Abstract
Introduction
Numerous studies have characterised trajectories of asthma and allergy in children using machine learning, but with different techniques and mixed findings. The present work aimed to summarise the evidence and critically appraise the methodology.
Methods
10 databases were searched. Screening, data extraction and quality assessment were performed in pairs. Trajectory characteristics were tabulated and visualised. Associated risk factor and outcome estimates were pooled using a random-effects meta-analysis.
Results
89 studies were included. Early-onset (infancy) persistent, mid-onset (∼2–5 years) persistent, early-onset early-resolving (within ∼2 years) and early-onset mid-resolving (by ∼3–6 years) wheezing and eczema, respectively, were the most commonly identified disease trajectories. Intermediate/transient trajectories were rare. Male sex was associated with a higher risk of most wheezing trajectories and possibly with early-resolving eczema, while being slightly protective against mid-onset persistent eczema. Parental disease/genetic markers were associated with persistent trajectories of wheezing and eczema, respectively. Prenatal (and less so postnatal) tobacco smoke exposure was associated with most wheezing trajectories, as were lower respiratory tract infections in infancy (particularly with the early-onset resolving patterns). Most studies (69%) were of low methodological quality (particularly in modelling approaches and reporting). Few studies investigated allergic multimorbidity, allergic rhinitis and food allergy.
Conclusions
Childhood asthma/wheezing and eczema can be characterised by a few relatively consistent trajectories, with some actionable risk factors such as pre-/postnatal smoke exposure. Improved computational methodology is warranted to better assess generalisability and elucidate the validity of intermediate/transient trajectories. Likewise, allergic multimorbidity and trajectories of allergic rhinitis and food allergy need to be further elucidated.
Shareable abstract
Trajectories of asthma and allergy are heterogeneous in children. However, relatively few longitudinal patterns are consistently identified in the literature, with actionable risk factors such as parental smoking. Improvement in methodology is warranted. https://bit.ly/3YnrN0s
Introduction
Asthma and allergic diseases are among the most common diseases in childhood [1–3]. These conditions are associated with substantial morbidity and impose a societal burden through increased healthcare utilisation, reduced academic performance, social life limitations and a reduced quality of life [4–7]. There is a wide range of clinical manifestations (phenotypes) of asthma and allergy, which can vary in terms of disease development (trajectories) [8–10]. Characterising trajectories is essential to identify risk factors, distinguish groups with similar outcomes/prognosis and optimise management. Machine learning (ML) is a promising and increasingly utilised tool for phenotyping longitudinal data, as it facilitates large-scale analysis in a data-driven and relatively hypothesis-free manner.
Many studies have used ML-based approaches to characterise the trajectories of asthma and allergy in children. Their findings, however, are mixed and fail to provide a clear picture, due to substantial variations in terms of follow-up time, assessment intervals, disease definitions and study populations. Furthermore, ML-based trajectory modelling depends on pre-processing, the nature of the input data and model settings. Given the diverse evidence on the topic, the aim of this work was three-fold, as follows: 1) summarise the identified trajectories of asthma and allergic disease in childhood, 2) critically assess computational methodologies, and 3) synthesise the subject characteristics and associated risk factors and outcomes of the identified trajectories. Ultimately, this work aims to provide a structured overview of the findings from recently published studies on the topic, thereby facilitating a detailed and systematic evaluation of their strengths, limitations, similarities, differences and patterns and highlighting opportunities for improvements in future studies.
Methods
This work was outlined in a protocol registered with the International Prospective Register of Systematic Reviews (PROSPERO; registration number CRD42023441691) and has been published [11]. Reporting of the results was done in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) [12] checklist (supplementary checklist 1) and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) [13] reporting guidelines (supplementary checklist 2). Deviations from the protocol are described in supplementary text 1.
Data sources and search strategy
The CAB Direct, CINAHL (Cumulative Index to Nursing and Allied Health Literature), Embase, Google Scholar, PsycInfo, PubMed, Scopus, Web of Science, World Health Organization Global Index Medicus and WorldCat Dissertations and Theses databases were searched. The search queries consisted of three blocks, as follows: 1) asthma and allergy, 2) trajectory analysis, and 3) age. An initial search query was piloted in PubMed, during which additional search terms were identified. The resulting search query was modified for use in the other databases. A fourth block was added for databases with >1000 (arbitrary threshold to reduce screening load) results and appropriate filters for age (excluding adult-only studies). The search queries are presented in tables S1A–S1J. Non-English articles were translated using Google Translate. References in included studies (and relevant reviews) were screened as an additional source. Relevant articles under embargo, conference abstracts and abstracts without available full text were included but not assessed further (data extraction, quality assessment, narrative synthesis or meta-analysis). To provide an overview of the most up-to-date and methodologically refined approaches and outputs, we restricted the search to the past decade.
The eligibility criteria were as follows:
Study design: observational studies with ≥2 assessments of asthma or allergy with ≥1 year between the first and last assessment.
Population: representative samples of children (aged ≤18 years at the last assessment; exception for studies with ≥2 childhood assessments).
Objective: identification/characterisation of trajectories of asthma, allergy (allergic rhinitis/rhinoconjunctivitis/conjunctivitis, atopic dermatitis or food allergy) or indirect measurement (e.g., allergic sensitisation), regardless of assessment method, data source or definition.
Publication date: past 10 years (2013–2023). Earlier original trajectory analyses were also eligible in cases of secondary analyses published within the past decade.
De-duplication, screening, and data extraction
De-duplication was manually performed in EndNote 21 (Clarivate Analytics, USA) using a semi-automated approach proposed by Bramer et al. [14]. The records were then transferred to Rayyan [15]. Screening was performed in two steps, as follows: 1) title/abstract/keyword screening and 2) full-text screening by two independent reviewers. After completion, the decisions were unblinded and differences discussed. A senior reviewer (B.I. Nwaru) arbitrated in edge cases. Data extraction was performed similarly, utilising a standardised Microsoft Excel (Microsoft Corp., USA) form (https://osf.io/tj4g8). Screening and data extraction were performed without the assistance of artificial intelligence (AI)-based tools. Missing/incomplete data were requested from the corresponding author and then co-authors if no response was received within 1 week.
Quality assessment
Given the paucity of specified tools, to assess methodological quality a custom tool was developed based on reviews and reporting guidelines (i.e., the Guidelines for Reporting on Latent Trajectory Studies (GRoLTS)) [16] and the structure/rating system of the Effective Public Health Practice Project (EPHPP) [17]. Seven domains were evaluated, as follows: 1) selection bias, 2) data collection methods, 3) withdrawals and dropouts, 4) pre-processing, 5) trajectory modelling, 6) associated risk factors and outcomes, and 7) evaluation and reporting of results. Quality assessment was performed as the previous steps (i.e., by two independent reviewers following by unblinding of ratings, discussion and arbitration where needed) without AI assistance. The robustness of the computational methodology was visually presented in further detail with a weighted score based on relevant items. Details on the quality assessment can be found at https://osf.io/ayf35 in the folder Documents/Screening, data extraction, and quality assessment/Quality assessment.
Data synthesis and statistical analysis
Extracted data were tabulated, plotted and narratively synthesised. Trajectories similar in terms of longitudinal disease pattern(s) (determined through consensus between B.I. Nwaru, D. Lisik, E. Goksör and G. Wennergren) were grouped and named. The definitions of “persistent”, “transient”, “early”, “mid” and “late” in the provided names were based on the original trajectory names, but occasionally modified to better fit with other similar trajectories. Disease (measure) probability/prevalence over time was extracted using WebPlotDigitizer (https://automeris.io/). Comparable data on associated risk factors and outcomes were synthesised using a random-effects meta-analysis with robust variance estimation. This method can account for nonindependent effect sizes and varying weighting schemes [18]. In meta-analyses with only independent effect sizes or Satterthwaite degrees of freedom <4, random-effects meta-analysis with a restricted maximum-likelihood estimator was utilised. As some studies investigated large numbers of risk factors/outcomes, we implemented false discovery rate correction on the p-values. Depending on the input data, the effect measure was odds ratio (OR) or risk ratio. Heterogeneity was assessed through I2 and τ2 statistics and further evaluated with subgroup analysis by sex and asthma/allergy heredity. Static trajectory characteristics (e.g., proportion of boys) were synthesised through a generalised linear mixed model with logit-transformed percentages, which is suitable for small samples and low/high proportions [19]. The corresponding 95% confidence interval was calculated through the Wilson score interval method without continuity correction. Sensitivity analysis was performed by excluding studies with a “weak” overall rating. Meta-analyses were presented as forest plots. All analyses were performed using the R statistical software (version 4.2.3; R Core Team).
