Abstract
Background
There are conflicting findings regarding the risk of acute kidney injury (AKI) and mortality with vancomycin/piperacillin-tazobactam combination (VPT) and vancomycin/meropenem (VM). The aim of this meta-analysis was to compare the risk of AKI and mortality between VPT and VM.
Methods
Observational studies reporting the incidence of AKI and mortality in patients receiving VPT or VM between January 2017 and September 2024 were retrieved from PubMed, the Cochrane Library, and Web of Science. The primary outcome of the analysis was the risk of AKI, and the secondary outcomes were the mortality rate, need for renal replacement therapy (RRT), and hospital length of stay (LOS). This meta-analysis was conducted using a random-effects model to estimate the odds ratios (OR) and 95% confidence intervals (CI) for AKI, mortality, and RRT or mean difference and 95% CI for the LOS.
Results
Seventeen studies involving a total of 80,595 patients were included in the analysis. The odds of developing AKI were higher among patients who received the VPT versus those who received the VM combination (OR = 2.02; 95%CI 1.56–2.62). There were no differences between VPT and VM in the mortality and hospital length of stay; however, the odds of requiring RRT were higher among VPT group versus VM group (OR = 1.55; 95%CI 1.23–1.96).
Conclusion
The findings suggest that the use of VPT is associated with a higher risk of AKI compared to VM and highlight the need for cautious antibiotic selection and monitoring of renal function in patients receiving these combinations.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-024-10227-0.
Keywords: Vancomycin, Beta-lactams, Piperacillin-tazobactam, Meropenem, Acute kidney injury, Nephrotoxicity
Introduction
Acute kidney injury (AKI) is a medical condition that is marked by an abrupt and pronounced decline in kidney function. The incidence of AKI can reach up to 22% in hospitalized patients, with a mortality rate of 11% [1]. Many risk factors have been linked to AKI, including age, race, elevated baseline serum creatinine (SCr) level, and medications [2]. Vancomycin is an antimicrobial agent used in hospitalized patients to treat gram-positive bacterial infections, such as methicillin-resistant staphylococcus aureus (MRSA) [3]. Vancomycin-induced AKI is a significant adverse effect that can be developed in patients treated with vancomycin. Patients who are receiving high doses of vancomycin, have high severity of illness, or are on concomitant nephrotoxic agents, including antimicrobial agents are at high risk of developing vancomycin-induced AKI [3].
Broad-spectrum antimicrobial agents that target Pseudomonas aeruginosa are commonly combined with vancomycin for empirical coverage in patients with suspected sepsis or nosocomial infections. Several observational studies have reported that the use of vancomycin/piperacillin-tazobactam combination (VPT) can increase the risk of AKI compared to vancomycin alone or an alternative beta-lactam agent, such as meropenem or cefepime. This has caused many clinicians to become hesitant to use VPT, and use an alternative agent instead to minimize the risk of AKI [4, 5].
Despite the lack of randomized controlled trials comparing the risk of AKI between VPT and vancomycin with meropenem (VM), recent observational studies have reported conflicting findings regarding which combination poses higher risk of developing AKI [6–22]. Therefore, the aim of this meta-analysis was to compare the risk of AKI between VPT and VM.
Materials and methods
Data source and search strategy
Observational studies reporting the incidence of AKI in patients receiving VPT or VM between January 2017 and April 2024 were retrieved from PubMed, the Cochrane Library, and Web of Science. The following terms were used in our search strategy: vancomycin, piperacillin, piperacillin/tazobactam, meropenem, beta-lactams, and acute kidney injury. Additionally, we reviewed the references of other systematic reviews and meta-analyses to identify any pertinent studies. The comprehensive search strategy used is available in Supplementary Material (Table S2).
Study selection, data extraction, and risk of bias assessment
Observational studies comparing the occurrence of AKI in adult patients (> 18 years) treated with VPT or VM for a minimum of 48 h were included. Studies unavailable in full-text, including abstracts, were excluded. We also excluded animal studies, pediatric studies, and studies that did not compare VPT with VM or compared VPT to vancomycin with meropenem or cefepime as one group. All studies were reviewed for eligibility, and data extraction was performed by two authors (AMA and AA), with confirmation by a third author (LA). The primary outcome of the analysis was the risk of AKI, and the secondary outcomes were the mortality rate, need for renal replacement therapy (RRT), and hospital length of stay (LOS). In addition to these outcomes, the author’s name, year of publication, country, study design, inpatient setting, sample size, intervention, comparator, and AKI definition were extracted for each study. The methodological quality of each study was assessed using the Newcastle-Ottawa Scale (NOS) by two independent investigators (AMA and AA) [23].
