Abstract
Background
Although pentatricopeptide repeat domain 1 (PTCD1) has been found to modulate mitochondrial metabolic and oxidative phosphorylation, its contribution in the growth of clear cell renal cell carcinoma (ccRCC) remains unknown.
Methods
The Cancer Genome Atlas (TCGA) dataset was utilized to examine the transcriptional alterations, patient characteristics, clinical outcomes, as well as pathway activation of PTCD1. The Weighted Gene Co-expression Network Analysis (WGCNA) was performed to investigate potential genes that associated with PTCD1. The researchers estimated the relationship between PTCD1, tumor immunology, and epithelial mesenchymal transition (EMT). Researchers studied how PTCD1 affects the functional behavior of tumor cells in vitro.
Results
PTCD1 expression was greater in ccRCC samples than in normal samples, and expression increased gradually as the stage increased. In TCGA cohorts, higher PTCD1 expression was substantially associated with a poorer clinical stage, histological grading, T stage, N stage, M stage, and survival outcomes. The results of multivariate analysis showed that PTCD1 was an independent variable affecting the survival outcomes of ccRCC patients (p < 0.001). PTCD1 regulated ccRCC progression via various cancer mechanisms including PI3K-Akt signaling, focal adhesion, PD-L1 expression, and PD-1 checkpoints in cancer. WGCNA discovered a significant relationship between PTCD1 and IARS2, LRPPRC, MT-ND2, MT-CO1, MT-CO2, MT-CYB, MT-ATP6, and MT-ND4. Furthermore, PTCD1 expression levels was closely associated with immune infiltrating, immunological checkpoint, EMT, immunotherapy responsiveness, and anti-tumor medication sensitivities. Upregulation of PTCD1 in ccRCC cells resulted in considerably increased cellular invasion and migration. Mechanistically, the upregulation of PTCD1 increased the phosphorylation of AKT at Ser473 and GSK-3β at Ser9, as well as enhanced activation of Wnt/β-catenin pathway.
Conclusion
Elevated expression of PTCD1 was associated with malignant biological behaviors and poor outcomes of ccRCC patients, and PTCD1 may accelerate tumor cells proliferation and invasion via the Akt/GSK3β/β-catenin pathway. Our findings indicated that PTCD1 had the potential to become a new target for predicting prognosis and targeted therapy.
Keywords: Pentatricopeptide repeat domain 1, Clear cell renal cell carcinoma, Biomarker, Prognosis, Immune microenvironment, Weighted Gene Coexpression Network Analysis
Introduction
Kidney cancer has become one of the most prevalent cancers of the urinary system, with over 70,000 new cases reported in the United States by 2021 [1]. Clear cell renal cell carcinoma (ccRCC) is the most frequent histologic subtype, representing for around 70%–80% of all cases [2]. At an early phase, more than 30% of ccRCC cases are identified with a local recurrence or metastatic disease [3]. Despite the detection and therapy for kidney cancer have made a breakthrough in latest years, the 5-year survival rate of individuals with progressive ccRCC has not risen significantly [2, 4]. Developing an innovative diagnostic indicator and therapeutic targets is beneficial to ccRCC individuals.
Being a member of pentatricopeptide repeat (PPR) proteins, pentatricopeptide repeat domain 1 (PTCD1) is an RNA-binding polypeptide with a characteristic thirty-five sequence repeating pattern [5]. Inside the body, there are 7 PPR peptides, all of which are in the mitochondrial and engaged in cell function, particularly RNA metabolism [6, 7]. During past few years, a growing amount of PPRs have really been recognized to have critical regulatory roles in tumor carcinogenesis. LRPPRC, for example, enhanced urinary cancer progression by modulating redox balance via the circANKHD1/FOXM1 pathway [7]. According to Black et al., FOXM1 transferred to mitochondrial and reduced mitochondria respiration and oxidative phosphorylation via boosting PTCD1, suggesting that it could be potential biological target [8]. Just like previously demonstrated, PTCD1 is much probably implicated in the formation and growth of cancer. Nevertheless, cancer mechanism of PTCD1 for ccRCC also isn't fully understood.
Within that study, transcription information from The Cancer Genome Atlas (TCGA) database was applied to determine potential variations in PTCD1 transcription and its connection of patients’ prognosis. The Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis were undertaken to reveal underlying mechanisms. The Weighted Gene Co-expression Network Analysis (WGCNA) was used to explored the possible genes associated with PTCD1. This relationship among PTCD1 transcription with the degree of immune cell infiltration were studied to determine the potential pathway of PTCD1-induced carcinogenesis and growth. Lastly, functional experiments and mechanism explorations were performed to confirm the effect of PTCD1 in ccRCC cell lines in vitro.
Materials and methods
Data collection for PTCD1 expression
The implementation process of this study was shown in Fig. 1, and this study followed the methods of Zhou et al. [9]. The TCGA-KIRC dataset was used to collect PTCD1 transcriptome and patient information. Age, gender, clinical stage, TNM stage, pathologic grade, survival status, and survival time were the key clinical statistics. The following these were exclusion criteria: (a) The pathologic assessment was inconsistent with the criteria for ccRCC; (b) Both PTCD1 transcriptome and clinical information were insufficient. Survival information was analyzed in R software using “survival” and “survminer” packages.
Fig. 1.

The implementation process of this study
Prognostic nomogram and functional analysis
Researchers built a predictive nomogram using the findings of multivariate Cox regression analysis to estimate the prognosis for ccRCC cases. Differential expression genes (DEGs; |log2FC|> 2, false discovery rate (FDR) < 0.001) were selected in the GO and KEGG pathways analysis using R package “clusterProfiler.” FDR q-value less than 0.05 and p adjustment less than 0.05 were considered to greatly enrich in these items.
Module identification of related with PTCD1 expression
WGCNA was implemented to discover the genetic cluster that was deeply linked with PTCD1 transcription with R package “WGCNA”. The module-trait connections were employed to calculate the correlations among components and ccRCC patients. The module eigengene (ME) was regarded as the primary core element of a particular component. Ultimately, important clinical components were discovered after analyzing the relationship among clinical characteristics with MEs. The intergenic interaction network was created using the Cytoscape 3.6.0 software.
