Abstract
Background
Cytoreductive radical prostatectomy (cRP) has emerged as a promising therapeutic approach for low-volume metastatic hormone-sensitive prostate cancer (mHSPC), but the best candidates for cRP are still unknown. This study aims to explore the potential value of 18F-PSMA-1007 PET/CT-derived quantitative volumetric tumor parameters in cRP treatment selection among patients with low-volume mHSPC.
Methods
A total of 122 patients with primary low-volume mHSPC who underwent 18F-PSMA-1007 PET/CT followed by systemic therapy alone or plus cRP were included. The whole-body PSMA-derived tumor volume (PSMA-TV) was defined as the total volume of whole-body PSMA-avid tumor lesions, and prostate PSMA-TV was defined as the volume of prostate PSMA-avid tumor lesions. Spearman’s correlation was used to analyze the relationships between whole-body PSMA-TV and clinicopathological characteristics. The primary endpoint was progression-free survival (PFS), and Cox regression analyses were performed to explore the independent predictors for PFS.
Results
Among 122 patients, 37 (30.32%) underwent systemic therapy plus cRP. The median and optimal cutoff values of the whole-body PSMA-TV were 71.68 cm3 (41.28–157.41 cm3) and 78.57 cm3, respectively. Whole-body PSMA-TV was positively correlated with prostate-specific antigen (PSA), and patients with nonregional lymph node (NRLN) metastases had a greater whole-body PSMA-TV (P = 0.001). Cox regression analyses revealed that cRP, lower whole-body PSMA-TV and the absence of NRLN metastases were associated with better PFS (all P < 0.05). Subgroup analyses revealed that patients with a low whole-body PSMA or no NRLN metastases had a significant improvement in PFS for cRP versus no cRP (HR: 8.26; 95% CI: 2.72–25.06, P = 0.001; HR: 2.71; 95% CI: 1.25–5.93, P = 0.018). Moreover, among patients with higher prostate PSMA-TV and prostate PSMA-TV/whole-body PSMA-TV, cRP also significantly prolonged PFS compared with those without cRP (HR: 3.49; 95% CI: 1.49–8.18, P = 0.004; HR: 8.54; 95% CI: 2.47–29.50, P = 0.013).
Conclusion
In management of primary low-volume mHSPC, whole-body and prostate PSMA-TV evaluations based on 18F-PSMA-1007 PET/CT could be helpful to identify the most suitable candidates for cRP.
Trial registration
Retrospectively registered.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-025-13482-9.
Keywords: 18F-PSMA-1007 PET/CT, Low-volume mHSPC, PSMA-TV, Nonregional lymph node metastases, Cytoreductive radical prostatectomy
Background
Prostate cancer (PCa) is the second most common malignancy in men worldwide, and ranks first in the developed countries [1, 2]. Among new cases of PCa, approximately 12% of patients present with metastatic hormone-sensitive PCa (mHSPC) and up to 20% in high-risk PCa [3]. In the past few years, the therapeutic landscape of mHSPC management has changed dramatically, and systemic therapy, including docetaxel and/or androgen receptor signaling inhibitor (ARPI) plus androgen deprivation therapy (ADT), has become the standard first-line treatment for primary mHSPC [4]. However, mHSPC is a highly heterogeneous disease with different prognosis, indicating that the optimal treatment regimen for mHSPC may vary from patient to patient.
Tumor burden stratified as low- vs. high-volume based on the definition of the CHAARTED trial has been shown to stratify mHSPC into different risk categories, which facilitates treatment selection and prognosis evaluation [5]. Owing to the interplay between the primary tumor and metastatic sites [6, 7], there has been increasing interest in evaluating the effect of local therapy as a part of the treatment strategy for mHSPC. The HORRAD trial and the STAMPEDE arm H trial recently reported that overall survival (OS) benefitted from the delivery of radiation therapy to the prostate for patients with a low metastatic burden; thus, a new standard of care for patients with low-volume mHSPC was established [8, 9]. In addition to radiation therapy, cytoreductive radical prostatectomy (cRP) has also been used in this setting, and several studies recently noted that cRP is a feasible, safe, and potentially effective therapy in well-selected primary low-volume mHSPC patients [10–12].
Prostate-specific membrane antigen (PSMA) is a transmembrane protein that is weakly expressed in healthy prostate tissue but strongly upregulated in higher-grade prostate tumor cells and metastatic lesions [13]. Therefore, molecular imaging PSMA PET/CT could improve the accuracy of initial staging due to its superior sensitivity and specificity in detecting metastatic lesions. Furthermore, PSMA PET/CT-derived parameters, including PSMA-derived tumor volume (PSMA-TV) and total lesion PSMA (TL-PSMA), have been shown to serve as surrogate imaging-based markers for tumor burden evaluation and have an excellent ability to assess treatment responses [14–19]. Given these findings, we hypothesize that whole-body and/or prostate tumor burden evaluation via PSMA PET/CT-derived parameters play an important role in guiding cRP treatment selection for patients with primary low-volume mHSPC, but the relevant studies are still lacking. To address this gap in knowledge, we analyzed a consecutive cohort of patients with primary low-volume mHSPC who received 18F-PSMA-1007 PET/CT followed by systemic therapy alone or combined with cRP and investigated the potential value of quantitative parameters from 18F-PSMA-1007 PET/CT in cRP treatment selection for primary low-volume mHSPC patients.
Methods
Study population
Following institutional review board approval, we performed a retrospective review of patients with primary low-volume mHSPC who underwent 18F-PSMA-1007 PET/CT before treatment between January 2019 and December 2022. Low-volume diseases were defined according to the CHAARTED criteria, which included patients with either fewer than four bone metastases and no visceral metastases on the basis of the findings of 18F-PSMA-1007 PET/CT [5]. Patients who had received local or systemic treatment before 18F-PSMA-1007 PET/CT, had prior invasive malignancy or any serious comorbidity, or had incomplete data and histologic features supporting a diagnosis of a pure neuroendocrine or small cell tumor were excluded. This study was conducted in accordance with the Declaration of Helsinki, and all subjects signed an informed consent form.
