Abstract
Objectives
Rheumatoid arthritis (RA) is a chronic autoimmune disease linked with metabolic dysfunction-associated steatotic liver disease (MASLD), which may increase cardiovascular (CV) risk. This study explores the association between liver fibrosis, assessed by the Fibrosis-4 (FIB-4) index, and CV risk factors in RA patients.
Methods
Cross-sectional data from the Franciscus Rheumatoid Arthritis and Cardiovascular Intervention Study (FRANCIS), a randomized, cardiovascular single center, intervention study involving RA patients without cardiovascular disease (CVD) or type 2 diabetes (T2DM), were analyzed. Liver fibrosis was assessed using FIB-4, with a cut-off point of ≥ 1.3 to define high fibrosis risk, and its relationship with CV risk factors, medication use, and subclinical atherosclerosis, measured by carotid intima-media thickness (cIMT), was evaluated.
Results
Among 326 patients (68.4% female, age 53 ± 11 years, BMI 26.5 ± 4.5 kg/m2), those with high FIB-4 (n = 49) had higher cIMT (p = 0.002), apolipoprotein B48 (p = 0.04), systolic blood pressure (p = 0.007), alkaline phosphatase (p = 0.002), and anti-CCP levels (p = 0.02). High FIB-4 was associated with lower leukocyte count and complement component 3. Statin use was linked to higher FIB-4 (OR = 4.49, p = 0.014), while hydroxychloroquine use was associated with lower FIB-4 (OR = 0.11, p = 0.004). Disease activity scores did not differ between low and high FIB-4 groups.
Conclusions
Elevated FIB-4 in RA patients is associated with increased cIMT, higher blood pressure, and elevated atherogenic remnants. Incorporating FIB-4 measurements into routine clinical care for RA populations could effectively identify individuals at the highest CV risk, enabling the implementation of more intensive CV risk management strategies.
|
Key Points • RA patients with liver fibrosis have higher cIMT, indicating greater risk of atherosclerosis. • RA patients with liver fibrosis show accumulation of circulating atherogenic chylomicron remnants, contributing to atherogenesis. • HCQ may provide a protective effect against liver fibrosis in RA patients. |
Supplementary Information
The online version contains supplementary material available at 10.1007/s10067-025-07364-5.
Keywords: Chylomicron remnants, FIB-4, Liver fibrosis, MASH, MASLD
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune and inflammatory disease which can lead to significant disability and reduced quality of life [1]. Beyond joint involvement, RA has notable extra-articular manifestations, impacting the lungs, skin, eyes, heart, kidneys, nervous system, and gastrointestinal tract [2, 3]. One potential clinical outcome of RA is liver involvement, which can manifest as autoimmune biliary disease, abnormal liver test results, or metabolic dysfunction-associated steatotic liver disease (MASLD) [4–6]. MASLD is characterized by excess fat accumulation in the liver along with at least one cardiometabolic risk factor, in the absence of secondary causes of hepatic steatosis or excessive alcohol consumption [7]. Approximately one-third of RA patients are affected by MASLD [8, 9], which can range from simple steatosis to more severe forms like metabolic dysfunction-associated steatohepatitis (MASH), potentially advancing to cirrhosis and hepatocellular carcinoma (HCC) [10]. Furthermore, MASLD is associated with an increased risk for CVD [11].
Treatment with disease-modifying antirheumatic drugs (DMARDs) may also contribute to the risk of MASLD in RA. Methotrexate (MTX), the most frequently used DMARD for RA, has been linked to various hepatic side effects, including elevated liver enzymes and an increased risk of liver fibrosis, especially with long-term use or higher doses [12, 13]. Other RA medications, such as biologicals, have also been associated with hepatotoxicity, although the extent of their contribution to liver fibrosis remains unclear [14]. Given the widespread use of these medications in RA management, it is crucial to evaluate their potential impact on liver health, particularly on the progression of liver fibrosis.
RA is closely linked to cardiovascular disease (CVD), primarily due to the combined effects of systemic inflammation, potentially causing harm to the arterial wall, and the presence of traditional CV risk factors, which are often undertreated in this population [15]. Patients with RA have a 1.5-fold increased risk of CVD and experience higher rates of CVD-related morbidity and mortality compared to the general population [16, 17]. The increased risk of premature mortality in RA patients is attributed to both persistent inflammation and inadequate management of traditional CV risk factors [18–22].
In addition to traditional CV risk factors, the carotid intima-media thickness (cIMT), a surrogate marker for subclinical atherosclerosis, has also been shown to be increased in RA patients [23, 24]. Recent studies also suggested that the increased CVD risk in RA may be associated with accumulation of atherogenic intestinal remnants carrying apolipoprotein (apo) B48 [25, 26].
Although CVD and MASLD are closely interconnected and inflammation plays a central role in both, this has not been studied in detail in a typical systemic inflammatory condition like RA. We hypothesized that the presence of MASLD, particularly liver fibrosis, a key determinant of MASLD prognosis, has been associated with increased CV risk [25].
