ABSTRACT
Polyamines play crucial roles in various biological processes, including cell proliferation and differentiation, immune response modulation, and signal transduction. Ornithine decarboxylase (ODC) initiates polyamine biosynthesis by catalyzing the conversion of ornithine to putrescine in a pyridoxal phosphate (PLP)-dependent manner. While the structures of mammalian and protozoan ODCs have been elucidated, fungal ODCs remain uncharacterized. In this study, AlphaFold2 was employed to simulate the structures of ODCs from four fungi: Kluyveromyces lactis, Candida albicans, Debaryomyces hansenii, and Schizosaccharomyces pombe. The results indicated that, although these ODCs share α/β-barrel and β-sheet domains, their active site conformations exhibit subtle differences. Additionally, substrate selectivity among ODCs and related decarboxylases varied depending on the distance between the Cα of aspartate or glutamate residues within the specificity helix and the C4α of PLP. Notably, the bacterial Campylobacter jejuni decarboxylase (CjCANSDC), which binds the largest substrate, exhibits the longest distance, whereas fungal ODC, which binds the smallest substrate, displays the shortest distance. Furthermore, significant differences in the composition of amino acid residues within the active sites were also observed. This study provides insights into the structural diversity and catalytic activity of ODCs across a broad range of organisms, advancing the understanding of structure-dependent evolutionary processes.
KEYWORDS: Candida albicans, Debaryomyces hansenii, Kluyveromyces lactis, ornithine decarboxylase, PLP-dependent enzyme, polyamine, Schizosaccharomyces pombe, specificity helix
Introduction
Polyamines, including putrescine, spermidine, and spermine, are polycationic alkylamines that are essential to a wide range of organisms, from prokaryotes to eukaryotes [1,2]. While the intracellular concentration of polyamines is tightly regulated at millimolar levels, the actual amount of free polyamines in the cell remains low due to their predominant binding with cellular anions [3]. These compounds play crucial roles in various cellular functions, such as cell division, differentiation, proliferation, DNA and protein synthesis, gene expression, homeostasis, signal transduction, immune response modulation, and the maintenance of chromatin structure [4]. However, understanding the physiological and pathophysiological implications of alterations in polyamine content is challenging due to the pleiotropic effects of polyamines on cell growth and development [5]. Ornithine decarboxylase (ODC), a pyridoxal phosphate (PLP)-dependent enzyme, initiates polyamine biosynthesis by catalyzing the decarboxylation of ornithine to putrescine, which is the rate-limiting step in this pathway [6]. Subsequently, spermidine synthase converts the generated putrescine into spermidine, which then triggers autophagy by suppressing protein acetylation [7].
To regulate polyamine levels, ODC is modulated by either antizyme (AZ) or antizyme inhibitor (AZIN) [8]. Polyamines regulate the synthesis of AZ1, one of the three isoforms of AZ, by inducing a ribosomal frameshift, thus acting as sensors for cellular polyamine levels [9,10]. Elevated levels of cellular polyamines facilitate the bypassing of the in-frame stop codon on AZ1 mRNA. This process relieves translational repression imposed by the N-terminal fragment of AZ1, promoting the translation of ODC’s inhibitor, AZ1 [11]. Upon interaction with AZ1, ODC undergoes conformational changes [12]. The affinity between AZ1 and ODC exceeds that of ODC monomers, resulting in the formation of inhibitory ODC-AZ heterodimers [13]. Subsequently, the 26S proteasome initiates rapid turnover of ODC without ubiquitination, demonstrating a unique degradation mechanism [5]. AZIN, a regulatory protein related to ODC, shows a higher affinity for AZ1 compared to ODC. As a result, AZIN displaces ODC from the ODC-AZ1 complex, restoring ODC activity by counteracting AZ1’s inhibitory effect and protecting ODC from proteolytic degradation [14].
ODC plays a role in inflammation by regulating MER proto-oncogene tyrosine kinase (MerTK). MerTK, a receptor located on the surface of macrophages, is crucial for recognizing and engulfing apoptotic cells (AC) through a process termed efferocytosis [15]. The interaction between MerTK and AC activates the MerTK-Erk1/2 signaling pathway, which promotes the expression of IL-10 [16]. Additionally, putrescine synthesized by ODC maintains the steady-state level of MerTK by regulating H3K9 di/trimethylation in MerTK mRNA [17]. Furthermore, putrescine directly mitigates inflammation by reducing the activity of M1 macrophages via histone modifications [18]. While M1 macrophages are essential for pathogen elimination and enhancing anti-tumor immunity, they also secrete tumor-promoting and pro-inflammatory cytokines [19]. Deficiencies in ODC can lead to increased susceptibility to diseases linked with chronic inflammation, such as chronic lung diseases and autoimmune disorders.
