Abstract
The pituitary gland is a key endocrine gland with various physiological functions including metabolism, growth, and reproduction. It comprises several distinct cell populations that release multiple polypeptide hormones. Although the major endocrine cell types are conserved across taxa, the regulatory mechanisms of gene expression and chromatin organization in specific cell types remain poorly understood. Here, we performed simultaneous profiling of the transcriptome and chromatin landscapes in the pituitary cells of the three-spined stickleback (Gasterosteus aculeatus), which represents a good model for investigating the genetic mechanisms underlying adaptive evolution. We obtained pairwise gene expression and chromatin profiles for 5184 cells under short- and long-day conditions. Using three independent clustering analyses, we identified 16 distinct cell clusters and validated their consistency. These results advance our understanding of the regulatory dynamics in the pituitary gland and provide a reference for future research on comparative physiology and evolutionary biology.
Subject terms: Physiology, Genetics
Background & Summary
As a crucial endocrine gland, the pituitary gland releases multiple hormones that control various physiological processes, such as growth, metabolism, reproduction, migration, osmoregulation, and stress responses in vertebrates. These hormones are synthesized in distinct cell populations and are regulated by both internal and external stimuli. The dynamic regulation of hormone production and chromatin organization in specific endocrine cell types in the pituitary gland has not been well characterized, especially in teleost species1.
Recent advances in next-generation sequencing (NGS), including bulk RNA sequencing (RNA-seq), have substantially enhanced our understanding of gene expression dynamics in the pituitary gland across taxa2–5. However, bulk RNA-seq can capture only a mixture of gene expression profiles from tissues or cell populations6. Particularly in the pituitary gland, which shows notable heterogeneity1,7, gene expression in rare cell populations or specific cell types may have been overlooked in the analysis. Recently, single-cell RNA-seq (scRNA-seq) has been developed to distinguish individual cells within a heterogeneous population of the pituitary gland across species, including humans, mice, chickens, and medaka7–10. These studies identified six conserved hormone cells, including lactotropes, corticotropes, somatotropes, thyrotropes, gonadotropes, melanotropes and somatolactotropes, with variations in gene expression profiles across species11. These intraspecific variations in the gene expression profiles of hormone cells indicate that we need to investigate the cellular variations in the pituitary glands in different species and under different environmental conditions to understand the divergent regulatory mechanisms. Gene expression is generally regulated by transcription factors (TFs) through interactions with regulatory regions located within or near target genes12. These interactions are influenced by epigenetic mechanisms such as chromatin accessibility13–15. However, our understanding of the epigenetic regulatory mechanisms underlying pituitary hormone synthesis remains incomplete. Further investigation of the TF-binding activities in different pituitary cell types will elucidate the regulation of hormone synthesis and help clarify whether different hormone-releasing cells share similar regulatory elements, such as TF-binding motifs. Although the single-cell Assay for Transposase-Accessible Chromatin (ATAC) sequencing technique (scATAC-seq) has recently been used to investigate the epigenetic mechanisms underlying early embryogenesis of zebrafish at the single-cell level16, the single-cell chromatin landscapes of the pituitary gland remain unknown in teleosts.
The three-spined stickleback (Gasterosteus aculeatus) is a good model for investigating the mechanisms of adaptive evolution. This species is primarily marine but has repeatedly colonized and adapted to freshwater environments after glacial retreats17–19. Recent genetic and genomic analyses have revealed the molecular mechanisms underlying the morphological, physiological, and behavioral diversification between marine and freshwater populations20–29. Moreover, different stickleback populations exhibit varied seasonal responses in terms of reproduction, salinity tolerance, migratory behavior, and growth patterns30–32. These variations are likely associated with the diversification in the regulation of pituitary hormones and their related genes31,33. For instance, changes in the expression of genes that control the activity of specific pituitary hormones can affect reproductive timing and success. Additionally, sticklebacks can adjust their osmoregulatory mechanisms to cope with different salinity levels, modulate migratory behavior, and alter growth patterns to optimize energy utilization under different environmental conditions34,35. However, heterogeneity within the pituitary gland presents considerable challenges in understanding the regulatory mechanisms of hormone production underlying these physiological diversifications in sticklebacks.
In the present study, we performed simultaneous profiling of transcriptome and chromatin landscapes in the pituitary gland of three-spined sticklebacks using single-cell multiome (scRNA-seq and scATAC-seq) analyses. Consequently, we generated more than 5000 high-quality profiles of cellular gene expression and chromatin landscapes under different photoperiods. Using three independent clustering analyses, we consistently identified and characterized 16 distinct cell clusters, including red blood cells, gonadotropes, somatotropes, somatolactotropes, lactotropes, thyrotropes, melanotropes, thyroid-stimulating hormone subunit beta 2 (TSHB2)-producing cells, endothelial cells, immune cells, and stem cells. We also performed differentially expressed genes (DEG) analysis and motif enrichment analyses of each cell cluster to further validate the data accuracy. Overall, this study provided a valuable resource for future research in comparative physiology and evolutionary biology.
Materials & Methods
Experimental animals (husbandry and fish population)
Anadromous three-spined stickleback fish were collected using minnow traps in the Bekanbeushi River, Akkeshi, Hokkaido, Japan, as previously described36–38. They were then maintained in the laboratory at 16 °C under long-day conditions (L: D = 16:8) to produce pure crosses using artificial fertilization. After hatching, the juveniles were initially reared for two to three months under long-day conditions, followed by a six to seven month period under short-day conditions (L: D = 8:16) with 10% seawater (salinity = 3.1‰) until the experiment. Sex was determined by PCR to detect sex-specific markers in the leucine-rich repeat containing 61 genes (LRRC61)17. Only males were included in this study. Six individuals were divided into two groups (short- and long-day) in an 8-L tank with 10% seawater at 16 °C under short- or long-day conditions. The long-day group was exposed to long-day conditions for an additional 1–2 days after the dissection of the short-day group. Before dissection, all fish were euthanized with an overdose of ethyl 3-aminobenzoate methanesulfonate (MS-222).
Tissue sampling and cell dissociation
The pituitary cells were dissociated immediately after dissection. Briefly, the pituitary gland was treated with 100 µL of 0.25% trypsin-EDTA (Thermo Fisher Scientific, MA, USA). The samples were gently pipetted for 15 min under a microscope, until no cell aggregates were visible. Subsequently, to inactivate the trypsin, 100 µL of Leibovitz’s L-15 Medium with L-Glutamine, Phenol Red, and Sodium Pyruvate (Wako, Osaka, Japan) supplemented with Fetal Bovine Serum (FBS) in a 9:1 ratio was added. Finally, the dissociated cells were filtered through a 100 µm strainer (Corning, NY, USA) to ensure a homogenous single-cell suspension. The cell numbers were calculated using a Millipore Scepter 2.0 (Merck, Darmstadt, Germany) with 60 µm sensors (PHCC60050). All the cells obtained from each individual (8433–21978 cells) were used for library preparation (Table 1). The cells from each individual were independently processed for library preparation to ensure proper capture of individual variations. Moreover, to preserve cellular integrity and ensure accurate gene expression and chromatin status landscapes, library preparation was performed immediately after cell dissociation, thereby minimizing any possible delay.
Table 1.
The standard length and cell number of each sample. SD: Short-day. LD: Long-day.
| Sample ID | Standard length | Input cell number | Cell number after 1st quality control | Cell number after 2nd quality control |
|---|---|---|---|---|
| SD1 | 35.62 | 15656 | 580 | 573 |
| SD2 | 39.28 | 8433 | 517 | 501 |
| SD3 | 38.2 | 14609 | 1151 | 1093 |
| LD1 | 36.06 | 8939 | 260 | 236 |
| LD2 | 45.23 | 20608 | 1477 | 1448 |
| LD3 | 45.01 | 21978 | 1401 | 1333 |
10x genomics single-cell multiome Library preparation and sequencing
Single-cell multiome libraries were constructed using the Chromium Next GEM Single-Cell Multiome ATAC + Gene Expression Kit (10x Genomics, Pleasanton, CA, USA) according to the manufacturer’s instructions (https://www.10xgenomics.com/support/single-cell-multiome-atac-plus-gene-expression/documentation/steps/library-prep/chromium-next-gem-single-cell-multiome-atac-plus-gene-expression-reagent-kits-user-guide). As the number of cells used for library preparation was less than 40,000, we used the low-cell-input nuclei isolation protocol provided in the Appendix (CG000365 RevB). After library construction, quality control and quantification were conducted using an Agilent Bioanalyzer High-Sensitivity chip (Agilent Technologies, Santa Clara, CA, USA), Agilent TapeStation with High-Sensitivity D1000, and Qubit (Thermo Fisher Scientific). Libraries from different individuals were labeled with different barcode adapters, pooled, and run on one lane of an Illumina HiSeq X with a 150 bp-paired end mode at Macrogen (Seoul, Korea).
