Abstract
Lung adenocarcinoma (LUAD) is the most common subtype of lung cancer. Despite advances in treatment, the prognosis remains poor due to late diagnosis. Cuproptosis (driven by copper ion accumulation) and disulfidptosis (driven by disulfide bond accumulation) are novel forms of programmed cell death, closely linked to tumor initiation, progression, and resistance. However, the specific roles of these mechanisms in LUAD remain inadequately studied. This study integrated multi-omics data from TCGA and GEO databases to systematically evaluate the differential expression and prognostic significance of copper and disulfide-related genes (DCRGs), identify two DCRG molecular subtypes, and construct a DCRG scoring model based on four key genes. Multi-omics analysis results revealed that the DCRG score not only accurately predicts prognosis in LUAD patients but is also closely associated with immune cell infiltration patterns and EGFR inhibitor responses. RT-qPCR validated the high expression of FURIN and RHOV in LUAD cells, supporting their role as potential therapeutic targets. Further Mendelian randomization analysis confirmed the causal relationship between FURIN and LUAD development. These findings provide novel biomarkers for the prognosis evaluation of LUAD based on cuproptosis and disulfidptosis mechanisms and offer a theoretical basis for targeting FURIN in LUAD treatment.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-90653-5.
Keywords: Lung adenocarcinoma, Programmed cell death, Prognostic biomarkers, Machine learning, Immune Microenvironment
Subject terms: Biomarkers, Cancer, Cancer, Cancer genomics, Cancer microenvironment, Cancer models
Introduction
Lung cancer is one of the leading causes of death worldwide, ranking first in both incidence and mortality rates1. Lung adenocarcinoma (LUAD) accounts for 50% of all lung cancer cases, with its incidence continually rising, reflecting the high prevalence of this subtype worldwide2. Despite recent advancements in treatment, most LUAD patients are still diagnosed at advanced stages, as early symptoms are subtle and effective screening methods are lacking, leading to delayed intervention and poor prognosis. Therefore, accurately predicting prognosis and improving treatment outcomes, particularly in early diagnosis and targeted therapy, is of paramount importance3.
In recent years, the study of cell death mechanisms has advanced, with novel pathways emerging as potential therapeutic targets for cancer treatment4. Cuproptosis, a programmed form of cell death triggered by copper ions, involves mitochondrial respiration and the Lipoic Acid (LA) pathway5. Copper ions interfere with the function of mitochondrial TCA cycle enzymes, particularly dihydrolipoamide dehydrogenase, inhibiting oxidative phosphorylation and resulting in insufficient ATP production and metabolic imbalance6. Additionally, copper ions interact with Fe-S cluster-containing enzymes, leading to the loss of their function, which further exacerbates metabolic disruption and ultimately triggers cell death6. Cuproptosis promotes tumor progression in cancer cells by affecting energy metabolism, increasing oxidative stress, altering the tumor microenvironment, and potentially contributing to immune evasion and drug resistance7–9. Therefore, drugs that regulate copper ion concentrations could not only directly inhibit tumor cell proliferation through inducing cuproptosis, but also suppress tumor growth by improving the redox state of the tumor microenvironment, thus offering a potential strategy for cancer therapy7,10. In particular, modulating the cuproptosis pathway in LUAD therapy may represent a novel approach to overcoming drug resistance and reducing tumor progression.
Disulfidptosis is another novel form of cell death characterized by accumulating excessive disulfide bonds under glucose deprivation, leading to cell death11. During this process, the intracellular redox balance is disrupted, depleting antioxidants such as glutathione, leading to abnormal disulfide bond formation, interfering with protein folding and function, and ultimately causing cellular dysfunction and death12. Studies have shown that disulfidptosis is closely related to the high oxidative stress status in the tumor microenvironment, but may also play a crucial role in tumor progression by regulating the metabolic adaptation of tumor cells13. Targeted regulation of key molecules in disulfidptosis or improving the cellular redox state may provide new strategies for cancer therapy and offer new directions for exploring the mechanisms of tumor cell death12. Therefore, targeted regulation of key molecules in disulfidptosis or improving the cellular redox state may provide a potential new strategy for the treatment of malignant tumors such as LUAD.
The tumor microenvironment (TME) is a complex system composed of tumor cells, stromal cells, immune cells, and other cellular components14. The TME plays a crucial role in tumor initiation and progression by altering intercellular interactions, secreting cytokines, and regulating immune responses, thereby promoting tumor growth, metastasis, immune escape, and drug resistance15. Immune cells within the TME influence immune surveillance and therapeutic responses in LUAD by altering the local immune environment, secreting immunosuppressive factors, and inhibiting the activity of effector T cells16. Recent studies have shown that the characteristics of immune cells in the TME and their interactions with tumor cells can serve as important indicators for predicting therapeutic outcomes in LUAD17. Targeting the composition and function of immune cells in the TME, particularly by modulating their interactions with tumor cells, may provide new strategies for immunotherapy in LUAD and help overcome drug resistance in conventional treatments16.
To further explore the mechanistic applications of cuproptosis and disulfidptosis in LUAD, this study collected 34 genes related to these processes (DCRGs) and identified differentially expressed genes using transcriptomic data from TCGA and GEO databases. Based on this, we identified two DCRG molecular subtypes and selected key prognostic genes to construct and validate a prognostic model. Additionally, we performed analyses from the perspectives of tumor mutations, immune microenvironment, and drug sensitivity, and experimentally validated the expression levels of key genes. Mendelian randomization analysis further confirmed the causal relationship of core genes, providing new insights for the early diagnosis and targeted therapy of LUAD.
Methods
Data collection
As depicted in the flowchart (Fig. 1), we conducted this study by obtaining comprehensive multi-omics data from multiple databases. Specifically, RNA sequencing data, clinical information, and tumor mutation burden (TMB) data were obtained from the TCGA database, comprising 541 LUAD samples and 59 normal lung tissue samples (https://portal.gdc.cancer.gov). In addition, we retrieved the GSE31210 dataset from the GEO database (https://www.ncbi.nlm.nih.gov/geo/), which includes 226 LUAD samples, providing clinical parameters such as age, pathological stage, survival time, and survival status. To integrate and align the data from TCGA and GEO, we processed the data using Perl software18. Additionally, we retrieved copy number variation (CNV) data for relevant genes from the UCSC Xena database (xenabrowser.net). Through a literature review, we collected 34 DCRGs19–21.
Fig. 1.
The Flow Chart Summarizes the Scheme Performed to Construct Prognostic Gene Signatures of Lung Adenocarcinoma (LUAD).
Expression differences and prognostic value analysis of DCRGs in LUAD
We used the processed data to generate box plots to reveal the differential expression levels of DCRGs between tumor and normal samples. Additionally, Cytoscape software was employed to construct an interaction network of DCRGs, depicting the interactions among genes. Using the “maftools” package in R, we analyzed the mutation frequency of DCRGs and visualized the data with waterfall plots22. The “barplot” and “RCircos” packages were utilized to visualize CNV alterations and their chromosomal distribution23. Furthermore, we integrated tumor samples from the TCGA and GSE31210 datasets, applying univariate Cox regression analysis and Kaplan-Meier survival curves to identify key genes significantly associated with tumor prognosis24. Finally, we visualized these prognosis-related DCRGs using the “igraph” package’s visualization functions25.
Identification of molecular subtypes in LUAD based on consensus clustering
Based on the mRNA expression levels of prognosis-related DCRGs, we applied unsupervised consensus clustering to LUAD samples using the “ConsensusClusterPlus” package to precisely stratify molecular subtypes, identify potential disease subtypes, and offer a new perspective on LUAD classification26. Subsequently, we validated the clustering results through principal component analysis (PCA) and survival analysis and visualized the findings using the “pheatmap” package in conjunction with clinical data27,28. Additionally, we explored functional and pathway enrichment differences among subtypes using gene set variation analysis (GSVA) and single-sample gene set enrichment analysis (ssGSEA), further analyzing differences in immune cell infiltration to reveal the biological heterogeneity of the disease and its impact on patient prognosis.
Screening and functional analysis of differentially expressed genes in DCRG subtypes
By setting a log2 fold change (log2FC) threshold greater than 0.585 and an adjusted p-value less than 0.05, we successfully identified differentially expressed genes (DEGs) within the DCRG subtypes. We performed Gene Ontology (GO) functional enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis on these DEGs to explore the biological differences among molecular subtypes29. Using univariate Cox regression analysis, we identified DEGs significantly associated with LUAD prognosis and performed unsupervised consensus clustering based on these genes. Kaplan-Meier survival curves and heatmaps visually demonstrated the correlation between different gene subtypes and LUAD patient prognosis and clinical characteristics. Boxplots clearly revealed the expression patterns of DCRGs across different gene subtypes.
