Abstract
Aggregation of microtubule-associated tau protein is a distinct hallmark of several neurodegenerative disorders such as Alzheimer’s disease (AD), dementia with Lewy bodies (DLB), and progressive supranuclear palsy (PSP). Tau oligomers are suggested to be the primary neurotoxic species that initiate aggregation and propagate prion-like structures. Furthermore, different diseases are shown to have distinct structural characteristics of aggregated tau, denoted as polymorphs. Here, we investigate the structural and functional differences of amplified brain-derived tau oligomers (aBDTOs) from AD, DLB, and PSP. Our results indicate that the aBDTOs possess different structural and morphological features that impact neuronal function, gene regulation, and ultimately disease progression. The distinct tau oligomeric polymorphs may thus contribute to the development of clinical phenotypes and shape the progression of diseases. Our results can provide insight into developing personalized therapy to target a specific neurotoxic tau polymorph.
Subject terms: Neurodegeneration, Alzheimer's disease
Different aggregation characteristics and stability profiles of tau oligomeric polymorphs highlight the importance of studying the tau polymorphs to shed light on the functional differences and neuropathological heterogeneity in tauopathies.
Introduction
The pathological aggregation of the microtubule-associated tau protein is implicated in a heterogeneous group of age-related neurodegenerative diseases, collectively known as tauopathies1,2. It is widely accepted that tau misfolding and aggregation play a crucial role in mediating neurodegeneration and cognitive impairment in tauopathies3,4. Different tauopathies and related diseases such as progressive supranuclear palsy (PSP), Alzheimer’s disease (AD), and dementia with Lewy bodies (DLB) are highly variable in their clinical presentations and phenotypes1. Tau lesions identified from different diseases also vary in their isoforms and their affected brain regions and cell types5,6. PSP is a primary 4R-tauopathy exhibiting neuronal and glial tau inclusions7,8, whereas AD is referred to as a secondary tauopathy, which is characterized by the presence of other amyloidogenic protein pathology, specifically, amyloid-β9,10. While DLB is not technically considered a tauopathy, tau is a commonly exhibited pathology in addition to its primary pathology, α-synuclein11,12. AD is often associated with microglial activation and increased amyloid plaques in the entorhinal cortex and hippocampus, while DLB is largely associated with cortical involvement and α-synuclein plaques in the striatum13–18.
Multiple studies suggested the concept of “prion-like” induction and spreading of pathogenic proteins for many neurodegenerative diseases19–21. It is proposed that tau aggregates act as prion-like cores due to their characteristics to seed and promote misfolding and aggregation of endogenous tau19–23. However, little is known about how tau accumulation and aggregation manifest into such pathological heterogeneity and phenotypes. Several structural studies identified different fibrillar tau conformers in AD and other tauopathies24,25. These structural variants, named “polymorphs”, led to the hypothesis that a disease-specific misfolded tau conformer shapes each tauopathy, suggesting a direct link between the tau polymorph and disease progression and phenotype26–28. To date, many studies on tau protein have been performed on fibrillar tau aggregates29–32. Although neurofibrillary tangles have been defined as the pathological hallmark of AD, recent evidence has identified soluble tau oligomers (TauO) as the more relevant and toxic species in the propagation of disease due to their ability to initiate tau misfolding4,33–38. Therefore, tau polymorphs in their toxic oligomeric states must be more thoroughly investigated.
In this study, we characterized tau oligomers derived from postmortem human brain with AD, DLB, and PSP. We demonstrated that tau oligomers derived from different diseases form distinct polymorphs that are characterized by unique structure, morphology, seeding propensity, stability, and digestion profile. Structural and conformational differences in the oligomers were characterized using conventional methods as well as a novel technique, Fluorescent Amyloid Multi Emission Spectra (FLAMES), which allows us to detect and profile different amyloid conformations by measuring the spectral shift of amyloid fluorescent dyes when bound to protein39,40. Furthermore, we demonstrated the variabilities in the different tau oligomeric polymorphs’ functions by analyzing seeding propensity, neuronal function, and gene regulation. The neuropathological heterogeneity seen across tauopathies challenges our understanding of the pathophysiology behind tau seeding and aggregation. The studies presented here highlight the importance of developing accurate diagnostic methods to detect and distinguish the disease-specific tau polymorphs at an early stage and further personalized medicine.
Results
The brain-derived tau oligomers differ in size, morphology, and secondary structure
We recently showed that tau oligomers amplified by BDTOs (aBDTOs) derived from AD, DLB, and PSP brain tissues could successfully be distinguished based on their immunological properties, protein stability, and internalization mechanism in primary neurons7,41. To gain insight into the structural features of the aBDTOs in different proteinopathies, we isolated and characterized tau oligomers derived from brain extracts of AD, DLB, and PSP patients (Fig. 1A). First, we immunoprecipitated tau oligomers from the brain homogenates of AD, DLB, and PSP. Three cases from each disease were used, and T22 antibody was used to isolate the BDTOs from PBS-soluble fraction of brain homogenates as described previously33. The isolated tau oligomers were used as seeds for amplification in vitro using purified recombinant monomeric tau (2N4R). These amplified oligomers will be referred to as ‘aBDTOs’ hereafter. The isolated BDTO seeds and aBDTO from the three pathologies were subjected to biochemical and biological testing to evaluate the successful templating of the seeds to aBDTOs.
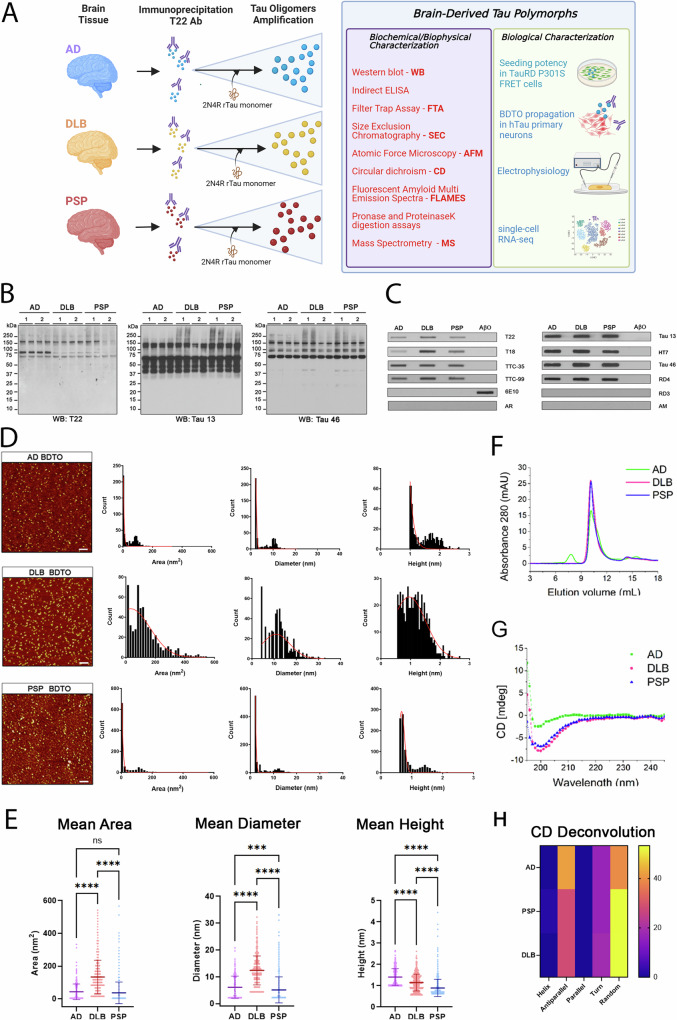
Fig. 1. Amplified brain-derived tau oligomers (aBDTOs) show differences in conformation, morphology, and structure.
A A schematic outlining the procedure for tau oligomer isolation and amplification from AD, DLB, or PSP brain tissues. Briefly, T22 antibody was used to immunoprecipitate the PBS soluble portion of brain homogenate. The purified oligomers were then used as seeds to amplify the BDTOs. The aBDTOs were characterized using biochemical, biophysical, and biological characterization to determine their structural and functional characteristics. B Western blot and C filter trap assay using T22 and other in-house tau antibodies (T18, TTC-35, and TTC-99) that recognize misfolded tau, as well as several generic tau antibodies (Tau 13, HT7, Tau 46, and Tau RD4) probing for different parts of tau regions show the differences in the conformation of aBDTOs. The controls anti-Aβ antibody, 6E10, Tau RD3 (for 3 R tau), and anti-mouse (AM) and anti-rabbit (AR) secondary antibodies only were used to exclude nonspecific probing. D Representative AFM images of AD, DLB and PSP aBDTOs with their corresponding area, diameter, and height distribution. The three aBDTOs show differences in their area, diameter, and height. Scale bar = 100 nm. E Area, diameter, and height distribution from AFM images were compared. One-way ANOVA with Tukey’s multiple comparison test was used to calculate the significance. Data are presented as mean ± SD (***p < 0.001, ****p < 0.0001) F Size exclusion chromatograms (SEC) of the AD, DLB, and PSP aBDTOs show the difference in the aggregate sizes. G Circular dichroism (CD) of the aBDTOs show different features in their secondary structures. H Heat map of the deconvoluted CD spectra to show the different secondary structure compositions. Importantly, AD shows 42% antiparallel sheet compared to 28.3% in PSP and DLB. AD also shows only 39.1% random coil compared to 53.3% for PSP and 53.1% for DLB.
We extensively characterized the aggregation pattern of AD, DLB, and PSP aBDTOs using western blot (WB) and filter trap assay (Fig. 1B, C). In WB, we used anti-oligomeric antibody T22, pan-tau antibody Tau 13 (sequence epitope: 15–25aa), and Tau 46 (sequence epitope: 404–441aa) (Fig. 1B). T22 immunoblotting showed aggregated tau exceeding 250 kDa, which appears as a smear, and potential trimeric tau at ~150 kDa in all samples except one case of PSP aBDTOs. Two cases of the AD aBDTOs also showed a prominent band at ~75 kDa that was absent in other cases (Fig. 1B). Immunoblotting with N-terminal Tau 13 and C-terminal Tau 46 displayed the difference in the aBDTOs prepared from the three pathologies. While Tau 13 only showed high molecular weight (HMW) tau in one DLB and two PSP cases, Tau 46-reactive HMW tau was visible in all the samples. Similarly, we confirmed the 4 R nature of the aBDTOs using filter trap assay, where the aBDTOs showed distinct immunoreactivity against an array of in-house and commercial anti-tau antibodies, including RD3 (3 R tau-specific) and RD4 (4 R tau-specific) (Fig. 1C). To exclude nonspecific probing, anti-Aβ antibody, 6E10, Tau RD3 (for 3 R tau), and anti-mouse (AM) and anti-rabbit (AR) secondary antibodies alone were used as controls (Fig. 1C).
To further characterize aBDTOs morphology and size, we performed atomic force microscopy (AFM). We observed different sizes between the aBDTOs when measuring their areas, diameters, and heights (Fig. 1D, E). There were two distinct populations of smaller (lower area, diameter, and height) and larger (higher area, diameter, and height) aggregates for AD aBDTOs, with the majority being the smaller aggregates (Fig. 1D). In contrast, DLB aBDTOs exhibited more heterogeneous sizes, although their average size was comparable to that of the smaller AD aBDTOs. The PSP aBDTOs were generally similar in size to the smaller AD aBDTOs, but the larger aggregates were completely absent. The mean area of DLB (132.9 nm2) was significantly higher than AD (42.9 nm2) or PSP (36.4 nm2), as well as the mean diameter with 12.4 nm for DLB compared to 6.1 nm for AD and 5.1 nm for PSP (Fig. 1E). The average height, on the other hand, was larger with AD (1.4 nm) compared to 1.2 nm for DLB and 0.90 nm for PSP (Fig. 1E). We observed a large variability in the three parameters between the aBDTOs, suggesting that different diseases have different morphology and size of tau aggregates. These results were comparable to those obtained from size exclusion chromatography (SEC). SEC result showed an early peak at lower elution volume for AD aBDTOs (Fig. 1F), suggesting the presence of larger aggregates in AD aBDTOs compared to DLB and PSP aBDTOs. The peaks shown around 10.5 mL elution volume overlapped for the three aBDTOs, indicating the presence of comparably sized AD, DLB, and PSP aBDTOs (Fig. 1F). To gain deeper insights, we performed circular dichroism (CD) to analyze the secondary structure composition of the aBDTOs (Fig. 1G, H). The CD spectra revealed a comparable profile for DLB and PSP aBDTOs, both of which displayed a higher proportion of random coils compared to AD aBDTOs (Fig. 1G). This was confirmed by the deconvoluted CD spectra, which only showed 39.1% random coil compared to 53.3% and 53.1% for PSP and DLB, respectively (Fig. 1H). The absence of random coil seemed to be attributable to an increase in antiparallel β-sheet as AD contained 42% compared to 28.3% in DLB and PSP (Fig. 1H).
