Abstract
Patients with Moyamoya disease (MMD) exhibit significant alterations in brain structure and function, but knowledge regarding gray matter networks is limited. The study enrolled 136 MMD patients and 99 healthy controls (HCs). Clinical characteristics and gray matter network topology were analyzed. Compared to HCs, MMD patients exhibited decreased clustering coefficient (Cp) (P = 0.006) and local efficiency (Eloc) (P = 0.013). Ischemic patients showed decreased Eloc and increased characteristic path length (Lp) compared to asymptomatic and hemorrhagic patients (P < 0.001, Bonferroni corrected). MMD patients had significant regional abnormalities, including decreased degree centrality (DC) in the left medial orbital superior frontal gyrus, left orbital inferior frontal gyrus, and right calcarine fissure and surrounding cortex (P < 0.05, FDR corrected). Increased DC was found in bilateral olfactory regions, with higher betweenness centrality (BC) in the right median cingulate, paracingulate fusiform gyrus, and left pallidum (P < 0.05, FDR corrected). Ischemic patients had lower BC in the right hippocampus compared to hemorrhagic patients, while hemorrhagic patients had decreased DC in the right triangular part of the inferior frontal gyrus compared to asymptomatic patients (P < 0.05, Bonferroni corrected). Subnetworks related to MMD and white matter hyperintensity volume were identified. There is significant reorganization of gray matter networks in patients compared to HCs, and among different types of patients. Gray matter networks can effectively detect MMD-related brain structural changes.
Keywords: Moyamoya Disease, Gray Matter Network, Graph theory, Gray Matter volume, Network-based statistic
Subject terms: Diseases, Medical research, Neurology, Neurological disorders, Neurovascular disorders
Introduction
Moyamoya disease (MMD) is a rare cerebrovascular disorder involving the progressive narrowing of the distal internal carotid arteries and proximal intracranial arteries. Collateral vessels arise from the base of the skull, which appears as a puff of smoke on digital subtraction angiography (DSA)1. Reduced cerebral blood flow in the major vessels can lead to transient ischemic attack, infarction, cerebral hemorrhage, and cognitive impairment2.
Even in the absence of overt structural brain damage, MMD patients exhibit subtle alterations in brain microstructure due to chronic hypoperfusion Neuroimaging research has uncovered significant changes in brain structure and networks in MMD. For example, MMD patients showed decreased gray matter volume and damaged white matter fiber integrity compared to healthy controls. Additionally, injury to specific brain regions has been linked to cognitive impairment3,4. Lei et al.5reported substantial reorganization of network topology in MMD patients using resting-state functional MRI. Brain functional network connectivity changes result in the brain requiring more energy to accomplish specific tasks6. Furthermore, patients with MMD who exhibit different symptoms also show variations in collateral compensatory blood vessels, cerebral perfusion, and brain structure indicating that the brain network may be affected7,8.
The human brain’s morphological data contains a wealth of information. Networks derived from the statistical correlations of morphological indices between brain regions can reveal the developmental coordination or synchronized maturation processes across different areas, thus characterizing the brain’s structural architecture9. A previous study found a reorganization of brain structural covariance networks in ischemic MMD compared to healthy controls10. However, this method can only be analyzed at the group level and loses individual brain morphology information. Fortunately, novel methodologies have been proposed to construct single-subject morphometric networks. Among these, the graynet approach achieves this by measuring the morphological similarity between different regions of the brain, thereby constructing morphological associations among brain areas for individual subjects, which retains individual differences. This similarity indicates potential structural and functional correlations among brain regions and have been demonstrated to be both meaningful and reliable11,12. Consequently, this approach provides further insights into the mechanisms of collaborative functioning across distinct brain areas.
This study analyzed the individual gray matter volume network of MMD patients and proposed the following hypotheses: (1) The network of patients differs from that of healthy controls. (2) Types of symptoms influence the topological properties of individual networks. (3) Individual networks have a particular potential for predicting patients and brain structural lesions.
Results
Demographics and group matching
As shown in Table 1, the age and sex ratio of patients and HCs were well-matched. The gray matter and white matter volumes of the patients were lower than those of the HCs. The difference was still significant after correction for total intracranial volume.
Table 1.
Demographics and clinical characteristics of MMD patients and healthy controls. GMV, gray matter volume; WMV, white matter volume; TIV, total intracranial volume.
| HC (99) | MMD (136) | t/χ² | p | |
|---|---|---|---|---|
| Age (years) | 45.40±8.84 | 44.19±10.68 | 0.923 | 0.357 |
| Gender (male) | 40(40.40%) | 51(37.50%) | 0.204 | 0.652 |
| Education level | ||||
| Primary | 5(5.05%) | 12(8.82%) | 1.603 | 0.449 |
| Median | 54(54.55%) | 66(48.53%) | ||
| Graduate | 40(40.40%) | 58(42.65%) | ||
| Hypertension | 30(30.30%) | 54(39.71%) | 2.205 | 0.138 |
| Diabete | 12(12.12%) | 18(13.24%) | 0.064 | 0.801 |
| Hyperlipid | 25(25.25%) | 25(18.38%) | 1.614 | 0.204 |
| GMV (ml) | 633.02±58.10 | 603.07±61.00 | 3.742 | <0.001 |
| WMV (ml) | 515.43±55.13 | 488.35±60.84 | 3.503 | <0.001 |
| GMV/TIV | 0.44±0.02 | 0.43±0.03 | 3.600 | <0.001 |
| WMV/TIV | 0.36±0.02 | 0.35±0.02 | 4.397 | <0.001 |
| TIV (ml) | 1433.22±135.80 | 1403.87±11.70 | 1.631 | 0.104 |
Changes in brain network properties between HC and MMD
In the defined threshold range, both groups exhibited a small-world topology as indicated by all γ > 1 with λ ≈ 1 or σ > 1 (Fig. 1). Patients showed a significant decrease in aEloc (p = 0.013) and aCp (p = 0.006) compared to HCs. No significant difference was observed in other global measures (Table 2).
