Abstract
Oral probiotics have significant potential for preventing and treating many diseases. Yet, their efficacy is often hindered by challenges related to survival and colonization within the gastrointestinal tract. Nanoparticles emerge as a transformative solution, offering robust protection and enhancing the stability and bioavailability of these probiotics. This review explores the innovative application of nanoparticle-armored engineered probiotics for precise disease treatment, specifically addressing the physiological barriers associated with oral administration. A comprehensive evaluation of various nano-armor probiotics and encapsulation methods is provided, carefully analyzing their respective merits and limitations, alongside strategies to enhance probiotic survival and achieve targeted delivery and colonization within the gastrointestinal tract. Furthermore, the review explores the potential clinical applications of nano-armored probiotics in precision therapeutics, critically addressing safety and regulatory considerations, and proposing the innovative concept of ‘probiotic intestinal colonization with nano armor’ for brain-targeted therapies. Ultimately, this review aspires to guide the advancement of nano-armored probiotic therapies, driving progress in precision medicine and paving the way for groundbreaking treatment modalities.
Graphical abstract
Keywords: Probiotics, Nanoparticles, Oral administration, Gut to brain, Precision therapy
Introduction
Probiotics, active microorganisms beneficial to the host, represent a category of organisms colonizing the human intestinal and reproductive systems [1]. They are crucial in producing precise health effects, enhancing the host’s microecological balance, and contributing to overall well-being. Among human intestinal flora, comprising commensal, conditionally pathogenic, and pathogenic bacteria, it is the commensal bacteria—probiotics—that predominantly generate beneficial substances for the body [2]. These specialized anaerobic bacteria, constituting over 99% of the total intestinal flora, serve as the backbone [3].
In contemporary probiotic research, these microorganisms find widespread application in addressing prevalent health issues such as rheumatism, aging, inflammation, cancer, obesity, hypertension, and diabetes [4–9]. The effectiveness of probiotics hinges on the dynamic gastrointestinal tract (GIT) environment, where they engage with internal factors, triggering the release of GIT-regulated pro- and anti-inflammatory agents. Studies highlight the success of genetically engineered probiotics localized in the colon, consistently secreting biopharmaceuticals like cytokines and therapeutic enzymes, notably effective in treating gastrointestinal disorders, particularly in animal models [10].
Nevertheless, oral probiotics are confronted with a substantial challenge. Furthermore, the harsh conditions within the gastrointestinal tract result in the inactivation of probiotics before reaching specific areas. The precise localization of probiotics is susceptible to the extensive mucosa in the gastrointestinal tract. Effectively locating probiotics in particular regions and ensuring their prolonged survival presents a significant challenge, compounded by various influencing factors. The gut faces challenges, including the widespread use of antibiotics or anti-inflammatory drugs during common ailments, disrupting the balance of intestinal flora [11]. Environmental pollution in food production further complicates probiotic efficacy [12]. Unhealthy dietary habits, work-related stress, and reduced gastrointestinal peristalsis impact intestinal health, compromising probiotic survival. Post-probiotic consumption, amylase in the mouth, and gastric acids can potentially deactivate probiotics, limiting their concentration at disease sites and diminishing their effectiveness [13]. Addressing these challenges is crucial for optimizing probiotic action.
Encapsulating probiotics with nanomaterials represents a promising strategy, ensuring the preservation of their integrity during oral delivery to targeted sites. Nanomaterials, characterized by their sub-stable properties, large specific surface area, small particle size, and unique electronic motion states, possess macroscopic quantum tunneling and quantum size effects, endowing them with exceptional qualities [14]. Probiotic delivery via nanocarriers offers several advantages. Firstly, these carriers can enter the bloodstream for precise drug delivery, enhancing probiotic bioavailability [15]. Secondly, the smaller particle size and increased specific surface area of nanocarriers improve probiotic solubility, mitigating co-solvent side effects observed in traditional drugs [16]. Thirdly, custom modifications of probiotic nanocarriers with targeting groups facilitate intestinal tissue-specific drug delivery, reducing both dosage and associated side effects [17, 18]. Fourthly, nanocarriers overcome physiological barriers, such as the highly acidic and alkaline environments of the gastrointestinal tract, ensuring probiotics reach intestinal tissues accurately, thereby augmenting their regulatory impact on the intestinal tract [19, 20].
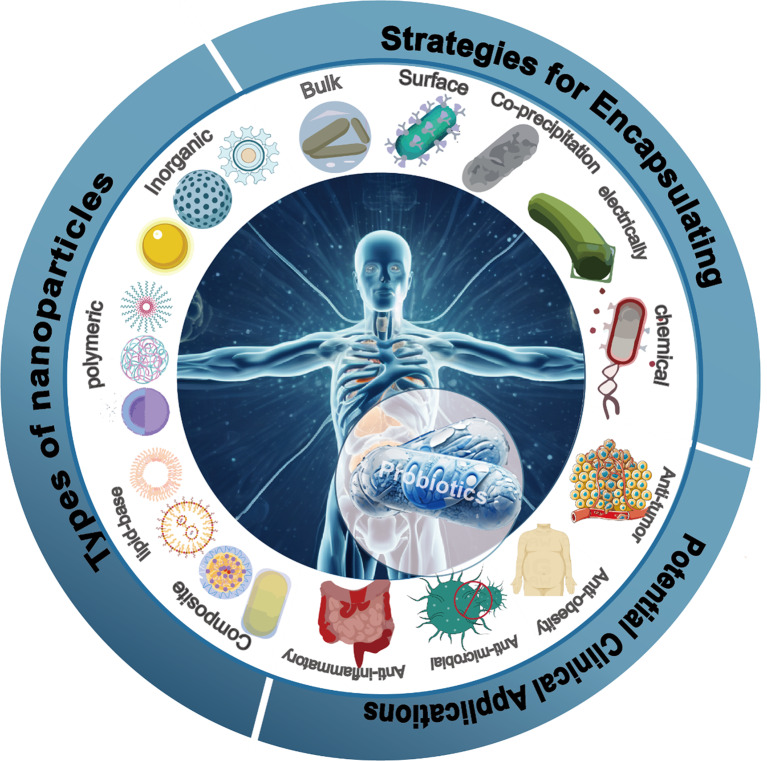
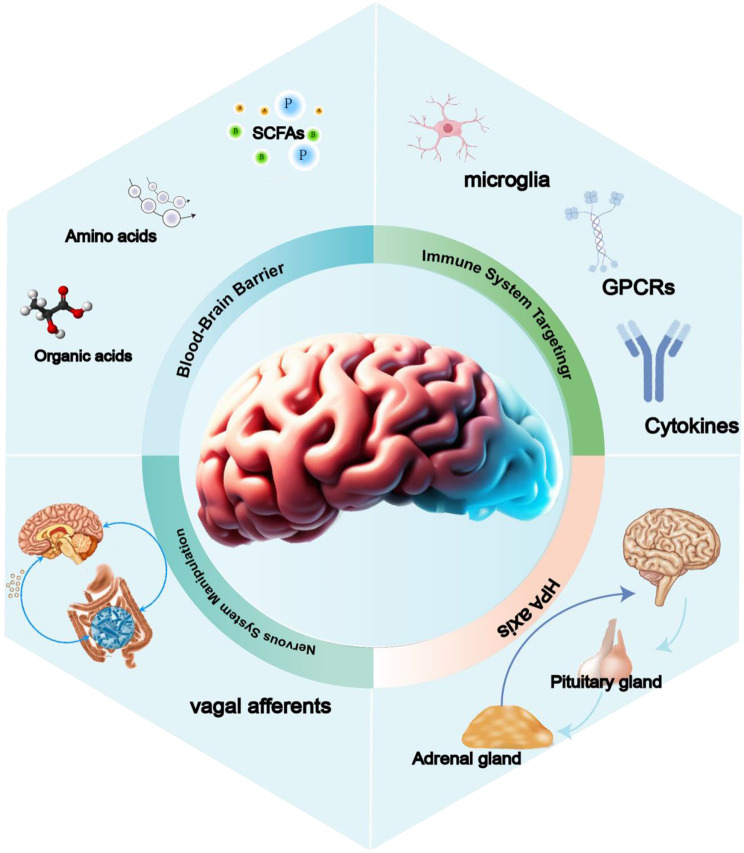
This review endeavors to delve into the pivotal role of nanocarriers in oral probiotic therapy, specifically focusing on tailored oral nano-probiotic capsules designed for precision treatment in intestinal tissues (Fig. 1). Firstly, enhancing stability and bioavailability: Nanoparticles serve as a protective shield, safeguarding probiotics against deleterious environments like gastric acid, thereby bolstering probiotic stability and augmenting their bioavailability within the digestive tract. Secondly, precision in treatment pathways: Leveraging the carrier function of nanoparticles, probiotics can be precisely guided to the location, thereby establishing meticulous treatment pathways. Such precision not only amplifies treatment efficacy but also mitigates impacts on other bodily systems. Thirdly, fostering therapeutic efficacy: Nanoparticles elevate the colonization rate of probiotics in the digestive tract, prolonging their residence time within the intestines and consequently amplifying their beneficial impact on the host. Fourthly, mitigating side effects: Nanoparticles not only shield probiotics but also facilitate their controlled release within target tissues, thereby intensifying therapeutic effects. This strategic modulation heightens treatment effectiveness and abbreviates its duration.
Fig. 1.
Precision disease treatment with tailored oral nano-armor-probiotics
The challenges of orally delivered probiotics
Probiotics, as active microbial supplements, play a crucial role in surviving the gastrointestinal tract, producing beneficial substances, and modulating the immune system. The most extensively studied genera include Bifidobacterium and Lactobacillus, alongside widely used Enterococcus, Yeast, and Escherichia coli Nissle 1917 (EcN). Recent advances in microbiology have highlighted the significant role of gut probiotics in maintaining and regulating human health. However, delivering probiotics orally presents several challenges. One major obstacle is the stomach’s harsh acidic environment, which can destroy many probiotic strains before they reach the intestines where they are beneficial. Additionally, bile salts and digestive enzymes further threaten the viability of probiotics. Formulation and encapsulation technologies aim to protect these microorganisms during their passage through the digestive system, but maintaining their stability during manufacturing, storage, and transportation adds another layer of complexity. Furthermore, individual variations in gut microbiota composition and function can influence the efficacy of probiotics, making it difficult to standardize treatment outcomes. These challenges necessitate ongoing research and innovation to improve the delivery and effectiveness of orally administered probiotics.
Overview of probiotics
The term “probiotic” comes from Greek, meaning “for life,” and refers to a variety of microorganisms, mainly bacteria but also including yeasts. As our understanding of how probiotics work and their effects on human health has grown, the definition has evolved. Initially described as live microbial supplements that improve the host’s intestinal microbial balance, probiotics are now defined as “live microorganisms which, when administered in adequate amounts, confer a health benefit on the host.” This means that probiotic products like yogurts and drinks contain live microorganisms that benefit the gastrointestinal tract [21, 22].
Probiotics contribute to digestive health by promoting the balance of beneficial bacteria in the gastrointestinal tract, which is essential for proper nutrient absorption and immune system modulation. These microorganisms have gained significant attention for their potential to alleviate various gastrointestinal issues, such as irritable bowel syndrome, diarrhea, and inflammatory bowel diseases [23–25].
Beyond their impact on gut health, probiotics have been studied for their potential systemic effects. Emerging research suggests that these microorganisms may influence immune function, metabolism, and even mental well-being [26, 27]. Probiotics are commonly found in fermented foods like yogurt, kefir, sauerkraut, and kimchi, as well as in dietary supplements [28, 29]. As interest in personalized nutrition grows, researchers are exploring the concept of precision probiotics, tailoring specific strains to individual needs based on factors such as age, genetics, and health conditions.
Despite their potential benefits, it’s crucial to note that not all probiotics are created equal. Strain specificity, viability, and dosage are critical considerations for ensuring the effectiveness of these microorganisms. Additionally, ongoing research is uncovering the intricate mechanisms through which probiotics exert their influence, expanding our understanding of their diverse roles in promoting health and well-being. In essence, the overview of probiotics reflects a dynamic and evolving field with vast implications for human health. As our understanding deepens, the integration of probiotics into mainstream healthcare and wellness practices is likely to grow, offering new avenues for maintaining and enhancing our overall well-being.
Understanding probiotics and their role in health and disease
Delving into the intricate world of probiotics unveils a profound exploration of their pivotal role in shaping both health and disease dynamics. Probiotics, those microscopic agents often described as “friendly” bacteria, emerge as central players in the delicate equilibrium governing our well-being [30]. At its essence, understanding probiotics is an odyssey through the intricate tapestry of the human microbiome, particularly the gut microbiota. These live microorganisms, predominantly hailing from the Lactobacillus and Bifidobacterium families, orchestrate a symphony within our digestive tracts, influencing a myriad of physiological processes [31, 32].
According to academic and popular sources, the history of probiotics traces back to early civilizations, when humans began consuming fermented foods. The earliest recorded use dates back to around 2000 BC, when bacteria and yeast were employed by the first food manufacturers to convert milk into fermented dairy products [33]. Historical accounts suggest that yeast was utilized in beverage production as early as 2000 BC [34, 35]. Ancient Egyptian artifacts from around 3500 BC indicate the consumption of dairy products such as “Laban Rayad” and “Laban Khed” (fermented products), which remain popular in the Middle East today [36, 37]. In ancient Indian Ayurvedic texts, the consumption of dairy products and milk is linked with health and longevity [37, 38].
In the early 1900s, Elie Metchnikoff, a Russian research scientist at the Pasteur Institute in Paris, proposed the idea that manipulating the gut microbiome (GM) with beneficial bacteria found in yogurt could enhance human health (Fig. 2) [39, 40]. This concept laid the foundation for the medical application of probiotics. Various bacteria have been identified as major probiotic candidates, with Lactobacilli and Bacillus garnering interest due to their potent antagonistic activity and availability. This interest is also attributed to the biosynthesis of extracellular enzymes commonly utilized by Ayurvedic practitioners in the subcontinent.
Fig. 2.
Timeline of selected items in the history of probiotic-related terms [40]. Copyright © 2019 Martín and Langella
Probiotics are typically categorized into three main groups, including Lactobacilli, Bifidobacteria, and others. Lactobacilli, a genus of rod-shaped bacteria first described in 1901, have long been integral to fermented foods and are naturally present in the human digestive tract and vaginal cavity [41–43]. Many probiotics in use today belong to the genus Lactobacillus, known colloquially as “Lactobacilli.” These microbes serve various functions, including extending the shelf life of foods (e.g., yogurt or cheese production) and enhancing health when consumed in probiotic foods or supplements [44, 45]. Recent reclassification efforts have resulted in the division of the genus Lactobacillus into 25 genera, including the revised Lactobacillus genera and 23 new genera [46]. This updated taxonomy aims to group species based on their physiological and metabolic properties, potentially shedding light on common mechanisms mediating the health benefits of probiotics.
Bifidobacterium, another important probiotic type, plays a vital role in human health by colonizing and metabolizing in the small and large intestines, producing probiotic factors, regulating intestinal health, and adapting to anaerobic conditions [47]. Bifidobacterium has been associated with inhibiting the growth of pathogenic bacteria, modulating inflammatory responses, maintaining intestinal barrier function, synthesizing vitamins and amino acids, enhancing calcium bioavailability, and exhibiting anti-tumor effects [48]. Additionally, other bacterial species like Enterococci, frequently utilized in the food industry, possess characteristics conducive to competing, surviving, and attaching to intestinal host cells. They exhibit resilience to varying pH and temperature levels, attributed to their robust bacteriocin production capabilities, making them valuable natural antimicrobials in food processing [49].
Saccharomyces cerevisiae, a well-known non-pathogenic probiotic strain, is extensively utilized in the commercial production of probiotic foods. This Yeast strain has been extensively studied for its probiotic effects, particularly in alleviating digestive disorders such as diarrhea, especially when used alongside antibiotic treatment [50, 51]. Yeast strains demonstrate high survivability through the digestive tract, aiding in maintaining a balanced intestinal microbiota and modulating immune responses during pathogenic infections or chronic diseases. Other commonly used probiotics include Bacillus, E. coli, and Streptococcus, whose effects and applications align with those of some prevalent probiotics [52].
The important role of nano-armor-probiotics
The vital role of nanoparticles as carriers extends far beyond their minute size, ushering in a transformative era in drug delivery and therapeutic interventions. As carriers, nanoparticles play a pivotal role in addressing challenges associated with traditional drug therapies, offering a myriad of advantages that significantly enhance the efficacy, precision, and safety of medical treatments. One of the cardinal aspects defining the importance of nanoparticles as carriers lies in their ability to encapsulate and protect therapeutic agents, such as drugs or probiotics, from the harsh environments encountered during their journey through the body [53, 54]. This protective shield not only ensures the stability and integrity of the loaded cargo but also shields it from premature degradation, enabling controlled and sustained release at the target site.
The versatility of nanoparticles as carriers is underscored by their diverse composition, ranging from natural to synthetic materials. Natural polymers, such as chitosan and gelatin, exhibit inherent biocompatibility, reducing the risk of adverse reactions within the body [55–57]. On the other hand, synthetic polymers, like polyethylene glycol (PEG) and poly(lactic-co-glycolic acid) (PLGA), offer tunable properties, allowing for precise control over drug release kinetics and carrier biodegradability [58, 59]. Moreover, the unique physicochemical properties of nanoparticles contribute to their exceptional capabilities. Nanoparticles possess a high surface area-to-volume ratio, enabling efficient drug loading and enhancing solubility—a common challenge in traditional drug formulations [60]. This characteristic not only facilitates the incorporation of a diverse range of therapeutic agents but also allows for the simultaneous delivery of multiple drugs, paving the way for combination therapies with synergistic effects.
The strategic design and modification of nanoparticles further amplify their importance as carriers. Surface functionalization with ligands or targeting moieties enables the specific delivery of therapeutic payloads to desired tissues or cells, reducing off-target effects and enhancing therapeutic precision. This targeted approach is particularly valuable in cancer therapy, where nanoparticles can selectively accumulate in tumor tissues through enhanced permeability and retention (EPR) effects. In the realm of nanocarriers, the utilization of various nanomaterials, including lipids, polymers, and metals, provides a versatile toolkit for tailored drug delivery systems. Lipid-based nanoparticles, such as liposomes and solid lipid nanoparticles, offer the advantage of encapsulating both hydrophobic and hydrophilic drugs, addressing the challenge of delivering diverse therapeutic agents with varying solubilities [61, 62].
Additionally, the importance of nanoparticles as carriers is exemplified by their role in overcoming physiological barriers [63]. Nanocarriers can navigate complex biological environments, including the gastrointestinal tract, and facilitate the transport of drugs or probiotics to specific sites of action. This capability is particularly significant in oral drug delivery, where challenges like enzymatic degradation and poor bioavailability are common. The crucial role of nanoparticles as carriers lies in their ability to revolutionize drug delivery, offering a versatile platform that enhances therapeutic outcomes. From protecting cargo to enabling targeted delivery and overcoming biological barriers, nanoparticles exemplify a groundbreaking paradigm in modern medicine, promising more effective, precise, and patient-friendly treatment modalities.
Limitations of oral probiotic administration in precision therapy
Exploring the limitations of probiotics in oral administration reveals intricate challenges that, while not diminishing their therapeutic potential, necessitate thoughtful consideration in the pursuit of precision therapy. The unique characteristics of probiotics, beneficial bacteria crucial for gut health, pose hurdles in their effective delivery and targeted action, particularly when administered orally. Firstly, due to their biological characteristics, probiotics require specific storage conditions when stored, and improper storage can lead to a substantial reduction in probiotic activity, affecting the clinical therapeutic effect (Fig. 3a).
Fig. 3.
Adverse environment encountered by probiotics during a storage, b gastrointestinal transport, and c functions of armor probiotics in vivo [72]. © 2024 Elsevier B.V. All rights are reserved, including those for text and data mining, AI training, and similar technologies
Secondly, one significant limitation lies in the vulnerability of probiotics to the harsh conditions of the gastrointestinal tract (Fig. 3b). The acidic environment of the stomach and the alkaline conditions of the small intestine can compromise the viability and stability of probiotics, leading to a decrease in their efficacy before reaching the targeted areas in the gut [64, 65]. This challenge becomes even more pronounced when dealing with specific strains that are particularly sensitive to variations in pH levels.
Furthermore, the intricate interplay of probiotics with the vast and diverse microbiota inhabiting the gastrointestinal tract presents a hurdle [66]. Probiotics face competition and potential displacement by the resident microbiota, limiting their ability to establish and exert their intended therapeutic effects [67], and achieving a balance that allows administered probiotics to flourish in this complex ecosystem while avoiding disruption of the existing microbial community remains a delicate challenge.
Precision therapy demands a nuanced understanding of individual variations, yet probiotics often exhibit strain-specific responses, introducing another layer of complexity. Variability in individual gut microbiomes and host factors can impact the effectiveness of probiotics, making it challenging to standardize their application across diverse populations. Identifying the most suitable probiotic strains for specific individuals or health conditions becomes crucial for achieving precision in therapy. The transit time through the gastrointestinal tract is another factor that contributes to the limitations of oral probiotic administration [66, 68]. The rapid transit time may not provide sufficient exposure for probiotics to interact with the intestinal mucosa and exert their therapeutic effects(Fig. 3c). Prolonged retention and sustained release are essential considerations to maximize the beneficial impact of probiotics.
Innovative strategies are emerging to address these limitations and enhance the precision of probiotic therapy. Nanotechnology, for instance, offers a promising avenue by encapsulating probiotics in protective nano-carriers, safeguarding them from harsh gastrointestinal conditions, and enabling targeted delivery to specific sites in the gut [69, 70]. Such advancements aim to overcome the challenges associated with probiotic stability and viability during oral administration.
Additionally, the development of genetically engineered probiotics holds potential for tailoring strains with enhanced resilience and therapeutic capabilities [71]. Customizing probiotics to withstand the hostile gastrointestinal environment, adhere efficiently to intestinal surfaces, and interact synergistically with the existing microbiota can pave the way for more precise and effective therapeutic interventions. While the limitations of probiotics in oral administration present formidable challenges, ongoing research and technological innovations hold the promise of overcoming these hurdles. Addressing issues related to probiotic stability, strain specificity, and the dynamic gut environment is crucial for advancing precision therapy, ensuring that probiotics can be harnessed to their full potential in promoting gut health and overall well-being.
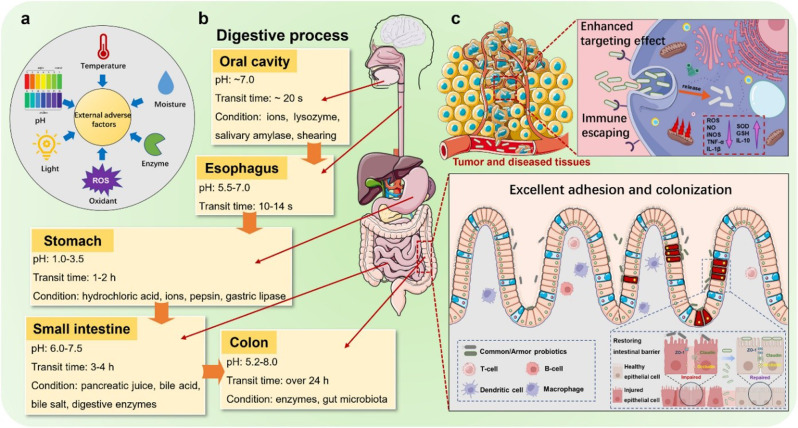
Challenges related to probiotic survival and viability in the harsh gastrointestinal environment are paramount considerations in the development of effective probiotic formulations (Fig. 4a) [73]. The gastrointestinal tract, characterized by acidic conditions and enzymatic activity, poses formidable obstacles to the successful transit and functionality of probiotics [74]. Understanding and addressing these challenges are crucial for ensuring the delivery of viable and active probiotics to the intended sites within the gut.
Fig. 4.
a schematic illustration of the barriers to oral drug delivery (pH variations, microbiota content, mucus thickness) between the stomach and colon [73]. © 2021 Elsevier B.V. All rights reserved. b The double-layered multinucleated microcapsules were prepared by mixing SN15-2 as probiotics with inner core microspheres and calcium alginate as the shell material [75]. Copyright ©2023 American Chemical Society.c Characterization of artificial-enzyme-armed probiotic [76]. Copyright © 2023, The Author(s), under exclusive licence to Springer Nature Limited
Elaborating further on the challenges posed by the acidic environment of the stomach for probiotic viability, it’s crucial to understand the intricacies of how low pH levels can impact the integrity of probiotic strains. The stomach’s acidic conditions serve as a natural defense mechanism, primarily designed to break down ingested food and eliminate potentially harmful pathogens [77]. However, this harsh environment becomes a formidable obstacle for probiotics, as the acidity can disrupt their structural components and compromise their functionality. The low pH in the stomach presents a two-fold challenge for probiotics: first, it can directly affect the viability of the microbial strains, leading to a reduction in their numbers; second, the acidic conditions may alter the molecular structure of the probiotics, diminishing their ability to survive and thrive in the subsequent stages of the digestive process.
To overcome these challenges, innovative strategies have been developed to enhance probiotic survival under acidic conditions. One such approach involves the utilization of acid-resistant coatings [70, 78]. These coatings act as a protective barrier around the probiotic cells, shielding them from the harsh acidity in the stomach. By preventing direct exposure to low pH levels, these coatings help maintain the structural integrity of probiotics, ensuring a higher percentage of viable cells reach the intestines. Encapsulation techniques represent another key strategy in addressing the impact of stomach acidity on probiotic viability [53]. Microencapsulation involves enveloping probiotic cells in protective materials, often in the form of microspheres or capsules [79, 80]. This encapsulation provides an additional layer of defense, safeguarding probiotics from the acidic environment during their journey through the stomach. As the encapsulated probiotics move into the intestines, the protective coating gradually dissolves, releasing the viable and active microbial cells at the desired location.
The acidic environment of the stomach poses a significant challenge to probiotic viability, impacting their integrity and reducing their effectiveness. However, with the implementation of innovative approaches such as acid-resistant coatings and encapsulation techniques, researchers aim to enhance the survival of probiotics through the acidic gastric milieu. These strategies not only contribute to overcoming the hurdles posed by low pH but also ensure that probiotics reach the intestines in a functional state, maximizing their potential health benefits for individuals consuming these probiotic formulations.
