Abstract
Introduction
Multimorbidity is increasing globally, emphasizing the need for effective self-management strategies. The Cumulative Complexity Model (CuCoM) offers a unique perspective on understanding self-management based on workload and capacity. This study aims to validate the CuCoM in multimorbid patients and identify tailored predictors of self-management.
Methods
This multicenter cross-sectional survey recruited 1920 multimorbid patients in five primary health centres and four hospitals in China. The questionnaire assessed workload (drug intake, doctor visits and follow-up, disruption in life, and health problems), capacity (social, environmental, financial, physical, and psychological), and self-management. Data were analyzed using latent profile analysis, chi-square, multivariate linear regression, and network analysis.
Results
d Patients were classified into four profiles: low workload-low capacity (10.2%), high workload-low capacity (7.5%), low workload-high capacity (64.6%), and high workload-high capacity (17.7%). Patients with low workload and high capacity exhibited better self-management (β = 0.271, p < 0.001), while those with high workload and low capacity exhibited poorer self-management (β=-0.187, p < 0.001). Social capacity was the strongest predictor for all profiles. Environmental capacity ranked second for ‘high workload-high capacity’ (R² = 3.26) and ‘low workload-low capacity’ (R² = 5.32) profiles. Financial capacity followed for the ‘low workload-high capacity’ profile (R² = 5.40), while psychological capacity was key in the ‘high workload-low capacity’ profile (R² = 6.40). In the network analysis, socioeconomic factors exhibited the central nodes (p < 0.05).
Conclusions
Personalized interventions designed to increase capacity and reduce workload are essential for improving self-management in multimorbid patients. Upstream policies promoting health equity are also crucial for better self-management outcomes.
Keywords: Workload, capacity, cumulative complexity model, multimorbidity, self-management
Introduction
Multimorbidity, the coexistence of two or more chronic diseases within an individual, has emerged as a worldwide concern with substantial effects on patients, caregivers, and society [1]. Globally, the overall prevalence of multimorbidity has up to 33.1% and exceeded 50% in adults aged 65 years and above [2]. Compared to patients with a single disease condition, those with multimorbidity are more likely to endure a higher burden of symptoms, increased psychological distress, experience a lower quality of life, suffer greater economic burden, and even die prematurely [3,4]. Actions to control and manage the increasing multimorbidity are highly desirable.
Self-management has been recognized as a cornerstone in managing multimorbidity, requiring substantial and consistent personal ongoing commitment from patients regarding time, effort, and financial resources [5]. This commitment encompasses adherence to prescribed medication regimens, continuous monitoring, symptom management, dietary adjustment, and regular clinical visits, collectively imposing a significant burden on patients [6]. For example, Vaure et al. revealed that patients with three chronic conditions needed to take 6–13 medicines daily, visit medical personnel 1.2–5.9 times monthly, and spend 49.6 to 71.0 h per month on health-related activities, resulting in an enormous workload [7]. These workloads or burdens will further and negatively impact adherence, compromise self-management, and worsen health outcomes, exacerbating additional intensive treatments and self-management interventions [8]. Such a negative cycle could eventually deteriorate patients’ health conditions and undermine the health system. Thus, minimally disruptive medicine, which emphasizes the ‘fit’ of health care with patients’ lives, is becoming increasingly prominent [9].
The cumulative complexity model (CuCoM), a patient-centred framework grounded in minimally disruptive medicine, provides a structured understanding of how external factors (e.g. social support and health care service) and internal factors (e.g. physical and psychological) interact with complicated patient care, providing a clear and applicable understanding of functional mechanisms of complexity at the patient level [10]. According to CuCoM, every patient encompasses various demands requiring time and energy, such as treatment and life responsibilities, constituting the ‘workload’ [11]. At the same time, patients possess various capacities to handle these demands, including abilities and resources, such as socioeconomic and social support [11]. The workload-capacity imbalances constitute the core mechanism driving patient complexity. Specifically, when the workload overwhelms the capacity, it can increase the treatment burden and potentially impair self-management ability. These adverse outcomes appear and accumulate over time, interacting with each other in emergent and even cyclical ways and eventually resulting in a deterioration of health [10].
Although previous studies have employed CuCoM as the core framework to explore the causal process of deterioration in self-management in qualitative studies [11–13] and experimental studies [14], the precise impact of different states of workload-capacity balance and imbalance on self-management is not well explicated in recent studies. Furthermore, given the wide range and diversity of workloads and capacities, it is also a great challenge for CuCoM-based interventions to effectively target multiple workloads and capacities simultaneously. Therefore, it is crucial to delineate the workload-capacity profiles, encompassing both balanced and imbalanced states, and identify tailored predictors of self-management across different profiles. This understanding is pivotal for developing personalized interventions for multimorbidity patients with complex care needs and achieving patient-centred, minimally disruptive medicine.
Latent profile analysis (LPA) is a person-centred statistical tool that uncovers hidden groups within observed data and implements clustering functionality [15]. It is a useful approach for identifying workload-capacity profiles. Network analysis is a powerful tool for understanding complex systems by constructing networks of interdependent elements. This approach facilitates the identification of key metrics, such as ‘central nodes’ and ‘bridge nodes,’ which assist in pinpointing the most interconnected and potentially influential factors of self-management, critical for targeted intervention [16,17]. Therefore, this study endeavours to utilize the CuCoM framework with the objective to
Picture the hidden profiles of patients’ workload and capacity characteristics;
Explore the relationship between these distinct profiles and self-management abilities;
Identify tailored predictors of self-management across different workload-capacity profiles, providing a foundation for developing targeted, empirically-driven interventions for personalized patient care.
