Abstract
Introduction
Reverse shoulder arthroplasty (RSA) is performed for various shoulder pathologies. Inadequate tensioning of the deltoid muscle can lead to complications, highlighting the need for optimization. Preoperative quantification of deltoid health may improve operative execution and outcomes. Shear wave elastography (SWE) quantifies stiffness as a marker for muscle health and tensioning. This study establishes normal reference values for the deltoid in healthy individuals.
Methods
40 participants were enrolled, 10 in each 18–30, 31–40, 41–55, and 55 + age groups. Two operators performed SWE in 3 deltoid regions, and the intra- and inter-operator agreement was assessed.
Results
Mean values were 23.2 ± 4.6, 26.4 ± 5.6, and 17.9 ± 5.2 kPa for the anterior, lateral, and posterior regions. The posterior region did not show any age dependency. However, there were significant differences between the age groups of 18–30 and 55 + years in the anterior (19.7 ± 3.7 vs. 26.7 ± 5.3 kPa) and lateral (22.0 ± 5.4 vs. 30.2 ± 8.8 kPa). Intra-operator reliability ranged from 0.45 to 0.60, and inter-reader agreement from 0.57 to 0.85.
Conclusion
This study provides reference values of deltoid stiffness which could serve as a comparison with clinical measurements for RSA operative planning and execution and prediction of surgical outcomes.
Keywords: Shear wave elastography, deltoid muscle, healthy volunteers, age, reverse shoulder arthroplasty
Introduction
Reverse shoulder arthroplasty (RSA) has become the procedure of choice for patients with cuff tear arthropathy, many patients with irreparable cuff tears, selected osteoarthritic shoulders with severe bone loss, and many displaced multifragmentary proximal humerus fractures. The number of RSAs implanted in the United States each year increased by 191.3% between 2011 and 2017, and RSA accounted for about 60% of shoulder arthroplasty cases in 2017. 1 It is projected that by 2025, the number of RSA cases may increase anywhere between 87.9% and 353% relative to the number of cases performed in 2017. 1 Other studies have also projected the incidence of RSAs to increase by 333% in 2030 relative to cases performed in 2011, and an increase of 122% by 2040.2–4 In rotator cuff-deficient shoulders treated with RSA, the prosthetic joint must rely on a functional deltoid muscle for shoulder elevation. 5 Inadequate tensioning of the deltoid in RSA may cause complications, highlighting the need to optimize deltoid tensioning.5–11 Insufficient tension has been demonstrated to contribute to dislocation, whereas excessive tension can result in pain and stiffness and may contribute to stress fractures of the acromion or scapular spine. Intraoperative assessment of deltoid tensioning is challenging and often must rely solely on subjective surgeon experience, underscoring the need for quantitative and reproducible assessments.5,7
Ultrasound-based shear wave elastography (SWE) measures shear wave propagation to estimate tissue shear modulus as a surrogate for tissue stiffness.12–15 In brief, SWE technique involves generating shear waves within a region of interest using acoustic radiation force, and tracking the shear wave velocity as the shear waves propagate in a direction perpendicular to the insonating ultrasound beam.16,17 Using the underlying assumptions that the interrogated tissue is incompressible, homogeneous, isotropic and has linear response to excitation, the calculated shear wave velocities (in m/s) are converted to stiffness values (in kPa) and displayed to the user for the interrogated region of interest.16,17 Typically, the region of interest with stiffness values is superimposed on the conventional grayscale ultrasound image providing anatomic specificity.16,17 While MRI-based elastography measurements can also be obtained, ultrasound-based elastography measurements offer advantages inherent to ultrasound imaging which include cost, portability, safety (for imaging with implants) and ability to perform intraoperative assessment. Therefore, we chose to utilize ultrasound imaging-based SWE in this study.
