Abstract
Nectar is an important source of food for adult mosquitoes, influencing their biological characteristics including longevity, fecundity, and flight range. Consequently, it can impact mosquitoes’ survival and efficiency in transmitting disease. Different mosquito species are known to show preferences for flower nectar from certain plants, yet despite the importance of these plant–mosquito associations, knowledge of such biotic interactions is sparse. Here, we present a systematic map to address the question: “Which nectar do mosquitoes feed on?.” The mapping process identified 49 articles (comprising 51 studies) meeting inclusion criteria, detailing 397 records of 74 mosquito species feeding on nectar from 145 plant species and 109 genera. Data extracted from the map were then analyzed to better understand if mosquitoes showed preferences for specific plant nectar. A key finding from this study is clear evidence supporting the hypothesis that mosquitoes exhibit preferences for nectar from particular plant species, including 77 species of plants and 58 genera for Aedes species, 18 species of plants and 17 genera for Anopheles species, and 16 species of plants and 16 genera for Culex species (all 3 genera belong in Diptera: Culicidae). Our study also highlighted the need for further field and laboratory work in time and space and using methods that randomly selects plant species for investigation. This would facilitate a better understanding of the relationship between mosquito feeding behavior and nectar seasonality and abundance; data that are critical for the development and improvement of new mosquito control methods to tackle vector-borne diseases.
Keywords: mosquito, nectar feeding, vector biology, vector control, meta-synthesis
Introduction
Nectar is a critical source of food for adult mosquitoes of both sexes. Plant-derived sugar is the only source of food for male mosquitoes and is required for their overall survival, to fly and copulate. If sugar-deprived, male mosquitoes may not achieve reproductive success (Foster 1995). Female mosquitoes also feed on nectar, but in many species, they need blood meals to provide additional nutrients to complete egg maturation (Valzania et al. 2019, Harrison et al. 2021). Nectar from flowers (floral nectar) appears to be the preferred food for both male and female mosquitoes, although sometimes they also obtain sugar from other plant sources, including extrafloral nectaries, fruits, and plant tissue, as well as honeydew from other sap feeding insects (Clements 1963, Foster 1995, Stone and Foster 2013).
Nectar is produced by the nectaries of plants present in the flower (flower nectaries) or outside the flower (extrafloral nectaries) and its composition can vary between different species of plants. While it primarily consists of water and sugars (sucrose, glucose, fructose), the exact ratio of these sugars, as well as the presence of other compounds like amino acids, vitamins, minerals, and secondary metabolites can differ significantly (Nicolson 2022). These variations can impact the attractiveness of nectar to specific insects. Both flower nectar and extrafloral nectar mainly attract and reward insects; however, they serve different ecological functions (González-Teuber and Heil 2009). Flower nectar is present in the flower, close to the reproductive organs, and serves as a reward for pollinators when transferring pollen from flower to flower. Extrafloral nectaries are most often found on the leaves and petioles of the plant, and are suggested to work as an indirect defense of the plant as they attract herbivores’ predators such as ants (Nepi et al. 2018).
Early studies of nectar feeding behavior usually linked the species of mosquitoes to the plants by observing where mosquitoes fed and rested in the field (Müller 1873, Knab 1907, Haeger 1955, Sandholm and Price 1962, Grimstad and DeFoliart 1974). However, recently developed laboratory techniques (Van Handel 1972, Schlein and Muller 1995, Burkett et al. 1999, Junnila et al. 2010) have also allowed the identification of the plant species and the part of the plant where mosquitoes have fed by analyzing the plant tissue and sugars in the mosquito’s gut (Gary Jr and Foster 2004, Impoinvil et al. 2004, Manda et al. 2007b, Gouagna et al. 2010). These laboratory-based methods not only save researchers time in the field but also allow for more observations and better control of variables. The main focus of these latter studies has tended to be on understanding the mechanisms used by mosquitoes to identify their preferred flowering plants including visual (Orbán and Plowright 2014, Bernáth et al. 2016, Dieng et al. 2018, Peach et al. 2019), olfactory (Manda et al. 2007a, Nyasembe et al. 2012, 2018, Gouagna et al. 2014, Nikbakhtzadeh et al. 2014, Yu et al. 2017, 2018, Lahondère et al. 2020), and gustatory (Ignell et al. 2010, Chen and Kearney 2015, Kessler et al. 2015) cues.
Understanding whether there is a preference of mosquitoes for certain plant types is an important question for a number of reasons: First, given the critical role of plant nectar in a mosquito’s life cycle, if there are particular species of plants that mosquitoes prefer to feed on, then a high concentration of these plants on a landscape could increase vector abundance (Russell et al. 2011, Moiroux et al. 2012, Padonou et al. 2012, Durnez and Coosemans 2013, Mwesigwa et al. 2017). Second, and equally important, the growing resistance to insecticides like pyrethroids and the shift in biting behavior reducing mosquito exposure to insecticide treated bednets or indoor residual spraying are challenging the effectiveness of these traditional control strategies (Moiroux et al. 2012, Durnez and Coosemans 2013, Hemingway et al. 2016, Ranson and Lissenden 2016, Mwesigwa et al. 2017, Van den Berg et al. 2021). As such, new mosquito control methods, such as attractive targeted sugar baits (ATSBs) (Müller and Schlein 2006, Schlein and Müller 2008, Müller et al. 2010, 2011, Sissoko et al. 2019), have been developed and are of interest in areas of high mosquito abundance. These novel interventions combine floral or fruit sugars with insecticide compounds targeted for mosquito control. As a method, this has shown successful results across multiple species of mosquitoes and in some environments (Schlein and Müller 2008, Müller et al. 2010, Gu et al. 2011, Traore et al. 2020). However, it is uncertain if large amounts of naturally occurring sugars in nectar from plant species that mosquitoes prefer to feed on, will influence the effectiveness of the ATSBs. Thus, it is important to better understand the impact of potential competition from naturally occurring nectar resources across different landscapes.
The importance of understanding this relationship between nectar resource availability and mosquito abundance has been acknowledged many times (Gary Jr. and Foster 2001, Okech et al. 2003, Gary Jr and Foster 2004, Impoinvil et al. 2004, Müller and Schlein 2006, Manda et al. 2007a, Schlein and Müller 2008, Müller et al. 2010, Gu et al. 2011, Beier et al. 2012, Stone et al. 2012, Qualls et al. 2013, Yu et al. 2016, Sissoko et al. 2019, Nyasembe et al. 2021), and there are now many published studies that have looked at individual aspects of this functional relationship involving various plant/mosquito genera. However, to our knowledge, the evidence yielded from these studies has not yet been synthesized to investigate whether there is overall evidence to show that certain species of mosquito feed on particular plant species. The alternative hypothesis is that all mosquitoes are generalist feeders and feed on any available nectar on the landscape.
In this study, we therefore reviewed the studies to date that have examined the relationship between mosquitoes and plant feeding, both in field and laboratory studies, to ascertain if collectively the data they contain can provide a better understanding on whether mosquitoes exhibit feeding preferences for specific plant species.
Materials and Methods
Our study involved creating a systematic map to assimilate the current knowledge on mosquito nectar feeding (see the section “Visualization of the Systematic Map”), followed by meta-analysis of the data extracted to determine whether mosquitoes exhibit preferences for specific plant nectars (see sections “Risk of Bias Assessment to Study Mosquito Nectar Preferences” and “Data Handling and Meta-analysis”).
Our systematic map followed the protocol published by the Collaboration for Environmental Evidence (CEE) (Pullin et al. 2022) and addressed the question: “Which nectar do mosquitoes feed on?” (see Supplementary Appendix 1). By following this protocol, we aimed to (i) identify relevant studies that contribute toward current knowledge on mosquito and plant nectar feeding and (ii) quantify knowledge gaps in the identified evidence. Key stages of the protocol are outlined below:
Searches
We conducted an initial bibliographic search in December 2020 and updated this with a later supplementary search in May 2022. We also conducted snowballing, whereby we identified earlier sources from the reference lists of selected papers (backward snowballing process) (Sayers 2007). To ensure that the search captured all literature pertaining to our research question, an iterative approach was applied and in accordance with systematic mapping practice, our research question was framed within the population (P), exposure (E) and outcome (O) (PEO) framework (Frampton et al. 2017), as shown in Table 1.
Table 1.
PEO framework
| Category | Definition | Description |
|---|---|---|
| P—Population | Populations of subject(s) of relevance to the review question. | Studies related to any mosquito species |
| E—Exposure | Environmental variable relevant to the Population. | Studies related to plant nectar (floral and extrafloral nectar) |
| O—Outcome | Documented record(s) of the Population and Exposure. | Studies including records of a mosquito feeding on a plant’s nectar (including any plant in any geographical area) |
We conducted searches across 3 bibliographic databases: Web of Science, Scopus, and CAB Abstracts (Table 2). The search terms were structured using the PEO framework and tested against a set of 19 relevant articles, known as the test library. Keywords were combined with Boolean operators (AND, OR) and iteratively refined until all articles from the test library were consistently retrieved. A full search was completed on 1 December 2020, and the retrieved articles were exported to the reference management software EndNote X9.3.3., where duplicates were removed. Finalized search terms and the test library are provided in Supplementary Appendix 1.
Table 2.
Database search
| Database | Type of literature | Description of search |
|---|---|---|
| Web of Science | Platform of bibliographic databases | All records included in the 1945–2022 databases |
| Scopus | Bibliographic database | All records to 2022 |
| Cab Abstracts | Bibliographic database | All records included in the 1910–2022 databases |
| Snowballing process | Grey literature | Relevant published articles cited and found in reference lists of our relevant studies (Sayers 2007) based on the assumption that these studies are relevant to our systematic map. |
Screening
After deduplication, articles were uploaded into Colandr (https://colandrcommunity.com), an open-access platform that uses machine learning for screening and data extraction (Kahili-Heede and Hillgren 2021). Initial screening was done at the title-abstract level. To ensure consistency, 100 articles were randomly selected for double screening, and agreement between reviewers was measured using Cohen’s Kappa (Cohen 1960), with a minimum score of 0.69 achieved (recommended threshold by CEE (Pullin et al. 2022) is > 0.6). Once consistency was evaluated, articles were divided into 3 batches for independent screening by reviewers, applying PEO eligibility criteria. Full-text screening was conducted by one reviewer, with details of exclusions provided in Supplementary Appendix 2.
