Combined spatial transcriptomics and single-cell profiling maps cellular ecosystems in gastric cancer, revealing heterogeneity, evolutionary trajectories, and ecosystem states associated with clinical outcomes and molecular subtypes.
Abstract
Gastric cancer is a major cause of global cancer mortality. To explore geospatial interactions in gastric tumors, we integrated 2,138 spatial transcriptomic regions of interest with 152,423 single-cell expression profiles across 226 cancer samples from 121 patients. We observed pervasive expression-based intratumor heterogeneity, recapitulating tumor progression through spatially localized and functionally ordered subgroups associated with specific immune microenvironments, checkpoint profiles, and genetic drivers (SOX9). Phylogenetic analysis revealed two separate evolutionary trajectories (branched evolution and internal diaspora evolution) associated with distinct molecular subtypes, clinical prognoses, and stromal neighborhoods, including VWF+ ACKR1+ endothelial cells. Spatial analysis of tumor–stroma interfaces across multiple gastric cancers highlighted new ecosystem states not attributable to mere tumor/stroma admixture, landmarked by increased GREM1 expression. Our results provide insights into how the cellular ecosystems of individual gastric cancers are sculpted by tumor-intrinsic and extrinsic selective pressures, culminating in individualized patient-specific cancer cartographies.
Significance:
Integration of spatial transcriptomic (GeoMx Digital Spatial Profiler) and single-cell RNA sequencing data from multiple gastric cancers identifies spatially resolved expression-based intratumoral heterogeneity, associated with distinct immune microenvironments. We uncovered two separate evolutionary trajectories associated with specific molecular subtypes, clinical prognoses, stromal neighborhoods, and genetic drivers. Tumor–stroma interfaces emerged as a unique state of tumor ecology.
Introduction
Gastric cancer is a major global health concern, ranking fifth in worldwide cancer incidence and mortality (4.8% of all cancer cases; 6.8% of cancer deaths; ref. 1). Gastric cancer has high prevalence in Asia, Central/Eastern Europe, and South America, with many current treatments challenged by intrinsic or acquired resistance, including immunotherapy (2–4). Histologically, gastric cancers can be classified into diffuse or intestinal subtypes (Lauren classification) and molecularly into chromosomal instability (CIN), genome stable (GS), microsatellite instability (MSI), and Epstein–Barr virus (EBV)-positive subtypes by The Cancer Genome Atlas (TCGA; ref. 5). Besides TCGA, other gastric cancer classification schemes have been proposed (6, 7), highlighting challenges for precision oncology (8).
Besides interpatient heterogeneity, intratumoral heterogeneity (ITH) also represents a significant barrier to outcomes of patients with gastric cancer, occurring within microregions in the same tumor, between primary and metastatic lesions, and between metastases (9–14). ITH has been linked to the emergence of tumor subclones and a source of therapeutic resistance (15). Previous gastric cancer ITH studies have leveraged DNA-based genomic sequencing to identify clonal and subclonal genomic alterations such as mutations and somatic copy-number alterations (sCNA), enabling phylogenetic reconstruction of tumor evolutionary trajectories (16–20). However, while analyses based on DNA-based tumor phylogenies can provide valuable insights into the chronology and architecture of ITH evolution, such approaches often do not fully capture functional differences in tumor cellular states associated with subclone evolution, nor how evolving subclones interact with the tumor microenvironment (TME; ref. 21). Moreover, recent studies have shown that clinically relevant ITH can arise through non–genomic/DNA-based mechanisms such as epigenetic reprogramming (22–24). Experimental strategies linking DNA-based evolutionary phylogenies to corresponding transcriptional cell states are thus emerging as an important area for translational cancer research (25, 26).
Single-cell RNA sequencing (scRNA-seq) technologies enable high-resolution cataloging of lineages and cellular states (11, 27–29), and sophisticated algorithms such as CopyKAT and inferCNV have been developed for scRNA-seq data to infer tumor phylogenies comparable with DNA-based methods (30–32). However, although scRNA-seq is a powerful tool, the technical process of cellular dissociation disrupts a tumor’s spatial context, limiting the ability to analyze interactions between tumor-associated epithelial cell states and their surrounding local TMEs. To address this limitation, recent spatial transcriptomic (ST) technologies have paved the way for exploring spatially resolved ITH (33, 34). Among the different ST platforms, the NanoString GeoMx Digital Spatial Profiler (GeoMx DSP) technology enables the guided selection of regions of interest (ROI) across a complete tumor tissue section followed by transcriptome analysis of individual ROIs across 18,000 protein-coding genes. This guided approach is particularly suited for exploring molecular changes in specific areas such as the tumor–stroma interface (TSI; refs. 34, 35). Additionally, GeoMx DSP can be performed on formalin-fixed paraffin-embedded (FFPE) samples, which enables the profiling of retrospective clinical datasets.
In this study, we applied both GeoMx DSP and scRNA-seq technologies to analyze 226 samples from 121 patients with gastric cancer, integrating data from >2,000 ROIs and >150,000 single cells. We identified spatially co-localized RNA-based intratumor subgroups within samples, linked to distinct spatially oriented TMEs and immune profiles. We delineated two separate evolutionary trajectories in gastric cancer associated with different prognostic outcomes. Analysis of the TSI provides evidence that they represent a unique state of tumor ecology. To our knowledge, this paired GeoMx DSP/scRNA-seq dataset represents one of the most extensive of its kind for gastric cancer and a notable expansion from previous studies (36–39), broadening our understanding of the spatial and molecular complexity of gastric cancer ITH and offering a valuable data resource for future exploration and discovery.
Results
Spatially Resolved Patterns of ITH in Gastric Cancer
To explore patterns of spatial ITH in gastric cancer, we integrated 2,138 GeoMx DSP ROIs and 152,423 scRNA-seq single-cell profiles across 226 samples from 121 individual patients with gastric cancer. These included a discovery cohort of 15 gastric cancers in which whole-section–based GeoMx DSP and paired scRNA-seq were both performed (1,063 ROIs, ∼ave 71 ROIs/patient; scRNA-seq of 75,807 cells, ∼ave 5,054 cells/patient), an independent whole-section–based GeoMx DSP validation cohort of six patients (235 ROIs, ∼ave 39 ROIs/patient), a GeoMx DSP tissue microarray (TMA) cohort of 180 gastric cancer samples from 86 patients in which each gastric cancer was represented by two areas: tumor core and tumor edge [840 ROIs, ∼ave 10 ROIs/patient; Singapore Gastric Cancer Consortium (SGCC TMA)], and 14 additional gastric cancers profiled by scRNA-seq (11 primary gastric cancers, 3 peritoneal metastases; scRNA-seq of 76,616 cells, ∼ave 5,473 cells/patient; Fig. 1A; Supplementary Tables S1 and S2).
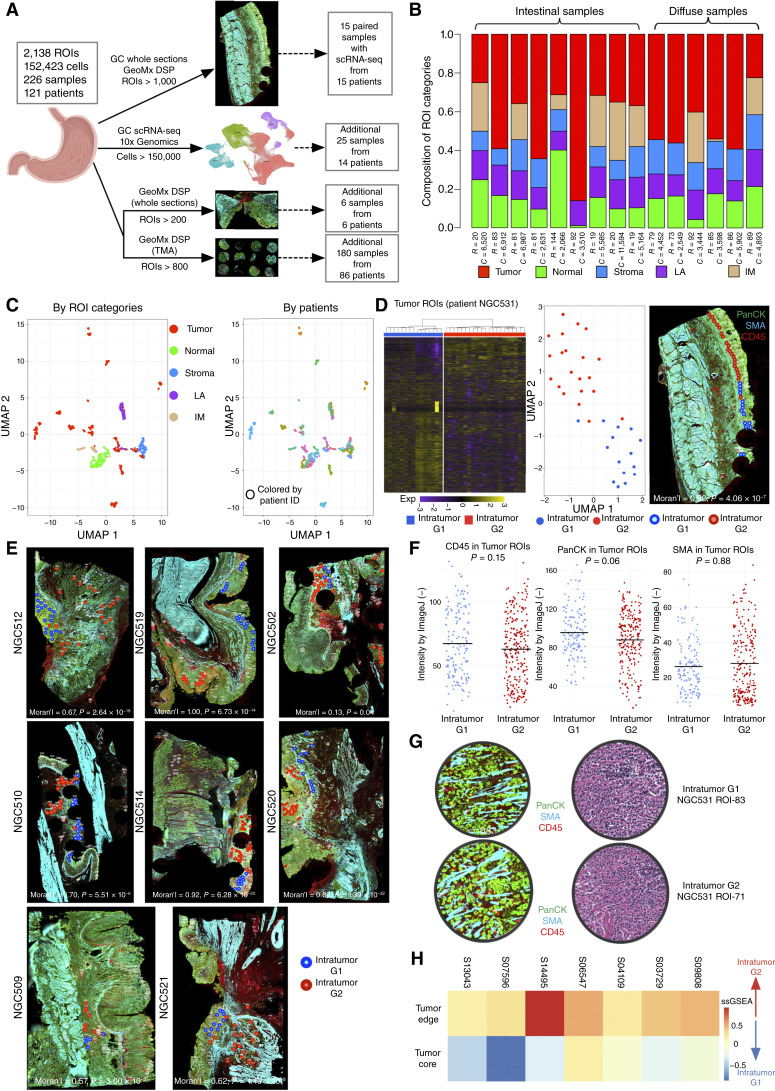
Figure 1.
Spatially resolved ITH in gastric cancer. A, Schematic depiction of study datasets. The study contains 2,138 GeoMx DSP ROIs and 152,423 scRNA-seq single-cell profiles across 226 samples from 121 individual patients with gastric cancer. Fifteen patients were analyzed by GeoMx DSP and paired scRNA-seq, 14 patients by scRNA-seq only, 6 patients from an additional GeoMx DSP validation set, and 86 patients on TMAs. B, Bar chart of the proportional distribution of ROI types across 15 samples (nine intestinal and six diffuse). ROIs were categorized into five types, namely tumor, normal, stroma, LA, and intestinal metaplasia (IM), based on morphology and cell type–specific markers from serial paired H&E-stained images. The number of ROIs (represented as “R”) and the number of paired scRNA-seq cells (represented as “C”) are shown below the proportion bar for each patient. C, UMAP projections of ROIs, distinguished by ROI categories (left). A separate color-coded representation of the same UMAP based on patients is on the right. Each UMAP point represents an ROI. D, Unsupervised heatmap clustering (left) displays two distinct intratumor subgroups based on gene expression (Exp) in patient NGC531, with each row representing a gene and each column an individual ROI. The UMAP projection (middle) further illustrates these subgroups, in which each point denotes an ROI. Right, The stained section is the same NGC531 image from A, onto which tumor ROIs have been overlaid as small circles with different colors indicating distinct intratumor subgroups. Spatial autocorrelation values (Moran’s I) and significance values for intratumor subgroups are shown at the bottom of the stained image (right). E, Stained GeoMx DSP slides annotated with intratumor subgroup labels derived from unsupervised clustering in eight more samples. Each circle within the stained slide represents a tumor ROI, with varied circle colors denoting different tumor subgroups. Spatial autocorrelation values (Moran’s I) and significance values for intratumor subgroups are shown for each sample. F, Intensity of CD45/PanCK/SMA IHC signals in tumor ROIs across samples. Blue dots represent G1 RNA-ITH ROIs, and red dots represent G2 RNA-ITH ROIs. P values were calculated using Wilcoxon rank-sum tests. G, Image showing H&E- and immunofluorescence-stained tissue sections showing representative G1 RNA-ITH and G2 RNA-ITH tumor areas. Both areas show scattered tumor cells in between immune cells and stroma. The morphology of the two areas is histologically similar. H, Heatmap of mapping scores based on signatures derived from GeoMx DSP intratumor subgroups applied to TMA data. Color intensities represent scaled average ssGSEA scores for each sample. Each square in the heatmap corresponds to an individual sample within the gastric cancer TMA cohort. GC, gastric cancer.
In the discovery cohort, each ROI (median 328 cells/ROI) was meticulously curated and independently annotated by two qualified pathologists (S. Srivastava and H.Y.J. Lum) as tumor, stroma, lymphoid aggregate (LA), intestinal metaplasia, and adjacent normal epithelial regions (interpathologist concordance = 97.6%). Our annotations were based on the predominant cell type within each ROI, identified by combining cell morphology [using serial paired hematoxylin and eosin (H&E) slides] and fluorescent immunohistochemical (IHC) markers (Fig. 1B; Supplementary Fig. S1A). Each ROI expression profile generated ∼3,800 measurable genes (IQR = 2,058–5,265; see “Methods”). To validate the ROI annotations, we evaluated published cell type–specific markers in the GeoMx DSP data and found a high reproducibility, such as expression of the tumor markers KRT8 and EPCAM (40, 41), in tumor ROIs (Supplementary Fig. S1B). The ROI categories were also highly correlated with cell type clusters determined by paired scRNA-seq data (Supplementary Fig. S1C). Comparisons of gene expression differences between tumor and adjacent normal epithelial ROIs confirmed significant upregulation in tumor ROIs of previously reported gastric cancer genes (e.g., CLDN4, FDR = 2.41 × 10−6 and CD44, FDR = 3.35 × 10−5 by Wilcoxon test; refs. 42, 43) and oncogenic pathways (e.g., epithelial–mesenchymal transition (EMT), FDR = 9.47 × 10−10 and angiogenesis, FDR = 2.18 × 10−4 by Kolmogorov–Smirnov test; Supplementary Fig. S1D and S1E; refs. 44, 45). Enriched gene programs for each ROI category are displayed in Supplementary Fig. S1F. When evaluated by Uniform Manifold Approximation and Projection (UMAP) for dimension projection, tumor ROIs exhibited patient-specific clustering, whereas nontumor ROIs (e.g., LAs, stroma, etc.) clustered by ROI category, similar to other studies where tumor expression profiles are often patient-specific due to individualized patterns of tumor aneuploidy (Fig. 1C; refs. 46, 47). We confirmed that batch effects in the GeoMx DSP data are minimal using three different batch correction methods (ComBat, Limma, and RUV4; Supplementary Table S3A and S3B; refs. 48–50).
Selecting a representative gastric cancer (NGC531), we performed clustering analysis on 36 tumor ROIs [Fig. 1D (left)]. Both unsupervised clustering and UMAP analysis confirmed two distinct expression subgroups, referred to as “G1” and “G2” [Fig. 1D (left and middle)]. Importantly, when mapped to their geospatial coordinates within gastric cancer NGC531, the subgroups mapped to distinct spatial regions by spatial autocorrelation analysis (Moran’s I = 0.92, P = 4.06 × 10−7) demonstrating the presence of spatially resolved expression-based ITH [RNA-ITH; Fig. 1D (right)]. Similar patterns of spatially resolved RNA-ITH were also observed in other gastric cancers (mean Moran’s I = 0.70, P < 0.05 for all samples; Fig. 1E). To determine if these RNA-ITH patterns are explainable by overt differences in histologic architecture, we examined proportions of pan-cytokeratin (PanCK), smooth muscle actin (SMA), and CD45-positive cells within the G1 and G2 ROIs (see “Methods”) and found no significant differences (P = 0.15 for CD45, P = 0.88 for SMA, and P = 0.06 for PanCK; Fig. 1F). Additionally, visual inspection of 33 G1/G2 ROI pairs by a pathologist (S. Srivastava) confirmed that G1 and G2 ROIs exhibited similar tumor cell compositions (Fig. 1G). These results suggest that the G1/G2 RNA-ITH patterns are not explained by overt differences in tumor histology and may be associated with cancer-intrinsic expression states.
We focused on characterizing the G1 and G2 RNA-ITH clusters, as these two clusters exhibited the most robust clustering statistics with the highest average Silhouette scores among 2 to 5 cluster ranges (Supplementary Table S4; ref. 51). To compare pathway differences between the G1 and G2 RNA-ITH regions, we performed gene set analysis identifying differentially expressed genes (DEG) between G1 and G2 ROIs. Across multiple patients, we found that G2 RNA-ITH regions commonly exhibited an upregulation of tumor pathways linked to aggressive clinical behavior such as EMT (Supplementary Fig. S2A), consistent with G2 regions representing a more invasive tumor subpopulation. Similar G1/G2 RNA-ITH and pathway differences were observed in the whole-section–based GeoMx DSP validation cohort (Supplementary Fig. S2B and S2C). We then analyzed GeoMx DSP data from the SGCC TMA (Supplementary Fig. S2D; Supplementary Table S5), where tumor core and tumor edge ROIs were manually selected by macroscopic and low-power microscopy by a pathologist (H.Y.J. Lum) during TMA assembly (Supplementary Fig. S2E). Mapping the G1 and G2 signatures onto the SGCC TMA (Fig. 1H), we found that tumor core ROIs resembled the G1 subgroup while tumor edge ROIs were correlated with the G2 subgroup. To validate that G2 RNA-ITH regions are also closer to tumor edges in the discovery GeoMx DSP data, we adopted a K-nearest neighbor method comparing spatial distances between G1 or G2 ROIs with TSI ROIs, in which the latter represents the tumor boundary containing both tumor and stroma cells. Consistent with the SGCC TMA results, we found that G2 ROIs are the major intratumor subtype closer to TSI ROIs (proportion = 67.7%, K = 10; P = 0.02).