While liver biopsy is considered the gold standard for diagnosing liver fibrosis, it is an invasive and costly procedure which is not routinely performed unless there is a clear clinical indication [27]. Therefore, we used the Fibrosis-4 (FIB-4) index, a non-invasive blood test recommended by the European Association for the Study of the Liver (EASL) guidelines, for the assessment of liver fibrosis [7, 28]. The aims of this study were to investigate the association between liver fibrosis, as estimated by the FIB-4, and CV risk factors, including hypertension, lipid levels, and cIMT in patients with RA. Additionally, it aimed to investigate the association between liver fibrosis and medication use in this patient population.
Materials and methods
Study design and study population
This is a post-hoc analysis of the Franciscus Rheumatoid Arthritis and Cardiovascular Intervention Study (FRANCIS), an open-label randomized controlled trial that aimed to investigate the effectiveness of a strict treatment of CV risk factors in RA [26, 29, 30]. The study population (n = 326) consisted of patients attending the outpatient clinic of the Department of Rheumatology at the Franciscus Gasthuis Hospital in Rotterdam, the Netherlands. Participants were included if they were less than 70 years of age and if they had been diagnosed with rheumatoid arthritis, defined according to the criteria of the American College of Rheumatology [31]. Patients were excluded if they had type 1 diabetes mellitus (T1DM) or T2DM at baseline, defined as a fasting glucose of > 7.0 mmol or HbA1c > 48 mmol/mol, or being treated for these conditions or if they had a history of CVD. The latter of which was defined as a prior myocardial infarction, cerebrovascular event, intermittent claudication, amputation due to peripheral artery disease (PAD), coronary artery bypass graft (CABG), or percutaneous transluminal coronary angioplasty (PTCA). Additionally, patients were excluded if they had kidney disease, defined as an estimated glomerular filtration rate (eGFR) of < 40 ml/min/1.73m2. The study was approved by the medical ethical committee of the Maasstad hospital in Rotterdam, the Netherlands, and was conducted in accordance with the Declaration of Helsinki (The Dutch Trial register, NTR3873; ABR no. NL32669.101.10) [32].
Data collection
Baseline data was collected during outpatient clinic visits between 2009 and 2012. Blood samples were obtained after an overnight fast. In addition, anthropomorphic measurements (e.g., weight, height, blood pressure) were recorded. Variables included for medication use were methotrexate (MTX), NSAIDs, biologicals, statins, antihypertensives, leflunomide, hydroxychloroquine (HCQ), prednisone, and anti-TNF therapy. Furthermore, RA disease activity was evaluated using the Disease Activity Score (DAS28) wherein 28 joints are evaluated [33].
Fibrosis-4 index
The FIB-4 was calculated according to the following formula: age (years) × AST(U/L)/platelet (PLT) (109/L) × √ALT(U/L). Low hepatic fibrosis risk was defined as a FIB-4 < 1.3 and high fibrosis risk as a FIB-4 ≥ 1.3. The FIB-4 has been extensively validated in a broad spectrum of MASLD subjects and in RA patients [7, 28].
Carotid intima media thickness
cIMT was measured by trained and experienced sonographers using the ART-LAB (Esaote, Italy) as described earlier [29]. Using B-mode ultrasounds which produced two echogenic lines, ultrasound images were made of the far wall of each common carotid artery (CCA). These lines represented the overall thickness of the media and intima layers of the arterial wall. Atherosclerotic plaque was defined as a lesion with a focal cIMT ≥ 1.0 mm.
Laboratory measurements
In each patient, a standard set of laboratory measurements was performed at the Department of Clinical Chemistry of the Franciscus Gasthuis hospital. Blood samples were drawn following the standard procedures. Liver enzymes and renal tests as well as total cholesterol, HDL-C, glucose, C-reactive protein (CRP), and triglycerides (TGs) were measured using DxC analyzers (Beckman Coulter, United States) or Synchron LX20 (Beckman Coulter, United States) [34, 35]. LDL-C was measured using the Friedewald formula if TGs were below 4.00 mmol/L. Complement C3 levels were measured using the Atellica® Solution (Siemens Healthineers, Germany). Furthermore, using an ELISA, apoB48 serum levels were quantified as previously described [36].
Statistical analysis
Data are presented as mean ± standard deviation (SD), unless otherwise specified. Normally distributed variables were analyzed using the independent T-test, while non-normally distributed parameters were assessed with the Mann–Whitney U test or Kruskal–Wallis test. Correlation analyses were conducted using Pearson’s or Spearman’s correlation coefficients, where appropriate. Multivariate binary logistic regression was performed with FIB-4 categories (low vs. high) as the dependent variable and medication use as the independent variables. Chi-square tests were also conducted. A multiple regression analysis was performed to investigate the association between FIB-4 (low vs. high) and cIMT adjusting for hypertension and lipid levels. All statistical analyses were performed using IBM SPSS Statistics version 28.0.0.0 (IBM SPSS Statistics, New York, United States), with statistical significance set at a p-value < 0.05.