Fig. 1.
Small-worldness properties of MMD and HC in the defined sparsity. (A) normalized clustering coefficients were larger than 1, (B) normalized characteristic path lengths were approximately equal to 1, and (C) small worldness coefficients were bigger than 1.
Table 2.
Comparisons of global network properties between HC and MMD. aEg = AUC of global efficiency; aEloc = AUC of local efficiency; aCp = AUC of clustering coefficient; aLp = AUC of characteristic path length; aγ = AUC of normalized clustering coefficient; aλ = AUC of normalized characteristic path length; aσ = AUC of small-worldness.
| HC (99) | MMD (136) | t | p | |
|---|---|---|---|---|
aEg (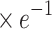 ) ) |
2.5±0.04 | 2.49±0.04 | 1.575 | 0.117 |
aEloc (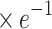 ) ) |
3.66±0.05 | 3.64±0.04 | 2.508 | 0.013 |
aCp ( ) ) |
3.14±0.05 | 3.12±0.05 | 2.790 | 0.006 |
aLp (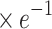 ) ) |
10.41±0.16 | 10.44±0.17 | −1.004 | 0.316 |
aγ ( ) ) |
9.49±0.58 | 9.47±0.67 | 0.189 | 0.85 |
aλ ( ) ) |
5.8±0.07 | 5.8±0.08 | −0.532 | 0.595 |
aσ (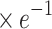 ) ) |
8.01±0.45 | 7.99±0.52 | 0.187 | 0.852 |
Figure 2 displays brain regions that showed significant between-group differences in at least one nodal metric in patients compared to HCs (FDR corrected, p < 0.05): nodal DC of bilateral olfactory cortex (OLF) and nodal BC of right median cingulate and paracingulate gyri (DCG.R), fusiform gyrus (FFG. R) and left pallidum (PAL.L) were significantly lower in HCs, whereas MMD patients showed significantly decreased nodal DC in left orbital part of inferior frontal gyrus (ORBinf.L), left medial orbital part of superior frontal gyrus (ORBsupmed.L) and right calcarine fissure and surrounding cortex (CAL.R).
Fig. 2.
Regions of significant differences in nodal degree centrality or betweenness centrality of gray matter morphological networks between MMD patients and healthy controls, showing both increases (red) and decreases (blue) in MMD compared to HC. OLF.L, left olfactory cortex; OLF.R, right olfactory cortex; DCG.R, right median cingulate and paracingulate gyri; FFG.R, fusiform gyrus; PAL.L, left pallidum; ORBinf.L, left orbital part of inferior frontal gyrus; ORBsupmed.L, left medial orbital superior frontal gyrus; CAL.R, right calcarine fissure and surrounding cortex.
Demographic and clinical characteristics in different types of MMD
MMD patients were divided into three groups according to their symptoms. The demographic and clinical characteristics of 136 patients are shown in Table 3. No significant difference was found among the three groups in gray matter volume, white matter volume, the ratio of white matter hyperintensity volume to total intracranial volume, white matter hyperintensity, and number of lacunes after Bonferroni correction.
Table 3.
Demographics and clinical characteristics of MMD patients with different symptoms. GMV, gray matter volume; WMV, white matter volume; TIV, total intracranial volume; WMH, white matter hyperintensity.