To validate that the G1 and G2 gene sets are expressed by cancer cells and not by other TME cell types, we used multiple approaches. First, harnessing paired scRNA-seq data to estimate cell type proportions in the ROIs using CIBERSORTx (52), we found that the average tumor cell proportion was >60% for 92.35% of the tumor ROIs, indicating that tumor cells are the major cell type within tumor ROIs (Supplementary Fig. S2F). Supporting the tumor-intrinsic nature of G1 and G2, we confirmed that even when restricted to tumor ROIs of very high tumor purity (>80%), we were still able to recapitulate the G1 and G2 expression differences (Supplementary Fig. S2G). Second, using the SGCC TMA, to confirm that the G1 and G2 RNA-ITH patterns are driven predominantly by cancer cell–intrinsic expression, we performed paired multiplex IHC (mIHC) on 380 TMA ROIs using five antibodies—four immune-related (CD3, CD20, CD68, and CD163) and one epithelial marker (CK/EpCAM). The mIHC results confirmed that there were no significant differences in the tumor epithelial cell proportion between G2-like tumor edge ROIs and G1-like tumor core ROIs (enriched in G1 signatures; P = 0.37; Supplementary Fig. S3A). Third, we interrogated bulk RNA-seq data of an in-house panel of gastric cancer cell lines (n = 62), which are composed entirely of tumor epithelial cells, and confirmed that the gastric cancer lines can be clearly separated into two subtypes based on the G1–G2 signature (Supplementary Fig. S3B). Fourth, to increase our spatial resolution beyond that achievable by GeoMx DSP, we performed spatial enhanced resolution omics sequencing (Stereo-seq), a recently available high-resolution ST platform (53), on two gastric cancer samples. Analyzing only tumor epithelial cells, we successfully identified G1 and G2 RNA-ITH subgroups in both Stereo-seq gastric cancer samples (Supplementary Fig. S3C), exhibiting enriched pathways highly similar to GeoMx DSP (Supplementary Fig. S3D). Also, consistent with the GeoMx DSP findings that G2-expressing ROIs are closer to the tumor edge than the tumor core, we found in the Stereo-seq data that compared with G1 tumor cells, G2 tumor cells are significantly closer to stroma cells (P < 2.20 × 10−16 in both samples, Supplementary Fig. S3E). Fifth, to functionally demonstrate in vitro that G1 and G2 are cancer cell–intrinsic cellular states, we treated a gastric cancer organoid with TGFB1 (a known driver of EMT) and analyzed the pre- and post-treated cells using Stereo-seq. We found that after TGFB1 treatment, G1-like gastric cancer organoid cells significantly shifted to a G2-like status (P < 2.20 × 10−16), with upregulation of EMT and TGF-β pathways (Supplementary Fig. S3F and S3G). These findings reveal the pervasive presence of spatially oriented tumor cell populations exhibiting RNA-ITH in gastric cancer, with likely functional relevance to tumor progression.
Besides tumor ROIs, we also investigated nontumor ROI categories (stromal and immune) for the presence of RNA-ITH. Referencing cell types from paired scRNA-seq data, we applied CIBERSORTx cell type deconvolution (43) on the stromal (n = 138) and LA ROIs (n = 128). Stromal ROIs were deconvoluted primarily to endothelial and fibroblast cells, whereas LA ROIs were deconvoluted to T cells, NK cells, B cells, plasma cells, and myeloid cells (Supplementary Fig. S3H and S3I). For stromal ROIs, we identified two subgroups by unsupervised clustering, with one subgroup showing a higher association of myogenesis and hypoxia pathways (Supplementary Fig. S3J). Leveraging paired scRNA-seq signatures, we found that this stromal subgroup was enriched in specific fibroblast subtypes (“Fib1” and “Fib2”) and a decreased proportion of endothelial cell subtypes. For LA ROIs, the correlation of cell type compositions within and across samples was very high (0.61–0.92, median = 0.78), suggesting that LA ROIs are more homogenous.
G2 RNA-ITH Regions Exhibit an Immunosuppressive TME
We explored if the G1 and G2 RNA-ITH subgroups might be associated with differences in their local TMEs. Leveraging the paired scRNA-seq annotated cell types, we focused on immune cell types and applied CIBERSORTx (43) on >300 tumor ROIs from 10 gastric cancers (∼average 38 tumor ROIs/gastric cancer). Analyzing the samples in an aggregated fashion, we observed significant decreases in T-cell proportions in G2 RNA-ITH regions compared with G1 regions (P = 4.21 × 10−7 by Wilcoxon test), along with significant increases in plasma cells (P = 5.97 × 10−6; Fig. 2A). Supporting the accuracy of CIBERSORTx deconvolution, we confirmed by orthogonal mIHC in the SGCC TMA data that G2-like tumor edge ROIs similarly exhibited decreased T-cell proportions (∼2.19 times decrease of CD3+ cells) compared with G1-like tumor core ROIs (Fig. 2B and C). We further tested two immune exhaustion signatures (54, 55), one containing genes such as LAG3, TIGIT, and PD1. Both signatures were significantly higher in G2 compared with G1 RNA-ITH regions, consistent with G2 regions being immunosuppressed (Fig. 2D; Supplementary Fig. S4A). To broaden this investigation, we then queried the expression levels of 24 previously identified inhibitory immune checkpoints (56). This analysis confirmed that G2 RNA-ITH regions exhibited significantly higher expression of 11 checkpoints (P < 0.05 by Wilcoxon test), lower expression of 2 checkpoints, and similar expression for the remaining checkpoints compared with G1 regions (Fig. 2E). The immune-suppressive nature of the G2 RNA-ITH regions was further supported by the elevated expression of 10 inhibitory chemokines (9/10 chemokines being significant, P < 0.05 by Wilcoxon test; ref. 56), such as CXCL16 (P = 4.24 × 10−11) and CXCL5 (P = 4.34 × 10−6; Fig. 2F), and also significantly elevated expression of 6 inhibitory cytokines (5/6 cytokines being significant, P < 0.05 by Wilcoxon test; ref. 56), including TGFB1 (P = 2.88 × 10−13) and TNFSF12 (P = 4.44 × 10−7; Supplementary Fig. S4B). Angiogenesis signature scores were also significantly upregulated in G2 RNA-ITH regions (P = 3.98 × 10−9; Supplementary Fig. S4C).
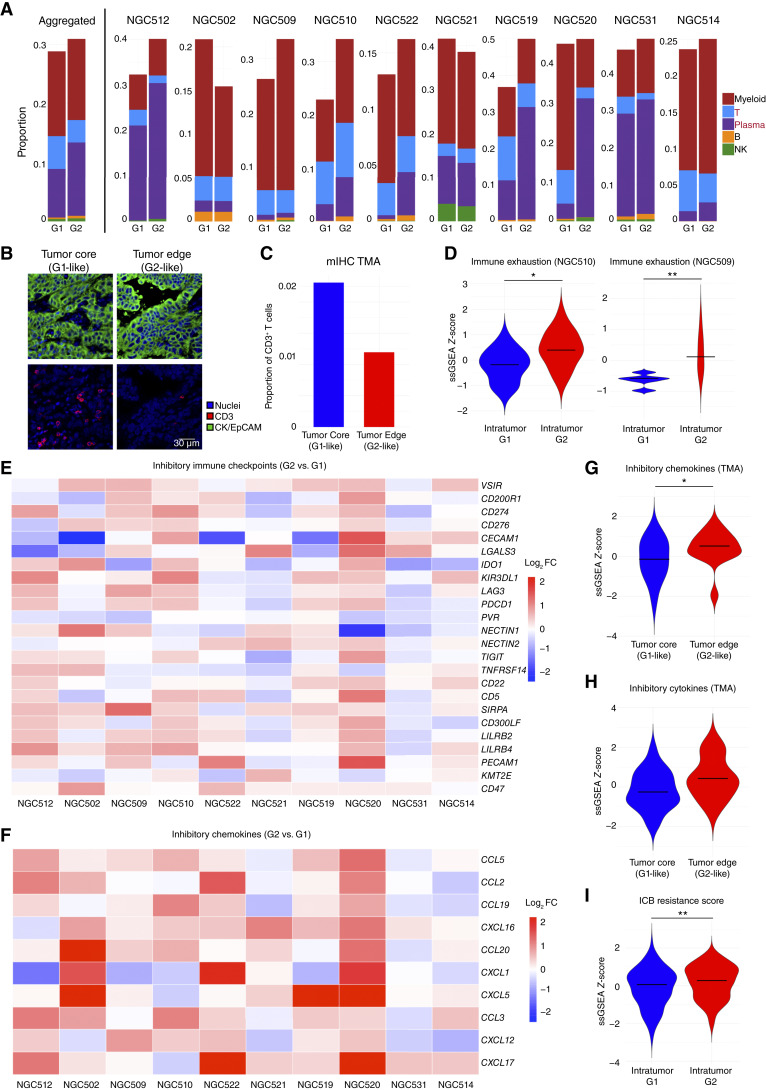
Figure 2.
Immune heterogeneity in spatial intratumor subgroups. A, Bar chart of the proportion of deconvoluted immune cell types between intratumor subgroups (G1 and G2) in tumor ROIs in aggregated 10 GeoMx DSP samples (left of the vertical line) and in each individual sample (right). Samples were selected based on the number of tumor ROIs. Colors represent five different immune cell types, including myeloid, T, plasma, B, and NK cells. B, Representative stained slides of mIHC for tumor core (left) and tumor edge regions (right) in TMA sample S03729. Blue represents nuclei, red represents CD3, and green represents CK/EpCAM. The length of the scale bar represents 30 μm. C, Bar plot of the proportion of CD3+ T cells in tumor core and tumor edge ROIs by TMA mIF. Colors represent different tumor regions. D, Violin plot of scaled ssGSEA scores of immune exhaustion signatures (comprising genes such as LAG3, TIGIT, and PD1) on G1 and G2 subregions in two representative samples. Stars represent the statistical significance of the Wilcoxon test. Bars represent median values of the scaled ssGSEA score. E, Heatmap of log2 fold changes (log2 FC) in expression of inhibitory immune checkpoints between intratumor subgroups (G2 vs. G1) in 10 GeoMx DSP samples. Each row represents an immune checkpoint, and each column represents a GeoMx DSP sample. Colors represent log2 FC values between G2 and G1. F, Heatmap of log2 FCs in expression of inhibitory chemokines between intratumor subgroups (G2 vs. G1) in 10 GeoMx DSP samples. Each row represents an immune checkpoint, and each column represents a GeoMx DSP sample. Colors represent log2 FC values between G2 and G1. G, Violin plot of scaled ssGSEA scores of inhibitory immune chemokines on tumor core and tumor edge ROIs using the gastric cancer TMA cohort. Stars represent statistical significance of the Wilcoxon test. Bars represent median values of scaled ssGSEA scores. H, Violin plot of scaled ssGSEA scores of inhibitory cytokines on tumor core and tumor edge ROIs using the gastric cancer TMA cohort. Stars represent statistical significance of the Wilcoxon test. Bars represent median values of scaled ssGSEA scores. I, Violin plot of scaled ssGSEA scores of an immune checkpoint blockade resistance signature on G1 and G2 subregions in aggregated 10 samples. Stars represent the statistical significance of the Wilcoxon test. Bars represent the median values of the scaled ssGSEA score. (*, P < 0.05; **, P < 0.01; ***, P < 0.001; absence of a star represents non-significance, P ≥ 0.05).
To validate these results in an independent cohort, we analyzed the same inhibitory immune checkpoints, inhibitory chemokines, and inhibitory cytokines between G1-like tumor core and G2-like tumor edge ROIs in the SGCC TMA data set. Our analysis revealed that tumor edge ROIs (G2-like) exhibited higher expression levels of inhibitory chemokines (P = 0.04 by Wilcoxon test; Fig. 2G; Supplementary Fig. S4D), inhibitory cytokines (P = 0.06 but similar trend; Fig. 2H; Supplementary Fig. S4E), and inhibitory immune checkpoints (P = 0.25 but similar trend; Supplementary Fig. S4F). Collectively, these findings reveal that spatially co-localized intratumor subgroups possess distinct TME immune profiles. Notably, the observed differences in immune exhaustion markers were specific to the TMEs of tumor ROIs and not observed in LA ROIs from the same gastric cancers (Supplementary Fig. S4G). These results demonstrate how spatially resolved analysis can enable a more refined understanding of local TME organization, which would likely have been missed by bulk or dissociated single-cell analyses.
To investigate potential therapeutic implications of the G2 RNA-ITH subregions, we then queried published therapy resistance signatures, including (i) an immune checkpoint blockade treatment resistance signature from a colorectal and pancreatic adenocarcinoma phase II trial (57), and (ii) a different immune checkpoint blockade treatment resistance signature from a pan-cancer study (PredictIO; ref. 58). Our analysis revealed that G2 RNA-ITH subregions demonstrated elevated resistance scores in both cases (P = 7.25 × 10−3, P = 0.05, respectively; Fig. 2I; Supplementary Fig. S4H). Exploring the expression levels of established and emerging therapeutic targets (59), G1 RNA-ITH subregions exhibited elevated CAPRIN1 expression (P = 1.14 × 10−4 by Wilcoxon test), a promising target reported in recent clinical trials (Supplementary Fig. S4I; ref. 60). Conversely, G2 RNA-ITH subregions were characterized by enhanced expression of targets such as DKK1 (P = 1.76 × 10−9) and CTNNB1 (P = 7.73 × 10−3; Supplementary Fig. S4I), indicative of Wnt pathway activation (61). This upregulation was confirmed by increases in Wnt pathway activity in G2 RNA-ITH subregions (P = 0.03; Supplementary Fig. S4J). These findings suggest a potential role for spatial RNA-ITH in gastric cancer therapy resistance and potential strategies for targeting ITH landscapes. However, as applying therapy-associated signatures to ST profiles should be carefully considered, the actual therapeutic implications of these signatures being enriched in G2 subregions will require further investigation.
G1 and G2 RNA-ITH Relationships Are Linked with Distinct Patterns of Within-Tumor Evolution
To explore potential mechanisms contributing to the immune-exhausted phenotype of G2 RNA-ITH subregions, we considered recent reports that cancers harboring CIN may induce regional immune suppression in a non-autonomous fashion through chronic cGAS-STING pathway activation (62). Indeed, we found that G2 RNA-ITH regions exhibited higher HET70 aneuploidy signature scores indicating higher levels of somatic copy-number variations (sCNAs; Supplementary Fig. S5A) and also significantly higher cGAS-STING pathway enrichment scores (P = 6.10 × 10−3 by Wilcoxon test) (Supplementary Fig. S5B). Consistent with elevated cGAS-STING activity, IFNα and IFNγ signatures were also upregulated in G2 RNA-ITH subregions (P = 3.11 × 10−4 and P = 1.09 × 10−6, respectively; Supplementary Fig. S5C). To validate higher levels of aneuploidy in G2 RNA-ITH subregions, we inferred sCNAs from scRNA-seq tumor epithelial cells from 29 tumor samples using CopyKAT and validated the sCNA calls using inferCNV (median rho = 0.55 using Spearman’s rank correlation, P < 2.20 × 10−16 in 29 samples; Fig. 3A; Supplementary Fig. S5D). We further validated our sCNA calls using matched bulk whole-exome sequencing (WES) data from five samples (median rho = 0.58 using Spearman’s rank correlation, P < 0.05 in four samples; Supplementary Fig. S5E). Unsupervised clustering of the scRNA-seq sCNA matrix delineated distinct intratumor subgroups exhibiting distinct sCNA patterns (Fig. 3B). A comparison of sCNA levels between the subgroups revealed one subgroup with significantly increased sCNA levels (P = 1.26 × 10−11) and associated with G2-like signatures (P < 2.20 × 10−16; Supplementary Fig. S5F; see “Methods”). We also inferred sCNAs in tumor cells from the two primary gastric cancer Stereo-seq samples (see “Methods”) and confirmed that G2 tumor cells have significantly higher sCNA levels compared with G1 tumor cells (P = 2.18 × 10−5 for S704T, P = 9.35 × 10−13 for S697T; Supplementary Fig. S5G and S5H).
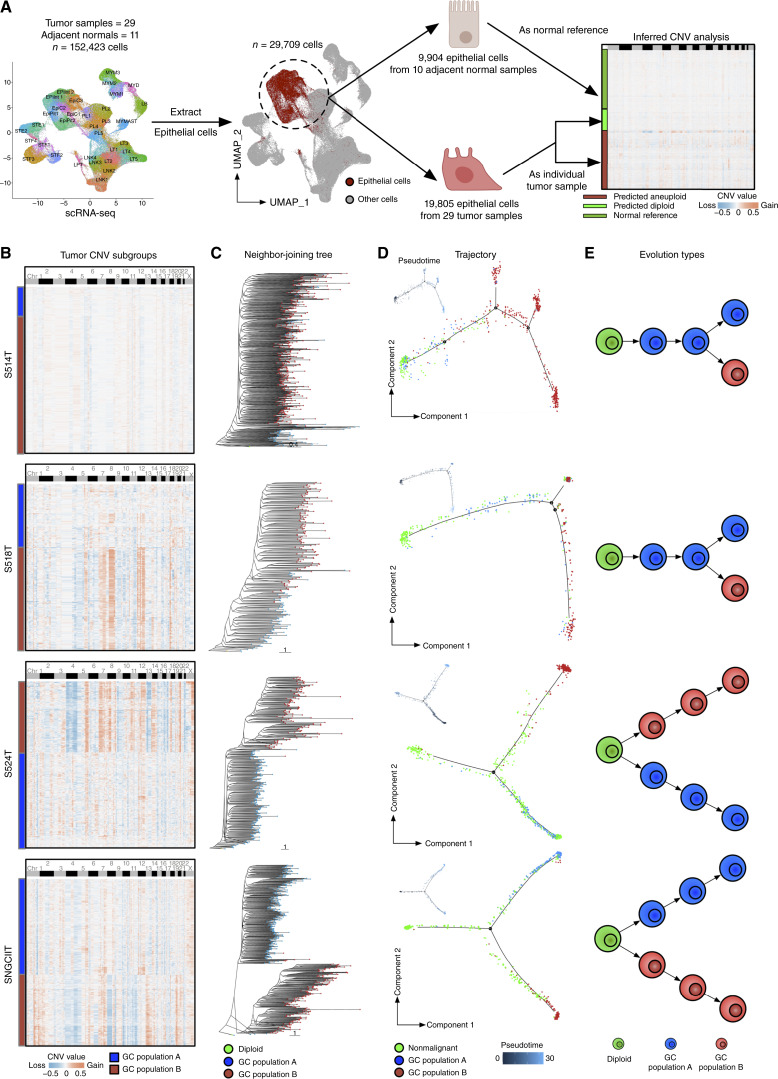
Figure 3.
Single-cell resolved evolutionary trajectories in gastric cancer. A, Workflow depiction for sCNA inference from scRNA-seq single-cell data. Adjacent normal epithelial cells from 10 adjacent normal samples were used as references (one normal sample was filtered out due to the lack of a matched tumor sample), and tumor epithelial cells from 29 patients were classified as diploid and aneuploid. B, Heatmap presenting inferred sCNA values for tumor subgroups within single-cell data for samples S514T, S518T, S524T, and SNGCIIT (from top to bottom). Rows represent individual cells, whereas columns correspond to genomic bin positions of 220 kb each. Color gradations indicate varying states of sCNA, with subgroups determined through unsupervised clustering based on Euclidean distances. C, Neighbor-joining tree constructed on the inferred sCNA values, with the tree rerooted to a diploid state for reference. Each dot on a tree branch represents a single cell, color-coded according to subgroup labels identified in B. D, Main panels show trajectory plots, whereas the top left panels show pseudotime graphics for nonmalignant cells and tumor cells from identified sCNA subgroups. Each dot represents a cell, color-coded by cell type. Solid lines in the trajectory plot are derived from single-cell expression data, indicating the developmental path of cells. Pseudotime analysis, with zero time points anchored to selected nonmalignant cells, use color gradations to represent estimated pseudotimes for each cell. E, The illustrated figure conceptualizes the two identified tumor evolution patterns in gastric cancer. Cells in green are depicted as diploids, whereas cells in blue and red symbolize two distinct tumor populations, each characterized by their sCNA patterns. GC, gastric cancer.
