Abstract
Cnidarians (sea anemones, corals, hydroids, and jellyfish) are a key outgroup for comparisons with bilateral animals to trace the evolution of genomic complexity and diversity within the animal kingdom, as they separated from most other animals 100 s of million years ago. Cnidarians have extensive diversity, yet the paucity of genomic resources limits our ability to compare genomic variation between cnidarian clades and species. Here, we report the genome for Edwardsia elegans, a sea anemone in the most specious genus of the family Edwardsiidae, a phylogenetically important family of sea anemones that contains the model anemone Nematostella vectensis. The E. elegans genome is 396 Mb in length and is predicted to encode approximately 49,000 proteins. We annotated a large conservation of macrosynteny between E. elegans and other Edwardsiidae anemones as well as conservation of both microRNAs and ultra-conserved noncoding elements previously reported in other cnidarians species. We also highlight microsyntenic variation of clustered developmental genes and ancient gene clusters that vary between species of sea anemones, despite previous research showing conservation between cnidarians and bilaterians. Overall, our analysis of the E. elegans genome highlights the importance of using multiple species to represent a taxonomic group for genomic comparisons, where genomic variation can be missed for large and diverse clades.
Keywords: cnidaria, genome sequencing, synteny, Anthozoa, microRNA, genomic diversity
Introduction
Sequencing the genomes of species in the phylum Cnidaria has proven to be useful for understanding the genomic evolution of animals (Putnam et al. 2007; Stefanik et al. 2014; Zimmermann et al. 2023). The first released cnidarian genome from the edwardsiid sea anemone Nematostella vectensis revealed unexpected complexity in gene content and structure (Putnam et al. 2007), providing novel insights into the cnidarian–bilaterian ancestor. Since then, most reported genomes currently generated for the cnidarian phylum have focused on Anthozoans (104 of 157 genomes), where the large majority (n = 94) are from Hexacorallia (stony corals and sea anemones). Within the Hexacorallia, only 20 of these species are sea anemones (Actinarians). The relative paucity of genomic data compared to the taxonomic and evolutionary diversity of sea anemones, as well as the phylum Cnidaria more broadly, limits our understanding of genomic evolution in this ancient group and how it relates to the diversity of life histories represented by the many species of cnidarians. The lack of genomic data for much of the phylum leaves the community with the need to strategically sequence, assemble, and annotate genomes from additional species to better resolve patterns and processes in genome evolution.
The family Edwardsiidae (Cnidaria, Anthozoan, and Actinaria) contains over 100 species of sea anemones in 13 accepted genera, many composed of only a few species (World Register of Marine Species, accessed 04/09/2024). Species in the family Edwardsiidae are distributed throughout the world from polar to tropical seas, ranging from deep seas to shallow coastal habitats (Daly 2002; McFadden et al. 2007, 2021; Daly et al. 2008, 2013; Daly and Ljubenkov 2008). Edwardsiids have been studied due to their unique structures (nemathybomes and nematosomes), their “simple” anatomy and “phylogenetic primitiveness” as adults, and challenges with resolving relationships of this family within Anthozoa (Daly 2002; Daly et al. 2002, 2008). Species of interest have included Scolanthus callimorphus (Zimmermann et al. 2023), the parasitic Edwardsiella lineata (Bumann and Puls 1996; Stefanik et al. 2014), the Antarctic ice-dwelling species Edwardsiella andrillae (Daly et al. 2013), and the model species for evolutionary developmental biology and genomics Nematostella vectensis (Darling et al. 2005; Layden et al. 2016; Al-Shaer et al. 2021). The most specious genus in this family is Edwardsia with more than 60 recognized species, which lacks any genomic data, limiting our ability to contextualize the genomic diversity and variation reported for existing genomes for N. vectensis and the recently published S. callimorphus (Zimmermann et al. 2023). The genus Edwardsia diverged from other genera in the family 100 s of million years ago (McFadden et al. 2021; Zimmermann et al. 2023), thus providing a comparable context for evolutionary separation between N. vectensis and S. callimorphus for genome content as well as organization.
To improve our understanding of the Edwardsia genus and increase the genomic resources for actinarians, we have sequenced and assembled a de novo genome for the edwardsiid anemone Edwardsia elegans Verrill 1869. E. elegans live in soft bottom habitats from the intertidal to 120 m depth along the North Atlantic coast of North America from Maine to North Carolina. The latitudinal range of E. elegans overlaps with other Edwardsiidae species, most notably the model cnidarian N. vectensis. The E. elegans range also coincides with some of the fastest-warming waters on Earth, where genome-enabled species can be utilized to monitor acclimation and adaptation to climate change (Kavanaugh et al. 2017; Aguirre-Liguori et al. 2021).
Here we used this genome to compare genomic variation between sea anemones, including two edwardsiid species, to compare patterns of representation for gene families, specific conserved features [microRNAs, Ultra Conserved Noncoding Elements (UCNEs)] and syntenic organization. Syntenic regions have developed into particularly useful regions of the genome to highlight evolutionary relationships (Schultz et al. 2023) in addition to their influence on gene regulation (Marques-Bonet et al. 2004; Farre et al. 2019). Altogether, we show that the addition of this genome from Edwardsia elegans provided an insightful comparison to better understand variation in genome content and organization for actiniarians including model species like Nematostella vectensis.
Methods
Specimen collection and culture
Adult E. elegans were collected with the assistance of the Gulf of Maine, Incorporated (www.gulfofme.com) from Cobscook Bay, Maine. They were shipped to the University of North Carolina at Charlotte, where they were housed in a recirculating water system and in finger bowls. The recirculating water system was set to a temperature of 16°C with artificial seawater (Instant Ocean) at 30 parts per thousand (ppt), with a ∼5% water change twice per week. E. elegans in finger bowls were kept in 30 ppt artificial seawater at room temperature with a full water change once a week. All anemones were cultured with sand substrate (Nature's Ocean Marine White #0) and regularly fed freshly hatched Artemia nauplii and pieces of mantle tissue from mussels (Mytilus edulis). Prior to DNA extraction, anemones from the recirculating tanks were placed in standing glass finger bowls with a small amount of sand substrate, held in a 16°C incubator in total darkness, and starved for 2 weeks to minimize DNA from food sources.
DNA extraction, library preparation and sequencing
A single individual E. elegans was used for DNA extraction for both Illumina and Oxford Nanopore sequencing. DNA was extracted using a protocol based on Smith et al (Smith et al. 2023), quantified using a Qubit (Q32857) with the dsDNA High Sensitivity kit (Q32851), following standard manufacturer protocols, and quality checked on a 1% agarose gel.
A library for Illumina sequencing was prepared with the Illumina DNA PCR-Free Prep kit (20041855). The library was quantified and quality checked using a Qubit (Q32857) and dsDNA Quantitation, High Sensitivity kit (Q32851). The resulting library was sequenced using NextSeq 2000 P3 (2 × 150 bp) on a NextSeq 2000 instrument at the University of North Carolina at Charlotte.
Four libraries were prepared for Nanopore sequencing using the ligation sequencing kit (LSK109; Oxford Nanopore Technologies) and were sequenced with a MinION sequencer (R10.4; Oxford Nanopore Technologies). All libraries were sequenced on a single flow cell for approximately 45 hours each, with the flow cell being washed between each new library following manufacturer protocols. Prior to assembly, raw nanopore reads were basecalled using Guppy v. 6.0.6 (Oxford Nanopore Technologies) with Cuda and a configuration file for the R10.3 flow cell and a minimum quality score of 7.