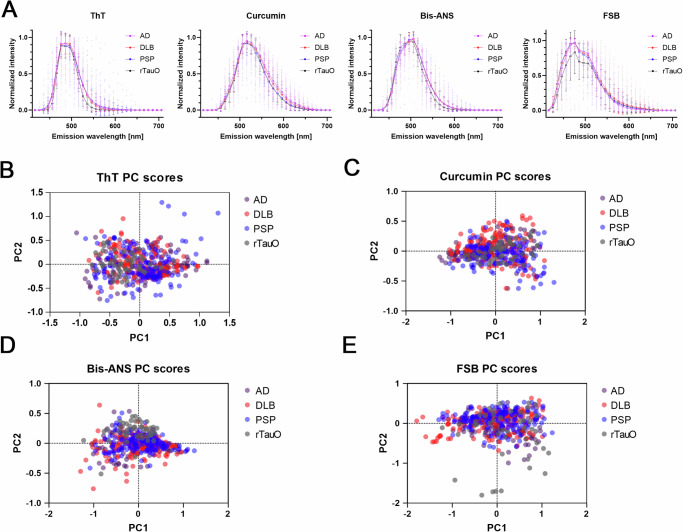
To further differentiate the conformational properties of the aBDTOs, we performed a novel technique known as FLAMES, developed to distinguish different amyloid conformations using confocal spectral imaging (Fig. 2)39. We examined the AD, DLB, and PSP aBDTOs using four commercially available fluorescent amyloid-binding dyes (ThT, curcumin, bis-ANS and FSB) to detect the spectral shift of each dye when bound to different tau polymorphs. Recombinant tau oligomers (rTauO) were included as controls alongside the aBDTOs. The aBDTOs and rTauO immobilized in agarose gel were stained with each dye (100 µM bis-ANS, 50 µM ThT, 20 µM curcumin, and 10 µM FSB) and then visualized and analyzed under confocal microscope. The fluorescent emission intensity of each oligomer was normalized to 1 and then averaged for easier comparison. None of the fluorescent emission spectra showed difference across diseases, apart from a lower average FSB intensity with rTauO (Fig. 2A). While the spectral data provide useful insights, plotting the variations between the aggregates offer more valuable information about their heterogeneity and conformational differences. Principal component analysis (PCA) of the fluorescent emission spectra was performed on the aBDTOs and rTauO, further supporting that none of the fluorescent dyes distinguished the different polymorphs (Fig. 2B–E). Bis-ANS somewhat discriminated rTauO from the aBDTOs, indicated by the separate clustering of rTauO (Fig. 2D). However, none of the aBDTOs were distinguishable from one another (Fig. 2D). When comparing the average peak intensities of ThT (486 nm) and curcumin (516 nm), AD showed a stronger intensity compared to PSP (Fig. S1a, b). ThT intensity for AD was higher but insignificant compared to DLB, and curcumin intensity was significantly higher than DLB (Fig. S1a, b). This correlates with the CD data (Fig. 1G, H), where AD oligomers show higher β-sheet content compared to DLB or PSP. To validate the viability of FLAMES in distinguishing different polymorphs, recombinant tau fibrils (rTauF) were synthesized and compared to the rTauO (Fig. S1c–g). Interestingly, PCA revealed that ThT, curcumin, and bis-ANS could distinguish rTauF from rTauO and AD aBDTO, while FSB could not. A similar analysis was also performed in brain sections (Fig. S2). The sections were stained with the four dyes (green) as well as a pan-tau antibody Tau 5 (red) to ensure the co-staining of the dye with the antibody. The co-stained loci were visualized using a confocal microscope, revealing that bis-ANS and curcumin mainly detected soluble aggregates while ThT and FSB highlighted filamentous aggregates. Furthermore, these loci were analyzed using fluorescent emission spectra, revealing that FSB showed some visible differences in PSP aBDTOs compared to AD and DLB aBDTOs, and curcumin showed the most visible differences between the three diseases. These results indicate structural variability between the three aBDTOs and rTauO, distinguishable by emission spectra using certain amyloid-binding dyes.
Fig. 2. Fluorescent Amyloid Multi Emission Spectra (FLAMES) data of aBDTOs and rTauO support their conformational differences.
A Fluorescent emission profile of ThT-, curcumin-, bis-ANS-, and FSB-bound to AD, DLB, and aBDTOs and recombinant tau oligomers (rTauO). The bars and error bars represent mean ± SD. N = 145–150 per case for the aBDTOs and n = 44–50 for rTauO. B–E PCA analysis performed on the emission spectra of aBDTOs and rTauO. Each circle in each panel represents the fluorescent signal from individual oligomers. The separation is not very prominent, with only bis-ANS bound to rTauO showing a bit of separate clustering from the rest.
Proteolytic stability reveals conformational differences in aBDTOs
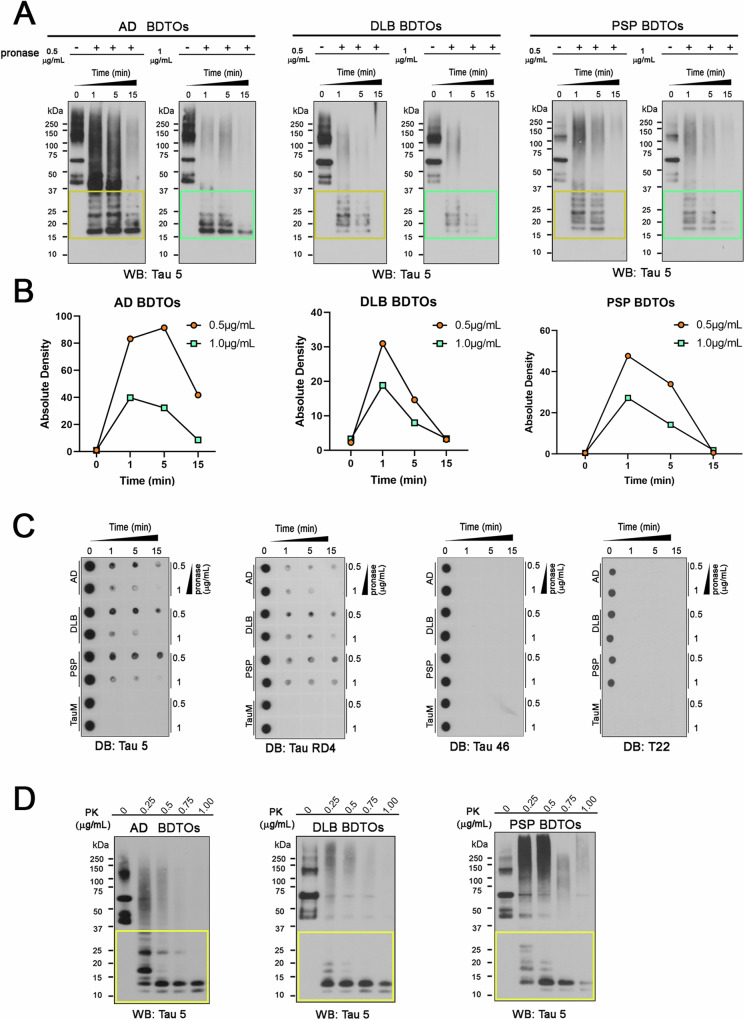
In vitro proteolysis is one of the most widely used methods to expose the core of amyloid proteins42,43. It is also shown that the core region of different tau aggregates exhibits variable protease stability44. Recently, our group also demonstrated that the conformational stability of aBDTOs, polymorphic α-synuclein oligomers, and cross-seeded recombinant tau oligomers can be successfully distinguished based on their proteolytic profiles7,35,41,45,46. As the different core structures and conformation of the proteins can be accountable for the different proteolytic stability, we investigated the proteolytic profiles of AD, PSP, and DLB aBDTOs. We subjected the aBDTOs to proteolysis using pronase and proteinase K (PK) enzymes. AD, DLB and PSP aBDTOs were exposed to 0.5 and 1 µg/mL of pronase for 1, 5, and 15 min and were evaluated by WB probed with Tau 5 antibody (Fig. 3A). Here, Tau 5 antibody was used after being experimentally determined to show the most staining of digested oligomers. This is most likely due to a disruption of epitopes for other total tau antibodies like Tau 13. They all yielded fragments upon exposure to the enzymes and showed an increased sensitivity to increased concentration of pronase and incubation time. AD aBDTOs showed more resistance to 0.5 µg/mL of pronase at 1 min compared to DLB and PSP aBDTO (Fig. 3A). On the contrary, DLB aBDTOs showed a strong degradation after 1 min, indicating a higher sensitivity to pronase activity. The degradation profiles of each aBDTOs were also graphically represented with quantification of the cleaved forms in terms of normalized density of the western blot. We clearly observed that each aBDTO present a different profile, suggesting that the three aBDTOs have variable pronase stability (Fig. 3B).
Fig. 3. Proteolytic stability of aBDTOs depict their conformational differences.
A AD, DLB and PSP aBDTOs were exposed to 0.5 and 1 μg/mL of pronase for 1, 5, and 15 min and were evaluated by Western blot probed with Tau 5 antibody. B Absolute density of cleaved tau forms is represented in a timeline graph for AD, DLB, and PSP aBDTOs exposed to 0.5 and 1 µg/mL of pronase for 1, 5, and 15 min C AD, DLB, and PSP aBDTOs and recombinant tau monomer were exposed to 0.5 and 1 μg/mL of pronase for 1, 5 and 15 min and were evaluated by dot blot probed with Tau 5, RD4, Tau 46 and T22. D AD, DLB and PSP aBDTOs were exposed to increasing concentrations of Proteinase K (PK) enzyme (0.25, 0.5, 0.75, and 1 μg/mL) and evaluated by Western blot probed with Tau 5. The aBDTOs showed diverse patterns of fragmentation from proteolysis, which indicates their conformational variability.
To explore deeper into the immunoreactivity of the digested proteins, the aBDTOs and recombinant tau monomer after pronase incubation were probed with Tau 5, RD4, Tau 46 and T22 (Fig. 3C). Density of aBDTOs with Tau 5 (sequence epitope: 210–241aa) was more present than with Tau 46 (sequence epitope: 404–441aa). This is as expected that pronase activity happens more readily in the N terminal of Tau than in the middle of the protein. Similarly, the reactivity with RD4 was comparable to that of Tau 5. No activity was seen with T22, suggesting that the oligomeric nature of aBDTOs was disrupted even at 1 min, as verified in Fig. 3A. We then conducted a proteolytic assay using a different enzyme, proteinase K (PK), to validate our findings (Fig. 3D). PK assay corroborated the observations made with pronase, indicating that AD aBDTOs exhibit the most resistance, while DLB aBDTOs are the most susceptible to proteolytic digestion. As an additional verification of our observations from pronase and PK digestions, we performed mass spectrometry (MS) analysis of trypsin digestion of aBDTOs. The amino acid sequence of human tau is shown along with MS intensities of the tryptic peptides of the aBDTOs in Fig. S3. All the experiments thus far evidence towards the different conformational characteristics of AD, DLB, and PSP aBDTOs.
Immunological characterization of aBDTO polymorphs using monoclonal antibodies targeting tau oligomers (TOMAs)
We evaluated the immunological properties of aBDTOs using tau oligomeric monoclonal antibodies (TOMAs). Representative WB images of AD, DLB, and PSP aBDTOs probed with TOMA clones and a generic total tau antibody, Tau 5. All four TOMAs exhibited distinct patterns between the diseases, indicating variations in their immunoreactivity across the studied pathologies (Fig. 4A). Notably, TOMA2 showed stronger binding to DLB aBDTOs, while TOMA 3 had the weakest binding to PSP aBDTOs at lower molecular weights. These results were compared and verified with the Tau 5 immunoreactivity, which showed a similar binding pattern to TOMA3 at higher molecular weights (Fig. 4B). A similar analysis was performed using filter trap assay, probed with Tau 5, TOMA clones (TOMA1, 2, 3 and 4), TTC-M antibodies, and two antibody controls: anti-Aβ antibody 6E10 and anti-mouse (AM) secondary antibody (Fig. 4C) Recombinant amyloid-beta oligomers (AβO) were used as negative control. TOMA1 and TTC-M1 showed the strongest immunoreactivity with AD aBDTOs, and TOMA4 showed the weakest reactivity with PSP aBDTOs (Fig. 4C). Filter trap assay confirmed the distinct immunoreactivities of AD, DLB, and PSP aBDTOs when probed with toxic-tau oligomer-targeting antibodies, TTCM1 and 2 in addition to the TOMA clones. For additional validation, we conducted ELISA analysis on aBDTO polymorphs probed with the oligomeric-specific tau antibodies T22 and the four TOMA clones (Fig. 4D). TOMA1 exhibited significant difference between the three aBDTOs, while TOMA2 and TOMA3 showed a more notable difference between AD and PSP (Fig. 4D). These three independent techniques consistently confirmed that the aBDTOs from AD, DLB, and PSP display distinct immunoreactivity profiles.