Data Coding
The items outlined in Table 3 were extracted from the studies, and results are presented in Supplementary Appendices 2 and 3. If the plant species was not identified but the genus was provided followed by “sp.”, it was treated as a species. However, when the genus was followed by “spp.”, it was counted as a genus. Articles often included multiple studies on mosquito nectar feeding and, in such cases, we added suffixes (a, b, or c) to the article codes to differentiate between studies. Additionally, each study could report multiple records of mosquitoes feeding on nectar.
Table 3.
Data extraction for studies that met the inclusion criteria in the full text.
| Coded elements | Description |
|---|---|
| Study metadata | Authors, Title, Journal, DOI, Year of publication and Abstract (Supplementary Appendix 2) |
| Study area metadata | Location of the study (Supplementary Appendix 3) |
| Methodology | Information about the study methods—including study design (field/lab based/semi-field, observation/experimental) (Supplementary Appendix 3) |
| Mosquito data | Mosquito species and sex (Supplementary Appendix 3) |
| Plant data | Plant species and family—including number of plants studied and plant nectar where mosquitoes fed on (Supplementary Appendix 3) |
| Study data | Information about the study design (controlled/not controlled, number of repetitions and measurements taken in the study) (Supplementary Appendix 3) |
| Selection of the plants | Reasons that researchers used to select the plants of the study (Supplementary Appendix 3) |
| Outcomes | Purposes of the authors when carrying the study out (Supplementary Appendix 3) |
Visualization of the Systematic Map
We applied the final data from the systematic map to create an interactive map using the Thalloo mapping framework (Martin 2017). In this we included filters to allow different elements of data (for example, plant family, geographic location, study design, and purpose of the study) to be displayed separately allowing a better comprehension of the underlying information. Data from the United States (U.S.A.) were presented by state instead of for the whole country, as there was a large number of records from this country. Due to their relevance in transmission of vector-borne diseases, some mosquito species have traditionally been subject to a greater scrutiny than others and have notably more records. Therefore, where data allowed, these species were studied individually. The remaining mosquito records were at genus level.
Risk of Bias Assessment to Study Mosquito Nectar Preferences
While all studies retrieved from our mapping process reported instances of mosquitoes feeding on nectar, only those employing a rigorous method were included for the analysis of potential nectar preferences. To select this subset of studies, we investigated a risk of bias assessment based on 3 critical criteria as follows:
i) Random plant selection: This criterion resulted in the exclusion of those studies where plant selection was non-random, for example, when plants were chosen based on prior results or specific plant characteristics.
ii) Multiple plant choices for mosquitoes: The presence of more than one plant that mosquitoes could feed on in the study was considered essential for a comprehensive understanding of mosquito preferences on nectar. Single-choice assay is an example of tests that only used one plant.
iii) Replication of studies: The inclusion of replicates in the study design was considered crucial, as studies lacking replicates were susceptible to bias. The absence of replicates raised the possibility of other variables influencing mosquito preferences, such as the location of the plants in the study.
The risk of bias was defined as ‘high’ when the response to any of the 3 critical criteria was negative. Information on the calculation of the risk of bias is included in Supplementary Appendix 4.
Data Handling and Meta-analysis
Determination of Mosquito Preferences for Specific Plant Nectar
Details of the total number of plants included in the studies classified as low risk of bias (see Supplementary Appendix 4) was constructed, and the total number of plants that mosquitoes fed on from these studies was then used to determine if mosquitoes showed preferences for specific plant nectar.
To explore these preferences, we calculated the ratio between the number of preferred plants and the total number of plants investigated in each study classified as low risk of bias (see Supplementary Appendix 4). We then used this ratio as an indicator of whether mosquitoes exhibited preferences for specific plant species. While ideally, we could expect this ratio to be 1 (100%) when mosquitos show no preference for specific nectar types, this outcome is unlikely to be met in every such case. Hence, and as a rule of thumb, a ratio falling below 0.7 would indicate a tendency for mosquitoes to favor certain plants during their feeding activities. The same methodology was applied for the 3 genera of mosquitoes for which we obtained the most records in our analysis (Aedes, Anopheles, and Culex—Diptera: Culicidae) to explore if different mosquito genera showed preferences for a particular plant nectar.
Statistical Analysis
Chi-squared tests were conducted in R version 4.3.0 (2023-04-21) on those studies classified as low risk of bias and where the number of observations was sufficient. As a general guideline, we ran tests on all studies when the expected mosquito counts per plant species were 5 or greater (McHugh 2013). We also kept the analyses separate by study, as each included a different number of plants and mosquitoes (Supplementary Appendix 5).
Representation of Mosquito Genus—Plant Family Preferences
To visualize the interactions between different mosquito genera with plant families, 2 chord diagrams were created in R version 4.3.0 (2023-04-21) using the package circlize 0.4.16 (Gu et al. 2014). The first one included all the records of mosquitoes feeding on plant nectar in the systematic map. The second chord diagram specifically included records from studies registered as having low risk of bias (as stated in ‘Determination of Mosquito Preferences for Specific Plant Nectar’), and thereby providing a representation of the preferences of different mosquito genera for particular plant families.
Results
Study Characteristics
The total number of articles retrieved from database searches was 1,671. After deduplication and title and abstract screening, 222 articles were selected. After reading the full text, 171 articles did not fulfill the PEO inclusion criteria and were not included in the systematic map. At the end of the selection process, 49 articles (all of them were journal articles) detailing 51 studies fulfilled all our inclusion criteria. These studies reported one or more records of mosquitoes feeding on plant nectar, amounting to a total of 397 records of different species of mosquitoes feeding on diverse plant species. The number of articles identified and included/excluded at each stage of the systematic mapping process is detailed in Figure 1. Individual records and their associated metadata may be viewed and filtered on the online visualization (Fig. 2): https://oxlel.github.io/evidencemaps/mosquito_map/. All references are included in Table 4.
Fig. 1.
Workflow of the systematic map showing number of sources identified at each stage of the mapping process.
Fig. 2.
Geographical map showing the spatial distribution of the 397 records of mosquitoes (field and laboratory studies) feeding on plant nectar that were included in the final systematic map dataset. The colors represent different species of mosquitoes, and the pie size is related to the number of studies found per country (or by state for U.S.A. records). More filters are available in the online version of the map: https://oxlel.github.io/evidencemaps/mosquito_map/
Table 4.
List of references retrieved from the systematic review process
| References | Location | Mosquito genera | Plant genera | Plant family |
|---|---|---|---|---|
| Twinn et al. (1948) | Canada | Aedes | Platanthera | Orchidaceae |
| Haeger (1955) | Florida, USA | Aedes, Psorophora | Avicennia, Conocarpus | Ancanthaceae, Combretaceae |
| Haeger (1960) | Florida, USA | Aedes | Avicennia, Bidens, Eupatorium | Ancanthaceae, Asteraceae |
| Sandholm and Price (1962) | Minnesota, USA | Aedes, Coquillettidia, Culex, Culiseta | Apocynum, Aquilegia, Asclepias, Aster, Catalpa, Chrysanthemum, Cirsium, Cornus, Dasiphora, Echinocystis, Eutrochium, Fraxinus, Heracleum, Hydrangea, Lilium, Melilotus, Monarda, Nepeta, Pastinaca, Philadelphus, Phlox, Physocarpus, Rosa, Rudbeckia, Solidago, Spiraea, Symphoricarpos, Syringa, Tamarix, Tanacetum, Trifolium, Verbena | Apocynaceae, Asteraceae, Bignoniaceae, Caprifoliaceae, Cornaceae, Cucurbitaceae, Fabaceae, Hydrangeaceae, Lamiaceae, Liliaceae, Oleaceae, Polemoniaceae, Ranunculaceae, Rosaceae, Tamaricaceae, Umbelliferae, Verbenaceae |
| Abdel-Malek (1964) | Egypt | Anopheles | Alhagi, Juncus, Salicornia | Fabaceae, Juncaceae, Amaranthaceae |
| Corbet (1964) | Canada | Aedes | Dryas | Rosaceae |
| McCrae et al. (1969) | Uganda | Aedes, Anopheles, Culex, Eretmapodites, Ficalbia, Mansonia, Toxorhynchites, Uranotaenia | Harungana | Hypericaceae |
| Thien (1969) | Wisconsin, USA | Aedes | Platanthera | Orchidaceae |
| Schaefer and Miura (1972) | California, USA | Culex | Helianthus, Heterotheca | Asteraceae |
| Grimstad and DeFoliart (1974) | Wisconsin, USA | Aedes, Anopheles, Coquillettidia, Culex, Culiseta | Achillea, Apocynum, Asclepias, Cornus, Eupatorium, Euphorbia, Leucanthemum, Liatris, Maianthemum, Monarda, Prunus, Salix, Silene, Solidago, Spiraea | Apocynaceae, Asparagaceae, Asteraceae, Caryophyllaceae, Cornaceae, Euphorbiaceae, Lamiaceae, Rosaceae, Salicaceae |
