Abstract
The two main glial cell types of the central nervous system (CNS), astrocytes and microglia, are responsible for neuroimmune homeostasis. Recent evidence indicates astrocytes can participate in removal of pathological structures by becoming phagocytic under conditions of neurodegenerative disease when microglia, the professional phagocytes, are impaired. We hypothesized that adenosine triphosphate (ATP), which acts as damage-associated molecular pattern (DAMP), when released at high concentrations into extracellular space, upregulates phagocytic activity of human astrocytes. This study is the first to measure changes in phagocytic activity and mitochondrial respiration of human astrocytic cells in response to extracellular ATP. We demonstrate that ATP-induced phagocytic activity of U118 MG astrocytic cells is accompanied by upregulated mitochondrial oxidative phosphorylation, which likely supports this energy-dependent process. Application of a selective antagonist A438079 provides evidence identifying astrocytic purinergic P2X7 receptor (P2X7R) as the potential regulator of their phagocytic function. We also report a rapid ATP-induced increase in intracellular calcium ([Ca2+]i), which could serve as regulator of both the phagocytic activity and mitochondrial metabolism, but this hypothesis will need to be tested in future studies. Since ATP upregulates interleukin (IL)-8 secretion by astrocytes but has no effect on their cytotoxicity towards neuronal cells, we conclude that extracellular ATP affects only specific functions of astrocytes. The selectivity of P2X7R-dependent regulation of astrocyte functions by extracellular ATP could allow targeting this receptor-ligand interaction to upregulate their phagocytic function. This could have beneficial outcomes in neurodegenerative disorders, such as Alzheimer’s disease, that are characterized by reactive astrocytes and defective phagocytic processes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11302-025-10066-x.
Keywords: Astrocytes, Extracellular adenosine triphosphate (ATP), Damage-associated molecular pattern (DAMP), Oxidative phosphorylation, Phagocytosis, Purinergic receptor P2X7
Introduction
Maintenance of a healthy central nervous system (CNS) requires continuous immune surveillance and removal of pathogens, cell debris, and abnormal molecular structures by phagocytosis-capable cells. Microglia, which represent mononuclear phagocytes in the CNS, are the only professional phagocytes among brain cells. Increasing evidence indicates that astrocytes, another type of glial cells, contribute to neuroimmune responses, including phagocytic processes [1]. Human and murine astrocytes have been shown to phagocytose synaptic structures [2], axons and axonal organelles [3, 4], apoptotic cells [5], cellular and myelin debris [6, 7], and abnormally aggregated proteins [8]. It has also been suggested that phagocytic activity of astrocytes is upregulated in pathological conditions, such as Alzheimer’s disease (AD), where microglia are unable to phagocytose efficiently [1, 9]. This implies the existence of endogenous CNS molecules capable of autocrine or paracrine regulation of the phagocytic activity of astrocytes. High concentrations of extracellular adenosine triphosphate (ATP) is one such candidate molecular signal.
ATP is a primary energy carrier used by all living cells. In the mammalian nervous system, it can also be found extracellularly where it plays physiological signaling roles by regulating embryonic and postnatal development, synaptic plasticity, and serving as a neurotransmitter and gliotransmitter (reviewed in [10]). In pathological contexts, such as brain injury, neuroinflammation, and neurodegenerative diseases, ATP operates as a mitochondria-derived damage-associated molecular pattern (DAMP): after its release from damaged or activated CNS cells, extracellular ATP can activate P2 purinergic receptors (P2Rs) including the ionotropic P2XRs and to a lesser extent the metabotropic P2YRs (reviewed in [10]). Binding of ATP to microglial P2X7R in particular can activate NOD-like receptor protein 3 (NLRP3) inflammasome and caspase-1, leading to the release of the pro-inflammatory cytokine interleukin (IL)−1β and exacerbation of neuroinflammation. Extracellular ATP affects multiple other functions of microglia including proliferation, directional migration toward damaged cells, release of extracellular vesicles, and phagocytic activity [11, 12]. While the effects of extracellular ATP on microglia have been extensively documented, its actions on astrocytes are less studied.
Previous research exploring extracellular ATP as a modulator of astrocyte functions has primarily focused on the release of pro-inflammatory cytokines, production of neurotrophic factors, generation of reactive oxygen species by nicotinamide adenine dinucleotide phosphate (NADPH)-dependent oxidase, synaptogenesis regulated by astrocyte-derived extracellular vesicles, as well as glutamate metabolism and signaling [13–16]. Several P2Y1R-mediated effects of ATP on astrocytes have been reported. For example, binding of extracellular ATP to this receptor subtype induces glutamate exocytosis from murine astrocytes and release of additional ATP, which can stimulate neighboring cells in paracrine manner [17]. It has been suggested that under several pathological conditions such as AD, brain injury, and ischemia, ATP engages P2Y1R to initiate reactive astrogliosis characterized by astrocyte hypertrophy, enhanced adhesion, and migration toward injury sites (reviewed in [18, 19]). However, the impact of ATP on phagocytic activity of human astrocytes remains a topic yet to be explored. Phagocytic activity in professional phagocytes such as microglia is supported by upregulated mitochondrial respiration [20], a phenomenon that has not been investigated in human astrocytic cells. Given the importance of mitochondrial metabolic reprogramming for mediating immune cell polarization and effector functions [21–23] and reported differences in immunometabolic responses between rodent and human cells [24], we performed a comprehensive analysis of mitochondrial bioenergetics in human astrocytic cells using high-resolution respirometry and recorded the effects of extracellular ATP on mitochondrial respiration.
P2XRs are expressed by all types of brain cells, including neurons, microglia and astrocytes (reviewed in [25, 26]). Among them, P2X2R, P2X4R, and P2X7R are primarily expressed in the CNS and their upregulation has been documented in the brains of individuals with AD (reviewed in [27, 28]). In this study we focused on P2X7R since both human and murine astrocytes express this receptor subtype (reviewed in [29]), which is also a well-established modulator of phagocytic activity in microglia and other professional human phagocytes such as monocytes and macrophages (reviewed in [30, 31]). For example, P2X7R mediates phagocytosis of latex beads and apoptotic cells by human monocytes [32]. Additionally, phagocytosis of Escherichia coli bioparticles by human microglia is regulated by binding of ATP to P2X7R [33]. Therefore, we hypothesized that phagocytic activity of human astrocytic cells can be modulated by extracellular ATP binding to P2X7R. We used human U118 MG astrocytic cells as models of human astrocytes given that their phagocytic activity has already been shown to be regulated by several inflammatory stimuli and DAMPs including lipopolysaccharide (LPS), interferon (IFN)-γ, and cytochrome c [34].
Materials and methods
Reagents and cells
The following reagents were purchased from Sigma Aldrich (Oakville, ON, Canada): ATP disodium salt hydrate, adenosine diphosphate (ADP), LPS from E. coli O55:B5, the competitive P2X7R antagonist A438079, digitonin, malate, pyruvate, succinate, carbonyl cyanide m-chlorophenyl hydrazone (CCCP), 3-(4,5-dimethyl-2-thiazolyl)−2,5-diphenyl-2H-tetrazolium bromide (MTT), bisbenzimide (Hoechst 33,258), Pluronic F-127, and poly-D-lysine (PDL). Fluo-4 AM, calf bovine serum (CBS), fetal bovine serum (FBS), Dulbecco’s modified Eagle medium nutrient mixture F-12 (DMEM-F12), and penicillin–streptomycin-amphotericin B were obtained from ThermoFisher (Waltham, MA, USA). Fluorescein isothiocyanate (FITC) surface-labeled fluorescent polystyrene latex beads (one µm diameter) were obtained from Bangs Laboratories (Fishers, IN, USA). ELISA kit for human IL-8 was purchased from PeproTech (Cranbury, NJ, USA). Rhodamine-labeled wheat germ agglutinin (WGA) was from Vector Laboratories (Newark, CA, USA). Goat anti-rabbit IgG CY5-labeled antibodies were from Jackson ImmunoResearch (West Grove, PA, USA). Rabbit anti-glial fibrillary acidic protein (GFAP) antibody was purchased from Agilent (#Z0334, Santa Clara, CA, USA). Mitochondrial respiration medium MiR05 was purchased from Oroboros Instruments (Innsbruck, Austria). The human U118 MG astrocytic cell line was purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). The human SH-SY5Y neuroblastoma cells were donated by Dr. T. Wenzel (University of Saskatchewan, Canada) and the BV-2 murine microglial cell line was donated by Dr. G Garden (University of Washington, Seattle, WA, USA). Murine primary astrocytes were isolated from the brains of postnatal day one to four pups of CD-1 mice (Charles River laboratories, Montreal, Quebec, Canada) as previously described [34]. All animal experiments were conducted in compliance with the guidelines of the Animal Care Committee at the University of British Columbia, BC, Canada, under protocol A20-0105.