The differing sCNA levels between G1 and G2 subregions motivated us to leverage published approaches harnessing DNA-based sCNA patterns to assess tumor evolutionary trajectories at the single-cell level using the paired scRNA-seq data (Fig. 3B; refs. 30–32). Notably, in some gastric cancers (N = 21), the sCNA subgroups exhibited a shared base of genomic gains/losses with few unique chromosomal changes. This pattern is suggestive of a “branching evolution” model in which tumors gradually accumulate sCNAs over time, leading to the eventual formation of various subgroups. However, in other gastric cancers (N = 8), the sCNA subgroups displayed more divergent profiles with distinct gains/losses dominating their genome (representative examples are provided in Fig. 3B). This second pattern is more reminiscent of an “internal diaspora” or rapid in situ evolution, where tumor subgroups rapidly acquire distinct sCNAs early in evolution, with each subgroup subsequently embarking on its own evolutionary trajectory. To further test the possibility of two seperate evolutionary tumor trajectories (branched evolution and internal diaspora evolution), we used neighbor-joining tree and maximum parsimony tree methods for phylogenetic analysis. We constructed phylogenetic trees for each gastric cancer sample (Fig. 3C; Supplementary Fig. S6A). The phylogeny trees confirmed that groups with more genomic similarities tended to branch off gradually from a diploid ancestor (branched evolution), whereas those with unique sCNA patterns demonstrated early divergence, establishing distinct branches from the onset (internal diaspora). This phylogenetic branching was further substantiated by expression-based trajectory and pseudotime analyses using multiple methods such as Monocle, Slingshot, and PAGA (Fig. 3D; Supplementary Fig. S6B and S6C; refs. 63–65). Notably, the presence of two principal evolutionary patterns revealed by single-cell sCNA analyses (Fig. 3E) is consistent with findings inferred from previous bulk WES studies of gastric cancers (66), thereby demonstrating coherence of tumor evolution patterns at both the macro and micro scales (see “Discussion”).
Clinical Impact of Internal Diaspora Evolution on Gastric Cancer Prognosis
The observation of two separate evolutionary trajectories by scRNA-seq analysis prompted us to investigate clinical and molecular features associated with branched evolution and internal diaspora evolution. We assigned each gastric cancer profiled by scRNA-seq to molecular subtypes defined by TCGA (CIN, GS, MSI, and EBV) (5). Correlation of the branched and internal diaspora evolution patterns to the TCGA molecular subtypes revealed that internal diaspora samples (N = 8) predominantly correlated with CIN (CIN = 6, others = 2, P = 0.04 by χ2 test), whereas branched evolution samples (N = 21) were fairly evenly spread across the various subtypes (GS = 9, MSI = 7, CIN = 5, P = 0.56; Fig. 4A). cGAS-STING pathway scores were higher in CIN tumor cells compared with GS tumor cells (P = 3.63 × 10−12; Supplementary Fig. S6D). No major differences were observed with Lauren histologic subtypes (intestinal, diffuse) between internal diaspora and branched evolution gastric cancer (Fig. 4B). None of the scRNA-seq samples were EBV-positive by Epstein-Barr Encoding Region (EBER) in situ hybridization (Supplementary Table S2).
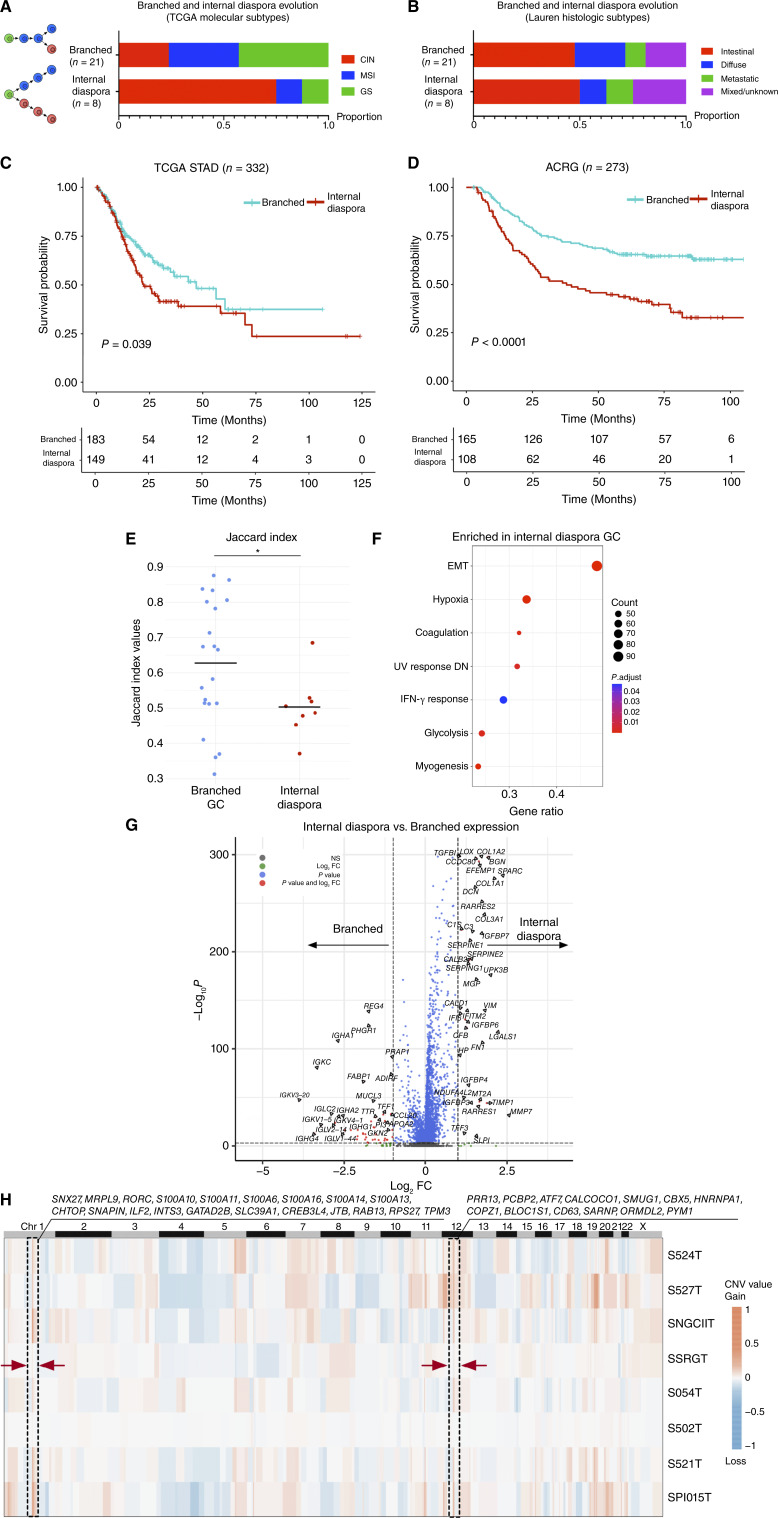
Figure 4.
Internal diaspora evolution in gastric cancer progression and prognosis. A, Bar plots showing the proportion of TCGA molecular subtypes across internal diaspora and branched evolution gastric cancer samples, denoted by sample counts on bars. B, Bar plots showing the proportion of Lauren histologic subtypes across internal diaspora and branched evolution gastric cancer samples, denoted by sample counts on bars. C, Kaplan–Meier survival plot comparing internal diaspora and branched evolution patterns in TCGA gastric cancer samples (n = 332), as classified by single-cell–derived signatures. The blue survival curve represents patients with tumors exhibiting a branched evolution pattern, whereas the red curve denotes patients with tumors exhibiting an internal diaspora evolution pattern. D, Kaplan–Meier survival plot of internal diaspora vs. branched evolution in the ACRG (n = 273) classified by single-cell–derived signatures. The blue survival curve represents patients with tumors exhibiting a branched evolution pattern, whereas the red curve denotes patients with tumors exhibiting an internal diaspora evolution pattern. E, Bar chart of Jaccard indexes for two sCNA evolution types. Jaccard indexes were calculated among intratumor subgroups using the sCNA matrix within each sample. Bars represent mean values in each group. The star * indicates statistical significance using t tests (P < 0.05). F, GSEA dot plot shows upregulated pathways in internal diaspora evolution samples compared with branched evolution samples. Dot dimensions denote gene overlap counts with the Hallmark database, whereas colors represent GSEA enrichment significance. “DN” represents “down-regulated”. G, Volcano plot comparing gene expression between internal diaspora and branched evolution samples at the single-cell level. Axes indicate Log2 fold changes (log2 FC) and significance levels. H, A sCNA heatmap showing the average sCNA pattern of aneuploid cells from internal diaspora samples. Arrows and boxes highlight common large copy-number variation events (Chr1 and Chr12) across internal diaspora samples. Genes in these regions are listed. GC, gastric cancer; STAD, stomach adenocarcinoma.
To investigate clinical implications of the two evolutionary trajectories, we applied the branched evolution and internal diaspora signatures from our samples to the TCGA gastric cancers (n = 332; https://portal.gdc.cancer.gov/projects/TCGA-STAD; see “Methods”). Patients with gastric cancers exhibiting internal diaspora evolution had significantly poorer survival outcomes compared with those with branched evolution (P = 0.04 by log-rank test, Kaplan–Meier estimator; Fig. 4C). Using multivariate Cox regression analysis adjusting for gender and tumor stage as confounders, we found that internal diaspora evolution is independently associated with a heightened risk of mortality (HR = 1.49; 95% CI, 1.05–2.11; P = 0.03). To validate this result, we then analyzed two other independent gastric cancer cohorts from the Asian Cancer Research Group (ACRG) GSE62254 (n = 273; ref. 6) and GASCAD (n = 83; ref. 67). Again, patients with internal diaspora gastric cancers exhibited significantly poorer prognosis compared with patients with branched evolution gastric cancers (P < 1.0 × 10−4 for ACRG, P < 0.05 for GASCAD; Fig. 4D; Supplementary Fig. S7A). Multivariate Cox regression analysis further supported this result, with internal diaspora evolution remaining significant for poor survival (HR = 1.88; 95% CI, 1.31–2.69; P = 6.44 × 10−4). For EBV gastric cancers, analysis of two EBV gastric cancer cohorts did not identify a survival association between the two evolution subtypes (Supplementary Fig. S7B; ref. 5).
Notably, while internal diaspora evolution was significantly associated with a worse prognosis in both the TCGA and GASCAD cohorts, CIN was not (P < 0.05 for internal diaspora; P = 0.252 and 0.254 for CIN; Supplementary Fig. S7C). This suggests that the poor clinical outcomes of internal diaspora gastric cancers are only partially explainable by DNA-based CIN. To elucidate molecular pathways responsible for the aggressive clinical behavior of internal diaspora gastric cancers, we considered previous studies highlighting elevated DNA-based ITH levels as another major contributor to gastric cancer treatment resistance (9–12). Analyzing the 29 scRNA-seq gastric cancers, we confirmed that internal diaspora gastric cancers exhibited significantly higher ITH levels (measured by lower Jaccard index values between intratumor subgroups) compared with branched evolution gastric cancers (P = 0.02 by t test; Fig. 4E). However, in a multivariate Cox regression analysis comparing both tumor evolution patterns and DNA-based ITH levels [measured by mutant allele tumor heterogeneity (MATH; ref. 68)] as covariates (TCGA stomach adenocarcinoma cohort), internal diaspora evolution remained significantly associated (P = 4.53 × 10−2) with worse prognosis whereas ITH MATH levels were not (P = 0.84). Thus, similar to CIN, the aggressive clinical behavior of internal diaspora gastric cancers cannot be fully attributed to DNA-based ITH levels alone. Subsequently, we performed differential gene expression analysis comparing tumor cell populations between branched evolution versus internal diaspora gastric cancers. This analysis revealed that internal diaspora tumor cells exhibited a marked upregulation of genes mapping to EMT and hypoxia pathways (Fig. 4F; FDR < 0.05). Examples of genes expressed in internal diaspora gastric cancer cells included collagen-related genes (COL1A1, COL1A2, and COL3A1), serpins (SERPINE1 and SERPINE2), and growth factor–binding proteins (IGFBP6 and IGFBP7; Fig. 4G; Supplementary Fig. S7D). Notably, as this expression analysis utilizes single-cell tumor epithelial profiles, the possibility that these signatures are being contributed by non-tumor stromal populations is excluded. When comparing consensus sCNA profiles with branched evolution gastric cancers, internal diaspora gastric cancers exhibited two major gained regions (CHR1q 21-23 and CHR12q 13) consistently across samples, involving genes such as RAB13 which have known roles in cancer cell migration (Fig. 4H; refs.69, 70). Taken collectively, these results demonstrate that internal diaspora tumor cells have distinct molecular signatures compared to branched evolution tumor cells.
Internal Diaspora Gastric Cancers Harbor a Specific Stromal Microenvironment – Evidence from scRNA-seq and GeoMx DSP
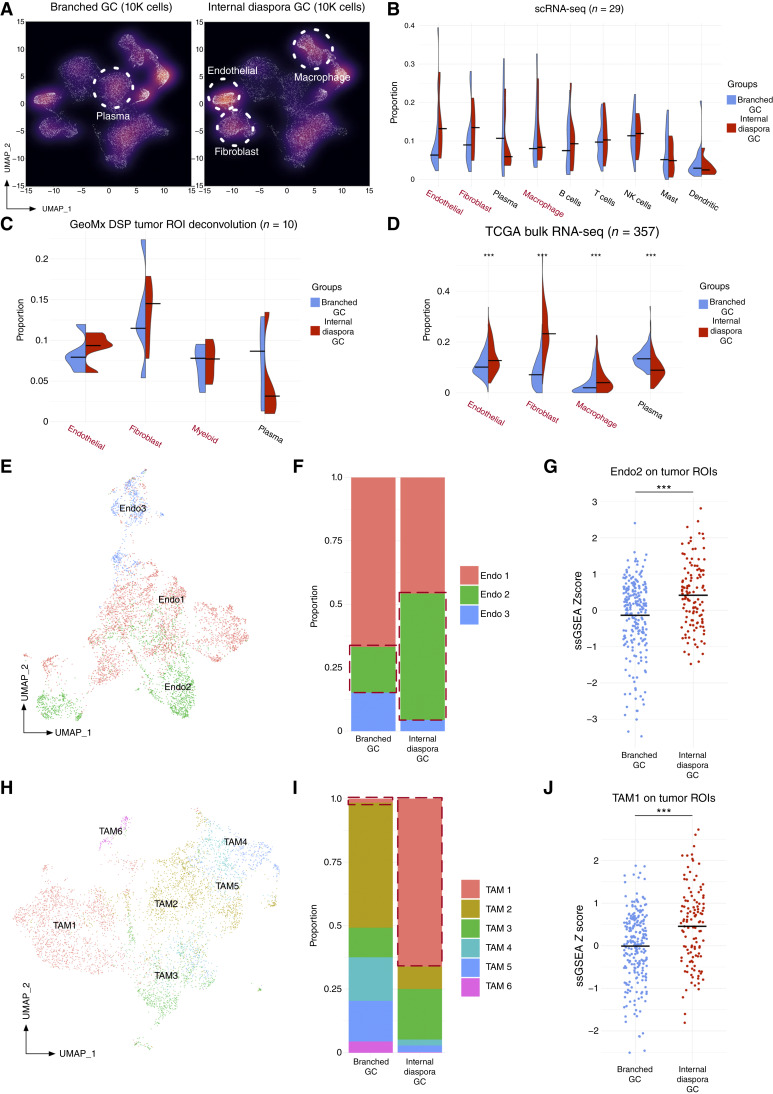
We explored whether gastric cancers exhibiting distinct evolutionary patterns are also associated with different TMEs. Internal diaspora gastric cancers were associated with a higher proportion of endothelial cells, fibroblasts, and macrophages, whereas branched evolution samples exhibited a higher proportion of plasma cells (Fig. 5A). scRNA-seq density plots were concordant with aggregated analysis (Fig. 5B; see Supplementary Fig. S8A for all cells), and further validated using GeoMx DSP data (Fig. 5C). To orthogonally validate these results in a bulk RNA-seq cohort, we performed CIBERSORTx cell type deconvolution on the TCGA gastric cancer dataset and found consistent TME differences between branched evolution and internal diaspora gastric cancers (n = 357; P < 1 × 10−3 by Wilcoxon test; Fig. 5D). These results suggest that gastric cancers with different tumor evolutionary trajectories are also associated with distinct tumor cell–extrinsic TMEs.
Figure 5.
Internal diaspora gastric cancer exhibits a unique TME. A, UMAP density plots comparing internal diaspora and branched gastric cancer samples. Each dot represents a single cell, with solid circles emphasizing predominant cell populations. As the x–y positioning of cell clusters in the UMAP density plots are often affected by the number of cells, to enable an intuitive visual comparison, we utilized 10,000 randomly downsampled cells for each type. B, Split violin plot of cell type proportions comparing branched evolution and internal diaspora evolution samples from gastric cancer scRNA-seq tumor samples (29 patients). Median values are indicated by bars. C, Split violin plot of deconvoluted cell type proportions comparing branched evolution and internal diaspora evolution samples from gastric cancer GeoMx DSP tumor ROIs (10 patients). Median values are indicated by bars. D, Split violin plot of cell type proportions comparing branched and internal diaspora evolution from TCGA samples mapped by single cell–derived signatures (357 patients). Median values are indicated by bars. Stars represent statistical significance of the Wilcoxon test between branched evolution and internal diaspora gastric cancer. E, UMAP of endothelial subclusters in gastric cancer scRNA-seq samples. Colors indicate different endothelial subtypes (Endo1–Endo3). F, Bar plot of proportions of distinct endothelial subclusters identified in gastric cancer scRNA-seq. The left bar represents these proportions in branched gastric cancer samples, whereas the right bar represents the proportions in internal diaspora gastric cancer samples. Different colors denote distinct endothelial subclusters. G, Dot plot of Endo2 signature mapping scores on GeoMx DSP tumor ROIs. Gastric cancers analyzed by GeoMx DSP were categorized by evolution type using matched scRNA-seq assignments. Scores were z-transformed ssGSEA scores using Endo2 signatures derived from scRNA-seq data. Colors of the dots represent the two evolution types. Stars represent Wilcoxon test statistical significance between branched and internal diaspora gastric cancer. Bars represent the median values of scaled ssGSEA scores. H, UMAP of TAM subclusters in the gastric cancer scRNA-seq dataset. Colors indicate different TAM subtypes (TAM1–TAM6). I, Bar plots of the distribution of various TAM subclusters within the gastric cancer scRNA-seq dataset. The left bar represents the proportion of each TAM subcluster in branched gastric cancer samples, whereas the right bar shows distributions in internal diaspora gastric cancer samples. Different colors in the chart distinguish between the various TAM subclusters. J, Dot plot of TAM1 signature mapping scores on GeoMx DSP tumor ROIs. Scores were z-transformed ssGSEA scores using TAM1 signatures derived from scRNA-seq data. Colors of the dots represent the two evolution types. Stars represent Wilcoxon test statistical significance between branched and internal diaspora gastric cancer. Bars represent median values of scaled ssGSEA scores. (*, P < 0.05; **, P < 0.01; ***, P < 0.001; without stars represents not significant, P ≥ 0.05). GC, gastric cancer.