Assembly
Prior to genome assembly, Illumina reads were used to estimate the genome size. Jellyfish v. 2.3.0 (Marcais and Kingsford 2011) was used to generate counts for 21-bp kmers and were visualized in GenomeScope (Vurture et al. 2017), estimating the genome to be roughly 332Mb with heterozygosity of 2.5%. Based on the high heterozygosity from the kmer estimates, we used the program wtdbg2 v. 2.5 (Ruan and Li 2020), for genome assembly using the basecalled Oxford Nanopore reads. All settings were default except “–edge-min” which was reduced to 2, sampling rate “-S” which was reduced to 2, and “–rescue-low-cov-edges” was added. After assembly, the genome was polished twice using Illumina raw reads with the program Pilon v. 1.24 with default settings (Walker et al. 2014).
Repeat content was determined using a combination of RepeatModeler v 2.0.2 (Smit and Hubley 2008–2015) and RepeatMasker v. 4.1.2 (Smit et al. 2013–2015). First, the assembled genome was run through RepeatModeler using default settings. This generated a masked genome that was used as an input for RepeatMasker, with default settings. The masked genome output from RepeatMasker was used for protein predictions.
Annotations
Genes in the final assembled genome were annotated using the program BRAKER2 v 2.1.5 (Bruna et al. 2021) which uses GeneMark and Augustus to predict gene models. Three data sources were used as inputs into BRAKER2: (1) raw RNA-Seq data from E. elegans (GenBank Accession GKWK00000000), (2) proteins from the confamilial species N. vectensis downloaded from SIMRbase (NV2, https://simrbase.stowers.org/starletseaanemone) and (3) the metazoan protein database available from ProtHint (https://bioinf.uni-greifswald.de/bioinf/partitioned_odb11/). Protein files from N. vectensis and the ProtHint metazoan database were concatenated into a single file. Outputs from BRAKER2 were then queried with BLASTp v2.11.0+ against proteins in databases from NCBI (accessed March 2022), Uniprot Swiss-Prot (accessed March 2022), and N. vectensis proteins using Diamond BLAST (Buchfink et al. 2014) to identify known genes for annotations. BLASTp matches were sorted by e-value and percent similarity, and top hits based on e-value and bit score were utilized for annotation for each predicted gene. Gene predictions were also annotated with functional domains for protein families using the program HmmrScan v 3.1 (Eddy 2009). Only hits with an E-value less than 1e-05 were retained and used for annotation.
BUSCO v. 5.1.3 (Manni et al. 2021) using the metazoa_odb10 ortholog set was used to assess quality based on the completeness of both gene predictions and the genome as a whole.
Orthologous proteins
Orthofinder v. 2.4.0 (Emms and Kelly 2019) was used to identify orthogroups, orthologs, and gene duplications between the newly assembled E. elegans genome and other anthozoans. Single-copy orthologs generated were then used for syntenic analysis (see below). We used proteins from the following anthozoans: Exaiptasia diaphana, Actinia tenebrosa, N. vectensis, S. callimorphus, and E. elegans. The hexacoral Acropora millepora and the octocoral Renilla reniformis were used as outgroups. E. elegans proteins were from this study, N. vectensis and S. callimorphus proteins were obtained from SIMRbase, and all other proteins were obtained from NCBI. Orthofinder was run with default settings.
Syntenic analysis
We compared both macro- and microsyntenic regions between our E. elegans genome and the other Edwardsiidae anemones species with high-quality genome assemblies, N. vectensis and S. callimorphus. For macrosyntenic analysis, reciprocal protein BLASTp were performed between E elegans protein predictions generated from BRAKER2 and N. vectensis protein predictions, as well as E. elegans and S. callimorphus. For Oxford Dot Plot comparisons, top hits for reciprocal BLASTp were taken and one-to-one hits between species were identified. Information on locations for each of these proteins was then taken. Genome location information for each gene was compared with the R (R Core Team 2022) program MacrosyntR (El Hilali and Copley 2023) to generate Oxford Dot Plot comparisons for pairwise species comparisons. For ribbon plot comparisons, orthogroups with only a single copy in all three Edwardsiidae anemones were identified and confirmed with reciprocal BLASTp searches. MacrosyntR was then used to generate ribbon plots.
For microsyntenic analysis, sequences for each region of interest were obtained and used for BLASTp searches against proteins annotated from BRAKER2 and then the locations of the top hits were found in the genomes of each target species. Top hits for each gene were taken and then sorted by starting location along each chromosome/scaffold, and then, gene order, proximity, and direction were compared between each species.
Ultra-conserved noncoding elements
To identify ultra-conserved noncoding elements (UCNE) in the E. elegans genome, known UCNEs annotated for N. vectensis and S. callimorphus were obtained from SIMRbase (https://simrbase.stowers.org/starletseaanemone); 143 UCNE were then searched for in the E. elegans genome with BLASTn. Hits higher than an e-value of 1e-05 and shorter than a length of 100 bp were removed, as all but two of the UCNEs identified in N. vectensis exceed 100 bp. Top hits from the E. elegans genome were annotated and used to generate an Oxford dot plot in MacrosyntR with UCNEs from N. vectensis and S. callimorphus.
Small-RNA sequencing and annotation of miRNAs
Total RNA was extracted from E. elegans polyps using TRIzol reagent (Thermo Fisher Scientific, USA), following the manufacturer's protocol. The extracted RNA was then selected for 18–30 nucleotides on a 15% denaturing urea polyacrylamide gel (Bio-Rad, USA). RNA was eluted overnight in 0.3 M NaCl. For library preparation, we used a modified version of the Illumina TruSeq small-RNA Cloning Protocol (Zamore lab, http://www.umassmed.edu/zamore/resources/protocols/, accessed April 2014). In brief, the small RNAs (sRNAs) were ligated to 3′ and 5′ adapters containing four random nucleotides at the ligation interface to minimize ligation bias. The ligation products were then reverse transcribed using SuperScript III Reverse Transcriptase (Thermo Fisher Scientific). The cDNA samples were PCR amplified using the KAPA Real-Time Library Amplification Kit (PeqLab, Germany). The amplified cDNA was purified on 2% low-melt agarose gels (Bio-Rad). The small-RNA library was validated on a High Sensitivity D1000 ScreenTape (Agilent, USA) Finally, the sRNA library was sequenced on NextSeq 500 (Illumina, USA), with read lengths of 50 nucleotides. miRNA analysis was performed using mirDeep2 (Friedländer et al. 2011). Initially, the sequencing data was pre-processed to remove 3′ end adapters using clip_adapters.pl script provided by miRDeep2. Next, the short sequences less than 18 nucleotides were discarded using mapper.pl. The miRDeep2 core algorithm was then used to identify miRNAs, with the sequenced E. elegans genome from the current study used as a reference. The identified miRNA candidates were validated manually based on specific criteria suggested for miRNA annotation in animals (Fromm et al. 2015), including a 2-nucleotide overhang on the 3′ end of precursor miRNA, a distinct length of ∼18–23 nucleotides, predicted folding of pre-miRNA transcript into a hairpin structure of ∼60 nucleotides, a clear signature for strand selection with a dominant guide strand, that contains a homogeneous 5′ end, guide/star ratio higher than two, and at least 16 nucleotide complementarities between mature and star strand. However, the terminal loop size of precursor miRNAs above eight nucleotides and the consistency of the star strand of miRNAs were not considered since cnidarian miRNAs do not seem to follow these rules (Praher et al. 2021). To identify conserved miRNA sequences across cnidarians, mature miRNA and miRNA precursors from ten cnidarian species were retrieved from previous miRNA studies (Liew et al. 2014; Moran et al. 2014; Gajigan and Conaco 2017; Baumgarten et al. 2018; Fridrich et al. 2020; Praher et al. 2021) and used as input in the miRDeep2 quantification algorithm. Output files from the miRDeep2 core algorithm and accepted miRNAs are provided in Supplementary Table 1.
