Abstract
Determining the ecology of fossil species presents considerable challenges due to the often fragmentary preservation of specimens. The mammaliaform Hadrocodium wui from the Jurassic of China is known only from the cranium and mandible but may have had a fossorial lifestyle. It shares morphological similarities with talpid moles and soricid shrews and is closely related to other fossorial mammaliaforms. However, the lack of postcranial elements has so far precluded a definitive assessment regarding its fossorial behavior. Using a combination of geometric morphometric analysis of the lower mandible and finite element analyses of the cranium, comparisons between H. wui and extant groups are made. H. wui resembles talpid moles more closely than shrews in mandible shape. However, there are more similarities between H. wui and semi‐fossorial/semi‐aquatic moles than fully fossorial moles. The skull of H. wui is particularly weak in every tested biomechanical scenario when compared to the related mammaliaform Morganucodon oehleri and talpid moles. This weakness is potentially a result of the reduction in thickness of the zygomatic arch. In contrast, the shrew crania show similar stress magnitudes and distribution. These results imply that H. wui was not fully fossorial and fed on a diet of softer invertebrates. Skull morphology may therefore not be sufficient to infer fossoriality in forelimb‐digging species.
Keywords: finite element analysis, geometric morphometrics, mammaliaformes, mammalian evolution
1. INTRODUCTION
Identifying the ecology of the fossil predecessors to modern mammals is crucial to understanding their evolution. Before the end‐Cretaceous extinction event, mammals and their precursors were thought to have largely filled insectivorous, nocturnal niches (Gerkema et al., 2013; Grossnickle & Polly, 2013). This tendency generally predates mammals, instead originating in non‐mammalian synapsids (Angielczyk & Schmitz, 2014). However, the last two decades have produced a number of new mammaliaform fossils with diverse ecological adaptations, ranging from terrestrial and arboreal insectivores and herbivores to semi‐aquatic carnivores, gliding omnivores, and fossorial food specialists (Luo, 2007). While fossil dietary inferences are largely based on tooth morphology and cranial modifications, the majority of these ecological inferences are based on the anatomy of the postcranial skeleton. This can be a problem, as fossil species are often incompletely preserved, disarticulated, or otherwise damaged and it is not uncommon that only parts of the skeleton are available after millions of years of fossilization. A prominent example highlighting this problem is the mammaliaform Hadrocodium wui, from the Early Jurassic Lufeng Formation of China (Luo et al., 2001). Based on different phylogenetic analyses, Hadrocodium is one of the closest known species to crown mammals (Bi et al., 2014; Close et al., 2015; Luo et al., 2001) (Figure 1), represented by a nearly complete cranium and mandible, but lacking postcranial remains.
FIGURE 1.

Phylogenetic context of H. wui and other species discussed in the text. Diet and fossorial adaptations for the fossil species indicated in the phylogeny. Simplified after Lautenschlager et al. (2023).
Many mammaliaforms and early mammals had small body sizes which helped them to hide from Mesozoic predators (Slater, 2013). H. wui is an extremely small species with original body mass estimates of two grams (Luo et al., 2001) and current estimates between 0.75 and 2.31 grams (Huang et al., 2024). Because of this, the potential for fossoriality among these species appears possible. Digging and taking shelter in burrows is known from less derived species such as Thrinaxodon from the Lower Triassic (notably without osteological features for burrowing) (Damiani et al., 2003) and small cynodonts from the Upper Triassic (Colombi et al., 2012). Fossoriality has also been suggested for the closely related docodont mammaliaform Haldanodon exspectatus (Martin, 2005) and the more derived early mammal Fruitafossor windsheffeli (Luo & Wible, 2005), both from the Late Jurassic. In both species, the fossorial ecology has been inferred from the well‐preserved appendicular skeleton, yet the cranial skeleton is incomplete or restricted to the mandible. This means that possible adaptations to a fossorial lifestyle cannot be traced on the skull in Haldanodon and Fruitafossor. Conversely, H. wui is known only from the cranium and mandible which presents challenges when researching its fossoriality.
The question remains whether the cranial skeleton alone can be used to determine fossoriality in extinct species. In order to address this question, the cranial morphology of H. wui needs to be compared to relevant extant analogues for which a fossorial function is known; in particular, species from the Talpidae (moles) and Soricidae (shrews) families are relevant in this context. There are clear similarities between these species' cranial morphologies and H. wui, including a flattened skull, a pointed rostrum, and an anteriorly sloping occipital. All three of these features are a pattern commonly found within fossorial taxa (Agrawal, 1967). There are also some similarities shared only by moles and H. wui, such as a zygomatic arch orientated at a similar angle, the low position of the zygomatic arch, and an angled (not rounded) squamosal (Lautenschlager et al., 2023).
Talpid moles are often fully subterranean, making them a good comparison for fossoriality, although it should be noted that moles do not use their heads as a tool when burrowing (Arlton, 1936). However, the skull may still play an important role in adaptation to a fossorial lifestyle (Vassallo et al., 2019). Similarly, some shrew species are known to take shelter underground and even burrow (He et al., 2015). Shrews (skull length = 15–20 mm) are considerably smaller than moles (skull length = 35–40 mm), and thus more similar to H. wui (skull length = 12 mm [Luo et al., 2001]). This reduction in body size has been correlated with an insectivorous diet (Lautenschlager et al., 2023). As cynodonts became more mammal‐like, cranial functional reorganization and a reduction in adductive mechanical advantage took place which is also correlated with insectivory (Lautenschlager et al., 2023).
All members of Talpidae and Soricidae are predominantly insectivores (Gunnell & Bloch, 2008) (we use insectivore instead of faunivore to avoid being too generalized. Henceforth the terms insectivore and insectivorous will include groups like annelids as well in this context). Both groups have a general diet of insects, insect larvae, earthworms, and vegetation (Funmilayo, 1977, 1979; Hisaw, 1923). The primary feature of a mole's diet is earthworms, shown through wear on the molars from soil inside and outside of the worm (Silcox & Teaford, 2002). There is some variation in diet across talpid mole species with different ecologies; fully subterranean moles feed predominantly on earthworms (Funmilayo, 1977) while other groups feed on insect larvae, isopods, fish, etc. (Catania & Remple, 2005; Dalquest & Orcutt, 1942; Oparina et al., 2013). The primary feature of a shrew's diet is insects (such as coleopterans) (Churchfield, 1982; Rychlik, 2002). H. wui was an insectivorous species (Luo et al., 2001), much like the majority of mammaliaforms and early mammals (Gill et al., 2014). The extent to which H. wui is a specialized feeder is unknown, but certainly possible based on dietary specialization in the mammaliaforms Morganucodon and Kuehenotherium (Gill et al., 2014).
In this study, we assess the viability of using only the skull morphology to determine whether a species is fossorial or not. To do this, the crania of H. wui, Morganucodon oehleri, and extant moles and shrews were subjected to finite element analysis (FEA). A morphological analysis was also conducted on the lower mandibles of these species and Fruitafossor windsheffeli with the aim of investigating whether H. wui shared ecological features with extant fossorial species.
2. METHODS
2.1. Jaw measurements
Images of the mandibles of H. wui, Morganucodon oehleri, 14 talpid mole species (and 1 golden mole), and 16 soricid shrew species were taken from the available literature (all data is available in the Supplementary Information). Measurements of the jaw were taken using tpsDig2 (version 2.31) whereby lengths were measured. The measurements were made into ratios to negate the effect of images not being to scale, allowing for comparison across all species. The ratio of the length and height of each mandible (Figure 2a) was calculated to establish the relative mandible height of each species. The length of the mandible is defined as the distance between the anterior‐most point of the mandible and the posterior‐most point of the condyloid process. The height of the mandible is defined as the distance between the dorsal‐most point of the coronoid process and the ventral‐most point of the dentary margin. The ratio of outlever and inlever lengths (Figure 2b) was calculated to determine the mechanical advantage for each species' lengths (Morales‐García et al., 2021). The outlever length is defined as the distance between the jaw joint and the bite point (in this case behind the first incisor on the lower mandible). The inlever length is defined as the distance between the jaw joint and the muscle attachment point.
FIGURE 2.