PTCD1 expressions, immune functions, EMT-related genes
The Wilcoxon rank sum test was employed to evaluate the correlations between PTCD1 expression and immune infiltration using “GSVA” and “GSEABase” in R software. We also used single-sample Gene Set Enrichment Analysis (ssGSEA) to calculate the correlation between the expression of PTCD1 and enrichment scores of T helper cells, eosinophils, Th17 cells, macrophages, Th2 cells, neutrophils, TReg and NK CD56 bright cell. Furthermore, Wilcoxon rank sum test was used to reveal the relationship between PTCD1 expression, immune checkpoints and epithelial mesenchymal transition (EMT)-related genes expression. p < 0.05 was considered statistically significant.
QRT-PCR
Total RNA was extracted using an RNA extraction kit (Mabio, Guangzhou, China). The concentration and quality of total RNA were detected using a spectrophotometer. cDNA synthesis was performed using 1 μg of total RNA in a 20 μl reaction volume using a cDNA synthesis kit (AG, Changsha, China). cDNA was involved in qRT-PCR analysis (Script SYBR Green PCR kit; AG, Changsha, China) based on the manufacturer's instructions. The gene expressions were obtained with the 2-ΔΔCt function normalized to GAPDH. Related primer sequences were listed:
PTCD1 Forward Primer (F): 5ʹ-ACATACCACGCGCTGCTGAAGA-3ʹ;
PTCD1 Reverse Primer (R): 5ʹ-GAGCAGGAAACTGAAGGTCTCC-3ʹ;
GAPDH F: 5ʹ-TGACTTCAACAGCGACACCCA-3ʹ;
GAPDH R: 5ʹ-CACCCTGTTGCTGTAGCCAAA-3ʹ.
Cell culture and transfection
One normal renal cell line (HK-2) and four ccRCC cell lines (ACHN, A498, Caki and 786o) were gained from Chinese Academy of Sciences (Shanghai, China). RPMI-1640 medium containing 10% FBS was used for cell culture (37 °C; 5% CO2). A lentiviral expression vector (pLV-PTCD1) [Genechem (Shanghai) Co., LTD.] was used for PTCD1 gene delivery and stable overexpression. ACHN and 786o cells were cultured in complete medium containing recombinant lentivirus (pLV-PTCD1 or pLV-NC) for 12 h; then, serum-free medium was replaced with normal medium. As observed by a fluorescence microscope, the proportion of GFP-positive cells represented the infection efficiency. The medium containing puromycin (Thermo Fisher, USA) was used to kill cells that were unsuccessfully transfected with virus.
Cell proliferation and survival assay
Transfected ccRCC cells were seeded in 12 well plates (1 × 104 cells/well). Cell numbers were estimated using a cytometer after 2, 4, 6, 8, and 10 days to analyze the proliferative capacity of tumor cells. For cell viability, transfected ccRCC cells were seeded on 12 well plates (1 × 105 cells/well), then, 8 h after seeding these plates, a concentration gradient of hydrogen peroxide was added, respectively. Cell numbers were analyzed using a cytometer after 8 h.
Cell invasion assays
A 24-well Transwell insert (Corning, USA) with 8-micron pores was used to detect cell invasiveness. 5 × 104 transfected cells floated in 200 µL serum-free medium were added into the upper part of a Transwell insert coated with Matrigel (BD Bioscience, USA). 600 ul medium containing 10% FBS was added to the lower part as an attractant. After 48 h of culture, the invading cells were fixed, stained, and the upper cells were cleared and observed by microscopy.
Cell wound‐healing assay
First, 8 × 105 tumor cells were seeded on a 6-well culture plate. After 8 h, each well was cut with a 1-mL sterile pipette tip to form four uniform wounds, which were replaced with serum-free medium for incubation. Cell migration was recorded with an inverted microscope at 0 and 12 h, respectively.
Colony formation assay
In total, 1000 cells per well were inoculated onto 6-well plates and then cultured for 1–2 weeks. The generated colonies were then fixed with 4% polyoxymethylene, and stained with 1% Giemsa to calculate the number and size of colonies.
Western blot
Total protein from cultured cells was harvested using RIPA buffer (Servicebio, Wuhan, China) and adding proteinase inhibitor (Roche, Shanghai, China) and phosphatase inhibitor (Roche, Shanghai, China). The BCA Protein Assay Kit (Servicebio, Wuhan, China) was used to quantify protein concentration. Antibody listed as follow: anti‐AKT (pan) (C67E7) (Cell Signaling Technology, 4691T), Anti‐phosphorylated AKT (P-AKT; Ser473) (Abmart, T56569), anti‐GSK3β (Proteintech, Ag17320), anti-phosphorylated GSK3β (P-GSK3β; Ser9) (Cell Signaling Technology, 9323T), anti‐β-catenin (Cell Signaling Technology, 8480T), anti‐PTCD1 (Abmart, ab121620) and anti‐GAPDH (Abmart, M20006).
Statistics analysis
R software (version 4.0.2) and GraphPad Prism v7.0 (GraphPad, San Diego, CA, United States) were used for all data analysis. Categorical indicators were expressed as proportions, and continuous indicators were expressed in mean ± standard deviation (SD). The Wilcoxon rank sum test was used to compare the difference of PTCD1 transcriptional level across several classified clinical indicators. The chi-square test was employed to assess the relationship of clinical characteristics in ccRCC participants across high- and low-PTCD1 subgroups. When p < 0.05, that results were judged meaningful.