Results
Screening and study characteristics
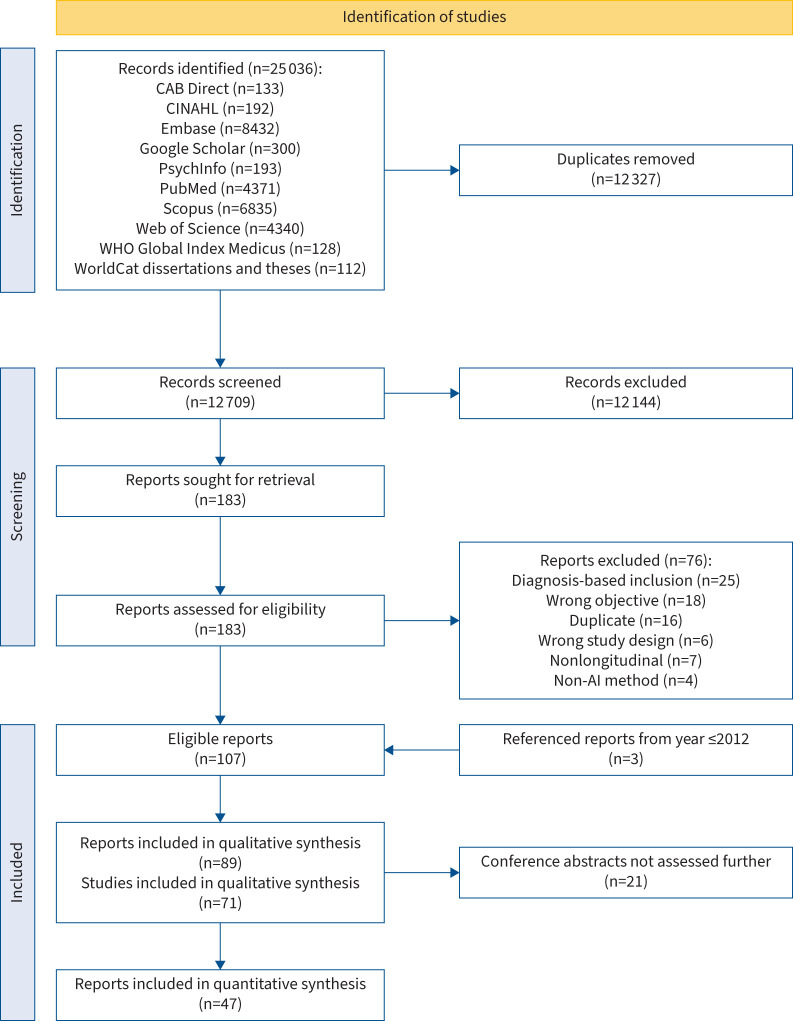
In total, 12 327 de-duplicated records were retrieved from the database searches. After the initial screening based on title/abstract/keywords, 183 records remained, which were assessed by full text (see table S2 for reasons for exclusion). Finally, 107 reports remained. Of these, three reports were secondary analyses of primary analyses (studies in which trajectory analyses were performed) published prior to 2013, which were also included, totalling 110 reports, of which 21 were conference abstracts (table S3) and 89 were full-text articles [20–108] based on 71 primary analyses (figure 1). A total of 47 reports were included in the 220 meta-analyses (details at https://osf.io/ayf35). Most studies were performed in high-income “Western” countries (figure S1) and were generally of low methodological quality (figures S2 and S3 and tables S4A and S4B), with little improvement over time (figure 2b). Trajectory modelling and reporting were the sections with the highest proportion of “weak” ratings. The number of assessments and length of follow-up varied widely but did not correlate clearly with the number of identified trajectories (figure S4).
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart. The “Referenced reports from year ≤2012” represents the papers published in 2012 or earlier in which trajectories were derived and which were referenced in secondary analyses (published within the defined timeframe (2013–2023)) included in this work. AI: artificial intelligence; CINAHL: Cumulative Index to Nursing and Allied Health Literature; WHO: World Health Organization.
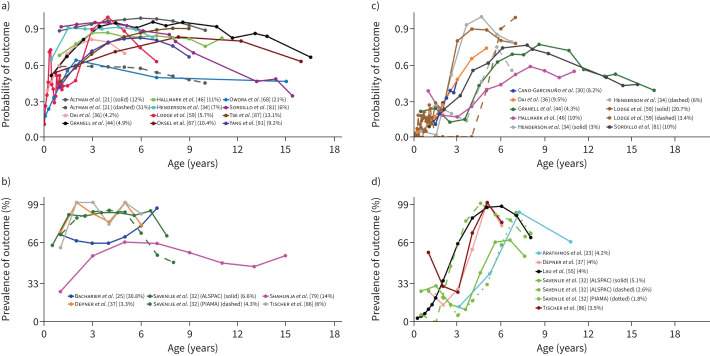
FIGURE 2.
Early-onset and mid-onset persistent wheezing. Each line represents a trajectory from a study. When multiple trajectories are included from the same study, different line types (e.g. solid or dashed) distinguish between them. Line colour indicates the specific study source for each trajectory. Alongside each study, a percentage shows the proportion of the study's total sample represented by that trajectory. The points along each line denote the time-points at which an assessment of the trajectory-defining variable(s) was done. The y-axis denotes the prevalence/probability, while the x-axis indicates the subject age. a) Early-onset persistent wheezing in studies [21, 34, 36, 44, 46, 59, 67, 68, 81, 87, 91] which presented trajectories by probability. b) Early-onset persistent wheezing in studies [25, 32, 37, 79, 86] which presented trajectories by prevalence. c) Mid-onset persistent wheezing in studies [30, 34, 36, 44, 46, 59, 81] which presented trajectories by probability. d) Mid-onset persistent wheezing in studies [23, 32, 37, 55, 86] which presented trajectories by prevalence.
Allergic multimorbidity
18 studies [21, 22, 25, 27–29, 38, 41, 42, 49, 50, 57, 69, 72, 73, 77, 80, 85] derived trajectories of two or more diseases or measures thereof (https://osf.io/xy58k). Most studies investigated unique sets of parameters over time and were not comparable. Two studies, however, assessed rhinitis, eczema, wheezing and nocturnal cough without concomitant cold/influenza (table S5). A trajectory was found in these studies of relatively persistent rhinitis and nocturnal cough (with somewhat prevalent wheezing and eczema). Meta-analyses did not reveal any significantly associated risk factor (table S5).
(Allergic) rhinitis and rhinoconjunctivitis
Only one study [60] derived trajectories of rhinitis (https://osf.io/xy58k).
Allergic sensitisation
12 studies [21, 25, 35, 40, 42, 43, 47, 53, 54, 56, 76, 78] derived trajectories of allergic sensitisation (https://osf.io/xy58k). While the investigated allergens varied substantially, eight similar longitudinal patterns appeared in at least two studies (table S6). Six of these trajectories were characterised by aeroallergen sensitisation, commonly increasing with time. Of these, two were primarily targeted at mites, namely 1) early-onset persistent mite-dominated sensitisation and 2) increasing mite-dominated sensitisation, while one trajectory exhibited sensitisation to most investigated aeroallergens and the three remaining trajectories were defined by mite or grass/tree pollen sensitisation with varying sensitisation to other aeroallergens. Finally, one trajectory was dominated by sensitisation to grass pollen with a notable component of tree and peanut sensitisation and one trajectory was characterised by persistent sensitisation to egg and milk. In terms of related outcomes, meta-analyses suggested an association with (allergic) rhinitis at the end of follow-up (pooled OR 2.99 (95% CI 1.77–5.05) for increasing mite-dominated sensitisation and pooled OR 7.27 (95% CI 4.14–12.77) for grass-dominated sensitisation with a notable component of late-onset tree and peanut sensitisation). The aforementioned trajectory was also associated with asthma at the end of follow-up (pooled OR 3.63 (95% CI 1.93–6.81)).