PET/CT imaging protocol
18F-PSMA-1007 was synthesized as described previously [20]. Reagent kits, the PSMA-1007 precursor, and the PSMA-1007 reference standard were purchased from ABX Advanced Biochemical Compounds GmbH, Germany. A whole-body PET/CT scan was conducted via the GEMINI TF64 PET/CT system (Philips Health Care). 18F-PSMA-1007 (3.7 MBq/kg body weight) was injected intravenously, and PET and CT scans were taken 90 min after the injection. Low-dose CT scans from the head to the proximal thighs (pitch 0.8 mm, 60 mA, 140 kV [peak], tube single turn rotation time 1.0 s, and 5-mm slice thickness) for PET attenuation were acquired (pitch 0.8 mm, automatic mA, 140 kV [peak], and 512 × 512 matrix). Whole-body PET scans were performed in three-dimensional mode (emission time: 90 s per bed position, scanned at a total of 7–10 beds).
Image analyses and quantification
All 18F-PSMA-1007 PET/CT images were analyzed using Fusion Viewer software in the Extended Brilliance Workstation (EBW, Philips, Netherlands). All PET/CT images were visually inspected and interpreted independently by 2 nuclear medicine specialists who were blinded to the patients’ medical record, and any discrepancies were resolved through discussion. All suspicious lesions with PSMA uptake higher than the background and not related to physiological uptake were considered pathological according to PSMA PET/CT reporting recommendations [21]. The PSMA-TV was obtained via a whole-body region of interest with a fixed threshold of SUV ≥ 3, as previously described [17]. The standardized uptake value (SUV) was normalized to the patient’s lean body mass, and the volumetric parameters were calculated. Semiquantitative parameters, including the highest maximum SUV (SUVmax), mean SUV (SUVmean), and whole-body PSMA tumor volume (PSMA-TV), defined as the sum of the PSMA-TV of all body lesions, and the total lesion PSMA uptake (TL-PSMA), defined as PSMA-TV × SUVmean, were measured. The prostate PSMA-TV was defined as the sum of the PSMA-TV of the lesion volume only within the prostate, and the prostate TL-PSMA was defined as the prostate PSMA-TV × SUVmean [16].
Initial treatment and cRP
Initial treatments included ADT monotherapy, enhanced systemic therapy, and systemic therapy plus cRP. Enhaced systemic therapy included ADT plus ARPI or docetaxel. cRP was performed via a robot-assisted laparoscopic transabdominal approach with extended pelvic lymph node dissection as previously described [22]. Patients with resectable prostate (stage ≤ T3) with or without pelvic lymph node metastases and having a good general performance status (ECOG score 0 to 1) were all recommended to receive cRP. In addition, docetaxel was offered to fit patients with low-volume mHSPC starting from September 2019. Abiraterone became available for low-volume mHSPC in March 2019, enzalutamide in March 2021, doralutamide in October 2021 and apalutamide in March 2022. The contemporary treatment regimen was in accordance with the European Association of Urology guidelines and every decision on therapy initiation or switch was made after discussion at a multidisciplinary urologic oncology board.
Follow-up and outcome measures
Patients were followed up every month for the first 3 months and every 3 months thereafter with prostate-specific antigen (PSA) tests and imaging. Imaging included chest, abdominal, and pelvic cross-sectional imaging (i.e., CT or MRI) and Tc-99 m bone scintigraphy or 18F-PSMA-1007 PET/CT. The endpoint of survival evaluation was progression-free survival (PFS), defined as the time from treatment beginning to biochemical and/or clinical/radiological progression (based on the results of conventional imaging or 18F-PSMA-1007 PET/CT) or death from any cause, whichever occurred first.
Statistical analysis
Patient characteristics and radiological data were summarized via descriptive statistics, which present categorical data as frequencies (percentages) and continuous variables as medians and interquartile ranges (IQRs) or mean ± standard deviation, as appropriate. Clinical features and radiological data between groups were compared with Student’s t test or the Mann‒Whitney U test and Pearson’s chi‒square test or Fisher’s exact test for continuous and categorical variables, respectively. The optimal cutoff points for the variables were calculated by receiver operating characteristic (ROC) curves based on the largest Youden index. The relationships between 18F-PSMA-1007 PET/CT-derived parameters and clinicopathological characteristics were determined by Spearman’s correlation coefficients. Cox regression analyses were performed to explore the independent predictors of PFS. Survival curves were generated via the Kaplan‒Meier method, and log-rank tests were used to compare the differences. Statistical analysis was performed using SPSS 23.0 for Windows (SPSS, Chicago, IL). All tests were 2-tailed, and p < 0.05 was considered to indicate statistical significance.
Results
Baseline characteristics of patients
In total, 122 patients were finally included in this study. The clinicopathologic characteristics and imaging data were summarized in Table 1. The mean age was 71.02 ± 8.64 years, and the median PSA level at diagnosis was 53.69 (20.59‒114.84) ng/mL. A total of 99 patients (81.15%) had a Gleason score of 8‒10. miT2, miT3 and miT4 diseases was present in 43 (35.24%), 41 (33.61%) and 38 (31.15%) patients, respectively. Pelvic lymph node metastases were detected in 83 patients (68.03%). Twelve patients (9.84%) received ADT monotherapy, 73 patients (59.84%) received enhanced systemic therapy, and 37 patients (30.32%) received systemic therapy plus cRP. Details of the initial treatment were described in Supplement Table 1. Among 37 patients who received systemic therapy plus cRP, 7 patients (5.74%) received ADT plus cRP, 16 patients (11.48%) received ADT plus docetaxel and cRP, and 14 patients (11.48%) received ADT plus ARPI and cRP. In addition, a total of six cycle of docetaxel were performed in patients who received ADT plus docetaxel with or without cRP. The median follow-up and PFS of the 122 patients were 29.57 ± 11.67 and 22.98 ± 10.31 months, respectively.
Table 1.
