Abstract
Selective breeding is a potent method for developing strains with enhanced traits. This study compared the growth performance and stress responses of the genetically improved Abbassa Nile tilapia strain (G9; GIANT-G9) with a local commercial strain over 12 weeks, followed by exposure to stressors including high ammonia (10 mg TAN/L), elevated temperature (37 °C), and both for three days. The GIANT-G9 showed superior growth, including greater weight gain, final weight, length gain, specific growth rate, and protein efficiency ratio, as well as a lower feed conversion ratio and condition factor compared to the commercial strain. The expression of growth hormone in the brain of the GIANT‐G9 increased significantly after 6 weeks, although it slightly decreased after 12 weeks. Growth hormone receptor 1 expression also increased significantly after 6 weeks. Muscle insulin-like growth factors (igf1 and igf2) levels up-regulated significantly only after 12 weeks in the GIANT‐G9. Under stress, serum enzymes (alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase (ALP)) were significantly higher in the GIANT‐G9, while the commercial strain had lower levels. No significant changes were observed in liver ALP activity among stressed strains. Under stress, the GIANT‐G9 exhibited marked upregulation of splenic Toll-like receptors (tlr2, tlr9, tlr21), myeloid differentiation primary response protein 88 (myd88), nuclear factor kappa B (nf-κB), interleukin (il) 1β, and il6. Notably, il6 expression was higher than il1β in the spleen, with the opposite pattern in the head kidney. In response to immune stimulation, globulin levels significantly increased in the GIANT‐G9 but with similar values to the stressed commercial strain. Myostatin expression increased in the spleen of the stressed GIANT‐G9. The commercial strain exhibited the best liver catalase and superoxide dismutase activities under stress, while the GIANT‐G9 showed increased liver glutathione-S-transferase (GST) activity after exposure to ammonia and temperature stress. Serum lysozyme activity increased in the stressed commercial strain and under temperature stress in the GIANT‐G9 but decreased under other stress conditions. Overall, the stressed commercial strain demonstrated higher survivability than the stressed GIANT‐G9. The study revealed significant interactions between strains and stress factors. The GIANT‐G9 exhibited higher growth rates but lower antioxidant and immune capacities compared to the commercial strain at the juvenile stage of life and production cycle.
Keywords: Abbassa strain, Ammonia stress, Growth, Nile tilapia, Temperature stress
Introduction
Tilapia (omnivorous cichlids) could be likened to aquatic chickens for their rapid and efficient growth. They possess a sweet and subtle flavour and are remarkably easy to cultivate. As one of the fastest-growing aquaculture products, tilapias may very well represent the future of the industry. Among the various tilapia species, the Nile tilapia (Oreochromis niloticus) has swiftly ascended the ranks of farmed fish (Cressey 2009). Following carp, tilapias stand as the second most popular farmed fish globally (Eknath and Hulata 2009; Yacout et al. 2016). The initial cultivation of tilapias took place in Kenya in 1924 (O. spilurus niger) and in Zaire in 1937 (Tilapia rendalli and O. nyasalapia macrochir) (Chimits 1957). According to FAO data, Egypt is one of the world’s leading producers of tilapia, with production expected to continue increasing in the future. From 2022 to 2023, both China and Egypt achieved a 5% growth in tilapia production (Darryl 2023; FAO 2022). Decades of research, both past and ongoing, aim to further enhance the productivity and health of Nile tilapia (Atef et al. 2024; Obirikorang et al. 2019; Ridha and Cruz 2003).
Tilapias boast numerous advantages over other cultured species, including their rapid growth and resistance to disease. Generally, some cultured species can tolerate concentrations of 0.1 mg/L of dissolved oxygen and 2.4 mg/L of unionized ammonia (Lovell 1998). While they originate from freshwater environments, tilapias are euryhaline, meaning they can thrive in and adapt well to saline water. They, however, cannot survive temperatures significantly below 12 °C (Lovell 1998).
Cultured fish face various stressors, including extreme temperatures, ammonia, heavy metal, and xenobiotic pollution, rendering them susceptible to diseases and reduced productivity. These factors can lead to an overproduction of reactive oxygen species (ROS), surpassing the body’s defensive antioxidant system, resulting in stress and disruptions to bodily functions (Abo-Al-Ela and Faggio 2021; Hamed et al. 2024).
ROS are endogenous, highly reactive, oxygen-bearing molecules that include two species: free radicals such as superoxide radical anion (O2˙−) and hydroxyl radicals (OH˙) and non-radicals such as hydrogen peroxide (H2O2), ozone (O3) and singlet oxygen (1O2) (Krumova and Cosa 2016). ROS or related reactive species can act as first messengers, in which ROS levels are transiently increasing, fulfill a signaling role that leads to physiological responses, or as second messengers, where ROS levels cause redox modulation, leading to physiological or pathophysiological responses and subsequent cell injury (Li et al. 2016). The latter potential pathway alters the expression of cellular products, such as inflammatory mediators and adhesion molecules (Li et al. 2016; Wu et al. 2017).
Animal breeding and selection serve as valuable tools for enhancing desired traits in animals. However, the process is time-consuming and exhibits gradual progress (Rezk et al. 2009; Thodesen et al. 2012). Ongoing research aims to refine the selection process, thereby improving the outcomes of selective breeding (de Verdal et al. 2022; Humanes et al. 2024). In Egypt, the selective breeding of Nile tilapia has demonstrated sustainable increases in harvest weight, with recorded gains of 6.64% and 6.96% over two consecutive years (Rezk et al. 2009). Similarly, in China, a six-generation multi-trait selection of the genetically improved farmed tilapia (GIFT breed) by the WorldFish Center resulted in significant growth improvements. This effort manifested in remarkable increases of 60–90% in body weights at harvest (Thodesen et al. 2011). In 2001, WorldFish Egypt initiated a selective breeding program to develop the genetically improved Abbassa Nile tilapia (GIANT) strain, utilizing the same technology used by Rezk et al. (2009) to produce a strain with enhanced growth rates and higher survival (Ibrahim et al. 2019).
It is noteworthy that the GIANT strain in Egypt and the GIFT strain in the Philippines, when reared in two distinct input environments, did not exhibit significant changes in family rankings, indicating a low genotype-environment interaction (Eknath et al. 2007; Khaw et al. 2009). This suggests that these strains may perform consistently across different environmental conditions, though it does not imply resilience of the production systems themselves to environmental variations (Eknath et al. 2007; Khaw et al. 2009).
Selective breeding involves the transmission of the desirable gene-associated traits from one generation to another, encompassing the genetic composition that, in turn, modulates the expression of genes and phenotype (Robinson et al. 2008; Tan et al. 2020; Yáñez et al. 2020). In aquaculture, genome-wide association studies have revealed that productive traits are often polygenic, influenced by multiple loci (Houston et al. 2020). Consequently, it is crucial to delve into the molecular events beyond the improvements resulting from selective breeding to comprehend the potential interaction between genetic elements and the key modulators of these traits.
This study aims to examine differences in growth performance and stress responses—specifically to elevated temperature and ammonia—between two distinct Nile tilapia strains: the 9th generation genetically improved Abbassa Nile tilapia (GIANT-G9) strain and a commercial local strain. The investigation focuses on growth performance, expression of growth- and immune-related genes, and potential changes in biochemical parameters.
Materials and methods
Fish rearing and management
All-male Nile tilapia were obtained from two distinct sources. A commercial local strain was obtained from a farm in West El-Qantara, Ismailia, Egypt, which distributes its production widely within the local market. The GIANT-G9 strain (commercially available) was acquired from WorldFish in Abbassa, Abou-Hammad, Sharkia, Egypt. The fish weights were 6.01 ± 0.08 g and 6.10 ± 0.09 g for the GIANT-G9 strain and the commercial local strain, respectively. They were hand-fed three times daily with a powdered fresh feed containing 30% crude protein, 5.70% crude fat, 7.10% ash, 90.35% dry matter, and 46.55% nitrogen-free extract (Grand Aqua®, Egypt).
The study was divided into two distinct experiments, as illustrated in Fig. 1. The first experiment focused on comparing the growth of two Nile tilapia strains, while the second experiment aimed to assess differences in stress responses between the strains.
Fig. 1.
Diagrammatic illustration of the experimental plan. TAN, total ammonium nitrogen
In the first experiment, a portion of the fish was stocked in 100-liter round plastic tanks, with four tanks designated for each strain and 20 fingerlings per tank. Before the experiment began, the fish were allowed a two-week acclimation period. The first experiment lasted for 84 days (12 weeks). For the first six weeks, the feeding rate was set at 5% of their total body weight per day, and for the remaining six weeks, it was reduced to 4%.
Throughout both experiments, continuous aeration and daily exchanges of fresh, dechlorinated tap water were provided to maintain water quality. Key water parameters were monitored regularly to ensure optimal conditions: dissolved oxygen (DO) and temperature were measured with a HANNA portable DO meter, pH levels were recorded using a HANNA portable pH meter, and ammonia levels were assessed with a HANNA medium-range ammonia reagent kit.
During the first part of the experiment, the water temperature, dissolved oxygen, pH, and ammonia levels remained within normal ranges, as shown in Table 1. During the second part of the experiment, which involved stress exposure, these parameters changed according to the type of stress, as detailed in Table 2.
Table 1.
Water quality criteria of the first experiment (growth)
| Starin | Water temperature (°C) | DO (mg/L) | pH | Ammonia (mg/L) |
|---|---|---|---|---|
| GIANT-G9 | 28.60 ± 0.000 | 5.870 ± 0.034 | 7.598 ± 0.043 | 0.0723 ± 0.006 |
| Commercial | 28.54 ± 0.000 | 6.033 ± 0.080 | 7.885 ± 0.019 | 0.0825 ± 0.009 |
The data expressed as mean ± SD
Table 2.