| Magnarelli (1977) | Connecticut, USA | Aedes | Achillea, Daucus, Gerardia, Hieracium, Leucanthemum, Malva, Rosa, Solidago, Taraxacum | Apiaceae, Asteraceae, Malvaceae, Orobanchaceae, Rosaceae |
| Magnarelli (1978) | Connecticut, USA | Aedes | Achillea, Daucus, Hieracium, Leucanthemum, Malva, Rosa, Solidago | Apiaceae, Asteraceae, Malvaceae, Rosaceae |
| Magnarelli (1979) | Connecticut, USA | Aedes | Achillea, Daucus, Hieracium, Leucanthemum, Malva, Rosa, Solidago | Apiaceae, Asteraceae, Malvaceae, Rosaceae |
| Magnarelli (1980) | Connecticut, USA | Aedes | Achillea, Hieracium, Leucanthemum, Solidago | Asteraceae |
| Magnarelli (1983) | Connecticut, USA | Psorophora | Aster | Asteraceae |
| Vargo and Foster (1984) | Ohio, USA | Aedes, Culex | Ageratina, Solidago | Asteraceae |
| Andersson and Jaenson (1987) | Sweden | Aedes, Anopheles, Coquillettidia, Culex, Culiseta | Tanacetum | Asteraceae |
| Healy and Jepson (1988) | UK | Anopheles | Achillea | Asteraceae |
| Jepson and Healy (1988) | UK | Aedes | Leucanthemum | Asteraceae |
| Mogi and Miyagi (1989) | Philippines | Topomyia | Mucuna, Psidium | Fabaceae, Myrtaceae |
| Andersson (1990) | Sweden | Aedes | Achillea, Aegopodium, Anthriscus, Myosotis, Rubus, Vaccinium | Apiaceae, Asteraceae, Boraginaceae, Ericaceae, Rosaceae |
| Jaenson and Ameneshewa (1991) | Sweden | Anopheles | Achillea, Tanacetum | Asteraceae |
| Gadawski and Smith (1992) | Aedes | Actaea, Amelanchier, Cornus, Crataegus, Leucanthemum, Prunus, Pyrus, Ranunculus, Rhamnus, Ribes, Rubus, Syringa, Taraxacum, Zanthoxylum | Asteraceae, Cornaceae, Grossulariaceae, Oleaceae, Ranunculaceae, Rhamnaceae, Rosaceae, Rutaceae | |
| Yee et al. (1992) | Ohio, USA | Aedes | Apocynum, Asclepias, Daucus, Leucanthemum | Apiaceae, Apocynaceae, Asteraceae |
| Smith and Gadawski (1994) | Canada | Aedes | Prunus | Rosaceae |
| Burkett et al. (1999) | Florida, USA | Aedes, Culex, Psorophora, Uranotaenia | Cassia | Fabaceae |
| Gary Jr and Foster (2004) | Ohio, USA | Anopheles | Manihot, Ricinus | Euphorbiaceae |
| Impoinvil et al. (2004) | Kenya | Anopheles | Ipomoea, Lantana, Ricinus | Convolvulaceae, Verbenaceae, Euphorbiaceae |
| Müller and Schlein (2006) | Israel | Aedes, Anopheles | Acacia | Fabaceae |
| Manda et al. (2007b) (a) | Kenya | Anopheles | Hamelia, Parthenium, Ricinus, Senna, Tecoma | Asteraceae, Bignoniaceae, Euphorbiaceae, Fabaceae, Rubiaceae |
| Manda et al. (2007b) (c) | Kenya | Anopheles | Hamelia, Lantana, Parthenium, Ricinus, Senna, Tecoma | Asteraceae, Bignoniaceae, Euphorbiaceae, Fabaceae, Rubiaceae, Verbenaceae |
| Manda et al. (2007b) (b) | Kenya | Anopheles | Parthenium, Ricinus, Senna, Tecoma | Asteraceae, Bignoniaceae, Euphorbiaceae, Fabaceae, |
| Schlein and Müller (2008) | Israel | Culex | Acacia, Polygonum, Tamarix | Fabaceae, Polygonaceae, Tamaricaceae |
| Gouagna et al. (2010) | Burkina Faso | Anopheles | Cassia, Delonix, Mangifera, Senna, Thevetia | Anacardiaceae, Apocynaceae, Fabaceae |
| Gu et al. (2011) | Israel | Anopheles | Acacia | Fabaceae |
| Dötterl et al. (2012) | Germany | Aedes, Anopheles, Culex | Silene | Caryophyllaceae |
| Gouagna et al. (2014) | Reunion | Anopheles | Duranta, Stachytarpheta | Verbenaceae |
| Chen and Kearney (2015) | Texas, USA | Aedes, Culex | Asclepias, Campis, Impatiens, Passiflora, Ricinus | Asclepiadaceae, Balsaminaceae, Bignoniaceae, Euphorbiaceae, Passifloraceae |
| Hien et al. (2016) | Burkina Faso | Anopheles | Barleria, Thevetia | Acanthaceae, Apocynaceae |
| Nikbakhtzadeh et al. (2016) | Ohio, USA | Anopheles | Lantana, Parthenium, Senna | Asteraceae, Fabaceae, Verbenaceae |
| Peach and Gries (2016) | Canada | Culex, Culiseta | Achillea, Leucanthemum, Tanacetum | Asteraceae |
| Kenney et al. (2017) | West Virginia, USA | Aedes | Cucurbita | Cucurbitaceae |
| Müller et al. (2017) | Mali | Anopheles | Prosopis | Fabaceae |
| Rewicz et al. (2017) | Poland | Culicidae | Epipactis | Orchidaceae |
| Nyasembe et al. (2018) | Kenya | Aedes, Anopheles | Hibiscus, Leonotis, Opuntia, Parthenium, Pithecellobium, Ricinus, Senna | Asteraceae, Cactaceae, Euphorbiaceae, Fabaceae, Lamiaceae, Malvaceae |
| Yu et al. (2018) | China | Culex | Abelia, Ligustrum, Nerium | Apocynaceae, Caprifoliaceae, Oleaceae |
| Sissoko et al. (2019) | Mali | Aedes | Acacia, Galphimia, Lantana, Prosopis | Fabaceae, Malpighiaceae, Verbenaceae |
| Pruett et al. (2020) | Florida, USA | Aedes, Culex | Impatiens | Balsaminaceae |
| Nyasembe et al. (2021) | Kenya | Aedes | Leonotis, Opuntia, Pithecellobium | Cactaceae, Fabaceae, Lamiaceae |
| Patil and Jagdale (2021) | India | Culex | Luffa | Cucurbitaceae |
| Rambey et al. (2021) | Indonesia | Aedes | Amorphophallus | Araceae |
The records included in our analyses (field and laboratory based) described observations of mosquitoes feeding on plant nectar in 17 different countries. The largest body of work on mosquito feeding behavior is found in North America (number of studies (n) = 24, 288 records). In comparison, far fewer studies have been carried out by researchers on the African continent (n = 13, 67 records), with studies in Kenya detailing the largest number of records of mosquito floral preferences in Africa (n = 6; 31 records). A total of 7 studies (29 records) were carried out in Europe, primarily reported from Sweden (n = 3, 21 records). Four studies were performed in Eastern Asia (7 records) and several research publications have also been based in Israel (Western Asia, n = 3, 6 records). Figure 3 shows the temporal and spatial distribution of the total number of records retrieved in the systematic process.
Fig. 3.
Number of records of published studies that have examined floral nectar feeding behavior (field and laboratory) and the regions where this research was undertaken.
Aedes was the most studied genus with 29 studies (233 records) and 30 different species. The most studied species were Aedes vexans (Meigen, 1830) (n = 5, 53 records), Aedes aegypti (Linnaeus, 1972) (n = 7, 19 records), Aedes cinereus (Meigen, 1818) (n = 4, 18 records), Aedes provocans (Walker, 1848) (n = 2, 21 records), Aedes communis (De Geer, 1776) (n = 3, 10 records) and Aedes sollicitans (Walker, 1856) (n = 2, 13 records). Note that genera Stegomyia and Ochlerotatus were also included as keywords in the search process to maximize the number of studies related to Aedes species, since these two genera names were formerly used to identify some Aedes species (eg Ae. aegypti was formerly known as Stegomyia aegypti, and Aedes taeniorhynchus (Wiedemann, 1821) was known as Ochlerotatus taeniorhynchus).
Twenty studies (60 records) were related to Anopheles species. A total of 11 species were found feeding on nectar of different plants, Anopheles gambiae Giles, 1900 being the most studied (n = 8, 34 records), followed by Anopheles sergentii (Theobald, 1907) (n = 3; 5 records).
Fourteen studies (47 records) reported a total of 13 Culex species feeding on different plants. Culex pipiens Linnaeus, 1972 was the most studied species (n = 7, 18 records), followed by Culex restuans Theobald, 1901 (n = 3, 15 records).
Other genera of mosquitoes that were observed feeding on plant nectar were: Psorophora (n = 3, 4 records); Coquillettidia (n = 3, 20 records), Culiseta (n = 4, 18 records), with Culiseta inornata (Williston, 1893) having the highest number of records within this genus (9 records), and Uranotaenia (n = 2, 2 records). Finally, the following mosquito genera were only reported in one study: Mansonia (n = 1, 7 records), Eretmapodites (n = 1, 1 record), Ficalbia (n = 1, 1 record), Topomyia (n = 1, 2 record), Toxorhynchites (n = 1, 1 record).
The selection of plants by researchers in the different studies was based on the following reasons: (i) plants were present and abundant in the study sites (n = 32, 327 records); (ii) specific characteristics of the plants (n = 7, 14 records); (iii) plants that other species of mosquitoes in previous studies fed on (n = 8, 24 records); (iv) observation of mosquitoes visiting different plants in the field (n = 2, 18 records); (v) observation of pollen on mosquitoes’ bodies (n = 1, 4 records); and (iv) plant DNA identification in mosquitoes’ guts (n = 1, 10 records). Studies that selected the plants based on previous studies without any additional information on the species of mosquitoes feeding on the plant were not included in the analysis.
Thirty published studies described results from field studies (320 records), most of them before the year 2000. Sixteen studies (55 records) were performed in laboratory conditions, mainly after the year 2000 with the development of new lab techniques. Three studies (17 records) used a combination of field and lab methodologies, and 2 studies (5 records) were carried out in semi-field conditions.
Most of the studies were based on observations of mosquitoes feeding on plant nectar (n = 29; 314 records). Other methodologies used by researchers were (i) a combination of single choice assay (mosquitoes only had one species of plant to feed on in the lab), with either the cold anthrone method to calculate the proportion of mosquitoes that have fed on the plant (n = 10; 32 records) or liquid/gas chromatography mass spectrometry (n = 2, 5 records), (ii) multiple choice assay where mosquitoes had different plants to choose to feed on (n = 2, 11 records), (iii) the use of markers on vegetation or on mosquitoes (n = 6, 22 records), (iv) detection of presence of pollen on the body of mosquitoes (n = 1; 4 records), and (vi) plant DNA extraction and identification from mosquitoes’ guts (n = 1; 10 records).