Baseline characteristic of patients
| Variable | Total (N = 122) |
|---|---|
| Age, years (mean ± SD) | 71.02 ± 8.64 |
| PSA at diagnosis, ng/mL (median, range) | 53.69 (20.59-114.84) |
| Gleason score, n (%) | |
| 6–7 | 23 (18.85%) |
| 8 | 41 (33.61%) |
| 9–10 | 58 (47.54%) |
| T stage, n (%) | |
| miT2 | 43 (35.24%) |
| miT3 | 41 (33.61%) |
| miT4 | 38 (31.15%) |
| N stage, n (%) | |
| miN0 | 39 (31.97%) |
| miN1 | 83 (68.03%) |
| Metastasis site, n (%) | |
| NRLN | 72 (59.02%) |
| Bone | 92 (75.41%) |
| SUVmax (median, range) | 21.14 (11.19–30.23) |
| SUVmean (median, range) | 7.68 (4.62–13.12) |
| Whole-body PSMA-TV, cm3 (median, range) | 71.68(41.28-157.41) |
| Whole-body TL-PSMA, cm3 (median, range) | 684.97 (202.49-1193.62) |
| Prostate PSMA-TV, cm3 (median, range) | 33.41 (17.22–57.73) |
| Prostate TL-PSMA, cm3 (median, range) | 270.57 (104.65–625.10) |
| Primary treatment modality, n (%) | |
| ADT monotherapy | 12 (9.84%) |
| Enhanced systemic therapy | 73 (59.84%) |
| Systemic therapy + cRP | 37 (30.32%) |
| Median PFS, months (mean ± SD) | 22.98 ± 10.31 |
| Median Follow-up, months (mean ± SD) | 29.57 ± 11.67 |
PSA: Prostate-specific antigen; NRLN: Non-regional lymph node; PSMA-TV: Prostate-specific membrane antigen-positive tumor volume; TL-PSMA: Total lesion prostate-specific membrane antigen; ADT: Androgen-deprivation therapy; cRP: cytoreductive radical prostatectomy; PFS: Progression-free survival
PSMA imaging findings and quantitative parameters
All patients presented with significantly overexpressing metastatic lesions on 18F-PSMA-1007 PET/CT. As shown in Table 1 and 72 patients (59.02%) had nonregional lymph node (NRLN) metastases, and 92 patients (75.41%) had bone metastases. For all patients, the median whole-body PSMA-TV, whole-body TL-PSMA, prostate PSMA-TV, prostate TL-PSMA, SUVmax and SUVmean were 71.68 cm3 (41.28–157.41 cm3), 684.97 cm3 (202.49–1193.62 cm3), 33.41 cm3 (17.22–57.73 cm3), 270.57 cm3 (104.65–625.10 cm3), 21.14 (11.19–30.23) and 7.68 (4.62–13.12), respectively.
Associations between whole-body PSMA-TV and clinicopathological characteristics
As shown in Fig. 1A-D, a higher whole-body PSMA-TV was correlated with a higher PSA (Spearman’s rho = 0.386, P = 0.0019), higher SUVmax (Spearman’s rho = 0.319, P = 0.0094), higher SUVmean (Spearman’s rho = 0.285, P = 0.021), and higher whole-body TL-PSMA (Spearman’s rho = 0.849, P = 0.001). The Gleason score was not associated with the whole-body PSMA-TV (P = 0.121) (Fig. 1E), but patients with NRLN metastases had a greater whole-body PSMA-TV (P = 0.001) (Fig. 1F).
Fig. 1.
Associations between whole-body PSMA-TV and clinical data. (A) PSA, (B) SUVmax, (C) SUVmean, (D) whole-body TL-PSMA, (E) Gleason score, (F) NRLN metastases
Prognostic factors for PFS in primary low-volume mHSPC patients
Univariate Cox regression analyses revealed that cRP was a statistically significant prognosticator of PFS (HR: 0.253; 95% CI: 0.125–0.512, P = 0.001). The same was true for NRLN metastases and whole-body PSMA-TV (P < 0.05) (Table 2). While, enhanced systemic therapy and systemic therapy plus cRP could also reduce the risk of progression compared with ADT monotherapy, but the differences were not significant (P > 0.05) (Table 2). In multivariable Cox regression accounting for NRLN metastases, whole-body PSMA-TV and cRP, all three factors were not significantly associated with PFS (all P > 0.05).
Table 2.
Cox regression analysis for associations of clinicopathological characteristic and PSMA PET/CT-derived parameters with PFS
| Variable | Univariable | Multivariable | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |||
| Age (Continuous) | 1.002 | 0.972–1.033 | 0.879 | |||||
| PSA at diagnosis (Continuous) | 0.986 | 0.944–1.029 | 0.825 | |||||
| Gleason score | ||||||||
| 8 vs.6–7 | 1.888 | 0.917–3.888 | 0.085 | |||||
| 9–10 vs.6–7 | 1.837 | 0.950–3.552 | 0.071 | |||||
| T stage | ||||||||
| miT3 vs. miT2 | 1.372 | 0.736–2.559 | 0.320 | |||||
| miT4 vs. miT2 | 1.653 | 0.912–2.993 | 0.097 | |||||
| N stage (miN1 vs. miN0) | 1.596 | 0.959–2.654 | 0.072 | |||||
| NRLN metastases (Absence vs. Presence) | 4.24 | 2.549–7.054 | 0.001 | 2.212 | 0.909–5.380 | 0.080 | ||
| Primary treatment modality | ||||||||
| Enhanced systemic therapy vs. ADT monotherapy | 0.538 | 0.180–1.611 | 0.268 | |||||
| Systemic therapy + cRP vs. ADT monotherapy | 0.415 | 0.164–1.052 | 0.064 | |||||
| SUVmax (Continuous) | 0.997 | 0.979–1.014 | 0.701 | |||||
| SUVmean (Continuous) | 0.995 | 0.952–1.040 | 0.818 | |||||
| Whole-body PSMA-TV (Continuous) | 1.004 | 1.002–1.007 | 0.002 | 1.001 | 0.997–1.004 | 0.640 | ||
| Whole-body TL-PSMA (Continuous) | 1.001 | 1.000-1.002 | 0.190 | |||||
| Prostate PSMA-TV (Continuous) | 1.004 | 0.897–1.009 | 0.057 | |||||
| Prostate TL-PSMA (Continuous) | 1.001 | 0.999–1.001 | 0.446 | |||||
| cRP (Yes vs. No) | 0.253 | 0.125–0.512 | 0.001 | 0.407 | 0.157–1.057 | 0.065 | ||
PSA: Prostate-specific antigen; NRLN: Non-regional lymph node; ADT: Androgen-deprivation therapy; PSMA-TV: Prostate-specific membrane antigen-positive tumor volume; TL-PSMA: Total lesion prostate-specific membrane antigen; cRP: cytoreductive radical prostatectomy; PFS: Progression-free survival