Increased levels of ODC and polyamines have been associated with cancer and other conditions characterized by unchecked cell proliferation [20]. ODC expression is tightly regulated in normal cells; however, neoplastic cells often exhibit persistently elevated levels of ODC expression and activity [21]. High cellular putrescine concentration facilitate interactions between HuR and c-Myc mRNA through Chk2-mediated phosphorylation of HuR, thereby promoting c-Myc translation [22]. Elevated c-Myc protein levels, in turn, activate ODC transcription by interacting with specific sequences in the ODC promoter region [23]. ODC and c-Myc mutually enhance each other’s expression, contributing to cancers associated with epithelial cells, such as skin and colorectal cancers [24,25]. Conversely, 2-difluoromethylornithine (DFMO), a well-known irreversible inhibitor of ODC, is employed in cancer treatment [26]. Given the correlation between excessive ODC expression and an increased risk of cancer, ongoing research is focused on utilizing DFMO as a therapeutic approach, particularly in the treatment of neuroblastoma [27].
The Structures of ODCs from humans, mammals, and protozoa have been extensively investigated. In contrast, the structures of ODCs in fungi have yet to be characterized. In this study, we simulated the three-dimensional structures of ODCs from four fungal species: Kluyveromyces lactis, Debaryomyces hansenii, Candida albicans, and Schizosaccharomyces pombe, using AlphaFold2 [28]. The results highlighted key structural features, including the binding sites for substrates and the cofactor pyridoxal 5’-phosphate (PLP). These findings expand our understanding of structural diversity across a wide range of organisms and contribute to the knowledge of structure-dependent evolutionary processes, with the potential to aid in the development of novel antifungal agents.
Materials and methods
Simulation of three-dimensional structures
AlphaFold2, developed by DeepMind, was employed to predict the structures of the five selected fungal ODC proteins [28]. AlphaFold2 utilizes an innovative machine learning approach that employs the multiple sequence alignment technique to integrate both physical and biological insights of protein structures into deep learning algorithms. AlphaFold2 accurately predicts inter-amino acid distances, facilitating the creation of precise protein models that faithfully represent their structures [29]. Each protein’s FASTA sequence was input into the AlphaFold2 program, and predictions were refined through three iterations to enhance accuracy.
Structural analysis and visualization
Structural analyses were conducted using Coot (Emsley and Cowtan 2004), and visualizations were performed using PyMOL (www.pymol.org) (Schrödinger Inc., New York, NY, USA). PyMOL, developed using robust and sophisticated graphic libraries, enables the accurate depiction of protein structures [30]. The PDB file of the simulated AlphaFold2 output for K. lactis ODC was uploaded to the DALI web server (https://ekhidna2.biocenter.hensinki.fi/dali/) for comparison with other homologous structures using the PDB search and pairwise tools outlined in Table 1.
Table 1.
Homologous structures of KlODC identified by the DALI servera.
| Species | Z-score | r.m.s.d. (Å) | Identity (%) | PDB code |
|---|---|---|---|---|
| Kluyveromyces lactis | – | – | – | – |
| Schizosaccharomyces pombe | 51.3 | 1.5 | 48 | – |
| Debaryomyces hansenii | 49.1 | 1.3 | 50 | – |
| Candida albicans | 47.3 | 1.3 | 50 | – |
| Homo sapiens | 46.3 | 1.4 | 49 | 1D7K |
| Trypanosoma brucei gambiense | 46.2 | 1.5 | 47 | 1NJJ |
| Mus musculus | 46.0 | 1.5 | 49 | 7ODC |
aThis server computes optimal and suboptimal structural alignments between two protein structures using the DaliLite-pairwise option.
Results
Prediction of fungal ODC protein structures
The three-dimensional structures of fungal ODC proteins were predicted using the AlphaFold2 web-based software [28,29]. Renowned for its high accuracy in predicting protein structures from amino acid sequences, AlphaFold2 serves as an invaluable resource in the fields of biology and medicine [31]. The predicted structures of ODC proteins from Kluyveromyces lactis (KlODC), Debaryomyces hansenii (DhODC), Candida albicans (CaODC), and Schizosaccaromyces pombe (SpODC) were visualized using the PyMOL graphic program (Figure 1). Overall, the predicted folds of these proteins, including α/β-barrel and β sheet domains, were largely similar. However, subtle differences were observed in the number of α helices as well as in the length and arrangement of secondary structures. Specifically, while all ODC proteins exhibited 16 β-sheets, KlODC exhibited 11 α-helices, whereas DhODC and SpODC had 12 α-helices each, while CaODC consists of 13 α-helices (Figure 1).
Figure 1.

The predicted three-dimensional structures of the KlODC, DhODC, CaODC, and SpODC proteins. The structures of the ODCs from four different fungi Kluyveromyces lactis (KlODC), Debaryomyces hansenii (DhODC), Candida albicans (CaODC), and schizosaccharomyces pombe (SpODC) were predicted using AlphaFold2 and visualized using PyMOL. While the number of β-strands was consistent, variations were observed in the number of α-helixes. The differing regions are highlighted using red dotted circles. The right panel shows a 90º rotated view of the N-terminal region of the structures.