Results
PTCD1 upregulation in ccRCC
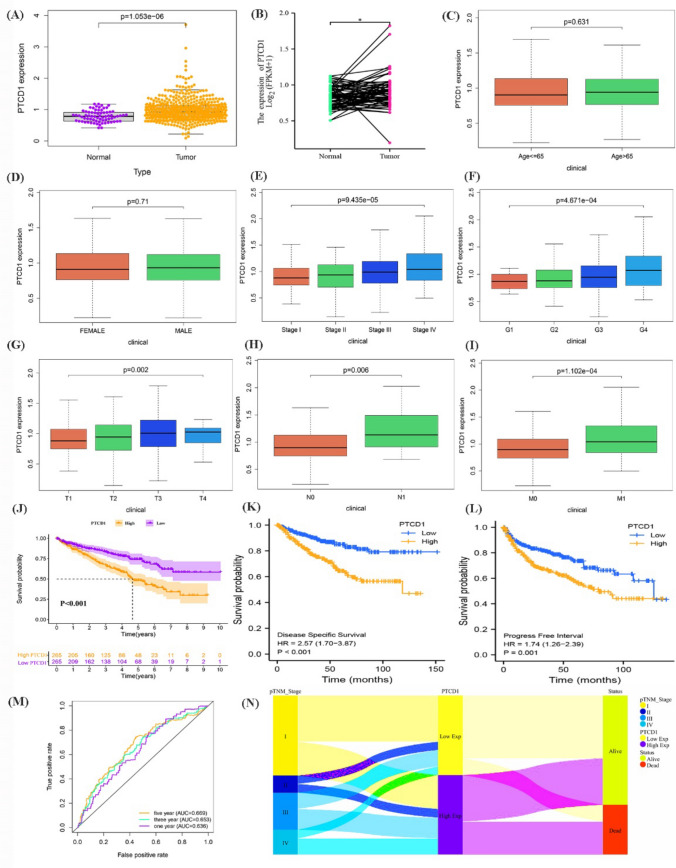
Initially, researchers did a transcriptome differential analysis and discovered that PTCD1 expression level for ccRCC samples was considerably greater than that in normal samples (p = 1.053e−06) (Fig. 2a). According to paired analysis, PTCD1 transcription in ccRCC samples was substantially greater than that in adjacent tissues (p < 0.05) (Fig. 2b). A stratified analysis discovered that PTCD1 transcription steadily rose with stage (p = 9.435e−05), grade (p = 4.671e−04), T stage (p = 0.002), N stage (p = 0.006), and M stage (p = 1.102e-04), while age (p = 0.631) or gender (p = 0.71) seemed to have no relationship with PTCD1 transcription level (Fig. 2c–i).
Fig. 2.
The PTCD1 expression level and survival analysis in ccRCC. a PTCD1 expression in normal tissues and ccRCC tissues; b PTCD1 expression in ccRCC paired tissues; Box plot evaluating PTCD1 expression according to different clinical characteristics including age (c), gender (d), stage (e), grade (f), T stage (g), N stage (h) and M stage (i); The OS (j), DSS (k), PFI (l) of high-PTCD1 versus low-PTCD1 in the TCGA dataset; ROC curve demonstrated the stability of PTCD1 in evaluating 1-, 3- and 5-year OS (m); Sankey diagram of the connection between TNM stage, PTCD1 expression, and survival status (N); *p < 0.05; **p < 0.01; ***p < 0.001
The association of PTCD1 expression and clinical outcomes
The TCGA-KIRC populations subsequently split into two groups based upon this median level of PTCD1 as the cutoff. Table 1 showed some characteristics of ccRCC populations, and we discovered that elevated PTCD1 expression related to much worse stage (p = 0.0016), grade (p = 0.0020), T stage (p = 0.0080), N stage (p = 0.0247), M stage (p = 0.0018), and survival condition (p < 0.0001). The Kaplan–Meier curves demonstrated that low-PTCD1 group exhibited significantly greater overall survival (OS) (Fig. 2j; p < 0.001), disease specific survival (DSS) (Fig. 2k; p < 0.001), and progress free interval (PFI) (Fig. 2l; p = 0.001) than high-PTCD1 group. The receiver operating characteristic (ROC) curve indicated that PTCD1 had a better reliability in predicting survival prognosis of ccRCC cases (Fig. 2m). The Sankey graph revealed a correlation among pTNM stage, PTCD1 expression, and surviving status, revealing that almost all individuals with low-PTCD1 transcription remained live (Fig. 2n). The subgroups assessment of OS has also been done on age (Fig. 3a, b), gender (Fig. 3c, d), grade (Fig. 3e, f), stage (Fig. 3g, h), T stage (Fig. 3i, j), and M stage (Fig. 3k, l) (all p < 0.05). The findings revealed that overexpression of PTCD1 seemed to have a stable influence on ccRCC individuals with varying clinical features.
Table 1.
The relationship between the expression of PTCD1 and various clinicopathological variables in the TCGA database
| Characteristics | Total | PTCD1 expression | p-value | ||
|---|---|---|---|---|---|
| Low | High | ||||
| Total | 530 | 265 | 265 | ||
| Age (years) | ≤ 65 | 384 | 177 (33.40%) | 171 (32.26%) | 0.6475 |
| > 65 | 182 | 88 (16.60%) | 94 (17.73%) | ||
| Sex | Male | 344 | 169 (31.89%) | 175 (33.02%) | 0.6491 |
| Female | 186 | 96 (18.11%) | 90 (16.98%) | ||
| Histologic grade | G1 | 14 | 8 (1.51%) | 6 (1.13%) | 0.0020 |
| G2 | 227 | 133 (25.09%) | 94 (17.74%) | ||
| G3 | 206 | 93 (17.55%) | 113 (21.32%) | ||
| G4 | 75 | 27 (5.09%) | 48 (9.06%) | ||
| Pathological stage | Stage I | 265 | 153 (28.87%) | 112 (21.13%) | 0.0016 |
| Stage II | 57 | 28 (5.28%) | 29 (5.47%) | ||
| Stage III | 123 | 54 (10.19%) | 69 (13.02%) | ||
| Stage IV | 82 | 29 (5.47%) | 53 (10.00%) | ||
| T stage | T1 | 271 | 155 (29.25%) | 116 (21.89%) | 0.0080 |
| T2 | 69 | 31 (5.85%) | 38 (7.17%) | ||
| T3 | 179 | 75 (14.15%) | 104 (19.62%) | ||
| T4 | 11 | 4 (0.75%) | 7 (1.32%) | ||
| N stage | N0 | 239 | 129 (24.34%) | 110 (20.75%) | 0.0247 |
| N1 | 16 | 4 (0.75%) | 12 (2.26%) | ||
| M stage | M0 | 420 | 226 (42.64%) | 194 (36.60%) | 0.0018 |
| M1 | 78 | 27 (5.09%) | 51 (9.62%) | ||
| Survival status | Alive | 364 | 209 (39.43%) | 155 (29.25%) | < 0.0001 |
| Dead | 166 | 56 (10.57%) | 110 (20.75%) | ||
| PTCD1 level (mean ± SD) | 0.98 ± 0.35 | 0.73 ± 0.14 | 1.23 ± 0.33 | < 0.0001 | |
SD, standard deviation. p < 0.05 are shown in bold
Fig. 3.
