Abstract
In addition to being linked to an excess of lipid accumulation in the liver, being overweight or obese can also result in disorders of lipid metabolism. There is limited understanding regarding whether different levels of protein intake within an energy-restricted diet affect liver lipid metabolism in overweight and obese rats and whether these effects differ by gender, despite the fact that both high protein intake and calorie restriction can improve intrahepatic lipid. The purpose of this study is to explore the effects and mechanisms of different protein intakes within a calorie-restricted diet on liver lipid metabolism, and to investigate whether these effects exhibit gender differences. The Sprague-Dawley rats, which were half female and half male, were used to construct a rat model of overweight and obesity attributed to a high-fat diet. They were then split up into five groups: the normal control (NC) group, the model control (MC) group, the calorie-restricted low protein (LP) group, the calorie-restricted normal protein (NP) group, and the calorie-restricted high protein (HP) group. Body weight was measured weekly. Samples of plasma and liver were obtained after eight weeks and analyzed for glucose, triglycerides, cholesterol, and hormones in the plasma as well as the liver fat and factors involved in the liver’s synthesis and degradation. For the male rats, compared to the HP group, the weight of liver fat in the LP and NP group was significantly higher (P < 0.05). However, for the female rats, there was no significant variation among the three calorie-restricted groups (P > 0.05). There was no significant variation in the concentration of total cholesterol (TC), very low density lipoprotein (VLDL), low density lipoprotein (LDL), and high density lipoprotein (HDL) among the three male calorie-restricted groups (P > 0.05), while the TC and VLDL concentrations in the female LP and NP group were significantly higher compared to those in the HP group (P < 0.05). Moreover, the trend of expression in the signaling pathways of adiponectin/phosphorylated AMP-activated protein kinase (p-AMPK) and adiponectin/peroxisome proliferators-activated receptor alpha (PPARα) in the liver was consistent with that of the liver fat content, and leptin acted in the same way as adiponectin. Compared with the three calorie-restricted groups, the expressions of nuclear sterol-regulatory element-binding protein-2 (nSREBP-2) and 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-CoA reductase) involved in cholesterol synthesis and low-density lipoprotein receptor (LDLR) and cholesterol 7-alpha hydroxylase (CYP7A1) involved in cholesterol clearance in the MC group were significantly lower (P < 0.05). A 40% energy restriction can significantly reduce the body weight, body fat, liver fat, and the blood concentration of TG in both male and female overweight and obese rats, but it can significantly increase the blood concentration of TC in overweight and obese male rats. At the same time of 40% calorie restriction, increasing dietary protein intake to twice the normal protein intake has a stronger effect on promoting hepatic triglyceride oxidation and reducing liver fat content in the male overweight and obese rats by increasing the levels of adiponectin and leptin in the blood, and can also significantly reduce the plasma cholesterol concentration in the female overweight and obese rats through inhibiting cholesterol synthesis most likely by increasing glucagon level in the blood.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-86596-6.
Keywords: Protein intake, Calorie restriction, Lipid metabolism, Liver, Overweight, Obesity, Rat
Subject terms: Obesity, Fatty acids, Fat metabolism, Obesity
Introduction
In the past 40 years, the prevalence of obesity has quadrupled, placing a significant strain on people’s health1. Obesity or overweight is not only associated with excess lipid accumulation in the liver2, but also can lead to abnormalities of lipid metabolism, resulting in cardiovascular and cerebrovascular diseases3. The liver is an important metabolic organ that carries out intrahepatic metabolism of lipid and glucose, which is crucial for controlling the plasma concentrations of lipid and glucose4. Therefore, regulating the metabolism of glucose and lipid in the liver can not only improve the fatty liver of overweight and obese people, but also reduce the risk of cardiovascular and cerebrovascular diseases.
Calorie restriction is currently recognized as an effective way to lose weight and improve intrahepatic lipid of overweight and obese people. The study by Johansson et al. indicated that providing a low-energy diet (800–1100 kcal/day, with carbohydrates accounting for 52%, protein for 25%, and fat for 21%) to ten obese women for 28 days could linearly reduce liver fat content and improve insulin resistance5. The study only restricted the energy intake of the obese women without adjusting the energy contribution ratio of protein, fat, and carbohydrates, hence the intakes of these three nutrients were all below the normal level. The results indicate that the increase in markers associated with fat breakdown and oxidation, such as non-esterified fatty acids and 3-hydroxybutyrate, demonstrates that low energy intake can reduce liver fat content by promoting the decomposition of liver fat. Furthermore, increasing protein intake while calorie restriction was shown to prevent the accumulation of liver fat in both rodent animals and humans. For example, an animal study with female obese Zucker rats (Leprfa+/fa+) showed that substituting dietary carbohydrate (from 76 to 56%) with protein (from 15 to 35%) can result in decrease in the amount of triglycerides (TG) present in the liver by lowing TG produced, especially in animals under 40% calorie restriction6. However, the Zucker rats in the study are obese-prone rats due to a mutation in the leptin receptor gene, whereas the most common cause of obesity in humans is excessive energy intake from diet, so this animal model does not adequately represent human obesity. It is necessary to establish a diet-induced obese rat model to conduct studies on energy restriction with high protein intake. However, we have not yet found any literature that reports on the study of liver lipid metabolism in rats with such an experimental design. Therefore, it is currently unclear whether the mechanisms of energy restriction without changing the nutrient composition and energy restriction with increasing protein intake have consistent effects on liver lipid metabolism in overweight and obese individuals. Additionally, the liver is a sexually dimorphic organ, and both its lipid metabolism and blood lipid profiles exhibit sexual dimorphism7. Both estrogen and androgen play a protective role in liver lipid metabolism. They inhibit the de novo synthesis of TG and cholesterol in the liver, increases fatty acid oxidation, and promotes the export of liver TG as very low-density lipoprotein (VLDL) out of the liver8,9. Our previously published article has reported that in the normal control group, model control group, calorie-restricted low protein group, calorie-restricted normal protein group and calorie-restricted high protein group, the levels of plasma testosterone and estradiol in the male Sprague-Dawley (SD) rats are higher than those in the female rats10. Based on this, we speculate that the amount of liver fat deposition in the male rats may be less than that in the female. However, whether energy restriction combined with increased protein intake will have a better improving effect on the liver lipid metabolism in the male rats is uncertain, which is also a problem we aim to clarify through this study.
In addition, there is a complex interrelationship among the three macronutrients of protein, fat, and carbohydrate in the biochemical metabolism process. Because they share receptor signaling and intermediate metabolites, the anabolic and catabolic pathways are challenging to distinguish from one another in the liver4. For the purpose of managing weight loss, it was discovered that the total amount of protein consumed mattered more than the proportion of calories11. Because if the protein intake is calculated according to the energy supply ratio, when the energy intake is very low, even if the protein supply ratio is at a normal level, the actual protein intake is lower than the physiological requirement. Thus, for overweight or obese individuals, real protein consumption during weight reduction through calorie restriction is more informative. But it is currently unclear whether different protein intakes—below, equal to, and above the normal protein intake—on the basis of energy restriction have different impacts on the anabolism and catabolism of liver lipids.