Genome and annotation
Raw reads were mapped to the Ensembl stickleback genome assembly BROAD S1 using archived annotation v.106 (https://apr2022.archive.ensembl.org/Gasterosteus_aculeatus/Info/Index). Specifically, to avoid redundant and overlapping annotations, non-protein-coding entries were removed from the annotation file by adding an extra filter argument–attribute = gene_biotype:protein_coding to the mkgtf function from the CellRanger ARC v2.0.239,40. Furthermore, we standardized the gene nomenclature derived from the annotation file by converting all letters to uppercase letters to ensure consistency in spelling (Supplementary Table 1).
Bioinformatics processing
Reads alignment and counting
Raw sequencing data were transformed into FASTA files using 10x Genomics Cell Ranger ARC v2.0.239,40 and then aligned to Ensembl stickleback genome assembly: BROAD S1 with archived annotation v.106 (https://apr2022.archive.ensembl.org/Gasterosteus_aculeatus/Info/Index) using STAR, which is embedded in Cell Ranger ARC to count reads41. Subsequently, the generated feature-barcode matrix for each sample was supplied into R (R version 4.3) packages Seurat v4.3 and Signac v1.1242,43 for downstream analyses.
Quality control (QC)
The joint cell calling process implemented by Cell Ranger ARC performed a preliminary cellular filtration by excluding the barcodes meeting three filtering criteria: (1) the barcodes whose fraction of fragments overlapping called peaks is lower than the fraction of genome in the peak; (2) putative gel bead doublets, where a partition contains one cell and two barcoded gel beads; and (3) the barcodes not being observed in either chromatin accessibility or gene expression libraries. Although preliminary cellular filtration was conducted, this process did not sufficiently exclude all noncellular barcodes. Therefore, we performed an additional QC step using the following criteria: (1) the detected number of genes was > 100 but < 3000; (2) the percentage of mitochondrial reads was < 20%; (3) the nucleosome signal was < 2; and (4) Transcription Starting Site (TSS) enrichment score was > 1.
Peak recalling
To further improve peak accuracy, we recalled peaks in each ATAC assay with an adapted effective genome size of the three-spined stickleback using MACS244.
Object merging
Because the previous peak calling was performed on each dataset independently, we created a unified but non-overlapping set of peaks across all samples using the R package GenomicRanges v1.54.145. The refined peak set with identical chromatin features was then quantified for each sample, and the biological replicates were merged using the standard merge function in Seurat v4.342.
Data Normalization and linear dimensional reduction
For gene expression (GEX) data, the feature expression matrix was normalized using the NormalizeData function with a scale factor of 10000. Subsequently, the top 3000 features that exhibited high cell-to-cell variations in the dataset were identified, followed by a linear transformation of these variable features. Next, we performed principal component analysis on the scaled data using the previously determined variable features as inputs.
For the ATAC-seq data, term frequency-inverse document frequency normalization was implemented to account for variations in sequencing depth across cells and to adjust across peaks to assign greater significance to infrequent peaks46. The low dynamics of single-cell ATAC-seq data pose additional challenges in the identification of variable chromatin features. Therefore, we performed singular value decomposition on the TD-IDF matrix using features with at least one occurrence46.
Data integration
To analyze data from different experimental conditions, we independently performed data integration for the ATAC and GEX assays. For the GEX profiles, we integrated the normalized expression matrix across all samples by identifying integration anchors, which were cross-dataset pairs of cells in a matched biological state. For the ATAC-seq data, we performed a similar identification of integration anchors using reciprocal latent semantic indexing (LSI) projection, during which the first LSI component was ignored because it predominantly captures sequencing depth rather than biological variation. Furthermore, because of the sparsity of chromatin matrices, we integrated low-dimensional cell embeddings (LSI coordinates) across the samples.
Cell clustering and non-linear dimensional reduction
In this study, we used the FindNeighbors function in Seurat to cluster cells based on three strategies: (1) gene expression profiles alone, (2) chromatin profiles alone, and (3) weighted nearest neighbor (WNN) analysis integrating multiple modalities (gene expression and chromatin status). In graph-based clustering based only on gene expression or chromatin profiles, a k-nearest neighbor (KNN) graph was created using Euclidean distance, followed by refinement of edge weights between cell pairs by evaluating the shared commonality of their local neighborhoods. For gene expression-based clustering, the first 20 principal components were supplied while the 2nd to the 50th LSI components were supplied for clustering based on chromatin profiles only. However, in the clustering based on the WNN analysis, a WNN graph representing a weighted combination of GEX and ATAC modalities was calculated. For all three strategies, the cells were grouped using the FindClusters function with an optimized resolution parameter until all expected endocrine cells were present, and visualized via Uniform Manifold Approximation and Projection for Dimension Reduction (UMAP).
Identification of differentially expressed genes (DEGs) and differentially accessible regions (DARs)
Given that cell clustering based on gene expression profiles showed the best separation of distinct cell groups, we used the cell identities assigned by the gene expression profiles to identify DEGs and DARs. The DEGs/DARs analysis was implemented using the FindAllMarkers function (by default settings), only testing features that were detected in a minimum fraction of 1% of cells in either of the cell populations. The photoperiod-responsive DEGs analysis was performed using the FindMarkers function (by default settings), only testing features that were detected in a minimum fraction of 1% of cells in either of the day length conditions. The Wilcoxon rank-sum test was used for the DEG test, whereas a logistic regression framework was adopted for the DAR test.
Motif analysis
For the cell type-specific motif test, position weight matrices of the vertebrate motifs were first extracted from JASPAR 2022 (https://jaspar2022.genereg.net/) and converted to PWMatrixList objects using the R package TFBSTOOLS v1.4.047,48. Motif enrichment analysis was performed to identify the motifs that were overrepresented within the cell type-specific DARs using the FindMotifs function in Signac with default settings. For differential motif analysis between short- and long-day conditions, a per-cell motif activity score was computed using the R package chromVAR v1.24.049, followed by the FinMarkers function in Seurat with default settings.
Ethics
All animal experiments were conducted at the University of Tokyo under the University of Tokyo’s Regulations regarding the Conduct of Animal Experiments, after obtaining approval for the animal experiment plan (B-21-4). Before dissection, all fish were euthanized with an overdose of ethyl 3-aminobenzoate methanesulfonate (MS-222).
Data Records
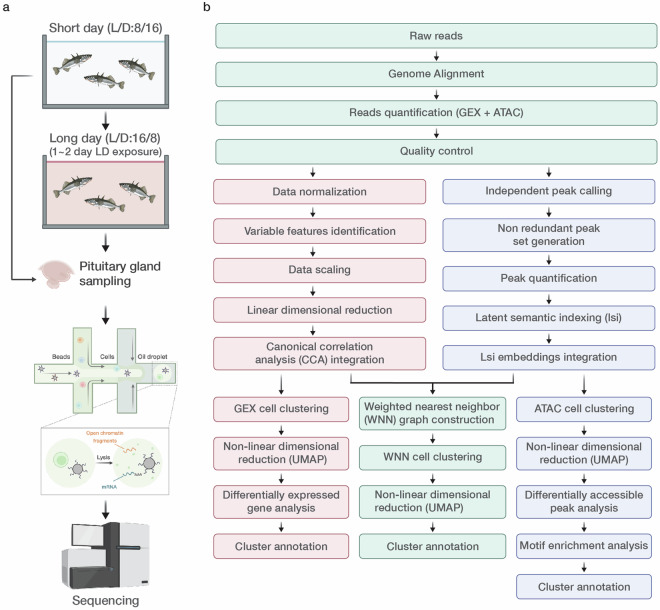
Figure 1 provides an overview of the experimental design (Fig. 1a) and the bioinformatics workflow (Fig. 1b). Table 1 summarizes the quality of each library. Raw sequence data are available from the DNA Data Bank of Japan (DDBJ) (Assession No., DRP011961)50. Preprocessed barcodes (*. tsv) and features (*. tsv), and gene expression matrices (*. mtx) can be accessed on Figshare via 10.6084/m9.figshare.2679532351.
Fig. 1.
Experimental and bioinformatics workflow of single-cell multiome ATAC + Gene Expression (GEX). (a) Experimental workflow. (b) Bioinformatics workflow. The acronym L/D refers to the light/dark cycle used during the experiment. Figure 1a was created with icons from biorender.com.