Plants That Mosquito Genera Feed on
Our search found 74 different species of mosquitoes (12 genera) feeding on 145 plant species (109 genera). A list of all the records of mosquitoes feeding on plants can be found in Supplementary Appendix 2.
Asteraceae had the most records reporting Aedes (n = 13, 67 records) and Culex (n = 6, 20 records species feeding on them, followed by Rosaceae only for the Aedes species (8 studies, 36 records). Fabaceae (n = 10, 14 records), Asteraceae (n = 9, 15 records), and Euphorbiaceae (n = 6, 7 records) were the families with the most records of Anopheles species feeding on them. Anopheles species located in Africa showed clear preferences for Fabaceae plants (n = 7, 10 records); however, Anopheles species not located in Africa demonstrated preferences for plants pertaining to the Asteraceae family (n = 5, 11 records).
At the species level for plants, Achillea millefolium L. (Asterales: Asteraceae; 15 records), Leucanthemum vulgare Lam. (Asterales: Asteraceae; 13 records), and Asclepias syriaca L. (Gentianales: Apocynaceae; 12 records) were the plants with the most records of Aedes species feeding on their nectar, followed by Tanacetum vulgare L. (Asterales: Asteraceae; 7 records), Solidago canadensis L. (Asterales: Asteraceae; 6 records), and Solidago spp. (5 records). The plants with the most records of Anopheles species feeding on them were Ricinus communis L. (Malpighiales: Euphorbiaceae; 6 records), Parthenium hysterophorus L. (Asterales: Asteraceae; 5 records), and Senna didymobotrya (Fresen.) H.S.Irwin & Barneby (Fabales: Fabaceae; 4 records). Anopheles species not located in Africa demonstrated preferences for plants pertaining to the Asteraceae family such as T. vulgare (3 records) and A. millefolium (3 records). Tanacetum vulgare (4 records), A. millefolium (3 records), Solidago spp. (3 records), and Harungana madagascariensis Poir. (Malpighiales: Hypericaceae; 3 records) were the species of plants with the most records of Culex species feeding on them.
Plants That Predominant Mosquito Vector Species Feed on
Aedes aegypti and Ae. albopictus are 2 key vectors of dengue, yellow fever, chikungunya, and Zika (Kraemer et al. 2019). Understanding which species they feed on is therefore of particular interest. Our analysis of the studies demonstrated that Aedes aegypti was observed feeding on 17 different plant species (19 records), with the Fabaceae family having the most records. Impatiens walleriana Hook.f. (Ericales: Balsaminaceae; 2 records) and Pithecellobium dulce (Roxb.) Benth. (Fabales: Fabaceae; 2 records) were the only plants with multiple records of Ae. aegypti feeding on them. In contrast, Ae. albopictus was recorded feeding on only 3 different plant species (3 records).
Among the main malaria vectors, An. gambiae was the most studied species and studies indicate that feed on at least 17 different plant species (34 records), with 7 belonging to the Fabaceae family. The plant species demonstrating the highest number of feeding observations of An. gambiae were R. communis (6 records), P. hysterophorus (5 records), S. didymobotrya (4 records), Lantana camara L. (Lamiales: Verbenaceae; 3 records), and Tecoma stans (L.) Juss. ex Kunth (Lamiales: Bignoniaceae; 3 records).
Other important malaria vectors include Anopheles arabiensis Patton, 1995 and Anopheles coluzzi Coetzee and Wilkerson, 2013 and again, interesting observations have been found. For example, Anopheles arabiensis was observed feeding on A. millefolium (1 record), Duranta erecta L. (Lamiales: Verbenaceae; 1 record), and Stachytarpheta urticifolia Sims (Lamiales: Verbenaceae; 1 record) and An. coluzzi showed preferences for Barleria lupulina Lindl. (Lamiales: Acanthaceae; 1 record) and Cascabela thevetia (L.) Lippold (Gentianales: Apocynaceae; 1 record). In comparison, no studies were found related to Anopheles funestus Giles, 1900 feeding behavior on plant nectar.
Culex pipiens, another significant vector known for transmitting West Nile virus, Saint Louis encephalitis, avian malaria, and filarial worms (Farajollahi et al. 2011), displayed a preference for plants from the Asteraceae family (5 records), particularly A. millefolium (2 records) and T. vulgare (2 records). Additionally, Cx. pipiens fed on 10 other plant species, with one record for each. See Supplementary Appendix 3 for more details.
Risk of Bias Assessment to Study Mosquito Nectar Preferences
The risk of bias assessment revealed that 36 studies (71%) were considered to have a high risk of bias, while 15 studies were classified as low risk. The results of the risk of bias assessments are available in Supplementary Appendix 4.
Only studies that selected the plants randomly, employed more than one plant where mosquitoes could feed on in the study, and included experimental repetitions, were classified as low risk of bias. It was these studies that were included in our analysis to study mosquito preferences for specific plant nectar. Conversely, high-risk studies demonstrated a propensity to exclusively include plants known a priori to be attractive to mosquitoes and often conducted single-choice assays, limiting mosquitoes to feeding on a sole plant species. The high-risk category also included studies with the primary objective of observing variations in mosquito survival, fecundity, or vectorial capacity depending on the plants they fed on. In these cases, plant selection was not randomized but rather influenced by prior studies or inherent plant characteristics. To investigate mosquito preferences, it is essential to include a diverse selection of plants that reflects the variety mosquitoes encounter in their natural environment, or at least does not intentionally limit their choices to a few selected species.
Results of Meta-analysis
Do Mosquitoes Show Preferences for Specific Plant Nectar?
A detailed analysis was specifically conducted on studies classified as having low risk of bias to better understand whether mosquitoes showed preferences for particular plants and identify those preferences. Two of the 15 low risk of bias studies were excluded due to lack of information on the total number of plant species included. From these 13 studies, we obtained a ratio below 0.5 in 10 out of 13 studies, and a ratio between 0.5 and 0.7 in 2 out of 13 studies. Only one study obtained a ratio of 1, indicating no preference of mosquitoes for specific plant nectar. This suggests, therefore, that from the studies carried out to date, mosquitoes do appear to show a preference for the nectar of certain types of plants.
Do Different Mosquito Genera Show Preferences for Specific Plant Species?
Our analysis included 7 low risk of bias studies to investigate whether Aedes mosquitoes exhibited preferences for specific plant nectar. All demonstrated ratios below 0.7 and 5 of these obtained a ratio lower than 0.5 indicating that this mosquito species preferred to feed in particular plants, specifically, Aedes preferred to feed on 77 plant species.
Other 7 studies were involved in determining preferences in Anopheles species, and 6 out of 7 such studies obtained a ratio lower than 0.5, again demonstrating preferences for specific species of plants—in this case for 18 plant species.
Same conclusions in terms of plant preferences were obtained for Culex species, with ratios lower than 0.5 in all the studies (3 in total). In particular, this species showed preferences for 16 plant species.
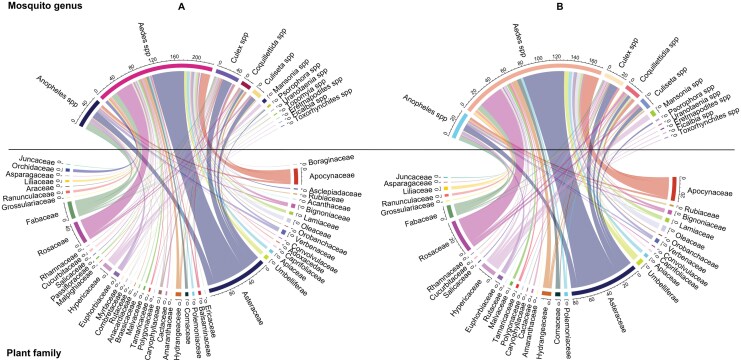
The representation of these genera-specific interactions is shown in Fig. 4B and includes a total of 274 records of mosquitoes feeding on 100 distinct plant species (73 genera). From our analysis of the data would appear that at the plant family level, Asteraceae and Rosaceae dominated in terms of records of Aedes species feeding on them. In addition, studies with Aedes species based in Africa also showed preferences for plants within the Fabaceae family.
Fig. 4.
Plant family preferences by different genera of mosquitoes. Panel (A) shows all records and Panel (B) records with low risk of bias.
For Anopheles species, the families Asteraceae, Fabaceae, and Euphorbiaceae had the most feeding records. Finally, Asteraceae was the family with the highest number of records of Culex species feeding on them.
Similar results were obtained consistent with the previous analysis that incorporated all studies (and therefore potential bias) (Fig. 4A).
At the plant species level, our findings revealed distinct preferences among mosquito genera: Aedes species displayed a preference for feeding on 77 species of plants pertaining to 58 genera (42 species of those were identified in the field by Sandholm and Price (1962); A. millefolium (n = 4, 12 records), A. syriaca (n = 3, 12 records, L. vulgare (n = 6, 11 records), and S. canadensis (n = 2, 5 records) were the plants with the most records of Aedes species feeding on them.
Anopheles species fed on 18 plant species (17 genera); R. communis (n = 4, 4 records), P. hysterophorus (n = 3, 3 records), and S. didymobotrya (n = 2, 2 records) remained the plants with the highest number of Anopheles feeding records, consistent with the previous analysis that included all records. For the studies on Anopheles species not conducted in Africa, A. syriaca (n = 1, 3 records), A. millefolium (n = 1, 2 records), and Solidago L. spp. (n = 1, 2 records) (Grimstad and DeFoliart 1974) were identified as the plants with highest number of records of Anopheles species feeding on them.
Finally, Culex species demonstrated preferences for 16 species of plants (16 genera); Solidago spp. (n = 1, 3 records) and H. madagascariensis (n = 1, 3 records) were the plants with more records of Culex species feeding on them. Following closely were A. millefolium (n = 1), A. syriaca (n = 2), and S. canadensis (n = 1) each with 2 records. These results can be found in Supplementary Appendix 4.