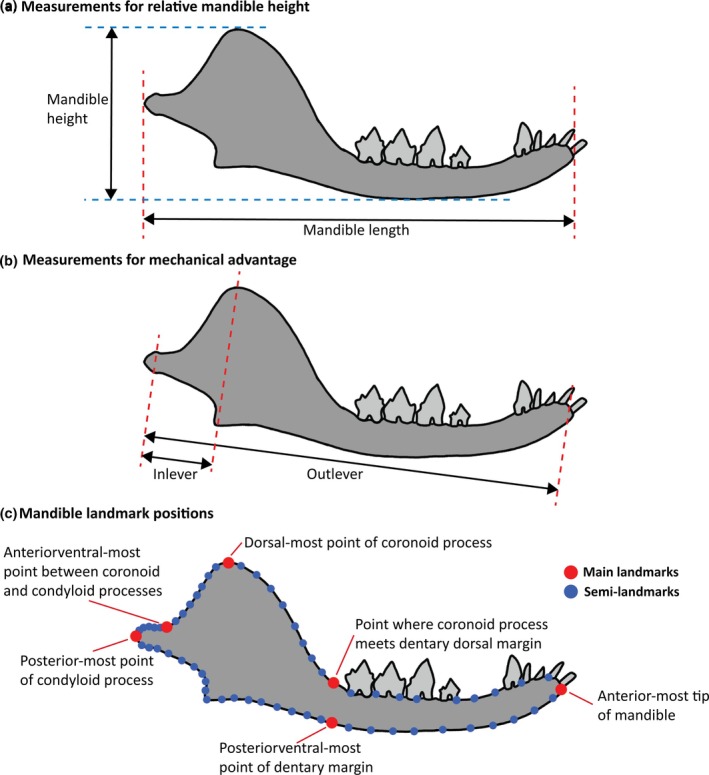
Measurements taken on the lower mandible shown on H. wui. (a) Relative mandible height measurements: Mandible length = anterior‐most point of mandible to posterior‐most point of condyloid process; mandible height = dorsal‐most point of coronoid process to ventral‐most point of dentary margin. (b) Mechanical advantage measurements: Outlever = distance between jaw joint and bite point; inlever = distance between jaw joint and muscle attachment point. (c) Landmark placements (red = landmark; blue = semi‐landmark) (Adapted from Morales‐García et al., 2021).
To compare the general shape of the jaws of each species, six landmarks were taken (Morales‐García et al., 2021): the anterior‐most point of the mandible; the point where the coronoid process meets the dentary dorsal margin; the dorsal‐most point of the coronoid process; the anterior ventral‐most point between the coronoid and the condyloid processes; the posterior‐most point of the condyloid process; and the posterior ventral‐most point of the dentary margin. These landmarks were joined together by semi‐landmarks (Figure 2c) for a total (including main landmarks) of 64. All landmarks and curves were completed using tpsDig2 (version 2.31) and imported into PAST (version 4.03) (Hammer et al., 2001) to perform a Procrustes (removes variation not related to shape by translating, scaling, and rotating the landmarks) and principal components analysis (PCA).
2.2. FEA
FEA is a tool commonly used in engineering but its use in paleontology has increased over recent years (Bright, 2014; Richmond et al., 2005). FEA calculates the stresses across an object when a particular load is applied. Digital skull models of six species underwent analysis (Figure 3): H. wui, Morganucodon oehleri, Talpa europaea, Talpa occidentalis, Neomys anomalus, and Crocidura leucodon (see Goswami, 2015a, 2015b; Lautenschlager, 2016a, 2016b; Mammal Research Institute PAS, Poland, 2021a, 2021b for digitization details) (models can be found in the Supplementary Information). The models were imported into HyperMesh (version 13.0.110) where they were meshed and given material properties, constraints, and loads. Material properties were based on the small hedgehog bone properties from Lautenschlager et al. (2023): E = 12GPa, ʋ = 0.30. For the models of the extant species, the teeth were not available as a separate component. Therefore, teeth were given the same material properties as the rest of the cranium. To negate the effects of skull size, all skull models were scaled to the same surface area as the Hadrocodium model. Constraints were applied at equal positions on either side of the skull, on the occipital condyles (to mimic constraints of where the skull attaches to the vertebral column), and the jaw joint on each squamosal (to mimic constraints of where the mandible attaches to the cranium).
FIGURE 3.
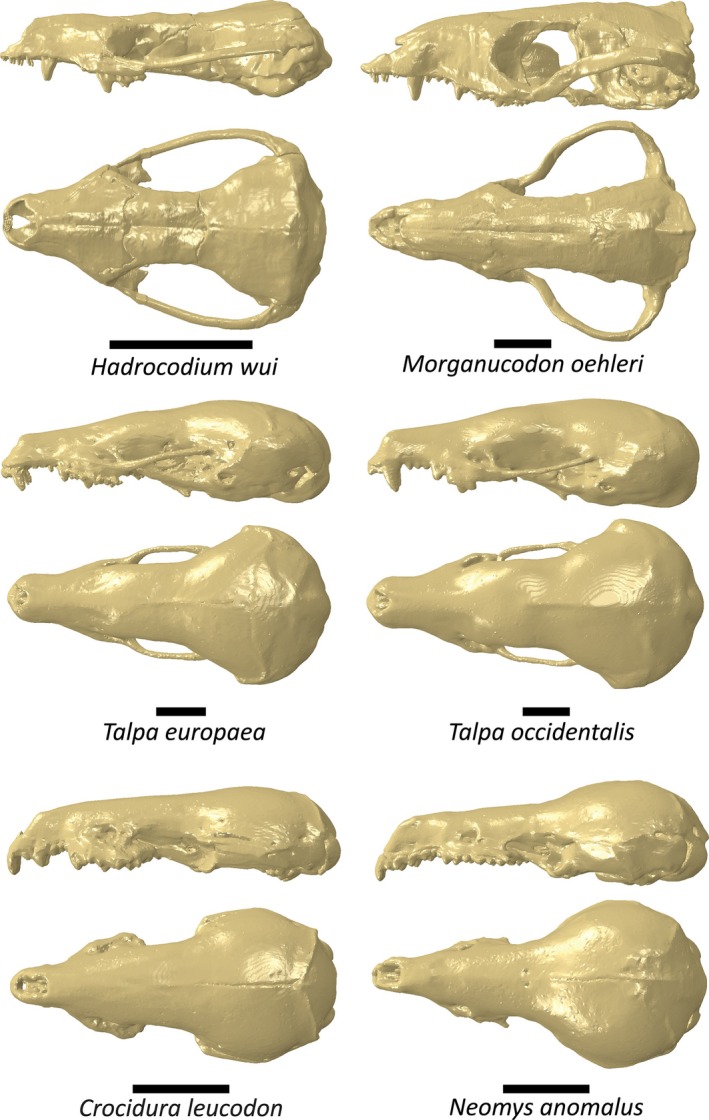
Models used for each species in FEA in left lateral and dorsal views. Scale bars = 5 mm.
Four separate point loads were applied to the crania to simulate different situations. These loads (Figure 4a) were applied to the teeth, nasal region, frontal region, and parietal region. Each load was given a magnitude of 5 N, which falls within the range of bite force values for Hadrocodium (Lautenschlager et al., 2023). The load onto the teeth represents a biting scenario where the canine is used for the mammaliaforms and moles, but the incisor (I1) is used for the shrews. The tooth chosen for each species was based on which is more likely to be used in catching/killing prey (Dötsch, 1986; Nagorsen, 1996, pp. 15–18). A direct load on the teeth was chosen over simulating the jaw adductor muscles as data for the latter was not readily available for all modeled species. Furthermore, the loss of the zygomatic arch in shrews results in a divergent muscle arrangement compared to the other tested species (Cornette et al., 2015), which could impact on the results which were aimed at testing different skull morphologies rather than soft‐tissues. Single point loads on the teeth also have a stronger effect than muscle loads which are spread across a larger part of the skull. The other three loads represent pressure encountered at different regions during burrowing. These regions (nasal, frontal, parietal) are typically enlarged or thickened in head‐digging mammals but also used by scratch diggers as bracing points (Hildebrand, 1985).
FIGURE 4.

(a) Positions at which point loads were applied during FEA shown on left lateral view of H. wui. (b) Landmarks on the cranium where stress was measured shown on dorsal view of H. wui: (1) anteriormost point of premaxilla; (2) right lateralmost point of premaxilla; (3) right lateralmost point where premaxilla meets maxilla; (4) right lateralmost point where maxilla meets the jugal; (5) right lateralmost point where zygoma meets squamosal; (6) right lateralmost point of parietal; (7) posteriormost point of parietal; (8) anteriormost point of nasal; (9) central point of frontal; (10) central point of parietal.