Technical Validation
Library preparation
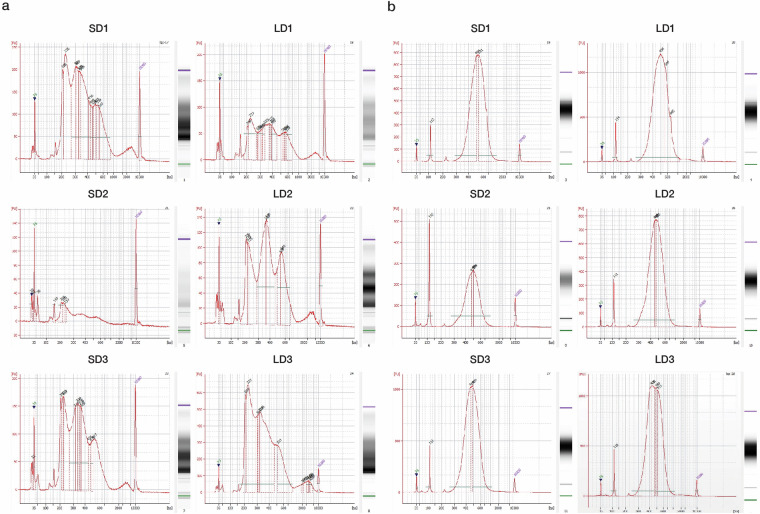
The cDNA and ATAC libraries were constructed using the 10x Genomics Chromium Next GEM Single-Cell Multiome GEM Kit according to the manufacturer’s instructions (https://www.10xgenomics.com/support/single-cell-multiome-atac-plus-gene-expression/documentation/steps/library-prep/chromium-next-gem-single-cell-multiome-atac-plus-gene-expression-reagent-kits-user-guide). Subsequently, fragment size distribution and density were evaluated using a DNA assay with a Bioanalyzer. In the ATAC-seq data, we observed distinctive peak patterns indicative of the periodic chromatin structure, displaying segments of nucleosome-free DNA, single nucleosomes, dinucleosomes, and multiple nucleosome assemblies. Notably, peaks occurred at a frequency of 150–180 bp, which aligned with the expected span of DNA wrapped around the individual nucleosomes (Fig. 2). In the GEX assay, we observed a well-defined peak pattern, indicating a high-quality library preparation. The Bioanalyzer trace displayed a dominant peak centered at approximately 300–600 bp, which corresponded to the expected fragment size distribution for a properly constructed sequencing library (Fig. 2). The presence of a single sharp peak without substantial secondary peaks or smearing suggested minimal RNA degradation and a homogeneous fragment size distribution. Additionally, the absence of smaller peaks or a high baseline indicated low levels of adapter dimers and other artifacts, further confirming proper library quality for downstream sequencing applications. Single-cell libraries were sequenced using the Illumina system once the quality was validated. We successfully generated an average of 1,295,087,942 and 680,594,782 sequence reads for ATAC and GEX assays respectively, corresponding to an average 53.1 gigabase pairs (Gbp) and 40.2 Gbp of sequence data per assay, as documented in Table 2. Following alignment to the stickleback genome, an average of over 85% of reads were accurately mapped, suggesting the high quality of our sequencing libraries. The average ratio of bases across samples with a phred quality score greater than and equal to 30 (Q30) was 94.13% for ATAC assay and 97.29% for GEX assay, indicating high sequencing fidelity (Table 2). For further genomic alignment, we achieved average mapping rates of 77.39% and 89.93% for the ATAC and GEX assays, respectively, confirming reliable sequence integrity (Table 3).
Fig. 2.
Quality assessment of ATAC and cDNA libraries. (a,b) Electropherogram profiles of ATAC and cDNA library quality, respectively. The integrity and size distribution were evaluated using a Bioanalyzer. SD: Short-day; LD: Long-day.
Table 2.
ATAC and cDNA library statistics for each sample.
| Type of library | Sample ID | Read | Total bases | Read count | GC (%) | Q20 (%) | Q30 (%) |
|---|---|---|---|---|---|---|---|
| ATAC | SD1 | Read1 | 22,034,323,100 | 440,686,462 | 48.84 | 99.08 | 97.19 |
| Read2 | 10,576,475,088 | 440,686,462 | 57.35 | 95.87 | 93.56 | ||
| Read3 | 21,593,636,638 | 440,686,462 | 49.05 | 93.82 | 88.74 | ||
| R1 + R2 + R3 | 54,204,434,826 | 1,322,059,386 | 50.58 | 96.36 | 93.12 | ||
| ATAC | SD2 | Read1 | 19,287,681,650 | 385,753,633 | 50.61 | 98.93 | 96.85 |
| Read2 | 9,258,087,192 | 385,753,633 | 55.84 | 97.1 | 95.41 | ||
| Read3 | 18,901,928,017 | 385,753,633 | 50.9 | 91.85 | 86.68 | ||
| R1 + R2 + R3 | 47,447,696,859 | 1,157,260,899 | 51.75 | 95.75 | 92.52 | ||
| ATAC | SD3 | Read1 | 21,502,300,600 | 430,046,012 | 49.74 | 98.99 | 96.9 |
| Read2 | 10,321,104,288 | 430,046,012 | 56.61 | 97.53 | 95.87 | ||
| Read3 | 21,072,254,588 | 430,046,012 | 49.95 | 95.87 | 91.94 | ||
| R1 + R2 + R3 | 52,895,659,476 | 1,290,138,036 | 51.17 | 97.46 | 94.72 | ||
| ATAC | LD1 | Read1 | 20,964,221,050 | 419,284,421 | 50.46 | 99.04 | 97.18 |
| Read2 | 10,062,826,104 | 419,284,421 | 58.46 | 96.97 | 95.24 | ||
| Read3 | 20,544,936,629 | 419,284,421 | 50.7 | 93.11 | 88.27 | ||
| R1 + R2 + R3 | 51,571,983,783 | 1,257,853,263 | 52.12 | 96.27 | 93.25 | ||
| ATAC | LD2 | Read1 | 22,223,949,950 | 444,478,999 | 51.73 | 99.09 | 97.28 |
| Read2 | 10,667,495,976 | 444,478,999 | 56.86 | 97.71 | 96.23 | ||
| Read3 | 21,779,470,951 | 444,478,999 | 51.92 | 96.1 | 92.65 | ||
| R1 + R2 + R3 | 54,670,916,877 | 1,333,436,997 | 52.81 | 97.63 | 95.23 | ||
| ATAC | LD3 | Read1 | 23,496,317,800 | 469,926,356 | 48.86 | 99.26 | 97.74 |
| Read2 | 11,278,232,544 | 469,926,356 | 56.85 | 97.94 | 96.5 | ||
| Read3 | 23,026,391,444 | 469,926,356 | 49.02 | 97.03 | 93.84 | ||
| R1 + R2 + R3 | 57,800,941,788 | 1,409,779,068 | 50.48 | 98.12 | 95.94 | ||
| cDNA | SD1 | Read1 | 10,210,837,196 | 364,672,757 | 45.24 | 99.23 | 97.79 |
| Read2 | 32,820,548,130 | 364,672,757 | 47.75 | 94.95 | 89.37 | ||
| R1 + R2 | 43,031,385,326 | 729,345,514 | 47.16 | 95.97 | 91.36 | ||
| cDNA | SD2 | Read1 | 6,635,570,984 | 236,984,678 | 45.29 | 98.74 | 96.62 |
| Read2 | 21,328,621,020 | 236,984,678 | 46.45 | 94.57 | 88.05 | ||
| R1 + R2 | 27,964,192,004 | 473,969,356 | 46.17 | 95.56 | 90.09 | ||
| cDNA | SD3 | Read1 | 10,323,097,624 | 368,682,058 | 45.57 | 99.09 | 97.49 |
| Read2 | 33,181,385,220 | 368,682,058 | 46.23 | 96.27 | 91.51 | ||
| R1 + R2 | 43,504,482,844 | 737,364,116 | 46.07 | 96.94 | 92.93 | ||
| cDNA | LD1 | Read1 | 9,353,078,028 | 334,038,501 | 45.05 | 99.13 | 97.48 |
| Read2 | 30,063,465,090 | 334,038,501 | 49.1 | 95.1 | 89.48 | ||
| R1 + R2 | 39,416,543,118 | 668,077,002 | 48.14 | 96.05 | 91.38 | ||
| cDNA | LD2 | Read1 | 9,487,918,888 | 338,854,246 | 45.68 | 98.58 | 96.71 |
| Read2 | 30,496,882,140 | 338,854,246 | 45.68 | 96.71 | 92.28 | ||
| R1 + R2 | 39,984,801,028 | 677,708,492 | 45.68 | 97.16 | 93.33 | ||
| cDNA | LD3 | Read1 | 11,159,458,940 | 398,552,105 | 45.71 | 99.11 | 97.55 |
| Read2 | 35,869,689,450 | 398,552,105 | 46.65 | 96.67 | 92.31 | ||
| R1 + R2 | 47,029,148,390 | 797,104,210 | 46.42 | 97.25 | 93.55 |
GC (%): GC content, representing the percentage of guanine and cytosine bases in the reads. Q20 (%) and Q30 (%): Percentages of bases with sequencing quality scores above Q20 (99% base call accuracy) and Q30 (99.9% base call accuracy), respectively. SD: Short-day. LD: Long-day.