Therefore, in the present study, diet-induced overweight and obese rats were used as the subjects, with the actual protein intakes as the basis for grouping in the energy restriction rats. The objectives were: (1) to investigate whether there are different effects on liver lipid metabolism under the state of energy intake restriction in overweight and obese rats with three levels of dietary protein intake—below, equal to, and above the normal dietary protein intake; (2) to determine if these impact exhibit sexual dimorphism.
Materials and methods
Animals and diets
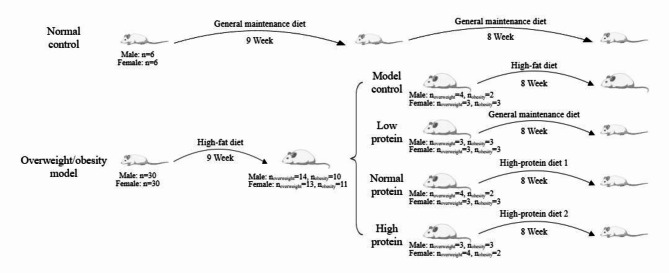
The animals used in this investigation and their diets matched those in our previous published article10. Briefly, thirty-six male and thirty-six female 6-week-old SD rats (Sippr-BK Laboratory Animals, Shanghai, China) were raised three per cage, with a light-dark cycle of twelve hours and a controlled temperature of 22 ± 2 °C and 55 ± 15% relative air humidity. As two normal controls (NC), six female rats and six male rats were chosen at random and given a normal maintenance diet12. A high-fat diet was given to the other rats for 9 weeks. After that, rats whose body weight was more than 10-20% of the average body weight of the NC group were classified as “overweight”, while those with a body weight over 20% of the average body weight of the NC group were classified as “obese”. Eight groups, each with six rats, were selected at random from the overweight and obese rats according to sex, ensuring that there was no statistical difference in body weight among the groups of the same gender: the model control groups (MC) for both sexes which were fed high-fat diets, the calorie-restricted low protein groups (LP) for both sexes which were fed normal maintenance diets, the calorie-restricted normal protein groups (NP) for both sexes which were fed high-protein diet 1 and the calorie-restricted high protein groups (HP) for both sexes which were fed high-protein diet 2 (Fig. 1). The composition, primary nutrient content, and energy content of the diets were all identical to those in our previous study10 (Appendix 1 and Appendix 2).
Fig. 1.
Experimental grouping schedule.
The feed intakes of rats were consistent with our previous study as well10. Briefly, free access to water and food was available to the rats in the NC and MC group. However, the calorie intake of the LP, NP and HP group was 60% of that in the NC group. The protein intake of LP, NP, and HP was 60%, 100%, and 200% of that of the NC group, respectively. On the first day, calorie intake for the NC group was computed. While the dietary intakes on the next day for the LP, NP and HP group were calculated according to the following formula (1):
 |
1 |
Iintervention: Intake of diet for the LP, NP, or HP group;
INC: Intake of diet for the NC group;
ECNC: Energy content of the diet in the NC group;
ECintervention: Energy content of the diet in the LP, NP, or HP group.
Weekly measurements of body weight were made. All rats were fasted for 12 h and given 20% Zoletil to sedate at the end of the 17th week. An abdominal aorta puncture was performed to obtain their blood. After being separated using low-speed centrifugation, the plasma was kept at -80 °C for storage. After being surgically excised, the liver and the white adipose from subcutaneously, retroperitoneally, around the groin and epididymis were weighed, instantly frozen in liquid nitrogen, and preserved at -80 ºC for further investigations.
Measurement of the plasma parameters and the liver fat
The concentration of glucose, total cholesterol (TC), TG, low density lipoprotein (LDL), high density lipoprotein (HDL), and VLDL in the plasma were measured with Hitachi 7020 automatic biochemical analyzer (Hitachi High-tech Co., Ltd., Japan). The concentration of insulin, glucagon, adiponectin and leptin in the plasma was measured using ELISA kits in accordance with the manufacturer’s instructions (Catalog NOs. HB127-Ra, HB112-Ra, HB057-Ra, HB349-Ra, respectively; Hengyuan Biotechnology Co., LTD, Shanghai, China). The content of fat in the liver was measured by the Soxhelt extraction method13.
Glycemic homeostasis was evaluated utilizing the homeostatic model assessment of insulin resistance (HOMA-IR) and β-cell function (HOMA-β), where14:
 |
2 |
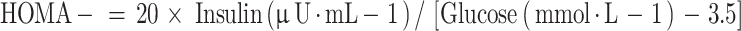 |
3 |
Measurement of the factors associated with TG metabolism in the liver
TG synthesis and degradation-related components in the liver were measuring using ELISA kits in accordance with the manufacturer’s instructions (Hengyuan Biotechnology Co., LTD, Shanghai, China). The details were as follows: the tissue was washed with pre-cooled PBS (0.01 M, pH = 7.4) to remove residual blood and then cut into pieces. The shredded tissue was weighed. Subsequently, the tissue and the corresponding volume of PBS (generally 1:9, such as 1 g tissue sample corresponding to 9 mL PBS) were mixed into glass homogenizer and well ground on ice. Finally, the homogenate was centrifuged at 5000 r for 5 ~ 10 min, and then the supernatant could be detected. Results multiplied by dilution multiple (×5). Factors associated with TG synthesis were: phosphorylated AMP-activated protein kinase (p-AMPK, Catalog NO. HB1291-Ra), nuclear sterol-regulatory element-binding protein-1c (nSREBP-1c, Catalog NO. HB1288-Ra), carbohydrate response element binding protein (ChREBP, Catalog NO. HB1402-Ra), acetyl-CoA carboxylase (ACC, Catalog NO. HB1289-Ra), fatty acid synthase (FAS, Catalog NO. HB058-Ra). Factors associated with the fatty acids oxidation were: peroxisome proliferators-activated receptor alpha (PPAR-α, Catalog NO. HB735-Ra), carnitine palmitoyl transterase-1 A (CPT-1 A, Catalog NO. HB404-Ra).
Measurement of the factors associated with TC metabolism in the liver
TG synthesis and degradation-related components in the liver were measuring using ELISA kits in accordance with the manufacturer’s instructions (Hengyuan Biotechnology Co., LTD, Shanghai, China). Factors associated with TC synthesis were: nuclear sterol-regulatory element-binding protein-2 (nSREBP-2, Catalog NO. HB865-Ra), 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-CoA reductase, Catalog NO. HB1078-Ra). Factors associated with TC degradation were: cholesterol 7-alpha hydroxylase (CYP7A1, Catalog NO. HB867-Ra), low-density lipoprotein receptor (LDLR, Catalog NO. HB846-Ra).
Statistical analysis
For all variables having a normal distribution, the quantitative data were shown as the mean ± standard deviation. Moreover, one-way analysis of variance was used. The least-square difference method was utilized for post hoc analysis if a significant difference (P < 0.05) was found. SPSS version 20 (IBM, Armonk, New York, USA) was utilized to analyze the data.
Ethical approval and consent to participate
All procedures and animal care were approved by the Ethical Committee for Laboratory Animals of the Yangzhou University (201809.001) and performed in accordance with ARRIVE guidelines, and the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health.
