsules n. ii. Glaucium flavum, Glaucium corniculatum and Chelidonium majus. Analele Științifice Ale Univ Al Cuza Din Iași Genet Și Biol Mol Ser Nouă Secțiunea II A. 2004;5:191. [Google Scholar]
- 136.Ubeda A, Montesinos C, Payá M, Terencio C, Alcaraz MJ, Halliwell B. Antioxidant action of benzylisoquinoline alkaloids. Free Radic Res Commun. 1993;18:167–75. [DOI] [PubMed] [Google Scholar]
- 137.Nawrot R, Wolun-Cholewa M, Bialas W, Wyrzykowska D, Balcerkiewicz S, Gozdzicka-Jozefiak A. Cytotoxic activity of proteins isolated from extracts of Corydalis cava tubers in human cervical carcinoma HeLa cells. BMC Complement Altern Med. 2010;10:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Xiang M-L, Hu B-Y, Qi Z-H, Wang X-N, Xie T-Z, Wang Z-J, et al. Chemistry and bioactivities of natural steroidal alkaloids. Nat Prod Bioprospecting. 2022;12:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Anwar M, Turner M, Farrell N, Zomlefer WB, McDougal OM, Morgan BW. Hikers poisoned: veratrum steroidal alkaloid toxicity following ingestion of foraged Veratrum parviflorum. Clin Toxicol. 2018;56:841–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Atta-ur-Rahman A, Akhtar MN, Choudhary MI, Tsuda Y, Sener B, Khalid A, et al. New steroidal alkaloids from Fritillaria imperialis and their cholinesterase inhibiting activities. Chem Pharm Bull. 2002;50:1013–6. [DOI] [PubMed] [Google Scholar]
- 141.Rajput H. Effects of Atropa belladonna as an anti-cholinergic. Nat Prod Chem Res. 2013;1:1000104. [Google Scholar]
- 142.Karch SB. Introduction to the forensic pathology of cocaine. Am J For Med Pathol. 1991;12:126. [DOI] [PubMed] [Google Scholar]
- 143.Chan TY, Tomlinson B, Tse LK, Chan JC, Chan WW, Critchley JA. Aconitine poisoning due to Chinese herbal medicines: a review. Vet Hum Toxicol. 1994;36:452–5. [PubMed] [Google Scholar]
- 144.Ameri A. The effects of Aconitum alkaloids on the central nervous system. Prog Neurobiol. 1998;56:211–35. [DOI] [PubMed] [Google Scholar]
- 145.Yin T, Cai L, Ding Z. A systematic review on the chemical constituents of the genus Consolida (Ranunculaceae) and their biological activities. RSC Adv. 2020;10:35072–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Olsen JD, Manners GD. Toxicology of diterpenoid alkaloids in rangeland larkspur (Delphinium spp.). Toxic Plant Orig. 1989;1:291–326. [Google Scholar]
- 147.Brown M, Vale W. Central nervous system effects of hypothalamic peptides. Endocrinology. 1975;96:1333–6. [DOI] [PubMed] [Google Scholar]
- 148.Markert A, Steffan N, Ploss K, Hellwig S, Steiner U, Drewke C, et al. Biosynthesis and accumulation of ergoline alkaloids in a mutualistic association between Ipomoea asarifolia (Convolvulaceae) and a Clavicipitalean Fungus. Plant Physiol. 2008;147:296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dhyani P, Quispe C, Sharma E, Bahukhandi A, Sati P, Attri DC, et al. Anticancer potential of alkaloids: a key emphasis to colchicine, vinblastine, vincristine, vindesine, vinorelbine and vincamine. Cancer Cell Int. 2022;22:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Colodel EM, Gardner DR, Zlotowski P, Driemeier D. Identification of swainsonine as a glycoside inhibitor responsible for Sida carpinifolia poisoning. Vet Hum Toxicol. 2002;44:177–8. [PubMed] [Google Scholar]
- 151.Barceloux DG. Potatoes, tomatoes, and solanine toxicity (Solanum tuberosum L., Solanum lycopersicum L.). Dis Mon. 2009;55:391–402. [DOI] [PubMed] [Google Scholar]
- 152.Leonard JB, Anderson B, Klein-Schwartz W. Does getting high hurt? Characterization of cases of LSD and psilocybin-containing mushroom exposures to national poison centers between 2000 and 2016. J Psychopharmacol. 2018;32:1286–94. [DOI] [PubMed] [Google Scholar]
- 153.Toennes SW, Harder S, Schramm M, Niess C, Kauert GF. Pharmacokinetics of cathinone, cathine and norephedrine after the chewing of khat leaves. Br J Clin Pharmacol. 2003;56:125–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ramadan LB, Zwawi A, Almaghour H. Toxicity and antioxidant of Arum Cyrenaicum hurby. Egypt J Forensic Sci Appl Toxicol. 2012;12:31–48. [Google Scholar]
- 155.Arrázola G, Grane N, Dicenta F. Quantification of cyanogenic compounds, amygdalin, prunasin, and hydrocyanic acid in almonds (Prunus dulcis Miller) for industrial uses. Rev Colomb Cienc Hortícolas. 2021;15.