Statistical analysis
This meta-analysis was conducted using a random-effects model using inverse variance method to estimate the odds ratios (OR) and 95% confidence intervals (CI) for the risk of AKI, mortality, and RRT between VPT and VM. The model was also used to report the mean difference in hospital LOS between the two groups. The model utilized restricted maximum-likelihood estimator for heterogeneity between the trials which was reported using the I2 test with 95% confidence interval. I2 values of 25–50%, 50–75%, and > 75% where used to indicate low, moderate, and high heterogeneity, respectively [24, 25]. Also, we estimated the prediction interval to further explore the heterogeneity [26]. Subgroup analyses were performed based on clinical setting, mortality definitions, and AKI severity. Moderate AKI was defined according to the following criteria: RIFLE Injury, KDIGO Stage II, and AKIN Injury, while RIFLE Failure, KDIGO Stage III, and AKIN Failure were classified as severe AKI in the subgroup. All statistical analyses were performed using the R language version 4.0.4. Publication bias was assessed via visual inspection of the funnel plot, Egger’s test (for ≥ 10 studies) (Figure S1), and the Duval and Tweedie Trim-and-Fill method (Figure S2) [27]. This systematic review and meta-analysis was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guideline [28].
Results
Study characteristics
The search yielded a total of 662 observational studies. However, 645 studies were excluded because they did not align with the inclusion criteria for this meta-analysis based on the population, interventions of interest, and measured outcomes (Fig. 1). Seventeen studies involving a total of 80,595 patients were included in the analysis [6–22]. The sample size in each study ranged between 76 and 61,529 patients. The majority of the studies were retrospective, with only one being prospective. Seven studies were conducted including patients from both critical (ICU) and non-critical care (non-ICU) settings, four included patients from ICU settings only, and six included patients from non-ICU settings only. To identify AKI events in the sixteen studies, nine used the Kidney Disease Improving Global Outcomes (KDIGO) guidelines [29], three used the Acute Kidney Injury Network (AKIN) criteria [30], four used the RIFLE criteria developed by the Acute Dialysis Quality Initiative group [31], and only two used study specific prespecified criteria (an increase of serum creatinine (Scr) by at least 0.5 mg/dL or 50% from baseline (Table 1). The quality assessment NOS score for all included studies was 7–9 (Table S3).
Fig. 1.
Flow diagram for selection of studies for the meta-analysis
Table 1.
Summary of studies that were included in the systematic review and meta-analysis
| First author | Year | Country | Type | AKI Definition | Setting | Sample Size | Incidence of AKI | p-value | |
|---|---|---|---|---|---|---|---|---|---|
| VPT | VM | ||||||||
| Alyami | 2017 | USA | Retrospective Cohort | KDIGO | ICU & Non-ICU | 183 |
8/108 (7.4%) |
4/75 (5.3%) |
0.400 |
| Balcı | 2018 | Turkey | Retrospective Cohort | AKIN | Non-ICU | 132 |
26/63 (41.3%) |
7/69 (10.1%) |
< 0.001 |
| Cannon | 2018 | USA | Retrospective Cohort | 0.5 mg/dL increase in SCr or > 50% from baseline | ICU & Non-ICU | 366 |
74/292 (25.3%) |
8/74 (9.5%) |
0.008 |
| Robertson | 2018 | USA | Retrospective Cohort | 0.5 mg/dL increase in SCr or > 50% from baseline | Non-ICU | 169 |
14/85 (16.5%) |
3/84 (3.6%) |
0.009 |
| Mullins | 2018 | USA | Prospective Cohort | AKIN | Non-ICU | 141 |
28/94 (29.8%) |
7/49 (14.3%) |
< 0.001 |
| Blevins | 2019 | USA | Retrospective Cohort | KDIGO | ICU | 758 |
144/366 (39.3%) |
92/392 (23.5%) |
< 0.0001 |
| Ide | 2019 | Japan | Retrospective Cohort | KDIGO | Non-ICU | 76 |
9/27 (33.3%) |
4/49 (8.2%) |
0.015 |
