Abstract
Traditional decision-making models conceptualize humans as adaptive learners utilizing the differences between expected and actual rewards (prediction errors, PEs) to maximize outcomes, but rarely consider the influence of violations of emotional expectations (emotional PEs) and how it differs from reward PEs. Here, we conducted a fMRI experiment (n = 43) using a modified Ultimatum Game to examine how reward and emotional PEs affect punishment decisions in terms of rejecting unfair offers. Our results revealed that reward relative to emotional PEs exerted a stronger prediction to punishment decisions. On the neural level, the left dorsomedial prefrontal cortex (dmPFC) was strongly activated during reward receipt whereas the emotions engaged the bilateral anterior insula. Reward and emotional PEs were also encoded differently in brain-wide multivariate patterns, with a more sensitive neural signature observed within fronto-insular circuits for reward PE. We further identified a fronto-insular network encompassing the left anterior cingulate cortex, bilateral insula, left dmPFC and inferior frontal gyrus that encoded punishment decisions. In addition, a stronger fronto-insular pattern expression under reward PE predicted more punishment decisions. These findings underscore that reward and emotional violations interact to shape decisions in complex social interactions, while the underlying neurofunctional PEs computations are distinguishable.
Subject terms: Morality, Human behaviour
Reward and emotional evaluations interact to guide decisions regarding the punishment of a social norm-violating proposer, with these processes being mediated by distinct neurofunctional computation.
Introduction
Scaling the discrepancy between actual and anticipated reward or punishment (prediction errors, PEs), critically guides adaptive social behavior, which is essential for survival and personal development1. Traditional learning and value-based decision-making models posit that individuals act as adaptive learners and utilize reward PEs to maximize their reward while minimizing costs2,3. This perspective is supported by convergent evidence from animal models that highlight the crucial role of dopamine reward PE signaling in driving approach learning towards rewarding stimuli4,5. However, in humans the emotional reaction towards unexpected gains and losses may additionally impact decision-making processes6. Indeed, a positive PE (i.e., obtaining a better outcome than expected) reflects a pleasant surprise, elicits hedonic experiences and motivates the future pursuit of rewards, whereas a negative PE (i.e., obtaining a worse outcome than expected) evokes negative emotions such as disappointment and frustration, ultimately leading to avoidance4. When making decisions, humans frequently engage in anticipation of the hedonic valence (pleasure or pain) associated with future outcomes. The accuracy of those prediction holds paramount importance, as an overestimation of pleasure pertaining to favorable outcomes can lead to risky choices, while an overestimation of the aversive experience for unfavorable outcomes may lead to avoidance and result in missed opportunities7.
Within an experimental context the corresponding complex decision process has been extensively examined using the classic social context-dependent Ultimatum Game (UG). In this economic exchange paradigm one player proposes a division of a sum of money and the other player (responder) can either accept or reject the offer (in which case neither the proposer nor responder receives any money). From an economic perspective, the rational decision would be to accept even small offers to maximize reward, yet humans frequently reject offers that they consider unfair. The underlying decision-making process has commonly been explained in terms of a negative reward PE signaling (i.e., receiving the (lower) actual offer than expected) and the behavior may serve to “punish” individuals who violate social fairness norms8. However, humans do not solely build their models about the environment on reward computations and accumulating evidence indicates that humans establish complex mental models to accurately predict their own and others’ emotional experience9. Recent supportive works have for instance demonstrated that the anticipation of regret strongly impacts decision-making10,11.
While numerous studies have employed computational models to determine the behavioral and neural dynamics of classic reward PEs during social learning, initial studies reconcile that the anticipated emotions also affect decisions. Within a reinforcement learning (RL) framework, the Rescorla-Wagner RL model appears suitable to explain social learning mechanisms7,12. For instance, the learning rate at which people recalibrate their social expectations quantifies the extent to which PEs are integrated into the updating of reward values13. In real-world social interactions, however, the basic RL model does not take into consideration the complex social contexts or associated emotional reactions. Sophisticated studies have begun to explore the impact of emotional experiences on RL-based learning in social contexts, demonstrating that empathy influences the prosocial learning rate14,15 and acquiring knowledge about others’ emotions facilitates inferences regarding the informational value of social cues16. A recent study by Heffner and colleagues utilized a modified version of the UG that required participants to report their anticipated and actual emotional state for (rather unfair) monetary offers and could demonstrate that emotional, rather than reward PEs are critical determinants for punishing unfair offers in an UG17. While these studies suggest a critical role of reward and emotional evaluations in social decisions, it remains unclear how the emotional and reward PEs are generated in the brain and uniquely shape decisions.
Although considerable evidence has outlined that a common ‘neural currency’ underlies evaluation of rewards18,19, it remains unknown if reward and emotional PEs are mediated by common or distinguishable neurofunctional computations. The striatum has been consistently identified as a key brain system for encoding reward PE during non-social learning20; however in the context of social reward learning entailing additional social and emotional processes a broader brain network is recruited. When socially learning about other’s actions and outcomes, RL-like PEs with respect to expectations formed about how others viewed the self are revealed in the activity of anterior insula (aINS), anterior cingulate cortex (ACC) and orbitofrontal cortex (OFC)21. Additionally, the aINS has been proposed to mediate approach and avoidance in response to social affective stimuli22,23, as well as the experience of aversive emotional states24. In social economic games such as the UG with inherently intertwined norm violations and aversive emotional states, activation of the aINS has been associated with the willingness to reject unfair offers25, possibly reflecting the involvement of the aINS in encoding both aversive and positive reward PEs26. Moreover, numerous studies have explored the computational function of the ACC for encoding expectations and PEs related to others’ decisions during social RL learning27, and further proved significant correlation of prosocial PE signals with levels of empathy28. Encoding of social reward-related PEs by ACC neurons may reflect the relevance of other individuals to one’s own emotional states, thereby eliciting changes in emotional arousal29. Finally, the OFC plays an important role in self-reference, including prioritized encoding of self-referenced rewards in PEs30. However, whether and how these brain systems process emotion and reward PEs as well as their distinct contributions to these two computational decision features remain to be explored.
Against this background we conducted a functional magnetic resonance imaging (fMRI) experiment (Fig. 1a) with a modified UG paradigm on N = 43 healthy participants to examine the cognitive and neural basis of reward and emotional PEs in social value-based decision-making. In the modified UG paradigm, participants were required to predict how much reward they would get from the proposer and then predict how they feel on two emotional dimensions (valence and arousal) while again reporting their emotional states when receiving the actual offer and finally deciding whether to accept or reject the proposer’s offer (Fig. 1b). When the offers are accepted, the monetary allocation will be made according to the offer, whereas when offers are rejected, neither the proposer nor responder receives any money (punishment decisions)17. On the behavioral level logistic mixed-effects regression models were employed to determine the predictive capacity of emotional and reward PEs to punishment decisions (Fig. 1c). On the neural level, we aim to: (1) investigate the distinct-neural systems that support the reward and emotion processing during prediction and experience-moments of money split, (2) reveal the multivariate neural patterns that are sensitive to capture variations of emotional and reward PEs and demonstrate whether these neural patterns are dissimilar using machine-learning based neural decoding approaches (given the higher precision of this approach to establish cognitive processes specific neural signatures31,32), (3) identify multivariate neural patterns of punishment decisions, and finally explore whether reward or emotional neurofunctional PEs representation predict the neurofunctional punishment decisions to reject an (unfair) offer (Fig. 1c).
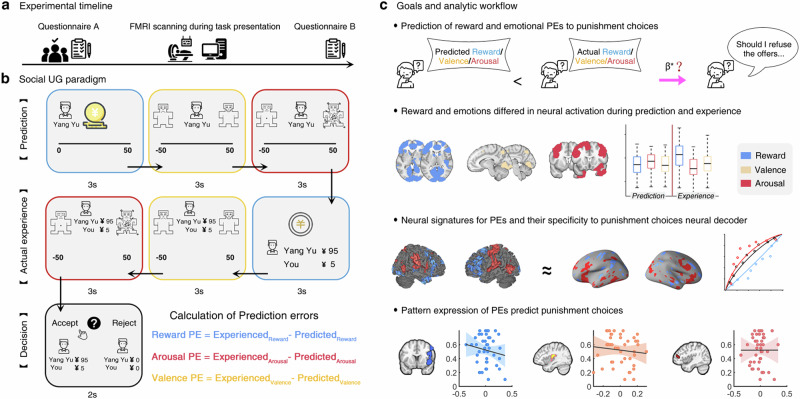
Fig. 1. Experimental protocol, task design, and main goals and corresponding analytic workflow.
a Experimental timeline. The questionnaire A and B include positive and negative affect scale, and state-trait anxiety inventory. b The modified Ultimatum Game task and PEs computation. Before each offer individuals predict how much money they anticipate to receive from the proposers, and how they would feel in terms of valence and arousal when receiving the anticipated offer. After receiving the actual offers, individuals report their current actual emotional experience in terms of arousal and valence and finally decide either to accept or to reject the offer. Main behavioral outcome where the computed reward and emotional PEs in terms of establishing trial-by-trial PEs: a reward PE (color coded in blue), an arousal PE (color coded in red) and a valence PE (color coded in yellow) scaling the difference between subjects’ prediction about the reward or emotion and their actual experience. c Major goals and analytic workflow of the current study. On the behavioral level, the predictive contribution of the reward and emotional PE to the decision to punish the proposer (i.e. rejecting the offers) is determined, modeling of the simultaneously acquired fMRI data aim at: i) determining the univariate activation profiles of reward and emotional experience during the prediction and experience period to find separable neural underpinnings of reward and emotional, ii) training distinct multivariate neural patterns of emotional and reward PEs, as well as of punishment decisions to further indicate which neural pattern of PEs is specific to that of punishment decisions, iii) finally examining the links between the multivariate neural patterns for punishment decisions and all PEs to elucidate the neural pathway underlying the effects of reward and emotional PEs in social choices. Image in a–c were obtained from Flaticon.com under the free license with attribution.