The stratified analysis of OS based on clinicopathological characteristics including age (a, b), gender (c, d), grade (e, f), stage (g, h), T stage (i, j) and M stage (k, l), which showed PTCD1 expression had a stable prognosis on ccRCC, respectively (all p < 0.05)
Nomogram construction and evaluation
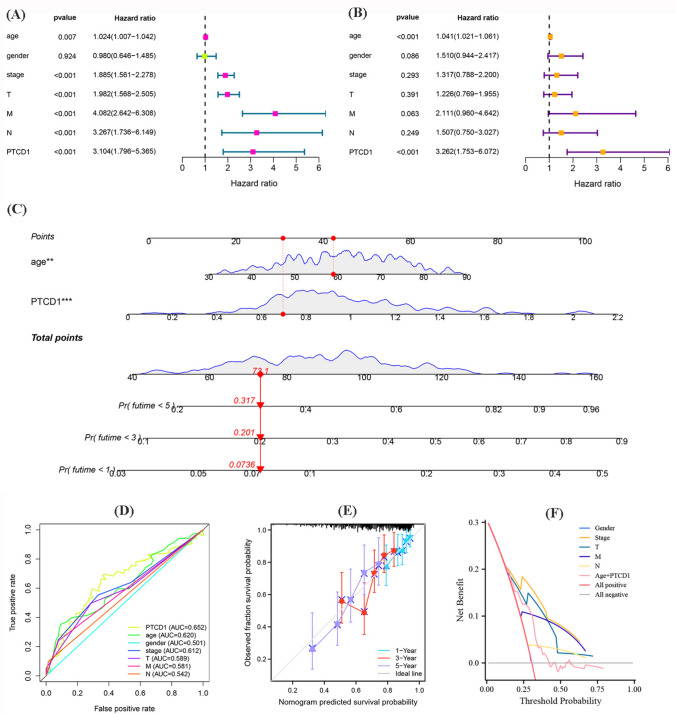
The univariate and multivariable Cox regression confirmed that PTCD1 expression and age were potential independent risk factors for ccRCC cases (Fig. 4a, b). PTCD1 transcription and age at diagnosis were used to develop a nomogram in R using the “rms” package to estimate for 1-, 3-, and 5-year OS of ccRCC cases (Fig. 4c). The ROC curve confirmed that PTCD1 expression was more accurate than some other clinical factors (Fig. 4d). The C-index = 0.654 and 1-, 3-, and 5-year curves demonstrated the reliability and accuracy of predictive nomogram (Fig. 4e). Using decision curve analysis revealed that nomogram was more accurate than other clinical factors in evaluating the prognosis of ccRCC (Fig. 4f).
Fig. 4.
a Univariate Cox regression analysis; b Multivariate Cox regression analysis; c Nomogram for predicting probability of patients with 1-, 3-, and 5-year OS; d The ROC of PTCD1 expression and clinicopathological characteristics; e Actual and predicted survivals by the calibration curves; f Decision curve analysis of nomogram and other clinical indicators
Investigation of functionality enriched between low- and high-PTCD1
The most common GO keywords were “neutrophil activation involved in immune response”, “RNA splicing”, “proteasomal protein catabolic process”, “cell-substrate junction” and “focal adhesion” (Fig. 5a). Additionally, KEGG pathways analysis indicated that DEGs were particularly linked to PI3K-Akt signaling, focal adhesion, PD-L1 expression, and PD-1 checkpoint pathway in cancer (Fig. 5b). The circle graph also displayed the top 15 significant GO and KEGG terms, as well as the corresponding genetic enrichment (Fig. 5c, d).
Fig. 5.
Functional enrichment analysis between low-PTCD1 group and high-PTCD1 group in TCGA dataset; a Enriched biological process, cellular component, and molecular function; b Enriched Kyoto Encyclopedia of Genes and Genomes pathways; c, d The circle plot of the top 15 most relevant terms in GO analysis and KEGG analysis
WGCNA and selection of module
To screen the modules linked with PTCD1, co-expression analysis was implemented to generate gene co-expression networks using DEGs datasets. To cluster tumor samples from the DEGs datasets, Pearson’s correlation and average linkage techniques were applied (Fig. 6a). There weren't any suspicious specimens found or discarded. Optimal = 12 (scale-free R2 = 0.9) was selected to guarantee that scale-free nets were constructed inside the DEGs dataset. With a threshold of 0.25 and a minimum module number of 30, seven modules (Fig. 6b) were retained for subsequent analysis. The scatterplot would be used to evaluate potential correlations among modules and traits, and it revealed that the pink component (cor = 0.62, p = 3.2e−67) was strongly linked with expression levels of PTCD1 (Fig. 6c).
Fig. 6.
Identification of the module related with PTCD1 in DEGs dataset. a Clustering dendrograms of samples as well as traits; b Cluster dendrogram of co-expression network modules based on the 1-TOM matrix; c High-PTCD1 expression was closely related to the pink module; d Co-expression network of PTCD1 in the pink module; e Dot heatmap of gene correlation in co-expression network
Generation of a co-expression connection and correlation
Researchers selected DEGs in the pink module that were closely related with PTCD1 to build a co-expression network using Cytoscape 3.6.0 software (Fig. 6d). The dot heatmap displayed its power of each gene's communication (Fig. 6e), revealing that PTCD1 had a favorable co-expression connection with MT-ND2 (r = 0.335), MT-CO1 (r = 0.316), MT-CO2 (r = 0.404), MT-CYB (r = 0.366), MT-ATP6 (r = 0.353), and MT-ND4 (r = 0.378), and a negative co-expression partnership with IARS (r = −0.316) and LRPPRC (r = −0.443) (all p < 0.001; Fig. 7a–h).