Construction and multidimensional validation of a prognostic risk assessment model for LUAD patients
Based on data integrated from the TCGA and GEO databases, samples were randomly divided into training and validation sets. In the training set, we employed least absolute shrinkage and selection operator (LASSO) regression analysis to identify DEGs significantly associated with patient prognosis3 and used multivariable Cox regression and proportional hazards models to estimate overall survival (OS). Samples were then stratified into high-risk and low-risk groups based on risk scores. Kaplan-Meier survival curves and receiver operating characteristic (ROC) curves were used to evaluate the statistical validity and predictive accuracy of the model. To visually integrate multidimensional data, alluvial plots were used to depict DEGs subtypes, DCRG subtypes, risk scores, and survival status. We further analyzed the relationship between key genes and patient clinical characteristics. DEGs between the high-risk and low-risk groups were displayed using heatmaps, while the constructed nomogram model provided scores for clinically significant characteristics. The predictive accuracy of the model was validated by comparing the area under the ROC curve (AUC) at different time points and using calibration curves to assess the consistency between predicted and actual survival rates.
Biological characteristics analysis of LUAD
We used CIBERSORT to analyze immune cell infiltration in LUAD patients30, assessing the association between immune cell types, risk scores, and key prognostic genes. Using the “estimate” R package, we scored the immune and stromal components of the tumor microenvironment and further analyzed the relationship between tumor stem cell index and risk scores31. Additionally, we retrieved immunotherapy biomarker data from the TCIA database32, calculated and visualized immunotherapy scores for different risk groups, and utilized the TIDE score from the TIDE database to evaluate the roles of immune dysfunction and immune exclusion in LUAD33, examining their association with prognosis. To further investigate the single-cell expression characteristics of key DCRGs, we obtained the GSE146100 LUAD single-cell dataset from the Tumor Immune Single-Cell Hub (TISCH; http://tisch.comp-genomics.org)34, performing detailed analyses and visualizations. We also quantified TMB to assess the genetic complexity of LUAD and explored the correlation between TMB, risk scores, and immune cell infiltration. In drug sensitivity analysis, we used the GDSC2 database to identify drugs with significant differences between the risk groups35. Finally, we retrieved the protein structural sequences of key prognostic genes from the Uniprot database36 and drug structural information from PubChem37 and conducted molecular docking analysis using CB-DOCK2 to reveal the interactions between key prognostic genes and drugs38.
Validation using external databases and cell line models
Using the Kaplan-Meier Plotter database and the Human Protein Atlas (HPA) database, we obtained survival curves and immunohistochemistry (IHC) results for key DCRGs in LUAD prognosis39,40. The A549 and H1650 human LUAD cell lines were used as LUAD models, with the BEAS-2B normal bronchial epithelial cell line as the control; all cell lines were provided by Shanghai Fuheng Biotechnology Co., Ltd., and routinely screened for contamination. A549 cells were cultured in F-12 K medium with 10% fetal bovine serum, H1650 cells in 1640 medium, and BEAS-2B cells in DMEM medium, incubated at 37 °C with 95% humidity. Total RNA was extracted using TRIzol reagent, reverse transcribed into cDNA with the PrimeScript RT reagent kit (TaKaRa), and gene expression quantified using SYBR Premix Ex Taq II (TaKaRa) by the 2 − ΔΔCT method. Primer sequences are listed in Supplementary Table 1.
Causal effect of FURIN protein on LUAD
In this study, we applied the Two-Sample Mendelian Randomization (MR) method41 to investigate the causal relationship between FURIN protein and LUAD. We used single nucleotide polymorphisms (SNPs) as instrumental variables (IVs), extracting FURIN protein-related exposure data (ID: prot-a-1150) and LUAD outcome data (ID: ieu-a-965) from the GWAS database42. Data analysis was conducted using the TwoSampleMR package.
Results
Transcriptional and genetic characteristics of DCRGs in LUAD
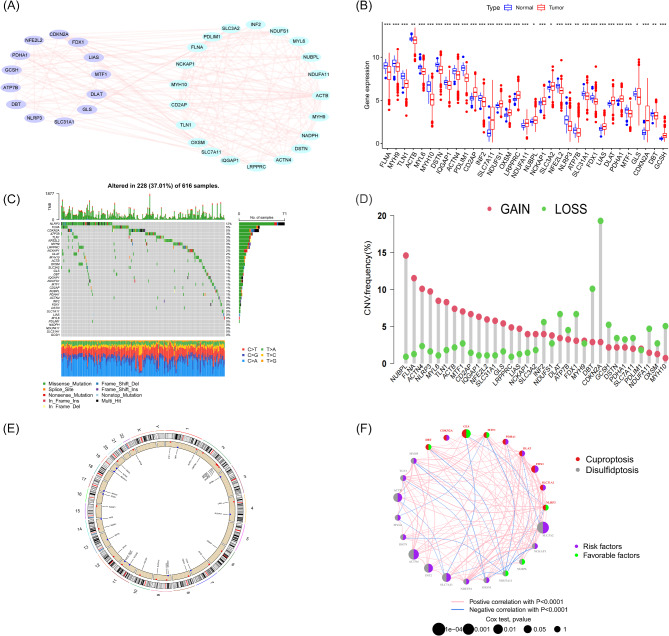
In this study, we focused on 34 DCRGs, exploring their interactions using the String online platform and using Cytoscape software to generate the gene co-expression network (Fig. 2A). By analyzing data from 541 LUAD patients and 59 normal lung tissue samples in the TCGA database, we observed significant differential expression of the 34 DCRGs in LUAD (Fig. 2B), with 19 genes showing decreased expression in tumor tissues and 15 genes showing increased expression. During the mutation analysis of 600 samples (Fig. 2C), we found that approximately 37% of the samples harbored DCRG mutations, with NLRP3 having the highest mutation rate (12%), while 11 DCRGs showed no mutations. In the CNV analysis (Fig. 2D), CNVs were observed in all DCRGs except NADPH in LUAD samples, with CDKN2A having the highest frequency of variations, and its corresponding chromosomal location was clearly identified (Fig. 2E). To further investigate the impact of DCRG expression levels on LUAD patient prognosis, we integrated data from the TCGA and GEO databases, analyzed clinical data from 762 LUAD samples, and identified 23 prognostically relevant DCRGs through univariate COX regression analysis and Kaplan-Meier survival curves (P < 0.05) (Figure Supplementary 1–2). The prognosis network clearly demonstrated the correlation between DCRGs protein expression levels and prognostic factors (Fig. 2F).
Fig. 2.
Overview of Genomic and Transcriptomic Analysis of DCRGs in LUAD. (A) Protein-protein interaction network of DCRGs generated based on the STRING database. (B) Boxplot comparing the expression levels of 34 DCRGs between normal individuals and LUAD patients. (C) Mutation status of 34 DCRGs in LUAD patients, including mutation types and their distribution across samples. (D) CNV frequencies of 33 DCRGs in LUAD patients, where red dots indicate gene amplifications and green dots indicate gene deletions. (E) Locations of CNV changes across the 23 chromosomes in LUAD patients. (F) Interaction network of the 23 prognostic-related DCRGs, displaying the risk and favorable genes, as well as the correlation strength and statistical significance between them.
DCRG Subtype classification results in LUAD
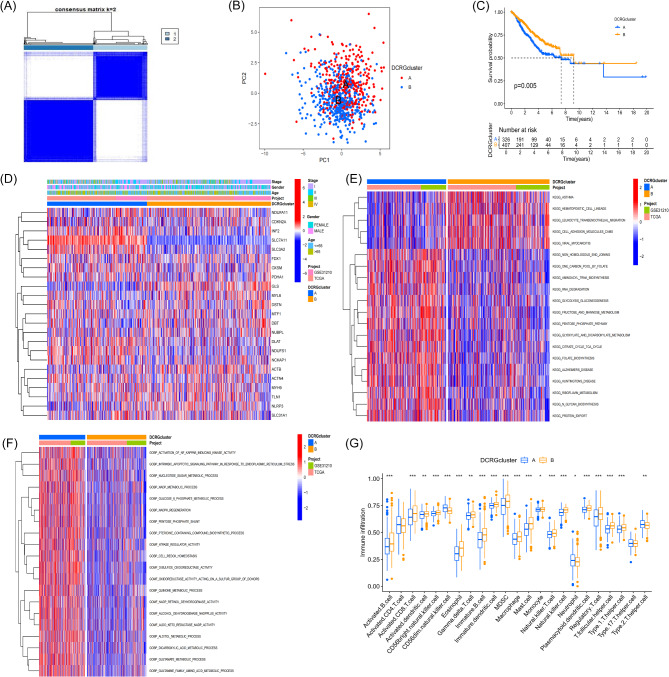
To reveal the potential differences between LUAD molecular subtypes, we performed unsupervised consensus clustering analysis on LUAD samples, dividing them into Group A (333 cases) and Group B (429 cases) (Fig. 3A). When the clustering number K was set to 2, we observed that the clustering results showed the most significant intra-group differences and the smallest inter-group differences (Figure Supplementary 3). PCA revealed a clear separation between the two molecular subtypes (Fig. 3B). Kaplan-Meier survival analysis showed significant differences in prognosis and survival between the two subtypes (P < 0.05), with patients in Group B having a markedly better prognosis than those in Group A (Fig. 3C). Heatmap visualization of clinical characteristics and DCRG expression levels revealed significant differences in gene expression in Group A, further highlighting the heterogeneity of gene expression between subtypes (Fig. 3D).