All models were then imported into Abaqus (version 6.14) for analysis. Both von Mises stress and absolute maximum principal stress are used as outputs. The von Mises stress shows low to high stresses across the model. The absolute max principal stress shows the magnitude of compressive and tensile stresses across the skull (positive values = tension; negative values = compression). The color map “Parula” is used for von Mises because of its contrast; it is easy to see high and low stress points even if printed in black and white (Lautenschlager, 2021). For absolute maximum principal stress, the color map “Polar” was altered to be darker (“DarkPolar”) so it had a higher contrast against a white background. This map was found to be useful because it makes the difference between compression and tension very clear (compression = blue; tension = red). All color maps used here can be found in the Supplementary Information.
To further quantify the results of the FE models, specific landmarks across the skull were chosen to measure the stress values for each load. These landmarks were chosen because each point can be identified on each species. Landmark positions are specified in Figure 4b. Measuring the stress value at each of these landmarks allows for easier comparison of species. A PCA was also performed on these stress values to further compare stress patterns across the skulls.
3. RESULTS
3.1. Results from mandible measurements
Mechanical advantage fluctuates across the species used in this study (Figure 5a). Shrews have a higher average mechanical advantage (31.80) than the talpid moles (28.08). Chrysospalax trevelyani (golden mole) has a higher mechanical advantage (34.77) than the majority of the talpid moles and shrews. The mechanical advantage for H. wui is closer to the talpid moles than the shrews with a value of 25.58. Uropsilinae, a sub‐family of Talpidae known as shrew moles, have an average (25.85) much closer to H. wui. Hadrocodium (25.58) and the early mammal Fruitafossor windsheffeli (27.63) have similar mechanical advantages while Morganucodon oehleri is notably higher (31.90).
FIGURE 5.

Results for (a) mechanical advantage and (b) relative mandible height for each species. Red = extinct species; gold = golden mole; green = Talpidae; blue = Soricidae; gray = Uropsilinae. See Supplementary Information for further detail.
Results for relative mandible height (Figure 5b) are more variable between groups. All three extinct species have higher relative mandible heights than any other species in this analysis. Talpid mole species have a greater average relative mandible height (231.91) than shrews (195.83). The relative mandible height of H. wui is 308.70, which is far closer to moles than shrews but still considerably higher. Morganucodon oehleri has the greatest relative mandible height (320.69). Uropsilinae have an average relative mandible height (194.21) much closer to shrews than to other moles. Talpa europaea was the extant species with the greatest relative mandible height (281.06), followed closely by the golden mole Chrysospalax trevelyani (276.09).
The jaw landmarks were subjected to a PCA (Figure 6) to quantify the similarity of the mandible shapes across all species. PC1 represents the length of the angular process and curvature of the dentary where lower values display a longer angular process and flatter dentary. PC2 represents the height of the coronoid process where lower values display a shorter coronoid process. Three groupings are observed for Talpidae, Soricidae, and the extinct species. These groups each have similar ranges of PC2 values but vary along PC1 indicating that there are distinct changes in the angular process length and curvature of the dentary. The talpid mole grouping has two distinct halves: the top and the bottom. The bottom half contains the fully subterranean moles from Talpini and Scalopini. The top half contains the moles that are not fully subterranean from Uropsilinae and Desmonini (Galemys pyrenaicus). There is some overlap between the Nectogalini shrews (Chodsigoa dabieshanensis and Chodsigoa hypsibia), and some of the Uropsilinae moles (and G. pyrenaicus). The golden mole Chrysospalax trevelyani lies outside of the talpid mole and Soricidae groupings. Of the extinct species, Fruitafossor windsheffeli lies the closest to the extant species, while H. wui is located furthest away. The talpid mole Euroscaptor subanura is located close to the extinct species.
FIGURE 6.

PCA plot for mandible shape across all species with convex hulls representing different groups: Green = Talpidae; blue = Soricidae; red = extinct species. Example mandibles shown in respective colors. See Supplementary Information for further detail.
3.2. Results from FEA
Results for the FEA are shown for two different stress types: von Mises and absolute maximum principal stress. In addition to the presentation of the contour plots, attaining the von Mises stresses at selected landmarks allows for easier comparison of the species. A principal component analysis (PCA) of these values indicates how similar the stress magnitudes are across the six species (Figure 7). Across all loads (Figure 7a), H. wui sits closest to the soricids. This shows that the magnitude of stresses across H. wui is most similar to the shrew species. Morganucodon oehleri is an isolated point on the plot (Figure 7a), indicating that its stress magnitudes are not similar to the other species here.
FIGURE 7.

PCA plot for stress values at each landmark on the cranium (Figure 4b) split into: (a) all loads; (b) parietal load; (c) frontal load; (d) nasal load; (e) teeth load.
3.2.1. Parietal load
For the first tested scenario, with the load on the center of the parietal, stress distribution varies considerably between the different species (Figures 7b and 8). Both moles, Talpa europaea and Talpa occidentalis, have very low stresses across the zygomatic arch, but in H. wui and Morganucodon oehleri stresses are much higher. In Morganucodon oehleri this stress in the zygoma is primarily located in the region where the zygoma attaches to the parietal (Figure 8c, landmark 5). For H. wui, these stresses are prominent along the entire zygomatic arch, particularly on the right lateral arch. Across the chosen landmarks, H. wui has a very similar pattern and magnitude of stress to the mole species which is further indicated by the PCA of the stress magnitudes with H. wui located very close to the moles. The shrew species, Crocidura leucodon and Neomys anomalus, have considerably higher stresses across the cranium than any other species. The nasal, maxilla, and premaxilla of all species have extremely low to no stress. Aside from landmarks 5 and 6, Morganucodon oehleri has comparably low overall stresses.
FIGURE 8.

Finite element analysis results for the parietal loads of each species with contour plots of the left lateral and dorsal views of (a) von Mises stress (yellow = higher stress) and (b) absolute maximum principal stress (red = tensile stress; blue = compressive stress. Scale bars = 5 mm. (c) Plot of von Mises stress values at landmarks across cranium.
Results for the absolute maximum principal stresses (Figure 8b) provide further details about the stress patterns. The compressive stress, experienced by all crania in the center of the parietal from the applied load, expands anteriorly into the frontal region of the skull. All species have an area of high tensile stress surrounding this compressive stress on the parietal. This tensile stress region is significantly reduced to the lateral sides of the parietal in Morganucodon oehleri. The shrew species have relatively high tensile stress around the parietal compared to the other species. There is minimal compressive and tensile stress across the zygomatic arches of Talpa europaea and Talpa occidentalis, whereas in H. wui areas of both compression and tension are higher. For Morganucodon oehleri, extremely high compressive stress is seen where the zygoma attaches to the parietal. Notably this point in H. wui has high tensile stress instead.
3.2.2. Frontal load
The second scenario tests a load on the center of the frontal part of the skull. Both H. wui and Morganucodon oehleri have distinctly higher von Mises stresses (Figure 9) compared to the shrews and particularly the moles. A far larger area surrounding the point where the load was applied exhibits extremely high stresses on Hadrocodium compared with every other species. Stress dissipation from this load extends across the parietal for both mammaliaforms but this is not seen in any extant species. Once again, both mole species have little to no stress in the zygomatic arch. In H. wui and Morganucodon oehleri, stress across the zygoma is high, particularly where the zygoma attaches to the parietal. The right lateral arch has increased stresses on H. wui. Overall, H. wui does not share similarities in stress patterns with any other species. This is further shown through the PCA (Figure 7c) where neither Hadrocodium nor Morganucodon are near to the cluster of extant species.
FIGURE 9.