Results
Genome assembly and annotation
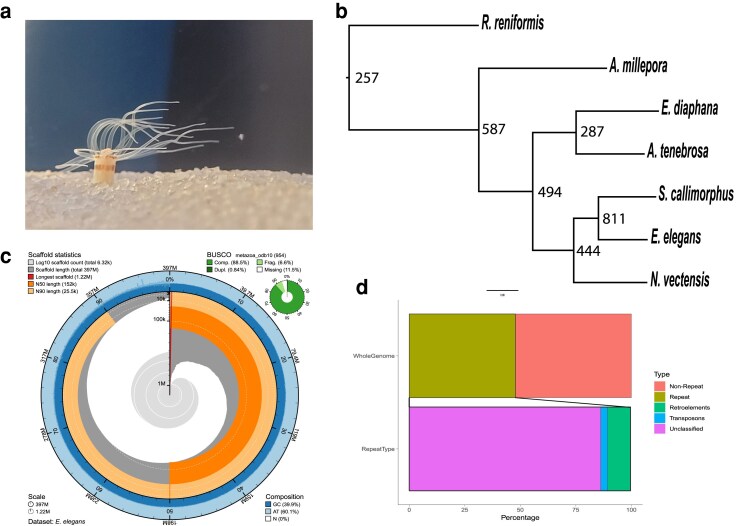
Nanopore sequencing of genomic DNA generated approximately 9.5 gigabytes of raw data. Raw nanopore reads were basecalled leading to 385,657 reads that were then assembled into 6,319 contigs. Illumina sequencing of genomic DNA resulted in 79,640,638 paired-end reads and was used to polish the 6,319 assembled contigs. This results in an E. elegans genome that is 396,821,203bps in length with an N50 of 151,884 bp. Genome quality was assessed with BUSCO and resulted in a score of 88% (Complete + Partial: 95.07%, Complete: 87.6%, Duplicate: 0.7%, Missing: 5.0%) based on the metazoan database. Guanine-Cytosine content is 39.9% and repeat content was calculated to be 47.8% of the genome (Fig. 1). Beside unclassified repeats, the most abundant type of repetitive elements was the LINE group, making up 3.78% of the genome. LTRs make up 1.09% of the genome, and DNA transposons were 1.49% of the genome. 49,837 protein-coding regions were predicted using Braker2 (Bruna et al. 2021).
Fig. 1.
a) Edwardsia elegans in the recirculating aquaria at UNC Charlotte. b) Phylogenetic tree with node number representing duplications. c) Snail plot of the E. elegans genome generated using BlobToolKit (Challis et al. 2020). d) Repetitive Regions of the genome.
Orthologous protein groups
Orthofinder (Emms and Kelly 2019) was used to identify and compare orthologous groups shared between E. elegans and other sea anemone species. Orthofinder generated 24,525 orthogroups, with 91.2% of total proteins from all species being placed into an orthogroup. There were 6,134 orthogroups (25%) containing proteins from all species, with 869 single-copy orthogroups shared between all species. All species had over 90% of their proteins placed into an orthogroup, except E. elegans and R. reniformis, which had 82.0 and 83.5% respectively. 74.7% of all orthogroups contained at least one E. elegans protein, the highest of any species examined here. We determined the number of gene duplications at each node (Fig. 1b). Focusing on the Edwardsiidae branch, there are 444 duplications for the node leading to the edwardsiid species and nearly double that number for the E. elegans/S. callimorphus node.
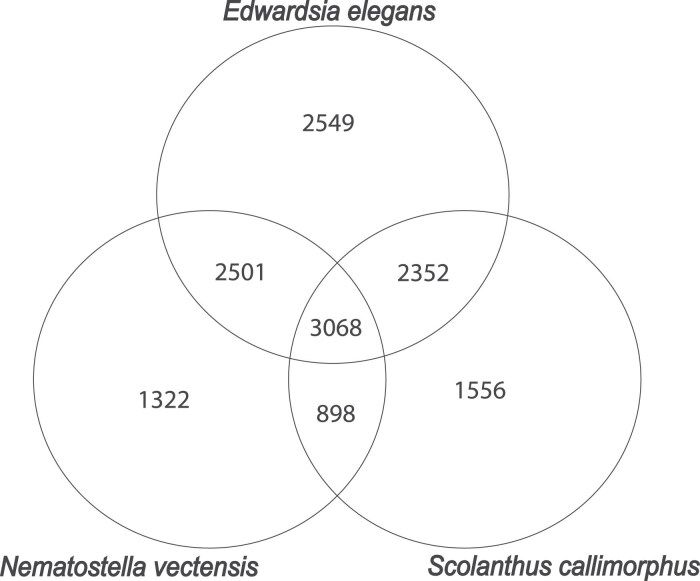
When comparing the number of single-copy orthologs in species in the Edwardsiidae family (i.e. E. elegans, N. vectensis, and S. callimorphus), we find 3,068 single-copy orthologs shared between all three Edwardsiidae species (Fig. 2). When comparing E. elegans to the other Edwardsiidae species, 5,569 single-copy orthologs are shared with N. vectensis, or approximately 11.7% of the predicted proteins in E. elegans. There are 5,420 single-copy orthologs shared with S. callimorphus, approximately 10.8% of the predicted proteins in E. elegans. Single-copy orthologs shared between all three Edwardsiidae anemones include several housekeeping genes such as heat shock proteins, guanosine monophosphate synthase, wnt, and glyceraldehyde-3-phosphate dehydrogenase. There were 2,501 single-copy orthologs shared between E. elegans and N. vectensis but not S. callimorphus, and 2,352 single-copy orthologs shared by E. elegans and S. callimorphus but not N. vectensis. The single-copy orthologs shared in all three species were then used to identify regions of synteny between E. elegans, S. callimorphus, and N. vectensis.
Fig. 2.
Venn Diagram showing the number of species specific and shared Single-Copy Orthologs found between the three edwardsiid species.
Syntenic regions of genomes
Macrosyntenic arrangements
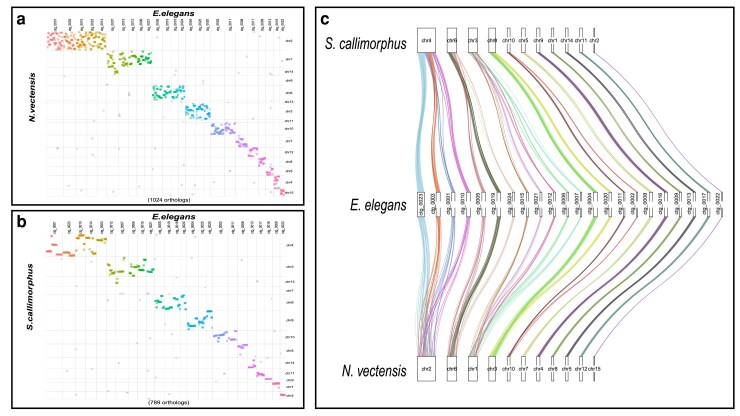
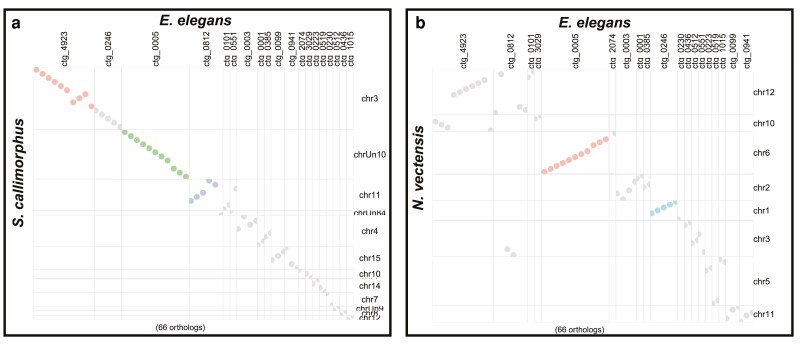
We identified numerous conserved macrosyntenic regions between E. elegans and the two other Edwardsiidae anemones, N. vectensis and S. callimorphus. Using reciprocal BLASTp across the largest 25 contigs in the E. elegans genome there were 1,024 one-to-one orthologs between E. elegans and N. vectensis and 789 one-to-one orthologs between E. elegans and S. callimorphus. These comparisons show many E. elegans contigs localizing to single chromosomes in S. callimorphus and N. vectensis (Fig. 3a and b, respectively). These macrosyntenic regions shared between E. elegans and S. callimorphus are more tightly distributed, as seen in more instances of straight lines in Fig. 3b. For example, E. elegans ctg_0001 has two syntenic regions of over 10 single-copy orthologs that are also located in the same order on chromosome 4 of S. callimorphus. However, these same proteins are more dispersed on chromosome 2 of N. vectensis, with only one conserved syntenic region of five proteins. We also show that E. elegans ctg_0023 has a syntenic region of 21 single-copy orthologs shared on chromosome 4 of S. callimorphus, while the same region on chromosome 2 of N. vectensis has only a small syntenic region of 5 proteins with the rest mixed across the chromosome.