Subgroup analyses
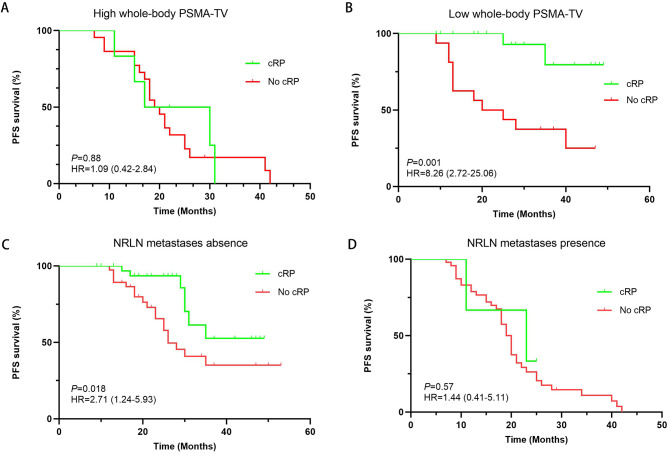
Based on the ROC curve results, the optimal cutoff point of whole-body PSMA-TV was 78.57 cm3, and 122 patients were subsequently divided into a high whole-body PSMA-TV group (whole-body PSMA-TV ≥ 78.57 cm3) and a low whole-body PSMA-TV group (whole-body PSMA-TV < 78.57 cm3). In patients with high whole-body PSMA-TV, cRP was not significantly associated with PFS (HR: 1.09, 95% CI: 0.42–2.84; P = 0.88) (Fig. 2A). However, cRP significantly prolonged PFS in patients with low whole-body PSMA-TV (HR: 8.26; 95% CI: 2.72–25.06, P = 0.001) (Fig. 2B). In patients without NRLN metastases, cRP was also associated with a better PFS (HR: 2.71; 95% CI: 1.24–5.93, P = 0.018) (Fig. 2C), but the impact of cRP on PFS in patients with NRLN metastases was not significant (HR: 1.44; 95% CI: 0.41–5.11, P = 0.57) (Fig. 2D).
Fig. 2.
Kaplan‒Meier estimates of the impact of cRP in (A) patients with high whole-body PSMA-TV, (B) patients with low whole-body PSMA-TV, (C) patients without NRLN metastases, and (D) patients with NRLN metastases
PSMA-TV spatial distribution pattern and cRP
Owing to cRP reducing the local tumor burden to delay progression and improve survival, we hypothesized that the maximal reduction in tumor burden caused by cRP might contribute to the better survival outcomes. Based on the ROC results, the optimal cutoff point for prostate PSMA-TV was 34.04 cm3. As shown in Fig. 3A, cRP significantly prolonged PFS in patients with high prostate PSMA-TV (HR: 3.49; 95% CI: 1.49–8.18, P = 0.004). However, even though cRP improved the PFS of patients with low prostate PSMA-TV, the difference was not significant (HR: 2.01; 95% CI: 0.79–5.05, P = 0.15) (Fig. 3B). Furthermore, we noted that patients with a high percentage of prostate PSMA-TV/whole-body PSMA-TV (≥ 66%) showed a significant improvement in PFS after cRP (HR: 8.54; 95% CI: 2.47–29.50, P = 0.013) (Fig. 3C), but the effect of cRP in patients with a low percentage of prostate PSMA-TV/whole-body PSMA-TV (< 66%) was limited (HR: 1.83; 95% CI: 0.91–3.66, P = 0.099) (Fig. 3D).
Fig. 3.
Kaplan‒Meier estimates of the impact of cRP in (A) patients with high prostate PSMA-TV, (B) patients with low prostate PSMA-TV, (C) patients with a high percentage of prostate PSMA-TV/whole-body PSMA-TV, and (D) patients with a low percentage of prostate PSMA-TV/whole-body PSMA-TV
Four representative patient cases were shown in Fig. 4. Patient 1 had a low whole-body tumor burden (whole-body PSMA-TV: 17.15 cm3) and prostate tumor burden (prostate PSMA-TV: 2.24 cm3, prostate PSMA-TV/whole-body PSMA-TV: 13.06%). Following ADT combined with 6 cycles of docetaxel, there was no evidence of tumor progression after 30 months of follow-up. Patient 2 had a low whole-body tumor burden (whole-body PSMA-TV: 31.87 cm3) but a higher percentage of prostate PSMA-TV/whole-body PSMA-TV (88.55%), and the PFS was only 12 months after ADT combined with 6 cycles of docetaxel. Patient 3 had a low whole-body tumor burden (whole-body PSMA-TV: 71.36 cm3) and high prostate tumor burden (prostate PSMA-TV: 61.50 cm3, prostate PSMA-TV/whole-body PSMA-TV: 86.19%). Surprisingly, there was no evidence of tumor progression during the 34-month follow-up after treatment with ADT combined with 6 cycles of docetaxel and cRP. Patient 4 had a high whole-body tumor burden (whole-body PSMA-TV: 123.46 cm3) and NRLN metastases, but the prostate tumor burden was lower (prostate PSMA-TV: 62.14 cm3, prostate PSMA-TV/whole-body PSMA-TV: 50.33%). Even after treatment with ADT combined with 6 cycles of docetaxel and cRP, the PFS was only 11 months.
Fig. 4.
18F-PSMA-1007 PET/CT maximum intensity projection images for four representative cases. Patient 1 had a low whole-body PSMA-TV (17.15 cm3), prostate PSMA-TV (2.24 cm3), and prostate PSMA-TV/whole-body PSMA-TV (13.06%). ADT combined with 6 cycles of docetaxel was given, and the PFS was 30 months. Patient 2 had a low whole-body PSMA-TV (31.87 cm3) and a higher percentage of prostate PSMA-TV/whole-body PSMA-TV (88.55%). The PFS was only 12 months after ADT combined with 6 cycles of docetaxel. Patient 3 had a low whole-body PSMA-TV (71.36 cm3) and a high percentage of prostate PSMA-TV/whole-body PSMA-TV (86.19%). Surprisingly, there was no evidence of tumor progression after 34 months of follow-up after treatment with ADT combined with 6 cycles of docetaxel and cRP. Patient 4 had a high whole-body PSMA-TV (123.46 cm3) and NRLN metastases, but the prostate PSMA-TV (62.14 cm3) and the percentage of prostate PSMA-TV/whole-body PSMA-TV (50.33%) were lower. The PFS was only 11 months even after receiving ADT combined with 6 cycles of docetaxel and cRP
Discussion
In this study, we found that tumor volume measured via 18F-PSMA-1007 PET/CT, especially for whole-body and prostate tumor volume evaluation, could assist urologists in identifying the most suitable candidates for cRP and thus pave the way for a more personalized treatment strategy for primary low-volume mHSPC disease.