Results
The study population included 223 women and 103 men, with an average age of 53 years (± 11) and an average BMI of 26.5 kg/m2 (± 4.5). The majority of the cohort had a low FIB-4 score (n = 277, 85.0%). Patients with an elevated FIB-4 score were significantly older and had higher systolic blood pressure, higher apoB48 concentrations, higher cIMT values, higher alkaline phosphatase, and higher anti-cyclic citrullinated peptide (anti-CCP) concentrations (Table 1; Fig. 1). In addition, they had statistically significant lower BMI, as well as lower levels of thrombocytes and a lower leukocyte count, particularly neutrophils and lymphocytes, and lower levels of complement C3. There was no difference in disease activity score between groups (Table 1).
Table 1.
Total group (n = 326). Baseline characteristics in the low FIB-4 versus high FIB-4 groups. p-value is for comparison between FIB-4 groups. Data are expressed as mean ± standard deviation, median (IQR), or n (%), as appropriate
| Low FIB-4 N = 277 |
High FIB-4 N = 49 |
p-value | |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years) | 52 ± 11 | 62 ± 7.2 | < 0.001 |
| Sex (m/f) | 83/194 (30/70) | 20/29 (41/59) | 0.132 |
| Smoking (no/yes) | 207/55 (79/21) | 39/9 (81/19) | 0.724 |
| Clinical characteristics | |||
| Body mass index (kg/m2) | 26.7 ± 4.6 | 25.2 ± 3.6 | 0.031 |
| Systolic blood pressure (mmHg) | 131 ± 18 | 139 ± 22 | 0.007 |
| Diastolic blood pressure (mmHg) | 78.9 ± 10 | 81 ± 12 | 0.142 |
| Total cholesterol (mmol/L) | 5.4 ± 1.1 | 5.6 ± 1.1 | 0.224 |
| HDL-C (mmol/L) | 1.5 ± 0.41 | 1.6 ± 0.43 | 0.069 |
| LDL-C (mmol/L) | 3.4 ± 0.94 | 3.4 ± 0.90 | 0.537 |
| Triglycerides (mmol/L) | 1.04 (0.72–1.55) | 1.05 (0.76–1.49) | 0.757 |
| ApoAI (g/L) | 1.68 ± 0.37 | 1.73 ± 0.37 | 0.441 |
| Apo B (g/L) | 1.00 ± 0.26 | 1.00 ± 0.25 | 1.000 |
| Apo B48 (mg/L) | 8.2 (5.0–12.1) | 9.6 (6.8–15.5) | 0.036 |
| Fasting glucose (mmol/L) | 5.5 ± 0.54 | 5.5 ± 0.63 | 0.350 |
| HbA1c (mmol/mol) | 35.3 ± 4.5 | 35.1 ± 3.9 | 0.839 |
| CRP (mg/L) | 3.0 (1.0–6.0) | 2.0 (1.0–5.0) | 0.181 |
| Hemoglobin (g/dL) | 8.5 ± 0.8 | 8.7 ± 0.5 | 0.002 |
| Thrombocytes (109/L) | 255 ± 63.2 | 172 ± 30.9 | < 0.001 |
| Leukocyte counts (109/L) | 6.7 ± 2.0 | 5.2 ± 1.5 | < 0.001 |
| Neutrophils (109/L) | 4.1 ± 1.7 | 3.1 ± 1.2 | < 0.001 |
| Lymphocytes (109/L) | 1.8 (1.4–2.28) | 1.5 (1.1–1.75) | 0.024 |
| Monocytes (109/L) | 0.55 ± 0.19 | 0.49 ± 0.18 | 0.057 |
| Eosinophils (109/L) | 0.16 ± 0.11 | 0.16 ± 0.16 | 0.979 |
| Basophils (109/L) | 0.03 ± 0.05 | 0.02 ± 0.04 | 0.128 |
| Complement C3 (g/L) | 1.19 ± 0.23 | 1.08 ± 0.18 | < 0.001 |
| Complement C4 (g/L) | 0.21 ± 0.06 | 0.19 ± 0.05 | 0.053 |
| Alkaline phosphatase (U/L) | 70.5 ± 23.1 | 83.1 ± 38.2 | 0.002 |
| Gamma-GT (U/L) | 21 (16–29) | 21 (16–32) | 0.399 |
| Creatinine (µmol/L) | 69.5 ± 14.7 | 73.3 ± 13.9 | 0.092 |
| Microalbumin (mg/L) | 7.0 (5.0–14) | 7.0 (5.0–15) | 0.249 |
| Uric acid (mmol/L) | 0.29 (0.25–0.34) | 0.32 (0.26–0.36) | 0.878 |
| TSH (mU/L) | 1.9 (1.3–2.7) | 2.0 (1.3–3.8) | 0.221 |
| Anti-CCP (U/mL) | 0.64 ± 0.48 | 0.80 ± 0.41 | 0.027 |
| DAS28CRP | 2.5 ± 1.1 | 2.2 ± 0.93 | 0.171 |
| DAS28BSE | 2.6 ± 1.3 | 2.4 ± 0.98 | 0.287 |
| ALAT (U/L) | 16 (13—20) | 23 (19.5—27) | < 0.001 |
| ASAT (U/L) | 25 (19–32) | 27 (20–35) | 0.086 |
| FIB-4 | 0.73 ± 0.28 | 1.75 ± 0.45 | < 0.001 |
| Medication use | |||
| Antihypertensives | 43 (15.6) | 10 (20.4) | 0.405 |
| Statins | 9 (3.3) | 6 (12.2) | 0.015 |
| Leflunomide | 3 (1.1) | 2 (4.1) | 0.166 |
| Hydroxychloroquine | 71 (25.8) | 2 (4.1) | < 0.001 |
| Anti-TNF | 97 (35.3) | 19 (38.8) | 0.747 |
| Prednisone | 34 (12.4) | 5 (10.2) | 0.814 |
| Methotrexate | 206 (74.9) | 38 (77.6) | 0.589 |
| NSAID | 120 (43.6) | 20 (40.8) | 0.755 |
HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, remnant-C remnant cholesterol, apoAI apolipoprotein AI, apoB apolipoprotein B, apoB48 apolipoprotein B48, HbA1c hemoglobin A1C, CRP C-reactive protein, Gamma-GT gamma-glutamyl transferase, TSH thyroid-stimulating hormone, anti-CCP anti-cyclic citrullinated peptide, DAS28CRP Disease Activity Score 28-joint count with C-Reactive Protein, DAS28BSE Disease Activity Score 28-joint count with Erythrocyte Sedimentation Rate, ALT alanine aminotransferase, AST aspartate aminotransferase, FIB-4 Fibrosis-4 index
Fig. 1.