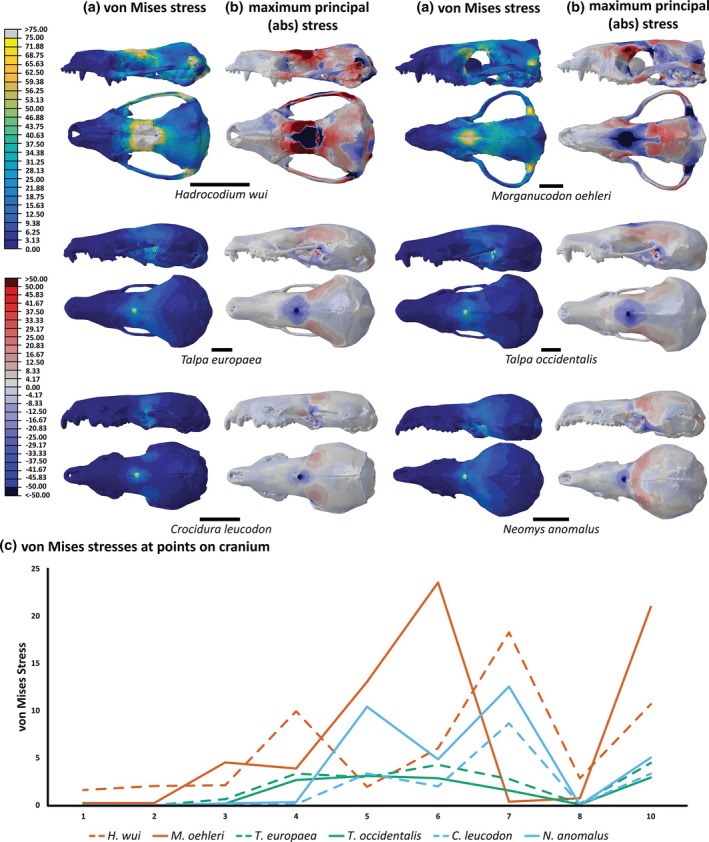
Finite element analysis results for the frontal loads of each species with contour plots of the left lateral and dorsal views of (a) von Mises stress (yellow = higher stress) and (b) absolute maximum principal stress (red = tensile stress; blue = compressive stress. Scale bars = 5 mm. (c) Plot of von Mises stress values at landmarks across cranium.
Absolute maximum principal stress (Figure 9b) reveals that the high stresses across the frontal of H. wui are extreme compression surrounded by extreme tension. Other species do not have this extreme tension. Neomys anomalus is distinct from the other extant species because it has a region of tensile stress spanning across the anterior edge of the parietal. This stress is reduced in Crocidura leucodon but directed across the lateral sides of the parietal for both mole species. In both mammaliaforms, there is relatively high tensile stress across most of the parietal. The area of the zygomatic arch where it connects to the parietal is of high compressive stress. In H. wui, this region is of high tensile stress on the right lateral side but high compressive stress on the right lateral side.
3.2.3. Nasal load
The nasal load causes generally higher von Mises stresses across the skulls of each of the species (Figure 10a) compared with the parietal and frontal loads. Again, the zygomatic arches of both mammaliaforms experience higher stresses than the moles. H. wui has a region of particularly high stress across the center of the frontal that is not present in any other species. The pattern of stress for the moles is similar across the landmarks (Figure 10c). The shrew species are alike as well, although not to the same extent. Morganucodon oehleri has distinctly different stress values at most of the landmarks, particularly at the rightmost point of the parietal (landmark six) where it is considerably higher than in all other species. H. wui is most similar to the shrews but has significantly higher stress values at many of the landmarks. The PCA plot (Figure 7d) shows H. wui to be far away from the other species but closer to the shrews than the moles.
FIGURE 10.

Finite element analysis results for the nasal loads of each species with contour plots of the left lateral and dorsal views of (a) von Mises stress (yellow = higher stress) and (b) absolute maximum principal stress (red = tensile stress; blue = compressive stress. Scale bars = 5 mm. (c) Plot of von Mises stress values at landmarks across cranium.
The results for absolute maximum principal stress (Figure 10b) reveal very similar stress patterns across the extant species. Most of the nasal and all of the frontal for each of these species is a region of tensile stress while the parietal is largely an area of compressive stress. In the mammaliaforms, tensile stress covers most of the dorsal side of the skull, including the parietal. H. wui has a region of compressive stress on the maxilla which is not present or significantly reduced in the other species. Patterns of compressive and tensile stress in the zygomas of H. wui and Morganucodon oehleri are similar to the previous loads.
3.2.4. Teeth load
This scenario represents a simplified biting simulation with the load centered on both canine teeth (or functional analogues). Since this load is directed onto the teeth on both sides of the skull, the force on the skull is effectively doubled. This causes higher stresses across every skull (Figure 11). H. wui and Neomys anomalus have similar levels of von Mises stress (Figure 11a) across the frontal, although the region of stress is shifted left laterally in H. wui. Stress across the zygomatic arch is similar to the previous loads, where it is increased in the mammaliaforms. Morganucodon oehleri has lower stress across much of the skull when compared with the other species. Talpa europaea experiences higher stresses than Talpa occidentalis. Overall, H. wui has similar stresses to the shrew species. This is further outlined in the PCA (Figure 7e) where H. wui is clustered with both shrew species while the moles and Morganucodon oehleri are in significantly different areas of the plot.
FIGURE 11.

Finite element analysis results for the teeth loads of each species with contour plots of the left lateral and dorsal views of (a) von Mises stress (yellow = higher stress) and (b) absolute maximum principal stress (red = tensile stress; blue = compressive stress. Scale bars = 5 mm. (c) Plot of von Mises stress values at landmarks across cranium.
Compressive stress (Figure 11b) encompasses most of the dorsal side of the skull on every species. The nasal region of H. wui has areas of tensile stress which is not present in any other species. Both mole species, and to some extent Neomys anomalus, have some tensile stress across the parietal which is not exhibited by the mammaliaforms. The area where the zygomatic arch attaches the parietal on the mammaliaforms (specifically the left lateral arch on H. wui) has high tensile stress. The rest of the zygoma for both species mostly exhibits compressive stress. Although stress is minimal in the zygomas of the mole species, Talpa europaea does show some tensile stress across the posterior half of the arch.
4. DISCUSSION
The lack of complete skeletal material for many cynodont and mammaliaform species often makes ecological reconstructions difficult. Although mammalian teeth (and those of their precursors) are highly indicative of diet and masticatory function (Ungar, 2010) and are vastly more abundant in many deposits than other fossil remains, they have only a very limited usefulness for predicting other ecological properties. Here, we tested whether the cranial anatomy can offer inferences on fossoriality using the mammaliaform Hadrocodium as a case study. We here focus on cranial features associated with forelimb digging as chisel‐tooth digging, scratch digging, and head‐lift digging are unlikely due to the incompatible incisor morphology (Doubell et al., 2020; Hopkins, 2005; Samuels & Valkenburgh, 2009) of Hadrocodium. Results from the conducted morphological and biomechanical analyses offer an ambiguous and non‐conclusive picture.
4.1. Morphological analyses
As found by the geometric morphometric analysis, the mandible shape of the mammaliaforms and early mammals investigated here is significantly different from modern moles and shrews. Furthermore, these two modern groups show little overlap. One reason for this may be that moles and shrews are far more derived than the extinct species represented in this study and the structure of the inner ear and jaw joint changed significantly across the transition from cynodonts to mammals (Lautenschlager et al., 2018). However, considering that all species share a similar insectivorous diet, a closer overlap would have been expected as found by Morales‐García et al. (2021) in a similar study. Although their study includes a wider range of extinct and extant species, Hadrocodium was not analyzed and only a single talpid (Parascalops breweri) and soricid (Blarina brevicauda) were included. They did, however, include Morganucodon, Fruitafossor, and Haldanodon, the latter being a fossorial species closely related to Hadrocodium. Interestingly, Fruitafossor was found to plot closely to the talpid species in the shape analyses of Morales‐García et al. (2021), and although classified as an omnivore, plotted within the same morphospace as herbivores. On the other hand, the insectivorous Haldanodon was found to occupy the same morphospace as extant carnivores in the same analyses. This mismatch between shape and inferred diet for the two extinct fossorial species could suggest that other factors than just diet play a role in shaping mandible morphology, and/or that our dietary classifications are uncertain. However, this may not necessarily hint at a fossorial signal as differences within the herbivorous categories (e.g., food toughness) are only poorly resolved for fossil species. In our study, Fruitafossor does not overlap with any of the moles. However, Luo and Wible (2005) described Fruitafossor as a forelimb‐digger but digging in the style of Monotremata rather than moles. Regardless of this interpretation, the lack of overlap between mandible shape of H. wui and mole and shrew species may not be significant enough to rule out a fossorial lifestyle for H. wui.
4.2. FEA
Across many of the FEA results, H. wui had significantly higher stresses implying that the cranial skeleton is weaker than other species overall. This may be a result of the enlarged braincase and posterior cranial expansion making the skull more susceptible to high stresses (Lautenschlager et al., 2023). However, the parietal load, and to some extent the teeth and nasal loads, showed shrew skulls having similar or higher stresses than H. wui. The structure of the cranium in shrews is visibly different from H. wui and moles; the zygomatic arch is absent, and the cranium is more cylindrical. Higher stress values appearing in shrews could be because forces dissipated differently across the skulls which lack a zygomatic arch.
