Abstract
This study examined the relationship between gallstones and estimated glucose disposal rate (eGDR) in people in the United States and the possible mediating function of body mass index (BMI). Data came from the National Health and Nutrition Examination Survey (NHANES), conducted between 2017 and March 2020. Logistic regression, subgroup analysis, smoothed curve fitting, and causal mediation studies were among the statistical techniques used to examine the participant data. There were 595 subjects with a gallstone diagnosis out of 5,656 total. A significant negative association between eGDR and gallstones was observed by fully adjusted multivariate logistic regression analysis with an OR of 0.90 and a 95% confidence interval (CI) of (0.83, 0.98). The mediation analysis indicated that BMI accounted for 58.58% of the relationship between eGDR and the occurrence of gallstones. This study identified a substantial non-linear negative relationship between the occurrence of gallstones and eGDR levels, with BMI acting as a mediating factor. A fresh viewpoint on gallstone therapy and prevention is offered by these findings.
Keywords: Gallstones, Body Mass Index, Estimated glucose disposal rate, National Health and Nutrition Examination Survey
Subject terms: Endocrinology, Gastroenterology
Introduction
Gallstones are a common digestive problem. The latest research shows that the pooled prevalence and incidence of gallstones in the 21st century are 6.1% and 0.47 cases per 100 person-years, respectively1. Geography, age, and gender all affect prevalence. Approximately 13.9% of people in the US have gallstones, compared to 5.13% and 4.00% in China and India, respectively2–4. However, gallstones are now more common in Asia due to lifestyle changes5. According to previous research, women are far more likely than males to develop gallstones, particularly during pregnancy and when using contraceptives6. Furthermore, the incidence of gallstones rises with age, with those over 50 experiencing a noticeable increase in gallstone incidence7. The majority of gallstones develop slowly; 20% of patients have indigestion, nausea, vomiting, and excruciating upper right abdominal discomfort. Acute cholecystitis, cholangitis, pancreatitis, gallstone intestinal blockage, and even potentially fatal sepsis and gallbladder perforation might result from them if treatment is not received8. Gallstones and their complications not only reduce patients’ quality of life but also pose significant public health risks due to the high costs associated with surgery and hospital stays. As a result, accurate and regulated clinical indicators are crucial for gallstone prediction, early identification, and therapy.
Insulin resistance (IR) is a decrease in the body’s sensitivity to insulin, resulting in the inability of insulin to effectively promote glucose uptake and utilization9. Numerous metabolic disorders, including obesity, hypertension, and fatty liver, are closely linked to this illness10–12. Prior research has demonstrated a substantial correlation between IR and gallstone formation13,14. This process is associated with changes in gallbladder dynamics and disrupted cholesterol metabolism, which occurs due to hyperinsulinemia related to insulin resistance15,16. The hyperinsulinemic euglycemic clamp (HEC) is considered the gold standard for assessing insulin resistance, but its high cost and invasiveness limit its clinical use17. Clinically, the estimated glucose disposal rate( eGDR) is a straightforward and accurate indicator of IR18.
Globally, obesity has emerged as one of the biggest public health issues. This is the highest number of obese persons in the world, according to the World Health Organization’s (WHO) 2023 report. Since 1975, the prevalence of obesity has nearly quadrupled globally. Not only can obesity lower a person’s quality of life, but it is also closely linked to several chronic illnesses. When evaluating obesity, the most often used metric is body mass index (BMI). Based on studies, the incidence of gallstones rises by 7–10% for every unit increase in BMI19. The link between eGDR and gallstones, or the moderating effect of BMI in a significant number of groups, has not yet been documented in any research. This research was based on the NHANES database to examine the link between eGDR and gallstones as well as the function that BMI plays as a mediator in this relationship.
Materials and methods
Data source and study population
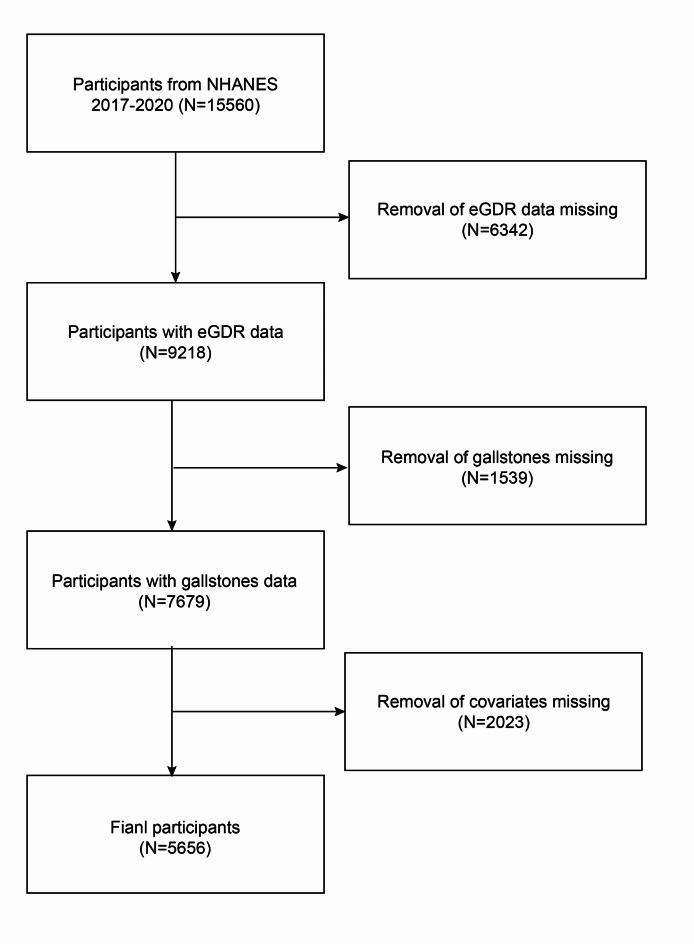
We use https://www.cdc.gov/nchs/nhanes/, which is provided by the National Health and Nutrition Examination Survey (NHANES) data in the database studied. For over two decades, NHANES has been a cross-sectional survey that is revised every two years, with each iteration encompassing around 10,000 respondents. Participants in the U.S. National Health Survey between 2017 and March 2020 who were at least 20 years old were included in the study. Individuals with no gallstone data, incomplete eGDR data, or known confounders were excluded from the study. The study involved a total of 5,656 individuals in the United States, of whom 595 self-reported having gallstones. Figure 1 depicts the filtering procedure.
