Abstract
Testicular ischemia-reperfusion (I/R) injury during testicular torsion is strongly influenced by oxidative stress caused by excessive accumulation of unscavenged reactive oxygen species. This study aimed to investigate the effects of intra-peritoneal administration of Mito-TEMPO (MT) on I/R injury in testicular torsion/detorsion (T/D) in mice. Forty-two male mice were divided into seven groups including 1 control and 6 treatment groups (360° T/D, 720° T/D, 360° T/D + 0.70 mg kg-1 MT, 360° T/D + 1.00 mg kg-1 MT, 720° T/D + 0.70 mg kg-1 MT, and 720° T/D + 1.00 mg kg-1 MT). After inducing 360° and 720° clockwise testicular torsions for 2 hr, sperm parameters, apoptosis-related genes expression, and in vivo fertility index were evaluated. The results showed that 720° T/D can lead to increased abnormal sperm morphology, sperm DNA damage, and Bax expression, while the Bcl-2 expression was reduced compared to the other groups. In addition, it also had negative effects on sperm total and progressive motilities as well as viability and plasma membrane functionality (PMF). The results also showed that administration of MT to T/D mice can result in a reduction in abnormal sperm morphology, DNA damage, and Bax expression. It could also increase sperm total and progressive motilities, viability and PMF, Bcl-2 expression, and in vivo fertility index. Based on our results, it is concluded that MT, when administered after spermatic cord torsion in mice, provides significant protection against acute testicular T/D injury.
Key Words: Mice, Reperfusion injury, Spermatic cord torsion, Testis
Introduction
Testicular torsion is a serious urologic surgical emergency occurring when the testicle twists and the spermatic cord becomes twisted. Delayed diagnosis and treatment can lead to testicular necrosis and atrophy. According to various studies, testicular torsion is the most common cause of testicular loss in newborns, children, and adolescent boys.1 Approximately 26.00% of people suffering from acute testicular problems suffer from testicular torsion.1 The degree and duration of torsion are the two most important indicators of testicular injury.2 If treated within 6 hr of pain onset, there is a significant chance of saving the affected testicle, with a survival rate of 90.00 - 100%. If treated within 6 - 12 hr, depending on the degree of torsion, 20.00 - 50.00 % of the testicles can be saved; while, if treated after 12 - 24 hr, the chance of saving drops to 0.00 - 10.00%.3 Quick surgery is needed to reverse the twisted spermatic cord and restore blood flow to the injured testicle. However, attempting to reperfused ischemic tissue can result in a severe testicular injury.3
The phenomenon of ischemia-reperfusion (I/R) injury can lead to persistent testicular degeneration even after the initial ischemia damage.3,4 In both the ischemia and reperfusion stages, pro-inflammatory cytokines are over-produced, promoting the migration of neutrophils and other leukocytes toward testicular tissue. This can lead to the production of reactive oxygen species (ROS) by neutrophils, harmful chemicals that can cause oxidative damage to testicular cells.5 Such changes including lipid peroxidation, pro-inflammatory cytokine over-production, and significant release of intracellular calcium (Ca2+) can lead to infertility.1 Oxidative stress can impair the ability of biological systems to detoxify reactive mediators and repair the damage caused by ROS over-generation. Cell swelling can produce peroxides and free radicals, which can damage all cell components, including proteins, lipids and DNA, and impair cell performance.6 Oxidative stress contributes to the development of several diseases and exacerbation of their symptoms.6
Reportedly, anti-oxidants with free radical scavenging properties can help to reduce I/R damage in various organs, including the testis.7 Anti-oxidants are chemicals preventing the oxidation of other molecules, which can lead to free radical formation. These free radicals can initiate chain reactions, but anti-oxidants can stop these reactions through displacing free radicals and preventing other oxidation reactions.8 To support tissue repair and prevent adverse effects of post-I/R testicular damage, combinations of enzymes and drugs have been used.1 Additionally, various methods have been used to reduce oxidative stress. Zinc aspartate, curcumin, and dexamethasone are effective in reducing I/R damage and increasing the activity of anti-oxidative enzymes.9
Plants and animals contain various anti-oxidants, such as glutathione, vitamin C, vitamin E, catalase, and superoxide dismutase. Mito-TEMPO (MT) is a compound composed of piperidine nitroxide and triphenyl-phosphonium cation as a lipophilic anti-oxidant.8 This cation can easily cross lipid bilayers and accumulate in mitochondria.10 The MT is a mitochondrion-targeted anti-oxidant protecting cells from oxidative damage in various pathological conditions, including sepsis-induced acute kidney injury, colitis, and endotoxin-induced liver injury.11 Several studies have demonstrated the protective effects of MT against oxidative damage in several diseases.12
To our knowledge, there are no previous reports concerning the effects of MT on testicular I/R injury. This study aimed to investigate the effects of intraperitoneally (IP) Mito-TEMPO administration on sperm parameters, apoptosis genes expression, and in vivo fertility index in adult mice following testicular I/R injury.
Materials and Methods
Chemicals. All chemical substances were sourced from reliable suppliers, including Sigma (St. Louis, USA).
Animals. Forty-two adult male mice weighing 20.00 - 25.00 g with the age of 6 - 8 weeks were provided from the Animal Resource Center of Urmia University, Urmia, Iran. Mice were placed in a standard location with regulated temperature (20.00 - 22.00 ˚C), ventilation, light (12 hr light/ dark), and relative humidity (50.00 ± 10.00 %). Animals were housed in plastic cages, fed ad libitum with conventional laboratory chow, and had unrestricted access to tap water. Before the experiment, they were acclimatized for 1 week.
