Abstract
Studies have confirmed that elevated glucose levels could lead to renal fibrosis through the process of ferroptosis. Liraglutide, a human glucagon-like peptide-1 (GLP-1) analogue, is a potential treatment option for diabetes. This study aimed to examine the potential of liraglutide (LIRA) in inhibiting ferroptosis and reducing high glucose-induced renal fibrotic injury in mice, and whether the Fsp1-CoQ10-NAD(P)H signal pathway is a mechanism for this effect. In our study, we used db/db mice to simulate Type 2 diabetes mellitus (T2DM). The mice were intraperitoneally injected with LIRA (200 µg/kg/d) daily for 6 weeks. Renal function, pathologic changes, lipid peroxidation levels, iron levels, and ferroptosis were assessed. First, LIRA ameliorated renal dysfunction and fibrosis in db/db mice. Second, LIRA inhibited lipid peroxidation by up-regulating T-SOD, GSH-Px, and GSH activities as well as down-regulating the levels of 8-OHDG, MDA, LPO, 4-HNE, 12-Lox, and NOX4 in db/db mice. In addition, LIRA attenuated iron deposition by decreasing the expression of TfR1 and increasing the expression of FPN1. Meanwhile, LIRA reduced high levels of high glucose-induced cell viability decline and intracellular lipid peroxidation. Furthermore, LIRA inhibited ferroptosis by adjusting the Fsp1-CoQ10-NAD(P)H pathway in vivo and in vitro. These findings suggested that LIRA attenuated kidney fibrosis injury in db/db mice by inhibiting ferroptosis through the Fsp1-CoQ10-NAD(P)H pathway.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-85658-z.
Keywords: High glucose, Lipid peroxidation, Iron overload, Fsp1-CoQ10-NAD(P)H pathway
Subject terms: Chronic kidney disease, Type 2 diabetes
Introduction
Type 2 diabetes mellitus (T2DM) is a prevalent metabolic disorder characterized by disturbances in glucose-lipid metabolism, primarily resulting from insufficient insulin production or resistance to insulin1. The global prevalence of diabetes has escalated significantly, leading to increased mortality rates and reduced life expectancy2. In patients with T2DM, persistent hyperglycemia plays a direct role in the development of substantial renal impairments, with diabetic kidney disease representing one of its most severe chronic complications3.
The main features of diabetic nephropathy are proteinuria and a gradual decline in renal function. The underlying pathology is characterized by the accumulation of extracellular matrix (ECM) and damage in the glomerular and tubular interstitium, which ultimately contributes to the development of renal fibrosis and subsequent disruption of renal structure and function4,5. Previous studies on hyperglycemia-mediated activation of renal fibrosis have mostly focused on oxidative stress, neurohumoral mechanisms and pro-inflammatory cascade responses6. However, recent studies have shown that ferroptosis appeared to play an important role in renal fibrotic injury7.
Ferroptosis is a regulated mechanism of cellular demise primarily distinguished by iron-dependent oxidative stress and lipid peroxidation8. Oxidative stress could lead to tubular and vascular endothelial cell damage and secretion of pro-fibrotic factors, ultimately leading to fibroblast/pericyte activation and fibroblast/macrophage recruitment9. Notably, a study has shown that HK-2 cells, which are human renal tubular cells, exhibit both proliferation and fibroblast activation during the process of ferroptosis10. There is empirical evidence that that lipid peroxidation products, such as 4-hydroxynonenal (4-HNE) and malondialdehyde (MDA), within the bloodstream of patients afflicted with chronic kidney disease. Additionally, chronic kidney disease patients exhibit a significant decrease in levels of glutathione peroxidase (GSH-Px) and glutathione (GSH). Furthermore, the presence of fibrosis in these patients amplifies the production of reactive oxygen species (ROS)9,11. Therefore, targeting ferroptosis may may be significant importance in the fight against fibrosis.
Glucagon-like peptide-1 (GLP-1) is widely used in the treatment of T2DM due to its ability to increase glucagon-dependent insulin release and decrease glucagon secretion12. Liraglutide (LIRA), a GLP-1 receptor agonist, has demonstrated a promising prognosis for chronic kidney disease in T2DM, outperforming other GLP-1 receptor agonists in terms of glycaemic control and overall patient tolerability13,14. Furthermore, previous research has demonstrated that LIRA possessed a beneficial effect on chronic renal failure, particularly in reducing renal fibrosis. However, the precise mechanism, by which LIRA exerts its inhibitory effect on fibrosis remains incompletely understood15,16. Recent studies have also indicated that LIRA was able to reduce oxidative stress levels in SH-SY5Y cells and inhibit iron overload in the hippocampus of diabetic mice17,18. Taken together, these findings provide valuable insights into the comprehensive mechanism of action of LIRA.
It is well known that mice with genetic defects in the leptin receptor (db/db) or leptin itself (ob/ob) have been widely used as models of T2DM, and although ob/ob and db/db mice are similar in terms of weight gain and obesity, db/db mice have a higher risk of diabetes, with an onset similar to that of T2DM kidney disease19. Therefore, we used the db/db mouse model to simulate T2DM to investigate the potential correlation between T2DM-induced kidney injury and ferroptosis, as well as the therapeutic effects of LIRA. The findings of our study could potentially contribute to the existing theoretical framework for managing diabetic kidney injury.
Results
Liraglutide alleviated glycometabolism disorders in db/db mice
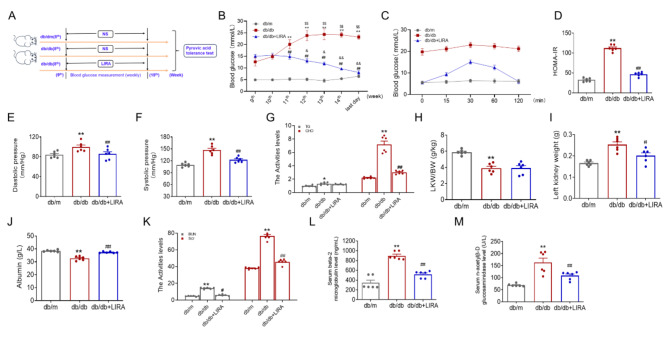
We used db/db mice to study the pathogenesis of T2DM and its associated complications. At the beginning of the 9th week, blood glucose was monitored in each group. Subsequently, the mice were treated with LIRA for five weeks while blood glucose levels were continuously monitored (Fig. 1A). The results showed a statistically significant increase in fasting blood glucose levels in db/db mice compared to db/m mice, with a continuous rise observed starting from the 11th week (Fig. 1B). Following LIRA administration, a notable reduction in elevated blood glucose levels was observed in db/db mice starting from the 12th week (Fig. 1B). At the end of the 14th week, tolerance to intraperitoneal pyruvate, a marker of gluconeogenesis, was assessed. Compared to db/m mice, pyruvate tolerance levels were significantly higher after 120 min, but decreased after LIRA treatment (Fig. 1C). The results also showed a significant increase in HOMA-IR in db/db mice, which was later reduced after LIRA administration (Fig. 1D). The db/db mice presented increased diastolic and systolic blood pressure, which was reduced after LIRA treatment (Fig. 1E-F). The triglycerides (TG) and cholesterol (CHO) levels were elevated in the db/db mice, and CHO levels were reduced after LIRA treatment (Fig. 1G). The kidney coefficient (KLW/BW) ratio (Fig. 1H) and left kidney weight (Fig. 1I) were all elevated in the db/db group, and the elevated weight was reduced to normal after LIRA treatment. The albumin content was increased (Fig. 1J), while, the urea nitrogen (BUN) (Fig. 1K), creatinine (Scr) (Fig. 1K), beta-2 microglobulin (beta 2-MG) (Fig. 1L), and n-acetyl-β-D-glucosidase (NAG) (Fig. 1M) levels were elevated in the db/db mice. These indicators returned to almost normal levels after LIRA treatment (Fig. 1J–M). Based on these findings, we have demonstrated that LIRA effectively improved glucose metabolism and insulin resistance in db/db mice.