Results
Prediction of reward and emotional PEs to punishment decisions
With the purpose of capturing participants’ punitive responses to unfair offers when expected rewards and emotions were violated in a dyadic social interaction, we employed a previously validated adaptation of the UG paradigm17. In this modified UG task, participant played as responders to interact with a proposer via: (1) firstly report how much money they would expect to obtain from the proposer and provide emotional ratings along the two dimensions (valence and arousal) to indicate their expected emotional responses if they receive the anticipated monetary split, and (2) secondly report the actual emotional states upon seeing the actual offers and finally decide whether to accept or reject the proposer’s offers. This design allowed to measure the violations of reward and emotion (i.e., reward and emotional PEs) by calculating the differences between the predicted versus actual money amount/ (un)pleasantness/ arousal.
In the realm of social interactions, complex social behaviors, such as the formation of alliances with peers, are theorized to be driven by the violation of expected outcomes including reward and emotions. We therefore tested the predictive role of reward and emotional PEs to the decisions about punishing a norm-violating proposer (i.e., rejecting the offers) via using a logistic mixed-effects regression model. Our results revealed that both reward and emotional PEs significantly predicted punishment decisions, such that participants showed higher punishment rates when experiencing less reward (β = −3.93 ± 0.46 (standard error), Z = −8.51, p < 0.001) and lower pleasant (β = −1.54 ± 0.29, Z = −5.27, p < 0.001) but higher intense feelings (β = 0.93 ± 0.20, Z = 4.71, p < 0.001) than anticipated (Fig. 2a). Moreover, we also employed β coefficient tests to compare the predictive power of reward and emotional PEs and found that the reward PE had a significantly stronger impact on motivating punishment choices than emotional PEs (reward PE vs valence PE, Z = −4.37, p < 0.001, reward PE vs arousal PE, Z = −8.89, p < 0.001).
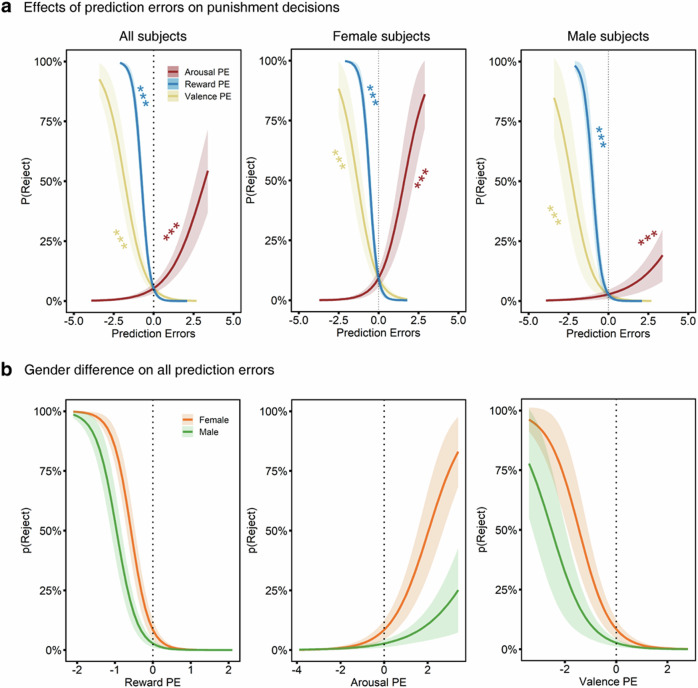
Fig. 2. Predictive role of the different prediction errors for the decision to punish a social norm-violating proposer.
a All PEs showed a significant predictive effect on punishment decisions such that participants showed higher punishment rates when they experienced less reward or lower valence but higher arousal than anticipated. b There was no significant gender difference on the reliance of all PEs to make punishment decisions. The lines reflect the probability of different choice pairs including rejecting versus accepting in the Ultimatum Game task, and the negative values represent negative PEs, suggesting less reward, less pleasantness, as well as less arousal than expected. Shaded areas reflect ± 1*standard errors. ***p < 0.001.
Given that men and women are differed in the behavioral or neural responses to reward and punishment33,34, as well as to the emotion evaluation35,36, further exploratory analyses examined potential sex differences were conducted by repeating the regression models in male and female individuals, separately. The results showed that all PEs robustly predicted punishment decisions (Female: reward PE, β = −4.03 ± 0.59, Z = −6.84, p < 0.001, valence PE, β = −1.71 ± 0.43, Z = −4.02, p < 0.001, arousal PE, β = 1.41 ± 0.38, Z = 3.68, p < 0.001; Male, reward PE, β = −3.93 ± 0.74, Z = −5.31, p < 0.001, valence PE, β = −1.47 ± 0.39, Z = −3.79, p < 0.001, arousal PE, β = 0.70 ± 0.18, Z = 3.83, p < 0.001, Fig. 2a) while the reward PE still had a higher predictive capacity than emotional PEs (Female, reward PE vs valence PE, Z = −3.19, p < 0.001, reward PE vs arousal PE, Z = −7.48, p < 0.001; Male, reward PE vs valence PE, Z = −2.94, p < 0.01, reward PE vs arousal PE, Z = −5.54, p < 0.001) across male and female subjects. This result was consistent with the subsequent finding that female and male subjects exhibited a comparable reliance on all PEs to make punishment choices (reward PE × gender, β = 0.40 ± 0.79, Z = 0.50; valence PE × gender, β = 0.23 ± 0.53, Z = 0.43; arousal PE × gender, β = −0.44 ± 0.39, Z = −1.14; all ps > 0.25, Fig. 2b). Therefore, given the absence of sex differences in the evaluation of various PEs and their influences on punishment decisions, sex was not considered in the following fMRI analyses.
Neural activation for reward and emotion during prediction and experience stages
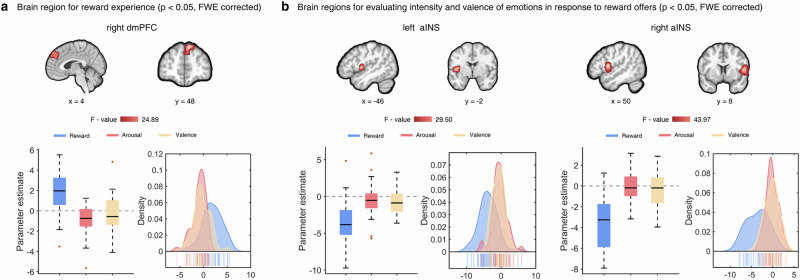
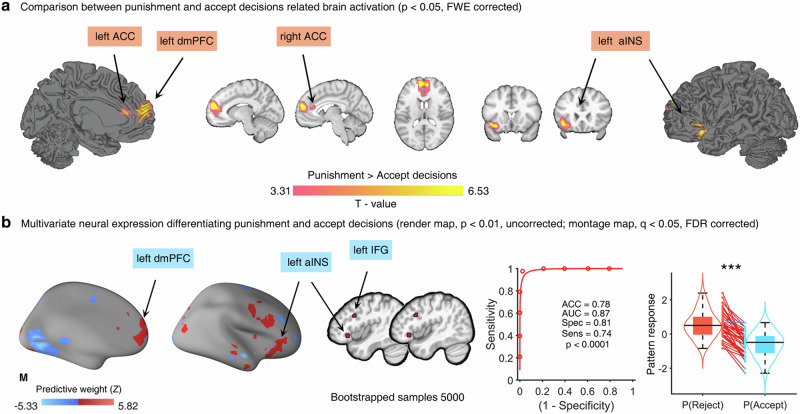
Assessment of reward and emotions typically involves common but partly distinct-neural networks: the fronto-striatal circuits are primarily engaged in processing motivational aspects of rewards37,38, while the prefronto-insula-amygdala pathways are directly implicated in the response to negative emotional stimuli31,32,39. However, whether this differentiation exists in dynamic social interactions remains unclear. We thus aimed to determine whether the anticipation and outcome evaluation on the reward and emotional level were supported by different brain systems by examining brain activation differences for reward and emotions separately for the prediction and experience stages of the experiment. While we did not observe that different regions were involved during predicting rewards and emotions, results suggested that the left dorsomedial prefrontal cortex (dmPFC, peak MNI coordinates, x/y/z = 10/48/46, F = 24.89, PFWE-peak < 0.001, k = 566, Fig. 3a) and bilateral aINS (left aINS, peak MNI coordinates, x/y/z = −46/−2/4, F = 29.50, PFWE-peak < 0.001, k = 267; right aINS, peak MNI coordinates, x/y/z = 52/6/10, F = 43.97, PFWE-peakr < 0.001, k = 653, Fig. 3b) were engaged differently during the reward and emotional evaluation stage. We next explored specific activation patterns for the experienced reward and actual emotional states via plotting the parameter estimates from the identified dmPFC and aINS regions (without computing further statistics on the regional parameter estimates to avoid circularity40,41.