Clinically, there is a growing interest in utilizing SWE to study the musculoskeletal system, specifically by first defining normal elasticity values of skeletal muscles.18–24 Normal values of stiffness in several regions of the deltoid muscles in fresh-frozen cadaveric shoulders, in both neutral and elongated states, have been previously reported. 24 If SWE measurements of the different regions of the deltoid muscle can be reliably obtained, then they may provide a quantitative marker for clinical assessment of the deltoid before, during, and after RSA. Before utilizing SWE measurements for clinical evaluation in patients, age-dependent reference values of deltoid stiffness need to be provided for comparisons and for intra- and inter-operator reliability to be evaluated. Therefore, the purpose of this study was to define the normal values of stiffness measurements of the deltoid muscle in healthy volunteers by age group, with the ultimate goal of using these reference values for comparison with clinical measurements for targeted RSA operative planning and execution as well as prediction of surgical outcomes.
Materials and methods
A prospective study was conducted after IRB approval on 40 healthy volunteers who provided informed consent to participate in this study. Ten volunteers were recruited in each of the following age cohorts: 18–30 years, 31–40 years, 41–55 years and >55 years. While most patients scheduled for RSA tend to be relatively older patients, for this study, in order to establish normal values of stiffness measurements of the deltoid muscle we considered recruiting all healthy adult volunteers (greater than 18 years of age) and grouped them according to the stated age groups. We did not account for the impact of individual age of volunteers but considered the following age groupings given prior published studies have shown that the relationship between age and muscle stiffness measured by SWE remains mixed.25–28 The data from this study will enable future assessments of older subjects and those scheduled for RTSA, allowing comparison with normative values. This comparison will help in the pre-surgical characterization of the tissue, as well as provide insights into potential post-surgical changes.
Recruitment was conducted via convenience sampling at our Institution's health and fitness facility. Exercise frequency of at least two times per week was used as an inclusion criterion as an indirect measure of a healthy volunteer based on the recommendations by the American College of Sports Medicine (ACSM). 29 Notably, patients with prior shoulder injury or surgery were excluded. The complete inclusion and exclusion criteria are provided in Table 1.
Table 1.
Inclusion and exclusion criteria for study participants.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
A GE LOGIQ E9 (GE Healthcare, Wauwatosa, WI) ultrasound scanner with SWE option was used for this study. All SWE data was acquired using a 9L transducer with the ‘MSK GEN’ preset configured on the Logiq E9 scanner. All study participants were in a seated position with the subject's arm resting at their side in a neutral position, i.e., without any internal or external rotation. Figure 1 shows a representative schematic for image and data acquisition. The following three regions of the deltoid were scanned: the anterior region, defined as the thickest portion of the anterior deltoid; the lateral region, defined as the lateral deltoid at 1–2 cm distal to the humeral head; and the posterior region, defined as the thickest portion of the posterior deltoid. Two operators (PGYIII and MS IV) performed the data acquisition; the primary operator had three years of training in ultrasonography, whereas the second operator had less than one year of training. Both operators underwent a structured training session in SWE with a research sonographer with over 20-year experience that also was available to assist with the scans if needed. The operators selected the predefined regions of interest from the acquired SWE images to extract the deltoid muscle tissue stiffness, excluding other soft tissue and bone (Figure 2). The primary operator acquired 10 anterior, 10 lateral, and 10 posterior SWE measurements for each study subject. The second operator acquired 10 SWE measurements per region of the deltoid in a subset of the subjects (n = 5, n = 3, n = 2, and n = 5 in the 18–30, 31–40, 41–55, and >55 years age groups, respectively). The two operators acquired the ultrasound data independently. The SWE acquisitions were performed within 10 min for each subject. Each of the studies was independently reviewed by ultrasound faculty (MD by training) to ensure study quality and correct placement of the ROI.
Figure 1.
Representative schematic for image acquisition from anterior (blue), lateral (yellow), and posterior (purple) regions of the deltoid muscle. The position of the ultrasound transducer is indicated by the dashed box.
Figure 2.
Data acquisition from the anterior (Fig. 2(a)), lateral (Fig. 2(b)) and posterior (Fig. 2(c)) regions of the deltoid muscle from a subject in the 41–55 years age group. The colored pixel values with a region of interest (yellow-dotted boundary) contributed to reported stiffness values.