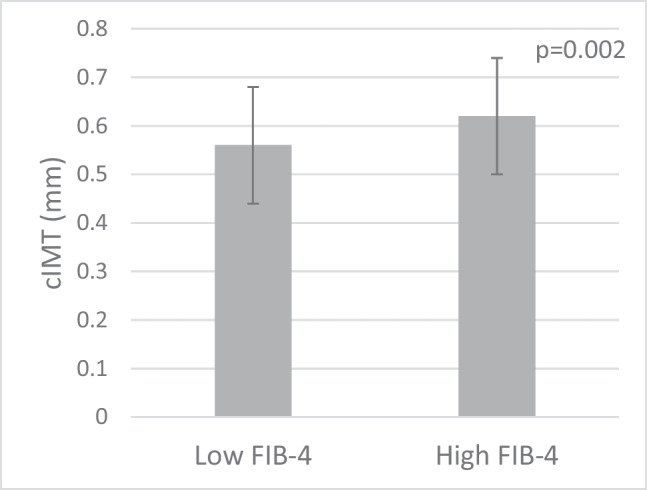
Mean carotid intima media thickness (cIMT) in the low FIB-4 versus high FIB-4 group; 0.57 mm ± 0.12 in the low FIB-4 group versus 0.62 mm ± 0.12 in the high FIB-4 group (p = 0.002)
Correlation analyses showed significant positive associations between FIB-4 and systolic blood pressure (rho = 0.301, p < 0.001), cIMT (rho = 0.409, p < 0.001), total cholesterol (rho = 0.212, p < 0.001), LDL-C (rho = 0.190, p < 0.001), and apoB48 (rho = 0.141, p = 0.011). In contrast, negative correlations were found between FIB-4 and complement C3 (rho = − 0.161; p = 0.004) and leukocyte count (rho = − 0.399, p < 0.001). There was no statistically significant correlation between FIB-4 and triglycerides (rho = 0.060, p = 0.278).
Logistic regression analysis showed that statin use was associated with high FIB-4, while HCQ use was associated with lower FIB-4 (Table 2). To further explore these associations, we conducted univariate logistic regression analyses for each medication separately (Supplementary Table S1). These analyses confirmed that statin use was significantly associated with high FIB-4, while HCQ use was significantly associated with lower FIB-4. No significant associations were observed for antihypertensives, leflunomide, anti-TNF agents, prednisone, MTX, NSAIDs, or other biologics (all p > 0.05). In terms of medication use, 12.2% of the patients in the high FIB-4 group used statins, compared to only 3.3% in the low FIB-4 group (Table 1). Similarly, HCQ use showed a statistically significant difference between the two groups, with 4.1% of the patients in the high FIB-4 group using HCQ, compared to 25.8% in the low FIB-4 group. These associations were found independently of the use of other medications included in the analysis. No significant differences were found for other medications, including antihypertensives, leflunomide, anti-TNF agents, prednisone, MTX, NSAIDs, and biologicals (all p > 0.05) (Table 1).
Table 2.