| Kang | 2019 | South Korea | Retrospective Cohort | KDIGO | ICU | 157 |
39/74 (52.7%) |
23/83 (27.7%) |
< 0.0001 |
| Rutter | 2019 | USA | Retrospective Cohort | RIFLE | Non-ICU | 10,236 |
2713/9898 (27.4%) |
52/338 (15.4%) |
< 0.001 |
| Schreier | 2019 | USA | Retrospective Cohort | AKIN | ICU | 1926 |
601/1540 (39.0%) |
135/386 (34.9%) |
0.49 |
| Aslan | 2021 | Turkey | Retrospective Cohort | RIFLE | ICU & Non-ICU | 100 |
20/50 (40.0%) |
13/50 (26.0%) |
0.028 |
| Lee | 2021 | USA | Retrospective Cohort | KDIGO | ICU & Non-ICU | 61,529 |
6939/56,396 (12.3%) |
465/5133 (9.1%) |
< 0.0001 |
| Liu | 2021 | China | Retrospective Cohort | KDIGO | ICU & Non-ICU | 526 |
12/79 (15.2%) |
27/447 (6.0%) |
0.004 |
| Rungkitwattanakul | 2021 | USA | Retrospective Cohort | KDIGO | Non-ICU | 141 |
16/74 (21.6%) |
5/67 (7.4%) |
0.002 |
| Tookhi | 2021 | Saudi Arabia | Retrospective Cohort | KDIGO | ICU & Non-ICU | 158 |
8/77 (10.3%) |
17/81 (20.9%) |
0.07 |
| Chen | 2023 | USA | Retrospective Cohort | KDIGO | ICU | 3648 |
334/1824 (18.3%) |
274/1824 (15.0%) |
0.007 |
| Wu | 2024 | Retrospective Cohort | RIFLE | ICU & Non-ICU | 349 |
36/154 (23.4%) |
21/195 (10.8%) |
0.002 | |
Abbreviations: AKI: Acute Kidney Injury; KDIGO: Kidney Disease: Improving Global Outcomes; SCr: Serum Creatinine; RIFLE: Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease; AKIN: Acute Kidney Injury Network; ICU: Intensive Care Unit; VPT: vancomycin and piperacillin/tazobactam; VM: vancomycin and meropenem
Outcomes from the comparison between VPT and VM combinations
AKI
Overall analysis
The pooled analysis of data from the 17 studies comparing the risk of AKI between VPT and VM combinations indicated that the odds of developing AKI were higher among patients who received the VPT versus those who received the VM combination (OR = 2.02; 95%CI 1.56–2.62; I2 = 72% (95%CI 54.9%, 82.9%), prediction interval (0.79, 5.16) (Fig. 2).
Fig. 2.
Risk of acute kidney injury (AKI) in vancomycin/piperacillin-tazobactam combination (VPT) [Experimental group] versus vancomycin with meropenem (VM) [control group]. I2 = 72.3% [CI 54.9%; 82.9%]
Subgroup analysis based on the clinical settings
When the data from the studies was analyzed in accordance with the clinical settings, critical care patients who received the VPT combination had higher odds of AKI than those who received the VM combination (OR = 1.62; 95%CI 1.12–2.35; I2 = 79% (95%CI 44.4%, 92.2%), prediction interval (0.31, 8.47). Moreover, patients in non-ICU settings who received the VPT combination had higher odds of AKI compared to those who received the VM combination (OR = 3.33; 95%CI 1.95–5.69; I2 = 50% (95%CI 0.0%, 81.7%), prediction interval (0.68, 16.35). The combined results of 9 studies conducted in both ICU and non-ICU settings revealed that the odds of developing AKI were also higher among patients who received the VPT combination (OR = 1.81; 95%CI 1.20–2.72; I2 = 64% (95%CI 21.9%, 83.0%), prediction interval (0.54, 6.03)) (Figures S3).
Subgroup analysis based on the AKI Severity
The pooled analysis of studies reporting moderate AKI found no statistically significant association between VPT combination and moderate AKI when compared to VM combination (OR = 1.46; 95%CI 0.96–2.24; I2 = 69% (95%CI 37.8%, 84.5%), prediction interval (0.44, 4.86)) (Figure S4). However, there was a statistically significant association between VPT combination and severe AKI when compared to VM combination (OR = 2.04; 95%CI 1.27–3.27; I2 = 39% (95%CI 0.0%, 72.1%), prediction interval (0.44, 4.86) (Figure S4).
Mortality
Overall analysis
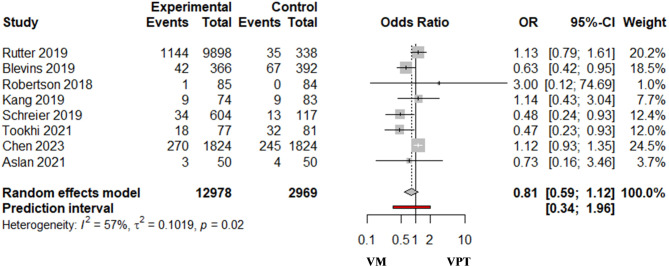
The pooled analysis of 8 studies that reported mortality indicated no difference in the odds of mortality between the VPT and VM combinations (OR = 0.81; 95%CI 0.59–1.12; I2 = 57% (95%CI 6.6%, 80.6%), prediction interval (0.34, 1.96)) (Fig. 3).