In contrast to the stress magnitudes, the stress distribution in Hadrocodium resembles the other species in all FEA plots to some extent. However, Morganucodon oehleri is considerably different in every plot, with high stress values for all loads where the zygoma attaches to the squamosal. This region of high stress is most likely a result of the reduction in thickness and height of the zygoma in mammaliaforms (Lautenschlager et al., 2023), which also explains the high stresses seen across the entire zygoma in H. wui. Regardless of the high zygoma stresses, Morganucodon oehleri exhibits generally lower stresses for all loads. The zygoma in Morganucodon oehleri might therefore be more effective for stress dissipation than in H. wui, or the skull is just overall stronger to compensate for a diet of harder foods (Gill et al., 2014).
The FEA results as a whole show that H. wui is most similar to shrews. Shrews do not use their head as a digging tool meaning that it is unlikely that Hadrocodium did either in the context of these results, particularly because of how generally weak the cranium of H. wui is to the loads. This weakness was further confirmed in Lautenschlager et al. (2023), where multiple loads were applied to the teeth of different species and, in every example, the stress values were higher in H. wui. This also rules out the option of chisel‐tooth digging, consistent with the fact that the incisor teeth are not prominently procumbent and enlarged and flattened for such a scenario (McIntosh & Cox, 2016b; Rodrigues et al., 2023). Several species of rodents use their ever‐growing incisors for chisel‐tooth digging, but mammaliaforms likely did not possess rootless and ever‐growing teeth, making such a digging style unlikely for Hadrocodium.
4.3. Skull morphology and fossoriality
The post‐cranial skeletons of fossorial mammals have characteristics that make forelimb digging more efficient, including an expanded olecranon and epicondylar processes of the forelimb elements, an enlarged scapula and humerus, and increased size of the digital and carpal flexors (Lessa et al., 2008; Vassallo et al., 2019). With only the skull and mandible known for H. wui, the osteological correlates for fossoriality in the cranial skeleton are of importance. Similarities between the crania of H. wui and mole species are visually present, but how much this extends to their fossoriality is unknown. Vassallo et al. (2019) emphasize the importance of the cranium in taking on forces during digging. Fossorial mammals often use their head as a shovel while digging/burrowing. Agrawal (1967) analyzed and identified morphological trends in the skull in fossorial rodent species (using their head to burrow): a flattened skull, a pointed rostrum, and a sloping occiput (toward anterior) are morphological features commonly found in fossorial rodents. All these features can be seen in H. wui, particularly the flattened skull (Lautenschlager et al., 2023).
Mole skulls have specific functional morphology for a subterranean lifestyle. Infraorbital foramen size and mandible and humerus shape have specific trends within the ecology of moles species (Crumpton & Thompson, 2012; Sansalone et al., 2019). Talpini and Scalopini are entirely subterranean talpid clades while all others have adaptations to either an aquatic, terrestrial or semi‐fossorial lifestyle (Chen et al., 2021; Goswami & Prochel, 2007; Shinohara et al., 2003; Xu et al., 2023). Sansalone et al. (2019) showed that fully fossorial mole species have a distinct mandible shape, likely because of changes in diet across different lifestyles (Funmilayo, 1977). This trend is supported here with Talpini and Scalopini moles clustering together (Figure 6). The proximity of H. wui to the fully fossorial versus the other groups is similar. This could indicate that H. wui was not fully fossorial, that this analysis cannot be extended outside of crown Mammalia, or that subterranean food sources were distinctly different during the Jurassic.
4.4. Zygomatic arch
The zygomatic arch is distinct in H. wui and Morganucodon oehleri and is retained by most mammals today. Stresses from the masticatory muscles are exerted onto the zygoma (Preuschoft & Witzel, 2002) and the shape of the zygoma varies with diet (Edmonds et al., 2023; Menegaz et al., 2010). For example, Jones and Law (2018) found that the zygomatic arch is wider in mice species with a dietary preference for harder foods.
Shrews are one of the few groups of mammals that have lost the zygomatic arch entirely (but retain the masseter muscle attaching further anteriorly on the maxilla [Pommerening & Martin, 2024]). They have a tube‐shaped skull to accommodate for masticatory stresses meaning the zygomatic arch became unnecessary for support (Preuschoft & Witzel, 2002). The teeth load is the closest to representing masticatory movement and, in the FEA, there are notably higher stresses in the shrew skulls (particularly in Neomys anomalus) than in the mole skulls. This may be a result of the loss of a zygomatic arch, though a more in‐depth analysis with full reconstruction of the masticatory muscles would provide a more definitive answer to this.
There were significant evolutionary changes in the zygoma shape and position across the species between cynodonts and mammals. The height of the zygomatic arch decreases toward mammalian species (Lautenschlager et al., 2023) with H. wui and Morganucodon oehleri having some of the lowest pre‐mammalian values. While a wider zygoma is associated with masticatory muscle force (Jones & Law, 2018), decreased height is associated with weaker masticatory muscles (Menegaz et al., 2010). In mammaliaforms, the zygoma is wide but the bone is thin, having less capacity for larger loads (Lautenschlager et al., 2023), implying a diminishing need for efficient stress dissipation in this way. High stresses are seen in the zygomatic arch of H. wui both in this analysis (particularly on the right lateral side) and in Lautenschlager et al. (2023). This may be a result of it being largely reconstructed but could also relate to the zygoma being generally weaker. However, the closely related mammaliaform Morganucodon possesses a similarly thin zygomatic arch but does not show the same overall stress susceptibility as Hadrocodium (Lautenschlager et al., 2023). While a more strongly built zygomatic arch could act as a mechanism to reduce some stress, it is unlikely that it would have reduced stresses in other parts of the cranium. Nevertheless, this weakness of the zygoma in H. wui may to some extent explain the similarities in the stress dissipation across the skull with the zygoma‐absent shrews.
4.5. Mammalian jaw joint
The PCA plot (Figure 6) shows a significant separation of H. wui and Morganucodon oehleri from the extant species. In Figure 6, Fruitafossor windsheffeli is a near‐perfect midpoint between the mammaliaforms and fully subterranean mole species. F. windsheffeli is a fossorial mammal from the Late Jurassic of Colorado that fed on insects and small invertebrates (Luo & Wible, 2005). In this attempt to understand the ecology of H. wui, F. windsheffeli appears to be an extremely useful species to use in comparison. Unfortunately, while the mandible and large parts of the postcranial skeleton have been found, the actual cranium of F. windsheffeli has not been discovered yet which limited its use in this report.
The position of F. windsheffeli on the mandible PCA plot (Figure 6) almost appears to bridge the gap between the mammaliaforms and fully fossorial talpid moles. This may be a result of the mandible morphology changing across the transition from cynodonts to modern mammals. The jaw joint in Morganucodon oehleri represents a transitional period, whereas in H. wui it has progressed to the mammalian jaw joint (Lautenschlager et al., 2016). During the Cretaceous, the mammalian jaw joint became more prevalent, and the middle ear progressed with it to become the structure seen in modern mammals (Anthwal & Tucker, 2022). As an early mammal, F. windsheffeli did not yet have this defined middle ear structure as it was still attached to the lower jaw (Luo & Wible, 2005). Rather than representing the fossoriality of moles and F. windsheffeli, the PCA plot could represent the transition from mammaliaforms to modern fossorial mammals.
In the relative mandible height plot (Figure 5b), all three extinct species have notably higher values than the moles and shrews. If F. windsheffeli had similar fossorial behaviors to moles and shrews, then this would show a lack of importance in the mandible height for digging. However, F. windsheffeli likely dug via a scraping method, with similar shoulder mobility to species of Monotremata (Luo & Wible, 2005). The lack of overlap on the plot (Figure 5b) may therefore be a result of different digging styles implying that H. wui could have used a different method of digging. Thus, studying the morphology of the mandible may not provide enough information on whether the animal is fossorial or not.
4.6. The middle ear
The detachment of the mammalian middle ear from the mandible led to increased hearing function (greater frequency range and attenuation) and adaptability (Le Maître et al., 2020; Luo, 2011). Original studies of H. wui determined the middle ear ossicles to be detached from the mandible (Luo et al., 2001, Luo, 2011; Rowe et al. 2011). However, this has since been corrected; the middle ear of H. wui is not fully detached from the mandible (Bi et al., 2014; Lautenschlager et al., 2023; Luo et al., 2016, 2022; Meng et al., 2016; Wang et al., 2019). Both Morganucodon oehleri and F. windsheffeli also have the mandibular mammalian middle ear (Luo & Wible, 2005; Rowe et al. 2011; Lautenschlager et al., 2023).