Statistical analyses were performed using SAS version 9.4 (SAS Institute; Cary, NC). The primary outcome was age-specific stiffness from healthy deltoid muscles (reported as mean ± standard deviation). The secondary outcome was to compare mean deltoid muscle stiffness between age groups and examine the intra- and inter-operator agreement. Comparisons of measurements among the four age groups were evaluated using one-way analysis of variance (ANOVA). Pairwise comparisons between age groups were conducted using Tukey's tests. The 10 anterior, lateral, and posterior stiffness values for the 15 participants with assessments by both the primary and secondary operators were averaged to evaluate inter-reader agreement. Intra- and inter-operator agreement were assessed using intraclass correlation coefficients (ICC3,1 in both cases), with values of 0.00–0.20, 0.21–0.40, 0.41–0.60, 0.61–0.80, and 0.81–0.99 indicating slight, fair, moderate, substantial, and almost perfect agreement, respectively. P-values <0.05 were considered statistically significant. Bonferroni corrections were used for multiple comparisons.
Results
Table 2 presents the stiffness values for the anterior, lateral, and posterior regions of the deltoid muscle as a function of the age group of study subjects obtained by the primary operator. The ANOVA test revealed a significant main effect of the age group on the stiffness values in the anterior and lateral regions of the deltoid muscle (p ≤ 0.031). However, the stiffness values in the posterior region of the deltoid muscle did not show any dependency on the age group (p = 0.66). Post-hoc tests showed that the stiffness values from the anterior region of the deltoid muscle were significantly different only between the age groups of 18–30 years and 55 + years (Table 2). A similar finding was noted for the stiffness values from the lateral region of the deltoid muscle (Table 2).
Table 2.
Stiffness values for the anterior, lateral, and posterior regions of the deltoid muscle as a function of the age group of study subjects obtained by the primary operator (mean values are provided along with standard deviation – SD, and 95% confidence interval (CI) values; * indicates statistically significant different between age groups with p < 0.05).
| Age-Group (years) | Anterior (SD; 95% CI) (kPa) | Lateral (SD; 95% CI) (kPa) | Posterior (SD; 95% CI) (kPa) |
|---|---|---|---|
| 18–30 (n = 10) | 19.7* (3.7; 17.4–22.0) | 22.0* (5.4; 18.7–25.3) | 17.8 (6.3; 13.9–21.7) |
| 31–40 (n = 10) | 23.5 (5.8; 19.9–27.1) | 26.2 (3.4; 24.1–28.3) | 19.6 (5.1; 16.4–22.8) |
| 41–55 (n = 10) | 22.8 (3.6; 20.6–25.0) | 27.2 (4.7; 24.3–30.1) | 17.7 (4.1; 15.2–20.2) |
| 55+ (n = 10) | 26.7* (5.3; 23.4–30.0) | 30.2* (8.8; 24.7–35.7) | 16.6 (5.1; 13.4–19.8) |
| All subjects (n = 40) | 23.2 (4.6; 21.8–24.6) | 26.4 (5.6; 24.7–28.1) | 17.9 (5.2; 16.3–19.5) |
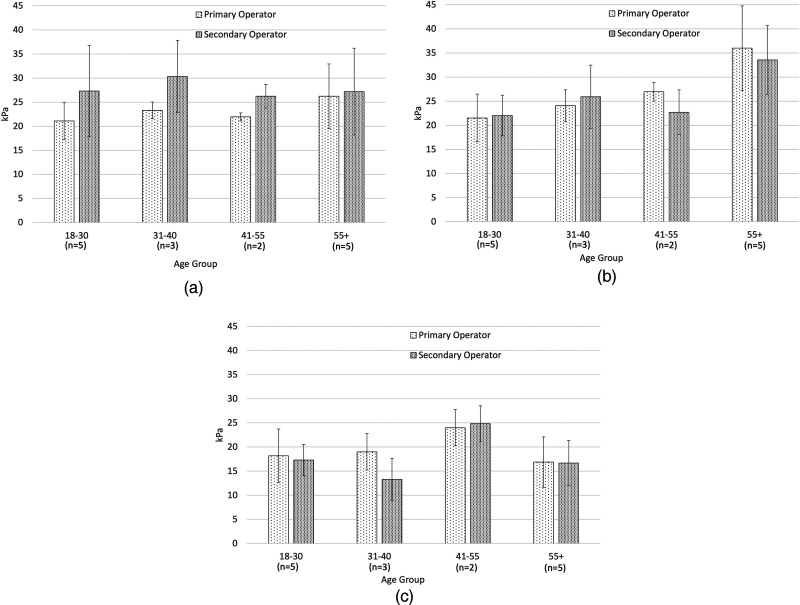