Experimental protocol. The mice were divided randomly into seven groups each having six animals as follows: Group 1 (sham-operated control group) under-went a sham operation without testicular I/R injury, Group 2 (360° torsion/detorsion [T/D]) experienced 2 hr of 360° torsion-induced ischemia, Group 3 (720° T/D) experienced 2 hr of 720° torsion-induced ischemia Group 4 (360° T/D + 0.70 mg kg-1 MT) experienced 2 hr of 360° torsion-induced ischemia, and 30 min before testicular detorsion, mice received MT (0.70 mg kg-1; IP),13 Group 5 (360° T/D + 1.00 mg kg-1 MT) experienced 2 hr of 360° torsion-induced ischemia, and 30 min before testicular detorsion, mice received MT (1.00 mg kg-1; IP),14 Group 6 (720° T/D + 0.70 mg kg-1 MT) experienced 2 hr of 720° torsion-induced ischemia, and 30 min before testicular detorsion, mice received MT (0.70 mg kg-1; IP),13 and Group 7 (720° T/D + 1.00 mg kg-1 MT) experienced 2 hr of 720° torsion-induced ischemia, and 30 min before testicular detorsion, mice received MT (1.00 mg kg-1; IP).14
Surgical procedure. The surgical procedure was performed under sterile conditions. The mice were anesthetized using intraperitoneal 80.00 mg kg-1ketamine (Alfasan, Woerden, The Netherlands) and 10.00 mg kg-1 xylazine (Alfasan). The testes were exposed through ventral midline laparotomy and rotated clockwise 360° (groups 2, 4, and 5) or 720° (groups 3, 6, and 7) following the surgical preparation of the testicular region, shaving, and cleaning with a 10.00 % povidone-iodine solution. The testis was then fixed in the torsion position with three simple single stitches (5/0 silk; Supa, Tehran, Iran), and detorsion was performed after 2 hr. The incision was then closed with a simple running suture technique (4/0 nylon; Supa). The right testis and its epididymis were removed after 30 days to assess the sperm parameters and apoptosis-related genes expression.15 The study was carried out in accordance with the regulations of the Animal Ethics Committee of Urmia University, Urmia, Iran (IR-UU-AEC-3/45).
Epididymal sperm collection. In each mouse, sperms were collected from the caudal epididymis. The caudal epididymis was quickly cut into small pieces and placed in a Petri dish containing 1.00 mL of human tubal fluid medium (2.00 mM CaCl2-2H2O, 2.50 mM glucose, 5.00 mM KCl, 0.40 mM KH2PO4, 0.20 mM MgSO4-7H2O, 100 mM NaCl, 25 mM NaCHO3, 18.50 mM sodium lactate, 0.30 mM sodium pyruvate, 0.20 mM penicillin G sodium salt, 0.30 mM streptomycin sulfate, 4.00 g L-1 bovine serum albumin, and 2.00 mg L-1 phenol red) for 30 min at 37.00 ˚C and 5.00 % CO2.16
Epididymal sperm count. The sperms were diluted in distilled water at a ratio of 1:5, devitalized, counted using a Neubauer hemocytometer (BrandTM, Berlin, Germany), and the average number of epididymal sperm per mL was determined.16
Epididymal sperm motility. The motility tests were performed at room temperature using a computer-assisted semen analysis system (Test Sperm 3.2; Videotest, St. Petersburg, Russia). The system measured total motility (%), progressive motility (%), curvilinear velocity (VCL; μm per sec), straight-line velocity (VSL; μm per sec), average path velocity (VAP; μm per sec), straightness (STR; %), linearity (LIN; %), amplitude of lateral head displacement (ALH; μm per sec), and beat-cross frequency (Hz).17 To examine the sperm, an aliquot of sperm suspension of approximately 10 μL was loaded onto a microscopic slide and observed using dark or phase-contrast field microscopy (Olympus, BX41, Tokyo, Japan). At least 500 spermatozoa were observed in five microscopic fields.18
Epididymal sperm viability and morphology. To evaluate sperm viability and morphology, the Eosin-Nigrosin staining method was used according to the World Health Organization protocol.19 Eosin and Nigrosin (Merck, Darmstadt, Germany) were mixed in distilled water. The combination of one volume of semen with two volumes of 1.00 % Eosin-Nigrosin was analyzed using a light microscope (Model CHT, Olympus Optics Co. Ltd., Tokyo, Japan) at a magnification of 400 ×. The non-viable sperms appeared red due to the staining, while the viable sperms remained colorless, allowing viability assessment. Additionally, Eosin-Nigrosin staining was used to determine the percentage of abnormal sperm. Sperms with obvious abnormalities, such as cytoplasmic remnants, were classified as abnormal. From each sample, 200 sperms were examined at 400× magnification, and the results were presented as a percentage.18
Epididymal sperm DNA damage. Acridine Orange (AO) staining was used to detect denatured DNA in sperm under low pH challenge. The denatured DNA showed the strongest fluorescence. Concentrated smears were soaked in a mixture of acetic acid and Carnoy’s fixative (1:3) for 2 hr and then, dried outdoors for 5 min. They were then added to a stock solution consisting of 1,000 mL purified water and 1.00 mg AO. The mixture was kept in a dark room at 4.00 ˚C for 5 min. After staining, the spermatozoa were examined under a fluorescence microscope (GS7; Nikon Co., Tokyo, Japan) at a magnification of 400×. Spermatozoa appearing red or yellow were considered damaged or aberrant.18
Sperm plasma membrane functionality (PMF). The hypo-osmotic swelling test identified sperms with PMF. To carry out the test, 100.00 μL of a hypo-osmotic solution consisting of fructose (1.351 g L-1) and sodium citrate (0.735 g L-1) was mixed with 10.00 μL of sperm sample and then, incubated at 37.00 ˚C for 1 hr. The functionality of sperm plasma membrane was evaluated using a phase- contrast microscope (BX41; Olympus Tokyo, Japan) at a magnification of 400×. The main indicators of the test were coiled or swollen tails.18
RNA extraction, cDNA synthesis, and real-time quantitative polymerase chain reaction (qRT-PCR). SinaSyber Blue HF-qPCR Mix (CinnaGen, Tehran, Iran) was used on a StepOne Real-Time PCR System (Applied Biosystems, Massachusetts, USA) using a 25.00 μL reaction system. The cDNA samples were used to measure the relative expression of the Bax and Bcl-2 genes compared to the 18S rRNA gene as a reference gene (Table 1). The qRT-PCR thermal cycle settings were as follows: A defatting cycle at 95.00 ˚C for 10 min, 45 three-step cycles each with 10 sec defatting at 95.00 ˚C, and 30 sec compounding at 60 ˚C at the stretching step at 72.00 ˚C for 30 sec. Gene expression in the samples was used to calculate relative expression using the following formula:
Table 1.