Fig. 1.
LIRA improved the disorders of glycol-metabolism in db/db mice. (A) The schematic diagram of the experimental flow. (B) The blood glucose of mice from the 9th to 15th week. (The data are shown as the means ± SEM. n = 6. **p < 0.01 vs. db/m group. ##p < 0.01 vs. db/db group. $$p < 0.01 vs. db/db group 9th week. &p < 0.05, &&p < 0.01 vs. LIRA group 9th week). (C) The intraperitoneal glucose tolerance test of mice with 120 min. (D) The HOMA-insulin resistance (HOMA-IR) of db/m, db/db and LIRA groups. (E–F) The diastolic and systolic pressure. (G) The triglyceride (TG) and cholesterol (CHO) levels in serum. (H–I) The ratio of LKW/BW and the left kidney weight of mice in each group. (J–M) The albumin, BUN, Scr, beta-2 microglobulin (beta 2-MG), and n-acetyl-β-D-glucosidase (NAG) content in serum of mice in different groups. The data are shown as the means ± SEM. n = 6. **p < 0.05 vs. db/m group. ##p < 0.01 vs. db/db group).
Liraglutide significantly attenuated kidney fibrosis damage in db/db mice
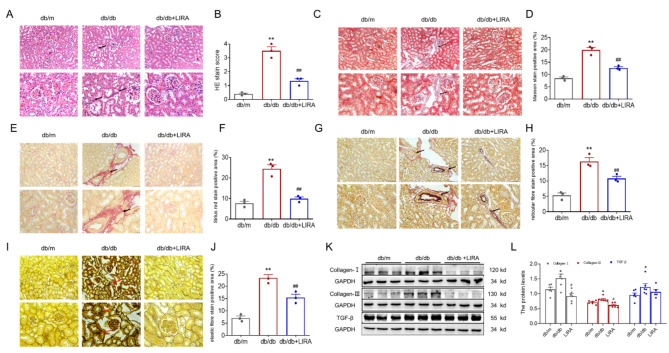
The pathological structure of the kidney was assessed by HE staining. In kidney sections from db/m mice, no discernible structural changes were observed. However, sections from db/db mice showed compensatory dilation of the renal tubules, variable sized vacuoles and partial atrophy of the glomeruli, as shown in Fig. 2A-B. We also evaluated renal fibrosis by Masson’s stain (Fig. 2C-D), Sirius red stain (Fig. 2E-F), reticulocyte fibre stain (Fig. 2G-H) and elastic fibre stain (Fig. 2I-J). These analyses showed a significant increase in the renal fibrosis area in db/db mice. In addition, the renal tissues of the db/db group showed a significant increase in the expression of Collagen-I, Collagen-III and transforming growth factor-β (TGF-β) as observed by western blotting analysis (Fig. 2K-L). The elevated levels of fibrosis were significantly reduced after LIRA administration (Fig. 2). The results indicated that db/db mice exhibited kidney damage along with fibrosis and that the use of LIRA resulted in an improvement in renal fibrosis.
Fig. 2.
The kidney dysfunction and fibrosis in db/db mice. (A–B) HE stain (n = 3). (C–D) Masson stain (n = 3). (E–F) Sirius red stain (n = 3). (G–H) Reticulocyte fibre stain (n = 3). (I–J) Elastic fibre stain (n = 3). (K–L) The expression and statistics of Collagen-I, Collagen-III and TGF-β proteins in kidney tissue by western blotting (n = 6). The results are presented as the mean ± SEM. The scale bar = 25 or 75 μm. *p < 0.05, **p < 0.01 vs. db/m group. #p < 0.05, ##p < 0.01 vs. db/db group.
Liraglutide markedly improved iron-dependent oxidative stress damage in db/db mice
An excess of iron intensifies the production of ROS and leads to lipid peroxidation injury. Therefore, we evaluated iron levels in db/db mice. Perls’ staining revealed iron levels increased in the kidneys of db/db mice compared to the db/m group, suggesting the presence of iron overload in db/db mice (Fig. 3A-B). In addition, the levels of iron-storage proteins, mainly ferritin heavy chain (FTH) and ferritin light chain (FTL), exhibited a significant rise in the kidney tissue of db/db mice (Fig. 3C-D). Afterwards, we proceeded to examine the expression of proteins involved in the control of iron absorption and release. Western blotting analysis showed that the db/db mice exhibited significantly lower levels of ferroportin 1 (FPN1, an iron export protein) and higher levels of divalent metal transporter 1 (DMT1, the divalent iron uptake protein) and transferrin receptor 1 (TfR1, the ferric iron uptake protein) (Fig. 3E-F). Notably, treatment with LIRA resulted in a significant reduction in kidney iron levels.
Fig. 3.
The iron levels in the kidney tissue of db/db mice. (A) Perls’ staining of kidney tissue (scale bar = 75 or 50 μm, n = 3). (B) The mean density of Fe content is shown in panel A. (C–D) The expression and statistics of FTL and FTH proteins (n = 6). (E–F) The expression and statistics of FPN1, DMT1, and TfR1 proteins by western blotting (n = 6). The results are presented as the mean ± SEM. *p < 0.05, **p < 0.01 vs. db/m group. #p < 0.05, ##p < 0.01 vs. db/db group.
At the same time, LIRA therapy effectively ameliorated iron-dependent oxidative stress damage in db/db mice. This was demonstrated by the lower levels of total superoxide dismutase (T-SOD) and catalase (CAT) activities in the renal tissue of db/db mice compared to db/m mice (Fig. 4A-B). In addition, the higher levels of serum 8-hydroxy-2-deoxyguanosine (8-OHdG), and elevated malonaldehyde (MDA) and lipid peroxidation (LPO) levels in renal tissue were also found in db/db mice (Fig. 4C-E). At the same time, NAD(P)H Oxidase 4 (NOX-4), 4-HNE and 12-Lox protein levels were all increased in the renal tissues of db/db mice as detected by western blotting (Fig. 4F-G). However, LIRA treatment increased T-SOD and CAT activities, while decreasing 8-OHdG, MDA, LPO, NOX-4, 4-HNE and 12-Lox levels compared to the db/db group (Fig. 4). These observations indicated that db/db mice exhibited elevated levels of iron and oxidative stress, which could be ameliorated by LIRA.
Fig. 4.
The oxidative stress levels in the kidney tissue of db/db mice. (A–B) The T-SOD and CAT activities in kidney tissue (n = 5). (C) The 8-hydroxy-2 deoxyguanosine (8-OHDG) in serum (n = 6). (D–E) The MDA and LPO content in kidney tissue (n = 5). (F–G) The expression and statistics of NOX-4, 4-HNE and 12-Lox proteins in kidney tissues (n = 6). The results are presented as the mean ± SEM. *p < 0.05, **p < 0.01 vs. db/m group. #p < 0 05, ##p < 0.01 vs. db/db group.
Liraglutide prevented ferroptosis in the kidneys of db/db mice
Studies have shown that ferroptosis is associated with increased levels of iron and lipid peroxidation. Electron microscopy revealed that the mitochondrial membranes of the db/db mice were wrinkled, the cristae were reduced or disappeared and the outer membrane ruptured (Fig. 5A). We then detected the activity of GSH, an indicator of ferroptosis, and observed a notable reduction in the levels of GSH and GSH-Px in db/db mice (Fig. 5B-C). In the db/db group, western blotting analysis revealed a significant decrease in the levels of GPX-4 and Fsp1 compared to the db/m group (Fig. 5D). In the meantime, immunohistochemical analysis also revealed a decrease in GPX-4 levels in the renal tissues of db/db mice (Fig. 5E). Furthermore, the ratio of NAD+/NADH and CoQ10(H2)/CoQ10 were respectively down-regulated in the kidney tissue of the db/db mice (Fig. 5F-G). However, LIRA treatment reversed all the trends (Fig. 5). These results suggested that LIRA could potentially reduce ferroptosis in the kidneys of db/db mice via the Fsp1-CoQ10-NADH pathway.
Fig. 5.