Intra-operator agreement among the 10 anterior, lateral, and posterior SWE measurements of the deltoid muscle for the primary operator is summarized in Table 3. Figure 3 shows the stiffness measurements obtained by both operators in 15 subjects. Inter-operator agreement for stiffness values obtained in 15 subjects (where both the operators acquired data) showed that the intraclass correlation coefficient values were 0.57, 0.85, and 0.78 for the anterior, lateral, and posterior regions of the deltoid muscle, respectively, indicating moderate to above-substantial levels of agreement.
Table 3.
Intra-operator agreement among the 10 anterior, lateral, and posterior SWE measurements of the deltoid muscle for the primary operator (intraclass correlation coefficient values are shown; values in the range of 0.00–0.20, 0.21–0.40, 0.41–0.60, 0.61–0.80, and 0.81–0.99 indicate slight, fair, moderate, substantial, and almost perfect agreement, respectively).
| Age-Group (years) | Anterior | Lateral | Posterior |
|---|---|---|---|
| 18–30 (n = 10) | 0.44 | 0.51 | 0.56 |
| 31–40 (n = 10) | 0.55 | 0.25 | 0.38 |
| 41–55 (n = 10) | 0.35 | 0.35 | 0.35 |
| 55+ (n = 10) | 0.50 | 0.73 | 0.51 |
| All subjects (n = 40) | 0.54 | 0.57 | 0.45 |
Figure 3.
Stiffness measurements from the anterior (Fig. 3(a)), lateral (Fig. 3(b)), and posterior (Fig. 3(c)) regions of the deltoid muscle obtained by both primary and secondary operators (mean values are shown with the error bars indicating ± one standard deviation).
Discussion and conclusion
This study established normative stiffness values for the various deltoid muscle subregions in healthy adults by age using shear wave elastography. SWE is a non-invasive technique that can directly measure muscle properties, such as stiffness, during passive and active joint positions. Importantly, while electromyography (EMG) has been used to evaluate muscle activation, SWE allows for the evaluation of individual muscle subregions preventing the risk of signal contamination due to cross-talk from adjacent muscle groups, as it can be seen on EMG. 30 A previous study reported average posterior deltoid stiffness as 17.8 kPa based on data acquired from a cohort of 18 healthy Japanese adults (average age: 23.4 years). 31 The data was acquired with the arm relaxed with 90° elbow flexion. This reported average value of 17.8 kPa is similar to the average value obtained in the present study for the age group of 18–30 years, lending credence to the normative values for the posterior deltoid obtained in the current study. However, in the prior study 31 other deltoid regions were not measured, the sample size was relatively small compared to the current study, and the distribution of the deltoid muscle stiffness across age groups was not considered. Patient positioning is an important factor in standardizing the measurement of muscle stiffness as arm position and muscle tensioning might affect the measured outcomes.22–24,32 However, while the arm positions were different between the current study (participants were in a seated position with the subject's arm resting at their side in a neutral position, i.e., without any internal or external rotation) and the other published study, 31 the deltoid muscle stiffness in the posterior region were similar. This can be the result of the elbow joint having a minor effect on the passive mechanical properties of the deltoid.