Fig. 1.
Flowchart for choosing participants.
Definition of the eGDR and gallstones
The eGDR was calculated by applying the formula: eGDR = 21.158 − (0.09∗WC) − (3.407∗HT)− (0.551∗HbA1c) [WC = waist circumference (cm), HT = hypertension (yes = 1/no = 0) and HbA1c = HbA1c (%)]. Hypertension was defined as follows: 1)systolic blood pressure ≥ 140 mm Hg and/or diastolic blood pressure ≥ 90 mm Hg; 2) self-reported hypertension;3) taking antihypertensive medication. According to eGDR levels, participants were divided into four groups based on predefined criteria (≤ 4, 4–6, 6–8, > 8 mg/kg/min)20. Additionally, participants were grouped into four quartiles (Q1, Q2, Q3, and Q4) based on their eGDR values for further analysis. Gallstone statistics were taken from the MCQ questionnaire, “Has DR ever said you have gallstones?” to ascertain whether gallstones are present.
Covariates
In this study, we selected covariates associated with gallstones, drawing on previous studies2,21,22. The selected covariates include demographic data (sex, age, race, education level, and poverty-to-income ratio [PIR]), examination data (body mass index [BMI]), laboratory data (alanine aminotransferase [ALT], aspartate aminotransferase [AST], total cholesterol [TC], and high-density lipoprotein cholesterol [HDL]), and questionnaire data. The questionnaire data covers smoking status (measured by the SMQ020, with participants who smoked at least 100 cigarettes classified as smokers), alcohol consumption (measured by ALQ151, with participants who reported having 4 or more drinks daily classified as alcohol drinkers), physical activity, coronary heart disease (CHD), asthma, and cancer (participants who answered “yes” on the questionnaire were identified as having these conditions).
Statistical analysis
R software (version 4.2.2) and Empower Stats (version 4.2) were used for the statistical analysis, and P < 0.05 was the significance threshold. The baseline demographic parameters are expressed as balanced percentages for categorical categories and as the average and standard deviation for continuous variables. Multifactor logistic regression analysis was used to investigate the relationship between gallstones and the eGDR. Model 1 was adjusted for no covariates; Model 2 was adjusted for age, gender, and race; Model 3 had additional adjustments to account for education, smoked, alcohol, physical activity, asthma, CHD, cancers, PIR, ALT, AST, TC, HDL, and BMI. To further investigate the relationship between gallstones and the eGDR, we performed a smoothed curve fitting study (penalized spline method). A two-segment linear regression model (segmented regression model) was used to fit each segment and calculate the threshold effect. Subgroup analyses were also carried out to see if there were any possible differences in eGDR and gallstones among particular groups according to variables such as age, gender, BMI, race, asthma, CHD, malignancies, smoked, and alcohol use. Age was split into ≥ 50 and <50 groups. BMI was segmented into ≥ 25 and < 25 categories. We can determine how many mediated effects BMI produces by using mediation analysis. These statistical techniques allow for a more thorough investigation of potential correlations between gallstone prevalence and eGDR. To verify the stability of the results, we interpolated the missing covariates. We used the mean to interpolate covariates that followed a normal distribution. For those that were non-normally distributed, we used the median.
Results
Baseline characteristics
A total of 5,656 adults participated in the study, of which 595 had gallstones, resulting in an overall gallstone prevalence of 10.52%. The baseline characteristics of the subjects, including whether or not they had gallstones, are compiled in Table 1. Gallstone sufferers were less educated and had lower PIR, and their BMI was greater than that of people without gallstones. Women, older persons, white people, smokers, those with coronary heart disease, and those with cancer are more likely to have gallstones. The baseline characteristics of individuals stratified by eGDR level are shown in Table 2. As eGDR levels increased, BMI decreased, HDL levels rose significantly, and the prevalence of gallstones, cancer, and coronary heart disease also decreased markedly.
Table 1.
Baseline characteristics of the gallstones group versus the non-gallstones.
| Characteristic | Nonstone formers (n = 5,061) |
Stone formers (n = 595) |
P-value |
|---|---|---|---|
| Age (years) | 47.08 ± 16.85 | 56.69 ± 15.14 | <0.001 |
| Gender | <0.001 | ||
| Men | 52.55 | 26.17 | |
| Women | 47.45 | 73.83 | |
| Race | |||
| Mexican American | 8.06 | 7.11 | 0.003 |
| White | 66.39 | 72.38 | |
| Black | 10.46 | 6.10 | |
| Other race | 15.09 | 14.40 | |
| Education level | 0.005 | ||
| Less than high school | 8.93 | 9.76 | |
| High school | 25.98 | 31.71 | |
| More than high school | 65.09 | 58.54 | |
| Smoked | <0.001 | ||
| Yes | 44.43 | 54.51 | |
| No | 55.57 | 45.49 | |
| Alcohol | 0.247 | ||
| Yes | 13.98 | 15.70 | |
| No | 86.02 | 84.30 | |
| Moderate activities | 0.125 | ||
| Yes | 51.34 | 48.06 | |
| No | 48.66 | 51.94 | |
| Asthma | 0.094 | ||
| Yes | 15.15 | 17.74 | |
| No | 84.85 | 82.26 | |
| CHD | 0.005 | ||
| Yes | 3.97 | 6.38 | |
| No | 96.03 | 93.62 | |
| Cancers | <0.001 | ||
| Yes | 10.45 | 18.06 | |
| No | 89.55 | 81.94 | |
| PIR | 3.24 ± 1.63 | 3.02 ± 1.55 | 0.002 |
| ALT(U/L) | 22.90 ± 17.38 | 22.39 ± 16.75 | 0.488 |
| AST(U/L) | 21.94 ± 12.83 | 21.55 ± 12.93 | 0.479 |
| TC (mmol/L) | 4.85 ± 1.05 | 4.89 ± 1.08 | 0.381 |
| HDL (mmol/L) | 1.39 ± 0.41 | 1.38 ± 0.41 | 0.540 |
| BMI (kg/m2) | 29.49 ± 6.86 | 33.13 ± 8.42 | <0.001 |
| eGDR (mg/kg/min) | 7.79 ± 2.70 | 6.13 ± 2.83 | <0.001 |
*CHD: coronary heart disease; PIR: the ratio of income to poverty; ALT: alanine aminotransferase; AST: aspartate aminotransferase; TC: total cholesterol; HDL: direct HDL-Cholesterol; BMI: body mass index; eGDR: estimated glucose disposal rate.