Comparison of the structure of ODC homologs
Structures homologous to ODC were identified using the web-based server DALI, which revealed ODC structures of Homo sapiens (HsODC; PDB code: 1D7K), Mus musculus (MmODC; PDB code: 7ODC), and Trypanosoma brucei gambiense (TbgODC; PDB code: 1NJJ) (Table 1) [32]. The similarities among these ODC homologs were assessed in relation to KlODC. The Z-score values for SpODC, HsODC, TbgODC, and MmODC were 51.3, 46.3, 46.2, and 46.2, respectively. Additionally, the root-mean-square deviation (r.m.s.d.) values for SpODC, HsODC, TbgODC, and MmODC were 1.5 Å, 1.4 Å, 1.5 Å, and 1.5 Å, respectively. The sequence identity for these homologs were 48%, 49%, 47%, and 49%, respectively, indicating marginal variations (Figure 2a). Among the fungal ODCs, SpODC exhibited the highest similarity to KlODC, while the non-fungal ODCs. HsODC, TbgODC, and MmODC displayed progressively lower levels of similarity (Figure 2b). The structures of HsODC, TbgODC, and MmODC, similar to the four fungal ODCs (KlODC, DhODC, CaODC, and SpODC), consist of α/β-barrel and β sheet-domains.
Figure 2.

Comparison of the homologous structures of ODCs. (a) Amino acid sequence alignment of ODCs from various organisms. ODC from Kluyveromyces lactis (kl), Debaryomyces hansenii (dh), Candida albicans (ca), schizosaccharomyces pombe (sp), Homo sapiens (hs), Mus musculus (mm) and Trypanosoma brucei gambiense (tbg) were compared. Among the six species, conserved residues with 100% identity are emphasized in red, and those with 75% identity are denoted by blue boxes. Small and big black dots above the sequences indicate every 10th- and 50th- residues, respectively. Conserved residues with 100% identity are highlighted in red, and those with 75% identity are marked using blue boxes. Black dots above the sequences indicate every 10th residue. (b) The structures of their homologs, HsODC (PDB ID: 1D7K), MmODC (PDB ID: 7ODC), and TbgODC (PDB ID: 1NJJ), were obtained from PDB. (c) The structures of KlODC, DhODC, CaODC, and SpODC were predicted using AlphaFold2. The three-dimensional structures of KlODC, DhODC, CaODC, and SpODC are compared with those of HsODC, MmODC, and TbgODC. Unique α-helices or β-strands specific to each structure are highlighted using red dotted circles, and regions with differing lengths or shapes are highlighted using blue dotted circles.
ODC exists as a dimer in solution, and a monomer of HsODC is composed of 13 α-helices and 16 β-sheets [33]. A comparison of the structure of HsODC with those of the four fungal ODCs revealed notable differences in the length of α1 and the N-terminal loop, as well as variations in the number of α-helices. In the α/β-barrel domain, both DhODC and CaODC feature a short α-helix (α10) (Figure 2c). Furthermore, unlike the four fungal ODCs, HsODC contains a short α-helix (α12) rather than a loop between α10 and β16 (Figure 2b). A monomer of MmODC is composed of 13 α-helices and 18 β-sheets [34]. Notably, MmODC and the four fungal ODCs differ in the length of α1 and the N-terminal loop, as well as in the number of α-helices and β-sheets. In MmODC, two β-strands (β1 and β2) are integrated within its β sheet domain (Figure 2b). A monomer of TbgODC contains 12 α-helices and 16 β-sheets [35]. TbgODC, along with the four fungal ODCs, shows variations in the length of α1, β15, and the N-terminal loop, in addition to differences in the number of α-helices. Moreover, unlike the four fungal ODCs, TbgODC features a short α-helix (α12) located between β11 and β12 (Figure 2b).
Comparison of PLP-binding sites
The interaction between PLP and various active site residues leads to the formation of a Schiff base with ornithine, thereby enabling enzymatic activity [34]. This highlights the significance of examining the PLP-binding site environment [36]. The pyridine ring of PLP is enveloped by an acidic cluster composed of two aspartate residues and one glutamate residue, while the phosphate group of PLP is situated in a pocket formed by basic residues, including lysine and arginine (Figure 3). While the residues in the PLP-binding pockets are conserved among the six ODC homologs, including KlODC, the shapes of these pockets exhibit slight variations that may influence catalytic efficiency. In the structure of KlODC, Lys 97 is essential for properly orienting the carboxyl group of ornithine to facilitate efficient catalysis and for forming a Schiff base with the aldehyde group of PLP (Figure 3). The acidic cluster, especially the Glu 300 residue, plays a crucial role in establishing an internal Schiff base and stabilizing the protonated pyridine nitrogen (N1) of PLP. Additionally, the phosphate group of PLP is stabilized through hydrogen bonding with Arg 303, which is coordinated by the glycine-rich loop (264–266) [37].
Figure 3.