Methods
Participants
Participants were recruited through convenience sampling from four general hospitals (one county, two municipal, one provincial) and five primary healthcare centres (two in rural areas and three in urban areas). The cross-sectional survey was conducted between July 2022 and May 2023, during which both the exposure variables (workloads and capacities) and the outcome variable (self-management) were collected simultaneously. Participants were eligible if they were (1) ≥18 years old and (2) diagnosed with ≥2 chronic diseases in electronic health records. The diagnosis was classified under the International Classification of Diseases, and we considered 46 major chronic diseases, such as hypertension, dyslipidaemia, diabetes, solid tumours, chronic pulmonary diseases, liver disease, stroke, renal disease, gastrointestinal disease, depression, anxiety, and asthma [18]. Patients were excluded if they (1) did not consent to participate, (2) had severe visual or auditory impairments, and (3) had a severe cognitive, psychiatric, or neurological disorder and were unable to cooperate. In the linear regression analysis, the sample size followed Kendall’s principle, requiring 5 to 10 times the number of variables, with at least 260 samples [19]. For network analysis, a minimum of 153 samples was needed, calculated using the formula N(N − 1)/2 for pairwise associations, where N is the number of nodes [20]. The participants were categorized into workload-capacity profiles for analysis in this study.
Data collection
This survey was conducted by nursing students with substantial site survey experience. Prior to data collection, uniform training was provided to the investigators on questionnaire administration and interviewing techniques. Face-to-face interviews were conducted during the data collection process, wherein the investigators offered a comprehensive explanation of the study’s aim and procedures to the participants and obtained their written consent. The participants completed the questionnaires independently, with the investigators offering assistance as needed. The questionnaires were collected and examined on-site. To enhance participation and completion rates, participants were provided with gifts as compensation for their involvement.
Measurements
Self-management
The dependent variable was self-management, as measured by the partners in health scale (PIH), which has been validated in Chinese patients [21]. The PIH reflects a definition of chronic condition self-management that embraces a ‘holistic’ approach to empower individuals through proactive strategies to manage both physical and psychosocial components, representing a suitable measure for chronic condition self-management [22]. The PIH consists of 12 items in four dimensions: knowledge (4 items), coping (3 items), management of symptoms (3 items), and adherence to treatment (2 items). The items were rated on a 9-point Likert scale (0: low self-management and 8: high self-management). The total score, treated as a continuous variable, was calculated by summing the equally weighted scores of the individual items, resulting in a range from 0 to 96. The higher scores indicated greater self-management. Cronbach’s α for PIH was 0. 898 in this study.
Workload
The workload was assessed using the treatment burden questionnaire (TBQ), which measures treatment burden without restricting its scope to a single condition or treatment context [23]. The Chinese version of TBQ contained 15 items and four dimensions: drug intake, doctor visits and follow-up, disruption in life, and health problems [24]. An 11-point Likert scale ranging from 0 (not a problem) to 10 (big problem) and ‘does not apply’ was used, with a global score ranging from 0 to 150 [23]. ‘Does not apply’ answers were coded as 0 scores. A higher score indicates a higher level of treatment burden. Cronbach’s α for this questionnaire was 0.866 in this study.
Capacity
Patient capacity contains five dimensions. First, physical capacity was evaluated using the physical functioning scale from SF-36 [25]. Second, social capacity was evaluated by four items, which were selected from the social support subscale of the Patient-reported Outcomes Measurement System due to their unidimensionality and strong correlations with the overall scale score [26]. Financial capacity was evaluated by ‘How satisfied are you with the current economic situation?’ based on previous studies [27]. Environmental capacity was evaluated by the questionnaire adapted from the physical activity neighbourhood environment survey core scale, which consists of items such as ‘You can walk to an exercise facility (such as the park, trail, sports field) from my home in 10-15 min’ (score range 1–5) [28]. Finally, psychological capacity was evaluated using five items selected from the stress adaption scale [29]. The items, score range, and Cronbach α coefficients of each dimension were described in Table S1.
Covariates
Demographic and clinical variables included: age, gender, place of residence, educational level, marital status, labour market attachment, civil living status, monthly household income, medical insurance, and comorbidities were assessed via the Charlson comorbidity index (CCI) [30].
Statistical analysis
Normal distribution was assessed conducted by Kolmogorov–Smirnov test. Demographic characteristics were presented as percentages or mean ± standard deviation, as appropriate. Missing values were imputed using multiple imputation mean imputation (1.0% missing). We used LPA to identify the optimal workload-capacity profiles (parameters were detailed in Supplementary Method), then used chi-square tests to compare characteristics within these subgroups. Univariate and multiple linear regressions were employed to analyze the association between workload-capacity profiles and self-management. A relative importance analysis was performed utilizing the lmg method from the ‘relaimpo’ package of R to identify key variables explaining differences in self-management variance among subgroups. This analysis aimed to partition the explained variance (R2) into the proportional contributions of each variable, considering both their direct effects on self-management and their indirect effects mediated through other variables [31]. This methodology enabled correlated independent variables to share each other’s contributions, with the edge metric ranging from 0 to 1. The covariates were also included in the relative importance analysis to account for their potential influence on the results. To further identify the key nodes in four workload-capacity profiles, network analysis using the ‘qgraph’ package of R visualized interactions between workloads and capacities, identifying key nodes in subgroups. The ‘bootnet’ package of R was applied to ensure the accuracy and stability of networks, and CS coefficients should be at least 0.25 and ideally over 0.50. Data analyses were performed using Mplus (version 7.1.4), SPSS (25.0 version), and R (version 4.2.1). P values <0.05 were considered significant.
Results
Sociodemographic characteristics of participants
A total of 1946 participants completed the questionnaire; 23 were excluded due to diagnosis with only one chronic illness, and 3 were excluded owing to incomplete questionnaire completion (<80%). The final sample consisted of 1920 multimorbid patients. Table 1 shows participants’ sociodemographic characteristics. The participants’ mean age (±SD) was 66.44 ± 11.72 years, and 47.2% were female. The vast majority are married (89.8%).
Table 1.