The current study also establishes that the deltoid muscle stiffness in the anterior and the lateral regions differed between the youngest (18–30 years) and the oldest (55 + years) subjects. Published literature on the relationship between age and muscle stiffness measured by SWE remains mixed..25–28 A study of 66 healthy adults (age range, 20 to 89 years) revealed that amongst the five upper extremity muscles studied, only the middle trapezius and sternocostal pectoralis major muscles showed increased stiffness with age. 26 Another study with 77 healthy adults (age range 20–94 years) demonstrated an inverse relationship between muscle stiffness and age for quadriceps, hamstrings, and biceps brachii muscles and a positive correlation between stiffness and muscle strength. 28 Other studies have shown an increase in biceps stiffness with age (n = 13; age range: 21 to 94 years) 25 and in supraspinatus muscle stiffness with age. 27 Specifically one study evaluated stiffness of biceps brachii measured with 90° elbow flexion and full extension and showed that shear wave modulus in older patients (> 60 years) was a function of sex and age; this relationship did not exist over the entire age range considered in the study (21–94 years old). 25 While the correlation between age and muscle stiffness has not been firmly established, the current study provides further evidence of the increasing deltoid muscle stiffness in the anterior and lateral regions as a function of age (and no changes in the posterior region as a function of age). It is important to note that stiffness outcomes are influenced by several factors, including the specific muscle subregion, arm position, and whether the muscle is assessed in an active or passive state. Stiffness alterations in the context of RTSA, based on these factors have yet to be thoroughly evaluated pre- and post-operatively, or following rehabilitation procedures. The relationship between these specific biomechanical changes and clinical outcomes remains unexplored but is a promising avenue for future research. Notably, previous cadaveric studies simulating RTSA showed a 27% increase in stiffness following a 10 mm muscle tensioning, leading to statistically significant differences from baseline measurements. However, further studies are needed to determine how these statistically significant results translate into clinical relevance. 24 It is also important to note that equipment brand, experience, muscle of interest, muscle activation, and joint position might affect outcomes, informing their important consideration when investigating muscle properties and comparing outcomes.
Prior studies have reported intra-operator reliability in the range of 0.72 to 0.95 for assessing muscle stiffness of different muscles24,33 as compared to the average intra-operator reliability for both the operators in our study to be in the range of 0.45 to 0.60, and inter-reader agreement to be in the range of 0.57 to 0.85 for the different regions of the deltoid muscle examined. This discrepancy between our intra- and inter-observer reliability and other published studies may be due to the inherent anatomic variability of deltoid morphology and overlying fat tissue that may have been confounded by small sample sizes in ours and other studies. Probe positioning, pressure applied with the probe, suitability of specific deltoid regions, and SWE training of the observers may be contributing factors to the discrepancy noted as published in the literature.20,33 Demographics of the different sample populations may also affect the reliability of SWE measurements.
Our results suggest that the lateral (also referred to as the middle or acromialis) portion of the deltoid may be the most suitable for SWE measurement. We found that this region demonstrated both a statistically significant difference in stiffness with age and the best intra- and inter-observer reliability of the three deltoid regions examined in this study. This finding is also supported by prior published studies reporting almost perfect intra- and inter-observer reliability when measuring the lateral aspect of the deltoid muscle.20,24 From a clinical perspective, current options for assessing deltoid tension (intra-operatively) are limited and generally qualitative. Establishing a patient's pre-operative deltoid tension on the contralateral side, if healthy, may be achieved using SWE to measure deltoid stiffness as a surrogate for tension. It has been demonstrated that RSA increases the stiffness of the deltoid muscle postoperatively because of deltoid tensioning and elongation.34,35 Given the reliance on the deltoid muscle for functional shoulder mobility after RSA, preoperative evaluation of the deltoid muscle of surgical candidates with quantitative methods may be beneficial. 36
Additionally, preoperative assessment of the deltoid can predict postoperative outcomes.37,38 Ultrasound-based SWE offers advantages due to the portability of the ultrasound scanner for intra-operative evaluation as well as assessment of deltoid stiffness in outpatient consults. The current study provides age-dependent stiffness values from the different regions of the deltoid muscle, albeit in healthy volunteers. These values would serve as a reference for future evaluations in a clinical setting (pre-, intra-, and post-operative assessment).