Phagocytic activity of human U118 MG astrocytic cells and murine primary astrocytes
Phagocytosis of fluorescent latex beads by astrocytic cells was quantified as previously described with minor modifications [34]. Human U118 MG astrocytic cells or murine primary astrocytes were plated in four-chambered glass-bottom Petri dishes at a density of 5 × 104 cells/ml in 500 μl of DMEM-F12 containing 10% FBS and antibiotics and allowed to adhere for 24 h. ATP (0.1 mM) and/or LPS (400 ng/ml) was added to the plate for 48 h, followed by three µl of suspension containing one µm diameter FITC surface-labeled latex beads (10 mg/ml) for one h (U118 cells) or two h (murine primary astrocytes). U118 MG cells were washed, then fixed in 500 μl of cold 70% ethanol for five min and bisbenzimide (2 μg/ml) was added to stain the nucleus prior to imaging. For fluorescent imaging (see Fig. 1C), U118 MG cell membranes were stained by adding rhodamine-labeled WGA (10 μg/ml) for 10 min prior to their fixation [35]. Murine primary astrocytes were fixed, permeabilized, and immunostained as previously described, using goat anti-rabbit CY5-lableled antibodies (1:1000) following the application of anti-GFAP antibody [34]. Bisbenzimide (2 μg/ml) was added to stain the nucleus prior to imaging. Astrocytic cells were imaged at 40 × magnification with a Zeiss AxioObserver.Z1 inverted widefield fluorescence microscope with ZEN 2 (Black edition) software. Three channels were used: 350/470 nm for bisbenzimide, 470/520 nm for fluorescent beads, 594/615 nm for GFAP-positive murine primary astrocytes, and differential interference contrast (DIC) for brightfield microscopy. Measurements of corrected total cell fluorescence intensities were performed by an investigator blinded to the experimental condition using the NIH ImageJ program (FIJI build). The engulfment of fluorescent beads by murine astrocytes was confirmed with a TCS SPE-II confocal microscope (Leica Microsystems, Concord, ON, Canada, see Fig. 1C).
Fig. 1.
Extracellular ATP upregulates the phagocytic activity of human U118 MG astrocytic cells (A) and murine primary astrocytes (B). Cells were exposed to ATP (0.1 mM), LPS (400 ng/ml), their combination, or vehicle solutions (Control) for 48 h, followed by addition of fluorescent latex beads for one h (A) or two h (B). Corrected total cell fluorescence values (means ± SEM) from 163–238 (A) or 152–162 (B) randomly selected cells from three to five (A) or four (B) independent experiments were measured and normalized to control values obtained in the absence of immune mediators. P and F values for the one-way ANOVA are displayed (A); * P < 0.05, ** P < 0.01, according to Tukey’s post-hoc tests (A) or the unpaired Student’s t-test (B). Representative fluorescent images demonstrating the uptake of latex beads (green) by human U118 MG astrocytic cells and murine primary astrocytes (C). U118 MG cell membranes were stained with rhodamine-labeled WGA (red), while murine astrocytes were visualized by sequential application of anti-GFAP antibodies and CY5-lableled (red) secondary antibodies. Nuclei of both cell types were stained with bisbenzimide (blue). The scale bars represent 50 μm
Calcium imaging
Calcium signaling in BV-2 murine microglia and U118 MG human astrocytic cells was visualized using Fluo-4 AM fluorescent dye by following a previously published procedure with minor modifications [36]. BV-2 and U118 MG cells were seeded on PDL-coated glass bottom dishes at a density of 4.5 × 103 cells per plate in 300 μl of DMEM-F12 containing 10% FBS and an antibiotic/antimycotic mixture. After 24 h, the culture medium was removed, cells were washed twice with Tyrode’s 2 mM calcium solution (140 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, and 10 mM glucose, pH = 7.4), and incubated in Tyrode’s solution with 5 μM Fluo-4 AM for 30 min at 37 °C (BV-2 cells) or 20 min at room temperature (U118 MG cells). After washing twice, cells were incubated in Tyrode’s solution for additional 20 min at 37 °C (BV-2 cells) or at room temperature (U118 cells), followed by another round of washing. Real-time fluorescence intensity was recorded every 2 s for 40 min at 40x (BV-2 cells) or 20x (U118 MG cells) magnification using a Zeiss AxioObserver.Z1 inverted widefield fluorescence microscope and imaged with Zen 2 software, using a 488 nm excitation and a 520 nm emission filters. ATP (1 mM) or deionized (DI) water was added to the dish seven (BV-2 cells) or five (U118 MG cells) min after the start of recording baseline fluorescence. Hyperstack images were manually analyzed by NIH ImageJ program (FIJI build). Regions of interest were assigned to individual BV-2 cells, and temporal Fluo-4 AM fluorescence intensity was calculated by subtracting mean background fluorescence.
High resolution respirometry of human U118 MG astrocytic cells
Mitochondrial respiration was measured using Oxygraph-2K (Oroboros Instruments) following the procedures recommended by the manufacturer. U118 MG human astrocytic cells were plated in 10 cm cell culture plates at a density of 1.5 × 105 cells/ml in 10 ml of DMEM-F12 containing 5% CBS and antibiotics. After 24 h incubation, the medium was refreshed and ATP (0.1 mM) and/or LPS (400 ng/ml) was added for 48 h. Due to the limitations of the available instrument allowing simultaneous recording from three samples only, two separate experiments were performed. First, it was established that LPS on its own did not affect mitochondrial respiration of U118 MG human astrocytic cells (Supplementary Fig. S1). Subsequently, the respiration rates were concurrently recorded for cells treated with ATP or ATP plus LPS, and for control cells exposed to the vehicle solutions only. Following stimulation, cells were gently removed using a cell lifter, centrifugated (400 g for 10 min at room temperature), resuspended in mitochondrial respiration medium MiR05, and counted using an automated cell counter (Countess, ThermoFisher). Mitochondrial respiration was determined in approximately 5 × 105 live U118 MG cells in 0.5 ml MiR05 maintained at 37℃ with constant stirring (750 rpm). Basal respiration was assessed in intact cells prior to permeabilization with digitonin (4.05 µM) and subsequent addition of malate (2 mM) and pyruvate (5 mM) for the determination of leak respiration. Complex I stimulated oxidative phosphorylation was then assessed in the presence of submaximal (50 µM) or saturating (5 mM) ADP. Maximal oxidative phosphorylation through complex I and II was achieved by addition of succinate (10 mM), followed by titrations with the uncoupler CCCP (0.5 µM steps) to determine maximal electron transfer capacity. Data were recorded and analyzed in DatLab version 7.4 (Oroboros Instruments).