Table 2.
Baseline characteristics of participants according to eGDR level.
| eGDR groups | ≤ 4 mg/kg/min | 4–6 mg/kg/min | 6–8 mg/kg/min | > 8 mg/kg/min | P-value |
|---|---|---|---|---|---|
| (n = 802) | (n = 1,241) | (n = 1,078) | (n = 2,535) | ||
| Age (years) | 55.31 ± 14.65 | 57.24 ± 15.38 | 51.16 ± 16.55 | 41.86 ± 15.52 | <0.001 |
| Gender | <0.001 | ||||
| Men | 58.80 | 55.31 | 45.55 | 46.92 | |
| Women | 41.20 | 44.69 | 54.45 | 53.08 | |
| Race | <0.001 | ||||
| Mexican American | 5.23 | 5.95 | 8.14 | 9.29 | |
| White | 69.91 | 70.19 | 65.71 | 65.63 | |
| Black | 15.75 | 10.27 | 10.96 | 8.25 | |
| Other race | 9.10 | 13.59 | 15.18 | 16.84 | |
| Education level | <0.001 | ||||
| Less than high school | 9.87 | 11.16 | 8.82 | 8.05 | |
| High school | 32.50 | 27.72 | 31.69 | 23.04 | |
| More than high school | 57.62 | 61.12 | 59.49 | 68.91 | |
| Smoked | <0.001 | ||||
| Yes | 55.51 | 48.42 | 51.70 | 39.95 | |
| No | 44.49 | 51.58 | 48.30 | 60.05 | |
| Alcohol | <0.001 | ||||
| Yes | 17.67 | 18.23 | 17.33 | 10.66 | |
| No | 82.33 | 81.77 | 82.67 | 89.34 | |
| Moderate activities | 0.109 | ||||
| Yes | 47.70 | 49.85 | 50.22 | 52.44 | |
| No | 52.30 | 50.15 | 49.78 | 47.56 | |
| Asthma | <0.001 | ||||
| Yes | 22.92 | 14.59 | 15.41 | 14.10 | |
| No | 77.08 | 85.41 | 84.59 | 85.90 | |
| CHD | <0.001 | ||||
| Yes | 9.74 | 9.07 | 4.16 | 1.13 | |
| No | 90.26 | 90.93 | 4.16 | 98.87 | |
| Cancers | <0.001 | ||||
| Yes | 16.31 | 15.46 | 12.70 | 8.00 | |
| No | 83.69 | 84.54 | 87.30 | 92.00 | |
| Gallstones | <0.001 | ||||
| Yes | 21.70 | 16.02 | 10.52 | 6.46 | |
| No | 78.30 | 83.98 | 89.48 | 93.54 | |
| PIR | 3.00 ± 1.60 | 3.28 ± 1.58 | 3.07 ± 1.60 | 3.29 ± 1.65 | <0.001 |
| ALT (U/L) | 28.45 ± 19.70 | 24.12 ± 14.94 | 24.17 ± 19.72 | 20.62 ± 16.29 | <0.001 |
| AST (U/L) | 23.66 ± 12.95 | 21.89 ± 10.24 | 22.75 ± 15.10 | 21.20 ± 12.81 | <0.001 |
| TC (mmol/L) | 4.65 ± 1.06 | 4.85 ± 1.07 | 4.97 ± 1.03 | 4.86 ± 1.04 | <0.001 |
| HDL (mmol/L) | 1.15 ± 0.29 | 1.31 ± 0.35 | 1.43 ± 0.46 | 1.46 ± 0.40 | <0.001 |
| BMI (kg/m2) | 39.52 ± 7.46 | 32.14 ± 5.72 | 30.71 ± 7.44 | 26.54 ± 4.60 | <0.001 |
*CHD: coronary heart disease; PIR: the ratio of income to poverty; ALT: alanine aminotransferase; AST: aspartate aminotransferase; TC: Total cholesterol; HDL: direct HDL-Cholesterol; BMI: body mass index; eGDR: estimated glucose disposal rate.