The present study has several limitations. First, our sample size of 40 volunteers, while larger than some previously cited work, 31 is not exhaustive and relied on the reporting by subjects regarding their health status (shoulder pain, etc.). Second, measurements were acquired by two operators. Inter-operator agreement needs to be assessed with a relatively greater number of operators.
Another limitation of this study is the categorization of participants into broad age groups without recording their specific ages. This approach means that within each age group, there could be significant variability in the ages of the participants. This variability could potentially influence the stiffness measurements of the deltoid muscle, as age-related changes in muscle properties might not be uniformly distributed across the entire age range.
The absence of recorded gender and BMI data for the participants in this study is important to note. Obesity can affect muscle stiffness measurements due to differences in muscle mass and fat distribution. 39 In addition, previous studies have indicated that muscle stiffness can be significantly influenced by both sex and age. However, as noted above, results of previous studies exploring the relationship between sex and age on muscle stiffness are mixed, with some studies showing an increase of stiffness with age and in females 25 while others show the opposite28,40 Similarly, an increase in stiffness does not necessarily indicate stronger muscles. These results are likely a product of examining different muscle groups with diverse biomechanical demands and the complex interaction of age-related muscle degeneration, hormone-dependent structural muscle composition, physical activity level, and measurement parameters. Future studies should aim to include detailed demographic data, including gender, BMI, and physical fitness, to better understand the relationship between muscle stiffness, strength, and age. This would allow for a more comprehensive analysis and help clarify the observed trends in muscle stiffness across different genders and age groups.
In conclusion, normal age-group-specific values of the anterior, lateral, and posterior regions of the deltoid muscle stiffness are reported in this study. Moderate to above-substantial levels of inter-operator agreement were noted for the SWE measurements of the deltoid. The ultrasound-based SWE measurements of the deltoid muscle may provide a quantitative marker for clinical assessment of the deltoid before, during, and after RSA – this remains to be investigated.
Acknowledgments
We would like to acknowledge CL for assistance with statistical analysis and composing the results.
Footnotes
Declaration conflicts of interest: None related to the work presented in this manuscript.
Data statement: Data available upon reasonable request.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Hugo Giambini https://orcid.org/0000-0002-3147-209X
Joaquin Sanchez-Sotelo https://orcid.org/0000-0003-3199-3247
Tobias Kummer https://orcid.org/0000-0002-2420-7001
References
- 1.Wagner ER, Farley KX, Higgins I, et al. The incidence of shoulder arthroplasty: rise and future projections compared with hip and knee arthroplasty. J Shoulder Elbow Surg 2020; 29: 2601–2609. [DOI] [PubMed] [Google Scholar]
- 2.Padegimas EM, Maltenfort M, Lazarus MD, et al. Future patient demand for shoulder arthroplasty by younger patients: national projections. Clin Orthop Relat Res 2015; 473: 1860–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palsis JA, Simpson KN, Matthews JH, et al. Current trends in the use of shoulder arthroplasty in the United States. Orthopedics 2018; 41: e416–e423. [DOI] [PubMed] [Google Scholar]
- 4.Zeng GJ, Sheng X, Lie DTT. Short- to medium-term outcomes and future direction of reverse shoulder arthroplasty: current concepts. J ISAKOS 2023; 8: 398–403. [DOI] [PubMed] [Google Scholar]