Sociodemographic characteristics of participants.
| Characteristics | Total sample (n = 1920) | Workload-capacity profiles |
||||
|---|---|---|---|---|---|---|
| Class1a (n = 196) | Class 2a (n = 144) | Class 3a (n = 1241) | Class 4a (n = 339) | P | ||
| Sex | .006 | |||||
| Male | 1013 (52.8) | 124 (63.3) | 84 (58.3) | 631 (50.8)b | 174 (52.3)b | |
| Female | 907 (47.2) | 72 (36.7) | 60 (41.7) | 610 (49.2)b | 165 (48.7)b | |
| Age (years) | <.001 | |||||
| <60 | 439 (22.9) | 25 (12.8) | 10 (6.9) | 291 (23.4)b | 113 (33.3)b | |
| 60–69 | 654 (34.1) | 53 (27.0) | 38 (26.4) | 457 (36.8)b | 106 (31.3) | |
| 70–79 | 614 (32.0) | 77 (39.3) | 65 (45.1)b | 371 (29.9) | 101 (29.8) | |
| ≥80 | 213 (11.1) | 41 (20.9) | 31 (21.5) | 122 (9.8)b | 19 (5.6)b | |
| Place of residence | <.001 | |||||
| City | 862 (44.9) | 70 (35.7) | 42 (29.2) | 615 (49.6)b | 135 (39.8) | |
| Town | 406 (21.1) | 48 (24.5) | 27 (18.8) | 256 (20.6) | 75 (22.1) | |
| Rural | 652 (34.0) | 78 (39.8) | 75 (52.1)b | 370 (29.8)b | 129 (38.1) | |
| Educational level | <.001 | |||||
| Primary school | 865 (45.1) | 122 (62.2) | 98 (68.1) | 506 (40.8)b | 139 (41.0)b | |
| Junior high school and senior high school | 902 (47.0) | 67 (34.2) | 39 (27.1) | 622 (50.1)b | 174 (51.3)b | |
| College and above | 153 (8.0) | 7 (3.6) | 7 (4.9) | 113 (9.1) | 26 (7.7) | |
| Marital status | . 408 | |||||
| Married | 1725 (89.8) | 178 (90.8) | 130 (90.3) | 1105 (89.0) | 312 (92.0) | |
| Widowed, divorced, and other | 195 (10.2) | 18 (9.3) | 14 (9.7) | 136 (11.0) | 27 (8.0) | |
| Labour market attachment | <.001 | |||||
| Working | 890 (46.4) | 94 (48.0) | 81 (56.3) | 510 (41.1) | 205 (60.5)b | |
| Not working | 1030 (53.6) | 102 (52.0) | 63 (43.8) | 731 (58.9) | 134 (39.5)b | |
| Civil living status | .306 | |||||
| With partner | 1793 (93.4) | 188 (95.9) | 136 (94.4) | 1150 (92.7) | 319 (94.1) | |
| Living alone | 127 (6.6) | 8 (4.1) | 8 (5.6) | 91 (7.3) | 20 (5.9) | |
| Monthly household income | <.001 | |||||
| <1000 | 324 (16.9) | 57 (29.1) | 60 (41.7)b | 134 (10.8)b | 73 (21.5) | |
| 1000 ∼ 5000 | 1101 (57.3) | 103 (52.6) | 72 (50.0) | 737 (59.4) | 189 (55.8) | |
| 5001 ∼ 10000 | 413 (21.5) | 32 (16.3) | 10 (6.9)b | 309 (24.8) | 62 (18.3) | |
| >10000 | 82 (4.3) | 4 (2.0) | 2 (1.4) | 61 (5.0) | 15 (4.4) | |
| Medical insurance | <.001 | |||||
| Urban employee basic medical insurance | 717 (37.3) | 85 (42.9) | 39 (27.1)b | 495 (38.8) | 98 (28.9)b | |
| Urban resident basic medical insurance | 748 (39.0) | 61 (31.1) | 48 (33.3) | 508 (40.9) | 131 (38.6) | |
| New rural cooperative medical scheme | 455 (23.7) | 50 (25.5) | 57 (39.6)b | 238 (18.5)b | 110 (32.4) | |
| Charlson comorbidity index (CCI) | <.001 | |||||
| 0 | 386 (20.1) | 11 (5.6) | 8 (5.6) | 333 (26.8)b | 34 (10.0) | |
| 1 | 587 (30.6) | 40 (20.4) | 31 (21.5) | 448 (36.1)b | 68 (20.1) | |
| 2 | 450 (23.4) | 54 (27.6) | 31 (21.5)b | 251 (20.2)b | 114 (33.6) | |
| 3 | 257 (13.4) | 46 (23.5) | 39 (27.1) | 108 (8.7)b | 64 (18.9) | |
| ≥4 | 240 (12.5) | 45 (23.0) | 35 (24.3) | 101 (8.1)b | 59 (17.4) | |
Class 1: low workload and low capacity; Class 2: high workload and low capacity; Class 3: low workload and high capacity; Class 4: high workload and high capacity.
Significant differences with Bonferroni correction (p<.05).
CCI: Charlson Comorbidity Index.
Clarification and configurations of workload-capacity profiles
Table S2 shows the goodness-of-fit indices for the LAP. In both workload and capacity datasets, the three-profile model showed a slight improvement in AIC, BIC, and aBIC compared to the two-profile model. However, the LMR was only significant for the two-profile models, indicating that the two-profile models had an advantage over the three-profile models. Ultimately, the two-profile models were selected as the best fit for workload and capacity. The plot of the scores (T-scores) for the LPA model is shown in Figure S1. Workload data was divided into ‘low’ (74.7%) and ‘high’ (25.3%) categories, while capacity data was split into ‘low’ (17.7%) and ‘high’ (82.3%) categories. Participants were then grouped into four profiles based on workload and capacity levels: ‘low workload-low capacity’ (10.2%), ‘high workload-low capacity’ (7.5%), ‘low workload-high capacity’ (64.6%), and ‘high workload-high capacity’ (17.7%). Demographic information for these profiles can be found in Table 1.
The relationship between workload-capacity profiles and self-management
In Table 2, univariate linear regression showed that all four workload-capacity profiles were significantly associated with self-management (p < 0.001). The multiple linear regression analyses, adjusted for sex, age, place of residence, educational level, labour market attachment, monthly household income, medical insurance, and CCI, further revealed that the ‘low workload-high capacity’ profile was an independent promoting factor contributing to self-management (β = 0.271, p < 0.001), while the ‘high workload-low capacity’ profile was identified as an independent barrier factor for self-management among patients with multimorbidity (β=-0.187, p < 0.001).