The role of cRP in patients with low-volume mHSPC is still unclear; cRP remains an experimental approach based on a few retrospective studies [23, 24]. In the Belgian multicenter LoMP registry study, 109 patients with low-volume mHSPC were included, and 48 patients received cRP. The authors reported that the OS and cancer-specific survival (CSS) of patients who received cRP were significantly better than those of patients without local treatment and were similar to those of patients who received radiotherapy to the prostate during a median follow-up of 22 months [10]. In addition, they reported that cRP was effective to prevent local adverse events induced by disease progression. Rajwa et al. recently conducted a multicenter study to explore the outcomes of cRP in patients with a small metastatic burden diagnosed by PSMA PET/CT and noted that the patients could also benefit from cRP, as the 2-year castration-resistant PCa-free survival and OS rates were 85.5% (95% CI: 78.5–93.7%) and 98.9% (95% CI: 96.8–100%), respectively [12]. In this study, we further noted that for patients with low-volume mHSPC, cRP significantly reduced the risk of disease progression (HR: 0.253; 95% CI: 0.125–0.512, P = 0.001). Among 37 patients who received cRP, ADT combined ARPI or docetaxel could reduce the risk of progression compared with ADT monotherapy, but the significant differences were not observed. In addition, the difference between ARPI and docetaxel was also not significant (Supplemental Fig. 1), indicating that the different systemic therapy regimens had no significant impact on the outcome of patients underwent cRP. However, after adjusting for clinical characteristics, a significant difference in cRP was not observed. This result pointed out that cRP might be a promising modality for the management of low-volume mHSPC, but the oncologic activity of cRP was limited by several confounding factors. Therefore, the most suitable candidates for cRP require further investigation.
Tumor burden assessment is essential for treatment selection and outcome prediction in clinical practice. Historically, the localization and number of metastases have been widely used to assess tumor burden, but focusing only on the number of metastases and their localization neglects the difference in intrinsic tumor biometrics, ultimately leading to limited and inaccurate tumor burden assessment. Barbato et al. reported that PSMA PET/CT-derived volume quantification delivery was comparable to the CHAARTED tumor burden discretion criteria, which indicated that the tumor burden evaluation by PSMA PET/CT also had an excellent ability to guide targeted or multimodal therapy in the management of mHSPC [16]. Furthermore, several studies have reported that PSMA-TV and TL-PSMA can predict treatment responses and survival outcomes in PCa patients [14, 17, 18]. To the best of our knowledge, this is the first study to explore the role of PSMA-TV and TL-PSMA and the spatial distribution pattern of PSMA-TV in cRP treatment selection among patients with primary low-volume mHSPC.
First, we analyzed the relationships between whole-body PSMA-TV and clinical risk factors and reported that a greater whole-body PSMA-TV was associated with greater PSA, as reported in previous studies [25–27]. In addition, the prognostic role of PSMA-TV in primary low-volume mHPSCs was also observed in the present study. Previously, Schmuck et al. retrospectively reviewed the data of 101 patients who underwent PSMA PET/CT and noted that PSMA-TV provided a quantitative imaging biomarker for whole-body tumor burden [15]. Moreover, a study conducted by Kluge et al. revealed that PSMA-TV could provide a highly granular and prognostic assessment of tumor burden across the spectrum of PCa disease progression [28]. These findings might explain why PSMA-TV could serve as a prognostic factor in the management of PCa, as it could more accurately assess the tumor burden and intrinsic metabolic activity of the tumor, which have a profound impact on treatment decisions and follow-up planning in clinical practice.
In the past, the number of metastases was the primary marker used to assess tumor burden, and only patients with a lower tumor burden benefitted from cRP [29]. As mentioned above, PSMA-TV can accurately assess tumor burden, indicating that PSMA-TV might play an important role in cRP treatment selection, but no data have been available until now. In this study, we first found that PSMA-TV could guide cRP treatment selection in primary low-volume mHSPC. For patients with low whole-body PSMA-TV (< 78.57 cm3), cRP could significantly improve PFS, but the value of cRP in patients with high whole-body PSMA-TV was not significant. In addition to whole-body PSMA-TV, prostate PSMA-TV could also guide cRP treatment selection. Among patients with high prostate PSMA-TV (≥ 34.04 cm3) or a high percentage of prostate PSMA-TV/whole-body PSMA-TV (≥ 66%), cRP could also significantly improve PFS, whereas the impact was limited to cRP in patients with low prostate PSMA-TV or a low percentage of prostate PSMA-TV/whole-body PSMA-TV. The main reason for this phenomenon was that among patients with high prostate PSMA-TV, cRP could maximize the reduction in the tumor burden by debulking the primary lesion, which might prevent tumor spread from the primary lesion and subsequently delay or reduce the risk of progression. Therefore, we deduced that whole PSMA-TV and prostate PSMA-TV evaluations could provide new insights for quantitatively assessing the tumor burden of the whole body and primary lesion, which could assist urologists in identifying more suitable candidates for cRP.
Nowadays, the optimal cut-off of the PSMA-TV was still unclear. Previously, in high-risk localized PCa, Andela et al. found that the optimal cut-off of PSMA-TV was 1.8 mL, and patients with higher PSMA-TV were at high risk for biochemical recurrence [30]. Subsequently, Ke et al. pointed out that the optimal cut-off of whole-body PSMA-TV was 79.1 mL for predicting biochemical response to abiraterone in metastatic castration-resistant prostate cancer (mCRPC) [31]. Moreover, Shagera et al. found that the optimal cut-off of whole-body PSMA-TV were 515 mL for PFS and 473 mL for OS in late stage mCRPC treated with cabazitaxel, and patients with lower PSMA-TV presented longer PFS and OS [18]. Thus, the optimal cut-off of PSMA-TV in different patients might be varied owing to different tumor burden at different stage of PCa. In this study, we first found that patients with higher whole-body, prostate PSMA-TV or percentages of prostate PSMA-TV/whole-body PSMA-TV could benefit more from cRP, which might help urologists to make treatment decision for cRP. However, owing to relatively small sample size and no external validation, further prospective multicenter studies are warranted.