Expanding on the challenge of enzymatic degradation within the gastrointestinal tract, it’s important to delve into the specific mechanisms by which proteolytic enzymes impact the structural integrity and functionality of probiotics [81]. The gastrointestinal tract is equipped with a variety of enzymes, particularly proteases, designed to break down proteins into smaller peptides and amino acids during the digestive process. Unfortunately, these enzymes can also target the protein structures of probiotics, potentially leading to their inactivation and reduced efficacy. The stomach, with its gastric juices rich in proteolytic enzymes, poses an initial hurdle for probiotics [82]. Proteases in the stomach can begin to break down the proteins of probiotic strains, potentially compromising their viability even before reaching the small intestine. The challenge continues in the small intestine, where additional proteolytic enzymes may further degrade the probiotics, limiting their ability to exert their beneficial effects.
To address this challenge, researchers and formulators are actively working on developing probiotic formulations that are resistant to enzymatic degradation. One approach involves the selection or engineering of probiotic strains with inherent resistance to specific proteases present in the gastrointestinal tract [83, 84]. By understanding the enzyme profiles within different regions of the digestive system, scientists can tailor probiotic strains that can withstand the enzymatic onslaught. Additionally, advanced encapsulation techniques offer additional protection against enzymatic degradation for probiotics. Microencapsulation, for instance, involves enveloping probiotic cells within a protective coating, shielding them from direct exposure to digestive enzymes. Li et al. demonstrated the efficacy of encapsulating probiotics in alginate microgels for co-administration with antibiotics, mitigating antimicrobial resistance in pathogens [85]. This encapsulation not only preserves probiotic viability during transit through the stomach and small intestine but also ensures controlled release in the intestines, where conditions are conducive to their survival and activity.
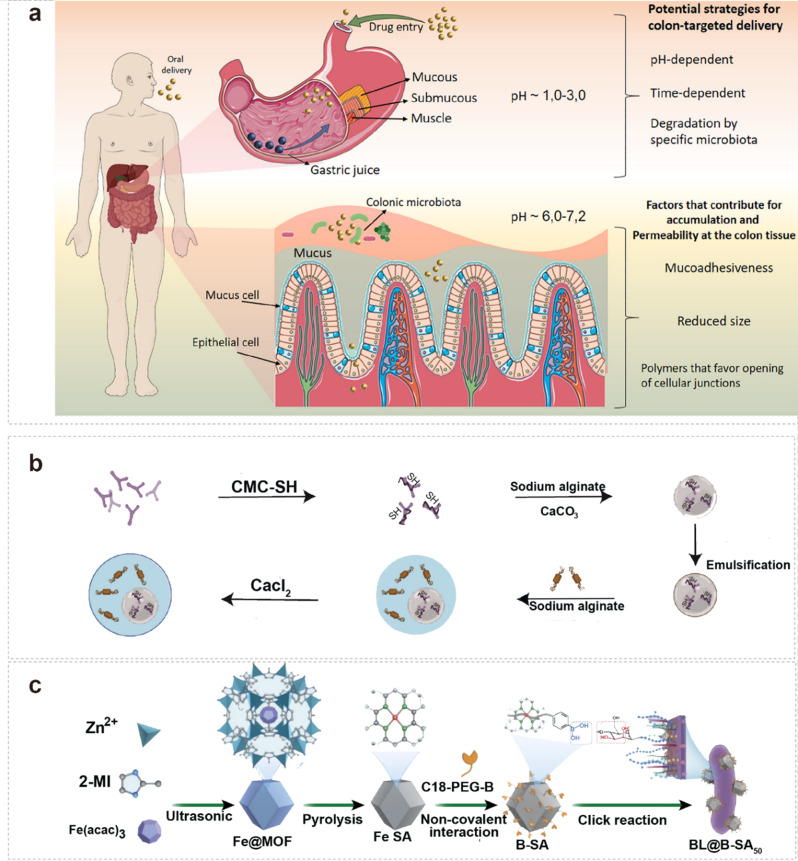
To enhance bifidobacteria’s ability to adhere to and colonize the gastrointestinal tract, Wu and Liu et al. screened Bifidobacterium adolescent FS2-3 (FS2-3) and Bacillus subtilis SN15-2 (SN15-2) [75]. FS2-3, selected as a probiotic, was embedded in core microspheres and combined with sulfhydryl-modified sodium carboxymethylcellulose (CMC-SH) to enhance adhesion. Core microspheres were crafted using endogenous emulsification. SN15-2 was then incorporated with the inner core microspheres to form probiotic bilayer multinucleated microcapsules, utilizing sodium alginate as the wall material through the extrusion method (Fig. 4b). Enzymatic degradation represents a significant challenge for probiotic functionality in the gastrointestinal tract. Developing probiotic formulations that are resistant to the proteolytic activity of digestive enzymes is crucial for maintaining the structural integrity of these beneficial microorganisms. By employing a combination of strain selection, genetic engineering, and advanced encapsulation techniques, researchers aim to enhance the resilience of probiotics, ensuring their effectiveness in promoting gut health and overall well-being throughout the digestive journey. The subsequent chapters will provide a comprehensive exploration of the topic.
Expanding on the challenge of competition with endogenous microflora in the gut, it’s essential to explore the intricate dynamics of the gastrointestinal ecosystem and how they impact the colonization and survival of probiotics. The gut is home to a diverse community of microorganisms, collectively known as the microbiota or microflora, which plays a crucial role in maintaining gut health and supporting various physiological functions [86]. When exogenously introduced probiotics enter the gastrointestinal tract, they encounter the existing microbial community, creating a competitive environment for colonization. The complex interplay between probiotics and the resident microflora may limit the establishment of exogenous strains, as the indigenous microbes have already adapted to the local conditions and developed mechanisms to maintain their niche.
To address this challenge, one strategy involves selecting probiotic strains with strong adhesion properties [87]. The ability of probiotics to adhere to the mucosal surfaces of the gut lining is critical for their colonization and persistence. Certain probiotic strains exhibit enhanced adhesion capabilities, allowing them to compete more effectively with the endogenous microflora for attachment sites in the intestines. This adhesion is often mediated by specific surface structures or molecules on the probiotic cells that interact with receptors in the gut lining. Furthermore, promoting the growth and activity of introduced probiotics through prebiotics, which are substances that selectively nourish beneficial microorganisms, can create a more favorable environment for their colonization [88]. Prebiotics, such as certain types of dietary fibers, serve as a source of nutrients for probiotics, encouraging their proliferation and reinforcing their presence in the gut [89].
Understanding the specific requirements of probiotic strains and their compatibility with the existing microbiota is essential for designing effective strategies to enhance their establishment in the gut. Additionally, research into the communication and interactions between probiotics and endogenous microflora can provide insights into developing formulations that foster a balanced and harmonious coexistence within the gastrointestinal ecosystem. The competition with endogenous microflora represents a significant challenge for probiotics in establishing themselves in the gut. Strategies focused on selecting probiotic strains with strong adhesion properties, combined with the use of prebiotics to support their growth, are pivotal for overcoming this challenge and ensuring the successful colonization and survival of exogenously introduced probiotics in the complex environment of the gastrointestinal tract.
Expanding on the importance of ensuring probiotic viability during storage, it is essential to delve into the specific factors and considerations that impact the stability of probiotic formulations over time. While the challenges within the gastrointestinal tract are significant, the conditions to which probiotics are exposed during storage can equally influence their effectiveness. Temperature, humidity, and oxygen exposure are key factors that can compromise the stability of probiotic formulations [90]. Elevated temperatures can accelerate the degradation of probiotics, potentially reducing their viability and rendering them less effective. Likewise, excessive humidity can create conditions conducive to microbial growth, leading to a decline in the probiotic population. Oxygen exposure poses a risk as well, as it may induce oxidative stress, damaging the delicate structures of probiotic cells.
To address these challenges, implementing proper storage conditions is paramount. Storing probiotics in a cool, dry environment helps mitigate the impact of temperature and humidity [91]. Refrigeration or even freezing can be particularly effective in preserving probiotic viability, slowing down the degradation processes, and extending their shelf life [92]. Additionally, airtight packaging that minimizes exposure to oxygen is crucial for maintaining the stability of probiotic formulations [93].
Technological approaches such as freeze-drying (lyophilization) and microencapsulation also play a crucial role in enhancing probiotic stability during storage [90, 94, 95]. Freeze-drying involves removing moisture from probiotic cells, reducing the risk of bacterial degradation due to water activity [96]. This process allows probiotics to be stored in a dormant state and reactivated upon consumption. Microencapsulation, as mentioned earlier, provides a protective shell around probiotic cells, shielding them from external factors and extending their shelf life [97].
Furthermore, research and development efforts are ongoing to explore novel technologies and formulations that can further enhance the stability of probiotics during storage. This includes the use of advanced protective coatings, stabilizing agents, and packaging innovations that can provide an additional layer of defense against environmental challenges.
Ensuring probiotic viability during storage is a critical consideration to maximize their efficacy [91, 98]. Proper storage conditions, including temperature and humidity control, along with the implementation of technologies like freeze-drying and microencapsulation, are essential for maintaining the stability of probiotic formulations over their shelf life. As the demand for probiotics continues to grow, ongoing advancements in storage technologies will contribute to the development of more robust and long-lasting probiotic products.
Strain selection and specificity
“Strain Selection and Specificity” is a pivotal aspect in the realm of probiotics, contributing significantly to their efficacy and targeted functionality [99]. The careful selection of probiotic strains plays a crucial role in determining their ability to regulate the balance of intestinal flora and positively impact gut health [100]. Each probiotic strain possesses unique characteristics, and its specific interactions with the host’s microbiota can influence the outcomes of probiotic interventions [101]. The deliberate choice of probiotic strains with well-defined characteristics ensures not only their survival and functionality in the gastrointestinal environment but also their ability to bring about positive changes in the host’s gut microbiota [102]. As the field of probiotics continues to advance, refining the understanding of strain specificity will contribute to the development of targeted and personalized probiotic interventions for improved overall well-being.
Strain selection
Probiotic strain selection is a fundamental aspect of harnessing the full potential of probiotics for promoting gut health and overall well-being [103]. With a diverse array of probiotic strains available, the process of choosing the right strains becomes a critical determinant in the effectiveness of probiotic interventions. Each strain exhibits unique characteristics, and understanding their specific attributes is essential for tailoring probiotic therapies to meet individual needs.
Several factors come into play during the selection of probiotic strains. These include the ability of strains to survive the harsh conditions of the gastrointestinal tract, their adhesion properties to intestinal surfaces, and their capacity to produce beneficial metabolites [104]. Additionally, the strain’s interactions with the host’s immune system and microbiota contribute to its overall impact on gut health [105, 106]. A holistic approach to strain selection considers these factors to ensure optimal outcomes.
Strain specificity is a key consideration in probiotic interventions [107]. Different strains within a probiotic species may have distinct effects on the host. Certain strains may exhibit stronger immunomodulatory properties, while others may excel in producing specific bioactive compounds. Understanding the unique characteristics of each strain allows for targeted applications based on specific health goals, contributing to the overall success of probiotic therapies.
The diverse nature of individuals’ gut microbiota underscores the importance of tailoring probiotics to meet specific needs. Through precise strain selection, probiotic formulations can be designed to address particular health concerns or conditions [108]. This personalized approach enhances the likelihood of probiotics positively influencing the host’s gut microbiota, supporting a balanced microbial environment, and promoting overall digestive health.
Ongoing advancements in probiotic strain research continually expand our understanding of the intricate interactions between specific strains and the host. This knowledge fuels the development of innovative probiotic formulations that can target precise health outcomes. As researchers delve deeper into the characteristics of probiotic strains, the potential for uncovering new therapeutic applications and optimizing probiotic interventions for diverse populations becomes increasingly promising.
Strain specificity
Probiotic strain specificity refers to the unique characteristics and properties exhibited by individual strains within the vast array of probiotic microorganisms [99]. It is a crucial factor in understanding how different strains can elicit specific responses within the host’s body. The significance lies in tailoring probiotic interventions to achieve precise health outcomes based on the distinctive attributes of each strain. In the quest for precision medicine, envisioning the potential of probiotics attaining a remarkable level of disease specificity emerges as a fascinating prospect. The inherent adaptability and versatility of these beneficial microorganisms suggest a future where they can be tailored with unprecedented precision to address specific diseases.
At the forefront of this vision is the notion of genetically engineered probiotic strains designed to target distinct diseases at the molecular level. Scientists could potentially manipulate the genetic makeup of probiotics to ensure they possess receptors and signaling mechanisms finely tuned to recognize and engage with specific pathological markers associated with various illnesses [109]. In this advanced scenario, probiotics would be crafted to navigate the complex journey through the gastrointestinal tract with enhanced efficiency, ensuring their arrival at the precise sites of disease manifestation. These engineered strains would exhibit an exceptional ability to adhere selectively to diseased tissues, distinguishing them from healthy counterparts and minimizing unintended consequences.
Moreover, disease-specific probiotics could be armed with the capability to produce bioactive compounds designed to counteract the molecular mechanisms underlying specific diseases. For instance, probiotics engineered for metabolic disorders might release substances targeting insulin resistance, while those designed for inflammatory conditions could modulate immune responses with unprecedented precision [110, 111]. As research continues to unravel the intricate relationship between the microbiome and various diseases, the potential for probiotics to become disease-specific therapeutic agents becomes increasingly plausible. Tailored probiotic interventions could revolutionize treatment strategies by addressing the root causes of diseases, offering a more nuanced and targeted approach to healthcare.
Strain specificity and therapeutic effects
The utilization of different genera of bacteria for various diseases represents a sophisticated approach to harnessing the therapeutic potential of probiotics [112]. In this intricate landscape, specific strains are identified and deployed based on their unique attributes, creating a targeted and effective intervention for diverse health conditions. For instance, in addressing eczema, food allergies, and immunity, strains like Bifidobacterium, Saccharomyces boulardii, and Clostridium casei play a pivotal role [113, 114]. These strains not only improve overall illness but also contribute to enhancing the quality of life for individuals grappling with these conditions. It highlights the nuanced relationship between specific bacterial genera and their impact on certain health aspects.
Moreover, when focusing on intestinal disorders, the importance of strain specificity becomes even more apparent. Strains such as Lactobacillus rhamnosus, Lactobacillus complex eosinophils, and Enterococcus faecalis demonstrate a targeted effect, addressing abdominal pain, bowel habits, and symptoms of diarrhea or constipation [115, 116]. Meanwhile, Bifidobacterium infantis emerges as a multifaceted solution, not only alleviating bloating and abdominal pain but also contributing to the reduction of inflammation within the intestines [117].
The specificity observed in the therapeutic effects of different bacterial strains emphasizes a personalized approach to probiotic interventions. Recognizing the distinct qualities of each strain allows for a tailored strategy, enhancing the therapeutic precision of probiotics. By customizing microorganisms that specifically target the intricacies of the intestinal flora associated with particular diseases, we unlock the potential to elevate the efficacy of probiotic therapies. The selective and specific nature of probiotic strains opens up new dimensions in the field of personalized medicine. Tailoring probiotics based on the unique requirements of each disease allows us to optimize therapeutic outcomes, providing individuals with targeted relief and contributing to the advancement of precision probiotic interventions for improved gut health and overall well-being.
Probiotic colonization
Successful colonization of the gastrointestinal tract with probiotics can greatly influence the efficiency of oral probiotic delivery. The colonization of probiotics in the gastrointestinal tract can firstly improve the function of the gastrointestinal tract by regulating the balance of the gastrointestinal flora and inhibiting the growth of harmful bacteria [118]. Secondly, after colonization, probiotics can activate the immune system and enhance the immune function of the body. They can regulate the proliferation of T-cells and promote the production of anti-inflammatory cytokines and immunoglobulins, thus improving the body’s resistance to disease [119]. They also form a biological barrier against harmful bacteria and toxins.
Successful colonization of probiotics requires strains that can resist the harsh intestinal environment, have suitable surface structures to attach to the intestinal epithelium or mucus, and produce metabolic molecules to mediate colonization resistance to other intestinal competitors. Intestinal epithelial cells secrete mucins to avoid pathogenic bacteria, and several Lactobacillus proteins have been shown to promote this adhesion, exhibiting surface adhesins that contribute to attachment to the mucus layer [120]. They also invoke mild immunogenicity to build immune tolerance, promote efficient intercellular communication to form symbiotic relationships, and mediate interactions with the host.TLRs are an evolutionarily conserved family of PRRs that recognize a wide range of microbial components. Probiotics can suppress intestinal inflammation by downregulating TLR expression, secreting metabolites that may inhibit the entry of TNF-α into blood monocytes, and inhibiting NF-κB signaling in enterocytes [121].
However, probiotics are inactivated by harmful bacteria that secrete large amounts of harmful substances. Immune cells sterilize the bacteria by releasing large amounts of reactive oxygen species, while probiotics that cannot adapt to oxidative stress die off. Therefore, nano-armor probiotics can act as an escort before the probiotic reaches the target site, ensuring that the probiotic is successfully anchored in the intestinal epithelium. For example, Cao et al. achieved the complexation of B-SA with Bifidobacterium bifidum under mild conditions by combining Fe/C-based single-atom nanoenzymes (B-SA) with multi-enzyme catalytic function, modifying phenylboronic acid moiety on B-SA, and taking advantage of the supramolecular interactions between boronic acid and bacterial surface polysaccharides-phthalocyanine diols to preserve the activities of the probiotics and the artificial enzymes to a maximum extent and to promote the release of the probiotics at the specific inflammatory sites (Fig. 4c) [122]. This shows that nanoarmor plays a crucial role in the effective utilization of probiotics.
Genetic engineering offers another effective method for targeted delivery. For instance, designing probiotics using genetic engineering techniques allows for the delivery of synthetic antigens into specific environments, such as the tumor microenvironment [123]. While promising, the toxicity and interactions of engineered probiotics with the complex microflora require further investigation.
Improving the efficiency of probiotics in targeting the gut and regulating gut microflora is crucial for their application. Considerations include tolerance in the gastrointestinal tract, adsorption to the intestinal mucosa, and the stability and toxicity of delivery vectors [104, 124]. Addressing these challenges is key to unlocking the full potential of probiotics in promoting gut health and preventing diseases. The intricacies of individual patient responses pose a significant challenge to the precision of probiotic-based therapies. The dynamic nature of patients’ responses to the same probiotic strains creates hurdles in the application of probiotics with consistent efficacy. Researchers at the Weizman Institute of Science have shed light on the substantial variations in the colonization of commonly used oral probiotic supplements within the human gut, emphasizing the need for a more tailored approach [67].
The genetically engineered E. Coli Nissle 1917 is one of the most well-established strains of probiotics currently under study, which can maintain the stability of the intestinal environment by enhancing the mucosal barrier, regulating host cytokines and participating in the regulation of intestinal flora [125]. Therefore, according to relevant studies, it plays a significant role in managing inflammatory bowel disease and serving as an adjuvant in antitumor therapy [126, 127]. Bifidobacterium adolescentum S2-1outcompetes Porphyromonas gingivalis by readily utilizing vitamin K and other essential growth factors, thus preventing infections through nutrient limitation, currently, it is primarily used for treating chronic inflammation and alleviating pain following fractures [128, 129, 130]. Lactobacillus and Bifidobacterium also interact with proteins that regulate the cell cycle and inhibit the proliferation of cancer cells, which are often intrinsically resistant to apoptosis [131]. As a result, numerous studies have demonstrated its therapeutic effects on gastric inflammation and cancer [132]. Bifidobacterium infantis subspecies 157 F, a novel strain of Bifidobacterium infantis, has been found to produce acetate, which combats Shiga toxin in E. coli O157:H7. Additionally, it has been shown to inhibit inflammation and promote intestinal homeostasis [133, 134].
Additionally, Lactobacillus reuteri has been found to safeguard keratin-forming cells by producing antimicrobial agents that inhibit the growth of Staphylococcus aureus [135]. It is now widely used in the treatment of cancer, gastrointestinal disorders, and psychiatric disorders [136–138]. Lactobacillus sakei JD10, isolated from the lungs, inhibits pathogenic bacteria proliferation, stimulates host immune defense [139]. Diale et al. demonstrated the potential of BLISs from Bacillus paracasei MHSD3 to resist the proliferation of Staphylococcus aureus, Staphylococcus epidermidis, Staphylococcus putrefaciens, and Escherichia coli [140]. Pivotal role for the health of the intestinal environment [141]. Probiotic microorganisms confer various health benefits to hosts and produce a wide array of beneficial substances to regulate organismal health. Lactobacillus plantarum has been found to synthesize folic acid in the presence of p-aminobenzoic acid (pABA), which also inhibits fungal colonization [142, 143]. Another study observed that Bifidobacterium strains isolated from the intestines of human infants produce indole-3-lactic acid can be used to prevent or treat pediatric diseases [144, 145]. Lactobacillus bulgaricus subspecies, Lactobacillus mucilaginosus, Lactobacillus fermentum and Lactobacillus plantarum CRL 2130 naturally synthesizes riboflavin to reduce inflammation and prevent the development of cancer [146].
Probiotics can positively modulate intestinal factor levels, thus mitigating intestinal barrier permeability. Studies utilizing Lactobacillus rhamnosus strain GG and Lactobacillus plantarum 299v demonstrated their ability to prevent E. coli adherence to human colon cells [147]. Most of them have a regulatory effect on the normal functioning of the immune system [148]. Additionally, 10-hydroxycis-12-octadecenoic acid (HYA) produced by Lactobacillus plantarum inhibits TJ permeability by modulating TNF receptor 2 expression, which has been found to manage the composition of the fecal flora and to prevent and treat irritable bowel syndrome, cancer, coronary heart disease, and certain gastrointestinal symptoms based on the results of the current study [149, 150]. The potential of Bifidobacterium dentium N8 in preserving the intestinal barrier was investigated using human colorectal cancer adenocarcinoma cells (Caco-2) as a model. B. dentium N8 up-regulated TJ protein expression, down-regulated inflammatory markers, inhibited Caco-2 permeability, and increased TEER levels, thereby reducing inflammation and enhancing intestinal barrier integrity.
Another study highlighted the role of surface layer proteins (Slps) from various lactic acid bacteria in maintaining intestinal barrier robustness. These findings underscore the crucial role of probiotics in intestinal barrier maintenance [151, 152]. Addressing these unavoidable differences in patient responses requires a multifaceted approach [153]. One avenue is to minimize differential therapeutic effects by designing probiotic oral medications with comprehensive risk assessments tailored to specific populations. This approach acknowledges the diversity in individual responses and aims to optimize probiotic interventions based on the unique characteristics of each patient.
The consideration of individual differences should be a paramount concern, especially when evaluating changes in drug efficacy. Strict adherence to the principle of randomization in clinical trials becomes essential to account for the vast variability in patient responses [154, 155]. As the application of probiotics moves beyond laboratory settings, recognizing and accommodating the diverse real-world factors influencing patient responses, including living environment, work and study pressures, and dietary habits, becomes pivotal.
While numerous studies are still in the laboratory stage, translating these findings into clinical trials has faced challenges due to the considerable individual variations observed [156]. The stability of laboratory environments and controlled diets in animal models contrasts with the diverse and dynamic conditions experienced by patients in clinical research phases. This divergence significantly impacts the accuracy of probiotic therapy outcomes. In the quest for precision and efficacy in probiotic therapies, an integrated approach that accounts for individual variability, risk assessments, and strict adherence to randomization principles is crucial [157]. As research progresses, a deeper understanding of these complexities will pave the way for more personalized and effective probiotic interventions, navigating the intricacies of individual patient responses with greater precision (Table 1).
Table 1.
Role and mechanism of different probiotics
| Probiotic strain | Mechanisms | Efficacy | References |
|---|---|---|---|
| E. Coli Nissle 1917 | Enhancement of the mucosal barrier, modulation of host cytokines, involvement in the regulation of intestinal flora | Inflammatory bowel disease and antitumor adjuvant therapy | [125, 126, 127] |
| Bifidobacterium adolescentis S2-1 | Competes with Porphyromonas gingivalis for beneficial growth factors such as vitamin K | Chronic colitis and attenuates fracture-induced systemic sequelae | [128, 129, 130] |
| Bifidobacteria and Lactobacilli | activation of pro-caspases and downregulation of the anti-apoptotic Bcl-2 and upregulation of pro-apoptotic Bax proteins | Gastrointestinal inflammation and carcinogenesis | [131, 132] |
| Bifidobacterium longum subsp. infantis 157 F | Produces acetate against E. coli O157:H7 Shiga toxin | suppress inflammation, improves intestinal barrier function and increases acetate production | [133, 134] |
| Lactobacillus reuteri |
Produces antimicrobial agents against Staphylococcus aureus Blocks the growth of lung pathogens |
Anti-tumor, prevent diarrhea and relieve depression | [135, 136–138] |
| Lactobacillus sakei JD10 | Produce carnosine to impede the growth of lung pathogens | Fight against pathogenic infection and regulate the host immune function | [139, 158] |
| Bacillus paranthracis MHSD3 | Produces BLISs against Staphylococcus aureus, Staphylococcus epidermidis, Staphylococcus hemolytic, and Escherichia coli and E. coli BLISs | The improvement of gastrointestinal tract health | [140, 141] |
| Lactiplantibacillus plantarum | Production of folic acid from p-aminobenzoic acid (pABA) | Fungal growth inhibition and mycotoxin removal. | [142, 143] |
| Bifidobacterium breve, and Bifidobacterium bifidum | Generation of Indole-3-lactic acid | the prevention/treatment of paediatric pathologies | [144, 145] |
| Lactobacillus delbrueckii subsp. bulgaricus, L. mucosae, L. fermentum, and L. plantarum CRL 2130 | Naturally, synthesize riboflavin | Prevent intestinal inflammation and cancer | [146] |
| Lactobacillus rhamnosus strain GG and Lactobacillus plantarum 299v | Prevents E. coli from adhering to human colon cells | Immune-Modulating effects | [147, 148] |
| L. Plantarum |
Produce HYA ↑ ZO-1, occludin, claudin-1 |
Management of the fecal flora composition, prevention and treatment of irritable bowel syndrome, cancer, coronary heart disease, and certain gastrointestinal symptoms | [149, 150] |
Please check and confirm if the inserted citation of Table 1 is correct. If not, please suggest an alternate citation. Please note that table should be cited in text.Thank you for your constructive suggestions.We have checked and corrected the relevant content citations that were missing from the text. And we have also made changes in the original text.