Fig. 3.
Macrosyntenic regions between E. elegans and a) N. vectensis and b) S. callimorphus. Each dot represents the best one-to-one reciprocal BLASTp hit for a protein between the two species. c) A ribbon plot of single-copy orthologs showing macrosynteny between E. elegans and S. callimorphus and N. vectensis.
We identified regions where E. elegans and S. callimorphus share longer stretches of genes in the same order that are not in synteny with N. vectensis. In these largest 25 contigs of the E. elegans genome, there were 13 regions of 10 or more genes in nearly identical gene order between E. elegans and S. callimorphus, where these same regions in N. vectensis are dispersed and are not larger than 9 protein-coding genes. The largest syntenic region between E. elegans and S. callimorphus in this analysis was 25 protein-coding genes found across approximately 450 kb in both species, whereas the largest syntenic region between E. elegans and N. vectensis was 9 coding genes across approximately 100 kb.
Using single-copy orthologs shared between all three Edwardsiidae species, we investigated how macrosyntenic regions have been rearranged (Fig. 3c). For example, syntenic blocks of genes located on E. elegans ctg_0003 and ctg_0010 correspond to 2 regions of chromosome 4 in S. callimorphus. However, these clustered genes were more dispersed in N. vectensis. Similarly, two syntenic regions on E. elegans ctg_0004 and ctg_0020 match chromosome 8 of S. callimorphus, while these gene regions are dispersed on chromosome 3 of N. vectensis. These regions are an indication of conservation of chromosomal gene content between these edwardsiid species, while also showing the synteny within chromosomes is less conserved in N. vectensis.
Microsyntenic trends
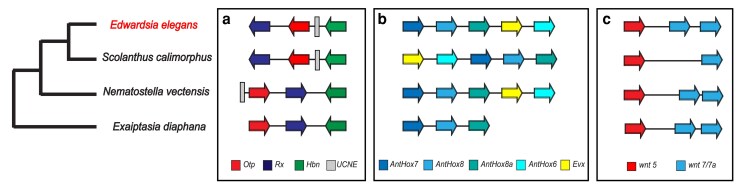
We compared the microsynteny of previously identified developmental genes that are clustered in Edwardsiidae anemones with the model anemone Exaiptasia diaphana (Baumgarten et al. 2015) as an outgroup. The first region we compared was a cluster of three Paired-class homeobox genes, Hbn–Rx–Otp, involved in animal development (Holland 2013) and previously shown to be clustered in N. vectensis (Mazza et al. 2010). All three genes are found in close association in all four of these anemone species; however, the genomic organization varies (Fig. 4a). E. elegans and S. callimorphus share the same order of Rx–Otp–Hbn and all genes are in the same transcriptional orientation. The gene order of these two species differs compared to N. vectensis and E. diaphana, where the orientation and transcriptional direction of the Otp and Rx genes are inverted with respect to Hbn. In addition, a UCNE was identified near the 5′ end of Otp in the edwardsiid species that also translocated with the inferred inversion (discussed more below).
Fig. 4.
Microsyntenic comparisons between E. elegans and the three other actinarians. a) Hbn–Otp–Rx and an UCNE b) HOX c) wnt5 and 7/7a.
Next, we compared regions of homeobox-related genes, which are critical for animal embryonic development and whose gene order impacts axial patterning (Gellon and McGinnis 1998; DuBuc et al. 2018). We identified 30 homeobox-related genes previously annotated in N. vectensis and other sea anemones (Chourrout et al. 2006; Ryan et al. 2007; Zimmermann et al. 2023). In E. elegans we find four clusters of three or more of these homeobox genes along with three sets of gene pairs. These clusters were not all located on the same contig as they are in other sea anemones, which may be a result of the fragmented assembly compared to the chromosome scale of the other anemones” genomes. The ParaHox cluster (xlox/cdx & gsx) is conserved in all four species with highly similar distances between genes. A cluster of hox genes which contains anthox7, anthox8, anthox8b, evx, and anthox6, is identical between E. elegans and N. vectensis, while this cluster is slightly rearranged in S. callimorphus, with evx and anthox6 in a different position relative to the other genes (Fig. 4b). The arrangement of this homeobox cluster found in E. elegans and N. vectensis is also found in the coral Acropora digitifera.(DuBuc et al. 2012). We also annotated a pair of nk genes, nk1 and nk5, that form a paired cluster in E. elegans on ctg_1036, as well as a cluster that contains msx, nk2c, and nk2d on ctg_0003. hlxe and gbx are arranged as a pair on ctg_0208. We also identified a cluster of anthox1, lbx, nk3, rough on ctg_0005.
E. elegans also has a wnt cluster that was identified in N. vectensis and other cnidarians (Ryan et al. 2006, 2007; Sullivan et al. 2007; Steinworth et al. 2023). Both E. elegans and S. callimorphus have a cluster of wnt-5 and wnt-7/7b. We confirmed the presence of both wnt7 and wnt7b in E. elegans and S. callimorphus through a tBLASTn of the exons from Sullivan et al. (2007). This wnt cluster spans a similar size of the genome in both N. vectensis and E. elegans while in S. callimorphus these genes are distributed over approximately 12,000 additional bases.
Ultra-conserved noncoding elements
Previous research in N. vectensis and S. callimorphus identified 143 ultra-conserved noncoding elements (UCNEs) present in the family Edwardsiidae (Zimmermann et al. 2023). Here, we show there are at least 92 UCNEs (of 143 total) that have previously been found in N. vectensis and S. callimorphus present in this new E. elegans genome. These UCNEs are distributed across 62 different contigs in the genome. We also observe UCNEs found near syntenic regions, such as UCNE 579 within the Otp-Rx-Hbn syntenic region, approximately 100 bp upstream fromOtp. This UCNE is found outside of this cluster in N. vectensis but is present inside the cluster in both E. elegans and S. callimorphus (Fig. 4a), presumably due to the same inversion event.