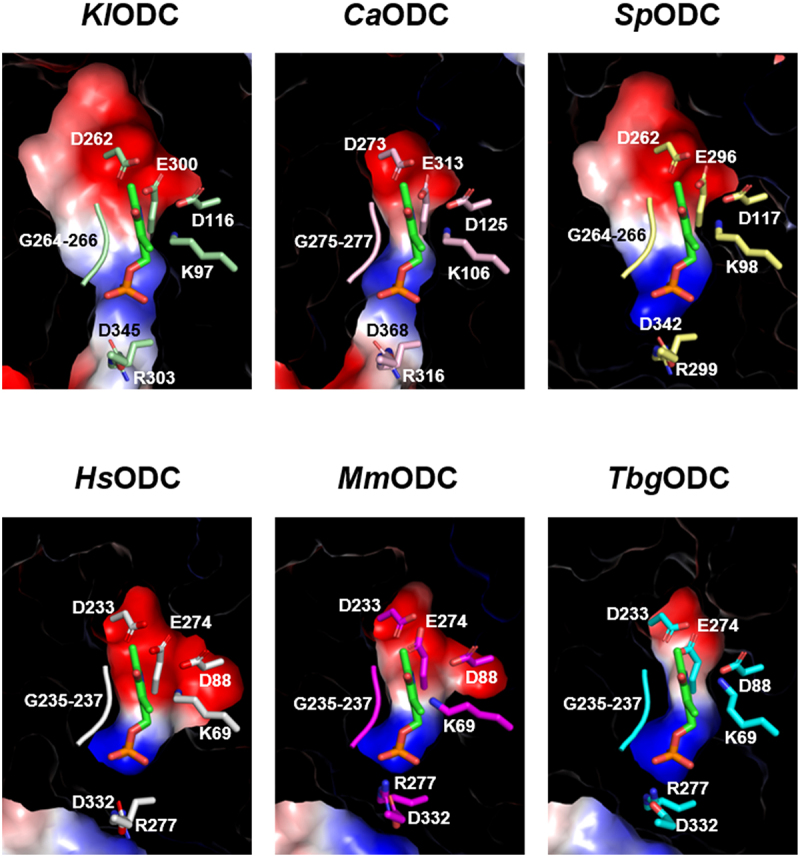
Discrepancy in electrostatic distribution and conformation at the plp-binding pocket. The plp-binding pockets of KlODC, CaODC, SpODC, HsODC, MmODC, and TbgODC. The red and blue surfaces represent acidic and basic residues, respectively.
Specificity helix-dependent substrate selectivity
Several PLP-dependent enzymes, such as ODC, arginine decarboxylase (ADC), diaminopimelate decarboxylases (DAPDC), L-lysine and L-ornithine decarboxylase (L/ODC), and carboxynorspermidine decarboxylase (CANSDC), are responsible for the decarboxylation of basic amino acids [38]. These enzymes are categorized as group IV decarboxylases and feature an α/β-barrel domain [39]. A notable feature of these enzymes is the specificity helix, which plays a crucial role in substrate selectivity [40].
To examine the properties of the specificity helix, the structures of Campylobacter jejuni CANSDC (CjCANSDC, PDB code: 3N29) and Paramecium bursaria chlorella virus ADC (PBCVADC, PDB code: 2NVA) were chosen for comparison. CjCANSDC facilitates the decarboxylation of carboxynorspermidine, yielding norspermidine, an important polyamine in Vibrio species (Figure 4a) [41]. The N3-amino group of the substrate forms a robust interaction with Asp 272 via a salt bridge within the specificity helix (α11) of CjCANSDC (Figure 4b) [42]. PBCVADC catalyzes the decarboxylation of L-arginine to produce agmatine and exhibits approximately 40% sequence similarity to eukaryotic ODC (Figure 4a) [43]. The guanidino group of the substrate engages with Glu 296 in the specificity helix, facilitating enzyme activity (Figure 4b) [44]. In the case of ODC, the Nϵ-amino group of ornithine, the substrate, is anchored by Asp 332 in the specificity helix (Figure 4a) [34]. Unlike the N3-amino group of carboxynorspermidine, the guanidino group of L-arginine, and the Nϵ-amino group of ornithine, the α-amino group of the substrate on the opposite side forms a covalent bond with the C4 of PLP, creating Schiff bases with lysine residues (Figure 4b) [45]. Therefore, the size of the substrate capable of binding to the active site is influenced by the distance between the Cα of the aspartate or glutamate residue and the C4-α of PLP, which is essential for substrate specificity [40].
Figure 4.

Comparison of the specificity helix sequences and substrate selectivity for CjCANSDC, PBCVADC, and fungal ODCs. (a) The substrates are listed in descending order of size: carboxynorspermidine for CjCANSDC, L-arginine for PBCVADC, and L-ornithine for ODC. (b) The dimer structures of CjCANSDC (PDB ID: 3N29), PBCVADC (PDB ID: 2NVA), and HsODC (PDB ID: 1D7K) are shown with an enlarged view of their PLP and substrate binding sites. The secondary structures and sequences of the specificity helices are shown. In the case of HsODC, the sequences of MmODC and TbgODC are included.
