Abstract
Background
Tumour deposits are a prognostic factor for overall survival and distant metastasis in lymph node-negative colorectal cancer. However, the current TNM staging system does not account for the presence of tumour deposits in lymph node-positive colorectal cancer, or for the presence of multiple deposits. This study aimed to investigate the prognostic effect of tumour deposit count in patients with colorectal cancer.
Methods
Patients who underwent curative surgery for colorectal cancer between 2016 and 2019 were identified nationwide from the Swedish Colorectal Cancer Registry. Patients with undisclosed tumour deposit status/count and stage IV disease were excluded. Univariable and multivariable Cox regression analyses were used to assess the prognostic effect of tumour deposit count on overall survival and distant metastasis adjusted for age, sex, neoadjuvant treatment, and number of positive lymph nodes.
Results
Of 18 913 patients assessed, 14 154 patients were analysed with tumour deposits (TDs) present in 1702 (12%) patients. Patients were stratified by tumour deposit count (0, 1, 2, 3, 4, and ≥5 TDs). Increased tumour deposit count was associated with decreased 5-year overall survival (79%, 70%, 61%, 66%, 50%, 49%) and increased 5-year risk for distant metastasis (14%, 26%, 35%, 41%, 48%, 54%) respectively. Tumour deposit count remained an independent negative prognostic factor after multivariable Cox regression analysis.
Conclusion
Tumour deposit count is a negative prognostic predictor of both overall survival and distant metastasis in colorectal cancer, independent of positive lymph nodes or neoadjuvant treatment. These findings suggest that tumour deposit count should be integrated into the TNM staging regardless of lymph nodes status to improve prognostic accuracy.
This study investigates the prognostic significance of multiple tumour deposits (TDs) in CRC using population-based data from the Swedish Colorectal Cancer Registry. Our findings demonstrate that TD count is a prognostic marker for overall survival and distant metastasis, independent of neoadjuvant treatment and number of positive lymph nodes.
Introduction
Colorectal cancer (CRC) is the third most common malignancy worldwide and is staged according to the TNM staging system, which is considered the single most important prognostic factor for overall survival, distant metastasis, and local recurrence1,2. Despite recent diagnostic and therapeutic advances, the 5-year mortality for CRC remains at 65%, and more accurate prognostic and therapeutic tools are called for3,4.
Tumour deposits are discrete, discontinuous tumour nodules within the colorectal mesentery without any identifiable lymphatic, vascular, or neural structures2,5. Tumour deposits are observed in roughly 15–25% of CRC, and multiple studies have shown a strong correlation with impaired survival and increased risk of distant metastasis in colon and rectal cancer cohorts5–11. Tumour deposits often exist synchronously with positive lymph nodes and other negative prognostic histopathological findings such as high tumour grade, perineural growth, and extramural vascular invasion (EMVI)12,13
With the introduction of the N1c-stage in the seventh TNM edition, the presence of tumour deposits was assigned prognostic value in lymph node-negative CRC2. However, the current TNM staging system does not account for the presence of tumour deposits in lymph node-positive CRC, or the presence of multiple deposits2. Consequently, important clinical information may be overlooked when determining treatment for patients with multiple tumour deposits and/or synchronous positive lymph nodes14,15.
The aim of this study was to evaluate if tumour deposit count is a negative prognostic factor for overall survival, distant metastasis, or local recurrence in a national cohort of patients treated for CRC. We hypothesized that an increased tumour deposit count would negatively impact long-term oncological outcomes.
Methods
Swedish Colorectal Cancer Registry
The Swedish Colorectal Cancer Registry is a national database that includes 99% of patients with CRC in Sweden and has a high level of data ascertainment16. The data set includes over 800 variables with validation of the registry routinely performed17. Recurrence data are collected continuously and in conjunction with standardized follow-up at three and five years.
Study population
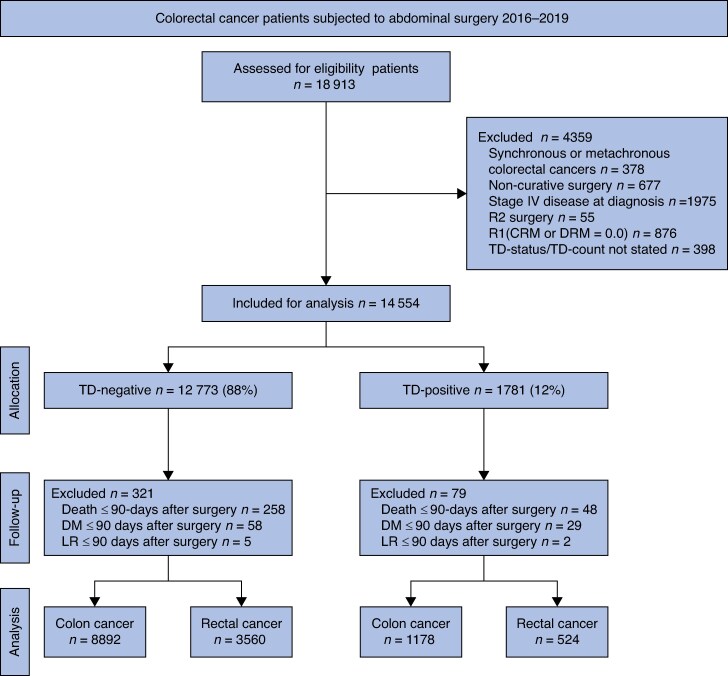
Data from the registry were extracted for patients who underwent resectional surgery for a solitary colon or rectal cancer with curative intent between January 2016 and December 2019. Survival data were collected from the Swedish Cause of Death Register on 23 January 2024. A flowchart of the inclusion and exclusion process is presented in Fig. 1. Early study outcomes (i.e. mortality or disease progression) within 90 days of surgery were excluded as they were believed to represent cases of postoperative mortality secondary to complications, undiagnosed metastases present at the time of surgery, or palliative surgical resection. After exclusions, patients were stratified by tumour deposit (TD) count (0, 1, 2, 3, 4, and ≥5 TDs).