We further observed that there are several regions where these UCNE are found in syntenic arrangements in all three edwardsiid species. These regions were also found to be nearly uniformly distributed across many regions between all three species (Fig. 5). Similar to the macrosynteny for genes in orthogroups, these UCNE regions were more contiguous between E. elegans and S. callimorphus (Fig. 5a), with more instances of rearrangement between E. elegans and N. vectensis (Fig. 5b).
Fig. 5.
Dot plot of UCNE regions between a) E. elegans and S. callimorphus and b) E. elegans and N. vectensis.
E. elegans miRNA repertoire
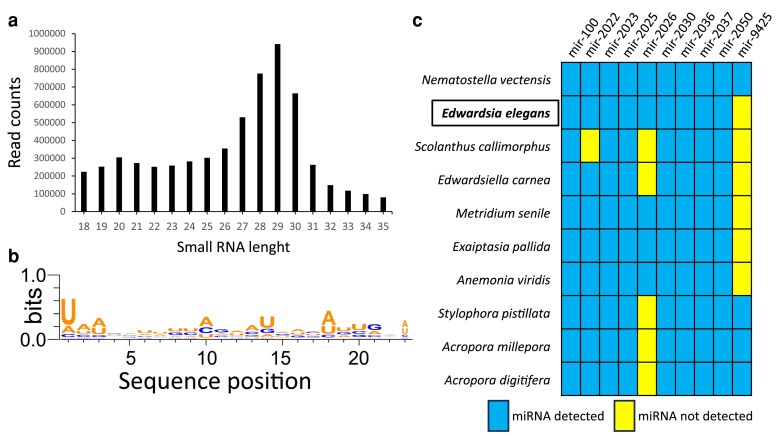
To explore miRNA repertoire of E. elegans, we prepared small-RNA libraries from the polyps and analyzed the data using miRDeep2 (Friedländer et al. 2011) (see Material and methods for details). The length distribution of total small-RNA reads revealed two major small-RNA populations with nucleotide lengths of 19–22 and 26–31 nucleotides (Fig. 6a). The first population represents putative miRNAs and small-interfering RNAs (siRNAs), whereas the second population represents putative P-element-induced wimpy testis-interacting RNAs (piRNAs) and constituted the majority of the sequenced small RNAs. The length distribution of the small RNAs and the enrichment of piRNA population over miRNAs is consistent with previous studies from other cnidarians (Liew et al. 2014; Moran et al. 2014; Gajigan and Conaco 2017; Baumgarten et al. 2018; Fridrich et al. 2020; Praher et al. 2021). The miRDeep2 predicted 137 miRNA candidates, of which 30 were identified as bona fide miRNAs based on specific criteria suggested for miRNA annotation in animals (see Material and methods for details). The analysis of the nucleotide composition of miRNA sequences identified here reveals a strong bias for U at the first position of the mature sequence, representing a characteristic feature of miRNAs (Fig. 6b). This bias is explained by the preference of Argonaute proteins for a U at the 5′ end of the miRNA, which is a known characteristic of miRNAs in bilaterian (Frank et al. 2010) and cnidarians (Moran et al. 2014; Praher et al. 2021). A comprehensive list of bona fide miRNAs of E. elegans and those who did not pass the criteria is available in Supplementary Table 1. Using miRDeep2's quantifier module, we also detected conserved miRNAs across cnidarians by providing sequences of previously identified cnidarian miRNAs (Liew et al. 2014; Moran et al. 2014; Gajigan and Conaco 2017; Baumgarten et al. 2018; Fridrich et al. 2020; Praher et al. 2021). Only 10 out of the 30 miRNAs were homologous in sequence to ones that had been annotated previously in one or more anthozoan species, with only seven being conserved among all the anthozoans for which there are data available (Fig. 6c).
Fig. 6.
Annotation of the E. elegans miRNA repertoire. a) Two distinct populations of small-RNA reads: putative siRNAs/miRNAs (19–23nt) and putative piRNAs (27–31nt). b) miRNA sequences exhibit a bias toward uridine at position one. WebLogo3 was utilized to create sequence logos (Crooks et al. 2004). c) The miRNAs that are evolutionarily conserved between E. elegans and other sequenced anthozoan species.
Discussion
The de novo genome for the sea anemone Edwardsia elegans is the first genome for a species in the Edwardsia genus. The genome is 396.8Mb in length and is predicted to contain 49,837 protein-coding regions, with a repeat content calculated to be approximately 47%. Comparative analysis of E. elegans to the edwardsiid anemones S. callimorphus and N. vectensis shows the conservation of thousands of single-copy orthologs, macrosyntenic structures, as well as variation in gene order of important developmentally related genes. This conservation is consistent with previous research on the family Edwardsiidae and strengthens our understanding of the relationship of edwardsiid sea anemones (Daly 2002; Rodriguez et al. 2014; Zimmermann et al. 2023).
Macrosynteny analyses have recently emerged as a particularly informative approach for inferring evolutionary timing of genomic rearrangements(Simakov et al. 2020; Schultz et al. 2023). We observe macrosyntenic regions of both proteins and UCNEs that are shared between all three Edwardsiidae species, but many of these regions are more contiguous and linear between E. elegans and S. callimorphus. We expect this in more closely related species that have had less evolutionary time to differentiate. Sequencing of other Edwardsiidae anemones, especially those in the Edwardsia genus, will be needed to determine if these regions are conserved more broadly in the genus. More genomic detail will also allow us to see how these regions differentiated in the family Edwardsiidae to discern if rearrangements in N. vectensis are unique in the Edwardsiidae family.
Gene clusters of evolutionarily conserved transcription factors (e.g. Hox genes) are hypothesized to be conserved over large phylogenetic distances due to functional constraints. The spatial arrangement of these genes can be related to the timing and location of expression during development (Gellon and McGinnis 1998). Cnidarians, particularly N. vectensis, have been insightful for the evolutionary history of the Hox, wnt, Paired-Class, and other genes due to the arrangement of these genes in the genome and their spatiotemporal expression patterns and their developmental functions (Kusserow et al. 2005; Ryan et al. 2006, 2007; Sullivan et al. 2007; DuBuc et al. 2018; Steinworth et al. 2023). Comparisons of E. elegans with other edwardsiid anemones show variation within these clusters, helping to clarify the evolutionary timing of these genomic rearrangement events. Previous research in N. vectensis has shown these gene clusters to be expressed at particular times and locations in development (Ryan et al. 2007; Sullivan et al. 2007; Mazza et al. 2010; Kraus et al. 2016; DuBuc et al. 2018; He et al. 2018; Holstein 2022), and thus, research into the developmental timing using E. elegans or S. callimorphus in comparison with N. vectensis would be needed to determine if the order of these genes influences their developmental timing in these edwardsiid species and possibly in other actinarians.
miRNAs are small noncoding RNAs that play a crucial role in various biological processes such as development and cell physiology in both plants and animals (Bartel 2009; Voinnet 2009; Modepalli et al. 2018). Despite extensive research, the evolution of miRNAs remains enigmatic, particularly with respect to whether they have a common origin in plants and animals due to their differences in biogenesis (Axtell et al. 2011; Moran et al. 2013, 2017; Tripathi et al. 2022; Edelbroek et al. 2024). The phylogenetic position of E. elegans in relation to the other edwardsiids helps to clarify the timing of miRNA evolution within Cnidaria. Here, we analyzed the miRNAs in E. elegans and identified 30 bonified miRNAs. By comparing miRNA sequences with other cnidarian species, we discovered nine miRNAs that were conserved with N. vectensis, a closely related species, and six that were shared with other anthozoan species. The findings not only revealed E. elegans-specific miRNAs but also supported our earlier report on miRNA sequence evolution in cnidarians (Praher et al. 2021), which highlighted rapid gains and losses of miRNAs in Cnidaria, indicating a higher miRNA turnover rate in cnidarians compared to bilaterians.