Fig. 3. Brain regions processing the reward experience and emotional response to monetary offers, respectively.
a The left dmPFC was strongly engaged during reward experiences, while emotion processing regions such as the bilateral aINS showed increased activation for evaluating the intensity and valence of emotions upon receiving the actual split of the monetary offer (b). For the purpose of illustrating the specific activation patterns for the reward experiences and the corresponding emotion evaluations, the parameter estimates extracted from spherical (radius: 8 mm) regions of interest in the identified dmPFC and aINS regions were presented with box and density plots. The error bars reflect minimum and maximum value of the data, while the thick lines inside the box represent median value of the data. The red points represent outliers. The shaded areas indicate the distribution of regional extracted parameters for reward experience and emotional response to monetary offers.
Examination of extracted parameter estimates (spherical masks, radius: 8 mm) revealed that the right dmPFC was strongly activated during reward experience (Fig. 3a), while the bilateral aINS was primarily activated during the subjective reflection on the valence and arousal of emotions induced by the violations of reward or emotional expectations (Fig. 3b). Overall, these results indicate that the dmPFC encodes the experience of reward while the aINS may underpin encoding the intensity and valence of emotions in response to (unfair) offers.
Neural signatures of reward and emotional PE
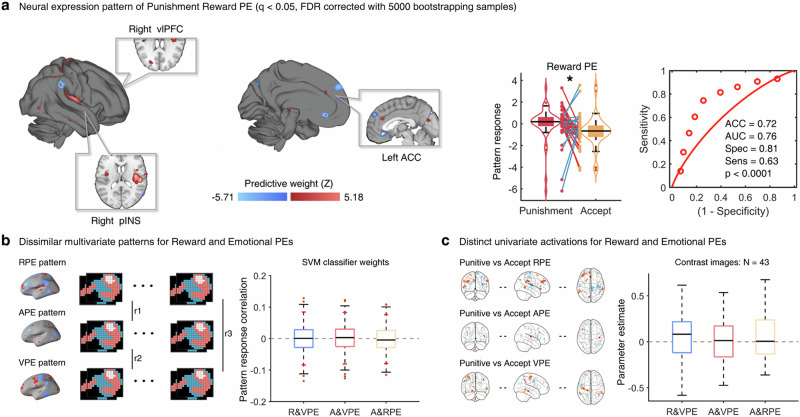
While we identified distinct-neural regions associated with reward experience and the associated emotional states, our experimental design enabled us to further explore whether the reward and emotional PEs are encoded by separate neural circuits. In addition, given the high sensitivity of multivariate pattern analysis42,43 with respect to segregating cognitive and emotional processes, we developed the support vector machine (SVM) classifiers for reward and emotional PEs separated for punishment and accept decisions. To ensure more specific models we initially modeled the reward, arousal and valence PE during each of the three time points (reward receipt, valence and arousal rating) in general linear models (GLM) and included the corresponding individual first-level GLM contrast into the SVM classifier models. Examination of the reward PE during the time period of reward receipt confirmed previous works21,25,26,44,45 such that multivariate predictive expressions in the ventromedial prefrontal cortex, dorsolateral prefrontal cortex and dmPFC predicted the reward PE under accept decisions (Supplementary Table 1), whereas multivariate expression in the right posterior insula (pINS), right ventrolateral prefrontal cortex (vlPFC), and left ACC classified reward PE during punishment decisions (accuracy, 0.72, sensitivity and specificity, 0.63, 0.81, separately, p < 0.0001, Fig. 4a). Classification accuracies for emotional PEs separated by punishment and accept decisions remained at chance level (arousal PE, mean ACC = 0.50; valence PE, mean ACC = 0.59, Supplementary Table 2) even though they were modeled at the corresponding time points of emotional ratings.
Fig. 4. Multivariate neural expressions of reward and emotion PEs and their distinction.
a Whole brain multivoxel pattern for differentiating punishment and accept reward PE included the right pINS, right vlPFC and left ACC as key contributors to the. The violin and box plots show the distributions of multivariate neural pattern map response to classify the reward PE separated by punishment and accept decisions, reflecting that the reward PE signature exhibited a stronger responses for the punishment decisions compared to accept decisions, t(42) = 2.16, p = 0.04, two-tailed, paired t-test. The box is bounded by the first and third quartiles, and the whiskers stretched to the greatest and lowest values within the median ± 1.5 interquartile range, while each colored line between dots represents each participant’s paired data (red line: correct classification; blue line, incorrect classification). The forced-choice classification accuracy was 0.72, p < 0.0001, two-tailed, binomial test. b Bootstrapping test results for SVM classifier weight correlations. The short red lines reflect 95% confidence intervals obtained from bootstrap tests (500 samples). No regions showed significant correlations between SVM classifier weights. (c) Group-level correlations between activation of contrast images for punishment and accept-decision separated reward or emotional PEs. No regions showed significant average correlations between activations of contrast values across participants. The error bars reflect minimum and maximum value of the data, while the thick lines inside the box represent median value of the data. The red points represent outliers. ACC accuracy, AUC area under curve, Spec specificity, Sens sensitivity, *p < 0.05.
To segregate the neural differences between reward and emotional PEs, a pattern similarity analysis based on multivariate neural patterns of all PEs and a group-level correlation analysis between activations of contrast images for PEs were employed. We found no significant correlation between the whole brain multivariate expression weights for reward and emotional PEs (95% confidence intervals (CI) obtained from bootstrap tests (500 samples) all included zero) or contrast images for reward and emotional PEs for the specific decisions (all ps > 0.05, Fig. 4b). Together the findings indicate a sensitive neural pattern for reward PE in the frontal-insular circuit which was distinct from emotional PEs.
Univariate activation and multivariate expression pattern for punishment decisions
In line with previous studies systematically mapping neural activation related to social decisions25,46, we examined the brain regional activation for the differences between punishment and accept decisions and observed a fronto-insular network encompassing clusters located in the left dmPFC (peak MNI coordinates, x/y/z = −10/56/16, T = 6.13, PFWE-cluster < 0.05, k = 996), bilateral ACC (peak MNI coordinates, left ACC: x/y/z = −10/48/14, T = 4.71, PFWE-cluster < 0.05, k = 143, right ACC: x/y/z = 6/48/14, T = 4.05, PFWE-cluster < 0.05, k = 76) and left aINS (peak MNI coordinates, x/y/z = −42/20/−10, T = 6.53, PFWE-cluster < 0.05, k = 549, Fig. 5a) showing increased activation for punishment decisions. Further analyses using machine-learning-based whole brain multivariate analyses47 via SVM classifier across individuals discriminated punishment and accept decisions with high accuracy (0.78), high specificity (0.81) and high sensitivity (0.74). This neural pattern encompassed the left dmPFC, left aINS, and left inferior frontal gyrus (bootstrapped 5000 samples, FDR corrected, p < 0.05, Fig. 5b), which showed robust contribution to predict the choices of rejecting unfair offers.
Fig. 5. Univariate activations and multivariate expression patterns for punishment decisions.
a Univariate activation for the difference between punishment and accept decisions showed that the bilateral ACC, left dmPFC and aINS were activated strongly for punishment choices (reject the unfair offer). b Whole-brain multivariate neural expression classifying the punishment and accept decisions suggested that regions such as the left dmPFC, aINS and inferior frontal gyrus made stable prediction to punishment decisions. The violin and box plots show the distributions of responses of the multivariate neural pattern in the classification of punishment and accept decisions, indicating a higher level of reactivity to the punishment compared to accept decisions, t(42) = 10.85, p < 0.001, two-tailed, paired t-test. The box is bounded by the first and third quartiles, and the whiskers stretched to the greatest and lowest values within the median ± 1.5 interquartile range, while each colored line between dots represents each participant’s paired data (red line: correct classification; blue line, incorrect classification). The forced-choice classification accuracy was 0.78, p < 0.001, two tailed, binomial test. ACC accuracy, AUC area under curve, Spec specificity, Sens sensitivity, ***p < 0.001.