Association between the eGDR and gallstones
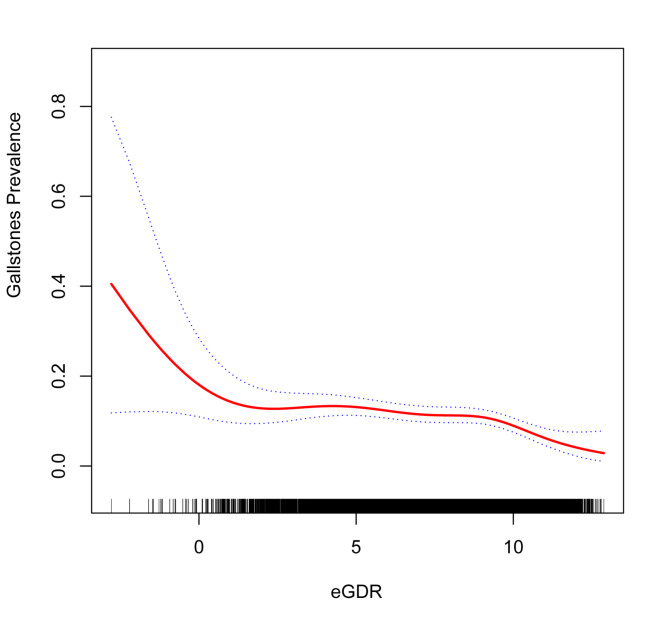
Table 3 illustrates the connection between gallstones and eGDR. The three models showed a significant negative connection (P < 0.05) between cholelithiasis and eGDR when eGDR was examined as a continuous variable. In the fully adjusted model, a 1 unit increase in eGDR was associated with a 10% reduction in gallstone prevalence (OR = 0.90, 95% CI: 0.83, 0.98). Further divided into four groups according to eDGR level, the negative correlation between eDGR and gallstones remained. In Model 3, subjects with eGDR values of 6–8 mg/kg/min and > 8 mg/kg/min had a 39% and 40% reduction in gallstone prevalence, respectively, compared with subjects with eGDR ≤ 4 mg/kg/min (OR = 0.61, 95%CI: 0.38, 0.96; OR = 0.60, 95% CI: 0.36, 1.00). In Model 3, the prevalence of gallstones was 38% lower in the third quartile compared to the lowest quartile of the eGDR. (OR = 0.62, 95%CI: 0.41, 0.93). Regardless of whether eGDR was considered a categorical or continuous variable, the risk of gallstones decreased as eGDR levels increased. We used smooth curve fitting to further examine the relationship between the eGDR index and gallstone prevalence. According to our research, there is a nonlinearly negative correlation between gallstone occurrence and eGDR (Fig. 2). Gallstone prevalence and the eGDR index threshold effect were ascertained using the likelihood ratio test, which had an ideal turning point at 9.81 (Table 4).
Table 3.
Logistic regression analysis between eGDR with gallstones prevalence.
| Characteristic | Model 1 OR (95%CI) | Model 2 OR (95%CI) | Model 3 OR (95%CI) |
|---|---|---|---|
| eGDR | 0.81 (0.77,0.85) | 0.81 (0.77,0.86) | 0.90 (0.83,0.98) |
| Categories | |||
| ≤ 4 mg/kg/min | 1.0 | 1.0 | 1.0 |
| 4–6 mg/kg/min | 0.69 (0.48,0.98) | 0.57 (0.40,0.83) | 0.87 (0.58,1.31) |
| 6–8 mg/kg/min | 0.42 (0.28,0.65) | 0.35 (0.23,0.53) | 0.61 (0.38,0.96) |
| > 8 mg/kg/min | 0.25 (0.17,0.36) | 0.25 (0.17,0.38) | 0.60 (0.36,1.00) |
| Categories | |||
| Q1 | 1.0 | 1.0 | 1.0 |
| Q2 | 0.53 (0.38,0.74) | 0.48 (0.34,0.68) | 0.70 (0.48,1.01) |
| Q3 | 0.37 (0.27,0.51) | 0.39 (0.27,0.56) | 0.62 (0.41,0.93) |
| Q4 | 0.21 (0.13,0.33) | 0.23 (0.14,0.40) | 0.58 (0.29,1.15) |
Model 1 was adjusted for no covariates; Model 2 was adjusted for age, gender, and race; Model 3 was adjusted for covariates in Model 2 + education, smoked, alcohol, physical activity, asthma, CHD, cancers, PIR, ALT, AST, TC, HDL and BMI were adjusted.
Fig. 2.
Density dose-response relationship between eGDR with gallstones prevalence.
Table 4.
Two-piecewise linear regression and logarithmic likelihood ratio test explained the threshold effect analysis of eGDR with gallstones prevalence.
| eGDR index | ULR test | PLR test | LRT test |
|---|---|---|---|
| OR (95%CI) | OR (95%CI) | P value | |
| <9.81 | 0.94(0.89,0.99) | 0.96(0.91,1.01) | 0.002 |
| ≥ 9.81 | 0.56(0.39,0.80) |
In mediation analyses, adjustments were made for age, gender, race, education, smoked, alcohol, physical activity, asthma, CHD, cancers, PIR, ALT, AST, TC, HDL and BMI.
Subgroup analysis
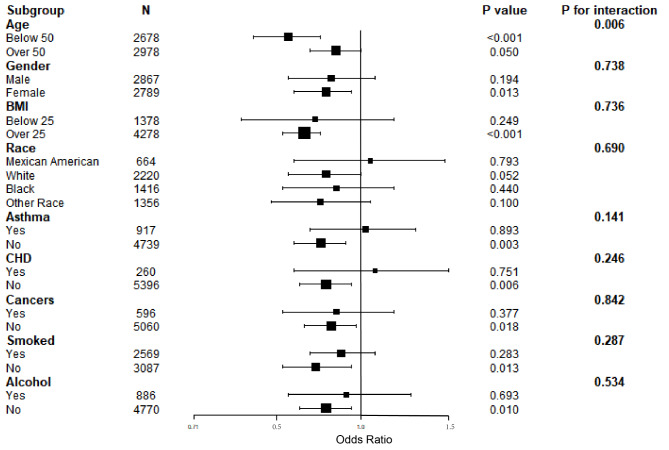
Figure 3 illustrates the subgroup analyses that evaluate the stability of the eGDR-gallstone prevalence association and potential variations across different populations. Notably, the subgroups of women, with BMI ≥ 25, no asthma, no coronary heart disease, no cancer, no smoked, and no alcohol consumption showed a more pronounced negative association between eGDR and gallstone prevalence(all P values < 0.05).In the interaction test, except for age, no associations were found for interaction p-values that reached statistical significance.