Fig. 1.
Study flowchart
TD, tumour deposit; CRM, circumferential resection margin; DRM, distal resection margin; DM, distant metastasis; LR, local recurrence
Tumour deposits were defined according to the seventh or eighth edition of the TNM staging system depending on the year of diagnosis2,5. For the seventh TNM edition, tumour deposits were defined as a foci of tumour cells discontinuous from the primary tumour located within the lymphatic drainage of the pericolic or perirectal fat tissue without evidence of lymphatic tissue5. In the eighth edition, tumour deposits should, in addition, not contain any neural or vascular tissue2. A detailed description of the variables used in the study is presented in Supplementary Methods. All histopathological specimens were examined by a gastrointestinal pathologist as part of the clinical routine.
Primary study outcomes
The primary study outcomes were overall survival, distant metastasis, and local recurrence. Overall survival was defined as the time from primary surgery to death from any cause. Distant metastasis was defined as recurrence of a tumour in the peritoneum or mesenteric lymph nodes remote from the original tumour as documented by clinical, radiological, or histopathological examination. Local recurrence was defined as the recurrence of local extraperitoneal tumour, lymph node tumour growth, intraluminal tumour recurrence, or peritoneal tumour growth below the peritoneal reflection, as documented by clinical, radiological, or histopathological examination. Distant metastasis and local recurrence-free survival was defined as the time from surgery to either the last follow-up, or the occurrence of the outcome event.
Statistical analysis
Categorical variables were presented as numbers and proportions in percentages. Numerical data were reported as median with interquartile range. Fisher’s exact test with Monte Carlo was used for intergroup comparisons where appropriate.
Kaplan–Meier curves were used to determine the 5-year cumulative overall survival, as well as the 5-year cumulative risk of distant metastasis and local recurrence, with differences investigated using the log-rank test. Uni- and multivariable Cox regression analyses were performed for relative survival, overall survival, distant metastasis, and local recurrence. Confounding variables for multivariable analysis were identified prior to data analysis with the aid of directed acyclic graphs (Fig. S1) and limited by the number of outcomes. Adjustments were performed for age, sex, neoadjuvant treatment, and positive lymph nodes. Relative survival was based on overall survival, and additionally adjusted for death of any cause in an age-matched group of citizens using data from the Human Mortality Database (Sweden, life table for men and female by age and year in 1-year intervals).
Sensitivity and subgroup analysis
Descriptive and Kaplan–Meier survival analyses of the effect of tumour deposit count on the primary outcomes based on tumour location (colon or rectal) were performed. For the combined CRC cohort, Cox regression sensitivity analysis was performed with additional adjustment for clinical stage, pathological stage, tumour grade, perineural growth, vascular/lymphatic invasion, EMVI, and the year of diagnosis. To enable sensitivity analysis, multivariable imputation of the EMVI variable was performed on patients operated in 2016 due to an update of the registry in 2017, where the histopathological variable ‘vascular/lymphatic invasion’ was subdivided into ‘vascular invasion’, ‘lymphatic invasion’, and ‘EMVI’. The imputation was based on sex, age, tumour location, clinical stage, neoadjuvant treatment, year of surgery, pathological stage, number of positive lymph nodes, tumour deposit count, perineural growth, tumour grade, vascular/lymphatic infiltration, vascular invasion, lymphatic invasion, EMVI, local recurrence, time to local recurrence, distant metastasis, time to distant metastasis, length of time in follow-up, and mortality rate. All available data from the data set prior to exclusion were included for the imputation process. Twenty imputations were performed using a seed value of 20 240 525.
All statistical analyses were performed using R version 4.2.118 with RStudio19. Kaplan–Meier curves and Cox regressions were created using the survival package20 and visualizations were generated using the ggplot2 package21. Imputation was performed using the mice package22 and relative survival was calculated using the relsurv package23. P < 0.05 was considered statistically significant.
Missing data
Patients with unspecified status due to emigration were censored for relative survival and overall survival in Kaplan–Meier and Cox regression analysis at the day of emigration. Patients with unspecified follow-up status due to missing data were censored for distant metastasis and local recurrence for Kaplan–Meier and Cox regression analysis at day zero.
Ethical considerations
This study was approved by the Swedish Ethical Review Authority (DNR: 2023-02433-02) and the registry steering committee. Contribution to the registry is voluntary with the possibility to delete personal health data at any time. Registration in the Cause of Death Register is mandatory for all citizens by law and does not require consent.
Results
Study population
Between January 2016 and December 2019, 18 913 patients from the registry database fulfilled the inclusion criteria and were evaluated for eligibility (Fig. 1). After exclusions, 14 154 (75%) patients remained, among whom 1702 (12%) had evidence of tumour deposits.
Baseline characteristics of tumour deposit-negative and tumour deposit-positive CRC patients are summarized in Table 1. Only 495 (29%) tumour deposit-positive patients were classified as N1c. While both TD-positive and TD-negative tumors were more commonly left-sided, the proportion of right-sided tumors was higher among TD-positive cases compared to TD-negative cases. These patients also tended to have higher clinical stage and were more likely to have undergone neoadjuvant therapy.
Table 1.