Fig. 7.
The association of PTCD1 with top eight core genes including IARS2 (a), LRPPRC (b), MT-ND2 (c), MT-CO1 (d), MT-CO2 (e), MT-CYB (f), MT-ATP6 (g) and MT-ND4 (h)
Potential association of PTCD1 transcription with immune infiltration
We collected the results of 530 ccRCC patients computed using various algorithms and analyzed all immune cell subtypes, which revealed that the percentage of partial infiltrating cell subtypes differed significantly between the two groups (Fig. 8). The ssGSEA analysis found that NK CD56 bright cell and Treg had a higher enrichment score in the high-PTCD1 group, while T helper cells, eosinophils, Th17 cells, macrophages, Th2 cells and neutrophils had a lower proportion in the high-PTCD1 group (all p < 0.05; Fig. 9a–h).
Fig. 8.
Using different algorithms to calculate immune cell infiltration in 530 ccRCC cases, demonstrating that the proportion of immune cell infiltration was significantly different between the two groups
Fig. 9.
The enrichment scores of eosinophils (a), macrophages (b), neutrophils (c), NK CD56 bright cell (d), T helper cells (e), Th17 cells (f), Th2 cells (g), Treg (h) between high-PTCD1 group and low-PTCD1 group; The immune checkpoint (i), EMT-related genes (j) of the high- and low-PTCD1 group for ccRCC patients in the TCGA cohorts. *p < 0.05; **p < 0.01; ***p < 0.001
The relationship of PTCD1 expression, immune checkpoints and EMT-related genes
Researchers explored the level of immune checkpoints-related genes transcription and identified that some markers (CTLA4, LAG3, CD27, TNFRSF18, CD244, TMIGD2, LAIR1, TIGIT, TNFRSF14, TNFRSF9, PDCD1, IDO2, TNFSF9, LGALS9, CD70, ADORA2A, BTNL2, TNFRSF25, TNFSF14, and TNFRSF8) were elevated in the high-PTCD1 group, and some biomarkers (NRP1, KIR3DL1, PDCD1LG2, IDO1, TNFSF18, and CD274) were negatively regulated in the high-PTCD1 group, indicating the existence of immunological and fatigued phenotype (Fig. 9i). The expressions of EMT-related genes in two groups were also analyzed and identified ENO2, LGALS1, VEGFA in the low-PTCD1 group was upregulated, and SFRP1, LOX, BDNF were downregulated, indicating some genetic epigenetic changes in the high-PTCD1 group (Fig. 9j, all p < 0.05). Based on these results, we observed that individuals in 2 groups exhibited dramatically distinct patterns of immune infiltration and EMT characteristics, which could result in varied prognosis.
Prediction of anti-tumor drug sensitivity and immunotherapy response
The results indicated that the high-PTCD1 group was more sensitive to GSK690693, Sorafenib, Cisplatin and Axitinib, as shown in Fig. 9a–d. Moreover, the low-PTCD1 group was more sensitive to Saracatinib, Pazopanib, Sunitinib and Rapamycin, as shown in Fig. 10e–h. The TCIA dataset was utilized to construct the IPS of ccRCC cases, which was a strong predictor of responsiveness to anti-CTLA-4 and anti-PD-1. The results identified that individuals in the high-PTCD1 group had a substantially lower reaction than patients in the low-PTCD1 group for CTLA-4 positive or both negative, which strongly indicated that patients with high-PTCD1 level would have a poorer immunological reaction (Fig. 10i, j). These findings will help us choose specific drugs based on anti-tumor drug sensitivity.
Fig. 10.
Analysis of anti-tumor drug sensitivity (a–h) and immunotherapy response (i, j) between high- and low-PTCD1 groups; (k) Normal tissues and Tumor tissues (l) on the protein levels of PTCD1 from HPA database
Immunohistochemistry
The Human Protein Atlas website (https://www.proteinatlas.org) provided IHC staining data, which was a collection focused on proteome, transcriptome, and systems biology that could map tumors, cells, and tissues. HPA achieved IHC staining of PTCD1 in cancer tissues and normal tissues and discovered that its protein levels of PTCD1 of ccRCC cases were considerably higher than normal tissues (Fig. 10k, l).
Upregulation of PTCD1 promoted the proliferation, migration and invasion of ccRCC cells
We transfected the PTCD1 vector into 786o and ACHN cells, which showed a relatively low expression of PTCD1, to upregulate PTCD1 expression (Fig. 11a, b). We found that upregulation of PTCD1 markedly increased the proliferation and viability of 786o and ACHN cells (Fig. 11c–f). Transwell invasion assay revealed that upregulation of PTCD1 also promoted the invasive ability of 786o and ACHN cells (Fig. 11g, h). Wound-healing assays showed that PTCD1 overexpression significantly increased 786o and ACHN cell migrations, compared to the negative control groups (Fig. 11i, j). Colony formation assays showed that upregulation of PTCD1 distinctly generated more colonies compared with the negative control cells (Fig. 11k, l). Thus, PTCD1 accelerated the proliferation, migration and invasion of ccRCC cells.
Fig. 11.
Overexpression of PTCD1 promoted the proliferation, viability, migration and invasion of ccRCC cells in vitro. Expression levels of PTCD1 mRNA in HK-2 and four ccRCC cell lines were detected by qRT-PCR (a). qRT-PCR revealed that PTCD1 was markedly upregulated by pLV-PTCD1 in the 786o and ACHN cells (b). Upregulation of PTCD1 markedly promoted the proliferation (c, d) and viability (e, f) of 786o and ACHN cells; Transwell invasion assays accessing the invasive ability of 786o and ACHN cells (g, h); Wound-healing assays accessing the invasive ability of 786o and ACHN cells (i, j); Colony formation assays showed the more colonies in pLV-PTCD1 cells than LV-NC cells (k, l); Upregulation of PTCD1 increased the level of β‐catenin, and contributed the phosphorylation of AKT at Ser473, GSK-3β at Ser9 (M). *p < 0.05; **p < 0.01; ***p < 0.001
PTCD1 was a positive regulator of the Akt/GSK‐3β/β-catenin pathway
To gain insight into the PTCD1-mediated effects in ccRCC, based on the TCGA cohort-associated pathway of enrichment, we assessed the role of PTCD1 in regulating Akt/GSK‐3β/β-catenin pathway in 786o and ACHN cell lines (Fig. 11m). The results identified that upregulation of PTCD1 strikingly increased the level of β‐catenin, which was reported that it can regulate TCF/LEF-mediated transcriptional activity. Upregulation of PTCD1 also increased the phosphorylation of AKT at Ser473 and GSK-3β at Ser9, indicating that PTCD1 promoted the activation of AKT/GSK-3β, which has been proved that it contributed significantly to tumor initiation and progression in ccRCC. Collectively, our findings implied that PTCD1 promotes the Wnt/β-catenin pathway by regulating AKT/GSK-3β.