Fig. 3.
Risk of mortality in vancomycin/piperacillin-tazobactam combination (VPT) [Experimental group] versus vancomycin with meropenem (VM) [control group]. I2 = 57.5% [Cl 6.6%; 80.6%]
Subgroup analysis based on the clinical settings
In the analysis of data from the studies based on the clinical settings, the results showed non-statistically significant difference in the odds of mortality among ICU patients (OR = 0.80; 95% CI 0.52–1.22; I2 = 72% (95%CI 21.6%, 90.2%), prediction interval (0.14, 4.58), or non-ICU patients (OR = 1.14; 95% CI 0.80–1.63; I2 = 0%) who received the VPT compared to those who received VM combinations (Figure S5). However, the results from two studies including patients from ICU and non-ICU settings indicated a 50% lower odd among patients who received VM than those who received VPT (OR = 0.50; 95%CI 0.27–0.95; I2 = 0%) (Figure S5).
Subgroup analysis based on the mortality definition
The pooled analysis based on morality definitions revealed no statistically significant difference in the odds of mortality between the VPT and VM combinations, with OR = 0.86; 95% CI 0.59–1.26; I2 = 66% (95%CI 11.5%, 87.0%), prediction interval (0.25, 2.92) for inpatient mortality, and OR = 1.00; 95% CI 0.44–2.3; I2 = 0% for 30-days mortality. Only one trial reported 90-days mortality, which indicated a statistically significant reduction in the risks of mortality in the VPT group over the VM group (OR = 0.48; 95% CI 0.24–0.93) (Figure S6).
RRT
Overall analysis
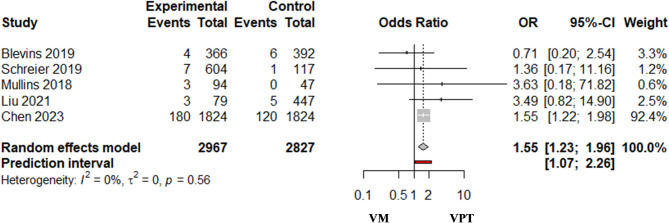
The pooled analysis of five studies that reported the need for RRT indicated that the odds of requiring RRT were higher among patients who received VPT versus those who received VM (OR = 1.55; 95%CI 1.23–1.96; I2 = 0.0% (95%CI 0.0%, 79.2%), Prediction interval (1.07, 2.26) (Fig. 4).
Fig. 4.
Renal replacement therapy in vancomycin/piperacillin-tazobactam combination (VPT) [Experimental group] versus vancomycin with meropenem (VM) [control group]. I2 = 0.0% [Cl 0.0%; 79.2%]
Subgroup analysis based on the clinical settings
The combined results of three studies conducted among critical care patients indicated 48% higher odds of RRT requirements among those who received VPT than those who received VM (OR = 1.48; 95%CI 1.07–2.04; I2 = 0% (95%CI 0.0%, 89.6%), Prediction interval (0.11, 19.30). A single study was conducted including patients from non-ICU settings (OR = 3.63; 95%CI 0.18–71.82), and another one conducted including patients from both ICU and non-ICU settings (OR = 3.49; 95%CI 0.82–14.9) showed non-significant difference between the VPT and VM combinations (Figure S7).
Hospital length of stay (LOS)
The mean difference (MD) in the length of hospital stay was not statistically different for patients who received the VPT compared to those who received the VM combination (MD = -0.06 days, 95%CI -0.72–0.60, I2 = 62.5% (95%CI 19.3%, 82.6%), Prediction interval (-1.76, 1.64) (Fig. 5).
Fig. 5.
Hospital length of stay in vancomycin/piperacillin-tazobactam combination (VPT) [Experimental group] versus vancomycin with meropenem (VM) [control group]. I2 = 62.5% [19.3%; 82.6%]
Publication bias analysis
The presence of publication bias was evaluated using Egger’s test, which indicated significant asymmetry in the funnel plot for AKI studies (p-value = 0.0086) (Figure S1). This result suggests potential small study bias within the included studies. To address publication bias, the Duval and Tweedie Trim-and-Fill method was applied (Figure S2). This method imputed six additional studies to account for potential missing data due to publication bias. After adjusting for these imputed studies, the corrected OR for AKI among patients who received the VPT versus those who received the VM combination was 1.54 (95% CI: 1.12–2.11), which is slightly lower than the initial estimate but remains statistically significant (Table S4).
Discussion
In this systematic review and meta-analysis, we evaluated the risk of AKI and mortality associated with the concomitant use of vancomycin with either piperacillin/tazobactam (VPT) or meropenem (VM) in a mixed population of critically and non-critically ill patients. A total of 17 studies met the inclusion criteria, providing a total sample size of 80,663 patients. The majority of the included studies were retrospective, with only one being prospective.