Water quality criteria in the second experiment (stress)
| Strain | Treatment | Water temperature (°C) | DO (mg/L) | pH | Ammonia (mg/L) |
|---|---|---|---|---|---|
| GIANT-G9 | Control | 28.667 ± 1.258 | 7.133 ± 0.321 | 8.033 ± 0.503 | 0.093 ± 0.137 |
| Ammonia | 29.167 ± 1.040 | 7.033 ± 0.208 | 8.033 ± 0.115 | 0.567 ± 2.466 | |
| Temperature | 35.833 ± 1.607 | 7.067 ± 0.351 | 8.233 ± 0.252 | 0.157 ± 0.150 | |
| Ammonia and Temperature | 36.667 ± 0.577 | 6.933 ± 0.513 | 8.067 ± 0.208 | 0.567 ± 1.443 | |
| Commercial | Control | 28.167 ± 1.040 | 6.900 ± 0.361 | 7.833 ± 0.305 | 0.077 ± 0.124 |
| Ammonia | 28.833 ± 0.289 | 6.867 ± 0.231 | 8.010 ± 0.100 | 0.500 ± 2.598 | |
| Temperature | 35.667 ± 1.258 | 6.967 ± 0.208 | 8.000 ± 0.200 | 0.123 ± 0.146 | |
| Ammonia and Temperature | 36.333 ± 1.040 | 6.733 ± 0.252 | 8.033 ± 0.252 | 0.533 ± 1.607 |
The data expressed as mean ± SD
Dead fish were regularly checked, and their survivability was determined as a percentage at the end of each experimental period.
Growth performance, feed utilization parameters, and survivability
The formulas for various growth and performance metrics are defined as follows:
Specific growth rate (SGR % day–1)) = (ln Wt– ln Wo) / t × 100, where ln represents the natural logarithm, Wt is the final weight, Wo is the initial weight, and t is the rearing duration in days.
Weight gain (WG; g) = Wt– Wo, where Wt is the final weight, Wo is the initial weight.
Length gain (LG; cm) = Lt– Lo, where Lt is the final length, and Lo is the initial length.
Feed conversion ratio (FCR) = Feed intake (FI; g) / Weight gain (WG; g).
Protein efficiency ratio (PER) = Wet weight gain (g) / Total protein intake (g).
Condition factor (CF) (%) = Total weight / Standard length3.
Hepato-somatic index (HIS) (%) = (Liver weight / Total body weight) × 100.
Stress exposure
At the end of the growth experiment, fish from each strain were divided into four experimental groups. The second experiment involved a total of 240 fish per strain, allocated into 12 tanks for each strain, with three replicates per treatment group. Following the first experiment, the fish acclimated for seven days. Each group was subjected to a different treatment: a control (no exposure), temperature stress at 37 °C, ammonia stress at 10 mg/L total ammonium nitrogen (TAN), or a combination of elevated temperature and high ammonia under the same conditions. This exposure experiment lasted for three days, during which the fish were fed the same commercial feed used throughout the experiment. The feed was administered at a rate of 3% of the fish’s body weight, twice daily at 08:00 am and 2:00 pm.
For the temperature stress, the temperature was increased by 2 °C every 2 h until reaching 37 °C. For the high ammonia concentration stress, an ammonium chloride (NH4Cl; Fisher Scientific, USA) solution was prepared to achieve 10 mg TAN/L, following the protocols of Hegazi et al. (2010) and Benli et al. (2008). Water quality maintenance procedures were consistently followed, including the use of freshly prepared ammonium chloride solution to maintain TAN at 10 mg/L and pre-warmed water at 37 °C. These measures ensured that the stress was solely induced by ammonia and/or temperature.
Sampling
For RNA extraction and gene expression analysis, samples were collected from both the brain and muscle at two time points: after six weeks and at the completion of the 12-week growth experiment. Three samples per replicate were taken at each time point. At the end of the second experiment, tissue samples (n = 5 from each tank) were taken from the head kidney and spleen. These samples were promptly frozen and stored at − 80ºC. Additionally, serum samples were collected for biochemical analysis, and liver samples were taken for the analysis of antioxidant parameters (n = 5 from each tank).
Gene expression analysis
RNA extraction was carried out using TRIzol reagent (Invitrogen™ Life Technologies, US). The extracted RNA’s quality and quantity were assessed using a nanodrop (NanoPhotometer® NP80, Implen, Germany) and by running it through 1% agarose gel electrophoresis. The synthesis of cDNA was performed using 2 µg of RNA and kits from Bioline, based in London, UK.
Real-time PCR was conducted using specific primers targeting growth hormone (gh), growth hormone receptor 1 (ghr1), insulin-like growth factor (igf) 1 and 2, Toll-like receptors (tlr) 2, 9, and 21, myeloid differentiation primary response protein 88 (myd88), nuclear factor kappa B (nf-κB), interleukin (il) 1β and 6, and myostatin (mstn), along with the reference genes elongation factor 1 alpha (ef1α) and ubiquitin-conjugating enzyme (ubce) (Table 3). The cycling parameters for the real-time PCR were set as follows: 95 °C for 3 min, followed by 40 cycles of 95 °C for 15 s, 60 °C for 1 min, and 72 °C for 30 s. The specificity of the PCR products was confirmed through dissociation analysis. Igf1 and Igf2 were analyzed in the muscle; gh and ghr1 were analyzed in the brain; tlr2, tlr9, tlr21, myd88, nf-κB, il1β, il6, and mstn were analyzed in the spleen; and nf-κB, il1β, and il6 were analyzed in the head kidney.
Table 3.
Primers used for real-time PCR
| Gene name | forward (sense, left) primer (5′−3′) | reverse (antisense, right) primer (5′−3′) | amplicon size (bp) | GenBank accession no. | References |
|---|---|---|---|---|---|
| gh | CTGTCTGTCTGTCTGTCAGTCGT | AGAGGAGACGCCCAAACAC | 60 | M26916; M84774 | Ber and Daniel (1992) and Rentier-Delrue et al. (1989) |
| ghr1 | TCTCAGCAGAACCGATTAATGA | TTTGATTTTGGGTGCAGGA | 60 | EF052861 | Herkenhoff et al. (2020) |
| igf1 | CCCGAACTTCCTCGACTTGA | CCTCAGCCAGACAAGACAAAAA | 101 | EU272149 | |
| igf2 | CCCCTGATCAGCCTTCCTA | GACAAAGTTGTCCGTGGTGA | 60 | EU272150 | |
| tlr2 | TGTCATGTGCCATCAGGTTT | TCTGCCTTATCTGTGCGTTG | 180 | JQ809459.1; XM_013264298.3 | The current study |
| tlr9 | CAGTTTTCGTGCTGTCCAGA | TGCACAATCGTTTTCTCAGC | 174 | MT338570.1; XM_031753215.2 | The current study |
| tlr21 | AACGGACTCACCGTTTTACCA | GGAGAAGTTCTGAATGCCCAT | 243 | KJ010824.1; NM_001311317.1 | Pang et al. (2017) |
| myd88 | TAAACGGATGGTGGTGGTGA | GCGTTTACTTCGAGCTCCAG | 106 | KJ130039 | Trung and Lee (2020) |
| nf-κB | CGACCACTACCTACACGCTC | GATGTCGTTTGAGGCATCGC | 96 | XM_019363515.2 | Yu et al. (2022) |
| il1β | TGCACTGTCACTGACAGCCAA | ATGTTCAGGTGCACTATGCGG | 113 | DQ061114.1 | Choi et al. (2007) |
| il6 | ACAGAGGAGGCGGAGATG | GCAGTGCTTCGGGATAGAG | 165 | XM_019350387 | Wei et al. (2018) |
| mstn | CACTGTGGACTTCGAGGACT | CTCTGGGGTTGGCTTTGTTC | 143 | KT987208.1 | The current study |
| ubce | CTCTCAAATCAATGCCACTTCC | CCCTGGTGGAGGTTCCTTGT | 130 | XM_003460024 | Yang et al. (2013) |
| ef1α | GCACGCTCTGCTGGCCTTT | GCGCTCAATCTTCCATCCC | 250 | AB075952 |
gh, growth hormone; ghr1, growth hormone receptor 1; igf, insulin-like growth factor; tlr, Toll-like receptor; myd88, myeloid differentiation primary response protein 88; nf-κB, nuclear factor kappa B; il, interleukin; mstn, myostatin; ubce, ubiquitin-conjugating enzyme; ef1α, elongation factor 1 alpha. The primers designed in this study were generated using Primer3web (https://bioinfo.ut.ee/primer3/) and subsequently underwent specificity testing using the BLAST™ tool for primers (https://www.ncbi.nlm.nih.gov/tools/primer-blast/)
Gene expression was normalized using the geometric mean of the two reference genes ef1α and ubce as described in Chervoneva et al. (2010) and Atef et al. (2024). The expression data for gh, ghr1, and igf1 and igf2 were presented as 2–ΔCt (Petakh et al. 2024; Rochman et al. 2014). However, the gene expression results of the second experiment were calculated using the Pfaffl (2001) method.
Biochemical analysis of sera and liver samples
The serum lysozyme activity was measured using a fish lysozyme ELISA kit (Sunlong Biotech Co., China) at a wavelength of 450 nm, following the manufacturer’s instructions. Serum biochemical parameters were determined colorimetrically using ready-made chemical kits, according to the manufacturers’ instructions: alkaline phosphatase at 510 nm, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and albumin (Biodiagnostic Co., Egypt), and total serum proteins (Spectrum Co., Egypt). Globulin content was calculated using the values of total proteins and albumin.