Cytotoxicity of human U118 MG astrocytic cells towards human SH-SY5Y neuronal cells and IL-8 secretion by astrocytic cells
Human U118 MG astrocytic cells were seeded in a 24-well plate at a density of 2 × 105 cells/ml in one ml of DMEM-F12 containing antibiotics and 10% FBS. After 24 h incubation, the medium in the plate was refreshed and ATP (0.1 mM) and/or LPS (400 ng/ml) added for 48 h. Human SH-SY5Y neuronal cells were seeded in a 24-well plate in 400 μl of DMEM-F12 containing antibiotics and 5% FBS at a density of 4 × 105 cells/ml. Following 24 h incubation, the medium of SH-SY5Y was removed, and 400 μl of the U118 MG cell supernatants were transferred to each corresponding well. The remaining U118 MG cell supernatants were collected and used for an ELISA, which was performed according to the instructions provided by the manufacturer (PeproTech). The MTT cell viability assay was performed on the U118 MG cultures at the time of supernatant transfer, and on the SH-SY5Y neuronal cell cultures at the end of a 72 h incubation period with the astrocytic cell supernatants according to the previously published procedure [34].
Statistical analyses
Statistical analyses and graphing were completed using Prism GraphPad software (version 10.1.0, GraphPad Software Inc., La Jolla, CA, USA). Data were analyzed by paired or unpaired Student’s t-test or using one-way analysis of variance (ANOVA) or randomized block design ANOVA, followed by Dunnett’s or Tukey’s post-hoc tests. Data collected from independent experiments performed on separate days are presented as means ± standard error of the mean (SEM). Significance was established at P < 0.05.
Results
Extracellular ATP upregulates the phagocytic activity of astrocytic cells from two different species
To test the hypothesis that the phagocytic activity of astrocytes is regulated by extracellular ATP, we conducted experiments with two different types of astrocytes: human U118 MG astrocytic cells and murine primary astrocytes. Since LPS has already been shown to upregulate phagocytic activity in both these cell types [34], it was used as a positive control in the experiments. 0.1 mM ATP was chosen for this study, based on the reported half-maximal effective concentration (EC50) for activation of P2X7R [37]. Preliminary experiments also showed that 0.1 mM ATP effectively upregulated phagocytic activity of U118 MG cells without causing cytotoxicity while the increased phagocytosis in the presence of 1 mM ATP was associated with a significant reduction in viability of astrocytic cells (Supplementary Fig. S2). Figure 1A confirms that both LPS and ATP (0.1 mM) alone and in combination significantly upregulated phagocytosis of fluorescent latex beads by human U118 MG cells. ATP at the same 0.1 mM concentration was also effective at inducing phagocytic activity of cultured murine astrocytes extracted from newborn animal brains (Fig. 1B). Figure 1C demonstrates uptake of fluorescent beads by U118 MG cells imaged by widefield fluorescence microscope and murine primary astrocytes imaged by a confocal microscope.
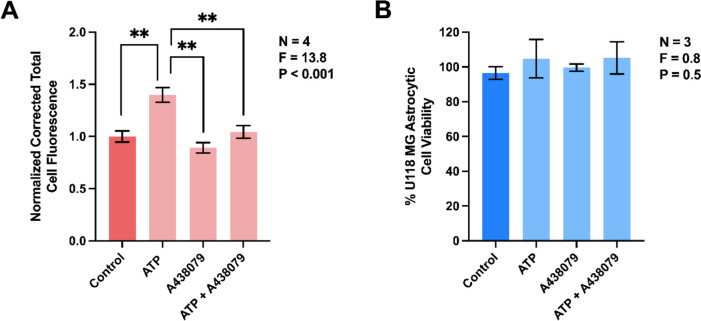
The extracellular ATP-induced phagocytic activity of human astrocytic cells is blocked by a selective antagonist of P2X7R
Previous studies indicate that ATP modulates phagocytic activity of microglia by interacting with their P2X7R (reviewed in [32, 38]). We hypothesized that this receptor also regulates the phagocytic activity of U118 MG astrocytic cells. Figure 2A demonstrates that preincubation of U118 MG cells with a competitive P2X7R antagonist A438079 blocked the ATP-induced upregulation of phagocytic activity. Notably, A438079 on its own did not inhibit this astrocytic function and neither ATP nor A438079 at the concentrations used in this study reduced the viability of U118 MG cells (Fig. 2B); therefore, the inhibitory effect of A438079 was not due to cytotoxicity of this antagonist.
Fig. 2.
Extracellular ATP upregulates the phagocytic activity of human U118 MG astrocytic cells in a P2X7R-dependent manner. A438079 (10 µM) or its vehicle solution was added to human U118 MG astrocytic cells for 30 min before their exposure to ATP (0.1 mM) or its vehicle solution for 48 h, followed by addition of fluorescent latex beads for one h. Corrected total cell fluorescence from 154–206 randomly selected cells from four independent experiments was measured (A) and normalized to control values obtained in the absence of immune mediators. The MTT assay was used to assess cell viability in three independent experiments (B). P and F values for the one-way ANOVA are displayed; ** P < 0.01, according to Tukey’s post-hoc tests
Extracellular ATP induces rapid increase in human U118 MG astrocytic cell cytoplasmic calcium
Intracellular calcium ([Ca2+]i) signaling is known to promote phagocytosis in microglia [39]. We hypothesized that [Ca2+]i in U118 MG cells increases in response to ATP stimulation. Murine BV-2 microglia were employed as positive controls since they are established professional phagocytes that are well known to respond to ATP stimulation with a rise in [Ca2+]i [36, 40]. Experiments presented in Fig. 3A,B confirm our hypothesis by demonstrating an increase in fluorescence of the calcium-sensitive Fluo-4 AM dye immediately after ATP application to human U118 MG astrocytic cells. This rapid monophasic rise in fluorescence signal is very similar to that observed after stimulation of murine BV-2 cells with extracellular ATP (Fig. 3C) and leads to significantly elevated [Ca2+]i in both cell types (Fig. 3B,D).
Fig. 3.
Extracellular ATP induces rapid increase in intracellular calcium concentration ([Ca2+]i) in human U118 MG astrocytic cells (A, B) and murine BV-2 microglia (C, D). To detect changes in [Ca2+]i, cells were loaded with Fluo-4 AM and real-time fluorescence intensities were measured for 25 min. A: ATP (1 mM, 265 cells monitored) or its vehicle solution (deionized water, 255 cells monitored) was added 5 min after signaling stabilized. C: ATP (1 mM, 114 cells monitored) or its vehicle solution (deionized water, 110 cells monitored) was added 2 min after the fluorescence signal had stabilized. B, D: The normalized maximum [Ca2+]i responses after the addition of ATP or deionized water were quantified. Data from six to eight independent experiments performed on three different days are presented as means ± SEM. ** P < 0.01, according to the unpaired Student’s t-test
Extracellular ATP stimulates mitochondrial respiration in human U118 MG astrocytic cells
In immune cells, Ca2+ influx induced by P2R-mediated signaling can stimulate mitochondrial metabolism [41, 42] and mitochondrial ATP production is required for NLRP3 inflammasome activation [43]. These observations, coupled with the reported importance of mitochondrial respiration for microglial phagocytic activity [20], led us to hypothesize that the increase in extracellular ATP-induced phagocytosis in astrocytic cells recorded in this study (Fig. 1A) would be accompanied by elevated rates of mitochondrial metabolism. In support of this hypothesis, we observed increased basal (Fig. 4A), leak (Fig. 4B), and submaximal ADP supported (Fig. 4C) respiration in U118 MG cells following stimulation with extracellular ATP. However, ADP supported respiration through complex I in the presence of saturating ADP concentrations (Fig. 4D), the maximal complex I + II-supported capacity for oxidative phosphorylation (Fig. 4E) and uncoupled respiration (Fig. 4F) were not significantly altered in the presence of extracellular ATP. When ATP was added to U118 MG cells in combination with LPS, all measured parameters of mitochondrial function, except uncoupled respiration, were significantly upregulated compared to unstimulated astrocytic cells. Notably, exposing U118 MG cells to the same concentration of LPS in the absence of ATP did not induce significant changes in mitochondrial respiration (Supplementary Fig. S1).
Fig. 4.