| Ischemic (62) | Hemorrhagic (12) | Asymptomatic (62) | F | p | |
|---|---|---|---|---|---|
| Age (years) | 44.39±10.75 | 43.67±12.5 | 44.1±10.42 | 0.027 | 0.973 |
| Gender (male) | 24(38.70%) | 4(33.30%) | 23(37.10%) | 0.132 | 0.966 |
| Disease Duration (months) | 10.11±8.02 | 6.08±2.78 | - | - | - |
| Education level | |||||
| Primary | 6(9.68%) | 1(8.33%) | 5(8.06%) | 1.175 | 0.882 |
| Median | 27(43.55%) | 6(50.00%) | 33(53.23%) | ||
| Graduate | 29(46.77%) | 5(41.67%) | 24(28.71%) | ||
| Hypertension | 30(48.39%) | 3(25.00%) | 21(33.87%) | 3.917 | 0.162 |
| Diabete | 9(14.52%) | 1(8.33%) | 8(12.90%) | 0.346 | 0.938 |
| Hyperlipid | 15(24.19%) | 0(0.00%) | 10(16.13%) | 4.308 | 0.143 |
| MRA stage left | |||||
| 1 | 3(4.84%) | 0(0.00%) | 5(8.06%) | 6.164 | 0.360 |
| 2 | 46(74.19%) | 8(66.66%) | 37(59.68%) | ||
| 3 | 10(16.13%) | 4(33.33%) | 19(30.65%) | ||
| 4 | 3(4.84%) | 0(0.00%) | 1(1.61%) | ||
| MRA stage right | |||||
| 1 | 3(4.84%) | 0(0.00%) | 4(6.45%) | 3.634 | 0.705 |
| 2 | 42(67.74%) | 7(58.33%) | 39(62.90%) | ||
| 3 | 14(22.58%) | 4(33.33%) | 18(29.03%) | ||
| 4 | 3(4.84%) | 1(8.33%) | 1(1.61%) | ||
| GMV/TIV | 0.43±0.03 | 0.43±0.02 | 0.43±0.02 | 0.82 | 0.443 |
| WMV/TIV | 0.35±0.02 | 0.34±0.03 | 0.35±0.02 | 3.442 | 0.035 |
| WMH (Lesion Volume, ml) | 2.15±3.94 | 3.57±3.07 | 1.43±2.77 | 2.222 | 0.112 |
| WMH (Number of Lesions) | 8±5.02 | 7.75±5.17 | 6.85±6.04 | 0.685 | 0.506 |
WMH(ml)/TIV(ml) ( ) ) |
0.15±0.29 | 0.25±0.21 | 0.1±0.21 | 1.999 | 0.14 |
| GMV (ml) | 599.17±60.08 | 606.53±56.4 | 607.15±63.39 | 0.279 | 0.757 |
| WMV (ml) | 484.65±57.51 | 474.63±58.68 | 494.7±64.59 | 0.755 | 0.472 |
| TIV (ml) | 1403.68±133.25 | 1413.07±111.21 | 1402.28±145.69 | 0.031 | 0.969 |
| Lacune (Number of Lesions) | 0.73±1.67 | 0.33±1.16 | 0.27±0.79 | 1.973 | 0.143 |
Alterations in brain network properties in different yypes of MMD
Table 4 shows the global network properties among the three types of MMD patients. The aEg of ischemic MMD patients was significantly lower than that of hemorrhagic and asymptomatic patients (p = 0.001, p = 0.003, respectively, Bonferroni corrected). The aLp showed a significant increase in ischemic patients compared to the other two types (p = 0.004, p = 0.003, respectively, Bonferroni corrected). The aλ of ischemic patients was significantly higher than that of hemorrhagic patients.
Table 4.
Comparisons of global network properties in MMD patients. aEg = AUC of global efficiency; aEloc = AUC of local efficiency; aCp = AUC of clustering coefficient; aLp = AUC of characteristic path length; aγ = AUC of normalized clustering coefficient; aλ = AUC of normalized characteristic path length; aσ = AUC of small-worldness.
| Ischemic (62) | Hemorrhagic (12) | Asymptomatic (62) | F | p | |
|---|---|---|---|---|---|
| aEg | 0.247 ±0.003 | 0.252 ±0.004 | 0.250 ±0.004 | 9.75 | <0.001 |
| aEloc | 0.363 ±0.004 | 0.365 ±0.005 | 0.365 ±0.005 | 2.175 | 0.118 |
| aCp | 0.312 ±0.005 | 0.313 ±0.005 | 0.313 ±0.005 | 0.668 | 0.514 |
| aLp | 1.050 ±0.017 | 1.033 ±0.017 | 1.040 ±0.016 | 8.67 | <0.001 |
| aγ | 0.958 ±0.070 | 0.913 ±0.088 | 0.943 ±0.059 | 2.603 | 0.078 |
| aλ | 0.582 ±0.008 | 0.576 ±0.010 | 0.580 ±0.007 | 3.611 | 0.030 |
| aσ | 0.806 ±0.057 | 0.778 ±0.067 | 0.797 ±0.043 | 1.611 | 0.204 |
For the regional network measures, as shown in Fig. 3, BC of the right hippocampus (HIP.R) was significantly increased in hemorrhagic patients compared to ischemic patients (p < 0.001, Bonferroni corrected). Moreover, hemorrhagic patients showed a significant decrease in DC of the right triangular part of the inferior frontal gyrus (IFGtriang.R) compared with asymptomatic patients (p < 0.001, Bonferroni corrected).
Fig. 3.
Regions exhibiting altered nodal degree centrality or betweenness centrality among MMD patients. IFGtriang.R, right triangular part of inferior frontal gyrus; HIP.R, right hippocampus; DC, degree centrality; BC, betweenness centrality.
Network-based statistic (NBS) prediction
NBS prediction was used to identify MMD patients from HCs based on the gray matter network AUC = 0.631 (95% CI: 0.623, 0.639, p < 0.001). The subnetworks included frontal, temporal, and occipital lobes. NBS-predict regression analysis was also used to predict white matter hyperintensity volume with Pearson’s correlation coefficients of 0.318 (95% CI: 0.286, 0.349, p < 0.001). The results are shown in Fig. 4.
Fig. 4.
Subnetworks identified using NBS prediction (A) Subnetworks with significant classification between MMD and HC. (B) NBS-based regression on volume of white matter hyperintensity.
Discussion
In this study, the individual brain network was constructed based on gray matter volume. Compared to HCs, MMD patients had significant brain atrophy, and the individual brain network of patients had significant changes in both global and local properties. Additionally, patients with diverse symptom patterns also have differences in the topological properties of brain networks. The NBS-predict method was also used to analyze the subnetworks in individual structural networks related to both MMD and white matter hyperintensity. These results suggest that individual gray matter volume networks respond to the pathophysiological processes of MMD.