Mechanisms for improved therapeutic efficacy
Over the past three decades, substantial strides have been achieved in unraveling the properties, classification, distribution, and nutritional significance of probiotics. These microscopic powerhouses have emerged as pivotal agents, wielding significant biological functions, including fortifying immune responses and demonstrating anti-tumor properties [159, 160]. While the benefits of probiotics for human well-being are well-established, the current focus of researchers lies in the comprehensive and efficient application of these remarkable entities [161, 162].
The evolution of engineered probiotics stands out as a beacon of progress, promising enhanced delivery and targeting efficiency [163, 164]. Ingeniously employing various types of nanoparticles to encapsulate probiotics represents a groundbreaking approach [165, 166]. This innovative strategy not only augments the abundance and biodiversity of intestinal probiotics but also strengthens their resilience against the challenging pH conditions within the gastrointestinal tract. Furthermore, the diversification of probiotics through distinct encapsulation methods has emerged as a pioneering avenue. This nuanced approach allows for the tailoring of probiotics, catering to their application in the treatment of diverse diseases. The exploration of such multifaceted techniques showcases the commitment of researchers to transcending traditional boundaries and maximizing the potential of probiotics in the realm of human health.
As we navigate the forefront of probiotic research, the refinement of delivery mechanisms and the strategic use of encapsulation technologies illuminate a path toward a more holistic and effective integration of probiotics into therapeutic strategies. The quest for a deeper understanding and the application of these advancements underscores the dedication of the scientific community to elevate the discourse on probiotics, ushering in an era of precision and efficiency in their utilization for human benefit.
Engineered nano-armor-probiotics
In the cutting-edge field of medical interventions, the use of engineered probiotics encapsulated in nanoparticles is a beacon of innovation, marking a pivotal stride toward enhancing precision, efficacy, and targeted therapeutic delivery [167]. Harnessing synthetic biology principles, engineered probiotics are strategically enclosed in nanoparticles, providing a protective shield that facilitates their passage through the intricate gastrointestinal environment [168]. This encapsulation augments probiotic abundance, ensuring viability despite extreme pH conditions. Additionally, it enables a controlled, slow-release mechanism, optimizing therapeutic impact with tailored nanocarriers [78, 169]. Advancements include double-layer coatings, reinforcing probiotic encapsulation, and ensuring acid resistance [170, 171]. The vast potential applications of these capsules offer a transformative approach to treating various intestinal diseases while mitigating side effects associated with traditional drugs [74]. In conclusion, the encapsulation of engineered probiotics within nanoparticles represents a groundbreaking frontier in therapeutic precision, promising to revolutionize medical interventions for the betterment of human health.
Types of nano-armor-probiotics
Oral medications, as a noninvasive and needle-free treatment modality, boast low temporal and financial costs alongside high patient acceptance [172]. Nevertheless, the formidable gastrointestinal barrier significantly impedes the bioavailability of oral drugs [173]. With the relentless progression of nanotechnology, a plethora of studies have showcased the potential of engineered oral nanomedicines in surmounting the gastric acid barrier, effectively traversing the intestinal mucus and epithelial barriers, and enhancing the drug’s solubility, safety, targeting, and half-life to facilitate successful delivery to the intended site [174, 175]. The efficacy of most oral solid drug delivery systems is intricately linked to particle size [176, 177]. Thanks to their diminutive dimensions and expansive specific surface area, drug nanoparticles exhibit heightened solubility and consequently improved bioavailability [178]. Nanoparticles fashioned from natural and synthetic polymers have garnered significant attention due to their capacity for targeted drug delivery, enhancement of bioavailability, provision of controlled release of single doses, and mitigation of drug degradation by endogenous enzymes [179]. Encapsulation of probiotics within various types of nanoparticles holds promise for delivering them to targeted sites, potentially maximizing the preservation of probiotic bioactivity and retention time in the gut, thereby addressing existing challenges associated with probiotic delivery into the body [108]. The utilization of nanoparticles harbors immense potential for the oral delivery of probiotics.
Inorganic nano-armor-probiotics
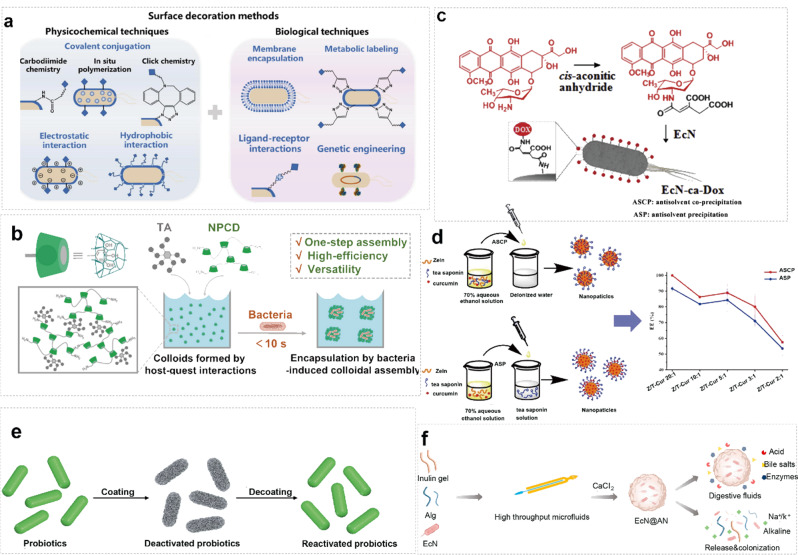
The encapsulation of probiotics within inorganic nanoparticles represents a promising avenue in the field of biotechnology. By leveraging the unique properties of inorganic nanoparticles, such as their tunable size, surface chemistry, and stability, we can enhance the viability, stability, and targeted delivery of probiotic microorganisms [68]. Inorganic nanoparticles offer several advantages for encapsulating probiotics [180, 181]. Their high surface area-to-volume ratio allows for efficient loading of probiotic cells, while their inert nature protects against harsh environmental conditions and enzymatic degradation in the gastrointestinal tract [182]. Additionally, inorganic nanoparticles can be engineered to possess specific surface functional groups, enabling targeted delivery to the desired site within the body [183]. Furthermore, inorganic nanoparticles can serve as carriers for controlled release formulations, allowing for sustained and prolonged release of probiotics, thereby maximizing their therapeutic efficacy [184]. Moreover, the use of inorganic nanoparticles facilitates the development of novel delivery systems that can overcome the challenges associated with conventional probiotic formulations, such as poor stability, low viability, and limited bioavailability.
Au NPs
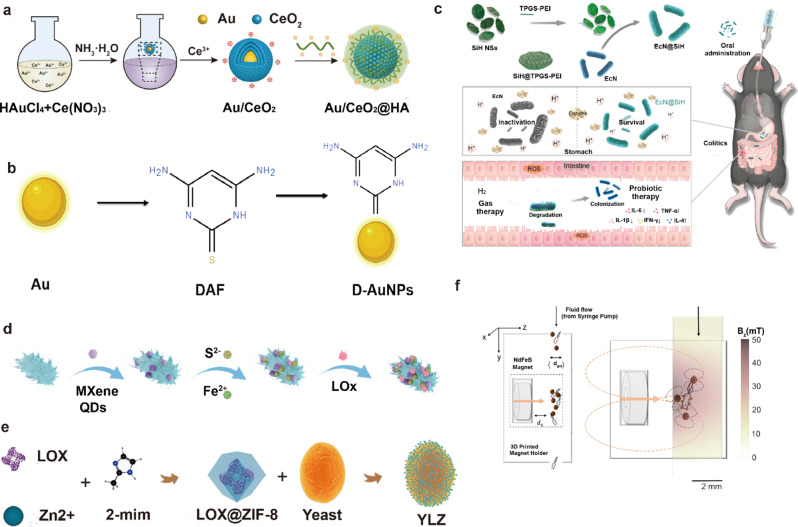
Due to their multiple surface compatibility, AuNPs tend to interact with biomolecules [191]. Various molecules with functional groups can be readily bound with high affinity to the surface of AuNPs. The capping agent around the AuNPs can be replaced by other functional thiols or adsorbed ligands through ligand exchange reactions. External functional groups will displace existing ligands on the AuNPs at equilibrium. Li et al. have reported that incorporating a cerium dioxide system as a core enhances the catalytic activity of superoxide dismutase (SOD) and catalase (CAT), effectively scavenges reactive oxygen species (ROS), and mitigates inflammation in mice (Fig. 5a) [185]. Au nanoparticles can be modified with polysaccharides to support their colonization in the gut, and Au nanoparticles can also be altered with polysaccharides to enable the release of engineered probiotics after targeting the gut to exert their excellent stability and anti-inflammatory properties (Fig. 5b) [186]. Due to widespread concerns about the toxicity of gold nanoparticles, the use of gold nanoparticles to deliver probiotics should be concerned about whether they will affect the activity of the probiotics as well as the safety and metabolic pathways of both in vivo.
Fig. 5.
a schematic illustration of the synthesis progress of Au/CeO2@HA [185]. © 2023 The Authors. Publishing services by Elsevier B.V. on behalf of KeAi Communications Co. Ltd. b Schematic illustration of synthesis progress of D-AuNPs [186]. c Schematic Illustration Showing the Construction Procedures of EcN@SiH System and Its Applications in Probiotic/Gas Dual-Mode Therapy for the Treatment of IBD [187]. Copyright ©2023 American Chemical Society.d Schematic illustration of P-bioHJ formation [188]. © 2024 Wiley-VCH GmbH. e Construction of the Yeast@LOX@ZIF-8 (YLZ) bioreactor [189]. © 2021 Elsevier Ltd. All rights reserved. f Synthetic magnetized BL21(DE3) E. coli cells expressing mScarlet to facilitate downstream quantification using flow cytometry. The color change in the tube in the right inset indicates the simulated magnetic field strength at a distance of z = 10–13 mm from the surface of the N52 magnet. The orange arrows indicate the direction of magnetization of the permanent magnet, while the orange dashed ellipses indicate the magnetic lines of force generated by the external magnet as well as the micro magnets inside the tube. The coordinate system is oriented so that gravity acts in the + y direction and the center of the magnet is located at (y = 40, z = 0) [190]. © 2021 Wiley-VCH GmbH
Silica nanoparticles
Silica nanoparticles offer numerous advantages, including high surface modification, biocompatibility, enzyme stability, and cost-effectiveness [192, 193]. Surface modification of probiotic nanocarriers with silica is a viable strategy to uphold probiotic bioavailability. Shi et al. pioneered the development of an inorganic nanomaterial-based probiotic system using copolymer-modified two-dimensional H-silylene nanomaterials, known as SiH@TPGS-PEI [187]. This innovation involved forming a protective layer on the bacterial surface, effectively shielding probiotics from erosion in the acidic gastric environment and enzymatic degradation in the stomach. Consequently, this approach achieved efficient intestinal delivery of probiotics via conventional oral administration. Furthermore, the SiH@TPGS-PEI layer exhibited rapid self-adaptive degradation in a neutral to weakly alkaline intestinal environment, releasing encapsulated bacteria and hydrogen. Compared to uncoated probiotics, E. coli Nissle 1917@SiH (ECN@SiH) bacteria demonstrated significantly prolonged survival time, enhanced intragastric survival, and improved intestinal colonization. Moreover, hydrogen production synergistically modulated the intestinal microenvironment, promoting an anti-inflammatory state and ameliorating colitis symptoms (Fig. 5c). However, the long-term biosafety of the rapidly degradable SiH@TPGS-PEI layer requires thorough investigation regarding its interaction with biomaterials in vivo, along with considerations for large-scale stable production.
Quantum dots
Quantum dots, ultra-small nanoparticles ranging from 1 to 10 nm, are commonly employed for cell imaging and drug delivery [194]. Huang’s team developed a probiotic heterojunction (P-bioHJ) composite, which is composed of Lactobacillus rhamnosus (LG) and MXene quantum dots (MQDs)/FeS heterojunction (Fig. 5d). The material has good antimicrobial properties, anti-biofilm properties, biosafety, and antioxidant properties [188]. Fabrication of ultrasmall nano-microbial nanocarriers can simplify the oral drug delivery process. However, the potential of utilizing such tiny nanoparticles for targeted delivery of engineered probiotics at deeper levels needs further validation. Meanwhile, the size of probiotics poses a challenge for encapsulation, suggesting that a synergistic approach combining the ultra-small size of nanoparticles with their unique physicochemical properties is the next frontier in probiotic nanocarrier encapsulation research.
Metal-organic frameworks (MOFs)
Encapsulating probiotics within MOFs presents an innovative approach in the realm of biotechnology. MOFs, known for their high surface area, tunable porosity, and versatile chemical properties, offer a promising platform for enhancing the stability, viability, and targeted delivery of probiotic microorganisms [195]. MOFs possess several key characteristics that make them well-suited for encapsulating probiotics. Their large surface area and porous structure allow for efficient loading of probiotic cells, ensuring high encapsulation efficiency. Additionally, the tunable pore size and surface chemistry of MOFs enable precise control over the release kinetics of probiotics, facilitating sustained and controlled release formulations. Furthermore, MOFs can protect encapsulated probiotics against harsh environmental conditions, such as acidic pH and enzymatic degradation in the gastrointestinal tract, thereby enhancing their stability and viability [196]. Moreover, the biocompatibility of MOFs ensures minimal cytotoxicity and immunogenicity, making them suitable carriers for the oral delivery of probiotics [197].
Qu et al. proposed, for the first time, the modification of Saccharomyces cerevisiae using lactate oxidase (LOX) encapsulated in a MOF material known as ZIF-8 to construct a Yeast@MOF bioreactor (YLZ) (Fig. 5e) [189]. This innovative approach effectively inhibits metabolically heterogeneous tumors by competitively depleting glucose and lactate in the tumor microenvironment. The utilization of MOFs for encapsulating probiotics introduces exciting possibilities for advancing probiotic formulations with enhanced therapeutic efficacy and targeted delivery. Leveraging the unique properties of MOFs enables researchers to overcome the limitations associated with conventional probiotic formulations, thereby paving the way for the development of novel probiotic-based therapeutics for diverse health applications.
Others
In addition to SiO2, Au, Quantum dots, and MOFs, various other inorganic nanoparticles hold the potential for encapsulating probiotics. These nanoparticles encompass a wide range of materials, each offering unique advantages and characteristics for probiotic delivery and protection. One example is calcium phosphate nanoparticles [198], which have been explored for their biocompatibility and ability to mimic the mineral composition of bone. These nanoparticles could provide a stable environment for probiotics while offering controlled release capabilities. Similarly, iron oxide nanoparticles have gained attention for their magnetic properties, allowing for targeted delivery and imaging of probiotics within the body (Fig. 5f) [190]. Their biocompatibility and ease of functionalization make them promising candidates for encapsulating and delivering probiotics to specific sites.
Zinc oxide nanoparticles have also shown promise due to their antimicrobial properties and biocompatibility [199]. These nanoparticles could not only protect encapsulated probiotics from degradation but also enhance their therapeutic efficacy through synergistic antimicrobial effects. Furthermore, titanium dioxide nanoparticles, known for their photocatalytic properties, could potentially be utilized for targeted delivery and controlled release of probiotics under specific light conditions. Overall, the exploration of various inorganic nanoparticles beyond SiO2, Au, MOFs, and Quantum dots offers a diverse array of options for encapsulating and delivering probiotics, each with its own unique set of advantages and applications in biomedicine. Continued research in this area holds the potential to further advance the field of probiotic therapy and improve health outcomes.
Polymeric nano-armor-probiotics
Encapsulating probiotics within polymeric nanoparticles represents a versatile and promising strategy in biotechnology. Various types of polymers can be utilized for this purpose, each offering unique advantages in terms of encapsulation efficiency, stability, and targeted delivery. Commonly employed polymers for encapsulating probiotics include natural polymers such as chitosan, alginate, and gelatin, as well as synthetic polymers like poly(lactic-co-glycolic acid) (PLGA), polyethylene glycol (PEG), and polylactic acid (PLA) [200, 201]. These polymers can be tailored to achieve specific properties such as biocompatibility, biodegradability, and controlled release kinetics.
The encapsulation of probiotics within polymeric nanoparticles can be achieved through various methods, including emulsion/solvent evaporation, nanoprecipitation, and electrostatic interactions. In emulsion/solvent evaporation, the probiotics are dispersed within a polymer solution, which is then emulsified in an organic solvent. Subsequent solvent evaporation leads to the formation of polymeric nanoparticles encapsulating the probiotics [202]. Nanoprecipitation involves the rapid mixing of a polymer solution with a non-solvent, resulting in the precipitation of polymer nanoparticles entrapping the probiotics [203]. Electrostatic interactions can be utilized for the encapsulation of probiotics within polyelectrolyte nanoparticles, where the opposite charges of the polymer and probiotics facilitate their association and encapsulation (Fig. 6a) [204].
Fig. 6.
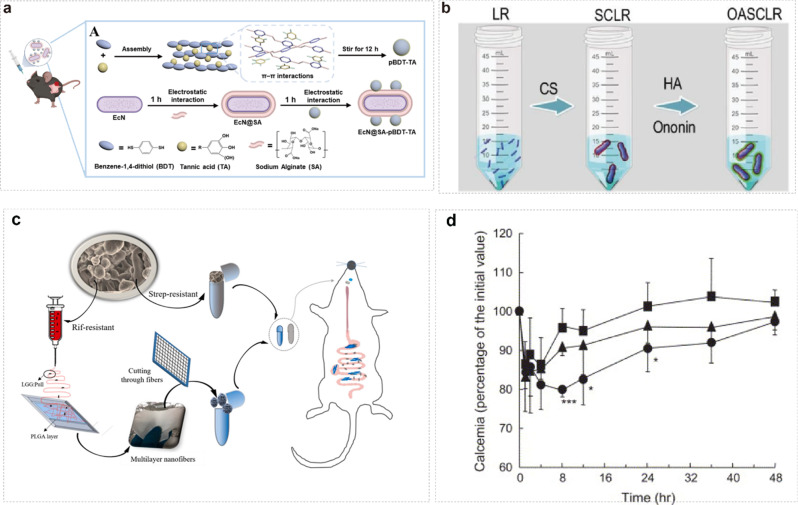
a schematic representation of pBDT-TA nanoparticles prepared by π-π interaction with BDT and TA. Escherichia coli Nissle 1917 (EcN) coated the sodium alginate (SA) layer and pBDT-TA onto the SA surface by electrostatic interactions [204]. Copyright ©2024 American Chemical Society. b Schematic representation of the formation of SCLR and OASCLR [205]. Copyright © 2022, © The Author(s) 2022. Published by Oxford University Press on behalf of China Science Publishing & Media Ltd. c Schematic representation of the procedure of loading LGG into nanofibres and filling the fibers with either Streptococcus-resistant spray-dried LGG (StrepR) or Rif-resistant LGG (RifR) (or opposite combinations) in capsules for a schematic representation of the procedure performed to perform a competition study between the two forms of LGG dosed into individual rats [206]. © 2021 Elsevier Ltd. All rights reserved. d Blood calcium levels after administration of elcatonin-loaded nanospheres (100 IU/kg) to the lungs of male guinea pigs (6 weeks). (■) Elcatonin solution; (▲) uncoated PLGA nanospheres; (●) chitosan-coated PLGA nanospheres. Data are expressed as mean ± S.D. (n = 5). p < 0.001, *p < 0.05 vs. Elcatonin solution [207]. Copyright © 2004 Elsevier B.V. All rights reserved
Once encapsulated within polymeric nanoparticles, probiotics are protected from harsh environmental conditions such as low pH and enzymatic degradation in the gastrointestinal tract. The polymeric matrix acts as a barrier, shielding the probiotics and enhancing their stability during transit through the digestive system. Additionally, polymeric nanoparticles can provide a controlled release of probiotics, allowing for sustained delivery and prolonged therapeutic effects. Several studies have demonstrated the efficacy of polymeric nanoparticles for encapsulating and delivering probiotics. For example, chitosan nanoparticles have been utilized for the oral delivery of probiotics, enhancing their survival and colonization in the gut (Fig. 6b) [205]. Similarly, PLGA nanoparticles have been employed for the targeted delivery of probiotics to specific sites within the gastrointestinal tract, improving their therapeutic efficacy in treating various gastrointestinal disorders (Fig. 6c) [206].
Yoshiaki K et al. have successfully developed viscous propylglycolide/ethylglycolide copolymer (PLGA) nanospheres for oral peptide delivery through surface modification with chitosan (CS) [207]. Their research demonstrated that these modified nanoparticles exhibit enhanced mucosal adhesion and facilitate peptide delivery to the lungs. Furthermore, they proposed a novel mechanism wherein CS enhances drug uptake by modulating tight junctions between lung epithelial cells (Fig. 6d). These findings underscore the potential application of CS-modified PLGA nanoparticles in improving peptide delivery via the lung pathway. Indeed, it is well-established that nanoparticles crafted from modified polymers hold promise in significantly enhancing therapeutic outcomes for specific diseases.
Polymeric nanoparticles offer a versatile and effective means of encapsulating and delivering probiotics, with potential applications in various fields including medicine, food, and agriculture [208]. Continued research in this area holds promise for the development of advanced probiotic formulations with improved stability, efficacy, and targeted delivery capabilities.
Lipid-based nano-armor-probiotics
Encapsulating probiotics within lipid-based nanoparticles holds tremendous potential for revolutionizing various fields, including biomedicine, food science, and agriculture. The versatility and efficacy of lipid-based nanoparticles make them a promising tool for enhancing probiotic delivery and efficacy. Expanding on the types of lipid-based nanoparticles, it’s worth noting that each type offers distinct advantages and applications. Liposomes, for instance, are well-known for their ability to encapsulate both hydrophilic and hydrophobic compounds, making them ideal carriers for a wide range of probiotic strains with different physicochemical properties [209]. Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) offer improved stability and controlled release capabilities, making them suitable for long-term probiotic delivery and sustained therapeutic effects [210]. Lipid-polymer hybrid nanoparticles combine the advantages of lipids and polymers, allowing for enhanced versatility and customization in probiotic delivery systems.
Moreover, the methods employed for encapsulating probiotics within lipid-based nanoparticles can be further diversified and optimized. Advanced techniques such as solvent evaporation, supercritical fluid technology, and electrospinning offer precise control over nanoparticle size, morphology, and encapsulation efficiency, thereby enhancing probiotic stability and bioavailability. Furthermore, the application of lipid-based nanoparticles extends beyond oral delivery. These nanoparticles can also be tailored for targeted delivery to specific sites within the gastrointestinal tract or other regions of the body, offering personalized therapeutic solutions for various health conditions (Fig. 7) [211]. In addition to their protective role against gastric acid and enzymatic degradation, lipid-based nanoparticles can be engineered to enhance probiotic survival and colonization in the gut microbiota. Surface modification with bioadhesive polymers or ligands targeting specific receptors in the gastrointestinal tract can improve nanoparticle retention and facilitate probiotic uptake by intestinal cells [212].
Fig. 7.
Strategies for efficient oral delivery via lipid-based nanocarriers [211]. a Improvement of the stability of nanocarriers in the harsh gastrointestinal environment that includes enzymes, salts, and microbiota. b Enhancement of mucoadhesion. Nanocarriers remain adhered to the mucus and thereby their residence time is increased. The cargo molecule may be released. c Enhancement of immunodiffusion. Nanocarriers diffuse through the mucus, increasing the chances for their interaction with the epithelium. d Inhibition of P-glycoprotein. Drug efflux may be decreased, increasing drug-effective absorption. e Active targeting. f Enhancement of lymphatic transport, transport pathway that avoids first pass effect. Copyright©2021, Controlled Release Society
While lipid-based nanoparticles offer numerous advantages for probiotic delivery, it’s important to address challenges such as scalability, stability during storage, and variability in drug loading efficiency. Collaborative efforts between researchers, industry stakeholders, and regulatory agencies are essential to overcome these hurdles and translate lipid-based probiotic delivery systems into clinically viable products. Lipid-based nanoparticles represent a versatile and promising platform for encapsulating and delivering probiotics, with wide-ranging applications in medicine, nutrition, and agriculture. Continued research and innovation in this field hold the key to unlocking the full potential of lipid-based probiotic delivery systems and improving human health and well-being.
Composite nano-armor-probiotics
Encapsulating probiotics within composite nanoparticles represents an innovative approach in biotechnology. Composite nanoparticles are defined as nanoscale structures composed of two or more distinct materials, typically combining the properties of each component to achieve desired functionalities [213]. Composite nanoparticles can be categorized into various types based on their composition and structure. Some common types include polymer-metal composites, polymer-ceramic composites, and lipid-polymer hybrid composites. Each type offers unique advantages for probiotic encapsulation and delivery.
To encapsulate probiotics within composite nanoparticles, several methods can be employed depending on the nanoparticle composition. For example, in polymer-metal composites, probiotics can be dispersed within a polymer matrix containing metal nanoparticles, such as silver or gold, through techniques like solvent evaporation or nanoprecipitation. In lipid-polymer hybrid composites, probiotics can be encapsulated within lipid nanoparticles embedded in a polymer matrix, utilizing methods like emulsification or solvent diffusion. Once encapsulated within composite nanoparticles, probiotics are shielded from harsh environmental conditions in the gastrointestinal tract, such as gastric acid and enzymatic degradation. The composite structure offers enhanced stability and protection, ensuring the survival and viability of probiotics during transit through the digestive system. Additionally, composite nanoparticles can provide a controlled release of probiotics, enabling sustained delivery and prolonged therapeutic effects.
Several studies have demonstrated the efficacy of composite nanoparticles for encapsulating and delivering probiotics. For instance, polymer-metal composites have been utilized for oral delivery, enhancing probiotic survival and colonization in the gut (Fig. 8a) [214]. Lipid-polymer hybrid composites have also shown promise in targeted delivery to specific gastrointestinal sites, improving therapeutic efficacy in treating related disorders. The utilization of proteins and polysaccharides in crafting nano-delivery carriers epitomizes a pivotal approach to augmenting the stability and bioavailability of active substances. Of particular note is the ongoing research by Yang et al., exploring the utilization of zein (Z), derived from corn alcohol-soluble protein, to fabricate Z nanoparticles (Z NPs) in conjunction with chitosan—a non-toxic, bacteriostatic, biocompatible, and biodegradable material renowned for its film-forming properties. Furthermore, goat casein insoluble aggregates serve as cross-linking agents in the preparation of supramolecular structured peptides, which, when complexed with Z and chitosan, give rise to a delivery carrier encapsulating Lutein (Lut), exemplifying the versatility of composite nanocarriers (Fig. 8b) [215].