Our findings regarding the association between the VPT use and AKI has been previously explored in meta-analysis, which found a greater risk of nephrotoxicity with VPT [32]. The overall incidence of AKI in patients receiving VPT varied widely, ranging between 7.4% and 40% [6, 15], which could be attributed to differences in the definitions of AKI across studies. Our analysis revealed a 100% higher risk of developing AKI in patients receiving VPT combination compared to those receiving VM combination, in both ICU and non-ICU settings. This finding aligns with existing data showing increased creatinine levels associated with VPT use.
It is important to note that while serum creatinine was predominantly used as a surrogate marker for kidney function and nephrotoxicity, this can be misleading [33]. VPT has been shown to increase serum creatinine concentration by competitively inhibiting tubular secretion of creatinine without necessary affecting glomerular filtration rate (GFR) [34]. This phenomenon supports the notion that some of the AKI reported in these studies might reflect pseudo-nephrotoxicity, rather than true nephron damage.
The exact mechanism of nephrotoxicity associated with the use of VPT remains poorly defined. Prospective clinical studies and data from animal studies that looked at other biomarkers such as cystatin-C found that piperacillin-tazobactam had a nephroprotective effect [34–38]. In one study, a decline in eGFR was observed among rats treated with vancomycin alone but not those who received VPT [36].
Identifying risk factors for AKI is essential in clinical practice. However, it remains challenging due to competing etiologies and the lack of accurate diagnostic tools [39]. Studies have shown that older age, chronic kidney disease, and the use of other nephrotoxic drugs such as non-steroidal anti-inflammatory drugs, vancomycin and piperacillin/tazobactam increased the susceptibility to drug induced AKI [40]. In a large pharmacoepidemiologic study investigating nephrotoxic drugs in ICU patients highlighted that confounding due to the presence of multiple nephrotoxic drugs, complicated the association between specific drug groups and AKI. This emphasizes the need for more comprehensive adjustment for confounding factors in AKI risk assessments in critically ill populations [41]. Understanding these risk factors allows for targeted preventative strategies, such as optimizing fluid management and intravenous magnesium sulfate, which can mitigate the risk of AKI high risk critically ill patients [42].
Our analysis did not show a significant difference in mortality between patients who received the VPT or VM combinations. The pooled analysis of eight studies reporting mortality outcomes demonstrated comparable odds of mortality in both groups. This suggests that while VPT may increase AKI incidence, it does not translate to higher mortality risk. These findings are supported by earlier studies showing that AKI associated with VPT is not always linked to increased mortality in critically ill patients [6, 32]. However, heterogenicity in the duration for the mortality outcomes, such as in-patient, 30-day, 90-day mortality, may have influenced these findings. Subgroup analysis based on clinical settings further indicated no significant difference in mortality between VPT and VM in both ICU and non-ICU populations. However, when combining ICU and non-ICU settings, we observed 50% lower odds of mortality among patients receiving VM compared to VPT, suggesting that VM may confer a mortality benefit in broader clinical settings. This outcome could be influenced by variations in patient severity, comorbidities, or antibiotic pharmacodynamics, which warrant further exploration in further studies.
The requirement for renal replacement therapy (RRT) was significantly higher among patients receiving VPT treatment. However, evidence from studies conducted in non-ICU settings or both ICU and non-ICU settings did not show a statistically significant difference in RRT requirements between the VPT and VM combinations. Nevertheless, the need for RRT was only evaluated in five studies in this meta-analysis. These results echo those of a previous meta-analysis [32]. This challenges the notion that piperacillin-tazobactam is a pseudo-nephrotoxin that increases creatinine without affecting the glomerular function filtration rate [20], indicating the possibility of true nephrotoxicity in certain patient populations.
We also assessed the length of hospital stay as another outcome measure, but our analysis did not find any difference in the length of hospital stay between VPT and VM groups. This suggests that antibiotic choice may not significantly impact the duration of hospitalization.