Fig. 3.
Results, Validation, and Enrichment Analysis of DCRG-Related Subtypes. (A) Clustering analysis using a consensus matrix (k = 2), clearly classifying DCRGs into two distinct molecular subtypes. (B) PCA showing the spatial distribution of the two molecular subtypes (A and B) derived from DCRGs. (C) Kaplan-Meier analysis illustrating the differences in survival probability between DCRG molecular subtypes A and B in LUAD patients. (D) Heatmap displaying the differences in gene expression levels under the two DCRG molecular subtypes (A and B) and their associations with various clinical characteristics, such as disease stage, gender, age, and data source project. (E-F): Heatmaps visualizing the GSVA results between the two DCRG molecular subtypes. (G) Box plot illustrating the differences in the levels of immune cell infiltration between the two DCRG molecular subtypes (A and B).
Functional enrichment analysis and immune microenvironment characteristics of DCRG subtypes
To elucidate the intrinsic heterogeneity of LUAD in greater depth, we conducted GSVA on the two DCRG subtypes. Pathway enrichment analysis indicated that subtype A was primarily associated with energy metabolism, DNA repair mechanisms, protein biosynthesis, and cellular signaling pathways. In contrast, subtype B was prominently implicated in pathophysiological and immune response-related pathways (Fig. 3E). Functional enrichment analysis revealed that subtype A LUAD samples were predominantly associated with redox reactions, metabolic pathways, and cellular metabolic processes (Fig. 3F). Utilizing ssGSEA, we observed profound discrepancies in immune cell infiltration types between the two DCRG subtypes. Specifically, CD56dim natural killer cells, neutrophils, and Th2 cells were more pervasive in subtype A. In contrast, activated B cells, activated CD8 T cells, activated dendritic cells, CD56bright natural killer cells, eosinophils, and immature B cells were more prevalently observed in subtype B (Fig. 3G). Notably, activated CD4 T cells and Th17 cells did not exhibit statistically significant differences between the two subtypes.
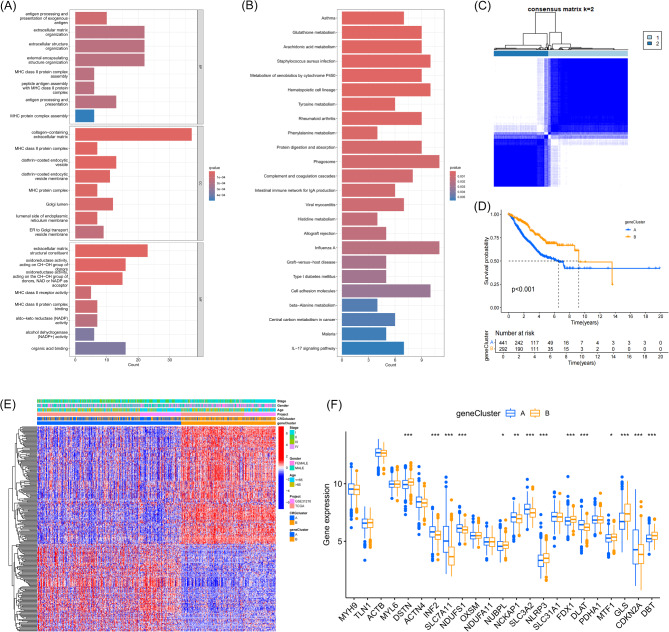
To further investigate potential biomarkers related to disulfidptosis and cuproptosis, we identified shared DEGs between the two subtypes. GO functional enrichment analysis indicated that these DEGs were implicated in the organization and structure of the extracellular matrix (Fig. 4A). KEGG pathway enrichment analysis further revealed that these genes were involved in fundamental metabolic pathways, pathophysiological mechanisms, and biochemical cascades (Fig. 4B). These findings are consistent with the results of GSVA and further underscore the pivotal role of these DEGs in the molecular heterogeneity of LUAD.
Fig. 4.
Enrichment Analysis of DEGs Between DCRG Molecular Subtypes, and Biological Functions, Prognostic Impact, and Gene Expression Differences Revealed by Unsupervised Consensus Clustering. (A) GO enrichment analysis bar plot showing enrichment in biological processes, cellular components, and molecular functions. (B) KEGG enrichment analysis bar plot showing enrichment in metabolic pathways, cellular signaling, and disease-related pathways. (C) Unsupervised consensus clustering analysis divided DEGs between the two DCRG molecular subtypes into two gene subtypes. (D) Kaplan-Meier survival curves show the significant survival differences between the two gene subtypes in LUAD patients. (E) Heatmap displaying the expression levels of DEGs between the two gene subtypes and their correlation with clinical characteristics (such as age, gender, pathological stage). (F) Box plot showing the expression levels of DCRGs in the two gene subtypes (A and B).
Identification of Gene subtypes using DEGs
Through univariate COX regression analysis, we identified 405 DEGs associated with the prognosis of LUAD patients. We then applied unsupervised consensus clustering to classify these genes, and the results showed that when the clustering number k = 2 (Fig. 4C) (Figure Supplementary 4), the optimal clustering effect for gene subtyping was achieved. Kaplan-Meier survival curve analysis revealed significant prognostic differences between the two gene subtypes, with subtype B showing a significantly better prognosis than subtype A (Fig. 4D). Heatmap analysis further revealed that patients classified under gene subtype A were predominantly composed of patients from DCRG subtype A, confirming the association and reliability between the two subtyping methods (Fig. 4E). Additionally, box plots demonstrated significant expression differences in most disulfidptosis and cuproptosis-related genes between the two gene subtypes (Fig. 4F).
Development and validation of a prognostic DCRG scoring system
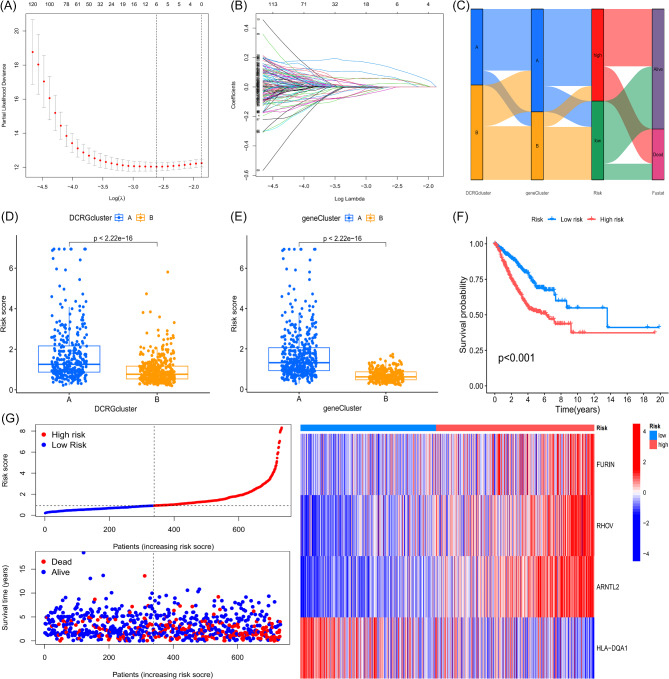
To develop and validate a prognostic scoring model based on DCRGs for LUAD patient survival, we initially employed R to randomly partition the dataset into a training cohort (n = 367) and a test cohort (n = 366) at a 1:1 ratio. Subsequently, we conducted multivariate COX regression and LASSO regression on the 405 prognostic DEGs (Fig. 5A-B), pinpointing four pivotal prognostic genes (FURIN, RHOV, ARNTL2, HLA-DQA1) through selection based on the optimal λ value and minimal partial likelihood deviance. Based on these genes, we developed the following DCRG scoring formula: Risk Score = (0.1342 × Expression of FURIN) + (0.1371 × Expression of RHOV) + (0.3308 × Expression of ARNTL2) − (0.1412 × Expression of HLA-DQA1).
Fig. 5.