Fig. 8.
a schematic illustration of the development of engineered probiotics for multipronged management of IBD (a) and the characterization of EcN-Fh [214]. Copyright©2024, American Chemical Society. b Preparation of carboxymethyl konjac glucan-chitosan (CMKGM-CS) nanogels and their biological effects [215]. © 2023 Elsevier Ltd. All rights reserved. c Schematic diagram of in vitro digestion behavior of alginate hydrogel beads coated W1/O/W2 double emulsions [216]. © 2022 Published by Elsevier Ltd
This composite nanocarrier confers multifaceted delivery advantages. Presently, Zhan et al. are employing carboxymethyl konjac glucan-chitosan (CMKGM-CS) nanogels to stabilize W1/O/W2 double emulsions, thereby encapsulating and safeguarding probiotics within the inner aqueous phase. Concurrently, the pH responsiveness of an alginate-calcium system is harnessed to construct hydrogel beads (ACGs) encapsulating probiotic-containing double-emulsion droplets, augmenting the sustained release capability of probiotics within the intestinal milieu (Fig. 8c) [216]. These endeavors underscore the intrinsic advantages of composite nanocarriers in probiotic delivery, effectively addressing the challenges associated with probiotics’ vulnerability to the intestinal environment and their low delivery efficiency. Additionally, composite nanoparticles can offer versatility and customization in probiotic delivery systems, allowing for tailored solutions to specific health conditions.
However, challenges such as complex synthesis processes, potential toxicity of certain materials, and variability in nanoparticle properties may need to be addressed. Additionally, thorough characterization and optimization of composite nanoparticles are essential to ensure safety and efficacy in biomedical applications. Composite nanoparticles present a promising platform for encapsulating and delivering probiotics, with potential applications in medicine, nutrition, and agriculture. Continued research and development efforts are crucial to harness the full potential of composite nanoparticle-based probiotic delivery systems and improve human health outcomes.
Other
Beyond inorganic nanoparticles, polymeric nanoparticles, lipid-based nanoparticles, and composite nanoparticles, there exists a myriad of other nanomaterials with potential for encapsulating probiotics. These alternative nanomaterials offer unique properties and characteristics that may be advantageous for probiotic delivery. One exemplary class of nanomaterials comprises carbon-based nanoparticles, encompassing carbon nanotubes, graphene, and fullerenes. Zhang et al. have documented Cu2+-doped carbon-based nanoparticles (Cu-cys CBNPs), which have significantly augmented CDT efficacy by depleting GSH via redox reactions while concurrently generating -OH within cells, thereby synergistically increasing intracellular ROS levels [217].
Meanwhile, Shi’s research team developed an oral probiotic delivery system based on calcium tungstate microgel (CTM) [218]. CTM selectively disrupts the ecological niche occupied by the abnormally expanding Enterobacteriaceae during colitis to promote probiotic colonisation. In addition, calcium-binding proteins, which are highly expressed in colitis, can efficiently extract calcium from CTM and release tungsten by displacing molybdenum in molybdenum enzyme to inhibit Enterobacteriaceae bacteria without affecting the delivered probiotics, effectively improving probiotic delivery and colonisation.
In addition, Xue et al. have also recently designed a bilayer polysaccharide hydrogel (DPH), which is a bilayer structure consisting of a carboxymethyl cellulose (CMCL) supramolecular inner layer and a double alginate alginate (DAA) cross-linked carboxymethyl chitosan (CMCS) outer layer [219]. This bilayer structure allows DPH to encapsulate and deliver probiotics in the body in a targeted manner.
nanogels, composed of cross-linked polymer networks, provide controlled release capabilities, facilitating sustained delivery of probiotics. Overall, the exploration of alternative nanomaterials for probiotic encapsulation holds great promise for advancing probiotic therapy and improving health outcomes. Continued research and innovation in this field are essential for unlocking the full potential of nanotechnology in probiotic delivery.
Strategies for encapsulating probiotics
Encapsulation of probiotics offers a promising strategy to address challenges in oral probiotic utilization [108]. However, current techniques face limitations such as low efficiency, toxic cross-linking agents, and complexity. Thus, developing a simpler, more effective strategy for enhancing oral probiotic bioavailability remains a significant challenge. The following provides a succinct overview of probiotic encapsulation technology. Presently, probiotic encapsulation mainly involves bulk and single-cell methods [54]. Bulk encapsulation immobilizes probiotics in micron-sized gel matrices like polysaccharides, proteins, and coated hydrogels. Single-cell encapsulation forms nanomembranes around individual probiotic cells using techniques like layer-by-layer encapsulation and self-assembled coatings.
Polysaccharides, notably alginate, are widely studied for probiotic encapsulation due to their water-absorbent properties and resistance to gastric acid. However, alginate’s large pore size can lead to premature release of probiotics and low stretchability. Protein hydrogel encapsulation confines probiotics within milk proteins, offering robust protection under acidic conditions. Yet, protein denaturation and interactions with probiotic components may affect delivery efficiency.
In single-cell encapsulation, materials such as silica, graphene, polydopamine, and metal polyphenol nanoshells provide biodegradable protection without compromising probiotic viability.
Layer-by-layer encapsulation, using cationic polysaccharides and anionic polymers, shows promise in enhancing probiotic protection and release in the gastrointestinal tract. Challenges remain in adhesion and large-scale implementation. Alternative strategies include self-generated biofilms and cell membrane camouflage. Bacillus subtilis-induced biofilms enhance probiotic adhesion and resistance, while erythrocyte membrane encapsulation offers prolonged circulation with low immunogenicity [220, 221]. Selecting suitable probiotic nanocarrier materials is crucial for stability and protection within the body. Further research is needed to refine encapsulation techniques and optimize delivery for improved therapeutic outcomes.
Surface modification
Surface modification refers to the use of physical, chemical, or biological techniques to coat or modify the surface of an active microbial material to introduce additional target functions that differ from its original properties [228]. Communication between bacteria and interactions between bacteria and their surroundings are closely linked to the structure and characterization of bacterial surfaces. Once the bacterial surface is changed, specific physiological signals and responses can be triggered to manipulate the biological behavior and function of the bacteria. Surface decoration is an important way to achieve improved and innovative material properties, giving new functions and features to the material, which ultimately determines the overall performance of the material (Fig. 9a) [222]. Recently, Liu’s research team took bacterial surface modification as an entry point and constructed polydopamine-based antigen-antibody combination nano-immunoactivators by designing a novel covalent modification strategy, which were further covalently combined with bacterial surfaces to prepare anti-tumour probiotics with triple immune activity (Fig. 10) [229]. The triple-immunoreactive probiotics constructed based on surface modification showed unique advantages in the regulation of tumour microenvironment.
Fig. 9.
a Methods of surface decoration [222]. © 2022 Elsevier B.V. All rights reserved. b Probiotic encapsulation by the bacteria-induced colloidal assembly [223]. Copyright©2023, American Chemical Society. c EcN-ca-DOX was obtained by conjugation of DOX molecules onto EcN [224]. ©2017 Elsevier B.V. All rights reserved. d Flow chart of preparation of nanoparticles by two different methods: antisolvent co-precipitation (M1-ASCP) and antisolvent precipitation (M2-ASP) [225]. © 2022 Elsevier Ltd. All rights reserved. e EcN deactivation by restraining inside mineralized coating and reactivation by removing the coating in response to physiological gastric acid stimulation [226]. © 2023 Wiley-VCH GmbH. f Mass production of EcN@AN with high-throughput microfluidics, gastrointestinal resistance, and on-demand delivery of encapsulated probiotics [227]. Copyright©2024, American Chemical Society
Fig. 10.
Schematic illustration of preparing tumor-resident living immunotherapeutics by decorating bacteria with triple immune nanoactivators [229]. ©2022Wiley-VCH GmbH. a Conjugation of tumor-specific antigen OVA and immune checkpoint inhibitor α-PD-1 to PDA nanoparticles, which are attached to the bacterial surface through in situ precipitation polymerization of dopamine. b Decorated bacteria-mediated reversal of the tumor immunosuppressive microenvironment via repolarization of TAMs, maturation of dendritic cells, and activation of cytotoxic T lymphocytes
Direct electrostatic adsorption
Bacterial cells contain large amounts of proteins, which are composed of amino acids. Amino acids are amphoteric ionized substances that dissociate in solution into positively charged amino groups and negatively charged carboxyl groups. The specific electrically charged nature of bacteria is related to the isoelectric point of the amino acid, the acidity or alkalinity (pH) of the environment in which they live [230]. When the pH is greater than the isoelectric point, bacteria are negatively charged. When the pH is equal to the isoelectric point, the bacteria are uncharged. When pH is less than the isoelectric point, bacteria are positively charged. In most cases, the pH of the environment in which bacteria live is usually greater than their isoelectric point, so their surfaces are usually negatively charged.
Optimal colloidal particles are crafted through the delicate balance of amino-modified poly-β-cyclodextrin and tannins, achieved through a synergy of mutual attraction via host-guest interactions and electrostatic repulsion between colloids. The negatively charged probiotic bacteria are drawn towards the positively charged colloidal particles through electrostatic forces, disrupting this equilibrium and swiftly facilitating the assembly of colloidal particles to encapsulate the probiotic bacteria. Han’s team has demonstrated that through this method, an impressive 97% of bacteria can be encapsulated within colloidal coatings within a mere 10 s, with a remarkable 91% utilization of colloidal particles. Moreover, probiotic bacteria nestled within the colloidal clamshell maintain their ability to proliferate normally, as the host-guest interaction remains dynamic and non-covalent, exhibiting some self-adaptive characteristics (Fig. 9b) [223].
This facile probiotic-induced colloidal encapsulation strategy can be extended to encapsulate other electronegative microorganisms as oral biologics for the treatment of a wide range of diseases. The most typical example of this application is Nissle 1917 (EcN),which can be genetically modified for the secretion of different cytokines, including interleukin 2, superoxide dismutase, etc., in order to target the treatment of specific diseases through the electrostatic adsorption assembly of multiple shells [231, 232]. it can undergo a two-layer bilayer electrostatic assembly technique to encapsulate EcN bacteria in a protective layer of mucosal adherent chitosan (CS) and immunomodulatory hyaluronic acid (HA) to produce HA-CS-EcN.These biomaterials confer resistance to environmental attack on the coated EcN and enhance mucosal adhesion in the gastrointestinal tract [233].
In addition to this, EcN can be genetically engineered to overexpress catalase and superoxide dismutase (EcN-pE) [234]. Chitosan and sodium alginate (potent biofilms) were used to encapsulate EcN-pE by a layer-by-layer electrostatic self-assembly strategy, and it was found that chitosan/sodium alginate encapsulation of ECN-pE (EcN-pE(C/A)2) was effective in alleviating inflammation and repairing epithelial barriers in the colon in different chemically induced IBD models in mice.
Additionally, Xie et al. immobilised Fe3 + on the surface of EcN by electrostatic adsorption, and then formed an Fe-TA network through cross-linking of galloyl and Fe3 + ions. Finally, due to the attraction of the large number of hydrogen bonds between the Fe-TA network and the mGN, a layer of mGN was deposited on top of the Fe-TA layer to form a modified “shield” based on modified prebiotics (Fe-TA@mGN). This strategy provides new insights for exploring the synergistic effects between prebiotics and probiotics, and also offers new ideas for future IBD treatments (Fig. 11) [235]. However, this mutual attraction between charges can be affected by the gastrointestinal tract when entering the in vivo environment, and the encapsulation is not specific, potentially encapsulating pathogenic microorganisms into the colloidal capsules.
Fig. 11.
Schematic illustration of the fabrication of EcN@Fe-TA@mGN and its alleviation process for DSS-induced colitis in mice [235]. Copyright © 2023, American Chemical Society. a Preparation of EcN@Fe-TA@mGN. First, bacterial suspension was stirred with TA and Fe3+ for 60 s to form a Fe-TA network layer on EcN. Then, they were encapsulated by mGN through simple agitation. b Probiotics exhibited a superior resistance to gastric acids and bile salts after being armed with an Fe-TA@mGN “shield” and remained intact in the upper digestive tract. Once EcN@Fe-TA@mGN reached the colon, the mGN layer would be degraded by gut microbiota and metabolized to SCFAs to synergize with EcN for the alleviation of colitis. The exposed Fe-TA layer would aid the colonization of EcN in colon for the realization of sustainable functions
Chemical modification
Surface modifications in addition to the electrostatic interactions covered above, there are also surface modifications that utilize chemical methods. These include amidation reactions between amino and carboxyl groups, in situ polymerization, click chemistry, and probiotics based on chemical modifications. The amidation reaction, facilitated by carbodiimide with the assistance of 1-(3-Dimethylaminopropyl)-3-ethyl carbodiimide hydrochloride (EDC)/N-hydroxysuccinimide (NHS), entails the chemical binding of amino and carboxyl groups derived from bacterial membrane proteins and targets. Li et al. have pioneered the development of drug-conjugated bacterial microsomes (ECN-CA-DOX) utilizing acid-unstable linkers, enabling targeted accumulation within bacteria and acid-responsive release of anticancer drugs within tumors. Adriamycin (DOX) undergoes carboxylation by cis-anhydride and subsequent covalent linkage to the amino group of the surface membrane protein of Escherichia coli Nissel 1917 (ECN) via a carbodiimide reaction (Fig. 9c). The heightened bacterial-mediated drug accumulation within tumors, coupled with efficient drug release triggered by bacterial proliferation and stimulation upon degradation of the acid-unstable linker, substantially enhances antitumor efficacy [224]. Carbodiimide cross-linking chemistry has thus emerged as a potent tool for designing biotic-abiotic hybrid systems to advance drug delivery and therapy.
In situ polymerization represents a sophisticated chemical encapsulation technique wherein bacteria are seamlessly integrated with reactive monomers and catalysts directly onto their surfaces to initiate polymerization [236]. Various materials, including Au NPs, metal-organic backbones (MOFs), dopamine (DOPA), calcium phosphate (Ca3(PO4)2), metal ion/polyphenol ligand complexes (e.g., Fe3+/tannins (TA)), polypropylene (PPy), silica, and titanium dioxide, can be employed to coat bacterial surfaces. Remarkably, tumor-specific antigens and checkpoint-blocking antibodies have been concurrently affixed to bacterial surfaces using polydopamine (PDA) nanoparticles formed through in situ co-deposition polymerization with dopamine [236].
Zhang et al. have pioneered the encapsulation of three bacteria within calcium alginate gel microspheres to achieve co-localization, subsequently polymerizing a layer of polydopamine nanomembrane onto the gel microspheres through in-situ polymerization technology. This nanomembrane exhibits selectivity, permitting only small molecules to permeate, thereby successfully establishing a bacterial microecosystem [237]. This groundbreaking technology holds the promise of revolutionizing orally administered probiotics, offering a novel and safe therapeutic option. The in situ polymerization preparation method is carried out under mild conditions and does not affect the activity of bacteria and their proliferation. It has made great progress in surface modification but still has the disadvantages of low coating coverage and hard shell after coating. Therefore, understanding the reaction mechanism of modified bacteria and optimizing the design and preparation of modified bacteria is the key to improving the performance of bacterial therapeutics.
Additionally, click chemistry reaction emphasizes the opening of new methods of combinatorial chemistry based on the synthesis of carbon-heteroatom bonds (C-X-C) and the simple and efficient access to molecular diversity with the help of these reactions (click reactions) [238]. A representative reaction of click chemistry is the copper-catalyzed Azide-Alkyne Cycloaddition. d-type amino acid metabolic labeling is combined with click chemistry for probiotic delivery. Specifically, the metabolic labeling enables probiotics and Enterobacteria to be modified with dibenzocyclooctyne (DBCO) and azide groups, respectively, and then the probiotics are adhered to Enterobacteria through the click chemistry reaction in the intestinal tract, thus enhancing the colonization ability of probiotics in the intestinal tract (Fig. 12a) [239].
Fig. 12.
a Schematic illustration of bioorthogonal-mediated bacterial delivery to enhance probiotics colonization in the gut [239]. Copyright © 2022 The Authors. Published by American Chemical Society. b Illustration of the prebiotics-encapsulated probiotics to regulate gut microbiota and suppress colon cancer. Bacteria specifically enriching in tumor tissues were screened. C. butyricum was then modified with prebiotic dextran by host–guest chemistry. The system that carried the chemotherapeutic drugs was used orally by mice for colon cancer treatment [240]. © 2020 Wiley-VCH GmbH
Finally, encapsulation of probiotic spores based on chemically modified prebiotics can achieve efficient enrichment of probiotics at the tumor site after oral administration (Fig. 12b) [240]. Probiotics and prebiotics are ideally biocompatible, and the combination of both can construct a safe microbiota-modulating material. Probiotic-encapsulated probiotic spores (spores-dex) were prepared by a host-guest chemical reaction between commercial Clostridium typhimurium and chemically modified probiotic dextran. The spores-dex were found to be specifically enriched in colon cancer after oral administration.
Co-precipitation
Co-precipitation is a widely used method of synthesizing nanoparticles, which is mainly done by mixing two or more precursor salts in solution and then forming a precipitate by adding a precipitant. The precipitate is formed by adding a precipitant. This method has the following advantages easy to implement, cost effective, and scalable. Zein, a hydrophobic protein extracted from the endosperm of corn kernels, constitutes 35–60% of the total protein content [241]. Renowned for its unique assembly properties and remarkable resistance to gastric acid and bile salts, zein stands out as a superior material for constructing active substance delivery systems and facilitating targeted release. However, the encapsulation of probiotics within zein, despite its advantages, has historically encountered challenges such as low encapsulation rates and poor dispersion.
To address the issue of zein’s limited water solubility for probiotic encapsulation, Liu et al. devised a method to produce zein nanoparticles (ZNPs) as the encapsulating material for probiotic microcapsules using the antisolvent precipitation technique (Fig. 13) [242]. The resulting nanocomposite carrier system, formed through electrostatic, hydrophobic, and hydrogen bonding interactions between zein and polysaccharides, synergistically enhances the stability of the transport carrier and the biocompatibility of the system.
Fig. 13.
a Anti-Solvent Precipitation of Zein. b Layer-by-Layer Assembled L. plantarum 550 Microcapsule Using Zein Nanoparticles and Pectin [242]. © 2023 Elsevier Ltd. All rights reserved
However, subsequent research revealed that nanoparticles prepared through the antisolvent co-precipitation method exhibit superior properties compared to those prepared via antisolvent precipitation alone. The co-precipitation method yields higher encapsulation efficiency, smaller particle size, and enhanced storage stability. Liu et al. further explored the potential of this approach by investigating the encapsulation efficiency and photostability of curcumin using zein-tea saponin nanoparticles [225]. Their findings demonstrated that after lyophilization and rehydration, the nanoparticles dispersed well in water. Hence, employing the antisolvent co-precipitation method for probiotic encapsulation may yield more significant efficacy and improved outcomes (Fig. 9d).
This research study will help in expanding the range of probiotic-based oral drug delivery methods and may lead to innovative drug effects also helps in expanding the range of probiotic-based oral drug delivery methods and may lead to innovative solutions to improve the efficacy and patient compliance of oral probiotic therapies.
Bionic mineralization
Bionic mineralization that is, the use of the template effect on biological macromolecules on the inorganic nucleation-crystallization process of the reaction kinetic control, to achieve the controlled synthesis of bionic materials, the construction of organic-inorganic composite materials [243]. Biomineralization is the combination of mineralized material and living organism to form a new type of living organism, using the structural and physicochemical properties of the material to achieve the purpose of the structure, function, and behavioral regulation of the living organism [244]. Materials are introduced on the surface or inside of probiotics to construct new functional lifeforms. Probiotic surface shell engineering can protect the organism from external environmental damage. Metal complexes, inorganic mineral materials, and polymers are often used to create rigid shells of organisms, such as MOF, CaP, SiO2, and natural or synthetic polymers, which are useful for protection against external stress, disease treatment, environmental protection, biocatalysis, and conductive sensing.
Bionic mineralization technology has emerged as a groundbreaking approach for oral probiotic delivery. Meng’s research group has pioneered the development of a multishell-layered colloidal capsule (MSC) platform, integrating biomimetic mineralization and microfluidics, to achieve sequential gastrointestinal resistance (Fig. 9e) [226]. Their findings reveal a remarkable 280-fold increase in the viability of encapsulated probiotic bacteria compared to free probiotic bacteria during long-term storage. The distinctive sequential gastrointestinal resistance properties of the MSC enable encapsulated probiotics to withstand extreme acidity, bile salts, and digestive enzymes. As a result, encapsulated probiotics exhibit significantly higher viability (61%) compared to free probiotics (EcN), which show no viability under similar conditions. This innovative approach holds tremendous promise for enhancing probiotic delivery and efficacy in oral applications.
The on-demand release of probiotics from the multicell colloidal capsule to the colonic site maintained the integrity of the intestinal barrier and regulated the balance of intestinal flora, significantly improving the therapeutic efficacy of the treatment for colitis in mice. This technology has significantly improved the efficiency of probiotic delivery and has promising clinical applications in the future.Recently, Liu et al. used polypyrrolidone-mediated biointerfacial mineralisation to put a calcium carbonate coat on probiotics, and due to the physical isolation of the coating, the encapsulated probiotics could substantially enhance their tolerance to complex environments, which provided a new way for the preparation of oral preparations of living bacteria (Fig. 14) [245]. In vivo experiments demonstrated that the number of live bacteria in the intestinal tract of mice increased more than 5 times after oral administration of mineralisation-coated probiotics. However, attention should be paid to the possible inhibition of probiotic activity and the stability of the synthesized shell layer when applying the mineralization technique.
Fig. 14.
Schematic illustration of biointerface mineralization that generates ultraresistant gut microbes as oral biotherapeutics [245]. a Preparation of mineral coating on bacterial surface. b Resistances of coated bacteria against environmental assaults. c Neutralization of gastric acid, adaptable release of coated bacteria, and calcium ions–triggered aggregation of bile acid by double-decomposition reaction of mineral coating in the gastrointestinal tract following oral ingestion
Others
Microfluidic-derived encapsulation strategies offer a promising solution to the challenge of effectively delivering and colonizing orally administered probiotics in a harsh gastrointestinal environment. Wang et al. introduced a pioneering synergistic delivery system, combining EcN Nissle 1917 with prebiotics such as sodium alginate and inulin gel, for the treatment of inflammatory bowel disease and colitis-associated colorectal cancer (Fig. 9f) [227]. Utilizing high-throughput microfluidics, probiotics were encapsulated alongside functional prebiotics, resulting in a significant enhancement of in situ colonization and therapeutic efficacy.
Encapsulation of polymeric nanoparticles (NPs) stands as a well-established approach for augmenting the oral bioavailability of both hydrophobic and hydrophilic compounds. This encapsulation method enhances solubility in aqueous media, shields the compounds from acid and enzymatic degradation in the gastrointestinal tract, and facilitates permeability through mucous membranes. By employing suitable materials, the size and surface properties of NPs can be tailored to engage specific mucus interaction mechanisms. Remarkably, owing to their easily modifiable surface properties and attributes such as biodegradability, biocompatibility, and non-toxicity, polymeric NPs hold immense potential for widespread application in the oral delivery of drugs within the industry.
Therefore, it has been investigated that probiotics using nano microencapsulated mucus biopolymers prolong their survival in the gut and continuously deliver live cells, thus maintaining microbial homeostasis in the gut [246]. This delivery method encapsulates the probiotics inside the NPs with the help of mucus adhesion in the organism, which well protects the activity of the probiotics and opens up a new way of oral delivery of probiotics. However, it is worth thinking about whether this approach based on biopolymer encapsulation can deliver probiotics to reach the intestinal site without being intercepted by other organismal active substances. After all, the body is covered with a large number of mucous membranes all capturing species of nano-microencapsulated probiotics.
Extracellular vesicles (EVs) are naturally released from prokaryotic and eukaryotic cells as nanoscale membranous vesicles containing components such as proteins, lipids and nucleic acids.Vesicles have excellent pharmacokinetic and immunological properties as well as the ability to penetrate physiological barriers, making them ideal vehicles for delivering active substances [247]. Probiotics are active substances that are difficult to deliver directly, so encapsulating probiotics in vesicles can significantly improve the delivery efficiency of probiotics.
Su et al. synthesised nanoscale probiotic membrane vesicles using ultrasound technology and applied them to encapsulate fucoidan [248]. The results showed that the engineered membrane vesicles yielded 150 times more than the naturally secreted vesicles of probiotics compared to the naturally secreted vesicles of probiotics.In addition, the engineered vesicles-fucoxanthin improved the gastrointestinal stability of fucoxanthin through effective scavenging of free radicals and inhibited H2O2-induced oxidative damage.
Not only that, but a recent study by Zhou et al. showed that probiotic nanoparticles can synergize copper-like bacterial death and immunomodulation to prevent titanium implant infections [249]. An acoustic-sensitive metal-organic framework consisting of Cu-TCPP (tetrakis(4-carboxyphenyl)porphyrin) nanosheets and probiotic-derived membrane vesicles (OMVs) doped with tinidazole eradicated implant infections by highly permeable sonodynamic therapy (SDT). Following infection clearance, outer membrane vesicles (omvs) derived from Lactobacillus animalis promoted the modulation of macrophage polarization toward a pro-healing phenotype, attenuated biofilm-induced inflammation, and facilitated the expression of osteogenic factors, which are essential for bone repair and enhanced implant integrity. From the above studies, it is evident that nanovesicles are valuable in the development and clinical translation of probiotic therapies.
Potential clinical applications of nano-encapsulated probiotics
The gut flora is the largest microbial ecosystem in the human body, playing a critical role in regulating the immune system and maintaining overall balance. Disruptions in this delicate microbial community can lead to various gut-related diseases [101]. Consequently, probiotics have gained significant attention as potential therapeutic agents, offering a promising approach for both preventing and treating these conditions. Effective delivery of probiotics to the gut can inhibit the growth of harmful bacteria and promote a healthy microbial balance. However, the effectiveness of probiotics is often affected by external factors, making them less resilient to environmental stressors and limiting their time within the gut. This reduced stability can hinder their clinical utility and effectiveness. In contrast, nano-coated probiotics show promise due to their ability to release slowly, which suggests the potential for more targeted delivery within the intestines. At the intersection of chemical biotechnology and microbiology, the integration of probiotics and nanotechnology is unlocking new possibilities for biomedical applications [250]. This section highlights the use of nanotechnology-encapsulated probiotics in the treatment of various diseases. These therapeutic effects are achieved through the encapsulation of diverse probiotic species using specialized nanomaterials and technologies, enabling precise targeting of specific diseases.