Results
Body weight, white adipose weight and liver weight of rats
Between the NC and MC groups, there was no significant difference in the initial body weight (P > 0.05). After modeling, compared to the NC group, the body weight of the MC group was significantly higher (P < 0.05). Among them, there were 14 overweight male rats, 13 overweight female rats, 10 obese male rats and 11 obese female rats (513.60 ± 16.21 g, 270.17 ± 6.00 g, 596.40 ± 40.02 g, 294.13 ± 8.39 g, respectively). Among the three calorie-restricted groups, there was no significant difference in weight (P > 0.05). Moreover, compared to the female rats, the body weight of the male rats was significantly higher (P < 0.05) (Fig. 2).
Fig. 2.
Changes in body weight of rats during intervention. (a) Body weight of male rats. (b) Body weight of female rats. Mean values of the same sex with different symbols (*, & and #) were significantly different (P < 0.05).
Table 1 showed the weight of white adipose, liver and liver fat and the proportion of the weight of liver fat to the weight of liver in different groups. For both the male and the female groups, the above four indexes in the MC group were notably higher compared with those in the other four groups (P < 0.05). And the above four indicators of the three male calorie-restricted groups were all significantly lower than those of the NC group (P < 0.05). However, for the calorie-restricted female rats, except for the weight of white fat which was significantly lower than that of the NC group (P < 0.05), the other three indicators showed no significant difference compared to that of the NC group (P > 0.05). Among the three energy-restricted groups, the weight of liver fat in the male HP group was significantly lower compared to that in the LP and NP groups (P < 0.05), and the same statistical difference was showed in the index of ratio of liver fat weight to liver weight (P < 0.05), but there was no significant differences in the weight of liver and liver fat for the three energy-restricted female groups (P > 0.05). Furthermore, the weights of white adipose, liver and liver fat of the male rats were all significantly higher compared with those of the female (P < 0.05).
Table 1.
Weight of white adipose tissue, liver and liver fat.
| White adipose tissue (g) | Liver (g) | Liver fat (mg) | Liver fat/ Liver (%) | ||
|---|---|---|---|---|---|
| NC | 27.28 ± 4.39b | 13.44 ± 1.32b | 113.83 ± 27.51c | 0.85 ± 0.20c | |
| MC | 46.28 ± 11.41c | 18.16 ± 2.27c | 647.19 ± 182.40d | 3.56 ± 1.00d | |
| Male | LP | 14.33 ± 4.12a | 10.36 ± 0.97a | 44.74 ± 6.52b | 0.43 ± 0.06b |
| NP | 15.47 ± 6.63a | 10.65 ± 0.91a | 42.72 ± 6.41b | 0.40 ± 0.06b | |
| HP | 12.25 ± 1.16a | 10.51 ± 0.42a | 23.34 ± 4.34a | 0.22 ± 0.04a | |
| NC | 10.09 ± 4.24b | 6.67 ± 0.79a | 22.70 ± 5.31a | 0.34 ± 0.08a | |
| MC | 16.92 ± 3.28c | 9.01 ± 0.69b | 103.43 ± 6.32b | 1.14 ± 0.09b | |
| Female | LP | 1.84 ± 0.87a | 5.53 ± 0.63a | 20.14 ± 1.22a | 0.36 ± 0.02a |
| NP | 3.80 ± 1.82a | 5.77 ± 0.53ab | 19.73 ± 2.49a | 0.34 ± 0.04a | |
| HP | 3.24 ± 1.14a | 5.97 ± 1.11ab | 19.28 ± 3.71a | 0.32 ± 0.06a |
NC: normal control group, MC: model control group, LP: calorie-restricted low protein group, NP: calorie-restricted normal protein group, HP: calorie-restricted high protein group. Values are the mean ± SD. Mean values of the same sex within a column with different superscript letters (a, b, c, d) were significantly different (P < 0.05).
Intakes of diets, calorie and macronutrients
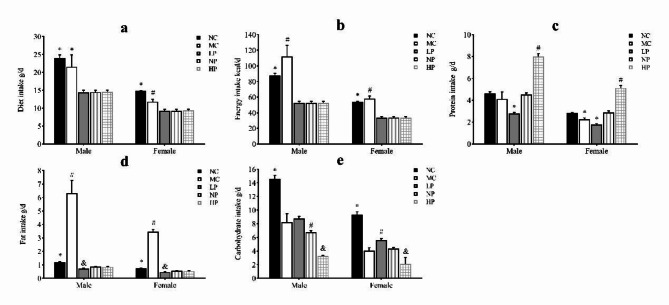
Compared with the three calorie-restricted groups, the intakes of diets, calorie and fat in the MC and NC groups were significantly higher (P < 0.05). As the protein intake increased, the intake of carbohydrate in the three groups declined. Moreover, compared with the female rats, the intakes of diets, calorie and macronutrients of the male rats were significantly higher (P < 0.05) (Fig. 3).
Fig. 3.
Intakes of diets, energy and macronutrients of rats in different groups. (a) Statistical analysis of diet intake. (b) Statistical analysis of energy intake. (c) Statistical analysis of protein intake. (d) Statistical analysis of fat intake. (e) Statistical analysis of carbohydrate intake. Mean values of the same sex with different superscript symbols (#, * and &) were significantly different (P < 0.05).
Plasma parameters
Table 2 showed the glucose metabolic profile in the plasma in each group. While there was no significant difference observed in the plasma glucose concentration for the female groups (P > 0.05), the plasma glucose concentration in the male MC group was significantly higher than that in the other groups (P < 0.05). The trend of insulin concentration in the male groups was similar to that in the female groups, that is, compared to the MC group, the insulin concentrations in the NP, HP, and NC groups were significantly higher (P < 0.05). Especially, the insulin concentration in the NP and HP groups had reached or was close to that of the NC group. For both the male and the female rats, HOMA-IR in the LP group was the lowest compared with that in the other groups, while the HOMA-IR of the NP and HP groups showed no significant difference compared to that of the NC group (P > 0.05). Moreover, the trend of HOMA-β was consistent in both sexes, i.e., compared to the other groups, HOMA-β in the MC group was significantly lower (P < 0.05) and the HOMA-β of the three calorie-restricted groups showed no significant difference compared to that of the NC group (P > 0.05). Compared to the other groups, glucagon concentration in the MC group of both sexes was significantly higher than that in other groups (P < 0.05). And for both sexes, although the glucagon concentration in the three calorie-restricted groups had decreased compared to that of the MC group, it still remained significantly higher than that in the NC group (P < 0.05).
Table 2.