18F-PSMA-1007 is a novel PSMA-based radiopharmaceutical that has emerged as an alternative tracer in addition to 68Ga-PSMA-11 for PCa detection. Compared to 68Ga, 18F has a longer physical half-life (110 vs. 68 minutes), allowing for large-scale radiosynthesis (cyclotron-produced 18F vs. generator-produced 68Ga), centralized production and distribution leading to cost saving, and higher quantities service for more patients at each production [32]. 18F-PSMA-1007 also offers a higher spatial resolution of image than 68Ga-PSMA-11 due to the relatively lower positron energy of 18F [33]. Moreover, the rapid blood clearance but lower renal excretion and urinary activity of 18F-PSMA-1007 also help urologists to assess prostate and pelvic lesion [32]. However, 18F-PSMA-1007 has nonspecific uptake in osseous structures and increase interobserver variability regarding bone metastases compared with 68Ga-PSMA-11 [34]. So, the results of 18F-PSMA-1007 should be interpreted cautiously in routine clinical practice. In this study, all PET/CT images were interpreted independently by 2 nuclear medicine specialists. Moreover, any discrepancies were resolved through discussion, and suspicious bone metastases were also validated by computerized tomography, magnetic resonance imaging and/or Tc-99 m bone scintigraphy in order to minimize the impact of nonspecific uptake in osseous structures of 18F-PSMA-1007. While, there were still need further study to confirm our findings and explore the difference in PSMA-TV between 18F-PSMA-1007 and 68Ga-PSMA-11.
Concomitant NRLN and bone metastases represented a high-risk feature in metastatic volume stratification. Heesterman et al. retrospectively reviewed the data of 14,087 patients with synchronous mHSPC and reported that the presence of concomitant NRLN and bone metastases was a poor prognostic indicator, especially in patients with low-volume mHSPC [35]. Our previous studies revealed that the presence of NRLN metastases was an independent prognostic factor for primary mHSPC, and the risk of disease progression increased 2-fold in patients with NLRN metastases [36]. Moreover, patients with low-volume plus NRLN metastases may be suitable for more intensive systemic treatment, such as early docetaxel chemotherapy [36]. In this study, we further revealed that NRLN metastases were associated with worse outcomes in patients with primary low-volume metastases. Furthermore, the benefit from the addition of cRP was greater in patients without NRLN metastases than in those with NRLN metastases. Thus, for patients with low-volume disease who present with NRLN metastases early, more intensive systemic treatments should be performed rather than cRP.
Despite these positive results, several limitations of this study should be recognized. First, this was a retrospective study from a single center and the sample size in the present study was relatively small, which might cause selection bias and limit the generalizability of our results. However, a rigorous inclusion and exclusion criteria were established to minimize selection bias in this study. In addition, double data entry and validation were also used in the data collections process in order to decrease potential biases and ensure the results’ reliability and validity. Second, only 3 patients (2.46%) died during follow-up, and none of these patients died from PCa. Because most patients were alive at the last follow-up, the impact of cRP on OS was not performed and a longer follow-up duration might produce more positive findings. Third, the optimal cutoff values of whole-body PSMA-TV and prostate PSMA-TV lacked external validation, and the relatively small sample was the main reason. So, the clinical applicability and reproducibility of PSMA-TV were still need to confirm. Therefore, further prospective multicenter studies are warranted to support our findings.
Conclusions
In this retrospective study, we explored the potential value of whole-body PSMA-TV and prostate PSMA-TV in cRP treatment selection among patients with primary low-volume mHSPC and found that whole-body PSMA-TV, NRLN metastases and cRP were the significant prognostic factors for PFS in primary low-volume mHSPC. Specifically, higher whole-body PSMA-TV and the presence of NRLN metastases were associated with lower PFS after cRP treatment. In addition, patients with higher prostate PSMA-TV or percentages of prostate PSMA-TV/whole-body PSMA-TV could benefit more from cRP, as more favorable survival outcomes have been observed. Thus, the PSMA PET/CT-derived parameters such as whole-body PSMA-TV and prostate PSMA-TV could help urologists to identify the most suitable candidates for cRP.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors appreciate Prof. Xinyang Wang from the Department of Urology, the First Affiliated Hospital of Xi’an Jiaotong University for helping with this paper.
Abbreviations
- PCa
Prostate cancer
- mHSPC
Metastatic hormone-sensitive prostate cancer
- ADT
Androgen deprivation therapy
- cRP
Cytoreductive radical prostatectomy
- mCRPC
metastatic castration-resistant prostate cancer
- PSA
Prostate-specific antigen
- PSMA
Prostate-specific membrane antigen
- PSMA-TV
PSMA-derived tumor volume
- TL-PSMA
Total lesion PSMA
- NRLN
Non-regional lymph node
- PFS
Progression-free survival
- ROC
Receiver operating characteristic
- OS
Overall survival
- CSS
Cancer-specific survival
- ARPI
androgen receptor pathway inhibitor
Author contributions
Conceptualization: XYD and KJW; Methodology: JJF, XYD and KJW; Data collection and analysis: JJF, KX, ZDJ, CSG, HS, GQG, GJW, QYK, LL and ZNW. Original draft preparation: JJF and KJW; Paper review and editing: DLH, XYD and KJW. All authors read and approved the final manuscript.All authors reviewed the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC 81202014 to KW), Key Research and Development Program of Shaanxi Province (2022SF-307 to KW), the Fundamental Research Funds for the Central Universities (to KW) and the Natural Science Foundation in Shaanxi Province (2023-JC-QN-0890 to JF).
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
All procedures performed in the study and involving human participants were performed in accordance with the ethical standards of the institutional and/or national research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. This retrospective analysis was approved by the Institutional Review Board of the First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, P.R. China. Informed consent was obtained from all individual participants included in the study.
Consent for publication
Written informed consent was obtained from the patient for publication of this study and accompanying images.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Junjie Fan are first author
1. References
- 1.Culp MB, Soerjomataram I, Efstathiou JA, Bray F, Jemal A. Recent global patterns in prostate Cancer incidence and mortality rates. Eur Urol. 2020;77(1):38–52. [DOI] [PubMed] [Google Scholar]
- 2.Prostate Cancer. [https://www.ncbi.nlm.nih.gov/books/NBK470550/].