Nucleotide sequences and product size of primers used in reverse transcription-polymerase chain reaction.
| Genes | Primers | Band size (bp) |
|---|---|---|
| Bcl-2 | Forward: 5- CTCGTCGCTACCGTCGTCACT TCG-3 Reverse: 5- CAGATGCCGGTTCAGGTACTCAGTC-3 |
242 |
| Bax | Forward: 5- ACC AGC TCTGAACAGATC ATG-3 | 424 |
| Reverse: 5- TGG TCT TGGATCCAGACAAG-3 | ||
| 18SrRNA | Forward: 5- GCAATTATTCCCCATGAACG - 3 Reverse: 5- GGCCTCACTAAACCATCCAA- 3 |
123 |
Relative expression = 2 - (SΔct-CΔct)
where, SΔct is derived by subtracting cycle threshold (ct) of the reference gene from ct of the tested gene, and the CΔct values of ct are internal control samples. To generate the normal distribution of the relative expression values, these numbers were fitted by logarithm of 10 and then examined.16
In vivo fertility index. In this study, six male mice per experimental group were placed in individual cages with female mice unfamiliar with the males at a ratio of 1:2. It was possible to mate for up to 72 hr. Vaginal swabs were collected at 24, 48, and 72 hr post-mating to verify sperm presence and determine pregnancy initiation. The mice were euthanized 17 days after confirmed pregnancy using a specific dosage of thiopental sodium (250 mg kg-1, IP; Exipental, Exir, Tehran, Iran).20,21 The reproductive organs were subsequently examined to calculate fertility indices.
Statistical analysis. The study data were analyzed using SPSS Software (version 26.0; IBM Corp., Armonk, USA). To determine significant differences between the studied groups, a one-way ANOVA was performed. Tukey’s post hoc analysis was used to identify which specific groups differed significantly. A p value of ≤ 0.05 was used to determine statistical significance.
Results
Epididymal sperm parameters. Based on the results, it was found that group 3 had significantly lower sperm concentration than the other groups (p ≤ 0.05; Table 2).
Table 2.
Epididymal sperm characteristics in different experimental groups. Values are expressed as mean ± SEM.
| Parameters | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | Group 7 |
|---|---|---|---|---|---|---|---|
| Sperm concentration (10 6 per mL) | 36.80 ± 31.32a | 35.00 ± 32.61b | 18.90 ± 25.91e | 35.30 ± 49.42b | 36.20 ± 12.56a | 24.60 ± 25.19d | 32.50 ± 30.34c |
| Total motility (%) | 79.54 ± 2.50a | 72.43 ± 2.05c | 45.27 ± 1.72f | 75.38 ± 2.84bc | 77.29 ± 2.50ab | 60.53 ± 2.15e | 68.72 ± 1.98d |
| Progressive motility (%) | 45.81 ± 1.17a | 38.91 ± 1.66b | 16.60 ± 1.15d | 42.26 ± 1.30a | 43.96 ± 1.29a | 34.81 ± 1.68c | 40.35 ± 1.44b |
| Average path velocity (μm sec -1 ) | 28.43 ± 1.61a | 26.60 ± 1.93ab | 15.95 ± 1.45d | 27.91 ± 1.13a | 28.29 ± 1.94a | 21.47 ± 1.12c | 25.73 ± 1.27b |
| Curvilinear velocity (μm sec -1 ) | 82.43 ± 3.36a | 76.61 ± 2.34bc | 46.39 ± 1.98e | 79.41 ± 2.25ab | 80.64 ± 3.57a | 63.50 ± 2.45d | 74.93 ± 1.85c |
| Straight-line velocity (μm sec -1 ) | 21.75 ± 1.15a | 19.05 ± 1.23a | 11.83 ± 1.12c | 20.27 ± 1.23a | 21.40 ± 1.41a | 16.27 ± 1.71b | 20.49 ± 1.39a |
| Linearity (%) | 0.18 ± 0.02a | 0.18 ± 0.01a | 0.17 ± 0.02a | 0.17 ± 0.03a | 0.18 ± 0.02a | 0.17 ± 0.03a | 0.17 ± 0.20a |
| Amplitude of lateral head displacement (μm sec -1 ) | 2.32 ± 0.03a | 2.04 ± 0.02a | 1.12 ± 0.01c | 2.18 ± 0.01a | 2.23 ± 0.02a | 1.75 ± 0.03b | 2.01 ± 0.03ab |
| Straightness (%) | 0.63 ± 0.02a | 0.63 ± 0.01a | 0.61 ± 0.01a | 0.63 ± 0.02a | 0.63 ± 0.02a | 0.62 ± 0.01a | 0.62 ± 0.02a |
| Beat-cross frequency (Hz) | 11.46 ± 1.39a | 10.47 ± 1.26ab | 6.45 ± 0.60c | 10.53 ± 1.71ab | 11.09 ± 1.47a | 9.72 ± 1.23b | 10.14 ± 1.12ab |
| Plasma membrane functionality (%) | 84.32 ± 2.92a | 76.75 ± 2.18c | 48.25 ± 1.10f | 79.62 ± 2.81bc | 82.53 ± 3.84ab | 64.25 ± 2.28e | 74.92 ± 2.50d |
| Sperm viability (%) | 93.19 ± 2.89a | 82.31 ± 3.92b | 53.83 ± 1.87e | 87.49 ± 2.48b | 90.27 ± 3.51a | 70.72 ± 3.38d | 81.50 ± 3.42c |
| Sperm DNA damage (%) | 3.30 ± 0.22e | 10.19±1.21cd | 43.85 ± 1.91a | 8.41 ± 0.62d | 5.65±0.29de | 27.74±1.49b | 12.36 ± 0.81c |
| Sperm abnormal morphology (%) | 6.07 ± 0.39c | 7.89 ± 0.26c | 35.24 ± 1.10a | 7.35 ± 0.46c | 6.40 ± 0.38c | 21.07 ± 0.40b | 8.36 ± 0.62c |
a-f Different superscripts within the same row demonstrate significant differences (p ≤ 0.05).