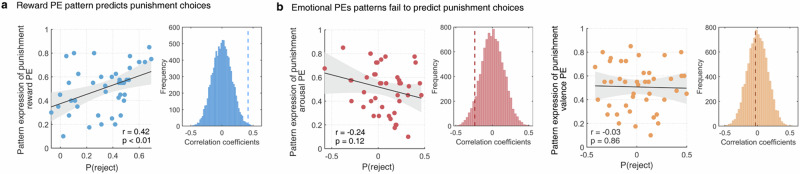
Given that reward and emotional PEs can directly predict the punishment decisions to reject the unfair offers on the behavioral level, we further explored whether the patterns expression of PEs neural signatures were related to the multivariate expression of punishment decisions by examining correlation between the group-average reward or emotional PEs signatures responses and the group-average neural response of punishment decisions. Results demonstrated – to a certain extent – the specificity of the reward PE pattern to predict the punishment decision expression (r = 0.29, p < 0.01, for emotional PEs, all ps > 0.05).
The neural signature of reward PEs predicts punishment decisions
Although we found high specificity of the reward PE (relative to emotional PEs) decoder to the neural expression of punishment decisions, the behavioral relevance remained unclear. We therefore tested whether the reward PE (relative to emotional PEs) decoder could directly predict punishment decisions on the behavioral level by employing the correlation analyses. Our results found that heightened patterns expressions of reward PE (as decoded under punishment decision condition) within the fronto-insular network were significantly correlated with an increased propensity for punishment decisions (higher rates at rejecting unfair offers, r = 0.42, p < 0.01, Fig. 6a), while this effect was not observed for the emotional PEs (all ps > 0.10, Fig. 6b). Taken together, combined with our observation of a higher sensitivity of the neural signature for reward PE (relative to emotional PEs), the associations of the reward PE on the neural and behavioral level with punishment decisions, findings underscore that the reward PE exerts a strong influence on the decision to punish a proposer allocating unfair offers.
Fig. 6. Exploratory correlation between neural signatures of PEs and punishment decisions.
a The scatter plots reflect that stronger pattern expression within frontal-insular network representing punishment reward PE was significantly correlated with increased number of punishment choices across participants, while this association was diminished for emotional PEs (b). The histograms show correlation coefficients from permutation tests, whereas the dashed lines represent the true correlation.
Discussion
Traditional neurobiological decision-making models have determined the critical role of reward PE signaling in fronto-striatal circuits in shaping choices and learning across contexts48. However, the accompanying emotional response scaling the discrepancy between the expected and actual experiences as well as the underlying neurocomputational mechanism and whether they differ and interact with reward PEs have not been systematically determined. Here we combined a recently developed and modified UG paradigm which allows to quantify differences between experienced and predicted monetary reward and emotion evaluations (along valence and arousal dimensions) in terms of reward and emotional PEs, respectively, with fMRI and multivariate predictive neurofunctional decoding to determine common and distinct influences of reward and emotional PEs on the punishment decision to reject an unfair offer and the underlying neurocomputational representations. Our behavioral results confirmed that both reward and emotional PEs could significantly predict punishment decisions, with participants punishing at higher rates when experiencing less reward or pleasantness or more arousal than expected. However, the reward PE exerted a comparably stronger impact on motivating punishment choices compared to both emotional PEs. On the neural level, reward and emotional processes exhibited distinct-neural representations across univariate and multivariate analyses. The experience of reward engaged the left dmPFC whereas the bilateral aINS was engaged during actual emotional response to money split offers. Moreover, reward and emotional PEs were encoded in distinguishable brain-wide neural patterns. During the decision stage a fronto-insular network including the bilateral ACC, left aINS, left dmPFC and IFG increased activation and neural expression for punishment decisions, while the multivariate signatures of these regions closely resembled the distributed reward, yet not emotional PE signatures. In support of this an exploratory correlation analysis further demonstrated that a higher fronto-insular pattern expression under punishment reward PE predicted the subsequent punishment decisions following unfair offers. Taken together, these findings shed lights on the computational and neural mechanism that distinguish emotional and rewards evaluation and how violations of expected rewards and emotions determine social decision-making.
Rewards serve as an essential motivational driver that shapes adaptive decisions to optimize reward outcomes and to avoid negative consequences49. Classical models of learning and decision-making have focused solely on reward and conceptualized individuals as optimal learners striving for maximizing expected rewards50–52. Despite the normative appeal of those models in describing how humans evaluate and decide based on the differences between expected and actual reward outcomes, these models do not provide a clear psychological mechanism to account for the accompanying affective processes (e.g., emotion) and their influences on decision-making. Punishment and uncooperative decisions are driven by a diverse array of negative emotions, including sadness and disappointed53. In relation to reward computations a positive reward PE signaling that the experienced reward exceeds expectations can evoke pleasant feelings while a negative reward PE may induce strong negative emotions such as disappointment or anger4. Utilizing computational modeling recent studies found that momentary emotions are not only explained by reward outcomes but rather by the combined influence of reward expectations and PEs that depend on these expectations51,54. Together, this suggests that reward PEs shape not only future decisions but also the momentary emotional state which in turn may impact the decisions. In line with this hypothesis, a recent study demonstrated that violations of expected emotions (in particular valence) linked with expected and experienced rewards predict punishment choices in response to unfair offers stronger than reward PEs17. Partly consistent with this work, our results suggest that emotional and reward PEs are intertwined to guide social decisions, such that both violations of reward and emotional predictions lead to punishment choices towards unfair offers.
On the neural level, we initially examined whether the emotional and reward processes are localized in separable neural systems and found that univariate activation in the dmPFC or aINS characterized reward experiences or emotional responses to monetary split proposals, respectively. Numerous previous neuroimaging studies have suggested an important role of the dmPFC in metacognitive effort cost valuation during reward decisions55,56. However, this view has been recently challenged by findings indicating that the dmPFC is a crucial site for deploying learnt reward values in action selection particularly during social inference57,58. Supporting this role of the dmPFC, a recent rodent study reported dmPFC neuronal activity accurately in predicting reward availability and during the initiation of conditioned reward seeking after cue-reward learning59. In contrast, the aINS exhibited a stronger engagement during the report of actual intensity and valence of emotions upon receiving the offer. In the context of unfair monetary treatments, people would generate negative emotions especially when they obtained less reward amount than expected. This may increase the activation of aINS that plays an important role in the evaluation and experience of aversive experiences, such as pain, disgust60, introception and approach-avoidance decisions in social contexts23,61 and witnessing unfair transactions or unmoral acts25,45.
Our further multivariate predictive modeling also revealed distinct distributed neural representations of emotional and reward PEs. However, we did not obtain a multivariate decoder that could robustly and sensitively capture variation of emotional PEs. Despite that, a recent study utilizing the data from this modified UG paradigm have determined that unfair offers indeed evoke a strong aversive emotional response within subcortical regions for avoidance responses (amygdala, PAG, thalamus, putamen) and cortical systems involved in emotional appraisal such as the insula, dorsal ACC and lateral frontal regions31. This may provide some inspiration for understanding the possible neural pathways underlie the emotional PEs and could further support spatial dissimilarity between emotional and reward PEs, since the reward PE was implicated in a concentrated frontal-insular network encompassing the left ACC, right vlPFC and pINS. These findings align with previous studies that negative reward PE followed by unfair offers are embedded in the typical brain regions engaged in generating PE-like signaling for unexpected reward outcomes (ACC)44 and anticipation of uncertain punishments or rewards with the same neural codes (vlPFC)62. Our results, however, extend more broadly to indicate that negative reward PE computed based on the unfair offers is also represented in the region for interoceptive processes63 and exerting regulatory top-down control over reward-related behaviors via its projections to the nucleus accumbens24.
We next determined that the neural responses of fronto-insular network encompassing the dmPFC, aINS, ACC and IFG represent or predict punishment decisions through both univariate and multivariate analytic approaches. The dmPFC and aINS exhibited the most stable predictive weights across univariate and multivariate analyses. These findings are congruent with the previously described role of the dmPFC in personal moral decisions, functioning as a conflict monitor in response to inequality of economic offers64,65 and self-disadvantageous unfairness66. During human cooperation, the dmPFC is important for modulating outcome value signals for oneself and others to guide behavior appropriate to the local social context67. Similar to the functions of dmPFC in social decisions, the aINS holds independent neural populations responding specifically to aversive states in a disadvantageous-inequality processing60,68 and showing heightened activity when rejecting unfair offers25,69. In particular, the functional integration between ACC and aINS is enhanced when individuals make dishonest decisions for a monetary reward, as it requires high need for emotional processing and conflicts detection70, which can also be generated during evaluating outcomes of self and proposers in our task. Significant activation of the aINS and IFG can be simultaneously observed when asking subjects to reappraise the proposers’ money dividing intentions as more negative and is linked with a greater number of unfair offers rejected71. However, currently the specific contributions of the separate systems in terms of punishment decisions during norm violations remain unclear. Our findings may bridge this gap by determining which PE representation significantly predict punishment choices. Within this context our predictive models demonstrated high specificity of fronto-insular neural predictive weights for the reward but not the emotional PEs to the multivariate response of punishment decisions. Moreover, we revealed that aINS and ACC generally engaged in unfairness perception and rejection of unfair offers given that they demonstrated increased pattern expression of punishment reward PE to significantly predict behavioral punishment choices. Overall, these findings underscore the critical role of reward PE coding in the fronto-insula system on decisions in response to unfairness.