The sequence of the specificity helix in HsODC, MmODC, and TbgODC is Asn-Cys-Ile-Leu-Tyr-Asp. The specificity helix (α11) of both HsODC and MmODC contains Asp 326 and Asp 332, while the specificity helix (α10) of TbgODC includes only Asp 332. In contrast, the sequence of the specificity helix in fungal ODCs is Asn-Cys-Ile-Leu-Phe-Asp, with Tyr replaced by Phe. The specificity helix (α11) of KlODC and SpODC contains Asp 345 and Asp 368, respectively, whereas CaODC has Asp 342 in its specificity helix (α12). Notable variations exist in the distance between the Cα of an aspartate or glutamate residue within the specificity helix and the C4-α of PLP among the enzymes: CjCANSDC shows the longest distance at 14.1 Å, followed by PBCVADC at 12.1 Å. Overall, the ODCs exhibit an average distance of 10.1 Å (Figure 4b). While the majority of residues in the specificity helix are conserved, several notable differences have been identified. For example, Asp 88 in HsODC corresponds to Asp 67 in PBCVADC, yet CjCANSDC features Thr 60 at this position. Furthermore, Arg 277 in HsODC is conserved as Arg 255 in PBCVADC, whereas in CjCANSDC, it is substituted with Glu 236 (Figure 4b). These variations may have significant implications for the substrate specificity of the enzymes.
Discussion
In most organisms, ODC plays a vital role in initiating polyamine biosynthesis by catalyzing the decarboxylation of ornithine to produce putrescine [46]. The enzymatic activation of ODC depends on the cofactor PLP [47]. Within the active site of ODC, the aldehyde group at the C4 position of PLP forms a Schiff base with the ε-amino group of lysine. Following this, decarboxylation occurs as the substrate’s amino group displaces the ε-amino group of lysine [48]. ODC typically functions as a homodimer, with the active site situated at the interface between the two monomers [49]. In this study, the purified KlODC, CaODC, and SpODC proteins were also observed to exist as dimers, as indicated by size exclusion chromatography profiles (Supplementary Figure S1). Extensive efforts were dedicated to optimizing crystallization conditions to elucidate the molecular structures of these fungal ODC proteins. However, despite numerous attempts, successful optimization of crystal formation and subsequent X-ray diffraction analyses were not achieved.
The structures of the fungal ODCs including KlODC, CaODC, and SpODC were predicted using AlphaFold2 [50]. Comparative analyses included HsODC, MmODC, and TbgODC proteins [33–35]. While variations were observed in the secondary structures, such as α-helices and β-strands, the classification into α/β-barrel and β sheet domains remained consistent across the analyzed proteins. Among PLP-dependent enzymes, ODC is categorized as fold-type IV, characterized by the formation of a Schiff base with lysine and interactions between the glycine-rich loop and the phosphate group of PLP [51,52]. An acidic cluster is located near PLP’s pyridine ring, consisting of HsODC: Asp 88, Asp 233, and Glu 274; KlODC: Asp 116, Glu 300, and Arg 303; CaODC: Asp 125, Glu 313, and Arg 316; SpODC: Asp 117, Glu 296, and Arg 299, based on the electrostatic distribution [53]. Additionally, the glycine-rich loop (HsODC: 235–237; KlODC: 264–266; CaODC: 275–277; SpODC: 264–266) interacts with the phosphate group of PLP. While the amino acid sequences of the PLP-binding pocket remain identical across different ODCs, slight conformational differences have been observed, which could potentially affect enzyme activity.
The specificity helices of CjCANSDC, PBCVADC, and ODC share two notable structural characteristics. Firstly, within the active site, the pyridine nitrogen of PLP forms a salt bridge with acidic residues in the specificity helix. This interaction leads to the protonation of the nitrogen, thereby stabilizing the structure [54]. Secondly, the specificity helix is implicated in substrate selectivity, which is influenced by the distance between the acidic residue and the C4-α of PLP. CjCANSDC, which catalyzes the conversion of carboxynorspermidine – the largest substrate – displays a distance of 14.1 Å. In contrast, PBCVADC, which acts on L-arginine, the second largest substrate, has a distance of 12.1 Å. ODC, accommodating the smallest substrate, L-ornithine, shows an average distance of 10.1 Å. These observations underscore the relationship between substrate specificity and the distance from the C-α of the Asp or Glu residue in the specificity helix to the C4-α of PLP.
Given the diverse environments of PLP-binding pockets and the varying distances to the specificity helix among ODC-related enzymes, further research is necessary to investigate the modulation of enzymatic activities. Such studies could expand our understanding of the molecular basis for ODC evolution and potentially contribute to the development of antifungal agents.
Supplementary Material
Acknowledgments
We thank Jeong Eun Lee and Soo Jin Lee for assisting protein purifications.