Glucose metabolic profile in the plasma.
| Glucose (mmol/L) |
Insulin (mU/L) |
HOMA-IR | HOMA-β | Glucagon (pg/ml) |
||
|---|---|---|---|---|---|---|
| NC | 10.00 0.53a 0.53a
|
27.88 0.33a 0.33a
|
12.93 ± 2.42a | 76.44 ± 11.22a | 24.26 8.75a 8.75a
|
|
| MC | 12.15 1.45b 1.45b
|
19.90 0.49b 0.49b
|
10.42 ± 1.81a | 45.07 ± 5.66b | 81.44 12.18b 12.18b
|
|
| Male | LP | 9.75 1.30a 1.30a
|
16.22 0.64c 0.64c
|
7.32 ± 1.38b | 55.30 ± 10.30ab | 52.56 8.48c 8.48c
|
| NP | 10.33 0.93a 0.93a
|
24.19 0.69d 0.69d
|
10.79 ± 0.64a | 70.29 ± 12.07a | 56.05 8.47c 8.47c
|
|
| HP | 9.93 1.12a 1.12a
|
23.08 0.83d 0.83d
|
11.43 ± 2.41a | 65.54 ± 22.41ab | 57.21 16.58c 16.58c
|
|
| NC | 7.76 1.17a 1.17a
|
20.76 1.12a 1.12a
|
7.14 ± 0.96a | 106.89 ± 44.07a | 30.54 2.34a 2.34a
|
|
| MC | 8.47 1.41a 1.41a
|
15.74 1.21b 1.21b
|
5.88 ± 0.70b | 62.66 ± 19.85b | 60.41 9.21b 9.21b
|
|
| Female | LP | 7.37 1.28a 1.28a
|
16.59 0.86b 0.86b
|
5.42 ± 0.90b | 96.32 ± 39.68ab | 40.84 7.27a, c 7.27a, c
|
| NP | 7.35 0.59a 0.59a
|
21.48 1.39a 1.39a
|
7.03 ± 0.86a | 113.60 ± 17.15a | 49.45 7.41c, d 7.41c, d
|
|
| HP | 8.13 0.58a 0.58a
|
21.02 1.11a 1.11a
|
7.59 ± 0.59a | 91.96 ± 12.88ab | 55.63 10.18d 10.18d
|
NC: normal control group, MC: model control group, LP: calorie-restricted low protein group, NP: calorie-restricted normal protein group, HP: calorie-restricted high protein group. Values are the mean ± SD. Mean values of the same sex within a column with different superscript letters (a, b, c, d) were significantly different (P < 0.05).
Table 3 showed the plasma concentrations of TG, TC, VLDL, LDL, HDL, adiponectin and leptin in different groups. For the male groups, there was no significant difference in all of the plasma lipid concentrations between the NC and MC groups (P > 0.05). The TG concentrations in the three calorie-restricted groups were all significantly lower than those in the NC and MC groups (P < 0.05), while the concentrations of TC, VLDL, LDL, and HDL in the three calorie-restricted groups were all significantly higher than those in the NC and MC groups (P < 0.05). For the female groups, the TG concentration in the MC group was significantly higher compared to that in the NC group (P < 0.05). However, there was no significant difference in the concentration of other lipids between the NC and MC group (P > 0.05). The TG concentrations in the three calorie-restricted groups all decreased to a level with no significant difference compared to that of the NC group (P > 0.05), which was consistent with the male rats. The concentrations of TC, VLDL and LDL in the MC group were not significantly different from those in the LP and HP group (P > 0.05). However, compared to the MC and NC groups, the concentrations of TC, VLDL, LDL, and HDL in the NP group were all significantly higher (P < 0.05). Furthermore, compared with the NP group, the concentrations of TC, VLDL, LDL, and HDL in the HP group were significantly lower (P < 0.05). As to the adiponectin, for both sexes, the concentration of the MC group was significantly lower than the other groups (P < 0.05). Moreover, in the energy-restricted groups, the adiponectin concentration in the female rats reached the level of the NC group (P > 0.05), and the adiponectin concentration in the male rats was significantly higher than that of the NC group (P < 0.05). Regarding the leptin, the concentration in both the male and the female MC groups was significantly higher than that in the other groups (P < 0.05), and energy restriction significantly reduced the leptin concentration for both sexes (P < 0.05), reaching or approaching the levels of the NC group.
Table 3.
Concentration of lipids, adiponectin and leptin in the plasma.
| TG (mmol/L) |
TC (mmol/L) |
VLDL (mmol/L) |
LDL (mmol/L) |
HDL (mmol/L) |
Adiponectin (µg/L) |
Leptin (µg/L) |
||
|---|---|---|---|---|---|---|---|---|
| NC | 1.16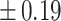 a, c a, c
|
1.45 0.06a 0.06a
|
0.20 a a
|
0.74 a a
|
0.51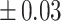 a a
|
80.56 ± 1.31a | 3.58 ± 0.58a | |
| MC | 1.24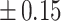 a a
|
1.35 0.40a 0.40a
|
0.14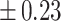 a a
|
0.76 0.08a 0.08a
|
0.45 0.10a 0.10a
|
57.07 ± 4.31b | 6.90 ± 0.77d | |
| Male | LP | 1.01 bc bc
|
2.19 b b
|
0.57 b b
|
0.95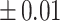 b b
|
0.67 b b
|
79.27 ± 7.11a | 4.06 ± 0.58a, b |
| NP | 0.97 0.10bc 0.10bc
|
2.21 b b
|
0.57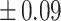 b b
|
0.98 b b
|
0.67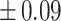 b b
|
87.82 ± 5.15c | 4.57 ± 0.60b, c | |
| HP | 0.93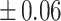 b b
|
2.03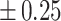 b b
|
0.46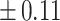 b b
|
0.95 0.10b 0.10b
|
0.62 b b
|
91.39 ± 4.83c | 5.05 ± 0.49c | |
| NC | 0.92 0.05a 0.05a
|
1.97 0.33ab 0.33ab
|
0.49 0.19b 0.19b
|
0.77 0.05a 0.05a
|
0.71 0.10 ab 0.10 ab
|
104.41 ± 3.50b | 5.28 ± 0.63a | |
| MC | 1.12 0.08b 0.08b
|
1.82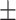 0.23ab 0.23ab
|
0.41 0.12ab 0.12ab
|
0.77 0.05a 0.05a
|
0.65 0.07a 0.07a
|
89.96 ± 5.77a | 6.95 ± 0.69b | |
| Female | LP | 0.94 0.03a 0.03a
|
2.14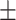 0.27bc 0.27bc
|
0.57 0.17bc 0.17bc
|
0.80 0.04ab 0.04ab
|
0.76 0.07b 0.07b
|
108.06 ± 12.17b | 4.95 ± 0.20a |
| NP | 0.93 0.03a 0.03a
|
2.37 0.32cd 0.32cd
|
0.67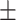 0.17c 0.17c
|
0.84 0.05b 0.05b
|
0.87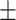 0.10c 0.10c
|
107.79 ± 10.08b | 5.03 ± 0.73a | |
| HP | 0.89 0.05a 0.05a
|
1.74 0.20a 0.20a
|
0.30 0.08a 0.08a
|
0.75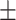 0.03a 0.03a
|
0.75 0.05b 0.05b
|
106.01 ± 5.16b | 5.64 ± 0.44a |
NC: normal control group, MC: model control group, LP: calorie-restricted low protein group, NP: calorie-restricted normal protein group, HP: calorie-restricted high protein group. Values are the mean ± SD. Mean values of the same sex within a column with different superscript letters (a, b, c, d) were significantly different (P < 0.05).