While the number of proteins predicted is higher than the other Edwardsiidae anemones (Zimmermann et al. 2023), high BUSCO scores and the fact that the majority of predicted proteins had significant BLAST hits give us confidence that they are transcribed portions of the genome. Efforts to reduce the number of genes with tools in the BRAKER pipeline resulted in the removal of genes previously annotated in E. elegans (e.g. toxin genes). Thus, we preferred to retain all predicted genes for transparency in the annotation process for this de novo genome. While the true number of proteins in the E. elegans genome is likely smaller than what we have predicted, we are leaving all predicted proteins rather than risk removing true proteins.
We have assembled and annotated the genome of E. elegans, which we have shown is a valuable tool for studying cnidarian genomic diversity and evolution. More genetic information for species within the actiniarians will improve our understanding of the evolutionary history of both actiniarians and cnidarians as a whole and can shed light on many processes that are shared between the two. Broad comparisons across taxonomic groups will benefit by increasing the available species that can be used for comparisons. Increasing the number of species with genomic resources is the first step and will also improve our understanding of genomic diversity and variation across a lineage. This genome will help aid in comparisons of the diversity of ecologically and phylogenetically important cnidarians, as well as a comparative investigation of the parallel evolution of cnidarians and bilaterians.
Supplementary Material
Acknowledgments
CIPHER Center for support with reagents and sequencing resources. UNC Charlotte High Performance Computing for technical support and access to computing resources. A huge thank you to Dr. Edward Smith for advice and assistance with both high molecular weight DNA extractions and bioinformatics. Thank you to Dr. Sydney Birch for your help with bioinformatics.
Contributor Information
Auston I Rutlekowski, Department of Biological Sciences, University of North Carolina at Charlotte, 9201 University City Blvd, Charlotte, NC 28223, United States; Center for Computational Intelligence to Predict Health and Environmental Risks, University of North Carolina at Charlotte, 9331 Robert D. Snyder Rd, Charlotte, NC 28223, United States.
Vengamanaidu Modepalli, Marine Biological Association of the UK, The Laboratory, Citadel Hill, Plymouth PL1 2PB, United Kingdom.
Remi Ketchum, Whitney Laboratory for Marine Bioscience, University of Florida, 9505 Ocean Shore Blvd, St Augustine, FL 32080, United States; Department of Genetics, University of North Carolina at Chapel Hill, 120 Mason Farm Rd, Chapel Hill, NC 27599, United States.
Yehu Moran, Department of Ecology, Evolution and Behavior, Alexander Silberman Institute of Life Sciences, Faculty of Science, The Hebrew University of Jerusalem, Edmond J. Safra Campus, Jerusalem 9190401, Israel.
Adam M Reitzel, Department of Biological Sciences, University of North Carolina at Charlotte, 9201 University City Blvd, Charlotte, NC 28223, United States; Center for Computational Intelligence to Predict Health and Environmental Risks, University of North Carolina at Charlotte, 9331 Robert D. Snyder Rd, Charlotte, NC 28223, United States.
Data availability
The genome and raw data sequences have been deposited on NCBI, accession number JBHDXZ000000000, Bioproject number PRJNA1147700, Biosample number SAMN43163413, and SRA number SRP533906. Codes for this study are available at github.com/austonrut/EelegansGenome. Genomes and proteins that were not generated as part of this study we accessed from NCBI.
Supplemental material available at G3 online.
Funding
This research was supported through incentive funds from the University of North Carolina at Charlotte. Small-RNA sequencing was supported by United States - Israel Binational Science Foundation program with the National Science Foundation grants 2020669 and 1536530 to Y.M. and A.M.R.
Author contributions
A.I.R. and A.M.R conceived of study. A.I.R. and R.K. generated sequence data. A.I.R. analyzed all data except miRNA, which was generated and analyzed by V.M. and Y.M. A.I.R., A.M.R., V.M., and Y.M wrote the manuscript. All authors edited the manuscript.
Literature cited
- Aguirre-Liguori JA, Ramirez-Barahona S, Gaut BS. 2021. The evolutionary genomics of species’ responses to climate change. Nat Ecol Evol. 5(10):1350–1360. doi: 10.1038/s41559-021-01526-9. [DOI] [PubMed] [Google Scholar]
- Al-Shaer L, Havrilak J, Layden MJ. 2021. Nematostella vectensis as a model system. In: Handbook of marine model organisms in experimental biology. CRC Press. p. 107–128. [Google Scholar]
- Axtell MJ, Westholm JO, Lai EC. 2011. Vive la différence: biogenesis and evolution of microRNAs in plants and animals. Genome Biol. 12(4):221. doi: 10.1186/gb-2011-12-4-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. 2009. MicroRNAs: target recognition and regulatory functions. Cell. 136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgarten S, Cziesielski MJ, Thomas L, Michell CT, Esherick LY, Pringle JR, Aranda M, Voolstra CR. 2018. Evidence for miRNA-mediated modulation of the host transcriptome in cnidarian–dinoflagellate symbiosis. Mol Ecol. 27(2):403–418. doi: 10.1111/mec.14452. [DOI] [PubMed] [Google Scholar]
- Baumgarten S, Simakov O, Esherick LY, Liew YJ, Lehnert EM, Michell CT, Li Y, Hambleton EA, Guse A, Oates ME, et al. 2015. The genome of Aiptasia, a sea anemone model for coral symbiosis. Proc Natl Acad Sci U S A. 112(38):11893–11898. doi: 10.1073/pnas.1513318112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruna T, Hoff KJ, Lomsadze A, Stanke M, Borodovsky M. 2021. BRAKER2: automatic eukaryotic genome annotation with GeneMark-EP+ and AUGUSTUS supported by a protein database. NAR Genom Bioinform. 3(1):lqaa108. doi: 10.1093/nargab/lqaa108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchfink B, Xie C, Huson DH. 2014. Fast and sensitive protein alignment using DIAMOND. Nat Methods. 12(1):59–60. doi: 10.1038/nmeth.3176. [DOI] [PubMed] [Google Scholar]
- Bumann D, Puls G. 1996. Infestation with larvae of the sea anemone Edwardsia lineata affects nutrition and growth of the ctenophore Mnemiopsis leidyi. Parasitology. 113(2):123–128. doi: 10.1017/S0031182000066361. [DOI] [Google Scholar]
- Challis R, Richards E, Rajan J, Cochrane G, Blaxter M. 2020. BlobToolKit—interactive quality assessment of genome assemblies. G3 (Bethesda). 10(4):1361–1374. doi: 10.1534/g3.119.400908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chourrout D, Delsuc F, Chourrout P, Edvardsen RB, Rentzsch F, Renfer E, Jensen MF, Zhu B, de Jong P, Steele RE, et al. 2006. Minimal ProtoHox cluster inferred from bilaterian and cnidarian hox complements. Nature. 442(7103):684–687. doi: 10.1038/nature04863. [DOI] [PubMed] [Google Scholar]
- Crooks GE, Hon G, Chandonia J-M, Brenner SE. 2004. WebLogo: a sequence logo generator. Genome Res. 14(6):1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly M. 2002. A systematic revision of Edwardsiidae (Cnidaria, Anthozoa). Invertebr Biol. 121(3):212–225. doi: 10.1111/j.1744-7410.2002.tb00061.x. [DOI] [Google Scholar]