Logistic regression analysis of medication effects on FIB-4 (low versus high)
| Variable | B | S.E | Wald | df | Sig | Exp(B) | 95% CI for Exp(B) |
|---|---|---|---|---|---|---|---|
| Constant | − 1.826 | 0.461 | 15.686 | 1 | < 0.001 | 0.161 | |
| Antihypertensives | 0.257 | 0.423 | 0.370 | 1 | 0.543 | 1.293 | 0.565–2.962 |
| Statins | 1.501 | 0.612 | 6.019 | 1 | 0.014 | 4.488 | 1.352–14.890 |
| Hydroxychloroquine | − 2.176 | 0.749 | 8.436 | 1 | 0.004 | 0.113 | 0.026–0.493 |
| Anti-TNF | − 0.070 | 0.343 | 0.041 | 1 | 0.839 | 0.933 | 0.476–1.828 |
| Prednisone | − 0.265 | 0.536 | 0.244 | 1 | 0.621 | 0.767 | 0.269–2.193 |
| Methotrexate | 0.403 | 0.420 | 0.921 | 1 | 0.337 | 1.497 | 0.657–3.411 |
| NSAID | − 0.096 | 0.335 | 0.082 | 1 | 0.774 | 0.909 | 0.472–1.750 |
Higher FIB-4 scores are associated with increased cIMT. This relationship persists even when adjusting for potential confounders, including systolic blood pressure and LDL-C (Table 3). While adjustments were also made for HDL-C and diastolic blood pressure, the linearity with these variables was weaker than that of those included in the final model.
Table 3.
Multiple regression analysis of cIMT and FIB-4, adjusted for confounding variables
| Variable | B | 95% CI | p-value |
|---|---|---|---|
| Model 1 | 0.055 | 0.021–0.089 | 0.002 |
| Model 2 | 0.037 | 0.005–0.068 | 0.024 |
Model 1, crude model; model 2, adjusted for systolic blood pressure and LDL-C
Discussion
The results from the current study have extended the findings from our previous observations reporting on the association between RA and CV risk factors, by also showing an association with liver fibrosis as estimated by FIB-4 [25, 26, 37]. The findings of the current study showed that RA patients with a high FIB-4 have higher cIMT values, even after adjusting for potential confounders, reinforcing the notion that liver fibrosis may independently contribute to CV risk in RA patients. This underscores FIB-4’s potential as a marker for both liver health and CV risk stratification in RA. Additionally, high systolic blood pressure was found to be significantly associated with increased cIMT, regardless of FIB-4 levels. This highlights the importance of managing blood pressure in RA patients, particularly in those with liver fibrosis, to reduce CV risk. There was no difference in disease activity score between those with low and high FIB-4, suggesting that liver fibrosis, as measured by FIB-4, may be independent of overall RA disease activity, which highlights the need to consider additional factors when assessing liver health in RA patients.
Previously, we reported elevated apoB48 concentrations in RA patients compared to controls [25, 26]. The present study expands that observation by also demonstrating an association with liver fibrosis. It is well known that chylomicrons and their remnants can directly infiltrate intact endothelium, become trapped, and induce an inflammatory response along with the formation of foam cells. This process plays a crucial role in the initiation and progression of atherosclerosis [37, 38]. This mechanism may explain the increased cIMT observed in RA patients with liver fibrosis. While the relationship between cIMT and liver fibrosis is evident, the pathophysiology of the accumulation of chylomicron remnants remains unclear. A key factor in the clearance of chylomicron remnants is a receptor-mediated pathway in the liver. Liver fibrosis could disrupt this process by reducing the number or function of these receptors. One could speculate that this impairment may lead to higher levels of circulating chylomicron remnants due to reduced hepatic clearance. Another important consideration is that RA patients with high FIB-4 levels also were more frequently on statins. Normally, statin use increases LDL-receptor expression, thereby enhancing the clearance of lipoproteins. This could either mean that these subjects had already been identified as having a higher CV risk and were therefore on appropriate treatment or that liver fibrosis might reduce statin effectiveness by impairing LDL-receptor activity, thus hindering the clearance of atherogenic lipoproteins despite statin treatment. At this stage, there is no clinical or experimental evidence supporting the latter hypothesis.
Another factor that may contribute to liver complications in RA is the presence of anti-CCP antibodies. While these antibodies are well-known for targeting various cell types in RA, their impact on liver cells is less understood. Recent laboratory studies have revealed that anti-CCP antibodies can influence hepatocytes by triggering the release of inflammatory proteins, impairing fat and glucose metabolism, and ultimately causing liver damage and apoptosis [39]. This mechanism could potentially explain the increased anti-CCP levels observed in patients with high FIB-4 scores.
Our study demonstrated a significant association between HCQ use and lower FIB-4 scores, a finding consistent with recent research suggesting that HCQ may have a protective effect against the development of MASLD [40]. HCQ is known to influence adiponectin levels, which play a key role in regulating metabolic processes. Higher adiponectin levels, linked to HCQ use, are associated with reduced systemic inflammation and improved insulin sensitivity, both of which can help mitigate liver damage [41]. This may explain the lower FIB-4 scores observed in patients using HCQ. However, it is important to consider that this association may be confounded by indication The protective effect of HCQ against liver fibrosis observed in this study may, in part, be attributed to its use in patients with lower RA disease activity. As low disease activity is inherently associated with reduced systemic inflammation, it is possible that the observed benefit reflects better-controlled RA rather than a direct pharmacological effect of HCQ. Given these considerations, the findings need to be interpreted with caution, and further studies are warranted to explore these potential mechanisms and establish the independent role of HCQ in liver fibrosis prevention.