Factors associated with TG metabolism in the liver
Figure 4 showed the concentrations of the factors associated with TG metabolism in the liver. As to the factors involved in TG synthesis, the concentration of the factors in the male and female rats showed the same trend, i.e., the concentration of p-AMPK in the three energy-restricted groups was significantly higher than that in the MC group (P < 0.05) and increased to a level where there was no significant difference compared to the NC group (P > 0.05); and the concentrations of ChREBP, nSREBP-1c, ACC and FAS in the three calorie-restricted groups were significantly lower than those in the MC group (P < 0.05) and reached or approached the level of the NC group. As to the factors involved in fatty acids oxidation, there was also the same trend in the concentrations of PPARα and CPT1A for both sexes, i.e., compared with those in the MC group, the concentrations in the three calorie-restricted groups were significantly higher (P < 0.05) and reached or exceeded the level of the NC group.
Fig. 4.
Concentration of factors associated with TG metabolism in the liver. (a)Statistical analysis of p-AMPK. (b)Statistical analysis of ChREBP. (c)Statistical analysis of n-SREBP-1c. (d)Statistical analysis of ACC. (e)Statistical analysis of FAS. (f)Statistical analysis of PPARα. (g)Statistical analysis of CPT1. (a, b, c, d, e)Associated with synthesis of TG. (f, g)Associated with oxidation of fatty acids. Mean values of the same sex with different superscript symbols (#, * and &) were significantly different (P < 0.05).
Factors associated with TC metabolism in the liver.
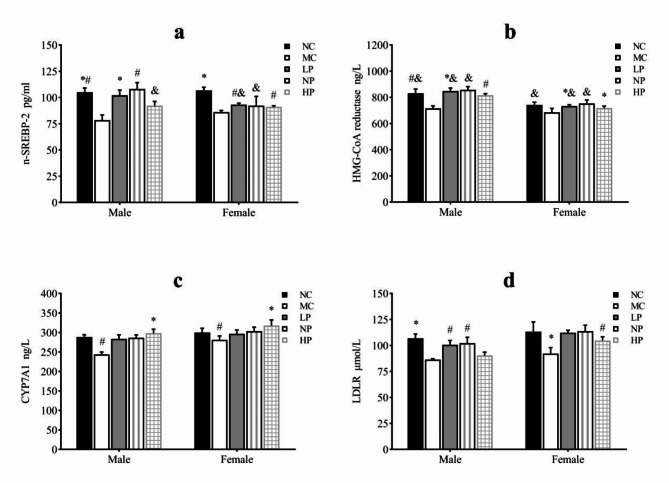
Figure 5 showed the concentrations of the factors involved in TC metabolism in the liver. There was the same trend in the concentrations of all of the factors related to the TC synthesis and degradation for the male and female rats, i.e., compared with the MC group, the concentrations of the factors in the three calorie-restricted groups were significantly higher (P < 0.05) and reached or approached the level of the NC group. Furthermore, compared with the LP and NP group, the LDLR concentration in the HP group was significantly lower (P < 0.05), while the CYP7A1 concentration in the HP group was significantly higher (P < 0.05) for both sexes.
Fig. 5.
Concentration of factors associated with TC metabolism in the liver. (a) Statistical analysis of n-SREBP-2. (b) Statistical analysis of HMG-CoA reductase. (c) Statistical analysis of CYP7A1. (d) Statistical analysis of LDLR. Mean values of the same sex with different superscript symbols (#, * and &) were significantly different (P < 0.05).
Discussion
The main tool for investigating the pathophysiology of overweight and obesity and its associated diseases is the animal model of overweight and obesity. The diet-induced overweight and obesity animal model has become one of the important models for the study of overweight and obesity, since it reproduces human overweight and obesity with greater reliability compared to genetic models15. Thus in the present study, the overweight and obesity model was created via feeding the rats a high-fat diet.
The liver is a central organ for synthesis, storage, metabolism and redistribution of lipids, carbohydrates and proteins. Furthermore, there is an intense connection between the metabolism of hepatic lipids and hepatic glucose16,17. Triglyceride decomposition in fatty tissue generates fatty acids that flow to the liver. Subsequently, fatty acids are oxidized to acetyl-coenzyme A, which is the substrate of cholesterol. Additionally, glucose may also be utilized to generate cholesterol and fatty acids. Glucose from a diet and gluconeogenesis is metabolized via glycolysis into pyruvic acid, and subsequently reduced to acetyl-coenzyme A which acted as a material for the synthesis of triglyceride and cholesterol17. Overweight and obesity are usually accompanied by increased plasma insulin levels and exotic fat deposits in the liver4, and the pathological accumulation of intrahepatocellular triglyceride often results in dysregulation in lipid metabolism4.
The present results demonstrated that restricting calorie intake not only significantly reduced the body weight of overweight and obese rats, but also significantly reduced their liver weight, with no sex difference in these effects. The above results are consistent with previous studies18,19. Additionally, the liver fat content in the male rats for the NC and MC groups was significantly higher than that in the female rats, which was contrary to our speculation and might be due to the fact that the male rats consume significantly more energy and fat than the female rats, leading to excessive liver fat accumulation. However, in the three energy-restricted groups, the liver fat content in the male rats dramatically decreased, significantly lower than that in the NC group; but this phenomenon was not observed in the female rats. There may be two reasons for this. First, the levels of testosterone and estradiol in the energy-restricted male rats were significantly higher than those in the female rats10, which enhanced the promotion of liver fat breakdown and inhibition of liver fat synthesis20. Second, the muscle mass of the energy-restricted male rats was significantly higher than that of the female10, hence the energy expenditure by muscles was also greater in the male rats, thereby reducing the accumulation of liver fat, which was consistent with the research by Uchida et al.21. At the same time of calorie restriction, the effect of the highest protein intake in the HP group on the reduction of liver fat in the male rats was significantly better compared to that in the LP and NP group, which is consistent with the study by Margolis et al.14. But no such result was found in the female rats. Since all of the processes are orchestrated by intracellular signaling pathways, a delicate interplay of hormones, transcription factors, and nuclear receptors in hepatic lipid metabolism16, we examined the hormone and cellular mechanism associated with hepatic lipid synthesis and lipidolysis to explore the reasons for the above results.
Insulin promotes de novo synthesis of fat in the liver22. Overweight and obesity often lead to hyperinsulinemia and insulin resistance which is often associated with liver steatosis. But in this present study, compared to the MC group, the insulin levels of the NC, NP and HP group were significantly higher for both sexes. Moreover, HOMA-IR in the MC group of both male and female rats was not higher than that in the NC group, indicating that the rats of both sexes in the present study did not develop insulin resistance. In addition, compared with the other groups, the blood concentrations of glucose and glucagon of the MC group were notably higher for both sexes. The reason is probably that the decrease in insulin levels in the MC group is mainly due to the impairment of pancreatic β cell function caused by hyperglycemia and hyperlipidemia, and the increase of glucagon level in the MC group is due to excessive intake of saturated fatty acids10. HOMA-β is an indicator which assesses pancreatic β-cell function from basal insulin and glucose concentrations and reflects pancreatic β-cell insulin secretion under non-stimulated conditions23. The trend of HOMA-β in our study is consistent with the plasma insulin level, and the HOMA-β of the energy-restricted groups had recovered to a level with no significant difference compared to the NC group, indicating that for both sexes calorie restriction can improve the function of pancreatic β cells in overweight and obese rats and even restore it to a normal state, but the amount of protein intake during calorie restriction has no effect on β cell function.