Asthma, wheezing and cough without concomitant cold/influenza
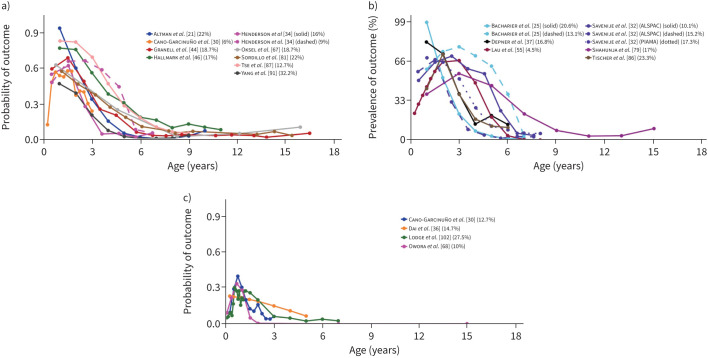
34 studies [21, 23, 25, 26, 30, 32, 34, 36, 37, 39, 44–46, 51, 55, 58, 59, 62, 63, 66–68, 70, 75, 79, 81, 82, 84, 86–89, 91, 92] derived trajectories of asthma, wheezing and cough without concomitant cold/influenza (https://osf.io/xy58k). Most studies investigated wheezing (typically any wheezing in the past year), while nine studies investigated asthma through various definitions (e.g., specific number of asthma-related clinic/hospital visits in the past year), among which one trajectory was reoccurring, namely early-onset mid-resolving asthma-related healthcare utilisation (table S7). Among the wheezing-based studies, six reoccurring trajectories were identified. Two of these were defined by persistent symptoms, namely 1) early-onset persistent wheezing and 2) mid-onset persistent wheezing (figure 2). Onset was seen at baseline/within first ∼1 year and by 3–5 years, respectively. The most prominent risk factors for early-onset persistent wheezing were lower respiratory tract infections (LRTIs) in infancy (pooled OR 8.84 (95% CI 3.65–21.37)), parental asthma (pooled risk ratio 2.98 (95% CI 2.14–4.15)) and male sex (pooled risk ratio 1.72 (95% CI 1.55–1.92)). Similar findings were seen for mid-onset persistent wheezing, as follows: LRTI in infancy (pooled risk ratio 3.2 (95% CI 1.68–6.12)), parental asthma (pooled risk ratio 2.05 (95% CI 1.38–3.05)) and male sex (pooled risk ratio 1.53 (95% CI 1.33–1.75)). An allergic component appeared to be prevalent in these trajectories, as allergic sensitisation at age 6–12 years was associated with early-onset persistent wheezing (pooled OR 3.56 (95% CI 2.87–4.42)) and at age 6–8 years to mid-onset persistent wheezing (pooled OR 6.8 (95% CI 5.37–8.6)). Two trajectories were characterised by resolving symptoms, namely 1) early-onset early-resolving wheezing and 2) early-onset mid-resolving wheezing, the former with remission by ∼3 years and the latter with symptoms into early school years (figure 3). LRTI in infancy was the most prominently associated risk factor (pooled risk ratio 2.25 (95% CI 1.68–3.02) for early-onset early-resolving wheezing and pooled risk ratio 8.34 (95% CI 5.89–11.82) for early-onset mid-resolving wheezing). Daycare attendance also constituted a risk factor (pooled risk ratio 1.45 (95% CI 1.16–1.82) for early-onset early-resolving wheezing and pooled risk ratio 1.38 (95% CI 1.18–1.61) for early-onset mid-resolving wheezing). For early-onset early-resolving wheezing, however, the association was not significant in subjects with heredity for asthma/allergy (pooled risk ratio 1.29 (95% CI 0.9–1.86)). Finally, two wheezing trajectories were defined by a transient, relatively short episode of wheezing, namely 1) mid-transient wheezing and 2) late-transient wheezing. These trajectories were identified in fewer studies than the two persistent/resolving trajectories and generally constituted lower proportions of the sample.
FIGURE 3.
Early-onset mid-resolving and early-onset early-resolving wheezing. Each line represents a trajectory from a study. When multiple trajectories are included from the same study, different line types (e.g. solid, dotted or dashed) distinguish between them. Line colour indicates the specific study source for each trajectory. Alongside each study, a percentage shows the proportion of the study's total sample represented by that trajectory. The points along each line denote the time-points at which an assessment of the trajectory-defining variable(s) was done. The y-axis denotes the prevalence/probability, while the x-axis indicates the subject age. a) Early-onset mid-resolving wheezing in studies [21, 30, 34, 44, 46, 67, 81, 87, 91] which presented trajectories by probability. b) Early-onset mid-resolving wheezing in studies [25, 32, 37, 55, 79, 86] which presented trajectories by prevalence. c) Early-onset early-resolving wheezing in studies [30, 36, 59, 68] which presented trajectories by probability.
Atopic dermatitis and eczema
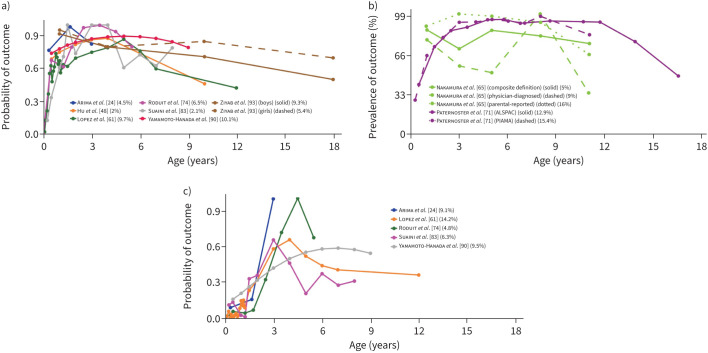
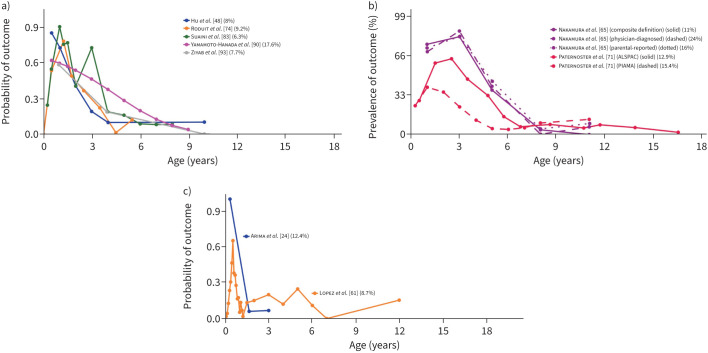
11 studies [20, 24, 48, 61, 64, 65, 71, 74, 83, 90, 93] derived trajectories of atopic dermatitis/eczema (https://osf.io/xy58k). Most commonly, disease was defined by parental/self-report of itchy rash (in age-typical locations) in the past year. Seven longitudinal patterns were identified in at least two studies (table S8). Two of these were persistent, namely 1) early-onset persistent eczema and 2) mid-onset (persistent) eczema, with onset in infancy in the former and by ∼3 years in the latter (figure 4). The most prominent risk factor for early-onset persistent eczema was parental allergy (pooled OR 2.6 (95% CI 1.22–5.55)). Male sex was associated with lower risk for mid-onset (persistent) eczema (pooled OR 0.62 (95% CI 0.45–0.87)), but the association was nonsignificant for early-onset persistent eczema (pooled OR 0.86 (95% CI 0.63–1.17)). Both trajectories were associated with an increased risk of atopic disease and related outcomes, e.g., allergic sensitisation, pooled OR 3.87 (95% CI 1.83–8.17) for early-onset persistent eczema (at 6–10 years) and pooled OR 1.87 (95% CI 1.25–2.82) for mid-onset (persistent) eczema (at 6–8 years). The five remaining patterns indicated remission, three of which had early onset, as follows: 1) early-onset early-resolving eczema, 2) early-onset mid-resolving eczema and 3) early transient eczema. In early-onset early-resolving eczema, remission was seen within ∼18 months, whereas early-onset mid-resolving eczema was commonly present until early school years (figure 5). Male sex was borderline associated with early-onset mid-resolving eczema (pooled OR 1.27 (95% CI 1.0–1.62); in sensitivity analysis this associated was weakened). Early transient eczema was characterised by a longitudinal pattern similar to early-onset mid-resolving eczema, albeit with somewhat later onset. Finally, two studies identified mid-onset late-resolving eczema and late-onset late-resolving eczema, respectively.