Extracellular ATP stimulates mitochondrial respiration in human U118 MG astrocytic cells. Mitochondrial respiration was assessed in cells stimulated for 48 h with ATP (0.1 mM) in the presence or absence of LPS (400 ng/ml). High resolution respirometry was used to measure mitochondrial respiration in the basal state (A; basal respiration), after cell permeabilization (B; leak respiration), and following addition of mitochondrial complex I substrates (2 mM malate and 5 mM pyruvate) in the presence of submaximal (C; 50 µM) and maximal (D; 5 mM) concentrations of ADP. Complex I + II supported respiration (E) was then assessed following addition of succinate (10 mM) prior to titrations with the uncoupler CCCP (0.5 µM steps) to determine maximal electron transfer capacity (F; uncoupled respiration). Data from five to six independent experiments are presented as means ± SEM. P and F values for the one-way ANOVA are displayed. * P < 0.05, ** P < 0.01, according to Tukey’s post-hoc tests
Extracellular ATP upregulates secretion of IL-8 by human U118 MG astrocytic cells, but has no effect on their cytotoxicity towards human SH-SY5Y neuronal cells
In response to specific immune stimuli and their combinations, human primary astrocytes and U118 MG astrocytic cells have been shown to secrete a broad range of inflammatory cytokines and chemokines as well as potentially cytotoxic molecules (reviewed in [44, 45]); however, the repertoire of cytokines secreted by astrocytes in response to stimulation with extracellular ATP is quite restricted and limited to only several prominent pro-inflammatory mediators including tumor necrosis factor (TNF), IL-6, and IL-8, which are also linked to AD pathogenesis [46, 47]. Furthermore, data obtained by using different types of astrocytes show that in some cases ATP on its own induces cytokine expression, while in other instances costimulation with other pro-inflammatory agents such as LPS or IL-1β is required [14, 48–50]; therefore, in this study we chose to test the effects of ATP and LPS alone and in combination on secretion of IL-8 by U118 MG astrocytic cells.
Figure 5A demonstrates that ATP, similar to LPS, an established inducer of cytokine expression by astrocytes [51], significantly upregulated IL-8 secretion by human U118 MG astrocytic cells. Stimulation with ATP and LPS alone or in combination was insufficient for the induction of cytotoxicity of U118 MG cells towards human SH-SY5Y neuronal cells (Fig. 5B). Neither of these stimuli were toxic to U118 MG astrocytic cells.
Fig. 5.
Extracellular ATP upregulates IL-8 secretion by human U118 MG astrocytic cells, but has no effect on their secretion of cytotoxins. Human astrocytic cells were treated with ATP (0.1 mM), LPS (400 ng/ml), their combination, or vehicle solutions for 48 h. Subsequently, an ELISA was used to measure the concentration of IL-8 in cell culture supernatants (A) and the MTT assay was used to assess the viability of astrocytic cells (C). Aliquots of U118 MG cell supernatants were also transferred to human SH-SY5Y neuronal cell cultures and their viability was measured by the MTT assay after 72 h incubation (B). Data from eight (A) or five (B, C) independent experiments are presented as means ± SEM. P and F values for the one-way randomized block ANOVA are displayed. * P < 0.05, according to Dunnett’s post-hoc tests. The limit of detection for the ELISA is shown as a dotted line (A)
Discussion
The pathophysiological significance of astrocyte phagocytic activity is becoming increasingly recognized; however, our knowledge regarding the endogenous to the CNS mediators that can induce and control this important protective cellular mechanism is very limited. Several neuropathology-linked molecules have been shown to modulate phagocytic activity of astrocytes, including Alzheimer amyloid β (Aβ) [52] and α-synuclein fibrils, which accumulate in Parkinson’s disease [53]. Additionally, proinflammatory cytokines secreted by reactive glial cells such as IFN-γ, IL-1β, and TNF, along with molecules originating from all CNS cell types, such as cytochrome c and ganglioside GM1 [34, 54] have been identified as regulators of astrocyte phagocytic activity. This study adds extracellular ATP as another DAMP-like molecule of the CNS that displays similar functional activity. We used human U118 MG astrocytic cells and murine primary astrocytes to demonstrate that ATP at a non-toxic 0.1 mM concentration significantly upregulated phagocytosis of latex beads by both these cell types. LPS was employed as a positive control since it has already been demonstrated to upregulate phagocytosis of latex beads by U118 MG cells and murine astrocytes [34] as well as phagocytosis of myelin and colloidal gold particles by mouse and rat astrocytes, respectively [55, 56]. While, to the best of our knowledge, regulation of the phagocytic activity by extracellular ATP has not been reported before for any human astrocytic cell type, our data showing its upregulation in human and murine cells differ from previous observations by Yamamoto et al. [57] who report that incubation of ddY-mouse astrocytes with 5 mM of ATP for one h decreases engulfment of latex beads. This study attributes this effect to the ATP-induced dissociation of non-muscle myosin heavy chain II A from the P2X7R complex [58]. However, they also note a significant reduction of astrocyte viability in the presence of 5 mM ATP, which aligns with our preliminary data showing significant toxicity of ATP at 1 mM towards U118 MG astrocytic cells. Compromised mitochondrial function induced by the presence of cytotoxic ATP concentrations could impair phagocytic activity, which requires considerable cellular energy expenditure. This could explain the inconsistency of the previously observed inhibitory outcome compared to our study, where ATP upregulated phagocytosis of latex beads at a non-toxic 0.1 mM concentration.
We used the competitive antagonist A438079 to demonstrate that the effect of extracellular ATP, when applied at 0.1 mM, on phagocytic activity of U118 MG astrocytic cells was mediated by P2X7R. Cultured human primary astrocytes and astrocytic cells express P2X7R (reviewed in [59, 60]). While ATP concentrations above 1 mM have been used previously to induce P2X7R activation, this receptor has already been demonstrated to mediate effects of 0.1 mM extracellular ATP on both murine and human astrocytes [61, 62]; however, it should be noted that responses to increasing concentrations of ATP or other agonists of P2XRs vary among different types of astrocytes and could involve not only P2X7R, but also P2X1-6 receptors [37, 63]. Nevertheless, ATP at 0.1 mM and higher concentrations is generally believed to evoke P2X7R activation (reviewed in [28, 64, 65]). Our observed P2X7R-dependent effect of ATP on astrocytes is also consistent with the previously reported regulatory role of P2X7R in various other cell types, including human neural precursor cells, neuroblasts, as well as murine and human primary microglia [33, 66, 67]. The precise molecular mechanisms responsible for the ATP-induced upregulation of astrocyte phagocytic activity will require additional studies, but they could be similar to those implicated in P2X7R-regulated phagocytosis in other cell types. It is known that stimulation of P2X7R leads to the activation of p38 mitogen-activated protein kinase (MAPK) and Rho-dependent signaling pathways, initiating membrane blebbing and actin reorganization in multiple cell types (reviewed in [68, 69]). It has already been demonstrated that phagocytic activity of macrophages can be modulated by p38 MAPK/Akt pathway [70]. Additionally, A438079 has an inhibitory effect on calcium influx in murine primary optic nerve head astrocytes and spinal cord astrocytes [14, 71]. Calcium is crucial for many steps of phagocytosis, such as solubilization of actin meshwork around phagosomes and fusion of phagosomes with lytic granules (reviewed in [72]); therefore, increased [Ca2+]i is another mechanism that could be responsible for ATP-induced and P2X7R-mediated increase in astrocyte phagocytic activity. Notably, elevated [Ca2+]i has been observed at phagocytic subcellular sites in murine primary microglia and astrocytes [7, 73].
Here we demonstrate instant upregulation of [Ca2+]i by extracellular ATP in U118 MG human astrocytic cells. Application of ATP triggered a monophasic – or in some cases biphasic – initial response, which was followed by a series of [Ca2+]i oscillations. This pattern was similar to the monophasic and sharp increase in [Ca2+]i caused by extracellular application of ATP to murine BV-2 microglia, which were used as control cells previously demonstrated to respond to ATP with upregulated [Ca2+]i [40]. Our data demonstrating ATP-induced [Ca2+]i increase are similar to the results obtained by Wang et al. [74] who recorded [Ca2+]i response in human fetal astrocytes induced by 0.1 mM ATP. Furthermore, Wakida et al. [7] report [Ca2+]i-dependent upregulation of astrocyte phagocytic activity in response to cell debris. These observations may indicate that increased [Ca2+]i regulates astrocyte phagocytic activity similar to its role in numerous other functional responses of astrocytes, including release of gliotransmitters, regulation of local blood flow, and synaptic plasticity (reviewed in [75, 76]). However, this hypothesis will require experimental proof.