Moreover, in our study, we did not find any significant associations between MTX use and liver fibrosis. Similarly, a recent multicenter study involving around 1000 patients with RA or psoriasis also found no association between the cumulative dose or duration of MTX exposure and liver fibrosis [42]. Although MTX has been previously linked to hepatotoxicity and an increased risk of liver fibrosis [13, 43], these newer findings suggest that the actual risk may be lower than previously believed. This discrepancy underscores the need for further research to clarify MTX’s impact on liver health and to better understand the factors that may influence these outcomes.
The lower inflammatory markers observed in patients with high FIB-4 levels could be attributed to the effects of advanced liver fibrosis. Advanced liver fibrosis often leads to portal hypertension and resultant hypersplenism, causing the sequestration of leukocytes and thrombocytes in the spleen, thereby reducing their levels in the bloodstream. Alternatively, the inverse relationship between leukocyte count and FIB-4 may also be attributed to MTX use, which can cause myelosuppression and reduce leukocyte counts, often leading to neutropenia, anemia, and thrombocytopenia [43, 44].
Additionally, in contrast with previous studies, our study did not find significant associations between glucocorticoid use and liver fibrosis [40, 45]. This may be due to differences in glucocorticoid dosage, treatment duration, or comorbidities within our cohort. Further research with larger samples and more detailed data on glucocorticoid use is needed to clarify its potential impact on liver fibrosis in patients with RA.
This study had some limitations. Firstly, the cross-sectional design limits our ability to determine causality and observe changes over time. Longitudinal studies are needed to explore these aspects further. Secondly, while FIB-4 provides valuable insights, it is not the gold standard for liver fibrosis assessment; liver biopsy remains the preferred method. Additionally, only a small portion of the study population had a high FIB-4. Another limitation is the lack of data on cumulative steroid use. As this information was not collected prospectively, it was not feasible to include it in our analysis. However, given the primary focus of this study on the associations between liver fibrosis and CVD risk factors, as well as medication use in patients with RA, we believe the absence of this data is unlikely to have impacted the validity of our key findings though it is an important consideration for future research. Moreover, some patients in this study were prescribed medications such as HCQ, prednisolone, and NSAIDs, which are not currently recommended as first-line treatments for rheumatoid arthritis. These prescriptions likely reflect individual clinical decisions, including intolerance or contraindications to recommended therapies like methotrexate, or historical prescribing practices. Such variations highlight the complexity of managing rheumatoid arthritis in real-world settings and should be considered when interpreting the study’s findings.
In conclusion, RA patients with a high FIB-4 showed increased cIMT, indicating higher CV risk. This relationship appears to be influenced by hypertension and the accumulation of intestinal atherogenic chylomicron remnants. The observed inverse relationship between FIB-4 with systemic inflammatory markers suggests a complex interaction that warrants further research. Hydroxychloroquine may have the potential to offer some protective effect against liver fibrosis in RA patients, though further research is needed to confirm this finding.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Preliminary data from this study were presented as a poster at the European Association for the Study of the Liver (EASL) Congress in Prague, 2023.
Author contribution
ANS and MCC were involved in the conception, design, and conduct of the study and the analysis and interpretation of the results. SPR contributed to the analysis. ANS wrote the first draft of the manuscript, and all authors, reviewed, edited, and approved the final version of the manuscript.
Funding
This article is based on the FRANCIS study, which was supported by research funds from the Franciscus Gasthuis Hospital and the Coolsingel Foundation in Rotterdam, the Netherlands.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study involves human participants, and the original study (FRANCIS) was approved by the medical ethical committee of the Maasstad hospital in Rotterdam, the Netherlands (The Dutch Trial register, NTR3873; ABR no. NL32669.101.10) and was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonisation Guidance for Good Clinical Practice. Participants gave informed consent to participate in the study before taking part.
Consent for publication
Not applicable.
Disclosures
None.