- 5.Boileau P, Watkinson DJ, Hatzidakis AMet al. et al. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg 2005; 14: 147S–161S. [DOI] [PubMed] [Google Scholar]
- 6.Wright TW, Roche CP, Wright L, et al. Reverse shoulder arthroplasty augments for glenoid wear. Comparison of posterior augments to superior augments. Bull Hosp Jt Dis (2013) 2015; 73: S124–S128. [PubMed] [Google Scholar]
- 7.Lädermann A, Williams MD, Melis B, et al. Objective evaluation of lengthening in reverse shoulder arthroplasty. J Shoulder Elbow Surg 2009; 18: 588–595. [DOI] [PubMed] [Google Scholar]
- 8.Hamilton MA, Diep P, Roche C, et al. Effect of reverse shoulder design philosophy on muscle moment arms. J Orthop Res 2015; 33: 605–613. [DOI] [PubMed] [Google Scholar]
- 9.Crosby LA, Hamilton A, Twiss T. Scapula fractures after reverse total shoulder arthroplasty: classification and treatment. Clin Orthop Relat Res 2011; 469: 2544–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheung EV, Sarkissian EJ, Sox-Harris A, et al. Instability after reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2018; 27: 1946–1952. [DOI] [PubMed] [Google Scholar]
- 11.Boileau P, Chuinard C, Le Huec J-C, et al. Proximal humerus fracture sequelae: impact of a new radiographic classification on arthroplasty. Clin Orthop Relat Res 2006; 442: 121–130. [DOI] [PubMed] [Google Scholar]
- 12.Ophir J. Elastography: a quantitative method for imaging the elasticity of biological tissues. Ultrason Imaging 1991; 13: 111–134. [DOI] [PubMed] [Google Scholar]
- 13.Scaperrotta G, Ferranti C, Costa C, et al. Role of sonoelastography in non-palpable breast lesions. Eur Radiol 2008; 18: 2381–2389. [DOI] [PubMed] [Google Scholar]
- 14.Guibal A, Boularan C, Bruce M, et al. Evaluation of shearwave elastography for the characterisation of focal liver lesions on ultrasound. Eur Radiol 2013; 23: 1138–1149. [DOI] [PubMed] [Google Scholar]
- 15.Ciledag N, Arda K, Aribas BK, et al. The utility of ultrasound elastography and MicroPure imaging in the differentiation of benign and malignant thyroid nodules. AJR Am J Roentgenol 2012; 198: W244–W249. [DOI] [PubMed] [Google Scholar]
- 16.Dietrich CF, Bamber J, Berzigotti A, et al. EFSUMB Guidelines and recommendations on the clinical use of liver ultrasound elastography, update 2017 (long version). Ultraschall Med 2017; 38: e16–e47. [DOI] [PubMed] [Google Scholar]
- 17.Taljanovic MS, Gimber LH, Becker GW, et al. Shear-wave elastography: basic physics and musculoskeletal applications. Radiographics 2017; 37: 855–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tas S, Onur MR, Yilmaz S, et al. Shear wave elastography is a reliable and repeatable method for measuring the elastic modulus of the rectus femoris muscle and patellar tendon. J Ultrasound Med 2017; 36: 565–570. [DOI] [PubMed] [Google Scholar]
- 19.Rosskopf AB, Ehrmann C, Buck FM, et al. Quantitative shear-wave US elastography of the supraspinatus muscle: reliability of the method and relation to tendon integrity and muscle quality. Radiology 2016; 278: 465–474. [DOI] [PubMed] [Google Scholar]
- 20.Kim K, Hwang H-J, Kim S-G, et al. Can shoulder muscle activity be evaluated with ultrasound shear wave elastography? Clin Orthop Relat Res 2018; 476: 1276–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeon M, Youn K, Yang S. Reliability and quantification of gastrocnemius elasticity at relaxing and at submaximal contracted condition. Med Ultrason 2018; 20: 342–347. [DOI] [PubMed] [Google Scholar]