To define more precise cell states associated with these TME differences, we performed detailed subclustering and annotation for endothelial cells and macrophages. Endothelial cells were stratified into three subtypes (Endo1–Endo3) using Seurat clustering (Fig. 5E; Supplementary Table S6). Of these three endothelial subtypes, Endo2 subcluster cells, characterized by high expression of VWF and ACKR1, exhibited significantly increased proportions in internal diaspora gastric cancers (Fig. 5F). Endo2 cells have previously been implicated in accelerating tumor angiogenesis (56), which may contribute to the aggressiveness of internal diaspora gastric cancers. This scRNA-seq result was also validated in GeoMx DSP data, in which tumor ROIs from internal diaspora gastric cancers exhibited higher Endo2 signatures compared with branched evolution samples (P = 4.30 × 10−5 by Wilcoxon test, one outlier excluded; Fig. 5G). Notably, the enrichment of Endo2 cells was also observed in GeoMx DSP stroma ROIs from internal diaspora gastric cancers (P = 4.78 × 10−5; Supplementary Fig. S8B).
For macrophages, clustering analysis delineated six tumor-associated macrophage (TAM) subtypes (TAM1–TAM6; Fig. 5H), each defined by distinct gene expression markers (Supplementary Table S7). Of these, internal diaspora gastric cancers exhibited higher proportions of TAM1 subtype macrophages characterized by expression of SPP1 and FN1 (Fig. 5I). TAM1 macrophages are known to contribute to the formation of tumor-immune barriers by interacting with cancer-associated fibroblasts (CAF; ref. 71) and by cross-talk with CD8+ exhausted T cells (72). TAM1 macrophages have also been associated with tumor metastasis (56). Similar to the Endo2 cells, we also observed a higher TAM1 enrichment in tumor ROIs (P = 2.16 × 10−5 by Wilcoxon test; Fig. 5J) and LA ROIs from internal diaspora gastric cancers (P = 7.72 × 10−6; Supplementary Fig. S8C). To orthogonally support these results, we leveraged deconvoluted cell proportions in GeoMx DSP samples using paired scRNA-seq data and again found that internal diaspora gastric cancers have a higher proportion of TAM1 cells compared with branched evolution gastric cancers (P = 0.04). This finding was not influenced by the few peritoneal samples in the dataset (three samples, 7,210 cells, 5.91% of all cells), as removing the peritoneal samples still resulted in internal diaspora gastric cancers exhibiting a significantly increased proportion of Endo2 cells (P < 2.20 × 10−16, χ2 test) and TAM1 cells (P < 2.20 × 10−16, χ2 test). These results reveal a unique stromal composition in internal diaspora evolution gastric cancers, marked by the presence of cell types known to promote tumor growth and metastasis, such as VWF+ ACKR1+ endothelial cells and SPP1+ FN1+ TAM cells.
SOX9 is a Candidate Internal Diaspora Driver
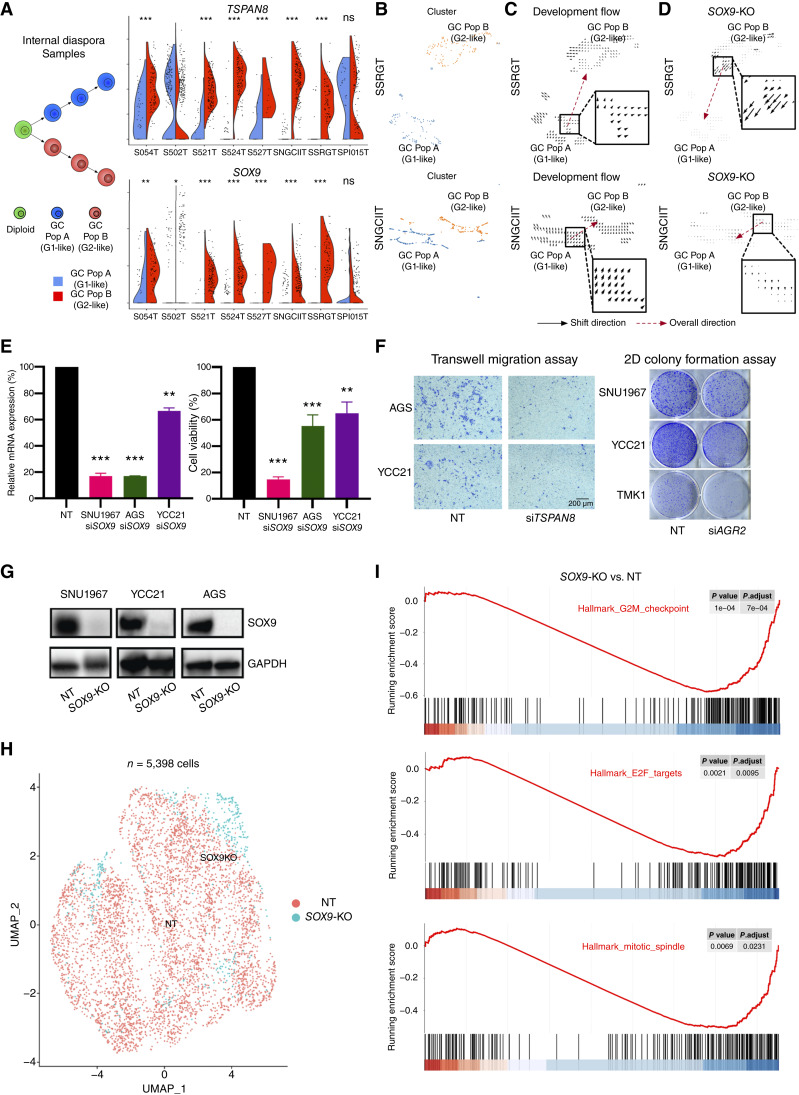
Both branched evolution and internal diaspora gastric cancers exhibited RNA-ITH reflected by G1-like and G2-like tumor populations (Fig. 1). We identified genes specifically upregulated in G2-like populations such as TSPAN8, SOX9, and AGR2 (Fig. 6A; Supplementary Fig. S8D). Of these genes, SOX9 is known to enhance TSPAN8 expression by binding to its promoter (73). SOX9 has also been linked to tumor progression and is an emerging driving factor for tumor progression and metastasis (74–76). To investigate a functional role for SOX9 in driving internal diaspora evolution, we used CellOracle, a recently described gene-regulatory network modeling approach which allows in silico knockouts (KO) of transcription factors (TF) in scRNA-seq data (77). As a positive control, we first validated CellOracle on a public dataset (78) by observing that myogenin (MYOG) KO can significantly shift the cell state of 72H-cultured primary human myoblasts to 0H-cultured cells, consistent with MYOG being a key driver for muscle development and myogenesis (Supplementary Fig. S8E). Next, to demonstrate the applicability of CellOracle to gastric cancer, we compared TP53 nontargeting (TP53-NT) and TP53 KO (TP53-KO) gastric cell lines (Supplementary Fig. S8F), conducting scRNA-seq to acquire data on 11,999 TP53-NT cells and 4,164 TP53-KO cells. Subsequent in silico KO of TP53 in the TP53-NT cell data resulted in a significant transition of cell states toward those observed in actual TP53-KO cells (Supplementary Fig. S8G). We then conducted in silico SOX9 KO analysis on internal diaspora gastric cancer tumor cells. CellOracle simulations confirmed that upon SOX9 KO, G2-like profiles shifted significantly toward that of the G1 state (Fig. 6B–D), a deviation that was markedly distinct from shifts induced by random TF KO simulations (Supplementary Fig. S8H).
Figure 6.
Candidate drivers of internal diaspora evolution. A, Split violin plots of TSPAN8 and SOX9 expression between gastric cancer population A (G1-like) and B (G2-like) in internal diaspora samples. The left subfigure depicts the evolution of the two populations in internal diaspora samples. Stars above the violin plots represent the significance of the Wilcoxon test between expression in G1 RNA-ITH and G2 RNA-ITH regions. B, UMAP of single cells in two representative internal diaspora samples (SSRGT and SNGCIIT). Cells were normalized, clustered using Scanpy, and colored by gastric cancer populations A (G1-like) and B (G2-like). C, Development vector field plots in two internal diaspora samples (SSRGT and SNGCIIT) using CellOracle. Arrows represent the direction of shift between cell states of gastric cancer population A (G1-like) to B (G2-like). D,SOX9 KO simulation vector field plots in two internal diaspora samples (SSRGT and SNGCIIT) using CellOracle. Arrows represent the direction of shift between cell states of gastric cancer population B (G2-like) to A (G1-like). E, Relative mRNA expression (left) and relative cell viability (right) of NT and SOX9 siRNA gastric epithelial cells (SNU1967, AGS, and YCC21). Statistical significance was determined using Student t test. F, Transwell migration assay after TSPAN8 siRNA in gastric epithelial cells (AGS and YCC21; left), and 2D colony formation assay after AGR2 siRNA in gastric epithelial cells (SNU1967, YCC21, and TMK1; right). G, Confirmation of SOX9 CRISPR KO at the protein level in NT and SOX9 CRISPR KO gastric epithelial cells (SNU1967, AGS, and YCC21; representative of three independent experiments). H, UMAP of integrated SOX9 KO and NT scRNA-seq (5,398 cells). Each dot on the UMAP represents a cell, and the color of the dot denotes a SOX9 KO (in blue) or NT cell (in red). I, GSEA enrichment score curves plot of the top enriched pathways between SOX9 KO and NT cells. G2M checkpoint, EF2 targets, and MITOTIC spindle pathway activity is downregulated in SOX9 KO cells compared with NT cells. (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, P ≥ 0.05). GC, gastric cancer; Pop, population.
In addition to in silico validation, we also performed in vitro functional validation. First, to validate the internal diaspora G2 markers SOX9, TSPAN8 and AGR2, we performed siRNA knockdown in multiple cell lines (SNU1967, AGS, YCC21, and TMK1), followed by functional assays including cell viability, cell migration, and/or 2D colony formation. We found that for all three genes, knockdown significantly reduced either cell viability, cell migration, or colony formation in at least two and often three lines, indicating that these functional effects are not specific to any one individual line (Fig. 6E and F; Supplementary Fig. S8I). Second, in addition to siRNA-mediated silencing, we also performed CRISPR-mediated KO experiments for SOX9 in three cell lines (SNU1967, AGS, and YCC21). For each cell line, SOX9 KO caused significantly reduced cell viability and cell migration compared with control (wild-type) cells (Fig. 6G; Supplementary Fig. S8J). Third, to further prove that SOX9 is a key driver, we performed single-cell sequencing of paired CRISPR-deleted SOX9 cells and negative control cells [total 5,398 cells after quality control (QC)] in YCC21 gastric cancer cells (Fig. 6H). We found that after SOX9 KO, tumor proliferation-related pathways such as G2M checkpoint, E2F targets, and mitotic spindle gene sets exhibited significant decreases compared with control cells (Fig. 6I), consistent with the experimentally observed SOX9 KO phenotype. These findings suggest a potential role for SOX9 in driving tumor progression in internal diaspora gastric cancers.
Spatial Analysis of the TSI Reveals a Unique TGF-β–Mediated State
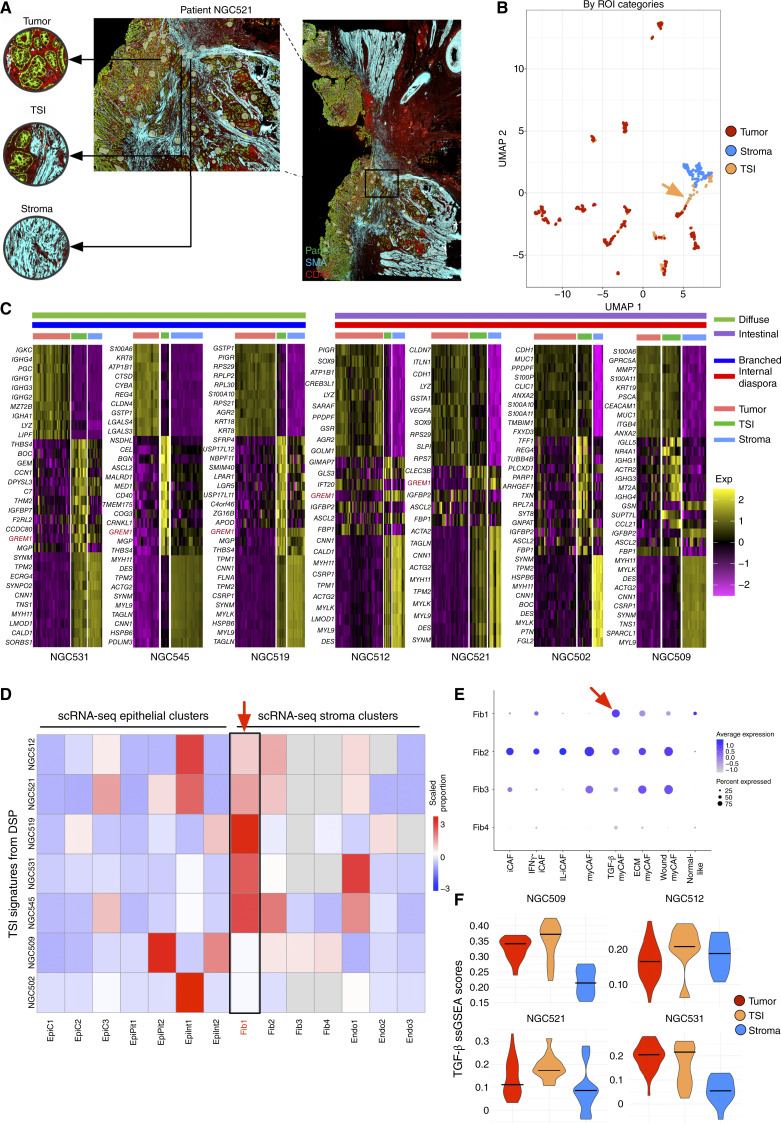
Finally, we leveraged our spatial dataset to probe a specific cancer landmark – the TSI. In cancer biology, the TSI is a site of functional interactions between tumor cells and surrounding normal tissues, providing a window into critical facets of tumor progression (34, 35). We closely examined 85 TSI ROIs in our data set, selected through high-power inspection of tumor samples and defined by regions showing an intermingling of PanCK-stained tumor cells and SMA-stained stroma within each ROI (Fig. 7A). Reflecting their admixture of tumor and stromal cells, the TSI ROIs were juxtaposed between tumor and stromal regions in UMAP space (Fig. 7B).
Figure 7.
TSIs represent a unique TGF-β–mediated cell state. A, Stained GeoMx DSP slide of a specific region from patient NGC521 showing the selection of tumor, TSIs, and stroma ROIs. This region is a zoom-in from the larger NGC521 section (right, black box) shown in Fig. 1E. B, UMAP visualization of the ROIs and tumor/stroma ROIs in the gastric cancer GeoMx DSP dataset. Each dot represents an ROI. Color variations represent ROI categories. TSI ROIs are highlighted with a dotted circumference. C, Expression heatmap of signature genes for tumor, TSI, and stroma ROIs in seven samples after filtering by the number of TSI ROIs. For the heatmap, each row represents a signature gene, and each column represents an individual ROI. The chromatic gradient on the heatmap designates normalized expression intensities of signature genes within individual ROIs. Lauren classification, evolution subtype, and ROI categories are colored as different annotation bars above the heatmap. GREM1 expression is highlighted in red. D, Heatmap displaying the proportions of mapped gastric cancer scRNA-seq cluster labels in corresponding GeoMx DSP samples, determined by CIBERSORTx. Each row corresponds to a GeoMx DSP sample, whereas columns represent different single-cell clusters within the sample. Color intensities reflect the scaled proportions of each cluster. E, Dot plot illustrating the expression of CAF subtype signatures in the gastric cancer scRNA-seq fibroblast clusters. The size of the dot represents the proportion of signature-consistent cells within a cluster, and the dot color represents the scaled average expression of different CAF subtypes. F, Violin plot showing expression of TGF-β gene programs in epithelial, TSI, and stroma ROIs in GeoMx DSP data. The y-axis represents the ssGSEA scores of TGF-β gene program enrichment in each ROI. Exp, expression; Fib, fibroblast.
We investigated the TSI ROIs for genes and pathways highly expressed in TSI regions but lowly expressed in both tumor ROIs and stroma ROIs (Fig. 7C). Analyzing each patient with their Lauren and evolution subtype labels, we identified TSI-associated signatures characterized by specific pathway activation rather than a simple blend of admixed tumor and stromal cells. In intestinal-type gastric cancers, TSI ROIs showed a significant involvement in cell-cycle regulation and extracellular matrix remodeling, whereas diffuse-type gastric cancer TSI ROIs exhibited pronounced activation of genes associated with EMT, coagulation, and hypoxic response (Supplementary Fig. S9A). Interestingly, Gremlin 1 (GREM1), a gene associated with TGF-β signaling and known to be expressed by CAFs (79), was often upregulated in TSI ROIs across both diffuse and intestinal gastric cancers (Supplementary Fig. S9B). When mapped onto scRNA-seq data, we found that the TSI signatures mapped predominantly to a myofibroblastic-like CAF (myCAF) cluster (Fib1) known for TGF-β–driven activity (Fig. 7D and E; Supplementary Table S8; ref. 80). The enrichment of myCAFs at TSIs was orthogonally validated using the AddModuleScore method in Seurat with TSI signatures derived from GeoMx DSP samples (Supplementary Fig. S9C). We also observed heightened TGF-β pathway activity in the TSI, consistent with the enrichment of Fib1 myCAFs (Fig. 7F). To experimentally validate these findings, we cocultured two GREM1+ CAF cell lines (PT13CAF and PT54CAF) with gastric cancer organoid cells (PT25GC) and observed significant upregulation of GREM1 expression by RT-PCR in both CAF lines (Supplementary Fig. S9D). We further analyzed the two Stereo-seq gastric cancer samples and successfully distinguished TSI bins from tumor and stroma bins (see “Methods”), in which TSI bins expressed both tumor-and stroma-specific markers (Supplementary Fig. S9E). Using single-sample gene set enrichment analysis (ssGSEA), we found that tumor regions closer to stroma cells have upregulated TGF-β pathway activity (P < 2.20 × 10−16; Supplementary Fig. S9F), supporting the GeoMx DSP results. Taken collectively, these data demonstrate unique interactions and gene programs occurring in a TSI-specific manner, with GREM1 and heightened TGF-β signaling as potential biomarkers to pinpoint these regions.