- 156.Cooke RD, de la Cruz EM. The changes in cyanide content of cassava (Manihot esculenta crantz) tissues during plant development. J Sci Food Agric. 1982;33:269–75. [Google Scholar]
- 157.Nartey F. Studies on cassava, Manihot utilissima Pohl—I Cyanogenesis: the biosynthesis of linamarin and lotaustralin in etiolated seedlings. Phytochemistry. 1968;7:1307–12. [Google Scholar]
- 158.Curto G, Dallavalle E, Nicola GRD, Lazzeri L. Evaluation of the activity of dhurrin and sorghum towards Meloidogyne incognita. 2012. 10.1163/156854112X627291.
- 159.Senthilkumaran S, Meenakshisundaram R, Thirumalaikolundusubramanian P. Plant Toxins and the Heart. In: Heart and toxins. Boston: Academic Press; 2015. p. 151–74. [Google Scholar]
- 160.Langford SD, Boor PJ. Oleander toxicity: an examination of human and animal toxic exposures. Toxicology. 1996;109:1–13. [DOI] [PubMed] [Google Scholar]
- 161.Shivkar YM, Kumar VL. Anthelmintic activity of latex of Calotropis procera. Pharm Biol. 2003;41:263–5. [Google Scholar]
- 162.Kumar R. Anti-nutritional factors, the potential risks of toxicity and methods to alleviate them. Legume trees fodd trees protein source livest FAO. Anim Prod Health Pap. 1992;102:145–60. [Google Scholar]
- 163.Hixson-Wallace J. Digoxin toxicity: a review. US Pharm. 2006;2:28–36. [Google Scholar]
- 164.Morimoto M, Tatsumi K, Yuui K, Terazawa I, Kudo R, Kasuda S. Convallatoxin, the primary cardiac glycoside in lily of the valley (Convallaria majalis), induces tissue factor expression in endothelial cells. Vet Med Sci. 2021;7:2440–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Üçüncü O, Baltaci C, Akar Z, Duzgun A, Cuce M, Kandemir A. Biological activities and phytochemical screening of ethanol extracts from Adonis paryadrica (Ranunculaceae). Farmacia. 2020;68:1068. [Google Scholar]
- 166.Cuny E. Bioactive Ingredients of Helleborus niger L (Christmas Rose): the renaissance of an old medicinal herb—a review. Nat Prod Commun. 2023;18:1934578X231201053. [Google Scholar]
- 167.Adams M, Berset C, Kessler M, Hamburger M. Medicinal herbs for the treatment of rheumatic disorders—a survey of European herbals from the 16th and 17th century. J Ethnopharmacol. 2009;121:343–59. [DOI] [PubMed] [Google Scholar]
- 168.Guo X, Mei N. Aloe vera: a review of toxicity and adverse clinical effects. J Environ Sci Health Part C. 2016;34:77–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Liu Y, An Z, He Y. The traditional uses, phytochemistry, pharmacology and toxicology of Bergenia purparescens: a review comments and suggestions. Heliyon. 2023;9:e22249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Boo YC. Arbutin as a skin depigmenting agent with antimelanogenic and antioxidant properties. Antioxidants. 2021;10:1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Weiler EW, Zenk MH. Radioimmunoassay for the determination of digoxin and related compounds in Digitalis lanata. Phytochemistry. 1976;15:1537–45. [Google Scholar]
- 172.Apel L, Kammerer DR, Stintzing FC, Spring O. Comparative metabolite profiling of triterpenoid saponins and flavonoids in flower color mutations of Primula veris L. Int J Mol Sci. 2017;18:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jia Z, Koike K, Nikaido T. Major triterpenoid saponins from Saponaria officinalis. J Nat Prod. 1998;61:1368–73. [DOI] [PubMed] [Google Scholar]
- 174.Idris S, Mishra A, Khushtar M. Phytochemical, ethanomedicinal and pharmacological applications of escin from Aesculus hippocastanum L. towards future medicine. J Basic Clin Physiol Pharmacol. 2020;31:5. [DOI] [PubMed] [Google Scholar]
- 175.Wilson CR, Sauer J-M, Hooser SB. Taxines: a review of the mechanism and toxicity of yew (Taxus spp.) alkaloids. Toxicon. 2001;39:175–85. [DOI] [PubMed] [Google Scholar]
- 176.Kleinwächter M, Schnug E, Selmar D. The Glucosinolate−myrosinase system in nasturtium (Tropaeolum majus L): variability of biochemical parameters and screening for clones feasible for pharmaceutical utilization. J Agric Food Chem. 2008;56:11165–70. [DOI] [PubMed] [Google Scholar]