Footnotes
Part of the preliminary data was previously presented as an abstract at the EASL SLD Summit 2023 held in Prague.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Di Matteo A, Bathon JM, Emery P (2023) Rheumatoid arthritis. Lancet 402(10416):2019–2033. 10.1016/s0140-6736(23)01525-8 [DOI] [PubMed] [Google Scholar]
- 2.Conforti A, Di Cola I, Pavlych V, Ruscitti P, Berardicurti O, Ursini F et al (2021) Beyond the joints, the extra-articular manifestations in rheumatoid arthritis. Autoimmun Rev 20(2):102735 [DOI] [PubMed] [Google Scholar]
- 3.Figus FA, Piga M, Azzolin I, McConnell R, Iagnocco A (2021) Rheumatoid arthritis: extra-articular manifestations and comorbidities. Autoimmun Rev 20(4):102776 [DOI] [PubMed] [Google Scholar]
- 4.Radovanović-Dinić B, Tešić-Rajković S, Zivkovic V, Grgov S (2018) Clinical connection between rheumatoid arthritis and liver damage. Rheumatol Int 38(5):715–724 [DOI] [PubMed] [Google Scholar]
- 5.Craig E, Cappelli LC (2018) Gastrointestinal and hepatic disease in rheumatoid arthritis. Rheum Dis Clin North Am 44(1):89–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sellami M, Saidane O, Mahmoud I, Tekaya AB, Tekaya R, Abdelmoula L (2020) Etiological features of liver involvement in rheumatoid arthritis. Curr Rheumatol Rev 16(4):332–336 [DOI] [PubMed] [Google Scholar]
- 7.EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J Hepatol 2024;81(3):492–542. [DOI] [PubMed]
- 8.Zamani M, Alizadeh-Tabari S, Chitkara P, Singh S, Loomba R (2023) Prevalence of nonalcoholic fatty liver disease in patients with rheumatoid arthritis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 21(11):2789–2796. 10.1016/j.cgh.2023.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erre GL, Castagna F, Sauchella A, Meloni P, Mangoni AA, Farina G et al (2021) Prevalence and risk factors of moderate to severe hepatic steatosis in patients with rheumatoid arthritis: an ultrasonography cross-sectional case-control study. Ther Adv Musculoskelet Dis 13:1759720x211042739. 10.1177/1759720x211042739 [DOI] [PMC free article] [PubMed]
- 10.Loomba R, Friedman SL, Shulman GI (2021) Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 184(10):2537–2564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aviña-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D (2008) Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum 59(12):1690–1697 [DOI] [PubMed] [Google Scholar]
- 12.Sakthiswary R, Chan GY, Koh ET, Leong KP, Thong BY (2014) Methotrexate-associated nonalcoholic fatty liver disease with transaminitis in rheumatoid arthritis. ScientificWorldJournal 2014:823763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mori S, Arima N, Ito M, Fujiyama S, Kamo Y, Ueki Y (2018) Non-alcoholic steatohepatitis-like pattern in liver biopsy of rheumatoid arthritis patients with persistent transaminitis during low-dose methotrexate treatment. PLoS ONE 13(8):e0203084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hernandez N, Bessone F (2022) Hepatotoxicity induced by biological agents: clinical features and current controversies. J Clin Transl Hepatol 10(3):486–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dijkshoorn B, Raadsen R, Nurmohamed MT (2022) Cardiovascular disease risk in rheumatoid arthritis Anno. J Clin Med 11(10):2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Semb AG, Ikdahl E, Wibetoe G, Crowson C, Rollefstad S (2020) Atherosclerotic cardiovascular disease prevention in rheumatoid arthritis. Nat Rev Rheumatol 16(7):361–379 [DOI] [PubMed] [Google Scholar]
- 17.Barbarroja N, Ruiz-Ponce M, Cuesta-López L, Perez-Sanchez C, Lopez-Pedrera C, Arias-de La Rosa I, Collantes-Estévez E (2022) Nonalcoholic fatty liver disease in inflammatory arthritis: relationship with cardiovascular risk. Front Immunol 13:997270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Breukelen-van der Stoep DF, van Zeben D, Klop B, van de Geijn GJ, Janssen HJ, van der Meulen N, et al (2016) Marked underdiagnosis and undertreatment of hypertension and hypercholesterolaemia in rheumatoid arthritis. Rheumatology (Oxford)55(7):1210–6 [DOI] [PubMed]
- 19.Nurmohamed MT, Heslinga M, Kitas GD (2015) Cardiovascular comorbidity in rheumatic diseases. Nat Rev Rheumatol 11(12):693–704 [DOI] [PubMed] [Google Scholar]
- 20.Bonek K, Głuszko P (2016) Cardiovascular risk assessment in rheumatoid arthritis - controversies and the new approach. Reumatologia 54(3):128–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung CP, Oeser A, Solus JF, Avalos I, Gebretsadik T, Shintani A et al (2008) Prevalence of the metabolic syndrome is increased in rheumatoid arthritis and is associated with coronary atherosclerosis. Atherosclerosis 196(2):756–763 [DOI] [PubMed] [Google Scholar]
- 22.Mulumba C, Lebughe P, Mbuyi-Muamba JM, Makulo JR, Lepira F, Mukaya J et al (2019) Prevalence and associated factors of subclinical atherosclerosis in rheumatoid arthritis at the university hospital of Kinshasa. BMC Rheumatol 3:37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajabzadeh F, Akhlaghipour I, Moosavi SS, Nasimi Shad A, Babazadeh Baghan A, Shariati-Sarabi Z et al (2023) Comparison of the intima-media thickness of the common carotid artery in patients with rheumatoid arthritis: a single-center cross-sectional case-control study, and a brief review of the literature. Health Sci Rep 6(11):e1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khaliq T, Shan S, Shah SA, Saleem S, Adil MH (2023) Carotid intimomedial thickness (CIMT) in patients with rheumatoid arthritis; the need for more aggressive cardiovascular screening in RA. J Coll Physicians Surg Pak 33(4):427–432 [DOI] [PubMed] [Google Scholar]