Although there was no significant difference between groups 1 and 5 (p > 0.05; Table 2), but administrations of 0.70 and 1.00 mg kg-1 MT (groups 6 and 7, respectively) increased sperm concentration following 720° T/D. The T/D had a negative impact on sperm motility. In group 3, total and progressive sperm motilities were significantly lower than those in the control group (p ≤ 0.05; Table 2). The results showed that groups 6 and 7 were able to increase total and progressive sperm motilities compared to group 3 (p ≤ 0.05; Table 2). The VCL, VSL, VAP, and ALH were significantly reduced in group 3 compared to the other groups (p ≤ 0.05, Table 2). However, these characteristics increased significantly in groups 6 and 7 compared to group 3. Furthermore, the results showed that there was no significant difference between groups in terms of STR and LIN (p > 0.05; Table 2).
The 720° T/D significantly reduced the sperm PMF and viability (p ≤ 0.05). However, administrations of MT at doses of 0.70 and 1.00 mg kg-1 restored the above-mentioned parameters. Furthermore, 720° T/D resulted in a significant increase in sperm DNA damage and abnormal morphology (p ≤ 0.05; Table 2). The MT treatments at 2 aforementioned doses were also effective in preserving sperm morphology and DNA (p ≤ 0.05; Table 2).
Bax and Bcl-2 genes expression. Using qRT‒PCR, the levels of Bcl-2 and Bax mRNA were determined. Group 3 had higher levels of Bax mRNA than the other groups (p ≤ 0.05; Fig. 1A). The results also showed that there was no significant difference between groups 1, 2, 4, 5, and 7 (p > 0.05; Fig. 1A). Furthermore, there were no significant differences in Bcl-2 mRNA levels between groups 1, 4, and 5 (p > 0.05; Fig. 1B). However, the control group expressed more Bcl-2 mRNA than the other groups (p ≤ 0.05; Fig. 1B).
Fig. 1.
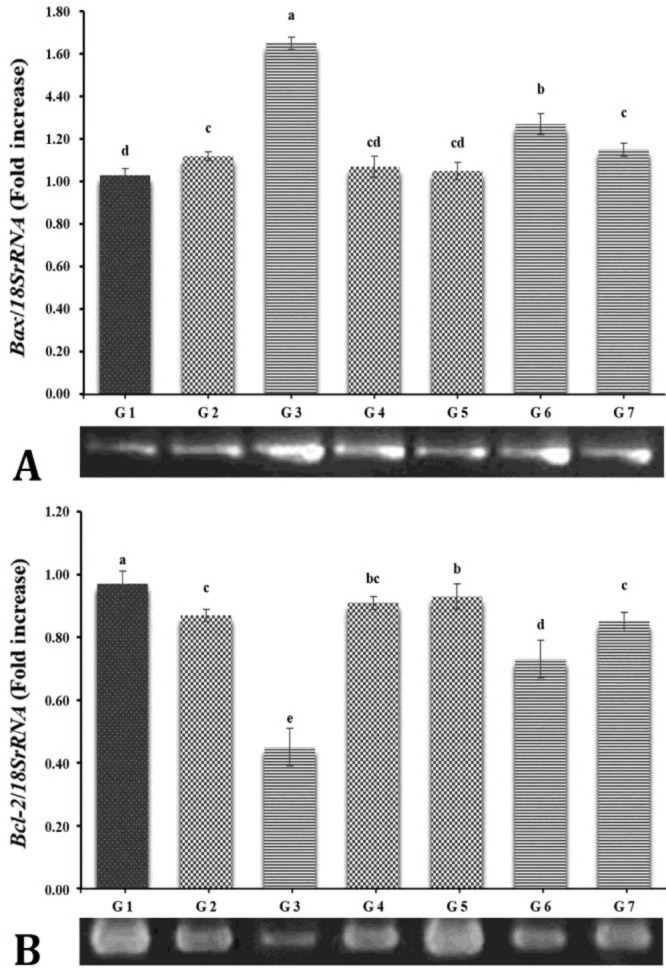
Reverse transcription-polymerase chain reaction findings in different experimental groups. G1: Control group; G2: 360° torsion and detorsion (T/D) group; G3: 720° T/D group; G4: 360° T/D + 0.70 mg kg-1 Mito-Tempo group; G5: 360° T/D + 1.00 mg kg-1 Mito-Tempo group; G6: 720° T/D + 0.70 mg kg-1 Mito-Tempo group; G7: 720° T/D + 1.00 mg kg-1 Mito-Tempo group. The densities of A) Bax and B) Bcl-2 (mRNA levels in testicular tissue were measured by densitometry and normalized to the 18SrRNA mRNA expression level. Significant differences between groups are indicated by different superscripts (p ≤ 0.05; mean ± SEM).