Table 2.
Linear regression results for predicting self-management among patients with multimorbidity.
| Univariate linear regression |
Multivariate linear regression |
|||||
|---|---|---|---|---|---|---|
| Variable | β | 95%CI | P | β | 95%CI | P |
| Low workload and low capacity | −0.157 | −11.407∼-6.393 | <0.001 | |||
| High workload and low capacity | −0.332 | −24.37∼-18.88 | <0.001 | −0.187 | −14.93∼-9.41 | <.001 |
| Low workload and high capacity | 0.404 | 13.04 ∼ 15.98 | <0.001 | 0.271 | 8.13 ∼ 11.32 | <.001 |
| High workload and high capacity | −0.153 | −8.87∼-0.49 | <0.001 | |||
Relative importance analysis in four workload-capacity profiles
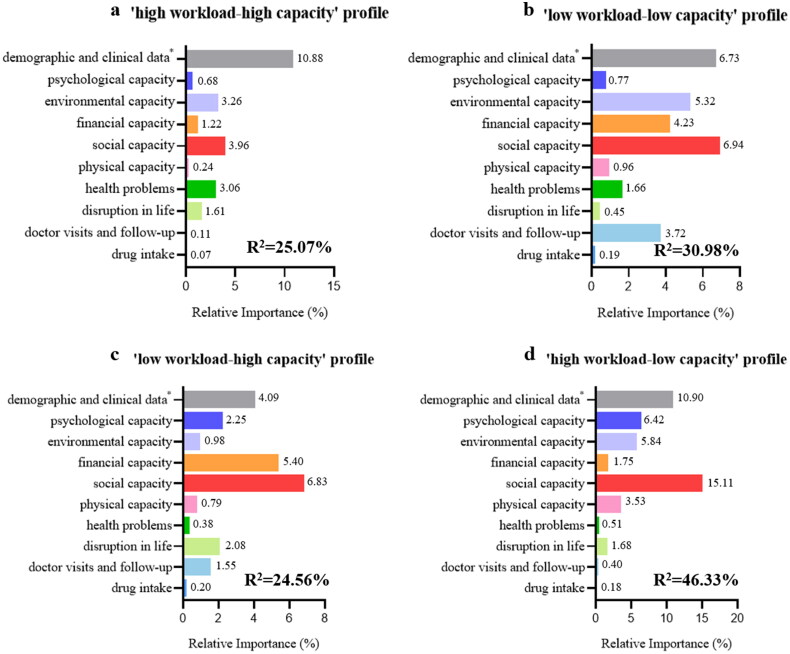
We conducted a relative importance analysis to clarify the impact of workloads and capacities on self-management, pinpointing precise intervention targets tailored for four distinct profiles. Figure 1 demonstrated that social capacity was the most robust predictor across all workload-capacity profiles, particularly evident in the ‘high workload-low capacity’ profile (R2=15.11). Environment capacity was the second strongest predictor of the ‘high workload-high capacity’ profile (R2=3.26) and the ‘low workload-low capacity’ profile (R2=5.32). In the ‘low workload-high capacity’ profile, financial capacity (R2 = 5.40) assumes the next significant predictor, while psychological capacity (R2 = 6.4) and environment capacity (R2 = 5.84) also played a key role in the ‘high workload-low capacity’ profile.
Figure 1.
Relative importance of 17 independent variables on (a) ‘high workload-high capacity’ profile, (b) ‘low workload-low capacity’ profile, (c) ‘low workload-high capacity’ profile, and (d) ‘high workload-low capacity’ profile. Note: the metric of ‘lmg’ (R2 partitioned by averaging over orders) is used to quantify the contribution of the independent variables to the dependent variable.
*age, sex, place of residence, educational level, labour market attachment, monthly household income, medical insurance, and Charlson comorbidity index.
Network analysis in four workload-capacity profiles
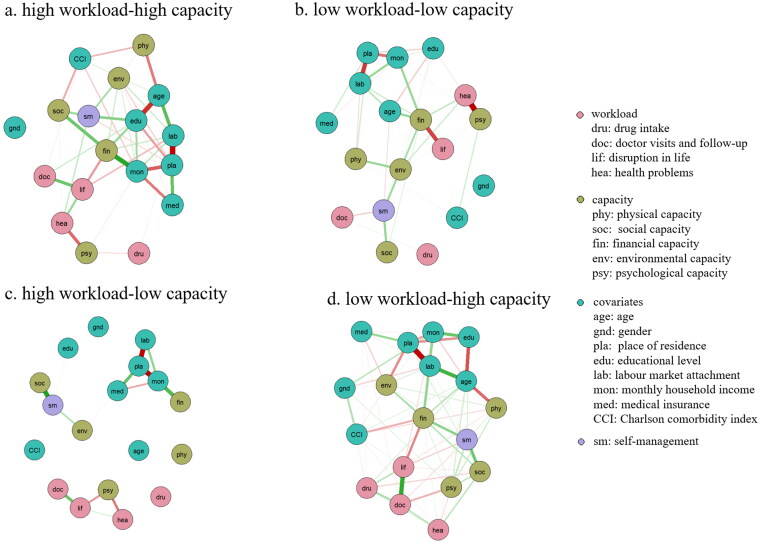
Network analysis was employed to delve into the complex interrelationships between predictors. As illustrated in Figure 2, Fig S2, and Table S3, financial capacity (rs: 0.88 ∼ 1.09) exhibited the highest strength centrality, followed by the place of residence (rs: 0.81 ∼ 1.09), labour market attachment (rs: 0.77 ∼ 0.97), and monthly income (rs: 0.68 ∼ 0.96) across various profiles. Despite the ‘high workload-low capacity’ profile indicating insufficient stability, place of residence (rs: 0.69) and labour market attachment (rs: 0.42) also evidenced the most robust trend in strength notes. Strength (CS coefficients >0.25) showed sufficient stability, whereas closeness and betweenness centrality fell below the recommended minimum threshold of >0.25, indicating poor stability. Therefore, we interpreted our findings primarily based on the strength indices. Bootstrapping showed a significant overlap for the confidence intervals of the edge weights (Fig. S3 and Fig. S4).
