1. Clinical Presentation
A 19‐year‐old woman developed severe postprandial colicky abdominal pain associated with vomiting and diarrhea. Examination revealed diffusely tender and distended abdomen associated with guarding, hyperactive bowel sounds, a low‐grade fever (37.6°C), and persistently elevated diastolic BP (> 95 mmHg). A month prior, she experienced a sudden left 6th cranial nerve palsy which spontaneously resolved after 3 days and with a normal brain MRI. Her medical history is otherwise unremarkable.
Laboratory tests revealed hemoglobin of 101 g/L, platelet of 445 × 109/L, white cell count of 19.1 × 109/L with neutrophilia (84.6%), and a C‐reactive protein (CRP) of 150 mg/L. ANA was positive; however, anti‐dsDNA, ENA, anti‐MPO/‐PR3, hepatitis B, and infectious work‐up were all negative. Contrast‐enhanced abdominal CT revealed small bowel obstruction necessitating urgent surgery (Figure 1A). Intraoperative macroscopic examination revealed extensive gangrenous small bowel with focal perforations (Figure 1B).
FIGURE 1.
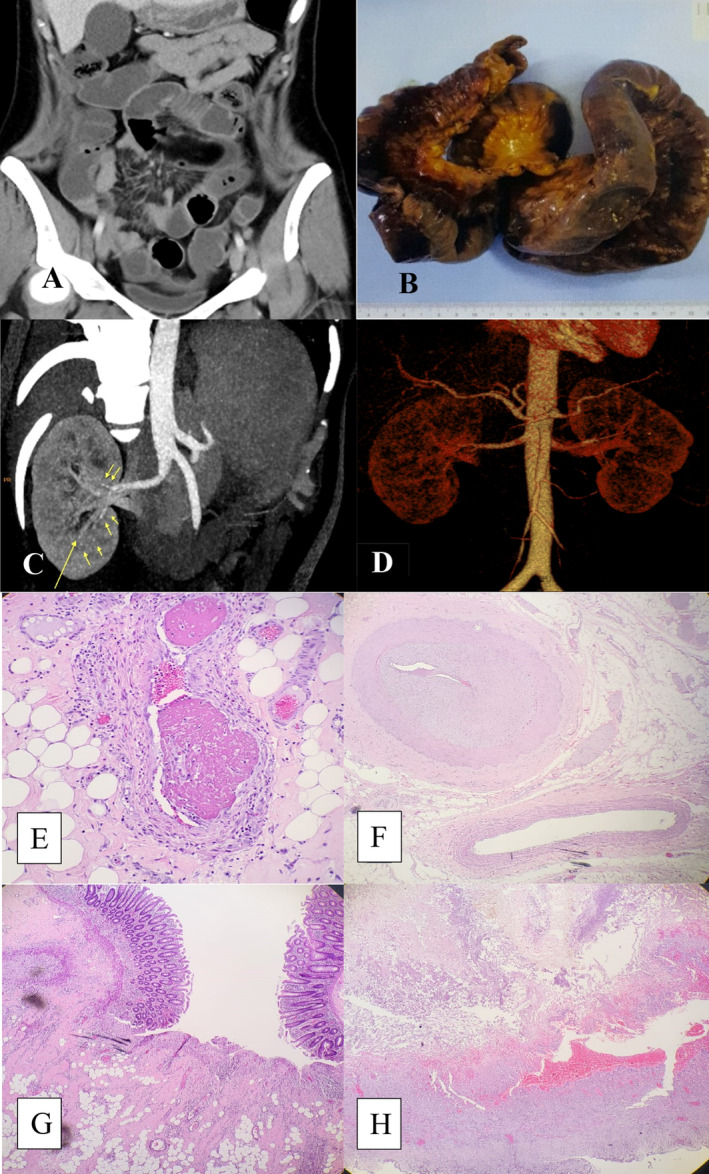
(A) Contrast‐enhanced abdominal CT reveals fluid in small bowel loops, ascending and transverse colon (B) Resected segment of small intestines showing extensive necrotic and gangrenous changes with multiple perforations (C) Reconstructed CT renal angiography demonstrating scattered small hyperdense “dots” along lobar and interlobar arteries (D) 3D‐virtual reconstructive CT angiography revealing large abdominal aorta branches with irregular outlines (E–H): Histopathology of the small intestine and caecum (E, F) Focal vasculitis with lymphocyte infiltration, thrombosed blood vessels, and intimal thickening causing lumen narrowing in medium‐sized vessel (G) Cecal mucosal ulceration with adjacent thickened medium‐sized vessel and vasculitis (H) small bowel transmural necrosis (20× magnification).