Discussion
ST is a rapidly evolving field combining the power of transcriptomic analysis while preserving the spatial context of tissue samples. Several ST technologies currently exist, broadly divided into sequencing-based (sST) and imaging-based ST (iST) platforms (81). sST methods such as Visium (10× Genomics) use spatially indexed surfaces with barcodes, with similar approaches adopted by other sST technologies such as Slide-seq (82) and Stereo-seq (83). In general, sST-based technologies have the advantage of whole-transcriptome analysis and compatibility with standard histologic samples. Alternatively, iST methods, including technologies such as RNAscope and MERFISH, utilize RNA molecules labeled with fluorescent probes that are sequentially imaged across multiple rounds for transcript identification and quantification, allowing direct visualization of RNA molecules. In this study, we utilized NanoString’s GeoMx DSP technology which was developed specifically for FFPE tissues, coupled with tandem 10× Genomics scRNA-seq. In recent years, the integration of single-cell sequencing with spatial technologies is emerging (36–38, 84). For example, Hwang and colleagues (84) combined data from 608 ROIs using the GeoMx DSP platform with 224,988 single-nucleus RNA-seq profiles from patients with pancreatic ductal adenocarcinoma. Park and colleagues (39) adopted integrated scRNA-seq and spatial profiling to study the influence of ACTA2 expression on survival in gastric cancer, whereas Sun and colleagues (38) performed integrative spatial lipidomics and metabolomics profiling of gastric cancer. Here, we applied GeoMx DSP and scRNA-seq technologies to analyze 226 samples from 121 patients with gastric cancer, including 2,138 ROIs from 201 samples across 107 patients. While representing, to the best of our knowledge, the largest GeoMx DSP data set for gastric cancer in the field, our study should be considered only a first step in fully elucidating the ST landscape of gastric cancer, paving the way for subsequent studies with more patients, samples, and ST technologies.
Selective pressures driving ITH in gastric cancer and other solid tumors remain largely ill-defined, and whether ITH evolution is neutral or under selection remains an active area of research. The interplay of tumor-intrinsic and extrinsic factors, including microenvironmental influences such as hypoxia, nutrient availability, and immune surveillance, are likely to exert functional influences on ITH evolution. However, the temporal dynamics of these selective pressures and their role in shaping the spatial distribution of tumor subclones remains poorly understood (85). Whereas some studies suggest that ITH arises primarily from neutral processes such as genetic drift and clonal expansion, others have reported the presence of positive selection for specific subclones that possess advantageous traits in the TME (86, 87). In this regard, RNA-ITH can be harnessed to provide information on the functional consequences of genomic alterations and cellular interactions within the TME, allowing for the identification of different cell states, immune interactions, and signaling pathways driving tumor progression. In this study, we found that G1 RNA-ITH subregions from tumor core regions were characterized by expression signatures reflecting insufficient oxygen supply and severe hypoxia, whereas G2 RNA-ITH subregions from tumor edge regions were associated with higher oxygenation and nutrient availability, advanced invasion ability, and EMT, which may grant tumor cells survival benefits throughout evolution and metastasis (88–90). These results, from the SGCC TMA dataset, demonstrate a concordance between the “tumor edge” macroscopic definition and the microscopic identification of ROIs and suggest that RNA-ITH is not merely a result of intrinsic genetic diversification but also of the selective pressures exerted by the external environment (91). Our spatial analysis also enabled insights into how different tumor-associated cell states interact with the TME. Specifically, in G2 RNA-ITH subregions, we observed features of an immunosuppressive TME likely linked to immune evasion. Notably, G2 RNA-ITH regions also expressed signatures of Wnt pathway activation, which may highlight opportunities for targeted therapies.
Diverse evolutionary phylogenies can exist within the same tumor type (10, 92–94). Specifically, in gastric cancer, a recent study analzying patterns of metastasis in gastric cancer using WES found that hematogenous metastasis originated directly from the primary tumor through blood vessels, whereas peritoneal metastasis was phylogenetically closer to the primary tumor than lymph node metastasis. Relevant to this study, the authors reported evolutionary patterns very similar to branched evolution and internal diaspora evolution, with the latter associated with high ITH and a poorer prognosis (66). Mirroring these findings, our analysis of primary gastric cancer samples utilizing single-cell data also yielded similar evolutionary trajectories (branched evolution and internal diaspora evolution), demonstrating consistency between DNA-ITH and RNA-ITH approaches to infer evolution patterns. Survival analysis demonstrated that internal diaspora evolution portended a significantly worse prognosis compared with gastric cancers with branched evolution in multiple independent cohorts. Multivariate analyses further confirmed that internal diaspora evolution was independently associated with an increased risk of gastric cancer mortality.
Using both spatial and scRNA-seq analysis, we identified a unique TME associated with internal diaspora samples. These included an enrichment in Endo2 cells, which highly express von Willebrand factor (VWF) and ACKR1 (Duffy antigen receptor for chemokines). VWF-expressed endothelial cells have been reported to be enriched in late-stage gastric cancers (95) and have been linked to myogenesis (56). ACKR1, specifically enriched in tumor endothelial cells, has been associated with a poorer prognosis in gastric cancer (96). These observations suggest that endothelial cell populations play a critical role in shaping the unique characteristics of the internal diaspora gastric cancer TME. Additionally, TMEs in internal diaspora gastric cancer were further characterized by the presence of TAM1 macrophages, marked by high expression of SPP1 and FN1. TAM1 macrophages have been associated with worse clinical outcomes due to their immune-suppressive properties (56, 71, 72). These findings emphasize the significance of considering the endothelial cell population and TME characteristics in developing targeted treatment strategies for internal diaspora gastric cancers.
To identify potential genetic drivers of tumor progression, we identified genes associated with G2 versus G1 tumor progression in internal diaspora gastric cancers, including SOX9 and TSPAN8. Computational in silico SOX9 knock-outs, applied to the scRNA-seq data, predicted that SOX9 loss significantly shifted G2-like states to G1, consistent with SOX9 being a potential G1/G2 driver. SOX9, a highly expressed TF in gastric cancer, has been reported to play significant roles in tumorigenesis and progression and regulated by multiple embryonic signaling pathways, including Notch, Wnt, and Hippo/YAP1. In gastric cancer, studies have demonstrated that SOX9 expression is associated with therapy resistance and reduced NK cell function, contributing to treatment failure and immune evasion (97).
The TSI region, in which tumor cells interact with stromal cells, is a critical area of investigation in current research (34, 35). Traditional tumor sequencing and profiling methodologies often face challenges in delineating and characterizing this region. In our study, we utilized GeoMx DSP to manually select 85 TSI ROIs, providing a valuable dataset for TSI research. Our findings are consistent with prior research from other cancers linking the tumor periphery to increased invasion (98–100). In the TSI, we uncovered a distinct cell state characterized by upregulated activity of the TGF-β pathway, potentially involving GREM1. GREM1 has been associated with bone morphogenesis and disorders of bone formation, and its expression has been observed in CAFs, driving EMT through the TGF-β pathway to promote tumor progression and metastasis (79). By mapping the TSI ROIs to single-cell clusters from paired samples, we identified a specific TGF-β MyCAF cluster called Fib1, characterized by high expression of RGS5, ACTA2, and NOTCH3. This cell population corresponds to the “VSMC_C03” cell population identified in a recent gastric cancer study (56) and has also been observed in other cancers, such as “vCAFs” in breast cancer (101), providing validation that the TSI represents a unique cell state across independent cohorts.
In summary, our study sheds light on the intricate landscape of gastric cancer, highlighting the importance of spatial heterogeneity and its impact on tumor progression and evolution. By combining spatial profiling with single-cell sequencing, we uncovered distinct spatial intratumor subgroups with unique expression-based molecular signatures, evolutionary trajectories, and TME characteristics. These findings not only deepen our understanding of gastric cancer heterogeneity but also unveil potential targets and spatial biomarkers for therapy response prediction. Looking ahead, the integration of single-cell and spatially based multiomic profiling holds great promise for unraveling the complexities of gastric cancer biology.
Methods
Ethics Declaration, Sample Collection, and Processing
This study was approved by the local ethics board [National Healthcare Group, Domain-Specific Review Board Ref Nos: 2005/00440 and 2016/00059]. Protocols were performed in accordance with the Declaration of Helsinki for human research. Patients diagnosed with gastric adenocarcinoma undergoing surgical resection or endoscopy at National University Hospital, Singapore, were enrolled after providing written informed consent. On-table endoscopic biopsies or surgical resection samples were collected along with matched normal gastric tissues from areas several centimeters away from the tumor site. Fifteen primary gastric cancers were analyzed by GeoMx Human Whole Transcriptome Atlas (GeoMx WTA) DSP and integrated with paired scRNA-seq (28), along with 14 additional patients profiled by scRNA-seq (11 primary and 3 peritoneal metastases, GSE183904; https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE183904, DOI: 10.1158/2159-8290.CD-21-0683). Clinical information of the gastric cancer samples, including tumor stage, HER2, EBV status, DNA Mismatch Repair (MMR), survival, gender, age, and other related information is presented in Supplementary Table S2. For validation cohorts, 6 patients with gastric cancer were analyzed by GeoMx DSP, and 86 patients with gastric cancer on TMAs were analyzed by GeoMx DSP. In cases in which surgical resections were available, samples from the same patient were obtained from the same resection. Five gastric cancers were also paired with WES data, detailed in Supplementary Table S1. For treatments, gastric cancers profiled by GeoMx DSP either did not receive chemotherapy or chemotherapy of differing types delivered in different regimens (e.g., adjuvant, perioperative, etc.). Organoid culture–related experiments used human tissue samples consented under Domain-Specific Review Board 2005/00440 and approved under NUS Institutional Review Board Reference number LH-19-070E.
GeoMx Digital Spatial Profiling
For GeoMx DSP, 16 FFPE blocks from 15 patients were sectioned into 5-μm slices and placed onto BOND Plus slides (Leica Biosystems). H&E staining was performed on one slide from each block, in which a pathologist (SS) delineated specific ROIs, including tumor, stroma, normal epithelial, intestinal metaplasia, and LAs. The adjacent slide was processed using the GeoMx WTA protocol (NanoString). ROI selection was guided by immunostaining with four markers: 4’, 6-diamidino-2-phenylindole (DAPI), CD45, PanCK, and SMA. Between 22 and 95 ROIs were selected per slide (∼71 ROIs per slide before QC). 92.61% of the ROIs were full ROIs rather than segmented ROIs using antibody markers. Libraries for sequencing were constructed using SeqCode reagents (NanoString) and sequenced on the Illumina platform.
TMA
The SGCC TMA was constructed using 2-mm cores by a qualified pathologist (JHYL). A core of benign gastric mucosa was included at least 5 mm away from the tumor. Tumor cores were obtained from the following locations: the mucosal–tumor interface, labeled as “tumor edge,” and superficial and deep tumor regions (if the tumor exceeded 5 mm in depth), labeled as “tumor core.” GeoMx DSP ROI selections (median 282 cells/ROI) and sample processing followed a similar protocol as whole section–based primary gastric cancers.
Single-cell Profiling
Chromium Next GEM Single Cell 3′ Kit v3.1 Library and Gel Bead Kit (10× Genomics) was used according to manufacturer protocols. Briefly, Gel Bead-in-EMulsion (GEM) assemblies were created by combining barcoded single-cell 3′ v3.1 Gel Beads, a Master Mix containing cells, and partitioning oil onto Chromium Next GEM Chip. The resulting GEMs contained barcoded, full-length cDNAs, which were then amplified via PCR. Subsequently, enzymatic fragmentation, size selection, and adapter ligation processes were used in preparation for sequencing. Sequencing libraries were generated with unique sample indexes, quantified using the Kapa library kit. Quantified libraries were sequenced using an Illumina Hiseq4000 sequencer.
GeoMx DSP Data Processing and Analysis
FASTQ files from GeoMx DSP were converted into count matrices using established protocols (102). Sequencing counts were deduplicated based on unique molecular identifiers and molecular target tag sequences, with single-probe genes reported as deduplicated count values. Data processing and normalization were performed utilizing the GeoMxTools R package v2.0 (RRID: SCR_023424). ROIs that did not meet quality standards—those with fewer than 1,000 raw reads, less than 75% read alignment, or sequencing saturation below 50%—were excluded from further analysis. The quantitation limit was established as two geometric SDs above the geometric mean of the negative control probes. ROIs in which fewer than 5% of panel genes surpassed the quantitation limit were discarded, along with genes detected in less than 10% of the remaining ROIs. Data normalization involved upper quartile (Q3) normalization. Following filtering, each ROI exhibited a median of more than 3,800 detected genes. Cell abundances within each ROI were estimated by CIBERSORTx (RRID: SCR_016955; docker version) using signatures derived from paired scRNA-seq samples (n_permutation = 1,000). Interpatient/sample batch effects were normalized by ComBat (RRID: SCR_010974), Limma (RRID: SCR_010943), and RUV4 (DOI: 10.1093/biostatistics/kxr034). To compare each ROI category, DEG analysis was performed utilizing the GeoMxTools R package v2.0 (RRID: SCR_023424). GSEA of DEGs was conducted using MSigDB Hallmark/GO/KEGG databases (https://www.gsea-msigdb.org/gsea/msigdb/, RRID: SCR_016863) through the R clusterProfiler package (RRID: SCR_016884). Top significant pathways for each ROI category were visualized by heatmaps drawn by the R pheatmap package (RRID: SCR_016418).
Intratumor subgroups within each sample were delineated through unsupervised clustering using the ward.D2 method and correlation distances calculated from log-transformed, Q3-normalized whole gene expression data. The resulting log-transformed NanoStringGeoMxSet data were transformed into a Seurat object using the built-in as.Seurat function, which facilitated subsequent analyses using the Seurat pipeline (RRID: SCR_016341). This pipeline included steps such as FindVariableFeatures, ScaleData, RunPCA, RunUMAP, FindNeighbors, and FindClusters, using the Leiden algorithm for cluster detection. Individual gene expression levels were examined using the processed Seurat object. Differential expression analysis was performed with DESeq2 (RRID: SCR_015687) utilizing raw count data. GSEA of DEGs was conducted using the MSigDB Hallmark database (https://www.gsea-msigdb.org/gsea/msigdb/, RRID: SCR_016863) through the R clusterProfiler package (RRID: SCR_016884). HET70 signature scores were computed using the AddModuleScore function in Seurat. Additional signatures and pathways were mapped onto GeoMx DSP data through ssGSEA using the R GSVA package (RRID: SCR_021058). Differential analysis for TSI ROIs involved comparisons with combined tumor and stroma ROIs using DESeq2 (RRID: SCR_015687). Deconvolution of TSI ROIs into corresponding single-cell clusters utilized CIBERSORTx (RRID: SCR_016955), applying cluster-specific signatures per sample (n_permutation = 1,000 without batch correction). The processing and analysis of TMA ROIs paralleled those for GeoMx DSP ROIs. Only TMA samples containing both tumor core and tumor edge ROIs were retained for the analysis. To generate signatures for G1/G2 RNA-ITH subregions, shared top DEGs in each subregion across samples were identified and aggregated into a composite signature. These signatures were subsequently mapped to TMA tumor core and tumor edge ROIs via ssGSEA. Proportions of PanCK/SMA/CD45 staining were calculated using serial immunostained slides for all tumor ROIs using ImageJ v2.3 (RRID: SCR_003070) following a previous GeoMx DSP study (103). Spatial autocorrelations were evaluated by Moran’s I between intratumor subregions using R package spdep v1.2 (RRID: SCR_019294). To evaluate whether G1 or G2 RNA-ITH subregions are closer to TSI ROIs, a K-nearest neighbor method was used, in which for each TSI ROI we calculated the proportion of the G1 and G2 subregions in the top K (K = 10) tumor ROIs that are spatially closest to the TSI ROIs.
scRNA-seq Data Processing and Analysis
QC and initial processing of scRNA-seq data were conducted using the Seurat package, adhering to methodologies outlined in our prior studies (28). CopyKAT (RRID: SCR_024512) was utilized to identify sCNAs across 29 tumor samples using a pool of 10 normal samples as a normal reference. For validation purposes, inferCNV (RRID: SCR_021140) was applied on the same samples. Correlations between CopyKAT-derived and WES-derived sCNAs were assessed at the chromosomal arm level following established protocols (11). Correlations between CopyKAT-derived and InferCNV-derived sCNAs was assessed at the per-gene level using Spearman’s correlation. Subsequent sCNA analysis was performed on CopyKAT-predicted sCNAs. Unsupervised clustering of sCNA matrices within each sample used ward.D2 and Euclidean distances, as recommended by the CopyKAT pipeline. Identified subclusters were subjected to phylogenetic analysis utilizing both neighbor-joining (based on Euclidean distances) and maximum parsimony tree methods (based on Hamming distances, using prebuilt UPGMA tree, trace = 0, minit = 1,000), with trees rerooted to an artificial diploid. This process was performed by the R packages ape (RRID: SCR_017343) and phangorn (RRID: SCR_017302). The event sCNA matrix for each sample was constructed from the CopyKAT sCNA matrix following a previously described approach (104). A consensus sCNA profile emerged by retaining consistent sCNA events across cells that exceeded a specified threshold (70%) within each chromosomal position. Common sCNA regions among internal diaspora samples were defined as intersecting regions from the profiles of individual samples. To connect sCNA levels to G1/G2-like features, we mapped G1/G2 GeoMx DSP signatures onto intratumor subgroups defined by scRNA-seq phylogenetic analysis. Overall sCNA burdens per cell were calculated using the sum of the squared sCNA values from the CopyKAT-called raw sCNA matrix for each genomic position.