Risk Scoring Model for DCRGs, Including Predicted Risk Scores, Survival Analysis, and the Relationship Between Gene Expression and Risk Levels. (A) LASSO regression coefficients. (B) Lambda path plot. (C) Sankey diagram showing the flow relationships from DCRG subtypes to intersecting DEG subtypes, risk scores, and final survival outcomes. (D) Differences in DCRG scores between the two DCRG subtypes (A and B). (E) Differences in DCRG scores between the two intersecting DEG subtypes (A and B). (F) Kaplan-Meier survival curves comparing the survival probabilities of high-risk and low-risk patient groups. (G) Scatter plot and heatmap showing the relationship between risk scores and survival time.
A Sankey diagram was employed to illustrate the intricate interconnections between DCRG subtypes, DEG subtypes, the prognostic model, and survival status in LUAD (Fig. 5C). Leveraging the median risk score, we dichotomized both the training and test cohorts into high-risk and low-risk categories, further evaluating the model’s prognostic accuracy. Scatter plots revealed that subtype A consistently exhibited higher risk scores than subtype B in both DCRG and DEG classifications (Fig. 5D-E). Kaplan-Meier survival curves demonstrated that high-risk group patients faced significantly worse prognoses (Fig. 5F). Risk plots elucidated that as risk scores escalated, mortality and incidence rates surged in parallel. The heatmap illustrated prognostic gene expression across all patient samples, with HLA-DQA1 demonstrating a pronounced negative correlation in the high-risk cohort (Fig. 5G). This trend aligned with the prognostic gene expression patterns observed in both the training and validation sets (Figure Supplementary 5).
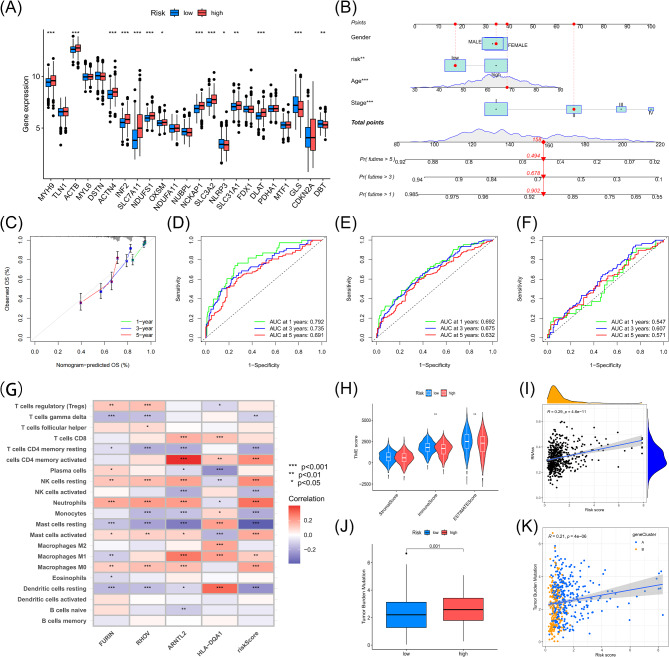
Boxplots underscored that most DCRGs displayed marked expression differences between the two risk groups (Fig. 6A). To further substantiate the predictive efficacy and reliability of the prognostic model, we constructed a nomogram and calibration plots, calculating patients’ total scores based on key prognostic factors such as gender, risk stratification, age, and disease stage (Fig. 6B). The calibration plot closely mirrored the ideal predictive model (Fig. 6C). ROC analysis indicated that the AUC values for 1-, 3-, and 5-year survival predictions in the training set were 0.792, 0.735, and 0.691, respectively, reflecting the robust predictive capacity of the model (Fig. 6D). Additionally, the AUC values for all patients (Fig. 6E) and the test set (Fig. 6F) consistently exceeded 0.5, reinforcing the model’s predictive accuracy.
Fig. 6.
Evaluation Metrics and Multidimensional Analysis of the LUAD Prognostic Model. (A) Box plot showing the expression differences of DCRGs between the high-risk and low-risk groups in LUAD patients. (B) Prognostic model nomogram calculating prognosis based on clinical-pathological factors such as gender, risk group, age, and pathological stage. (C) Calibration curve assessing the accuracy of the nomogram’s predicted 1-year, 3-year, and 5-year OS by comparing predicted and observed survival rates. (D-F): ROC curve analysis showing the performance of the DCRG-based prediction model in assessing 1-year, 3-year, and 5-year survival predictions in the training set, all patients, and the test set for LUAD. (G) Correlation between key prognostic genes and 21 types of immune cells. (H) Violin plot showing the TME score analysis between different risk groups. (I) Relationship between DCRG score and CSC. (J) Differences in TMB between the different risk groups. (K) Scatter plot showing the correlation between DCRG scores and TMB.
Analysis of immunity, Gene Mutations, and drug response driven by DCRG Scoring
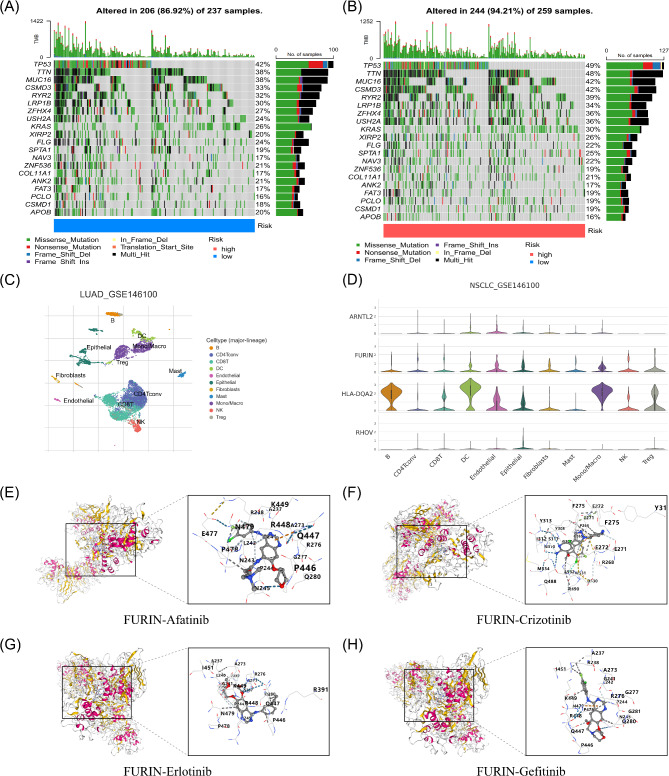
CIBERSORT algorithm analysis revealed that the DCRG score negatively correlated with γδ T cells, resting mast cells, activated NK cells, monocytes, resting memory CD4 + T cells, and resting dendritic cells, while positively correlating with activated memory CD4 + T cells, M1 macrophages, neutrophils, resting NK cells, M0 macrophages, and activated mast cells (Figure Supplementary 6). A heatmap demonstrated a positive correlation between RHOV and activated memory CD4 + T cells and a negative correlation with resting mast cells (Fig. 6G). A violin plot indicated significant differences in tumor microenvironment scores between high- and low-risk LUAD patients, particularly in ImmuneScore and ESTIMATEScore (Fig. 6H). A scatter plot revealed a positive correlation between risk score and stemness index (Fig. 6I). TCIA and TIDE database analyses (Figure Supplementary 7A-D) indicated that the low-risk group had significantly higher CTLA4 and PD1 immune response scores compared to the high-risk group, suggesting a stronger response to immune checkpoint inhibitors in low-risk patients. In contrast, the high-risk group potentially exhibited a more active immune evasion mechanism (Figure Supplementary 7E). Box plots indicated higher TMB in the high-risk group (Fig. 6J), with a positive correlation between risk score and TMB (Fig. 6K). Mutation analysis demonstrated a higher mutation rate in the high-risk group (94.21%), with TP53 mutations exhibiting specific patterns, including nonsense mutations (Fig. 7A-B).
Fig. 7.
Tumor Mutation Frequency in Different Risk Groups and Results of Key Prognostic Gene Single-Cell Analysis and Molecular Docking. (A) Waterfall plot of tumor mutation frequencies in the low-risk group. (B) Waterfall plot of tumor mutation frequencies in the high-risk group. (C) Single-cell clustering map from the LUAD_GSE146100 dataset. (D) Expression of the four key prognostic DCRGs in different cell types in LUAD. (E) Molecular docking result of FURIN with Afatinib. (F) Molecular docking result of FURIN with Crizotinib. (G) Molecular docking result of FURIN with Erlotinib. (H) Molecular docking result of FURIN with Gefitinib.