Fig. 3.
Subgroup analyses of the association between eGDR and gallstones.
The intermediary role of BMI
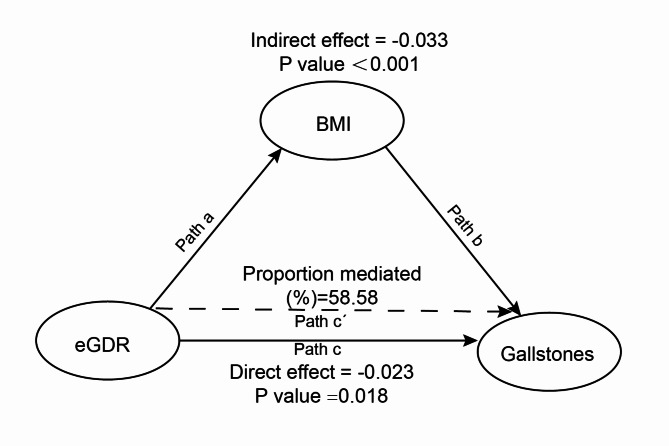
The possible mediating function of BMI in eGDR and gallstone incidence was examined using causal mediation analysis. Gallstones are the dependent variable, BMI is the mediator, and eGDR is the independent variable in the mediation analysis model and pathway depicted in Fig. 4. The results indicated that eGDR had a significant indirect influence on gallstone incidence through BMI (β=-0.033, 95% CI:-0.045, -0.021) after controlling for all possible confounders. This suggests that BMI partially mediates the role of eGDR in connection to gallstone occurrence. Additionally, a direct association between eGDR and gallstones was still statistically significant even after controlling for BMI (β=-0.023, 95% CI:-0.041, -0.002). This implies that eGDR and gallstone incidence are impacted by BMI in both direct and indirect ways. The impact of eGDR on gallstones was mediated by BMI to the extent of around 58.58%. Table 5 displays the findings of the mediation study.
Table 5.
Mediation analysis for the associations between eGDR and gallstones.
| Independent variable | Mediator | Total effect | Indirect effect | Direct effect | Proportion mediated,% | |||
|---|---|---|---|---|---|---|---|---|
| Coefficient(95% CI) | Pvalue | Coefficient(95% CI) | Pvalue | Coefficient(95% CI) | Pvalue | |||
| eGDR | BMI | -0.057(-0.070,-0.042) | <0.001 | -0.033(-0.045, -0.021) | <0.001 | -0.023(-0.041, -0.002) | 0.018 | 58.58 |
Fig. 4.
Mediated analysis model path diagram. Notes eGDR was defined as the independent variable; gallstones as the dependent variable; and BMI as the mediating variable. Path a represents the regression coefficient of the association between eGDR and BMI. Path b represents the regression coefficient of the association between BMI and gallstones. Path c represents the simple total effect of eGDR on gallstones. Path c’ represents the direct effect of eGDR on gallstones when controlling for BMI.
Sensitivity analysis
We performed a sensitivity analysis with missing covariates interpolated. The results of the sensitivity analysis were consistent with those of the main analysis. The eGDR, both as a continuous and categorical variable, was negatively associated with gallstones in sensitivity analyses. In the fully adjusted model, eGDR as a continuous variable was negatively associated with gallstones (OR = 0.93, 95% CI: 0.87, 1.00). eGDR values of 6–8 and the third quartile of eGDR were less likely for participants to have gallstones (OR = 0.61, 95% CI: 0.41, 0.91; OR = 0.61; OR = 0.69, 95% CI: 0.49, 0.99) (Table 6). Therefore, the results of sensitivity analyses showed that the conclusions drawn from this study were stable and reliable.
Table 6.
Logistic regression analysis between eGDR and gallstone prevalence in the sensitivity analysis.
| Characteristic | Model 1 OR (95%CI) | Model 2 OR (95%CI) | Model 3 OR (95%CI) |
|---|---|---|---|
| eGDR | 0.82 (0.78,0.85) | 0.83 (0.78,0.87) | 0.93 (0.87,1.00) |
| Categories | |||
| ≤ 4 mg/kg/min | 1.0 | 1.0 | 1.0 |
| 4–6 mg/kg/min | 0.62 (0.45,0.84) | 0.54 (0.39,0.75) | 0.84 (0.59,1.19) |
| 6–8 mg/kg/min | 0.38 (0.27,0.55) | 0.34 (0.23,0.49) | 0.61 (0.41,0.91) |
| > 8 mg/kg/min | 0.24 (0.18,0.33) | 0.26 (0.18,0.38) | 0.66 (0.43,1.01) |
| Categories | |||
| Q1 | 1.0 | 1.0 | 1.0 |
| Q2 | 0.52 (0.39,0.70) | 0.49 (0.36,0.66) | 0.74 (0.53,1.03) |
| Q3 | 0.37 (0.28,0.49) | 0.41 (0.30,0.55) | 0.69 (0.49,0.99) |
| Q4 | 0.23 (0.15,0.34) | 0.27 (0.17,0.43) | 0.72 (0.40,1.27) |
Model 1 was adjusted for no covariates; Model 2 was adjusted for age, gender, and race; Model 3 was adjusted for covariates in Model 2 + education, smoked, alcohol, physical activity, asthma, CHD, cancers, PIR, ALT, AST, TC, HDL and BMI were adjusted.