- 177.Majak W. Review of toxic glycosides in rangeland and pasture forages. Rangel Ecol Manag Range Manag Arch. 2001;54:494–8. [Google Scholar]
- 178.Dhawan K, Dhawan S, Sharma A. Passiflora: a review update. J Ethnopharmacol. 2004;94:1–23. [DOI] [PubMed] [Google Scholar]
- 179.Adebowale KO, Lawal OS. Functional properties and retrogradation behaviour of native and chemically modified starch of mucuna bean (Mucuna pruriens). J Sci Food Agric. 2003;83:1541–6. [Google Scholar]
- 180.Sudarkina OJ, Kurmanova AG, Kozlov JV. Production and characterization of the B chains of mistletoe toxic lectins. Mol Biol. 2007;41:601–8. [PubMed] [Google Scholar]
- 181.Lin J, Zhou X, Wang J, Jiang P, Tang K. Purification and characterization of curcin, a toxic lectin from the seed of Jatropha Curcas. Prep Biochem Biotechnol. 2010;40:107–18. [DOI] [PubMed] [Google Scholar]
- 182.Weder JKP, Telek L, Vozári-Hampe M, Saini HS. Antinutritional factors in Anasazi and other pinto beans (Phaseolus vulgaris L.). Plant Foods Hum Nutr. 1997;51:85–98. [DOI] [PubMed] [Google Scholar]
- 183.Turner NJ, Szczawinski AF. Common poisonous plants and mushrooms of North America. 1991.
- 184.Romeis J, Babendreier D, Wäckers FL. Consumption of snowdrop lectin (Galanthus nivalis agglutinin) causes direct effects on adult parasitic wasps. Oecologia. 2003;134:528–36. [DOI] [PubMed] [Google Scholar]
- 185.Samardzic K, Rodgers KJ. Cell death and mitochondrial dysfunction induced by the dietary non-proteinogenic amino acid l-azetidine-2-carboxylic acid (Aze). Amino Acids. 2019;51:1221–32. [DOI] [PubMed] [Google Scholar]
- 186.Kamboj A, Saluja AK. Phytopharmacological review of Xanthium strumarium L. (Cocklebur). Int J Green Pharm IJGP. 2014;4:3. [Google Scholar]
- 187.Liyasova MS, Schopfer LM, Lockridge O. Cresyl saligenin phosphate, an organophosphorus toxicant, makes covalent adducts with histidine, lysine, and tyrosine residues of human serum albumin. Chem Res Toxicol. 2012;25:1752–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lake BG. Coumarin metabolism, toxicity and carcinogenicity: relevance for human risk assessment. Food Chem Toxicol. 1999;37:423–53. [DOI] [PubMed] [Google Scholar]
- 189.Eason CT, Parke DV, Clark B, Smith DA. The mechanism of hepatotoxicity of a chromone carboxylic acid (FPL 52757) in the dog. Xenobiotica. 1982;12:155–64. [DOI] [PubMed] [Google Scholar]
- 190.Ramírez-Pelayo C, Martínez-Quiñones J, Gil J, Durango D. Coumarins from the peel of citrus grown in Colombia: composition, elicitation and antifungal activity. Heliyon. 2019. 10.1016/j.heliyon.2019.e01937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Fathima A, Rao JR. Selective toxicity of Catechin—a natural flavonoid towards bacteria. Appl Microbiol Biotechnol. 2016;100:6395–402. [DOI] [PubMed] [Google Scholar]
- 192.Gregoretti B, Stebel M, Candussio L, Crivellato E, Bartoli F, Decorti G. Toxicity of Hypericum perforatum (St. John’s wort) administered during pregnancy and lactation in rats. Toxicol Appl Pharmacol. 2004;200:201–5. [DOI] [PubMed] [Google Scholar]
- 193.Du G-H. Natural small molecule drugs from plants. Singapore: Springer Singapore; 2018. [Google Scholar]
- 194.Unlu U, Kocabaş A. Dieffenbachia plant poisoning cases and effects on human health. Anatol J Bot. 2020;4:65–8. [Google Scholar]
- 195.Lee ST, Davis TZ, Gardner DR, Colegate SM, Cook D, Green BT, et al. Tremetone and structurally related compounds in white snakeroot (Ageratina altissima): a plant associated with trembles and milk sickness. J Agric Food Chem. 2010;58:8560–5. [DOI] [PubMed] [Google Scholar]
- 196.Uwai K, Ohashi K, Takaya Y, Ohta T, Tadano T, Kisara K, et al. Exploring the structural basis of neurotoxicity in c17-polyacetylenes isolated from water hemlock. J Med Chem. 2000;43:4508–15. [DOI] [PubMed] [Google Scholar]
- 197.Kochanowski M, Kała M. Tetrahydrocannabinols in clinical and forensic toxicology. Przegl Lek. 2005;62:576–80. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data are available in the manuscript.