Patient and preoperative characteristics for tumour deposit-negative and tumour deposit-positive patients
| Total (n = 14 154) | Tumour deposit-negative (n = 12 452) | Tumour deposit-positive (n = 1702) | |
|---|---|---|---|
| Sex | |||
| Male | 7358 (52.0) | 6452 (51.8) | 906 (53.2) |
| Age | |||
| Years | 73 (65–79) | 73 (66–80) | 71 (64–78) |
| BMI | |||
| kg/m2 | 26 (23–29) | 26 (23–29) | 26 (23–29) |
| Tumour location | |||
| Colon | 10 070 (71.1) | 8892 (71.4) | 1178 (69.2) |
| Left colon | 6409/10 070 (63.6) | 5763/8892 (64.8) | 646/1178 (54.8) |
| Right colon | 3643/10 070 (36.2) | 3112/8892 (35.0) | 531/1178 (45.1) |
| Rectum | 4084 (28.9) | 3560 (28.6) | 524 (30.8) |
| ASA score | |||
| ASA 1–2 | 9013 (63.7) | 7877 (63.3) | 1136 (66.7) |
| ASA 3 | 4438 (31.4) | 3948 (31.7) | 490 (28.8) |
| ASA 4 | 349 (2.5) | 308 (2.5) | 41 (2.4) |
| ASA 5 | 9 (0.1) | 8 (0.1) | 1 (0.1) |
| Missing | 345 (2.4) | 311 (2.5) | 34 (2.0) |
| Time to surgery | |||
| Days | 35 (24–55) | 35 (24–55) | 35 (24–55) |
| cT stage | |||
| T1–T2 | 4140 (29.2) | 3872 (31.1) | 268 (15.7) |
| T3 | 5989 (42.3) | 5119 (41.1) | 870 (51.1) |
| T4 | 1917 (13.5) | 1584 (12.7) | 333 (19.6) |
| TX | 1871 (13.2) | 1671 (13.4) | 200 (11.8) |
| Missing | 237 (1.7) | 206 (1.7) | 31 (1.8) |
| cN stage | |||
| N0 | 7333 (51.8) | 6775 (54.4) | 558 (32.8) |
| N1-2 | 5780 (40.8) | 4794 (38.5) | 986 (57.9) |
| NX | 847 (6.0) | 717 (5.8) | 130 (7.6) |
| Missing | 194 (1.4) | 166 (1.3) | 28 (1.6) |
| cStage | |||
| Stage I | 3317 (23.4) | 3129 (25.1) | 188 (11.0) |
| Stage II | 3025 (21.4) | 2722 (21.9) | 303 (17.8) |
| Stage III | 5812 (41.1) | 4815 (38.7) | 997 (58.6) |
| Missing | 2000 (14.1) | 1786 (14.3) | 214 (12.6) |
| Discussed at a preoperative multidisciplinary conference | |||
| Yes | 12 942 (91.4) | 11 440 (91.9) | 1502 (88.2) |
| Neoadjuvant therapy | |||
| Yes | 2645 (18.7) | 2236 (18.0) | 409 (24.0) |
Continuous values are presented as median (i.q.r.). Categorical values are presented as frequency (%). c = clinical.
Histopathological characteristics of tumour deposit-negative and tumour deposit-positive CRC patients are presented in Table 2. Tumour deposit-positive patients more often exhibited synchronous negative prognostic histopathological findings, such as circumferential resection margin involvement, lymphovascular invasion, EMVI, perineural growth, and high tumour grade. Subsequently, tumour deposit-positive patients more often received adjuvant therapy compared to tumour deposit-negative patients (56% versus 21% respectively). The median (i.q.r.) follow-up was slightly longer for tumour deposit-negative patients (63 (51–71) months) compared to tumour deposit-positive patients (58 (37–75) months).
Table 2.
Postoperative histopathology for tumour deposit-negative and tumour deposit-positive patients
| Total (n = 14 154) | Tumour deposit-negative (n = 12 452) | Tumour deposit-positive (n = 1702) | |
|---|---|---|---|
| pT stage | |||
| pT0 | 46 (0.3) | 45 (0.4) | 1 (0.1) |
| pT1 | 1155 (8.2) | 1138 (9.1) | 17 (1.0) |
| pT2 | 3034 (21.4) | 2933 (23.6) | 101 (5.9) |
| pT3 | 7706 (54.4) | 6674 (53.6) | 1032 (60.6) |
| pT4 | 2212 (15.6) | 1661 (13.3) | 551 (32.4) |
| Missing | 1 (0.0) | 1 (0.0) | 0 (0) |
| pN stage | |||
| N0 | 8947 (63.2) | 8947 (71.9) | 0 (0) |
| N1a | 1682 (11.9) | 1408 (11.3) | 274 (16.1) |
| N1b | 1503 (10.6) | 1138 (9.1) | 365 (21.4) |
| N1c | 495 (3.5) | 0 (0) | 495 (29.1) |
| N2a | 865 (6.1) | 566 (4.5) | 299 (17.6) |
| N2b | 536 (3.8) | 277 (2.2) | 259 (15.2) |
| NX | 126 (0.9) | 116 (0.9) | 10 (0.6) |
| pStage | |||
| Stage I | 3463 (24.5) | 3463 (27.8) | 0 (0) |
| Stage II | 5542 (39.2.) | 5542 (44.5) | 0 (0) |
| Stage III | 5144 (36.3) | 3442 (27.6) | 1702 (100) |
| Positive lymph nodes | |||
| Number of lymph nodes | 0 (0–1) | 0 (0–1) | 2 (0–4) |
| Less than 12 examined lymph nodes | |||
| Yes | 897 (6.3) | 801 (6.4) | 96 (5.6) |
| Tumour deposits | |||
| None | 12 452 (88.0) | 12 452 (100) | 0 (0) |
| 1 | 845 (6.0) | 0 (0) | 845 (49.6) |
| 2 | 380 (2.7) | 0 (0) | 380 (22.3) |
| 3 | 178 (1.3) | 0 (0) | 178 (10.5) |
| 4 | 101 (0.7) | 0 (0) | 101 (5.9) |
| ≥5 | 198 (1.4) | 0 (0) | 198 (11.6) |
| CRM (mm) | |||
| 0.1–1.0 mm | 566 (4.0) | 397 (3.2) | 169 (9.9) |
| > 1.0 mm | 12 884 (91.0) | 11 414 (91.7) | 1470 (86.4) |
| Missing* | 704 (5.0) | 641 (5.1) | 63 (3.7) |
| Lymphovascular invasion | |||
| Yes | 4142 (29.3) | 3112 (25.0) | 1030 (60.5) |
| EMVI | |||
| Yes | 1685 (11.9) | 1149 (9.2) | 536 (31.5) |
| Missing† | 1182 (8.4) | 890 (7.1) | 292 (17.2) |
| Perineural growth | |||
| Yes | 2407 (17.0) | 1686 (13.5) | 721 (42.4) |
| Tumour grade | |||
| High-grade | 2584 (18.3) | 2172 (17.4) | 412 (24.2) |
| Adjuvant therapy | |||
| Yes | 3571 (25.2) | 2618 (21.0) | 953 (56.0) |
Continuous values are presented as median (i.q.r.). Categorical values are presented as frequency (%). c = clinical. p = histopathological. *Negative circumferential resection margin (CRM) but missing distance measurement. †Based on patient from 2016 with positive ‘lymphovascular invasion’ or patients from any year with unknown ‘lymphovascular invasion’ status.