In vivo fertility index. Table 3 summarizes the in vivo fertility follow-up findings. The copulation and gestation indices were not significantly different between the treatment groups and the control group, as shown in Table 3 (p > 0.05). According to the results, the fertility rate in group 3 was lower than that in the other groups, but administrations of 0.70 and 1 mg kg-1 MT respectively to groups 6 and 7 increased the fertility index compared to that in group 3 (p ≤ 0.05; Table 3). The fertility rates of these groups were significantly lower than those of groups 1, 2, 4, and 5 (p ≤ 0.05; Table 3).
Table 3.
Fertility indices in different experimental groups.
| Parameters | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | Group 7 |
|---|---|---|---|---|---|---|---|
| Number of females | 12 | 12 | 12 | 12 | 12 | 12 | 12 |
| Number of females mated | 12 | 12 | 12 | 12 | 12 | 12 | 12 |
| Number of males mated | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| Number of males impregnating females | 6 | 5 | 2 | 6 | 6 | 4 | 5 |
| Number of pregnant females | 12 | 10 | 3 | 11 | 12 | 7 | 10 |
| Female mating index (%)* | 100a | 100a | 100a | 100a | 100a | 100a | 100a |
| Male mating index (%)** | 100a | 100a | 100a | 100a | 100a | 100a | 100a |
| Pregnancy index (%)*** | 100a | 83.33a | 25.00c | 91.66a | 100a | 58.33b | 83.33 |
| Male fertility index (%)**** | 100a | 83.33a | 33.33c | 100a | 100a | 66.66b | 83.33a |
* Female mating index = number of females mated/number of females × 100
** Male mating index = number of males mated/number of males × 100
*** Pregnancy index = number of pregnant females /number of females mated × 100
**** Male fertility index = number of males impregnating females/number of males mated × 100
a-c Different superscripts within the same row demonstrate significant differences (p ≤ 0.05).
Discussion
In testicular torsion, the blood supply of the organ is severely disrupted, leading to testicular atrophy and degeneration.22 The only effective treatment is to perform surgery as soon as possible after damage occurs.23 The main cause of the pathophysiological effects of testicular torsion is the delay in detorsion, leading to the death of germ cells due to a lack of reperfusion being necessary to maintain the function of the ischemic tissue.
The accumulation of harmful compounds, like ROS, and the decrease in oxygen levels during ischemia also contribute to germ cell death.24 The use of exogenous anti-oxidants may help to reduce the severity of oxidative stress and tissue damage caused by T/D.25 Several drugs, like omeprazole, hesperidin, and diclofenac are effective in protecting the testes from I/R injury.26 This study examined the effectiveness of IP administration of MT in mice as a protective agent against testicular T/D. The results of this study showed that MT therapy could increase sperm quantity and quality, and improve in vivo fertility index and apoptosis following experimental testicular T/D.
It was observed that 720° T/D had a significant impact on the seminiferous tubules, resulting in spermatogenesis impairment. The group that underwent 720° T/D had a lower sperm count, PMF, and motility than the other groups. When assessing sperm fertilizing potential, motility is a crucial factor;27 sperm migration in female reproductive system depends on progressive motility, the ability of sperm to move forward in a specific direction. Sperm motility is regulated by several metabolic and regulatory pathways, like Ca2+ pathway and cyclic adenosine mono-phosphate pathway-dependent protein kinase or protein kinase A.28 Mitochondria play a crucial role in maintaining normal cellular function and energy metabolism through oxidative phosphorylation and adenosine triphosphate (ATP) synthesis.29 Studies have found that cell mitochondria are sensitive to T/D, which can impair ATP transport and reduce sperm motility.30 Studies have also shown sperm damage after 4 hr of ischemia in testicular tissue, resulting in reduced sperm concentration as well as normal morphology and motility.31 It has been observed that sperm abnormalities can lead to changes in sperm motility and gene mutations. Oxidative stress caused by ROS can lead to sperm damage during reperfusion,32 causing DNA, membrane lipid, and cellular protein oxidation.33 Various studies have shown a positive correlation between the percentage of progressive sperm motility and sperm morphology.34 It has been suggested that oxidative damage and elevated lipid peroxide levels can cause DNA damage in the genome of sperm and germ cells, impair sperm motility and trigger apoptosis in testicular tissue.35 However, a recent study found that the use of MT in sperm extenders can increase sperm viability.36 Moreover, research on ram semen extenders has shown that the use of MT can improve sperm total and progressive motilities, VAP, PMF, and survival after the thawing process.37 Our study supports these results and showed that MT administration could alleviate sperm DNA damage and abnormal morphology caused by 720° T/D and improve sperm total and progressive motilities.
Multiple kinases and cysteine proteases, called caspases, are involved in apoptosis, a form of planned cell death. It is a genetically regulated suicide not cause inflammation and is characterized by DNA cleavage.38 Several studies have shown that testicular I/R leads to germ cell apoptosis being triggered by neutrophil-produced ROS.39 The degradation of DNA and membrane proteins and chromatin condensation are morphological changes caused by caspase-3, also known as the main executor caspase.40 It was found in this study that after T/D, the relative mRNA expression levels of Bax and Bcl-2 were respectively higher and lower in the T/D group compared to the control group. This is consistent with previous findings reporting that 720° T/D results in germ cell-specific apoptosis in a rat model.41 The Bcl-2 is a protein with anti-apoptotic properties42 preventing caspase activation and delaying the onset of apoptosis.