Table 3.
Statistics of raw sequencing data. SD: Short-day. LD: Long-day.
| Sample ID | Estimated number of cells | ATAC | GEX | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Confidently mapped read pairs | Mean raw read pairs per cell | Median high-quality fragments per cell | Non-nuclear read pairs | ATAC Valid barcodes | Mean raw reads per cell | Median UMI counts per cell | Median genes per cell | Reads mapped to genome | Total genes detected | Valid UMIs | Valid barcodes | ||
| SD1 | 580 | 0.7737 | 759804.2448 | 9943 | 0.0006 | 0.9466 | 628746.1328 | 751.5 | 472 | 0.9068 | 14615 | 0.9995 | 0.955 |
| SD2 | 516 | 0.7265 | 747584.5601 | 5139.5 | 0.0015 | 0.9704 | 459272.6318 | 287 | 119 | 0.8557 | 12430 | 0.9996 | 0.9653 |
| SD3 | 1151 | 0.7855 | 373628.1599 | 15644 | 0.0014 | 0.9811 | 320314.5595 | 709 | 506 | 0.9222 | 16727 | 0.9994 | 0.9465 |
| LD1 | 260 | 0.7588 | 1612632.389 | 14494 | 0.0016 | 0.963 | 1284763.465 | 853.5 | 486 | 0.9097 | 15931 | 0.9997 | 0.973 |
| LD2 | 1477 | 0.8091 | 300933.6486 | 9501 | 0.0018 | 0.9809 | 229420.6134 | 293 | 166 | 0.8793 | 16487 | 0.9996 | 0.9582 |
| LD3 | 1400 | 0.79 | 335661.6829 | 13911.5 | 0.0012 | 0.9824 | 284680.075 | 820 | 577 | 0.9222 | 17548 | 0.9995 | 0.9436 |
Technical quality control
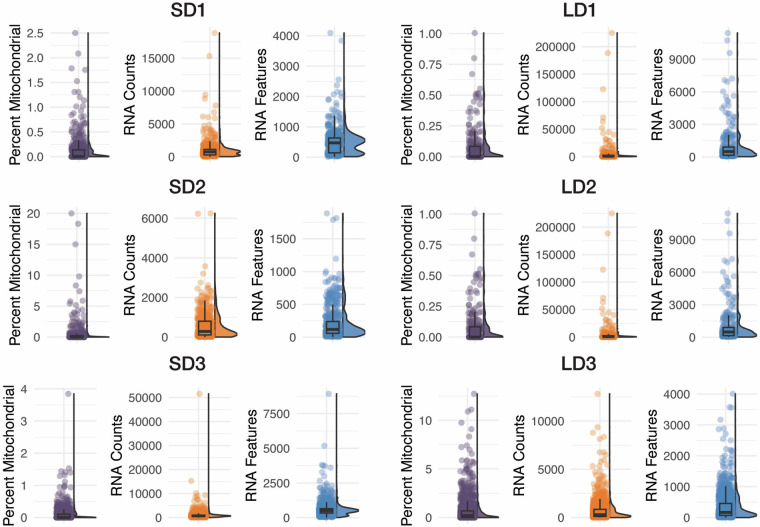
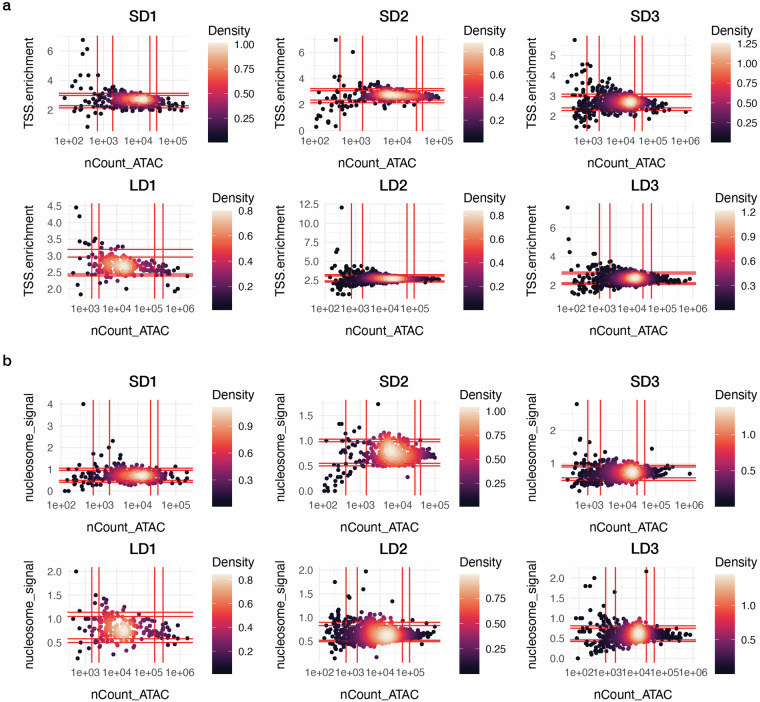
We successfully obtained 260–1477 cells of good quality in terms of both gene expression and chromatin profiles (Table 1). Although preliminary QC was performed using CellRanger ARC, some debris cells remained in the dataset. Therefore, after obtaining the feature-barcode matrix for each sample, we performed additional QC to ensure that the selected cells reliably reflected chromatin states and transcriptional activity (see details in M and M). We first excluded cells with fewer than 100 or more than 3000 detected genes in the GEX assay to avoid aberrations, such as cellular stress or the presence of doublets (Fig. 3). Mitochondrial reads were limited to less than 20% to eliminate dying or damaged cells, ensuring the preservation of cellular integrity in our dataset (Fig. 3). Additionally, we retained the cells exhibiting a TSS enrichment score above 1 to ensure concentrated transcription initiation, which is reflective of active gene expression (Fig. 4a). Furthermore, we selected cells with a nucleosome signal, which refers to the ratio of fragments between 147 and 294 bp (mononucleosome) to fragments < 147 bp (nucleosome-free), lower than 2, to enhance the inclusion of cells with open chromatin conducive to transcriptional activity (Fig. 4b). These stringent selection criteria culminated in a refined cell population of 5184 cells for downstream analyses, as detailed in Table 1.
Fig. 3.
GEX quality control metrics. Percent Mitochondrial: Percent of mitochondrial reads detected in each cell. RNA Counts: Count of GEX reads detected in each cell. RNA Features: Number of genes/features detected in each cell.
Fig. 4.
ATAC quality control metrics. (a) Transcription Start Site (TSS) enrichment. (b) Nucleosome Signals. nCount_ATAC: ATAC reads detected in each cell. The red vertical and horizontal lines indicate the 5th, 10th, 90th, and 95th quantiles of the data distribution (left to right/bottom to top).