FIGURE 4.
Early-onset and mid-onset persistent eczema. Each line represents a trajectory from a study. When multiple trajectories are included from the same study, different line types (e.g. solid or dashed) distinguish between them. Line colour indicates the specific study source for each trajectory. Alongside each study, a percentage shows the proportion of the study's total sample represented by that trajectory. The points along each line denote the time-points at which an assessment of the trajectory-defining variable(s) was done. The y-axis denotes the prevalence/probability, while the x-axis indicates the subject age. a) Early-onset persistent eczema in studies [24, 48, 61, 74, 83, 90, 93] which presented trajectories by probability. b) Early-onset persistent eczema in studies [65, 71] which presented trajectories by prevalence. c) Mid-onset persistent eczema in studies [24, 61, 74, 83, 90] which presented trajectories by probability.
FIGURE 5.
Early-onset mid-resolving and early-onset early-resolving eczema. Each line represents a trajectory from a study. When multiple trajectories are included from the same study, different line types (e.g. solid or dashed) distinguish between them. Line colour indicates the specific study source for each trajectory. Alongside each study, a percentage shows the proportion of the study's total sample represented by that trajectory. The points along each line denote the time-points at which an assessment of the trajectory-defining variable(s) was done. The y-axis denotes the prevalence/probability, while the x-axis indicates the subject age. a) Early-onset mid-resolving eczema in studies [48, 74, 83, 90, 93] which presented trajectories by probability. b) Early-onset mid-resolving eczema in studies [65, 71] which presented trajectories by prevalence. c) Early-onset early-resolving eczema in studies [24, 61] which presented trajectories by probability.
Computational methodology
No clear trends were seen in methodology across the past 10 years. The most common (utilised in ∼50% of studies) modelling approach was latent class analysis. Other naïve techniques (not explicitly modelling by time/age), e.g., longitudinal k-means and partitional clustering algorithms, were also common. Few studies assessed modelling stability/overfitting or reported sufficient information for assessments of internal homogeneity and model performance in comparison to other possible solutions. Practically none of the studies provided analysis code (https://osf.io/ny8qu).
Discussion
In this work, characteristics and associated risk factors/outcomes of asthma and allergy trajectories in children were synthesised. Although most studies defined asthma and allergic disease similarly (parental/self-reported disease/symptoms in the past year), substantial heterogeneity was seen in methodology, e.g., modelling approaches, missing data management, subject age, number of assessments and assessment intervals. Nevertheless, most studies of wheezing and eczema identified several similar trajectories. These trajectories were associated with multiple risk factors, e.g., sex, parental asthma/allergy and early-life exposure, as well as several outcomes, including allergic sensitisation and allergy/asthma at the end of follow-up. Subgroup and sensitivity analyses mostly revealed comparable albeit occasionally slightly weaker associations.
The comprehensive search strategy and intricate methodology assessment and trajectory characterisation provide a rich overview of the literature. Furthermore, the 220 meta-analyses contribute clinical context and an indication of the pathophysiological mechanisms involved. However, this work has some limitations. Relevant articles may have been published following the database searches. Nevertheless, we believe, given the publication rate in recent years (figure 2b), that the findings from more recent papers would likely not differ substantially from our data, while older findings are of reasonably similar or weaker methodological quality. Meta-analyses typically included few studies, which limits precision and interpretability, particularly given the between-study heterogeneity, notable classification uncertainty [67] and insufficient follow-up time. This also hampered sensitivity/subgroup analysis and rendered publication bias assessment impractical. Longitudinal studies are costly and most included studies were performed in high-income countries, restricting generalisability. While we developed the quality assessment tool based on multiple guidelines and recommendations of experts in the field (https://osf.io/kc3wt), it is possible that factors of importance have been overlooked and the rating system was ultimately subjectively constructed. Finally, the definition and naming of the trajectories are subjective and some trajectories could theoretically be categorised to other groups.
The wheezing patterns that were commonly found in the included studies correspond with clinically recognisable phenotypes. The association with male sex is in accordance with studies reporting that asthma is more prevalent in boys until puberty, after which a switch between boys and girls is seen. Likewise, physiological differences in early childhood, e.g., smaller airway diameter in relation to lung volume in boys compared to girls, as well as (epi)genetic differences [109] may explain the higher prevalence of asthmatic symptoms in boys during this age [110], reflecting the findings in our meta-analyses. As most of the included studies did not follow subjects beyond puberty onset, male sex was associated with a higher risk of most trajectories. LRTI in infancy was strongly associated with both persistent and resolving wheezing trajectories, reflecting that respiratory viral infections are related to not only transient viral wheezing, but also wheezing associated with eczema/allergic sensitisation, so-called “true asthma”. However, LRTI and other factors related to respiratory (cross)infections, e.g., older siblings and daycare attendance, were more strongly associated with resolving wheezing trajectories, likely reflecting the infection-related wheezing seen in early childhood. Although daycare attendance was significantly associated with an increased risk of wheezing trajectories, as found in a previous related meta-analysis [111], our meta-analyses did not replicate significant protective associations for breastfeeding. The mid-transient wheezing trajectory was identified in four studies but was rare in the study populations. It is possible, given the likeness to the early-onset early-resolving wheezing trajectory, that the former group constitutes a subgroup of the latter, albeit with more persistent and pronounced symptoms.
For eczema, the commonly identified trajectories also reflect clinically recognisable phenotypes. Male sex being associated with borderline higher risk of early-onset mid-resolving eczema and slightly lower risk of mid-onset (persistent) eczema is consistent with previous reviews and likely reflects sex-related mechanisms similar to those in asthma, but switching earlier on [112]. Early transient eczema was identified and differentiated from early-onset early-resolving eczema and early-onset mid-resolving eczema in three studies. As these trajectories are relatively similar, with the onset in early-transient wheezing only months later than the two aforementioned trajectories, it is possible that this subgroup constitutes those with eczema triggered by certain factors with a later exposure window. The other transient trajectories (mid-onset late-resolving eczema and late-onset late-resolving eczema) were rare and whether these constitute generalisable/clinically meaningful entities is unclear.
The allergic sensitisation trajectories are difficult to interpret/generalise, given the varying assessment methods and allergens assessed, as well as the central role of allergen prevalence and exposure to sensitisation, by means of e.g., follow-up length and geographical region [113].
Follow-up time, number of assessments and intervals in-between have been reported to substantially influence the discoverability of smaller classes [114]. Our findings, however, mostly indicate a pattern between studies investigating single diseases versus allergic multimorbidity, with larger number of trajectories found in the latter. This is in line with simulation studies suggesting that higher number of indicators enables discovery of larger number of classes [115]. The transient trajectories were relatively uncommon and varied widely in longitudinal patterns, but may represent clinically relevant subgroups. To discern the validity of such subgroups, more robust modelling approaches are needed. External validation, relatively rare in studies published thus far, is pivotal to ascertain overall generalisability of disease trajectories. Translating these findings to clinical practice will require even more, not least characterisation of subjects at different stages and use of prediction models to further our understanding of parameters prospectively indicative of development of specific disease trajectories. On a related note, reporting of computational choices and outputs in general and of modelling metrics and classification uncertainty in particular needs to be more detailed for evaluation and comparison of results between studies. At present, well-established frameworks and guidelines for unsupervised trajectory analyses are lacking, although recent checklists such as GRoLTS contribute a well-needed foundation [16]. Finally, while it is clear that asthma and allergic diseases are intricately related (supported by the strong association between identified trajectories and allergic comorbidity), more longitudinal phenotyping studies of allergic rhinitis and food allergy and studies encompassing multiple diseases/measures are warranted to comprehensively elucidate the frequency and characteristics of longitudinal patterns of asthma and allergy.