- 25.Burggraaf B, van Breukelen-van der Stoep DF, van Zeben J, van der Meulen N, van de Geijn GM, Liem A, et al (2018) Evidence for increased chylomicron remnants in rheumatoid arthritis. Eur J Clin Invest 48(2). 10.1111/eci.12873 [DOI] [PubMed]
- 26.Burggraaf B, van Breukelen-van der Stoep DF, de Vries MA, Klop B, van Zeben J, van de Geijn GM, et al. Progression of subclinical atherosclerosis in subjects with rheumatoid arthritis and the metabolic syndrome. Atherosclerosis. 2018;271:84–91. [DOI] [PubMed]
- 27.Tokushige K, Ikejima K, Ono M, Eguchi Y, Kamada Y, Itoh Y et al (2021) Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis 2020. J Gastroenterol 56(11):951–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD) (2024). J Hepatol 81(3):492–542. 10.1016/j.jhep.2024.04.031 [DOI] [PubMed]
- 29.van Breukelen-van der Stoep DF, van Zeben D, Klop B, van de Geijn GJ, Janssen HJ, Hazes MJ, et al. Association of cardiovascular risk factors with carotid intima media thickness in patients with rheumatoid arthritis with low disease activity compared to controls: a cross-sectional study. PLoS One. 2015;10(10):e0140844. 10.1371/journal.pone.0140844 [DOI] [PMC free article] [PubMed]
- 30.Burggraaf B, van Breukelen-van der Stoep DF, de Vries MA, Klop B, Liem AH, van de Geijn GM, et al. Effect of a treat-to-target intervention of cardiovascular risk factors on subclinical and clinical atherosclerosis in rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis. 2019;78(3):335-41. 10.1136/annrheumdis-2018-214075 [DOI] [PubMed]
- 31.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS et al (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31(3):315–324 [DOI] [PubMed] [Google Scholar]
- 32.Association WM (2013) World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 310(20):2191–2194 [DOI] [PubMed] [Google Scholar]
- 33.Wells G, Becker JC, Teng J, Dougados M, Schiff M, Smolen J et al (2009) Validation of the 28-joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann Rheum Dis 68(6):954–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klop B, van de Geijn GJ, Bovenberg SA, van der Meulen N, Elte JW, Birnie E et al (2013) Erythrocyte-bound apolipoprotein B in relation to atherosclerosis, serum lipids and ABO blood group. PLoS ONE 8(9):e75573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bovenberg SA, Klop B, Alipour A, Martinez-Hervas S, Westzaan A, van de Geijn GJ et al (2012) Erythrocyte-associated apolipoprotein B and its relationship with clinical and subclinical atherosclerosis. Eur J Clin Invest 42(4):365–370 [DOI] [PubMed] [Google Scholar]
- 36.Ambrosino P, Lupoli R, Di Minno A, Tasso M, Peluso R, Di Minno MN (2015) Subclinical atherosclerosis in patients with rheumatoid arthritis. A meta-analysis of literature studies. Thromb Haemost 113(5):916–30 [DOI] [PubMed] [Google Scholar]
- 37.Alipour A, Elte JW, van Zaanen HC, Rietveld AP, Cabezas MC (2007) Postprandial inflammation and endothelial dysfuction. Biochem Soc Trans 35(Pt 3):466–469 [DOI] [PubMed] [Google Scholar]
- 38.Gui Y, Zheng H, Cao RY (2022) Foam cells in atherosclerosis: novel insights into its origins, consequences, and molecular mechanisms. Front Cardiovasc Med 9:845942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arias-de la Rosa I, Ruiz-Ponce M, Cuesta-López L, Pérez-Sánchez C, Leiva-Cepas F, Gahete MD et al (2023) Clinical features and immune mechanisms directly linked to the altered liver function in patients with rheumatoid arthritis. Eur J Intern Med 118(49):48–58 [DOI] [PubMed] [Google Scholar]
- 40.Meng CC, Chen DY, Chen YH, Huang WN, Chen HH (2024) Antirheumatic drugs and the risk of nonalcoholic fatty liver disease in patients with rheumatoid arthritis: a nationwide, population-based cohort study. Int J Rheum Dis 27(1):e15003 [DOI] [PubMed] [Google Scholar]
- 41.Pagano C, Soardo G, Esposito W, Fallo F, Basan L, Donnini D et al (2005) Plasma adiponectin is decreased in nonalcoholic fatty liver disease. Eur J Endocrinol 152(1):113–118 [DOI] [PubMed] [Google Scholar]
- 42.Atallah E, Grove JI, Crooks C, Burden-Teh E, Abhishek A, Moreea S et al (2023) Risk of liver fibrosis associated with long-term methotrexate therapy may be overestimated. J Hepatol 78(5):989–997 [DOI] [PubMed] [Google Scholar]
- 43.Mori S, Hidaka M, Kawakita T, Hidaka T, Tsuda H, Yoshitama T et al (2016) Factors associated with myelosuppression related to low-dose methotrexate therapy for inflammatory rheumatic diseases. PLoS ONE 11(4):e0154744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gonzalez-Ibarra F, Eivaz-Mohammadi S, Surapaneni S, Alsaadi H, Syed AK, Badin S et al (2014) Methotrexate induced pancytopenia. Case Rep Rheumatol 2014:679580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woods CP, Hazlehurst JM, Tomlinson JW (2015) Glucocorticoids and non-alcoholic fatty liver disease. J Steroid Biochem Mol Biol 154:94–103 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