Figure 2.
Estimated regularized network structure of (a) ‘high workload-high capacity’ profile, (b) ‘low workload-low capacity’ profile, (c) ‘low workload-high capacity’ profile, and (d) ‘high workload-low capacity’ profile. The edges are colour-coded to indicate partial correlations, with green representing positive and red representing negative. Thicker lines represent stronger connections.
Discussion
CuCoM emphasized that an imbalance between workload and capacity – where workloads exceed capacities—drives disruptions in self-management and adverse health outcomes [10]. Based on this framework, the present study identified four distinct workload-capacity profiles among multimorbid patients, underscoring that high workload and low capacity impeded self-management, whereas low workload coupled with high capacity facilitated it. Surprisingly, a balanced workload-capacity status (either low workload/low capacity or high workload/high capacity) did not significantly affect patients’ self-management ability. Among these profiles, social capacity emerged as the most influential factor, and the ‘high workload-low capacity’ profile was also strongly predicted by psychological and environmental capacity, while the ‘low workload-high capacity’ profile was distinguished by financial capacity. Notably, although the most effective indicators of self-management varied across profiles, socioeconomic indicators (e.g. place of residence, labour market attachment) consistently emerged as central nodes in network analysis.
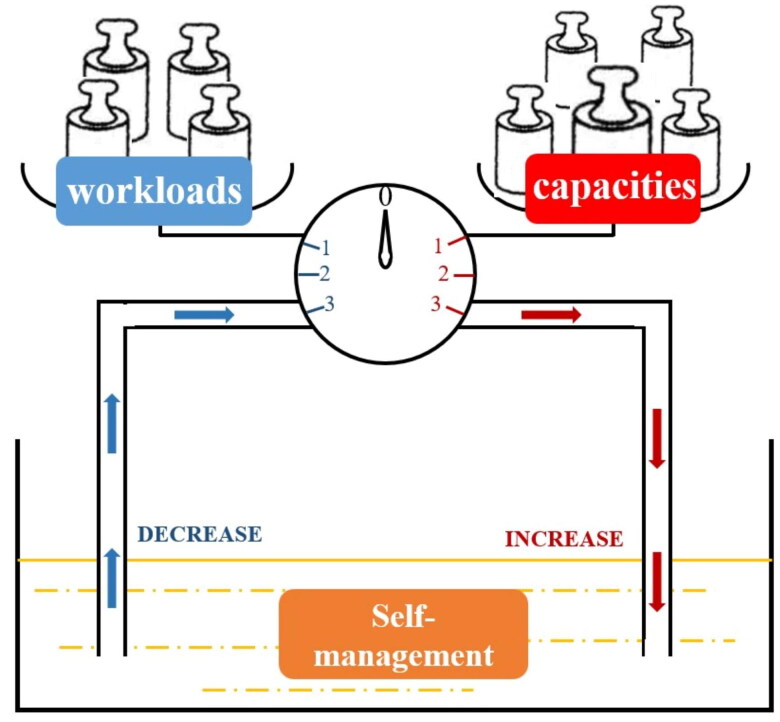
Significant disparities in the effect of four workload-capacity profiles on self-management were the predominant finding. Drawing on Conservation of Resources Theory (CRT), the concepts of ‘gain spiral’ and ‘loss spiral’ help to elucidate the potential mechanisms [10,32,33]. Gain spiral refers to a process in which individuals possess or gain sufficient resources to cope with loss, and that initial resource gain begets further gain [34]. For instance, to meet lower self-management demand, patients with high capacity could leverage their social capacity to delegate self-management tasks and easily access medical services with environmental capacity advantages. This strategic coordination of their self-management demand could enhance their self-efficacy, further enhancing their self-management ability. In contrast, patients with a high workload and low capacity often face a stretch in protecting themselves, leading to a spiral of loss, leading to a loss spiral. With a continuously depleting resource pool, slight stimuli could manifest as significant challenges, and poor access and nonadherence increase in momentum [34], which might explain the negative relationship between the ‘high workload-low capacity’ profile and self-management. Similarly, a mixed-methods study demonstrated that diabetes patients with limited capacity face significant treatment burdens due to challenges such as navigating healthcare services and affording medications, which ultimately diminishes their well-being [35]. For those with balanced workload-capacity profiles, their capacity was ‘just enough’ to encounter the losses caused by the workload, safeguarding resources from being lost or threatened. As a result, changes in self-management behaviours may not be noticeable. To intuitively present this mechanism, we proposed a self-management reservoir schematic in Figure 3. This schematic might illustrate the complex and dynamic mechanisms underlying self-management.
Figure 3.
Proposed self-management reservoir schematic.
Self-management (yellow pool) is regarded as a dynamic resource. When patient capacities overwhelm workloads, the pointer will move towards the right, increasing the resource of self-management (red arrow). Note: initial workload-capacity imbalance (capacities > workloads) might allow individuals to invest further, rendering them less vulnerable to loss and loss spirals. When patient workloads overwhelm capacities, the pointer will move towards the left, decreasing the resource of self-management (blue arrow). Initial workload-capacity imbalance (workload > capacities) might bring individuals caught in a loss spiral.
In this study, social support was the significant predictor of self-management, while there are tailored targets for different profiles. Multimorbidity is a major life event that evolved, filled with numerous recurrent stressful situations, overwhelming patients with complex medication regimens, dramatic lifestyle changes, and emotion dysregulation [36]. Social support provides knowledge, practical help, and emotional support to help patients gradually adapt to stress and challenges, acting as the priority and direct role in self-management [37]. In the ‘high workload-low capacity’ profile, psychological and environmental capacity were secondary determinants of self-management. Multimorbidity-induced stress can threaten a patient’s independence and autonomy, surpassing their capacity to cope and resulting in psychological distress and depression [38]. Psychology resilience, for instance, mitigates or buffers this stress, transforming it into a constructive learning experience and promoting proactive self-management [39]. Moreover, environmental factors may directly affect healthcare access, exacerbating self-management challenges, especially for patients with high medical resource needs. For patients with low workload and low capacity, environmental capacity, financial capacity, and doctor visits and follow-up were significant to self-management. It’s possible that they experienced a lower treatment burden and less urgent medical needs, leading them more likely to delay or abandon self-management when faced with tasks that challenge their capacities. In the ‘low workload-high capacity’ profile, financial capacity may facilitate self-management in relatively low-stress conditions by enhancing self-control [40].