Trajectory and pseudotime analyses were conducted on raw expression matrices using Monocle v2.22 (RRID: SCR_016339), Slingshot v2.10 (RRID: SCR_017012), and PAGA (built within Scanpy v1.8 under Python v3.7, RRID: SCR_018139) under default parameter settings. Differential expression analysis used the FindMarkers function within Seurat. GSEA on DEGs was performed using the MSigDB Hallmark database through the R clusterProfiler package (RRID: SCR_016884). Jaccard indices were calculated among intratumor subgroups using R, and average values were calculated when the sample had more than two subgroups. To construct density plots of TMEs between internal diaspora and branched evolution gastric cancers, 10,000 cells from each category were randomly selected and visualized using a custom Python script. The raw matrix, inclusive of cell type labels from scRNA-seq, served as the foundation for CIBERSORTx to develop tailored cell type signatures, which were then applied to deconvolute the TCGA stomach adenocarcinoma bulk RNA data into nine distinct subtypes. For analyses focusing on stromal and macrophage cell types, matched cells were first isolated from the overall cell population using the subset function in Seurat, and normalization, scaling, dimensionality reduction, clustering, and annotation were performed to accurately characterize each subpopulation. Signatures for each identified subcluster were derived using the FindAllMarkers function.
WES Data Processing and Analysis
Exome sequencing reads were aligned to the human reference genome using BWA MEM (RRID: SCR_010910). Subsequent preprocessing included marking duplicates, locally realigning reads, and recalibrating base quality scores using Picard (RRID: SCR_006525) and the Genome Analysis Toolkit (GATK, RRID: SCR_001876). These steps produced analysis-ready BAM files. sCNAs within the exome data were identified using the GATK ACNV method, which utilized a panel of more than 200 normal samples from a comprehensive, in-house dataset.
In silico KO Stimulations
CellOracle (DOI: 10.1038/s41586-022-05688-9) was initially validated using a publicly available dataset GSE52529 (DOI: 10.1038/nbt.2859; ref. 78) in which 0 hour–cultured and 72 hour–cultured primary human myoblasts were extracted. An in silico KO of MYOG was then simulated on this dataset using the standard CellOracle pipeline, referencing a default human single-cell ATAC sequencing dataset. For cell line scRNA-seq data, QC and preprocessing steps were conducted as previously outlined, resulting in 11,999 TP53 TP53-NT cells and 4,164 TP53-KO cells. In silico TP53 KOs were performed on these cells using the CellOracle standard pipeline. Finally, within each gastric cancer scRNA-seq sample, G1 RNA-ITH and G2 RNA-ITH cells were extracted, and an in silico SOX9 KO was performed using the standard CellOracle pipeline.
mIHC Experiment and Scoring
mIHC staining was performed on 4-μm-thick gastric cancer FFPE TMA sections using a Leica Bond Max autostainer (Leica Biosystems), Bond Refine Detection Kit (Leica Biosystems), and Panovue 7-plex TSA IHC Kit (Panovue; refs. 105–109). FFPE tissue sections were deparaffinized, rehydrated, and thereafter subjected to repeated cycles of heat-induced epitope retrieval, incubation with primary antibody, incubation with secondary antibodies conjugated with polymeric HRP (Bond Refine Kit), and tyramide signal amplification (Panovue). After all markers were applied, spectral DAPI (Panovue) was utilized as the nuclear counterstain. Lastly, slides were mounted with ProLong Diamond Anti-fade Mountant (Molecular Probes, Life Technologies) and cured in the dark at room temperature for 24 hours. Images were captured using a Zeiss Axioscan 7 slide scanner (Zeiss).
We performed cell segmentation and feature measurement on the multiplex IHC images using automated tissue image analysis software (cellXpress v2.4.0, Bioinformatics Institute, Singapore; ref. 110). After all the cells from a TMA core were identified and quantified, ROIs selected for DSP analysis within the TMA core were annotated on the mIHC images as polygons using cellXpress. TMA cores that were mismatched or with artifacts were excluded. Mean staining intensity levels of CD3, CD20, CD68, and CD163 across whole cells and CK intensities in cytoplasmic regions were used to assess marker positivity.
Positive cells for each marker were identified using a semi-automated multiplex image scoring system (ImmunoThresholdTM; ImmunoQs Pte. Ltd.) written in R (version 4.3.2). For each marker, the system used Gaussian mixture modeling (mixtools v2.0.0; ref. 111) to determine a decision threshold separating between cells with (“positive”) or without (“negative”) staining of the marker. For threshold determination, the system removed cells with the lowest 1st-percentile mean marker intensity levels, log2-transformed the mean intensity values, and ran the GMM algorithm for 20,000 iterations until convergence was attained. All the thresholds were manually checked based on expected phenotypes, appearances, and coexpression of other related markers in the positive cells. If a threshold was found to be imprecise, the threshold was then manually tuned based on the aforementioned criteria. To ensure consistency, all ROIs from the same TMA cores used the same threshold values to identify positive cells for the same markers. Positions and feature values for all the individual positive and negative cells were exported as .csv files.
Primary antibodies used were as follows:
CD3 (DAKO, clone M0452)
CD20 (Agilent, Cat. # M0755, RRID: AB_2282030)
CD68 (Agilent, Cat. # M0876, RRID: AB_2074844)
CD163 (Cell Marque, Cat. # 163M-16, RRID: AB_1159122)
PanCK (Agilent, Cat. # M3515, RRID: AB_2132885)
Stereo-seq Profiling
For Stereo-seq, gastric cancer tumor tissues were embedded in cryomold (4566, Sakura) with Tissue-Tek optimum cutting temperature compound (4583, Sakura) and kept frozen at long-term storage in −80°C. Gastric cancer tumor tissue and organoid-containing optimum cutting temperature blocks were cryosectioned at 10 and 12 μm thickness, respectively. Sectioned slices were placed onto a Stereo-seq capture chip and fixed with methanol for 30 minutes at −20°C. Tissues on the capture chip were stained with Qubit ssDNA reagent (Invitrogen), and chips were mounted with glycerol and imaged using a fluorescent microscope (Olympus). Next, tissues on the chips were processed according to the manufacturer’s protocol (Stereo-seq Transcriptomics T Kit). Briefly, sections were permeabilized using Permeabilization Mix containing HCl (Sigma) for an optimized time determined using Stereo-seq Permeabilization Kit. After reverse transcription, cDNA products were released from the chips by cDNA Release Enzyme Mix. The cDNA mix was collected, purified by 0.8× beads (AMPure) selection, amplified by cDNA Amplification Mix with cDNA primers, and purified by 0.6× beads (AMPure). Purified cDNA samples were used for cDNA library preparation following the manufacturer’s protocol for Library Preparation Kit. cDNA libraries were sequenced on a MGI DNBSEQ-T7 Sequencer (BGI).
Stereo-seq Data Processing and Analysis
FASTQ files from Stereo-seq data were converted into count matrices using the SAW pipeline v7 (RRID: SCR_025001; ref. 112). Sequencing counts were first preprocessed using the Stereopy package v0.12 (bioRxiv 2023.12.04.569485) under Python v3.8. Bin50 was selected as the basic “spot” unit, and each spot was filtered for genes/features shared by three or more cells and spots showing 500 or more features. Bin50 spots with mitochondrial RNA percentages of >20 were filtered out. The Stereopy object (with Bin50 counts after QC) was then converted to a Seurat object using the st.io.stereo_to_anndata function from Stereopy. Subsequent analysis using Seurat (RRID: SCR_016341) was similar to the GeoMx DSP data analysis. Spot types were first defined by the expression of cell-specific markers used in scRNA-seq annotation and then optimized based on the expression pattern of top signatures for each cell type by DEG analysis. For sCNA analysis, inferCNV (RRID: SCR_021140) was applied to the tumor spots in the gastric cancer samples. Immune cells were selected as references for inferCNV using a hard threshold of 0.05 for denoising the results. TSI spots were defined as spots expressing both tumor and stroma markers and without expressing immune markers. Spatial distances between G1-like/G2-like tumor spots and stroma spots were measured using mean pairwise Euclidian distances.
Gastric Cell Lines
GES1 cells (RRID: CVCL_EQ22) were provided by Dr. Alfred Cheng from the Chinese University of Hong Kong in 2016. SNU1967 cells were obtained from the Korean Cell Line Bank in 2013. AGS cells (RRID: CVCL_0139) were obtained from the ATCC in 2013. YCC21 cells (RRID: CVCL_9654) were a gift from Yonsei University College of Medicine, Seoul, in 2013. TMK1 cells (RRID: CVCL_4384) were obtained from the Health Science Research Resource Bank in 2016. Culture medium/Supplements for GES1, SNU1967, and AGS are RPMI 1640 + 10% FBS + 1% penicillin–streptomycin, for YCC21 are MEM + 10% FBS + 1% penicillin–streptomycin, and for TMK1 are RPMI1640 + 15% FBS + 1% penicillin–streptomycin. The latest Mycoplasma testing date for GES1, SNU1967, and AGS was on July 31, 2024, and for YCC21 and TMK1 was on September 30, 2024. All lines were negative for Mycoplasma contamination, as assessed by MycoAlert Mycoplasma Detection Kit (Lonza). The selection of gastric cancer cell lines for functional validation was based on a combination of factors, including ease of growth, transfection efficiency, and expression level of the gene of interest. Average time between cell thawing and profiling/assay completion was within 6 weeks.
siRNA Gene Knockdowns
ON-TARGETplus Human siRNA SMARTpools targeting SOX9 (L-021507-00-0005), TSPAN8 (L-010219-00-0005), or AGR2 (L-003626-00-0005) or ON-TARGETplus Nontargeting Pool (D-001810-10) was used to transfect gastric cancer cells using Lipofectamine RNAiMAX (Thermo Fisher Scientific). To assess knockdown efficiency, total RNA was extracted following manufacturer’s protocols (Qiagen) and reverse transcribed using iScript Reverse Transcriptase Supermix (Bio-Rad). qPCR analysis was performed using SYBR Green PCR Master Mix (Life Technologies) and the ViiA7 Real Time PCR system. Primers used to quantify relative gene expression are as follows:
SOX9-F-5′-AGGAAGCTCGCGGACCAGTAC-3′
SOX9-R-5′-GGTGGTCCTTCTTGTGCTGCAC-3′
AGR2-F-5′-GGTGACCAACTCATCTGGACTC-3′
AGR2-R-5′-TGACTGTGTGGGCACTCATCCA-3′
TSPAN8-F-5′-CATAGGCTTGCTTCTGATCCTGC-3′
TSPAN8-R-5′-CTTTCCCCTGTGGCGCTCAAAA-3′
Generation of Genome-Edited KO Cells
To generate KO cells, the following single-guide RNA–containing oligos were cloned into lentiCRISPR v2 backbones:
NT gRNA#1 Forward: 5′-CACCGGATGCCCGGCTTCCACTTCG-3′
NT gRNA#1 Reverse: 5′-AAACCGAAGTGGAAGCCGGGCATCC-3′
NT gRNA#2 Forward: 5′-CACCGAAAACAGGACGATGTGCGGC-3′
NT gRNA#2 Reverse: 5′-AAACGCCGCACATCGTCCTGTTTTC-3′
NT gRNA#3 Forward: 5′-CACCGAAAACATCGACCGAAAGCGT-3′
NT gRNA#3 Reverse: 5′-AAACACGCTTTCGGTCGATGTTTTC-3′
NT gRNA#4 Forward: 5′-CACCGAAAATAGCAGTAAACTCAAC-3′
NT gRNA#4 Reverse: 5′-AAACGTTGAGTTTACTGCTATTTTC-3′
TP53 gRNA#1 Forward: 5′-CACCGCCATTGTTCAATATCGTCCG-3′
TP53 gRNA#1 Reverse: 5′-AAACCGGACGATATTGAACAATGGC-3′
TP53 gRNA#2 Forward: 5′-CACCGGAGCGCTGCTCAGATAGCGA-3′
TP53 gRNA#2 Reverse: 5′-AAACTCGCTATCTGAGCAGCGCTCC-3′
TP53 gRNA#3 Forward: 5′-CACCGATGTGTAACAGTTCCTGCAT-3′
TP53 gRNA#3 Reverse: 5′-AAACATGCAGGAACTGTTACACATC-3′
TP53 gRNA#4 Forward: 5′-CACCGCAGAATGCAAGAAGCCCAGA-3′
TP53 gRNA#4 Reverse: 5′-AAACTCTGGGCTTCTTGCATTCTGC-3′
SOX9 gRNA#1 Forward: 5′-CACCGCATGAAGATGACCGACGAGC-3′
SOX9 gRNA#1 Reverse: 5′-AAACGCTCGTCGGTCATCTTCATGC-3′
SOX9 gRNA#2 Forward: 5′-CACCGCGTGTTCTCGGTGTCCGAGC-3′
SOX9 gRNA#2 Reverse: 5′-AAACGCTCGGACACCGAGAACACGC-3′
SOX9 gRNA#3 Forward: 5′-CACCGCTCACCCGAGTGCTCGCCGG-3′
SOX9 gRNA#3 Reverse: 5′-AAACCCGGCGAGCACTCGGGTGAGC-3′
SOX9 gRNA#4 Forward: 5′-CACCGTTCACCGACTTCCTCCGCCG-3′
SOX9 gRNA#4 Reverse: 5′-AAACCGGCGGAGGAAGTCGGTGAAC-3′
To generate TP53 or SOX9 KO cells, cells were infected with pools of four TP53- or SOX9-targeting single-guide RNAs containing lentiviral particles. For wild-type controls, cells infected with lentiviral particles containing a pool of four NT single-guide RNAs. Infected cells were selected with 2 μg/mL puromycin for 2 weeks before other analyses.
Western Blotting
Cells were lyzed for 10 minutes on ice using RIPA buffer (Sigma) supplemented with protease inhibitors. Protein concentrations were determined using Pierce BCA Protein Assay Kit. Samples were subsequently incubated at 95°C for 10 minutes prior to gel electrophoresis. The following antibodies were used for Western blotting: p53 (Santa Cruz Biotechnology, Cat. # sc-126, RRID: AB_628082), GAPDH (Proteintech, Cat. # 60004-1-Ig, RRID: AB_2107436), and SOX9 (Cell Signaling Technology, Cat. # D8G8H).
Cell Proliferation Assay
For cell proliferation assays, 1,000 to 3,000 cells were seeded in 96-well plates and measured using Cell Counting Kit-8 (Dojindo) at 3 and 5 day time points. Ten μL Cell Counting Kit-8 solution was added to each well and incubated for 2 hours in a humidified incubator. Absorbance readings were measured using a Tecan Infinite M200 microplate reader, and readings for each day were normalized to day 0 to generate growth curves.
Cell Migration Assay
To evaluate cell migration, 5 × 104 cells were suspended in 0.1 mL serum-free medium and added to the top of a Transwell insert (Corning Costar 6.5-mm Transwell with 8.0-μm Pore Polycarbonate Membrane Inserts, 3422, Corning). Medium containing 10% FBS was added to the bottom well as a chemoattractant. After incubation for 24 hours at 37°C in a 5% CO2 incubator, cells were washed twice with 1 × PBS and permeabilized with 100% methanol. Cells were stained with 0.5% crystal violet and washed trice with 1 × PBS. Nonmigrated cells were scraped off from the upper surface of the membrane with cotton swabs. The number of migrated cells was represented as the total area of migrated cells against the Transwell membrane area and calculated using ImageJ software (RRID: SCR_003070).
Coculture of CAFs and Gastric Cancer Organoids
CAF lines and gastric cancer organoids were isolated from patients with primary gastric cancer and cultured in organoid medium (advanced DMEM/F12 medium containing WNT3A, RSPO-1, FGF, EGF, B27, gastrin, etc). The medium was refreshed every 2 days. CAF cells were passaged every 4 days, and gastric cancer organoids were passaged every 7 days. CAF lines and gastric cancer organoids were cocultured in Corning transwell plates (CLS3422). Organoids were seeded in the insert well, and fibroblast cells were seeded in the bottom well. After 3-day coculture, fibroblast cells were collected for subsequent RT-qPCR measurements.
RT-qPCR Assay
RT-qPCR assays were performed to test GREM1 expression in CAF lines. RNA was extracted from fibroblast lines following the manufacturer’s protocols (Qiagen Rneasy Mini Kit 74106) and reverse transcribed using iScript Reverse Transcriptase Supermix (Bio-Rad 1708841BUN). qPCR analysis was conducted using SsoFast EvaGreen Supermix (Bio-Rad 1725202). Samples were analyzed using the Applied Biosystems 7900HT system. Primers used are as follows:
GREM1_F2: TCATCAACCGCTTCTGTTACG
GREM1_R2: GGCTGTAGTTCAGGGCAGTT
Survival Analysis
Samples from the TCGA stomach adenocarcinoma cohort were classified into branched evolution or internal diaspora evolution categories based on their expression of top markers (n = 100) demonstrating consistent patterns. Samples yielding ambiguous mapping scores, indicative of no clear categorization into either branched or internal diaspora gastric cancer, were excluded from subsequent analysis. For the remaining 332 samples, Kaplan–Meier survival analysis was conducted, with significance determined via the log-rank test. Multivariate Cox regression analysis incorporated evolution types, patient gender, and tumor stages as confounding variables. Parallel survival analyses utilizing both Kaplan–Meier and Cox regression methodologies were similarly applied to an additional dataset from the ACRG GSE62254 (DOI: 10.1038/nm.3850; n = 273; ref. 6). To perform multivariate Cox regression analysis comparing both evolution patterns and ITH levels on the TCGA stomach adenocarcinoma data, previously called mutations in MAF file format of the TCGA stomach adenocarcinoma data were used. MATH scores for each patient was calculated to reflect ITH levels using the R maftools V2.14 package (RRID: SCR_024519).
Statistical Analysis
Statistical analyses were conducted using the R software (version 4.1.2). Significance was determined with a threshold set at P < 0.05, adjusted for multiple testing where applicable. Continuous variables, such as gene expression and cell proportion, were assessed using the Wilcoxon rank-sum test. ssGSEA scores for gene programs were z-transformed across samples and analyzed using the Wilcoxon rank-sum test. An outlier, NGC502, was excluded from ssGSEA analysis. Associations between CopyKAT-called sCNAs and those called by InferCNV or WES were evaluated using Spearman’s rank correlation test. χ2 tests were employed to examine relationships between sCNA evolution subtypes and TCGA molecular or Lauren histologic subtypes. Differential gene expression analysis was conducted using DESeq2 for GeoMx DSP data or data obtained from TCGA/Gene Expression Omnibus (GEO), whereas Seurat’s FindMarkers function was used for scRNA-seq data. Adjustments for multiple comparisons were made using the respective default settings of DESeq2 and FindMarkers. Survival outcomes were analyzed using Kaplan–Meier curves with log-rank statistics to compare overall survival, and HRs were computed using a multivariate Cox proportional hazards model. All tests of statistical significance were two-sided in this study.