Her abdominal symptoms and low‐grade pyrexia unfortunately recurred several days after surgery and progressively worsened despite antibiotic therapy. A repeat infectious work‐up including blood, urine, and stool cultures and swab cultures from the surgical wound were normal or negative. Parenteral nutrition was commenced after the extensive intestinal resection surgery as she was losing weight with persistent loose motion due to the resultant short bowel syndrome.
Following rheumatology consultation, coupled with insights from our radiologist, due to a high index of suspicion for vasculitis, a reconstructed CT renal angiography (RA) was performed from the preoperative abdominal CT images. It showed small hyperdense “dots” scattered along the lobar and interlobar arteries signifying microaneurysms (arrows; Figure 1C). 3D‐virtual reconstructive‐CTRA demonstrated large abdominal aorta branches with irregular outlines (Figure 1D).
In view of the surgical (extensive necrotizing and gangrenous small intestine) and imaging findings, and that septic parameter had been covered with intravenous antibiotic, our patient received three‐1 g‐pulses of intravenous methylprednisolone followed by high‐dose oral steroid taper (over several months), and mycophenolate mofetil (MMF) 500 mg twice daily with good clinical response. Histology results followed after the induction therapy and was consistent with medium‐size vessel vasculitis (Figure 1E–H). Constellation of clinical (including clinical response to treatment), radiological and histopathological findings were in keeping with systemic polyarteritis nodosa (sPAN) causing extensive necrotizing intestinal vasculitis.
The patient was followed up very closely due to the severity and extent of the disease; nevertheless, unfortunately very recently she developed a significant vasculitic neurological manifestation in the form of a stroke which required immunosuppressive escalation from MMF to cyclophosphamide.
Systemic PAN has an insidious course with mononeuritis multiplex affecting up to 70% of patients and gastrointestinal manifestation although uncommon, is an independent predictor of death [1, 2]. Albeit limited, data on 3D‐virtual reconstructive‐CTRA assisting timely PAN diagnosis is promising [3, 4]. This case demonstrates the diagnostic challenge of sPAN resulting in treatment delay and subsequent life‐threatening complication. Recognizing sPAN from unexplained gastrointestinal symptom(s) with raised CRP, persistently elevated BP assisted by appropriate imaging, and histology is crucial as this prognosticate the condition.
We acknowledge our limitation of having a single case report; and consequently, definitive conclusions are limited. Notwithstanding, the major strength of this case is the use of 3D‐reconstructive CT derived from the original preoperative images as an impressive, timely, and promising addition in sPAN, particularly in visualizing vascular abnormalities characteristic of sPAN.
Further studies exploring larger patient cohorts including collaborative efforts may provide conclusive insights into the role of 3D‐imaging in diagnosing sPAN and other types of vasculitis. Additionally, comparative studies analyzing timely diagnostic accuracy between 3D‐reconstruction and other imaging modalities such as PET‐CT or MRI in vasculitis would be invaluable. Longitudinal studies assessing long‐term outcomes of vasculitis patients receiving prompt intervention from early diagnosis via advanced imaging technique are also warranted.
Author Contributions
W.S., F.A., M.M., and Y.H.C. drafted the manuscript and reviewed the literature. W.S., F.A., and O.P.S. critically revised the manuscript. R.S. provided the histology slide and interpretation. C.O.T.O. provided the radiology findings and interpretation. All authors were in agreement at submission.
Consent
Obtained (attached).
Conflicts of Interest
The authors declare no conflicts of interest.
Funding: The authors received no specific funding for this work.
Contributor Information
Wahinuddin Sulaiman, Email: wahinuddin@unikl.edu.my.
Fahd Adeeb, Email: fahd_adeeb@yahoo.com.
Data Availability Statement
The manuscript has not been previously published, nor is it under consideration for publication elsewhere.
References
- 1. Halabi C., Williams E. K., Morshed R. A., et al., “Neurological Manifestations of Polyarteritis Nodosa: A Tour of the Neuroaxis by Case Series,” BMC Neurology 21 (2021): 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Karadag O., Bolek E. C., Ayan G., et al., “Clinical Characteristics and Outcomes of Polyarteritis Nodosa: An International Study,” Arthritis & Rhematology 76 (2024): 1120–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jain N., Shankar B., Mitra S., and Singh Y. P., “Polyarteritis Nodosa: Resolution of Radiological Changes,” Rheumatology (Oxford) 60 (2021): 3481–3482. [DOI] [PubMed] [Google Scholar]
- 4. Scatarige J. C., Urban B. A., Hellmann D. B., and Fishman E. K., “Three‐Dimensional Volume‐Rendering CT Angiography in Vasculitis: Spectrum of Disease and Clinical Utility,” Journal of Computer Assisted Tomography 25 (2001): 598–603. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The manuscript has not been previously published, nor is it under consideration for publication elsewhere.