- Daly M, Chaudhuri A, Gusmao L, Rodriguez E. 2008. Phylogenetic relationships among sea anemones (Cnidaria: Anthozoa: Actiniaria). Mol Phylogenet Evol. 48(1):292–301. doi: 10.1016/j.ympev.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Daly M, Lipscomb DL, Allard MW. 2002. A simple test: evaluating explanations for the relative simplicity of the Edwardsiidae (Cnidaria: Anthozoa). Evolution. 56(3):502–510. doi: 10.1111/j.0014-3820.2002.tb01361.x. [DOI] [PubMed] [Google Scholar]
- Daly M, Ljubenkov JC. 2008. Edwardsiid sea anemones of California (Cnidaria: Actiniaria: Edwardsiidae), with descriptions of eight new species. Zootaxa. 1860(1):1–27. doi: 10.11646/zootaxa.1860.1.1. [DOI] [Google Scholar]
- Daly M, Rack F, Zook R. 2013. Edwardsiella andrillae, a new species of sea anemone from Antarctic ice. PLoS One. 8(12):e83476. doi: 10.1371/journal.pone.0083476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling JA, Reitzel AR, Burton PM, Mazza ME, Ryan JF, Sullivan JC, Finnerty JR. 2005. Rising starlet: the starlet sea anemone, Nematostella vectensis. Bioessays. 27(2):211–221. doi: 10.1002/bies.20181. [DOI] [PubMed] [Google Scholar]
- DuBuc TQ, Ryan JF, Shinzato C, Satoh N, Martindale MQ. 2012. Coral comparative genomics reveal expanded Hox cluster in the cnidarian-bilaterian ancestor. Integr Comp Biol. 52(6):835–841. doi: 10.1093/icb/ics098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBuc TQ, Stephenson TB, Rock AQ, Martindale MQ. 2018. Hox and Wnt pattern the primary body axis of an anthozoan cnidarian before gastrulation. Nat Commun. 9(1):2007. doi: 10.1038/s41467-018-04184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy SR. 2009. A new generation of homology search tools based on probabilistic inference. Genome Inform. 23(1):205–211. doi: 10.1142/9781848165632_0019. [DOI] [PubMed] [Google Scholar]
- Edelbroek B, Kjellin J, Biryukova I, Liao Z, Lundberg T, Noegel AA, Eichinger L, Friedländer MR, Söderbom F. 2024. Evolution of microRNAs in Amoebozoa and implications for the origin of multicellularity. Nucleic Acids Res. 52(6):3121–3136. doi: 10.1093/nar/gkae109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hilali S, Copley RR. 2023. macrosyntR: Drawing automatically ordered Oxford Grids from standard genomic files in R. bioRxiv 525673. 10.1101/2023.01.26.525673, preprint: not peer reviewed. [DOI]
- Emms DM, Kelly S. 2019. OrthoFinder: phylogenetic orthology inference for comparative genomics. Genome Biol. 20(1):238. doi: 10.1186/s13059-019-1832-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farre M, Kim J, Proskuryakova AA, Zhang Y, Kulemzina AI, Li Q, Zhou Y, Xiong Y, Johnson JL, Perelman PL, et al. 2019. Evolution of gene regulation in ruminants differs between evolutionary breakpoint regions and homologous synteny blocks. Genome Res. 29(4):576–589. doi: 10.1101/gr.239863.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank F, Sonenberg N, Nagar B. 2010. Structural basis for 5′-nucleotide base-specific recognition of guide RNA by human AGO2. Nature. 465(7299):818–822. doi: 10.1038/nature09039. [DOI] [PubMed] [Google Scholar]
- Fridrich A, Modepalli V, Lewandowska M, Aharoni R, Moran Y. 2020. Unravelling the developmental and functional significance of an ancient Argonaute duplication. Nat Commun. 11(1):6187. doi: 10.1038/s41467-020-20003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedländer MR, Mackowiak SD, Li N, Chen W, Rajewsky N. 2011. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 40(1):37–52. doi: 10.1093/nar/gkr688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm B, Billipp T, Peck LE, Johansen M, Tarver JE, King BL, Newcomb JM, Sempere LF, Flatmark K, Hovig E, et al. 2015. A uniform system for the annotation of vertebrate microRNA genes and the evolution of the Human microRNAome. Annu Rev Genet. 49(1):213–242. doi: 10.1146/annurev-genet-120213-092023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajigan AP, Conaco C. 2017. A microRNA regulates the response of corals to thermal stress. Mol Ecol. 26(13):3472–3483. doi: 10.1111/mec.14130. [DOI] [PubMed] [Google Scholar]
- Gellon G, McGinnis W. 1998. Shaping animal body plans in development and evolution by modulation of Hox expression patterns. Bioessays. 20(2):116–125. doi:. [DOI] [PubMed] [Google Scholar]
- He S, Del Viso F, Chen C-Y, Ikmi A, Kroesen AE, Gibson MC. 2018. An axial Hox code controls tissue segmentation and body patterning in Nematostella vectensis. Science. 361(6409):1377–1380. doi: 10.1126/science.aar8384. [DOI] [PubMed] [Google Scholar]
- Holland PW. 2013. Evolution of homeobox genes. Wiley Interdiscip Rev Dev Biol. 2(1):31–45. doi: 10.1002/wdev.78. [DOI] [PubMed] [Google Scholar]
- Holstein TW. 2022. The role of cnidarian developmental biology in unraveling axis formation and wnt signaling. Dev Biol. 487:74–98. doi: 10.1016/j.ydbio.2022.04.005. [DOI] [PubMed] [Google Scholar]
- Kavanaugh MT, Rheuban JE, Luis KMA, Doney SC. 2017. Thirty-Three years of ocean benthic warming along the U.S. Northeast continental shelf and slope: patterns, drivers, and ecological consequences. J Geophys Res Oceans. 122(12):9399–9414. doi: 10.1002/2017JC012953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus Y, Aman A, Technau U, Genikhovich G. 2016. Pre-bilaterian origin of the blastoporal axial organizer. Nat Commun. 7(1):11694. doi: 10.1038/ncomms11694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusserow A, Pang K, Sturm C, Hrouda M, Lentfer J, Schmidt HA, Technau U, von Haeseler A, Hobmayer B, Martindale MQ, et al. 2005. Unexpected complexity of the Wnt gene family in a sea anemone. Nature. 433(7022):156–160. doi: 10.1038/nature03158. [DOI] [PubMed] [Google Scholar]
- Layden MJ, Rentzsch F, Rottinger E. 2016. The rise of the starlet sea anemone Nematostella vectensis as a model system to investigate development and regeneration. Wiley Interdiscip Rev Dev Biol. 5(4):408–428. doi: 10.1002/wdev.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew YJ, Aranda M, Carr A, Baumgarten S, Zoccola D, Tambutté S, Allemand D, Micklem G, Voolstra CR. 2014. Identification of MicroRNAs in the coral Stylophora pistillata. PLoS One. 9(3):e91101. doi: 10.1371/journal.pone.0091101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manni M, Berkeley MR, Seppey M, Simao FA, Zdobnov EM. 2021. BUSCO update: novel and streamlined workflows along with broader and deeper phylogenetic coverage for scoring of eukaryotic, prokaryotic, and viral genomes. Mol Biol Evol. 38(10):4647–4654. doi: 10.1093/molbev/msab199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcais G, Kingsford C. 2011. A fast, lock-free approach for efficient parallel counting of occurrences of k-mers. Bioinformatics. 27(6):764–770. doi: 10.1093/bioinformatics/btr011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques-Bonet T, Caceres M, Bertranpetit J, Preuss TM, Thomas JW, Navarro A. 2004. Chromosomal rearrangements and the genomic distribution of gene-expression divergence in humans and chimpanzees. Trends Genet. 20(11):524–529. doi: 10.1016/j.tig.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Mazza ME, Pang K, Reitzel AM, Martindale MQ, Finnerty JR. 2010. A conserved cluster of three PRD-class homeobox genes (homeobrain, rx and orthopedia) in the Cnidaria and Protostomia. Evodevo. 1(1):3. doi: 10.1186/2041-9139-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden CS, Daly M, Brugler MR, Cartwright P, Collins AG, Dawson MN, Fautin DG, France SC, Opresko DM, Rodriguez E, et al. 2007. The phylum cnidaria: a review of phylogenetic patterns and diversity 300 years after Linnaeus. Zootaxa. 1668:127–182. [Google Scholar]