Biological validation
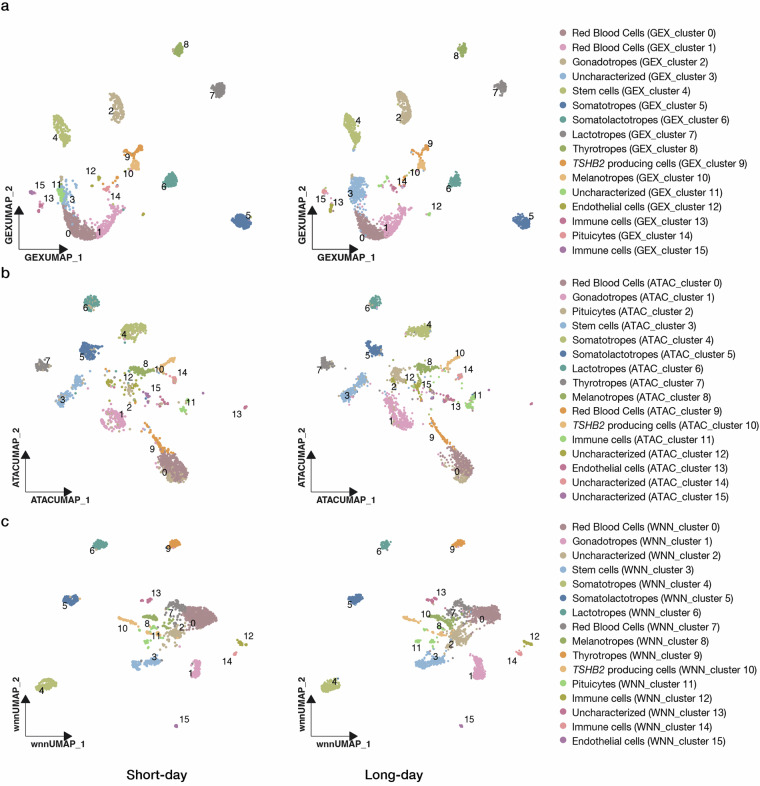
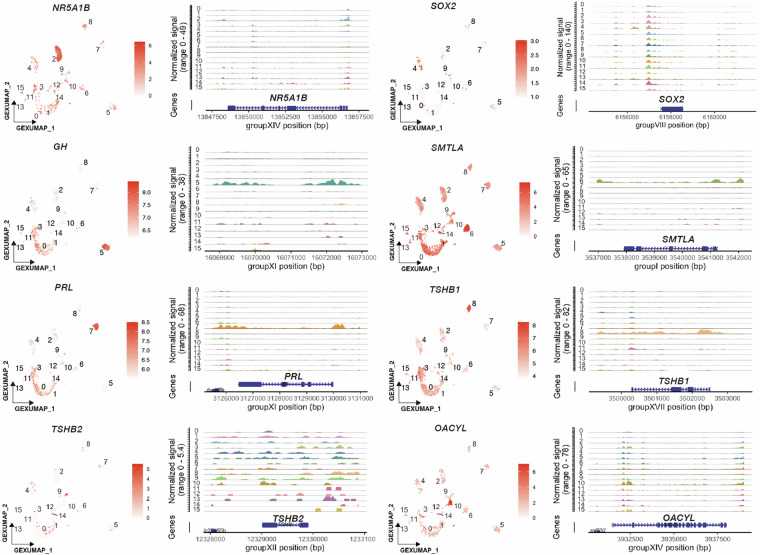
To validate the data quality of each assay, we first performed independent clustering based on the gene expression profiles and chromatin landscapes. In the cluster analysis based on gene expression profiles alone, 16 cell clusters were identified under both short- and long-day conditions (Fig. 5a). Subsequently, we optimized the clustering resolution to achieve the same number of cell clusters based solely on the chromatin profiles (Fig. 5b). Given the recognized heterogeneity of the pituitary gland, which includes different cell types that synthesize unique peptide hormones, we used the genes encoding these hormones as markers for the annotation of cell clusters. Specifically, GEX_clusters 0 and 1 showed unique expression of HBAE5 (ENSGACG00000014492), which encodes hemoglobin alpha embryonic 5 (Supplementary Figure 1). Thus, we defined these two clusters as red blood cells. GEX_cluster 2, showing the co-expression of CGA (glycoprotein hormone alpha polypeptide, ENSGACG00000009153), NR5A1B (nuclear receptor subfamily 5 group A member 1, ENSGACG00000018317), LHB (luteinizing hormone beta subunit, ENSGACG00000011475), and AR (androgen receptor beta, ENSGACG00000020332), were identified as gonadotropes (Fig. 6, Supplementary Figure 1)52. GEX_cluster 4 was annotated as potential stem cells because of the high expression of two homeobox genes LHX3 (LIM homeobox 3, ENSGACG00000016592), PROP1 (PROP paired-like homeobox 1, ENSGACG00000002587), SOX2 (SRY-box transcription factor 2, ENSGACG00000006100) and NFIB (nuclear factor I B, ENSGACG00000019677) (Fig. 6, Supplementary Figure 1)53. GEX_cluster 5 was defined as somatotropes, as it showed a unique expression of GH (growth hormone, ENSGACG00000014829)10,52 (Fig. 6), while GEX_cluster 6 with a unique expression of SMTLA (somatolactin alpha, ENSGACG00000006593) was characterized as somatolactotropes (Fig. 6)54,55. In addition, GEX_cluster 7 was identified as lactotropes because of the strong and distinct expression of PRL (prolactin, ENSGACG00000006561) (Fig. 6)10,52. GEX_cluster 8 exhibited a strong expression pattern of CGA (glycoprotein hormone alpha polypeptide, ENSGACG00000009153), GATA2A (GATA binding protein 2a, ENSGACG00000009608) and TSHB1 (thyroid-stimulating hormone subunit beta 1, ENSGACG00000005276) (Fig. 6, Supplementary Figure 1)10,52,56 and was thus annotated as thyrotropes. Notably, TSHB2 (ENSGACG00000009897) encoding thyroid-stimulating hormone subunit beta 2, which is a paralog of TSHB157 was not co-expressed with TSHB1 within GEX_cluster 8, but markedly displayed strong expression in GEX_cluster 9 (Fig. 6). This observation, consistent with the spatial expression pattern in Atlantic salmon, led to the definition of GEX_cluster 9 as TSHΒ2-producing cells58. We also identified GEX_cluster 10 as melanotropes because it shares the expression of POMC (proopiomelanocortin a, ENSGACG00000009521), and OACYL (O-acyltransferase like, ENSGACG00000016460) (Fig. 6, Supplementary Figure 1)52,59. In addition to endocrine cells, GEX_clusters 12, 13/15, and 14 were categorized as pituicytes, immune cells, and endothelial cells, respectively, based on the cluster-specific expression of LHX2B (LIM homeobox 2b, ENSGACG00000003520), PTPRC (protein tyrosine phosphatase receptor type C, ENSGACG00000007843), and PECAM1 (platelet/endothelial cell adhesion molecule 1, ENSGACG00000013350), respectively53 (Supplementary Figure 1). However, GEX_clusters 3 and 11 were defined as uncharacterized owing to the absence of clear cell markers.
Fig. 5.
Clustering and annotation of gene expression and chromatin accessibility of the stickleback pituitary under short- (left) and long-day conditions (right). Independent analyses based on (a) gene expression profiles, (b) chromatin accessibility, and (c) Weighted Nearest Neighbor (WNN) analysis. Cells are visualized by Uniform Manifold Approximation and Projection for Dimension Reduction (UMAP). Cell annotation and their corresponding GEX_, ATAC_ and WNN_cluster ID are listed in Table 4.
Fig. 6.
Gene expression and chromatin accessibility of representative cell markers. Uniform Manifold Approximation and Projection for Dimension Reduction (UMAP) visualizations represent the expression of the marker genes. The adjacent genomic track plots display chromatin accessibility near the marker genes. Cluster numbers, corresponding to the gene expression-based analysis, are noted to the left of the genomic tracks.
Accessibility of DNA is a prerequisite for gene transcription. To evaluate the correlation between gene expression and chromatin accessibility, we examined the accessibility of the genomic regions proximal to these marker genes within each GEX_cluster. A strong correlation between cell type-specific gene expression and corresponding DNA accessibility levels was observed for all previously defined cell types (Fig. 6). Subsequently, we annotated clusters (ATAC_cluster) based on chromatin landscapes using the same set of biomarkers as in gene expression-based analysis. This analysis confirmed the presence of all previously recognized cell types demonstrating strong consistency across independent clustering methods (Fig. 5b). To validate data reliability and consistency between clustering based on gene expression and the chromatin landscape, we compared independent clustering with integrative clustering by combining both ATAC and GEX assays (WNN analysis). These analyses confirmed that the same cell types consistently appeared in the three independent analyses (Fig. 5c). Both independent and integrative analyses underscored the robustness of our dataset, validating data quality and reinforcing the reliability of our cell-type annotations.
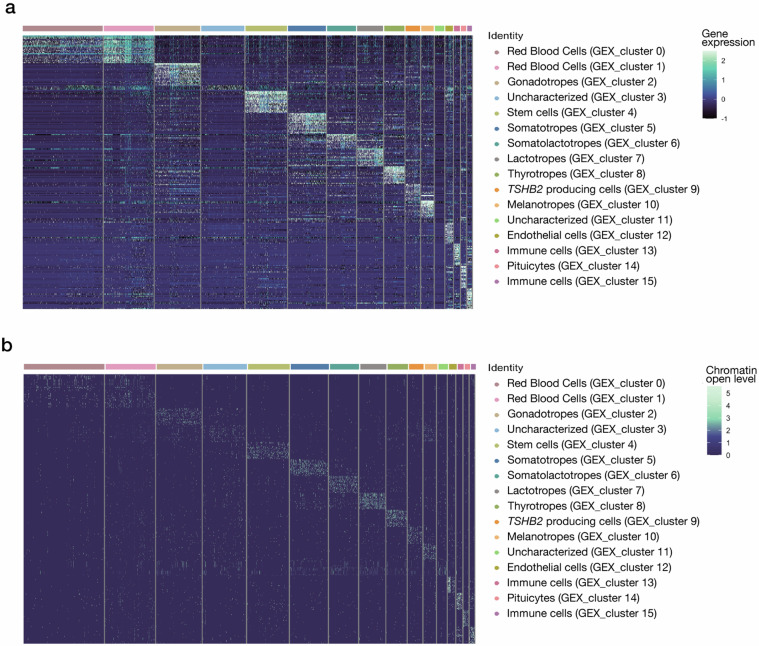
To evaluate the accuracy of the cell clustering results, we identified DEGs and accessible genomic regions within each GEX_cluster (Supplementary Tables 2, 3). Distinct cell type-specific patterns were observed across different clusters in both assays, which is consistent with the reliability of our data (Fig. 7). Subsequently, we conducted Gene Ontology (GO) enrichment analysis of the top 20 DEGs (ordered by average log2 fold change) within each GEX_cluster to investigate their functional enrichment. In GEX_clusters 0 and 1, which represent red blood cells, significant enrichment was observed in GO terms associated with oxygen and gas transport, hemoglobin complex assembly, oxygen binding, and oxygen carrier activity (Supplementary Table 4). GEX_cluster 4, which comprises stem cells, was primarily enriched in GO terms related to cellular development and differentiation processes (Supplementary Table 4). Furthermore, in endocrine cell populations such as somatotropes, heightened hormone activity was observed (Supplementary Table 4). Simultaneously, enrichment of GO terms linked to immune responses was detected in the corresponding immune cell clusters (Supplementary Table 4).