The ferroptosis in kidney tissue of db/db mice. (A) The ultrastructure of mitochondria observed by TEM. (B–C) The activity of GSH and GSH-Px in the kidney tissue (n = 5). (D) The expression and statistics of GPX-4 and Fsp1 proteins (n = 6). (E) The immunohistochemical staining of GPX-4 protein (scale bar = 100 μm, n = 3). (F–G) The ratio of NAD+/NADH and CoQ10 (H2)/CoQ10 (n = 4). The results are presented as the mean ± SEM. *p < 0.05, **p < 0.01 vs. db/m group. #p < 0.05, ##p < 0.01 vs. db/db group.
Liraglutide inhibited the HG-induced ferroptosis in HK-2 cells
To further verify whether LIRA suppresses ferroptosis, we selected HK-2 cells with high glucose (HG) to mimic hyperglycaemia in vitro. HK-2 cells were treated with different concentrations of HG for 24 and 48 h (Fig. 6A-B). Therefore, we chose a concentration of 30 mM HG for 48 h as the condition for later cell culture. Then, cells were divided into: Con, HG, RSL-3 (5 µM), LIRA (HG + 500 nM LIRA), and Fer-1 (HG + 5 µM Fer-1) groups. Cell viability was significantly reduced in the HG and RSL-3 groups compared to the control group, as shown in Fig. 6C. Furthermore, the cell viability recovered in the LIRA group was comparable to that in the Fer-1 group (Fig. 6C-E). Next, we observed the ultrastructural characteristics of mitochondria and mitochondrial membrane potential (MMP) in each group. In the HG group, the mitochondria were smaller with fragmented ridges and localized fat infiltration (Fig. 6F), accompanying with the decline of MMP levels (Fig. 6G), which was similarly to the results in the RSL-3 group. The administration of LIRA and Fer-1 improved the mitochondrial ultrastructure and MMP, as shown in Fig. 6F-G. In addition, the MFI of BODIPY 581/591 C11 was elevated in the HG group, and the alterations were reversed by LIRA treatment (Fig. 6H-I). Western blotting analysis revealed that GPX-4 and Fsp1 expression levels were decreased in the HG group, aligning with the in vivo findings (Fig. 6J). In contrast, the LIRA and Fer-1 groups displayed contrasting outcomes (Fig. 6J). Meanwhile, the immunofluorescence double-label staining revealed a notable rise in the expression of 4-HNE protein and a decline in the expression of Fsp1 protein in HK-2 cells following exposure to HG, which could be improved by LIRA and Fer-1 (Fig. 6K). Furthermore, the ratio of NAD+/NADH and CoQ10(H2)/CoQ10 were respectively down-regulated in the HG group, and it was reversed by LIRA and Fer-1 treatment (Fig. 6L-M). These results further confirmed that LIRA could inhibit ferroptosis induced by HG.
Fig. 6.
Liraglutide inhibited HG-induced ferroptosis in HK-2 cells. (A–B) The cell viability of HK-2 cells treated with HG with 0, 10, 20, 30, and 50 mM for 24 h and 48 h respectively (n = 5, **p < 0.01 vs. Con group). (C) The cell viability of HK-2 cells treated HG (30 mM), RSL3 (5 µM), liraglutide (LIRA, 500 nM), and Fer-1 (5 µM) (n = 6). (D–E) The cell viability of HK-2 cells detected by flow cytometry (n = 3). (F) The ultrastructure of HK-2 cells mitochondria was observed by TEM (scale bar = 1.0 μm, n = 3). (G) The JC-1 staining of HK-2 cells (scale bar = 100 μm, n = 3). (H–I) The fluorescence absorption spectrum of BODIPY 581/591 C11 (scale bar = 100 μm, n = 5). (J) The expression and statistics of GPX-4 and Fsp1 proteins (n = 3). (K) The immunofluorescence double label staining of Fsp1 and 4-HNE (scale bar = 50 μm, n = 3). (L–M) The ratio of NAD+/NADH and CoQ10 (H2)/CoQ10 (n = 4). The results are presented as the mean ± SEM. *p < 0.05, **p < 0.01 vs. Con group. #p < 0.05, ##p < 0.01 vs. HG group.
Liraglutide inhibited HG-induced ferroptosis in HK-2 cells via Fsp1-CoQ10
To determine the inhibitory effect of LIRA on ferroptosis via the Fsp1-CoQ10 signaling pathway, we selected iFsp1, an inhibitor of Fsp1, for validation in vitro. The cells were divided into HG, HG + iFsp1, and HG + iFsp1 + Fer-1 groups. The CCK8 results showed a decrease in cell viability with the addition of iFsp1 compared to the HG group. However, the addition of Fer-1 was able to restore cell viability (Fig. 7A). The results obtained from the BODIPY 581/591 C11 probe indicated a significant decrease in lipid peroxidation levels in the HG + iFsp1 + Fer-1 group compared to the HG + iFsp1 group (Fig. 7B-C).
Fig. 7.
Liraglutide inhibited HG-induced ferroptosis via Fsp1-CoQ10. (A) The cell viability of HK-2 cells (n = 6, **p < 0.01 vs. HG group, #p < 0.05 vs. HG + iFsp1 group). (B–C) The fluorescence absorption spectrum of BODIPY 581/591 C11 (scale bar = 100 μm, n = 3, **p < 0.01 vs. HG group, ##p < 0.01 vs. HG + iFsp1 group). (D) The cell viability of HK-2 cells (n = 6, **p < 0.01 vs. HG + LIRA group). (E–F) The fluorescence absorption spectrum of BODIPY 581/591 C11 (scale bar = 100 μm, n = 3, **p < 0.01 vs. HG + LIRA group). (G) Flow cytometry results of BODIPY 581/591 C11 probe. (H) The immunofluorescence double label staining of Fsp1 and 4-HNE (scale bar = 50 μm, n = 3). (I–J) The expression and statistics of GPX-4 and Fsp1 proteins (n = 3, **p < 0.01 vs. HG + LIRA group). (K–L) The ratio of NAD+/NADH and CoQ10 (H2)/CoQ10 (n = 4, *p < 0.05 vs. HG + LIRA group). The results are presented as the mean ± SEM.
To further verify whether the Fsp1-CoQ10 pathway was involved in the protection of LIRA, the cells were divided into the HG + LIRA and HG + iFsp1 + LIRA groups. The CCK8 results demonstrated a decrease in cell viability in the HG + iFsp1 + LIRA in comparison to the HG + LIRA group (Fig. 7D). Meanwhile, there was a significant elevation in lipid peroxidation levels within the HG + iFsp1 + LIRA group compared to the HG + LIRA group (Fig. 7E-G). Immunofluorescence double label staining indicated Fsp1 levels decreased and 4-HNE levels increased in the HG + iFsp1 + LIRA group compared to the HG + LIRA group (Fig. 7H). Western blotting analysis results further validated that GPX-4 and Fsp1 expression levels were all decreased in the HG + iFsp1 + LIRA group in comparison to the HG + LIRA group (Fig. 7I-J). Furthermore, the ratio of NAD+/NADH and CoQ10(H2)/CoQ10 were respectively down-regulated in the HG + iFsp1 + LIRA group (Fig. 7K-L). These findings suggested that LIRA might have a role in inhibiting ferroptosis by upregulating Fsp1 expression, and this inhibitory function of LIRA was partially hindered by the administration of iFsp1.
Discussion
The global prevalence of T2DM is increasing significantly due to rapid economic development and urbanisation20. The primary pathological features of T2DM are insulin resistance and hyperinsulinemia, with excessive release of glucose into the bloodstream being a major contributor to fasting hyperglycemia21. In normal physiology, the kidneys exhibit both glucose consumption and release, constituting approximately 20% of the overall endogenous glucose release. There is a notable deviation in renal glucose release, which is linked to heightened renal glycogenolysis in T2DM22. Organismal blood glucose levels are consistently regulated, with gluconeogenesis serving as a crucial metabolic pathway for synthesizing glucose from non-hexose precursors to maintain glucose homeostasis23. Renal gluconeogenesis contributes to 40% of endogenous gluconeogenesis and is regulated by insulin, cellular glucose levels, and stress hormones, highlighting the kidney’s significant role in preserving glucose levels during diverse stress conditions24.