The network analysis illuminated that financial capacity, place of residence, labour market attachment, and monthly income were central nodes, indicating socioeconomic status might be the upstream factor for self-management, regardless of profile. Previous studies revealed that socioeconomic status significantly impacted both the prevalence and prognosis of multimorbidity [41,42]. Similarly, socioeconomic factors such as employment and financial status have been identified as robust factors predicting poor adherence, unhealthy lifestyles, and insufficient self-monitoring [43,44]. The underlying mechanism may be that adverse socioeconomic status increases workloads, including time and travel burden; these ‘cumulative disadvantages’ lead to loss of autonomous living and loneliness [45]. Furthermore, the compounding effect of weakened capacities due to this status, such as inadequate support and limited access to medical services, results in adopting avoidant coping strategies, thereby hindering proactive self-management [45]. In the ‘high workload-low capacity’ profile, although socioeconomic indicators, such as place of residence, exhibited high strength and betweenness centrality, the strength centrality of financial capacity was relatively low, potentially due to its indirect influence on workloads and self-management through interactions with other factors such as monthly income. Patients perceived their financial capacity as influenced by comparisons with their peers in the surrounding community [46]. Even though they perceive their financial situation as relatively favourable, the actual constraints imposed by limited income hinder their ability to manage the disease burden effectively. The low betweenness centrality of monthly income and labour market attachment may be due to the limited sample size, which resulted in a dispersed node distribution and constrained the calculation of shortest paths among node pairs [47], thereby hindering an accurate representation of betweenness centrality and introducing potential bias.
The major contributions of this study lie in two folds. Theoretically, this study broadens the interpretation scope of CuCoM and illustrates the diverse workload-capacity profiles in shaping complex self-management in patients with multimorbidity. Additionally, the proposed self-management reservoir schematic delves into the mechanisms of how workload-capacity imbalances cumulatively impact self-management, providing deeper insights into the underlying processes from the perspective of CRT. Practically, the findings offer specific targets for personalized care. Healthcare professionals could implement shared decision-making prior to interventions or treatment to ensure patients comprehend the potential benefits and burdens [48]. Then, the tailored targets identified across different patient profiles in this study contribute to targeted and personalized interventions. For instance, healthcare professionals could provide robust support systems, and optimize the availability of social and health resources for multimorbid patients with low workload and capacity. Meanwhile, cognitive behavioural therapy and positive psychological interventions are also indispensable for patients dealing with high workloads and limited capacities, as they help address the multifaceted stressors and psychological challenges stemming from multimorbidity conditions. Furthermore, policymakers should be aware that socioeconomic status is the upstream factor affecting health and develop policies that achieve common prosperity, promote health equity, and narrow health disparity.
This study features several notable strengths. Firstly, this is a pioneering work to examine and refine the CuCoM among multimorbid patients in China quantitatively, and it further develops the self-management reservoir schematic to provide a rigorous analytical framework for elucidating self-management. Secondly, we applied a novel approach (e.g. LPA and network analysis) to explore complex interplays among workloads and capacities, identifying key factors and crucial interactive pathways in the complex system. However, there are also several limitations. Firstly, the convenience sampling method may limit the generalizability of our findings. Nevertheless, efforts were made to improve the representativeness of the sample by conducting surveys across nine healthcare institutions at different levels, including provincial, municipal, and county hospitals, as well as community health centres in both rural and urban areas. The sample size was also considerably larger than the minimum required to enhance representativeness further. Secondly, the sample size for the ‘high workload-low capacity’ profile was limited, potentially leading to bias in the network analysis results. It is necessary to expand the sample size in future studies to validate these findings. In addition, it is not currently possible to define specific cutoff values for classifying workload and capacity using the LPA method. Besides, the current study’s cross-sectional design precludes causality determination. Future studies should test whether interventions focused on tailored targets identified in this study can causally improve self-management. In addition, it is also necessary to construct a complex dynamic network that captures the trajectory of interactions between workloads and capacities over time.
Conclusions
Based on the CuCoM framework, this study identified four distinct ‘workload-capacity’ profiles of multimorbid patients, revealing that lower workloads combined with higher capacities could accumulatively enhance self-management outcomes. Furthermore, tailored predictors vary across different ‘workload-capacity’ profiles, with socioeconomic factors consistently acting as foundational influences. These results emphasize the need for targeted, personalized interventions to support minimally disruptive medicine and patient-centred care. Upstream, policy efforts should focus on achieving common prosperity and prompting health equity to mitigate the impact of socioeconomic disparities on health outcomes.
Supplementary Material
Funding Statement
This work was supported by the National Natural Science Foundation of China [grant numbers 72004193], Zhejiang Provincial Natural Science Foundation of China [grant numbers LY23G030008] and Fundamental Research Funds for the Central Universities [grant numbers S20230009].
Ethical approval
This study was approved by the Ethics Committee of Zhejiang University (2022-006). The study was performed in accordance with the Declaration of Helsink. Informed written consent was obtained from all the participants before the start of this study. By completing a consent form, the participants were informed about the purpose and method of the study. The participants were also informed that the researchers were committed to answering their questions and that their information would be kept confidential.