The metabolic activity of astrocytes has been studied extensively under homeostatic conditions where both glycolysis and oxidative phosphorylation are used as their energy sources; however, the contribution and regulation of oxidative phosphorylation in astrocyte reactivity induced by immune stimulants is not well understood (reviewed in [76–78]). Our data demonstrate that treatment with extracellular ATP and the combination of ATP plus LPS upregulated three and five distinct respiration states of U118 MG astrocytic cells, respectively. ATP alone or in combination with LPS increased basal respiration, which represents endogenous mitochondrial metabolism of intact live cells [79]. Both treatments also upregulated leak respiration, which largely reflects mitochondrial oxygen consumption compensating for proton leak across the inner mitochondrial membrane. Furthermore, both ATP and ATP plus LPS upregulated the complex I-supported respiration in the presence of submaximal (50 µM) ADP concentrations that are reflective of physiological ADP levels within the cell. On the other hand, maximal complex I-supported respiration with saturating ADP (5 mM) was increased by combined ATP plus LPS stimulation only. Similarly, maximal complex I and II-supported respiration (i.e., maximal oxidative phosphorylation capacity) was upregulated in U118 MG astrocytic cells treated with ATP plus LPS but not ATP alone. The improvement in submaximal ADP-supported respiration without alternations in maximal oxidative phosphorylation suggests an enhanced sensitivity of mitochondrial respiration to cellular ADP in cells treated with extracellular ATP alone. Finally, the maximal capacity for electron transfer independent of ATP synthesis – reflected by uncoupled respiration – was not significantly altered in response to extracellular ATP or LPS. This indicates that the increased mitochondrial activity induced by ATP was due to enhanced proton leak and/or capacity for oxidative phosphorylation.
Our observations of ATP-induced upregulation of basal respiration rate in human astrocytic cells are similar to the previously reported increase of the same parameter in murine primary astrocytes in response to stimulation with the pro-inflammatory cytokines IL-1β or TNF [80, 81]. Both these studies also detected significantly upregulated maximal respiration after stimulation with both cytokines, while in our experiments, U118 MG cells exposed to extracellular ATP alone demonstrated only a trend towards a significant increase in this parameter of oxidative phosphorylation. In contrast to ATP, IL-1β, and TNF, LPS did not affect any of the parameters of mitochondrial functions studied, despite being a powerful immune stimulant. Notably, extracellular ATP and LPS acted synergistically on maximal complex I- and complex I and II-supported respiration, since only when combined, they induced significant increase in this parameter. To the best of our knowledge, this study is the first to report upregulation of oxidative phosphorylation of astrocytic cells of any type by extracellular ATP and a lack of similar effect in response to LPS stimulation, which nevertheless was able to upregulate the phagocytic activity of astrocytes. The inability of LPS to regulate oxidative phosphorylation of astrocytes contrasts its reported almost two-fold downregulation of maximal mitochondrial respiration in murine microglia [82]. Our data and other very limited studies on astrocytes indicate that metabolic reprogramming responses to immune stimulants in this cell type could be radically different from analogous processes in microglia and other mononuclear phagocytes, which are known to shift their metabolism from oxidative phosphorylation to glycolysis under inflammatory conditions (reviewed in [77, 83]). Additional research using targeted inhibition of distinct components of the respiratory chain is needed to further elucidate the importance of mitochondrial ATP production for astrocyte activation, such as increased phagocytic capacity, in response to extracellular ATP. For example, inhibition of complex I, II, or V using rotenone, malonate, or oligomycin, respectively, in the presence of various substrate combinations could provide insight into complex- and substrate-specific respiration in ATP stimulated cells.
Our novel observations of enhanced mitochondrial respiration in extracellular ATP-exposed astrocytic cells indicate that ATP, by interacting with P2X7R, stimulates oxidative metabolism.
Although extracellular ATP-induced assembly of NLRP3 inflammasomes in macrophages has been shown to depend on mitochondrial activity [84], to the best of our knowledge only two immune stimulants, IL-1β and TNF, have been reported to upregulate basal and maximal mitochondrial respiration in cultured murine primary astrocytes and our study is the first to explore this mechanism in human astrocytic cells. This stimulatory ability of extracellular ATP could be an essential component of its DAMP-like activity, which is necessary for enabling phagocytic processes in astrocytic cells. ATP-induced upregulation of mitochondrial metabolism is likely mediated by elevated [Ca2+]i that has been shown to activate mitochondrial enzymes, such as tricarboxylic acid cycle dehydrogenases [85]. Importantly, an increase in mitochondrial ATP production in response to extracellular ATP could represent a feed-forward mechanism used to sustain astrocytic phagocytic activity by engaging autocrine purinergic signaling, which has been demonstrated in other immune cells [41]. Our findings also align with the previously reported importance of mitochondrial ATP production for NLRP3 inflammasome activation in immune cells [43] and microglial phagocytosis [20], though we are the first to demonstrate this in human astrocytic cells.
We also hypothesized that extracellular ATP serves as a modulator of only specific neuroimmune functions of astrocytes. This hypothesis was supported by our data showing that ATP at the non-toxic 0.1 mM concentration, similar to its effects on phagocytic activity, upregulated secretion of IL-8 by human U118 MG astrocytic cells but did not induce their cytotoxicity towards human SH-SY5Y neuronal cells. A similar observation was made by Adzic et al. [18] who report that, in murine primary astrocytes, extracellular ATP upregulates superoxide dismutase (SOD) activity, increases glutathione (GSH) content, and induces both cellular proliferation and shape remodeling, but has no effect on IL-1β release or migration of activated astrocytes. Extracellular ATP is an already established trigger for the release of pro-inflammatory cytokines, such as IL-1β, IL-6, TNF, IL-18, at the sites of brain injury following trauma, ischemia, or epilepsy, thereby potentially contributing to neuropsychiatric disorders and neurodegenerative diseases (reviewed in [29, 86]). Human adult astrocytes are known to increase IL-8 gene expression in response to LPS, and the combination of LPS plus ATP upregulates IL-8 secretion by human U251 MG astrocytic cells [48, 87]. Our experiments with U118 MG cells produced similar data showing that ATP and LPS effectively upregulated IL-8 secretion, but neither ATP nor LPS induced cytotoxicity of astrocytic cells towards neuron-like cells, akin to previous reports demonstrating the inability of LPS alone to trigger cytotoxic responses of human primary astrocytes [88]. The same study identified IFN-γ as a cytokine capable of inducing the secretion of cytotoxins by human astrocytic cells. Given that only specific astrocyte functions are modulated after binding of extracellular ATP to their P2X7R, this ligand-receptor interaction could be targeted to prevent or ameliorate astrocyte reactivity and regulate their phagocytic activity in numerous neuropathologies that are driven by neuroinflammatory processes.
Conclusion
The observed increase in the phagocytic activity of human astrocytic cells in response to extracellular ATP should be explored further since defective P2X7R-mediated phagocytosis has been reported in AD [32]. Use of human glia culture systems will be important as extracellular ATP has been shown to inhibit in P2X7R-dependent manner phagocytic activity of human microglia [33] and therefore may have opposing effects on phagocytosis by the two main glial cell types in humans. Nevertheless, strategies that either upregulate or restore this function in at least one glial cell type could have beneficial effects in AD and other neurodegenerative diseases.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank all members of the Laboratory of Cellular and Molecular Pharmacology for their valuable discussions and comments on the manuscript. We also thank Z. Mohammad and K. Wiens for their assistance with phagocytosis quantification, and S. Simtchouk for help with fluorescence imaging of cells.