- 22.Itoigawa Y, Sperling JW, Steinmann SP, et al. Feasibility assessment of shear wave elastography to rotator cuff muscle. Clin Anat 2015; 28: 213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hatta T, Giambini H, Uehara K, et al. Quantitative assessment of rotator cuff muscle elasticity: reliability and feasibility of shear wave elastography. J Biomech 2015; 48: 3853–3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hatta T, Giambini H, Sukegawa K, et al. Quantified mechanical properties of the deltoid muscle using the shear wave elastography: potential implications for reverse shoulder arthroplasty. PLoS One 2016; 11: e0155102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eby SF, Cloud BA, Brandenburg JE, et al. Shear wave elastography of passive skeletal muscle stiffness: influences of sex and age throughout adulthood. Clin Biomech (Bristol, Avon) 2015; 30: 22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chodock E, Hahn J, Setlock CAet al. et al. Identifying predictors of upper extremity muscle elasticity with healthy aging. J Biomech 2020; 103: 109687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baumer TG, Dischler J, Davis L, et al. Effects of age and pathology on shear wave speed of the human rotator cuff. J Orthop Res 2018; 36: 282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alfuraih AM, Tan AL, O’Connor P, et al. The effect of ageing on shear wave elastography muscle stiffness in adults. Aging Clin Exp Res 2019; 31: 1755–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garber CE, Blissmer B, Deschenes MR, et al. American College of sports medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011; 43: 1334–1359. [DOI] [PubMed] [Google Scholar]
- 30.Disselhorst-Klug C, Schmitz-Rode T, Rau G. Surface electromyography and muscle force: limits in sEMG-force relationship and new approaches for applications. Clin Biomech (Bristol, Avon) 2009; 24: 225–235. [DOI] [PubMed] [Google Scholar]
- 31.Umehara J, Hasegawa S, Nakamura M, et al. Effect of scapular stabilization during cross-body stretch on the hardness of infraspinatus, teres minor, and deltoid muscles: an ultrasonic shear wave elastography study. Musculoskelet Sci Pract 2017; 27: 91–96. [DOI] [PubMed] [Google Scholar]
- 32.Hoshikawa K, Yuri T, Mura N, et al. Coordination of the sub-regions of the supraspinatus and deltoid muscles during shoulder scaption: a shear wave elastography study. Muscles Ligaments Tendons J 2021; 11: 569. [Google Scholar]
- 33.Alfuraih AM, O’Connor P, Hensor E, et al. The effect of unit, depth, and probe load on the reliability of muscle shear wave elastography: variables affecting reliability of SWE. J Clin Ultrasound 2018; 46: 108–115. [DOI] [PubMed] [Google Scholar]
- 34.Ishikawa H, Muraki T, Sekiguchi Y, et al. Noninvasive assessment of the activity of the shoulder girdle muscles using ultrasound real-time tissue elastography. J Electromyogr Kinesiol 2015; 25: 723–730. [DOI] [PubMed] [Google Scholar]
- 35.Ishikawa H, Muraki T, Morise S, et al. Changes in shoulder muscle activities and glenohumeral motion after rotator cuff repair: an assessment using ultrasound real-time tissue elastography. J Shoulder Elbow Surg 2021; 30: 2577–2586. [DOI] [PubMed] [Google Scholar]
- 36.Lädermann A, Edwards TB,, Walch G. Arm lengthening after reverse shoulder arthroplasty: a review. Int Orthop 2014; 38: 991–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wiater BP, Baker EA, Salisbury MR, et al. Elucidating trends in revision reverse total shoulder arthroplasty procedures: a retrieval study evaluating clinical, radiographic, and functional outcomes data. J Shoulder Elbow Surg 2015; 24: 1915–1925. [DOI] [PubMed] [Google Scholar]
- 38.Yoon JP, Seo A, Kim JJ, et al. Deltoid muscle volume affects clinical outcome of reverse total shoulder arthroplasty in patients with cuff tear arthropathy or irreparable cuff tears. PLoS One 2017; 12: e0174361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshiko A, Ando R, Akima H. Passive muscle stiffness is correlated with the intramuscular adipose tissue in young individuals. Eur J Appl Physiol 2023; 123: 1081–1090. [DOI] [PubMed] [Google Scholar]
- 40.Wang D, De Vito G, Ditroilo Met al. et al. Effect of sex and fatigue on muscle stiffness and musculoarticular stiffness of the knee joint in a young active population. J Sports Sci 2017; 35: 1582–1591. [DOI] [PubMed] [Google Scholar]