Extreme adaptations are not apparent in the middle ear of extant fossorial mammals, although trends like a larger stapes footplate and an increased interampullar width are present (Crumpton et al., 2015; Mason, 2001). While the modern mammalian middle ear has definite improvements in hearing sensitivity (Lautenschlager et al., 2018; Luo, 2011), the lack of extreme middle ear adaptations to a fossorial lifestyle means that we cannot rule out the possibility of H. wui being fossorial. Trends that do appear in the middle ear of fossorial mammals are more prevalent in fully fossorial species (Crumpton et al., 2015), decreasing the likelihood that H. wui was fully fossorial.
4.7. Diet
The main dietary component of fully fossorial talpid moles is earthworms (Funmilayo, 1979; Hisaw, 1923; Silcox & Teaford, 2002). Soricidae species also feed on earthworms, but these are not necessarily their primary dietary component as they feed on other invertebrates, both larvae and adults (Churchfield, 1982; Nagorsen, 1996, pp. 15–18; Rychlik, 2002). H. wui has a similar mechanical advantage to moles while Morganucodon oehleri is more like the shrew species. This could imply that H. wui fed preferentially on softer invertebrates like earthworms.
Stem‐ and crown‐mammals from the Mesozoic have largely been thought to be generalist insectivores. However, there is clear evidence of more specialization than this. In Gill et al. (2014), another species of Morganucodon (Morganucodon watsoni) was found to feed on harder invertebrates while the mammaliaform Kuehneotherium praecursoris was shown to feed on softer invertebrates. Both the bite forces of H. wui and Morganucodon oehleri are low in comparison to other species across the cynodont‐mammal transition (Lautenschlager et al., 2018, 2023). It is therefore likely that H. wui fed on similar food items to morganucodontids, but potentially preferred softer invertebrates.
Results for the simulated bite (teeth load) in the FEA display clear similarities in the stress pattern of H. wui and the shrew species with the only exceptions being landmarks four and five. While the parallels in stress patterns could be coincidental, it is more likely that the zygomatic arch in the H. wui skull is representative of a transitional period in which the zygoma significantly reduced in thickness (Lautenschlager et al., 2023). Because of this reduction, the zygoma is weaker and less useful for stress dissipation, therefore causing the dissipation to appear more like the zygoma‐absent shrews.
Insectivorous rodents generally have a thin and narrow zygoma, regardless of diet (Samuels, 2009). Other dietary groups of rodents feeding on harder foods correlate with a wider zygoma (Jones & Law, 2018; Samuels, 2009). Morganucodon oehleri has a relatively wider zygomatic arch and a larger bite force (Lautenschlager et al., 2018, 2023) than H. wui and likely had a diet of harder foods (Gill et al., 2014). Overall, this implies that H. wui had an insectivorous diet with a preference for soft invertebrates.
4.8. Can functional morphology predict fossorial behavior?
H. wui shares similarities in cranial morphology with both shrews and moles (and extinct species) but also has some major differences. The skull is far weaker under each load tested here (and in Lautenschlager et al., 2023) which could be a result of being less derived than modern mammals. H. wui is a transitional species between cynodonts and mammals meaning structural changes are significant and may not be in place at this evolutionary stage. There are significant similarities in stress dissipation across the cranium in H. wui and the shrew species. The weakness that each of these crania exhibited indicates a likely inability to use the head during digging. Apart from lacking adaptation of the incisor teeth, for head‐shovel digging and chisel‐tooth digging, the skull must be significantly more robust to withstand the pressure. Some of this weakness may be a result of the loads being directed onto a single point rather than across a larger area. Our results do not provide definitive evidence for the lifestyle of H. wui. However, this does not mean that functional morphology, specifically of the cranial skeleton, cannot predict fossoriality in extinct species. The similarities that appear between H. wui and extant mole and shrew species are clearly not as present in Morganucodon oehleri. This may indicate a higher likelihood of fossoriality in H. wui than in Morganucodon oehleri, or a semi‐fossorial lifestyle. Further research should expand into species (e.g., mole‐rats) with different fossorial styles, including chisel‐tooth digging, head‐shovel digging, scratch digging, etc. (Becerra et al., 2012; Doubell et al., 2020; Gasc et al., 1985; Hopkins, 2005; Kley & Kearney, 2006; McIntosh & Cox, 2016a; McIntosh & Cox, 2019).
5. CONCLUSIONS
This project attempted to understand the ecology of H. wui by comparing its cranial skeleton with that of extant mole and shrew species, with a specific focus on fossoriality. While no definitive evidence of fossoriality in H. wui has been presented here, similarities with both shrews and moles have appeared. The shape of the mandible of H. wui is more like that of talpid moles than shrews but the stress dissipation across the cranium is more similar to shrews than moles (with the exception of the parietal load). The stress dissipation across the cranium being more like shrews may be because the zygomatic arch is not providing much support during mastication. This lack of support also suggests that H. wui had a diet of softer invertebrates which minimized deformation of the skull. Since the mandible of all fully fossorial talpid moles has adapted toward a similar shape, the lack of overlap with H. wui implies that H. wui was not fully fossorial. However, this does not exclude the possibility that H. wui was semi‐fossorial for which further research is required.
AUTHOR CONTRIBUTIONS
Molly Tumelty: Investigation; writing – original draft; methodology; validation; visualization; writing – review and editing; formal analysis. Stephan Lautenschlager: Conceptualization; writing – review and editing; supervision; resources.
Supporting information
Supplementary Information 1: Spreadsheet consisting of mandible measurements (mandible height, mandible length, outlever length, inlever length) for every species with referenced literature for each.
Supplementary Information 2: Landmark data before Procrustes.
Supplementary Information 3: Color map codes for Abaqus. Includes ‘Parula,’ ‘Polar,’ and ‘DarkPolar.’
Supplementary Information 4: Hypermesh files for each species. All models are meshed with material properties, constraints, loads, and load steps.
ACKNOWLEDGMENTS
We would like to thank the school of Geography, Earth and Environmental Sciences at the University of Birmingham for funding this work and providing access to the software used in this project. We thank the two reviewers for their useful suggestions to better the manuscript.