An interesting outcome arising from our analysis on mosquito nectar preferences is the discovery that several mosquito genera fed on nectar of certain plants. For example, 8 mosquito genera (15 species) fed on H. madagascariensis, 5 mosquito genera fed on A. milefollium (16 mosquito species), A. syriaca (16 mosquito species), and on Solidago spp. (14 mosquito species), and 4 mosquito genera fed on Spiraea latifolia (Aiton) Borkh. (Rosales: Rosaceae, 6 mosquito species) and Philadelphus caucasicus Koehne (Cornales: Hydrangeaceae, 6 mosquito species). Twenty more species of plants were attractive to several genera of mosquitoes.
Do Different Mosquito Species Present Specific Plant Preferences?
A total of 15 Chi-squared tests, involving 10 different mosquito species, were performed to statistically assess whether mosquito species exhibited preferences for specific plants. The tests were conducted using low-bias studies and only when the expected mosquito counts per plant species were 5 or greater. The results confirmed that different mosquito species showed preferences for specific plant traits (see Table 5 and Supplementary Appendix 5).
Table 5.
Chi-squared results using low risk of bias studies when the expected mosquito counts per plant species were 5 or greater.
| Mosquito species | No Plant species | Study ID | Chi-squared results |
|---|---|---|---|
| Aedes canadensis | 5 | Grimstad_1974_112 | X-squared = 32.375, df = 4, P-value <0.001 |
| Aedes vexans | 35 | Sandholm_1962_66 | X-squared = 6129, df = 34, P-value <0.001 |
| Aedes sollicitans | 17 | Magnarelli_1977_105 | X-squared = 313.56, df = 16, P-value <0.001 |
| Aedes sollicitans | 11 | Magnarelli_1979_43 | X-squared = 1898.3, df = 10, P-value <0.001 |
| Aedes cantator | 15 | Magnarelli_1978_33 | X-squared = 741.89, df = 14, P-value <0.001 |
| Aedes cantator | 11 | Magnarelli_1979_43 | X-squared = 1121.9, df = 10, P-value <0.001 |
| Anopheles earlei | 4 | Grimstad_1974_112 | X-squared = 8.6552, df = 3, P-value <0.05 |
| Anopheles gambiae | 8 | Impoinvil_2004_206 | X-squared = 212.51, df = 7, P-value <0.001 |
| Anopheles gambiae | 13 | Manda_2007_42a | X-squared = 126.89, df = 12, P-value <0.001 |
| Anopheles gambiae | 13 | Manda_2007_42b | X-squared = 124.15, df = 12, P-value <0.001 |
| Anopheles sergentii | 40 | Abdel-Malek_1964_226 | X-squared = 427.84, df = 39, P-value <0.001 |
| Culex pipiens | 10 | Schlein_2008_13 | X-squared = 6503.2, df = 9, P-value <0.001 |
| Coquillettidia perturbans | 10 | Sandholm_1962_66 | X-squared = 1012.7, df = 9, P-value <0.001 |
| Coquillettidia perturbans | 63 | Grimstad_1974_112 | X-squared = 146460, df = 62, P-value <0.001 |
When studying the main mosquito vectors, our results indicated that, Ae. aegypti fed on Hibiscus heterophyllus Vent. (Malvales: Malvaceae; 1 record), P. dulce (1 record), and Senna uniflora (Mill.) H.S.Irwin & Barneby (Fabales: Fabaceae; 1 record) (Nyasembe et al. 2018). The feeding preferences of An. gambiae also showed consistent preferences with this species primarily feeding on R. communis (4 records), P. hysterophorus (3 records), S. didymobotrya (2 records), L. camara (2 records), and T. stans (2 records). Similarly, Cx. pipiens was observed feeding on a few different plants, demonstrating preferences for Acacia saligna (Labill.) Wendl. (Fabales: Fabaceae; 1 record), Polygonum equisetiforme Sm. (Caryophyllales: Polygonaceae; 1 record), and Tamarix jordanis Boiss. (Caryophyllales: Tamaricaceae; 1 record).
Discussion
When addressing the question ‘Which nectar do mosquitoes feed on?’ using a systematic map, we found 74 mosquito species across 12 genera, feeding on 145 plant species (109 genera). Further analysis using a subset of studies with more stringent criteria to avoid potential bias demonstrated that mosquito families and genera, appear to show preferences for obtaining nectar from certain groups of plants. In particular, Aedes species exhibited preferences for 77 plant species (58 genera), Anopheles species favored 18 plant species (17 genera), and Culex species displayed preferences for 16 plant species (16 genera).
Our meta-analysis of the data from the systematic map indicated that Asteraceae, Rosaceae and Fabaceae are the families with the most records of mosquitoes feeding on them and in these, there was a sub-set of 18 plant species in the Asteraceae, 20 plant species in the Rosaceae and 11 plant species in the Fabaceae that clearly demonstrated a feeding preference. Further research is now needed to understand whether there are common traits within these families and species that might make them more attractive to mosquitoes.
Some caution, however, must be taken when making assumptions about these preferred plant families. First, Asteraceae and Fabaceae are two of the most species-rich and abundant plant families globally (Panero and Crozier 2016). Second, mosquitoes’ preferences in some cases seem to be country-specific, at least from the evidence available to date. For example, Asteraceae and Rosaceae emerged prominently in records of Aedes species feeding on them in North America (Sandholm and Price 1962, Grimstad and DeFoliart 1974, Magnarelli 1977, 1978, 1979, 1980, 1983, Gadawski and Smith 1992, Yee et al. 1992). In contrast, studies conducted in Africa with Aedes species did not show preferences for these two families (McCrae et al. 1969, Nyasembe et al. 2018). Also, while Asteraceae and Fabaceae were prevalent in records associated with Anopheles species, Fabaceae dominated for Anopheles species in Africa (Abdel-Malek 1964, Manda et al. 2007b, Nyasembe et al. 2018) (primarily An. gambiae), whereas Asteraceae reported more records of Anopheles species feeding on them in other continents (Grimstad and DeFoliart 1974, Andersson and Jaenson 1987, Healy and Jepson 1988, Jaenson and Ameneshewa 1991).
The absence of findings regarding Anopheles species in Africa feeding on plants within the Rosaceae family could be attributed to the difference in abundance of this plant family in Africa (it is much more widespread as a family in northern temperate regions (Liu et al. 2021)) but this needs further investigation. These regional differences in the preferred plant families, however, also emphasizes the importance of considering geographical factors such as local vegetation in future work to understand mosquito-plant interactions.
When analyzing the plants preferred by major mosquito vectors, we identified several plants that these mosquitoes tend to feed on. First, Anopheles gambiae, the most extensively studied, displayed a preference for R. communis, P. hysterophorus, T. stans, S. didymobotrya, and H. patens. Previous work has suggested that An. gambiae may also have a preference for L. camara. Our analysis of the literature suggest that this remains controversial, with some studies suggesting it is one of An. gambiae’s preferred plants (Impoinvil et al. 2004) while others indicating the opposite (Manda et al. 2007b). In particular, these contrasting results suggest that attractiveness may depend on specific ecological contexts or experimental designs. Second, DNA extraction and sequencing techniques have identified H. heterophyllus, P. dulce, and S. uniflora as preferred plants of Ae. aegypti in the field in Kenya (Nyasembe et al. 2018). Finally, Cx. pipiens showed preferences for 3 out of 10 plant species, with T. jordanis being the most visited.
Our findings are also of relevance when considering future scenarios of climate change and globalization. With these changes occurring across the globe, it is widely expected that the disappearance of certain plant species from their natural habitats will occur, and they will be replaced by different species, especially those with invasive traits (Bhagwat et al. 2012, Agha et al. 2021, Tabe Ojong et al. 2021). It is likely therefore that certain of the plant species already identified as preferred to An. gambiae, such as R. communis, P. hysterophorus, L. camara, and T. stans, which are invasive and becoming widespread across landscapes (Emily et al. 2014, Nyasembe et al. 2015, Müller et al. 2017, Stone et al. 2018), could potentially increase their distribution and abundance, thereby increasing the availability of this nectar resource across mosquito-prone landscapes.
Limitations and Further Work
Our systematic map search included all the continents; however, we only included articles written in English. This might impact our results—especially those from gray literature. For example, no papers were found in South America related to our review question. Given that around 98% of peer-reviewed scientific studies are published in English nowadays (Gordin 2015), we believe the overall impact is low, although it could be regionally moderate.
We excluded studies on fruits, seedpods, plant tissue and honeydew, since our question was only related to nectar, the main sugar source of mosquitoes. We also excluded papers related to olfactory, gustatory, and visual attraction if they did not include observations of mosquitoes feeding on nectar. Several papers related to this topic were found during the review process. It would be interesting to build on our work further to carry out additional systematic reviews related to this topic.
Conclusion
Our systematic map includes 397 records of mosquitoes feeding on various plant species, retrieved from 51 studies. The interactive map provided details the temporal and spatial distribution of these studies, their methodologies, criteria for plant selection, study designs, and main objectives. This information is valuable for researchers planning future work in this field and for practitioners developing mosquito control methods. Our further meta-analysis of a selected set of studies confirmed that mosquitoes prefer certain plants. Asteraceae and Rosaceae were frequently associated with Aedes species, Asteraceae and Fabaceae with Anopheles species, and Asteraceae with Culex species. However, the number of studies is still low, and many are based on laboratory trials. More studies combining field observations with laboratory research are needed to identify mosquito preferences in various natural environments and assess changes over time and space depending on local vegetation.
Our study also highlighted a critical need for more long-term studies, planned according to the biology of the species of mosquito (nocturnal/diurnal and urban/peri-urban/rural). In particular, there is a need for greater observations and collections of mosquitoes feeding on plants in the field, including invasive species of mosquitoes and plants. This needs to be combined with robust sampling methods which include randomized selection of plants in the field, study replication, and multiple plant choice assays. Subsequent laboratory work, to confirm mosquito feeding on these plants and identify their nectar compounds are also crucial for better understanding mosquito feeding behavior in the field. This will help in the design and development of more targeted mosquito control methods, considering mosquito preferences as well as the spatial and temporal presence and abundance of their preferred plants.
Supplementary material
Supplementary material is available at Environmental Entomology online.