- 3.Yaxley JW, Raveenthiran S, Nouhaud FX, Samaratunga H, Yaxley WJ, Coughlin G, Yaxley AJ, Gianduzzo T, Kua B, McEwan L, et al. Risk of metastatic disease on (68) gallium-prostate-specific membrane antigen positron emission tomography/computed tomography scan for primary staging of 1253 men at the diagnosis of prostate cancer. BJU Int. 2019;124(3):401–7. [DOI] [PubMed] [Google Scholar]
- 4.Matsumura N, Fujita K, Nishimoto M, Minami T, Tahara H, Yoshimura K, Uemura H. Current status and future perspectives of the managements of metastatic hormone-sensitive prostate cancer. World J Urol. 2023;41(8):2063–8. [DOI] [PubMed] [Google Scholar]
- 5.Kyriakopoulos CE, Chen YH, Carducci MA, Liu G, Jarrard DF, Hahn NM, Shevrin DH, Dreicer R, Hussain M, Eisenberger M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate Cancer: long-term survival analysis of the Randomized Phase III E3805 CHAARTED trial. J Clin Oncol. 2018;36(11):1080–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438(7069):820–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McAllister SS, Gifford AM, Greiner AL, Kelleher SP, Saelzler MP, Ince TA, Reinhardt F, Harris LN, Hylander BL, Repasky EA, et al. Systemic endocrine instigation of indolent tumor growth requires osteopontin. Cell. 2008;133(6):994–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boevé LMS, Hulshof M, Vis AN, Zwinderman AH, Twisk JWR, Witjes WPJ, Delaere KPJ, Moorselaar R, Verhagen P, van Andel G. Effect on survival of androgen deprivation therapy alone compared to Androgen Deprivation Therapy Combined with Concurrent Radiation Therapy to the prostate in patients with primary bone metastatic prostate Cancer in a prospective Randomised Clinical Trial: data from the HORRAD Trial. Eur Urol. 2019;75(3):410–8. [DOI] [PubMed] [Google Scholar]
- 9.Parker CC, James ND, Brawley CD, Clarke NW, Hoyle AP, Ali A, Ritchie AWS, Attard G, Chowdhury S, Cross W, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018;392(10162):2353–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lumen N, De Bleser E, Buelens S, Verla W, Poelaert F, Claeys W, Fonteyne V, Verbeke S, Villeirs G, De Man K, et al. The role of Cytoreductive Radical Prostatectomy in the treatment of newly diagnosed low-volume metastatic prostate Cancer. Results from the local treatment of metastatic prostate Cancer (LoMP) Registry. Eur Urol open Sci. 2021;29:68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sooriakumaran P, Wilson C, Rombach I, Hassanali N, Aning J, A DL, Cathcart P, Eden C, Ahmad I, Rajan P, et al. Feasibility and safety of radical prostatectomy for oligo-metastatic prostate cancer: the testing radical prostatectomy in men with prostate cancer and oligo-metastases to the bone (TRoMbone) trial. BJU Int. 2022;130(1):43–53. [DOI] [PubMed] [Google Scholar]
- 12.Rajwa P, Robesti D, Chaloupka M, Zattoni F, Giesen A, Huebner NA, Krzywon A, Miszczyk M, Moll M, Stando R, et al. Outcomes of Cytoreductive Radical Prostatectomy for oligometastatic prostate Cancer on prostate-specific membrane Antigen Positron Emission Tomography: results of a Multicenter European Study. Eur Urol Oncol; 2023. [DOI] [PubMed]
- 13.Maurer T, Eiber M, Schwaiger M, Gschwend JE. Current use of PSMA-PET in prostate cancer management. Nat Rev Urol. 2016;13(4):226–35. [DOI] [PubMed] [Google Scholar]
- 14.Schmidkonz C, Cordes M, Schmidt D, Bäuerle T, Goetz TI, Beck M, Prante O, Cavallaro A, Uder M, Wullich B, et al. (68)Ga-PSMA-11 PET/CT-derived metabolic parameters for determination of whole-body tumor burden and treatment response in prostate cancer. Eur J Nucl Med Mol Imaging. 2018;45(11):1862–72. [DOI] [PubMed] [Google Scholar]
- 15.Schmuck S, von Klot CA, Henkenberens C, Sohns JM, Christiansen H, Wester HJ, Ross TL, Bengel FM, Derlin T. Initial experience with volumetric (68)Ga-PSMA I&T PET/CT for Assessment of Whole-Body Tumor Burden as a quantitative imaging biomarker in patients with prostate Cancer. J Nucl Med. 2017;58(12):1962–8. [DOI] [PubMed] [Google Scholar]
- 16.Barbato F, Fendler WP, Rauscher I, Herrmann K, Wetter A, Ferdinandus J, Seifert R, Nader M, Rahbar K, Hadaschik B, et al. PSMA-PET for the assessment of metastatic hormone-sensitive prostate cancer volume of disease. J Nucl Med. 2021;62(12):1747–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shagera QA, Artigas C, Karfis I, Critchi G, Chanza NM, Sideris S, Peltier A, Paesmans M, Gil T, Flamen P. (68)Ga-PSMA PET/CT for Response Assessment and Outcome Prediction in metastatic prostate Cancer patients treated with taxane-based chemotherapy. J Nucl Med. 2022;63(8):1191–8. [DOI] [PubMed] [Google Scholar]
- 18.Shagera QA, Karfis I, Sideris S, Guiot T, Woff E, Martinez-Chanza N, Roumeguere T, Gil T, Flamen P, Artigas C. Tumor volume on PSMA PET as a prognostic biomarker in prostate Cancer patients treated with Cabazitaxel. Clin Nucl Med. 2023;48(9):775–80. [DOI] [PubMed] [Google Scholar]
- 19.Kim M, Seifert R, Fragemann J, Kersting D, Murray J, Jonske F, Pomykala KL, Egger J, Fendler WP, Herrmann K, et al. Evaluation of thresholding methods for the quantification of [(68)Ga]Ga-PSMA-11 PET molecular tumor volume and their effect on survival prediction in patients with advanced prostate cancer undergoing [(177)Lu]Lu-PSMA-617 radioligand therapy. Eur J Nucl Med Mol Imaging. 2023;50(7):2196–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cardinale J, Martin R, Remde Y, Schäfer M, Hienzsch A, Hübner S, Zerges AM, Marx H, Hesse R, Weber K et al. Procedures for the GMP-Compliant production and Quality Control of [(18)F]PSMA-1007: a Next Generation Radiofluorinated Tracer for the detection of prostate Cancer. Pharmaceuticals (Basel) 2017, 10(4). [DOI] [PMC free article] [PubMed]