Liver homogenates were prepared using phosphate-buffered saline for the determination of superoxide dismutase (SOD) at 560 nm, catalase, and alkaline phosphatase (ALP) at 510 nm, and glutathione-S-transferase (GST) at 340 nm, following the kit instructions from Biodiagnostic Co., Egypt.
Statistical analysis
The data were analyzed using IBM SPSS version 20. In the first experiment (growth), a t-test was employed to evaluate growth performance, feed utilization, carcass composition, and gene expression, with the tank mean as the data point. In the second experiment (stress), individual data points were used. A two-way ANOVA was conducted on the stress data to assess the interaction effect between strain and stress factor. Additionally, a one-way ANOVA followed by Tukey’s multiple comparison test was used to examine the results within the same strain. GraphPad Prism 9 (La Jolla, California, USA) was used for statistical analysis. Data are expressed as mean ± SD, with significance tested at the 0.05 level.
Results
Growth performance and feed utilization parameters
The GIANT-G9 exhibited consistent marked growth throughout the experiment, with a significant WG increase by 3.35 g compared to the commercial strain (Fig. 2). This growth was reflected in the fish’s FW and LG, with increases of 3.46 g and 3.03 cm, respectively, compared to the commercial strain (Table 4). The SGR was higher, while the FCR was lower in the GIANT‐G9 compared to the commercial strain. Additionally, the GIANT‐G9 had a higher PER and a lower CF % than the commercial strain (Table 4). FI and HIS did not differ significantly between the two strains.
Fig. 2.
Average weights for two Nile tilapia strains (commercial strain and GIANT-G9), recorded every two weeks during the first experimental period (84 days; 12 weeks)
Table 4.
Growth performance of two Nile tilapia strains (commercial strain and GIANT-G9) reared for 84 days (12 weeks)
| Growth parameters | GIANT-G9 | Commercial strain | P value |
|---|---|---|---|
| IW (g) | 6.01 ± 0.08 | 6.10 ± 0.09 | 0.193 |
| WG (g) | 12.08 ± 0.22 | 8.725 ± 0.26 | < 0.0001 |
| FW (g) | 18.16 ± 0.32 | 14.70 ± 0.34 | < 0.0001 |
| LG (cm) | 9.03 ± 0.05 | 6.00 ± 0.14 | < 0.0001 |
| FI (g/tank) | 454.36 ± 52.73 | 461.168 ± 53.509 | 0.756 |
| SGR (%) | 0.57 ± 0.01 | 0.46 ± 0.01 | < 0.0001 |
| FCR | 1.65 ± 0.05 | 2.30 ± 0.08 | < 0.0001 |
| PER | 1.95 ± 0.06 | 1.40 ± 0.08 | < 0.0001 |
| CF (%) | 0.54 ± 0.01 | 0.89 ± 0.02 | < 0.0001 |
| HIS (%) | 0.88 ± 0.46 | 0.91 ± 0.61 | 0.930 |
IW, initial weight; WG, weight gain; FW, final weight; LG, length gain; FI, feed intake; SGR, specific growth rate; FCR, feed conversion ratio; PER, protein efficiency ratio; CF, condition factor; HIS, hepato-somatic index. The data expressed as mean ± SD
Expression of growth-related genes
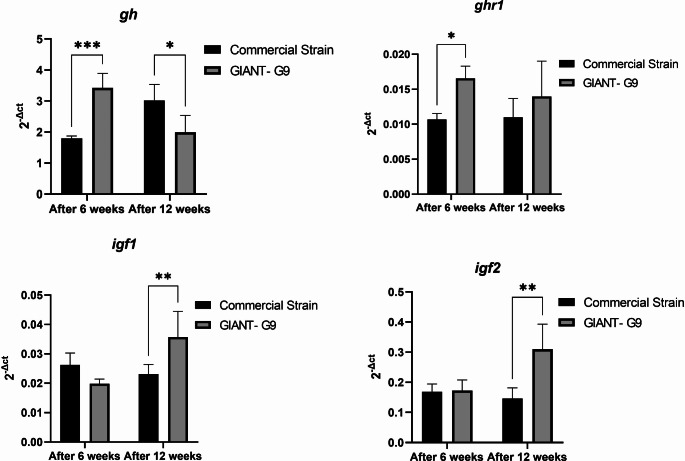
In the GIANT-G9 strain, brain gh expression was significantly higher than in the commercial strain after 6 weeks, but showed a slight decrease after 12 weeks (Fig. 3). The expression of ghr1 in the brain of the GIANT-G9 was significantly increased after 6 weeks, with nonsignificant increases after 12 weeks (Fig. 3). However, the expression of muscle igf1 and igf2 was significantly increased only after 12 weeks in the GIANT‐G9 compared to the commercial strain (Fig. 3).
Fig. 3.
Gene expression results of growth hormone (gh) and growth hormone receptor 1 (ghr1) in the brain, and insulin-like growth factors 1 and 2 (igf1 and igf2) in the muscle of two Nile tilapia strains (a commercial strain and the GIANT-G9) after 45 days and 90 days during the first experimental period (84 days; 12 weeks). The expression levels were normalized using the geometric mean of two reference genes, ef1α and ubce, and are presented as 2–ΔCt. Data are expressed as mean ± SD
Survivability
No fish deaths were recorded during the first experiment (growth). However, in the second experiment (stress), the survivability rates for the GIANT-G9 were 95% for the control group (no stress), 75% for the ammonia stress group, 85% for the temperature stress group, and 40% for the group exposed to both ammonia and temperature stress. For the commercial strain, the survivability rates were 95% for the control group (no stress), 85% for the ammonia stress group, 90% for the temperature stress group, and 60% for the group exposed to both ammonia and temperature stress.
Serum and liver biochemical parameters
Ammonia stress significantly increased the serum levels of ALT and AST in both strains and among other stress groups (Table 5). However, there was no significant difference between ammonia and temperature stress in the GIANT-G9. Notably, ALT and AST levels were significantly higher in the GIANT‐G9 compared to the commercial strain. Interestingly, exposure to both ammonia and temperature stressors decreased the serum levels of ALT and AST, but these changes were not significantly different from the control of each strain. The serum level of ALP increased in response to ammonia and decreased in response to temperature in the commercial strain (Table 5). In contrast, in the GIANT‐G9, ALP increased after exposure to ammonia and both stressors, with these increases being more pronounced than those observed in the commercial strain.
Table 5.
Biochemical changes in the blood of two Nile tilapia strains (commercial strain and GIANT-G9) exposed to different stressors (ammonia at 10 mg/L TAN/L, temperature at 37ºC, both stressors combined) and a control group (no stress)
| Parameters | Commercial Strain | GIANT-G9 | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Ammonia | Temperature | Ammonia & Temperature | Control | Ammonia | Temperature | Ammonia & Temperature | Strain ⨉ Stress factor | |
| ALT (U/L) | 22.87 ± 0.37a | 39.00 ± 0.58c, ** | 24.00 ± 0.24a | 15.23 ± 0.19d | 19.57 ± 0.55ab | 48.00 ± 1.73c, ** | 19.93 ± 0.18abc | 15.47 ± 0.32a | 0.0010 |
| AST (U/L) | 25.39 ± 0.20ab | 41.27 ± 1.18c | 29.94 ± 0.08b, * | 20.40 ± 0.31a, ** | 25.71 ± 0.19a | 40.92 ± 0.74b | 37.27 ± 0.86b, * | 29.54 ± 0.72a, ** | 0.005 |
| ALP (U/L) | 20.20 ± 0.25a | 29.36 ± 0.48c, **** | 21.32 ± 0.42a, * | 17.10 ± 0.15d, **** | 20.23 ± 0.23a | 39.71 ± 0.31b, **** | 19.18 ± 0.43a, * | 39.75 ± 0.29b, **** | < 0.0001 |
| Total protein (g/dL) | 6.48 ± 0.20abc, ** | 5.96 ± 0.09a | 6.68 ± 0.12a, b, c, * | 6.30 ± 0.16a, b | 4.54 ± 0.18a, ** | 6.18 ± 0.15ab | 5.08 ± 0.06abc, * | 5.49 ± 0.22bc | 0.009 |
| Albumin (g/dL) | 3.73 ± 0.25abc | 3.08 ± 0.04a | 3.71 ± 0.07abc | 3.71 ± 0.23ab, * | 3.13 ± 0.07abc | 2.43 ± 0.26a | 2.94 ± 0.10abc | 2.52 ± 0.24ab, * | 0.47 |
| Globulin (g/dL) | 2.75 ± 0.09ab, **** | 2.87 ± 0.07abc, *** | 2.97 ± 0.09bc, *** | 2.58 ± 0.08a, * | 1.40 ± 0.18a, **** | 3.75 ± 0.12b, *** | 2.14 ± 0.04c, *** | 2.97 ± 0.07d, * | < 0.0001 |
| Lysozyme activity (µg/mL) | 4.41 ± 0.37c |
7.59 ± 0.66ab, *** |
7.07 ± 0.81a |
7.74 ± 0.32 ab, *** |
4.91 ± 0.11d |
2.14 ± 0.21a, *** | 7.32 ± 0.87c | 2.59 ± 0.15a, *** | 0.0001 |
ALT, alanine aminotransferase, AST; aspartate aminotransferase; ALP, alkaline phosphatase. Values sharing no common lowercase letters are significantly different within the same strain, while asterisks denote significance within the same stress factor across different strains. * = significant difference at p < 0.05, ** = significant difference at p < 0.01, *** = significant difference at p < 0.001. The data expressed as mean ± SD
Changes in serum total protein appeared to be due to variations in the globulin fraction (Table 5). Although there was a slight increase in albumin in both strains, these values were not significantly different from the control group within the same strain. In the commercial strain, increases in serum globulin were observed with ammonia and temperature stress alone; however, significant increases were noted in all groups compared to the control in the GIANT-G9. Overall, globulin levels were higher in the GIANT‐G9 than in the commercial strain, including in the control group without stress.