Author contributions
BZ contributed to conceptualization, methodology, writing, and revision. YF collected data, refined methodology, and participated in writing. JW, EX, and CL contributed to data collection and methodology. DC, QW, JY, and CL assisted in data collection and writing. ZY introduced the paper’s concepts. JS contributed to conceptualization, review/editing, and secured research funding. All authors have read and approved the final work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Hassen CB, Fayosse A, Landré B, et al. Association between age at onset of multimorbidity and incidence of dementia: 30 year follow-up in Whitehall II prospective cohort study. BMJ. 2022;376:e068005. doi: 10.1136/bmj-2021-068005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen H, Manolova G, Daskalopoulou C, et al. Prevalence of multimorbidity in community settings: a systematic review and meta-analysis of observational studies. J Comorb. 2019;9:2235042X19870934. doi: 10.1177/2235042X19870934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jani BD, Hanlon P, Nicholl BI, et al. Relationship between multimorbidity, demographic factors and mortality: findings from the UK Biobank cohort. BMC Med. 2019;17(1):74. doi: 10.1186/s12916-019-1305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sathya T, Selvamani Y, Nagarajan R, et al. Association between multimorbidity and psychological distress among older adults in India: the moderating role of elder abuse. Clin Gerontol. 2024;0(0):1–11. doi: 10.1080/07317115.2024.2309942. [DOI] [PubMed] [Google Scholar]
- 5.Doyle J, Murphy E, Gavin S, et al. A digital platform to support self-management of multiple chronic conditions (ProACT): findings in relation to engagement during a one-year proof-of-concept trial. J Med Internet Res. 2021;23(12):e22672. doi: 10.2196/22672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liddy C, Blazkho V, Mill K.. Challenges of self-management when living with multiple chronic conditions: systematic review of the qualitative literature. Can Fam Physician. 2014;60(12):1123–1133. [PMC free article] [PubMed] [Google Scholar]
- 7.Buffel Du Vaure C, Ravaud P, Baron G, et al. Potential workload in applying clinical practice guidelines for patients with chronic conditions and multimorbidity: a systematic analysis. BMJ Open. 2016;6(3):e010119. doi: 10.1136/bmjopen-2015-010119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mendoza-Quispe D, Perez-Leon S, Alarcon-Ruiz CA, et al. Scoping review of measures of treatment burden in patients with multimorbidity: advancements and current gaps. J Clin Epidemiol. 2023;159:92–105. doi: 10.1016/j.jclinepi.2023.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skou ST, Mair FS, Fortin M, et al. Multimorbidity. Nat Rev Dis Primers. 2022;8(1):48. doi: 10.1038/s41572-022-00376-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shippee ND, Shah ND, May CR, et al. Cumulative complexity: a functional, patient-centered model of patient complexity can improve research and practice. J Clin Epidemiol. 2012;65(10):1041–1051. doi: 10.1016/j.jclinepi.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Forsyth F, Blakeman T, Burt J, et al. Cumulative complexity: a qualitative analysis of patients’ experiences of living with heart failure with preserved ejection fraction. Eur J Cardiovasc Nurs. 2023;22(5):529–536. doi: 10.1093/eurjcn/zvac081. [DOI] [PubMed] [Google Scholar]
- 12.Corbett T, Lee K, Cummings A, et al. Self-management by older people living with cancer and multi-morbidity: a qualitative study. Support Care Cancer. 2022;30(6):4823–4833. doi: 10.1007/s00520-022-06892-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mbokazi N, van Pinxteren M, Murphy K, et al. Ubuntu as a mediator in coping with multimorbidity treatment burden in a disadvantaged rural and urban setting in South Africa. Soc Sci Med. 2023;334:116190. doi: 10.1016/j.socscimed.2023.116190. [DOI] [PubMed] [Google Scholar]
- 14.Ariza-Mateos MJ, Cabrera-Martos I, López-López L, et al. Effects of a patient-centered program including the cumulative-complexity model in women with chronic pelvic pain: a randomized controlled trial. Maturitas. 2020;137:18–23. doi: 10.1016/j.maturitas.2020.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Bauer J. A primer to latent profile and latent class analysis. In: Goller M, Kyndt E, Paloniemi S, Damşa C, editors. Methods for researching professional learning and development: challenges, applications and empirical illustrations. Switzerland: Springer International Publishing; 2022. p. 243–268. doi: 10.1007/978-3-031-08518-5_11. [DOI] [Google Scholar]
- 16.Kaiser T, Herzog P, Voderholzer U, et al. Unraveling the comorbidity of depression and anxiety in a large inpatient sample: network analysis to examine bridge symptoms. Depress Anxiety. 2021;38(3):307–317. doi: 10.1002/da.23136. [DOI] [PubMed] [Google Scholar]
- 17.McNally RJ. Network analysis of psychopathology: controversies and challenges. Annu Rev Clin Psychol. 2021;17(1):31–53. doi: 10.1146/annurev-clinpsy-081219-092850. [DOI] [PubMed] [Google Scholar]
- 18.Koller D, Schön G, Schäfer I, et al. Multimorbidity and long-term care dependency—a five-year follow-up. BMC Geriatr. 2014;14(1):70. doi: 10.1186/1471-2318-14-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weber E, Kendall M . Multivariate analysis. Charles Griffin b Co. LTD. London, High Wycombe 1975. 210 s., 9 Abb., 27 Tab., 1 Anhang, £6,80. Biometrical J. 1977;19(4):309–309. doi: 10.1002/bimj.4710190413. [DOI] [Google Scholar]
- 20.Network-based statistic: identifying differences in brain networks. NeuroImage. 2010;53(4):1197–1207. doi: 10.1016/j.neuroimage.2010.06.041. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Jiang Y, Kang X, et al. Reliability and validity of the Chinese version of the partners in health scale in patients with chronic heart failure. Chin Gener Pract. 2022;25(04):497–504. [Google Scholar]
- 22.Smith D, Harvey P, Lawn S, et al. Measuring chronic condition self-management in an Australian community: factor structure of the revised Partners in Health (PIH) scale. Qual Life Res. 2017;26(1):149–159. doi: 10.1007/s11136-016-1368-5. [DOI] [PubMed] [Google Scholar]