Discussion
This study analyzed a sample of 5,656 U.S. adults from the NHANES 2017–2020 database. For the first time, it reveals the correlation between eGDR and gallstones and the mediating role of BMI in it. The results indicated that the prevalence of gallstones in the U.S. population is about 10.52%. The eGDR was negatively associated with gallstone, showing that each unit increase in eGDR correlated with a 10% decrease in gallstone prevalence in a fully adjusted model. Curve fitting and threshold effect analysis revealed a nonlinear correlation between eGDR and gallstone, identifying a turning point of 9.81. Subgroup analyses showed that the negative correlation between eGDR and gallstones was more pronounced in the subgroups of females, those with a BMI ≥ 25, those without asthma, those without coronary artery disease, those without cancer, and those who were nonsmokers and nonalcohol drinkers. There was no interaction between the subgroups except for age. Overall, eGDR was negatively associated with the occurrence of gallstones, and this negative association was partially mediated by BMI. Importantly, eGDR measurements are readily available, making them valuable for predicting and diagnosing gallstones early.
Gallstones are solid particles formed by the coalescence of cholesterol, bilirubin, or calcium salts in the bile in the gallbladder or bile ducts. Gallstone formation is influenced by abnormal bile composition, gallbladder dysfunction, and metabolic disorders. With the prevalence of obesity rising sharply, the relationship between IR, obesity, and gallstones has become a hot research topic in recent years23. Wang et al. reported a linear positive correlation between the metabolic index score of insulin resistance (METS-IR) and the prevalence of gallstones, and that for every 1-unit increase in the METS-IR index, the age of the first gallbladder stone surgery was advanced by 0.26 years14. Wirth et al. demonstrated that healthy lifestyles, especially the maintenance of healthy weight, are quite important for the prevention of symptomatic gallstones through 2 prospective cohort studies24. Fu et al. further combined insulin resistance indexes with obesity indexes, to investigate the correlation between Triglyceride glucose (TyG ) index-related indexes and gallstone disease, and the results showed a positive correlation between TyG- BMI, TyG-WC, TyG-WHtR, and gallstone disease25. Moreover, hepatic insulin resistance and visceral obesity are likely key factors in the mechanisms by which insulin resistance promotes gallstone development. Previous animal experiments have shown that hepatic IR increases both the expression of the biliary cholesterol transport proteins Abcg5 and Abcg8, leading to increased biliary cholesterol secretion, and decreases the expression of bile acid synthase (especially Cyp7b1), leading to a rock bile salt-producing profile, which in turn leads to an increased susceptibility to cholesterol gallstones in mice suffering from hepatic IR26. Building on this foundation, Aydin et al. further explored the finding that hepatic IR is associated with the development of gallstones among Native Americans in the southwestern United States and provides a critical link between gallstone susceptibility and metabolic syndrome27. A retrospective cohort study showed that IR was associated with gallstone formation in postmenopausal Korean women with abdominal obesity, stratified by obesity28. Furthermore, in guinea pigs with IR, the use of insulin-sensitizing drugs (pioglitazone), such as metformin or thiazolidinediones (e.g., pioglitazone), prevented gallstone formation, liver injury, and gallbladder injury29. The mechanism may be related to improving insulin sensitivity and reducing the effects of hyperinsulinemia, which in turn reduces cholesterol supersaturation. A systematic evaluation demonstrated that prophylactic oral ursodeoxycholic acid (UDCA) after bariatric surgery reduced the risk of developing gallstones from 38 to 8% and reduced the need for cholecystectomy30. All of the above reports indicate that IR and obesity play a key role in the development of gallbladder stones and that improving IR and maintaining a good body image is important in the prevention and treatment of gallstones. eGDR is a simple index for assessing IR. In recent years, a large number of studies have shown that eGDR is closely related to cardiovascular and cerebrovascular diseases and renal diseases31–34. To date, no academic studies have reported a potential relationship between eGDR and gallstones. Our results suggest that low eGDR levels are associated with an increased rate of patients with gallstones.
Our findings are largely in line with other research that has demonstrated that obesity, metabolic syndrome, advanced age, and female gender are risk factors for gallstone prevalence35. We discovered that age had an impact on the negative correlation between gallstone prevalence and eGDR in the research using the interaction test. In a similar vein, Su et al. discovered that obesity and metabolic abnormalities were linked to a higher incidence of gallstones in young people36. This suggests that, in addition to concentrating on the senior population for gallstone prediction and prevention, we should also consider the young population with obesity and metabolic abnormalities. Numerous studies have supported the theory that the primary cause of women’s higher prevalence of gallstones than males is estrogen’s increased release of cholesterol in the liver, which causes the bile to become oversaturated with cholesterol14,37,38. Nonetheless, research continues to demonstrate that metabolic syndrome is linked to cholecystectomy and gallstone disease in men but not in women39. Li et al. discovered that among those with a BMI of more than 25, the triglyceride-glucose (TyG) index and the occurrence of gallstones were positively correlated40. Another study concluded that the metabolic imbalance associated with obesity is exacerbated by the interaction between gallstones and hepatic insulin resistance, which results in lipid and energy metabolism41.
Based on these findings, it is necessary to perform abdominal ultrasound for low eGDR, especially in young, female, and overweight populations, to screen for and diagnose gallstone disease at an early stage.
Our study’s strength is that it used a sample of adult Americans and adhered to a strict research process, which guaranteed the correctness of the findings. To ensure our results are applicable to a larger population, we also performed subgroup analyses and adjusted for confounding factors.
Limitations
Our study has several limitations. First, our cross-sectional study could not establish a link between eGDR and gallstones, highlighting the need for future prospective studies or clinical trials to validate this relationship. Second, a questionnaire that is susceptible to recall bias was used to diagnose gallbladder stones. Lastly, we did not analyze the associations between specific therapies and patients’ medication histories. Despite these limitations, we present evidence for the first time showing a correlation between eGDR and gallstone incidence. This suggests that eGDR may be a useful predictor of gallstone development.
Conclusion
This study demonstrates a correlation between low eGDR levels and a higher prevalence of gallstones, with BMI playing a mediating role. This implies that maintaining eGDR in the lower range and maintaining a good body image is critical for gallstone prevention. These studies still need to be validated in additional large prospective cohorts.