Regarding lysozyme activity, stressors significantly increased this enzyme’s activity in the commercial strain, with slight differences among stress groups (Table 5). In the GIANT-G9, lysozyme activity increased under temperature stress but markedly decreased after exposure to ammonia and both stressors. A significant interaction between strain and stress was observed for all parameters except albumin.
The liver catalase activity decreased after ammonia stress but markedly increased after temperature stress and exposure to both stressors. These increases were significant in the GIANT-G9 under the same stress factor (Fig. 4). Specifically, in the GIANT‐G9, liver catalase activity increased with ammonia exposure and under both stressors. In the commercial strain, SOD levels increased after exposure to all stressors compared to the control, with no significant differences among the stressors (Fig. 4). For the GIANT‐G9, a significant increase in SOD was observed only in the ammonia group, which was significantly different from the commercial strain. Liver GST activity significantly increased after ammonia exposure in the commercial strain but decreased non-significantly in other stress groups compared to the control (Fig. 4). In the GIANT‐G9, liver GST activity significantly increased with temperature stress and under both stressors. The variations between strains were significantly different under the same stress conditions. Liver ALP activity significantly increased in fish exposed to ammonia stress in both strains compared to the control, with no significant difference between the strains (Fig. 4). A significant interaction between strain and stress was observed for these enzyme activities. parameters.
Fig. 4.
Liver enzyme activities of superoxide dismutase (SOD), catalase, glutathione-S-transferase (GST), and alkaline phosphatase (ALP) in two Nile tilapia strains (a commercial strain and the GIANT-G9) after exposure to control (no stress), ammonia (TAN at 10 mg/L), temperature (37ºC), and both ammonia and temperature for three days. Data are expressed as mean ± SD. Values sharing no common lowercase letters are significantly different within the same strain, while asterisks denote significance within the same stress factor across different strains
Expression of immune-related genes after exposure to stressors
The expression of tlr2, tlr9, and tlr21 in the spleen was markedly down-regulated in the commercial fish exposed to stress, particularly ammonia and temperature. However, these genes were markedly up-regulated in the GIANT-G9 (Fig. 5). A similar trend was observed for splenic myd88, except in fish exposed to both ammonia and temperature, where it was up-regulated in the commercial strain (Fig. 5).
Fig. 5.
Relative expression levels of Toll-like receptors (tlr) 2, 9, and 21, and myeloid differentiation primary response protein 88 (myd88) genes in the spleen of two Nile tilapia strains (a commercial strain and the GIANT-G9) after exposure to control (no stress), ammonia (TAN at 10 mg/L), temperature (37ºC), and both ammonia and temperature for three days. The expression levels were normalized using the geometric mean of two reference genes, ef1α and ubce, following the Pfaffl (2001) method. Data are expressed as mean ± SD. Values sharing no common lowercase letters are significantly different within the same strain, while asterisks denote significance within the same stress factor across different strains
The splenic expression of nf-κB was down-regulated in the commercial strain, with particularly lower expression after temperature exposure (Fig. 6). In the GIANT-G9, the splenic expression of nf-κB was massively increased in fish exposed to ammonia and both stressors, while it was massively down-regulated in fish exposed to temperature alone. The splenic expression of il1β was up-regulated in response to stress in all groups, with massive expression in the GIANT‐G9 (Fig. 6). Similarly, splenic il6 was up-regulated in the GIANT‐G9 (Fig. 6). However, in the commercial strain, il6 was down-regulated in fish exposed to ammonia or temperature stress and up-regulated in those exposed to both stressors. The splenic expression of mstn was markedly up-regulated in the GIANT‐G9 exposed to stress and down-regulated in the commercial strain exposed to ammonia or temperature stress. Significant interaction effects between strain and stress factors were observed in gene expression in the spleen.
Fig. 6.
Relative expression levels of nuclear factor kappa B (nf-κB), interleukin (il) 1β and 6, and myostatin (mstn) genes in the spleen of two Nile tilapia strains (a commercial strain and the GIANT-G9) after exposure to control (no stress), ammonia (TAN at 10 mg/L), temperature (37ºC), and both ammonia and temperature for three days. The expression levels were normalized using the geometric mean of two reference genes, ef1α and ubce, following the Pfaffl (2001) method. Data are expressed as mean ± SD. Values sharing no common lowercase letters are significantly different within the same strain, while asterisks denote significance within the same stress factor across different strains
In the head kidney, the expression of nf-κB was down-regulated in both strains under stress, with markedly lower values in the GIANT-G9 (Fig. 7). The downregulation followed a descending trend from ammonia, temperature, to both stressors. However, in the commercial strain, fish exposed to temperature stress showed a lesser degree of downregulation. The expression of il1β was up-regulated in all groups under stress, except for a downregulation in the commercial strain exposed to ammonia (Fig. 7). Notably, in the head kidney, il1β expression showed 2- to 4-fold increases for temperature stress and both stressors, respectively, in the commercial strain, while showing near 55-, 400-, and 300-fold increases in the GIANT‐G9. The expression of il6 in the head kidney was down-regulated similarly in both strains, except in those exposed to both stressors, where lower downregulation was observed in the commercial strain (Fig. 7). Significant interaction effects between strain and stress factors were observed in gene expression in head kidney, except for il6 expression.
Fig. 7.

Relative expression levels of nuclear factor kappa B (nf-κB) and interleukin (il) 1β and 6 genes in the head kidney of two Nile tilapia strains (a commercial strain and the GIANT-G9) after exposure to control (no stress), ammonia (TAN at 10 mg/L), temperature (37ºC), and both ammonia and temperature for three days. The expression levels were normalized using the geometric mean of two reference genes, ef1α and ubce, following the Pfaffl (2001) method. Data are expressed as mean ± SD. Values sharing no common lowercase letters are significantly different within the same strain, while asterisks denote significance within the same stress factor across different strains
Discussion
Achieving optimal growth performance along with balanced or enhanced health is the ultimate goal in animal production, particularly in aquaculture. The findings indicated that the GIANT-G9 demonstrated significantly better growth performance compared to a commercial strain. The brain expression of gh and ghr1 increased significantly during the initial raising period but tended to decrease afterward, with ghr1 levels remaining higher than those of the commercial strain. After 6 weeks, gh expression in the brain was higher in the GIANT‐G9. In contrast, the expression of igf1 and igf2 in the muscle changed significantly only at 12 weeks, with notable increases in the GIANT‐G9.
The growth hormone-insulin-like growth factor (Gh-Igf) system is a key modulator of growth and health in vertebrates (Fuentes et al. 2013; Gabillard et al. 2003). It has been found that igf1ra mRNA is most abundant in the skeletal muscle (Mohammed-Geba et al. 2016). Additionally, igf1ra expression increases while gh decreases in response to Igf1 stimulation (Mohammed-Geba et al. 2016). Fuentes et al. (2012) explored the remarkably slow growth of fine flounder and found an inherent systemic and muscle Gh resistance, with relatively high levels of plasma Gh and low plasma Igf1, as well as a higher content of truncated Ghr compared to full-length Ghr in the skeletal muscle. This was accompanied by impaired function of the Janus kinase 2 (JAK2)-signal transducers and activators of transcription 5 (STAT5) signaling pathway and decreased igf1 expression (Fuentes et al. 2012). This study also elucidated that the interplay between gh and igf1 is a key factor in regulating growth. These findings are supported by evidence from other species such as Atlantic halibut (Hippoglossus hippoglossus) and rainbow trout (Oncorhynchus mykiss) (Fuentes et al. 2013; Hildahl et al. 2008). In transgenic zebrafish that constantly overexpress full-length Ghr1, but not truncated Ghr1, there was increased expression of igf1 and higher growth rates compared to the control (Ishtiaq Ahmed et al. 2011). Notably, in the current study, the expression of ghr1 remained at higher levels in the GIANT-G9 compared to the commercial strain, regardless of the variations in gh, igf1, and igf2 expression.
Although Igf1 plays essential roles in protein synthesis and muscle growth in fish (Duran et al. 2022). While Igf1, but not Igf2, synergizes and interacts with Gh, Igf2 has a stronger effect on myocyte proliferation (Rius-Francino et al. 2011). The effect of Gh on the expression of igf1 and igf2 is modulated by insulin and cortisol (Pierce et al. 2011). Although igf2 does not seem to affect the body size of fish during the early stages of life (Schrader and Travis 2012), it is positively linked to growth in later stages. Notably, variations in the genetic makeup of igf2 have been associated with significant effects on male growth in the GIFT strain (Juhua et al. 2010), as well as total length and body weight in European sea bass (Dicentrarchus labrax) (Ozcan-Gokcek et al. 2020), and growth traits in Spotted sea bass (Lateolabrax maculatus) (Fan et al. 2023) and pikeperch (Sander lucioperca) (Teng et al. 2020).
The GIANT-G9 appears to have more positive regulation of gh, ghr1, igf1, and igf2, leading to better growth performance across various life stages compared to the commercial strain.
The GH-IGF system plays a crucial role in regulating the body’s response to stress (Gabillard et al. 2003). Zhong et al. (2022) proposed that Igf1 modulates the expression network related to complement and coagulation cascades in tilapia, highlighting its involvement in disease defense. Additionally, changes in rearing temperature affect the muscle expression of igf1 and igf2 without influencing growth. However, temperature affects growth by stimulating hepatic Igf1 secretion under the influence of Gh (Gabillard et al. 2003).