Research has demonstrated that kidney disease is a prevalent chronic complication of T2DM, with a morbidity of approximately 30–40%25. Several studies have provided evidence that elevated glucose levels could induce oxidative stress and pathological alterations, including diffuse glomerulosclerosis, tubulointerstitial fibrosis, and fibrosis in renal mesangial cells and tubular cells26. Furthermore, sustained hyperglycemia results in the buildup of advanced glycation end-product precursors, ultimately leading to apoptosis of glomerular and tubular cells27,28. GLP-1 is the body’s main incretin hormone and its receptor agonist can stimulate the ability of patients with T2DM to secrete insulin, reduce the expression of inflammatory markers such as CD68 in the kidney, and reduce the risk of kidney damage and worsening of diabetes-induced albuminuria. Glomerular endothelial cells are protected by inhibition of Ang II in the signaling pathway at phosphorylated c-Raf (Ser338)29–31. In addition, there is increasing evidence that GLP-1 also plays a role in renal protection during acute kidney injury (AKI), including alleviation of apoptosis and inhibition of renal interstitial fibrosis32,33. When the kidney is damaged, excessive connective tissue accumulation during repair or reaction leads to renal interstitial fibrosis, and activated myofibroblasts are the main matrix-secreting cell type34, with TGF-β as its major regulator35. Several studies have confirmed the suppressive effect of LIRA on the progression of fibrosis in the liver of db/db mice36 and the renal fibrosis induced by unilateral ureteral obstruction (UUO) by inhibiting the TGF-β/Smad3 signaling pathway16. Our findings demonstrated that db/db mice exhibited significantly heightened levels of blood creatinine and urea nitrogen, accompanied by renal fibrosis, elevated TGF-β, and collagen fibres. However, the administration of LIRA significantly alleviated these manifestations. Studies have shown that ferroptosis plays a crucial part in the advancement of renal fibrosis37. This association is supported by the shared metabolic pathways of ferroptosis and fibrosis, such as glycolysis and glutamine catabolism. Importantly, enhanced glutamine catabolism is pivotal in the activation of fibroblasts, and the subsequent facilitation of the tricarboxylic acid cycle further contributes to the initiation of ferroptosis38,39. Renal tubular epithelial cells are highly susceptible to ferroptosis, leading to the release of pro-fibrotic mediators, specifically TGF-β and connective tissue growth factor (CTGF), and these mediators then regulate the proliferation and differentiation of mesenchymal fibroblasts through paracrine signaling10.
Additionally, ferroptosis leads to a decline in the intracellular GSH level and a rise in lipid peroxidation40. Conversely, the application of ferroptosis blockers, like Fer-1 or desferrioxamine (DFO), has been found to impede the paracrine impacts of pro-fibrotic factors in epithelial cells40,41. Moreover, the study also has reported that the hypoxia inducible factor-1α/heme oxygenase 1 (HIF-1α/HO-1) pathway exacerbated the progression of renal tubular injury and fibrosis induced by renal ferroptosis in a mouse model of diabetes42. Moreover, the administration of an xCT inhibitor has been demonstrated to exert a protective effect against renal fibrosis in rats with UUO by efficiently decreasing the quantities of TGF-β, ROS, and lipid peroxides43,44. The protective mechanism is accomplished by the facilitation of ferritin degradation and modulation of the xCT/GPX-4 axis45. Consequently, the suppression of ferroptosis presents a promising approach for alleviating renal fibrotic damage.
The occurrence of ferroptosis in the renal system is believed to be primarily driven by impaired iron metabolism, accumulation of lipid peroxides, and an imbalance in antioxidant capacity46. Iron overload has been identified as a contributing factor to ferroptosis, playing a secondary role in both T2DM patients47 and db/db mice48. The elevated cellular iron levels may lead to pancreatic β-cell dysfunction49 and kidney damage50,51, attributed to the exacerbation of oxidative stress through the Fenton reaction50,51. Furthermore, prolonged episodes of elevated blood glucose levels possess the capability to stimulate an increase in the utilization of oxygen by mitochondria, hinder mitochondrial functionality, activate NAD(P)H activity, and subsequently intensify the production of ROS. This process further exacerbates the dysfunction of β-cells and insulin resistance52. A previous study has demonstrated the GSH-GPX-4 pathway of ferroptosis, with phospholipid peroxides (PLOOHs), a lipid derived from ROS, playing a pivotal role in this inhibition53.
The cystine/glutamate reverse transporter (system Xc−) facilitates the entry of cystine into the cell, where it undergoes reduction to cysteine through either the GSH-dependent or thioredoxin reductase 1 (TXNRD1)-dependent cystine reduction pathway. This pathway facilitates the synthesis of GSH, a highly efficient reductant that acts as a coenzyme for GPX-4. GSH plays an essential part in the elimination of lipid oxides by converting them into non-harmful alcohols, which is crucial for scavenging free radicals and safeguarding cells from oxidative stress54. In our study, we observed a significant decrease in the levels of GSH and GPX-4 and an increase in the levels of LPO in the kidney tissue of db/db mice. These observations suggest an imbalance in the Xc− system in hyperglycaemia (Fig. 8).
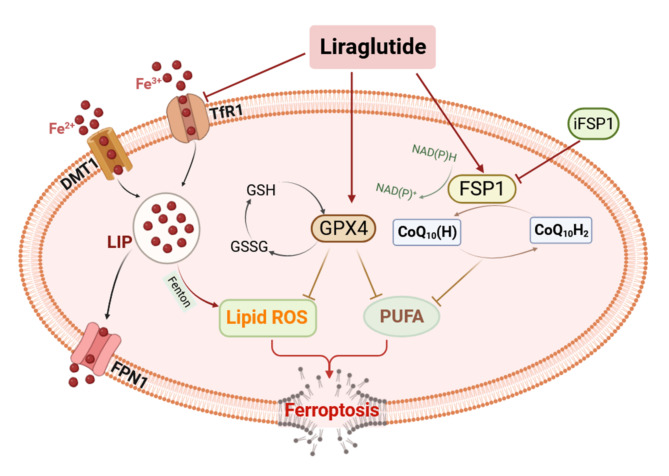
Fig. 8.
The schematic diagram illustrates the involvement of ferroptosis in the kidneys of db/db mice. HG has been found to induce renal fibrosis and ferroptosis in diabetic mice. This is attributed to the increased levels of TfR1 and DMT1, as well as the decreased level of FPN1, which results in elevated LIP in the kidney. The excess of labile iron pool (LIP), primarily consisting of Fe2+, exacerbates the generation of lipid ROS in response to the Fenton reaction. Furthermore, the reduced expression of Fsp1 hinders the CoQ10 to its reduced form, CoQ10(H2), thereby impeding its antioxidant effects. Additionally, the imbalance in the Xc− system disrupts the exchange and synthesis of GSH, leading to the inhibition of GSH-dependent antioxidant defences and ultimately resulting in ferroptosis. Liraglutide has the potential to enhance the expression of Fsp1 and ameliorate the Xc− system, thereby mitigating the ferroptosis response in the renal tissue of db/db mice.