Fig. 7.
Differentially expressed genes (DEGs) and differentially accessible regions (DARs) in each cell cluster. (a) Expression patterns of the top 20 DEGs in each cluster. (b) Accessibility patterns for top 20 DARs in each cluster.
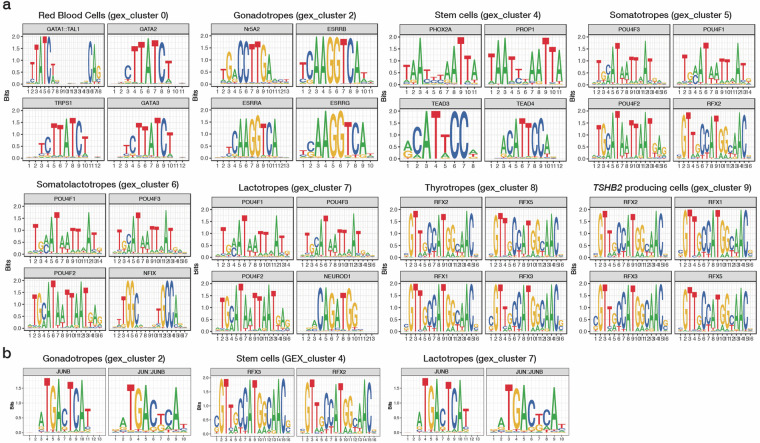
Next, to further assess the reliability of the data for comparison with previously published literature, we performed motif enrichment analysis to identify overrepresented motifs within the cell type-specific DARs (Supplementary Table 5). The binding motifs of the GATA transcription factor family, which are known to be key regulators of hematopoiesis, including red blood cell differentiation60–62, were significantly enriched in the DARs of GEX_cluster 0, defined as red blood cells (Fig. 8a). In addition, NR5A2, a key regulator of LHB and FSHB transcription63, exhibited an overrepresented binding motif, specifically in GEX_cluster 2, identified as gonadotropes (Fig. 8a). We also observed enrichment of RFX family transcription factor-binding motifs in GEX_cluster 8 and GEX_cluster 9, which are defined as thyrotropes and TSHΒ2-producing cells, respectively (Fig. 8a). This result is consistent with the interaction between RFX family transcription factors and POU1F1 in driving thyrotropic fate64. Similarly, POU domain protein motifs, which are essential for the differentiation and proliferation of somatotropes, lactotropes, and thyrotropes65, were enriched in GEX_clusters 5, 6, and 7, which were identified as somatotropes, somatolactotropes, and lactotropes, respectively (Fig. 8a). Furthermore, transcription factors that play fundamental roles in pituitary stem cell development, including TEADs and PROP166,67 showed significant motif enrichment in GEX_cluster 4, defined as stem cells (Fig. 8a). The binding motif of B-cell generation-associated transcription factors SPI-B and ELF1, which are involved in the transactivation of T cell-specific genes, was overrepresented in immune cell clusters (GEX_clusters 13 and 15) (Supplementary Figure 2)68,69.
Fig. 8.
Motif enrichment across cell clusters and those under different photoperiodic conditions. (a) Top four enriched motifs in representative cell clusters: red blood cells, progenitor cells, and major hormone producing cells. (b) Binding motifs for transcription factors showing changes in their enrichment in DARs in response to photoperiodic shifts to long-day conditions. The corresponding transcription factors (TFs) are shown at the top of each subplot. The cluster numbers correspond to the cell cluster identities determined by gene expression-based clustering (Fig. 5a).
To validate the data reliability through comparison with known regulatory patterns of the pituitary, we extracted the top 20 motifs enriched in each GEX_cluster (ranked by fold enrichment score) and analyzed their intersections (Supplementary Table 5). The binding motifs of the POU4F family proteins were enriched in GEX_clusters 5, 6, and 7, which were defined as somatotropes, somatolactotropes, lactotropes, and thyrotropes, respectively. In contrast, other POU domain TFs, such as POU1F1, POU3F4, and POU5F1B, were exclusively enriched in GEX_clusters 6 and 8, which were identified as somatolactotropes and thyrotropes, respectively. These enriched motif patterns aligned with the lineage-specific expression of POU1F1 in GEX_clusters 6 and 8 (Supplementary Figure 1). Certain TFs also exhibited cell type-specific putative binding activities; for example, motifs of Estrogen-Related Receptors (ESRRA, ESRRB, and ESRRG) were enriched solely in GEX_cluster 2 (Gonadotropes) as expected. This distinction between shared and cell-specific motifs supports the validity of the data, as it aligns with the expected balance of common and specialized regulation in pituitary hormone production.
Moreover, photoperiodic responses of the pituitary gland have been extensively documented across various species57,70,71. To further validate the current dataset, we compared it with previous reports by examining cell-type-specific responses in both gene expression (Supplementary Table 6) and putative chromatin-binding activities (Fig. 8b, Supplementary Table 7). For example, ATP2B2 was upregulated in GEX_cluster 2 (gonadotropes) and GEX_cluster 5 (somatotropes) under short-day conditions (Supplementary Table 6), which is consistent with a previous report in sheep72. VMO1B demonstrated higher expression levels in GEX_cluster 4 (stem cells) under long-day conditions (Supplementary Table 6), further supported by the photoperiodic responses of gene expression in sheep pituitary73. Notably, NR5A1B exhibited a consistent upregulation among several cell clusters: GEX_cluster 6 (somatolactotropes), GEX_cluster 7 (lactotropes), and GEX_cluster 8 (thyrotropes) under long-day conditions (Supplementary Table 6), which is also consistent with seasonal changes in gene expression in the white goose pituitary74. Under short-day conditions, both TSHB2 and the short-day marker CHGA75 showed increased expression in GEX_cluster 9 (TSHB2-producing cells) (Supplementary Table 6).
To validate the data accuracy of the ATAC seq data under different photoperiodic conditions, we also performed photoperiod-responsive motif enrichment analysis. The analysis revealed enrichment patterns of transcription factor binding motifs specific to daylength conditions, consistent with findings from previous studies. For instance, GEX_cluster 4 (stem cells) showed enriched binding motifs for RFX family TFs (RFX3 and RFX2) under long-day conditions (Fig. 8b, Supplementary Table 7). A previous report indicated that RFX family TFs were enriched in the DARs of the pituitary during the brood stage of geese71, which is consistent with marine sticklebacks generally starting their reproduction under long-day conditions31. In GEX_cluster 2 (gonadotropes) and GEX_cluster 7 (lactotropes), JUN family transcription factors (TFs) were enriched (Fig. 8b, Supplementary Table 7), aligning with the detection of JUN family TF binding motifs in the DARs of male quail exposed to long-day conditions76. These consistent results demonstrate the accuracy of our data.
In summary, we simultaneously profiled the transcriptome and chromatin landscapes of pituitary cells using multifaceted validation. This robust dataset may deepen our understanding of the regulatory mechanisms underlying the critical physiological functions of the pituitary gland in three-spined sticklebacks and provide a valuable reference for future studies on comparative physiology and evolutionary biology .
Table 4.