In tilapia, Gh treatment has been shown to increase igf1 mRNA levels in the head kidney more than in the liver, and to increase igf2 mRNA in the liver, as well as igf1 and igf2 in the spleen, while decreasing tnfα expression in both the head kidney and spleen (Shved et al. 2011). A similar trend was observed in the head kidney leukocytes of Atlantic salmon, where Gh treatment down-regulated tnfα and il8 while upregulating il1β expression (Pontigo and Vargas-Chacoff 2021). Cortisol, the primary stress hormone, and Tnfα, have been found to increase the expression of suppressors of cytokine signaling, inhibit Gh signaling via the JAK2/STAT5 pathway, and suppress gh-stimulated igf1 mRNA abundance (Jiang et al. 2020; Philip and Vijayan 2015).
Antioxidant and immune systems are the first lines of defense against stress, helping to maintain normal body physiology. Toll-like receptors are among their tools, recognizing pathogen-associated molecular patterns and thereby triggering immune responses (Sundaram et al. 2012; Yao et al. 2023). These receptors also respond to environmental changes such as salinity, temperature, and dietary alterations (Schmitz et al. 2017; Yao et al. 2023; Zhang et al. 2023).
In the current study, splenic tlr expression was markedly up-regulated in the GIANT-G9 exposed to stress, while it was down-regulated in the stressed commercial strain. This was accompanied by upregulation of splenic myd88, nf-κB, il1β, and il6 in the stressed GIANT‐G9. This could be linked to the function of Tlr in activating a variety of proinflammatory cytokine and chemokine production, the crucial role of Nf-κB in regulating inflammatory responses (Qiu et al. 2020), and the central role of Myd88 in the Tlr/Il1R activation cascade (Chen et al. 2020; Trung and Lee 2020). Additionally, in the GIANT‐G9, the expression of il6 was markedly higher than il1β in the spleen, while the reverse was observed in the head kidney, with extensive increases in il1β expression, suggesting differential tissue responses to the same stressors. In the commercial strain, il1β expression was mildly up-regulated compared to the GIANT‐G9. In response to immune stimulation in the GIANT‐G9, globulin levels were significantly increased. However, the globulin levels in the commercial strain remained similar to those in the stressed GIANT‐G9. This difference could be explained by the upregulation of splenic mstn in the spleen of the stressed GIANT‐G9, as Mstn likely plays a role in immune signaling and defense capacity (Wu et al. 2020). Conversely, dietary supplementation with Mstn inhibitory peptides improved the immune capacity of sea bass (Lateolabrax maculatus) (Yating et al. 2017).
Interestingly, the downregulation or upregulation of most genes followed a descending or ascending trend according to the fish strain, from ammonia, temperature, to both stressors. Based on this, the question is: if the level of stress increases, does the expression of immune-related genes also increase? This could be correct if we consider that excessive immune response, particularly cytokine production, is an indicator of immune dysregulation and could hinder the physiological state of the organism (Jarczak and Nierhaus 2022). It has been found that up-regulated expression of immune genes is not necessarily associated with enhanced defense and readiness (Abo-Al-Ela 2019; Abo-Al-Ela et al. 2017). Similarly, in GIFT strain, exposure to polystyrene microplastics caused increased levels of Il1β and Tnfα in the brain and gills. This overproduction was accompanied by oxidative stress, indicating that an excessive immune response disrupts overall fish health (Zheng et al. 2023). Additionally, overactivation of Toll-like receptors causes excessive triggering of various cytokine productions, leading to detrimental inflammatory responses (Huang et al. 2020). Further evidence supports these findings, showing that suppressive proteins, such as suppressors of cytokine signaling, help mitigate and control excessive immune responses to curb harmful inflammation and tissue damage that may result from an overactive immune response (Hao and Sun 2016; Huang et al. 2020).
Exposure to ammonia and/or elevated temperature stimulated the antioxidant response, with the commercial strain demonstrating the highest activities of liver catalase and SOD. Additionally, serum lysozyme activity increased in the commercial strain under stress. Under temperature stress, lysozyme activity also increased, whereas it decreased under other stress conditions in the GIANT-G9. The individual variations between the two strains in modulating antioxidant and immune systems in response to stress indicate a more functional response and lower stress levels in the commercial strain compared to the GIANT‐G9.
The increased immune stimulation, combined with higher ROS levels, may have hindered the physiological status, resulting in higher mortality in the GIANT-G9 compared to the commercial strain. Rapid growth rates may reduce the energy and nutrients available for normal immune and antioxidant functions, leading to some degree of immune suppression. Fish that are rapidly growing or in specific physiological states have high metabolic activity, which elevates ROS production (Birnie-Gauvin et al. 2017; Mortelette et al. 2010). This was also observed through greater liver injury, indicated by higher serum levels of ALT, AST, and ALP.
Exposure to stress triggers various physiological processes that activate the body’s antioxidant defense and immune system to maintain balance. However, this can sometimes lead to oxidative stress and system failure. Therefore, ensuring a balanced response and functional body systems is crucial for preserving organism performance and life.
Selective breeding is a well-established tool with a substantial impact on the aquaculture sector and will continue to drive its growth in the present and future (Cavatti Neto et al. 2023; Janssen et al. 2017). Worldwide, researchers and various countries are working to develop and implement tilapia strains with high growth performance at minimal cost (Basiao et al. 2005; Cavatti Neto et al. 2023; Janssen et al. 2017). These efforts focus on enhancing physiological traits and stress resistance in strains, including tolerance to temperature extremes, low oxygen levels, and disease resistance. Additionally, adaptability traits, which enable fish to better cope with environmental changes, are a key consideration (Hu et al. 2024).
Whole-genome selective breeding, or genomic selection, is a powerful tool that can help identify strains with more balanced characteristics, such as improved stress resistance, while also shortening the breeding cycle (Hu et al. 2024; Yáñez et al. 2023). For instance, genome-wide association studies and genomic prediction have successfully identified quantitative trait loci (QTLs) for acute hypoxia tolerance in rainbow trout (Prchal et al. 2023).
Selective breeding has also produced favorable outcomes in population resilience, as shown by coral strains with improved heat tolerance (Humanes et al. 2024), enhancing genomic selection rates among candidates. However, selective breeding may unintentionally overlook traits like temperature tolerance. For example, Rezk and Kamel (2011), reported variability in cold tolerance selection effects between O. niloticus and O. aureus, highlighting the importance of considering a comprehensive set of traits in breeding programs.
Similarly, studies on red tilapia, Genomar Supreme Tilapia (GST; GIFT-derived and developed further in the Philippines), and GIFT strains revealed comparable growth patterns when reared at 22 °C. However, at 30 °C, GIFT and GST exhibited superior growth compared to red tilapia (Santos et al. 2013). These findings underscore the role of temperature as a critical modulator of fish growth, even among different strains. Moreover, under high stocking densities, improved strains such as the sixth generation of GIFT and the 13th-generation FaST selected line (Freshwater Aquaculture Center Selected Tilapia) showed significantly higher growth compared to non-improved commercial strains (Ridha 2006).
Environmental conditions, such as freshwater versus brackish water, also influence growth performance. For instance, the GIFT strain demonstrated superior average weight gain compared to four other improved tilapia strains: BIG NIN (sourced from Asia), Chitralada (sourced from Asia), “Ruvu Farm” (sourced from Uganda, East Africa), and Silver YY (sourced from Europe) (Moses et al. 2021). Additionally, the GIFT strain exhibited excellent growth performance, and the genotype-by-environment interaction was minimal and not significant enough to warrant prioritization in breeding programs (Moses et al. 2021).
Under various rearing conditions, the specific growth rate of the GIFT strain ranked highest, followed by FaST, control stock NIFI (National Inland Fisheries Institute, Thailand), and SEAFDEC-selected stocks (Southeast Asian Fisheries Development Center, Philippines). These strains were evaluated under both controlled tank-rearing (farm conditions) and lake-based cage systems (uncontrolled conditions) for Nile tilapia, with no significant differences in performance between the two rearing environments (Romana-Eguia et al. 2010). The variation in growth observed among the Nile tilapia stocks, particularly under controlled tank farming conditions, was primarily attributed to genetic factors, specifically differences between stocks (Romana-Eguia et al. 2010).
GIANT, a tilapia strain developed by WorldFish in Egypt, has demonstrated excellent growth performance and net profits compared to commercial strains (Ibrahim et al. 2019). Since its establishment in 2002, the Abbassa strain has maintained high levels of genetic diversity (Nayfa et al. 2020), indicating a robust genetic structure. However, in its juvenile stages, the GIANT-G9 appeared to be more susceptible to stress. Maintaining a balanced physiological response throughout an organism’s lifespan can be complex, but future research on different life stages of the GIANT‐G9 should provide more insight into its stress tolerance capabilities. Including larger experimental populations will also offer a clearer understanding. Generally, it is recommended to consider strain improvements based on selecting organisms that are more immune and stress-resistant, in addition to achieving the highest growth rates.
Conclusion
Selective breeding is a powerful, long-term, and labor-intensive tool for developing aquaculture stocks with desirable characteristics. The GIANT-G9 exhibited better growth performance compared to the commercial strain. However, the commercial strain demonstrated a more balanced and functional antioxidative response to stress at the certain stage of the rearing cycle. It is highly recommended to conduct more research on different stages of the production cycle and to focus on maintaining relatively balanced characteristics to develop a stronger strain.
Acknowledgements
Not applicable.
Author contributions
Conceptualization: Said M.M., Abo-Al-Ela H.G. and Nasr-Allah A.M. Data Curation: Abo-Al-Ela H.G, Bedir E.A. and Al wakeel R.A. Formal analysis: Abo-Al-Ela H.G and Bedir E.A. Investigation: Abo-Al-Ela H.G, Bedir E.A., Said M.M. and Al wakeel R.A. Methodology: Abo-Al-Ela H.G, Bedir E.A. and Al wakeel R.A. Writing– original draft: Abo-Al-Ela H.G. Writing– review & editing: Abo-Al-Ela H.G. All authors discussed the results and commented on the manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). Not applicable.