Our study has some limitations that need to be addressed in future studies. The included studies were predominantly retrospective, which may introduce potential biases, including publication bias. While the Duval and Tweedie Trim-and-Fill method was applied mitigate the issue, it could not fully eliminate the influence of small study bias. The interplay between drug exposure and underlying conditions such as chronic diseases and hemodynamic instability, may serve as indications or contraindications to the drugs themselves, creating potential confounding. It was not reported in the included studies, but concurrent use of nephrotoxic drugs such as non-steroidal anti-inflammatory drugs, proton-pump-inhibitors, and diuretics could act as confounders, complicating the interpretation of our findings. Additionally, the heterogeneity in study design, patient populations, and outcome definitions across the studies, which could influence the pooled results. The substantial heterogeneity reported as I2 in the overall AKI (72%) was analyzed utilizing subgroup analysis in the clinical context, which lowered I2 to 50% in non-ICU settings and 64% in both ICU and non-ICU settings. However, only three studies in the ICU subgroup were included, with a high heterogeneity level of I2 = 79% and a broad prediction interval for the point estimate, indicating that about two-thirds of the studies may demonstrate a greater incidence of AKI in the VPT group. Furthermore, subgroup analysis based on the AKI severity reduced heterogeneity to I2 = 69% with prediction interval (0.44–4.86), indicating a higher proportion of studies that estimate a higher risk of moderate AKI in the VPT group. The heterogeneity was similarly reduced to I2 = 39% for severe AKI subgroup with prediction interval (0.66–6.30) demonstrating a greater proportion of studies estimating a higher risk of the severe AKI in VPT group. Nevertheless, the methodological quality of the included studies (NOS scores 7–9) supports the overall validity of our conclusions.
Conclusions
In conclusion, our findings suggest that VPT use is associated with a higher risk of AKI compared to VM. though no significant difference was observed in mortality or length of hospital stay between the two regimens. These results highlight the need for cautious antibiotic selection and monitoring of renal function in critically ill patients receiving these combinations. Further research, including well-designed prospective studies, is warranted to confirm these findings and clarify the underlying mechanisms contributing to the observed associations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to extend their appreciation to Princess Nourah bint Abdulrahman University Researchers Supporting Project for funding this work through the Researcher Supporting Project (PNURSP2024R418).
Author contributions
Conceptualization, M.S.A. and A.M.A.; methodology, A.M.A. and A.R.A.; software, A.R.A.; validation, A.R.A., M.S.A. and O.A.A.; formal analysis, A.R.A.; investigation, M.S.A.; resources, M.S.A.; writing—original draft preparation, A.M.A., A.A., L.A., and A.M.B ; writing—review and editing, A.M.A, M.S.A., O.A.A., A.R.A. and O.M.A.; supervision, M.S.A., O.A.A., and A.R.A.; project administration, M.S.A., O.A.A., A.R.A. and A.M.A.; funding acquisition, A.M.B.
Funding
This work was supported by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2024R418), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Data availability
The data supporting the findings of this review will be made available by the corresponding author, upon request.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Levey A.S. Defining AKD: the Spectrum of AKI, AKD, and CKD. Nephron. 2022;146(3):302–5. 10.1159/000516647. [DOI] [PubMed] [Google Scholar]
- 2.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A. Acute kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sawada A, Kawanishi K, Morikawa S, Nakano T, Kodama M, Mitobe M, Taneda S, Koike J, Ohara M, Nagashima Y, et al. Biopsy-proven Vancomycin-induced acute kidney injury: a case report and literature review. BMC Nephrol. 2018;19(1):72. 10.1186/s12882-018-0845-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim T, Kandiah S, Patel M, Rab S, Wong J, Xue W, Easley K, Anderson AM. Risk factors for kidney injury during Vancomycin and piperacillin/tazobactam administration, including increased odds of injury with combination therapy. BMC Res Notes. 2015;8(1):579. 10.1186/s13104-015-1518-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Contreiras C, Legal M, Lau TT, Thalakada R, Shalansky S, Ensom MH. Identification of risk factors for nephrotoxicity in patients receiving extended-duration, high-trough Vancomycin therapy. Can J Hosp Pharm. 2014;67(2):126–32. 10.4212/cjhp.v67i2.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al Yami MS. Comparison of the incidence of acute kidney injury during treatment with Vancomycin in combination with piperacillin-tazobactam or with meropenem. J Infect Public Health. 2017;10(6):770–3. 10.1016/j.jiph.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Balcı C, Uzun Ö, Arıcı M, Hayran SA, Yüce D, Ünal S. Nephrotoxicity of piperacillin/tazobactam combined with Vancomycin: should it be a concern? Int J Antimicrob Agents. 2018;52(2):180–4. 10.1016/j.ijantimicag.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 8.Cannon JM, Douce RW, Grubbs ER et al. Comparison of Acute Kidney Injury During Treatment with Vancomycin and either Piperacillin-Tazobactam or Meropenem. Spartan Med Res J. 2017;2(2):6440. Published 2017 Dec 19. 10.51894/001c.6440 [DOI] [PMC free article] [PubMed]