Tumelty, M. , & Lautenschlager, S. (2025). Is cranial anatomy indicative of fossoriality? A case study of the mammaliaform Hadrocodium wui . The Anatomical Record, 308(9), 2371–2391. 10.1002/ar.25630
REFERENCES
- Agrawal, V. C. (1967). Skull adaptations in fossorial rodents. Mammalia, 31(2), 300– 312. [Google Scholar]
- Angielczyk, K. D. , & Schmitz, L. (2014). Nocturnality in synapsids predates the origin of mammals by over 100 million years. Proceedings of the Royal Society B: Biological Sciences, 281(1793), 20141642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthwal, N. , & Tucker, A. S. (2022). Evolution and development of the mammalian jaw joint: Making a novel structure. Evolution & Development, 25(1), 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlton, A. V. (1936). An ecological study of the mole. Journal of Mammalogy, 17(4), 349. [Google Scholar]
- Becerra, F. , Casinos, A. , & Vassallo, A. I. (2012). Biting performance and skull biomechanics of a chisel tooth digging rodent (Ctenomys tuconax; Caviomorpha; Octodontoidea). Journal of Experimental Zoology Part A: Ecological Genetics and Physiology, 319(2), 74–85. [DOI] [PubMed] [Google Scholar]
- Bi, S. , Wang, Y. , Guan, J. , Sheng, X. , & Meng, J. (2014). Three new Jurassic euharamiyidan species reinforce early divergence of mammals. Nature, 514(7524), 579–584. [DOI] [PubMed] [Google Scholar]
- Bright, J. A. (2014). A review of paleontological finite element models and their validity. Journal of Paleontology, 88(4), 760–769. [Google Scholar]
- Catania, K. C. , & Remple, F. E. (2005). Asymptotic prey profitability drives star‐nosed moles to the foraging speed limit. Nature, 433(7025), 519–522. [DOI] [PubMed] [Google Scholar]
- Chen, Z. , He, S.‐W. , Hu, W. , Song, W.‐Y. , Onditi, K. O. , Li, X. , & Jiang, X. (2021). Morphology and phylogeny of scalopine moles (Eulipotyphla: Talpidae: Scalopini) from the eastern Himalayas, with descriptions of a new genus and species. Zoological Journal of the Linnean Society, 193(2), 432–444. [Google Scholar]
- Churchfield, S. (1982). Food availability and the diet of the common shrew, Sorex araneus, in Britain. The Journal of Animal Ecology, 51(1), 15. [Google Scholar]
- Close, R. A. , Friedman, M. , Lloyd, G. T. , & Benson, R. B. J. (2015). Evidence for a mid‐Jurassic adaptive radiation in mammals. Current Biology, 25(16), 2137–2142. [DOI] [PubMed] [Google Scholar]
- Colombi, C. E. , Fernández, E. , Currie, B. S. , Alcober, O. A. , Martínez, R. , & Correa, G. (2012). Large‐diameter burrows of the Triassic Ischigualasto Basin, NW Argentina: Paleoecological and paleoenvironmental implications. PLoS One, 7(12), e50662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornette, R. , Tresset, A. , & Herrel, A. (2015). The shrew tamed by Wolff's law: Do functional constraints shape the skull through muscle and bone covariation? Journal of Morphology, 276(3), 301–309. [DOI] [PubMed] [Google Scholar]
- Crumpton, N. , Kardjilov, N. , & Asher, R. J. (2015). Convergence vs. specialization in the ear region of moles (Mammalia). Journal of Morphology, 276(8), 900–914. [DOI] [PubMed] [Google Scholar]
- Crumpton, N. , & Thompson, R. S. (2012). The holes of moles: Osteological correlates of the trigeminal nerve in Talpidae. Journal of Mammalian Evolution, 20(3), 213–225. [Google Scholar]
- Dalquest, W. W. , & Orcutt, D. R. (1942). The biology of the least shrew‐mole, Neurotrichus gibbsii Minor. American Midland Naturalist, 27(2), 387. [Google Scholar]
- Damiani, R. , Modesto, S. , Yates, A. , & Neveling, J. (2003). Earliest evidence of cynodont burrowing. Proceedings of the Royal Society of London. Series B: Biological Sciences, 270(1525), 1747–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dötsch, C. (1986). Mastication in the musk shrew, Suncus murinus (Mammalia, Soricidae). Journal of Morphology, 189(1), 25–43. [DOI] [PubMed] [Google Scholar]
- Doubell, N. S. , Sahd, L. , & Kotzé, S. H. (2020). Comparative forelimb morphology of scratch‐digging and chisel‐tooth digging African mole‐rat species. Journal of Morphology, 281(9), 1029–1046. [DOI] [PubMed] [Google Scholar]
- Edmonds, H. M. , Daly, E. S. , & Smail, I. E. (2023). Zygomatic arch root position in relation to dietary type in Haplorhine primates. Anatomical Record‐Advances in Integrative Anatomy and Evolutionary Biology, 307, 2065–2083. [DOI] [PubMed] [Google Scholar]
- Funmilayo, O. (1977). Distribution and abundance of moles (Talpa europaea L.) in relation to physical habitat and food supply. Oecologia, 30(3), 277–283. [DOI] [PubMed] [Google Scholar]
- Funmilayo, O. (1979). Food consumption, preferences and storage in the mole. Acta Theriologica, 24(27), 379–389. [Google Scholar]
- Gasc, J. P. , Renous, S. , Casinos, A. , Laville, E. , & Bou, J. (1985). Comparison of diverse digging patterns in some small mammals. Fortschritte der Zoologie, 30, 35–38. [Google Scholar]
- Gerkema, M. P. , Davies, W. I. L. , Foster, R. G. , Menaker, M. , & Hut, R. A. (2013). The nocturnal bottleneck and the evolution of activity patterns in mammals. Proceedings of the Royal Society B: Biological Sciences, 280(1765), 20130508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill, P. G. , Purnell, M. A. , Crumpton, N. , Brown, K. R. , Gostling, N. J. , Stampanoni, M. , & Rayfield, E. J. (2014). Dietary specializations and diversity in feeding ecology of the earliest stem mammals. Nature, 512(7514), 303–305. [DOI] [PubMed] [Google Scholar]
- Goswami, A. (2015a). Talpa europaea (European mole). [online] Phenome10K: A free online repository for 3‐D scans of biological and palaeontological specimens. Available at: https://www.phenome10k.org/talpa-europaea-7 [Accessed 20 Oct. 2023]
- Goswami, A. (2015b). Talpa occidentalis (Spanish mole). [online] Phenome10K: A free online repository for 3‐D scans of biological and palaeontological specimens. Available at: https://www.phenome10k.org/talpa-occidentalis/ [Accessed 12 Apr. 2023]
- Goswami, A. , & Prochel, J. (2007). Ontogenetic morphology and allometry of the cranium in the common European mole (Talpa europaea). Journal of Mammalogy, 88(3), 667–677. [Google Scholar]
- Grossnickle, D. M. , & Polly, P. D. (2013). Mammal disparity decreases during the cretaceous angiosperm radiation. Proceedings of the Royal Society B: Biological Sciences, 280(1771), 20132110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnell, G. F. , & Bloch, J. I. (2008). Insectivorous mammals summary. In Janis C. M., Gunnell G. F., & Uhen M. D. (Eds.), Evolution of tertiary mammals of North America volume 2: Small mammals, Xenarthrans, and marine mammals (pp. 49–62). Cambridge University Press. [Google Scholar]
- Hammer, Ø. , Harper, D. A. T. , & Ryan, P. D. (2001). PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica, 4(1), 1– 9. [Google Scholar]
- He, K. , Woodman, N. , Boaglio, S. , Roberts, M. , Supekar, S. , & Maldonado, J. E. (2015). Molecular phylogeny supports repeated adaptation to burrowing within small‐eared shrews genus of Cryptotis (Eulipotyphla, Soricidae). PLoS One, 10(10), e0140280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand, M. (1985). Chapter 6. Digging of quadrupeds. In Functional vertebrate morphology (pp. 89–109). Harvard University Press. [Google Scholar]
- Hisaw, F. L. (1923). Feeding habits of moles. Journal of Mammalogy, 4(1), 9. [Google Scholar]
- Hopkins, S. S. B. (2005). The evolution of fossoriality and the adaptive role of horns in the Mylagaulidae (Mammalia: Rodentia). Proceedings of the Royal Society B: Biological Sciences, 272(1573), 1705–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, E. J. , Wilson, J. D. , Bhullar, B.‐A. S. , & Bever, G. S. (2024). High‐precision body mass predictors for small mammals: A case study in the Mesozoic. Palaeontology, 67(2), e12692. [Google Scholar]
- Jones, K. , & Law, C. J. (2018). Differentiation of craniomandibular morphology in two sympatric Peromyscus mice (Cricetidae: Rodentia). Mammal Research, 63(3), 277–283. [Google Scholar]
- Kley, N. J. , & Kearney, M. (2006). Chapter 17: Adaptations for digging and burrowing. In Hall B. K. (Ed.), Fins into limbs: Evolution, development, and transformation (pp. 284–309). University of Chicago Press. [Google Scholar]
- Lautenschlager, S. (2016a). Hadrocodium wui 3D Model. [online] Sketchfab. Available at: https://sketchfab.com/3d‐models/hadrocodium‐wui‐e7e6aa382c87446d8ad5c320e14dc559 [Accessed 22 Feb. 2023]
- Lautenschlager, S. (2016b). Morganucodon oehleri 3D Model. [online] Sketchfab. Available at: https://sketchfab.com/3d‐models/morganucodon‐oehleri‐e0e360be45204c8db27acae3a7740ff6 [Accessed 12 Apr. 2023]