Fig. 4. Immunological characterization of aBDTOs by tau oligomer monoclonal antibodies (TOMAs).
A Representative WB images of AD, DLB, and PSP aBDTOs (N = 4) probed with TOMA clones: TOMA 1, TOMA 2, TOMA 3, and TOMA 4. B Representative WB images of AD, DLB, and PSP aBDTOs (N = 4) probed with a generic total tau antibody, Tau 5. C Analyses of aBDTOs by filter trap assay probed with Tau 5, TOMA clones (TOMA1, 2, 3, and 4), and the toxic tau conformational monoclonal antibodies, TTC-M1 and TTC-M2. Recombinant amyloid-beta oligomer (AβO) was used as a negative control for sample, and anti-Aβ antibody, 6E10 and anti-mouse (AM) secondary antibody were used as negative controls for the antibodies. D ELISA analyses of oligomeric aBDTOs probed with T22 and the four TOMA clones. E A schematic depicting immunodepletion of aBDTOs with TOMAs 1–4 and the seeding activity of the aBDTO/TOMA complexes. F–H Tau biosensor cells were exposed to immunodepleted AD, DLB, and PSP aBDTO/TOMAs complexes mixed with lipofectamine 2000 as vehicle for 24 h. aBDTOs without TOMA immunodepletion were also used as controls. Images were taken with 40x magnification. The scale bar = 25 μm.
Next, we assessed the ability of TOMAs to immunodeplete aBDTOs by treating biosensor cells with immunodepleted aBDTOs. The schematic illustrating the process of immunodepleting the aBDTOs with TOMAs (1–4) and the resulting seeding activity of the aBDTO/TOMA complexes is shown in Fig. 4E. Tau biosensor cells were exposed to immunodepleted AD, DLB, and PSP aBDTO/TOMAs complexes mixed with the vehicle lipofectamine 2000 for 24 h. The seeding activity of the complexes was visualized through Förster Resonance Energy Transfer (FRET) signal via immunofluorescence microscopy. The aBDTOs without TOMA immunodepletion were also used as controls and showed numerous FRET aggregates as expected. The immunodepleted aBDTOs with TOMAs failed to trigger any FRET signal, indicating that TOMAs diminish seeding capacity of aBDTOs (Fig. 4F–H). The images were taken with 40x magnification. Additional experiments were conducted to demonstrate immunoreactivity of TOMAs in human tissue. AD, DLB, and PSP brain slices were co-immunostained with TOMAs. In AD tissues, TOMA1 and TOMA4 exhibited a spotted distribution, while TOMA2 and TOMA3 displayed a more diffused cellular signal (Fig. S4a). The overlapped image revealed some co-localized spots in white, but it also highlighted the differences in each TOMA fluorescence channel (Fig. S4a). DLB tissue presented a spotted distribution for all TOMAs, and aggregates were partially overlapped (Fig. S4b). PSP presented a strong overlap between TOMA3 and TOMA4 (Fig. S4c). Comparing PSP with AD and DLB, only a few colocalized points were observed in the human brain, suggesting a more heterogenous population of tau oligomers in PSP brain. Additionally, large TOMA1 and TOMA2-positive aggregates were observed, and they did not show any colocalized spots (Fig. S4c).
The aBDTOs modulate seeding potency and synaptic function differently
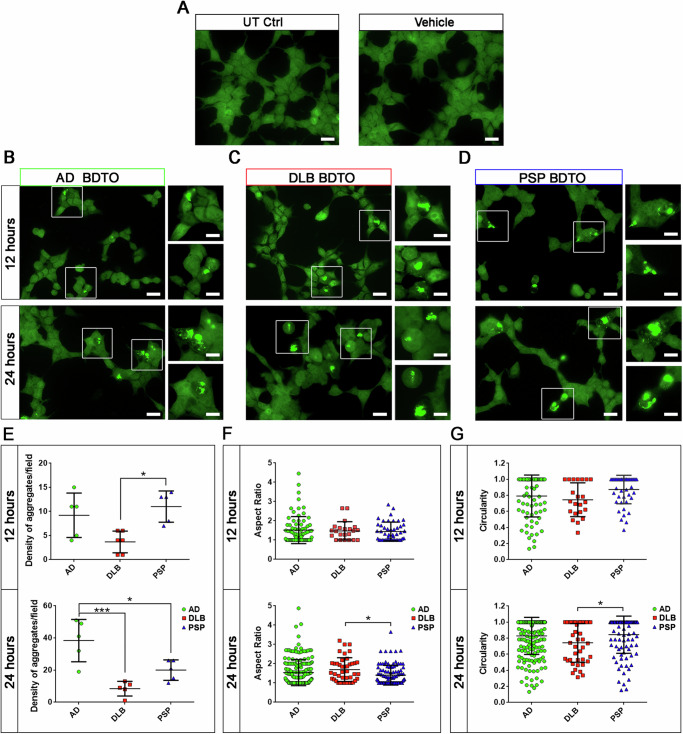
Tau RD P301S biosensor cells were used to determine the seeding activity of aBDTOs. Consistent with our previous study37, we observed FRET signal by immunofluorescence microscopy whose results indicate tau inclusion formation in the biosensor cells when exposed to the three aBDTOs (Fig. 5). Briefly, we treated the cells with the aBDTOs for 12 and 24 h and then observed a time-dependent increase in tau inclusion formation. The untreated (UT) or vehicle (liposome alone) treated control cells did not show any FRET signal as expected (Fig. 5A). FRET-positive cells showed bright green signals indicating tau aggregate formation in AD, DLB, and PSP aBDTO treated groups (Fig. 5B–D). Inset images are presented as higher magnification for better visualization. To evaluate the morphometric parameters in aBDTOs biosensor cells, we quantified the density of aggregates per field, aspect ratio, and circularity for each aBDTO treated group at 12 and 24 h (Fig. 5E–G). Statistical significance of data in Fig. 5E–G were analyzed using one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test. We observed a higher number of aggregates in AD and PSP compared to DLB after 12 h. Furthermore, the density of the aggregates among the aBDTOs after 24 h was the highest in AD, intermediate in PSP, and the lowest in DLB. The PSP aBDTOs showed a lower density at 24 h compared to the AD aBDTOs. We did not observe significant differences either in aspect ratio or circularity between the aBDTOs at 12 h. However, a significant difference was observed between DLB and PSP at 24 h (*p < 0.05) in parameters. PSP aBDTOs-treated cells exhibited less circular and more amorphous aggregates compared those treated with DLB aBDTOs. These observations in vitro regarding the heterogeneity of aBDTOs support that the AD, DLB, and PSP aBDTOs have different polymorphs, which drive different seeding capacities.
Fig. 5. Seeding potency of the BDTOs is modulated by their conformational heterogeneity.
Tau RD P301S biosensor cells were exposed to AD, DLB, or PSP aBDTOs at 100 nM concentration with lipofectamine 2000 as a vehicle for 12 and 24 h. A Cells that were untreated or treated with vehicle only were used as controls. FRET-positive cells showed bright green signals, indicating tau aggregate formation in B AD, C DLB, and D PSP aBDTO)-treated groups. Insets are presented for higher magnification of image. E Quantification of density of aggregates per field, F aspect ratio, and G circularity for each aBDTO-treated cell at 12 and 24 h are presented. Images were taken from 40x magnification of three random fields in triplicate. Data in (E, F, G) were compared by one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test: *p < 0.05; ***p < 0.001. Bars and error bars represent the mean ± SD. Scale bar = 20 µm.
We also evaluated the effects of aBDTOs on synaptic function. We observed that aBDTOs from different tauopathies impair neuronal functions to different magnitudes. Hippocampal slices were treated with the oligomeric samples for 1 h at 50 nM and washed with artificial cerebrospinal fluid (ACSF) for 10 min prior to recording (Fig. 6A). Input-output curve showed that AD and PSP aBDTOs, but not DLB aBDTOs, decreased basal neuronal transmission in comparison to the vehicle (Fig. 6B). These data indicated that AD and PSP aBDTOs only impair baseline transmission mechanisms compared to vehicle. Further, AD and PSP aBDTOs impaired baseline transmission to different extents, highlighting disease-specific differences in the synaptic toxicity of these aggregates. Paired-pulse ratios revealed no significant impairment of paired-pulse facilitation by the aBDTOs, although there was a trend toward a reduction in paired-pulse facilitation by the DLB aBDTOs (Fig. 6C). Long-term potentiation (LTP) profiles and analyses showed that LTP was abolished by DLB aBDTOs whereas it was impaired to a similar extent by AD and PSP aBDTOs (Fig. 6D, E). Linear regression fit was used in Fig. 6B with solid lines representing the linear fit and dotted lines representing 95% confidence bands. Two-way ANOVA was used in Fig. 6B followed by Dunnett’s multiple comparisons test to determine differences in fEPSP slope at 100 µV. One-way ANOVA was used in Fig. 6C, and E followed by Tukey’s multiple comparisons test. n = 3–6 mouse hippocampal slices per treatment group (*p < 0.05, ***p < 0.001, ****p < 0.0001).
Fig. 6. aBDTOs from different tauopathies impair neuronal functions to different magnitudes.
A An illustration indicating the regions of stimulation and recording. B Input-output curves showed that AD and PSP aBDTOs, but not DLB aBDTOs, have a significant decrease in basal neuronal transmission compared to the vehicle. C Paired pulse facilitation of the three aBDTOs showed no significant change in paired-pulse ratio compared to the vehicle. D The LTP profile and E LTP profile of the last 10 min of measurement only showed significant decrease of fEPSP slope in the three aBDTOs, with the highest significance in DLB aBDTOs. Linear regression fit was used in (B) with solid lines representing the linear fit and dotted lines representing 95% confidence bands. Two-way ANOVA was used in (B) followed by Dunnett’s multiple comparisons test to determine differences in fEPSP slope at 100 µV. One-way ANOVA was used in (C, E) followed by Tukey’s multiple comparisons test. n = 3–6 mouse hippocampal slices per treatment group (*p < 0.05, ***p < 0.001, ****p < 0.0001).
Propagation of aBDTOs in hTau primary neurons was evaluated to test differences in their capacity to seed human tau in a neuronal in vitro model (Fig. S5). A schematic representation of aBDTOs treatments and propagation in three successive passages (P0, P1, and P2) is presented in Fig. S5a–c. In each passage, neurons underwent concurrent treatment for immunostaining, and the cell lysates were collected for WB analysis. WB of total tau in UT, AD, DLB, and PSP has been performed from cell lysates at P0, P1, and P2 (Fig. S5d–f). Relative quantifications of tau (50 kDa band, Fig. S5g–i) and of tau (75–250 kDa bands, Fig. S5j–l) are reported for P0, P1, and P2. The capacity to seed between generations was also studied in the FRET biosensor cells for Generation 1 and Generation 2 (Fig. S6). The DLB polymorphs, compared to those in AD and PSP, exhibited a faster seeding capacity in vitro and a more homogeneous morphology. These characteristics are associated with a stronger impact on neuronal activity, particularly in abolishing LTP. In contrast, AD and PSP polymorphs, which are morphologically less homogeneous, impair not only LTP but also basal neuronal transmission. This evidence demonstrates that different polymorphs, characterized by unique seeding and morphological properties, exert varying impact on neuronal transmission.