Discussion
Unlike other PPR domain proteins, PTCD1, a mitochondrial matrix protein with eight PPR domains, is a low abundance protein linked with leucine tRNA and leucine tRNA-containing precursor RNAs [10, 11]. PTCD1 has been demonstrated to bind and deplete leucine mitochondrial tRNA, culminating in the suppression of mitochondrially encoded protein translation, and these anomalies can contribute to a variety of illnesses, including metabolic disorders, overweight, and cancers [6, 8, 10, 12–14]. Nonetheless, a function of PTCD1 overexpression in the prediction and diagnostic for individuals with ccRCC was unknown.
Initially, we estimated the PTCD1 transcriptional level in ccRCC using the TCGA database. Subsequently, we investigated the connection between PTCD1 transcriptional levels in ccRCC and clinicopathological characteristics. These findings demonstrated that the expression level of PTCD1 in ccRCC samples was greater than that in normal tissues, and it steadily rose with the progress of stage, grade, T stage, N stage, and M stage. Kaplan–Meier curves indicated that PTCD1 overexpression was associated with poor outcomes of ccRCC patients, that has been expressed in the latter clinical characteristics of tumor psychopathic stage, histopathologic grade, and metastasis to lymph nodes.
Multivariate analysis confirmed that elevated PTCD1 expression might be an independent prognostic index for OS in patients with ccRCC. These findings implied that PTCD1 played a tumor-promoting function in ccRCC. Throughout this study, we observed that TNM stage, which represented growth, invasion, and metastasis, had a significant influence on the expression of PTCD1. Combined with subsequent functional analysis, PTCD1 may impact tumor growth and distant metastasis of tumor cells through regulating the immune microenvironment. Considering the basic characteristics of ccRCC patients, a majority of those in the high-PTCD1 cohort were high-grade malignancies, which might be a contributing factor in predicting malignancy grade. In the experimental validation section, qRT-PCR identified that PTCD1 was ubiquitously highly expressed in ccRCC cell lines. Upregulation of PTCD1 in 786o and ACHN significantly enhanced the proliferation, survival, invasion, migration abilities of tumor cells, which also demonstrated the cancer promoting effect of PTCD1 in ccRCC.
To investigate the mechanism of PTCD1 in ccRCC deeper, functional annotation was performed by using enrichment analysis. Using the GO analysis, PTCD1-related DEGs were enriched in multiple terms including “neutrophil activation involved in immune response”, “RNA splicing”, “cell-substrate junction” and “focal adhesion”. Furthermore, KEGG pathway analysis reported that PTCD1-related DEGs were notably associated with “PI3K-Akt signaling pathway”, “focal adhesion”, “PD-L1 expression and PD-1 checkpoint pathway in cancer”. A growing number of studies indicated that the PI3K pathway was altered in tumorigenesis that played a crucial role in cancer oncogenesis [15–19]. The PI3K/Akt signaling pathway, which integrated a series of external signals to regulate downstream genes associated with cell growth, differentiation and angiogenesis, promoted ccRCC cell growth proliferation, migrantion, and invasion, ultimately leading to ccRCC tumorigenesis, metastatic spread, and therapeutic resistance [20–27]. Additionally, we proved the mechanism of PTCD1 in regulating the biological behavior of cancer cells (786o and ACHN). The results demonstrated that upregulation of PTCD1 significantly increased the level of β-catenin, and increased the phosphorylation of Akt at ser 473 and GSK3β at ser 9, suggesting that PTCD1 promoted the activation of Akt/GSK3β. Collectively, PTCD1 regulated Wnt/β-catenin by promotion of Akt/GSK-3β pathway, which in turn played a promoting role for ccRCC.
Tumor immune cells infiltration was critical for the prognosis of ccRCC and interacted with the responsiveness to immunotherapy [28–31]. Therefore, infiltration rates of immune cells with low- and high-PTCD1 expression were investigated subsequently, suggesting that PTCD1 expression was closely associated with immune infiltrations, especially for NK cells, Treg, T helper cells, eosinophils, Th17 cells, macrophages, Th2 cells and neutrophils. To have a better knowledge of the immunological landscape of ccRCC, we assessed the expression of immune checkpoints between the two groups, reporting that immune checkpoints were revealed to be significantly and heterogeneously expressed in the ccRCC immune microenvironment. Taken together, we believed that PTCD1 may influence the development and progression of ccRCC via immune cells. Moreover, IC50 analysis indicated that the high-PTCD1 group was more susceptible to Axetinib, Cisplatin, Sorafenib, and GSK690693. The low-PTCD1 group was more sensitive to Rapamycin, Saracatinib, Pazopanib, and Sunitinib. Patients with high-PTCD1 group were strongly significantly lower responses than that in the low-PTCD1 group for CTLA-4 positive or both negative, which powerfully predicted that patients with high-PTCD1 had a worse immunotherapy response. Summarizing the above results, PTCD1 expression can assess the prognosis, immune status, immunotherapy response and drug sensitivity of ccRCC cases.
The alteration of EMT-related genes served a position in the control of cancer incidence and progression by regulating the transcription of important genes, and has lately been a focus of research [32]. And previous studies have shown that abnormal expression of EMT-related genes may affect the biological behavior of tumor cells [33–35]. The differential expression of six EMT controllers identified in this study were highly dependent on PTCD1 transcriptional level of ccRCC. Downregulation of PTCD1 for ccRCC was probable to result in aberrant modulation of EMT-related regulators, although additional research was needed to confirm the assumption.