Funding Statement
This research was funded by supported by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science and ICT [grant number, 2022R1F1A1073775].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Author contributions
Conceptualization: JHC; methodology: MJK, JHC; formal analysis: MJK, JHC; investigation: MJK, JHC; writing – original draft preparation: MJK; writing – review and editing: MJK, JHC; supervision: JHC; funding acquisition: JHC. All authors have carefully read and approved the manuscript.
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding authors upon reasonable request.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/19420889.2025.2458872
References
- [1].Hussain T, Tan B, Ren W, et al. Exploring polyamines: functions in embryo/fetal development. Anim Nutr. 2017. Mar;3(1):7–10. doi: 10.1016/j.aninu.2016.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lian J, Liang Y, Zhang H, et al. The role of polyamine metabolism in remodeling immune responses and blocking therapy within the tumor immune microenvironment. Front Immunol. 2022;13:912279. doi: 10.3389/fimmu.2022.912279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Murray-Stewart TR, Woster PM, Casero RA Jr.. Targeting polyamine metabolism for cancer therapy and prevention. Biochem J. 2016. Oct 1;473(19):2937–2953. doi: 10.1042/BCJ20160383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Handa AK, Fatima T, Mattoo AK.. Polyamines: Bio-molecules with diverse functions in plant and human health and disease. Front Chem. 2018;6:10. doi: 10.3389/fchem.2018.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pegg AE, Casero RA Jr.. Current status of the polyamine research field. Methods Mol Biol. 2011;720:3–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhou XE, Suino-Powell K, Schultz CR, et al. Structural basis of binding and inhibition of ornithine decarboxylase by 1-amino-oxy-3-aminopropane. Biochem J. 2021. Dec 10;478(23):4137–4149. doi: 10.1042/BCJ20210647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kim S, Chang JH. Structural analysis of spermidine synthase from Kluyveromyces lactis. Molecules. 2023. Apr 13;28(8):3446. doi: 10.3390/molecules28083446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Silander KM, Pihlajamaa P, Sahu B, et al. Characterization of an androgen-responsive, ornithine decarboxylase-related protein in mouse kidney. Biosci Rep. 2017. Aug 31;37(4). doi: 10.1042/BSR20170163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bercovich Z, Snapir Z, Keren-Paz A, et al. Antizyme affects cell proliferation and viability solely through regulating cellular polyamines. J Biol Chem. 2011. Sep 30;286(39):33778–33783. doi: 10.1074/jbc.M111.270637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kahana C. The antizyme family for regulating polyamines. J Biol Chem. 2018. Nov 30;293(48):18730–18735. doi: 10.1074/jbc.TM118.003339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wu HY, Chen SF, Hsieh JY, et al. Structural basis of antizyme-mediated regulation of polyamine homeostasis. Proc Natl Acad Sci USA. 2015. Sep 8;112(36):11229–11234. doi: 10.1073/pnas.1508187112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Li MM, MacDonald MR. Polyamines: small molecules with a big role in promoting virus infection. Cell Host Microbe. 2016. Aug 10;20(2):123–124. doi: 10.1016/j.chom.2016.07.012 [DOI] [PubMed] [Google Scholar]
- [13].Ray RM, Bhattacharya S, Bavaria MN, et al. Antizyme (AZ) regulates intestinal cell growth independent of polyamines. Amino Acids. 2014. Sep;46(9):2231–2239. doi: 10.1007/s00726-014-1777-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hsieh JY, Liu YC, Cheng IT, et al. Critical factors in human antizymes that determine the differential binding, inhibition, and degradation of human ornithine decarboxylase. Biomolecules. 2019. Dec 12;9(12):864. doi: 10.3390/biom9120864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cai B, Kasikara C, Doran AC, et al. MerTK signaling in macrophages promotes the synthesis of inflammation resolution mediators by suppressing CaMKII activity. Sci Signal. 2018. Sep 25;11(549). doi: 10.1126/scisignal.aar3721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cai B, Thorp EB, Doran AC, et al. MerTK receptor cleavage promotes plaque necrosis and defective resolution in atherosclerosis. J Clin Invest. 