Disease-derived tau oligomeric polymorphs impair gene expression differently in primary excitatory neurons
Electrophysiological analysis revealed that each aBDTOs impact neuronal function to a different extent. The AD, DLB, and PSP aBDTOs affect basal neuronal transmission and LTP differently, indicating that disease-related polymorphs exert distinct toxic effects on neurons. To further explore their differential effects on neurons, we investigated transcriptional regulation of genes involved in neuronal activity and function when exposed to the aBDTOs. We isolated primary cortical neurons from hTau mice and exposed them to each aBDTOs for 24 h. After treatment, we performed single-cell RNA sequencing using 10X Genomics technology to identify genes commonly and specifically regulated by each aBDTOs. Our analysis focused on the subpopulation of excitatory neurons, given their known vulnerability to degeneration in these diseases.
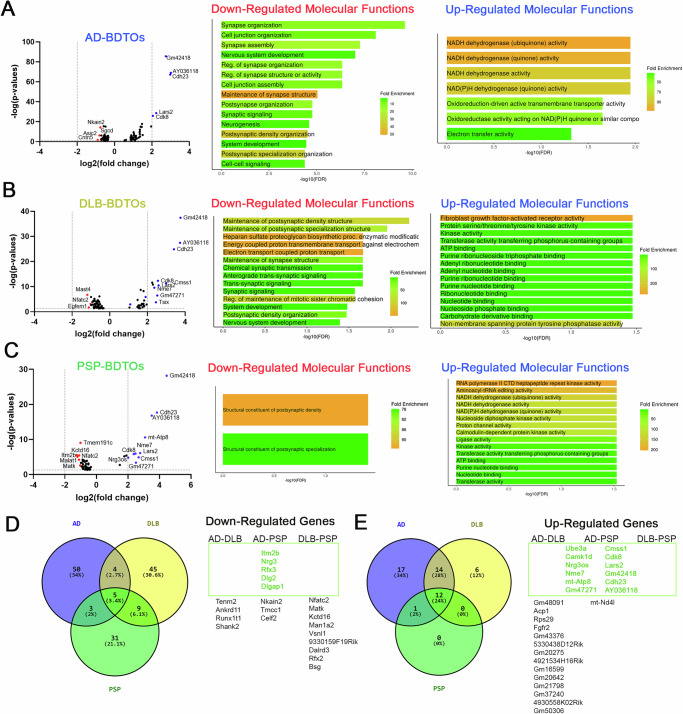
Figure 7 summarizes the genes that were significantly down and upregulated, as well as the gene ontologies (molecular function term) derived from the scRNA-seq analysis. AD aBDTOs down-regulated genes involved in maintaining synaptic structure and postsynaptic density organization (Fig. 7A). Additionally, genes associated with cell junctions, neurogenesis, and neuronal development were also downregulated. Similar patterns of downregulation were observed in DLB and PSP aBDTOs (Fig. 7B, C). The downregulation of synaptic genes is consistent with our previous electrophysiological observations, which showed functional impairments. Notably, neurons exposed to DLB aBDTOs exhibited unique downregulation in domains such as trans-synaptic signaling, heparan sulfate proteoglycan biosynthesis, energy-coupled proton transmembrane transport, and chemical synaptic transmission (Fig. 7B). The downregulation of synaptic genes corroborates our electrophysiological findings, where AD and PSP aBDTOs impaired both basal neuronal transmission and LTP, while DLB aBDTOs did not affect basal neuronal transmission but completely abolished LTP (Fig. 6).
Fig. 7. The aBDTOs from different proteinopathies modulate gene expression differently in excitatory neurons.
Volcano plot of genes down and upregulated in excitatory neurons and their gene ontology when treated with A AD aBDTOs, B DLB aBDTOs, and C PSP aBDTOs exhibit clear differences between them. These results were combined into a Venn diagram of D downregulated and E upregulated genes in AD, DLB, and PSP aBDTOs treated neurons. Several genes were commonly modulated, while many more were specifically modulated by each polymorph, especially genes that were downregulated.
Moreover, both AD and PSP aBDTOs induced the upregulation of genes involved in NADH dehydrogenase activity (Fig. 7A, C). Individually, AD aBDTOs upregulated oxidoreduction-driven active transmembrane transporter activity and electron transfer activity, while PSP aBDTOs upregulated genes implicated in RNA polymerase II CTD kinase activity, aminoacyl-tRNA editing activity, and Calmodulin-dependent protein kinase activity. In contrast, DLB aBDTOs upregulated genes mainly implicated in fibroblast growth factor-activated receptor activity and non-membrane spanning protein tyrosine phosphatase activity (Fig. 7B). Additionally, DLB aBDTOs increased the expression of genes involved in kinase activity and ATP binding.
We also identified five genes that were commonly downregulated: Itm2b, Nrg3, Rfx3, Dlg2, and Glgap1 (Fig. 7D). Itm2b is known to inhibit APP processing, and its interaction with APP has been reported to reduce amyloid peptide secretion47. Nrg3 (Neuregulin 3) promotes excitatory synapse formation in hippocampal neurons, playing a crucial role in synaptogenesis and synaptic functions48. Rfx3 is of particular interest because variants in this gene, along with other RFX genes, have been associated with autism spectrum disorder (ASD) and intellectual disability49. Dlg2 is also associated with ASD, and its dysregulation can affect the excitatory/inhibitory balance in the brain50,51. Glgap1 is a neuroprotective gene52, thus its downregulation is not surprising.
On the other hand, 12 genes were commonly upregulated, most of which are long non-coding RNAs (e.g., Gm42418 and AY036118) (Fig. 7E). Highly expressed protein-coding genes included Cdh23, Cdk8, Lars2, Cmss1, and Ube3a. We also observed that 14 additional genes were upregulated in both AD and DLB, while none were shared between AD-PSP and DLB-PSP (Fig. 7E). These findings suggest that aBDTOs share a common impact on gene expression, particularly regarding synapse organization, function, and purine ribonucleoside metabolism. Meanwhile, they also exhibit a distinct gene regulation profile. The differences in the downregulated genes underscore the diverse toxic effects that the aBDTOs exert on excitatory neurons.
Discussion
The importance of the structural and conformational differences of amyloid polymorphs in different neurodegenerative diseases are now more widely acknowledged and understood24–28. Yet, it is astounding that a single tau protein can manifest such pathological heterogeneity in tauopathies, which comprise almost 20 different diseases accompanied with diverse clinical representations. Several groups have used either whole brain homogenates from different brain pathologies or tau fibrils to demonstrate their spreading in animals19,53–56. Many studies, including ours, have shown that smaller aggregates, such as soluble oligomers, are the true toxic species that initiate the propagation of aggregated materials33,36,57–59. Hence, estimating the extent of the toxic effects exerted by the different oligomeric amyloid polymorphs is critical to the understanding of the biological differences between the proteinopathies. Here, we demonstrated that amplified tau oligomers derived from AD, DLB, and PSP pathologies exhibit differences in their structures, morphologies, immunological properties, and bioactivities.
While there is ongoing debate regarding whether templated amyloids accurately represent their biological seeds, they undoubtedly provide unparalleled means for studying amyloids in their most closely replicated forms60,61. Following previously published methods to isolate and amplify amyloid oligomers from AD brain tissues8,33,62–68, we successfully isolated, amplified, and characterized tau oligomers using brain tissues from PSP, a primary tauopathy, as well as from AD and DLB, mixed pathologies. Our characterization of amplified PSP, AD, and DLB aBDTOs resulted in differences in size, morphology (AFM, western blot, and filter trap assay) and conformation (CD and FLAMES). We further observed that aBDTOs presented a unique proteolytic stability pattern in Pronase and PK assays. DLB aBDTOs were the most susceptible to PK and Pronase activities, suggesting that aBDTOs derived from DLB have a more dynamic core than those derived from AD and PSP. This is also consistent with changes observed in biosensor cells, where aBDTOs from DLB formed less FRET aggregates than from AD and PSP.
These conformational and structural differences subsequently translate to functional variations among the three pathologies, as evidenced by the impact on neuronal activity. Indeed, the aBDTOs from different proteinopathies exhibited unique and disease-specific impairment of neuronal activity. AD and PSP aBDTOs reduced basal neuronal transmission to varying extents and similarly impaired LTP. In contrast, DLB aBDTOs showed no significant effect on basal neuronal transmission but exhibited a trend toward a reduction of paired-pulse facilitation and completely abolished LTP. This is correlated with the scRNA-seq data, where neuronal transmission deficiency as well as downregulation of genes involved in energy transport chain were observed in DLB aBDTOs-treated brains. Markedly, it has been previously demonstrated that amyloid proteins like amyloid-β and α-synuclein can trigger tau aggregation and vice versa10,64,68. It has also been shown that co-expression of tau and α-synuclein increased cellular toxicity, showing their synergistic effects69. Thus, the manifestation of both tau and α-synuclein in DLB may underlie the enhanced toxicity and functional impairment observed in the disease, providing critical insights into their combined pathological impact.
Despite substantial structural and biophysical evidence supporting the existence of the distinct AD, DLB, and PSP polymorphs, PCA analyzed from FLAMES results was not sufficient to clearly distinguish between the polymorphs using the four commercially available fluorescent amyloid-binding dyes. Some dyes lacked the ability to discriminate between aBDTOs, while others showed a limited potential. Notably, curcumin could clearly differentiate the oligomers from the fibrils (Fig. S1c), but it did not distinguish between the different oligomeric polymorphs (Fig. 2C). These observations suggest a promising research direction for the development of novel small molecule amyloid dyes that can effectively differentiate the different polymorphs. Furthermore, while crystal structures of different tau fibrillar polymorphs have been determined24,25,60, there is still a lack of structural understanding of the different oligomeric polymorphs at the molecular level. Thus, their structural determination using techniques such as cryo-EM could significantly enhance our understanding of the aggregation mechanism.
The differential mechanisms of action of Tau oligomeric polymorphs could spam from structural, intracellular signaling to transcriptional and disease context effects. Variations in conformation across oligomers from diseases like AD, DLB, and PSP lead to unique surface-exposed epitopes that dictate their interactions with cellular components, such as synaptic proteins, intracellular machinery, organelles, or nucleic acid. These structural differences influence binding specificity and affinity, which in turn drive disease-specific effects. For instance, distinct targets, such as neuronal receptors or intracellular organelles, may preferentially interact with particular polymorphs, resulting in unique patterns of cellular dysfunction.
Differential interactions with intracellular machinery, such as the proteasome70, cytoskeleton71, or chromatin72, could lead to disease-specific patterns of dysfunction. The tau oligomers may induce distinct patterns of kinase activation or phosphorylation cascades, altering cellular responses such as synaptic plasticity or energy metabolism73. For example, here we showed that the upregulation of genes related to NADH dehydrogenase activity by AD and PSP aBDTOs, but not DLB aBDTOs, suggests disease-specific mitochondrial or metabolic disruptions. Disease-specific tau oligomers may differentially impact chromatin structure or transcription factor activity, leading to distinct transcriptional profiles74,75. This is evident in the unique sets of upregulated and downregulated genes identified in our scRNA-seq analysis. The selective dysregulation of genes such as Itm2b, Nrg3, and Dlg2 could result from the differential nuclear localization or chromatin-binding properties of the oligomers.
The differential impact of tau oligomeric polymorphs on synaptic function and neuronal health may be influenced by both disease-specific microenvironments and interactions with other cellular pathways or by differential internalization pathways showed by our previous study7. For instance, DLB oligomers uniquely impair long-term potentiation (LTP) without affecting basal synaptic transmission, suggesting a selective disruption of synaptic plasticity mechanisms, whereas AD and PSP oligomers disrupt both basal transmission and LTP. These effects could be further shaped by the local neuronal and glial microenvironment, where co-pathologies such as amyloid plaques in AD or α-synuclein aggregation in DLB modulate the cellular response to tau oligomers. Additionally, distinct molecular partners, such as heparan sulfate proteoglycans or fibroblast growth factor receptors, may mediate specific toxic pathways, underscoring the polymorph-specific nature of cellular damage in tauopathies.
The structural and biochemical diversity of tau oligomers likely drives their differential mechanisms of action. These differences are reflected in unique gene expression profiles, synaptic functional impairments, and toxic pathways in neurons. Understanding these mechanisms, using mechanisms-based approaches, can inform the design of targeted therapeutic interventions tailored to the specific polymorphs associated with different tauopathies. Our study provides a compelling of evidence that tau oligomeric polymorphs from three different proteinopathies vary in their structural and functional attributes. More specifically, amplified tau oligomers derived from AD, DLB, and PSP brains form distinct polymorphs that contribute the pathological heterogeneity. These findings could explain the clinical and neuropathological heterogeneity observed in the different proteinopathies and guide further research regarding the BDTO-specific proteotoxic pathways involved in neuronal degeneration and death.