- Lautenschlager, S. (2021). True colours or red herrings?: Colour maps for finite‐element analysis in palaeontological studies to enhance interpretation and accessibility. Royal Society Open Science, 8(11), 211357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenschlager, S. , Fagan, M. J. , Luo, Z.‐X. , Bird, C. M. , Gill, P. , & Rayfield, E. J. (2023). Functional reorganisation of the cranial skeleton during the cynodont–mammaliaform transition. Communications Biology, 6(1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenschlager, S. , Gill, P. , Luo, Z.‐X. , Fagan, M. J. , & Rayfield, E. J. (2016). Morphological evolution of the mammalian jaw adductor complex. Biological Reviews, 92(4), 1910–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenschlager, S. , Gill, P. G. , Luo, Z.‐X. , Fagan, M. J. , & Rayfield, E. J. (2018). The role of miniaturization in the evolution of the mammalian jaw and middle ear. Nature, 561(7724), 533–537. [DOI] [PubMed] [Google Scholar]
- Le Maître, A. , Grunstra, N. D. S. , Pfaff, C. , & Mitteroecker, P. (2020). Evolution of the mammalian ear: An evolvability hypothesis. Evolutionary Biology, 47(3), 187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessa, E. P. , Vassallo, A. I. , Verzi, D. H. , & Mora, M. S. (2008). Evolution of morphological adaptations for digging in living and extinct ctenomyid and octodontid rodents. Biological Journal of the Linnean Society, 95(2), 267–283. [Google Scholar]
- Luo, Z. , Bhullar, B.‐A. , Crompton, A. , Neander, A. , & Rowe, T. (2022). Reexamination of the mandibular and dental morphology of the early Jurassic mammaliaform Hadrocodium wui . Acta Palaeontologica Polonica, 67(1), 95–113. [Google Scholar]
- Luo, Z.‐X. (2007). Transformation and diversification in early mammal evolution. Nature, 450(7172), 1011–1019. [DOI] [PubMed] [Google Scholar]
- Luo, Z.‐X. (2011). Developmental patterns in Mesozoic evolution of mammal ears. Annual Review of Ecology, Evolution, and Systematics, 42(1), 355–380. [Google Scholar]
- Luo, Z.‐X. , Crompton, A. W. , & Sun, A.‐L. (2001). A new mammaliaform from the early Jurassic and evolution of mammalian characteristics. Science, 292(5521), 1535–1540. [DOI] [PubMed] [Google Scholar]
- Luo, Z.‐X. , Schultz, J. A. , & Ekdale, E. G. (2016). Evolution of the middle and inner ears of mammaliaforms: The approach to mammals. Evolution of the Vertebrate Ear, 59, 139–174. [Google Scholar]
- Luo, Z.‐X. , & Wible, J. R. (2005). A late Jurassic digging mammal and early mammalian diversification. Science, 308(5718), 103–107. [DOI] [PubMed] [Google Scholar]
- Mammal Research Institute PAS, Poland . (2021a). Crocidura leucodon (Bicolored Shrew). sketchfab.com
- Mammal Research Institute PAS, Poland . (2021b). Neomys anomalus (Mediterranean Water Shrew). sketchfab.com
- Martin, T. (2005). Postcranial anatomy of Haldanodon exspectatus (Mammalia, Docodonta) from the late Jurassic (Kimmeridgian) of Portugal and its bearing for mammalian evolution. Zoological Journal of the Linnean Society, 145(2), 219–248. [Google Scholar]
- Mason, M. J. (2001). Middle ear structures in fossorial mammals: A comparison with non‐fossorial species. Journal of Zoology, 255(4), 467–486. [Google Scholar]
- McIntosh, A. F. , & Cox, P. G. (2016a). The impact of digging on craniodental morphology and integration. Journal of Evolutionary Biology, 29(12), 2383–2394. [DOI] [PubMed] [Google Scholar]
- McIntosh, A. F. , & Cox, P. G. (2016b). The impact of gape on the performance of the skull in chisel‐tooth digging and scratch digging mole‐rats (Rodentia: Bathyergidae). Royal Society Open Science, 3(10), 160568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh, A. F. , & Cox, P. G. (2019). The impact of digging on the evolution of the rodent mandible. Journal of Morphology, 280(2), 176–183. [DOI] [PubMed] [Google Scholar]
- Menegaz, R. A. , Sublett, S. V. , Figueroa, S. D. , Hoffman, T. J. , Ravosa, M. J. , & Aldridge, K. (2010). Evidence for the influence of diet on cranial form and robusticity. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology, 293(4), 630–641. [DOI] [PubMed] [Google Scholar]
- Meng, J. , Bi, S. , Zheng, X. , & Wang, X. (2016). Ear ossicle morphology of the Jurassic euharamiyidan Arboroharamiya and evolution of mammalian middle ear. Journal of Morphology, 279(4), 441–457. [DOI] [PubMed] [Google Scholar]
- Morales‐García, N. M. , Gill, P. G. , Janis, C. M. , & Rayfield, E. J. (2021). Jaw shape and mechanical advantage are indicative of diet in Mesozoic mammals. Communications Biology, 4(1), 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagorsen, D. W. (1996). Opossums, shrews and moles of British Columbia (pp. 15–18). UBC Press. [Google Scholar]
- Oparina, O. S. , Filinova, E. I. , Sonina, E. E. , Malinina, Y. A. , & Oparin, M. L. (2013). Current status of the Russian desman habitats in small rivers of the Don River basin in Saratov oblast and the abundance of this species. Biology Bulletin, 40(10), 854–861. [Google Scholar]
- Pommerening, S. D. , & Martin, T. (2024). Chewing shrews: Examining the morphology and function of the masticatory musculature in Soricidae via diffusible iodine‐based contrast‐enhanced computed tomography. Journal of Morphology, 285(9), e21761. [DOI] [PubMed] [Google Scholar]
- Preuschoft, H. , & Witzel, U. (2002). Biomechanical investigations on the skulls of reptiles and mammals. Senckenbergiana Lethaea, 82(1), 207–222. [Google Scholar]
- Richmond, B. G. , Wright, B. W. , Grosse, I. , Dechow, P. C. , Ross, C. F. , Spencer, M. A. , & Strait, D. S. (2005). Finite element analysis in functional morphology. The Anatomical Record Part A: Discoveries in Molecular, Cellular, and Evolutionary Biology, 283A(2), 259–274. [DOI] [PubMed] [Google Scholar]
- Rodrigues, H. G. , Šumbera, R. , Hautier, L. , & Herrel, A. (2023). Digging up convergence in fossorial rodents: Insights into burrowing activity and morpho‐functional specializations of the masticatory apparatus. In Convergent evolution: Animal form and function (pp. 37–63). Springer International Publishing. [Google Scholar]
- Rowe, T. B ., Macrini T. E., & Luo Z. X. (2011). Fossil evidence on origin of the mammalian brain. Science, 332(6032), 955–957. [DOI] [PubMed] [Google Scholar]
- Rychlik, L. (2002). Prey size, prey nutrition, and food handling by shrews of different body sizes. Behavioral Ecology, 13(2), 216–223. [Google Scholar]
- Samuels, J. X. (2009). Cranial morphology and dietary habits of rodents. Zoological Journal of the Linnean Society, 156(4), 864–888. [Google Scholar]
- Samuels, J. X. , & Valkenburgh, B. V. (2009). Craniodental adaptations for digging in extinct burrowing beavers. Journal of Vertebrate Paleontology, 29(1), 254–268. [Google Scholar]
- Sansalone, G. , Colangelo, P. , Loy, A. , Raia, P. , Wroe, S. , & Piras, P. (2019). Impact of transition to a subterranean lifestyle on morphological disparity and integration in talpid moles (Mammalia, Talpidae). BMC Evolutionary Biology, 19(1), 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara, A. , Campbell, K. L. , & Suzuki, H. (2003). Molecular phylogenetic relationships of moles, shrew moles, and desmans from the new and old worlds. Molecular Phylogenetics and Evolution, 27(2), 247–258. [DOI] [PubMed] [Google Scholar]
- Silcox, M. , & Teaford, M. F. (2002). The diet of worms: An analysis of mole dental microwear. Journal of Mammalogy, 83(3), 804–814. [Google Scholar]
- Slater, G. J. (2013). Phylogenetic evidence for a shift in the mode of mammalian body size evolution at the cretaceous‐Palaeogene boundary. Methods in Ecology and Evolution, 4(8), 734–744. [Google Scholar]
- Ungar, P. S. (2010). Mammal teeth: Origin, evolution, and diversity. JHU Press. [Google Scholar]
- Vassallo, A. I. , Becerra, F. , Echeverría, A. I. , Díaz, A. O. , Longo, M. V. , Cohen, M. , & Buezas, G. N. (2019). Analysis of the form‐function relationship: Digging behavior as a case study. Journal of Mammalian Evolution, 28(1), 59–74. [Google Scholar]
- Wang, H. , Meng, J. , & Wang, Y. (2019). Cretaceous fossil reveals a new pattern in mammalian middle ear evolution. Nature, 576(7785), 102–105. [DOI] [PubMed] [Google Scholar]
- Xu, D. , Sun, M. , Gao, Z. , Zhou, Y. , Wang, Q. , & Chen, L. (2023). Comparison and phylogenetic analysis of mitochondrial genomes of Talpidae animals. Animals, 13(2), 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information 1: Spreadsheet consisting of mandible measurements (mandible height, mandible length, outlever length, inlever length) for every species with referenced literature for each.
Supplementary Information 2: Landmark data before Procrustes.
Supplementary Information 3: Color map codes for Abaqus. Includes ‘Parula,’ ‘Polar,’ and ‘DarkPolar.’
Supplementary Information 4: Hypermesh files for each species. All models are meshed with material properties, constraints, loads, and load steps.