Overall survival, distant metastasis, and local recurrence
The overall survival, distant metastasis, and local recurrence rates based on tumour deposit count by tumour location (colon or rectum) are presented in Table 3. Colon cancer patients generally had lower overall survival compared to rectal cancer patients, although they less frequently experienced distant metastasis or local recurrence. Multiple tumour deposits were consistently associated with a higher risk for mortality, distant metatasis, and local recurrence in both colon and rectal cancers compared to a single tumour deposit (P < 0.001).
Table 3.
The effect of tumour deposit count on overall survival, distant metastasis, and local recurrence for colon and rectal cancer
| Colon cancer | 0 (n = 8892) | 1 (n = 583) | 2 (n = 271) | 3 (n = 121) | 4 (n = 65) | ≥5 (n = 138) | Total (n = 10 070) |
|---|---|---|---|---|---|---|---|
| Mortality | |||||||
| Alive at follow-up | 6454 (72.6) | 387 (66.4) | 160 (59.0) | 76 (62.8) | 28 (43.1) | 58 (42.0) | 7163 (71.1) |
| Deceased | 2410 (27.1) | 194 (33.3) | 111 (41.0) | 45 (37.2) | 37 (56.9) | 79 (57.2) | 2876 (28.6) |
| Emigrated | 28 (0.3) | 2 (0.3) | 0 (0) | 0 (0) | 0 (0) | 1 (0.7) | 31 (0.3) |
| Distant metastasis | |||||||
| Yes | 771 (8.7) | 120 (20.6) | 76 (28.0) | 45 (37.2) | 24 (36.9) | 63 (45.7) | 1099 (10.9) |
| Local recurrence | |||||||
| Yes | 163 (1.8) | 24 (4.1) | 15 (5.5) | 11 (9.1) | 3 (4.6) | 8 (5.8) | 224 (2.2) |
| Rectal cancer | 0 (n = 3560) | 1 (n = 262) | 2 (n = 109) | 3 (n = 57) | 4 (n = 36) | ≥5 (n = 60) | Total (n = 4084) |
|---|---|---|---|---|---|---|---|
| Mortality | |||||||
| Alive at follow-up | 2874 (80.7) | 172 (65.6) | 63 (57.8) | 36 (63.2) | 16 (44.4) | 28 (46.7) | 3189 (78.1) |
| Deceased | 678 (19.0) | 90 (34.4) | 46 (42.2) | 21 (36.8) | 20 (55.6) | 32 (53.3) | 887 (21.7) |
| Emigrated | 8 (0.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 8 (0.2) |
| Distant metastasis | |||||||
| Yes | 431 (12.1) | 76 (29.0) | 39 (35.8) | 20 (35.1) | 17 (47.2) | 30 (50.0) | 613 (15.0) |
| Local recurrence | |||||||
| Yes | 100 (2.8) | 11 (4.2) | 11 (10.1) | 5 (8.8) | 2 (5.6) | 8 (13.3) | 137 (3.4) |
Categorical values are presented as frequency (%).