Materials and methods
Statistics and reproducibility
All statistical analyses were performed using GraphPad Prism version 10.3 with statistical significance as p < 0.05. All specific statistical methods used, and the number of replicates is provided in each section of methods. All raw data are available in the supplementary data file and are also available upon request from the corresponding authors.
Brain homogenate preparation
Postmortem brain tissues from Alzheimer’s disease (AD), progressive nuclear palsy (PSP) and dementia with Lewy bodies (DLB) were obtained from Oregon Health and Science University, the Institute for Brain Aging and Dementia (University of California–Irvine, Irvine, California, USA) and the Brain Resource Center at Johns Hopkins. Neuropathological assessment conformed to National Institute on Aging/Reagan Institute consensus criteria. The following information was available for the cases used in this study: diagnosis, age at death, gender, post-mortem index, brain area, and Braak stage (Table S1). Each brain was homogenized in 1X phosphate-buffered saline (PBS) with a protease inhibitor cocktail (Roche; 11836145001), using a 1:3 dilution of brain: 1X PBS (w/v). Samples were centrifuged at 10,000 rpm for 10 min at 4 °C. Supernatants were then aliquoted, snap-frozen, and stored at −80 °C until use.
Immunoprecipitation of Tau oligomers from human brain tissues
Immunoprecipitation (IP) experiments were performed as previously described36. Briefly, tosyl-activated magnetic Dynabeads (Dynal Biotech, Lafayette Hill, PA) were coated with 20 μg of T22 antibody (1.0 mg/mL) diluted in 0.1 M borate, pH 9.5, overnight at 37 °C. Beads were washed (0.2 M Tris, 0.1% bovine serum albumin, pH 8.5) and then incubated with either AD, DLB, or PSP brain homogenate with rotation at room temperature (RT) for 1 h. Beads were then washed three times with 1X PBS and eluted using 0.1 M glycine, pH 2.8. The pH of each eluted fraction was adjusted using 1 M Tris pH 8.0. Fractions were then centrifuged in a micron centrifugal filter device with a molecular weight cut-off of 10 kDa (Millipore; 42415) at 14,000 g for 25 min at 4 °C. Oligomers were then resuspended in sterile 1X PBS. Total protein concentration was determined using PierceTM BCA protein assay kit (Thermo Scientific; 23225). The samples were again centrifuged in a micron centrifugal filter device with a cut-off of 10 kDa at 14,000 g for 25 min at 4 °C. Brain-derived Tau Oligomers (BDTOs) were then resuspended in 1X PBS to obtain the desired concentration (0.1–0.5 mg/mL) and kept at −20 °C. No oligomers were found in the IP from control brains as previously reported by our group and others7,41.
Preparation of recombinant Tau species
Recombinant tau protein (Tau-441 (2N4R) MW 45.9 kDa) was expressed and purified as previously described38,76. Tau pellet was treated with 8 M urea followed by overnight dialysis against 1X PBS pH 7.4. Tau concentration was measured using PierceTM BCA protein assay kit (Thermo Scientific; 23225) and normalized to 1 mg/mL by adding 1X PBS. Aliquots of Tau 4 R monomer in 1X PBS were stored at −20 °C until use.
For recombinant tau oligomer formation, the rTauM was first dissolved in 30% hexafluoroisopropanol (HFIP). The rTauM solution was aliquoted in a siliconized Eppendorf tube with holes placed on top of the cap to allow slow evaporation of HFIP. The samples were let stirred at 500 rpm using a Teflon-coated micro stir bar for 48 h at RT in a fume hood.
For recombinant tau fibril (rTauF) formation, the rTauM was first dissolved in 1X PBS. Heparin solution (50 mg/mL) was added to the solution to achieve a final 1:4 molar ratio of heparin to rTauM. The solution was then vortexed 5 times for 3 s each before being aliquoted out to 500 μL in siliconized Eppendorf tube. The aliquots were then vortexed 20 times for 3 s each at the same time. The Eppendorf tube was sealed with parafilm to avoid unwanted evaporation and incubated with rotation at 37 °C for 5 days. The rTauF was purified by centrifugation and qualified immediately using AFM.
In vitro amplification assay
Lyophilized pellets of recombinant Tau 4 R monomer were dissolved in 1X PBS at 1 mg/mL concentration38,77 and were seeded with BDTOs isolated from AD, DLB, or PSP brain tissues as previously described60,61,76. BDTOs-TauM mixture was made at a ratio of 1:100 (w/w) with gentle agitation at RT for 2 days. After seeding, aliquots were taken and immediately used for Western blotting using T22 and Tau 13 antibodies as well as AFM analysis for quality control. Total protein concentration was determined using PierceTM BCA protein assay kit (Thermo Scientific; 23225) and stored at −20 °C until use. To ensure an oligomeric population throughout the experiment, any insoluble aggregates such as fibrils that may have formed while handling were removed by centrifugation (10,000 rpm, 5 min) before every experiment.
Preparation of Aβ oligomers
Aβ oligomers (AβO) were prepared as previously described78. 0.3 mg of Aβ pellet was dissolved in 200 μL of HFIP and incubated for 10–20 min at RT. The resulting solution was added to 700 μL of ddH2O in a siliconized Eppendorf tube with holes placed on top of the cap to allow the slow evaporation of HFIP. The samples were then stirred at 500 rpm using a Teflon-coated micro stir bar for 48 h at RT in a fume hood.
Western blot
3 µg of each sample was resolved on a pre-cast NuPAGE 4–12% Bis-Tris Gels for SDS-PAGE (NP0335BOX, Invitrogen) and transferred to nitrocellulose membranes. Then, membranes were blocked with 10% non-fat milk in Tris-buffered saline with 0.01% tween (TBS-T) overnight at 4 °C. After blocking, membranes were probed with sequence-specific tau antibodies: Tau 13 (1:50000; 835204, BioLegend), Tau 46 (1:5000; 806601, BioLegend), Tau 5 (1:10000; 806402, BioLegend), and oligomeric-specific tau antibody T22 (1:250), each diluted in 5% non-fat milk for 1 h at RT. Membranes probed with Tau Oligomer Monoclonal Antibodies TOMA1 (1:100), TOMA 2 (1:200), TOMA 3 (1:100), and TOMA 4 (1:100) diluted in 5% non-fat milk, were incubated for overnight at 4°C. Membranes were then incubated with HRP-conjugated IgG anti-mouse or anti-rabbit (1:10,000, GE Healthcare) secondary antibody. ECL plus (GE Healthcare) was used for signal detection.
Filter trap assay
Filter Trap assay was performed using Bio-Dot SF Microfiltration Apparatus (Bio-Rad), as previously described41,77. Briefly, 1 μg of each end-product reaction was applied onto nitrocellulose membranes, previously pre-wetted with TBS-T, using a vacuum-based bio-slot apparatus. Membranes were then blocked with 10% non-fat milk in TBS-T overnight at 4 °C. Next day, membranes were probed with the oligomer-specific tau antibody, T22 (1:250; in-house), misfolded tau aggregates antibody T18 (1:5000; in-house), toxic tau conformation antibody TTC-35 (1:250; in-house), TTC-99 (1:250; in-house), and sequence-specific tau antibodies, Tau 13 (1:50000; 835204, BioLegend), HT7 (1:5000; Thermo Fisher, MN1000), Tau 46 (1:5000; 806601, BioLegend), Tau RD4 (1:1000, 05-804, Millipore Sigma), Tau RD3 (1:1000, 05-803, Millipore Sigma), Tau 5 (1:10,000, 806402, BioLegend) and anti-amyloid beta, 6E10 (1:1000, 803011, BioLegend), diluted in 5% non-fat milk for 1 h at RT. Membranes probed with TOMA clones TOMA1 (1:100), TOMA 2 (1:200), TOMA 3 (1:100), TOMA 4 (1:100) or Toxic Tau Conformational Monoclonal antibodies TTC-M1 (1:200) and TTC-M2 (1:1000), diluted in 5% non-fat milk, were incubated for overnight at 4 °C. Membranes were then incubated with HRP-conjugated IgG anti-rabbit (1:10000) or anti-mouse (1:10,000) secondary for 1 h at RT. ECL plus (GE Healthcare) was used for signal detection.
Morphological analysis of aBDTOs by Atomic Force Microscopy (AFM)
aBDTOs were characterized by AFM as previously described7,36,38,41,77,79. Briefly, samples were prepared by adding 5 µL onto freshly cleaved mica and allowed to adsorb to the surface. Mica was then washed three times with deionized water to remove unbound protein and impurities and then air-dried. Samples were then imaged with a Multimode 8 AFM machine (Veeco, CA) using a non-contact tapping method (ScanAsyst-Air). AFM analyses were performed using the particle analysis tool of the NanoScope Analysis v1.20rl AFM data processing software. All experiments were performed in duplicate.
Size Exclusion Chromatography (SEC)
aBDTOs were analysed using AKTA Explorer system fitted with a Superdex 200 Increase 10/300 GL Column as previously described7,41,79. Degassed deionized water was used as mobile phase with a flowrate of 0.5 mL/min. Gel filtration standard (Bio-Rad 51-1901) was used for calibrations. Samples were resolved using absorbance at 280 nm.
Circular Dichroism (CD)
The Jasco-720 (Jasco, Inc) machine with a temperature controller was used to measure the CD spectra of the aBDTOs. The spectra were recorded with a scan speed of 20 nm/min at 0.2 nm interval using a 1 mm pathlength quartz cell. Protein concentration of 0.1 mg/ml was mixed well in 1× PBS (pH 7.4), and measurements were made from 195 to 250 nm with an average of three iterations for each spectrum. The quartz cell was washed with water and ethanol between every use. The spectra were analyzed using K2D3 software80 and plotted using OriginPro 8.5 (OriginLab). The spectra were deconvoluted using the BeStSel algorithm81.
Immobilization of Tau oligomers and aBDTOs in agarose Gel
Recombinant 4 R TauO (rTauO) and AD, PSP, and DLB aBDTOs were immobilized in agarose gel as previously described with minor modifications39. 4 μg of samples were mixed with a solution of 0.8% low melting point agarose (Sigma A9414) in double-distilled water and then pipetted in 96-well microplate (Grenier Bio-One 675096). After gel formation, samples were incubated with fluorescent amyloid-binding dyes (100 µM bis-ANS, 50 µM ThT, 10 µM FSB, and 20 µM curcumin) for 30 min in an orbital shaker at RT. Following dyes incubation, immobilized proteins were washed twice with distilled water for 30 min. Gels were kept hydrated with distilled water at 4 °C for storage and imaging.
Immunofluorescence (IF)
IF was performed as previously described7,37,82 using frozen sections from AD, PSP, and DLB brains. Frozen sections were fixed in chilled methanol followed by three washes with 1X PBS. Sections were then incubated in autofluorescence and lipofuscin eliminator TrueBlack (Biotium, 23007) for 5 min, washed three times in 1X PBS, and blocked in blocking buffer (5% BSA and 5% normal goat serum in 1X PBS supplemented with 0.25% Triton X-100 (PBST)) for 1 h. Sections were washed three times with 1X PBS and incubated with primary antibody, Tau 5 (1: 500), diluted in 5% BSA in PBS-T overnight at 4 °C. The following day, sections were washed in 1X PBS three times for 10 min each and incubated with goat anti-mouse IgG Alexa-568 (1:1000, Life Technologies) for 1 h at RT. After three washes in 1X PBS, sections were incubated with the fluorescent dye (75 µM bis-ANS, 50 µM ThT, 10 µM FSB, and 20 µM curcumin) for 1 h at RT. Sections were then washed in 1X PBS three times for 10 min each and mounted using ProLong Diamond antifade mounting media without DAPI (Invitrogen, P36970).
Confocal microscopy and principal component analysis
For immobilized tau oligomers and fibrils, images were captured with Nikon 63x oil immersion objective on Zeiss LSM 880 confocal microscope using 405 nm diode laser. Images were collected using ZEN Black software, under lambda mode. Collection range was set to 410 nm–710 nm, with 10 nm increments. The emission spectrum of dye bound to each oligomer was resolved using unmixing tool and used in PCA. Data collected was normalized, graphed, and analysed using PCA. All post-collection data processing was conducted using GraphPad Prism 10.1.2. The immunofluorescence images were captured with Nikon 63x oil immersion objective of Zeiss LSM 880 confocal microscope using 405 nm diode laser and argon laser 458/488/514 nm. High-resolution images were obtained with ZEN Black Lite Software.