Both healthy controls and MMD patients exhibited brain networks with small-world properties, indicating well-established networks. Information can be processed in local regions and the whole network with low wiring costs, which means that networks with small-world topography show high efficiency for functional segregation and integration.
However, the global properties, including Cp and Eloc, show differences between patients and HCs. Cp reflects functional integration, while Eloc reflects the efficiency of local communication. Eloc and Cp decreased in patients, indicating that the ability to combine specialized information from distributed brain regions rapidly was impaired in MMD patients, and the network was less error-tolerant than HCs. This evidence supports a transition towards a “weaker small-world” pattern in the individual brain networks of MMD patients, which is supported by other research5,10.
Similar changes were observed in patients with different symptoms. We observed decreased Eloc and increased aLp in ischemic MMD patients. The Lp represents the average shortest path length between all pairs of nodes in the network. The shorter the Lp, the less energy is consumed when integrating local information. This can be explained by the viewpoint that asymptomatic patients were considered at an early stage of MMD8and hemorrhagic MMD patients without apparent brain structural damage had relatively good collateral circulation and higher bran perfusion13,14. Therefore, compared to ischemic MMD, patients with asymptomatic and hemorrhagic MMD may have better brain functional compensation.
In addition to changes in global network characteristics, alterations in regional topological measures of several nodes were also observed. Regional DC values of left orbital part of inferior frontal gyrus, left medial orbital part of superior frontal gyrus, and right calcarine fissure significantly decreased in MMD patients after FDR correlation. The left orbital part of inferior frontal gyrus is associated with semantic retrieval and produces sustained activity of semantic representations, thus playing a core role in the semantic attentional system15. Hu et al. found lower white matter fibers and functional connectivity between the left supplementary motor area and the left orbital part of inferior frontal gyrus in MMD. Additionally, the functional connectivity was correlated with cognitive function16. The left medial orbital part of superior frontal gyrus is a vital node in the default mode network which is involved in cognitive control, learning and memory17. The calcarine fissure and surrounding cortex cortex contains most primary visual cortex and is associated with visual processing and spatial memory18. In mild cognitive impairment patients, both the structural and metabolic status of right calcarine fissure were changed compared to HCs19. The altered topological properties of these nodes may account for the severe memory and attention deficits in MMD20.
Compared to healthy controls, patients with MMD showed increased DC of bilateral olfactory cortex and BC of right median cingulate and paracingulate gyri, right fusiform gyrus, and left pallidum. The olfactory cortex has close connections with the insula, hypothalamus, and hippocampus, which are essential for learning and memory21. The aberration of the surface area of the olfactory cortex has been reported as a biomarker for cognitive dysfunction in patients with Parkinson’s disease22. The median cingulate and paracingulate gyri connects various regions in the fronto-parietal network and is also a critical part of the limbic system, which is essential for cognitive integration and emotion processing23. The fusiform gyrus is another important node in the visual ventral stream, which participates in various visual cognitive functions, including face perception, word recognition, and semantic processing24. As mentioned above, the importance of the calcarine fissure and surrounding cortex was found to be reduced in MMD patients. The study results suggest that MMD patients experience functional remodeling of the visual cortex. However, further investigation is needed to determine the relationship between disease pathophysiology and visual cognition in MMD. As a component of the basal ganglia, the pallidum is responsible for controlling muscle contractions and motor processes. The pallidum has been reported to receive inputs from regions in the limbic system, such as the prefrontal cortex, hippocampus, and amygdala, which regulate motivation. Moreover, the cortex–striatum–pallidum–thalamus–cortex loop is associated with the reward system and may be involved in reinforcement learning25. The increased importance of the above nodes in the grey matter brain networks of MMD may imply compensatory alterations for the maintenance of the corresponding functions. The exact mechanisms behind these alterations need to be further explored using multimodal brain networks and cognitive assessments.
Changes in local network properties were observed among MMD patients with different symptomatic phenotypes. Specifically, the BC of the right hippocampus was decreased in ischemic patients compared to hemorrhagic patients, while the DC of the right triangular part of the inferior frontal gyrus was decreased in hemorrhagic patients compared to asymptomatic patients. The HIP encodes, stores, and retrieves memories26, while the triangular part of the inferior frontal gyrus belongs to the execution control network. Sun et al. found that the triangular part of the inferior frontal gyrus is a key node related to acroparesthesia in MMD patients27. Hu et al. revealed that hemorrhagic MMD patients have increase in cortical thickness in several brain regions compared with ischemic patients and HCs. The increased cortical thickness is associated with higher cerebral blood flow and integrity of white matter fiber7. Given that asymptomatic MMD may be in the early stages with relatively stable hemodynamic status8, and hemorrhagic MMD patients tend to have well compensated cerebral perfusion13,14, it is reasonable to infer that the cerebral perfusion is the main reason for the difference in local network properties among MMD patients.
The individual brain network of ischemic MMD patients tended to deteriorate compared to asymptomatic patients and hemorrhagic patients without significant cerebral structural damage. This phenomenon was hypothesized to be closely linked to variations in cerebral hemodynamic compensation among patients. The load on the collateral vessels in hemorrhagic MMD is often excessive, as frequently observed in patients with abnormally dilated choroidal anastomotic arteries28,29. The combination of low overall cerebral blood flow and relatively high local blood flow constitutes a distinct pathophysiological condition in hemorrhagic MMD, leading to unique alterations in the topological properties of the cerebral network in these patients that differ from those observed in ischemic or asymptomatic patients. Future research should include studies of cerebral perfusion, together with a comprehensive investigation of angiographic features and genetic variation, to elucidate the pathophysiological mechanisms underlying different symptomatic types of MMD and their impact on brain networks.