- 23.Tran VT, Harrington M, Montori VM, et al. Adaptation and validation of the treatment burden questionnaire (TBQ) in English using an internet platform. BMC Med. 2014;12(1):109. doi: 10.1186/1741-7015-12-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Z, Wang H, Ou W, et al. Current status of treatment burden of patients with noninfectious chronic diseases based on ordinal logistic regression. Chin Gener Pract. 2019;22(5):559–563. doi: 10.12114/j.issn.1007-9572.2018.00.206. [DOI] [Google Scholar]
- 25.Li L, Wang HM, Shen Y.. Chinese SF-36 health survey: translation, cultural adaptation, validation, and normalisation. J Epidemiol Community Health. 2003;57(4):259–263. doi: 10.1136/jech.57.4.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Broderick JE, DeWitt EM, Rothrock N, et al. Advances in patient-reported outcomes: the NIH PROMIS® measures. EGEMS (Wash DC). 2013;1(1):1015. doi: 10.13063/2327-9214.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hendriks I, Lubbers M, Scheepers P.. A panel study of attitudes toward ethnic minorities and the role of changes in individuals’ economic situations. Int J Public Opin R. 2022;34(1):edac003. doi: 10.1093/ijpor/edac003. [DOI] [Google Scholar]
- 28.Zhou R, Fu H, Luo J, et al. Evaluation on reliability and validity of Chinese Walkable Environment Scale for urbancommunity residents. Chin J Publ Heal. 2011;27(7):841–843. [Google Scholar]
- 29.Fu Y, Wu J, Zhao B, et al. Development of a Chinese version of the Stress Adaption Scale and the assessment of its reliability and validity among Chinese patients with multimorbidity. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2023;52(3):361–370. doi: 10.3724/zdxbyxb-2022-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 31.Groemping U. Relative importance for linear regression in R: the Package relaimpo. J Stat Software. 2007;17:1–27. doi: 10.18637/jss.v017.i01. [DOI] [Google Scholar]
- 32.Boehmer KR, Shippee ND, Beebe TJ, et al. Pursuing minimally disruptive medicine: disruption from illness and health care-related demands is correlated with patient capacity. J Clin Epidemiol. 2016;74:227–236. doi: 10.1016/j.jclinepi.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 33.Lin L, Bai Y.. The dual spillover spiraling effects of family incivility on workplace interpersonal deviance: from the conservation of resources perspective. J Bus Ethics. 2023;184(3):725–740. doi: 10.1007/s10551-022-05123-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hobfoll SE, Tirone V, Holmgreen L, et al. Chapter 7 - Conservation of resources theory applied to major stress. In: Fink G, editor. Stress: concepts, cognition, emotion, and behavior. Cambridge, MA: Academic Press; 2016. p. 65–71. doi: 10.1016/B978-0-12-800951-2.00007-8. [DOI] [Google Scholar]
- 35.Spencer-Bonilla G, Serrano V, Gao C, et al. Patient work and treatment burden in type 2 diabetes: a mixed-methods study. Mayo Clin Proc Innov Qual Outcomes. 2021;5(2):359–367. doi: 10.1016/j.mayocpiqo.2021.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Houtum L, Rijken M, Heijmans M, et al. Self-management support needs of patients with chronic illness: do needs for support differ according to the course of illness? Patient Educ Couns. 2013;93(3):626–632. doi: 10.1016/j.pec.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 37.Li H, Jiang Y F, Lin CC.. Factors associated with self-management by people undergoing hemodialysis: a descriptive study. Int J Nurs Stud. 2014;51(2):208–216. doi: 10.1016/j.ijnurstu.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 38.Ghanei Gheshlagh R, Sayehmiri K, Ebadi A, et al. Resilience of patients with chronic physical diseases: A systematic review and meta-analysis. Iran Red Crescent Med J. 2016;18(7):e38562. doi: 10.5812/ircmj.38562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jin Y, Bhattarai M, Kuo W, et al. Relationship between resilience and self-care in people with chronic conditions: a systematic review and meta-analysis. J Clin Nurs. 2023;32(9-10):2041–2055. doi: 10.1111/jocn.16258. [DOI] [PubMed] [Google Scholar]
- 40.Inequalities in health . 36 | Classic and contemporary readings in Soc; 2024. [cited April 21]. Available from https://www.taylorfrancis.com/chapters/edit/10.4324/9781315840154-36/inequalities-health-peter-townsend-nick-davidson
- 41.Schäfer I, Hansen H, Kaduszkiewicz H, et al. Health behaviour, social support, socio-economic status and the 5-year progression of multimorbidity: results from the MultiCare Cohort Study. J Comorb. 2019;9:2235042X19883560. doi: 10.1177/2235042X19883560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xue B, Xue Y, Dong F, et al. The impact of socioeconomic status and sleep quality on the prevalence of multimorbidity in older adults. Front Public Health. 2022;10:959700. doi: 10.3389/fpubh.2022.959700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tøttenborg SS, Lange P, Johnsen SP, et al. Socioeconomic inequalities in adherence to inhaled maintenance medications and clinical prognosis of COPD. Respir Med. 2016;119:160–167. doi: 10.1016/j.rmed.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 44.Gast A, Mathes T.. Medication adherence influencing factors—an (updated) overview of systematic reviews. Syst Rev. 2019;8(1):112. doi: 10.1186/s13643-019-1014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Wilder L, Pype P, Mertens F, et al. Living with a chronic disease: insights from patients with a low socioeconomic status. BMC Fam Pract. 2021;22(1):233. doi: 10.1186/s12875-021-01578-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoebel J, Lampert T.. Subjective social status and health: multidisciplinary explanations and methodological challenges. J Health Psychol. 2020;25(2):173–185. doi: 10.1177/1359105318800804. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Q, Deng R, Ding K, et al. Structural analysis and the sum of nodes’ betweenness centrality in complex networks. Chaos, Solitons Fractals. 2024;185:115158. doi: 10.1016/j.chaos.2024.115158. [DOI] [Google Scholar]
- 48.Cross SP, Alvarez-Jimenez M.. The digital cumulative complexity model: a framework for improving engagement in digital mental health interventions. Front Psychiatry. 2024;15:1382726. doi: 10.3389/fpsyt.2024.1382726. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.