2017. Feb 1;127(2):564–568. doi: 10.1172/JCI90520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yurdagul A Jr., Kong N, Gerlach BD, et al. ODC (Ornithine Decarboxylase)-dependent putrescine synthesis maintains MerTK (MER tyrosine-protein kinase) expression to drive resolution. Arterioscler Thromb Vasc Biol. 2021. Mar;41(3):e144–e159. doi: 10.1161/ATVBAHA.120.315622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hardbower DM, Asim M, Luis PB, et al. Ornithine decarboxylase regulates M1 macrophage activation and mucosal inflammation via histone modifications. Proc Natl Acad Sci USA. 2017. Jan 31;114(5):E751–E760. doi: 10.1073/pnas.1614958114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Monteleone G, Pallone F, Stolfi C. The dual role of inflammation in colon carcinogenesis. Int J Mol Sci. 2012;13(9):11071–11084. doi: 10.3390/ijms130911071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Park MG, Kim SY, Lee CJ. Dmso-tolerant ornithine decarboxylase (ODC) tandem assay optimised for high-throughput screening. J Enzyme Inhib Med Chem. 2023. Dec;38(1):309–318. doi: 10.1080/14756366.2022.2150186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Elmets CA, Athar M. Targeting ornithine decarboxylase for the prevention of nonmelanoma skin cancer in humans. Cancer Prev Res (Phila). 2010. Jan;3(1):8–11. doi: 10.1158/1940-6207.CAPR-09-0248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Liu L, Rao JN, Zou T, et al. Polyamines regulate c-myc translation through Chk2-dependent HuR phosphorylation. Mol Biol Cell. 2009. Dec;20(23):4885–4898. doi: 10.1091/mbc.e09-07-0550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cheng Y, Chaturvedi R, Asim M, et al. Helicobacter pylori-induced macrophage apoptosis requires activation of ornithine decarboxylase by c-Myc. J Biol Chem. 2005. Jun 10;280(23):22492–22496. doi: 10.1074/jbc.C500122200 [DOI] [PubMed] [Google Scholar]
- [24].Hayes CS, DeFeo-Mattox K, Woster PM, et al. Elevated ornithine decarboxylase activity promotes skin tumorigenesis by stimulating the recruitment of bulge stem cells but not via toxic polyamine catabolic metabolites. Amino Acids. 2014. Mar;46(3):543–552. doi: 10.1007/s00726-013-1559-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Luk GD, Moshier JA, Ehrinpreis MN. Ornithine decarboxylase as a marker for colorectal polyps and cancer. Prog Clin Biol Res. 1988;279:227–239. [PubMed] [Google Scholar]
- [26].LoGiudice N, Le L, Abuan I, et al. Alpha-Difluoromethylornithine, an irreversible inhibitor of polyamine biosynthesis, as a Therapeutic strategy against hyperproliferative and infectious diseases. Med Sci (Basel). 2018. Feb 8;6(1):12. doi: 10.3390/medsci6010012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sagar NA, Tarafdar S, Agarwal S, et al. Polyamines: functions, metabolism, and role in human disease management. Med Sci (Basel). 2021. Jun 9;9(2):44. doi: 10.3390/medsci9020044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Jumper J, Evans R, Pritzel A, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021. Aug;596(7873):583–589. doi: 10.1038/s41586-021-03819-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Senior AW, Evans R, Jumper J, et al. Improved protein structure prediction using potentials from deep learning. Nature. 2020. Jan;577(7792):706–710. doi: 10.1038/s41586-019-1923-7 [DOI] [PubMed] [Google Scholar]
- [30].Rosignoli S, Paiardini A. Boosting the full potential of PyMOL with structural biology plugins. Biomolecules. 2022. Nov 27;12(12):1764. doi: 10.3390/biom12121764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yang Z, Zeng X, Zhao Y, et al. AlphaFold2 and its applications in the fields of biology and medicine. Signal Transduct Target Ther. 2023. Mar 14;8(1):115. doi: 10.1038/s41392-023-01381-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Holm L. Dali server: structural unification of protein families. Nucleic Acids Res. 2022. Jul 5;50(W1):W210–W215. doi: 10.1093/nar/gkac387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Almrud JJ, Oliveira MA, Kern AD, et al. Crystal structure of human ornithine decarboxylase at 2.1 å resolution: structural insights to antizyme binding. J Mol Biol. 2000. Jan 7;295(1):7–16. doi: 10.1006/jmbi.1999.3331 [DOI] [PubMed] [Google Scholar]
- [34].Kern AD, Oliveira MA, Coffino P, et al. Structure of mammalian ornithine decarboxylase at 1.6 Å resolution: stereochemical implications of plp-dependent amino acid decarboxylases. Structure. 1999. May;7(5):567–581. doi: 10.1016/S0969-2126(99)80073-2 [DOI] [PubMed] [Google Scholar]
- [35].Jackson LK, Goldsmith EJ, Phillips MA. X-ray structure determination of Trypanosoma brucei ornithine decarboxylase bound to D-ornithine and to G418: insights into substrate binding and ODC conformational flexibility. J Biol Chem. 2003. Jun 13;278(24):22037–22043. doi: 10.1074/jbc.M300188200 [DOI] [PubMed] [Google Scholar]
- [36].Rastogi S, Chandra A. Free energy landscapes of the tautomeric interconversion of pyridoxal 5′-phosphate aldimines at the active site of Ornithine Decarboxylase in aqueous media. J Phys Chem B. 2023. Sep 28;127(38):8139–8149. doi: 10.1021/acs.jpcb.3c04142 [DOI] [PubMed] [Google Scholar]