NBS-predict is an algorithm developed based on network-based statistics30. NBS applies traditional clustering statistics to graph theory analysis to identify connected structures or components in a network, enhancing the statistical validity of this method. By combining graph theory with machine learning algorithms, NBS-predict effectively mitigates the curse of dimensionality and makes results more generalizable.
The NBS-predict method was utilized to differentiate between MMD patients and HCs. The subnetwork discovered involved the limbic, vision, sensory, and motor modules and the default network, which had a moderate differentiating effect. Additionally, the correlation analysis with white matter hyperintensity volume indicated that most of the work nodes were affected, which is consistent with the diffuse nature of the lesion. Therefore, individual brain networks were found to be associated with MMD-related lesions.
The individual brain network constructed based on cortical morphology similarity is consistent with networks constructed using other modalities, such as functional and white matter fiber networks. Intercortical morphology similarity contains anatomical, functional, and genetically relevant information. The similarity in cytoarchitectural categorization may be the anatomical basis for morphology similarity connectivity. The classification of morphologically similar brain regions will likely be the same11. Additionally, regions with similar cortical thickness may have more white matter fiber connections and exhibit greater functional coherence and correlation with cognitive performance31,32. Thus, individual brain networks may contain more biological information.
Abnormalities in individual networks have been shown to be associated with cognitive impairment diseases11. Furthermore, individual network abnormalities have been reported in cerebrovascular diseases such as cerebral small-vessel disease and carotid stenosis33,34. This study investigated the individual brain networks of patients with MMD, a cerebrovascular disease commonly associated with cognitive impairment. The study provides new information on the reorganization of brain structure and networks in MMD patients.
There are several limitations to this study. First, the structural brain images of the MMD patients included in this study were acquired by two MRI scans of the same model and used the same sequence settings. Only gray matter volume was analyzed in this study, and the data were normalized for subsequent analyses, thus minimizing the effects caused by differences in MRI equipment. Second, this study could not explore the factors associated with network reorganization in patients with MMD due to a lack of information on cognitive function and genetic data. Third, because most patients with hemorrhagic MMD have undergone ventricular puncture and hematoma removal surgery, resulting in significant structural brain damage, the number of patients with hemorrhagic MMD included in this study was relatively small. Hemorrhagic MMD patients are unique in terms of cerebral perfusion, and the course of the disease, and the study of the brain networks of such patients still needs more data.
In conclusion, our findings suggest that there is a reorganization of individual brain networks in both HCs and MMD patients, as well as among different types of MMD patients. In addition, it has been observed that individual brain networks are associated with MMD-related brain structure alternation.
Methods
Participants
This study was approved by the ethics committee of Beijing Tiantan Hospital, Capital Medical University (KY2023-275-03) and all methods were performed in accordance with relevant guidelines and regulations. All MMD patients and healthy control participants were volunteers and provided informed consent. The information of 140 patients diagnosed with MMD in our hospital from January 2019 to September 2023 was reviewed. The inclusion criteria were as follows: (1) diagnosed with MMD according to the criteria of the Research Committee on Spontaneous Occlusion of the Circle of Willis35; (2) over 18 years of age; (3) no evidence of brain lesions on T1-weighted images larger than 15 mm in diameter, and no significant structural damage to the gray matter was identified; 4)no cranial surgery (including, but not limited to, ventricular puncture drainage and intracerebral hematoma removal) has been performed prior to recruitment; 5) no history of other diseases with cognitive impairment or use of drugs that may alter cognitive function; 6) no MRI contraindications. 100 healthy controls (HCs) matched for age, sex, and educational background were recruited using the following criteria: (1) no history of neurological, psychiatric, or cognitive disease; (2) no history of drug use that could alter cognitive function; (3) no MRI contraindications.
Participants with incomplete clinical information and MRI data that did not meet quality control standards were excluded. 136 patients with MMD and 99 HCs were finally selected for the study. The MRA stage of MMD was recorded1. Lacune was defined as a round or ovoid subcortical cavity of cerebrospinal fluid signal with a diameter of 3 to 15 mm, and the number of lacunes was recorded36.
Study design
The baseline clinical information of all participants was recorded, including details such as age, gender, and education. T1-MPRAGE, fluid-attenuated inversion recovery (FLAIR), and time-of-flight magnetic resonance angiography (TOF-MRA) images were subsequently examined. Gray matter volume, white matter volume, and total brain volume were derived from T1-MPRAGE images, accompanied by the construction of individual gray matter volume networks. FLAIR was employed to evaluate lacunes and white matter hyperintensity lesions, while MRA served as the diagnostic criteria for MMD and its stage assessment. The study compared alterations in individual networks among patients and investigated sub-networks associated with MMD.