Cell annotations and corresponding clusters.
| Annotation | GEX_cluster ID | ATAC_cluster ID | WNN_cluster ID |
|---|---|---|---|
| Gonadotropes | 2 | 1 | 1 |
| Somatotropes | 5 | 4 | 4 |
| Somatolactotropes | 6 | 5 | 5 |
| Lactotropes | 7 | 6 | 6 |
| Thyrotropes | 8 | 7 | 9 |
| TSHB2 producing cells | 9 | 10 | 10 |
| Melanotropes | 10 | 8 | 8 |
| Red Blood Cells | 0 &1 | 0 & 9 | 0 & 7 |
| Uncharacterized | 3 & 11 | 12, 14 & 15 | 2 & 13 |
| Stem cells | 4 | 3 | 3 |
| Endothelial cells | 12 | 13 | 15 |
| Immune cells | 13 & 15 | 11 | 12 & 14 |
| Pituicytes | 14 | 2 | 11 |
Supplementary information
Supplementary Figure 1, Supplementary Figure 2
Acknowledgements
This research was supported by JSPS KAKENHI grants 23K23934, 22H04983, 20H04873, 19H03277, JST FOREST 21467575, the Mitsubishi foundation, the Toray Science and Technology Grant, National Institute for Basic Biology 23NIBB429, 21–358 (to A. I.), JST SPRING, Grant Number JPMJSP2108 (to L. L.), and the Graduate School of Frontier Sciences, The University of Tokyo, the Challenging New Area Doctoral Research Grant (Project No. C2308, to L. L.). Computations were partially performed on the NIG supercomputer at ROIS National Institute of Genetics. We also thank Ms. S. Kajiwara and Dr. Yo Yamasaki for their efforts in fish maintenance, and all Ishikawa Lab members for fish care and discussion.
Author contributions
L.L. and A.I. conceived and designed the study. J.K. and A.I. provided fish. A.I. and S.S. performed experiments. L.L. performed data analysis. L.L. and A.I. wrote the first draft of the manuscript with J.K. and S.S. making significant contributions to editing. All authors agree to be held accountable for the content therein and approve the final version of the manuscript.
Code availability
All codes were adapted from the standard pipeline of CellRanger ARC (https://www.10xgenomics.com/support/software/cell-ranger-arc/latest) and R packages Seurat (https://satijalab.org/seurat/) and Signac (https://stuartlab.org/signac/). The full R code used in this study was deposited in the GitHub repository (https://github.com/Liang6292/CO-PROFILING-OF-SINGLE-CELL-GENE-EXPRESSION-AND-CHROMATIN-LANDSCAPES-IN-STICKLEBACK-PITUITARY).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41597-025-04376-3.
References
- 1.Rahmad Royan, M. et al. Functional and developmental heterogeneity of pituitary lactotropes in medaka. Gen. Comp. Endocrinol.330, 114144 (2023). [DOI] [PubMed] [Google Scholar]
- 2.Chen, Z. et al. Pituitary transcriptome profile from laying period to incubation period of changshun green-shell laying hens. BMC Genomics25, 309 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeng, Z. et al. Transcriptome analysis of pigeon pituitary gland: Expression changes of genes encoding protein and peptide hormones at different breeding stages. Poult. Sci.103, 103742 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang, C. & Ma, W. Hypothalamic and pituitary transcriptome profiling using RNA-sequencing in high-yielding and low-yielding laying hens. Sci. Rep.9, 10285 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ager-Wick, E. et al. An RNA-seq time series of the medaka pituitary gland during sexual maturation. Sci. Data10, 62 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li, X. & Wang, C.-Y. From bulk, single-cell to spatial RNA sequencing. Int. J. Oral Sci.13, 1–6 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang, S. et al. Single-cell transcriptomics identifies divergent developmental lineage trajectories during human pituitary development. Nat. Commun. 2020 11111, 1–16 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang, J. et al. Single-cell RNA sequencing analysis of chicken anterior pituitary: A bird’s-eye view on vertebrate pituitary. Front. Physiol. 12, (2021). [DOI] [PMC free article] [PubMed]
- 9.Siddique, K., ager-Wick, E., Fontaine, R., Weltzien, F. & Henkel, C. V. Characterization of hormone-producing cell types in the teleost pituitary gland using single-cell RNa-seq. 10.1038/s41597-021-01058-8. [DOI] [PMC free article] [PubMed]
- 10.Ho, Y. et al. Single-cell transcriptomic analysis of adult mouse pituitary reveals sexual dimorphism and physiologic demand-induced cellular plasticity. Protein Cell11, 565 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fontaine, R. et al. Pituitary multi-hormone cells in mammals and fish: History, origin, and roles. Front. Neuroendocrinol.67, 101018 (2022). [DOI] [PubMed] [Google Scholar]
- 12.Latchman, D. S. Transcription factors: An overview. Int. J. Exp. Pathol.74, 417–422 (1993). [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu, F. et al. The interaction landscape between transcription factors and the nucleosome. Nature562, 76–81 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rudnizky, S., Khamis, H., Malik, O., Melamed, P. & Kaplan, A. The base pair-scale diffusion of nucleosomes modulates binding of transcription factors. Proc. Natl. Acad. Sci.116, 12161–12166 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lickwar, C. R., Mueller, F., Hanlon, S. E., McNally, J. G. & Lieb, J. D. Genome-wide protein–DNA binding dynamics suggest a molecular clutch for transcription factor function. Nature484, 251–255 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin, X. et al. Single-nucleus chromatin landscapes during zebrafish early embryogenesis. Sci. Data10, 464 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishikawa, A. et al. A key metabolic gene for recurrent freshwater colonization and radiation in fishes. Science364, 886–889 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Jones, F. C. et al. The genomic basis of adaptive evolution in threespine sticklebacks. Nature484, 55–61 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reid, K., Bell, M. A. & Veeramah, K. R. Threespine stickleback: A model system for evolutionary genomics. Annu. Rev. Genomics Hum. Genet.22, 357–383 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barber, I. Sticklebacks as model hosts in ecological and evolutionary parasitology. Trends Parasitol.29, 556–566 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Barber, I. & Scharsack, J. P. The three-spined stickleback-schistocephalus solidus system: An experimental model for investigating host-parasite interactions in fish. Parasitology137, 411–424 (2010). [DOI] [PubMed] [Google Scholar]
- 22.Barghi, N., Hermisson, J. & Schlötterer, C. Polygenic adaptation: A unifying framework to understand positive selection. Nat. Rev. Genet.21, 769–781 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Barrett, R. D. H. & Schluter, D. Adaptation from standing genetic variation. Trends Ecol. Evol.23, 38–44 (2008). [DOI] [PubMed] [Google Scholar]
- 24.Bengston, S. E. et al. Genomic tools for behavioural ecologists to understand repeatable individual differences in behaviour. Nat. Ecol. Evol.2, 944–955 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cresko, W. A., McGuigan, K. L., Phillips, P. C. & Postlethwait, J. H. Studies of threespine stickleback developmental evolution: Progress and promise. Genetica129, 105–126 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Hendry, A. P., Bolnick, D. I., Berner, D. & Peichel, C. L. Along the speciation continuum in sticklebacks. J. Fish Biol.75, 2000–2036 (2009). [DOI] [PubMed] [Google Scholar]
- 27.Huntingford, F. A. & Ruiz-Gomez, M. L. Three-spined sticklebacks gasterosteus aculeatus as a model for exploring behavioural biology. J. Fish Biol.75, 1943–1976 (2009). [DOI] [PubMed] [Google Scholar]
- 28.McKinnon, J. S. & Rundle, H. D. Speciation in nature: The threespine stickleback model systems. Trends Ecol. Evol.17, 480–488 (2002). [Google Scholar]
- 29.Peichel, C. L. & Marques, D. A. The genetic and molecular architecture of phenotypic diversity in sticklebacks. Philos. Trans. R. Soc. Lond. B. Biol. Sci.372, 20150486 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitano, J., Ishikawa, A., Kume, M. & Mori, S. Physiological and genetic basis for variation in migratory behavior in the three-spined stickleback, gasterosteus aculeatus. Ichthyol. Res.59, 293–303 (2012). [Google Scholar]
- 31.Ishikawa, A. & Kitano, J. Diversity in reproductive seasonality in the three-spined stickleback, gasterosteus aculeatus. J. Exp. Biol. 223, (2020). [DOI] [PubMed]
- 32.Bell, M. A. & Foster, S. A. The Evolutionary Biology of the Threespine Stickleback. (Oxford University Press, 1994).