Single-cell analysis from the TISCH GSE146100 dataset classified cells into 18 clusters, annotated into 11 cell types (Fig. 7C). ARNTL2 and RHOV exhibited low expression across most cell types. In contrast, FURIN showed higher expression in CD4 T regulatory cells, CD8 T cells, epithelial cells, and dendritic cells, and HLA-DQA2 was highly expressed in B cells and monocytes/macrophages, indicating its role in antigen presentation and immune response (Figure Supplementary 8, Fig. 7D). Drug sensitivity analysis (Figure Supplementary 9) revealed that the low-risk group showed higher sensitivity to Afatinib, Erlotinib, Gefitinib, and Crizotinib compared to the high-risk group. Based on the four prognostic genes (ARNTL2, FURIN, HLA-DQA1, and RHOV) identified in the prognostic model, we further conducted molecular docking studies to analyze the interaction between proteins encoded by these genes and the four targeted drugs (Afatinib, Crizotinib, Erlotinib, Gefitinib) (Figure Supplementary 10–12, Fig. 7E-H). The results demonstrated significant differences in binding energies among the proteins and drugs. Specifically (Table Supplementary 2), FURIN exhibited the lowest binding energy across all four drugs, particularly with Crizotinib (-9.6 kJ/mol), suggesting higher binding affinity. In contrast, ARNTL2 showed relatively higher binding energies, ranging from − 6.6 to -7.6 kJ/mol across the drugs. Additionally, HLA-DQA1 and RHOV displayed similar binding energies, ranging from − 6.6 to -7.9 kJ/mol. These findings suggest that ARNTL2, FURIN, HLA-DQA1, and RHOV may offer varying potentials for targeted therapy in LUAD, with FURIN’s lowest binding energy with Crizotinib indicating its potential as a more valuable therapeutic target.
Expression of key genes in single-cell datasets, external datasets, and RT-qPCR validation
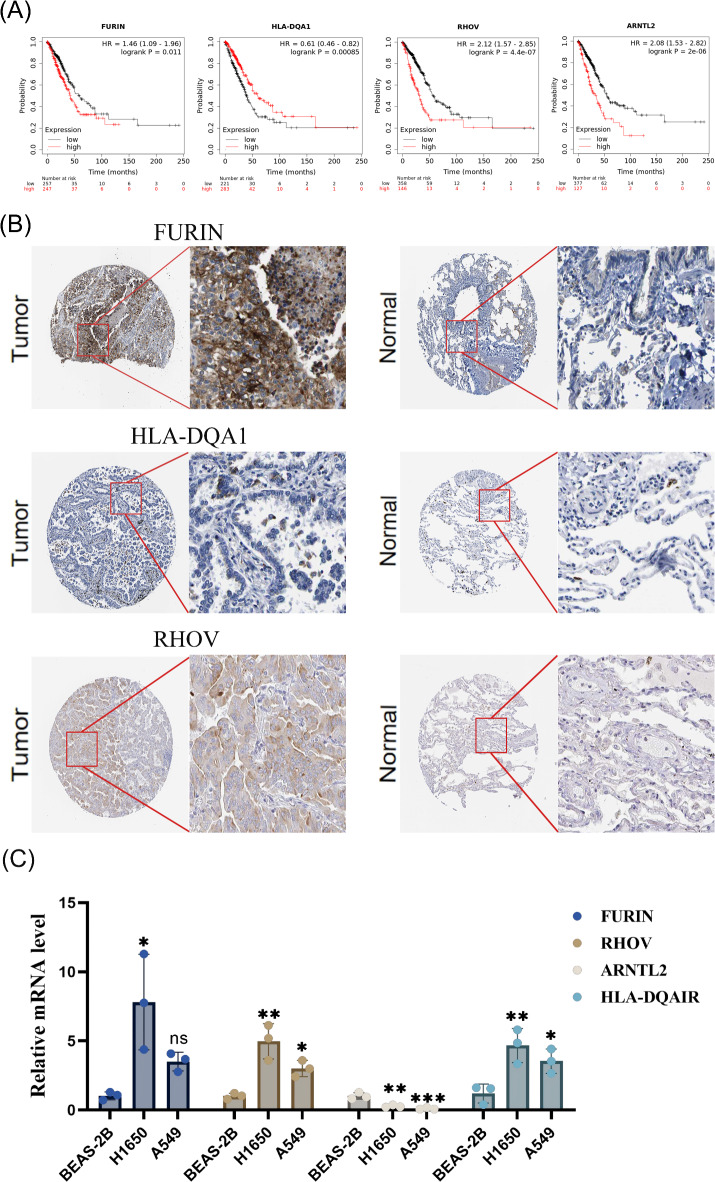
Using the Kaplan-Meier Plotter database (accessed on March 23, 2024), we validated the prognostic significance of four key DCRGs (Fig. 8A). ARNTL2 (HR = 2.08 [1.53–2.82], p < 0.001), FURIN (HR = 1.46 [1.09–1.96], p = 0.011), and RHOV (HR = 2.12 [1.57–2.85], p < 0.001) were associated with poor prognosis. In contrast, HLA-DQA1 served as a protective prognostic factor (HR = 0.61 [0.46–0.82], p = 0.00085). We also retrieved immunohistochemistry data for several key prognostic genes from the Human Protein Atlas (HPA) database, examining their expression in both normal and tumor tissues. In lung adenocarcinoma (LUAD) samples, FURIN and RHOV proteins were highly stained and widely upregulated, whereas HLA-DQA1 protein expression was markedly downregulated. Unfortunately, ARNTL2 expression data was not available in the HPA database (Fig. 8B).
Fig. 8.
External Database and In Vitro Cell Line Validation. (A) Correlation between the expression levels of four key prognostic genes in LUAD and patient survival. (B) Protein expression of three key prognostic genes in tumor tissues and corresponding normal lung tissues. (C) Comparative mRNA Expression Profiles of Key DCRGs Across Distinct Cell Lines.
To further validate the expression levels of key prognostic DCRGs in LUAD, we used A549 and H1650 cell lines as lung adenocarcinoma models, with the BEAS-2B cell line serving as a normal control. RT-qPCR results indicated a significant upregulation of FURIN and RHOV in A549 and H1650 cells (p < 0.05), while ARNTL2 expression showed no significant differences across the three cell lines (p > 0.05) (Fig. 8C). Notably, HLA-DQA1 was significantly upregulated in H1650 cells but showed no significant change in A549 cells, which contradicts the expectation that this protective prognostic gene should exhibit lower expression in cancer cells.
Causal effect of FURIN on LUAD
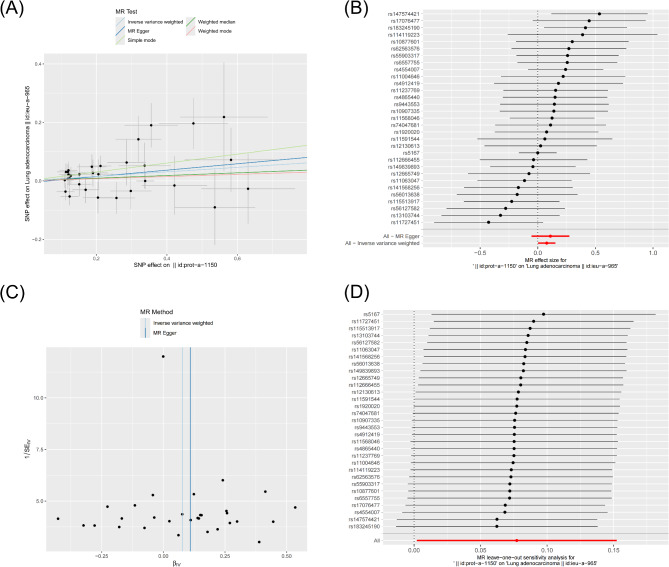
Based on the results from the GWAS database, we only identified information related to the FURIN protein, prompting further exploration of the causal relationship between FURIN and lung adenocarcinoma. First, we selected SNPs with a genome-wide statistical significance threshold of less than 5 × 10^−8 as instrumental variables (IVs). However, only a few SNPs showed significant associations with LUAD at this threshold. To obtain more comprehensive data, we applied a second threshold (less than genome-wide significance level, 1 × 10^−5) to identify additional SNPs. We selected these as the second set of IVs to investigate more potential causal links. Five Mendelian randomization (MR) methods were employed for the analysis: MR Egger, weighted median, inverse variance weighted (IVW), simple mode, and weighted mode. Overall effect estimates indicated a significant causal relationship between FURIN and LUAD (Fig. 9A) (Table Supplementary 3). A forest plot further confirmed this finding, showing consistent effect directions for most SNPs with overlapping confidence intervals, suggesting the consistency and reliability of the overall effect (Fig. 9B) (Table Supplementary 4). The funnel plot revealed no substantial publication bias (Fig. 9C), and leave-one-out sensitivity analysis demonstrated the robustness of the results (Fig. 9D). Heterogeneity tests indicated that neither the MR Egger method nor the IVW method showed significant heterogeneity (P-values of 0.3823 and 0.4230, respectively), suggesting no notable heterogeneity among the SNP effects (Table Supplementary 5). Pleiotropy testing revealed a P-value of 0.6668 for the MR Egger intercept, indicating no significant pleiotropic interference (Table Supplementary 6). These findings demonstrate the robustness and reliability of the causal relationship between FURIN protein and LUAD.