Survival analysis
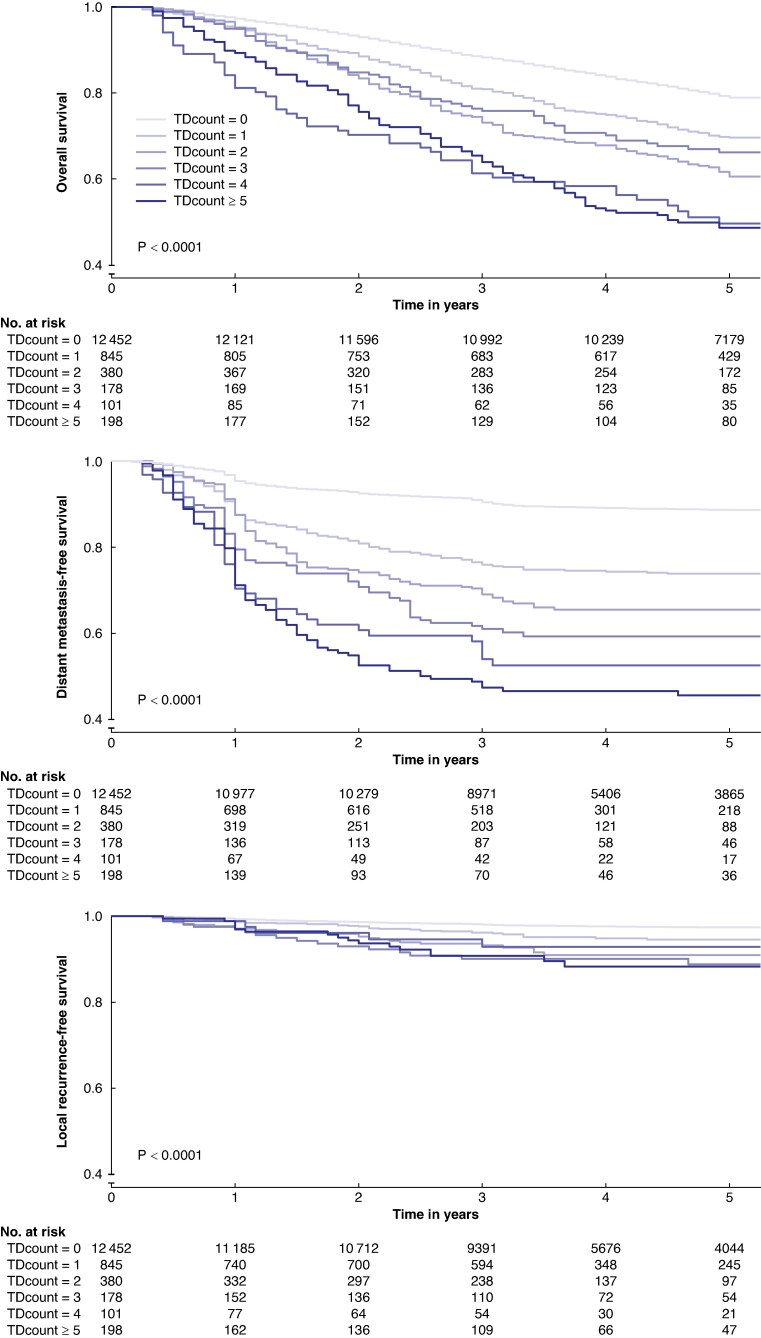
Figure 2 illustrates the association between the tumour deposit count and 5-year overall survival, as well as the 5-year risk of distant metastasis and local recurrence. For 5-year overall survival, the risk of death escalated with an increasing tumour deposit count, ranging from 21% (no tumour deposits) to 51% (≥5 tumour deposits). Similarly, there was a linear stepwise increase in the 5-year risk of distant metastasis, ranging from 11% (no tumour deposits) to 54% (≥5 tumour deposits). For local recurrence the disparity was smaller, ranging from 3% (no tumour deposits) to 12% (≥5 tumour deposits). Subgroup analysis of colon and rectal cancer patients revealed similar incidence in outcomes between groups (Fig. S2).
Fig. 2.
Kaplan–Meier curves of 5-year overall survival, distant metastasis-free survival, and local recurrence-free survival based on tumour deposit count (0, 1, 2, 3, 4 and ≥5) for patients with colorectal cancer
Note that the y-axis is offset at 40%
Table 4 presents uni- and multivariable Cox regression analyses for relative survival, overall survival, distant metastasis, and local recurrence based on tumour deposit count. In the univariable Cox regression analysis, the presence of a single tumour deposit was associated with reduced relative and overall survival, along with increased risk of distant metastasis and local recurrence. Multiple tumour deposits significantly reduced relative and overall survival, and increased distant metastasis and local recurrence compared to both the absence of tumour deposits and a singular tumour deposit. These associations persisted, albeit partially attenuated, in the adjusted Cox regression analysis.
Table 4.
Hazard ratio of the unadjusted and adjusted effect of tumour deposit count on relative survival, overall survival, distant metastasis and local recurrence
| Relative survival | Overall survival | Distant metastasis | Local recurrence | |
|---|---|---|---|---|
| TD count* (n = 14 154) | EHR | HR | HR | HR |
| 0 | 1 | 1 | 1 | 1 |
| 1 | 1.6 (1.5–1.9) | 1.5 (1.3–1.7) | 2.6 (2.3–3.1) | 2.0 (1.4–2.9) |
| 2 | 2.4 (2.1–2.9) | 1.9 (1.7–2.3) | 3.6 (3.0–4.3) | 3.5 (2.4–5.3) |
| 3 | 2.5 (1.9–3.2) | 1.7 (1.3–2.1) | 4.6 (3.5–5.8) | 4.7 (2.8–7.7) |
| 4 | 4.2 (3.2–5.4) | 3.2 (2.5–4.2) | 6.0 (4.4–8.2) | 3.0 (1.3–7.3) |
| ≥5 | 3.8 (3.1–4.6) | 3.0 (2.5–3.6) | 7.0 (5.7–8.6) | 4.6 (2.8–7.6) |
| TD count† (n = 14 040) | EHR | HR | HR | HR |
| 0 | 1 | 1 | 1 | 1 |
| 1 | 1.5 (1.3–1.6) | 1.4 (1.3–1.6) | 2.4 (2.1–2.8) | 1.9 (1.3–2.7) |
| 2 | 1.9 (1.6–2.3) | 1.9 (1.6–2.2) | 3.0 (2.5–3.7) | 3.1 (2.1–4.6) |
| 3 | 1.8 (1.4–2.3) | 1.8 (1.4–2.3) | 3.6 (2.8–4.6) | 3.8 (2.3–6.4) |
| 4 | 3.1 (2.4–4.9) | 3.1 (2.3–4.0) | 4.6 (3.4–6.3) | 2.4 (1.0–5.8) |
| ≥5 | 2.5 (2.1–3.0) | 2.4 (2.0–2.9) | 4.4 (3.6–5.5) | 3.3 (2.0–5.5) |
TD = tumour deposit. EHR = excess hazard ratio. All data are presented as (excessive) HR (95% c.i.).
*Unadjusted.
†Adjusted for age, sex, number of positive lymph nodes and neoadjuvant treatment.