Adiponectin and leptin are both derived from adipose tissue. Adiponectin is negatively associated with obesity, which can bind to receptors and activate downstream signaling pathways to regulate lipid metabolism in the liver24. There are two important signaling pathways of adiponectin: AMPK pathway and PPARα pathway. In the liver, adiponectin activates AMPK via attaching itself to the receptor, and turned-on AMPK inhibits the activity of ChREBP and SREBP-1c25,26. At the level of transcription, ChREBP and SREBP-1c synergistically induce the expression of ACC and FAS16. While FAS is the key enzyme of endogenous fatty acid synthesis, ACC is the rate-limiting enzyme of fatty acid synthesis. Therefore, when ChREBP and SREEP-1c are inhibited by adiponectin activated AMPK, the gene expression of ACC and FAS is inhibited, and the synthesis of liver fat is reduced27. In the present study, the plasma adiponectin concentration and p-AMPK expression in the liver were significantly increased in the three calorie-restricted groups compared with those in the MC group for both sexes. Correspondingly, the expressions of ChREBP, n-SREBP-1c, ACC and FAS in the liver was significantly decreased for both sexes. These findings imply that 40% calorie restriction can decrease hepatic triglycerides synthesis by activating the adiponectin-AMPK pathway, inhibiting the expression of ChREBP and SREBP-1c, and down-regulating their target proteins FAS and ACC, and this effect does not exhibit sex differences. In addition, adiponectin binding to receptors can also activate the PPARα signal pathway. As a key transcription factor, PPARα can regulate lipid metabolism in animals. Many of its downstream target genes are associated with the oxidation of fatty acid, such as CPT-1 (a rate-limiting enzyme for mitochondrial fatty acid oxidation), acyl coenzyme A oxidase and fatty acid-binding protein4,24. Our findings suggested that, compared with the three calorie-restricted groups, the expressions of PPARα and CPT-1 A (the liver isoform of CPT-1 that catalyzes the rate-limiting process of converting acyl-coenzyme As into acyl-carnitines, which can then cross membranes to get into the mitochondria28) in the liver of the MC group was significantly lower for both sexes, which was in accordance with the changes of adiponectin. Furthermore, the high expression of PPARα in the livers of the male HP rats is probably a reason why the liver fat content of the male rats in the HP group is significantly lower than that in the LP and NP group, indicating that high protein intake which is twice of the normal protein intake can significantly enhance the fatty acids oxidation in the liver of the male rats.
Leptin is a peptide hormone which synthesized mainly in white adipose tissue29. It acts on the hypothalamus and prevent lipogenesis and activate fatty acid β-oxidation by activating the sympathetic nerve30,31. Moreover, leptin decreases the expression of genes associated with the synthesis of fatty acid such as SREBP1, ChREBP, ACC and FAS for hepatic inhibition of lipogenesis32,33. Through inducing PPARα expression, leptin primarily induces the β-oxidation of fatty acid in the liver34. Therefore, the effects of leptin and adiponectin associated with the hepatic lipid metabolism are consistent. In this present study, compared with the other groups, the leptin concentration in the MC group was significantly higher for both sexes, which was consistent with the results in the white adipose tissue weight. However, the leptin concentration decline for both the male and the female rats in the three calorie-restricted groups was not as dramatic as the decline in the white adipose tissue, and the reasons were not clear, might be the higher insulin level in the calorie-restricted groups that can stimulate the white adipose tissue to produce more leptin31. Moreover, with the increasing intake of protein, the leptin concentration showed an increasing trend. Especially in the male rats, compared to the NC group, the leptin concentration in the HP group increased to a notably higher level. So we speculate that the synergistic effect of leptin and adiponectin is likely to be the main reason for the lowest liver fat content in the male HP group.
The liver is the most important organ for de novo cholesterol synthesis, accounting for 70–80% of the body’s cholesterol synthesis. The liver is also the main site of cholesterol conversion into bile acids35. SREBPs which include SREBP-1 (SREBP-1a, SREBP-1c) and SREBP-2 are essential lipogenesis-regulating transcription factors. SREBP-1 mainly stimulates fatty acid synthesis while SREBP-2 targets mostly genes associated with cellular cholesterol biosynthesis36,37. nSREBP-2 translocates to the nucleus and attaches to the sterol regulatory element to trigger the expression of the target genes, such as HMG-CoA reductase (a rate-limiting enzyme in the cholesterol synthesis by liver cells) and LDLR (involving in mediating the endocytosis of cholesterol-rich LDL by the liver and then conversion cholesterol into bile)38,39. In the liver, CYP7A1 acts as a rate-limiting enzyme that catalyzes the conversion of cholesterol to 7α-hydroxyl cholesterol, thereby promoting bile acid synthesis40. Therefore, both LDLR and CYP7A1 are involved in the catabolism of cholesterol in the liver. In this present study, compared with the three calorie-restricted groups, the expressions of nSREBP-2, HMG-CoA reductase, LDLR and CYP7A1 in the MC group were all significantly lower, suggesting that calorie restriction can not only significantly increase de novo synthesis of liver cholesterol, but also enhance the clearance of cholesterol from the liver. However, the data in Table 3 showed that TC in the MC group was significantly lower compared with that in the three calorie-restricted groups (except the female HP group), indicating that cholesterol synthesis in the liver was greater than the clearance under the 40% calorie-restricted state. This result is in comparison with the study of Dawson et al., which found that 40% calorie restriction increased hepatic cholesterol synthesis and concentration of LDL in blood in female Zucker rats6. However, studies showed that calorie restriction of 20-30% can significantly reduce blood LDL concentration41, indicating that the degree of calorie restriction is likely to be an important factor affecting cholesterol metabolism. We speculated that excessive calorie restriction would increase the catabolism of fats and proteins, and produce a large amount of acetyl-CoA, which is the material of cholesterol synthesis, thus leading to an increase in cholesterol synthesis, which is consistent with the increase in the expression of n-SREBP-2 and HMG-COA reductase in the three calorie-restricted groups in our study. Furthermore, owing to the low intake of carbohydrates in the HP group, the fat and protein mainly underwent gluconeogenesis to maintain the stability of blood glucose, which correspondingly reduced the production of acetyl-CoA, resulting in the synthesis of cholesterol less than that in the LP and NP groups. However, compared to the male rats, we cannot quite explain why the plasma concentration of TC in the HP group is significantly lower in the female rats, which may be because the level of glucagon in the female HP group was significantly increased, which could deactivate HMG-CoA reductase through chemical modification and inhibit cholesterol synthesis, while the change was not observed in the male HP group. But the exact mechanism needs further study.