This is achieved by preventing the release of cytochrome c and the entry of the Bax protein into the mitochondrial membrane. The results of this study showed that administration of MT following testicular T/D increased Bcl-2 expression and decreased Bax expression, indicating reduced apoptotic processes in the mouse testis. Trnka et al., showed that MT inhibits intracellular ROS generation and mitochondrial ROS production, maintains mitochondrial integrity, and reduces stress-induced necrosis and apoptosis.43 As a result, the structure of MT, being comparable to that of hydroxylamine, can prevent the over-flow and over-production of ROS during the T/D process. Also, MT generates nitroxide radicals functioning similar to superoxide dismutase to maintain the integrity of membrane bilayer phospholipids and stability of the electron transport chain.43 In addition, MT is thought to prevent mitochondrial Bax translocation, suggesting that it may be a useful strategy to preserve mitochondrial function and viability during the damage processes.44
Testicular torsion results in damage to the blood-testis barrier, resulting in the release of cytokines causing extensive apoptosis in the germinal epithelium of the contralateral testis. Due to the high frequency of infertility in individuals with testicular torsion, it is likely that repeated apoptotic events are responsible.45 The pregnancy rate and number of embryos after testicular T/D were studied previously and it was found that the pregnancy rate and number of embryos were significantly lower in the testicular T/D group. According to Shokoohi et al., this was likely caused by a decline in serum testosterone levels and sperm quality.46 To increase sperm viability and motility to reach the site of fertilization, exogenous anti-oxidants administration in T/D patients has been suggested.47 In our study, subsequent fertility rates decreased significantly after 720° T/D, and increased fertility was observed following MT therapy. Our research findings supported a previous report declaring that MT, as a sperm extender, can increase the fertility rate.36 The high integrity of the plasma membrane could be the cause of this improved fertility.48 Accordingly, the improvement of sperm motility, viability, and membrane integrity could be associated with the improvement of mitochondrial and anti-oxidative enzymes activities. According to Lu et al.,49 and Zhang et al.,50 this improvement could be related to the sperm fertilizing ability. This was supported by the results of our study, showing that the use of MT can improve sperm characteristics and testicular tissue cyto-architecture, which can increase fertility rates in mice.
In conclusion, our results suggest that MT could act as a powerful anti-oxidant, improving sperm parameters and in vivo fertility index, as well as decreasing apoptosis-linked genes expression. One limitation of our study was the absence of specific details regarding the best timing and dosage of MT administration for maximal effective-ness. The dosage and timing used in our research were chosen from available literature, and to determine the most effective dosage and timing more researches is needed.
Acknowledgments
This paper has been extracted from the thesis of Doctor of Veterinary Science in Veterinary Surgery of Dr. Zohreh Mostahsan carried out at Urmia University, Urmia, Iran, and the authors would like to sincerely thank the members of the Faculty of Veterinary Medicine, and Urmia University Research Council, Urmia, Iran, for the approval and support of this research.
Conflict of interest
Authors disclose no potential conflict of interest.
References
- 1.Karaguzel E, Kadihasanoglu M, Kutlu O. Mechanisms of testicular torsion and potential protective agents. Nat Rev Urol. 2014;11(7):391–399. doi: 10.1038/nrurol.2014.135. [DOI] [PubMed] [Google Scholar]
- 2.Sessions AE, Rabinowitz R, Hulbert WC, et al. Testicular torsion: direction, degree, duration and disinformation. J Urol. 2003;169(2):663–665. doi: 10.1097/01.ju.0000047381.36380.0e. [DOI] [PubMed] [Google Scholar]
- 3.Raisi A, Davoodi F. Testicular torsions in veterinary medicine. Vet Res Commun. 2022;46(2):303–313. doi: 10.1007/s11259-021-09873-5. [DOI] [PubMed] [Google Scholar]
- 4.Raisi A, Davoodi F, Mohammadi R. Protective effects of rosmarinic acid on testicular torsion-detorsion in an animal model. Iran J Vet Surg. 2022;17(2):80–86. [Google Scholar]
- 5.Volpe CMO, Villar-Delfino PH, Dos Anjos PMF, et al. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018;9(2):119 . doi: 10.1038/s41419-017-0135-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arroyo S, de la Morena A. Life-threatening adverse events of antiepileptic drugs. Epilepsy Res. 2001;47(1-2):155–174. doi: 10.1016/s0920-1211(01)00306-0. [DOI] [PubMed] [Google Scholar]
- 7.Ayan M, Tas U, Sogut E, et al. Protective effect of thymoquinone against testicular torsion induced oxidative injury. Andrologia. 2016;48(2):143–151. doi: 10.1111/and.12424. [DOI] [PubMed] [Google Scholar]
- 8.Conti M, Morand PC, Levillain P, et al. Improved fluorometric determination of malonaldehyde. Clin Chem. 1991;37(7):1273–1275. [PubMed] [Google Scholar]