Data Availability
Raw sequencing data of GeoMx WTA DSP profiles have been deposited at the European Genome-phenome Archive (EGA) under accession number EGAS50000000345 (https://ega-archive.org/studies/EGAS50000000345). Raw sequencing data of TMA GeoMx DSP data generated by the SGCC has been deposited at the EGA under accession number EGAS50000000640 (https://ega-archive.org/studies/EGAS50000000640). Gastric cancer scRNA-seq data has been uploaded to GEO under accession number GSE183904 (DOI: 10.1158/2159-8290.CD-21-0683). Additional validation GeoMx WTA DSP, Stereo-seq gastric cancer/organoid data, TP53/SOX9-KO cell line scRNA-seq data, and in-house gastric cancer line bulk mRNA-seq data were generated by Cancer and Stem Cell Biology Duke-NUS medical school and are available from the corresponding author (P. Tan) on request. Bulk mRNA-seq expression data (normalized) generated by TCGA on primary stomach adenocarcinoma were downloaded from NCI Cancer Genomic Data Commons (https://gdc.cancer.gov). ACRG GSE62254 (DOI: 10.1038/nm.3850) data were downloaded from the GEO database (https://www.ncbi.nlm.nih.gov/geo/, RRID: SCR_005012; ref. 6). GASCAD data is available under EGA accession number EGAS00001006397 (DOI: 10.1136/gutjnl-2022-328332). The public scRNA-seq dataset GSE52529 (DOI: 10.1038/nbt.2859) used for CellOracle validation was downloaded from the GEO database (https://www.ncbi.nlm.nih.gov/geo/, RRID: SCR_005012; ref. 78).
Supplementary Material
(1) Supplementary Table 1 shows GeoMx DSP ROI and scRNA-seq characteristics of samples profiled; (2) Supplementary Table 2 shows clinical characteristics of GeoMx DSP ROI and scRNA-seq samples profiled; (3) Supplementary Table 3A shows consistency between before and after batch effect removal on top enriched pathways in various ROI categories, 3B shows consistency between before and after batch effect removal on G1 and G2 labels on Tumor ROIs; (4) Supplementary Table 4 shows Silhouette scores of using different number of clusters in Tumor ROI hierarchical clustering; (5) Supplementary Table 5 shows detailed information of GeoMx ROIs in the TMA cohort; (6) Supplementary Table 6 shows top DEGs for endothelial cell subtypes (Endo1-Endo3); (7) Supplementary Table 7 shows top DEGs for macrophage cell subtypes (TAM1-TAM6); (8) Supplementary Table 8 shows top DEGs for fibroblast cell subtypes (Fib1-Fib4).
(1) Supplementary Figure 1 shows morphologies of different ROI categories and their representative genes, pathways and similarities with paired scRNA-seq data. (2) Supplementary Figure 2 shows validation of findings from the discovery GeoMx DSP cohort in the whole-section GeoMx DSP cohort and also the TMA GeoMx DSP cohort. (3) Supplementary Figure 3 shows experiments validating that intratumor G1/G2 subregion expression is tumor-intrinsic using multiplexed IHC, Stereoseq spatial transcriptomics, and in vitro Stereoseq drug treatment profiling. (4) Supplementary Figure 4 shows expression of individual immune checkpoints, chemokines, cytokines, molecular signatures (immune exhaustion, intracellular signaling) and emerging therapeutic targets in G1 and G2 subregions in the discovery and validation cohort. (5) Supplementary Figure 5 shows experiments validating that G2 RNA-ITH subregions have higher CNV burdens than G1 subregions using signature scores, CNV calling from scRNA-seq, and validation using WES and Stereoseq data. (6) Supplementary Figure 6 shows phylogenetic analysis and trajectory analysis of scRNA-seq tumor subgroups using various methods. (7) Supplementary Figure 7 shows survival analysis and multi-variate analysis validating that internal diaspora GC is associated with worse prognosis in various cohorts. (8) Supplementary Figure 8 shows functional validation experiments for the potential G2 markers SOX9, AGR2 and TSPAN8 using CellOracle, RNAi and CRISPR knock out assays. (9) Supplementary Figure 9 shows typical pathways, markers and cell types for tumor-stroma interface ROIs.
Acknowledgments
This research was supported by the National Research Foundation, Singapore, and Singapore Ministry of Health’s National Medical Research Council under its Open Fund-Large Collaborative Grant (; MOH-OFLCG18May-0003). This work was also supported by the National Medical Research Council grant (MOH-000967, CIRG23Jul-0035, and MOH-00062). We acknowledge support by the Ministry of Education, Singapore, under its MOE Academic Research Tier 3 (RIE2025; MOE-MOET32021-0004). Funding was also provided by the Cancer Science Institute of Singapore, National University of Singapore, supported by the National Research Foundation of Singapore and the Singapore Ministry of Education under its Research Centres of Excellence initiative. This research was also supported by the Duke-NUS Core funding. We thank Dr. Alfred Cheng from the Chinese University of Hong Kong for GES-1 cells. We thank all the patients, families, and investigators from NUH, SGCC, NUHS Tissue Repository, Duke-NUS Genome Biology Facility, and staff of participating laboratories and research institutes. We thank Dr. Lit Hsin Loo from ImmunoQs for mIHC scoring. We especially thank MGI Tech Singapore’s STOmics team in Singapore for providing Stereo-seq reagents and guidance on experiments [RCA 2022-2295 (Cancer and Stem Cell Biology)].
Footnotes
Note: Supplementary data for this article are available at Cancer Discovery Online (http://cancerdiscovery.aacrjournals.org/).
Authors’ Disclosures
J.J. Zhao reports grants from the National University Health System Seed Fund (NUHSRO/2024/008/RO5+6/Seed-Sep23/01), National University Hospital Junior Research Award 2023 (JRA/Sep23/002), Chan Heng Leong Education & Research Fund 2024 award by the National University Hospital Singapore, 2025 Conquer Cancer Merit Award by Conquer Cancer, the ASCO Foundation, and Dean’s Research Development Award awarded by the Yong Loo Lin School of Medicine, National University of Singapore, outside the submitted work. R. Sundar reports grants from the National Medical Research Council during the conduct of the study and other support from Astellas, AstraZeneca, Bayer, BeiGene, Bristol Myers Squibb, Daiichi Sankyo, DKSH, Eisai, GSK, Merck, MSD, Novartis, Pierre-Fabre, Sanofi, Taiho, Tavotek, Eli Lilly, Ipsen, Roche, CytoMed, Paxman, and Teladoc outside the submitted work. No disclosures were reported by the other authors.
Authors’ Contributions
H. Ma: Conceptualization, resources, data curation, formal analysis, supervision, funding acquisition, investigation, visualization, methodology, writing–original draft, writing–review and editing. S. Srivastava: Conceptualization, resources, data curation, supervision, funding acquisition, validation, investigation, methodology, writing–original draft, project administration. S.W.T. Ho: Conceptualization, data curation, formal analysis, validation, investigation, visualization, methodology, writing–original draft. C. Xu: Data curation, validation, investigation. B.S.X. Lian: Data curation, validation. X. Ong: Data curation, validation. S.T. Tay: Data curation, validation. T. Sheng: Data curation, investigation. H.Y.J. Lum: Resources, data curation. S.A.B. Abdul Ghani: Data curation, validation, investigation. Y. Chu: Resources, data curation, validation. K.K. Huang: Data curation, validation. Y.T. Goh: Data curation, validation, project administration. M. Lee: Data curation, validation, investigation, Project administration. T. Hagihara: Validation, investigation, project administration. C.S.Y. Ng: Resources, validation, investigation, project administration. A.L.K. Tan: Resources, validation, investigation, project administration. Y. Zhang: Resources, validation, investigation. Z. Ding: Resources, investigation, project administration. F. Zhu: Resources, validation, investigation. M.S.W. Ng: Resources, validation. C.R.C. Joseph: Resources, validation, project administration. H. Chen: Resources, validation. Z. Li: Resources, validation, project administration. J.J. Zhao: Resources, validation. S.Y. Rha: Resources, validation. M. Teh: Resources, validation. J. Yeong: Resources, validation. W.P. Yong: Resources. J.B.-Y. So: Resources, validation. R. Sundar: Conceptualization, resources, supervision, funding acquisition, investigation, methodology, writing–original draft, project administration. P. Tan: Conceptualization, resources, supervision, funding acquisition, validation, methodology, writing–original draft, writing–review and editing.
References
- 1. Morgan E, Arnold M, Camargo MC, Gini A, Kunzmann AT, Matsuda T, et al. The current and future incidence and mortality of gastric cancer in 185 countries, 2020–40: a population-based modelling study. EClinicalMedicine 2022;47:101404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, et al. Cancer statistics for the year 2020: an overview. Int J Cancer 2021;149:778–89. [DOI] [PubMed] [Google Scholar]
- 3. Alsina M, Arrazubi V, Diez M, Tabernero J. Current developments in gastric cancer: from molecular profiling to treatment strategy. Nat Rev Gastroenterol Hepatol 2023;20:155–70. [DOI] [PubMed] [Google Scholar]
- 4. Kelly RJ, Bever K, Chao J, Ciombor KK, Eng C, Fakih M, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immunotherapy for the treatment of gastrointestinal cancer. J Immunother Cancer 2023;11:e006658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. The Cancer Genome Atlas Research Network . Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cristescu R, Lee J, Nebozhyn M, Kim K-M, Ting JC, Wong SS, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med 2015;21:449–56. [DOI] [PubMed] [Google Scholar]
- 7. Ho SWT, Sheng T, Xing M, Ooi WF, Xu C, Sundar R, et al. Regulatory enhancer profiling of mesenchymal-type gastric cancer reveals subtype-specific epigenomic landscapes and targetable vulnerabilities. Gut 2023;72:226–41. [DOI] [PubMed] [Google Scholar]
- 8. Nakamura Y, Kawazoe A, Lordick F, Janjigian YY, Shitara K. Biomarker-targeted therapies for advanced-stage gastric and gastro-oesophageal junction cancers: an emerging paradigm. Nat Rev Clin Oncol 2021;18:473–87. [DOI] [PubMed] [Google Scholar]
- 9. von Loga K, Woolston A, Punta M, Barber LJ, Griffiths B, Semiannikova M, et al. Extreme intratumour heterogeneity and driver evolution in mismatch repair deficient gastro-oesophageal cancer. Nat Commun 2020;11:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Röcken C, Amallraja A, Halske C, Opasic L, Traulsen A, Behrens H-M, et al. Multiscale heterogeneity in gastric adenocarcinoma evolution is an obstacle to precision medicine. Genome Med 2021;13:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang R, Dang M, Harada K, Han G, Wang F, Pool Pizzi M, et al. Single-cell dissection of intratumoral heterogeneity and lineage diversity in metastatic gastric adenocarcinoma. Nat Med 2021;27:141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pectasides E, Stachler MD, Derks S, Liu Y, Maron S, Islam M, et al. Genomic heterogeneity as a barrier to precision medicine in gastroesophageal adenocarcinoma. Cancer Discov 2018;8:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kock Am Brink M, Dunst LS, Behrens H-M, Krüger S, Becker T, Röcken C. Intratumoral heterogeneity affects tumor regression and Ki67 proliferation index in perioperatively treated gastric carcinoma. Br J Cancer 2023;128:375–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dagogo-Jack I, Shaw AT. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol 2018;15:81–94. [DOI] [PubMed] [Google Scholar]
- 15. Ramón Y Cajal S, Sesé M, Capdevila C, Aasen T, De Mattos-Arruda L, Diaz-Cano SJ, et al. Clinical implications of intratumor heterogeneity: challenges and opportunities. J Mol Med (Berl) 2020;98:161–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhu Z, Fu H, Wang S, Yu X, You Q, Shi M, et al. Whole-exome sequencing identifies prognostic mutational signatures in gastric cancer. Ann Transl Med 2020;8:1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang J, Qiu W, Liu H, Qian C, Liu D, Wang H, et al. Genomic alterations in gastric cancers discovered via whole-exome sequencing. BMC cancer 2018;18:1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee Y-S, Cho YS, Lee GK, Lee S, Kim Y-W, Jho S, et al. Genomic profile analysis of diffuse-type gastric cancers. Genome Biol 2014;15:R55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Coorens THH, Spencer Chapman M, Williams N, Martincorena I, Stratton MR, Nangalia J, et al. Reconstructing phylogenetic trees from genome-wide somatic mutations in clonal samples. Nat Protoc 2024;19:1866–86. [DOI] [PubMed] [Google Scholar]
- 20. Deshwar AG, Vembu S, Yung CK, Jang GH, Stein L, Morris Q. PhyloWGS: reconstructing subclonal composition and evolution from whole-genome sequencing of tumors. Genome Biol 2015;16:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vitale I, Sistigu A, Manic G, Rudqvist N-P, Trajanoski Z, Galluzzi L. Mutational and antigenic landscape in tumor progression and cancer immunotherapy. Trends Cell Biol 2019;29:396–416. [DOI] [PubMed] [Google Scholar]
- 22. Cassidy T, Nichol D, Robertson-Tessi M, Craig M, Anderson AR. The role of memory in non-genetic inheritance and its impact on cancer treatment resistance. PLoS Comput Biol 2021;17:e1009348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bell CC, Gilan O. Principles and mechanisms of non-genetic resistance in cancer. Br J Cancer 2020;122:465–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shlyakhtina Y, Moran KL, Portal MM. Genetic and non-genetic mechanisms underlying cancer evolution. Cancers (Basel) 2021;13:1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Quinn JJ, Jones MG, Okimoto RA, Nanjo S, Chan MM, Yosef N, et al. Single-cell lineages reveal the rates, routes, and drivers of metastasis in cancer xenografts. Science 2021;371:eabc1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang D, Jones MG, Naranjo S, Rideout WM III, Min KHJ, Ho R, et al. Lineage tracing reveals the phylodynamics, plasticity, and paths of tumor evolution. Cell 2022;185:1905–23.e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu F, Fan J, He Y, Xiong A, Yu J, Li Y, et al. Single-cell profiling of tumor heterogeneity and the microenvironment in advanced non-small cell lung cancer. Nat Commun 2021;12:2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kumar V, Ramnarayanan K, Sundar R, Padmanabhan N, Srivastava S, Koiwa M, et al. Single-cell atlas of lineage states, tumor microenvironment, and subtype-specific expression programs in gastric cancer. Cancer Discov 2022;12:670–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ushijima T, Clark SJ, Tan P. Mapping genomic and epigenomic evolution in cancer ecosystems. Science 2021;373:1474–9. [DOI] [PubMed] [Google Scholar]
- 30. Gao R, Bai S, Henderson YC, Lin Y, Schalck A, Yan Y, et al. Delineating copy number and clonal substructure in human tumors from single-cell transcriptomes. Nat Biotechnol 2021;39:599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Serin Harmanci A, Harmanci AO, Zhou X. CaSpER identifies and visualizes CNV events by integrative analysis of single-cell or bulk RNA-sequencing data. Nat Commun 2020;11:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fan J, Lee H-O, Lee S, Ryu D-e, Lee S, Xue C, et al. Linking transcriptional and genetic tumor heterogeneity through allele analysis of single-cell RNA-seq data. Genome Res 2018;28:1217–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Erickson A, He M, Berglund E, Marklund M, Mirzazadeh R, Schultz N, et al. Spatially resolved clonal copy number alterations in benign and malignant tissue. Nature 2022;608:360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ferri-Borgogno S, Zhu Y, Sheng J, Burks JK, Gomez JA, Wong KK, et al. Spatial transcriptomics depict ligand-receptor cross-talk heterogeneity at the tumor-stroma interface in long-term ovarian cancer survivors. Cancer Res 2023;83:1503–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shen K, Luk S, Hicks DF, Elman JS, Bohr S, Iwamoto Y, et al. Resolving cancer-stroma interfacial signalling and interventions with micropatterned tumour–stromal assays. Nat Commun 2014;5:5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wu Y, Yang S, Ma J, Chen Z, Song G, Rao D, et al. Spatiotemporal immune landscape of colorectal cancer liver metastasis at single-cell level. Cancer Discov 2022;12:134–53. [DOI] [PubMed] [Google Scholar]
- 37. Cui Zhou D, Jayasinghe RG, Chen S, Herndon JM, Iglesia MD, Navale P, et al. Spatially restricted drivers and transitional cell populations cooperate with the microenvironment in untreated and chemo-resistant pancreatic cancer. Nat Genet 2022;54:1390–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sun C, Wang A, Zhou Y, Chen P, Wang X, Huang J, et al. Spatially resolved multi-omics highlights cell-specific metabolic remodeling and interactions in gastric cancer. Nat Commun 2023;14:2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Park S, Karalis JD, Hong C, Clemenceau JR, Porembka MR, Kim I-H, et al. ACTA2 expression predicts survival and is associated with response to immune checkpoint inhibitors in gastric cancer. Clin Cancer Res 2023;29:1077–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fang J, Wang H, Liu Y, Ding F, Ni Y, Shao S. High KRT 8 expression promotes tumor progression and metastasis of gastric cancer. Cancer Sci 2017;108:178–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Warneke VS, Behrens H-M, Haag J, Krüger S, Simon E, Mathiak M, et al. Members of the EpCAM signalling pathway are expressed in gastric cancer tissue and are correlated with patient prognosis. Br J Cancer 2013;109:2217–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen X, Zhao J, Li A, Gao P, Sun J, Song Y, et al. Clinicopathological significance of claudin 4 expression in gastric carcinoma: a systematic review and meta-analysis. Onco Targets Ther 2016;9:3205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang W, Dong L-P, Zhang N, Zhao C-H. Role of cancer stem cell marker CD44 in gastric cancer: a meta-analysis. Int J Clin Exp Med 2014;7:5059–66. [PMC free article] [PubMed] [Google Scholar]
- 44. Oh SC, Sohn BH, Cheong J-H, Kim S-B, Lee JE, Park KC, et al. Clinical and genomic landscape of gastric cancer with a mesenchymal phenotype. Nat Commun 2018;9:1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lei Z-N, Teng Q-X, Tian Q, Chen W, Xie Y, Wu K, et al. Signaling pathways and therapeutic interventions in gastric cancer. Signal Transduct Target Ther 2022;7:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Taylor AM, Shih J, Ha G, Gao GF, Zhang X, Berger AC, et al. Genomic and functional approaches to understanding cancer aneuploidy. Cancer cell 2018;33:676–89.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Joanito I, Wirapati P, Zhao N, Nawaz Z, Yeo G, Lee F, et al. Single-cell and bulk transcriptome sequencing identifies two epithelial tumor cell states and refines the consensus molecular classification of colorectal cancer. Nat Genet 2022;54:963–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 2007;8:118–27. [DOI] [PubMed] [Google Scholar]