Multivariable Cox regression sensitivity analysis of the effect of tumour deposit count is presented in Table S3. All regressions, except for relative survival, were based on pooled data from the imputation analysis. In the sensitivity analysis, tumour deposit count remained significantly associated with worse hazard ratios for relative survival, overall survival, and distant metastasis, but not for local recurrence.
Discussion
The present study is the first study to show a strong negative association between tumour deposit count and long-term oncological outcomes, independent of lymph node status. These findings advocate for considering both the presence of tumour deposits in lymph node-positive CRC, and the occurrence of multiple tumour deposits in CRC staging protocols.
This study contained a large sample of patients, of which 12% had tumour deposits. This is slightly less than the reported prevalence of 20%, which may be reflective of the stricter definition implemented in TNM86. Nearly all patients were discussed at a preoperative multidisciplinary conference. Only 29% of patients with tumour deposits were staged as N1c, emphasizing the effect of omitting tumour deposits in lymph node-positive patients.
A stepwise negative association between tumour deposit count and relative survival, overall survival, distant metastasis, and local recurrence in univariable and adjusted models was observed. Tumour deposit counts were divided into groups (0, 1, 2, 3, 4, and ≥5) to intuitively depict how a stepwise increase in tumour deposit count affects the risk of adverse outcomes. Although the relationship between tumour deposit count and the risk of local recurrence was not as prominent as for the other outcomes, these findings indicate that multiple tumour deposits are associated with worse prognosis for all investigated oncological outcomes. Additionally, the presence of tumour deposits emerged as a negative prognostic factor even in the presence of positive lymph nodes.
The importance of tumour deposits has been demonstrated both in colon and rectal cancer cohorts8,9. In this study, we found that tumour deposit count is prognostic even after adjusting for age, sex, neoadjuvant treatment, and number of positive lymph nodes. When accounting for other histopathological high-risk factors in the sensitivity analysis, the hazard ratio for all outcomes was notably reduced but remained significant for relative survival, overall survival, and distant metastasis, but not for local reccurence. This suggests that tumour deposits may not directly influence the risk of local recurrence but are instead indirectly linked with local recurrence by their association with other adverse histopathological risk factors, which is in line with earlier studies and the proposed metastatic pathway for tumour deposits8,10,24,25. However, this observation may also be attributed to the limited number of local recurrences, which restricts statistical strength. Notably, 80% of all recurrences occur within 3 years of curative surgery, indicating that the follow-up period should not have been a limiting factor26. Instead, the low number of local recurrences might be reflective of the effectiveness of current treatments in reducing the risk of local recurrence, supported by the consistently low rates across all investigated groups. This highlights the need to focus on pathways for distant metastasis to improve outcomes. Tumour deposit count was also shown to have equivalent associated effects on colon and rectal cancer patients. CRC was previously seen as a homogenous group, but recent research has challenged this view and called for separate analysis27. This study shows that not only presence of tumour deposits but the number is important in both colon and rectal cancer cohorts, expanding on results from earlier studies8,9.
The Swedish Colorectal Cancer Registry is a comprehensive database but faces challenges with missing data. Some gaps arise from updates in the variable list, with older, broader variables being replaced by more precise subclassifications. In the current data set, subclassification of lymphatic/vascular infiltration, such as EMVI, was missing for patients operated on in 2016. To address this, imputation was performed in consultation with a professional statistician, ensuring rigorous statistical integrity. Other missing unregistered data, such as smoking status, family history, and red meat consumption, can introduce unobserved confounders. User errors, like misreporting weight and height, also contributed to missing data. In this study clear errors were corrected and unclear errors excluded. Lastly, as histopathological data from the registry were retrieved from all hospitals in Sweden, re-review by a dedicated pathologist was not possible.
This study spans four years (2016–2019), during which time there was a transition from the seventh to the eighth edition of the TNM staging system, as well as changes in CRC treatment protocols. To mitigate potential bias stemming from more effective staging or treatments, the year of surgery was included in the sensitivity analysis. Finally, as this study included patients up until 2019, 5-year follow-up was not feasible for all patients at the time of data extraction, resulting in a significant number of censored patients in the fourth and fifth year in the Kaplan–Meier curves, potentially limiting long-term insights.
The current TNM staging system does not incorporate valuable prognostic information related to tumour deposits. This gap is consistent with prior research and underscores a growing call for a revised staging system that integrates these data to enhance prognostic accuracy and better guide adjuvant treatment decisions6–8,15,28–30. Efforts to stratify tumour deposit counts into distinct prognostic categories have shown potential to refine CRC staging, but a consensus on optimal stratification has yet to be reached31–37. Discrepancies between studies may stem from limitations in data sets, variations in outcomes studied, or inconsistencies in diagnostics.
The present study utilizes histologically diagnosed tumour deposits as a prognostic factor, and therefore any impact on oncological outcomes may have already occurred by the time of surgery. Recent studies have explored the potential of preoperative radiological diagnosis of tumour deposits with promising results38–40. Early detection of tumour deposits through imaging could deepen our understanding of their role in metastasis, potentially enabling personalized treatment.
Although preoperative detection of tumour deposits holds promise for improving prognostic assessment, it may not yet translate into better patient outcomes within the framework of currently available oncological treatments. The high incidence of adverse outcomes observed in this study suggests that existing treatments are insufficient to mitigate the risks associated with tumour deposits. Effective treatment strategies remain challenging, partly due to the unknown aetiology of tumour deposits which limits both diagnostic and therapeutic options41. Genetic and epigenetic research could help unravel the origins of tumour deposits and identify novel oncological targets, potentially improving outcomes for this patient group.
This study demonstrated that tumour deposit count is a negative prognostic factor for overall survival and distant metastasis in CRC, independent of the number of positive lymph nodes. These findings challenge the omission of tumour deposits in lymph node-positive CRC in the current TNM system, and advocate for integration into future staging systems.