- 9.Mogilner JG, Elenberg Y, Lurie M, et al. Effect of dexamethasone on germ cell apoptosis in the contralateral testis after testicular ischemia-reperfusion injury in the rat. Fertil Steril. 2006;85(Suppl 1):1111–1117. doi: 10.1016/j.fertnstert.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 10.Smith RA, Murphy MP. Mitochondria-targeted antioxidants as therapies. Discov Med. 2011;11(57):106–114. [PubMed] [Google Scholar]
- 11.Masoudi R, Asadzadeh N, Sharafi M. Effects of freezing extender supplementation with mitochondria-targeted antioxidant Mito-TEMPO on frozen-thawed rooster semen quality and reproductive performance. Anim Reprod Sci. 2021;225:106671 . doi: 10.1016/j.anireprosci.2020.106671. [DOI] [PubMed] [Google Scholar]
- 12.Choumar A, Tarhuni A, Lettéron P, et al. Lipopoly saccharide-induced mitochondrial DNA depletion. Antioxid Redox Signal. 2011;15(11):2837–2854. doi: 10.1089/ars.2010.3713. [DOI] [PubMed] [Google Scholar]
- 13.Ni R, Cao T, Xiong S, et al. Therapeutic inhibition of mitochondrial reactive oxygen species with mito-TEMPO reduces diabetic cardiomyopathy. Free Radic Biol Med. 2016;90:12–23. doi: 10.1016/j.freeradbiomed.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jamshidi HR, Emami A, Golmohammadi H, et al. Protective Effect of Mito-TEMPO on sodium valproate-induced hepatotoxicity in mice. Acta Med Iran. 2020;58(7):352–357. [Google Scholar]
- 15.Davoodi F, Taheri S, Raisi A, et al. Investigating the sperm parameters, oxidative stress and histopathological effects of salvia miltiorrhiza hydroalcoholic extract in the prevention of testicular ischemia reperfusion damage in rats. Theriogenology. 2020;144:98–106. doi: 10.1016/j.theriogenology.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Soleimanzadeh A, Pourebrahim M, Delirezh N, et al. Ginger ameliorates reproductive toxicity of formaldehyde in male mice: evidences for Bcl-2 and Bax. J Herbmed Pharmacol. 2018;7(4):259–266. [Google Scholar]
- 17.Cao W, Aghajanian HK, Haig-Ladewig LA, et al. Sorbitol can fuel mouse sperm motility and protein tyrosine phosphorylation via sorbitol dehydrogenase. Biol Reprod. 2009;80(1):124–133. doi: 10.1095/biolreprod.108.068882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramazani N, Mahd Gharebagh F, Soleimanzadeh A, et al. The influence of L‐proline and fulvic acid on oxidative stress and semen quality of buffalo bull semen following cryopreservation. Vet Med Sci. 2023;9(4):1791–1802. doi: 10.1002/vms3.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO. WHO laboratory manual for the examination and processing of human semen. 6th ed. Geneva, Switzerland: Department of reproductive health and research; 2021. [Google Scholar]
- 20.Taheri S, Davoodi F, Raisi A, et al. Co‐administration of Salvia miltiorrhiza and verapamil inhibits detrimental effects of torsion/detorsion on testicular tissue in rats. Andrologia. 2021;53(6):e14049. doi: 10.1111/and.14049. [DOI] [PubMed] [Google Scholar]
- 21.Sarlak M, Roumiani E, Kheradmand A, et al. Evaluating the effects of betaine on testicular ischemia/ reperfusion injury induced by torsion/detorsion in the rat. Andrologia. 2022;54(10):e14559. doi: 10.1111/and.14559. [DOI] [PubMed] [Google Scholar]
- 22.Webb A, Bond R, McLean P, et al. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc Natl Acad Sci U S A. 2004;101(37):13683–13688. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kazaz IO, Mentese A, Demir S, et al. Berberine inhibits the ischemia-reperfusion induced testicular injury through decreasing oxidative stress. Am J Emerg Med. 2020;38(1):33–37. doi: 10.1016/j.ajem.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Mogilner JG, Lurie M, Coran AG, et al. Effect of diclofenac on germ cell apoptosis following testicular ischemia-reperfusion injury in a rat. Pediatr Surg Int. 2006;22(1):99–105. doi: 10.1007/s00383-005-1580-9. [DOI] [PubMed] [Google Scholar]
- 25.Aitken RJ, Roman SD. Antioxidant systems and oxidative stress in the testes. Oxid Med Cell Longev. 2008;1(1):15–24. doi: 10.4161/oxim.1.1.6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Güney C, Coşkun KA, Tutar Y. ATPase inhibition by omeprazole reveals role of heat shock proteins on testicular torsion. Andrologia. 2020;53(2):e13929. doi: 10.1111/and.13929. [DOI] [PubMed] [Google Scholar]
- 27.Puja IK, Sawitri NM, Maharani N, et al. Preservation of semen from Kintamani Bali dogs by freezing method. J Adv Vet Anim Res. 2019;6(2):158–162. doi: 10.5455/javar.2019.f326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramazani N, Mahd Gharebagh F, Soleimanzadeh A, et al. Reducing oxidative stress by κ-carrageenan and C60HyFn: the post-thaw quality and antioxidant status of Azari water buffalo bull semen. Cryobiology. 2023;111:104–112. doi: 10.1016/j.cryobiol.2023.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Ruiz‐Pesini E, Díez‐Sánchez C, López‐Pérez MJ, et al. The role of the mitochondrion in sperm function: is there a place for oxidative phosphorylation or is this a purely glycolytic process? Curr Top Dev Biol. 2007;77:3–19. doi: 10.1016/S0070-2153(06)77001-6. [DOI] [PubMed] [Google Scholar]