Fig. 9.
Mendelian Randomization Analysis of FURIN Protein and LUAD. (A) MR analysis results between FURIN and LUAD. (B) Forest plot showing the effect estimates of each SNP on LUAD in the MR analysis. (C) Effect estimates analysis using MR-Egger and inverse variance weighted methods. (D) Leave-one-out sensitivity analysis.
Discussion
LUAD is the most common subtype of lung cancer, with its incidence closely associated with genetic susceptibility, tobacco smoke, and air pollution exposure43. Surgical treatment has shown favorable outcomes for early-stage LUAD. However, the prognosis for patients with advanced-stage disease remains poor44. Chemotherapy and radiotherapy are the conventional first-line treatments for LUAD, but in recent years, significant advancements have been made in targeted therapy and immunotherapy45. Despite advances in treatment, drug resistance and limited therapeutic options remain major challenges, necessitating the exploration of novel therapeutic targets and strategies. Programmed cell death mechanisms have become a focal point in cancer research, with previous studies primarily focusing on the impact of individual cell death pathways on tumor prognosis or pathogenesis. In this context, our study integrates the exploration of how disulfidptosis-related and cuproptosis-related genes jointly influence the prognosis of LUAD. This study aims to fill the gap in current research by revealing the synergistic role of these genes in LUAD progression and their potential impact on patient prognosis, providing new molecular targets and therapeutic strategies for cancer treatment.
In this study, we identified 34 DCRGs with significantly differential expression between LUAD patients and healthy individuals, confirming their close interconnections using the STRING database. A multi-dimensional analysis of gene expression, TMB, and CNV was conducted to explore the genomic variations and their potential functional impacts in LUAD. While some DCRGs exhibited expression changes in tumor tissues, their CNV status remained mostly stable, suggesting that the changes in mRNA expression are primarily driven by factors other than CNV.
The univariate Cox analysis identified 23 DCRGs significantly associated with prognosis, which were used to classify LUAD into two subtypes, A and B, based on differential gene expression. Survival curve analysis indicated that subtype B exhibited a better prognosis compared to subtype A. GSVA analysis revealed that subtype A was enriched in pathways associated with endoplasmic reticulum stress and oxidative stress-induced programmed cell death, which may contribute to its poor prognosis. In contrast, ssGSEA analysis of subtype B showed significant immune cell infiltration, suggesting that immune responses may help suppress tumor progression, leading to a more favorable prognosis. PCA analysis further confirmed the molecular differences between subtypes A and B.
We explored the transcriptomic differences and biological functions between LUAD subtypes, identifying two gene subtypes with distinct transcriptional profiles through the intersection of DEGs and secondary clustering of DCRG subtypes. Based on this, we constructed a LUAD prognostic scoring model incorporating four key prognostic genes: FURIN, RHOV, ARNTL2, and HLA-DQA1, validating their reliability as independent prognostic factors. A nomogram was used to assess the relationship between clinical features, risk scores, and survival outcomes. ROC curve analysis indicated that the DCRG score effectively predicted the 1-year, 3-year, and 5-year survival outcomes of LUAD patients, with AUC values above 0.6 for most time points, although the 1-year and 5-year AUC values were below 0.6. Nevertheless, the practical utility of this scoring model in clinical monitoring and treatment decision-making requires further validation.
Immune infiltration analysis revealed that the key prognostic genes are closely associated with most immune cells. The low-risk group exhibited higher ImmuneScore and ESTIMATEScore, suggesting a greater content of immune cells and stroma in the tumor microenvironment. This may enhance immune responses, suppress tumor progression, and improve treatment outcomes, ultimately correlating with a better prognosis. TMB analysis indicated that high TMB values may enhance tumor heterogeneity, aiding immune system recognition of the tumor, but do not directly determine the extent of immune cell infiltration. Comparison of mutation frequencies between high- and low-risk groups showed that TP53 (49%) and TTN (48%) mutations were the most common, while the largest difference between the two groups was observed for USHA2 (12%). Previous studies have indicated that TP53 mutations are a negative prognostic factor in LUAD46. Tumor stem cells, with their robust self-renewal and pluripotent differentiation abilities, represent the most aggressive and treatment-resistant cell population within tumors47. An increase in risk score was associated with a higher stem cell index, indicating its correlation with tumor invasiveness and treatment resistance.
FURIN, a key member of the proprotein convertase (PC) family, primarily facilitates the cleavage of various biological precursor proteins and is widely regarded as a “master switch” regulating tumor growth and progression48. Recent studies have confirmed that FURIN overexpression in numerous LUAD samples is closely associated with increased tumor invasiveness and poorer patient prognosis49, a finding supported by our research. In this research, RT-qPCR results revealed that, compared to the normal control BEAS-2B cells, FURIN was significantly upregulated in the H1650 cell line (p < 0.05). In contrast, its expression showed no significant difference in the A549 cell line (ns). This discrepancy may be related to the specific genetic mutation backgrounds of the cell lines: H1650 cells harbor an EGFR mutation, while A549 cells possess a KRAS mutation, potentially leading to differences in FURIN regulation via distinct signaling pathways. Additionally, H1650 cells may rely more on FURIN-mediated pathways to enhance proliferation and invasiveness, whereas A549 cells could utilize alternative mechanisms. Consistent with GSVA, FURIN promotes the remodeling of the tumor microenvironment by activating growth factors, receptors, and matrix metalloproteinases, thereby facilitating angiogenesis and immune evasion50–52. FURIN enhances tumor angiogenesis and metastasis by cleaving precursor proteins, such as vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMPs)53,54. FURIN cleaves proVEGF-C to generate its mature form (21-kDa VEGF-C), enhancing its binding to VEGFR-2 and VEGFR-3, thereby promoting both angiogenesis and lymphangiogenesis, which accelerates tumor progression55. FURIN cleaves and activates MT1-MMP, which in turn activates MMP-2, facilitating extracellular matrix degradation and enhancing tumor cell migration and invasion56. Furthermore, FURIN promotes immune evasion by upregulating the cytokine TGF-β, which suppresses immune cell activity, thereby further enhancing the tumor cells’ ability to escape immune surveillance57,58. FURIN plays a crucial role in the tumor immune microenvironment, making it an important target for immunotherapy. By modulating the phenotype of tumor-associated macrophages, FURIN promotes the formation of an immunosuppressive environment, thereby weakening the anti-tumor immune response59. Recent studies have shown that combining FURIN inhibitors with immune checkpoint inhibitors and other therapies may offer more effective treatment strategies60. To further explore the potential role of FURIN in LUAD, we conducted MR analysis, utilizing existing GWAS data to investigate the causal relationship between FURIN and LUAD. The analysis provides compelling evidence supporting a causal association between FURIN and the development of LUAD, highlighting its potential as a therapeutic target.
Studies have found that low expression levels of HLA-DQA1 are associated with poor prognostic outcomes, yet in LUAD, it is typically expressed at higher levels, making it a potential protective prognostic marker61–63. Our predictive model further corroborates this finding. As a component of the major histocompatibility complex class II, HLA-DQA1 plays a critical role in presenting antigens to CD4 + T cells64. Additionally, polymorphisms in HLA-DQA1 have been linked to various autoimmune diseases and malignancies65,66. However, during RT-qPCR experiments, we observed a significant increase in HLA-DQA1 expression in the H1650 cell line, while no noticeable difference was detected in the A549 cell line. This finding contradicts our initial hypothesis, suggesting that the expression of HLA-DQA1 in LUAD may be influenced by cell line or subtype-specific factors, or that its role in tumors may be complex and multifaceted. Further research is needed to elucidate the protective mechanisms of HLA-DQA1 in different LUAD subtypes and its potential role in the tumor immune microenvironment.
Previous studies have confirmed that the expression of RHOV significantly inhibits the growth, migration, invasion, and tumor formation of LUAD cells, further establishing RHOV’s core role in LUAD cell growth and dissemination67. RHOV accelerates LUAD cell growth, migration, invasion, and metastasis by activating the JNK pathway, and may play a critical role in tumor metastasis through the regulation of the epithelial-mesenchymal transition mechanism68,69. In our in vitro experiments, we observed a significant increase in RHOV expression in LUAD cell lines A549 (p < 0.05) and H1650 (p < 0.01) compared to the normal BEAS-2B control group, further confirming RHOV’s key role in LUAD cell proliferation and invasion. Moreover, RHOV depletion enhanced LUAD cell resistance to gefitinib67. In our drug sensitivity and molecular docking analysis, RHOV showed binding energies ranging from − 6.6 to -7.9 kJ/mol with various sensitive drugs, indicating its high affinity with these drugs. These findings further corroborate the literature regarding RHOV’s crucial role in modulating LUAD cell sensitivity to the EGFR inhibitor gefitinib.