In conclusion, our findings indicate the presence of relatively few reproducible trajectories of wheezing and eczema in children. These trajectories were clinically recognisable with multiple associated (actionable) risk factors. Suboptimal modelling and reporting limited critical assessment and comparability. More studies are needed to characterise food allergy and allergic rhinitis.
Questions for future research
Assessment of the robustness and clinical validity of intermediate and late childhood-onset trajectories of asthma and allergic disease.
Investigation of longitudinal patterns of allergic rhinitis and food allergy in children.
Exploration of trajectories of asthma or allergy based on severity, e.g. healthcare utilisation and perceived symptoms.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0160-2024.supplement (1.4MB, pdf)
Footnotes
Provenance: Submitted article, peer reviewed.
Author contributions: D. Lisik supported the study conceptualisation, drafted and revised the search queries and data extraction/quality assessment forms, performed the searches, performed screening, performed data extraction and quality assessment, performed the meta-analyses, interpreted the findings, and drafted and revised the manuscript. S.S. Özuygur Ermis performed screening, performed data extraction and quality assessment, and revised the manuscript. G.P. Milani supported the study conceptualisation, revised the search queries and data extraction/quality assessment forms, performed data extraction and quality assessment, and revised the manuscript. E. Goksör interpreted the findings and revised the manuscript. R. Basna supported the study conceptualisation, revised the search queries and data extraction/quality assessment forms, and revised the manuscript. G. Wennergren interpreted the findings and revised the manuscript. H. Kankaanranta interpreted the findings and revised the manuscript. B.I. Nwaru conceived the study idea, supported the study conceptualisation, revised the search queries and data extraction/quality assessment forms, interpreted the findings, and revised the manuscript. The remaining authors (G.C.I. Spolidoro, S. Ercan, M. Salisu, F. Odetola, D.G. Ghiglioni and D. Pylov) performed data extraction and quality assessment, and revised the manuscript.
Conflict of interest: H. Kankaanranta reports personal fees for lectures and consulting from AstraZeneca, Boehringer-Ingelheim, Chiesi Pharma, GSK, MSD, Novartis, Orion Pharma, and Sanofi Genzyme outside the current work. The remaining authors report that they have no conflict of interest.
Support statement: Supported by ALF (Swedish: Avtal om Läkarutbildning och Forskning), Herman Krefting Foundation for Allergy and Asthma Research, Swedish Asthma and Allergy Foundation, Swedish Heart-Lung Foundation, and Swedish Research Council. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the manuscript. Funding information for this article has been deposited with the Crossref Funder Registry.
Data availability
The R scripts and data are available at https://osf.io/ayf35.
References
- 1.Loh W, Tang MLK. The epidemiology of food allergy in the global context. Int J Environ Res Public Health 2018; 15: 2043. doi: 10.3390/ijerph15092043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.García-Marcos L, Asher MI, Pearce N, et al. The burden of asthma, hay fever and eczema in children in 25 countries: GAN phase I study. Eur Respir J 2022; 60: 2102866. doi: 10.1183/13993003.02866-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dharmage SC, Perret JL, Custovic A. Epidemiology of asthma in children and adults. Front Pediatr 2019; 7: 246. doi: 10.3389/fped.2019.00246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warren CM, Jiang J, Gupta RS. Epidemiology and burden of food allergy. Curr Allergy Asthma Rep 2020; 20: 6. doi: 10.1007/s11882-020-0898-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scadding GK, Smith PK, Blaiss M, et al. Allergic rhinitis in childhood and the new EUFOREA algorithm. Front Allergy 2021; 2: 706589. doi: 10.3389/falgy.2021.706589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrante G, La Grutta S. The burden of pediatric asthma. Front Pediatr 2018; 6: 186. doi: 10.3389/fped.2018.00186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Na CH, Chung J, Simpson EL. Quality of life and disease impact of atopic dermatitis and psoriasis on children and their families. Children (Basel) 2019; 6: 133. doi: 10.3390/children6120133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gabryszewski SJ, Hill DA. One march, many paths: insights into allergic march trajectories. Ann Allergy Asthma Immunol 2021; 127: 293–300. doi: 10.1016/j.anai.2021.04.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dharmage SC, Lowe AJ, Tang MLK. Revisiting the atopic march current evidence. Am J Respir Crit Care Med 2022; 206: 925–926. doi: 10.1164/rccm.202206-1219ED [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maiello N, Comberiati P, Giannetti A, et al. New directions in understanding atopic march starting from atopic dermatitis. Children (Basel) 2022; 9: 450. doi: 10.3390/children9040450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lisik D, Milani GP, Salisu M, et al. Machine learning-derived phenotypic trajectories of asthma and allergy in children and adolescents: protocol for a systematic review. BMJ Open 2024; 14: e080263. doi: 10.1136/bmjopen-2023-080263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 2000; 283: 2008–2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 14.Bramer WM, Giustini D, de Jonge GB, et al. De-duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc 2016; 104: 240–243. doi: 10.3163/1536-5050.104.3.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan–a web and mobile app for systematic reviews. Syst Rev 2016; 5: 210. doi: 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van de Schoot R, Sijbrandij M, Winter SD. The GRoLTS-checklist: guidelines for reporting on latent trajectory studies. Struct Equ Model 2017; 24: 451–467. doi: 10.1080/10705511.2016.1247646 [DOI] [Google Scholar]
- 17.Armijo-Olivo S, Stiles CR, Hagen NA, et al. Assessment of study quality for systematic reviews: a comparison of the Cochrane collaboration risk of bias tool and the effective public health practice project quality assessment tool: methodological research. J Eval Clin Pract 2012; 18: 12–18. doi: 10.1111/j.1365-2753.2010.01516.x [DOI] [PubMed] [Google Scholar]
- 18.Hedges LV, Tipton E, Johnson MC. Robust variance estimation in meta-regression with dependent effect size estimates. Res Synth Methods 2010; 1: 39–65. doi: 10.1002/jrsm.5 [DOI] [PubMed] [Google Scholar]
- 19.Lin L, Xu C, Chu H. Empirical comparisons of 12 meta-analysis methods for synthesizing proportions of binary outcomes. J Gen Intern Med 2022; 37: 308–317. doi: 10.1007/s11606-021-07098-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abuabara K, Ye M, Margolis DJ, et al. Patterns of atopic eczema disease activity from birth through midlife in 2 British birth cohorts. JAMA Dermatol 2021; 157: 1191–1199. doi: 10.1001/jamadermatol.2021.2489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altman MC, Calatroni A, Ramratnam S, et al. Endotype of allergic asthma with airway obstruction in urban children. J Allergy Clin Immunol 2021; 148: 1198–1209. doi: 10.1016/j.jaci.2021.02.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Apel K, Costet N, Chapron A, et al. Home environment: respiratory and allergic phenotypes from birth to age six in the PELAGIE cohort. NPJ Prim Care Respir Med 2019; 29: 29. doi: 10.1038/s41533-019-0141-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arathimos R, Granell R, Henderson J, et al. Sex discordance in asthma and wheeze prevalence in two longitudinal cohorts. PLoS One 2017; 12: e0176293. doi: 10.1371/journal.pone.0176293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arima T, Shiko Y, Kawasaki Y, et al. Phenotypes of atopic dermatitis up to 36 months of age by latent class analysis and associated factors in Japan. Asia Pac Allergy 2022; 12: e2. doi: 10.5415/apallergy.2022.12.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bacharier LB, Beigelman A, Calatroni A, et al. Longitudinal phenotypes of respiratory health in a high-risk urban birth cohort. Am J Respir Crit Care Med 2019; 199: 71–82. doi: 10.1164/rccm.201801-0190OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belgrave DCM, Simpson A, Semic-Jusufagic A, et al. Joint modeling of parentally reported and physician-confirmed wheeze identifies children with persistent troublesome wheezing. J Allergy Clin Immunol 2013; 132: 575–583. doi: 10.1016/j.jaci.2013.05.041 [DOI] [PubMed] [Google Scholar]