Acknowledgments
We thank Oxford Systematic Reviews LLP (OxSREV) for their contribution to the protocol, search strategy and data collection process. Our thanks go as well to Dr Paul Johnson, Senior Lecturer in the School of Biodiversity, One Health & Veterinary Medicine at the University of Glasgow, for his valuable advice on the statistical analysis.
Contributor Information
Eva Herreros-Moya, Department of Biology, University of Oxford, Oxford, UK.
Marianne Sinka, Department of Biology, University of Oxford, Oxford, UK.
Angela F Harris, Innovative Vector Control Consortium, Liverpool School of Tropical Medicine, Liverpool, UK.
Julian Entwistle, Innovative Vector Control Consortium, Liverpool School of Tropical Medicine, Liverpool, UK.
Andrew C Martin, Scott Polar Research Institute, Department of Geography, University of Cambridge, Cambridge, UK.
Kathy J Willis, Department of Biology, University of Oxford, Oxford, UK.
Conflicts of interest. The authors declare that they have no competing interests.
Funding
This study was funded by Innovative Vector Control Consortium (IVCC) through support from the Bill & Melinda Gates Foundation (grant: INV-007509), the Swiss Agency for Development and Cooperation (SDC) (grant: 81067480) and UK Aid (grant: 30041-105). The findings and conclusions contained within are those of the authors and do not necessarily reflect positions or policies of the Bill & Melinda Gates Foundation, SDC, UK Aid or IVCC.
Ethics Statement
The authors declare that approval by an ethics committee was not required for this study.
Data Availability
The data underlying this article are available in the article and in its online supplementary material.
References
- Abdel-Malek AA. 1964. Study of the feeding habits of male Anopheles sergenti Theo. at Siwa oasis using radiophosphorus. Bull. World Health Organ. 30:137–139. [PMC free article] [PubMed] [Google Scholar]
- Agha SB, Alvarez M, Becker M, et al. 2021. Invasive alien plants in Africa and the potential emergence of mosquito-borne arboviral diseases—a review and research outlook. Viruses 13, 32. https://doi.org/ 10.3390/v13010032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson IH. 1990. Nectar feeding activity of Aedes mosquitoes, with special reference to Aedes communis females. Journal of the American Mosquito Control Association. 6:482–489. [PubMed] [Google Scholar]
- Andersson IH, Jaenson TGT.. 1987. Nectar feeding by mosquitoes in Sweden, with special reference to Culex pipiens and Cx. torrentium. Med. Vet. Entomol. 1:59–64. https://doi.org/ 10.1111/j.1365-2915.1987.tb00323.x [DOI] [PubMed] [Google Scholar]
- Beier JC, Müller GC, Gu W, et al. 2012. Attractive toxic sugar bait (ATSB) methods decimate populations of Anopheles malaria vectors in arid environments regardless of the local availability of favoured sugar-source blossoms. Malar. J. 11:31. https://doi.org/ 10.1186/1475-2875-11-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernáth B, Anstett V, Guerin PM.. 2016. Anopheles gambiae females readily learn to associate complex visual cues with the quality of sugar sources. J. Insect Physiol. 95:8–16. https://doi.org/ 10.1016/j.jinsphys.2016.08.011 [DOI] [PubMed] [Google Scholar]
- Bhagwat SA, Breman E, Thekaekara T, et al. 2012. A battle lost? Report on two centuries of invasion and management of Lantana camara L. in Australia, India and South Africa. PLoS One 7:e32407. https://doi.org/ 10.1371/journal.pone.0032407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkett DA, Kline DL, Carlson DA.. 1999. Sugar meal composition of five north central Florida mosquito species (Diptera: Culicidae) as determined by gas chromatography. J. Med. Entomol. 36:462–467. https://doi.org/ 10.1093/jmedent/36.4.462 [DOI] [PubMed] [Google Scholar]
- Chen Z, Kearney CM.. 2015. Nectar protein content and attractiveness to Aedes aegypti and Culex pipiens in plants with nectar/insect associations. Acta Trop. 146:81–88. https://doi.org/ 10.1016/j.actatropica.2015.03.010 [DOI] [PubMed] [Google Scholar]
- Clements AN. 1963. The physiology of mosquitoes - adult feeding. In: Clements AN, editor. The physiology of mosquitoes. London: Pergamon Press, p. 128–150. [Google Scholar]
- Cohen J. 1960. A coefficient of agreement for nominal scales. Educ. Psychol. Measurement. 20:37–46. https://doi.org/ 10.1177/001316446002000104 [DOI] [Google Scholar]
- Corbet PS. 1964. Autogeny and oviposition in Arctic mosquitoes. Nature 203:669–669. https://doi.org/ 10.1038/203669a0 [DOI] [Google Scholar]
- Dieng H, Satho T, Binti Arzemi NA, et al. 2018. Exposure of a diurnal mosquito vector to floral mimics: foraging responses, feeding patterns, and significance for sugar bait technology. Acta Trop. 185:230–238. https://doi.org/ 10.1016/j.actatropica.2018.05.019 [DOI] [PubMed] [Google Scholar]
- Dötterl S, Jahreiß K, Jhumur US, et al. 2012. Temporal variation of flower scent in Silene otites (Caryophyllaceae): a species with a mixed pollination system. Bot. J. Linn. Soc. 169:447–460. https://doi.org/ 10.1111/j.1095-8339.2012.01239.x [DOI] [Google Scholar]
- Durnez L, Coosemans M.. 2013. Residual transmission of malaria: an old issue for new approaches. In Manguin S, editor.. Anopheles mosquitoes—New insights into malaria vectors.p. 671–704. https://doi.org/ 10.5772/55925 [DOI]
- Emily W, Agnes L, Jenipher B, et al. 2014. A roadside survey of the invasive weed Parthenium hysterophorus (Asteraceae) in East Africa. J. East African Nat. Hist. 103:49–57. https://doi.org/ 10.2982/028.103.0105 [DOI] [Google Scholar]
- Farajollahi A, Fonseca DM, Kramer LD, et al. 2011. ‘Bird biting’ mosquitoes and human disease: a review of the role of Culex pipiens complex mosquitoes in epidemiology. Infect. Genet. Evol. 11:1577–1585. https://doi.org/ 10.1016/j.meegid.2011.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster WA. 1995. Mosquito sugar feeding and reproductive energetics. Annu. Rev. Entomol. 40:443–474. https://doi.org/ 10.1146/annurev.en.40.010195.002303 [DOI] [PubMed] [Google Scholar]
- Frampton GK, Livoreil B, Petrokofsky G.. 2017. Eligibility screening in evidence synthesis of environmental management topics. Environ. Evidence 6:27. https://doi.org/ 10.1186/s13750-017-0102-2 [DOI] [Google Scholar]
- Gadawski RM, Smith SM.. 1992. Nectar sources and age structure in a population of Aedes provocans (Diptera: Culicidae). J. Med. Entomol. 29:879–886. https://doi.org/ 10.1093/jmedent/29.5.879 [DOI] [PubMed] [Google Scholar]
- Gary RE Jr., Foster WA.. 2001. Effects of available sugar on the reproductive fitness and vectorial capacity of the malaria vector Anopheles gambiae (Diptera: Culicidae). J. Med. Entomol. 38:22–28. https://doi.org/ 10.1603/0022-2585-38.1.22 [DOI] [PubMed] [Google Scholar]
- Gary RE Jr, Foster WA.. 2004. Anopheles gambiae feeding and survival on honeydew and extra-floral nectar of peridomestic plants. Med. Vet. Entomol. 18:102–107. https://doi.org/ 10.1111/j.0269-283X.2004.00483.x [DOI] [PubMed] [Google Scholar]
- González-Teuber M, Heil M.. 2009. Nectar chemistry is tailored for both attraction of mutualists and protection from exploiters. Plant Signal. Behav. 4:809–813. https://doi.org/ 10.4161/psb.4.9.9393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordin ME. 2015. Scientific Bable. The University of Chicago Press. [Google Scholar]
- Gouagna LC, Poueme RS, Dabiré KR, et al. 2010. Patterns of sugar feeding and host plant preferences in adult males of An. gambiae (Diptera: Culicidae). J Vector Ecol. 35:267–276. https://doi.org/ 10.1111/j.1948-7134.2010.00082.x [DOI] [PubMed] [Google Scholar]
- Gouagna LC, Kerampran R, Lebon C, et al. 2014. Sugar-source preference, sugar intake and relative nutritional benefits in Anopheles arabiensis males. Acta Trop. 132:S70–S79. https://doi.org/ 10.1016/j.actatropica.2013.09.022 [DOI] [PubMed] [Google Scholar]
- Grimstad PR, DeFoliart GR.. 1974. Nectar sources of Wisconsin mosquitoes. J. Med. Entomol. 11:331–341. https://doi.org/ 10.1093/jmedent/11.3.331 [DOI] [PubMed] [Google Scholar]
- Gu W, Müller G, Schlein Y, et al. 2011. Natural plant sugar sources of Anopheles mosquitoes strongly impact malaria transmission potential. PLoS One 6:e15996. https://doi.org/ 10.1371/journal.pone.0015996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Gu L, Eils R, et al. 2014. Circlize implements and enhances circular visualization in R. Bioinformatics 30:2811–2812. https://doi.org/ 10.1093/bioinformatics/btu393 [DOI] [PubMed] [Google Scholar]
- Haeger JS. 1955. The non-blood feeding habits of Aedes taeniorhynchus (Diptera: Culicidae) on Sanibel Island, Florida. Mosquito News. 15:21–26. [Google Scholar]
- Haeger JS. 1960. Behavior preceding migration in the salt-marsh mosquito, Aedes taeniorhynchus (Wiedemann). Mosquito News. 20:136–147. [Google Scholar]
- Harrison RE, Brown MR, Strand MR.. 2021. Whole blood and blood components from vertebrates differentially affect egg formation in three species of anautogenous mosquitoes. Parasites Vect. 14:119. https://doi.org/ 10.1186/s13071-021-04594-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy TP, Jepson PC.. 1988. The location of floral nectar sources by mosquitoes: the long-range responses of Anopheles arabiensis Patton (Diptera: Culicidae) to Achillea millefolium flowers and isolated floral odour. Bull. Entomol. Res. 78:651–657. https://doi.org/ 10.1017/s0007485300015509 [DOI] [Google Scholar]
- Hemingway J, Ranson H, Magill A, et al. 2016. Averting a malaria disaster: will insecticide resistance derail malaria control? Lancet (London, England). 387:1785–1788. https://doi.org/ 10.1016/S0140-6736(15)00417-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hien DFS, Dabiré KR, Roche B, et al. 2016. Plant-mediated effects on mosquito capacity to transmit human malaria. PLoS Pathog. 12:e1005773. https://doi.org/ 10.1371/journal.ppat.1005773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignell R, Okawa S, Englund J-E, et al. 2010. Assessment of diet choice by the yellow fever mosquito Aedes aegypti. Physiol. Entomol. 35:274–286. https://doi.org/ 10.1111/j.1365-3032.2010.00740.x [DOI] [Google Scholar]
- Impoinvil DE, Kongere JO, Foster WA, et al. 2004. Feeding and survival of the malaria vector Anopheles gambiae on plants growing in Kenya. Med. Vet. Entomol. 18:108–115. https://doi.org/ 10.1111/j.0269-283X.2004.00484.x [DOI] [PubMed] [Google Scholar]
- Jaenson TGT, Ameneshewa B.. 1991. Prehibernation diet and reproductive condition of female Anopheles messeae in Sweden. Med. Vet. Entomol. 5:243–252. https://doi.org/ 10.1111/j.1365-2915.1991.tb00547.x [DOI] [PubMed] [Google Scholar]