Biographies
Sijie (Shirley) Yang
holds both a BSc and MSc from the University of British Columbia, where her master’s research focused on neuroimmunology, inflammation, and neurodegenerative diseases. Currently a researcher at the Vancouver Coastal Health Research Institute, she is dedicated to uncovering disease mechanisms and advancing approaches to improve human health.

Noah A. H. Brooks
is a Graduate Student in the Department of Biology at the University of British Columbia Okanagan. His research is focused on the role that damage-associated molecular patterns (DAMPs) have in microglia-mediated neurodegeneration.
Dylan E. Da Silva (BSc)
is a medical student at the University of British Columbia with research interest in the pathophysiology of chronic disease.
Dr Julien Gibon
is an Adjunct Professor in the Department of Biology at the University of British Columbia Okanagan, where his research explores the intricate communication between cells in the nervous system and its role in memory formation.
Dr. Hashim Islam
is an Assistant Professor in the School of Health and Exercise Sciences at the University of British Columbia Okanagan. His research program focuses on mitochondrial function within different human cells and tissues, and the associated impacts of exercise, nutrition, and cardiometabolic disease.
Dr. Andis Klegeris
is a Professor at the University of British Columbia Okanagan. His research explores neuroimmune processes in the central nervous system and the pathophysiology of glial cells.
Authors’ contributions
Conceptualization, S.Y., J.G., H.I., A.K.; methodology, S.Y., N.A.H.B., D.E.D., J.G., H.I.; formal analysis, S.Y., N.A.H.B., D.E.D., H.I.; investigation, S.Y., N.A.H.B., D.E.D., H.I.; writing—original draft preparation, S.Y., A.K.; writing—review and editing, S.Y., N.A.H.B., D.E.D., J.G., H.I., A.K.; supervision, J.G., H.I., A.K.; funding acquisition, J.G., H.I., A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Jack Brown and Family Alzheimer’s Disease Research Foundation (A.K.), the Natural Sciences and Engineering Research Council of Canada (J.G., H.I., A.K.), and the University of British Columbia Okanagan Campus (A.K.).
Data availability
The datasets that support the findings of this study are available from the corresponding authors upon reasonable request.
Compliance with ethical standards
Ethics approval and consent to participate
All animal experiments were conducted in compliance with the guidelines of the Animal Care Committee at the University of British Columbia, BC, Canada, under protocol A20-0105.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hashim Islam, Email: hashim.islam@ubc.ca.
Andis Klegeris, Email: andis.klegeris@ubc.ca.
References
- 1.Konishi H, Koizumi S, Kiyama H (2022) Phagocytic astrocytes: Emerging from the shadows of microglia. Glia 70:1009–1026. 10.1002/glia.24145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung WS, Clarke LE, Wang GX et al (2013) Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 504:394–400. 10.1038/nature12776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis CHO, Kim KY, Bushong EA et al (2014) Transcellular degradation of axonal mitochondria. Proc Natl Acad Sci U S A 111:9633–9638. 10.1073/pnas.1404651111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen JV, Soto I, Kim KY et al (2011) Myelination transition zone astrocytes are constitutively phagocytic and have synuclein dependent reactivity in glaucoma. Proc Natl Acad Sci U S A 108:1176–1181. 10.1073/pnas.1013965108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damisah EC, Hill RA, Rai A et al (2020) Astrocytes and microglia play orchestrated roles and respect phagocytic territories during neuronal corpse removal in vivo. Sci Adv 6:3239. 10.1126/sciadv.aba3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ponath G, Ramanan S, Mubarak M et al (2017) Myelin phagocytosis by astrocytes after myelin damage promotes lesion pathology. Brain 140:399–413. 10.1093/brain/aww298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wakida NM, Gomez-Godinez V, Li H et al (2020) Calcium dynamics in astrocytes during cell injury. Front Bioeng Biotechnol 8:912. 10.3389/fbioe.2020.00912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wyss-Coray T, Loike JD, Brionne TC et al (2003) Adult mouse astrocytes degrade amyloid-β in vitro and in situ. Nat Med 9:453–457. 10.1038/nm838 [DOI] [PubMed] [Google Scholar]
- 9.Konishi H, Okamoto T, Hara Y et al (2020) Astrocytic phagocytosis is a compensatory mechanism for microglial dysfunction. EMBO J 39:e104464. 10.15252/embj.2020104464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burnstock G, Dale N (2015) Purinergic signalling during development and ageing. Purinergic Signal 11:277–305. 10.1007/s11302-015-9452-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drago F, Lombardi M, Prada I et al (2017) ATP modifies the proteome of extracellular vesicles released by microglia and influences their action on astrocytes. Front Pharmacol 8:910. 10.3389/fphar.2017.00910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elliott MR, Chekeni FB, Trampont PC et al (2009) Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 461:282–286. 10.1038/NATURE08296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacques-Silva MC, Rodnight R, Lenz G et al (2004) P2X7 receptors stimulate AKT phosphorylation in astrocytes. Br J Pharmacol 141:1106–1117. 10.1038/sj.bjp.0705685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munoz FM, Patel PA, Gao X et al (2020) Reactive oxygen species play a role in P2X7 receptor-mediated IL-6 production in spinal astrocytes. Purinergic Signal 16:97–107. 10.1007/S11302-020-09691-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vignoli B, Canossa M (2017) Glioactive ATP controls BDNF recycling in cortical astrocytes. Commun Integr Biol 10:e1277296. 10.1080/19420889.2016.1277296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang JM, Wang HK, Ye CQ et al (2003) ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron 40:971–982. 10.1016/S0896-6273(03)00717-7 [DOI] [PubMed] [Google Scholar]
- 17.Domercq M, Brambilla L, Pilati E et al (2006) P2Y1 receptor-evoked glutamate exocytosis from astrocytes: control by tumor necrosis factor-alpha and prostaglandins. J Biol Chem 281:30684–30696. 10.1074/jbc.M606429200 [DOI] [PubMed] [Google Scholar]
- 18.Adzic M, Stevanovic I, Josipovic N et al (2017) Extracellular ATP induces graded reactive response of astrocytes and strengthens their antioxidative defense in vitro. J Neurosci Res 95:1053–1066. 10.1002/jnr.23950 [DOI] [PubMed] [Google Scholar]
- 19.Lagos-Cabré R, Burgos-Bravo F, Avalos AM, Leyton L (2020) Connexins in astrocyte migration. Front Pharmacol 10:1546. 10.3389/fphar.2019.01546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fairley LH, Lai KO, Wong JH et al (2023) Mitochondrial control of microglial phagocytosis by the translocator protein and hexokinase 2 in Alzheimer’s disease. Proc Natl Acad Sci U S A 120:e2209177120. 10.1073/PNAS.2209177120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marques E, Kramer R, Ryan DG (2024) Multifaceted mitochondria in innate immunity. NPJ Metab Health Dis 2:6. 10.1038/s44324-024-00008-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mills EL, Kelly B, Logan A et al (2016) Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell 167:457-470.e13. 10.1016/j.cell.2016.08.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van den Bossche J, Baardman J, Otto NA et al (2016) Mitochondrial dysfunction prevents repolarization of inflammatory macrophages. Cell Rep 17:684–696. 10.1016/J.CELREP.2016.09.008 [DOI] [PubMed] [Google Scholar]