- 27.Belgrave DC, Granell R, Simpson A, et al. Developmental profiles of eczema, wheeze, and rhinitis: two population-based birth cohort studies. PLoS Med 2014; 11: e1001748. doi: 10.1371/journal.pmed.1001748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bougas N, Just J, Beydon N, et al. Unsupervised trajectories of respiratory/allergic symptoms throughout childhood in the PARIS cohort. Pediatr Allergy Immunol 2019; 30: 315–324. doi: 10.1111/pai.13027 [DOI] [PubMed] [Google Scholar]
- 29.Bui DS, Lodge CJ, Perret JL, et al. Trajectories of asthma and allergies from 7 years to 53 years and associations with lung function and extrapulmonary comorbidity profiles: a prospective cohort study. Lancet Respir Med 2021; 9: 387–396. doi: 10.1016/s2213-2600(20)30413-6 [DOI] [PubMed] [Google Scholar]
- 30.Cano-Garcinuño A, Mora-Gandarillas I. Wheezing phenotypes in young children: an historical cohort study. Prim Care Respir J 2014; 23: 60–66. doi: 10.4104/pcrj.2014.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caudri D, Savenije OE, Smit HA, et al. Perinatal risk factors for wheezing phenotypes in the first 8 years of life. Clin Exp Allergy 2013; 43: 1395–1405. doi: 10.1111/cea.12173 [DOI] [PubMed] [Google Scholar]
- 32.Savenije OE, Granell R, Caudri D, et al. Comparison of childhood wheezing phenotypes in 2 birth cohorts: ALSPAC and PIAMA. J Allergy Clin Immunol 2011; 127: 1505–1512. doi: 10.1016/j.jaci.2011.02.002 [DOI] [PubMed] [Google Scholar]
- 33.Collin SM, Granell R, Westgarth C, et al. Associations of pet ownership with wheezing and lung function in childhood: findings from a UK birth cohort. PLoS One 2015; 10: e0127756. doi: 10.1371/journal.pone.0127756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henderson J, Granell R, Heron J, et al. Associations of wheezing phenotypes in the first 6 years of life with atopy, lung function and airway responsiveness in mid-childhood. Thorax 2008; 63: 974–980. doi: 10.1136/thx.2007.093187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Custovic A, Sonntag HJ, Buchan IE, et al. Evolution pathways of IgE responses to grass and mite allergens throughout childhood. J Allergy Clin Immunol 2015; 136: 1645–1652. doi: 10.1016/j.jaci.2015.03.041 [DOI] [PubMed] [Google Scholar]
- 36.Dai R, Miliku K, Gaddipati S, et al. Wheeze trajectories: determinants and outcomes in the CHILD cohort study. J Allergy Clin Immunol 2022; 149: 2153–2165. doi: 10.1016/j.jaci.2021.10.039 [DOI] [PubMed] [Google Scholar]
- 37.Depner M, Fuchs O, Genuneit J, et al. Clinical and epidemiologic phenotypes of childhood asthma. Am J Respir Crit Care Med 2014; 189: 129–138. doi: 10.1164/rccm.201307-1198OC [DOI] [PubMed] [Google Scholar]
- 38.Dharma C, Lefebvre DL, Tran MM, et al. Patterns of allergic sensitization and atopic dermatitis from 1 to 3 years: effects on allergic diseases. Clin Exp Allergy 2018; 48: 48–59. doi: 10.1111/cea.13063 [DOI] [PubMed] [Google Scholar]
- 39.Divaret-Chauveau A, Mauny F, Hose A, et al. Trajectories of cough without a cold in early childhood and associations with atopic diseases. Clin Exp Allergy 2023; 53: 429–442. doi: 10.1111/cea.14257 [DOI] [PubMed] [Google Scholar]
- 40.Farraia M, Mendes FC, Sokhatska O, et al. Sensitization trajectories to multiple allergen components in a population-based birth-cohort. Pediatr Allergy Immunol 2023; 34: e13963. doi: 10.1111/pai.13963 [DOI] [PubMed] [Google Scholar]
- 41.Forster F, Ege MJ, Gerlich J, et al. Trajectories of asthma and allergy symptoms from childhood to adulthood. Allergy 2022; 77: 1192–1203. doi: 10.1111/all.15075 [DOI] [PubMed] [Google Scholar]
- 42.Gabet S, Rancière F, Just J, et al. Asthma and allergic rhinitis risk depends on house dust mite specific IgE levels in PARIS birth cohort children. World Allergy Organ J 2019; 12: 100057. doi: 10.1016/j.waojou.2019.100057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garden FL, Simpson JM, Marks GB. Atopy phenotypes in the Childhood Asthma Prevention Study (CAPS) cohort and the relationship with allergic disease: clinical mechanisms in allergic disease. Clin Exp Allergy 2013; 43: 633–641. doi: 10.1111/cea.12095 [DOI] [PubMed] [Google Scholar]
- 44.Granell R, Henderson AJ, Sterne JA. Associations of wheezing phenotypes with late asthma outcomes in the Avon Longitudinal Study of Parents and Children: a population-based birth cohort. J Allergy Clin Immunol 2016; 138: 1060–1070.e1011. doi: 10.1016/j.jaci.2016.01.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haider S, Fontanella S, Ullah A, et al. Evolution of eczema, wheeze, and rhinitis from infancy to early adulthood: four birth cohort studies. Am J Respir Crit Care Med 2022; 206: 950–960. doi: 10.1164/rccm.202110-2418OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hallmark B, Wegienka G, Havstad S, et al. Chromosome 17q12-21 variants are associated with multiple wheezing phenotypes in childhood. Am J Respir Crit Care Med 2021; 203: 864–870. doi: 10.1164/rccm.202003-0820OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hose AJ, Depner M, Illi S, et al. Latent class analysis reveals clinically relevant atopy phenotypes in 2 birth cohorts. J Allergy Clin Immunol 2017; 139: 1935–1945.e1912. doi: 10.1016/j.jaci.2016.08.046 [DOI] [PubMed] [Google Scholar]
- 48.Hu C, Duijts L, Erler NS, et al. Most associations of early-life environmental exposures and genetic risk factors poorly differentiate between eczema phenotypes: the Generation R Study. Br J Dermatol 2019; 181: 1190–1197. doi: 10.1111/bjd.17879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khan S, Ouaalaya EH, Chauveau AD, et al. Whispers of change in preschool asthma phenotypes: findings in the French ELFE cohort. Respir Med 2023; 215: 107263. doi: 10.1016/j.rmed.2023.107263 [DOI] [PubMed] [Google Scholar]
- 50.Kilanowski A, Thiering E, Wang G, et al. Allergic disease trajectories up to adolescence: characteristics, early-life, and genetic determinants. Allergy 2023; 78: 836–850. doi: 10.1111/all.15511 [DOI] [PubMed] [Google Scholar]
- 51.Kotecha SJ, Watkins WJ, Lowe J, et al. Comparison of the associations of early-life factors on wheezing phenotypes in preterm-born children and term-born children. Am J Epidemiol 2019; 188: 527–536. doi: 10.1093/aje/kwy268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kotsapas C, Nicolaou N, Haider S, et al. Early-life predictors and risk factors of peanut allergy, and its association with asthma in later-life: population-based birth cohort study. Clin Exp Allergy 2022; 52: 646–657. doi: 10.1111/cea.14103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simpson A, Tan VYF, Winn J, et al. Beyond atopy. Am J Respir Crit Care Med 2010; 181: 1200–1206. doi: 10.1164/rccm.200907-1101OC [DOI] [PubMed] [Google Scholar]