Nano-armor-probiotics precision treatment
The disadvantages of using probiotics directly to treat or prevent diseases are becoming more and more obvious. It is difficult for orally consumed probiotics to resist the attack of the gastrointestinal tract and the interference of the immune system, and it is not possible to fully utilize the efficacy of probiotics. Therefore, nano-armor protection of probiotics has emerged. Nano-armor not only protects probiotics to reach the mucosa of intestinal epithelial cells and successfully colonize but also helps probiotics to reach the target site to play a specific therapeutic effect. In this section, we discuss the current practical applications of nano-armor-probiotic precision therapy, including anticancer, antibacterial, anti-inflammatory, and treatment of obesity (Fig. 15).
Fig. 15.
Schematic diagram of the action of nano-armor-probiotics in the treatment of diseases
Please check and confirm if the inserted citation of fig. 15 is correct. If not, please suggest an alternate citation. Please note that figures should be cited in text.Thank you very much for your valuable suggestions. The diagram was drawn by ourselves in conjunction with the chapter synopses, so its meant as a summarizing graphic description.
Anti-tumor
Probiotics have emerged as promising agents for targeting tumors, yet their potential within tumor microenvironments (TMEs) as platforms for cancer therapy remains relatively underexplored [251]. r Tan et al. harnessed Lactobacillus acidophilus (LA) coupled with two-dimensional CoCuMo-LDH to unveil a novel approach (Fig. 16a) [252]. This strategy revealed that TME-induced in situ amorphization of CoCuMo-LDH nanosheets, triggered by 1270 nm laser irradiation, remarkably amplified its ROS generation activity by approximately 42.8-fold compared to crystalline CoCuMo-LDH nanosheets (Fig. 16c). This enhancement significantly augments the response of the tumor microenvironment to probiotic nanocapsules, thereby expanding the horizon of cancer therapeutic interventions.
Fig. 16.
a the preparation of LA&LDH [252]. © 2023 Wiley-VCH GmbH. b Synthesis schemes of CS-ID@NMs [253]. © 2022 Wiley-VCH GmbH.c Fluorescence intensity of SOSG [252]. © 2023 Wiley‐VCH GmbH. d GSH concentrations [253]. © 2023 Wiley-VCH GmbH. e B16-F0 tumor growth curve of Rag−/− mice that received an adoptive cell transfer (ACT) of 5 × 105 CD8 T cells from WT donor mice 1 day before TCE and were treated with orally administered Lr or PBS daily starting 1-day pTCE (n = 3 per group) [254]. © 2023 Elsevier Inc. f B16-F0 tumor growth of mice orally administered Lr or PBS daily starting on day 5 pTCE (tumor size ∼100 mm3) and treated with intraperitoneal injections (IP) of 50 µg αPD-L1 or isotype control (iso. ctrl.) on days 5, 7, 9, and 12 pTCE (n = 4–6 mice/group) [254]. © 2023 Elsevier Inc
Furthermore, Xiao et al. pioneered the construction of chitosan and sodium alginate hydrogel-encapsulated nanomedicine-carrying motors (CS-ID@NMs) (Fig. 16b) [253]. Under the dual influence of ultrasound and TME, these nanomotors efficiently traversed mucus barriers and penetrated deep into tumor tissues (Fig. 16d). Within the tumor microenvironment, stimulated by tumor cell conditions, ultrasound facilitated the nanomotor’s lysosomal escape a nd subsequent release of mitochondria. The presence of divalent manganese in the motor catalyzed the production of hydroxyl radicals via the Fenton reaction, thereby promoting lipid peroxidation within the tumor. This cascade of events synergistically enhanced the tumor immunosuppressive microenvironment and effectively inhibited both primary and metastatic colon cancer.
These findings underscore the ability of probiotics to serve as carriers for anticancer drugs. Moreover, surface modification of probiotics with nanomaterials enhances their resilience in the gastrointestinal tract, thereby maximizing their survival rate and efficacy in reaching tumor sites. Excitingly, research from the University of Pittsburgh highlights the potential of a common probiotic, Lactobacillus reuteri (Lr), to migrate from the intestinal tract to extraintestinal tumor sites following oral administration (Fig. 16e). Lr secretes indole-3-carboxaldehyde, which selectively targets the CD8+ T-cell receptor, significantly augmenting tumor suppression and potentiating the efficacy of immune checkpoint inhibitor (ICI) therapy (Fig. 16f) [254]. These multifaceted findings underscore the versatility of probiotics in cancer therapy, extending beyond intestinal cancers to metastatic tumors. Leveraging engineered probiotics as drug carriers for intestinal tumor treatment not only directly targets colon cancer cells but also harnesses probiotic activity to modulate intestinal dysregulation post-treatment, thereby mitigating drug-related side effects. This integrated approach holds immense promise in enhancing the efficacy of cancer therapy while minimizing its adverse effects.
Anti-obesity
The escalating prevalence of obesity poses a significant challenge to public health, profoundly impacting the quality of life and fueling the rise of chronic metabolic disorders [255]. As the global population continues to grapple with excessive weight gain, understanding the intricate role of the intestinal flora in obesity and its associated complications becomes paramount. Emerging research sheds light on the mechanisms through which gut microbiota influence fat storage and alter the composition of fatty acids, thereby contributing to the development and progression of obesity-related disorders [256].
Central to this understanding is the connection between human obesity and diminished peripheral levels of 5-hydroxytryptamine (5-HT), a neurotransmitter crucial for regulating appetite and mood. Remarkably, the intestinal flora emerges as a primary contributor to the production of intestinal 5-HT [257]. Studies in germ-free mice have revealed a striking correlation between impaired colonic 5-HT synthesis and reduced blood 5-HT concentrations, underscoring the pivotal role of gut microbiota in modulating 5-HT biosynthesis [258]. This regulatory mechanism is intricately linked to the production of short-chain fatty acids and certain secondary bile acids, such as deoxycholic acid, generated through the microbial metabolism of bile acids.
Further elucidation of the therapeutic potential of probiotics in combating obesity has been brought to light through studies involving Lactobacillus griseus SBT2055. Sato M et al. conducted research wherein rats were fed fermented milk containing Lactobacillus casei strain SBT2055 (LG2055), revealing reduced adipocyte cell size and serum leptin levels [259]. Subsequent animal studies further demonstrated that, in addition to inhibiting the uptake of dietary triglycerides by the host, strain SBT2055 (LG2055) also increased energy expenditure through carbohydrate oxidation, improved glucose tolerance, and reduced inflammation [259]. Administration of this probiotic strain to lean Zucker rats, a prevalent model of hereditary obesity, resulted in significant reductions in mesenteric tissue mass, serum leptin levels, and adipocyte volume. These findings underscore the potential of utilizing probiotic nanocarriers to modulate gut 5-HT and short-chain fatty acid levels, thereby offering a compelling avenue for managing obesity.
In essence, harnessing the intricate interplay between gut microbiota and host metabolism presents a novel frontier in the battle against obesity. By leveraging probiotic interventions to target key neurotransmitter pathways and metabolic processes within the gut, there exists the potential to devise more effective and tailored approaches for combating obesity and its attendant health complications. Through continued research and innovation in probiotic delivery systems, we stand poised to unlock new avenues for obesity treatment and pave the way toward healthier futures for individuals worldwide.
Anti-microbial
Antibiotics are frequently prescribed to combat bacterial infections, yet their indiscriminate action not only targets harmful pathogens but also disrupts beneficial gut bacteria, leading to antibiotic-associated diarrhea (AAD) and potentially exacerbating intestinal inflammation [260]. The depletion of healthy intestinal flora can pave the way for the unchecked proliferation of pathogenic microorganisms, precipitating severe localized or systemic inflammatory responses in the intestines.
Among probiotics, Bacillus licheniformis stands out as a key player in restoring microbial balance. Its repertoire of functions includes the modulation of dysbiosis, induction of antibacterial compounds, suppression of pathogenic bacteria, and secretion of anti-inflammatory substances [261].
While oral probiotic supplementation presents a promising approach to mitigate antibiotic-induced side effects and curb the growth of harmful bacteria, the efficacy of unprotected probiotics is compromised by antibiotic-mediated destruction within the intestinal tract. Consequently, these probiotics struggle to colonize effectively, resulting in suboptimal therapeutic outcomes.
Recent research endeavors have been dedicated to bolstering the viability of oral probiotics, particularly among individuals undergoing antibiotic treatment. Guo et al. introduced a pioneering “nano-armor” encapsulation technology, designed to encapsulate probiotics within individual cells via a supramolecular network material derived from natural polyphenols (Fig. 17) [262]. Tests have demonstrated that this innovative “armor” affords exceptional protection for probiotics against six commonly used clinical antibiotics. Moreover, it substantially enhances the survival rate of orally administered probiotics within the digestive tract of antibiotic-treated animals. This nanomaterial encapsulation shields probiotics from bacterial cell uptake, thereby mitigating the indiscriminate effects of antibiotics. Moreover, it facilitates the sequestration of antibiotics around probiotic cells, creating a microenvironment characterized by lower antibiotic concentrations conducive to probiotic survival. This “nano-armor” confers long-lasting protection, heralding significant potential for clinical application in enhancing probiotic efficacy amidst antibiotic therapy.
Fig. 17.
Natural polyphenol-based single-cell coating (nanoarmor) for the protection of bacteria from antibiotics in the gastrointestinal (GI) tract [262]. Copyright © 2022, The Author(s). a The nanoarmor enables a rapid and highly biocompatible single-cell encapsulation that protects from a wide range of antibiotics with different molecular structures and properties. b Armored probiotic bacteria can be freeze-dried and filled into enteric capsules designed for oral delivery. c The enteric capsule remains intact during the low pH of gastric transit and releases the armored probiotics in the gut. d The poor specificity of antibiotics normally depletes healthy commensals in the gut and hinders probiotic treatments. e The nanoarmor provides a safe and transient coating to the beneficial bacteria from antibiotics, facilitating healthy microbe repopulation
Anti-inflammatory
Inflammatory bowel disease (IBD) poses a complex challenge, influenced by a multitude of genetic, environmental, and immunological factors, necessitating multifaceted therapeutic approaches. Shi et al. have pioneered the development of a nanoparticle designed to address this complexity by scavenging cfDNA and ROS (Fig. 18) [263]. These nanoparticles, comprising mesoporous organosilicon nanoparticles (MONs) conjugated with polyethyleneimine (PEI) and the antioxidant diselenide, exhibit remarkable affinity for cell-free DNA (cfDNA) and responsively degrade reactive oxygen species (ROS). Upon oral administration, MON-PEI effectively interrupts the cfDNA-induced TLR9-MyD88-NF-κB signaling pathway while mitigating the ROS-mediated pro-inflammatory response. Consequently, the administration of MON-PEI mitigates colonic and peritoneal inflammation, thereby attenuating the tissue damage characteristic of IBD.
Fig. 18.
MON-PEI reduces cfDNA- and ROS-induced inflammation in vitro [263]. a Schematic of the design of a biodegradable nanomedicine with cfDNA- and ROS-scavenging activity for IBD therapy. b Transmission electron microscopy images of MON before and after a 1-day incubation in simulated body fluid solution containing 100 µM H2O2. c DNA binding efficiency of MON, PEI, and MON-PEI at different nanoparticle: DNA mass ratios at 37 °C. d Viability of Caco-2 cells treated for 24 h with various concentrations of MON, PEI, and MON-PEI. e Activation of HEK-TLR9 reporter cells by IBD patient sera in the absence or presence of MON, PEI, and MON-PEI for 24 h. The corresponding SEAP activity in supernatants from each group was determined with a QUANTI-Blue assay at OD620. f RAW 264.7 macrophages were stimulated with IBD patient sera in the absence or presence of MON, PEI, and MON-PEI for 24 h. Supernatants were assayed for TNF-α by ELISA. g Relative fluorescence intensity of oxidized DCF in Caco-2 cells after incubation with different formulations in the presence or absence of 100 µM H2O2 for 4 h. h The viability of Caco-2 cells was measured after treatment with different formulations in the presence of 100 µM H2O2 for 24 h. Data are means ± SEM (n = 3 independent experiments; *P < 0.05, **P < 0.01, and ***P < 0.001 by one-way ANOVA with Tukey’s multiple comparison test)
Concurrently, nano-encapsulation emerges as a promising strategy for treating colitis, particularly through the encapsulation of probiotics and anti-inflammatory agents such as 5-aminosalicylic acid (5-ASA) within gastrointestinal microenvironment-responsive alginate polysaccharides [264]. The acid-resistant alginate coating serves to safeguard probiotics from the harsh milieu of the gastrointestinal tract, ensuring their viability and efficacy. The synergistic action of probiotics and 5-ASA restores organismal biological activity, modulates microbiota abundance and diversity, suppresses pro-inflammatory cytokine expression, and fortifies the intestinal barrier, culminating in colitis alleviation. However, further investigation is warranted to ascertain the stability of probiotics and 5-ASA nanocarriers in the gastrointestinal environment and to address concerns regarding premature release of 5-ASA leading to off-target effects. Moreover, the strategic modification of probiotic-secreted therapeutics presents a compelling avenue for targeted colitis therapy. By leveraging probiotics as drug carriers, interventions can be tailored to the colitis locus, effectively alleviating oxidative stress, mitigating endoplasmic reticulum stress, suppressing intestinal epithelial cell apoptosis, curtailing inflammatory cell infiltration, restoring intestinal barrier integrity, and rebalancing the intestinal microbiota [265]. This multifaceted approach holds promise for advancing the management of IBD, offering targeted and efficacious therapeutic interventions with the potential to alleviate disease burden and enhance patient outcomes.
Other
Diabetes, a pervasive chronic condition affecting millions worldwide, takes a heavy toll on human health, with nearly 4 million individuals succumbing to diabetes-related complications annually [266]. Despite the widespread adoption of insulin injection therapy as the primary treatment modality, patients still grapple with issues of poor compliance, exorbitant costs, and injection site discomfort. However, recent strides in nanotechnology offer a promising avenue for revolutionizing diabetes management. Researchers, cognizant of the limitations of conventional therapies, have turned to nanomaterials to deliver oral insulin, thereby circumventing the drawbacks associated with needle-based administration. Innovations like NU-1000, a zirconium-based porous MOF material with high pH stability for controlled insulin release [267]. Additionally, novel formulations like oral glucose-responsive polymeric nanoparticles offer on-demand insulin release, minimizing systemic toxicity [268]. These demonstrate the potential of nanotechnology in diabetes.
Moreover, emerging strategies aim to bolster endogenous insulin production through gluconeogenesis facilitation or the controlled secretion of insulin in response to blood glucose fluctuations. Incorporating insulin into engineered probiotics, encapsulated within nano-probiotic capsules, holds promise for precise, targeted diabetes treatment. By leveraging the inherent targeting capabilities of probiotics and the controlled release of insulin, these nano-probiotic capsules present a compelling approach for clinical application [269]. Meanwhile, cardiovascular diseases stand as a leading cause of global mortality, underscoring the urgency for innovative therapeutic interventions [270]. Although traditional oral medications like aspirin and statins are commonplace, oral nanomedicine applications remain underexplored. Future research endeavors aim to surmount gastrointestinal barriers by engineering nanomedicines for enhanced tissue accumulation. Probiotic interventions offer a holistic approach to cardiovascular disease management, mitigating risk factors associated with metabolic syndrome [271]. Oral nano-probiotic capsules, customized to target lesions and harness the cardiovascular benefits of probiotics, hold immense potential in combatting cardiovascular ailments.
Simultaneously, the escalating prevalence of neurodegenerative diseases underscores the imperative for novel treatment modalities [272]. Nanomaterials present an avenue for preserving drug efficacy in physiological environments, while Probiotic nanocarriers offer a means to traverse the blood-brain barrier and modulate neuroinflammation. By inhibiting the aggregation of inflammatory factors and restoring intestinal flora balance, Probiotic nanocarriers hold promise for arresting the progression of neurological disorders [273]. The convergence of nanotechnology and probiotics heralds a new era in disease management, offering tailored, precise interventions across a spectrum of ailments, from diabetes and cardiovascular diseases to neurodegenerative disorders. Through continued research and innovation, these interdisciplinary approaches hold the potential to revolutionize healthcare, offering hope for improved patient outcomes and enhanced quality of life.
Safety and regulatory approval challenges
The safety and regulatory approval of nano-armored probiotics pose significant challenges. Given the intricate interplay between nanotechnology, probiotics, and neurological health, ensuring the safety and efficacy of this innovative approach is paramount. Primary concerns revolve around the biocompatibility and potential toxicity of the nano-armor materials used to encapsulate probiotic metabolites. While these materials are designed to enhance stability and targeted delivery, rigorous evaluation of their biocompatibility and long-term safety profiles is essential to mitigate any adverse effects on patients. Furthermore, the interaction between nano-armored probiotics and the complex ecosystem of the gut microbiota raises questions regarding unintended consequences on gastrointestinal health. Given the crucial role of gut-brain communication in neurological function, any disruption to the delicate balance of gut microbes could have unforeseen implications for neurological disorders.
Navigating the regulatory landscape presents another formidable challenge. Regulators must grapple with the novel nature of this therapeutic approach, requiring a nuanced understanding of both nanotechnology and probiotics. Clear guidelines and robust regulatory frameworks are needed to ensure the safe and timely translation of this innovative strategy from the laboratory to clinical practice (Fig. 19) [274]. Moreover, addressing safety and regulatory concerns is imperative to unlock the full potential of nano-armored probiotics for brain-targeted therapeutic strategies. Through multidisciplinary collaboration, rigorous preclinical testing, and transparent regulatory oversight, researchers can surmount these challenges and pave the way for the development of safe and effective treatments for neurological disorders. Techniques related to taxonomic identification, safety assessment, and performance evaluation are essential in the development of novel strains. Improving the performance of employed probiotics can be achieved through protective, enhancing, and gene-editing strategies. Future scope to address the major confusion of the probiotic market: guidelines of probiotics evaluation, definition and category of probiotics, manufacturing process, and trademark regulation of products.
Fig. 19.
Related strategies and future scope to address the potential issues regarding probiotic limitations and market confusion [274]. Rights managed by Taylor & Francis
Safety concerns of nano-encapsulated probiotics
The application of nanotechnology in probiotics presents potential safety concerns, particularly regarding its impact on the human body and the environment. First, the effect of nanoparticles on the intestinal microbiota is noteworthy. Certain nanoparticles can disrupt the gut microflora and metabolic processes, potentially inducing inflammation or excessively depleting beneficial microbes, thereby significantly impairing the immune system. Second, there is a potential threat to probiotics themselves. In the complex intestinal environment, nanoparticles may adversely affect the survival and activity of probiotics, undermining their intended benefits. Lastly, the antimicrobial properties of nanoparticles raise concerns about drug resistance. While nanoparticles can effectively interact with bacterial biofilms, their widespread use may inadvertently promote the emergence of drug-resistant strains in harmful bacteria, posing a new challenge to antimicrobial strategies.
The integration of nanotechnology with probiotics presents certain safety concerns, but these risks can be effectively managed and minimized through targeted mitigation strategies. Below are the key approaches:
Selection of Safe Embedding Materials: Employ natural protein- or polysaccharide-based materials, such as sodium alginate and chitosan. These biocompatible substances can shield probiotics from environmental stressors while ensuring safety.
Optimization of the Nano-Encapsulation Layer: Create a multifunctional protective layer around individual probiotic cells using cross-linking reactions and polymer self-assembly. This enhances their resistance, stability, and bioavailability during gastrointestinal transit.
Precise Control of Nanoparticle Size and Shape: Research has demonstrated that the size and shape of nanoparticles significantly affect their biocompatibility and safety. By carefully controlling these parameters, the in vivo toxicity of nanoparticles can be minimized.
Comprehensive Safety Assessments: Before applying nanotechnology to probiotics, conduct thorough in vitro and in vivo safety evaluations. These assessments should examine potential toxicity, biocompatibility, and metabolic pathways of the nanoparticles.
The application of nanotechnology in probiotics offers significant promise, particularly in advancing new methods for treating intestinal diseases. By continuously optimizing nano-embedding materials and techniques, the stability and bioavailability of probiotics can be further improved, thereby enhancing their clinical efficacy [275].
In conclusion, while safety concerns exist in the application of nanotechnology to probiotics, these can be effectively addressed through well-designed mitigation strategies. With ongoing technological advancements and deeper research, the integration of nanotechnology in probiotics is poised for a broader and more impactful future.
Regulatory approval process
The efficacy of probiotics as acquired microorganisms is intricately linked to the number of viable organisms, making it imperative to maintain high viability throughout processing, transport, storage, and gastrointestinal transit to ensure the quality of probiotic preparations. In future clinical trials, particular attention should be paid to the long-term safety of probiotics in various populations, including adolescents, immunosuppressed individuals, critically ill patients, and those in need of supplementation.
Several challenges hinder the approval of probiotics
Internationally, the regulation of probiotics is primarily overseen by the U.S. Food and Drug Administration (FDA) and the Federal Trade Commission (FTC). The FDA regulates products based on their category, such as foods, drugs, dietary supplements, medical devices, and cosmetics, while the FTC focuses on advertising and marketing practices. Unlike drugs, foods and dietary supplements do not require FDA premarket approval. By law, probiotic foods and dietary supplements must meet safety standards. However, manufacturers are allowed to self-determine whether their products qualify for the “Generally Recognized As Safe” (GRAS) designation, often based on historical use. Notably, manufacturers are not required to disclose specific details on product labels, such as the strains of probiotics used or the number of viable microorganisms present at the end of the product’s shelf life. While manufacturers must validate their label claims, they are not obligated to submit this information to the FDA. This regulatory framework underscores the importance of clearly defining the safety and quality of marketed probiotic products to ensure consumer protection and trust [276].
Secondly, current laws and FDA regulations pose significant challenges for food and dietary supplement manufacturers aiming to research the therapeutic benefits of probiotic products without classifying them as drugs. Conducting in-depth research on therapeutic effects requires manufacturers to submit an Investigational New Drug (IND) application. This process is not only lengthy and complex but also demands access to proprietary information from suppliers, creating further obstacles. These regulatory barriers hinder the approval of probiotic products and limit the ability to provide robust confirmation of their therapeutic efficacy.
Third, the accuracy of the labelling of commercial probiotic products currently on the market is not always reliable, with the Italian study observing that 42% of the products analysed contained at least a declared number of bacteria from one of the labelled strains. In addition, 17 per cent of the products had no live microorganisms and 8 per cent were contaminated with Enterococcus faecalis [277]. The presence of undisclosed microorganisms with possible pathogenic properties poses a significant risk to the health of the host. This study demonstrates the need for specific legislation that requires the accurate identification and characterisation of probiotic strains in commercial products and comprehensive testing of all available products.
In China, the issue is further compounded by the lack of standardized definitions for probiotics, leading to inconsistent use of the term. Establishing national standards for probiotics based on current research is essential to regulate their use, standardize evaluation processes, and develop clear specifications for probiotic strains. Standardization would not only enhance product reliability but also support the systematic advancement of probiotic applications in health and wellness.
Finally, the filing of probiotic raw materials is not yet perfect, and the approval process for probiotic health food products cannot keep up with the market demand for new products. China is gradually establishing a dual-track system of registration and filing for probiotic health food.
However, international and domestic challenges remain, including lengthy stability tests, difficulties in evaluating probiotic strain materials, standardization of probiotic production, and a slow approval process.
Moving forward, a comprehensive approach is needed to regulate probiotics effectively
Firstly, in the international arena, it is recommended to maintain continuous assessment of probiotic bioactivity, clarify product classification, and formulate unified assessment standards for “probiotics”.FDA should classify the production licenses of probiotic raw materials accordingly, and optimize the review and approval procedures for probiotic dietary supplements. Second, the country should draw on international standards and scientific consensus on probiotics, and join hands with relevant departments to formulate national standards for the systematic evaluation of probiotic strains and guidelines for the scientific application of probiotics in food. Third, ensuring the traceability of probiotic production, strict quality control of nano probiotic capsules, as well as continuous monitoring of their safety and efficacy after marketing are necessary steps for effective regulation of nano probiotic capsules.
Ultimately, a comprehensive assessment of the use and efficacy of nano probiotic capsules requires ongoing research and disease information registries in the population. By addressing these challenges and implementing strong regulatory measures, the safe and effective use of nanoprobiotic capsules can be ensured for the benefit of public health and the advancement of medical science.
Nano-armor-probiotics for brain-targeted therapeutic strategies
One of the major challenges in treating central nervous system (CNS) disorders is effectively delivering therapies across the blood-brain barrier (BBB). Various delivery methods have been explored, including both invasive and non-invasive techniques. Invasive methods such as deep brain stimulation, intracerebral transplantation, direct brain injection, and intrathecal delivery carry significant risks and discomfort. Consequently, there has been a growing focus on non-invasive approaches. Non-invasive techniques include receptor-mediated transcellular delivery and the use of neuroviruses, exosomes, and nanoparticles [278]. Given the risks and discomfort associated with invasive methods, non-invasive strategies are increasingly emphasized. This section explores innovative strategies for using probiotics to address brain disorders while leveraging nano-armor for protection. Key approaches include modulating BBB permeability through probiotic metabolism, utilizing the body’s immune cells to target the brain, and bypassing the BBB via the vagus nerve (Fig. 20) [279].
Fig. 20.
Pathways of communication along the gut-microbiota-brain axis [279]. A complex interplay of epithelial, immune, and neural cell signaling networks is involved in sensing and communicating changes in microbial metabolites in the gut and the brain involving both circulatory and neural routes
Modulating blood-brain barrier permeability
The blood-brain barrier (BBB) serves as a highly selective osmotic barrier that separates circulating blood from the brain’s extracellular fluid. This barrier is constructed and maintained by a combination of the basement membrane, endothelial cells, and surrounding brain cells such as pericytes and astrocytes, forming both enzymatic and physical defenses [280, 281]. Gut flora metabolites can influence the permeability of both the intestinal epithelial barrier and the BBB. Dysbiosis, or an imbalance in gut flora, can lead to increased levels of helper T-cells, pro-inflammatory cytokines, and lipopolysaccharides (LPS). These changes can enhance the permeability of the intestinal and BBB, potentially resulting in systemic or central nervous system inflammation. Research has shown a close relationship between gut flora imbalances and the onset of Parkinson’s and Alzheimer’s diseases [282].