Similarly, there were some limitations in this work. Firstly, because of the retrospective data and the limits of clinical information from the TCGA, more long-term survival results of clinical samples were necessary to confirm these results. Second, the levels of PTCD1 protein expression in tumor tissues should be investigated further. Third, knockdown of PTCD1 was required in our experimental section for reverse validation. Finally, more research was needed to investigate the connections between PTCD1 overexpression and immune infiltration by basic experimental validation.
Conclusions
Elevated expression of PTCD1 was associated with malignant biological behaviors and poor outcomes of ccRCC patients, and PTCD1 may accelerate tumor cells proliferation and invasion via the Akt/GSK3β/β-catenin pathway. Our findings indicated that PTCD1 had the potential to become a new target for predicting prognosis and targeted therapy.
Acknowledgements
The authors have no acknowledgments to declare.
Abbreviations
- PTCD1
Pentatricopeptide repeat domain 1
- ccRCC
Clear cell renal cell carcinoma
- TCGA
The Cancer Genome Atlas
- WGCNA
Weighted Gene Coexpression Network Analysis
- OS
Overall survival
- PPR
Pentatricopeptide repeat
- LRPPRC
Leucine-rich pentatricopeptide repeat motif-containing protein
- GSEA
Gene Set Enrichment Analysis
- DEGs
Differential expression genes
- FDR
False discovery rate
- GO
Gene Ontology
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- CC
Cellular component
- MF
Molecular function
- EMT
Epithelial-to-mesenchymal transition
- m6A
N6-methyladenosine
Author contributions
All authors constructed this study. Z.Z.B., L.Y.L., C.Y.M. performed the data analysis, figures plotted, and writing. Y.P. and Z.Y. were responsible for the critical reading of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Beijing Municipal Administration of Hospitals’ Ascent Plan, Code: DFL20190502; Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support, Code: ZYLX201820.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
HPA database and TCGA database belong to public databases. The patients involved in the database have obtained ethical approval.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhongbao Zhou, Yulong Li and Yumeng Chai contributed equally to this study as co-first authors.
Contributor Information
Yong Zhang, Email: doctorzhangyong2@163.com.
Pu Yan, Email: yanpu2024@163.com.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. [DOI] [PubMed]
- 2.Ljungberg B, Albiges L, Abu-Ghanem Y, Bedke J, Capitanio U, Dabestani S, Fernández-Pello S, Giles RH, Hofmann F, Hora M, et al. European Association of Urology Guidelines on renal cell carcinoma: the 2022 update. Eur Urol. 2022;82:399–410. [DOI] [PubMed]
- 3.Creighton CJ. Proteomic signatures of clear cell renal cell carcinoma. Nat Rev Nephrol. 2020;16(3):133–4. [DOI] [PubMed] [Google Scholar]
- 4.Campi R, Stewart GD, Staehler M, Dabestani S, Kuczyk MA, Shuch BM, Finelli A, Bex A, Ljungberg B, Capitanio U. Novel liquid biomarkers and innovative imaging for kidney cancer diagnosis: what can be implemented in our practice today? A systematic review of the literature. Eur Urol Oncol. 2021;4(1):22–41. [DOI] [PubMed] [Google Scholar]
- 5.Lightowlers RN, Chrzanowska-Lightowlers ZM. Human pentatricopeptide proteins: only a few and what do they do? RNA Biol. 2013;10(9):1433–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perks KL, Ferreira N, Richman TR, Ermer JA, Kuznetsova I, Shearwood AJ, Lee RG, Viola HM, Johnstone VPA, Matthews V, et al. Adult-onset obesity is triggered by impaired mitochondrial gene expression. Sci Adv. 2017;3(8): e1700677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei WS, Wang N, Deng MH, Dong P, Liu JY, Xiang Z, Li XD, Li ZY, Liu ZH, Peng YL, et al. LRPPRC regulates redox homeostasis via the circANKHD1/FOXM1 axis to enhance bladder urothelial carcinoma tumorigenesis. Redox Biol. 2021;48: 102201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Black M, Arumugam P, Shukla S, Pradhan A, Ustiyan V, Milewski D, Kalinichenko VV, Kalin TV. FOXM1 nuclear transcription factor translocates into mitochondria and inhibits oxidative phosphorylation. Mol Biol Cell. 2020;31(13):1411–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Z, Zhou Y, Zhou X, Huang Y, Cui Y, Zhang Y. Downregulation of PTCD1 in bladder urothelial carcinoma predicts poor prognosis and levels of immune infiltration. J Oncol. 2022;2022:1146186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rackham O, Davies SM, Shearwood AM, Hamilton KL, Whelan J, Filipovska A. Pentatricopeptide repeat domain protein 1 lowers the levels of mitochondrial leucine tRNAs in cells. Nucleic Acids Res. 2009;37(17):5859–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perks KL, Rossetti G, Kuznetsova I, Hughes LA, Ermer JA, Ferreira N, Busch JD, Rudler DL, Spahr H, Schöndorf T, et al. PTCD1 is required for 16S rRNA maturation complex stability and mitochondrial ribosome assembly. Cell Rep. 2018;23(1):127–42. [DOI] [PubMed] [Google Scholar]
- 12.Schild C, Hahn D, Schaller A, Jackson CB, Rothen-Rutishauser B, Mirkovitch J, Nuoffer JM. Mitochondrial leucine tRNA level and PTCD1 are regulated in response to leucine starvation. Amino Acids. 2014;46(7):1775–83. [DOI] [PubMed] [Google Scholar]