- 30.Fang L, Bai C, Chen Y, et al. Inhibition of ROS production through mitochondria-targeted antioxidant and mitochondrial uncoupling increases post-thaw sperm viability in yellow catfish. Cryobiology. 2014;69(3):386–393. doi: 10.1016/j.cryobiol.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 31.Sertkaya Z, Öztürk Mİ, Koca O, et al. Prohylactic effects of verapamil in testicular ischemia-reperfusion damage in rats. Haydarpasa Numune Med J. 2020;60(3):279–283. [Google Scholar]
- 32.Agarwal A, Said TM. Oxidative stress, DNA damage and apoptosis in male infertility: a clinical approach. BJU Int. 2005;95(4):503–507. doi: 10.1111/j.1464-410X.2005.05328.x. [DOI] [PubMed] [Google Scholar]
- 33.Camhi SL, Lee P, Choi AM. The oxidative stress response. New Horiz. 1995;3(2):170–182. [PubMed] [Google Scholar]
- 34.Soleimanzadeh A, Saberivand A, Ahmadi A. Effect of α-tocopherol on spermatozoa of rat semen after the freeze-thawing process [Persian] Urmia Med J. 2014;25(9):826–834. [Google Scholar]
- 35.Filho DW, Torres MA, Bordin AL, et al. Spermatic cord torsion, reactive oxygen and nitrogen species and ischemia-reperfusion injury. Mol Aspects Med. 2004;25(1-2):199–210. doi: 10.1016/j.mam.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 36.Masoudi R, Asadzadeh N, Sharafi M. The mitochondria-targeted antioxidant Mito-TEMPO conserves rooster’s cooled semen quality and fertility potential. Theriogenology. 2020;156:236–241. doi: 10.1016/j.theriogenology.2020.07.018. [DOI] [PubMed] [Google Scholar]
- 37.Zarei F, Daghigh-Kia H, Masoudi R. Supplementation of ram’s semen extender with Mito-TEMPO II: quality evaluation and flow cytometry study of post-thawed spermatozoa. Andrologia. 2022;54(1):e14299. doi: 10.1111/and.14299. [DOI] [PubMed] [Google Scholar]
- 38.Dhulqarnain AO, Takzaree N, Hassanzadeh G, et al. Pentoxifylline improves the survival of spermatogenic cells via oxidative stress suppression and upregulation of PI3K/AKT pathway in mouse model of testicular torsion-detorsion. Heliyon. 2021;7(4):e06868. doi: 10.1016/j.heliyon.2021.e06868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lysiak JJ, Nguyen QA, Kirby JL, et al. Ischemia-reperfusion of the murine testis stimulates the expression of proinflammatory cytokines and activation of c-jun N-terminal kinase in a pathway to E-selectin expression. Biol Reprod. 2003;69(1):202–210. doi: 10.1095/biolreprod.102.013318. [DOI] [PubMed] [Google Scholar]
- 40.D’amelio M, Cavallucci V, Cecconi F. Neuronal caspase-3 signaling: not only cell death. Cell Death Differ. 2010;17(7):1104–1114. doi: 10.1038/cdd.2009.180. [DOI] [PubMed] [Google Scholar]
- 41.Turner TT, Tung KS, Tomomasa H, et al. Acute testicular ischemia results in germ cell-specific apoptosis in the rat. Biol Reprod. 1997;57(6):1267–1274. doi: 10.1095/biolreprod57.6.1267. [DOI] [PubMed] [Google Scholar]
- 42.Ozen OA, Kus MA, Kus I, et al. Protective effects of melatonin against formaldehyde-induced oxidative damage and apoptosis in rat testes: an immunohistochemical and biochemical study. Syst Biol Reprod Med. 2008;54(4-5):169–176. doi: 10.1080/19396360802422402. [DOI] [PubMed] [Google Scholar]
- 43.Trnka J, Blaikie FH, Logan A, et al. Antioxidant properties of MitoTEMPOL and its hydroxylamine. Free Radic Res. 2009;43(1):4–12. doi: 10.1080/10715760802582183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu H, Li M. Mitochondria-targeted antioxidant mitotempo protects mitochondrial function against amyloid beta toxicity in primary cultured mouse neurons. Biochem Biophys Res Commun. 2016;478(1):174–180. doi: 10.1016/j.bbrc.2016.07.071. [DOI] [PubMed] [Google Scholar]
- 45.Hadziselimovic F, Geneto R, Emmons LR. Increased apoptosis in the contralateral testes of patients with testicular torsion as a factor for infertility. J Urol. 1998;160(3 Pt 2):1158–1160. doi: 10.1097/00005392-199809020-00053. [DOI] [PubMed] [Google Scholar]
- 46.Shokoohi M, Olad Saheb Madarek E, Khaki A, et al. Investigating the effects of onion juice on male fertility factors and pregnancy rate after testicular torsion/ detorsion by intrauterine insemination method. Int J Womens Health Reprod Sci. 2018;6(4):499–505. [Google Scholar]
- 47.Qamar AY, Naveed MI, Raza S, et al. Role of antioxidants in fertility preservation of sperm - a narrative review. Anim Biosci. 2023;36(3):385–403. doi: 10.5713/ab.22.0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mehmood A, Anwar M, Naqvi SMS. Motility, acrosome integrity, membrane integrity and oocyte cleavage rate of sperm separated by swim-up or Percoll gradient method from frozen-thawed buffalo semen. Anim Reprod Sci. 2009;111(2-4):141–148. doi: 10.1016/j.anireprosci.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 49.Lu X, Zhang Y, Bai H, et al. Mitochondria-targeted antioxidant MitoTEMPO improves the post-thaw sperm quality. Cryobiology. 2018;80:26–29. doi: 10.1016/j.cryobiol.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 50.Zhang X, Lu X, Li J, et al. Mito-Tempo alleviates cryodamage by regulating intracellular oxidative metabolism in spermatozoa from asthenozoospermic patients. Cryobiology. 2019;91:18–22. doi: 10.1016/j.cryobiol.2019.11.005. [DOI] [PubMed] [Google Scholar]