The medical history of each patient was collected in detail. Patients with transient limb weakness, hemiparesis, and aphasia that can recover without symptoms, and no cerebral infarction observed on brain MRI, were classified as ischemic type. Hemorrhagic MMD was diagnosed based on clinical presentation, including severe headache and consciousness disturbance, and confirmed by computed tomography (CT) scan, with a time interval between symptom onset and the first CT scan of less than 24 h. Asymptomatic MMD was defined as patients without a history of cerebrovascular events such as transient ischemic attack, cerebral infarction, intracranial hemorrhage, seizure, or involuntary movement.
MRI data acquisition
MRI data were acquired using an Ingenia 3.0 Tesla scanner (Philip Medical Systems, Best, Netherlands) equipped with a 32-channel head coil. A T1-weighted MPRAGE sequence with the following parameters was used for all scans: repetition time (TR) / echo time (TE) = 6.84/3.09 ms, flip angle = 8°, field of view (FOV) = 240 × 240 mm2, acquisition matrix = 240 × 240, slice thickness = 1.0 mm, voxel size 1.0 × 1.0 × 1.0 mm3.
A T2-weighted fluid-attenuated inversion recovery sequence with the following parameters was used for all scans: TR/TE/inversion time (TI) = 4800/340/1650 ms, flip angle = 90°, slice thickness = 1 mm, voxel size = 1.0 × 1.0 × 1.0 mm3.
A TOF-MRA sequence with the following parameters was used for all scans: TR/TE = 22/3.5 ms, flip angle = 18°, acquisition matrix = 384 × 250, slice thickness = 1 mm, voxel size 1.0 × 1.0 × 1.0 mm3.
Structural image preprocessing
All T1 images were manually checked for scanning artifacts and then subjected to standard preprocessing using voxel-based morphometry (VBM) based on statistical parametric mapping 12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/). The VBM analysis steps were: (1) The segmentation tool was first used to segment individual structural data to obtain gray matter images. (2) A study-specific template based on Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL) was created, which is based on the gray matter images of all the participants37. (3) The gray matter images of each participant were warped into the template and then normalized to the standard Montreal Neurological Institute (MNI) standard space. (4) Voxel values in individual gray matter images were then modulated and resampled to a resolution of 2 mm3. (5) Finally, all gray matter images were smoothed with a 15 mm full width at half maximum (FWMH) Gaussian kernel38. In addition, the volumes of gray matter, white matter, and total intracranial volume were also calculated for further analysis.
Construction of individual cortical thickness networks
The graynet toolbox was used to construct individual networks39. Network nodes were defined based on the Automated Anatomical Labeling (AAL) 90 atlas. Histogram-weighted networks (HiWeNet) based on gray matter volume were constructed. For each node according to the AAL90 atlas, the voxel-wise gray matter volume was transformed into a histogram and 5% outliers from the distribution were discarded. The histogram was further binned into uniformly spaced N = 100 bins and the histogram counts were then normalized for k = 1: N. The network edges were calculated as the histogram distance between two nodes (Fig. 5). A detailed description of the method can be found in the original articles12. The weight of the edge was thus defined as the statistical similarity of the morphological distributions and rescaled to [0, 1] using the min-max scaling. Since the weight of the edge was negatively correlated with the morphological similarity between the two nodes, the network matrix was subtraction normalized, with each edge subtracted by 1 to generate the traditional structural connectivity matrix33.
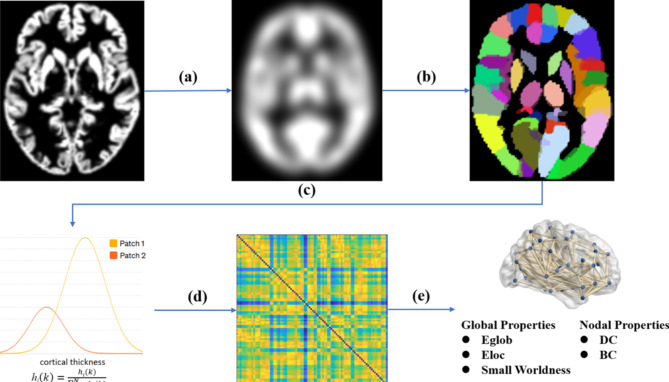
Fig. 5.
Flowchart for the construction of gray matter morphological networks using T1-weighted MRI. (a) Gray matter volume maps created using a routine VBM procedure were smoothed with a 15 mm FWHM Gaussian kernel. (b) The gray matter volume maps were divided into 90 regions based on the AAL atlases. (c) Individual gray matter volume connectivity was constructed using the Graynet software. (d) The histogram (hi) distance between each pair of nodes was calculated, resulting in a morphological similarity. (e) The network properties of gray matter morphological networks were finally calculated at both the global and nodal levels.
White matter hyperintensity
White matter hyperintensity burden was automatically segmented by the lesion prediction algorithm as implemented in the Lesion Segmentation Tool (LST) version 3.0.0 (www.statistical-modelling.de/lst.html) for SPM40. The total number and size (ml) of white matter hyperintensity were generated.
Network properties
All network properties of each brain network were calculated using the GRETNA toolbox in MATLAB41. According to a previous study34, the sparsity range was set from 10 to 60% with an interval of 5%. The global and nodal network metrics were calculated at each sparsity level, and the area under the curve (AUC) for each property across the entire sparsity range was used for statistical analysis.