Probiotics play a role in synthesizing various neurotransmitters, including gamma-aminobutyric acid (GABA), 5-hydroxytryptamine (5-HT), dopamine, and short-chain fatty acids (SCFAs) [283]. SCFAs, in particular, are effective in preventing dopaminergic neuron loss, reducing neuroinflammation, modulating microglial function, maintaining BBB integrity, and supporting synaptic growth and neurotrophic factor secretion. To address these issues, Zheng et al. have developed a nano-armor designed to protect probiotics from gastric juices and digestive enzymes (Fig. 21) [284]. This nano-armor encapsulates probiotics and enhances their ability to continuously produce GABA in vivo. The GABA produced can then travel through the bloodstream to the brain, helping to restore BBB function, regulate dopaminergic neuron activity, and reduce brain inflammation, thus alleviating symptoms of Parkinson’s disease. In summary, probiotic metabolites can be leveraged to treat brain disorders. Once nano-armor delivers probiotics to their target location, the probiotics release metabolites such as propionate, which can then modulate BBB permeability. This increased permeability allows more therapeutic metabolites to cross the BBB, potentially improving symptoms of central nervous system diseases.
Fig. 21.
Illustration of the bionic dormant body timed-triggering to treatment PD by suppressing p38MAPK/NF-κB-mediated signaling pathway in microglia [284]. aL. plantarum is coated with L30D-55 and taken orally to avoid the insult of gastric fluid and decipher in the intestine. b GABA synthesized by L. plantarum in the intestine enters the brain by the blood-brain barrier (BBB) and reduces neuroinflammation by inhibiting the p38MAPK/NF-κB-mediated signaling pathway in the microglia to rescue the loss of dopaminergic neurons for treatment PD. Copyright © 2022, American Chemical Society
Modulating immune cell targeting brain
Gut microbial metabolites play a pivotal role in regulating immune cells by interacting with the host immune system. Key metabolites, including short-chain fatty acids (SCFAs), secondary bile acids, and tryptophan derivatives, influence immune cell differentiation and function through specific signaling pathways. For instance, SCFAs promote the development of regulatory T cells (Tregs) by activating G protein-coupled receptors (GPCRs) or modulating histone deacetylase (HDAC) activity, while simultaneously suppressing the release of pro-inflammatory cytokines [285]. Beyond their immunomodulatory effects within the intestinal environment, these metabolites also impact central nervous system (CNS) immune regulation through signaling along the gut-brain axis [286].
Gut microbial metabolites can cross the blood-brain barrier or transmit signals via the vagus nerve, thereby modulating immune responses in the CNS. In neuroinflammatory conditions such as Alzheimer’s disease and Parkinson’s disease, these metabolites play a crucial role by regulating the activity of brain-resident immune cells, including microglia and astrocytes. For example, SCFAs like butyrate can attenuate excessive microglial activation and reduce the release of inflammatory mediators, thereby mitigating neuroinflammation. Additionally, certain gut-derived metabolites enhance the anti-inflammatory capacity of immune cells, offering promising avenues for the treatment of neurological disorders.
Harnessing the regulatory mechanisms of gut microbial metabolites offers a novel strategy for treating brain immune-related disorders. Targeted interventions, such as modulating gut microbiota composition or supplementing specific metabolites through probiotics, prebiotics, or metabolite analogs, enable precise control of microbial metabolism. These approaches not only restore immune balance in the gut but also influence brain inflammation and immune homeostasis via the gut-brain axis. This innovative strategy holds significant potential for managing neuroinflammatory diseases and other CNS immune dysfunctions. Recently, Li et al. developed premium armored probiotics with inflammation-targeting capabilities to enhance the efficacy and timely action of bacterial therapies against inflammatory bowel disease (IBD), a targeted approach that enhances the ability to maintain intestinal barrier homeostasis, ameliorate pathological inflammation, and reshape intestinal dysbiosis to synergistically improve IBD pathology [287]. Similarly, if the immune response is directed to neurons or nerve cells in the brain, thereby potentially prevents or even treats brain disorders (Fig. 22).
Fig. 22.
Modulation of intestinal bacteria by EcN@PC-Fe/HA during colitis treatment [287]. © 2024 Wiley-VCH GmbH. Comparison of alpha diversity assessed by a Chao1 index, b Shannon index, and c observed species. d Clustering of gut microbial communities for different experimental groups based on the PCoA plot with Weighted_Unifrac distance. e Column diagram of the relative abundance of gut microbiome at the phylum level. f Effects of EcN@PC-Fe/HA on microbial composition at order levels using ternary plot method. g Cladogram based on Linear discriminant analysis effect size (LEfSe) analysis showing community composition of the gut microbiota in mice. h Distribution histogram based on linear discriminant analysis (LDA). LDA score higher than 3 indicates a higher relative abundance in the corresponding group than that in other groups. LDA (log10) > 4.0, P < 0.05. i Heatmap of the functional prediction of altered gut microbiota based on KEGG pathways. Data are presented as means ± SD (n = 5). Statistical analysis was performed using Student’s t-test. *P < 0.05, **P < 0.01
Please check and confirm if the inserted citation of fig. 22 is correct. If not, please suggest an alternate citation. Please note that figures should be cited in text.Thank you for your insightful suggestions. We have labeled the text with the citation for Fig.22.
Nervous system manipulation to bypass the blood-brain barrier
Probiotic metabolites play a crucial role in the bidirectional communication within the microbial-gut-brain axis. Probiotics that successfully colonize the gut can produce metabolites capable of bypassing the blood-brain barrier, delivering active compounds directly to the brain, and potentially enhancing the treatment of central nervous system disorders. While the exact mechanisms through which gut microbes influence the brain remain unclear, it is evident that changes in brain chemistry, are induced by alterations in gut microbiota. This suggests that soluble gut-derived microbial metabolites contribute significantly to communication along the gut-brain axis.
This bidirectional communication is facilitated by afferent connections that link the gut wall to specific brainstem nuclei. These nuclei can be activated by shifts in microbiota composition [288]. Additionally, efferent connections allow sympathetic glutamatergic neurons in the central nervous system to interact with the vagus nerve in the gut, regulating gastrointestinal function and influencing gut microbiota composition. Such interactions underscore the potential for vagal nerve-mediated communication to significantly advance brain disease treatments. The use of nano-armored protected probiotics capable of targeted delivery of gut metabolites is a promising approach. A recent study by Yang et al. demonstrated the ability to deliver butyric acid secreted by genetically engineered probiotics via the vagus nerve, thereby alleviating depressive behavior in mice (Fig. 23) [289]. This advanced approach ensures that the specific probiotic releases relevant neurometabolites at the target site, which then travels directly across the vagus nerve to modulate neuronal activity. This targeted delivery method is expected to achieve the goal of treating neurological disorders.
Fig. 23.
The schematic illustration of mechanism exploration on gut bacterial metabolites in MGB using synthetic living delivery bacteria [289]. a The schematic illustration of mechanism exploration on gut bacterial metabolites in MGB using synthetic living delivery bacteria.b Overview of the experimental design and treatment schedule. C57BL/6J mice were exposed to CUMS for 8 weeks (marked as “CUMS” under the black line). From the onset of the 5th week to the end of CUMS exposure, depressed mice were dosed with saline (abbreviated as “SAL”), wild-type EcN (marked as “EcN”), and a butyrate-overproducing recombinant EcN M3P2TA (abbreviated as “eEcN”), respectively. The healthy mice were marked as “CON”. c-e Bar plots of the results in behavioral tests were shown, including ` c sucrose preference test, d forced swim test, e tail suspension test. n = 8–9 per group. Data are shown as mean ± SEM
Others
The hypothalamic-pituitary-adrenal (HPA) axis is a key neuroendocrine system that orchestrates various physiological processes in response to both psychological and physical stressors. It ensures an effective reaction to external challenges through its components: the paraventricular nucleus (PVN) of the hypothalamus, the anterior pituitary gland, and the adrenal glands. Emerging evidence highlights a bidirectional communication between the neuroendocrine system and the gut microbiota—a diverse community of microorganisms residing in the mammalian gut—that can directly impact the neuroendocrine system [290].
Corticosterone, a hormone produced by the adrenal glands within the HPA axis, has been studied in the context of probiotics. For instance, administering the probiotic Lactobacillus rhamnosus (JB-1) to non-stressed adult male BALB/c mice over 28 days resulted in reduced corticosterone levels, decreased depressive-like behaviors in the forced swim test, and diminished anxiety-like behaviors in the elevated plus maze (EPM) [291]. Similarly, Bifidobacterium infantis was found to alleviate maternal separation-induced depressive-like behaviors, exhibit anti-inflammatory effects, and influence levels of central norepinephrine and 5-hydroxyindoleacetic acid (5-HT metabolites) [292]. These findings suggest that probiotic metabolites can significantly enhance hormone regulation within the neuroendocrine system, thereby influencing the gut-brain axis. Probiotics, particularly those protected by nano-armor, are crucial for facilitating gut-brain axis communication. Nano-armor enables these probiotics to effectively reach the intestinal epithelial mucosa, produce targeted metabolites, and precisely regulate the nervous system through HPA axis transmission. This approach not only supports normal endocrine hormone secretion but also ensures the harmonious functioning of the organism.
Future and outlook
Nano-armored probiotics present immense potential for the future of precision medicine, especially as technological advancements continue to redefine the landscape of oral therapeutic interventions. The integration of nanotechnology into probiotic delivery systems opens up opportunities for more sophisticated and customized treatments, addressing various challenges in disease management. Current research on innovative nanomaterials aims to enhance the stability, bioavailability, and targeted delivery of probiotics. Cutting-edge technologies such as stimulus-responsive nanocarriers—which react to specific gastrointestinal conditions like pH, temperature, or enzymes—show promise in improving delivery accuracy and therapeutic effectiveness. Furthermore, incorporating biocompatible and biodegradable materials will help alleviate concerns about nanotoxicity, supporting the development of safer clinical applications.
The convergence of genetic engineering and nanotechnology for probiotics is also expected to accelerate. In the future, genetically engineered probiotic strains could be designed to produce therapeutic molecules for specific diseases, such as neurodegenerative disorders or cancer, and delivered to precise sites in the body via nanocapsules. For instance, brain-targeted therapies using nano-armored probiotics could revolutionize the treatment of neurodegenerative diseases like Parkinson’s and Alzheimer’s, possibly through the modulation of the gut-brain axis. Artificial intelligence (AI) will play an increasingly critical role in optimizing the design and functionality of nano-armored probiotics. Machine learning algorithms can process vast datasets to identify optimal nanoparticle compositions, predict probiotic survival rates in various gastrointestinal conditions, and even propose modifications to enhance efficacy. Additionally, AI could aid in the development of personalized probiotics tailored to an individual’s microbiome and specific health needs, marking a significant leap forward in precision therapy.
As this field evolves, transitioning nano-armored probiotics from laboratory research to clinical practice will require robust clinical trials and regulatory frameworks. Ensuring the safety, efficacy, and scalability of these technologies is crucial. Regulatory bodies will need to establish specific guidelines to assess the long-term effects of nanoparticles in probiotic therapies, including toxicity, biodegradability, and their impact on the host microbiome. Collaboration among academia, industry, and regulatory agencies will be essential to streamline the approval process and accelerate the clinical adoption of these innovative therapies. Although much of the focus on nano-armored probiotics has been on gastrointestinal disorders, their potential extends far beyond. Future research may explore their role in treating systemic diseases, including metabolic, cardiovascular, and autoimmune disorders. As our understanding of the relationship between gut microbiota and overall health deepens, it may become possible to develop targeted probiotic therapies for these conditions, leveraging nano-encapsulation technologies to ensure effective delivery and colonization.
In addressing the global health landscape, it is crucial to consider the scalability and cost-effectiveness of nano-armored probiotics. Their accessibility in low- and middle-income countries will depend on reducing production costs. Collaborative efforts should aim to ensure that these advanced therapies reach diverse populations, addressing global health disparities and contributing to the fight against chronic and infectious diseases worldwide.
In conclusion, the combination of nanotechnology and probiotic therapies represents a frontier in precision medicine. Future research and development could yield groundbreaking treatment strategies that address key health challenges while advancing the paradigm of personalized medicine. By continuously overcoming the physiological and technical barriers associated with oral probiotic administration, nano-armored probiotics have the potential to revolutionize disease treatment, paving the way for more targeted, effective, and safer therapeutic interventions.
Author contributions
The manuscript was written with the contributions of all authors. All authors have reviewed and approved the final version of the manuscript. All authors read and approved the final manuscript.
Funding
This work was partially supported by the Starting Research Fund from the National Natural Science Foundation of China (No. 32401173), Sichuan Academy of Medical Sciences & Sichuan Provincial People’s Hospital (No. 30420220004), Sichuan Science and Technology Program (24GJHZ0072), Sichuan Province Science and Technology Activities Funding for Returned Overseas Scholars, Department of Science and Technology of Sichuan Province (grant no: 2023NSFSC0106), the 15th Batch of Basic Research Funds for Central Universities and Colleges Frontier Cultivation Project (ZYGX2024J026), Open Project of the National Key Laboratory for Neuro-Oncology Drug Development (SKLSIM-F-2024) and Jiangsu Hansoh Pharmaceutical Group Co., Ltd. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors gave their consent for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yuhong Jiang, Email: yuhong.jiang@swjtu.edu.cn.
Jiahong Li, Email: jiahongljh@163.com.
Yang-Bao Miao, Email: miaoyangbao@uestc.edu.cn.
References
- 1.Stojanov S, Berlec A, Štrukelj B. The influence of Probiotics on the Firmicutes/Bacteroidetes ratio in the Treatment of Obesity and inflammatory bowel disease [J]. Microorganisms, 2020. [DOI] [PMC free article] [PubMed]
- 2.Zou X, Wang L, Xiao L, et al. Gut microbes in cerebrovascular diseases: gut flora imbalance, potential impact mechanisms and promising treatment strategies [J]. Front Immunol. 2022;13:975921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tian C-M, Yang M-F, Xu H-M, et al. Emerging role of bacterial outer membrane vesicle in gastrointestinal tract [J]. Gut Pathogens. 2023;15(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charbonneau MR, Isabella VM, Li N, et al. Developing a new class of engineered live bacterial therapeutics to treat human diseases [J]. Nat Commun. 2020;11(1):1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease [J]. Nat Rev Microbiol. 2021;19(1):55–71. [DOI] [PubMed] [Google Scholar]
- 6.Haran JP, Mccormick BA. Aging, frailty, and the microbiome—how dysbiosis influences human aging and disease [J]. Gastroenterology. 2021;160(2):507–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veiga P, Suez J, Derrien M, et al. Moving from probiotics to precision probiotics. Nat Microbiol. 2020;5:878–80. [Z]. [DOI] [PubMed] [Google Scholar]
- 8.Zaiss MM, Joyce Wu H-J, Mauro D, et al. The gut–joint axis in rheumatoid arthritis [J]. Nat Rev Rheumatol. 2021;17(4):224–37. [DOI] [PubMed] [Google Scholar]
- 9.Zhou S, Gravekamp C, Bermudes D, et al. Tumour-targeting bacteria engineered to fight cancer [J]. Nat Rev Cancer. 2018;18(12):727–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan X, Liu X-Y, Zhang D, et al. Construction of a sustainable 3-hydroxybutyrate-producing probiotic Escherichia coli for treatment of colitis [J]. Cell Mol Immunol. 2021;18(10):2344–2357. [DOI] [PMC free article] [PubMed]
- 11.Maier L, Goemans CV, Wirbel J, et al. Unravelling the collateral damage of antibiotics on gut bacteria [J]. Nature. 2021;599(7883):120–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Średnicka P, Juszczuk-Kubiak E, Wójcicki M, et al. Probiotics as a biological detoxification tool of food chemical contamination: a review [J]. Food Chem Toxicol. 2021;153:112306. [DOI] [PubMed]
- 13.Jiang Z, Li M, Mcclements DJ, et al. Recent advances in the design and fabrication of probiotic delivery systems to target intestinal inflammation [J]. Food Hydrocolloids. 2022;125:107438. [Google Scholar]
- 14.Gao J, Zong X, Yue X, et al. Exploring the properties of silica nanomaterials on supporting bimetallic PtZn sites for Direct Propane dehydrogenation [J]. ACS Appl Nano Mater. 2024;7(7):7018–27. [Google Scholar]
- 15.Liang D, Liu C, Li J, et al. Engineering probiotics-derived membrane vesicles for encapsulating fucoxanthin: evaluation of stability, bioavailability, and biosafety [J]. Food and Function; 2023;14(8):3475–87. [DOI] [PubMed]
- 16.Çanga EM, Dudak FC. Improved digestive stability of probiotics encapsulated within poly (vinyl alcohol)/cellulose acetate hybrid fibers [J]. Carbohydr Polym. 2021;264:117990. [DOI] [PubMed] [Google Scholar]
- 17.Ye N, Zhao P, Ayue S, et al. Folic acid-modified lactoferrin nanoparticles coated with a laminarin layer loaded curcumin with dual-targeting for ulcerative colitis treatment [J]. Int J Biol Macromol. 2023;232:123229. [DOI] [PubMed] [Google Scholar]
- 18.Quan K, Zhang Z, Ren Y, et al. Homogeneous distribution of magnetic, antimicrobial-carrying nanoparticles through an infectious biofilm enhances biofilm-killing efficacy [J]. ACS Biomaterials Sci Eng. 2019;6(1):205–12. [DOI] [PubMed] [Google Scholar]
- 19.Yang X, Wang C, Wang Q, et al. Armored probiotics for oral delivery [J]. Smart Med. 2023;2(4):e20230019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gheorghita R, Anchidin-Norocel L, Filip R, et al. Applications of biopolymers for drugs and probiotics delivery. Polymers. 2021;13:2729. [Z]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crhanova M, Karasova D, Juricova H, et al. Systematic culturomics shows that half of chicken caecal microbiota members can be grown in vitro except for two lineages of Clostridiales and a single lineage of Bacteroidetes [J]. Microorganisms. 2019;7(11):496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bodke H, Jogdand S. Role of probiotics in human health [J]. Cureus. 2022;14(11). [DOI] [PMC free article] [PubMed]
- 23.Goodoory VC, Ford AC. Antibiotics and probiotics for irritable bowel syndrome [J]. Drugs. 2023. [DOI] [PubMed]
- 24.Scott B M, Gutiérrez-Vázquez C, Sanmarco LM et al. Self-tunable engineered yeast probiotics for the treatment of inflammatory bowel disease [J]. Nat Med. 2021. [DOI] [PubMed]
- 25.Gou H, Zhang Y, Ren L et al. How do intestinal probiotics restore the intestinal barrier? Front Microbiol. 2022;13:929346. [DOI] [PMC free article] [PubMed]
- 26.Zhang Z, Tang H, Chen P, et al. Demystifying the manipulation of host immunity, metabolism, and extraintestinal tumors by the gut microbiome [J]. Signal Transduction and Targeted Therapy; 2019. [DOI] [PMC free article] [PubMed]
- 27.Pennisi E. Gut bacteria linked to mental well-being and depression [J]. Science, 2019. [DOI] [PubMed]
- 28.Hadjimbei E, Botsaris G, Chrysostomou S. Beneficial Effects of Yoghurts and Probiotic Fermented Milks and Their Functional Food Potential [J]. Foods, 2022. [DOI] [PMC free article] [PubMed]
- 29.Ilango S, Antony U. Probiotic microorganisms from non-dairy traditional fermented foods [J]. Trends in Food Science & Technology; 2021.
- 30.Górska A, Przystupski D, Niemczura MJ, et al. Probiotic bacteria: a promising tool in cancer prevention and therapy [J]. Curr Microbiol. 2019;76:939–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao Y, Zhai Q, Zhang H, et al. Gut colonization mechanisms of Lactobacillus and Bifidobacterium: an argument for personalized designs [J]. Annual Rev food Sci Technol. 2021;12:213–33. [DOI] [PubMed] [Google Scholar]
- 32.El-Dalatony MM, LI X. Introduction to Probiotics and Their Potential Health Benefits [J]. Gut Remediation of Environmental Pollutants: Potential Roles of Probiotics and Gut Microbiota. 2020:81–108.
- 33.Maftei N-M, Raileanu CR, Balta AA, et al. The potential impact of Probiotics on Human Health: an update on their health-promoting properties [J]. Microorganisms. 2024;12(2):234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gasbarrini G, Bonvicini F, Gramenzi A. Probiotics history [J]. J Clin Gastroenterol. 2016;50:S116–9. [DOI] [PubMed] [Google Scholar]
- 35.Tamang JP, Cotter PD, Endo A, et al. Fermented foods in a global age: East meets West [J]. Compr Rev Food Sci Food Saf. 2020;19(1):184–217. [DOI] [PubMed] [Google Scholar]
- 36.Reque PM, Brandelli A. An introduction to probiotics [M]. Probiotics Elsevier. 2022: 1–17.
- 37.Renuka B, Borse B. Delivery of Probiotics in the Food Industries [M]. Novel Processing methods for plant-based Health foods. Apple Acad Press. 2023: 285–310.
- 38.Tamang JP. History and culture of Indian ethnic fermented foods and beverages [J]. Ethnic fermented foods and beverages of India: Science history and culture. 2020:1–40.
- 39.Leeuwendaal NK, Stanton C, O’Toole PW, et al. Fermented foods, health and the gut microbiome [J]. Nutrients. 2022;14(7):1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martín R, Langella P. Emerging health concepts in the probiotics field: streamlining the definitions. Front Microbiol. 2019;10:1047. Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Savitri LP. Probiotics for Human Health [M]//GOEL G, KUMAR A. Advances in Probiotics for Sustainable Food and Medicine. Singapore; Springer Singapore. 2021:181–212.