- 33.Hoar, W. S. Hormones and the reproductive behaviour of the male three-spined stickleback (gasterosteus aculeatus). Anim. Behav.10, 247–266 (1962). [Google Scholar]
- 34.Manzon, L. A. The role of prolactin in fish osmoregulation: A review. Gen. Comp. Endocrinol.125, 291–310 (2002). [DOI] [PubMed] [Google Scholar]
- 35.Blanco, A. M. Hypothalamic- and pituitary-derived growth and reproductive hormones and the control of energy balance in fish. Gen. Comp. Endocrinol.287, 113322 (2020). [DOI] [PubMed] [Google Scholar]
- 36.Ravinet, M., Takeuchi, N., Kume, M., Mori, S. & Kitano, J. Comparative analysis of japanese three-spined stickleback clades reveals the pacific ocean lineage has adapted to freshwater environments while the japan sea has not. PLOS ONE9, e112404 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kitano, J., Mori, S. & Peichel, C. L. Phenotypic divergence and reproductive isolation between sympatric forms of japanese threespine sticklebacks. Biol. J. Linn. Soc.91, 671–685 (2007). [Google Scholar]
- 38.Ravinet, M. et al. The genomic landscape at a late stage of stickleback speciation: High genomic divergence interspersed by small localized regions of introgression. PLOS Genet.14, e1007358 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Satpathy, A. T. et al. Massively parallel single-cell chromatin landscapes of human immune cell development and intratumoral T cell exhaustion. Nat. Biotechnol.37, 925–936 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng, G. X. Y. et al. Massively parallel digital transcriptional profiling of single cells. Nat. Commun.8, 14049 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dobin, A. et al. STAR: Ultrafast universal RNA-seq aligner. Bioinforma. Oxf. Engl.29, 15–21 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell184, 3573–3587.e29 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stuart, T., Srivastava, A., Madad, S., Lareau, C. A. & Satija, R. Single-cell chromatin state analysis with signac. Nat. Methods18, 1333–1341 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang, Y. et al. Model-based analysis of ChIP-seq (MACS). Genome Biol.9, R137 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lawrence, M. et al. Software for computing and annotating genomic ranges. PLOS Comput. Biol.9, e1003118 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cusanovich, D. A. et al. Multiplex single-cell profiling of chromatin accessibility by combinatorial cellular indexing. Science348, 910–914 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan, G. & Lenhard, B. TFBSTools: An R/bioconductor package for transcription factor binding site analysis. Bioinformatics32, 1555–1556 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Castro-Mondragon, J. A. et al. JASPAR 2022: The 9th release of the open-access database of transcription factor binding profiles. Nucleic Acids Res.50, D165–D173 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schep, A. N., Wu, B., Buenrostro, J. D. & Greenleaf, W. J. chromVAR: Inferring transcription-factor-associated accessibility from single-cell epigenomic data. Nat. Methods14, 975–978 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DNA Data Bank of Japan.https://ddbj.nig.ac.jp/search/entry/sra-study/DRP011961 (2024).
- 51.Liu, L., Kitano, J., Shigenobu, S. & Ishikawa, A. Co-profiling of single-cell gene expression and chromatin landscapes in stickleback pituitary. figshare. Dataset.10.6084/m9.figshare.26795323 (2024). [DOI] [PMC free article] [PubMed]
- 52.Cheung, L. Y. M. et al. Single-cell RNA sequencing reveals novel markers of male pituitary stem cells and hormone-producing cell types. Endocrinology159, 3910–3924 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang, S. et al. Single-cell transcriptomics identifies divergent developmental lineage trajectories during human pituitary development. Nat. Commun.11, 5275 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weltzien, F.-A., Hildahl, J., Hodne, K., Okubo, K. & Haug, T. M. Embryonic development of gonadotrope cells and gonadotropic hormones–lessons from model fish. Mol. Cell. Endocrinol.385, 18–27 (2014). [DOI] [PubMed] [Google Scholar]
- 55.Fabian, P. et al. Lineage analysis reveals an endodermal contribution to the vertebrate pituitary. Science370, 463–467 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Quiroz, Y. et al. The HMG-box transcription factor Sox4b is required for pituitary expression of gata2a and specification of thyrotrope and gonadotrope cells in zebrafish. Mol. Endocrinol. Baltim. Md26, 1014–1027 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kitano, J. et al. Adaptive divergence in the thyroid hormone signaling pathway in the stickleback radiation. Curr. Biol.20, 2124–2130 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fleming, M. S. et al. Functional divergence of thyrotropin beta-subunit paralogs gives new insights into salmon smoltification metamorphosis. Sci. Rep.9, 4561 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weltzien, F.-A., Andersson, E., Andersen, Ø., Shalchian-Tabrizi, K. & Norberg, B. The brain–pituitary–gonad axis in male teleosts, with special emphasis on flatfish (Pleuronectiformes). Comp. Biochem. Physiol. A. Mol. Integr. Physiol.137, 447–477 (2004). [DOI] [PubMed] [Google Scholar]
- 60.Mimoto, M. S., Kwon, S., Song Green, Y., Goldman, D. & Christian, J. L. GATA2 regulates wnt signaling to promote primitive red blood cell fate. Dev. Biol.407, 1–11 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Briegel, K. et al. Regulation and function of transcription factor GATA-1 during red blood cell differentiation. Dev. Camb. Engl.122, 3839–3850 (1996). [DOI] [PubMed] [Google Scholar]
- 62.Chen, D. & Zhang, G. Enforced expression of the GATA-3 transcription factor affects cell fate decisions in hematopoiesis. Exp. Hematol.29, 971–980 (2001). [DOI] [PubMed] [Google Scholar]
- 63.Fortin, J. et al. NR5A2 Regulates Lhb and Fshb Transcription in Gonadotrope-Like Cells In Vitro, but Is Dispensable for Gonadotropin Synthesis and Fertility In Vivo. PLoS ONE8, e59058 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Daly, A. Z. et al. Multi-omic profiling of pituitary thyrotropic cells and progenitors. BMC Biol.19, 76 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pang, A. L.-Y., Martin, M. M., Martin, A. L. A. & Chan, W.-Y. Chapter 22 - molecular basis of diseases of the endocrine system. in Molecular Pathology (eds. Coleman, W. B. & Tsongalis, G. J.) 435–463. 10.1016/B978-0-12-374419-7.00022-6 (Academic Press, San D iego, 2009).
- 66.Lodge, E. J., Russell, J. P., Patist, A. L., Francis-West, P. & Andoniadou, C. L. Expression analysis of the hippo cascade indicates a role in pituitary stem cell development. Front. Physiol.7, 114 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pérez Millán, M. I., Brinkmeier, M. L., Mortensen, A. H. & Camper, S. A. PROP1 triggers epithelial-mesenchymal transition-like process in pituitary stem cells. eLife5, e14470 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Horiuchi, S. et al. SpiB regulates the expression of B-cell-related genes and increases the longevity of memory B cells. Front. Immunol.14, 1250719 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rellahan, B. L. et al. Elf-1 regulates basal expression from the T cell antigen receptor ζ-chain gene Promoter1. J. Immunol.160, 2794–2801 (1998). [PubMed] [Google Scholar]
- 70.Irachi, S. et al. Photoperiodic regulation of pituitary thyroid-stimulating hormone and brain deiodinase in atlantic salmon. Mol. Cell. Endocrinol.519, 111056 (2021). [DOI] [PubMed] [Google Scholar]
- 71.Chang, J. et al. Transcriptomic and chromatin landscape analysis reveals that involvement of pituitary level transcription factors modulate incubation behaviors of magang geese. Genes14, 815 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu, Q. et al. Screening of differentially expressed genes and miRNAs in hypothalamus and pituitary gland of sheep under different photoperiods. Genes13, 1091 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xia, Q. et al. Identification of photoperiod-induced LncRNAs and mRNAs in pituitary pars tuberalis of sheep. Front. Vet. Sci. 8, (2021). [DOI] [PMC free article] [PubMed]
- 74.Zhao, W. et al. Seasonal differences in the transcriptome profile of the zhedong white goose (anser cygnoides) pituitary gland. Poult. Sci.100, 1154–1166 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wood, S. H. et al. Binary switching of calendar cells in the pituitary defines the phase of the circannual cycle in mammals. Curr. Biol.25, 2651–2662 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chang, J. et al. The dynamic landscape of chromatin accessibility and active regulatory elements in the mediobasal hypothalamus influences the seasonal activation of the reproductive axis in the male quail under long light exposure. BMC Genomics25, 197 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- DNA Data Bank of Japan.https://ddbj.nig.ac.jp/search/entry/sra-study/DRP011961 (2024).
- Liu, L., Kitano, J., Shigenobu, S. & Ishikawa, A. Co-profiling of single-cell gene expression and chromatin landscapes in stickleback pituitary. figshare. Dataset.10.6084/m9.figshare.26795323 (2024). [DOI] [PMC free article] [PubMed]
Supplementary Materials
Supplementary Figure 1, Supplementary Figure 2
Data Availability Statement
All codes were adapted from the standard pipeline of CellRanger ARC (https://www.10xgenomics.com/support/software/cell-ranger-arc/latest) and R packages Seurat (https://satijalab.org/seurat/) and Signac (https://stuartlab.org/signac/). The full R code used in this study was deposited in the GitHub repository (https://github.com/Liang6292/CO-PROFILING-OF-SINGLE-CELL-GENE-EXPRESSION-AND-CHROMATIN-LANDSCAPES-IN-STICKLEBACK-PITUITARY).