In summary, our results indicate that energy restriction combined with doubling the protein intake to that of a normal diet exerts different effects on male and female SD rats. The effect is particularly pronounced in reducing TG in male rats, while in female rats it is manifested in a significant decrease in TC. Although we conducted mechanistic studies from the perspective of lipid metabolism pathways, the pathways we investigated were limited and could not fully elucidate the complete mechanisms underlying the observed sex differences. Cara L et al.42 utilized multi-omics approach to study the similarities and differences in the liver genomics, metabolomics, and lipidomics of mice of different sexes and strains when consuming an ad libitum low-protein diet. They found that the molecular mechanisms by which the male and female mice responded to a low-protein diet differed. For example, they found fibroblast growth factor 21 (FGF21), a hormone secreted in response to nutrient stress that initiates adaptive changes in metabolism and feeding and is induced by low protein diets in mice, rats, and humans, regulated some of the responses to a low-protein diet in a sex-dependent manner. However, the study was conducted under conditions of adequate energy intake, which differs from our calorie-restricted diet. Adamo Valle et al.43 analyzed the comprehensive impact of sex and energy restriction on the liver proteome expression profile of Wistar rats and showed that female rats have higher levels of mitochondrial enzymes involved in lipid catabolism and amino acid breakdown in the liver. However, the study did not set different levels of protein intake under energy restriction, so it cannot reflect the impact of protein intake on lipid metabolism. Therefore, current researches are not yet able to fully elucidate the mechanisms by which energy restriction and protein intake affect liver lipid metabolism in rats of different sexes.
There are certain limitations in the present study. First, Since SD rats can be divided into two subpopulations based on their tendency to gain weight on a high-fat or high-energy diet, some are diet-resistant and less likely to become overweight or obese44. So in the present study, only 80% of the rats fed with a high-fat diet developed into overweight or obese rats. Due to the limited number of rats that met the weight requirements, we were unable to further divide these rats into an overweight group and an obese group. Second, the energy restriction period of eight weeks for the rats was too long, resulting in their body weight being lower than that of the NC group, particularly the excessively low liver fat content in the male rats, which could potentially affect their health. Third, we did not euthanize the rats during the same phase of their estrous cycle, which could lead to inconsistent levels of sex hormones and might have influenced their lipid metabolism.
Conclusion
A 40% energy restriction can significantly reduce the body weight, body fat, liver fat, and the blood concentration of TG in both male and female overweight and obese rats, but it can significantly increase the blood concentration of TC in overweight and obese male rats. At the same time of 40% calorie restriction, increasing dietary protein intake to twice the normal protein intake has a stronger effect on promoting hepatic triglyceride oxidation and reducing liver fat content in the male overweight and obese rats by increasing the levels of adiponectin and leptin in the blood, and can also significantly reduce the plasma cholesterol concentration in the female overweight and obese rats through inhibiting cholesterol synthesis most likely by increasing glucagon level in the blood.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- NC
normal control
- MC
model control
- LP
low protein
- NP
normal protein
- HP
high protein
- TG
triglycerides
- TC
total cholesterol
- VLDL
very low density lipoprotein
- LDL
low density lipoprotein
- LDLR
low-density lipoprotein receptor
- HDL
high density lipoprotein
- HOMA-IR
homeostatic model assessment of insulin resistance
- HOMA-β
homeostatic model assessment of β-cell function
- p-AMPK
phosphorylated AMP-activated protein kinase
- nSREBP-1c
nuclear sterol-regulatory element-binding protein-1c
- ChREBP
carbohydrate response element binding protein
- ACC
acetyl-CoA carboxylase
- FAS
fatty acid synthase
- PPAR-α
peroxisome proliferators-activated receptor alpha
- CPT-1A
carnitine palmitoyl transterase-1 A
- nSREBP-2
nuclear sterol-regulatory element-binding protein-2
- HMG-CoA reductase
3-hydroxy-3-methylglutaryl coenzyme A reductase
- CYP7A1
cholesterol 7-alpha hydroxylase
Author contributions
Ying Tian designed and instructed this study and wrote the manuscript and Jiawei Gong, Zhiyan He, Suwen Peng, Yuping Huan and Hongpeng Cao performed the experiments and data analysis, interpreted the results.
Funding
This work was supported by the National Natural Science Foundation of China (81472963).
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Loos, R. J. F. & Yeo, G. S. H. The genetics of obesity: from discovery to biology. Nat. Rev. Genet.23, 120–133. 10.1038/s41576-021-00414-z (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drummen, M. et al. Long-term effects of increased protein intake after weight loss on intrahepatic lipid content and implications for insulin sensitivity: a PREVIEW study. Am. J. Physiol. Endocrinol. Metab.315, 885–891. 10.1152/ajpendo.00162.2018 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Yu, H., Nie, R. & Shen, C. The role of bile acids in regulating glucose and lipid metabolism. Endocr. J.70, 359–374. 10.1507/endocrj.EJ22-0544 (2023). [DOI] [PubMed] [Google Scholar]
- 4.Nagarajan, S. R., Cross, E., Sanna, F. & Hodson, L. Dysregulation of hepatic metabolism with obesity: factors influencing glucose and lipid metabolism. Proc. Nutr. Soc.81, 1–11. 10.1017/s0029665121003761 (2022). [DOI] [PubMed] [Google Scholar]
- 5.Johansson, H. E. et al. Energy restriction in obese women suggest linear reduction of hepatic fat content and time-dependent metabolic improvements. Nutr. Diabetes. 9, 34. 10.1038/s41387-019-0100-2 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawson, M. A. et al. Replacement of dietary carbohydrate with protein increases fat mass and reduces hepatic triglyceride synthesis and content in female obese Zucker rats. Physiol. Rep.11, e15885. 10.14814/phy2.15885 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sosic-Jurjevic, B. et al. Differences in Cholesterol Metabolism, Hepato-Intestinal aging, and hepatic endocrine milieu in rats as affected by the sex and age. Int. J. Mol. Sci.24, 12624. 10.3390/ijms241612624 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bentanachs, R. et al. Adipose tissue protects against Hepatic Steatosis in Male Rats Fed a High-Fat Diet plus Liquid Fructose: sex-related differences. Nutrients15, 3909. 10.3390/nu15183909 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maseroli, E. et al. Testosterone treatment is associated with reduced adipose tissue dysfunction and nonalcoholic fatty liver disease in obese hypogonadal men. J. Endocrinol. Investig.44, 819–842. 10.1007/s40618-020-01381-8 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian, Y. et al. Effects of protein intake from an energy-restricted diet on the skeletal muscle composition of overweight and obese rats. Sci. Rep.12, 20396. 10.1038/s41598-022-24961-5 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westerterp-Plantenga, M. S. et al. Dietary protein, weight loss, and weight maintenance. Annu. Rev. Nutr.29, 21–41. 10.1146/annurev-nutr-080508-141056 (2009). [DOI] [PubMed] [Google Scholar]
- 12.Standardization Administration of China. Vol. GB 14924.3–2010 (China Standard, 2010).