- 42.Drell T, Lillsaar T, Tummeleht L, et al. Characterization of the vaginal micro-and mycobiome in asymptomatic reproductive-age Estonian women [J]. PLoS ONE. 2013;8(1):e54379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chee WJY, Chew SY, Than LTL. Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health [J]. Microb Cell Fact. 2020;19(1):203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Behera SS, Ray RC, Zdolec N. Lactobacillus plantarum with functional properties: an approach to increase safety and shelf-life of fermented foods [J]. BioMed research international; 2018;2018(1):9361614. [DOI] [PMC free article] [PubMed]
- 45.Champagne CP, Da Cruz AG, Daga M. Strategies to improve the functionality of probiotics in supplements and foods [J]. Curr Opin Food Sci. 2018;22:160–6. [Google Scholar]
- 46.Zheng J, Wittouck S, Salvetti E, et al. A taxonomic note on the genus Lactobacillus: description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae [J]. Int J Syst Evol MicroBiol. 2020;70(4):2782–858. [DOI] [PubMed] [Google Scholar]
- 47.Tian P, Wang G, Zhao J, et al. Bifidobacterium with the role of 5-hydroxytryptophan synthesis regulation alleviates the symptom of depression and related microbiota dysbiosis [J]. J Nutr Biochem. 2019;66:43–51. [DOI] [PubMed] [Google Scholar]
- 48.He B-L, Xiong Y, Hu T-G, et al. Bifidobacterium spp. as functional foods: a review of current status, challenges, and strategies [J]. Crit Rev Food Sci Nutr. 2023;63(26):8048–65. [DOI] [PubMed] [Google Scholar]
- 49.Ben Braiek O, Smaoui S. Enterococci: between emerging pathogens and potential probiotics [J]. Biomed Res Int. 2019;2019(1):5938210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Htwe K, Yee KS, Tin M, et al. Effect of Saccharomyces boulardii in the treatment of acute watery diarrhea in Myanmar children: a randomized controlled study [J]. Am J Trop Med Hyg. 2008;78(2):214–6. [PubMed] [Google Scholar]
- 51.Hatoum R, Labrie S, Fliss I. Antimicrobial and probiotic properties of yeasts: from fundamental to novel applications [J]. Front Microbiol. 2012;3:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sen S, Mansell TJ. Yeasts as probiotics: mechanisms, outcomes, and future potential [J]. Fungal Genet Biol. 2020;137:103333. [DOI] [PubMed] [Google Scholar]
- 53.Xu C, Ban Q, Wang W, et al. Novel nano-encapsulated probiotic agents: encapsulate materials, delivery, and encapsulation systems [J]. J Controlled Release. 2022;349:184–205. [DOI] [PubMed] [Google Scholar]
- 54.Centurion F, Basit AW, Liu J, et al. Nanoencapsulation for probiotic delivery [J]. ACS Nano. 2021;15(12):18653–60. [DOI] [PubMed] [Google Scholar]
- 55.Yasmin R, Shah M, Khan SA, et al. Gelatin nanoparticles: a potential candidate for medical applications [J]. Nanatechnol Reviews. 2017;6(2):191–207. [Google Scholar]
- 56.Guadarrama-Escobar OR, Serrano-Castañeda P, Anguiano-Almazán E, et al. Chitosan nanoparticles as oral drug carriers [J]. Int J Mol Sci. 2023;24(5):4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Azizian S, Hadjizadeh A, Niknejad H. Chitosan-gelatin porous scaffold incorporated with Chitosan nanoparticles for growth factor delivery in tissue engineering [J]. Carbohydr Polym. 2018;202:315–22. [DOI] [PubMed] [Google Scholar]
- 58.Sagoe PN, K, Velázquez EJM, Espiritusanto YM, et al. Fabrication of PEG-PLGA microparticles with tunable sizes for controlled drug release application [J]. Molecules. 2023;28(18):6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schmitt V, Kesch C, Jackson JK, et al. Design and characterization of injectable poly (lactic-co-glycolic acid) pastes for sustained and local drug release [J]. Pharm Res. 2020;37:1–13. [DOI] [PubMed] [Google Scholar]
- 60.Nikzamir M, Akbarzadeh A, Panahi Y. An overview on nanoparticles used in biomedicine and their cytotoxicity [J]. J Drug Deliv Sci Technol. 2021;61:102316. [Google Scholar]
- 61.Mirchandani Y, Patravale VB, Brijesh S. Solid lipid nanoparticles for hydrophilic drugs [J]. J Controlled Release. 2021;335:457–64. [DOI] [PubMed] [Google Scholar]
- 62.Wang G, Wang J, Wu W, et al. Advances in lipid-based drug delivery: enhancing efficiency for hydrophobic drugs [J]. Expert Opin Drug Deliv. 2015;12(9):1475–99. [DOI] [PubMed] [Google Scholar]
- 63.Thomas OS, Weber W. Overcoming physiological barriers to nanoparticle delivery—are we there yet? [J]. Front Bioeng Biotechnol. 2019;7:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kowalska E, Ziarno M, Ekielski A, et al. Materials used for the microencapsulation of probiotic bacteria in the food industry [J]. Molecules. 2022;27(10):3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ziarno M, Zaręba D, Jamiołkowska D. Studia Nad Czynnikami determinującymi przeżywalność LAB w warunkach symulujących układ pokarmowy [J]. Bromatol Chem Toksykol. 2009;3(42):990–4. [Google Scholar]
- 66.Han S, Lu Y, Xie J, et al. Probiotic gastrointestinal transit and colonization after oral administration: a long journey [J]. Front Cell Infect Microbiol. 2021;11:609722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zmora N, Zilberman-Schapira G, Suez J et al. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features [J]. Cell. 2018;174(6):1388 – 405. e21. [DOI] [PubMed]
- 68.Yao M, Li B, Ye H, et al. Enhanced viability of probiotics (Pediococcus pentosaceus Li05) by encapsulation in microgels doped with inorganic nanoparticles [J]. Food Hydrocolloids. 2018;83:246–52. [Google Scholar]
- 69.Ghorbani S, Maryam A. Encapsulation of lactic acid bacteria and bifidobacteria using starch-sodium alginate nanofibers to enhance viability in food model [J]. J Food Process Preserv. 2021;45(12):e16048. [Google Scholar]
- 70.Fan Q, Zeng X, Wu Z et al. Nanocoating of lactic acid bacteria: Properties, protection mechanisms, and future trends [J]. Crit Rev Food Sci Nutr. 2023: 1–16. [DOI] [PubMed]
- 71.Goh YJ, Barrangou R. Harnessing CRISPR-Cas systems for precision engineering of designer probiotic lactobacilli [J]. Curr Opin Biotechnol. 2019;56:163–71. [DOI] [PubMed] [Google Scholar]
- 72.Zhao R, Yu T, Li J et al. Single-cell encapsulation systems for probiotic delivery: Armor probiotics [J]. Adv Colloid Interface Sci. 2024;103270. [DOI] [PubMed]
- 73.Dos Santos AM, Carvalho SG, Meneguin AB, et al. Oral delivery of micro/nanoparticulate systems based on natural polysaccharides for intestinal diseases therapy: challenges, advances and future perspectives [J]. Journal of Controlled Release; 2021. [DOI] [PubMed]
- 74.Yao M, Xie J, Du H, et al. Progress in microencapsulation of probiotics: a review [J]. Compr Rev Food Sci Food Saf. 2020;19(2):857–74. [DOI] [PubMed] [Google Scholar]
- 75.Zhang H, Liu Z, Fang H, et al. Construction of Probiotic double-layered Multinucleated microcapsules based on sulfhydryl-modified Carboxymethyl Cellulose Sodium for Increased Intestinal Adhesion of Probiotics and Therapy for intestinal inflammation Induced by Escherichia coli O157:H7 [J]. ACS Applied Materials & Interfaces; 2023. [DOI] [PubMed]
- 76.Cao F, Jin L, Gao Y et al. Artificial-enzymes-armed Bifidobacterium longum probiotics for alleviating intestinal inflammation and microbiota dysbiosis [J]. Nat Nanotechnol, 2023. [DOI] [PubMed]
- 77.Panwar H, Rokana N, Aparna S, et al. Gastrointestinal stress as innate defence against microbial attack [J]. J Appl Microbiol. 2021;130(4):1035–61. [DOI] [PubMed] [Google Scholar]
- 78.Talebian S, Schofield T, Valtchev P, et al. Biopolymer-based Multilayer Microparticles for Probiotic Delivery to Colon [J]. Adv Healthc Mater. 2022;11(11):2102487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Popović M, Stojanović M, Veličković Z, et al. Characterization of potential probiotic strain, L. Reuteri B2, and its microencapsulation using alginate-based biopolymers [J]. Int J Biol Macromol. 2021;183:423–34. [DOI] [PubMed] [Google Scholar]
- 80.Wang X, Gao S, Yun S, et al. Microencapsulating alginate-based polymers for probiotics delivery systems and their application [J]. Pharmaceuticals. 2022;15(5):644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang J, Ji H. Influence of probiotics on dietary protein digestion and utilization in the gastrointestinal tract [J]. Curr Protein Pept Sci. 2019;20(2):125–31. [DOI] [PubMed] [Google Scholar]
- 82.Cook MT, Tzortzis G, Charalampopoulos D, et al. Microencapsulation of probiotics for gastrointestinal delivery [J]. J Controlled Release. 2012;162(1):56–67. [DOI] [PubMed] [Google Scholar]
- 83.Zeise KD, Woods RJ, Huffnagle GB. Interplay between Candida albicans and lactic acid bacteria in the gastrointestinal tract: impact on colonization resistance, microbial carriage, opportunistic infection, and host immunity [J]. Clin Microbiol Rev. 2021;34(4):e00323–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mathipa-Mdakane MG, Thantsha MS. Lacticaseibacillus rhamnosus: a suitable candidate for the construction of novel bioengineered probiotic strains for targeted pathogen control [J]. Foods. 2022;11(6):785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li Z, Behrens AM, Ginat N et al. Probiotics: Biofilm-Inspired Encapsulation of Probiotics for the Treatment of Complex Infections (Adv. Mater. 51/2018) [J]. Advanced Materials. 2018. [DOI] [PubMed]
- 86.Li J, Hu FB. Research digest: reshaping the gut microbiota [J]. The Lancet Diabetes & Endocrinology; 2019. [DOI] [PubMed]
- 87.Topçu KC, Kaya M, Kaban G. Probiotic properties of lactic acid bacteria strains isolated from pastırma [J]. Lwt. 2020;134:110216. [Google Scholar]
- 88.Wang X, Huang M, Yang F, et al. Rapeseed polysaccharides as prebiotics on growth and acidifying activity of probiotics in vitro [J]. Carbohydr Polym. 2015. [DOI] [PubMed]
- 89.Szlufman C, Shemesh M. Role of probiotic Bacilli in developing synbiotic food: challenges and opportunities [J]. Frontiers in Microbiology; 2021. [DOI] [PMC free article] [PubMed]
- 90.Liu H, Cui SW, Chen M, et al. Protective approaches and mechanisms of microencapsulation to the survival of probiotic bacteria during processing, storage and gastrointestinal digestion: a review [J]. Crit Rev Food Sci Nutr. 2019;59(17):2863–78. [DOI] [PubMed] [Google Scholar]
- 91.Tripathi MK, Giri SK. Probiotic functional foods: survival of probiotics during processing and storage [J]. J Funct Foods. 2014;9:225–41. [Google Scholar]
- 92.Fonseca F, Cenard S, Passot S. Freeze-drying of lactic acid bacteria [J]. Cryopreservation and freeze-drying protocols. 2015;477 – 88. [DOI] [PubMed]
- 93.Cruz AGD, Castro W, Faria J, D A F, et al. Stability of probiotic yogurt added with glucose oxidase in plastic materials with different permeability oxygen rates during the refrigerated storage [J]. Food Res Int. 2013;51(2):723–8. [Google Scholar]
- 94.Rajam R, Subramanian P. Encapsulation of probiotics: past, present and future [J]. Beni-Suef Univ J Basic Appl Sci. 2022;11(1):46. [Google Scholar]
- 95.Ge S, Han J, Sun Q, et al. Research progress on improving the freeze-drying resistance of probiotics: a review [J]. Trends in Food Science & Technology; 2024. p. 104425.
- 96.Iaconelli C, Lemetais G, Kechaou N, et al. Drying process strongly affects probiotics viability and functionalities [J]. J Biotechnol. 2015;214:17–26. [DOI] [PubMed] [Google Scholar]
- 97.De Prisco A, Mauriello G. Probiotication of foods: a focus on microencapsulation tool [J]. Trends Food Sci Technol. 2016;48:27–39. [Google Scholar]
- 98.Meybodi NM, Mortazavian AM, Arab M, et al. Probiotic viability in yoghurt: a review of influential factors [J]. Int Dairy J. 2020;109:104793. [Google Scholar]
- 99.Mcfarland LV, Evans CT, Goldstein EJ. Strain-specificity and disease-specificity of probiotic efficacy: a systematic review and meta-analysis [J]. Front Med. 2018;5:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Azad MAK, Sarker M, Li T, et al. Probiotic species in the modulation of gut microbiota: an overview [J]. Biomed Res Int. 2018;2018(1):9478630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sanders ME, Merenstein DJ, Reid G, et al. Probiotics and prebiotics in intestinal health and disease: from biology to the clinic [J]. Nat Reviews Gastroenterol Hepatol. 2019;16(10):605–16. [DOI] [PubMed] [Google Scholar]
- 102.Chua JC, Hale JD, Silcock P, et al. Bacterial survival and adhesion for formulating new oral probiotic foods [J]. Crit Rev Food Sci Nutr. 2020;60(17):2926–37. [DOI] [PubMed] [Google Scholar]
- 103.Grumet L, Tromp Y, Stiegelbauer V. The development of high-quality multispecies probiotic formulations: from bench to market [J]. Nutrients. 2020;12(8):2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yao M, Xie J, Du H, et al. Progress in microencapsulation of probiotics: a review [J]. Comprehensive Reviews in Food Science and Food Safety; 2020. [DOI] [PubMed]
- 105.Yoo JY, Groer M, Dutra S, V O, et al. Gut microbiota and immune system interactions [J]. Microorganisms. 2020;8(10):1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pickard JM, Zeng MY, Caruso R, et al. Gut microbiota: role in pathogen colonization, immune responses, and inflammatory disease [J]. Immunol Rev. 2017;279(1):70–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bubnov RV, Babenko LP, Lazarenko LM, et al. Specific properties of probiotic strains: relevance and benefits for the host [J]. EPMA J. 2018;9:205–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Luo Y, De Souza C, Ramachandran M, et al. Precise oral delivery systems for probiotics: a review [J]. J Controlled Release. 2022;352:371–84. [DOI] [PubMed] [Google Scholar]
- 109.Yadav R, Kumar V, Baweja M, et al. Gene editing and genetic engineering approaches for advanced probiotics: a review [J]. Crit Rev Food Sci Nutr. 2018;58(10):1735–46. [DOI] [PubMed] [Google Scholar]
- 110.Zhou Z, Chen X, Sheng H, et al. Engineering probiotics as living diagnostics and therapeutics for improving human health [J]. Microb Cell Fact. 2020;19:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kan L, Zheng Z, Fu W, et al. Recent progress on engineered micro/nanomaterials mediated modulation of gut microbiota for treating inflammatory bowel disease [J]. J Controlled Release. 2024;370:43–65. [DOI] [PubMed] [Google Scholar]
- 112.Yadav M, Mandeep, Shukla P. Probiotics of diverse origin and their therapeutic applications: a review [J]. J Am Coll Nutr. 2020;39(5):469–79. [DOI] [PubMed] [Google Scholar]
- 113.Forouhandeh H, Soofiyani SR, Hosseini K, et al. Modulation of the Immune System mechanisms using probiotic Bacteria in allergic diseases: focus on allergic retinitis and food allergies [J]. Recent Adv Inflamm Allergy Drug Discovery. 2024;18(1):11–26. [DOI] [PubMed] [Google Scholar]
- 114.Shu S-A, Yuen AW, Woo E, et al. Microbiota and food allergy [J]. Clin Rev Allergy Immunol. 2019;57:83–97. [DOI] [PubMed] [Google Scholar]
- 115.Kim J, Cho K, Kim JS, et al. Probiotic treatment induced change of inflammation related metabolites in IBS-D patients/double-blind, randomized, placebo-controlled trial [J]. Food Sci Biotechnol. 2020;29:837–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Goswami N. Effect of Microgravity Environment on Gut Microbiome and Angiogenesis [J]. 2021. [DOI] [PMC free article] [PubMed]
- 117.O’Mahony L, Mccarthy J, Kelly P et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles [J]. Gastroenterology. 2005. [DOI] [PubMed]
- 118.Gou H-Z, Zhang Y-L, Ren L-F, et al. How do intestinal probiotics restore the intestinal barrier? [J]. Front Microbiol. 2022;13:929346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yousefi B, Eslami M, Ghasemian A, et al. Probiotics importance and their immunomodulatory properties [J]. J Cell Physiol. 2019;234(6):8008–18. [DOI] [PubMed] [Google Scholar]
- 120.Celebioglu HU, Svensson B. Dietary nutrients, proteomes, and adhesion of probiotic lactobacilli to mucin and host epithelial cells [J]. Microorganisms. 2018;6(3):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Das TK, Pradhan S, Chakrabarti S, et al. Current status of probiotic and related health benefits [J]. Appl Food Res. 2022;2(2):100185. [Google Scholar]
- 122.Cao F, Jin L, Gao Y, et al. Artificial-enzymes-armed Bifidobacterium longum probiotics for alleviating intestinal inflammation and microbiota dysbiosis [J]. Nat Nanotechnol. 2023;18(6):617–27. [DOI] [PubMed] [Google Scholar]
- 123.Chowdhury S, Castro S, Coker C et al. Programmable bacteria induce durable tumor regression and systemic antitumor immunity [J]. Nat Med. 2019. [DOI] [PMC free article] [PubMed]
- 124.Gómez-Guillén MC, Montero MP. Enhancement of oral bioavailability of natural compounds and probiotics by mucoadhesive tailored biopolymer-based nanoparticles: a review [J]. Food Hydrocolloids. 2021;118:106772. [Google Scholar]
- 125.Zhao Z, Xu S, Zhang W, et al. Probiotic Escherichia coli NISSLE 1917 for inflammatory bowel disease applications [J]. Food & Function. 2022;13(11):5914–24. [DOI] [PubMed]
- 126.Schultz M. Clinical use of E. Coli Nissle 1917 in inflammatory bowel disease [J]. Inflamm Bowel Dis. 2008;14(7):1012–8. [DOI] [PubMed] [Google Scholar]
- 127.Yu X, Lin C, Yu J, et al. Bioengineered Escherichia coli Nissle 1917 for tumour-targeting therapy [J]. Microb Biotechnol. 2020;13(3):629–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hojo K, Nagaoka S, Murata S et al. Reduction of vitamin K concentration by salivary Bifidobacterium strains and their possible nutritional competition with Porphyromonas gingivalis [J]. J Appl Microbiol. 2007. [DOI] [PubMed]
- 129.Fan L, Si J, Chen S. Su514 Bifidobacterium Adolescentis ameliorates Chronic Colitis by regulating Treg response [J]. Gastroenterology. 2021. [DOI] [PMC free article] [PubMed]
- 130.Roberts JL, Liu G, Darby TM, et al. Bifidobacterium adolescentis supplementation attenuates fracture-induced systemic sequelae [J]. Biomed Pharmacother. 2020. [DOI] [PMC free article] [PubMed]
- 131.Nowak A, Paliwoda A, Błasiak J. Anti-proliferative, pro-apoptotic and anti-oxidative activity of Lactobacillus and Bifidobacterium strains: a review of mechanisms and therapeutic perspectives [J]. Crit Rev Food Sci Nutr. 2019;59(21):3456–67. [DOI] [PubMed] [Google Scholar]
- 132.Sánchez B. Bile acid–microbiota crosstalk in gastrointestinal inflammation and carcinogenesis: a role for bifidobacteria and lactobacilli? [J]. Nat Rev Gastroenterol Hepatol. 2018. [DOI] [PubMed]
- 133.Arvidsson I, Tontanahal A, Johansson K, et al. Apyrase decreases phage induction and shiga toxin release from E. Coli O157:H7 and has a protective effect during infection [J]. Gut Microbes. 2022. [DOI] [PMC free article] [PubMed]
- 134.Henrick BM, Hutton AA, Palumbo MC, et al. Elevated fecal pH indicates a profound change in the breastfed infant gut microbiome due to reduction of Bifidobacterium over the past century [J]. MSphere. 2018;3(2). [DOI] [PMC free article] [PubMed]
- 135.Prince T, Mcbain AJ, O’neill C A. Lactobacillus reuteri protects epidermal keratinocytes from Staphylococcus aureus-induced cell death by competitive exclusion [J]. Appl Environ Microbiol. 2012;78(15):5119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yu Z, Chen J, Liu Y, et al. The role of potential probiotic strains Lactobacillus reuteri in various intestinal diseases: new roles for an old player [J]. Front Microbiol. 2023;14:1095555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang Z, Li L, Wang S, et al. The role of the gut microbiota and probiotics associated with microbial metabolisms in cancer prevention and therapy [J]. Front Pharmacol. 2022;13:1025860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang S, Ishima T, Zhang J, et al. Ingestion of Lactobacillus intestinalis and Lactobacillus reuteri causes depression-and anhedonia-like phenotypes in antibiotic-treated mice via the vagus nerve [J]. J Neuroinflamm. 2020;17:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Idrees M, Imran M, Atiq N, et al. Probiotics, their action modality and the use of multi-omics in metamorphosis of commensal microbiota into target-based probiotics [J]. Front Nutr. 2022;9:959941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Diale MO, Abrahams A, Serepa-Dlamini MH. Isolation and characterization of bacteriocin-like substances from Bacillus paranthracis strain MHSD3, a potential probiotic [J]. 2022.
- 141.Diale MO, Kayitesi E, Serepa-Dlamini MH. Genome in silico and in vitro analysis of the probiotic properties of a bacterial endophyte, Bacillus paranthracis strain MHSD3 [J]. Front Genet. 2021;12:672149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Greppi A, Hemery Y, Berrazaga I, et al. Ability of lactobacilli isolated from traditional cereal-based fermented food to produce folate in culture media under different growth conditions [J]. LWT. 2017;86:277–84. [Google Scholar]
- 143.Li Q, Zeng X, Fu H, et al. Lactiplantibacillus plantarum: a comprehensive review of its antifungal and anti-mycotoxic effects [J]. Trends Food Sci Technol. 2023;136:224–38. [Google Scholar]
- 144.Sakurai T, Odamaki T, Xiao J-Z. Production of Indole-3-Lactic Acid by Bifidobacterium Strains Isolated fromHuman Infants [J]. Microorganisms. 2019. [DOI] [PMC free article] [PubMed]
- 145.Bozzi Cionci N Baffonil, Gaggìa F, et al. Therapeutic microbiology: the role of Bifidobacterium breve as food supplement for the prevention/treatment of paediatric diseases [J]. Nutrients. 2018;10(11):1723. [DOI] [PMC free article] [PubMed]
- 146.Levit R, De Giori GS, De Leblanc ADM et al. Increasing B vitamins in foods to prevent intestinal inflammation and cancer [M]. Nutrients in dairy and their implications on Health and Disease. Elsevier. 2017: 193–204.
- 147.Magryś A, Pawlik M. Postbiotic Fractions of Probiotics Lactobacillus plantarum 299v and Lactobacillus rhamnosus GG Show Immune-Modulating Effects [J]. Cells. 2023. [DOI] [PMC free article] [PubMed]
- 148.Magryś A, Pawlik M. Postbiotic fractions of probiotics Lactobacillus plantarum 299v and Lactobacillus rhamnosus GG show immune-modulating effects [J]. Cells. 2023;12(21):2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Miyamoto J, Mizukure T, Park S-B et al. A gut microbial metabolite of linoleic acid, 10-hydroxy-cis-12-octadecenoic acid, ameliorates intestinal epithelial barrier impairment partially via GPR40-MEK-ERK pathway [J]. J Biol Chem, 2015. [DOI] [PMC free article] [PubMed]
- 150.Le B, Yang SH. Efficacy of Lactobacillus plantarum in prevention of inflammatory bowel disease [J]. Toxicol Rep. 2018;5:314–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Liu Q, Yu Z, Tian F, et al. Surface components and metabolites of probiotics for regulation of intestinal epithelial barrier [J]. Microbial Cell Factories; 2020. [DOI] [PMC free article] [PubMed]
- 152.Wang H, Zhang Q, Niu Y et al. Surface-layer protein from Lactobacillus acidophilus NCFM attenuates tumor necrosis factor-α-induced intestinal barrier dysfunction and inflammation [J]. Int J Biol Macromol. 2019. [DOI] [PubMed]
- 153.Swann J, Rajilic-Stojanovic M, Salonen A, et al. Considerations for the design and conduct of human gut microbiota intervention studies relating to foods [J]. Eur J Nutr. 2020;59:3347–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Schmedes M, Brejnrod AD, Aadland EK, et al. The effect of lean-seafood and non‐Seafood diets on fecal metabolites and gut microbiome: results from a randomized crossover intervention study [J]. Molecular nutrition & food research. 2019;63(1):1700976 [DOI] [PubMed]
- 155.Beaumont M, Portune KJ, Steuer N, et al. Quantity and source of dietary protein influence metabolite production by gut microbiota and rectal mucosa gene expression: a randomized, parallel, double-blind trial in overweight humans [J]. Am J Clin Nutr. 2017;106(4):1005–19. [DOI] [PubMed] [Google Scholar]
- 156.Greenhalgh K, Meyer KM, Aagaard KM, et al. The human gut microbiome in health: establishment and resilience of microbiota over a lifetime [J]. Environ Microbiol. 2016;18(7):2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jones R, Zhu X, Moan E, et al. Inter-niche and inter-individual variation in gut microbial community assessment using stool, rectal swab, and mucosal samples. Sci Rep. 2018;8:4139. [DOI] [PMC free article] [PubMed]
- 158.Li Z, Li Y, Xiao C, et al. Genomic and metabolic features of the Lactobacillus sakei JD10 revealed potential probiotic traits [J]. Microbiological Research; 2021. [DOI] [PubMed]
- 159.Abavisani M, Ebadpour N, Khoshrou A et al. Boosting vaccine effectiveness: the groundbreaking role of probiotics [J]. J Agric Food Res. 2024;101189.
- 160.Chattopadhyay I, Nandi D, Nag A. The pint-sized powerhouse: Illuminating the mighty role of the gut microbiome in improving the outcome of anti-cancer therapy; proceedings of the Seminars in cancer biology, F, 2021 [C]. Elsevier. [DOI] [PubMed]
- 161.Suez J, Zmora N, Segal E, et al. The pros, cons, and many unknowns of probiotics [J]. Nat Med. 2019;25(5):716–29. [DOI] [PubMed] [Google Scholar]
- 162.Trush EA, Poluektova EA, Beniashvilli AG, et al. The evolution of human probiotics: challenges and prospects [J]. Probiotics Antimicrob Proteins. 2020;12:1291–9. [DOI] [PubMed] [Google Scholar]
- 163.Ma J, Lyu Y, Liu X, et al. Eng Probiotics [J] Microb cell Factories. 2022;21(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fooladi S, Rabiee N, Iravani S. Genetically engineered bacteria: a new frontier in targeted drug delivery [J]. J Mater Chem B. 2023;11(42):10072–87. [DOI] [PubMed] [Google Scholar]
- 165.Dangi P, Chaudhary N, Chaudhary V, et al. Nanotechnology impacting probiotics and prebiotics: a paradigm shift in nutraceuticals technology [J]. Int J Food Microbiol. 2023;388:110083. [DOI] [PubMed] [Google Scholar]
- 166.Mohamadzadeh M, Fazeli A, Shojaosadati SA. Polysaccharides and proteins-based bionanocomposites for microencapsulation of probiotics to improve stability and viability in the gastrointestinal tract: a review [J]. Int J Biol Macromol. 2024: 129287. [DOI] [PubMed]
- 167.Shen H, Aggarwal N, Wun KS, et al. Engineered microbial systems for advanced drug delivery [J]. Adv Drug Deliv Rev. 2022;187:114364. [DOI] [PubMed] [Google Scholar]
- 168.Lee H, Kim N, Rheem HB, et al. A decade of advances in single-cell nanocoating for mammalian cells [J]. Adv Healthc Mater. 2021;10(13):2100347. [DOI] [PubMed] [Google Scholar]
- 169.Liu J, Wang Y, Heelan WJ, et al. Mucoadhesive probiotic backpacks with ROS nanoscavengers enhance the bacteriotherapy for inflammatory bowel diseases [J]. Sci Adv. 2022;8(45):eabp8798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhang H, Wei S, Yan J, et al. Development of double layer microcapsules for enhancing the viability of Lactobacillus casei LC2W in simulated gastrointestinal fluids [J]. LWT. 2021;145:111319. [Google Scholar]
- 171.Zaeim D, Sarabi-Jamab M, Ghorani B, et al. Double layer co-encapsulation of probiotics and prebiotics by electro-hydrodynamic atomization [J]. Lwt. 2019;110:102–9. [Google Scholar]
- 172.Brown TD, Whitehead KA, Mitragotri S. Materials for oral delivery of proteins and peptides [J]. Nat Reviews Mater. 2020;5(2):127–48. [Google Scholar]
- 173.Viswanathan P, Muralidaran Y, Ragavan G. Challenges in oral drug delivery: a nano-based strategy to overcome [M]. Nanostructures for oral medicine. Elsevier. 2017: 173–201.