- Jepson PC, Healy TP.. 1988. The location of floral nectar sources by mosquitoes: an advanced bioassay for volatile plant odours and initial studies with Aedes aegypti (L.) (Diptera: Culicidae). Bull. Entomol. Res. 78:641–650. https://doi.org/ 10.1017/s0007485300015492 [DOI] [Google Scholar]
- Junnila A, Müller GC, Schlein Y.. 2010. Species identification of plant tissues from the gut of An. sergentii by DNA analysis. Acta Trop. 115:227–233. https://doi.org/ 10.1016/j.actatropica.2010.04.002 [DOI] [PubMed] [Google Scholar]
- Kahili-Heede M, Hillgren KJ. 2021. Colandr. Journal of the Medical Library Association 109:523–525. https://doi.org/ 10.5195/jmla.2021.1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney A, Cusick A, Payne J, et al. 2017. The potential for flower nectar to allow mosquito to mosquito transmission of Francisella tularensis. PLoS One 12:e0175157. https://doi.org/ 10.1371/journal.pone.0175157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S, Vlimant M, Guerin PM.. 2015. Sugar-sensitive neurone responses and sugar feeding preferences influence lifespan and biting behaviours of the Afrotropical malaria mosquito, Anopheles gambiae. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 201:317–329. https://doi.org/ 10.1007/s00359-015-0978-7 [DOI] [PubMed] [Google Scholar]
- Knab F. 1907. Mosquitoes as flower visitors. J. N. Y. Entomol. Soc. 15:215–219. [Google Scholar]
- Kraemer MUG, Reiner RC, Brady OJ, et al. 2019. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat. Microbiol. 4:854–863. https://doi.org/ 10.1038/s41564-019-0376-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahondère C, Vinauger C, Okubo RP, et al. 2020. The olfactory basis of orchid pollination by mosquitoes. Proc. Natl. Acad. Sci. U.S.A. 117:708–716. https://doi.org/ 10.1073/pnas.1910589117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Huang M, Cline AR, et al. 2021. Rosaceae, Brassicaceae and pollen beetles: exploring relationships and evolution in an anthophilous beetle lineage (Nitidulidae, Meligethes-complex of genera) using an integrative approach. Front. Zool. 18:9. https://doi.org/ 10.1186/s12983-021-00390-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnarelli LA. 1977. Nectar feeding by Aedes sollicitans and its relation to gonotrophic activity. Environ. Entomol. 6:237–242. https://doi.org/ 10.1093/ee/6.2.237 [DOI] [Google Scholar]
- Magnarelli LA. 1978. Bionomics of the salt-marsh mosquito, Aedes cantator (Diptera: Culicidae). Environ. Entomol. 7:512–517. https://doi.org/ 10.1093/ee/7.4.512 [DOI] [Google Scholar]
- Magnarelli LA. 1979. Diurnal nectar-feeding of Aedes cantator and A. sollicitans (Diptera: Culicidae). Environ. Entomol. 8:949–955. https://doi.org/ 10.1093/ee/8.5.949 [DOI] [Google Scholar]
- Magnarelli LA. 1980. Bionomics of Psorophora ferox (Diptera: Culicidae): seasonal occurrence and acquisition of sugars. J. Med. Entomol. 17:328–332. https://doi.org/ 10.1093/jmedent/17.4.328 [DOI] [Google Scholar]
- Magnarelli LA. 1983. Nectar sugars and caloric reserves in natural populations of Aedes canadensis and Aedes stimulans (Diptera: Culicidae). Environ. Entomol. 12:1482–1486. https://doi.org/ 10.1093/ee/12.5.1482 [DOI] [Google Scholar]
- Manda H, Gouagna LC, Foster WA, et al. 2007a. Effect of discriminative plant-sugar feeding on the survival and fecundity of Anopheles gambiae. Malar. J. 6:113. https://doi.org/ 10.1186/1475-2875-6-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manda H, Gouagna LC, Nyandat E, et al. 2007b. Discriminative feeding behaviour of Anopheles gambiae s.s. on endemic plants in western Kenya. Med. Vet. Entomol. 21:103–111. https://doi.org/ 10.1111/j.1365-2915.2007.00672.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AC. 2017. Thalloo Evidence-Mapping: A Jekyll Theme. Available online: https://github.com/AndrewIOM/thalloo [Google Scholar]
- McCrae AWR, Ssenkubuge Y, Manuma P, Mawejje C, Kitama A.. 1969. Mosquito and tabanid activity at plant sugar sources. Report of the East African Virus Research Institute. 18:96–102
- McHugh ML. 2013. The chi-square test of independence. (1330-0962 (Print)) [DOI] [PMC free article] [PubMed]
- Mogi M, Miyagi I.. 1989. Sugar feeding of Topomyia pseudobarbus (Diptera: Culicidae) in Nature. J. Med. Entomol. 26:370–371. https://doi.org/ 10.1093/jmedent/26.4.370 [DOI] [Google Scholar]
- Moiroux N, Gomez MB, Pennetier C, et al. 2012. Changes in Anopheles funestus Biting Behavior Following Universal Coverage of Long-Lasting Insecticidal Nets in Benin. J. Infect. Dis. 206:1622–1629. https://doi.org/ 10.1093/infdis/jis565 [DOI] [PubMed] [Google Scholar]
- Müller H. 1873. Befruchtung der Blumen durch Insekten. Wilhelm Engelmann. [Google Scholar]
- Müller G, Schlein Y.. 2006. Sugar questing mosquitoes in arid areas gather on scarce blossoms that can be used for control. Int. J. Parasitol. 36:1077–1080. https://doi.org/ 10.1016/j.ijpara.2006.06.008 [DOI] [PubMed] [Google Scholar]
- Müller GC, Beier JC, Traore SF, et al. 2010. Field experiments of Anopheles gambiae attraction to local fruits/seedpods and flowering plants in Mali to optimize strategies for malaria vector control in Africa using attractive toxic sugar bait methods. Malar. J. 9:262–262. https://doi.org/ 10.1186/1475-2875-9-262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller GC, Xue R-D, Schlein Y.. 2011. Differential attraction of Aedes albopictus in the field to flowers, fruits and honeydew. Acta Trop. 118:45–49. https://doi.org/ 10.1016/j.actatropica.2011.01.009 [DOI] [PubMed] [Google Scholar]
- Müller GC, Junnila A, Traore MM, et al. 2017. The invasive shrub Prosopis juliflora enhances the malaria parasite transmission capacity of Anopheles mosquitoes: a habitat manipulation experiment. Malar. J. 16:237. https://doi.org/ 10.1186/s12936-017-1878-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwesigwa J, Achan J, Di Tanna GL, et al. 2017. Residual malaria transmission dynamics varies across The Gambia despite high coverage of control interventions. PLoS One 12:e0187059. https://doi.org/ 10.1371/journal.pone.0187059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepi M, Grasso DA, Mancuso S.. 2018. Nectar in plant-insect mutualistic relationships: from food reward to partner manipulation. Front. Plant Sci. 9:1063–1063. https://doi.org/ 10.3389/fpls.2018.01063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson SW. 2022. Sweet solutions: nectar chemistry and quality. Philos. Trans. R. Soc. London, Ser. B 377:20210163. https://doi.org/ 10.1098/rstb.2021.0163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikbakhtzadeh MR, Terbot Ii JW, Otienoburu PE, et al. 2014. Olfactory basis of floral preference of the malaria vector Anopheles gambiae (Diptera: Culicidae) among common African plants. J. Vector Ecol. 39:372–383. https://doi.org/ 10.1111/jvec.12113 [DOI] [PubMed] [Google Scholar]
- Nikbakhtzadeh MR, Terbot JW II, Foster WA.. 2016. Survival value and sugar access of four East African plant species attractive to a laboratory strain of sympatric Anopheles gambiae (Diptera: Culicidae). J. Med. Entomol. 53:1105–1111. https://doi.org/ 10.1093/jme/tjw067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyasembe VO, Teal PEA, Mukabana WR, et al. 2012. Behavioural response of the malaria vector Anopheles gambiae to host plant volatiles and synthetic blends. Parasites Vectors. 5:234. https://doi.org/ 10.1186/1756-3305-5-234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyasembe VO, Cheseto X, Kaplan F, et al. 2015. The invasive American weed Parthenium hysterophorus can negatively impact malaria control in Africa. PLoS One 10:e0137836. https://doi.org/ 10.1371/journal.pone.0137836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyasembe VO, Tchouassi DP, Pirk CWW, et al. 2018. Host plant forensics and olfactory-based detection in Afro-tropical mosquito disease vectors. PLoS Negl.Trop. Dis. 12:e0006185. https://doi.org/ 10.1371/journal.pntd.0006185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyasembe VO, Tchouassi DP, Muturi MN, et al. 2021. Plant nutrient quality impacts survival and reproductive fitness of the dengue vector Aedes aegypti. Parasites Vectors. 14:4. https://doi.org/ 10.1186/s13071-020-04519-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okech BA, Gouagna LC, Killeen GF, et al. 2003. Influence of sugar availability and indoor microclimate on survival of Anopheles gambiae (Diptera: Culicidae) under semifield conditions in western Kenya. J. Med. Entomol. 40:657–663. https://doi.org/ 10.1603/0022-2585-40.5.657 [DOI] [PubMed] [Google Scholar]
- Orbán L, Plowright CMS.. 2014. Getting to the start line: how bumblebees and honeybees are visually guided towards their first floral contact. Insectes Soc. 61:325–336. https://doi.org/ 10.1007/s00040-014-0366-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padonou GG, Gbedjissi G, Yadouleton A, et al. 2012. Decreased proportions of indoor feeding and endophily in Anopheles gambiae s.l. populations following the indoor residual spraying and insecticide-treated net interventions in Benin (West Africa). Parasites Vectors. 5:262. https://doi.org/ 10.1186/1756-3305-5-262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panero JL, Crozier BS.. 2016. Macroevolutionary dynamics in the early diversification of Asteraceae. Mol. Phylogenet. Evol. 99:116–132. https://doi.org/ 10.1016/j.ympev.2016.03.007 [DOI] [PubMed] [Google Scholar]
- Patil AH, Jagdale S.. 2021. Nocturnal insect pollinator diversity and species richness in Ridge gourd, Luffa acutangula. J Appl Nat Sci. 13:463–469. https://doi.org/ 10.31018/jans.v13i2.2625 [DOI] [Google Scholar]
- Peach DAH, Gries G.. 2016. Nectar thieves or invited pollinators? A case study of tansy flowers and common house mosquitoes. Arthropod Plant Interact. 10:497–506. https://doi.org/ 10.1007/s11829-016-9445-9 [DOI] [Google Scholar]
- Peach DAH, Ko E, Blake AJ, et al. 2019. Ultraviolet inflorescence cues enhance attractiveness of inflorescence odour to Culex pipiens mosquitoes. PLoS One 14:e0217484. https://doi.org/ 10.1371/journal.pone.0217484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruett G, Hawes J, Varnado W, et al. 2020. The readily transformable Impatiens walleriana efficiently attracts nectar feeding with Aedes and Culex mosquitoes in simulated outdoor garden settings in Mississippi and Florida. Acta Tropica. (1873-6254 (Electronic)). 210:105624. https://doi.org/ 10.1016/j.actatropica.2020.105624 [DOI] [PubMed] [Google Scholar]
- Pullin A, Frampton G, Livoreil B, Petrokofsky G.. 2022. Collaboration for environmental evidence. Guidelines and standards for evidence synthesis in environmental management.