- 13.Guntarti, A., Ahda, M., Kusbandari, A. & Prihandoko, S. W. Analysis of Lard in Sausage using Fourier transform Infrared Spectrophotometer combined with Chemometrics. J. Pharm. Bioallied Sci.11, 594–600. 10.4103/jpbs.JPBS_209_19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margolis, L. M. et al. Calorie restricted high protein diets Downregulate Lipogenesis and Lower Intrahepatic triglyceride concentrations in male rats. Nutrients8, 571. 10.3390/nu8090571 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Moura, E. D. M. et al. Diet-induced obesity in animal models: points to consider and influence on metabolic markers. Diabetol. Metab. Syndr.13, 32. 10.1186/s13098-021-00647-2 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bechmann, L. P. et al. The interaction of hepatic lipid and glucose metabolism in liver diseases. J. Hepatol.56, 952–964. 10.1016/j.jhep.2011.08.025 (2012). [DOI] [PubMed] [Google Scholar]
- 17.Bai, L. & Li, H. Innate immune regulatory networks in hepatic lipid metabolism. J. Mol. Med. (Berl). 97, 593–604. 10.1007/s00109-019-01765-1 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Vilar-Gomez, E. et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology149, 367–378. 10.1053/j.gastro.2015.04.005 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Smith, G. I. et al. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J. Clin. Invest.130, 1453–1460. 10.1172/jci134165 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kur, P., Kolasa-Wolosiuk, A., Misiakiewicz-Has, K. & Wiszniewska, B. Sex hormone-dependent physiology and diseases of Liver. Int. J. Environ. Res. Public Health. 17, 2620. 10.3390/ijerph17082620 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uchida, K. et al. Endogenous testosterone reduces hepatic lipid accumulation in protein-restricted male rats. Nutrition8510.1016/j.nut.2020.111130 (2021). [DOI] [PubMed]
- 22.Loomba, R., Friedman, S. L. & Shulman, G. I. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell184, 2537–2564. 10.1016/j.cell.2021.04.015 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu, X., Wang, Y., Jia, Y., Liu, J. & Wang, G. Risk factors for nonalcoholic fatty liver disease with different insulin resistance in a nonobese Chinese population. J. Diabetes Res.2022 (9060405). 10.1155/2022/9060405 (2022). [DOI] [PMC free article] [PubMed]
- 24.Ishtiaq, S. M., Rashid, H., Hussain, Z., Arshad, M. I. & Khan, J. A. Adiponectin and PPAR: a setup for intricate crosstalk between obesity and non-alcoholic fatty liver disease. Rev. Endocr. Metab. Disord. 20, 253–261. 10.1007/s11154-019-09510-2 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Li, Y. et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell. Metab.13, 376–388. 10.1016/j.cmet.2011.03.009 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iizuka, K., Bruick, R. K., Liang, G., Horton, J. D. & Uyeda, K. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc. Natl. Acad. Sci. U S A. 101, 7281–7286. 10.1073/pnas.0401516101 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen, L. et al. Hepatic differentiated embryo-chondrocyte-expressed gene 1 (Dec1) inhibits sterol regulatory element-binding protein-1c (Srebp-1c) expression and alleviates fatty liver phenotype. J. Biol. Chem.289, 23332–23342. 10.1074/jbc.M113.526343 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlaepfer, I. R. & Joshi, M. CPT1A-mediated fat oxidation, mechanisms, and therapeutic potential. Endocrinology16110.1210/endocr/bqz046 (2020). [DOI] [PubMed]
- 29.Pereira, S., Cline, D. L., Glavas, M. M., Covey, S. D. & Kieffer, T. J. Tissue-specific effects of leptin on glucose and lipid metabolism. Endocr. Rev.42, 1–28. 10.1210/endrev/bnaa027 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang, L. et al. Leptin receptor-expressing neuron Sh2b1 supports sympathetic nervous system and protects against obesity and metabolic disease. Nat. Commun.11, 1517. 10.1038/s41467-020-15328-3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang, W., Dedousis, N., Bandi, A., Lopaschuk, G. D. & O’Doherty, R. M. Liver triglyceride secretion and lipid oxidative metabolism are rapidly altered by leptin in vivo. Endocrinology147, 1480–1487. 10.1210/en.2005-0731 (2006). [DOI] [PubMed] [Google Scholar]
- 32.Huynh, F. K. et al. A role for hepatic leptin signaling in lipid metabolism via altered very low density lipoprotein composition and liver lipase activity in mice. Hepatology57, 543–554. 10.1002/hep.26043 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Denechaud, P. D., Dentin, R., Girard, J. & Postic, C. Role of ChREBP in hepatic steatosis and insulin resistance. FEBS Lett.582, 68–73. 10.1016/j.febslet.2007.07.084 (2008). [DOI] [PubMed] [Google Scholar]
- 34.Liang, C. P. & Tall, A. R. Transcriptional profiling reveals global defects in energy metabolism, lipoprotein, and bile acid synthesis and transport with reversal by leptin treatment in ob/ob mouse liver. J. Biol. Chem.276, 49066–49076. 10.1074/jbc.M107250200 (2001). [DOI] [PubMed] [Google Scholar]
- 35.Cho, A. R. et al. Effects of alternate day fasting and exercise on cholesterol metabolism in overweight or obese adults: a pilot randomized controlled trial. Metabolism93, 52–60. 10.1016/j.metabol.2019.01.002 (2019). [DOI] [PubMed] [Google Scholar]
- 36.Rong, S., McDonald, J. G. & Engelking, L. J. Cholesterol auxotrophy and intolerance to ezetimibe in mice with SREBP-2 deficiency in the intestine. J. Lipid Res.58, 1988–1998. 10.1194/jlr.M077610 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun, Y. et al. Targeting cholesterol homeostasis through inhibiting SREBP-2: an Achilles’ heel for glioblastoma. Neuro Oncol.25, 2100–2102. 10.1093/neuonc/noad145 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xue, L. et al. Targeting SREBP-2-regulated mevalonate metabolism for cancer therapy. Front. Oncol.10, 1510. 10.3389/fonc.2020.01510 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang, Y. N. et al. The berberine-enriched gut commensal Blautia producta ameliorates high-fat diet (HFD)-induced hyperlipidemia and stimulates liver LDLR expression. Biomed. Pharmacother. 155, 113749. 10.1016/j.biopha.2022.113749 (2022). [DOI] [PubMed] [Google Scholar]
- 40.Duan, R. et al. Flavonoids from whole-grain oat alleviated High-Fat Diet-Induced Hyperlipidemia via regulating bile acid metabolism and gut microbiota in mice. J. Agric. Food Chem.69, 7629–7640. 10.1021/acs.jafc.1c01813 (2021). [DOI] [PubMed] [Google Scholar]
- 41.Xu, R., Cao, Y., Wang, P. Y., Chen, X. L. & Tao, D. Intermittent energy restriction vs. continuous energy restriction on cardiometabolic risk factors in patients with metabolic syndrome: a meta-analysis and systematic review. Front. Nutr.10, 1090792. 10.3389/fnut.2023.1090792 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Green, C. L. et al. Sex and genetic background define the metabolic, physiologic, and molecular response to protein restriction. Cell Metabol.34, 209–226. 10.1016/j.cmet.2021.12.018 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valle, A., Silvestri, E., Moreno, M., Chambery, A. & Oliver, J. Combined effect of gender and caloric restriction on liver proteomic expression Profile. J. Proteome Res.7, 2872–2881. 10.1021/pr800086t (2008). [DOI] [PubMed] [Google Scholar]
- 44.Archer, Z. A., Rayner, D. V., Rozman, J., Klingenspor, M. & Mercer, J. G. Normal distribution of body weight gain in male Sprague-Dawley rats fed a high-energy diet. Obes. Res.11, 1376–1383. 10.1038/oby.2003.186 (2003). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information files.