Data availability
The majority of the data supporting the findings of this study are included within the manuscript. Other relevant datasets can be obtained from the corresponding author upon reasonable request.
Declarations
Ethics approval
The experiment and animal management protocols were approved by the Faculty of Fish Resources at Suez University, Suez, Egypt (Approval number 07/05/2024/10). The study was conducted in accordance with national and international regulations and guidelines, including the ARRIVE guidelines (https://arriveguidelines.org/).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests, except that Dr. Ahmed Nasr-Allah is the Country Director of WorldFish– Egypt. We affirm that this competing interest does not compromise the integrity of the research findings presented in the manuscript.
Footnotes
Eman Ahmed Bedir and Haitham G. Abo-Al-Ela contributed equally to this work and share first authorship.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abo-Al-Ela HG (2019) Does vitamin C mitigate the detrimental effect of androgens on immunity? Res J Vet Sci 125:43–44. 10.1016/j.rvsc.2019.05.011 [DOI] [PubMed] [Google Scholar]
- Abo-Al-Ela HG, Faggio C (2021) MicroRNA-mediated stress response in bivalve species. Ecotoxicol Environ Saf 208:111442. 10.1016/j.ecoenv.2020.111442 [DOI] [PubMed] [Google Scholar]
- Abo-Al-Ela HG, El-Nahas AF, Mahmoud S, Ibrahim EM (2017) Vitamin C modulates the immunotoxic effect of 17α-methyltestosterone in Nile tilapia. Biochemistry 56:2042–2050. 10.1021/acs.biochem.6b01284 [DOI] [PubMed] [Google Scholar]
- Atef S, Ahmed OM, Said MM, Abo-Al-Ela HG (2024) Dietary Bacillus species modulate lipid metabolism-related parameters, growth, water quality, and bacterial load in Nile tilapia (Oreochromis niloticus). Anim Feed Sci Technol 310:115943. 10.1016/j.anifeedsci.2024.115943 [Google Scholar]
- Basiao ZU, Arago AL, Doyle RW (2005) A farmer-oriented Nile tilapia, Oreochromis niloticus L., breed improvement in the Philippines. Aquac Res 36:113–119. 10.1111/j.1365-2109.2004.01053.x [Google Scholar]
- Benli AÇK, Köksal G, Özkul A (2008) Sublethal ammonia exposure of Nile tilapia (Oreochromis niloticus L.): effects on Gill, liver and kidney histology. Chemosphere 72:1355–1358. 10.1016/j.chemosphere.2008.04.037 [DOI] [PubMed] [Google Scholar]
- Ber R, Daniel V (1992) Structure and sequence of the growth hormone-encoding gene from Tilapia nilotica. Gene 113:245–250. 10.1016/0378-1119(92)90402-B [DOI] [PubMed] [Google Scholar]
- Birnie-Gauvin K, Costantini D, Cooke SJ, Willmore WG (2017) A comparative and evolutionary approach to oxidative stress in fish: a review. Fish Fish 18:928–942. 10.1111/faf.12215 [Google Scholar]
- Cavatti Neto A, Alvarenga ÉRd, Toral FLB, Leite NR, da Costa FFB, Goulart LQ, Correa RDS, da Silva MA, dos Santos BD, Fernandes AFA, Turra EM (2023) Impact of selection for growth and stocking density on Nile tilapia production in the biofloc system. Aquaculture 577:739908. 10.1016/j.aquaculture.2023.739908 [Google Scholar]
- Chen L, Zheng L, Chen P, Liang G (2020) Myeloid differentiation primary response protein 88 (MyD88): the central hub of TLR/IL-1R signaling. J Med Chem 63:13316–13329. 10.1021/acs.jmedchem.0c00884 [DOI] [PubMed] [Google Scholar]
- Chervoneva I, Li Y, Schulz S, Croker S, Wilson C, Waldman SA, Hyslop T (2010) Selection of optimal reference genes for normalization in quantitative RT-PCR. BMC Bioinformatics 11:253. 10.1186/1471-2105-11-253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimits P (1957) The Tilapia and their culture, a second review and bibliography. FAO Fish Bull 10:1–24 [Google Scholar]
- Choi K, Lehmann DW, Harms CA, Law JM (2007) Acute hypoxia–reperfusion triggers immunocompromise in Nile tilapia. J Aquat Anim Health 19:128–140. 10.1577/H06-010.1 [DOI] [PubMed] [Google Scholar]
- Cressey D (2009) Aquaculture: future fish. 458:398–400. 10.1038/458398a [DOI] [PubMed]
- Darryl J (2023) Annual farmed finfish production survey: A modest supply decline for 2023 and a predicted return to growth in 2024. Global Seafood Alliance. https://www.globalseafood.org/advocate/annual-farmed-finfish-production-survey-a-modest-supply-decline-for-2023-and-a-predicted-return-to-growth-in-2024/#:~:text=Global%20production%20of%20farmed%20tilapia,%2C%20Indonesia%2C%20Egypt%20and%20Brazil. Accessed January 2024
- de Verdal H, Haffray P, Douchet V, Vandeputte M (2022) Impact of a divergent selective breeding programme on individual feed conversion ratio in Nile tilapia Oreochromis Niloticus measured in groups by video-recording. Aquaculture 548:737572. 10.1016/j.aquaculture.2021.737572 [Google Scholar]
- Duran BOS, Zanella BTT, Perez ES, Mareco EA, Blasco J, Dal-Pai-Silva M, Garcia de la serrana D (2022) Amino acids and IGF1 regulation of fish muscle growth revealed by transcriptome and microRNAome integrative analyses of Pacu (Piaractus mesopotamicus) myotubes. Int J Mol Sci 23:1180. 10.3390/ijms23031180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eknath AE, Hulata G (2009) Use and exchange of genetic resources of Nile tilapia (Oreochromis niloticus). Rev Aquacult 1:197–213. 10.1111/j.1753-5131.2009.01017.x [Google Scholar]
- Eknath AE, Bentsen HB, Ponzoni RW, Rye M, Nguyen NH, Thodesen J, Gjerde B (2007) Genetic improvement of farmed tilapias: composition and genetic parameters of a synthetic base population of Oreochromis niloticus for selective breeding. Aquaculture 273:1–14. 10.1016/j.aquaculture.2007.09.015 [Google Scholar]
- Fan S, Wang P, Zhao C, Yan L, Zhang B, Qiu L (2023) Molecular cloning, screening of single nucleotide polymorphisms, and analysis of growth-associated traits of igf2 in spotted sea bass (Lateolabrax Maculatus). Animals 13:982. 10.3390/ani13060982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO (2022) The state of world fisheries and aquaculture 2022. Towards blue transformation. Food and Agriculture Organization of the United Nations, Rome, Italy. 10.4060/cc0461en [Google Scholar]
- Fuentes EN, Einarsdottir IE, Valdes JA, Alvarez M, Molina A, Björnsson BT (2012) Inherent growth hormone resistance in the skeletal muscle of the fine flounder is modulated by nutritional status and is characterized by high contents of truncated GHR, impairment in the JAK2/STAT5 signaling pathway, and low IGF-I expression. Endocrinology 153:283–294. 10.1210/en.2011-1313 [DOI] [PubMed] [Google Scholar]
- Fuentes EN, Valdés JA, Molina A, Björnsson BT (2013) Regulation of skeletal muscle growth in fish by the growth hormone– insulin-like growth factor system. Gen Comp Endocrinol 192:136–148. 10.1016/j.ygcen.2013.06.009 [DOI] [PubMed] [Google Scholar]
- Gabillard J-C, Weil C, Rescan P-Y, Navarro I, Gutiérrez J, Le Bail P-Y (2003) Effects of environmental temperature on IGF1, IGF2, and IGF type I receptor expression in rainbow trout (Oncorhynchus mykiss). Gen Comp Endocrinol 133:233–242. 10.1016/S0016-6480(03)00167-9 [DOI] [PubMed] [Google Scholar]
- Hamed S, El-Kassas S, Abo-Al-Ela HG, Abdo SE, Al Wakeel RA, Abou-Ismail UA, Mohamed RA (2024) Interactive effects of water temperature and dietary protein on Nile tilapia: growth, immunity, and physiological health. BMC Vet Res 20:349. 10.1186/s12917-024-04198-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao L-x, Sun L (2016) Comparative analysis of the expression patterns of eight suppressors of cytokine signaling in tongue sole, Cynoglossus semilaevis. Fish Shellfish Immunol 55:595–601. 10.1016/j.fsi.2016.06.034 [DOI] [PubMed] [Google Scholar]
- Hegazi MM, Attia ZI, Ashour OA (2010) Oxidative stress and antioxidant enzymes in liver and white muscle of Nile tilapia juveniles in chronic ammonia exposure. Aquat Toxicol 99:118–125. 10.1016/j.aquatox.2010.04.007 [DOI] [PubMed] [Google Scholar]
- Herkenhoff ME, Ribeiro AO, Costa JM, Oliveira AC, Dias MAD, Reis Neto RV, Hilsdorf AWS, Pinhal D (2020) Expression profiles of growth-related genes in two Nile tilapia strains and their crossbred provide insights into introgressive breeding effects. Anim Genet 51:611–616. 10.1111/age.12944 [DOI] [PubMed] [Google Scholar]