- 21.Fendler WP, Eiber M, Beheshti M, Bomanji J, Calais J, Ceci F, Cho SY, Fanti S, Giesel FL, Goffin K, et al. PSMA PET/CT: joint EANM procedure guideline/SNMMI procedure standard for prostate cancer imaging 2.0. Eur J Nucl Med Mol Imaging. 2023;50(5):1466–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poelaert F, Verbaeys C, Rappe B, Kimpe B, Billiet I, Plancke H, Decaestecker K, Fonteyne V, Buelens S, Lumen N. Cytoreductive prostatectomy for metastatic prostate Cancer: First lessons learned from the multicentric prospective local treatment of metastatic prostate Cancer (LoMP) trial. Urology. 2017;106:146–52. [DOI] [PubMed] [Google Scholar]
- 23.Culp SH, Schellhammer PF, Williams MB. Might men diagnosed with metastatic prostate cancer benefit from definitive treatment of the primary tumor? A SEER-based study. Eur Urol. 2014;65(6):1058–66. [DOI] [PubMed] [Google Scholar]
- 24.Sooriakumaran P, Karnes J, Stief C, Copsey B, Montorsi F, Hammerer P, Beyer B, Moschini M, Gratzke C, Steuber T, et al. A multi-institutional analysis of Perioperative outcomes in 106 men who underwent radical prostatectomy for distant metastatic prostate Cancer at Presentation. Eur Urol. 2016;69(5):788–94. [DOI] [PubMed] [Google Scholar]
- 25.Zschaeck S, Andela SB, Amthauer H, Furth C, Rogasch JM, Beck M, Hofheinz F, Huang K. Correlation between quantitative PSMA PET parameters and clinical risk factors in non-metastatic primary prostate Cancer patients. Front Oncol. 2022;12:879089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gafita A, Bieth M, Krönke M, Tetteh G, Navarro F, Wang H, Günther E, Menze B, Weber WA, Eiber M. qPSMA: Semiautomatic Software for whole-body tumor Burden Assessment in prostate Cancer using (68)Ga-PSMA11 PET/CT. J Nucl Med. 2019;60(9):1277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seifert R, Herrmann K, Kleesiek J, Schäfers M, Shah V, Xu Z, Chabin G, Grbic S, Spottiswoode B, Rahbar K. Semiautomatically quantified tumor volume using (68)Ga-PSMA-11 PET as a Biomarker for Survival in patients with advanced prostate Cancer. J Nucl Med. 2020;61(12):1786–92. [DOI] [PubMed] [Google Scholar]
- 28.Kluge K, Einspieler H, Haberl D, Spielvogel C, Stoiber S, Vraka C, Papp L, Wunsch S, Egger G, Kramer G, et al. Examining the relationship and prognostic significance of cell-free DNA levels and the PSMA-Positive tumor volume in men with prostate Cancer: a retrospective-prospective [(68)Ga]Ga-PSMA-11 PET/CT study. J Nucl Med. 2024;65(1):63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yanagisawa T, Rajwa P, Kawada T, Bekku K, Laukhtina E, Deimling MV, Majdoub M, Chlosta M, Karakiewicz PI, Heidenreich A, et al. An Updated Systematic and Comprehensive Review of Cytoreductive Prostatectomy for metastatic prostate Cancer. Curr Oncol (Toronto Ont). 2023;30(2):2194–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bela Andela S, Amthauer H, Furth C, Rogasch JM, Beck M, Mehrhof F, van den Ghadjar P, Klatte T, Tahbaz R, et al. Quantitative PSMA-PET parameters in localized prostate cancer: prognostic and potential predictive value. Radiation Oncol (London England). 2024;19(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ke ZB, Chen JY, You Q, Sun JB, Xue YT, Ye XJ, Chen SH, Xue XY, Sun XL, Chen DN, et al. Low TLR and PSMA-TV predict biochemical response to abiraterone acetate in metastatic prostate cancer patients developing castration resistance after chemohormonal therapy at hormone-sensitive stage. J Cancer Res Clin Oncol. 2023;149(8):5071–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts MJ, Maurer T, Perera M, Eiber M, Hope TA, Ost P, Siva S, Hofman MS, Murphy DG, Emmett L, et al. Using PSMA imaging for prognostication in localized and advanced prostate cancer. Nat Rev Urol. 2023;20(1):23–47. [DOI] [PubMed] [Google Scholar]
- 33.Giesel FL, Hadaschik B, Cardinale J, Radtke J, Vinsensia M, Lehnert W, Kesch C, Tolstov Y, Singer S, Grabe N, et al. F-18 labelled PSMA-1007: biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur J Nucl Med Mol Imaging. 2017;44(4):678–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hagens MJ, Oprea-Lager DE, Vis AN, Wondergem M, Donswijk ML, Meijer D, Emmett L, van Leeuwen PJ. Reproducibility of PSMA PET/CT imaging for primary staging of Treatment-Naïve prostate Cancer patients depends on the Applied Radiotracer: a retrospective study. J Nucl Med. 2022;63(10):1531–6. [DOI] [PubMed] [Google Scholar]
- 35.van der Heesterman BL, Schoots IG, Mehra N, Aben KKH. Prognostic importance of concomitant non-regional lymph node and bone metastases in men with newly diagnosed metastatic prostate cancer. BJU Int. 2022;130(2):217–25. [DOI] [PubMed] [Google Scholar]
- 36.Jiang Z, Fan J, Gan C, Dong X, Gao G, Wang Z, He D, Li L, Duan X, Wu K. Impact of non-regional lymph node metastases accurately revealed on (18)F-PSMA-1007 PET/CT in the clinical management of metastatic hormone-sensitive prostate cancer. EJNMMI Res. 2023;13(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the current study are available from the corresponding author on reasonable request.