- 54.Lau HX, Chen Z, Chan YH, et al. Allergic sensitization trajectories to age 8 years in the Singapore GUSTO cohort. World Allergy Organ J 2022; 15: 100667. doi: 10.1016/j.waojou.2022.100667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lau HX, Chen Z, Van Bever H, et al. Clinical predictors of wheeze trajectories and associations with allergy in Asian children. Ann Allergy Asthma Immunol 2023; 131: 466–473. doi: 10.1016/j.anai.2023.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lazic N, Roberts G, Custovic A, et al. Multiple atopy phenotypes and their associations with asthma: similar findings from two birth cohorts. Allergy 2013; 68: 764–770. doi: 10.1111/all.12134 [DOI] [PubMed] [Google Scholar]
- 57.Lee SY, Kim S, Kang MJ, et al. Phenotype of atopic dermatitis with food allergy predicts development of childhood asthma via gut Wnt signaling. Allergy Asthma Immunol Res 2022; 14: 674–686. doi: 10.4168/aair.2022.14.6.674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lewis KM, De Stavola BL, Cunningham S, et al. Socioeconomic position, bronchiolitis and asthma in children: counterfactual disparity measures from a national birth cohort study. Int J Epidemiol 2023; 52: 476–488. doi: 10.1093/ije/dyac193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lodge CJ, Zaloumis S, Lowe AJ, et al. Early-life risk factors for childhood wheeze phenotypes in a high-risk birth cohort. J Pediatr 2014; 164: 289–294. doi: 10.1016/j.jpeds.2013.09.056 [DOI] [PubMed] [Google Scholar]
- 60.Loo EXL, Liew TM, Yap GC, et al. Trajectories of early-onset rhinitis in the Singapore GUSTO mother–offspring cohort. Clin Exp Allergy 2021; 51: 419–429. doi: 10.1111/cea.13803 [DOI] [PubMed] [Google Scholar]
- 61.Lopez DJ, Lodge CJ, Bui DS, et al. Establishing subclasses of childhood eczema, their risk factors and prognosis. Clin Exp Allergy 2022; 52: 1079–1090. doi: 10.1111/cea.14139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu Y, Wang Y, Wang J, et al. Early-life antibiotic exposure and childhood asthma trajectories: a national population-based birth cohort. Antibiotics (Basel) 2023; 12: 314. doi: 10.3390/antibiotics12020314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McCready C, Haider S, Little F, et al. Early childhood wheezing phenotypes and determinants in a South African birth cohort: longitudinal analysis of the Drakenstein Child Health Study. Lancet Child Adolesc Health 2023; 7: 127–135. doi: 10.1016/s2352-4642(22)00304-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mulick AR, Mansfield KE, Silverwood RJ, et al. Four childhood atopic dermatitis subtypes identified from trajectory and severity of disease and internally validated in a large UK birth cohort. Br J Dermatol 2021; 185: 526–536. doi: 10.1111/bjd.19885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakamura T, Haider S, Fontanella S, et al. Modelling trajectories of parentally reported and physician-confirmed atopic dermatitis in a birth cohort study. Br J Dermatol 2022; 186: 274–284. doi: 10.1111/bjd.20767 [DOI] [PubMed] [Google Scholar]
- 66.Ödling M, Wang G, Andersson N, et al. Characterization of asthma trajectories from infancy to young adulthood. J Allergy Clin Immunol Pract 2021; 9: 2368–2376. doi: 10.1016/j.jaip.2021.02.007 [DOI] [PubMed] [Google Scholar]
- 67.Oksel C, Granell R, Haider S, et al. Distinguishing wheezing phenotypes from infancy to adolescence. A pooled analysis of five birth cohorts. Ann Am Thorac Soc 2019; 16: 868–876. doi: 10.1513/AnnalsATS.201811-837OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Owora AH, Becker AB, Chan-Yeung M, et al. Wheeze trajectories are modifiable through early-life intervention and predict asthma in adolescence. Pediatr Allergy Immunol 2018; 29: 612–621. doi: 10.1111/pai.12922 [DOI] [PubMed] [Google Scholar]
- 69.Panico L, Stuart B, Bartley M, et al. Asthma trajectories in early childhood: identifying modifiable factors. PLoS One 2014; 9: e111922. doi: 10.1371/journal.pone.0111922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pape K, Cowell W, Sejbaek CS, et al. Adverse childhood experiences and asthma: trajectories in a national cohort. Thorax 2021; 76: 547–553. doi: 10.1136/thoraxjnl-2020-214528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Paternoster L, Savenije OEM, Heron J, et al. Identification of atopic dermatitis subgroups in children from 2 longitudinal birth cohorts. J Allergy Clin Immunol 2018; 141: 964–971. doi: 10.1016/j.jaci.2017.09.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peng Z, Kurz D, Weiss JM, et al. Latent classes of atopic dermatitis and food allergy development in childhood. Pediatr Allergy Immunol 2022; 33: e13881. doi: 10.1111/pai.13881 [DOI] [PubMed] [Google Scholar]
- 73.Rancière F, Nikasinovic L, Bousquet J, et al. Onset and persistence of respiratory/allergic symptoms in preschoolers: new insights from the PARIS birth cohort. Allergy 2013; 68: 1158–1167. doi: 10.1111/all.12208 [DOI] [PubMed] [Google Scholar]
- 74.Roduit C, Frei R, Depner M, et al. Phenotypes of atopic dermatitis depending on the timing of onset and progression in childhood. JAMA Pediatr 2017; 171: 655–662. doi: 10.1001/jamapediatrics.2017.0556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sbihi H, Koehoorn M, Tamburic L, et al. Asthma trajectories in a population-based birth cohort. Impacts of air pollution and greenness. Am J Respir Crit Care Med 2017; 195: 607–613. doi: 10.1164/rccm.201601-0164OC [DOI] [PubMed] [Google Scholar]
- 76.Schmidt F, Hose AJ, Mueller-Rompa S, et al. Development of atopic sensitization in Finnish and Estonian children: a latent class analysis in a multicenter cohort. J Allergy Clin Immunol 2019; 143: 1904–1913.e1909. doi: 10.1016/j.jaci.2018.12.1014 [DOI] [PubMed] [Google Scholar]
- 77.Schoos AM, Chawes BL, Rasmussen MA, et al. Atopic endotype in childhood. J Allergy Clin Immunol 2016; 137: 844–851.e844. doi: 10.1016/j.jaci.2015.10.004 [DOI] [PubMed] [Google Scholar]
- 78.Schoos AM, Chawes BL, Melén E, et al. Sensitization trajectories in childhood revealed by using a cluster analysis. J Allergy Clin Immunol 2017; 140: 1693–1699. doi: 10.1016/j.jaci.2017.01.041 [DOI] [PubMed] [Google Scholar]
- 79.Shahunja KM, Sly PD, Chisti MJ, et al. Trajectories of asthma symptom presenting as wheezing and their associations with family environmental factors among children in Australia: evidence from a national birth cohort study. BMJ Open 2022; 12: e059830. doi: 10.1136/bmjopen-2021-059830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shi YY, Wei Q, Ma X, et al. Maternal affective and stress-related factors during pregnancy affect the occurrence of childhood allergic diseases: a Shanghai MCPC study. J Psychosom Res 2023; 165: 111142. doi: 10.1016/j.jpsychores.2022.111142 [DOI] [PubMed] [Google Scholar]
- 81.Sordillo JE, Coull BA, Rifas-Shiman SL, et al. Characterization of longitudinal wheeze phenotypes from infancy to adolescence in Project Viva, a prebirth cohort study. J Allergy Clin Immunol 2020; 145: 716–719.e718. doi: 10.1016/j.jaci.2019.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Su YT, Tsai Y, Chuang CY. Asthma phenotypes in the first three years of life and correlation with active asthma at 6 years of age: a national population-based study in Taiwan. Pediatric Pulmonol 2017; 52: S105. doi: 10.1002/ppul.23731 [DOI] [Google Scholar]
- 83.Suaini NHA, Yap GC, Bui DPT, et al. Atopic dermatitis trajectories to age 8 years in the GUSTO cohort. Clin Exp Allergy 2021; 51: 1195–1206. doi: 10.1111/cea.13993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tan DJ, Lodge CJ, Walters EH, et al. Longitudinal asthma phenotypes from childhood to middle-age: a population-based cohort study. Am J Respir Crit Care Med 2023; 208: 132–141. doi: 10.1164/rccm.202208-1569OC [DOI] [PubMed] [Google Scholar]