This work extends traditional learning and economic theories50,52 by highlighting the crucial contribution of both violations of expected emotion and rewards to the decisions. The prevailing theories posit that humans make decisions via assigning values to prospective gains or loss52, and have only implicated the activation within frontal-striatal circuits in representing value of anticipated or received rewards48. We updated this view by demonstrating that the neurofunctional computation of reward and emotional states are supported by distinguishable brain systems, such that the dmPFC encoded the receipt of the actual reward whereas the aINS encoded the intensity and valence of emotions in response to money split proposal. Utilizing a more rigorous machine-learning based neural decoding method, the multivariate neural patterns between reward and emotional PEs were also found dissimilar. In general, our findings extend influential theories of decisions which mainly focused on reward effects on choice behaviors and emphasize the importance of considering emotions in economic models, especially how these factors are dissociable from neural pathways during social decisions.
It is worth highlighting potential limitations of the present study. Although we demonstrated consistent behavioral results via the use of multiple logistic regression models17, the dynamic changes of subjects’ reliance on those PEs to make final decisions are not clear. This concern could be well resolved if future studies employed computational modeling to track the trial-wise updates of PEs and the relevant decision variations. Additionally, no direct modulation of the underlying neural processes was included and future studies may consider employing pharmacological approaches to regulate associated signaling systems such as the dopamine72, oxytocin73 or angiotensin74,75 and to determine whether separable signaling systems underlie emotional and reward PEs.
In conclusion, our study supports the dissociable contribution of emotion and reward evaluations to social decisions by identifying distinct brain regions engaged in emotion and reward processing during reward receipt, and proving dissimilar multivariate neural patterns between PEs generated from those experiences. Despite the contribution of both emotional and reward PEs to social decision-making, the latter could strongly guide social decisions given that the frontal-insular predictive neural expression of reward PE specifically correlates with multivariate pattern of punishment decisions and predict behavioral punishment choices. As such, our findings pave the way towards more precise understanding about how emotions and rewards distinctively represented in the brain to affect social decisions, and may have further implications on clinical disorders with deficient reward and emotion processing in complex social contexts.
Materials and methods
Participants
The study was approved by the Research Ethics Committee of the University of Electronic Science and Technology of China (1061422101024711) and adhered to the latest revision of the Declaration of Helsinki. We employed an fMRI experimental design with N = 50 (25 Female, 25 Male) individuals. Exclusion criteria for the enrollment were: (1) an excessive head movement (>2 mm translation or 2° rotation), (2) a current or a history of psychiatric, neurological, or other medical disorders, (3) current or regular use of psychotropic substances including nicotine, (4) a body mass index < 18 or > 24.9, (5) visual or motor impairments, and (6) contraindications for MRI. A total of 7 subjects were excluded due to fMRI data acquisition failure (fMRI technique issue and withdraw during the experiment, n = 3) and no punishment decisions (not rejecting the unfair offer, n = 4), leading to a final sample of N = 43 (23 Female, Mean ± SD, age = 21.57 ± 2.15 years; 20 Male, age = 20.90 ± 2.07 years) included into main analyses. Both female and male participants were comparable with respect to the sociodemographic and pre-fMRI mood indices arguing against possible gender bias on the following results (all ps > 0.06, Supplementary Table 1).
Experimental paradigm
An adapted and validated reward-emotion UG paradigm was employed17 (Fig. 1). Prior to this task, we asked participants to perform a practice protocol with an anonymous stranger during which they familiarized themselves with the paradigm. Participants reported understanding the conceptions of approaching rewards and emotions (i.e., valence—how unpleasant/pleasant you would feel if you obtained expected money, arousal - how calm/excited you would be if you obtained expected money) and showed a corresponding response pattern for expected rewards in the practice trial. All participants had the opportunity to ask questions and request further classification if necessary. At the start of the experiment, each participant was informed that they would play an economic-cooperation game with a proposer in complete anonymity. The participants were assigned the role of responder who would decide of whether accept or reject an offer made by the proposer17. Given that the characteristics of proposer such as facial trustworthiness76,77 and physical attractiveness78,79 can influence a responder’s decisions, participants did not meet the proposer and were informed that there was a another participant acting as a proposer in the experimental rooms of the lab. Moreover, to avoid confounding effects of gender77,80,81, participants were assigned to a proposer with a female or male name (same gender as the participant). The proposer’s name would be presented in the initial introduction screen, as well as in each trial to ensure that participants would clearly know who was deciding the amount of money they will receive. Followed by the introduction presentation, participant would give the following responses via button press before each offer: 1) judging how much money (within a range of ¥2.5 to ¥50, 20 offers) they would expect to receive from the proposer on a 50-point scale (indicating ¥0 to ¥50); and 2) rating the emotions that they expected to feel if they obtained anticipated monetary split along the two dimensions, valence (i.e., positive vs negative emotion) and arousal (i.e., intensity/strength of emotion) that was based on a scale ranging from −50 (Very negative/calm) to 50 (Very positive/aroused). Following the offer all individuals reported: (1) their actual emotional experience upon receiving the offer; and (2) decided whether to accept or reject the proposer’s offers. A total of 40 task trials – dispersed across two fMRI runs with 20 trials each (2 trials per actual offer) were presented. Each task trial began with a fixation cross presented for a jitter interval of 500 ms followed by the presentation of expected money (3 s) and anticipated emotion (3 s), actual offer (3 s) and evaluation of current emotional experiences (3 s), as well as the decisions periods (2 s) prior to a jittered intertrial interval (mean duration 2 s). Our trial duration was consistent with recommendations for event-related fMRI designs82–84 and several previous studies employed the identical trial periods in tasks investigating social and monetary reward learning85,86. The offers followed a uniform distribution allowing each participant to respond to the full range of fair (¥50 vs ¥50) to unfair offers (¥97.5 vs ¥2.5), whereas the order of the offers was randomized.
MRI acquisition, preprocessing and first level analysis
MRI data were acquired on a 3 T GE Discovery MR system (General Electric Medical System, Milwaukee, WI, USA) and preprocessed using standard workflow in SPM 12 (Statistical Parametric Mapping; http://www.fil.ion.ucl.ac.uk/spm/; Welcome Trust Centre for Neuroimaging) (see Supplemental Methods).
To explore the brain regions involved in anticipated and experienced reward/emotion, and punishment/accept decisions, we firstly establish a general linear model (GLM) incorporating separate onsets of prediction or experience of reward, arousal or valence, respectively, as well as the decision period, while the six head motion parameters were included as covariates. To further determine the specific neural expressions for reward and emotional PEs in the context of accept or punishment decisions, we designed separate parametric GLMs for the conditions of reward experience and emotional response to monetary split proposals in the present paradigm. These GLM models distinguished between events related to reward receipt, valence and arousal ratings, with each event modulated separately by reward, valence and arousal PEs. By incorporating each PE as a parametric modulator at different time points corresponding to reward receipt and emotional ratings, we were able to conduct a more nuanced analysis of neural activity, facilitating the identification of dissociable neural activation patterns associated with reward, valence, and arousal PEs.
Behavioral analyses
With the purpose of evaluating predictive contribution of all PEs to punishment decisions simultaneously we employed a logistic mixed-effects regression model with three PEs as independent variables and punishment choices as the dependent variable via the lme4 R package (https://cran.r-project.org/). The computation of PEs was based on the disparity between the actual experience at the time of offers and prior expectation. It should be noted that a zero value of PEs indicated instances where participants’ actual experiences corresponded with their expectations, resulting in the absence of errors. As such all PEs were firstly scaled (i.e., PE/ sqrt(sum(PE^2)/length(PE)-1)) but not mean-centered before being included to the regression model to ensure that β coefficients could be comparable. To account for multicollinearity in regression models we first estimated the variance inflation factor (VIF)87 (similar approach see Xu et al., 2020, Li et al., 88,89) with results arguing against problematic collinearity (VIFReward_PE = 1.52, VIFArousal_PE = 1.60, VIFValence_PE = 1.21). We also performed separate regression analyses in female and male subjects to avoid possible gender bias on PEs’ predictive role to punishment decisions.