ARNTL2 (Aryl hydrocarbon receptor nuclear translocator-like 2), a transcription factor from the bHLH-PAS (basic helix-loop-helix-PER-ARNT-SIM) protein family, acts as a biological cofactor for circadian rhythms70. Through its bHLH domain, ARNTL2 forms dimers with other proteins and, via its PAS domain, senses environmental signals to regulate downstream gene expression71. This complex participates in various signaling pathways, especially those related to cell growth regulation and stress response71–73. Recent studies have linked the expression of ARNTL2 to the progression of various cancers. In LUAD, overexpression of ARNTL2 is strongly associated with disease severity and poor patient prognosis, suggesting its crucial role in tumor cell proliferation and survival71,74. Based on predictions, ARNTL2 expression was expected to be elevated in LUAD cell lines. However, RT-qPCR results in this study revealed a decreasing trend in ARNTL2 expression in LUAD cell lines (A549 and H1650) compared to the normal BEAS-2B cell line (p > 0.05). This result contradicts the prediction and may indicate that ARNTL2 expression is influenced by cell type-specific regulatory mechanisms, warranting further investigation into its role and regulatory pathways in LUAD.
In summary, RT-qPCR analysis confirmed that FURIN and RHOV are significantly upregulated in LUAD cell lines, highlighting their potential as therapeutic targets. In contrast, ARNTL2 and HLA-DQA1 expression patterns deviated from initial predictions, suggesting cell type-specific regulatory mechanisms that warrant further investigation.
Despite providing valuable insights into the roles of DCRGs in LUAD, several limitations remain. First, our data primarily originate from TCGA and GEO databases, and although external datasets and cell line experiments offer some validation, further verification in clinical samples is required. Second, the predictive capability of the DCRG scoring model for longer-term survival, particularly 5-year survival predictions, needs improvement. Future studies could integrate more multi-omics data, such as metabolomics and proteomics, to further explore the molecular mechanisms of these genes in LUAD. Additionally, developing drugs targeting key genes like FURIN will be an essential direction for future research, with the potential for improved outcomes in personalized therapy.
Conclusion
This study developed a prognostic model for LUAD based on DCRGs, identifying FURIN as a key prognostic gene. Two-sample Mendelian randomization analysis confirmed, for the first time, a causal relationship between FURIN and LUAD, further underscoring its potential as a therapeutic target. Molecular docking analysis revealed high binding affinity between FURIN and several targeted drugs, such as Crizotinib, suggesting its promising application in LUAD targeted therapy. In our experimental validation, RT-qPCR analysis showed significant upregulation of FURIN and RHOV in LUAD cell lines, supporting their critical roles in LUAD progression. Additionally, we identified ARNTL2, RHOV, and HLA-DQA1 as supplementary prognostic genes, with their expression levels closely associated with responses to EGFR inhibitors, as demonstrated in drug sensitivity analysis. These findings offer valuable insights for optimizing personalized treatment strategies in LUAD. Despite these important discoveries, further clinical validation is essential to establish the broader applicability of the model. Integrating multi-omics data will also be critical to elucidate the molecular mechanisms of these genes in LUAD, potentially enhancing the model’s predictive accuracy and clinical utility.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to express our gratitude to the Shandong Provincial Key Medical and Health Laboratory of Perioperative Precise Anesthesia and Organ Protection Mechanism Research, Rizhao Key Laboratory of Basic Research on Anesthesia and Respiratory Intensive Care for their invaluable support and contributions to our research.
Author contributions
Concept and design of the study, J. You, Q. Yu, and T. Zhao; Acquisition of data (laboratory or clinical), J. You, R. Chen, and J. Li; Data analysis and/or interpretation, J. You, Q. Yu, R. Chen, and Z. Lu; Drafting of the manuscript and/or critical revision, J. You, J. Li, and Q. Yu; Approval of the final version of the manuscript, R. Chen, J. Li, Z. Lu, and T. Zhao. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Young Experts of Taishan Scholars (tsqn202211380), the China Postdoctoral Science Foundation (2023M741864), and Medical and Health Technology Project of Shandong Province (202318001632).
Data availability
The code supporting the findings of this study is available upon request from the corresponding author. The relevant data for this study are publicly accessible in the TCGA (https://portal.gdc.cancer.gov), GEO (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi? acc=GSE31210), STRING (https://cn.string-db.org/), UCSC Xena (https://xenabrowser.net/datapages/), Kaplan-Meier Plotter (https://kmplot.com/analysis/), and The Human Protein Atlas (https://www.proteinatlas.org/) databases. The KEGG pathway data used in this study are available from the KEGG database (https://www.kegg.jp/) and permission for use has been obtained from the Kanehisa laboratory.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
No animals or humans were involved in this study.
Consent for publication
All authors consent the publication of this work.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tao Zhao, Email: zttlwj@126.com.
Zhong Lu, Email: luzhong@sdsmu.edu.cn.
References
- 1.Nadia et al. The effect of advances in lung-Cancer treatment on Population Mortality. N. Engl. J. Med.383, 640–649 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hyuna et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. Cancer J. Clin.71, 209–249 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Robert, T., Tibshirani, R., THE LASSO METHOD FOR & VARIABLE SELECTION IN THE COX MODEL. Stat. Med.16, 385–395 (1997). [DOI] [PubMed] [Google Scholar]
- 4.Xuhui et al. Targeting cell death pathways for cancer therapy: recent developments in necroptosis, pyroptosis, ferroptosis, and cuproptosis research. J. Hematol. Oncol.15, 174 (2022). [DOI] [PMC free article] [PubMed]
- 5.Peter et al. Mitochondrial metabolism promotes adaptation to proteotoxic stress. Nat. Chem. Biol.15, 681–689 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsvetkov, P. et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Sci. (N Y N Y). 375, 1254–1261 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voli, F. et al. Intratumoral copper modulates PD-L1 expression and influences tumor immune evasion. Cancer Res.80, 4129–4144 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Vo, T. T. T. et al. The crosstalk between copper-induced oxidative stress and cuproptosis: a novel potential anticancer paradigm. Cell. Commun. Signal. : CCS. 22, 353 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang, C., Huang, T. & Li, L. Targeting cuproptosis for cancer therapy: mechanistic insights and clinical perspectives. J. Hematol. Oncol.17, 68 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dongdong et al. Novel copper complexes that inhibit the proteasome and trigger apoptosis in Triple-negative breast Cancer cells. ACS Med. Chem. Lett.10, 1328–1335 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiaoguang et al. Actin cytoskeleton vulnerability to disulfide stress mediates disulfidptosis. Nat. Cell Biol.25, 404–414 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiang, H., Shen, B., Zhang, C. & Li, R. Bioactive nanoliposomes for enhanced sonodynamic-triggered disulfidptosis-like cancer cell death via lipid peroxidation. Int. J. Nanomed.19, 8929–8947 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng, P., Zhou, C., Ding, Y. & Duan, S. Disulfidptosis: a new target for metabolic cancer therapy. J. Exp. Clin. Cancer Res. : CR. 42, 103 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aadhya et al. Tumor microenvironment: barrier or opportunity towards effective cancer therapy. J. Biomed. Sci.29, 83 (2022). [DOI] [PMC free article] [PubMed]
- 15.Wentao et al. Functional polarization of tumor-associated macrophages dictated by metabolic reprogramming. J. Experimental Clin. cancer Research: CR42, 245 (2023). [DOI] [PMC free article] [PubMed]
- 16.Genova, C. et al. Therapeutic implications of tumor microenvironment in lung cancer: focus on immune checkpoint blockade. Front. Immunol.12, 799455 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu, J. et al. A single-cell characterised signature integrating heterogeneity and microenvironment of lung adenocarcinoma for prognostic stratification. eBioMedicine102, 105092 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stephen, J. et al. FunctSNP: an R package to link SNPs to functional knowledge and dbAutoMaker: a suite of Perl scripts to build SNP databases. BMC Bioinform.11, 311–311 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hengrui, L. & Tao Tang. Pan-cancer genetic analysis of disulfidptosis-related gene set. Cancer Genet.278–279, 91–103 (2023). [DOI] [PubMed] [Google Scholar]
- 20.Tianbing et al. Disulfidptosis classification of hepatocellular carcinoma reveals correlation with clinical prognosis and immune profile. Int. Immunopharmacol.120, 110368–110368 (2023). [DOI] [PubMed] [Google Scholar]
- 21.Hengrui Liu. Pan-cancer profiles of the cuproptosis gene set. Am. J. Cancer Res.12, 4074-4081 (2022). [PMC free article] [PubMed]
- 22.Anand et al. Maftools: efficient and comprehensive analysis of somatic variants in cancer. Genome Res.28, 1747–1756 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emil, K. et al. ggtranscript: an R package for the visualization and interpretation of transcript isoforms using ggplot2. Bioinformatics (2022). 10.1093/bioinformatics/btac409 [DOI] [PMC free article] [PubMed]
- 24.Frank, E. et al. Prognostic/Clinical Prediction Models: Multivariable Prognostic Models: Issues in Developing Models, Evaluating Assumptions and Adequacy, and Measuring and Reducing Errors. 223–249 (2005). 10.1002/0470023678.ch2b(i)
- 25.Gábor Csárdi, Csárdi, G., Tamás, N. & Nepusz, T. The igraph software package for complex network research. 1695, (2006).