- 49. Gagnon-Bartsch JA, Speed TP. Using control genes to correct for unwanted variation in microarray data. Biostatistics 2012;13:539–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 2015;43:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rousseeuw PJ. Silhouettes: a graphical aid to the interpretation and validation of cluster analysis. J Comput Appl Math 1987;20:53–65. [Google Scholar]
- 52. Newman AM, Steen CB, Liu CL, Gentles AJ, Chaudhuri AA, Scherer F, et al. Determining cell type abundance and expression from bulk tissues with digital cytometry. Nat Biotechnol 2019;37:773–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Xia K, Sun H-X, Li J, Li J, Zhao Y, Chen L, et al. The single-cell stereo-seq reveals region-specific cell subtypes and transcriptome profiling in Arabidopsis leaves. Dev Cell 2022;57:1299–310.e4. [DOI] [PubMed] [Google Scholar]
- 54. Zhang Z, Chen L, Chen H, Zhao J, Li K, Sun J, et al. Pan-cancer landscape of T-cell exhaustion heterogeneity within the tumor microenvironment revealed a progressive roadmap of hierarchical dysfunction associated with prognosis and therapeutic efficacy. EBioMedicine 2022;83:104207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity 2016;44:989–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang R, Song S, Qin J, Yoshimura K, Peng F, Chu Y, et al. Evolution of immune and stromal cell states and ecotypes during gastric adenocarcinoma progression. Cancer Cell 2023;41:1407–26.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Parikh AR, Szabolcs A, Allen JN, Clark JW, Wo JY, Raabe M, et al. Radiation therapy enhances immunotherapy response in microsatellite stable colorectal and pancreatic adenocarcinoma in a phase II trial. Nat Cancer 2021;2:1124–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bareche Y, Kelly D, Abbas-Aghababazadeh F, Nakano M, Esfahani PN, Tkachuk D, et al. Leveraging big data of immune checkpoint blockade response identifies novel potential targets. Ann Oncol 2022;33:1304–17. [DOI] [PubMed] [Google Scholar]
- 59. Zhao JJ, Ong C-AJ, Srivastava S, Chia DKA, Ma H, Huang K, et al. Spatially resolved niche and tumor microenvironmental alterations in gastric cancer peritoneal metastases. Gastroenterology 2024;167:1384–98.e4. [DOI] [PubMed] [Google Scholar]
- 60. Okano F, Saito T, Minamida Y, Kobayashi S, Ido T, Miyauchi Y, et al. Identification of membrane-expressed CAPRIN-1 as a novel and universal cancer target, and generation of a therapeutic anti-CAPRIN-1 antibody TRK-950. Cancer Res Commun 2023;3:640–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chu HY, Chen Z, Wang L, Zhang Z-K, Tan X, Liu S, et al. Dickkopf-1: a promising target for cancer immunotherapy. Front Immunol 2021;12:658097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Li J, Hubisz MJ, Earlie EM, Duran MA, Hong C, Varela AA, et al. Non-cell-autonomous cancer progression from chromosomal instability. Nature 2023;620:1080–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Qiu X, Mao Q, Tang Y, Wang L, Chawla R, Pliner HA, et al. Reversed graph embedding resolves complex single-cell trajectories. Nat Methods 2017;14:979–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Street K, Risso D, Fletcher RB, Das D, Ngai J, Yosef N, et al. Slingshot: cell lineage and pseudotime inference for single-cell transcriptomics. BMC Genomics 2018;19:477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wolf FA, Hamey FK, Plass M, Solana J, Dahlin JS, Göttgens B, et al. PAGA: graph abstraction reconciles clustering with trajectory inference through a topology preserving map of single cells. Genome Biol 2019;20:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lee JE, Kim KT, Shin S-J, Cheong J-H, Choi YY. Genomic and evolutionary characteristics of metastatic gastric cancer by routes. Br J Cancer 2023;129:672–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Xu C, Huang KK, Law JH, Chua JS, Sheng T, Flores NM, et al. Comprehensive molecular phenotyping of ARID1A-deficient gastric cancer reveals pervasive epigenomic reprogramming and therapeutic opportunities. Gut 2023;72:1651–63. [DOI] [PubMed] [Google Scholar]
- 68. Mroz EA, Rocco JW. MATH, a novel measure of intratumor genetic heterogeneity, is high in poor-outcome classes of head and neck squamous cell carcinoma. Oral Oncol 2013;49:211–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chrisafis G, Wang T, Moissoglu K, Gasparski AN, Ng Y, Weigert R, et al. Collective cancer cell invasion requires RNA accumulation at the invasive front. Proc Natl Acad Sci U S A 2020;117:27423–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chen P, Chen G, Wang C, Mao C. RAB13 as a novel prognosis marker promotes proliferation and chemotherapeutic resistance in gastric cancer. Biochem Biophys Res Commun 2019;519:113–20. [DOI] [PubMed] [Google Scholar]
- 71. Liu Y, Xun Z, Ma K, Liang S, Li X, Zhou S, et al. Identification of a tumour immune barrier in the HCC microenvironment that determines the efficacy of immunotherapy. J Hepatol 2023;78:770–82. [DOI] [PubMed] [Google Scholar]
- 72. Du Y, Lin Y, Gan L, Wang S, Chen S, Li C, et al. Potential crosstalk between SPP1+ TAMs and CD8+ exhausted T cells promotes an immunosuppressive environment in gastric metastatic cancer. J Transl Med 2024;22:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Li J, Chen X, Zhu L, Lao Z, Zhou T, Zang L, et al. SOX9 is a critical regulator of TSPAN8-mediated metastasis in pancreatic cancer. Oncogene 2021;40:4884–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Panda M, Tripathi SK, Biswal BK. SOX9: an emerging driving factor from cancer progression to drug resistance. Biochim Biophys Acta Rev Cancer 2021;1875:188517. [DOI] [PubMed] [Google Scholar]
- 75. Ma Y, Shepherd J, Zhao D, Bollu LR, Tahaney WM, Hill J, et al. SOX9 is essential for triple-negative breast cancer cell survival and metastasis. Mol Cancer Res 2020;18:1825–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Fan Y, Li Y, Yao X, Jin J, Scott A, Liu B, et al. Epithelial SOX9 drives progression and metastases of gastric adenocarcinoma by promoting immunosuppressive tumour microenvironment. Gut 2023;72:624–37. [DOI] [PubMed] [Google Scholar]
- 77. Kamimoto K, Stringa B, Hoffmann CM, Jindal K, Solnica-Krezel L, Morris SA. Dissecting cell identity via network inference and in silico gene perturbation. Nature 2023;614:742–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Trapnell C, Cacchiarelli D, Grimsby J, Pokharel P, Li S, Morse M, et al. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat Biotechnol 2014;32:381–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhu D, Zhao D, Wang N, Cai F, Jiang M, Zheng Z. Current status and prospects of GREM1 research in cancer (Review). Mol Clin Oncol 2023;19:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kieffer Y, Hocine HR, Gentric G, Pelon F, Bernard C, Bourachot B, et al. Single-cell analysis reveals fibroblast clusters linked to immunotherapy resistance in cancer. Cancer Discov 2020;10:1330–51. [DOI] [PubMed] [Google Scholar]
- 81. Tian L, Chen F, Macosko EZ. The expanding vistas of spatial transcriptomics. Nat Biotechnol 2023;41:773–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Rodriques SG, Stickels RR, Goeva A, Martin CA, Murray E, Vanderburg CR, et al. Slide-seq: a scalable technology for measuring genome-wide expression at high spatial resolution. Science 2019;363:1463–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Chen A, Liao S, Cheng M, Ma K, Wu L, Lai Y, et al. Spatiotemporal transcriptomic atlas of mouse organogenesis using DNA nanoball-patterned arrays. Cell 2022;185:1777–92.e21. [DOI] [PubMed] [Google Scholar]
- 84. Hwang WL, Jagadeesh KA, Guo JA, Hoffman HI, Yadollahpour P, Reeves JW, et al. Single-nucleus and spatial transcriptome profiling of pancreatic cancer identifies multicellular dynamics associated with neoadjuvant treatment. Nat Genet 2022;54:1178–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Fowler JC, Jones PH. Somatic mutation: what shapes the mutational landscape of normal epithelia? Cancer Discov 2022;12:1642–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Swanton C, Bernard E, Abbosh C, André F, Auwerx J, Balmain A, et al. Embracing cancer complexity: hallmarks of systemic disease. Cell 2024;187:1589–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. McGranahan N, Swanton C. Clonal heterogeneity and tumor evolution: past, present, and the future. Cell 2017;168:613–28. [DOI] [PubMed] [Google Scholar]
- 88. Vitale I, Shema E, Loi S, Galluzzi L. Intratumoral heterogeneity in cancer progression and response to immunotherapy. Nat Med 2021;27:212–24. [DOI] [PubMed] [Google Scholar]
- 89. Dongre A, Weinberg RA. New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nat Rev Mol Celll Biol 2019;20:69–84. [DOI] [PubMed] [Google Scholar]
- 90. Bagaev A, Kotlov N, Nomie K, Svekolkin V, Gafurov A, Isaeva O, et al. Conserved pan-cancer microenvironment subtypes predict response to immunotherapy. Cancer Cell 2021;39:845–65.e7. [DOI] [PubMed] [Google Scholar]
- 91. Risom T, Glass DR, Averbukh I, Liu CC, Baranski A, Kagel A, et al. Transition to invasive breast cancer is associated with progressive changes in the structure and composition of tumor stroma. Cell 2022;185:299–310.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Guo L, Yi X, Chen L, Zhang T, Guo H, Chen Z, et al. Single-cell DNA sequencing reveals punctuated and gradual clonal evolution in hepatocellular carcinoma. Gastroenterology 2022;162:238–52. [DOI] [PubMed] [Google Scholar]
- 93. Davis A, Gao R, Navin N. Tumor evolution: linear, branching, neutral or punctuated? Biochim Biophys Acta Rev Cancer 2017;1867:151–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Venkatesan S, Swanton C. Tumor evolutionary principles: how intratumor heterogeneity influences cancer treatment and outcome. Am Soc Clin Oncol Educ Book 2016;36:e141–9. [DOI] [PubMed] [Google Scholar]
- 95. Yang X, Sun H-J, Li Z-R, Zhang H, Yang W-J, Ni B, et al. Gastric cancer-associated enhancement of von Willebrand factor is regulated by vascular endothelial growth factor and related to disease severity. BMC cancer 2015;15:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Li Y, Hu X, Lin R, Zhou G, Zhao L, Zhao D, et al. Single-cell landscape reveals active cell subtypes and their interaction in the tumor microenvironment of gastric cancer. Theranostics 2022;12:3818–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Song S, Ajani JA, Honjo S, Maru DM, Chen Q, Scott AW, et al. Hippo coactivator YAP1 upregulates SOX9 and endows esophageal cancer cells with stem-like properties. Cancer Res 2014;74:4170–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Venkataramani V, Yang Y, Schubert MC, Reyhan E, Tetzlaff SK, Wißmann N, et al. Glioblastoma hijacks neuronal mechanisms for brain invasion. Cell 2022;185:2899–917.e31. [DOI] [PubMed] [Google Scholar]
- 99. Huang Y, Qi L, Kogiso M, Du Y, Braun FK, Zhang H, et al. Spatial dissection of invasive front from tumor mass enables discovery of novel microRNA drivers of glioblastoma invasion. Adv Sci (Weinh) 2021;8:2101923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Arora R, Cao C, Kumar M, Sinha S, Chanda A, McNeil R, et al. Spatial transcriptomics reveals distinct and conserved tumor core and edge architectures that predict survival and targeted therapy response. Nat Commun 2023;14:5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Cords L, Tietscher S, Anzeneder T, Langwieder C, Rees M, de Souza N, et al. Cancer-associated fibroblast classification in single-cell and spatial proteomics data. Nat Commun 2023;14:4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Merritt CR, Ong GT, Church SE, Barker K, Danaher P, Geiss G, et al. Multiplex digital spatial profiling of proteins and RNA in fixed tissue. Nat Biotechnol 2020;38:586–99. [DOI] [PubMed] [Google Scholar]
- 103. Suwatthanarak T, Thanormjit K, Suwatthanarak T, Acharayothin O, Methasate A, Chinswangwatanakul V, et al. Spatial transcriptomic profiling of tetraspanins in stage 4 colon cancer from primary tumor and liver metastasis. Life (Basel) 2024;14:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Lu L, Wang JR, Henderson YC, Bai S, Yang J, Hu M, et al. Anaplastic transformation in thyroid cancer revealed by single-cell transcriptomics. J Clin Invest 2023;133:e169653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Yeong J, Thike AA, Lim JCT, Lee B, Li H, Wong S-C, et al. Higher densities of Foxp3+ regulatory T cells are associated with better prognosis in triple-negative breast cancer. Breast Cancer Res Treat 2017;163:21–35. [DOI] [PubMed] [Google Scholar]
- 106. Lim JCT, Yeong JPS, Lim CJ, Ong CCH, Wong SC, Chew VSP, et al. An automated staining protocol for seven-colour immunofluorescence of human tissue sections for diagnostic and prognostic use. Pathology 2018;50:333–41. [DOI] [PubMed] [Google Scholar]
- 107. Yeong J, Lim JCT, Lee B, Li H, Chia N, Ong CCH, et al. High densities of tumor-associated plasma cells predict improved prognosis in triple negative breast cancer. Front Immunol 2018;9:1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Yeong J, Lim JCT, Lee B, Li H, Ong CCH, Thike AA, et al. Prognostic value of CD8+ PD-1+ immune infiltrates and PDCD1 gene expression in triple negative breast cancer. J Immunother Cancer 2019;7:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Ng HHM, Lee RY, Goh S, Tay ISY, Lim X, Lee B, et al. Immunohistochemical scoring of CD38 in the tumor microenvironment predicts responsiveness to anti-PD-1/PD-L1 immunotherapy in hepatocellular carcinoma. J Immunother Cancer 2020;8:e000987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Laksameethanasan D, Tan R, Toh G, Loo L-H. cellXpress: a fast and user-friendly software platform for profiling cellular phenotypes. BMC Bioinformatics 2013;14(Suppl 16):S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Benaglia T, Chauveau D, Hunter DR, Young DS. mixtools: an R package for analyzing finite mixture models. J Stat Softw 2009;32:1–29. [Google Scholar]
- 112. Gong C, Li S, Wang L, Zhao F, Fang S, Yuan D, et al. SAW: an efficient and accurate data analysis workflow for Stereo-seq spatial transcriptomics. GigaByte 2024;2024:gigabyte111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(1) Supplementary Table 1 shows GeoMx DSP ROI and scRNA-seq characteristics of samples profiled; (2) Supplementary Table 2 shows clinical characteristics of GeoMx DSP ROI and scRNA-seq samples profiled; (3) Supplementary Table 3A shows consistency between before and after batch effect removal on top enriched pathways in various ROI categories, 3B shows consistency between before and after batch effect removal on G1 and G2 labels on Tumor ROIs; (4) Supplementary Table 4 shows Silhouette scores of using different number of clusters in Tumor ROI hierarchical clustering; (5) Supplementary Table 5 shows detailed information of GeoMx ROIs in the TMA cohort; (6) Supplementary Table 6 shows top DEGs for endothelial cell subtypes (Endo1-Endo3); (7) Supplementary Table 7 shows top DEGs for macrophage cell subtypes (TAM1-TAM6); (8) Supplementary Table 8 shows top DEGs for fibroblast cell subtypes (Fib1-Fib4).
(1) Supplementary Figure 1 shows morphologies of different ROI categories and their representative genes, pathways and similarities with paired scRNA-seq data. (2) Supplementary Figure 2 shows validation of findings from the discovery GeoMx DSP cohort in the whole-section GeoMx DSP cohort and also the TMA GeoMx DSP cohort. (3) Supplementary Figure 3 shows experiments validating that intratumor G1/G2 subregion expression is tumor-intrinsic using multiplexed IHC, Stereoseq spatial transcriptomics, and in vitro Stereoseq drug treatment profiling. (4) Supplementary Figure 4 shows expression of individual immune checkpoints, chemokines, cytokines, molecular signatures (immune exhaustion, intracellular signaling) and emerging therapeutic targets in G1 and G2 subregions in the discovery and validation cohort. (5) Supplementary Figure 5 shows experiments validating that G2 RNA-ITH subregions have higher CNV burdens than G1 subregions using signature scores, CNV calling from scRNA-seq, and validation using WES and Stereoseq data. (6) Supplementary Figure 6 shows phylogenetic analysis and trajectory analysis of scRNA-seq tumor subgroups using various methods. (7) Supplementary Figure 7 shows survival analysis and multi-variate analysis validating that internal diaspora GC is associated with worse prognosis in various cohorts. (8) Supplementary Figure 8 shows functional validation experiments for the potential G2 markers SOX9, AGR2 and TSPAN8 using CellOracle, RNAi and CRISPR knock out assays. (9) Supplementary Figure 9 shows typical pathways, markers and cell types for tumor-stroma interface ROIs.
Data Availability Statement
Raw sequencing data of GeoMx WTA DSP profiles have been deposited at the European Genome-phenome Archive (EGA) under accession number EGAS50000000345 (https://ega-archive.org/studies/EGAS50000000345). Raw sequencing data of TMA GeoMx DSP data generated by the SGCC has been deposited at the EGA under accession number EGAS50000000640 (https://ega-archive.org/studies/EGAS50000000640). Gastric cancer scRNA-seq data has been uploaded to GEO under accession number GSE183904 (DOI: 10.1158/2159-8290.CD-21-0683). Additional validation GeoMx WTA DSP, Stereo-seq gastric cancer/organoid data, TP53/SOX9-KO cell line scRNA-seq data, and in-house gastric cancer line bulk mRNA-seq data were generated by Cancer and Stem Cell Biology Duke-NUS medical school and are available from the corresponding author (P. Tan) on request. Bulk mRNA-seq expression data (normalized) generated by TCGA on primary stomach adenocarcinoma were downloaded from NCI Cancer Genomic Data Commons (https://gdc.cancer.gov). ACRG GSE62254 (DOI: 10.1038/nm.3850) data were downloaded from the GEO database (https://www.ncbi.nlm.nih.gov/geo/, RRID: SCR_005012; ref. 6). GASCAD data is available under EGA accession number EGAS00001006397 (DOI: 10.1136/gutjnl-2022-328332). The public scRNA-seq dataset GSE52529 (DOI: 10.1038/nbt.2859) used for CellOracle validation was downloaded from the GEO database (https://www.ncbi.nlm.nih.gov/geo/, RRID: SCR_005012; ref. 78).