- Hildahl J, Power DM, Björnsson BT, Einarsdóttir IE (2008) Involvement of growth hormone-insulin-like growth factor I system in cranial remodeling during halibut metamorphosis as indicated by tissue- and stage-specific receptor gene expression and the presence of growth hormone receptor protein. Cell Tissue Res 332:211–225. 10.1007/s00441-007-0568-2 [DOI] [PubMed] [Google Scholar]
- Houston RD, Bean TP, Macqueen DJ, Gundappa MK, Jin YH, Jenkins TL, Selly SLC, Martin SAM, Stevens JR, Santos EM, Davie A, Robledo D (2020) Harnessing genomics to fast-track genetic improvement in aquaculture. Nat Rev Genet 21:389–409. 10.1038/s41576-020-0227-y [DOI] [PubMed] [Google Scholar]
- Hu J, Yang J, Liao H (2024) Progress on stress resistance breeding in fish. Reprod Breed 4:267–278. 10.1016/j.repbre.2024.09.004 [Google Scholar]
- Huang S, Liu K, Cheng A, Wang M, Cui M, Huang J, Zhu D, Chen S, Liu M, Zhao X, Wu Y, Yang Q, Zhang S, Ou X, Mao S, Gao Q, Yu Y, Tian B, Liu Y, Zhang L, Yin Z, Jing B, Chen X, Jia R (2020) SOCS proteins participate in the regulation of innate immune response caused by viruses. Front Immunol 11. 10.3389/fimmu.2020.558341 [DOI] [PMC free article] [PubMed]
- Humanes A, Lachs L, Beauchamp E, Bukurou L, Buzzoni D, Bythell J, Craggs JRK, de la Torre Cerro R, Edwards AJ, Golbuu Y, Martinez HM, Palmowski P, van der Steeg E, Sweet M, Ward A, Wilson AJ, Guest JR (2024) Selective breeding enhances coral heat tolerance to marine heatwaves. Nat Commun 15:8703. 10.1038/s41467-024-52895-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim NA, Mohamed Nasr-Allah A, Charo-Karisa H (2019) Assessment of the impact of dissemination of genetically improved Abbassa Nile tilapia strain (GIANT-G9) versus commercial strains in some Egyptian governorates. Aquac Res 50:2951–2959. 10.1111/are.14249 [Google Scholar]
- Ishtiaq Ahmed AS, Xiong F, Pang S-C, He M-D, Waters MJ, Zhu Z-Y, Sun Y-H (2011) Activation of GH signaling and GH-independent stimulation of growth in zebrafish by introduction of a constitutively activated GHR construct. Transgenic Res 20:557–567. 10.1007/s11248-010-9439-9 [DOI] [PubMed] [Google Scholar]
- Janssen K, Chavanne H, Berentsen P, Komen H (2017) Impact of selective breeding on European aquaculture. Aquaculture 472:8–16. 10.1016/j.aquaculture.2016.03.012 [Google Scholar]
- Jarczak D, Nierhaus A (2022) Cytokine Storm—Definition, causes, and implications. Int J Mol Sci 23:11740. 10.3390/ijms231911740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, He M, Bai J, Chan CB, Wong AOL (2020) Signal transduction for TNFα-induced type II SOCS expression and its functional implication in growth hormone resistance in carp hepatocytes. Front Endocrinol 11. 10.3389/fendo.2020.00020 [DOI] [PMC free article] [PubMed]
- Juhua Y, Xuefeng C, Jianlin L, Yongkai T, Hongxia L, Pao X, Zaijie D (2010) Isolation of IGF2 and association of IGF2 polymorphism with growth trait in genetically improved farmed tilapias, Oreochromis niloticus L. Aquac Res 41:e743–e750. 10.1111/j.1365-2109.2010.02540.x [Google Scholar]
- Khaw HL, Bovenhuis H, Ponzoni RW, Rezk MA, Charo-Karisa H, Komen H (2009) Genetic analysis of Nile tilapia (Oreochromis niloticus) selection line reared in two input environments. Aquaculture 294:37–42. 10.1016/j.aquaculture.2009.05.025 [Google Scholar]
- Krumova K, Cosa G (2016) Chap. 1 Overview of Reactive Oxygen Species. In: Singlet Oxygen: Applications in Biosciences and Nanosciences, Volume 1, vol 1. The Royal Society of Chemistry, pp 1–21. 10.1039/9781782622208-00001
- Li YR, Jia Z, Trush MA (2016) Defining ROS in Biology and Medicine. React Oxyg Species 1:9–21. 10.20455/ros.2016.803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell T (1998) Feeding Tilapias. In: Lovell T (ed) Nutrition and Feeding of Fish. Springer US, Boston, MA, pp 215–225. 10.1007/978-1-4615-4909-3_12 [Google Scholar]
- Mohammed-Geba K, Martos-Sitcha JA, Galal-Khallaf A, Mancera JM, Martínez-Rodríguez G (2016) Insulin-like growth factor 1 (IGF-1) regulates prolactin, growth hormone, and IGF-1 receptor expression in the pituitary gland of the gilthead sea bream Sparus aurata. Fish Physiol Biochem 42:365–377. 10.1007/s10695-015-0144-8 [DOI] [PubMed] [Google Scholar]
- Mortelette H, Moisan C, Sébert P, Belhomme M, Amérand A (2010) Fish as a model in investigations about the relationship between oxygen consumption and hydroxyl radical production in permeabilized muscle fibers. Mitochondrion 10:555–558. 10.1016/j.mito.2010.05.002 [DOI] [PubMed] [Google Scholar]
- Moses M, Chauka LJ, de Koning DJ, Palaiokostas C, Mtolera MSP (2021) Growth performance of five different strains of Nile tilapia (Oreochromis niloticus) introduced to Tanzania reared in fresh and brackish waters. Sci Rep 11:11147. 10.1038/s41598-021-90505-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayfa MG, Jones DB, Benzie JAH, Jerry DR, Zenger KR (2020) Comparing genomic signatures of selection between the Abbassa strain and eight wild populations of Nile tilapia (Oreochromis niloticus) in Egypt. Front Genet 11. 10.3389/fgene.2020.567969 [DOI] [PMC free article] [PubMed]
- Obirikorang KA, Agbo NW, Obirikorang C, Adjei-Boateng D, Ahiave SE, Skov PV (2019) Effects of water flow rates on growth and welfare of Nile tilapia (Oreochromis niloticus) reared in a recirculating aquaculture system. Aquac Int 27:449–462. 10.1007/s10499-019-00342-0 [Google Scholar]
- Ozcan-Gokcek E, Isik R, Karahan B, Gamsiz K (2020) Genetic variation of insulin-like growth factor II (IGF-II) gene and its associations with growth traits in European sea bass (Dicentrarchus labrax). Turk J Fish Quat Sci 20:541–548. 10.4194/1303-2712-v20_7_04 [Google Scholar]
- Pang J-c, Gao F-y, Wang M, Zhao J-l, Lu M-x (2017) Isolation and characterization of toll-like receptor 21 and 22 genes from Nile tilapia, Oreochromis niloticus (Linnaeus). Aquac Res 48:3528–3544. 10.1111/are.13179 [Google Scholar]
- Petakh P, Kamyshna I, Kamyshnyi A (2024) Gene expression of protein kinase AMP-activated catalytic subunit alpha 1 (PRKAA1), solute carrier family 2 member 1 (SLC2A1) and mechanistic target of rapamycin (MTOR) in metformin-treated type 2 diabetes patients with COVID-19: impact on inflammation markers. Inflammopharmacol 32:885–891. 10.1007/s10787-023-01341-7 [DOI] [PubMed] [Google Scholar]
- Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res 29:e45–e45. 10.1093/nar/29.9.e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip AM, Vijayan MM (2015) Stress-immune-growth interactions: cortisol modulates suppressors of cytokine signaling and JAK/STAT pathway in rainbow trout liver. PLoS ONE 10:e0129299. 10.1371/journal.pone.0129299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce AL, Breves JP, Moriyama S, Hirano T, Grau EG (2011) Differential regulation of Igf1 and Igf2 mRNA levels in tilapia hepatocytes: effects of insulin and cortisol on GH sensitivity. J Endocrinol 211:201–210. 10.1530/joe-10-0456 [DOI] [PubMed] [Google Scholar]
- Pontigo JP, Vargas-Chacoff L (2021) Growth hormone (GH) and growth hormone release factor (GRF) modulate the immune response in the SHK-1 cell line and leukocyte cultures of head kidney in Atlantic salmon. Gen Comp Endocrinol 300:113631. 10.1016/j.ygcen.2020.113631 [DOI] [PubMed] [Google Scholar]
- Prchal M, D’Ambrosio J, Lagarde H, Lallias D, Patrice P, François Y, Poncet C, Desgranges A, Haffray P, Dupont-Nivet M, Phocas F (2023) Genome-wide association study and genomic prediction of tolerance to acute hypoxia in rainbow trout. Aquaculture 565:739068. 10.1016/j.aquaculture.2022.739068 [Google Scholar]
- Qiu W, Hu J, Magnuson JT, Greer J, Yang M, Chen Q, Fang M, Zheng C, Schlenk D (2020) Evidence linking exposure of fish primary macrophages to antibiotics activates the NF-kB pathway. Environ Int 138:105624. 10.1016/j.envint.2020.105624 [DOI] [PubMed] [Google Scholar]
- Rentier-Delrue F, Swennen D, Philippart JC, Lion CLh M, Benrubi O, Martial JA (1989) Tilapia growth hormone: molecular cloning of cDNA and expression in Escherichia coli. DNA 8:271–278. 10.1089/dna.1.1989.8.271 [DOI] [PubMed] [Google Scholar]
- Rezk M, Kamel E (2011) Cold tolerance of selective breeding of Oreochromis niloticus and oreochromis aureus. Egypt J Aquat 1:43–53. 10.21608/eja.2011.32049 [Google Scholar]