Global network properties, including small worldness and network efficiency, were analyzed. Five metrics: clustering coefficient (Cp), characteristic path length (Lp), normalized clustering coefficient (γ), normalized characteristic path length (λ), and small worldness (σ) indicate the degree of small-world organization. Cp is the average clustering coefficient of all nodes and reflects the degree of local interconnectivity. Lp is the average shortest path length between any two nodes in a network. γ and λ refer to normalized Cp and normalized Lp, respectively. σ is calculated as aCp/aLp. A brain network with γ > 1 and λ ≈ 1 or σ > 1 has a small-world property, reflecting an optimal balance of integration and segregation for a network. Network efficiency includes global efficiency (Eglob) and local efficiency (Eloc). Eglob measures the efficiency of parallel information transfer in the network. Eloc reflects the communication efficiency in the neighborhood of the nodes, which reflects the fault tolerance level of the network.
Node degree centrality (DC) and betweenness centrality (BC) were chosen for local topological characteristics. DC reflects the ability to communicate information, and BC captures the importance of a given node in the flow of information. Detailed definitions of the above metrics and the formulas can be found in Rubinov’s review42.
Network-based statistic (NBS) prediction
The NBS prediction method was used to test the effect of MMD and white matter hyperintensity on the individual gray matter volume networks30. Parameters were selected as recommended: 40-fold, 50 repeated cross-validation (CV) procedures, hyperparameters with the grid search algorithm, and auto-optimization for classification and regression algorithms. The initial p value was 0.01, and the edge weight threshold was 0.8 to visualize a subnetwork with relevant edges.
Statistical analysis
Analysis of demographic and clinical data was performed using IBM SPSS Statistics 25.0. Continuous variables underwent comparison using two-tailed independent samples t-test and analysis of variance (ANOVA), with Bonferroni’s correction applied for post hoc comparisons. Categorical variables were assessed using the Chi-squared test.
Network metrics were statistically examined through the GRETNA toolbox (https://www.nitrc.org/projects/gretna/) at a significance threshold of p < 0.05. Age, gender, and education level were adjusted for as confounding variables. The False Discovery Rate (FDR) was employed to adjust for multiple comparisons. Classification algorithms such as logistic regression, support vector classification, decision tree classification, and linear discriminant analysis were employed for identifying MMD-related subnetworks. Regression algorithms including linear regression, support vector regression, and decision tree regression were utilized to identify the subnetwork linked to white matter hyperintensity. This part of the statistical analysis was based on the NBS-Predict toolbox (https://www.nitrc.org/projects/nbspredict/), with the most effective algorithms automatically selected.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Contract grant number: 82371915).
Author contributions
H.Z. and P.W. conceived and designed the research. H.Z. performed statistical analysis and drafted the manuscript. W.L., Q.Z., C.Z. and T.L. enrolled patients and acquired brain magnetic resonance imaging. T.Y., X.L., Q.Z., J.Z. and Y.Z. critically revised the manuscript.
Data availability
The raw data that support the findings of this study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Research Committee on the Pathology and Treatment of Spontaneous Occlusion of the Circle of Willis & Health Labour Sciences Research Grant for Research on Measures for Infractable Diseases. Guidelines for diagnosis and treatment of moyamoya disease (spontaneous occlusion of the circle of Willis). Neurol. Med. Chir. (Tokyo). 52, 245–266 (2012). [DOI] [PubMed] [Google Scholar]
- 2.Karzmark, P., Zeifert, P. D., Bell-Stephens, T. E., Steinberg, G. K. & Dorfman, L. J. Neurocognitive impairment in adults with moyamoya disease without stroke. Neurosurgery70, 634–638 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Kazumata, K. et al. Combined structural and diffusion tensor imaging detection of ischemic injury in Moyamoya disease: relation to disease advancement and cerebral hypoperfusion. J. Neurosurg.134, 1155–1164 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Zuo, Z. et al. Atrophy in subcortical gray matter in adult patients with moyamoya disease. Neurol. Sci.44, 1709–1717 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lei, Y. et al. Reconfigured functional network dynamics in adult moyamoya disease: a resting-state fMRI study. Brain Imaging Behav.14, 715–727 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Zheng, G. et al. Changes in Brain Functional Network Connectivity in Adult Moyamoya diseases. Cogn. Neurodyn.15, 861–872 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu, J. et al. Moyamoya Disease with initial ischemic or hemorrhagic attack shows different brain structural and functional features: a pilot study. Front. Neurol.13, 871421 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun, H. et al. Angiographic and Hemodynamic Features in asymptomatic hemispheres of patients with Moyamoya Disease. Stroke53, 210–217 (2022). [DOI] [PubMed] [Google Scholar]
- 9.Alexander-Bloch, A., Giedd, J. N. & Bullmore, E. Imaging structural co-variance between human brain regions. Nat. Rev. Neurosci.14, 322–336 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang, P. et al. Reorganization of the Brain Structural Covariance Network in Ischemic Moyamoya Disease revealed by Graph Theoretical Analysis. Front. Aging Neurosci.14, 788661 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai, M. et al. Individual-level brain morphological similarity networks: current methodologies and applications. CNS Neurosci. Ther.29, 3713–3724 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pr, R. & Sc, S. Does size matter? The relationship between predictive power of single-subject morphometric networks to spatial scale and edge weight. Brain Struct. Function225, 2475–2493 (2020). [DOI] [PubMed]