- McFadden CS, Quattrini AM, Brugler MR, Cowman PF, Duenas LF, Kitahara MV, Paz-García DA, Reimer JD, Rodríguez E. 2021. Phylogenomics, origin, and diversification of Anthozoans (Phylum cnidaria). Syst Biol. 70(4):635–647. doi: 10.1093/sysbio/syaa103. [DOI] [PubMed] [Google Scholar]
- Modepalli V, Fridrich A, Agron M, Moran Y. 2018. The methyltransferase HEN1 is required in Nematostella vectensis for microRNA and piRNA stability as well as larval metamorphosis. PLoS Genet. 14(8):e1007590. doi: 10.1371/journal.pgen.1007590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran Y, Agron M, Praher D, Technau U. 2017. The evolutionary origin of plant and animal microRNAs. Nat Ecol Evol. 1(3):27. doi: 10.1038/s41559-016-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran Y, Fredman D, Praher D, Li XZ, Wee LM, Rentzsch F, Zamore PD, Technau U, Seitz H. 2014. Cnidarian microRNAs frequently regulate targets by cleavage. Genome Res. 24(4):651–663. doi: 10.1101/gr.162503.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran Y, Praher D, Fredman D, Technau U. 2013. The evolution of microRNA pathway protein components in cnidaria. Mol Biol Evol. 30(12):2541–2552. doi: 10.1093/molbev/mst159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praher D, Zimmermann B, Dnyansagar R, Miller DJ, Moya A, Modepalli V, Fridrich A, Sher D, Friis-Møller L, Sundberg P, et al. 2021. Conservation and turnover of miRNAs and their highly complementary targets in early branching animals. Proc Biol Sci. 288(1945):20203169. doi: 10.1098/rspb.2020.3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam NH, Srivastava M, Hellsten U, Dirks B, Chapman J, Salamov A, Terry A, Shapiro H, Lindquist E, Kapitonov VV, et al. 2007. Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science. 317(5834):86–94. doi: 10.1126/science.1139158. [DOI] [PubMed] [Google Scholar]
- R Core Team . 2022. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Rodriguez E, Barbeitos MS, Brugler MR, Crowley LM, Grajales A, Gusmão L, Häussermann V, Reft A, Daly M. 2014. Hidden among sea anemones: the first comprehensive phylogenetic reconstruction of the order Actiniaria (Cnidaria, Anthozoa, Hexacorallia) reveals a novel group of hexacorals. PLoS One. 9(5):e96998. doi: 10.1371/journal.pone.0096998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan J, Li H. 2020. Fast and accurate long-read assembly with wtdbg2. Nat Methods. 17(2):155–158. doi: 10.1038/s41592-019-0669-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan JF, Burton PM, Mazza ME, Kwong GK, Mullikin JC, Finnerty JR. 2006. The cnidarian-bilaterian ancestor possessed at least 56 homeoboxes: evidence from the starlet sea anemone, Nematostella vectensis. Genome Biol. 7(7):R64. doi: 10.1186/gb-2006-7-7-r64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan JF, Mazza ME, Pang K, Matus DQ, Baxevanis AD, Martindale MQ, Finnerty JR. 2007. Pre-bilaterian origins of the Hox cluster and the Hox code: evidence from the sea anemone, Nematostella vectensis. PLoS One. 2(1):e153. doi: 10.1371/journal.pone.0000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz DT, Haddock SHD, Bredeson JV, Green RE, Simakov O, Rokhsar DS. 2023. Ancient gene linkages support ctenophores as sister to other animals. Nature. 618(7963):110–117. doi: 10.1038/s41586-023-05936-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simakov O, Marletaz F, Yue J-X, O'Connell B, Jenkins J, Brandt A, Calef R, Tung C-H, Huang T-K, Schmutz J, et al. 2020. Deeply conserved synteny resolves early events in vertebrate evolution. Nat Ecol Evol. 4(6):820–830. doi: 10.1038/s41559-020-1156-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit A, Hubley R. RepeatModeler Open-1.0. 2008–2015.
- Smit A, Hubley R, Green P. RepeatMasker Open-4.0. 2013–2015.
- Smith EG, Surm JM, Macrander J, Simhi A, Amir G, Sachkova MY, Lewandowska M, Reitzel AM, Moran Y. 2023. Micro and macroevolution of sea anemone venom phenotype. Nat Commun. 14(1):249. doi: 10.1038/s41467-023-35794-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik DJ, Lubinski TJ, Granger BR, Byrd AL, Reitzel AM, DeFilippo L, Lorenc A, Finnerty JR. 2014. Production of a reference transcriptome and transcriptomic database (EdwardsiellaBase) for the lined sea anemone, Edwardsiella lineata, a parasitic cnidarian. BMC Genomics. 15(1):71. doi: 10.1186/1471-2164-15-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinworth BM, Martindale MQ, Ryan JF. 2023. Gene loss may have shaped the cnidarian and bilaterian Hox and ParaHox complement. Genome Biol Evol. 15(1):evac172. doi: 10.1093/gbe/evac172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JC, Ryan JF, Mullikin JC, Finnerty JR. 2007. Conserved and novel Wnt clusters in the basal eumetazoan Nematostella vectensis. Dev Genes Evol. 217(3):235–239. doi: 10.1007/s00427-007-0136-5. [DOI] [PubMed] [Google Scholar]
- Tripathi AM, Admoni Y, Fridrich A, Lewandowska M, Surm JM, Aharoni R, Moran Y. 2022. Functional characterization of a ‘plant-like’ HYL1 homolog in the cnidarian Nematostella vectensis indicates a conserved involvement in microRNA biogenesis. Elife. 11:e69464. doi: 10.7554/eLife.69464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voinnet O. 2009. Origin, biogenesis, and activity of plant microRNAs. Cell. 136(4):669–687. doi: 10.1016/j.cell.2009.01.046. [DOI] [PubMed] [Google Scholar]
- Vurture GW, Sedlazeck FJ, Nattestad M, Underwood CJ, Fang H, Gurtowski J, Schatz MC. 2017. GenomeScope: fast reference-free genome profiling from short reads. Bioinformatics. 33(14):2202–2204. doi: 10.1093/bioinformatics/btx153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BJ, Abeel T, Shea T, Priest M, Abouelliel A, Sakthikumar S, Cuomo CA, Zeng Q, Wortman J, Young SK, et al. 2014. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS One. 9(11):e112963. doi: 10.1371/journal.pone.0112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann B, Montenegro JD, Robb SMC, Fropf WJ, Weilguny L, He S, Chen S, Lovegrove-Walsh J, Hill EM, Chen C-Y, et al. 2023. Topological structures and syntenic conservation in sea anemone genomes. Nat Commun. 14(1):8270. doi: 10.1038/s41467-023-44080-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The genome and raw data sequences have been deposited on NCBI, accession number JBHDXZ000000000, Bioproject number PRJNA1147700, Biosample number SAMN43163413, and SRA number SRP533906. Codes for this study are available at github.com/austonrut/EelegansGenome. Genomes and proteins that were not generated as part of this study we accessed from NCBI.
Supplemental material available at G3 online.