- Rezk MA, Ponzoni RW, Khaw HL, Kamel E, Dawood T, John G (2009) Selective breeding for increased body weight in a synthetic breed of Egyptian Nile tilapia, Oreochromis niloticus: response to selection and genetic parameters. Aquaculture 293:187–194. 10.1016/j.aquaculture.2009.03.019 [Google Scholar]
- Ridha MT (2006) Comparative study of growth performance of three strains of Nile tilapia, Oreochromis niloticus, L. at two stocking densities. Aquac Res 37:172–179. 10.1111/j.1365-2109.2005.01415.x [Google Scholar]
- Ridha MT, Cruz EM (2003) Effect of different schedules for broodstock exchange on the seed production of Nile tilapia Oreochromis Niloticus (L.) in freshwater. Aquac Int 11:267–276. 10.1023/a:1024853107029 [Google Scholar]
- Rius-Francino M, Acerete L, Jiménez-Amilburu V, Capilla E, Navarro I, Gutiérrez J (2011) Differential effects on proliferation of GH and IGFs in sea bream (Sparus aurata) cultured myocytes. Gen Comp Endocrinol 172:44–49. 10.1016/j.ygcen.2011.03.024 [DOI] [PubMed] [Google Scholar]
- Robinson N, Goddard M, Hayes B (2008) Use of gene expression data for predicting continuous phenotypes for animal production and breeding. Animal 2:1413–1420. 10.1017/S1751731108002632 [DOI] [PubMed] [Google Scholar]
- Rochman CM, Kurobe T, Flores I, Teh SJ (2014) Early warning signs of endocrine disruption in adult fish from the ingestion of polyethylene with and without sorbed chemical pollutants from the marine environment. Sci Total Environ 493:656–661. 10.1016/j.scitotenv.2014.06.051 [DOI] [PubMed] [Google Scholar]
- Romana-Eguia MRR, Ikeda M, Basiao ZU, Taniguchi N (2010) Growth comparison of Asian Nile and red tilapia strains in controlled and uncontrolled farm conditions. Aquacult Int 18:1205–1221. 10.1007/s10499-010-9334-7 [Google Scholar]
- Santos VBD, Mareco EA, Dal Pai Silva M (2013) Growth curves of Nile tilapia (Oreochromis niloticus) strains cultivated at different temperatures. Acta Sci-Anim Sci 35:235–242. 10.4025/actascianimsci.v35i3.19443
- Schmitz M, Baekelandt S, Bequet S, Kestemont P (2017) Chronic hyperosmotic stress inhibits renal toll-like receptors expression in striped catfish (Pangasianodon Hypophthalmus, Sauvage) exposed or not to bacterial infection. Dev Comp Immunol 73:139–143. 10.1016/j.dci.2017.03.020 [DOI] [PubMed] [Google Scholar]
- Schrader M, Travis J (2012) Embryonic IGF2 expression is not associated with offspring size among populations of a placental fish. PLoS ONE 7:e45463. 10.1371/journal.pone.0045463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shved N, Berishvili G, Mazel P, Baroiller JF, Eppler E (2011) Growth hormone (GH) treatment acts on the endocrine and autocrine/paracrine GH/IGF-axis and on TNF-α expression in bony fish pituitary and immune organs. Fish Shellfish Immunol 31:944–952. 10.1016/j.fsi.2011.08.012 [DOI] [PubMed] [Google Scholar]
- Sundaram AYM, Consuegra S, Kiron V, Fernandes JMO (2012) Positive selection pressure within teleost toll-like receptors tlr21 and tlr22 subfamilies and their response to temperature stress and microbial components in zebrafish. Mol Biol Rep 39:8965–8975. 10.1007/s11033-012-1765-y [DOI] [PubMed] [Google Scholar]
- Tan K, Zhang H, Zheng H (2020) Selective breeding of edible bivalves and its implication of global climate change. Rev Aquacult 12:2559–2572. 10.1111/raq.12458 [Google Scholar]
- Teng T, Zhao X, Li C, Guo J, Wang Y, Pan C, Liu E, Ling Q (2020) Cloning and expression of IGF-I, IGF-II, and GHR genes and the role of their single-nucleotide polymorphisms in the growth of pikeperch (Sander lucioperca). Aquacult Int 28:1547–1561. 10.1007/s10499-020-00542-z [Google Scholar]
- Thodesen J, Rye M, Wang Y-X, Yang K-S, Bentsen HB, Gjedrem T (2011) Genetic improvement of tilapias in China: genetic parameters and selection responses in growth of Nile tilapia (Oreochromis niloticus) after six generations of multi-trait selection for growth and fillet yield. Aquaculture 322–323:51–64. 10.1016/j.aquaculture.2011.10.010 [Google Scholar]
- Thodesen J, Rye M, Wang Y-X, Bentsen HB, Gjedrem T (2012) Genetic improvement of tilapias in China: genetic parameters and selection responses in fillet traits of Nile tilapia (Oreochromis niloticus) after six generations of multi-trait selection for growth and fillet yield. Aquaculture 366–367:67–75. 10.1016/j.aquaculture.2012.08.028 [Google Scholar]
- Trung NB, Lee P-T (2020) Functional characterization of myeloid differentiation factor 88 in Nile tilapia (Oreochromis niloticus). Comp Biochem Physiol B-Biochem Mol Biol 250:110485. 10.1016/j.cbpb.2020.110485 [DOI] [PubMed] [Google Scholar]
- Wei X, Li B, Wu L, Yin X, Zhong X, Li Y, Wang Y, Guo Z, Ye J (2018) Interleukin-6 gets involved in response to bacterial infection and promotes antibody production in Nile tilapia (Oreochromis niloticus). Dev Comp Immunol 89:141–151. 10.1016/j.dci.2018.08.012 [DOI] [PubMed] [Google Scholar]
- Wu P, Liu Y, Jiang W-D, Jiang J, Zhao J, Zhang Y-A, Zhou X-Q, Feng L (2017) A comparative study on antioxidant system in fish hepatopancreas and intestine affected by choline deficiency: different change patterns of varied antioxidant enzyme genes and Nrf2 signaling factors. PLoS ONE 12:e0169888. 10.1371/journal.pone.0169888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Li Y, Xu Y, Wang L, Ma X, Dong C, Zhao X, Tian X, Li X, Kong X (2020) The roles of two myostatins and immune effects after inhibition in Qi river crucian carp (Carassius auratus). Fish Shellfish Immunol 98:710–719. 10.1016/j.fsi.2019.11.015 [DOI] [PubMed] [Google Scholar]
- Yacout DMM, Soliman NF, Yacout MM (2016) Comparative life cycle assessment (LCA) of Tilapia in two production systems: semi-intensive and intensive. Int J Life Cycle Assess 21:806–819. 10.1007/s11367-016-1061-5 [Google Scholar]
- Yáñez JM, Joshi R, Yoshida GM (2020) Genomics to accelerate genetic improvement in tilapia. Anim Genet 51:658–674. 10.1111/age.12989 [DOI] [PubMed] [Google Scholar]
- Yáñez JM, Barría A, López ME, Moen T, Garcia BF, Yoshida GM, Xu P (2023) Genome-wide association and genomic selection in aquaculture. Rev Aquac 15:645–675. 10.1111/raq.12750 [Google Scholar]
- Yang CG, Wang XL, Tian J, Liu W, Wu F, Jiang M, Wen H (2013) Evaluation of reference genes for quantitative real-time RT-PCR analysis of gene expression in Nile tilapia (Oreochromis niloticus). Gene 527:183–192. 10.1016/j.gene.2013.06.013 [DOI] [PubMed] [Google Scholar]
- Yao T, Wang R, Han P, Liu X, Wang X (2023) Identification of olive flounder (Paralichthys olivaceus) toll-like receptor genes: involvement in immune response to temperature stress and Edwardsiella tarda infection. Fish Shellfish Immunol 138:108841. 10.1016/j.fsi.2023.108841 [DOI] [PubMed] [Google Scholar]
- Yating L, Mengli T, Huanhuan X, Tianyu G, Jin N, Yongjian L, Lixia T (2017) Effects of myostatin inhibitory peptides supplementation on growth performance, body composition, serum biochemical indices, serum and liver immune indices of sea bass (Lateolabrax japonicas). Chin J Anim Nutr 29:519–528 [Google Scholar]
- Yu N-t, Zeng W-w, Xiong Z, Liu Z-x (2022) A high efficacy DNA vaccine against Tilapia lake virus in Nile tilapia (Oreochromis niloticus). Aquac Rep 24:101166. 10.1016/j.aqrep.2022.101166 [Google Scholar]
- Zhang X, Wang A, Chang E, Han B, Xu J, Fu Y, Dong X, Miao S (2023) Effects of dietary tryptophan on the antioxidant capacity and immune response associated with TOR and TLRs/MyD88/NF-κB signaling pathways in northern snakehead, Channa argus (Cantor, 1842). Front Immunol 14. 10.3389/fimmu.2023.1149151 [DOI] [PMC free article] [PubMed]
- Zheng Y, Addotey TNA, Chen J, Xu G (2023) Effect of polystyrene microplastics on the antioxidant system and immune response in GIFT (Oreochromis niloticus). Biology 12:1430. 10.3390/biology12111430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Lou C, Ren B, Zhou Y (2022) Insulin-like growth factor 1 injection changes gene expression related to amino acid transporting, complement and coagulation cascades in the stomach of tilapia revealed by RNA-seq. Front Immunol 13. 10.3389/fimmu.2022.959717 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The majority of the data supporting the findings of this study are included within the manuscript. Other relevant datasets can be obtained from the corresponding author upon reasonable request.