- [37].Jackson LK, Brooks HB, Osterman AL, et al. Altering the reaction specificity of eukaryotic ornithine decarboxylase. Biochemistry. 2000. Sep 19;39(37):11247–11257. doi: 10.1021/bi001209s [DOI] [PubMed] [Google Scholar]
- [38].Lee J, Michael AJ, Martynowski D, et al. Phylogenetic diversity and the structural basis of substrate specificity in the β/α-barrel Fold basic amino acid decarboxylases. J Biol Chem. 2007. Sep 14;282(37):27115–27125. doi: 10.1074/jbc.M704066200 [DOI] [PubMed] [Google Scholar]
- [39].Tran JU, Brown BL. Structural basis for allostery in plp-dependent enzymes. Front Mol Biosci. 2022;9:884281. doi: 10.3389/fmolb.2022.884281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Son HF, Kim KJ. Structural basis for substrate specificity of meso-diaminopimelic acid decarboxylase from Corynebacterium glutamicum. Biochem Biophys Res Commun. 2018. Jan 8;495(2):1815–1821. doi: 10.1016/j.bbrc.2017.11.097 [DOI] [PubMed] [Google Scholar]
- [41].Keating TA, Marshall CG, Walsh CT. Vibriobactin biosynthesis in Vibrio cholerae: VibH is an amide synthase homologous to nonribosomal peptide synthetase condensation domains. Biochemistry. 2000. Dec 19;39(50):15513–15521. doi: 10.1021/bi001651a [DOI] [PubMed] [Google Scholar]
- [42].Deng X, Lee J, Michael AJ, et al. Evolution of substrate specificity within a diverse family of β/α-barrel-fold basic amino acid decarboxylases. J Biol Chem. 2010. Aug 13;285(33):25708–25719. doi: 10.1074/jbc.M110.121137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Shah R, Coleman CS, Mir K, et al. Paramecium bursaria chlorella virus-1 encodes an unusual arginine decarboxylase that is a close homolog of eukaryotic ornithine decarboxylases. J Biol Chem. 2004. Aug 20;279(34):35760–35767. doi: 10.1074/jbc.M405366200 [DOI] [PubMed] [Google Scholar]
- [44].Shah R, Akella R, Goldsmith EJ, et al. X-ray structure of paramecium bursaria chlorella virus arginine decarboxylase: insight into the structural basis for substrate specificity. Biochemistry. 2007. Mar 13;46(10):2831–2841. doi: 10.1021/bi6023447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Bisello G, Longo C, Rossignoli G, et al. Oxygen reactivity with pyridoxal 5′-phosphate enzymes: biochemical implications and functional relevance. Amino Acids. 2020. Aug;52(8):1089–1105. doi: 10.1007/s00726-020-02885-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wu D, Kaan HY, Zheng X, et al. Structural basis of Ornithine Decarboxylase inactivation and accelerated degradation by polyamine sensor Antizyme1. Sci Rep. 2015. Oct 7;5(1):14738. doi: 10.1038/srep14738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Jackson LK, Brooks HB, Myers DP, et al. Ornithine decarboxylase promotes catalysis by binding the carboxylate in a buried pocket containing phenylalanine 397. Biochemistry. 2003. Mar 18;42(10):2933–2940. doi: 10.1021/bi026795z [DOI] [PubMed] [Google Scholar]
- [48].Wu F, Christen P, Gehring H. A novel approach to inhibit intracellular vitamin B6-dependent enzymes: proof of principle with human and plasmodium ornithine decarboxylase and human histidine decarboxylase. FASEB J. 2011. Jul;25(7):2109–2122. doi: 10.1096/fj.10-174383 [DOI] [PubMed] [Google Scholar]
- [49].Lee CY, Liu YL, Lin CL, et al. Functional roles of the dimer-interface residues in human ornithine decarboxylase. PLOS ONE. 2014;9(8):e104865. doi: 10.1371/journal.pone.0104865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Varga JK, Schueler-Furman O. Who binds better? let Alphafold2 Decide! Angew Chem Int Ed Engl. 2023. Jul 10;62(28):e202303526. doi: 10.1002/anie.202303526 [DOI] [PubMed] [Google Scholar]
- [51].Grishin NV, Phillips MA, Goldsmith EJ. Modeling of the spatial structure of eukaryotic ornithine decarboxylases. Protein Sci. 1995. Jul;4(7):1291–1304. doi: 10.1002/pro.5560040705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Osterman AL, Brooks HB, Jackson L, et al. Lysine-69 plays a key role in catalysis by ornithine decarboxylase through acceleration of the Schiff base formation, decarboxylation, and product release steps. Biochemistry. 1999. Sep 7;38(36):11814–11826. doi: 10.1021/bi9906221 [DOI] [PubMed] [Google Scholar]
- [53].Takatsuka Y, Yamaguchi Y, Ono M, et al. Gene cloning and molecular characterization of lysine decarboxylase from selenomonas ruminantium delineate its evolutionary relationship to ornithine decarboxylases from eukaryotes. J Bacteriol. 2000. Dec;182(23):6732–6741. doi: 10.1128/JB.182.23.6732-6741.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lin YL, Gao J. Internal proton transfer in the external pyridoxal 5′-phosphate Schiff base in dopa decarboxylase. Biochemistry. 2010. Jan 12;49(1):84–94. doi: 10.1021/bi901790e [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding authors upon reasonable request.