- 174.Deng B, Liu S, Wang Y, et al. Oral nanomedicine: challenges and opportunities [J]. Adv Mater. 2024;36(6):2306081. [DOI] [PubMed] [Google Scholar]
- 175.Onoue S, Yamada S, Chan H-K. Nanodrugs: pharmacokinetics and safety [J]. Int J Nanomed. 2014;1025–37. [DOI] [PMC free article] [PubMed]
- 176.Rostami E, Kashanian S, Azandaryani AH, et al. Drug targeting using solid lipid nanoparticles [J]. Chem Phys Lipids. 2014;181:56–61. [DOI] [PubMed] [Google Scholar]
- 177.Zur Mühlen A, Schwarz C, Mehnert W. Solid lipid nanoparticles (SLN) for controlled drug delivery–drug release and release mechanism [J]. Eur J Pharm Biopharm. 1998;45(2):149–55. [DOI] [PubMed] [Google Scholar]
- 178.Harder BG, Blomquist MR, Wang J, et al. Developments in blood-brain barrier penetrance and drug repurposing for improved treatment of glioblastoma [J]. Front Oncol. 2018;8:462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Badar A, Pachera S, Ansari A, et al. Nano based drug delivery systems: present and future prospects [J]. Nanomed Nanotechnol J. 2019;2(1):121. [Google Scholar]
- 180.Wang M, Li Y, Yang J, et al. Effects of food-grade inorganic nanoparticles on the probiotic properties of Lactobacillus plantarum and Lactobacillus fermentum [J]. LWT. 2021;139:110540. [Google Scholar]
- 181.Dai H, Hosseinpour S, Hua S, et al. Advances in porous inorganic nanomaterials for bone regeneration [J]. Nano TransMed. 2022;1(1):9130005. [Google Scholar]
- 182.Liu Q, Kim YJ, Im GB, et al. Inorganic nanoparticles applied as functional therapeutics [J]. Adv Funct Mater. 2021;31(12):2008171. [Google Scholar]
- 183.Zou Y, Huang B, Cao L, et al. Tailored mesoporous inorganic biomaterials: assembly, functionalization, and drug delivery engineering [J]. Adv Mater. 2021;33(2):2005215. [DOI] [PubMed] [Google Scholar]
- 184.Huang H, Feng W, Chen Y, et al. Inorganic nanoparticles in clinical trials and translations [J]. Nano Today. 2020;35:100972. [Google Scholar]
- 185.Li M, Liu J, Shi L, et al. Gold nanoparticles-embedded ceria with enhanced antioxidant activities for treating inflammatory bowel disease [J]. Bioactive Mater. 2023;25:95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hao W, Cha R, Wang M, et al. Ligand-modified gold nanoparticles as mitochondrial modulators: regulation of intestinal barrier and therapy for constipation [J]. ACS Nano. 2023;17(14):13377–92. [DOI] [PubMed] [Google Scholar]
- 187.Zhu Y-X, You Y, Chen Z, et al. Inorganic nanosheet-shielded probiotics: a self-adaptable oral delivery system for intestinal disease treatment [J]. Nano Lett. 2023;23(10):4683–92. [DOI] [PubMed] [Google Scholar]
- 188.Qin M, Zhang X, Ding H et al. Engineered Probiotic bio-heterojunction with Robust Antibiofilm Modality via Eating Extracellular Polymeric substances for Wound regeneration [J]. Adv Mater. 2024;2402530. [DOI] [PubMed]
- 189.Wang W, Zhang L, Deng Q, et al. Yeast@ MOF bioreactor as a tumor metabolic symbiosis disruptor for the potent inhibition of metabolically heterogeneous tumors [J]. Nano Today. 2022;42:101331. [Google Scholar]
- 190.Buss MT, Ramesh P, English MA et al. Spatial Control of Probiotic Bacteria in the gastrointestinal tract assisted by magnetic particles [J]. Adv Mater, 2021. [DOI] [PubMed]
- 191.Ramalingam V. Multifunctionality of gold nanoparticles: plausible and convincing properties [J]. Adv Colloid Interface Sci. 2019;271:101989. [DOI] [PubMed] [Google Scholar]
- 192.Ahmadi F, Sodagar-Taleghani A, Ebrahimnejad P, et al. A review on the latest developments of mesoporous silica nanoparticles as a promising platform for diagnosis and treatment of cancer [J]. Int J Pharm. 2022;625:122099. [DOI] [PubMed] [Google Scholar]
- 193.Sharma A, Yan J, Siwakoti P, et al. Morphology and temporal interactions of silica particles influence the chemotherapeutic cancer cell death [J]. Nano TransMed. 2024;3:100053. [Google Scholar]
- 194.Kundu S, Ghosh M, Sarkar N. State of the art and perspectives on the biofunctionalization of fluorescent metal nanoclusters and carbon quantum dots for targeted imaging and drug delivery [J]. Langmuir. 2021;37(31):9281–301. [DOI] [PubMed] [Google Scholar]
- 195.Petrovic S, Bita B, Barbinta-Patrascu M-E. Nanoformulations in Pharmaceutical and Biomedical Applications: Green perspectives [J]. Int J Mol Sci. 2024;25(11):5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhou Y, Chen Z, Zhao D, et al. A pH-Triggered Self‐Unpacking Capsule Containing Zwitterionic Hydrogel‐Coated MOF nanoparticles for efficient oral Exendin‐4 delivery [J]. Adv Mater. 2021;33(32):2102044. [DOI] [PubMed] [Google Scholar]
- 197.Lawson HD, Walton SP, Chan C. Metal–organic frameworks for drug delivery: a design perspective [J]. ACS Appl Mater Interfaces. 2021;13(6):7004–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhou Y, Hu Z, Jin W, et al. Intrafibrillar mineralization and immunomodulatory for synergetic enhancement of bone regeneration via calcium phosphate nanocluster scaffold [J]. Adv Healthc Mater. 2023;12(12):2201548. [DOI] [PubMed] [Google Scholar]
- 199.Singh TA, Sharma A, Tejwan N, et al. A state of the art review on the synthesis, antibacterial, antioxidant, antidiabetic and tissue regeneration activities of zinc oxide nanoparticles [J]. Adv Colloid Interface Sci. 2021;295:102495. [DOI] [PubMed] [Google Scholar]
- 200.Asgari S, Pourjavadi A, Licht TR, et al. Polymeric carriers for enhanced delivery of probiotics [J]. Adv Drug Deliv Rev. 2020;161:1–21. [DOI] [PubMed] [Google Scholar]
- 201.Grewal AK, Salar RK. Chitosan nanoparticle delivery systems: an effective approach to enhancing efficacy and safety of anticancer drugs [J]. Nano TransMed. 2024;3:100040. [Google Scholar]
- 202.Alves VD, Torres CA, Freitas F. Bacterial polymers as materials for the development of micro/nanoparticles [J]. Int J Polym Mater Polym Biomaterials. 2016;65(5):211–24. [Google Scholar]
- 203.Dutta H. Potential of polysaccharide nanoparticles in foods [M]. Nanotechnology Horizons in food process Engineering. Apple Acad Press. 2023;85–155.
- 204.Hu Q, Li J, Wang T, et al. Polyphenolic nanoparticle-modified Probiotics for Microenvironment Remodeling and targeted therapy of inflammatory bowel disease [J]. ACS nano. 2024. [DOI] [PubMed]
- 205.Fu J, Liu X, Cui Z, et al. Probiotic-based nanoparticles for targeted microbiota modulation and immune restoration in bacterial pneumonia [J]. Natl Sci Rev. 2023;10(2):nwac221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ajalloueian F, Guerra PR, Bahl MI, et al. Multi-layer PLGA-pullulan-PLGA electrospun nanofibers for probiotic delivery [J]. Food Hydrocolloids. 2022;123:107112. [Google Scholar]
- 207.Yamamoto H, Kuno Y, Sugimoto S, et al. Surface-modified PLGA nanosphere with Chitosan improved pulmonary delivery of calcitonin by mucoadhesion and opening of the intercellular tight junctions [J]. J Controlled Release. 2005;102(2):373–81. [DOI] [PubMed] [Google Scholar]
- 208.Hassan S, Prakash G, Ozturk AB, et al. Evolution and clinical translation of drug delivery nanomaterials [J]. Nano Today. 2017;15:91–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Riccardi D, Baldino L, Reverchon E. Liposomes, transfersomes and niosomes: production methods and their applications in the vaccinal field [J]. J Translational Med. 2024;22(1):339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Tenchov R, Bird R, Curtze AE, et al. Lipid nanoparticles from liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement [J]. ACS Nano. 2021;15(11):16982–7015. [DOI] [PubMed] [Google Scholar]
- 211.Plaza-Oliver M, Santander-Ortega MJ, Lozano MV. Current approaches in lipid-based nanocarriers for oral drug delivery [J]. Drug Delivery Translational Res. 2021;11:471–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zhao P, Xia X, Xu X, et al. Nanoparticle-assembled bioadhesive coacervate coating with prolonged gastrointestinal retention for inflammatory bowel disease therapy [J]. Nat Commun. 2021;12(1):7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Li Z, Yu C. Nanostructured materials: Physicochemical Fundamentals for Energy and Environmental applications [M]. Elsevier. 2023.
- 214.Chen T, Meng W, Li Y et al. Probiotics Armed with in situ mineralized nanocatalysts and targeted Biocoatings for Multipronged Treatment of Inflammatory Bowel Disease [J]. Nano Lett, 2024. [DOI] [PubMed]
- 215.Du X, Wang S, Lou Z, et al. Preparation, characterization and functional properties of ternary composite nanoparticles for enhanced water solubility and bioaccessibility of lutein [J]. Food Hydrocolloids. 2023;144:109039. [Google Scholar]
- 216.Ding X, Xu Y, Wang Y, et al. Carboxymethyl Konjac Glucomannan-Chitosan complex nanogels stabilized double emulsions incorporated into alginate hydrogel beads for the encapsulation, protection and delivery of probiotics [J]. Carbohydr Polym. 2022;289:119438. [DOI] [PubMed] [Google Scholar]
- 217.Lu J, Jiang Z, Ren J, et al. One-Pot synthesis of multifunctional carbon‐based nanoparticle‐supported dispersed Cu2 + disrupts redox homeostasis to enhance CDT [J]. Angew Chem Int Ed. 2022;61(4):e202114373. [DOI] [PubMed] [Google Scholar]
- 218.Yang J, Peng M, Tan S, et al. Calcium Tungstate Microgel enhances the delivery and colonization of Probiotics during Colitis via Intestinal ecological niche occupancy [J]. ACS Cent Sci. 2023;9(7):1327–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Huang W-C, Wang W, Wang W, et al. A double-layer Polysaccharide Hydrogel (DPH) for the enhanced intestine-targeted oral delivery of probiotics [J]. Engineering. 2024;34:187–94. [Google Scholar]
- 220.Rajasekharan SK, Paz-Aviram T, Galili S, et al. Biofilm formation onto starch fibres by Bacillus subtilis governs its successful adaptation to chickpea milk [J]. Microb Biotechnol. 2021;14(4):1839–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wang S, Jiang H, Wang J, et al. Superior in vitro anticancer effect of biomimetic paclitaxel and triptolide co-delivery system in gastric cancer [J]. J Biomedical Res. 2021;35(4):327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Wu F, Liu J. Decorated bacteria and the application in drug delivery [J]. Adv Drug Deliv Rev. 2022;188:114443. [DOI] [PubMed] [Google Scholar]
- 223.Zhang C, Gao X, Ren X, et al. Bacteria-Induced Colloidal Encapsulation for probiotic oral delivery [J]. ACS Nano. 2023. [DOI] [PubMed]
- 224.Xie S, Zhao L, Song X et al. Doxorubicin-conjugated Escherichia coli Nissle 1917 swimmers to achieve tumor targeting and responsive drug release [J]. J Controlled Release, 2017. [DOI] [PubMed]
- 225.Liu C, Xu B, Mcclements DJ, et al. Properties of curcumin-loaded zein-tea saponin nanoparticles prepared by antisolvent co-precipitation and precipitation [J]. Food Chemistry. 2022. [DOI] [PubMed]
- 226.Xie X, Li Q, Jia L, et al. Multishell Colloidosome platform with sequential gastrointestinal resistance for On-Demand probiotic delivery [J]. Adv Healthc Mater. 2023;12(12):2202954. [DOI] [PubMed] [Google Scholar]
- 227.Yin Y, Li Z, Gao H, et al. Microfluidics-Derived Microparticles with Prebiotics and Probiotics for enhanced in situ colonization and immunoregulation of colitis [J]. Nano Lett. 2024;24(4):1081–9. [DOI] [PubMed] [Google Scholar]
- 228.Bazaka K, Jacob MV, Crawford RJ, et al. Plasma-assisted surface modification of organic biopolymers to prevent bacterial attachment [J]. Acta Biomater. 2011;7(5):2015–28. [DOI] [PubMed] [Google Scholar]
- 229.Li J, Xia Q, Guo H, et al. Decorating Bacteria with Triple Immune Nanoactivators generates Tumor-Resident living immunotherapeutics [J]. Angewandte Chemie International Edition. 2022. [DOI] [PubMed]
- 230.Liang Y, Xu J, Sun Z, et al. Research progress on the correlation between bacterial biofilm microenvironment and charge regulation [J]. IEEE Trans Dielectr Electr Insul. 2022;29(4):1540–5. [Google Scholar]
- 231.Li M, Liu N, Zhu J, et al. Engineered probiotics with sustained release of interleukin-2 for the treatment of inflammatory bowel disease after oral delivery [J]. Biomaterials. 2024;309:122584. [DOI] [PubMed] [Google Scholar]
- 232.Liu J, Li W, Wang Y, et al. Biomaterials coating for on-demand bacteria delivery: selective release, adhesion, and detachment [J]. Nano Today. 2021;41:101291. [Google Scholar]
- 233.Wang Y, Liu J, Yuan S et al. Double-layer probiotic encapsulation for enhanced bacteriotherapy against inflammatory bowel disease [J]. Nano Res. 2024: 1–8.
- 234.Zhou J, Li M, Chen Q, et al. Programmable probiotics modulate inflammation and gut microbiota for inflammatory bowel disease treatment after effective oral delivery [J]. Nat Commun. 2022;13(1):3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Xie A, Ji H, Liu Z, et al. Modified prebiotic-based shield armed probiotics with enhanced resistance of gastrointestinal stresses and prolonged intestinal retention for synergistic alleviation of colitis [J]. ACS Nano. 2023;17(15):14775–91. [DOI] [PubMed] [Google Scholar]
- 236.Zhang A, Zhao S, Tyson J et al. Applications of synthetic polymers directed toward living cells [J]. Nat Synthesis. 2024.
- 237.Zheng D-W, Pan P, Chen K-W, et al. An orally delivered microbial cocktail for the removal of nitrogenous metabolic waste in animal models of kidney failure [J]. Nat Biomedical Eng. 2020;4(9):853–62. [DOI] [PubMed] [Google Scholar]
- 238.Long Y, Ma M, Wang Y et al. Sequence-controlled polymers constructed by alkyne-based polymerizations [J]. Giant, 2023:100168.
- 239.Song W-F, Yao W-Q, Chen Q-W, et al. In situ bioorthogonal conjugation of delivered bacteria with gut inhabitants for enhancing probiotics colonization [J]. ACS Cent Sci. 2022;8(9):1306–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Zheng DW, Li RQ, An JX, et al. Prebiotics-encapsulated probiotic spores regulate gut microbiota and suppress colon cancer [J]. Adv Mater. 2020;32(45):2004529. [DOI] [PubMed] [Google Scholar]
- 241.He Yuanqing HY, Ma Chaoyue MC, Pan Ye PY et al. Bioavailability of corn gluten meal hydrolysates and their effects on the immune system [J]. 2018.
- 242.Liu B, Hu J, Yao H, et al. Improved viability of probiotics encapsulated by layer-by-layer assembly using zein nanoparticles and pectin [J]. Food Hydrocolloids. 2023;143:108899. [Google Scholar]
- 243.Fu Q, Wei C, Wang M, Transition-Metal-Based, Nanozymes. Synthesis, mechanisms of therapeutic action, and applications in Cancer treatment [J]. ACS Nano. 2024;18(19):12049–95. [DOI] [PubMed] [Google Scholar]
- 244.Wang Q, Hu L, Wang X, et al. Expanding from materials to biology inspired by biomineralization [J]. Interdisciplinary Materials; 2024.
- 245.Geng Z, Wang X, Wu F, et al. Biointerface mineralization generates ultraresistant gut microbes as oral biotherapeutics [J]. Sci Adv. 2023;9(11):eade0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Wang K, Chen Q, Ding L, et al. Mucoadhesive probiotic-based oral microcarriers with prolonged intestinal retention for inflammatory bowel disease therapy [J]. Nano Today. 2023;50:101876. [Google Scholar]
- 247.Yang C, Zhang W, Bai M, et al. Edible plant-derived extracellular vesicles serve as promising therapeutic systems [J]. Nano TransMed. 2023;2(2):100004. [Google Scholar]
- 248.Liang D, Liu C, Li Y, et al. Engineering fucoxanthin-loaded probiotics’ membrane vesicles for the dietary intervention of colitis [J]. Biomaterials. 2023;297:122107. [DOI] [PubMed] [Google Scholar]
- 249.Gao Y, Deng Y, Geng W, et al. Infectious and inflammatory Microenvironment Self-Adaptive Artificial Peroxisomes with Synergetic Co‐Ru Pair Centers for Programmed Diabetic Ulcer Therapy [J]. Adv Mater. 2024;36(38):2408787. [DOI] [PubMed] [Google Scholar]
- 250.Li Z, Wang Y, Liu J, et al. Chemically and biologically engineered bacteria-based delivery systems for emerging diagnosis and advanced therapy [J]. Adv Mater. 2021;33(38):2102580. [DOI] [PubMed] [Google Scholar]
- 251.Cheng W, He L, Ren W, et al. Bacteria-nanodrug cancer treatment system: the combination of dual swords and the confrontation of needle tips [J]. Nano TransMed. 2023;2(2):100008. [Google Scholar]
- 252.Yang Y, Hu T, Bian Y, et al. Coupling probiotics with 2D CoCuMo-LDH nanosheets as a tumor‐microenvironment‐responsive platform for precise NIR‐II photodynamic therapy [J]. Adv Mater. 2023;35(23):2211205. [DOI] [PubMed] [Google Scholar]
- 253.Cao Y, Liu S, Ma Y et al. Oral nanomotor-enabled mucus Traverse and Tumor Penetration for targeted chemo-sono-immunotherapy against Colon cancer [J]. Small. 2022. [DOI] [PubMed]
- 254.Bender MJ, Mcpherson AC, Phelps CM et al. Dietary tryptophan metabolite released by intratumoral Lactobacillus reuteri facilitates immune checkpoint inhibitor treatment [J]. Cell. 2023. [DOI] [PMC free article] [PubMed]
- 255.Wang Y, Zhao L, Gao L, et al. Health policy and public health implications of obesity in China [J]. The lancet Diabetes & endocrinology. 2021;9(7):446–61. [DOI] [PubMed]
- 256.Takeuchi T, Kameyama K, Miyauchi E et al. Fatty acid overproduction by gut commensal microbiota exacerbates obesity [J]. Cell metabolism. 2023;35(2):361 – 75.e9. [DOI] [PubMed]
- 257.Breisch ST, Zemlan FP, Hoebel BG. Hyperphagia and obesity following serotonin depletion by intraventricular p-chlorophenylalanine [J]. Science. 1976;192(4237):382–5. [DOI] [PubMed] [Google Scholar]
- 258.Jones LA, Sun EW, Martin AM, et al. The ever-changing roles of serotonin [J]. Int J Biochem Cell Biol. 2020;125:105776. [DOI] [PubMed] [Google Scholar]
- 259.Sato M, Uzu K, Yoshida T, et al. Effects of milk fermented byLactobacillusgasseri SBT2055 on adipocyte size in rats [J]. Br J Nutr. 2008;99(5):1013–7. [DOI] [PubMed] [Google Scholar]
- 260.Ribeiro CFA, Silveira G, G D O S, Candido EDS, et al. Effects of antibiotic treatment on gut microbiota and how to overcome its negative impacts on human health [J]. ACS Infect Dis. 2020;6(10):2544–59. [DOI] [PubMed] [Google Scholar]
- 261.Ramirez-Olea H, Reyes-Ballesteros B, Chavez-Santoscoy RA. Potential application of the probiotic Bacillus licheniformis as an adjuvant in the treatment of diseases in humans and animals: a systematic review [J]. Front Microbiol. 2022;13:993451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Pan J, Gong G, Wang Q, et al. A single-cell nanocoating of probiotics for enhanced amelioration of antibiotic-associated diarrhea [J]. Nat Commun. 2022;13(1):2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Shi C, Dawulieti J, Shi F, et al. A nanoparticulate dual scavenger for targeted therapy of inflammatory bowel disease [J]. Sci Adv. 2022;8(4):eabj2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Peng P, Feng T, Yang X, et al. Gastrointestinal Microenvironment Responsive Nanoencapsulation of Probiotics and drugs for synergistic therapy of Intestinal diseases [J]. ACS Nano. 2023. [DOI] [PubMed]
- 265.Xu J, Xu J, Shi T, et al. Probiotic-inspired nanomedicine restores intestinal homeostasis in colitis by regulating redox balance, immune responses, and the gut microbiome [J]. Adv Mater. 2023;35(3):2207890. [DOI] [PubMed] [Google Scholar]
- 266.Miller V, Jenkins DA, Dehghan M, et al. Associations of the glycaemic index and the glycaemic load with risk of type 2 diabetes in 127 594 people from 20 countries (PURE): a prospective cohort study [J]. The Lancet Diabetes & Endocrinology. 2024;12(5): 330–8. 5. [DOI] [PubMed]
- 267.Chen Y, Li P, Modica JA, et al. Acid-resistant mesoporous metal–organic framework toward oral insulin delivery: protein encapsulation, protection, and release [J]. J Am Chem Soc. 2018;140(17):5678–81. [DOI] [PubMed] [Google Scholar]
- 268.Hunt NJ, Lockwood GP, Heffernan SJ et al. Oral nanotherapeutic formulation of insulin with reduced episodes of hypoglycaemia [J]. Nat Nanotechnol, 2024: 1–11. [DOI] [PMC free article] [PubMed]
- 269.Takeuchi T, Kubota T, Nakanishi Y, et al. Gut microbial carbohydrate metabolism contributes to insulin resistance [J]. Nature. 2023;621(7978):389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Amini M, Zayeri F, Salehi M. Trend analysis of cardiovascular disease mortality, incidence, and mortality-to-incidence ratio: results from global burden of disease study 2017 [J]. BMC Public Health. 2021;21:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Pavlidou E, Fasoulas A, Mantzorou M, et al. Clinical evidence on the potential beneficial effects of probiotics and prebiotics in cardiovascular disease [J]. Int J Mol Sci. 2022;23(24):15898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Kumar D, Ashraf GM, Bilgrami AL, et al. Emerging therapeutic developments in neurodegenerative diseases: a clinical investigation [J]. Drug Discovery Today. 2022;27(10):103305. [DOI] [PubMed] [Google Scholar]
- 273.Sanmarco LM, Rone JM, Polonio CM, et al. Lactate limits CNS autoimmunity by stabilizing HIF-1α in dendritic cells [J]. Nature. 2023;620(7975):881–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Liang D, Wu F, Zhou D, et al. Commercial probiotic products in public health: current status and potential limitations [J]. Crit Rev Food Sci Nutr. 2024;64(19):6455–76. [DOI] [PubMed] [Google Scholar]
- 275.Liu J, Yuan S, Bremmer A, et al. Convergence of Nanotechnology and Bacteriotherapy for Biomedical applications [J]. Adv Sci. 2024;11(16):2309295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Hoffmann DE, Fraser CM, Palumbo F, et al. Probiotics: achieving a better regulatory fit [J]. Food Drug Law J. 2014;69(2):237. [PMC free article] [PubMed] [Google Scholar]
- 277.Toscano M, Vecchi ED, Rodighiero V, et al. Microbiological and genetic identification of some probiotics proposed for medical use in 2011 [J]. J Chemother. 2013;25(3):156–61. [DOI] [PubMed] [Google Scholar]
- 278.Gong Y, Liu Z, Zhou P, et al. Biomimetic nanocarriers harnessing microbial metabolites usher the path for brain disease therapy [J]. Nano TransMed. 2023;2(4):100020. [Google Scholar]
- 279.Parker A, Fonseca S, Carding SR. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health [J]. Gut Microbes. 2020;11(2):135–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Wu D, Chen Q, Chen X, et al. The blood–brain barrier: structure, regulation, and drug delivery [J]. Signal Transduct Target Therapy. 2023;8(1):217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Abbott NJ, Patabendige AA, Dolman DE, et al. Structure and function of the blood–brain barrier [J]. Neurobiol Dis. 2010;37(1):13–25. [DOI] [PubMed] [Google Scholar]
- 282.Hang Z, Lei T, Zeng Z, et al. Composition of intestinal flora affects the risk relationship between Alzheimer’s disease/Parkinson’s disease and cancer [J]. Biomedicine & Pharmacotherapy. 2022;145:112343. [DOI] [PubMed]
- 283.Skonieczna-Żydecka K, Jakubczyk K, Maciejewska-Markiewicz D, et al. Gut biofactory—neurocompetent metabolites within the gastrointestinal tract. Scoping Rev [J] Nutrients. 2020;12(11):3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Guo M, Yang C, Li B, et al. Bionic dormant body of timed wake-up for bacteriotherapy in vivo [J]. ACS Nano. 2022;16(1):823–36. [DOI] [PubMed] [Google Scholar]
- 285.Suda K, Matsuda K. How microbes affect depression: underlying mechanisms via the gut–brain axis and the modulating role of probiotics [J]. Int J Mol Sci. 2022;23(3):1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Ju S, Shin Y, Han S, et al. The gut–brain axis in schizophrenia: the implications of the gut microbiome and SCFA production [J]. Nutrients. 2023;15(20):4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Zhu L, Yu T, Wang W, et al. Responsively degradable nanoarmor-assisted Super Resistance and stable colonization of Probiotics for enhanced inflammation‐targeted delivery [J]. Adv Mater. 2024;36(18):2308728. [DOI] [PubMed] [Google Scholar]
- 288.Gershon MD, Margolis KG. The gut, its microbiome, and the brain: connections and communications [J]. J Clin Investig. 2021;131(18). [DOI] [PMC free article] [PubMed]
- 289.Wang S, Zhou X, Ma Y et al. Gut-to-brain neuromodulation by synthetic butyrate-producing commensal bacteria [J]. Innov Life, 2024: 100082–1.
- 290.Agirman G, Hsiao EY, SnapShot. The microbiota-gut-brain axis [J]. Cell. 2021;184(9):2524. e1. [DOI] [PubMed] [Google Scholar]
- 291.Bravo JA, Forsythe P, Chew MV et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve [J]. Proceedings of the National Academy of Sciences. 2011;108(38):16050-5. [DOI] [PMC free article] [PubMed]
- 292.De Santa F, Strimpakos G, Marchetti N, et al. Effect of a multi-strain probiotic mixture consumption on anxiety and depression symptoms induced in adult mice by postnatal maternal separation [J]. Microbiome. 2024;12(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.