Supplementary Material
Acknowledgements
Anna Åkesson, statistician at Clinical Studies Sweden—Forum Söder, Skåne University Hospital, for assistance with statistical analyses.
Contributor Information
Simon Lundström, Department of Surgery, Skåne University Hospital, Malmö, Sweden; Department of Clinical Sciences, Lund University, Lund, Sweden.
Erik Agger, Department of Surgery, Skåne University Hospital, Malmö, Sweden; Department of Clinical Sciences, Lund University, Lund, Sweden.
Marie-Louise Lydrup, Department of Surgery, Skåne University Hospital, Malmö, Sweden; Department of Clinical Sciences, Lund University, Lund, Sweden.
Fredrik Jörgren, Department of Clinical Sciences, Lund University, Lund, Sweden; Department of Surgery, Helsingborg Hospital, Lund University, Helsingborg, Sweden.
Pamela Buchwald, Department of Surgery, Skåne University Hospital, Malmö, Sweden; Department of Clinical Sciences, Lund University, Lund, Sweden.
Funding
The study was financed by grants from the Swedish state under the agreement between the Swedish government and the county councils (the ALF agreement), from the Swedish Research Council and from the Swedish Cancer Society.
Disclosure
The authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS online.
Data availability
All data used are available through the Swedish Colorectal Cancer Registry.
Ethics approval
The study was approved by the Swedish Ethical Review Authority (DNR: 2023-02433-02)
Previous communication
Abstract presented at the Swedish Surgical Week 2024 in Karlstad, Sweden (19–23 August 2024) and at the European Society of Coloproctology’s 19th Scientific and Annual Conference in Thessaloniki, Greece (25–27 September 2024).
Author contributions
Simon Lundström (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Visualization, Writing—original draft, Writing—review & editing), Erik Agger (Conceptualization, Investigation, Methodology, Supervision, Writing—review & editing), Marie-Louise Lydrup (Conceptualization, Methodology, Supervision, Writing—review & editing), Fredrik Jörgren (Conceptualization, Investigation, Methodology, Supervision, Writing—original draft), and Pamela Buchwald (Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing—review & editing)
References
- 1. Eileen M, Melina A, Gini A, Lorenzoni V, Cabasag CJ, Mathieu L et al. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut 2023;72:338–344 [DOI] [PubMed] [Google Scholar]
- 2. Brierley J GM, Wittekind C. TNM Classification of Malignant Tumours (8th edn). Chichester: Wiley-Blackwell, 2017 [Google Scholar]
- 3. Jiang Y, Yuan H, Li Z, Ji X, Shen Q, Tuo J et al. Global pattern and trends of colorectal cancer survival: a systematic review of population-based registration data. Cancer Biol Med 2021;19:175–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hemminki K, Försti A, Hemminki A. Survival in colon and rectal cancers in Finland and Sweden through 50 years. BMJ Open Gastroenterol 2021;8:e000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours (7th edn). Chichester: Wiley-Blackwell, 2009 [Google Scholar]
- 6. Ueno H, Nagtegaal ID, Quirke P, Sugihara K, Ajioka Y. Tumor deposits in colorectal cancer: refining their definition in the TNM system. Ann Gastroenterol Surg 2023;7:225–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nagtegaal ID, Knijn N, Hugen N, Marshall HC, Sugihara K, Tot T et al. Tumor deposits in colorectal cancer: improving the value of modern staging—a systematic review and meta-analysis. J Clin Oncol 2017;35:1119–1127 [DOI] [PubMed] [Google Scholar]
- 8. Jörgren F, Agger E, Lydrup M-L, Buchwald P. Tumour deposits in colon cancer predict recurrence and reduced survival in a nationwide population-based study. BJS Open 2023;7:zrad122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Agger E, Jörgren F, Jöud A, Lydrup M-L, Buchwald P. Negative prognostic impact of tumor deposits in rectal cancer—a national study cohort. Ann Surg 2022;278:e526–e533 [DOI] [PubMed] [Google Scholar]
- 10. Lundström S, Agger E, Lydrup ML, Jörgren F, Buchwald P. Adverse impact of tumor deposits in lymph node negative rectal cancer—a national cohort study. Int J Colorectal Dis 2023;38:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Doroudian S, Osterman E, Glimelius B. Risk factors for recurrence after surgery for rectal cancer in a modern, nationwide population-based cohort. Ann Surg Oncol 2024;31:5570–5584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nagtegaal ID, Quirke P. Colorectal tumour deposits in the mesorectum and pericolon; a critical review. Histopathology 2007;51:141–149 [DOI] [PubMed] [Google Scholar]
- 13. Betge J, Pollheimer MJ, Lindtner RA, Kornprat P, Schlemmer A, Rehak P et al. Intramural and extramural vascular invasion in colorectal cancer. Cancer 2012;118:628–638 [DOI] [PubMed] [Google Scholar]
- 14. Lino-Silva LS, Xinaxtle DL, Salcedo-Hernández RA. Tumor deposits in colorectal cancer: the need for a new “pN” category. Ann Transl Med 2020;8:733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu W, Zeng S, Zhang X, Liu P, Qiu T, Li S et al. The value of tumor deposits in evaluating colorectal cancer survival and metastasis: a population-based retrospective cohort study. World J Surg Oncol 2022;20:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moberger P, Sköldberg F, Birgisson H. Evaluation of the Swedish Colorectal Cancer Registry: an overview of completeness, timeliness, comparability and validity. Acta Oncologica 2018;57:1611–1621 [DOI] [PubMed] [Google Scholar]