- 85.Tang HH, Teo SM, Belgrave DC, et al. Trajectories of childhood immune development and respiratory health relevant to asthma and allergy. eLife 2018; 7: e35856. doi: 10.7554/eLife.35856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tischer C, Täubel M, Kirjavainen PV, et al. Early-life residential exposure to moisture damage is associated with persistent wheezing in a Finnish birth cohort. Pediatr Allergy Immunol 2022; 33: e13864. doi: 10.1111/pai.13864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tse SM, Rifas-Shiman SL, Coull BA, et al. Sex-specific risk factors for childhood wheeze and longitudinal phenotypes of wheeze. J Allergy Clin Immunol 2016; 138: 1561–1568.e1566. doi: 10.1016/j.jaci.2016.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weber P, Jarvis D, Baptista Menezes AM, et al. Wheezing trajectories from childhood to adulthood in a population-based cohort. Allergol Int 2022; 71: 200–206. doi: 10.1016/j.alit.2021.09.002 [DOI] [PubMed] [Google Scholar]
- 89.Wu TJ, Pan SC, Chen BY, et al. Different seasonal effect on asthma trajectories: a population-based birth cohort study. Pediatr Allergy Immunol 2018; 29: 873–877. doi: 10.1111/pai.12973 [DOI] [PubMed] [Google Scholar]
- 90.Yamamoto-Hanada K, Yang L, Saito-Abe M, et al. Four phenotypes of atopic dermatitis in Japanese children: a general population birth cohort study. Allergol Int 2019; 68: 521–523. doi: 10.1016/j.alit.2019.02.010 [DOI] [PubMed] [Google Scholar]
- 91.Yang L, Narita M, Yamamoto-Hanada K, et al. Phenotypes of childhood wheeze in Japanese children: a group-based trajectory analysis. Pediatr Allergy Immunol 2018; 29: 606–611. doi: 10.1111/pai.12917 [DOI] [PubMed] [Google Scholar]
- 92.Zhou JX, Guo Y, Teng YZ, et al. Maternal anxiety during pregnancy and children's asthma in preschool age: the Ma'anshan birth cohort study. J Affect Disord 2023; 340: 312–320. doi: 10.1016/j.jad.2023.08.037 [DOI] [PubMed] [Google Scholar]
- 93.Ziyab AH, Mukherjee N, Zhang H, et al. Sex-specific developmental trajectories of eczema from infancy to age 26 years: a birth cohort study. Clin Exp Allergy 2022; 52: 416–425. doi: 10.1111/cea.14068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hu C, Nijsten T, Pasmans S, et al. Associations of eczema phenotypes with emotional and behavioural problems from birth until school age. The Generation R Study. Br J Dermatol 2020; 183: 311–320. doi: 10.1111/bjd.18705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hu C, Nijsten T, van Meel ER, et al. Eczema phenotypes and risk of allergic and respiratory conditions in school age children. Clin Transl Allergy 2020; 10: 7. doi: 10.1186/s13601-020-0310-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hu C, Duijts L, van Meel ER, et al. Association between nasal and nasopharyngeal bacterial colonization in early life and eczema phenotypes. Clin Exp Allergy 2021; 51: 716–725. doi: 10.1111/cea.13869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Duijts L, Granell R, Sterne JA, et al. Childhood wheezing phenotypes influence asthma, lung function and exhaled nitric oxide fraction in adolescence. Eur Respir J 2016; 47: 510–519. doi: 10.1183/13993003.00718-2015 [DOI] [PubMed] [Google Scholar]
- 98.Galobardes B, Granell R, Sterne J, et al. Childhood wheezing, asthma, allergy, atopy, and lung function: different socioeconomic patterns for different phenotypes. Am J Epidemiol 2015; 182: 763–774. doi: 10.1093/aje/kwv045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Granell R, Henderson AJ, Timpson N, et al. Examination of the relationship between variation at 17q21 and childhood wheeze phenotypes. J Allergy Clin Immunol 2013; 131: 685–694. doi: 10.1016/j.jaci.2012.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hanson C, Rifas-Shiman SL, Shivappa N, et al. Associations of prenatal dietary inflammatory potential with childhood respiratory outcomes in project Viva. J Allergy Clin Immunol Pract 2020; 8: 945–952. doi: 10.1016/j.jaip.2019.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kotecha SJ, Lowe J, Granell R, et al. The effect of catch-up growth in the first year of life on later wheezing phenotypes. Eur Respir J 2020; 56: 2000884. doi: 10.1183/13993003.00884-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lodge CJ, Lowe AJ, Allen KJ, et al. Childhood wheeze phenotypes show less than expected growth in FEV1 across adolescence. Am J Respir Crit Care Med 2014; 189: 1351–1358. doi: 10.1164/rccm.201308-1487OC [DOI] [PubMed] [Google Scholar]
- 103.Lodge CJ, Lowe AJ, Abramson MJ, et al. Transient childhood wheeze is associated with less atopy in adolescence. Pediatr Allergy Immunol 2020; 31: 913–919. doi: 10.1111/pai.13304 [DOI] [PubMed] [Google Scholar]
- 104.Savenije OE, Mahachie John JM, Granell R, et al. Association of IL33-IL-1 receptor-like 1 (IL1RL1) pathway polymorphisms with wheezing phenotypes and asthma in childhood. J Allergy Clin Immunol 2014; 134: 170–177. doi: 10.1016/j.jaci.2013.12.1080 [DOI] [PubMed] [Google Scholar]
- 105.Shahunja KM, Sly PD, Huda MM, et al. Trajectories of neighborhood environmental factors and their associations with asthma symptom trajectories among children in Australia: evidence from a national birth cohort study. J Environ Health Sci Eng 2022; 20: 835–847. doi: 10.1007/s40201-022-00824-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tutino M, Granell R, Curtin JA, et al. Dog ownership in infancy is protective for persistent wheeze in 17q21 asthma-risk carriers. J Allergy Clin Immunol 2023; 151: 423–430. doi: 10.1016/j.jaci.2022.10.012 [DOI] [PubMed] [Google Scholar]
- 107.Ramratnam SK, Lockhart A, Visness CM, et al. Maternal stress and depression are associated with respiratory phenotypes in urban children. J Allergy Clin Immunol 2021; 148: 120–127. doi: 10.1016/j.jaci.2021.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Clark H, Granell R, Curtin JA, et al. Differential associations of allergic disease genetic variants with developmental profiles of eczema, wheeze and rhinitis. Clin Exp Allergy 2019; 49: 1475–1486. doi: 10.1111/cea.13485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chowdhury NU, Guntur VP, Newcomb DC, et al. Sex and gender in asthma. Eur Respir Rev 2021; 30: 210067. doi: 10.1183/16000617.0067-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fuseini H, Newcomb DC. Mechanisms driving gender differences in asthma. Curr Allergy Asthma Rep 2017; 17: 19. doi: 10.1007/s11882-017-0686-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Owora AH, Zhang Y. Childhood wheeze trajectory-specific risk factors: a systematic review and meta-analysis. Pediatr Allergy Immunol 2021; 32: e13313. doi: 10.1111/pai.13313 [DOI] [PubMed] [Google Scholar]
- 112.Pyun BY. Natural history and risk factors of atopic dermatitis in children. Allergy Asthma Immunol Res 2015; 7: 101–105. doi: 10.4168/aair.2015.7.2.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chiu C-Y, Huang Y-L, Tsai M-H, et al. Sensitization to food and inhalant allergens in relation to atopic diseases in early childhood: a birth cohort study. PLoS One 2014; 9: e102809. doi: 10.1371/journal.pone.0102809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Oksel C, Granell R, Mahmoud O, et al. Causes of variability in latent phenotypes of childhood wheeze. J Allergy Clin. Immunol. 2019; 143: 1783–1790.e11. doi: 10.1016/j.jaci.2018.10.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wurpts IC, Geiser C. Is adding more indicators to a latent class analysis beneficial or detrimental? Results of a Monte-Carlo study. Front Psychol 2014; 5: 920. doi: 10.3389/fpsyg.2014.00920 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERR-0160-2024.supplement (1.4MB, pdf)
Data Availability Statement
The R scripts and data are available at https://osf.io/ayf35.