Acknowledgements
We thank all the editors and reviewers for their contributions to this paper!
Author contributions
Data analysis and manuscript writing: SY, YL. Study design and statistical advice: SY, YL, SM. Manuscript editing: SY, YL, SM. Validation and review: ZX. Quality control: SM. All authors reviewed the manuscript.
Funding
This work was supported by the Special Scientific Research Project of “Double First-class” Chinese Medicine in Henan Province (HSRP-DFCTCM-2023-3-20).
Data availability
Publicly available data sets were analyzed in this study. These data can be found at https://www.cdc.gov/nchs/nhanes.
Declarations
Competing interests
The authors declare no competing interests.
Informed consent
Informed consent was obtained from all subjects involved in the study.
Ethics Statement
The NCHS Research Ethics Review Committee approved the NHANES survey protocol (https://www.cdc.gov/nchs/nhanes/irba98.htm), and all participants of the study provided informed written consent. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
ShuaipengYuan and Yuexia Lu contributed equally to this work.
References
- 1.Wang, X. et al. Global Epidemiology of Gallstones in the 21st Century: A systematic review and Meta-analysis. Clin. Gastroenterol. Hepatol.22 (8), 1586–1595. 10.1016/j.cgh.2024.01.051 (2024). [DOI] [PubMed] [Google Scholar]
- 2.Unalp-Arida, A. & Ruhl, C. E. Increasing gallstone disease prevalence and associations with gallbladder and biliary tract mortality in the US. Hepatology77 (6), 1882–1895. 10.1097/HEP.0000000000000264 (2023). [DOI] [PubMed] [Google Scholar]
- 3.Song, Y. et al. Age, gender, geographic and clinical differences for gallstones in China: A nationwide study. Ann. Transl Med.10 (13), 735. 10.21037/atm-21-6186 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel, A. M., Yeola, M. & Mahakalkar, C. Demographic and risk factor Profile in patients of Gallstone Disease in Central India. Cureus14 (5), e24993. 10.7759/cureus.24993 (2022). Published 2022 May 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xia, Q., Liu, Q. & Ma, X. Intestinal microbiota in biliary diseases. Curr. Opin. Gastroenterol.39 (2), 95–102. 10.1097/MOG.0000000000000910 (2023). [DOI] [PubMed] [Google Scholar]
- 6.Lammert, F. et al. Gallstones. Nat. Rev. Dis. Primers. 2, 16024. 10.1038/nrdp.2016.24 (2016). Published 2016 Apr 28. [DOI] [PubMed] [Google Scholar]
- 7.Stinton, L. M., Myers, R. P. & Shaffer, E. A. Epidemiology of gallstones. Gastroenterol. Clin. North. Am.39 (2), 157–vii. 10.1016/j.gtc.2010.02.003 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Shabanzadeh, D. M. Incidence of gallstone disease and complications. Curr. Opin. Gastroenterol.34 (2), 81–89. 10.1097/MOG.0000000000000418 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Echouffo-Tcheugui, J. B. et al. Insulin resistance and N-Terminal Pro-B-Type natriuretic peptide among healthy adults. JAMA Cardiol.8 (10), 989–995. 10.1001/jamacardio.2023.2758 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill, M. A. et al. Insulin resistance, cardiovascular stiffening, and cardiovascular disease. Metabolism119, 154766. 10.1016/j.metabol.2021.154766 (2021). [DOI] [PubMed] [Google Scholar]
- 11.Tagi, V. M., Mainieri, F. & Chiarelli, F. Hypertension in patients with insulin resistance: Etiopathogenesis and management in children. Int. J. Mol .Sci.23(10), 5814. (2022). 10.3390/ijms23105814 [DOI] [PMC free article] [PubMed]
- 12.Buzzetti, E., Pinzani, M. & Tsochatzis, E. A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism65 (8), 1038–1048. 10.1016/j.metabol.2015.12.012 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Nervi, F. et al. Gallbladder disease is associated with insulin resistance in a high risk hispanic population. J. Hepatol.45 (2), 299–305. 10.1016/j.jhep.2006.01.026 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Wang, J. et al. Association of METS-IR index with prevalence of gallbladder stones and the age at the first gallbladder stone surgery in US adults: A cross-sectional study. Front. Endocrinol. (Lausanne). 13, 1025854. 10.3389/fendo.2022.1025854 (2022). Published 2022 Oct 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Erpecum, K. J. Pathogenesis of cholesterol and pigment gallstones: An update. Clin. Res. Hepatol. Gastroenterol.35 (4), 281–287. 10.1016/j.clinre.2011.01.009 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Shaffer, E. A. Gallstone disease: Epidemiology of gallbladder stone disease. Best Pract. Res. Clin. Gastroenterol.20 (6), 981–996. 10.1016/j.bpg.2006.05.004 (2006). [DOI] [PubMed] [Google Scholar]
- 17.Xiao, D. et al. Assessment of six surrogate insulin resistance indexes for predicting cardiometabolic multimorbidity incidence in Chinese middle-aged and older populations: Insights from the China health and retirement longitudinal study. Diabetes Metab. Res. Rev.40 (1), e3764. 10.1002/dmrr.3764 (2024). [DOI] [PubMed] [Google Scholar]
- 18.Orchard, T. J. et al. Insulin resistance-related factors, but not glycemia, predict coronary artery disease in type 1 diabetes: 10-year follow-up data from the Pittsburgh Epidemiology of Diabetes complications Study. Diabetes Care. 26 (5), 1374–1379. 10.2337/diacare.26.5.1374 (2003). [DOI] [PubMed] [Google Scholar]
- 19.Shabanzadeh, D. M., Sørensen, L. T. & Jørgensen, T. Determinants for gallstone formation - A new data cohort study and a systematic review with meta-analysis. Scand. J. Gastroenterol.51 (10), 1239–1248. 10.1080/00365521.2016.1182583 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Zabala, A. et al. Estimated glucose disposal rate and risk of stroke and mortality in type 2 diabetes: A nationwide cohort study. Cardiovasc. Diabetol.20(1), 202. 10.1186/s12933-021-01394-4 (2021). [DOI] [PMC free article] [PubMed]