- 13.Yin, H. et al. A Novel Staging System to evaluate cerebral hypoperfusion in patients with Moyamoya Disease. Stroke49, 2837–2843 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Liu, Z. W. et al. Collateral circulation in Moyamoya Disease: a New Grading System. Stroke50, 2708–2715 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Matchin, W. G. A neuronal retuning hypothesis of sentence-specificity in Broca’s area. Psychon Bull. Rev.25, 1682–1694 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu, J. et al. Abnormal brain functional and structural connectivity between the left supplementary motor area and inferior frontal gyrus in moyamoya disease. BMC Neurol.22, 179 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buckner, R. L. & DiNicola, L. M. The brain’s default network: updated anatomy, physiology and evolving insights. Nat. Rev. Neurosci.20, 593–608 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Yuan, Z. et al. Altered functional connectivity of the right caudate nucleus in chronic migraine: a resting-state fMRI study. J. Headache Pain. 23, 154 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei, C. et al. A comparative study of structural and metabolic brain networks in patients with mild cognitive impairment. Front. Aging Neurosci.13, 774607 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi, Z., Wen, Y. J., Huang, Z., Yu, L. B. & Zhang, D. Different aspects of cognitive function in adult patients with moyamoya disease and its clinical subtypes. Stroke Vasc Neurol.5, 86–96 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bothwell, A. R., Resnick, S. M., Ferrucci, L. & Tian, Q. Associations of olfactory function with brain structural and functional outcomes. A systematic review. Ageing Res. Rev.92, 102095 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li, Y. et al. Baseline cerebral structural morphology predict freezing of gait in early drug-naïve Parkinson’s disease. NPJ Parkinsons Dis.8, 176 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang, X., Zhang, M., Li, B., Shang, H. & Yang, J. Structural and functional brain abnormalities in idiopathic cervical dystonia: a multimodal meta-analysis. Parkinsonism Relat. Disord.103, 153–165 (2022). [DOI] [PubMed] [Google Scholar]
- 24.Zhang, W. et al. Functional organization of the fusiform gyrus revealed with connectivity profiles. Hum. Brain Mapp.37, 3003–3016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tachibana, Y. & Hikosaka, O. The primate ventral pallidum encodes expected reward value and regulates motor action. Neuron76, 826–837 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lisman, J. et al. Viewpoints: how the hippocampus contributes to memory, navigation and cognition. Nat. Neurosci.20, 1434–1447 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun, R. et al. White matter structural and network topological changes in moyamoya disease with limb paresthesia: a study based on diffusion kurtosis imaging. Front. Neurosci.16, 1029388 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujimura, M. et al. Intrinsic development of choroidal and thalamic collaterals in hemorrhagic-onset moyamoya disease: case-control study of the Japan Adult Moyamoya Trial. J. Neurosurg.130, 1453–1459 (2019). [DOI] [PubMed] [Google Scholar]
- 29.Zhang, Q. et al. Hemorrhagic patterns and their risk factors in patients with moyamoya disease. Eur. J. Neurol.27, 2499–2507 (2020). [DOI] [PubMed] [Google Scholar]
- 30.Serin, E., Zalesky, A., Matory, A., Walter, H. & Kruschwitz, J. D. NBS-Predict: a prediction-based extension of the network-based statistic. Neuroimage244, 118625 (2021). [DOI] [PubMed] [Google Scholar]
- 31.Sun, L. et al. Structural insight into the individual variability architecture of the functional brain connectome. Neuroimage259, 119387 (2022). [DOI] [PubMed] [Google Scholar]
- 32.Gong, G., He, Y., Chen, Z. J. & Evans, A. C. Convergence and divergence of thickness correlations with diffusion connections across the human cerebral cortex. Neuroimage59, 1239–1248 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Ren, J. et al. Asymptomatic carotid stenosis is associated with both edge and network reconfigurations identified by single-subject cortical thickness networks. Front. Aging Neurosci.14, 1091829 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao, Y. et al. Disrupted Gray Matter Networks Associated with Cognitive Dysfunction in Cerebral Small Vessel Disease. Brain Sci.13, 1359 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.KURODA, S. et al. Diagnostic criteria for Moyamoya Disease – 2021 revised version. Neurol. Med. Chir. (Tokyo). 62, 307–312 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wardlaw, J. M. et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol.12, 822–838 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ashburner, J. A fast diffeomorphic image registration algorithm. Neuroimage38, 95–113 (2007). [DOI] [PubMed] [Google Scholar]
- 38.Li, Y. et al. Surface-based single-subject morphological brain networks: effects of morphological index, brain parcellation and similarity measure, sample size-varying stability and test-retest reliability. NeuroImage235, 118018 (2021). [DOI] [PubMed] [Google Scholar]
- 39.Reddy Raamana, P. & Strother, C. Graynet: single-subject morphometric networks for neuroscience connectivity applications. JOSS3, 924 (2018). [Google Scholar]
- 40.Schmidt, P. Bayesian Inference for Structured Additive Regression Models for large-scale Problems with Applications to Medical Imaging (Ludwig-Maximilians-Universität, 2017). [Google Scholar]
- 41.Wang, J. et al. GRETNA: a graph theoretical network analysis toolbox for imaging connectomics. Front. Hum. Neurosci.9, (2015). [DOI] [PMC free article] [PubMed]
- 42.Rubinov, M. & Sporns, O. Complex network measures of brain connectivity: uses and interpretations. NeuroImage52, 1059–1069 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data that support the findings of this study are available from the corresponding author on reasonable request.