- 24.Vijayan V, Pradhan P, Braud L et al (2019) Human and murine macrophages exhibit differential metabolic responses to lipopolysaccharide - A divergent role for glycolysis. Redox Biol 22:101147. 10.1016/J.REDOX.2019.101147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abbracchio MP, Ceruti S (2006) Roles of P2 receptors in glial cells: Focus on astrocytes. Purinergic Signal 2:595–604. 10.1007/s11302-006-9016-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin MM, Liu N, Qin ZH, Wang Y (2022) Mitochondrial-derived damage-associated molecular patterns amplify neuroinflammation in neurodegenerative diseases. Acta Pharmacol Sin 43:2439–2447. 10.1038/s41401-022-00879-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castillo C, Saez-Orellana F, Godoy PA, Fuentealba J (2022) Microglial activation modulated by P2X4R in ischemia and repercussions in Alzheimer’s disease. Front Physiol 13:814999. 10.3389/fphys.2022.814999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jarvis MF, Khakh BS (2009) ATP-gated P2X cation-channels. Neuropharmacology 56:208–215. 10.1016/j.neuropharm.2008.06.067 [DOI] [PubMed] [Google Scholar]
- 29.Illes P, Rubini P, Ulrich H et al (2020) Regulation of microglial functions by purinergic mechanisms in the healthy and diseased CNS. Cells 9:1108. 10.3390/cells9051108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ni J, Wang P, Zhang J et al (2013) Silencing of the P2X7 receptor enhances amyloid-β phagocytosis by microglia. Biochem Biophys Res Commun 434:363–369. 10.1016/J.BBRC.2013.03.079 [DOI] [PubMed] [Google Scholar]
- 31.Savio LEB, de Andrade MP, da Silva CG, Coutinho-Silva R (2018) The P2X7 receptor in inflammatory diseases: Angel or demon? Front Pharmacol 9:52. 10.3389/fphar.2018.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gu BJ, Wiley JS (2018) P2X7 as a scavenger receptor for innate phagocytosis in the brain. Br J Pharmacol 175:4195–4208. 10.1111/bph.14470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janks L, Sharma CVR, Egan TM (2018) A central role for P2X7 receptors in human microglia. J Neuroinflammation 15:325. 10.1186/s12974-018-1353-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang S, Simtchouk S, Gibon J, Klegeris A (2023) Regulation of the phagocytic activity of astrocytes by neuroimmune mediators endogenous to the central nervous system. PLoS ONE 18:e0289169. 10.1371/JOURNAL.PONE.0289169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nielsen HM, Mulder SD, Beliën JAM et al (2010) Astrocytic Aβ1-42 uptake is determined by Aβ-aggregation state and the presence of amyloid-associated proteins. Glia 58:1235–1246. 10.1002/glia.21004 [DOI] [PubMed] [Google Scholar]
- 36.Gilbert DF, Stebbing MJ, Kuenzel K et al (2016) Store-operated Ca2+ entry (SOCE) and purinergic receptor-mediated Ca2+ homeostasis in murine BV2 microglia cells: early cellular responses to ATP-mediated microglia activation. Front Mol Neurosci 9:111. 10.3389/fnmol.2016.00111 [DOI] [PMC free article] [PubMed]
- 37.Surprenant A, Rassendren F, Kawashima E et al (1996) The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science 272:735–738. 10.1126/science.272.5262.735 [DOI] [PubMed] [Google Scholar]
- 38.Campagno KE, Mitchell CH (2021) The P2X7 receptor in microglial cells modulates the endolysosomal axis, autophagy, and phagocytosis. Front Cell Neurosci 15:645244. 10.3389/FNCEL.2021.645244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Umpierre AD, Li B, Ayasoufi K et al (2024) Microglial P2Y6 calcium signaling promotes phagocytosis and shapes neuroimmune responses in epileptogenesis. Neuron 112:1959–1977. 10.1016/j.neuron.2024.03.017 [DOI] [PMC free article] [PubMed]
- 40.Chun BJ, Stewart BD, Vaughan DD et al (2019) Simulation of P2X-mediated calcium signalling in microglia. J Physiol 597:799–818. 10.1113/JP277377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bao Y, Ledderose C, Seier T et al (2014) Mitochondria regulate neutrophil activation by generating ATP for autocrine purinergic signaling. J Biol Chem 289:26794–26803. 10.1074/jbc.M114.572495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ledderose C, Liu K, Kondo Y et al (2018) Purinergic P2X4 receptors and mitochondrial ATP production regulate T cell migration. J Clin Invest 128:3583–3594. 10.1172/JCI120972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Billingham LK, Stoolman JS, Vasan K et al (2022) Mitochondrial electron transport chain is necessary for NLRP3 inflammasome activation. Nat Immunol 23:692–704. 10.1038/s41590-022-01185-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jha MK, Kim JH, Song GJ et al (2018) Functional dissection of astrocyte-secreted proteins: Implications in brain health and diseases. Prog Neurobiol 162:37–69. 10.1016/J.PNEUROBIO.2017.12.003 [DOI] [PubMed] [Google Scholar]
- 45.Murray TE, Wenzel TJ, Simtchouk S et al (2022) Extracellular cardiolipin modulates select immune functions of astrocytes in toll-like receptor (TLR) 4-dependent manner. Mediators Inflamm 2022:9946439. 10.1155/2022/9946439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu Y, Chai YL, Hilal S et al (2017) Serum IL-8 is a marker of white-matter hyperintensities in patients with Alzheimer’s disease. Alzheimers Dement 7:41–47. 10.1016/J.DADM.2017.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu WT, Howell JC, Ozturk T et al (2019) CSF cytokines in aging, multiple sclerosis, and dementia. Front Immunol 10:480. 10.3389/FIMMU.2019.00480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Braganhol E, Kukulski F, Lévesque SA et al (2015) Nucleotide receptors control IL-8/CXCL8 and MCP-1/CCL2 secretions as well as proliferation in human glioma cells. Biochim Biophys Acta Mol Basis Dis 1852:120–130. 10.1016/j.bbadis.2014.10.014 [DOI] [PubMed] [Google Scholar]
- 49.Xia M, Zhu Y (2013) FOXO3a involvement in the release of TNF-α stimulated by ATP in spinal cord astrocytes. J Mol Neurosci 51:792–804. 10.1007/S12031-013-0067-8/FIGURES/9 [DOI] [PubMed] [Google Scholar]
- 50.John GR, Simpson JE, Woodroofe MN et al (2001) Extracellular nucleotides differentially regulate interleukin-1β signaling in primary human astrocytes: Implications for inflammatory gene expression. J Neurosci 21:4134–4142. 10.1523/JNEUROSCI.21-12-04134.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li L, Acioglu C, Heary RF, Elkabes S (2021) Role of astroglial toll-like receptors (TLRs) in central nervous system infections, injury and neurodegenerative diseases. Brain Behav Immun 91:740–755. 10.1016/j.bbi.2020.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanchez-Mico MV, Jimenez S, Gomez-Arboledas A et al (2021) Amyloid-β impairs the phagocytosis of dystrophic synapses by astrocytes in Alzheimer’s disease. Glia 69:997–1011. 10.1002/glia.23943 [DOI] [PubMed] [Google Scholar]
- 53.Chou TW, Chang NP, Krishnagiri M et al (2021) Fibrillar α-synuclein induces neurotoxic astrocyte activation via RIP kinase signaling and NF-κB. Cell Death Dis 12:756. 10.1038/s41419-021-04049-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sokolova TV, Rychkova MP, Basova NE et al (2021) Phagocytic activity of rat primary astrocytes is regulated by insulin and ganglioside GM1. J Evol Biochem Physiol 57:1072–1080. 10.1134/S0022093021050094 [Google Scholar]
- 55.Kalmár B, Kittel Á, Lemmens R et al (2001) Cultured astrocytes react to LPS with increased cyclooxygenase activity and phagocytosis. Neurochem Int 38:453–461. 10.1016/S0197-0186(00)00090-5 [DOI] [PubMed] [Google Scholar]
- 56.Wan T, Zhu W, Zhao Y et al (2022) Astrocytic phagocytosis contributes to demyelination after focal cortical ischemia in mice. Nat Commun 13:1134. 10.1038/s41467-022-28777-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamamoto M, Kamatsuka Y, Ohishi A et al (2013) P2X7 receptors regulate engulfing activity of non-stimulated resting astrocytes. Biochem Biophys Res Commun 439:90–95. 10.1016/j.bbrc.2013.08.022 [DOI] [PubMed] [Google Scholar]