Pronase digestion
Procedures for pronase digestion were performed as previously described with minor modifications20,25,83. Briefly, molecular grade water, Tris HCl 1 M, and sodium chloride 50 mM were added in an Eppendorf tube. Next, aBDTOs and TauM were added to the solution following treatment with pronase enzyme at 0, 0.5, and 1 μg/mL and incubation at 37 °C for 0, 1, 5, and 15 min. The enzymatic reaction was stopped by adding 0.2% protease inhibitor and samples were transferred onto ice to stop the cleavage reaction. Samples were then ready to be used or stored at −80 °C until use. Pronase-treated and untreated aBDTOs were loaded into 4–12% Bis-Tris pre-cast gel (Invitrogen) for SDS-PAGE gel electrophoresis. Western blot analysis with the sequence-specific tau antibody, Tau 5, was performed to visualize the digested samples. Pronase-treated samples (aBDTOs and TauM) were spotted onto a nitrocellulose membrane to evaluate immunoreactivity for sequence-specific and oligomeric tau antibodies. Briefly, dot blot was performed as previously described77,79. Briefly, 1.5 μL of each end-product reaction was applied onto nitrocellulose membranes and then blocked with 10% non-fat milk in TBS-T overnight at 4 °C. The next day, membranes were probed with Tau 5 (1:10,000), Tau RD4 (1:1000), Tau 46 (1:5000) and T22 (1:250) diluted in 5% non-fat milk for 1 h at RT. Membranes were then incubated with HRP-conjugated IgG anti-rabbit (1:10,000) to detect T22 and anti-mouse (1:10,000) secondary antibody to detect Tau 5, Tau 46, and Tau RD4. Blots were then washed three times in TBS-T and ECL plus (GE Healthcare) was used for protein expression detection. All experiments were performed in triplicate.
Proteinase K (PK) digestion
Procedures for PK digestion were previously described35,40,46,84. Briefly, in an Eppendorf tube, molecular grade water, Tris HCl, and sodium chloride were added so that the final concentrations for these two buffers became 100 mM and 5 mM, respectively. Next, aBDTOs were added and treated with proteinase K enzyme at 0, 0.25, 0.5, 0.75, or 1 μg/mL and incubated at 37 °C for 1 h. The enzymatic reaction was stopped by adding 1X LDS sample buffer (Invitrogen) followed by incubation at 95 °C for 5 min. Samples were then immediately transferred onto ice to stop the cleavage reaction. Samples were loaded into 4–12% Bis-Tris pre-cast gel (Invitrogen) for SDS-PAGE gel electrophoresis or stored at −80 °C. Western blot analysis with the sequence-specific Tau antibody, Tau 5, was performed to visualize the digested samples. All experiments were performed in triplicate.
aBDTOs trypsin digestion and desalting
Trypsin digestion was performed with 30 µg of each sample, diluted to 400 µL with 50 mM ammonium bicarbonate at pH 8.0 in 0.7 mL low-retention tubes as described earlier85. Each sample was then treated with 10 µL of 100 mM dithiothreitol (DTT) and incubated for 30 min at RT to reduce the disulfide bonds and denature the proteins. Then, 20 µL of 100 mM Iodoacetamide was added into each sample and incubated in the dark at RT for 1 h for capping and alkylation of the SH bonds of cysteine residues to ensure complete denaturing of the proteins. 10 μL of trypsin solution (0.10 µg/µL, sequencing grade modified trypsin, lot#00004671181) was added to each sample and incubated at 37 °C for 1 h to selectively cleave the positively charged C-terminal Lysine (K) and Arginine (R) to be detected by MS. After 1 h, 100 µL of the tryptic digested sample was transferred into another 0.7 mL tube, where 10 µL of 10% trifluoroacetic acid (TFA) was added into each sample to stop the activity of the trypsin enzyme. The remaining tryptic digest was put back into 37 °C incubator and incubated for 2 h. Then, 100 µL of the tryptic digest was transferred to a new 0.7 mL tube, to which 10 µL of 10% TFA was added. All samples were desalted using the ZipTip C18 procedure86, reconstituted in 0.1% TFA solution in water, and stored at 4 °C for liquid chromatography-tandem mass spectrometry analysis (LC-MS/MS).
Mass spectrometry
The desalted peptides were reconstituted in 20 μL 4% ACN/0.1% formic acid. All peptide samples were separated on an online nanoflow Easy nLC1000 UHPLC system (Thermo Scientific) and analyzed on a Q Exactive Orbitrap mass spectrometer (Thermo Scientific, San Jose, CA). 10 µL of the sample was injected onto a capillary peptide trap column (Acclaim® Pepmap 100, 75 µm × 2 cm, C18, 3 µm, 100 Å, Thermo Scientific). After sample injection, the peptides were separated on a 25-cm UHPLC reversed-phase column (Acclaim® Pepmap 100, 75 µm × 25 cm, C18, 2 µm, 100 Å, Thermo Scientific) at a flow rate of 300 nL/min. A 2 h linear gradient from 2% solvent A (0.1% formic acid in H2O) to 35% solvent B (0.1% formic acid in ACN) was used for each LC-MS/MS run. A data-dependent acquisition was performed using the Xcalibur 2.3 software in a positive ion mode at a spray voltage of 2.1 kV. Survey spectra were acquired in the Orbitrap with a resolution of 70,000, the maximum injection time of 20 ms, an automatic gain control (AGC) of 1e6, and a mass range from 350 to 1600 m/z. The top 15 ions in each survey scan were selected for higher-energy collisional dissociation scans with a resolution of 17,500. For all higher-energy collisional dissociation scans, collision energy was set to 28, the maximum inject time was 200 ms, and the AGC was 1e5. Ions selected for MS/MS were dynamically excluded for 30 s after fragmentation. Mass spectra were analyzed using MaxQuant software (version 1.5.2.8)87. The initial maximum allowed mass deviation was set to 10 ppm for monoisotopic precursor ions and 0.5 Da for MS/MS peaks. Enzyme specificity was set to trypsin, defined as C-terminal arginine and lysine excluding proline, and a maximum of two missed cleavages was allowed. Carbamidomethylcysteine was set as a fixed modification and methionine oxidation as variable modifications. The spectra were searched by the Andromeda search engine against the Human SWISSPORT sequence database (containing 20,193 human protein entries) combined with 248 common contaminants and concatenated with the reversed versions of all sequences. Quantification was performed with the label-free algorithms in Maxquant88. The ‘match between runs’ feature of MaxQuant was used to transfer identifications to other LC-MS/MS runs based on their masses and retention time (maximum deviation 0.7 min), and this was also used in quantification experiments. The required false positive rate for identification was set to 1% at the peptide level, and 1% at the protein level and the minimum required peptide length was set to 6 amino acids. Contaminants, reverse identification, and proteins only identified by modified peptides were excluded from further data analysis. We used the Perseus platform89 to analyze the Maxquant output, including statistical analysis. Reversed identifications and proteins identified only by site modification were strictly excluded from further analysis. For proteomics analysis, proteins identified only by site modification were excluded from further analysis. After filtering (3 valid values in at least one group), the remaining missing values were imputed from a normal distribution (width: 0.3 of standard deviation; downshift: 1.8 of standard deviation). The abundance of each tau peptide was normalized with total tau. Then, multiple sample ANOVA test with permutation-based FDR correction was performed to identify the significantly differentially expressed tau peptides.
Tau RD P301S biosensor cell culture and treatment
Tau RD P301S biosensor cells (ATCC, CRL-3275) were cultured in DMEM supplemented with 10% FBS, 100 μg/mL penicillin, and 100 μg/mL streptomycin as previously described35,41,46. Cultures were maintained in a humidified atmosphere of 5% CO2 at 37 °C. For prion-like activity assay, the cells were plated on poly-L-lysine-coated coverslips at a density of 1 × 105 cells/well in a 24-well plate. After 24 h, cells were transduced with aBDTOs/ Lipofectamine 2000 (Invitrogen, 11668-027) mixture in Opti-Mem (Gibco, 31985-070) (100 nM aBDTO + 6 μL Lipofectamine 2000 + Opti-Mem] for a total volume of 50 μL/well. BTDO/liposome mixture was incubated at RT for 30 min before adding it to cells. Cells were incubated with transduction complexes for 12 and 24 h. After treatment, cells were washed multiple times with 1X PBS, fixed with 4% formaldehyde for 15 min at RT, and mounted with Prolong Gold mounting media. Slides were then dried in the fume hood. Cells were imaged with a Keyence BZ-800 Microscope using standard filters for DAPI, GFP, and Texas Red. Nikon 40X objective was used to capture images of three random fields in triplicate. Images for quantification of density of aggregates/field, aspect ratio and circularity for each condition at 12 and 24 h were analyzed using BZ-X Analyzer.
Indirect ELISA
ELISA assay was conducted as previously described35,77. Briefly, 96 well plates (Nunc Immobilizer, Amino Plates and Modules, 436006, Thermo Fisher Scientific) were previously coated with 1.5 μL of aBDTOs using 50 μL of 1X PBS, pH 7.4, as coating buffer. The following day, plates were washed three times with TBS-T then blocked for 2 h at RT with 120 µL of 10% non-fat milk in TBS-T. Plates were then washed three times with TBS-T, and probed with 100 μL of primary antibodies for overnight at 4 °C, TOMA1 (1:100), TOMA2 (1:200), TOMA3 (1:100), TOMA4 (1:50), and T22 (1:250). Plates were then washed three times with TBS-T and incubated with 100 μL of HRP-conjugated anti-rabbit or anti-mouse IgG, diluted 1:10,000 in 5% non-fat milk in TBS-T, for 1 h at RT. Plates were washed three times with TBS-T and developed with 3,3,5,5-tetramethylbenzidine (TMB + Substrate- Chromogen, S1599, Dako) The reaction was stopped using 100 μL of 1 M HCl and absorbance was read at 450 nm using POLARstar OMEGA plate reader. All experiments were performed in triplicate.
Fluorescence labeling of Tau Oligomer Monoclonal Antibodies (TOMA)
TOMA clones were labeled with Alexa Fluor™ (AF) Protein/Antibody labeling Kit (Invitrogen) according to the manufacturer’s guideline. TOMA1, TOMA2, TOMA3, and TOMA4 were labeled with AF-350 (#A10170), −488 (#A10235), −647 (#A20173), and −568 (#A10238), respectively. Briefly, a volume of 50 µL of 1 M sodium bicarbonate was added to each TOMA antibody to make the final optimal concentration of 2 mg/mL. The antibody solution was then added to the dye and the mixture was rotated for 1 h at RT. The reaction mixture was then carefully loaded onto the purification column and the elution buffer was added until the labeled antibody has been eluted from the column.
Immunofluorescence using TOMA AF-labeled
IF were performed using frozen sections from human AD, PSP, and DLB brain. Frozen sections were fixed in chilled methanol followed by three washes with 1X PBS. Sections were then incubated in autofluorescence and lipofuscin eliminator TrueBlack (Biotium, #23007) for 5 min, washed three times in 1X PBS, followed by blocking in blocking buffer for 1 h. Sections were then washed three times with 1X PBS and incubated with TOMA AF-labeled primary antibodies (TOMA1, TOMA2, TOMA3 and TOMA4) for overnight at 4 °C. The following day, sections were washed in 1X PBS three times for 10 min each and mounted using ProLong Diamond antifade mounting media without DAPI (Invitrogen, P36970). Sections were then dried in the fume hood. Images were captured with a Keyence BZ-800 Microscope using standard filters for DAPI, GFP, and Texas Red. Nikon 40X objective was used to capture images.