- 13.Fleck D, Phu L, Verschueren E, Hinkle T, Reichelt M, Bhangale T, Haley B, Wang Y, Graham R, Kirkpatrick DS, et al. PTCD1 is required for mitochondrial oxidative-phosphorylation: possible genetic association with Alzheimer’s Disease. J Neurosci. 2019;39(24):4636–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pa J, Andrews SJ, Swerdlow RH. Mitochondria and Alzheimer’s: is PTCD1 the smoking gun? Trends Neurosci. 2019;42(11):759–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang D, Yang L, Cai J, Li H, Xing Z, Hou Y. Phosphoinositide 3-kinase/Akt and its related signaling pathways in the regulation of tumor-associated macrophages polarization. Mol Cell Biochem. 2022;477(10):2469–80. [DOI] [PubMed]
- 16.Zhang D, Deng T, Yuan W, Chen T, Jiang S. Glaucocalyxin A induces apoptosis of NSCLC cells by inhibiting the PI3K/Akt/GSK3β pathway. Clin Exp Pharmacol Physiol. 2022. [DOI] [PubMed]
- 17.Deng S, Leong HC, Datta A, Gopal V, Kumar AP, Yap CT. PI3K/AKT signaling tips the balance of cytoskeletal forces for cancer progression. Cancers. 2022;14(7):1652. [DOI] [PMC free article] [PubMed]
- 18.Braglia L, Zavatti M, Vinceti M, Martelli AM, Marmiroli S. Deregulated PTEN/PI3K/AKT/mTOR signaling in prostate cancer: still a potential druggable target? Biochim Biophys Acta. 2020;1867(9): 118731. [DOI] [PubMed] [Google Scholar]
- 19.Wang L, Song Y, Wang H, Liu K, Shao Z, Shang Z. MiR-210-3p-EphrinA3-PI3K/AKT axis regulates the progression of oral cancer. J Cell Mol Med. 2020;24(7):4011–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu M, Guo Q, Liu X, Wu L. SPRR3, a novel miR-338-3p target, regulates the malignant progression of clear cell renal cell carcinoma in vitro via the PI3K/Akt signaling pathway. Exp Ther Med. 2022;23(5):317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding XF, Chen J, Ma HL, Liang Y, Wang YF, Zhang HT, Li X, Chen G. KIR2DL4 promotes the proliferation of RCC cell associated with PI3K/Akt signaling activation. Life Sci. 2022;293: 120320. [DOI] [PubMed] [Google Scholar]
- 22.Gu W, Gong L, Wu X, Yao X. Hypoxic TAM-derived exosomal miR-155-5p promotes RCC progression through HuR-dependent IGF1R/AKT/PI3K pathway. Cell Death Discov. 2021;7(1):147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang A, Zeng P, Li Y, Lu W, Lai Y. LY294002 is a promising inhibitor to overcome Sorafenib resistance in FLT3-ITD mutant AML cells by interfering With PI3K/Akt signaling pathway. Front Oncol. 2021;11: 782065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miricescu D, Balan DG, Tulin A, Stiru O, Vacaroiu IA, Mihai DA, Popa CC, Papacocea RI, Enyedi M, Sorin NA, et al. PI3K/AKT/mTOR signalling pathway involvement in renal cell carcinoma pathogenesis (Review). Exp Ther Med. 2021;21(5):540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tumkur Sitaram R, Landström M, Roos G, Ljungberg B. Significance of PI3K signalling pathway in clear cell renal cell carcinoma in relation to VHL and HIF status. J Clin Pathol. 2021;74(4):216–22. [DOI] [PubMed] [Google Scholar]
- 26.Guo H, German P, Bai S, Barnes S, Guo W, Qi X, Lou H, Liang J, Jonasch E, Mills GB, et al. The PI3K/AKT pathway and renal cell carcinoma. J Genet Genomics. 2015;42(7):343–353. [DOI] [PMC free article] [PubMed]
- 27.Sourbier C, Lindner V, Lang H, Agouni A, Schordan E, Danilin S, Rothhut S, Jacqmin D, Helwig JJ, Massfelder T. The phosphoinositide 3-kinase/Akt pathway: a new target in human renal cell carcinoma therapy. Can Res. 2006;66(10):5130–42. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Liu S, Chen Y, Zhu B, Xing Q. survival prognosis, tumor immune landscape, and immune responses of PPP1R18 in kidney renal clear cell carcinoma and its potentially double mechanisms. World J Oncol. 2022;13(1):27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee MH, Järvinen P, Nísen H, Brück O, Ilander M, Uski I, Theodoropoulos J, Kankainen M, Mirtti T, Mustjoki S, et al. T and NK cell abundance defines two distinct subgroups of renal cell carcinoma. Oncoimmunology. 2022;11(1):1993042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Au L, Hatipoglu E, Robert de Massy M, Litchfield K, Beattie G, Rowan A, Schnidrig D, Thompson R, Byrne F, Horswell S, et al. Determinants of anti-PD-1 response and resistance in clear cell renal cell carcinoma. Cancer Cell. 2021;39(11):1497–518.e1411. [DOI] [PMC free article] [PubMed]
- 31.Matsuki M, Hirohashi Y, Nakatsugawa M, Murai A, Kubo T, Hashimoto S, Tokita S, Murata K, Kanaseki T, Tsukahara T, et al. Tumor-infiltrating CD8(+) T cells recognize a heterogeneously expressed functional neoantigen in clear cell renal cell carcinoma. Cancer Immunol Immunother. 2022;71(4):905–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greaves D, Calle Y. Epithelial mesenchymal transition (EMT) and associated invasive adhesions in solid and haematological tumours. Cells. 2022;11(4):649. [DOI] [PMC free article] [PubMed]
- 33.Han X, Piao L, Yuan X, Wang L, Liu Z, He X. Knockdown of NSD2 suppresses renal cell carcinoma metastasis by inhibiting epithelial-mesenchymal transition. Int J Med Sci. 2019;16(10):1404–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou WM, Wu GL, Huang J, Li JG, Hao C, He QM, Chen XD, Wang GX, Tu XH. Low expression of PDK1 inhibits renal cell carcinoma cell proliferation, migration, invasion and epithelial mesenchymal transition through inhibition of the PI3K-PDK1-Akt pathway. Cell Signal. 2019;56:1–14. [DOI] [PubMed] [Google Scholar]
- 35.Liu W, Li H, Wang Y, Zhao X, Guo Y, Jin J, Chi R. MiR-30b-5p functions as a tumor suppressor in cell proliferation, metastasis and epithelial-to-mesenchymal transition by targeting G-protein subunit α-13 in renal cell carcinoma. Gene. 2017;626:275–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.