- Qualls WA, Xue RD, Beier JC, et al. 2013. Survivorship of adult Aedes albopictus (Diptera: Culicidae) feeding on indoor ornamental plants with no inflorescence. Parasitol. Res. 112:2313–2318. https://doi.org/ 10.1007/s00436-013-3396-1 [DOI] [PubMed] [Google Scholar]
- Rambey R, Rangkuti AB, Onrizal, Susilowaty A, Wijayanto N, Siregar IZ.. 2021. Phenology of the bunga bangkai (Amorphophallus gigas) in North Padang Lawas, North Sumatra Province, Indonesia. IOP Conf Series. 782:032019. https://doi.org/ 10.1088/1755-1315/782/3/032019 [DOI] [Google Scholar]
- Ranson H, Lissenden N.. 2016. Insecticide resistance in African Anopheles mosquitoes: a worsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 32:187–196. https://doi.org/ 10.1016/j.pt.2015.11.010 [DOI] [PubMed] [Google Scholar]
- Rewicz A, Jaskuła R, Rewicz T, Tończyk G.. 2017. Pollinator diversity and reproductive success of Epipactis helleborine (L.) Crantz (Orchidaceae) in anthropogenic and natural habitats. (2167-8359 (Print)) [DOI] [PMC free article] [PubMed]
- Russell TL, Govella NJ, Azizi S, et al. 2011. Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar. J. 10:80. https://doi.org/ 10.1186/1475-2875-10-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandholm HA, Price RD.. 1962. Field observations on the nectar feeding habits of some Minnesota mosquitoes. Mosquito News. 22:346–349. [Google Scholar]
- Sayers A. 2007. Tips and tricks in performing a systematic review. (0960-1643 (Print)) [DOI] [PMC free article] [PubMed]
- Schaefer CH, Miura T.. 1972. Sources of energy utilized by natural populations of the mosquito, Culex tarsalis, for overwintering. J. Insect Physiol. 18:797–805. https://doi.org/ 10.1016/0022-1910(72)90204-1 [DOI] [PubMed] [Google Scholar]
- Schlein Y, Muller G.. 1995. Assessment of plant tissue feeding by sand flies (Diptera: Psychodidae) and mosquitoes (Diptera: Culicidae). J. Med. Entomol. 32:882–887. https://doi.org/ 10.1093/jmedent/32.6.882 [DOI] [PubMed] [Google Scholar]
- Schlein Y, Müller GC.. 2008. An approach to mosquito control: using the dominant attraction of flowering Tamarix jordanis Trees Against Culex pipiens. J. Med. Entomol. 45:384–390. https://doi.org/ 10.1603/0022-2585(2008)45[384:aatmcu]2.0.co;2 [DOI] [PubMed] [Google Scholar]
- Sissoko F, Junnila A, Traore MM, et al. 2019. Frequent sugar feeding behavior by Aedes aegypti in Bamako, Mali makes them ideal candidates for control with attractive toxic sugar baits (ATSB). PLoS One 14:e0214170. (). https://doi.org/ 10.1371/journal.pone.0214170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Gadawski RM.. 1994. Nectar feeding by the early-spring mosquito Aedes provocans. Med. Vet. Entomol. 8:201–213. https://doi.org/ 10.1111/j.1365-2915.1994.tb00499.x [DOI] [PubMed] [Google Scholar]
- Stone CM, Jackson BT, Foster WA.. 2012. Effects of plant-community composition on the vectorial capacity and fitness of the malaria mosquito Anopheles gambiae. Am. J. Trop. Med. Hyg. 87:727–736. https://doi.org/ 10.4269/ajtmh.2012.12-0123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone CM, Foster WA.. 2013. Plant-sugar feeding and vectorial capacity. In: Takken W, Koenraadt CJM, editors. Ecology of parasite-vector interactions. Wageningen Academic Publishers. p. 35–79. [Google Scholar]
- Stone CM, Witt ABR, Walsh GC, et al. 2018. Would the control of invasive alien plants reduce malaria transmission? A review. Parasites & Vectors 11:76. https://doi.org/ 10.1186/s13071-018-2644-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabe Ojong MP, Jr., Alvarez M, Ihli HJ, Becker M, Heckelei T.. 2021. Action on invasive species: control strategies of Parthenium hysterophorus L. on Smallholder Farms in Kenya. (1432-1009 (Electronic)) [DOI] [PMC free article] [PubMed]
- Thien LB. 1969. Mosquito pollination of Habenaria obtusata (Orchidaceae). Am. J. Bot. 56:232–237. https://doi.org/ 10.2307/2440711 [DOI] [Google Scholar]
- Traore MM, Junnila A, Traore SF, et al. 2020. Large-scale field trial of attractive toxic sugar baits (ATSB) for the control of malaria vector mosquitoes in Mali, West Africa. Malar. J. 19:72. https://doi.org/ 10.1186/s12936-020-3132-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twinn CR, Hocking B, McDuffie WC, et al. 1948. A preliminary account of the biting flies at Churchill, Manitoba. Can. J. Res. 26d:334–357. https://doi.org/ 10.1139/cjr48d-025 [DOI] [PubMed] [Google Scholar]
- Valzania L, Mattee MT, Strand MR, et al. 2019. Blood feeding activates the vitellogenic stage of oogenesis in the mosquito Aedes aegypti through inhibition of glycogen synthase kinase 3 by the insulin and TOR pathways. Dev. Biol. 454:85–95. https://doi.org/ 10.1016/j.ydbio.2019.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berg H, da Silva Bezerra HS, Al-Eryani S, et al. 2021. Recent trends in global insecticide use for disease vector control and potential implications for resistance management. Sci. Rep. 11:23867. https://doi.org/ 10.1038/s41598-021-03367-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Handel E. 1972. The detection of nectar in mosquitoes. Mosquito News. 32:458–458. [Google Scholar]
- Vargo AM, Foster WA.. 1984. Gonotrophic state and parity of nectar-feeding mosquitos. Mosquito News. 44:6–10. [Google Scholar]
- Yee WL, Foster WA, Howe MJ, et al. 1992. Simultaneous field comparison of evening temporal distributions of nectar and blood feeding by Aedes vexans and Ae. trivittatus (Diptera: Culicidae) in Ohio. J. Med. Entomol. 29:356–360. https://doi.org/ 10.1093/jmedent/29.2.356 [DOI] [PubMed] [Google Scholar]
- Yu BT, Ding YM, Mo XC, et al. 2016. Survivorship and fecundity of Culex pipiens pallens feeding on flowering plants and seed pods with differential preferences. Acta Trop. 155:51–57. https://doi.org/ 10.1016/j.actatropica.2015.12.015 [DOI] [PubMed] [Google Scholar]
- Yu BT, Huang SQ, Ding YM, et al. 2017. Laboratory evaluation of differential attraction of Culex pipiens pallens to the volatiles of flowers, fruits, and seed pods. J. Asia-Pac. Entomol. 20:1372–1376. https://doi.org/ 10.1016/j.aspen.2017.06.010 [DOI] [Google Scholar]
- Yu BT, Hu Y, Ding YM, et al. 2018. Feeding on different attractive flowering plants affects the energy reserves of Culex pipiens pallens adults. Parasitol. Res. 117:67–73. https://doi.org/ 10.1007/s00436-017-5664-y [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.