To further determine the effectiveness of the current paradigm in eliciting reward- and emotion-related responses, as well as to determine the impact of these factors on punishment decisions, we incorporated both expected and actual reward and emotional ratings as predictors in the regression model. Our results indicated that expected reward was a significant positive predictor of punishment decisions (β = 1.11 ± 0.29, Z = 3.82, p < 0.001), whereas no such effect was observed for expected emotions (valence, β = −0.03 ± 0.09, Z = −0.38, p = 0.70; arousal, β = 0.02 ± 0.08, Z = 0.27, p = 0.79). However we revealed significant predictions of both actual reward and emotions to punishment decisions, such that participants made more punishment decisions when they received less reward (β = −7.00 ± 0.84, Z = −8.37, p < 0.001) and perceived less positive (β = −2.47 ± 0.34, Z = −7.21, p < 0.001) but more arousing emotional states (β = 0.89 ± 0.26, Z = 3.40, p < 0.001). Together these findings may reflect that for both reward and emotional reactions the differences in terms of PEs may weight stronger compared to the general baseline expectations. In addition, we also scaled the difference between proposer’s and responder’s reward amounts in terms of unfairness (e.g., ¥92.5 − ¥2.5 = ¥90) and included it with gender in another regression model to predict punishment choices. Unfairness significantly predicted punishment decisions with the effect being equal in female and male subjects (Gender × Unfairness, β = 0.03, z = 0.05, p = 0.96, Supplementary Fig. S1). This confirms a close association between the unfairness manipulation and the punishment decision across both genders.
Univariate voxel-wise analyses
Considering the pivotal role of reward and emotions in guiding decisions, we aim to identify whether the neural activations of reward and emotion were distinct during prediction and experience moments. Given the strengths of univariate voxel-wise analyses in terms of determining the spatial localization of mental processes90, we initially employed this method to examine brain regions that were engaged for reward and emotions (i.e., arousal and valence) during prediction and experience periods, respectively. Specifically, the first-level contrasts corresponding to prediction (Predictionreward, Predictionarousal, Predictionvalence) and outcome periods (Experiencereward, Experiencearousal, Experiencevalence) were included into two separate voxel-wise one-way analyses of variance models. All resulting maps from the whole brain analyses were thresholded at the peak level of family-wise error (FWE) correction at p < 0.05.
Furthermore, to assess the neural activations differences between accepting and punishing decisions, we utilized the univariate voxel-wise one sample t-test on the first level contrast (i.e., Punishment decision > Accept decision) with the resulting t-value maps being thresholded at the cluster level of FWE correction at p < 0.05 (initial cluster threshold, p < 0.001, uncorrected; see recommendations in Slotnick91).
Multivariate voxel pattern analyses
Compared to the traditional univariate analyses, the machine-learning-based multivariate pattern analyses can provide more comprehensive and precise neural representations of cognitive and mental processes31,32. Therefore we utilized a linear support vector machine (SVM, C = 1, linear kernel) classifier to distinct between the neural response to reward and emotional PEs separated by punishment and accept decisions (using the Canlabcore tools, https://github.com/canlab/CanlabCore). However, given that the inherently higher number of unfair offers in our paradigm may lead to unbalanced trials we employed SVM classifier models on single-subject first-level GLM contrast images (given that the larger number of subject-level images may better account for the imbalance). In support of this approach previous studies have shown that linear SVM models reach robust performance in distinguishing two events (e.g., rewarding versus loss outcomes, physical pain versus social rejection) based on subject-level images even though the trials of those events are not balanced38,92,93. Here, we developed a linear SVM classifier on single subject first-level GLM contrast images to differentiate between neural responses to the e.g., reward PE under punishment decisions and responses to the reward PE under accept decisions (in line with the arousal and valence PEs). To facilitate the robustness of the analyses we employed a leave-one-out cross-validation (LOOCV) procedure, during which N-1 subjects were included for training while the remaining subjects were used for testing. The cross-validated neural predictive pattern of PEs thus consisted of the weights of each voxel in predicting the reward PE under punishment or accept decisions plus the intercept. Finally, we performed a bootstrap test which took 5000 samples with replacement from the training dataset and repeated the prediction process with each bootstrap sample to determine the voxels that contributed reliably to the classification. The resulting distribution was next converted into a z-value at each voxel and thresholded based on a voxel’s corresponding p value, FDR corrected p < 0.05 (In line with procedures used in our prior works31,32).
Moreover, we also assessed whether the neural expressions of reward and emotional PEs were similar via conducting pattern similarity analyses between multivariate neural patterns of all PES and calculating group-level correlations between activations of contrast images for PEs based on bootstrap tests with 500 iterations. We evaluated the significance of the pattern (dis-)similarity based on that: (1) 95% confidence intervals (CI) obtained from bootstrap tests (500 samples) included zero, and (2) the group-level correlation analysis between activations of contrast images for PEs did not reach statistical significance (p > 0.05, two-tailed).
While focusing on the decision period in our primary analyses, we also utilized the same linear SVM classifier with a LOOCV procedure to distinguish punishment and accept decisions. A bootstrap test that involved taking 5000 samples with replacement was performed to provide P-values for voxel weights in order to threshold the classifier weights for interpretation (FDR corrected p < 0.05).
Exploratory correlation analysis
The behavioral and fMRI results suggested a pronounced predictive role of reward PE to punishment decisions. We thus conducted an exploratory correlation analysis with permutation tests (10,000 permutations) to determine whether multivariate pattern expressions for reward PE generated before punishment decisions predict punishment decision behaviors. For the sake of completeness and increasing transparency, we also examined the correlations between punishment decisions and the multivariate pattern expressions for emotional PEs signaling.
Statistics and reproducibility
Statistical analyses were implemented via the use of Rstudio (with R version 4.0.5). To examine the predictive contribution of all PEs to punishment decisions, logistic mixed-effects regression models were employed via the lme4 R package and the resulting graphs were plotted using the ggplot2 R package. To account for multicollinearity in regression models the variance inflation factor of the predictors was estimated by using the vif function in the car R package. For exploratory correlation analyses, we randomized the distribution of independent variables 10,000 times using randperm function and next conducted Pearson correlation analyses by using the corr function in MATLAB. The p-value for the observed correlation was computed as the proportion of permuted correlations that were equal to or more extreme than the observed correlation. All results were considered significant if the p-value was less than 0.05.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Acknowledgements
This work was supported by the China MOST2030 Brain Project (Grant No. 2022ZD0208500), National Natural Science Foundation of China (Grants No. 32250610208, 82271583), and National Key Research and Development Program of China (Grant No. 2018YFA0701400) and a start-up grant from The University of Hong Kong. Disclaimer: Any opinions, findings, conclusions or recommendations expressed in this publication do not reflect the views of the Government of the Hong Kong Special Administrative Region or the Innovation and Technology Commission. We truly thank all colleagues for the important discussions that helped us to improve the manuscript and the help during the fMRI data acquisition, and all volunteers who participated in our study.
Author contributions
T.X. and B.B. designed the study. T.X., K.F., L.W., R.Z., C.L. conducted the experiment and collected the data. T.X. performed the data analysis with advices provided by L.Z., F.Z., X.G. and Z.C. T.X and B.B wrote the original manuscript draft which was further revised by L.Z., Z.C., K.K. and D.Y.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Christina Karlsson Rosenthal.
Data availability
The data for behavioral analyses and generating figures are available at: https://github.com/Tasia21/Distinct-neural-computations-for-RPE-and-EPE94, while the fMRI data used to for univariate and multivariate fMRI analyses are available at: https://figshare.com/articles/dataset/EPE/2810526895.
Code availability
The code for behavioral analyses and generating figures are available at: https://github.com/Tasia21/Distinct-neural-computations-for-RPE-and-EPE, while the code for fMRI analysis is available at: https://github.com/canlab and https://github.com/cocoanlab/cocoanCORE.
Competing interests
L.Z. is an Editorial Board Member for Communications Biology, but was not involved in the editorial review of, nor the decision to publish this article. The other authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-025-07561-7.