- 58.Gu BJ, Rathsam C, Stokes L et al (2009) Extracellular ATP dissociates nonmuscle myosin from P2X7 complex: This dissociation regulates P2X7 pore formation. Am J Physiol Cell Physiol 297:C430–C439. 10.1152/AJPCELL.00079.2009 [DOI] [PubMed] [Google Scholar]
- 59.Gehring MP, Pereira TCB, Zanin RF et al (2012) P2X7 receptor activation leads to increased cell death in a radiosensitive human glioma cell line. Purinergic Signal 8:729–739. 10.1007/s11302-012-9319-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Narcisse L, Scemes E, Zhao Y et al (2005) The cytokine IL-1beta transiently enhances P2X7 receptor expression and function in human astrocytes. Glia 49:245–258. 10.1002/glia.20110.The [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gandelman M, Peluffo H, Beckman JS et al (2010) Extracellular ATP and the P2X7receptor in astrocyte-mediated motor neuron death: Implications for amyotrophic lateral sclerosis. J Neuroinflammation 7:33. 10.1186/1742-2094-7-33/FIGURES/3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muller MS, Taylor CW (2017) ATP evokes Ca2+ signals in cultured foetal human cortical astrocytes entirely through G protein-coupled P2Y receptors. J Neurochem 142:876–885. 10.1111/JNC.14119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao YF, Ren WJ, Zhang Y et al (2022) High, in contrast to low levels of acute stress induce depressive-like behavior by involving astrocytic, in addition to microglial P2X7 receptors in the rodent hippocampus. Int J Mol Sci 23:1904. 10.3390/IJMS23031904/S1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Trang M, Schmalzing G, Müller CE, Markwardt F (2020) Dissection of P2X4 and P2X7 receptor current components in BV-2 microglia. Int J Mol Sci 21:8489. 10.3390/IJMS21228489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ronning KE, Déchelle-Marquet PA, Che Y et al (2023) The P2X7 receptor, a multifaceted receptor in Alzheimer’s disease. Int J Mol Sci 24:11747. 10.3390/IJMS241411747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fang KM, Yang CS, Sun SH, Tzeng SF (2009) Microglial phagocytosis attenuated by short-term exposure to exogenous ATP through P2X7 receptor action. J Neurochem 111:1225–1237. 10.1111/J.1471-4159.2009.06409.X [DOI] [PubMed] [Google Scholar]
- 67.Lovelace MD, Gu BJ, Eamegdool SS et al (2015) P2X7 receptors mediate innate phagocytosis by human neural precursor cells and neuroblasts. Stem Cells 33:526–541. 10.1002/stem.1864 [DOI] [PubMed] [Google Scholar]
- 68.Martínez-Cuesta MÁ, Blanch-Ruiz MA, Ortega-Luna R et al (2020) Structural and functional basis for understanding the biological significance of P2X7 receptor. Int J Mol Sci 21:8454. 10.3390/ijms21228454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pfeiffer ZA, Aga M, Prabhu U et al (2004) The nucleotide receptor P2X7 mediates actin reorganization and membrane blebbing in RAW 264.7 macrophages via p38 MAP kinase and Rho. J Leukoc Biol 75:1173–1182. 10.1189/JLB.1203648 [DOI] [PubMed] [Google Scholar]
- 70.Xin C, Quan H, Kim JM et al (2019) Ginsenoside Rb1 increases macrophage phagocytosis through p38 mitogen-activated protein kinase/Akt pathway. J Ginseng Res 43:394–401. 10.1016/j.jgr.2018.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Beckel JM, Argall AJ, Lim JC et al (2014) Mechanosensitive release of adenosine 5′-triphosphate through pannexin channels and mechanosensitive upregulation of pannexin channels in optic nerve head astrocytes: A mechanism for purinergic involvement in chronic strain. Glia 62:1486–1501. 10.1002/glia.22695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nunes P, Demaurex N (2010) The role of calcium signaling in phagocytosis. J Leukoc Biol 88:57–68. 10.1189/jlb.0110028 [DOI] [PubMed] [Google Scholar]
- 73.Lim HM, Woon H, Han JW et al (2017) UDP-induced phagocytosis and ATP-stimulated chemotactic migration are impaired in STIM1 -/- microglia in vitro and in vivo. Mediators Inflamm 2017:8158514. 10.1155/2017/8158514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Y-X, Kang X-N, Cao Y et al (2020) Porphyromonas gingivalis induces depression via downregulating p75NTR-mediated BDNF maturation in astrocytes. Brain Behav Immun 81:523–534. 10.1016/j.bbi.2019.07.012 [DOI] [PubMed] [Google Scholar]
- 75.Goenaga J, Araque A, Kofuji P, Herrera Moro Chao D (2023) Calcium signaling in astrocytes and gliotransmitter release. Front Synaptic Neurosci 15:1138577. 10.3389/FNSYN.2023.1138577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jackson JG, Robinson MB (2018) Regulation of mitochondrial dynamics in astrocytes: Mechanisms, consequences, and unknowns. Glia 66:1213–1234. 10.1002/GLIA.23252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Preeti K, Sood A, Fernandes V (2021) Metabolic regulation of glia and their neuroinflammatory role in Alzheimer’s disease. Cell Mol Neurobiol 42:2527–2551. 10.1007/S10571-021-01147-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rose J, Brian C, Pappa A et al (2020) Mitochondrial metabolism in astrocytes regulates brain bioenergetics, neurotransmission and redox balance. Front Neurosci 14:536682. 10.3389/FNINS.2020.536682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Divakaruni AS, Jastroch M (2022) A practical guide for the analysis, standardization, and interpretation of oxygen consumption measurements. Nat Metab 4:978–994. 10.1038/s42255-022-00619-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jiang T, Cadenas E (2014) Astrocytic metabolic and inflammatory changes as a function of age. Aging Cell 13:1059–1067. 10.1111/acel.12268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kabiraj P, Grund EM, Clarkson BDS et al (2022) Teriflunomide shifts the astrocytic bioenergetic profile from oxidative metabolism to glycolysis and attenuates TNFα-induced inflammatory responses. Sci Rep 12:3049. 10.1038/s41598-022-07024-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Baik SH, Kang S, Lee W et al (2019) A breakdown in metabolic reprogramming causes microglia dysfunction in Alzheimer’s disease. Cell Metab 30:493-507.e6. 10.1016/j.cmet.2019.06.005 [DOI] [PubMed] [Google Scholar]
- 83.Suhail H, Nematullah M, Rashid F et al (2023) An early glycolysis burst in microglia regulates mitochondrial dysfunction in oligodendrocytes under neuroinflammation. iScience 26:107921. 10.1016/j.isci.2023.107921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sadatomi D, Nakashioya K, Mamiya S et al (2017) Mitochondrial function is required for extracellular ATP-induced NLRP3 inflammasome activation. J Biol Chem 161:503–512. 10.1093/JB/MVW098 [DOI] [PubMed] [Google Scholar]
- 85.Duchen MR (2000) Mitochondria and calcium: from cell signalling to cell death. J Physiol 529:57–68. 10.1111/j.1469-7793.2000.00057.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.von Mücke-Heim IA, Martin J, Uhr M et al (2023) The human P2X7 receptor alters microglial morphology and cytokine secretion following immunomodulation. Front Pharmacol 14:1148190. 10.3389/fphar.2023.1148190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bsibsi M, Persoon-Deen C, Verwer RWH et al (2006) Toll-like receptor 3 on adult human astrocytes triggers production of neuroprotective mediators. Glia 53:688–695. 10.1002/glia.20328 [DOI] [PubMed] [Google Scholar]
- 88.Hashioka S, Klegeris A, Schwab C, McGeer PL (2009) Interferon-γ-dependent cytotoxic activation of human astrocytes and astrocytoma cells. Neurobiol Aging 30:1924–1935. 10.1016/j.neurobiolaging.2008.02.019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets that support the findings of this study are available from the corresponding authors upon reasonable request.