- 17. Osterman E, Hammarström K, Imam I, Osterlund E, Sjöblom T, Glimelius B. Completeness and accuracy of the registration of recurrences in the Swedish Colorectal Cancer Registry (SCRCR) and an update of recurrence risk in colon cancer. Acta Oncologica 2021;60:842–849 [DOI] [PubMed] [Google Scholar]
- 18. R Core Team . R Core: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2023. https://www.R-project.org/ [Google Scholar]
- 19. RStudio Team . RStudio: Integrated Development for R. Boston, MA: RStudio, PBC, 2020. http://www.rstudio.com/ [Google Scholar]
- 20. Therneau T. A Package for Survival Analysis in R. R package version 3.7-0, 2022. https://CRAN.R-project.org/package=survival [Google Scholar]
- 21. Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlag, 2016. https://ggplot2.tidyverse.org [Google Scholar]
- 22. van Buuren S, Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Softw 2011;45:1–67 [Google Scholar]
- 23. Perme MP, Pavlič K. Nonparametric relative survival analysis with the R package relsurv. J Stat Softw 2018;87:1–27 [Google Scholar]
- 24. Lord AC, Knijn N, Brown G, Nagtegaal ID. Pathways of spread in rectal cancer: a reappraisal of the true routes to distant metastatic disease. Eur J Cancer 2020;128:1–6 [DOI] [PubMed] [Google Scholar]
- 25. Brouwer NPM, Oguz Erdogan AS, van Vliet S, Rutgers N, Knijn N, van Lijnschoten G et al. Unraveling the routes to distant metastases in colorectal cancer: tumor deposits and lymph node metastases as the gateway. Cancer Communications 2024;44:1209–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ryu HS, Kim J, Park YR, Cho EH, Choo JM, Kim JS et al. Recurrence patterns and risk factors after curative resection for colorectal cancer: insights for postoperative surveillance strategies. Cancers (Basel) 2023;15:5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tamas K, Walenkamp AM, de Vries EG, van Vugt MA, Beets-Tan RG, van Etten B et al. Rectal and colon cancer: not just a different anatomic site. Cancer Treat Rev 2015;41:671–679 [DOI] [PubMed] [Google Scholar]
- 28. Long Q, Xu Y, Ma G, Mao W. Prognostic value of tumor deposit counts in patients with stage III colorectal cancer: a population-based study. J Invest Surg 2022;35:1502–1509 [DOI] [PubMed] [Google Scholar]
- 29. Delattre J-F, Selcen Oguz Erdogan A, Cohen R, Shi Q, Emile J-F, Taieb J et al. A comprehensive overview of tumour deposits in colorectal cancer: towards a next TNM classification. Cancer Treat Rev 2022;103:102325. [DOI] [PubMed] [Google Scholar]
- 30. Moon JY, Lee MR, Ha GW. Prognostic value of tumor deposits for long-term oncologic outcomes in patients with stage III colorectal cancer: a systematic review and meta-analysis. Int J Colorectal Dis 2022;37:141–151 [DOI] [PubMed] [Google Scholar]
- 31. Pricolo VE, Steingrimsson J, McDuffie TJ, McHale JM, McMillen B, Shparber M. Tumor deposits in stage III colon cancer: correlation with other histopathologic variables, prognostic value, and risk stratification-time to consider “N2c”. Am J Clin Oncol 2020;43:133–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wu WX, Zhang DK, Chen SX, Hou ZY, Sun BL, Yao L et al. Prognostic impact of tumor deposits on overall survival in colorectal cancer: based on surveillance, epidemiology, and end results database. World J Gastrointest Oncol 2022;14:1699–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang S, Guan X, Ma M, Zhuang M, Ma T, Liu Z et al. Reconsidering the prognostic significance of tumour deposit count in the TNM staging system for colorectal cancer. Sci Rep 2020;10:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pei JP, Zhang CD, Fu X, Ba Y, Yue S, Zhao ZM et al. A modified tumor-node-metastasis classification for stage III colorectal cancers based on treating tumor deposits as positive lymph nodes. Front Med (Lausanne) 2020;7:571154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Karbanowicz E, Fuchs TL, Chou A, Sioson L, Sheen A, Ahadi MS et al. What are the problems with the current staging of discontinuous tumour nodules (DTNs) in colorectal carcinoma? Is there a better way? Pathology 2022;54:848–854 [DOI] [PubMed] [Google Scholar]
- 36. Pei J-P, Zhang C-D, Liang Y, Zhang C, Wu K-Z, Li Y-Z et al. A modified pathological N stage including status of tumor deposits in colorectal cancer with nodal metastasis. Front Oncol 2020;10:548692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu F, Zhao J, Li C, Wu Y, Song W, Guo T et al. The unique prognostic characteristics of tumor deposits in colorectal cancer patients. Ann Transl Med 2019;7:769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. D'Souza N, Shaw A, Lord A, Balyasnikova S, Abulafi M, Tekkis P et al. Assessment of a staging system for sigmoid colon cancer based on tumor deposits and extramural venous invasion on computed tomography. JAMA Netw Open 2019;2:e1916987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lord AC, Moran B, Abulafi M, Rasheed S, Nagtegaal ID, Terlizzo M et al. Can extranodal tumour deposits be diagnosed on MRI? Protocol for a multicentre clinical trial (the COMET trial). BMJ Open 2020;10:e033395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lord AC, D’Souza N, Shaw A, Rokan Z, Moran B, Abulafi M et al. MRI-diagnosed tumor deposits and EMVI status have superior prognostic accuracy to current clinical TNM staging in rectal cancer. Ann Surg 2022;276:334–344 [DOI] [PubMed] [Google Scholar]
- 41. Lord A, Brown G, Abulafi M, Bateman A, Frankel W, Goldin R et al. Histopathological diagnosis of tumour deposits in colorectal cancer: a Delphi consensus study. Histopathology 2021;79:168–175 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used are available through the Swedish Colorectal Cancer Registry.