- 9.Robertson AD, Li C, Hammond DA, Dickey TA. Incidence of Acute kidney Injury among patients receiving the combination of Vancomycin with Piperacillin-Tazobactam or Meropenem. Pharmacotherapy. 2018;38(12):1184–93. 10.1002/phar.2179. [DOI] [PubMed] [Google Scholar]
- 10.Mullins BP, Kramer CJ, Bartel BJ, Catlin JS, Gilder RE. Comparison of the nephrotoxicity of Vancomycin in Combination with Cefepime, Meropenem, or Piperacillin/Tazobactam: a prospective, Multicenter Study. Ann Pharmacother. 2018;52(7):639–44. 10.1177/1060028018757497. [DOI] [PubMed] [Google Scholar]
- 11.Blevins AM, Lashinsky JN, McCammon C, Kollef M, Micek S, Juang P. Incidence of Acute kidney Injury in critically ill patients receiving vancomycin with concomitant Piperacillin-Tazobactam, Cefepime, or Meropenem. Antimicrob Agents Chemother. 2019;63(5):e02658–18. 10.1128/AAC.02658-18. Published 2019 Apr 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ide N, Sato S, Sawaguchi K. Risk of Acute kidney Injury in patients treated with vancomycin and Piperacillin/Tazobactam Compared to Vancomycin and Meropenem or Doripenem: a retrospective cohort study risk of Acute kidney Injury in patients treated with vancomycin and Piperacillin/Tazobactam Compared to Vancomycin and Meropenem or Doripenem: a retrospective cohort study. Yakugaku Zasshi. 2019;139:1609–14. [DOI] [PubMed] [Google Scholar]
- 13.Kang S, Park J, Yu YM, Park MS, Han E, Chang MJ. Comparison of acute kidney injury and clinical prognosis of Vancomycin Monotherapy and combination therapy with beta-lactams in the intensive care unit. PLoS ONE. 2019;14(6):e0217908. 10.1371/journal.pone.0217908. Published 2019 Jun 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rutter WC, Burgess DS. Incidence of Acute Kidney Injury among Patients Treated with Piperacillin-Tazobactam or Meropenem in Combination with Vancomycin. Antimicrob Agents Chemother. 2018;62(7):e00264-18. Published 2018 Jun 26. 10.1128/AAC.00264-18 [DOI] [PMC free article] [PubMed]
- 15.Schreier DJ, Kashani KB, Sakhuja A, et al. Incidence of Acute kidney Injury among critically ill patients with brief empiric use of Antipseudomonal β-Lactams with Vancomycin. Clin Infect Dis. 2019;68(9):1456–62. 10.1093/cid/ciy724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aslan AT, Pashayev T, Dağ O, Akova M. Comparison of teicoplanin versus Vancomycin in combination with piperacillin-tazobactam or meropenem for the risk of acute kidney injury. Eur J Clin Microbiol Infect Dis. 2021;40(9):1953–61. 10.1007/s10096-021-04258-7. [DOI] [PubMed] [Google Scholar]
- 17.Lee JD, Heintz BH, Mosher HJ, Livorsi DJ, Egge JA, Lund BC. Risk of Acute kidney Injury and Clostridioides difficile infection with Piperacillin/Tazobactam, Cefepime, and Meropenem with or without Vancomycin. Clin Infect Dis. 2021;73(7):e1579–86. 10.1093/cid/ciaa1902. [DOI] [PubMed] [Google Scholar]
- 18.Liu K, Zhang Y, Xu X, et al. Comparative prevalence of Acute kidney Injury in Chinese patients receiving vancomycin with concurrent β-Lactam antibiotics: a retrospective cohort study. Clin Ther. 2021;43(10):e319–51. 10.1016/j.clinthera.2021.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Rungkitwattanakul D, Ives AL, Harriott NG, Pan-Chen S, Duong L. Comparative incidence of acute kidney injury in patients on Vancomycin therapy in combination with cefepime, piperacillin-tazobactam or meropenem. J Chemother. 2022;34(2):103–9. 10.1080/1120009X.2021.1965334. [DOI] [PubMed] [Google Scholar]
- 20.Tookhi RF, Kabli NA, Huntul MA, Thabit AK. Impact of combining Vancomycin with piperacillin/tazobactam or with meropenem on Vancomycin-induced nephrotoxicity. Intern Emerg Med. 2021;16(4):975–9. 10.1007/s11739-020-02624-5. [DOI] [PubMed] [Google Scholar]
- 21.Chen AY, Deng CY, Calvachi-Prieto P, et al. A large-scale Multicenter Retrospective Study on Nephrotoxicity Associated with Empiric Broad-Spectrum antibiotics in critically ill patients. Chest. 2023;164(2):355–68. 10.1016/j.chest.2023.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu D, Wang X, Li G, et al. Risk of acute kidney injury in patients receiving Vancomycin and concomitant piperacillin-tazobactam or carbapenem: a multicenter, retrospective cohort study. Expert Opin Drug Saf Published Online August. 2024;19. 10.1080/14740338.2024.2393263. [DOI] [PubMed]
- 23.Wells G, Shea B, O’Connell D, Peterson j, Welch V, Losos M, Tugwell P. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. ᅟ 2000, ᅟ.