References
- 1.Cohen, J. Y., Haesler, S., Vong, L., Lowell, B. B. & Uchida, N. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature482, 85–88 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sambrook, T. D. & Goslin, J. A neural reward prediction error revealed by a meta-analysis of ERPs using great grand averages. Psychol. Bull.141, 213 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Tymula, A. et al. Dynamic prospect theory: Two core decision theories coexist in the gambling behavior of monkeys and humans. Sci. Adv.9, eade7972 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schultz, W. Reward prediction error. Curr. Biol.27, R369–R371 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Schultz, W. Dopamine reward prediction-error signalling: a two-component response. Nat. Rev. Neurosci.17, 183–195 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paulus, M. P. & Angela, J. Y. Emotion and decision-making: affect-driven belief systems in anxiety and depression. Trends Cogn. Sci.16, 476–483 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.FeldmanHall, O. & Nassar, M. R. The computational challenge of social learning. Trends Cogn. Sci.25, 1045–1057 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.t Wout, M., Kahn, R. S., Sanfey, A. G. & Aleman, A. Affective state and decision-making in the ultimatum game. Exp. Brain Res.169, 564–568 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Thornton, M. A. & Tamir, D. I. Mental models accurately predict emotion transitions. Proc. Natl Acad. Sci. USA114, 5982–5987 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coricelli, G. et al. Regret and its avoidance: a neuroimaging study of choice behavior. Nat. Neurosci.8, 1255–1262 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Mellers, B. A. & McGraw, A. P. Anticipated emotions as guides to choice. Curr. Direct. Psychol. Sci.10, 210–214 (2001). [Google Scholar]
- 12.Frolichs, K. M., Rosenblau, G. & Korn, C. W. Incorporating social knowledge structures into computational models. Nat. Commun.13, 6205 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang, L., Lengersdorff, L., Mikus, N., Gläscher, J. & Lamm, C. Using reinforcement learning models in social neuroscience: frameworks, pitfalls and suggestions of best practices. Soc. Cogn. Affect. Neurosci.15, 695–707 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lockwood, P. L., Apps, M. A., Valton, V., Viding, E. & Roiser, J. P. Neurocomputational mechanisms of prosocial learning and links to empathy. Proc. Natl Acad. Sci. USA113, 9763–9768 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Connell, K., Walsh, M., Padgett, B., Connell, S. & Marsh, A. A. Modeling variation in empathic sensitivity using go/no-go social reinforcement learning. Affect. Sci.3, 603–615 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaki, J., Kallman, S., Wimmer, G. E., Ochsner, K. & Shohamy, D. Social cognition as reinforcement learning: feedback modulates emotion inference. J. Cogn. Neurosci.28, 1270–1282 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Heffner, J., Son, J. Y. & FeldmanHall, O. Emotion prediction errors guide socially adaptive behaviour. Nat. Hum. Behav.5, 1391–1401 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruff, C. C. & Fehr, E. The neurobiology of rewards and values in social decision making. Nat. Rev. Neurosci.15, 549–562 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Arabadzhiyska, D. H. et al. A common neural account for social and nonsocial decisions. J. Neurosci.42, 9030–9044 (2022). [DOI] [PMC free article] [PubMed]
- 20.Pagnoni, G., Zink, C. F., Montague, P. R. & Berns, G. S. Activity in human ventral striatum locked to errors of reward prediction. Nat. Neurosci.5, 97–98 (2002). [DOI] [PubMed] [Google Scholar]
- 21.Olsson, A., Knapska, E. & Lindström, B. The neural and computational systems of social learning. Nat. Rev. Neurosci.21, 197–212 (2020). [DOI] [PubMed] [Google Scholar]
- 22.Rogers-Carter, M. M. et al. Insular cortex mediates approach and avoidance responses to social affective stimuli. Nat. Neurosci.21, 404–414 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng, C. et al. Common brain networks underlying human social interactions: Evidence from large-scale neuroimaging meta-analysis. Neurosci. Biobehav. Rev.126, 289–303 (2021). [DOI] [PubMed] [Google Scholar]
- 24.Gehrlach, D. A. et al. Aversive state processing in the posterior insular cortex. Nat. Neurosci.22, 1424–1437 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Sanfey, A. G., Rilling, J. K., Aronson, J. A., Nystrom, L. E. & Cohen, J. D. The neural basis of economic decision-making in the ultimatum game. Science300, 1755–1758 (2003). [DOI] [PubMed] [Google Scholar]
- 26.Corlett, P. R., Mollick, J. A. & Kober, H. Meta-analysis of human prediction error for incentives, perception, cognition, and action. Neuropsychopharmacology47, 1339–1349 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Behrens, T. E., Hunt, L. T. & Rushworth, M. F. The computation of social behavior. Science324, 1160–1164 (2009). [DOI] [PubMed] [Google Scholar]
- 28.Apps, M. A., Rushworth, M. F. & Chang, S. W. The anterior cingulate gyrus and social cognition: tracking the motivation of others. Neuron90, 692–707 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lockwood, P. L. & Wittmann, M. K. Ventral anterior cingulate cortex and social decision-making. Neurosci. Biobehav. Rev.92, 187–191 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang, S. W., Gariépy, J.-F. & Platt, M. L. Neuronal reference frames for social decisions in primate frontal cortex. Nat. Neurosci.16, 243–250 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gan, X. et al. A neurofunctional signature of subjective disgust generalizes to oral distaste and socio-moral contexts. Nat. Hum. Behav.8, 1–20 (2024). [DOI] [PubMed]
- 32.Zhou, F. et al. A distributed fMRI-based signature for the subjective experience of fear. Nat. Commun.12, 6643 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dhingra, I. et al. Sex differences in neural responses to reward and the influences of individual reward and punishment sensitivity. BMC Neurosci.22, 1–14 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warthen, K. G. et al. Sex differences in the human reward system: convergent behavioral, autonomic and neural evidence. Soc. Cogn. Affect. Neurosci.15, 789–801 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gard, M. G. & Kring, A. M. Sex differences in the time course of emotion. Emotion7, 429 (2007). [DOI] [PubMed] [Google Scholar]
- 36.Markovits, H., Trémolière, B. & Blanchette, I. Reasoning strategies modulate gender differences in emotion processing. Cognition170, 76–82 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Averbeck, B. & O’Doherty, J. P. Reinforcement-learning in fronto-striatal circuits. Neuropsychopharmacology47, 147–162 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Speer, S. P. et al. A multivariate brain signature for reward. NeuroImage271, 119990 (2023). [DOI] [PubMed] [Google Scholar]
- 39.Shiba, Y. et al. Converging prefronto-insula-amygdala pathways in negative emotion regulation in marmoset monkeys. Biol. Psychiatry82, 895–903 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Button, K. S. Double-dipping revisited. Nat. Neurosci.22, 688–690 (2019). [DOI] [PubMed] [Google Scholar]
- 41.Kriegeskorte, N., Simmons, W. K., Bellgowan, P. S. & Baker, C. I. Circular analysis in systems neuroscience: the dangers of double dipping. Nat. Neurosci.12, 535–540 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kragel, P. A., Koban, L., Barrett, L. F. & Wager, T. D. Representation, pattern information, and brain signatures: from neurons to neuroimaging. Neuron99, 257–273 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woo, C.-W., Chang, L. J., Lindquist, M. A. & Wager, T. D. Building better biomarkers: brain models in translational neuroimaging. Nat. Neurosci.20, 365–377 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bryden, D. W., Johnson, E. E., Tobia, S. C., Kashtelyan, V. & Roesch, M. R. Attention for learning signals in anterior cingulate cortex. J. Neurosci.31, 18266–18274 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schaich Borg, J., Lieberman, D. & Kiehl, K. A. Infection, incest, and iniquity: Investigating the neural correlates of disgust and morality. J. Cogn. Neurosci.20, 1529–1546 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gabay, A. S., Radua, J., Kempton, M. J. & Mehta, M. A. The Ultimatum Game and the brain: A meta-analysis of neuroimaging studies. Neurosci. Biobehav. Rev.47, 549–558 (2014). [DOI] [PubMed] [Google Scholar]
- 47.Kohoutová, L. et al. Toward a unified framework for interpreting machine-learning models in neuroimaging. Nat. Protoc.15, 1399–1435 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rangel, A., Camerer, C. & Montague, P. R. Neuroeconomics: The neurobiology of value-based decision-making. Nat. Rev. Neurosci.9, 545 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frömer, R., Dean Wolf, C. K. & Shenhav, A. Goal congruency dominates reward value in accounting for behavioral and neural correlates of value-based decision-making. Nat. Commun.10, 4926 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kording, K. Decision theory: what” should” the nervous system do? Science318, 606–610 (2007). [DOI] [PubMed] [Google Scholar]
- 51.Kubanek, J. Optimal decision making and matching are tied through diminishing returns. Proc. Natl Acad. Sci. USA114, 8499–8504 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kahneman, D. A psychological perspective on economics. Am. Econ. Rev.93, 162–168 (2003). [Google Scholar]