To date, advancements concerning experimental pharmaceuticals targeting ferroptosis in animal or cellular models of diabetes, various interventions such as hypoglycemic agents, antihypertensive agents, and traditional Chinese herbal medicines have shown efficacy in inhibiting ferroptosis55. Among these interventions, hypoglycemic drugs have been widely utilized, with dapagliflozin being identified as a mitigating agent for diabetic kidney disease (DKD) through ferroptosis inhibition. Furthermore, empagliflozin has the potential to improve cardiac function by reducing ferroptosis. Additionally, empagliflozin has demonstrated the ability to enhance cardiac function by decreasing ferroptosis through NLRP3-MyD88 pathways in mice treated with doxorubicin56,57. LIRA was approved for managing T2DM and obesity58. LIRA has demonstrated its ability to reduce non-alcoholic fatty liver disease linked to T2DM by activating the AMPK/ACC pathway and inhibiting ferroptosis59. In addition, LIRA could attach to the GLP-1 receptor, leading to the enhancement of hepatic lipid homeostasis by activating hepatic fibroblast growth factor 21 signaling pathway60. According to our previous investigation, LIRA triggered the Nrf2/HO-1 pathway, resulting in decreased levels of oxidative stress in the liver and brain of db/db mice, thereby inhibiting ferroptosis36,61. It’s clear that the main regulators of ferroptosis in well-known metabolic pathways have been extensively studied, but there is an urgent need to investigate other novel mechanisms involved in ferroptosis.
Undoubtedly, GPX plays a crucial role in the regulation of ferroptosis. However, recent research has identified three GPX-4-independent pathways, namely Fsp1/CoQ10, GCH1, and DHODH, which are involved in this process46. Extensive research has been carried out to investigate key regulators of ferroptosis within established pathways, leading to the need for further exploration of innovative mechanisms contributing to ferroptosis. Recent genetic screening studies have successfully identified the Fsp1/CoQ10 system as a comprehensive safeguard against ferroptosis induced by pharmacological inhibition of GPX-4 or GPX-4 knockdown. This discovery implies the presence of regulatory mechanisms that operate independently of the GPX-4/GSH axis, thereby offering protection against ferroptosis within the organism62,63. Our results also found the inhibition of the GPX-4/GSH axis as showed by LIRA (Fig. 8).
Fsp1, a highly conserved NAD(P)H-dependent ubiquinone oxidoreductase, is found in both bacterial and mammalian systems64. Following translation, Fsp1 undergoes carbamylation modification, which serves to anchor it to the cellular membrane65. This modification enables the oxidation of NAD(P)H to NAD(P)+, utilizing flavin adenine dinucleotide (FAD) acting as a cofactor. Furthermore, Fsp1 facilitates the conversion of reducing CoQ10 at the plasma membrane to CoQ10(H2), leading to the reduction of toxic lipid peroxides to non-toxic lipids and exhibiting anti-ferroptosis effects66. Evidence suggests that Fsp1 plays a role in maintaining the normal structure and function of renal tissues. Specifically, the lack of Fsp1 in renal tubular epithelial cells has been linked to tubular necrosis67, whereas an upregulation of Fsp1 expression in these cells has been observed to impede cellular ferroptosis and alleviate acute kidney injury68. Moreover, the ubiquitination of Fsp1 appeared to have a role in the occurrence of tubular ferroptosis during episodes of acute kidney injury69. Our study revealed that LIRA enhanced the Fsp1 expression while enhancing the CoQ10(H2)/CoQ10 levels in the kidney tissue of db/db mice (Fig. 8). Similarly, these observations were replicated in HK-2 cells cultured under HG conditions, indicating the suppression of LIRA by the Fsp1-CoQ10 pathway.
Collectively, these combined results indicated that ferroptosis played a role in renal fibrosis and contributed to kidney injury in db/db mice, possibly because of the increased levels of ROS, lipid peroxidation, and iron accumulation. Notably, our research suggested that LIRA might offer a protective effect against ferroptosis, which was not solely reliant on GPX-4 but also involved the Fsp1-CoQ10 pathway. However, it was important to note that these initial findings might serve as a foundation for future investigations into the therapeutic potential of LIRA in the management of renal ferroptosis and fibrotic injury.
Methods
Ethical approval
All the animal experiments were performed in compliance with the guidelines of ARRIVE and Committee of Experimental Animal Welfare Ethics of the Hebei University of Chinese Medicine. The Committee of Experimental Animal Welfare Ethics of the Hebei University of Chinese Medicine approved all the experimental protocols (DWLL2020089).
Animals
The non-diabetic littermate db/m mice (C57BLKS, SPF grade, male, 8 weeks) and the db/db mice (C57BLKS, SPF grade, male, 8 weeks) were obtained from Changzhou Cavens Experimental Animal Co., Ltd (License number: SCXK (Su) 2021-0013). All the mice were housed in the animal center that maintained a controlled temperature range of 22–24 °C and humidity level of 55-60%. Following a week of adaptive feeding, the db/db mice (n = 18) were randomly allocated into two groups: the db/db group and the LIRA group. The mice in the LIRA group received intraperitoneal injections of LIRA (200 mg/kg/d, MCE, HY-P0014) from the 9th to the 15th week, while the control group (db/m) group was administered equivalent quantities of saline. Once a week, the blood glucose levels were examined using a blood glucose meter (S59400839789, Sinocare). Finally, the mice were anesthetized and sacrificed by intraperitoneal injection of sodium pentobarbital and then blood and kidney tissue was collected to further analyses. The body weight and kidney weight of the mice in each group were counted, and the kidney coefficient (kidney coefficient = kidney weight/body weight, KLW/BW) was calculated.
Intraperitoneal pyruvic acid tolerance test
On the penultimate day before the culmination of the modelling process, an intraperitoneal pyruvate tolerance test was administered. Following a period of 14 h of fasting, the mice were subjected to an intraperitoneal injection of sodium pyruvate at a dosage of 2 g/kg. Utilizing the tail-blood collection technique, blood glucose levels were assessed at intervals of 15, 30, 60, and 120 min. Afterwards, the calculation of the area under the dynamic glucose curve (AUC) was performed.
Blood pressure
Tail artery pressure was measured in each group using the non-invasive tail sleeve method. The experimental platform was preheated for 30 min, awake mice were fixed to the experimental platform with a magnetic fixator, the mouse tail was passed through the sleeve and fixed to the experimental platform with tape, the rubber sleeve was pressed onto the root of the mouse tail and the measurement began after 5–10 min of adaptation.
Pathological staining
Pathological changes in the kidney were analyzed using HE stain. The paraffin sections of the kidneys were deparaffinized by a gradient of alcohol. Subsequently, the sections were subjected to hematoxylin stain solution, followed by ethanol-hydrochloric acid differentiation, eosin re-staining, another round of gradient alcohol dehydration, and xylene for clarification. Finally, the slices were sealed with neutral resin. Renal fibrosis was evaluated by Masson stain, Sirius red stain solution, reticular fibre stain solution, and elastic fibre stain solution.
ELISA
beta 2-MG (E-EL-M2411, Elabscience), 8-OHdG (E-EL-0028), and NAG (E-BC-K064-M, Elabscience) in serum were measured by ELISA. Mice serum was collected and measured according to the product instructions. Finally, the absorbance of beta 2-MG, 8-OHdG and NAG were detected separately using a multifunctional microplate reader (Varioskan LUX, Thermo Fisher Scientific) at the wavelength of 450 nm, 450 nm and 400 nm. The corresponding activity or concentration was calculated according to the standard curve.
Biochemical analysis
Serum levels of BUN (S03036, Shenzhen Leidu Technology, China), SCr (S03076, Shenzhen Leidu Technology), albumin (ALB, S03043, Shenzhen Leidu Technology), TG (S03027, Shenzhen Leidu Technology) and CHO (S03042, Shenzhen Leidu Technology) were determined using a biochemical analyser (Chemray 800, Shenzhen Leidu Technology). Mice serum was collected and the appropriate volume was added to be measured by fully automated detection. The concentration was calculated the according to their formulas.
Transmission electron microscope
The ultrastructure of mitochondria in HK-2 cells and mouse kidney tissues was observed by TEM. After centrifugation to collect cells or tissue precipitates for fixation, samples were embedded, polymerised and ultrathin sectioned at a thickness of 60–80 nm. Sections were stained in the dark with a 2% uranyl acetate saturated alcohol solution to enhance contrast. Images were examined using a HITACHI HT7700 electron microscope.