- 24.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanriver-Ayder E, Faes C, van de Casteele T, McCann SK, Macleod MR. Comparison of commonly used methods in random effects meta-analysis: application to preclinical data in drug discovery research. BMJ Open Sci. 2021;5(1):e100074. 10.1136/bmjos-2020-100074. Published 2021 Feb 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borenstein MICHAEL, Cooper HM, Hedges LV, Valentine JC. (2019). Heterogeneity in meta-analysis. The handbook of research synthesis and meta-analysis, 3, 453–470.
- 27.Page MJ, Higgins JPT, Sterne JAC. Chapter 13: Assessing risk of bias due to missing evidence in a meta-analysis [last updated August 2024]. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.5. Cochrane, 2024.
- 28.Page MJ, McKenzie JE, Bossuyt PM et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. Published 2021 Mar 29. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed]
- 29.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 Clinical Practice Guideline for the management of glomerular diseases. Kidney Int. 2021;100(4S):S1–276. 10.1016/j.kint.2021.05.021. [DOI] [PubMed] [Google Scholar]
- 30.Mehta RL, Kellum JA, Shah SV, et al. Acute kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204–12. 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alshehri AM, Alzahrani MY, Abujamal MA, et al. Comparative risk of Acute Kidney Injury Following Concurrent Administration of Vancomycin with Piperacillin/Tazobactam or Meropenem: a systematic review and Meta-analysis of Observational studies. Antibiot (Basel). 2022;11(4):526. 10.3390/antibiotics11040526. Published 2022 Apr 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. 2012;2(1):1-138. https://kdigo.org/wp-content/uploads/2016/10/KDIGO-2012-AKI-Guideline-English.pdf
- 34.Pais GM, Liu J, Avedissian SN, et al. Lack of synergistic nephrotoxicity between Vancomycin and piperacillin/tazobactam in a rat model and a confirmatory cellular model. J Antimicrob Chemother. 2020;75(5):1228–36. 10.1093/jac/dkz563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miano TA, Hennessy S, Yang W, et al. Association of Vancomycin plus piperacillin–tazobactam with early changes in creatinine versus cystatin C in critically ill adults: a prospective cohort study. Intensive Care Med. 2022;48(9):1144–55. 10.1007/s00134-022-06811-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang J, Pais GM, Valdez K, Marianski S, Barreto EF, Scheetz MH. Glomerular function and urinary biomarker changes between Vancomycin and Vancomycin plus Piperacillin-Tazobactam in a translational rat model. Antimicrob Agents Chemother. 2022;66(3):e02132–21. 10.1128/aac.02132-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hayashi T, Watanabe Y, Kumano K, et al. Protective effect of piperacillin against nephrotoxicity of cephaloridine and gentamicin in animals. Antimicrob Agents Chemother. 1988;32(6):912–8. 10.1128/AAC.32.6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hayashi T, Watanabe Y, Kumano K, et al. Protective effect of piperacillin against the nephrotoxicity of cisplatin in rats. Antimicrob Agents Chemother. 1989;33(4):513–8. 10.1128/AAC.33.4.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen JJ, Kuo G, Hung CC, et al. Risk factors and prognosis assessment for acute kidney injury: the 2020 consensus of the Taiwan AKI Task Force. J Formos Med Assoc. 2021;120(7):1424–33. 10.1016/j.jfma.2021.02.013. [DOI] [PubMed] [Google Scholar]
- 40.Yousif ZK, Koola JD, Macedo E, et al. Clinical characteristics and outcomes of Drug-Induced Acute kidney Injury cases. Kidney Int Rep. 2023;8(11):2333–44. 10.1016/j.ekir.2023.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yasrebi-de Kom IAR, Dongelmans DA, Abu-Hanna A, et al. Acute kidney injury associated with nephrotoxic drugs in critically ill patients: a multicenter cohort study using electronic health record data. Clin Kidney J. 2023;16(12):2549–58. 10.1093/ckj/sfad160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khalili H, Rahmani H, Mohammadi M, Salehi M, Mostafavi Z. Intravenous magnesium sulfate for prevention of Vancomycin plus piperacillin-tazobactam induced acute kidney injury in critically ill patients: an open-label, placebo-controlled, randomized clinical trial. DARU J Pharm Sci. 2021;29(2):341–51. 10.1007/s40199-021-00411-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this review will be made available by the corresponding author, upon request.