Electrophysiology
Acute brain slice methods were used as previously described33,35. In brief, 5-month-old hTau mice (n = 5 per treatment and both male and female) were deeply anaesthetized with isoflurane followed by transcardial perfusion with 25–30 mL of carbogen (95% O2 and 5% CO2 gas mixture) -N-methyl-D-gluconate-artificial cerebrospinal fluid RT. Transverse brain sections of 375 μm containing Schaffer collateral synapses were generated using the Compresstome VF-300 (Precisionary Instruments, Greenville, NC, USA). Slices were recovered in NMDG-HEPES cutting solution at 32–34 °C for 10 min and were then transferred to carbogen bubbling HEPES-artificial cerebrospinal fluid solution at RT for the rest of the experiment time. Slices were treated with aBDTOs (50 nM final concentration) diluted in carbogen bubbling HEPES-artificial cerebrospinal fluid solution at RT for 1 h before recording. After treatment, the slices were briefly (5–10 min) washed by placing them in an oligomer-free recovery artificial cerebrospinal fluid before placing them on the recording stage. Two slices at a time were transferred to the recording chamber and perfused with carbogen bubbling room temperature normal artificial cerebrospinal fluid at a rate of approximately 3 mL/min for recordings. Recording electrodes were pulled from borosilicate glass capillaries using a horizontal P-97 Flaming/Brown micropipette puller (Sutter Instruments, Novato, CA). Evoked field excitatory post-synaptic potentials (fEPSPs) in the CA1 were obtained by stimulating Schaffer collateral. A stable baseline was obtained by delivering single-pulse stimulation at 20 s inter-stimulus intervals. Long-term potentiation (LTP) was induced by exposing the slices to high-frequency stimulation (HFS; 3 × 100 Hz, 20 s) using Digidata 1550B (Molecular Devices, Sunnyvale, CA, USA), and measured using an Axon MultiClamp 700B differential amplifier (Molecular Devices). Clampex 10.6 software (Molecular Devices) was used. All data are represented as percent change from the initial average baseline fEPSP slope, which was defined as the average slope obtained for 10 min before HFS.
Primary neuron cell culture
Primary cortical neurons were prepared from hTau mice (Jackson Laboratory; stock #004808) at embryonic days 13–16, as described previously7,37,90. Briefly, cells were dissociated from cortical tissues by treatment with Accutase and gentle trituration with fire-polished glass pasture pipets. Cells were seeded onto poly-L-lysine–coated coverslips or six-well plates. Neuronal culture medium was prepared with Neurobasal Medium (Gibco; 12348017), B-27™ plus supplement (Gibco; A3582801), GlutaMax (Gibco; 35050–061), and Antibiotic–Antimycotic solution (Gibco; 15240062). After every 3 days, half the medium was replaced with fresh culture medium, and at days 10–13 of in vitro culture, cells were used for the indicated experiments.
Generation of single-cell suspensions for RNA sequencing
Cortical brain regions were dissected as described in primary neuron cell culture section. Briefly, neurons were dissociated from cortical tissues by treatment with Accutase and gentle trituration with fire-polished glass pasture pipets. Cells were seeded onto poly-L-lysine–coated coverslips or six-well plates. Neuronal culture medium was prepared with Neurobasal Medium. After 10 days in culture, neurons were treated for 24 h with 0.1 µM of TauO. After, the neurons have been removed with cell scrapers from the well and they have been filtered through a 70 µm cell strainer (BD Biosciences) to remove the clusters of cells. Before the generation of single-cell suspension we measured cell viability (Trypan Blues assay) and number using automatic Cell Counter (Invitrogen). Moreover, we inspect the cell suspension under a microscope to ensure it was clean and fully dissociated. At this point cell suspension was used as input for the single cell gene expression assay. The first step is to create a master mix for the downstream reverse transcription (RT) and combine that with the cell suspension. Next, the final master mix will be loaded into the Next GEM Chip together with barcoded gel beads and partitioning oil. Running the chip on the ChromiumX instrument (10x Genomics) will generate an emulsion whereby one individual gel bead will be partitioned together with a single cell surrounded by the RT master mix. These GEMs (Gel Beads-in-emulsion) are then transferred to a strip tube, and the reverse transcription reaction will take place on a thermal cycler. We generated cDNA with the resulting RT products carry unique barcodes to their cells of origin. The barcoded cDNA recovered from broken emulsions and cleaned using Dynabead cleanup. After cDNA amplification, was cleaned again and sequenced by Next Generation Sequencing core at UTMB.
Single cell RNA-seq analysis
The subsequent processing and visual representation of the scRNA-seq data were conducted utilizing the Seurat package (version 4.3.0)91 in R (version 4.3.0). For the preliminary quality control (QC) stage, cells that expressed fewer than 200 genes or exceeded 5000 genes were excluded. Cells with total read counts surpassing 10,000 and genes detected in fewer than three cells were also omitted. Additionally, cells with mitochondrial reads comprising more than 15% of total reads were removed from all samples. Following the application of these QC parameters, a total of 73,630 single cells and 21,158 genes were retained for subsequent analyses. The data were then normalized by scaling to 10,000 transcripts per cell and transformed to logarithmic space using Seurat’s LogNormalize method. The dataset’s highly variable genes were identified based on their dispersion and mean values. PCA was executed on the top 2000 variable genes, and the top 30 principal components were utilized to construct a k-nearest-neighbors cell-to-cell graph with k equal to 30 neighbors. All samples were batch-corrected and integrated using the Harmony package92 in R. The integrated dataset was then projected onto a two-dimensional space using Uniform Manifold Approximation and Projection (UMAP)93 for dimension reduction, based on the top 30 principal components. Clusters were identified using the Louvain graph-clustering algorithm, setting the resolution parameter to 0.3. The significance of differences was determined using a Wilcoxon Rank Sum test with Bonferroni correction, considering genes with an adjusted p-value of less than 0.05 as significantly altered. Over-representation enrichment analysis was performed to identify associated Gene Ontology terms using the Enrichr R package94, employing the libraries of Gene Ontology95 and Reactome96 pathway. The p-value from EnrichR is calculated with Fisher’s exact test and adjusted using the Benjamini-Hochberg method.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
We thank the members of the Kayed laboratory for their support and help. We thank Dr. Arar Sharif (University of Jordan) for MassSpec analysis. This work was supported by the National Institutes of Health (grant numbers RO1AG054025, RF1AG077484, U24AG072458, RF1AG055771, R01AG077253, RF1AG060718) R.K.; the Mitchell Center for Neurodegenerative Diseases R.K.; The Alzheimer’s Association Research Fellowship (AARF-21-720991) support M.M.
Abbreviations
- AD
Alzheimer’s Disease
- DLB
Dementia with Lewy Bodies
- PSP
Progressive Supranuclear Palsy
- Aβ
Amyloid-β
- α-Syn
α-Synuclein
- BDTOs
Brain-Derived Tau Oligomers
- aBDTOs
Amplified Brain-Derived Tau Oligomers
- LTP
Long-Term Potentiation
- AFM
Atomic Force Microscopy
- SEC
Size Exclusion Chromatography
- FLAMES
FLuorescent Amyloid Multi Emission Spectra
- PCA
Principal Component Analysis
Author contributions
Conceptualization, F.LC., M.M., and R.K.; Methodology, F.L.C., S.P., A.B., U.S., C.W., H.F., Q.M., Y.Z., M.M., and R.K.; Investigation, F.LC., S.P., U.S., N.P., N.B., N.S., C.J., N.M., A.B., R.X., Y.Z., C.W., H.F., Q.M.; Writing – Original Draft, F.LC., S.P., and U.S., M.M.; Writing – Review & Editing, F.LC., S.P., U.S., N.P., N.B., N.S., C.J., N.M., A.B., R.X., Y.Z., C.W., H.F., Q.M., M.M.; Funding Acquisition, M.M. and R.K.; Resources, R.K.; Supervision, M.M. and R.K.
Peer review
Peer review information
Communications Biology thanks Dmitry Kurouski and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Benjamin Bessieres.
Data availability
All relevant raw data supporting the findings of this study are available within the paper and its Supplementary Information. The source data behind the graphs in the paper can be found in Supplementary Data. All other data are available from the corresponding authors. The processed data in this study from single-cell RNA-sequencing are deposited in Gene Expression Omnibus public repository under the following code: GSE277105. Graphics in Figs. 1A, 4E, 6A, and S5a–c were generated with BioRender. Venn Diagrams were generated using Venny 2.1.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mauro Montalbano, Email: mamontal@utmb.edu.
Rakez Kayed, Email: rakayed@utmb.edu.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-025-07499-w.
References
- 1.Kovacs, G. G. Tauopathies. Handb. Clin. Neurol.145, 355–368 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Arendt, T., Stieler, J. T. & Holzer, M. Tau and tauopathies. Brain Res. Bull.126, 238–292 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Masters, C. L. et al. Alzheimer’s disease. Nat. Rev. Dis. Primer1, 15056 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Sengupta, U. & Kayed, R. Tau oligomers as pathogenic seeds: preparation, characterization, and propagation in vitro and in vivo. in Tau Protein: Methods and Protocols (ed. Smet-Nocca, C.) 147–183 10.1007/978-1-0716-3629-9_9 (Springer, 2024). [DOI] [PubMed]
- 5.Majounie, E. et al. Variation in tau isoform expression in different brain regions and disease states. Neurobiol. Aging34, 1922.e7–1922.e12 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang, H., Cao, Y., Ma, L., Wei, Y. & Li, H. Possible mechanisms of Tau spread and toxicity in Alzheimer’s disease. Front. Cell Dev. Biol.9, 707268 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puangmalai, N. et al. Internalization mechanisms of brain-derived tau oligomers from patients with Alzheimer’s disease, progressive supranuclear palsy and dementia with Lewy bodies. Cell Death Dis11, 314 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerson, J. E. et al. Characterization of tau oligomeric seeds in progressive supranuclear palsy. Acta Neuropathol. Commun.2, 73 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montalbano, M., Majmundar, L., Sengupta, U., Fung, L. & Kayed, R. Pathological tau signatures and nuclear alterations in neurons, astrocytes and microglia in Alzheimer’s disease, progressive supranuclear palsy, and dementia with Lewy bodies. Brain Pathol. Zurich Switz.33, e13112 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clinton, L. K., Blurton-Jones, M., Myczek, K., Trojanowski, J. Q. & LaFerla, F. M. Synergistic interactions between Abeta, tau, and alpha-synuclein: acceleration of neuropathology and cognitive decline. J. Neurosci.30, 7281–7289 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chin, K. S. et al. Tau in dementia with Lewy bodies. Aust. N. Z. J. Psychiatry58, 175–182 (2024). [DOI] [PubMed] [Google Scholar]
- 12.Chin, K. S., Yassi, N., Churilov, L., Masters, C. L. & Watson, R. Prevalence and clinical associations of tau in Lewy body dementias: a systematic review and meta-analysis. Parkinsonism Relat. Disord.80, 184–193 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Yap, C. X. et al. Brain cell-type shifts in Alzheimer’s disease, autism, and schizophrenia interrogated using methylomics and genetics. Sci. Adv.10, eadn7655 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Igarashi, K. M. Entorhinal cortex dysfunction in Alzheimer’s disease. Trends Neurosci.46, 124–136 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donaghy, P. C. & McKeith, I. G. The clinical characteristics of dementia with Lewy bodies and a consideration of prodromal diagnosis. Alzheimers Res. Ther.6, 46 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frigerio, R. et al. Incidental lewy body disease: do some cases represent a preclinical stage of Dementia with Lewy Bodies? Neurobiol. Aging32, 857–863 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halliday, G. M., Song, Y. J. C. & Harding, A. J. Striatal β-amyloid in dementia with Lewy bodies but not Parkinson’s disease. J. Neural Transm.118, 713–719 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Jellinger, K. A. & Attems, J. Does striatal pathology distinguish Parkinson disease with dementia and dementia with Lewy bodies? Acta Neuropathol.112, 253–260 (2006). [DOI] [PubMed] [Google Scholar]
- 19.Sanders, D. W. et al. Distinct tau prion strains propagate in cells and mice and define different tauopathies. Neuron82, 1271–1288 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaufman, S. K. et al. Tau prion strains dictate patterns of cell pathology, progression rate, and regional vulnerability in vivo. Neuron92, 796–812 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holmes, B. B. et al. Proteopathic tau seeding predicts tauopathy in vivo. Proc. Natl Acad. Sci. USA111, E4376–E4385 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strang, K. H. et al. Distinct differences in prion-like seeding and aggregation between Tau protein variants provide mechanistic insights into tauopathies. J. Biol. Chem.293, 2408–2421 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parra Bravo, C., Naguib, S. A. & Gan, L. Cellular and pathological functions of tau. Nat. Rev. Mol. Cell Biol. 1–20 10.1038/s41580-024-00753-9 (2024). [DOI] [PubMed]
- 24.Scheres, S. H., Zhang, W., Falcon, B. & Goedert, M. Cryo-EM structures of tau filaments. Curr. Opin. Struct. Biol.64, 17–25 (2020). [DOI] [PubMed] [Google Scholar]