Antioxidant and lipid peroxidation products
The mouse kidney tissues were subjected to homogenization at a concentration of 10% (g/V), and subsequent measurements were conducted following the guidelines provided by the kit manufacturer. The activities of T-SOD (A001-1-2, Nanjing Jiancheng Bioengineering Institute), GSH (A006-2-1, Nanjing Jiancheng Bioengineering Institute), GSH-Px (A005-1-2, Nanjing Jiancheng Bioengineering Institute), and CAT (A007-1-1, Nanjing Jiancheng Bioengineering Institute) were assessed to evaluate the antioxidant capacity. MDA (A003-1-2, Nanjing Jiancheng Bioengineering Institute) and LPO (A106-1-2, Nanjing Jiancheng Bioengineering Institute) were used as by-products of lipid peroxidation.
Perls’ staining
Perls’ staining was achieved by combining potassium ferricyanide and hydrochloric acid, which reacts with Fe3+ to generate soluble colouring. The sections went through dewaxing and were washed three times in phosphate buffer (PBS, 0.01 M, pH 7.4). Subsequently, they were exposed to 3% H2O2 for 20 min at room temperature (RT). Subsequently, the staining solution was immersed for six hours at RT. After staining, the sections were DAB enhanced, re-stained with hematoxylin, and sealed using neutral glue. Positive cells were observed under the microscope.
Immunohistochemistry
After deparaffinisation and rehydration, the sections were incubated with 3% H2O2 to remove endogenous peroxidase activity. Subsequently, antigen retrieval at high temperature was carried out, and the sections were incubated with 10% goat serum at 37 °C for 1 h. Following this, GPX-4 (ET1706-45, Huabio) and Fsp1 (20886-1-AP, Proteintech) were incubated overnight at 4 °C. Subsequently, the sections were incubated with secondary antibodies labelled with HRP label for 1 h at 37 °C. Finally, the sections were stained using a DAB kit and sealed with a sealer. The average density of positive proteins was calculated using IPP 6.0 software.
Cell culture
Human Renal Tubular Epithelial Cells, HK-2 Cells, was purchased from Wuhan Pricella Biotechnology Co., Ltd (CL-0109). HK-2 cells were cultured in penicillin, streptomycin, 10% fetal bovine serum and MEM medium at 37 °C and 5% CO2. To simulate diabetes, the cells were exposed to HG conditions. HK-2 cells were cultured with different concentrations of HG for 48 h to evaluate the protective effect of LIRA. At the same time, the cells were treated individually with liraglutide, RSL-3 (a ferroptosis activator, MCE, HY-100218 A), Ferrostatin-1 (Fer-1, an inhibitor of ferroptosis, MCE, HY-100579), and iFsp1 (an inhibitor of Fsp1, MCE, HY-136057). Following the treatment, cell viability and western blotting analysis were conducted for assessment.
Cell viability
Cell viability was assessed using the Cell Counting Kit-8 (CCK-8). HK-2 cells were seeded into 96-well culture plates with a density of 1 × 104 cells per well. After treatment with or without HG, CCK-8 reagents were added to the plates and incubated at 37 °C for 2 h. Absorbance was measured at a wavelength of 450 nm using a multifunctional microplate reader (Varioskan LUX, Thermo Fisher Scientific).
After the treatment of cells in each group, the adherent cells were digested with EDTA-free trypsin, washed twice with pre-cooled PBS, and centrifuged to collect the precipitates. The cells were resuspended with 500 µL binding buffer, then 5 µL annexin V and PI were added and incubated for 15 min in the dark. Finally, apoptosis was detected by flow cytometry (FC 500 MCL, Beckman Coulter).
JC-1 staining
JC-1 staining was used to measure the MMP levels. Cells were seeded on 6 cm cell culture at a density of 2 × 105/per well. Before staining, the cells were prepared by removing the supernatant and washing them three times with PBS. JC-1 staining solution was then added according to the instructions and the cells were incubated for 30 min at RT in the dark. Finally, the fluorescence signals were observed under a fluorescence microscope.
Lipid peroxidation assay
After inoculation the HK-2 cells into a 24-well plate, the lipid peroxidation sensor BODIPY 581/591 C11 (10 µM, D3861, Invitrogen) was added and incubated for 30 min at 37 °C. Afterwards,
the culture medium was removed and the cells were rinsed three times with PBS. Fluorescence measurements were performed at two different wavelengths: one with an excitation/emission of 581/591 nm and the other at 488/510 nm. The ratio of the fluorescence intensities emitted at 590 nm and 510 nm provides a quantitative assessment of lipid peroxidation within the cells.
NAD/NADH assay
NAD/NADH levels in mouse kidney tissue and HK-2 cells were measured using the NAD/NADH Assay Kit (MM-44925M1/MM-45162M2, MEIMIAN). Tissues or cells were washed with cold PBS, homogenised in NAD/NADH extraction buffer and assayed according to the kit instructions. The concentrations of NAD/NADH were calculated from a standard curve.
CoQ10 (H2)/CoQ10 assay
CoQ10(H2)/CoQ10 levels in mouse kidney tissue and HK-2 cells were detected using the CoQ10/CoQ10(H2) Assay Kit (MM45967M2/MM-45496M2, MEIMIAN). Tissues or cells were washed with cold PBS and homogenised in extraction buffer, and then the assay procedure was performed according to the kit instructions. The CoQ10(H2)/CoQ10 levels were calculated from the standard curve.
Western blotting analysis
Kidney tissue or HK-2 cells were prepared separately for homogenisation with cooled PIRA lysate. The supernatant was collected and the amount of total protein was measured. The proteins were then separated by SDS-PAGE electrophoresis and transferred to PVDF membranes. The blots were incubated with 5% skimmed milk at RT for 2 h and then washed with TBST. The blots were then incubated with primary antibodies overnight at 4 °C, including Collagen-I (GB112543, Servicebio), Collagen-III (GB11023, Servicebio), TGF-β(bs-0086R, Bioss), NOX-4 (A00403, Boster), 4-HNE (ARG23717, Arigo), MDA (ab243066, Abcam), TfR1(3-6800, Invitrogen), DMT1(abs112967, Absin), FPN1(MTP11-A, Alpha Diagnostic International), FTH(ab183781, Abcam), FTL(ab218400, Abcam), GPX-4, Fsp1, GAPDH (GB15002, Servicebio), β-actin (GB15001, Servicebio), α-Tubulin (GTX628802, GeneTex). On the second day, blots were incubated with secondary antibodies conjugated with HRP at RT. The ECL method was used to determine the immunoreactive protein, while the mean grey value was determined using Image J software.
Statistical analysis
The data were analyzed using SPSS 23.0 statistical software and presented as mean ± SEM. The data was analyzed using a one-way ANOVA, followed by a post hoc LSD test. Significance was determined at p < 0.05. Graphs were generated using Prism 9.0 software.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Thanks to all authors for their contributions. This work was supported by the Hebei Province medical science research project (NO. 20231577, 20241868).
Author contributions
Conceptualization: Yashuo Zhao, Qi Chen, Ji-Xian Song, Miao Tan; Methodology: Qi Chen, Ji-Xian Song, Yu-Jing Gou and Zhi Zhang; Resources: Qi Chen, Ji Xian Song, Ji-Ren An, Miao Tan; Data Curation: Ji-Xian Song, Ji-Ren An, Qi Chen, and Zhi Zhang; Writing-Original Draft: Ji-Xian Song, Qi Chen; Writing- Review & Editing: Yashuo Zhao and Miao Tan; Visualization: Ji-Xian Song, Ji-Ren An, Qi Chen, Yu-Jing Gou; Supervision: Yashuo Zhao, and Miao Tan; Funding acquisition: Yashuo Zhao and Miao Tan. All authors contributed to the article and approved the submitted version.
Data availability
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. Further inquiries can be directed to the corresponding author.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qi Chen, Ji-Xian Song and Zhi Zhang have contributed equally to this work.
Contributor Information
Miao Tan, Email: 13731195253@163.com.
Yashuo Zhao, Email: zys870207@126.com.