- 21.Cheng, Q. et al. Association of non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) and gallstones among US adults aged ≤ 50 years: A cross-sectional study from NHANES 2017–2020. Lipids Health Dis.23 (1), 265. 10.1186/s12944-024-02262-2 (2024). Published 2024 Aug 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du, W. et al. Diabetes Mellitus mediates the Relationship between Atherogenic Index of plasma and gallstones: A Population-based cross-sectional study. Diabetes Metab. Syndr. Obes.17, 317–332. 10.2147/DMSO.S449562 (2024). Published 2024 Jan 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Méndez-Sánchez, N. et al. Metabolic syndrome as a risk factor for gallstone disease. World J. Gastroenterol.11 (11), 1653–1657. 10.3748/wjg.v11.i11.1653 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wirth, J. et al. A healthy lifestyle pattern and the risk of symptomatic gallstone disease: Results from 2 prospective cohort studies. Am. J. Clin. Nutr.112 (3), 586–594. 10.1093/ajcn/nqaa154 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu, C. et al. Association between triglyceride glucose index-related indices with gallstone disease among US adults. Lipids Health Dis.23 (1), 203. 10.1186/s12944-024-02194-x (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biddinger, S. B. et al. Hepatic insulin resistance directly promotes formation of cholesterol gallstones. Nat. Med.14 (7), 778–782. 10.1038/nm1785 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aydin, B. N. et al. Hepatic insulin resistance increases risk of Gallstone Disease in Indigenous americans in the Southwestern United States. Clin. Transl. Gastroenterol.. 2110.14309/ctg.0000000000000763 (2024). [DOI] [PMC free article] [PubMed]
- 28.Kim, S. S. et al. Insulin resistance as a risk factor for gallbladder stone formation in Korean postmenopausal women. Korean J. Intern. Med.26 (3), 285–293. 10.3904/kjim.2011.26.3.285 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han, T. et al. Pioglitazone prevents cholesterol gallstone formation through the regulation of cholesterol homeostasis in guinea pigs with a lithogenic diet. Lipids Health Dis.18 (1), 218. 10.1186/s12944-019-1159-4 (2019). Published 2019 Dec 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma, A., Shanti, H., Nageswaran, H., Best, L. M. J. & Patel, A. G. Role of Ursodeoxycholic Acid in the Prevention of gallstones formation in bariatric Patients-A systematic review and meta-analysis of randomised trials. Obes. Surg.33 (12), 4115–4124. 10.1007/s11695-023-06893-9 (2023). [DOI] [PubMed] [Google Scholar]
- 31.Zhang, Z., Zhao, L., Lu, Y., Xiao, Y. & Zhou, X. Insulin resistance assessed by estimated glucose disposal rate and risk of incident cardiovascular diseases among individuals without diabetes: Findings from a nationwide, population based, prospective cohort study. Cardiovasc. Diabetol.23 (1), 194. 10.1186/s12933-024-02256-5 (2024). Published 2024 Jun 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu, Z. et al. Insulin resistance estimated by estimated glucose disposal rate predicts outcomes in acute ischemic stroke patients. Cardiovasc Diabetol.22(1), 225. 10.1186/s12933-023-01925-1 (2023). [DOI] [PMC free article] [PubMed]
- 33.Ebert, T. et al. Outcomes with finerenone in patients with chronic kidney disease and type 2 diabetes by baseline insulin resistance. Diabetes Care. 47 (3), 362–370. 10.2337/dc23-1420 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Penno, G. et al. Insulin resistance, diabetic kidney disease, and all-cause mortality in individuals with type 2 diabetes: A prospective cohort study. BMC Med.19 (1), 66. 10.1186/s12916-021-01936-3 (2021). Published 2021 Mar 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun, H. et al. Factors influencing gallstone formation: A review of the literature. Biomolecules12 (4), 550. 10.3390/biom12040550 (2022). Published 2022 Apr 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su, P. Y., Hsu, Y. C., Cheng, Y. F., Kor, C. T. & Su, W. W. Strong association between metabolically-abnormal obesity and gallstone disease in adults under 50 years. BMC Gastroenterol.19(1), 117 10.1186/s12876-019-1032-y (2019). [DOI] [PMC free article] [PubMed]
- 37.Wang, J. et al. Relationship of triglyceride-glucose index to gallstone prevalence and age at first gallstone surgery in American adults. Sci. Rep.14 (1), 16749. 10.1038/s41598-024-67883-0 (2024). Published 2024 Jul 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lavoie, J. M. Dynamics of hepatic and intestinal cholesterol and bile acid pathways: The impact of the animal model of estrogen deficiency and exercise training. World J. Hepatol.8 (23), 961–975. 10.4254/wjh.v8.i23.961 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baratta, F. et al. Sex-related differences in the Association between Metabolic Syndrome and Gallstone Disease. Int. J. Environ. Res. Public. Health. 18 (4), 1958. 10.3390/ijerph18041958 (2021). Published 2021 Feb 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li, H. & Zhang, C. Association between triglyceride-glucose index and gallstones: A cross-sectional study. Sci. Rep.14 (1), 17778. 10.1038/s41598-024-68841-6 (2024). Published 2024 Aug 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cortés, V. A., Barrera, F. & Nervi, F. Pathophysiological connections between gallstone disease, insulin resistance, and obesity. Obes. Rev.21 (4), e12983. 10.1111/obr.12983 (2020). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Publicly available data sets were analyzed in this study. These data can be found at https://www.cdc.gov/nchs/nhanes.