- 53.Heffner, J. & FeldmanHall, O. A probabilistic map of emotional experiences during competitive social interactions. Nat. Commun.13, 1718 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Villano, W. J., Otto, A. R., Ezie, C., Gillis, R. & Heller, A. S. Temporal dynamics of real-world emotion are more strongly linked to prediction error than outcome. J. Exp. Psychol. Gen.149, 1755 (2020). [DOI] [PubMed] [Google Scholar]
- 55.Skvortsova, V., Palminteri, S. & Pessiglione, M. Learning to minimize efforts versus maximizing rewards: computational principles and neural correlates. J. Neurosci.34, 15621–15630 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kurniawan, I. T., Guitart-Masip, M., Dayan, P. & Dolan, R. J. Effort and valuation in the brain: the effects of anticipation and execution. J. Neurosci.33, 6160–6169 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hauser, T. U. et al. Temporally dissociable contributions of human medial prefrontal subregions to reward-guided learning. J. Neurosci.35, 11209–11220 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rushworth, M. F., Noonan, M. P., Boorman, E. D., Walton, M. E. & Behrens, T. E. Frontal cortex and reward-guided learning and decision-making. Neuron70, 1054–1069 (2011). [DOI] [PubMed] [Google Scholar]
- 59.Grant, R. I. et al. Specialized coding patterns among dorsomedial prefrontal neuronal ensembles predict conditioned reward seeking. Elife10, e65764 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Corradi-Dell’Acqua, C., Tusche, A., Vuilleumier, P. & Singer, T. Cross-modal representations of first-hand and vicarious pain, disgust and fairness in insular and cingulate cortex. Nat. Commun.7, 10904 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yao, S. et al. Oxytocin facilitates approach behavior to positive social stimuli via decreasing anterior insula activity. Int. J. Neuropsychopharmacol.21, 918–925 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jezzini, A., Bromberg-Martin, E. S., Trambaiolli, L. R., Haber, S. N. & Monosov, I. E. A prefrontal network integrates preferences for advance information about uncertain rewards and punishments. Neuron109, 2339–2352. e2335 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuehn, E., Mueller, K., Lohmann, G. & Schuetz-Bosbach, S. Interoceptive awareness changes the posterior insula functional connectivity profile. Brain Struct. Funct.221, 1555–1571 (2016). [DOI] [PubMed] [Google Scholar]
- 64.Greene, J. D., Nystrom, L. E., Engell, A. D., Darley, J. M. & Cohen, J. D. The neural bases of cognitive conflict and control in moral judgment. Neuron44, 389–400 (2004). [DOI] [PubMed] [Google Scholar]
- 65.Buckholtz, J. W. et al. The neural correlates of third-party punishment. Neuron60, 930–940 (2008). [DOI] [PubMed] [Google Scholar]
- 66.Civai, C., Miniussi, C. & Rumiati, R. I. Medial prefrontal cortex reacts to unfairness if this damages the self: a tDCS study. Soc. Cogn. Affect. Neurosci.10, 1054–1060 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zoh, Y., Chang, S. W. & Crockett, M. J. The prefrontal cortex and (uniquely) human cooperation: a comparative perspective. Neuropsychopharmacology47, 119–133 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gao, X. et al. Distinguishing neural correlates of context-dependent advantageous-and disadvantageous-inequity aversion. Proc. Natl Acad. Sci. USA115, E7680–E7689 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bellucci, G., Feng, C., Camilleri, J., Eickhoff, S. B. & Krueger, F. The role of the anterior insula in social norm compliance and enforcement: Evidence from coordinate-based and functional connectivity meta-analyses. Neurosci. Biobehav. Rev.92, 378–389 (2018). [DOI] [PubMed] [Google Scholar]
- 70.Dupont, L., Santangelo, V., Azevedo, R. T., Panasiti, M. S. & Aglioti, S. M. Reputation risk during dishonest social decision-making modulates anterior insular and cingulate cortex activity and connectivity. Commun. Biol.6, 475 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grecucci, A., Giorgetta, C., Van’t Wout, M., Bonini, N. & Sanfey, A. G. Reappraising the ultimatum: an fMRI study of emotion regulation and decision making. Cereb. Cortex23, 399–410 (2013). [DOI] [PubMed] [Google Scholar]
- 72.Chowdhury, R. et al. Dopamine restores reward prediction errors in old age. Nat. Neurosci.16, 648–653 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Martins, D., Lockwood, P., Cutler, J., Moran, R. & Paloyelis, Y. Oxytocin modulates neurocomputational mechanisms underlying prosocial reinforcement learning. Prog. Neurobiol.213, 102253 (2022). [DOI] [PubMed] [Google Scholar]
- 74.Xu, T. et al. The central renin–angiotensin system: A genetic pathway, functional decoding, and selective target engagement characterization in humans. Proc. Natl Acad. Sci. USA121, e2306936121 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu, T. et al. Angiotensin blockade enhances motivational reward learning via enhancing striatal prediction error signaling and frontostriatal communication. Mol. Psychiatry28, 1692–1702 (2023). [DOI] [PubMed] [Google Scholar]
- 76.Pan, Y. et al. Beauty affects fairness: facial attractiveness alters neural responses to unfairness in the ultimatum game. Brain Imaging Behav.16, 2497–2505 (2022). [DOI] [PubMed] [Google Scholar]
- 77.Wu, Y. et al. Effects of facial trustworthiness and gender on decision making in the Ultimatum Game. Soc. Behav. Personality: Int. J.46, 499–516 (2018). [Google Scholar]
- 78.Bhogal, M. S., Galbraith, N. & Manktelow, K. Physical attractiveness, altruism and cooperation in an ultimatum game. Curr. Psychol.36, 549–555 (2017). [Google Scholar]
- 79.Ma, Q. & Hu, Y. Beauty matters: social preferences in a three-person ultimatum game. PLoS ONE10, e0125806 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fabre, E. F., Causse, M., Pesciarelli, F. & Cacciari, C. The responders’ gender stereotypes modulate the strategic decision-making of proposers playing the ultimatum game. Front. Psychol.7, 12 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Youssef, F. F., Bachew, R., Bissessar, S., Crockett, M. J. & Faber, N. S. Sex differences in the effects of acute stress on behavior in the ultimatum game. Psychoneuroendocrinology96, 126–131 (2018). [DOI] [PubMed] [Google Scholar]
- 82.Chen, G., Adleman, N. E., Saad, Z. S., Leibenluft, E. & Cox, R. W. Applications of multivariate modeling to neuroimaging group analysis: a comprehensive alternative to univariate general linear model. Neuroimage99, 571–588 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gorgolewski, K. J., Storkey, A. J., Bastin, M. E., Whittle, I. & Pernet, C. Single subject fMRI test–retest reliability metrics and confounding factors. Neuroimage69, 231–243 (2013). [DOI] [PubMed] [Google Scholar]
- 84.Valente, G., Kaas, A. L., Formisano, E. & Goebel, R. Optimizing fMRI experimental design for MVPA-based BCI control: Combining the strengths of block and event-related designs. NeuroImage186, 369–381 (2019). [DOI] [PubMed] [Google Scholar]
- 85.Schönberg, T., Daw, N. D., Joel, D. & O’Doherty, J. P. Reinforcement learning signals in the human striatum distinguish learners from nonlearners during reward-based decision making. J. Neurosci.27, 12860–12867 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang, L. & Gläscher, J. A brain network supporting social influences in human decision-making. Sci. Adv.6, eabb4159 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tay, R. Correlation, variance inflation and multicollinearity in regression model. J. East. Asia Soc. Transportation Stud.12, 2006–2015 (2017). [Google Scholar]
- 88.Li, J. et al. Common and dissociable contributions of alexithymia and autism to domain-specific interoceptive dysregulations: A dimensional neuroimaging approach. Psychother. Psychosom.88, 187–189 (2019). [DOI] [PubMed] [Google Scholar]
- 89.Xu, X. et al. Intrinsic connectivity of the prefrontal cortex and striato-limbic system respectively differentiate major depressive from generalized anxiety disorder. Neuropsychopharmacology46, 791–798 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Davis, T. et al. What do differences between multi-voxel and univariate analysis mean? How subject-, voxel-, and trial-level variance impact fMRI analysis. Neuroimage97, 271–283 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Slotnick, S. D. Cluster success: fMRI inferences for spatial extent have acceptable false-positive rates. Cogn. Neurosci.8, 150–155 (2017). [DOI] [PubMed] [Google Scholar]
- 92.Woo, C.-W. et al. Separate neural representations for physical pain and social rejection. Nat. Commun.5, 1–12 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wager, T. D. et al. A Bayesian model of category-specific emotional brain responses. PLoS Comput. Biol.11, e1004066 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Distinct-neural-computations-for-RPE-and-EPE behavioral dataset. Gitubhttps://github.com/Tasia21/Distinct-neural-computations-for-RPE-and-EPE (2024).
- 95.Distinct-neural-computations-for-RPE-and-EPE fMRI dataset. figsharehttps://figshare.com/articles/dataset/EPE/28105268 (2024).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data for behavioral analyses and generating figures are available at: https://github.com/Tasia21/Distinct-neural-computations-for-RPE-and-EPE94, while the fMRI data used to for univariate and multivariate fMRI analyses are available at: https://figshare.com/articles/dataset/EPE/2810526895.
The code for behavioral analyses and generating figures are available at: https://github.com/Tasia21/Distinct-neural-computations-for-RPE-and-EPE, while the code for fMRI analysis is available at: https://github.com/canlab and https://github.com/cocoanlab/cocoanCORE.