References
- 1.Ahmad, E., Lim, S., Lamptey, R., Webb, D. R. & Davies, M. J. Type 2 diabetes. Lancet400, 1803–1820. 10.1016/S0140-6736(22)01655-5 (2022). [DOI] [PubMed] [Google Scholar]
- 2.Collaborators, G. B. D. D. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the global burden of Disease Study 2021. Lancet402, 203–234. 10.1016/S0140-6736(23)01301-6 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michos, E. D. & Tuttle, K. R. GLP-1 receptor agonists in diabetic kidney disease. Clin. J. Am. Soc. Nephrol.16, 1578–1580. 10.2215/CJN.18771220 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leaf, I. A. & Duffield, J. S. What can target kidney fibrosis? Nephrol. Dial Transpl.32, i89–i97. 10.1093/ndt/gfw388 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Kuppe, C. et al. Decoding myofibroblast origins in human kidney fibrosis. Nature589, 281–286. 10.1038/s41586-020-2941-1 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tuleta, I. & Frangogiannis, N. G. Diabetic fibrosis. Biochim. Biophys. Acta Mol. Basis Dis.1867, 166044. 10.1016/j.bbadis.2020.166044 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang, Y. et al. Therapeutic implications of ferroptosis in renal fibrosis. Front. Mol. Biosci.9, 890766. 10.3389/fmolb.2022.890766 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang, X., Stockwell, B. R. & Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell. Biol.22, 266–282. 10.1038/s41580-020-00324-8 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daenen, K. et al. Oxidative stress in chronic kidney disease. Pediatr. Nephrol.34, 975–991. 10.1007/s00467-018-4005-4 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Zhang, B. et al. Liproxstatin-1 attenuates unilateral ureteral obstruction-induced renal fibrosis by inhibiting renal tubular epithelial cells ferroptosis. Cell Death Dis.1210.1038/s41419-021-04137-1 (2021). [DOI] [PMC free article] [PubMed]
- 11.Soulage, C. O. et al. Two toxic lipid aldehydes, 4-hydroxy-2-hexenal (4-HHE) and 4-hydroxy-2-nonenal (4-HNE), Accumulate in patients with chronic kidney disease. Toxins (Basel). 12. 10.3390/toxins12090567 (2020). [DOI] [PMC free article] [PubMed]
- 12.Drucker, D. J. GLP-1 physiology informs the pharmacotherapy of obesity. Mol. Metab.57, 101351. 10.1016/j.molmet.2021.101351 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaman, A. M. et al. Effect of the Glucagon-Like Peptide-1 receptor agonists semaglutide and Liraglutide on kidney outcomes in patients with type 2 diabetes: Pooled analysis of SUSTAIN 6 and LEADER. Circulation145, 575–585. 10.1161/CIRCULATIONAHA.121.055459 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mann, J. F. E. et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl. J. Med.377, 839–848. 10.1056/NEJMoa1616011 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Skov, J. et al. Short-term effects of liraglutide on kidney function and vasoactive hormones in type 2 diabetes: A randomized clinical trial. Diabetes Obes. Metab.18, 581–589. 10.1111/dom.12651 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Li, Y. K. et al. The glucagon-like peptide-1 (GLP-1) analog liraglutide attenuates renal fibrosis. Pharmacol. Res.131, 102–111. 10.1016/j.phrs.2018.03.004 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Panagaki, T., Michael, M. & Holscher, C. Liraglutide restores chronic ER stress, autophagy impairments and apoptotic signalling in SH-SY5Y cells. Sci. Rep.7, 16158. 10.1038/s41598-017-16488-x (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qi, L. et al. Liraglutide reduces oxidative stress and improves energy metabolism in methylglyoxal-induced SH-SY5Y cells. Neurotoxicology92, 166–179. 10.1016/j.neuro.2022.08.007 (2022). [DOI] [PubMed] [Google Scholar]
- 19.Azushima, K., Gurley, S. B. & Coffman, T. M. Modelling diabetic nephropathy in mice. Nat. Rev. Nephrol.14, 48–56. 10.1038/nrneph.2017.142 (2018). [DOI] [PubMed] [Google Scholar]
- 20.Cao, R. et al. Signaling pathways and intervention for therapy of type 2 diabetes mellitus. MedComm (2020). 4, e283. 10.1002/mco2.283 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Group, G. S. R. et al. Glycemia reduction in type 2 diabetes - glycemic outcomes. N Engl. J. Med.387, 1063–1074. 10.1056/NEJMoa2200433 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas, M. C. et al. Diabetic kidney disease. Nat. Rev. Dis. Primers. 1, 15018. 10.1038/nrdp.2015.18 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Legouis, D., Faivre, A., Cippa, P. E. & de Seigneux, S. Renal gluconeogenesis: An underestimated role of the kidney in systemic glucose metabolism. Nephrol. Dial Transpl.37, 1417–1425. 10.1093/ndt/gfaa302 (2022). [DOI] [PubMed] [Google Scholar]
- 24.Verissimo, T. et al. Decreased renal gluconeogenesis is a Hallmark of chronic kidney disease. J. Am. Soc. Nephrol.33, 810–827. 10.1681/ASN.2021050680 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tuttle, K. R. et al. Incidence of chronic kidney disease among adults with diabetes, 2015–2020. N Engl. J. Med.387, 1430–1431. 10.1056/NEJMc2207018 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furuichi, K. et al. Nationwide multicentre kidney biopsy study of Japanese patients with type 2 diabetes. Nephrol. Dial Transpl.33, 138–148. 10.1093/ndt/gfw417 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Yin, W. et al. Protein kinase C and protein kinase A are involved in the protection of recombinant human glucagon-like peptide-1 on glomeruli and tubules in diabetic rats. J. Diabetes Investig.10, 613–625. 10.1111/jdi.12956 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhuang, A. & Forbes, J. M. Diabetic kidney disease: A role for advanced glycation end-product receptor 1 (AGE-R1)? Glycoconj. J.33, 645–652. 10.1007/s10719-016-9693-z (2016). [DOI] [PubMed] [Google Scholar]
- 29.Mima, A., Nomura, A. & Fujii, T. Current findings on the efficacy of incretin-based drugs for diabetic kidney disease: A narrative review. Biomed. Pharmacother165, 115032. 10.1016/j.biopha.2023.115032 (2023). [DOI] [PubMed] [Google Scholar]
- 30.Mima, A. et al. Effects of incretin-based therapeutic agents including tirzepatide on renal outcomes in patients with type 2 diabetes: A systemic review and meta-analysis. Metabol Open.17, 100236. 10.1016/j.metop.2023.100236 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mima, A. et al. Protective effects of GLP-1 on glomerular endothelium and its inhibition by PKCbeta activation in diabetes. Diabetes61, 2967–2979. 10.2337/db11-1824 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katagiri, D. et al. Protection of glucagon-like peptide-1 in cisplatin-induced renal injury elucidates gut-kidney connection. J. Am. Soc. Nephrol.24, 2034–2043. 10.1681/ASN.2013020134 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katagiri, D. et al. Interstitial renal fibrosis due to multiple cisplatin treatments is ameliorated by semicarbazide-sensitive amine oxidase inhibition. Kidney Int.89, 374–385. 10.1038/ki.2015.327 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Li, J. et al. MicroRNA-10a/b inhibit TGF-beta/Smad-induced renal fibrosis by targeting TGF-beta receptor 1 in diabetic kidney disease. Mol. Ther. Nucleic Acids28, 488–499. 10.1016/j.omtn.2022.04.002 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carlisle, R. E. et al. TDAG51 induces renal interstitial fibrosis through modulation of TGF-beta receptor 1 in chronic kidney disease. Cell Death Dis.12, 921. 10.1038/s41419-021-04197-3 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song, J. X. et al. Liraglutide attenuates hepatic iron levels and ferroptosis in db/db mice. Bioengineered13, 8334–8348. 10.1080/21655979.2022.2051858 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu, Y. & Chen, Y. Research progress on ferroptosis in diabetic kidney disease. Front. Endocrinol.13, 945976. 10.3389/fendo.2022.945976 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henderson, N. C., Rieder, F. & Wynn, T. A. Fibrosis: from mechanisms to medicines. Nature587, 555–566. 10.1038/s41586-020-2938-9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng, J. & Conrad, M. The metabolic underpinnings of Ferroptosis. Cell. Metab.32, 920–937. 10.1016/j.cmet.2020.10.011 (2020). [DOI] [PubMed] [Google Scholar]