- 25.Fitzpatrick, A. W. P. et al. Cryo-EM structures of tau filaments from Alzheimer’s disease. Nature547, 185–190 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim, C. et al. Distinct populations of highly potent TAU seed conformers in rapidly progressing Alzheimer’s disease. Sci. Transl. Med.14, eabg0253 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hromadkova, L., Siddiqi, M. K., Liu, H. & Safar, J. G. Populations of Tau conformers drive prion-like strain effects in Alzheimer’s disease and related dementias. Cells11, 2997 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Condello, C. & Stöehr, J. Aβ propagation and strains: Implications for the phenotypic diversity in Alzheimer’s disease. Neurobiol. Dis.109, 191–200 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Shi, Y. et al. Structure-based classification of tauopathies. Nature598, 359–363 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelson, R. et al. Structure of the cross-beta spine of amyloid-like fibrils. Nature435, 773–778 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Falcon, B. et al. Novel tau filament fold in chronic traumatic encephalopathy encloses hydrophobic molecules. Nature568, 420–423 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schweighauser, M. et al. Structures of α-synuclein filaments from multiple system atrophy. Nature585, 464–469 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lasagna-Reeves, C. A. et al. Alzheimer brain-derived tau oligomers propagate pathology from endogenous tau. Sci. Rep.2, 700 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meraz-Ríos, M. A., Lira-De León, K. I., Campos-Peña, V., De Anda-Hernández, M. A. & Mena-López, R. Tau oligomers and aggregation in Alzheimer’s disease. J. Neurochem.112, 1353–1367 (2010). [DOI] [PubMed] [Google Scholar]
- 35.Bittar, A. et al. Neurotoxic tau oligomers after single versus repetitive mild traumatic brain injury. Brain Commun1, fcz004 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lasagna-Reeves, C. A. et al. Identification of oligomers at early stages of tau aggregation in Alzheimer’s disease. FASEB J.26, 1946–1959 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Puangmalai, N. et al. Lysine 63-linked ubiquitination of tau oligomers contributes to the pathogenesis of Alzheimer’s disease. J. Biol. Chem.298, 101766 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghag, G. et al. Soluble tau aggregates, not large fibrils, are the toxic species that display seeding and cross-seeding behavior. Protein Sci.27, 1901–1909 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Condello, C. et al. Structural heterogeneity and intersubject variability of Aβ in familial and sporadic Alzheimer’s disease. Proc. Natl Acad. Sci. USA115, E782–E791 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang, H. et al. EMBER multidimensional spectral microscopy enables quantitative determination of disease- and cell-specific amyloid strains. Proc. Natl Acad. Sci. USA120, e2300769120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lo Cascio, F. et al. Modulating disease-relevant tau oligomeric strains by small molecules. J. Biol. Chem.295, 14807–14825 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Legname, G. et al. Strain-specified characteristics of mouse synthetic prions. Proc. Natl Acad. Sci. USA102, 2168–2173 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghaemmaghami, S. et al. Conformational transformation and selection of synthetic prion strains. J. Mol. Biol.413, 527–542 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tepper, K. et al. Oligomer formation of tau protein hyperphosphorylated in cells. J. Biol. Chem.289, 34389–34407 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moore, K., Sengupta, U., Puangmalai, N., Bhatt, N. & Kayed, R. Polymorphic Alpha-Synuclein Oligomers: characterization and differential detection with novel corresponding antibodies. Mol. Neurobiol.60, 2691–2705 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sengupta, U. et al. Polymorphic α-Synuclein strains modified by dopamine and docosahexaenoic acid interact differentially with tau protein. Mol. Neurobiol.57, 2741–2765 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baron, B. W., Baron, R. M. & Baron, J. M. The ITM2B (BRI2) gene is a target of BCL6 repression: Implications for lymphomas and neurodegenerative diseases. Biochim. Biophys. Acta BBA - Mol. Basis Dis.1852, 742–748 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Müller, T. et al. Neuregulin 3 promotes excitatory synapse formation on hippocampal interneurons. EMBO J.37, e98858 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harris, H. K. et al. Disruption of RFX family transcription factors causes autism, attention-deficit/hyperactivity disorder, intellectual disability, and dysregulated behavior. Genet. Med.23, 1028–1040 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanders, B. et al. Transcriptional programs regulating neuronal differentiation are disrupted in DLG2 knockout human embryonic stem cells and enriched for schizophrenia and related disorders risk variants. Nat. Commun.13, 27 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoo, T. et al. A DLG2 deficiency in mice leads to reduced sociability and increased repetitive behavior accompanied by aberrant synaptic transmission in the dorsal striatum. Mol. Autism11, 19 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Batista, A. F., Bodart-Santos, V., De Felice, F. G. & Ferreira, S. T. Neuroprotective actions of Glucagon-Like Peptide-1 (GLP-1) Analogues in Alzheimer’s and Parkinson’s Diseases. CNS Drugs33, 209–223 (2019). [DOI] [PubMed] [Google Scholar]
- 53.Clavaguera, F. et al. ‘Prion-like’ templated misfolding in tauopathies. Brain Pathol. Zurich Switz.23, 342–349 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bernis, M. E. et al. Prion-like propagation of human brain-derived alpha-synuclein in transgenic mice expressing human wild-type alpha-synuclein. Acta Neuropathol. Commun.3, 75 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prusiner, S. B. et al. Evidence for α-synuclein prions causing multiple system atrophy in humans with parkinsonism. Proc. Natl Acad. Sci. USA112, E5308–E5317 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Holec, S. A. M. et al. Multiple system atrophy prions transmit neurological disease to mice expressing wild-type human α-synuclein. Acta Neuropathol.144, 677–690 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Castillo-Carranza, D. L. et al. Specific targeting of tau oligomers in Htau mice prevents cognitive impairment and tau toxicity following injection with brain-derived tau oligomeric seeds. J. Alzheimers Dis.40, S97–S111 (2014). [DOI] [PubMed] [Google Scholar]
- 58.Mudher, A. et al. What is the evidence that tau pathology spreads through prion-like propagation? Acta Neuropathol. Commun.5, 99 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nath, A. et al. The conformational ensembles of α-synuclein and tau: combining single-molecule FRET and simulations. Biophys. J.103, 1940–1949 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lövestam, S. et al. Assembly of recombinant tau into filaments identical to those of Alzheimer’s disease and chronic traumatic encephalopathy. eLife11, e76494 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tarutani, A. et al. Cryo-EM structures of tau filaments from SH-SY5Y cells seeded with brain extracts from cases of Alzheimer’s disease and corticobasal degeneration. FEBS Open Bio13, 1394–1404 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gerson, J. E., Mudher, A. & Kayed, R. Potential mechanisms and implications for the formation of tau oligomeric strains. Crit. Rev. Biochem. Mol. Biol.51, 482–496 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sengupta, U., Carretero-Murillo, M. & Kayed, R. Preparation and characterization of Tau Oligomer Strains. Methods Mol. Biol.1779, 113–146 (2018). [DOI] [PubMed] [Google Scholar]
- 64.Castillo-Carranza, D. L., Guerrero-Muñoz, M. J., Sengupta, U., Gerson, J. E. & Kayed, R. α-Synuclein Oligomers Induce a Unique Toxic Tau Strain. Biol. Psychiatry84, 499–508 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Patterson, K. R. et al. Characterization of prefibrillar Tau oligomers in vitro and in Alzheimer disease. J. Biol. Chem.286, 23063–23076 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang, Y. et al. The release and trans-synaptic transmission of Tau via exosomes. Mol. Neurodegener.12, 5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takeda, S. et al. Neuronal uptake and propagation of a rare phosphorylated high-molecular-weight tau derived from Alzheimer’s disease brain. Nat. Commun.6, 8490 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sengupta, U. et al. Pathological interface between oligomeric alpha-synuclein and tau in synucleinopathies. Biol. Psychiatry78, 672–683 (2015). [DOI] [PubMed] [Google Scholar]
- 69.Zabrocki, P. et al. Characterization of α-synuclein aggregation and synergistic toxicity with protein tau in yeast. FEBS J.272, 1386–1400 (2005). [DOI] [PubMed] [Google Scholar]
- 70.Myeku, N. et al. Tau-driven 26S proteasome impairment and cognitive dysfunction can be prevented early in disease by activating cAMP-PKA signaling. Nat. Med.22, 46–53 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Elie, A. et al. Tau co-organizes dynamic microtubule and actin networks. Sci. Rep.5, 9964 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rico, T. et al. Tau stabilizes chromatin compaction. Front. Cell Dev. Biol.9, 740550 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lauretti, E., Li, J.-G., Di Meco, A. & Praticò, D. Glucose deficit triggers tau pathology and synaptic dysfunction in a tauopathy mouse model. Transl. Psychiatry7, e1020–e1020 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mansuroglu, Z. et al. Loss of Tau protein affects the structure, transcription and repair of neuronal pericentromeric heterochromatin. Sci. Rep.6, 33047 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Montalbano, M. et al. Tau Modulates mRNA transcription, alternative polyadenylation profiles of hnRNPs, chromatin remodeling and spliceosome complexes. Front. Mol. Neurosci.14, 742790 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Margittai, M. & Langen, R. Template-assisted filament growth by parallel stacking of tau. Proc. Natl Acad. Sci. USA101, 10278–10283 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lo Cascio, F. et al. Toxic Tau Oligomers modulated by novel curcumin derivatives. Sci. Rep.9, 19011 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kayed, R. et al. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science300, 486–489 (2003). [DOI] [PubMed] [Google Scholar]
- 79.Lasagna-Reeves, C. A., Castillo-Carranza, D. L., Guerrero-Muoz, M. J., Jackson, G. R. & Kayed, R. Preparation and characterization of neurotoxic tau oligomers. Biochemistry49, 10039–10041 (2010). [DOI] [PubMed] [Google Scholar]
- 80.Louis-Jeune, C., Andrade-Navarro, M. A. & Perez-Iratxeta, C. Prediction of protein secondary structure from circular dichroism using theoretically derived spectra. Proteins80, 374–381 (2012). [DOI] [PubMed] [Google Scholar]
- 81.Micsonai, A. et al. Disordered–ordered protein binary classification by circular dichroism spectroscopy. Front. Mol. Biosci.9, 863141 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Farmer, K. M. et al. P53 aggregation, interactions with tau, and impaired DNA damage response in Alzheimer’s disease. Acta Neuropathol. Commun.8, 132 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Arakhamia, T. et al. Posttranslational modifications mediate the structural diversity of tauopathy strains. Cell180, 633–644.e12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Montalbano, M. et al. TDP-43 and Tau Oligomers in Alzheimer’s disease, amyotrophic lateral sclerosis, and frontotemporal dementia. Neurobiol. Dis.146, 105130 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang, Y., Sun, H., Zhang, J., Brasier, A. R. & Zhao, Y. Quantitative assessment of the effects of trypsin digestion methods on affinity purification-mass spectrometry-based protein-protein interaction analysis. J. Proteome Res.16, 3068–3082 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tubaon, R. M., Haddad, P. R. & Quirino, J. P. Sample clean-up strategies for ESI mass spectrometry applications in bottom-up proteomics: trends from 2012 to 2016. Proteomics17, 1700011 (2017). [DOI] [PubMed]
- 87.Cox, J. & Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol.26, 1367–1372 (2008). [DOI] [PubMed] [Google Scholar]
- 88.Cox, J. et al. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol. Cell. Proteomics MCP13, 2513–2526 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tyanova, S. et al. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods13, 731–740 (2016). [DOI] [PubMed] [Google Scholar]
- 90.Gaikwad, S. et al. Nasal tau immunotherapy clears intracellular tau pathology and improves cognitive functions in aged tauopathy mice. Sci. Transl. Med.16, eadj5958 (2024). [DOI] [PubMed] [Google Scholar]
- 91.Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell184, 3573–3587.e29 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Korsunsky, I. et al. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods16, 1289–1296 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Becht, E. et al. Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol.37, 38–44 (2019). [DOI] [PubMed] [Google Scholar]
- 94.Kuleshov, M. V. et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res.44, W90–W97 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gene Ontology Consortium, The et al. The Gene Ontology knowledgebase in 2023. Genetics224, iyad031 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Milacic, M. et al. The Reactome Pathway Knowledgebase 2024. Nucleic Acids Res.52, D672–D678 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
All relevant raw data supporting the findings of this study are available within the paper and its Supplementary Information. The source data behind the graphs in the paper can be found in Supplementary Data. All other data are available from the corresponding authors. The processed data in this study from single-cell RNA-sequencing are deposited in Gene Expression Omnibus public repository under the following code: GSE277105. Graphics in Figs. 1A, 4E, 6A, and S5a–c were generated with BioRender. Venn Diagrams were generated using Venny 2.1.