- 40.Kim, S. et al. Characterization of ferroptosis in kidney tubular cell death under diabetic conditions. Cell Death Dis.1210.1038/s41419-021-03452-x (2021). [DOI] [PMC free article] [PubMed]
- 41.Ide, S. et al. Ferroptotic stress promotes the accumulation of pro-inflammatory proximal tubular cells in maladaptive renal repair. Elife1010.7554/eLife.68603 (2021). [DOI] [PMC free article] [PubMed]
- 42.Feng, X. et al. Ferroptosis enhanced diabetic renal tubular injury via HIF-1alpha/HO-1 pathway in db/db mice. Front. Endocrinol.12, 626390. 10.3389/fendo.2021.626390 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mustafa, M., Wang, T. N., Chen, X., Gao, B. & Krepinsky, J. C. SREBP inhibition ameliorates renal injury after unilateral ureteral obstruction. Am. J. Physiol. Ren. Physiol.311, F614–625. 10.1152/ajprenal.00140.2016 (2016). [DOI] [PubMed] [Google Scholar]
- 44.Cao, J. et al. Artesunate attenuates unilateral ureteral obstruction-induced renal fibrosis by regulating the expressions of bone morphogenetic protein-7 and uterine sensitization-associated gene-1 in rats. Int. Urol. Nephrol.48, 619–629. 10.1007/s11255-016-1232-0 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Zhang, B. et al. Dihydroartemisinin attenuates renal fibrosis through regulation of fibroblast proliferation and differentiation. Life Sci.223, 29–37. 10.1016/j.lfs.2019.03.020 (2019). [DOI] [PubMed] [Google Scholar]
- 46.Wang, H. et al. Emerging role of ferroptosis in diabetic kidney disease: Molecular mechanisms and therapeutic opportunities. Int. J. Biol. Sci.19, 2678–2694. 10.7150/ijbs.81892 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang, X. et al. Genetic support of a causal relationship between Iron Status and Type 2 diabetes: A mendelian randomization study. J. Clin. Endocrinol. Metab.106, e4641–e4651. 10.1210/clinem/dgab454 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Altamura, S. et al. Iron aggravates hepatic insulin resistance in the absence of inflammation in a novel db/db mouse model with iron overload. Mol. Metab.51, 101235. 10.1016/j.molmet.2021.101235 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang, X. D. & Yang, Y. Y. Ferroptosis as a novel therapeutic target for diabetes and its complications. Front. Endocrinol.13, 853822. 10.3389/fendo.2022.853822 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang, L., Wang, F., Li, D., Yan, Y. & Wang, H. Transferrin receptor-mediated reactive oxygen species promotes ferroptosis of KGN cells via regulating NADPH oxidase 1/PTEN induced kinase 1/acyl-CoA synthetase long chain family member 4 signaling. Bioengineered12, 4983–4994. 10.1080/21655979.2021.1956403 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bayir, H., Dixon, S. J., Tyurina, Y. Y., Kellum, J. A. & Kagan, V. E. Ferroptotic mechanisms and therapeutic targeting of iron metabolism and lipid peroxidation in the kidney. Nat. Rev. Nephrol.19, 315–336. 10.1038/s41581-023-00689-x (2023). [DOI] [PubMed] [Google Scholar]
- 52.Zhang, P. et al. Oxidative stress and diabetes: Antioxidative strategies. Front. Med.14, 583–600. 10.1007/s11684-019-0729-1 (2020). [DOI] [PubMed] [Google Scholar]
- 53.Stockwell, B. R. et al. Ferroptosis: A regulated cell death Nexus linking metabolism, redox biology, and disease. Cell171, 273–285. 10.1016/j.cell.2017.09.021 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seibt, T. M., Proneth, B. & Conrad, M. Role of GPX4 in ferroptosis and its pharmacological implication. Free Radic Biol. Med.133, 144–152. 10.1016/j.freeradbiomed.2018.09.014 (2019). [DOI] [PubMed] [Google Scholar]
- 55.Wang, Y. et al. Ferroptosis involves in renal tubular cell death in diabetic nephropathy. Eur. J. Pharmacol.88810.1016/j.ejphar.2020.173574 (2020). [DOI] [PubMed]
- 56.Quagliariello, V. et al. The SGLT-2 inhibitor empagliflozin improves myocardial strain, reduces cardiac fibrosis and pro-inflammatory cytokines in non-diabetic mice treated with doxorubicin. Cardiovasc. Diabetol.20, 150. 10.1186/s12933-021-01346-y (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang, B., Wen, W. & Ye, S. Dapagliflozin ameliorates renal tubular ferroptosis in diabetes via SLC40A1 stabilization. Oxid. Med. Cell. Longev.2022 (9735555). 10.1155/2022/9735555 (2022). [DOI] [PMC free article] [PubMed]
- 58.Juhl, C. B. et al. Bedtime administration of NN2211, a long-acting GLP-1 derivative, substantially reduces fasting and postprandial glycemia in type 2 diabetes. Diabetes51, 424–429. 10.2337/diabetes.51.2.424 (2002). [DOI] [PubMed] [Google Scholar]
- 59.Guo, T. et al. Liraglutide attenuates type 2 diabetes mellitus-associated non-alcoholic fatty liver disease by activating AMPK/ACC signaling and inhibiting ferroptosis. Mol. Med.29, 132. 10.1186/s10020-023-00721-7 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu, D. et al. Hepatic fibroblast growth factor 21 is involved in mediating functions of Liraglutide in mice with Dietary Challenge. Hepatology74, 2154–2169. 10.1002/hep.31856 (2021). [DOI] [PubMed] [Google Scholar]
- 61.An, J. R. et al. Liraglutide alleviates cognitive deficit in db/db mice: Involvement in oxidative stress, iron overload, and ferroptosis. Neurochem Res.47, 279–294. 10.1007/s11064-021-03442-7 (2022). [DOI] [PubMed] [Google Scholar]
- 62.Doll, S. et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature575, 693–698. 10.1038/s41586-019-1707-0 (2019). [DOI] [PubMed] [Google Scholar]
- 63.Bersuker, K. et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature575, 688–692. 10.1038/s41586-019-1705-2 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu, M., Xu, L. G., Li, X., Zhai, Z. & Shu, H. B. AMID, an apoptosis-inducing factor-homologous mitochondrion-associated protein, induces caspase-independent apoptosis. J. Biol. Chem.277, 25617–25623. 10.1074/jbc.M202285200 (2002). [DOI] [PubMed] [Google Scholar]
- 65.Nakamura, T. et al. Phase separation of FSP1 promotes ferroptosis. Nature619, 371–377. 10.1038/s41586-023-06255-6 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang, M. et al. Involvement of FSP1-CoQ(10)-NADH and GSH-GPx-4 pathways in retinal pigment epithelium ferroptosis. Cell Death Dis.13, 468. 10.1038/s41419-022-04924-4 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tonnus, W. et al. Dysfunction of the key ferroptosis-surveilling systems hypersensitizes mice to tubular necrosis during acute kidney injury. Nat. Commun.12, 4402. 10.1038/s41467-021-24712-6 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guo, J., Wang, R. & Min, F. Ginsenoside Rg1 ameliorates sepsis-induced acute kidney injury by inhibiting ferroptosis in renal tubular epithelial cells. J. Leukoc. Biol.112, 1065–1077. 10.1002/JLB.1A0422-211R (2022). [DOI] [PubMed] [Google Scholar]
- 69.Ma, Y. et al. CD36 promotes tubular ferroptosis by regulating the ubiquitination of FSP1 in acute kidney injury. Genes Dis.11, 449–463. 10.1016/j.gendis.2022.12.003 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. Further inquiries can be directed to the corresponding author.