- 26.Matthew, D. et al. ConsensusClusterPlus: a class discovery tool with confidence assessments and item tracking. Bioinformatics26, 1572–1573 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ulla, B. et al. Evaluating Random Forests for Survival Analysis using prediction error curves. J. Stat. Softw.50, 1–23 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reija et al. Tensorial Principal Component Analysis in detecting temporal trajectories of purchase patterns in loyalty Card Data: Cohort Study (Preprint). J. Med. Internet. Res.10.2196/44599 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.KEGG. kyoto encyclopedia of genes and genomes - PMC. https://pmc.ncbi.nlm.nih.gov/articles/PMC148090/ [DOI] [PMC free article] [PubMed]
- 30.Aaron, M. et al. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods. 12, 453–457 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Win Sen et al. Lung cancer stem cells: origin, features, maintenance mechanisms and therapeutic targeting. Biochem. Pharmacol.160, 121–133 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Pornpimol et al. Pan-cancer immunogenomic analyses reveal genotype-immunophenotype relationships and predictors of response to checkpoint blockade. Cell. Rep.18, 248–262 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Jingxin et al. Large-scale public data reuse to model immunotherapy response and resistance. Genome Med.12, 21 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ya et al. TISCH2: expanded datasets and new tools for single-cell transcriptome analyses of the tumor microenvironment. Nucleic Acids Res.10.1093/nar/gkac959 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wanjuan et al. Genomics of Drug Sensitivity in Cancer (GDSC): a resource for therapeutic biomarker discovery in cancer cells. Nucleic Acids Res.41, 955–961 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alex et al. UniProt: the Universal protein knowledgebase in 2023. Nucleic Acids Res.10.1093/nar/gkac1052 (2022). [Google Scholar]
- 37.Kim, S. et al. PubChem 2023 update. Nucleic Acids Res.10.1093/nar/gkac956 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu, Y. et al. CB-Dock2: improved protein-ligand blind docking by integrating cavity detection, docking and homologous template fitting. Nucleic Acids Res.10.1093/nar/gkac394 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.András Lánczky, Lánczky, A., Balázs, G. & Győrffy, B. Web-based Survival Analysis Tool tailored for Medical Research (KMplot): development and implementation. J. Med. Internet. Res.23, 7 (2021). [DOI] [PMC free article] [PubMed]
- 40.Peter Thul, Thul, P., Cecilia, Lindskog & Lindskog, C. The human protein atlas: a spatial map of the human proteome. Protein Sci.27, 233–244 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Widding-Havneraas, T. W. H. T. Henrik Daae Zachrisson, & Henrik Daae Zachrisson. A Gentle introduction to instrumental variables. J. Clin. Epidemiol.10.1016/j.jclinepi.2022.06.022 (2022). [DOI] [PubMed] [Google Scholar]
- 42.Gibran et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife 7, (2018). [DOI] [PMC free article] [PubMed]
- 43.Jonathan, M. et al. LUNG CANCER IN NEVER SMOKERS: CLINICAL EPIDEMIOLOGY AND ENVIRONMENTAL RISK FACTORS. Clin. Cancer Res.15, 5626–5645 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Onkar, V. et al. Survival after Sublobar Resection versus Lobectomy for Clinical Stage IA Lung Cancer: an analysis from the National Cancer Data Base. J. Thorac. Oncol.10, 1625–1633 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paul, E. et al. Targeted therapy and checkpoint immunotherapy combinations for the treatment of Cancer. Trends Immunol.37, 462–476 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Xiao-Dong, J. et al. The prognostic value of TP53 and its correlation with EGFR mutation in advanced non-small cell lung cancer, an analysis based on cBioPortal data base. Lung Cancer. 123, 70–75 (2018). [DOI] [PubMed] [Google Scholar]
- 47.Najafi, M., Farhood, B. & Mortezaee, K. Cancer stem cells (CSCs) in cancer progression and therapy. J. Cell. Physiol.234, 8381–8395 (2019). [DOI] [PubMed] [Google Scholar]
- 48.Daniel, E. et al. Proprotein convertases: Master switches in the regulation of tumor growth and progression. Mol. Carcinog.44, 151–161 (2005). [DOI] [PubMed] [Google Scholar]
- 49.Bolun Zhou, Zhou, B. & Gao, S. Shugeng Gao Pan-cancer Analysis of FURIN as a potential prognostic and immunological biomarker. Front. Mol. Biosci.8, 648402 (2021). [DOI] [PMC free article] [PubMed]
- 50.Alexia et al. Reprogramming immune cells activity by furin-like enzymes as emerging strategy for enhanced immunotherapy in cancer. Br. J. Cancer. 128, 1189–1195 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He, Z. The proprotein convertase furin in cancer: more than an oncogene. (2022). [DOI] [PubMed]
- 52.Chenxing et al. Furin-instructed molecular self-assembly actuates endoplasmic reticulum stress-mediated apoptosis for cancer therapy. Nanoscale12, 12126–12132 (2020). [DOI] [PubMed] [Google Scholar]
- 53.Jaaks, P. & Bernasconi, M. The proprotein convertase furin in tumour progression. Int. J. Cancer. 141, 654–663 (2017). [DOI] [PubMed] [Google Scholar]
- 54.Varshavsky, A. et al. Semaphorin-3B is an angiogenesis inhibitor that is inactivated by furin-like pro-protein convertases. Cancer Res.68, 6922–6931 (2008). [DOI] [PubMed] [Google Scholar]
- 55.Siegfried, G. et al. The secretory proprotein convertases furin, PC5, and PC7 activate VEGF-C to induce tumorigenesis. J. Clin. Invest.111, 1723–1732 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tanaka, N. & Sakamoto, T. MT1-MMP as a key regulator of metastasis. Cells12, 2187 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rose, M., Duhamel, M., Rodet, F. & Salzet, M. The role of proprotein convertases in the regulation of the function of immune cells in the oncoimmune response. Front. Immunol.12, 667850 (2021). [DOI] [PMC free article] [PubMed]
- 58.Braun, E. & Sauter, D. Furin-mediated protein processing in infectious diseases and cancer. Clin. Transl Immunol.8, e1073 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rou et al. Furin-instructed aggregated gold nanoparticles for re-educating tumor associated macrophages and overcoming breast cancer chemoresistance. Biomaterials275, 120891–120891 (2021). [DOI] [PubMed] [Google Scholar]
- 60.Rose, M., Duhamel, M., Rodet, F. & Salzet, M. The role of proprotein convertases in the regulation of the function of immune cells in the oncoimmune response. Front. Immunol.12, 667850 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zitao et al. Molecular subtyping based on immune cell marker genes predicts prognosis and therapeutic response in patients with lung adenocarcinoma. BMC Cancer. 10.1186/s12885-023-11579-7 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fabíola, A. et al. Low expression of HLA-DRA, HLA-DPA1, and HLA-DPB1 is associated with poor prognosis in pediatric adrenocortical tumors (ACT). Pediatr. Blood Cancer. 61, 1940–1948 (2014). [DOI] [PubMed] [Google Scholar]
- 63.Yu et al. Cell trajectory-related genes of Lung Adenocarcinoma Predict Tumor Immune Microenvironment and Prognosis of patients. Front. Oncol.12, 911401 (2022). [DOI] [PMC free article] [PubMed]
- 64.Sergio et al. HLA DRB1*, DQB1*, DPA1*, and DPB1* and their association with the pathogenesis of leukemia in the population of Venezuela HLA DRB1*, DQB1*, DPA1* y DPB1* y su asociación con la patogénesis de las leucemias en población. Rev. Alerg. Mex.10.29262/ram.v63i3.147 (2016). [DOI] [PubMed] [Google Scholar]
- 65.Majid et al. HLA-DRB1,-DQA1 and -DQB1 allele and haplotype frequencies in female patients with early onset breast Cancer. Pathol. Oncol. Res.18, 49–55 (2012). [DOI] [PubMed] [Google Scholar]
- 66.Geno et al. The immunogenetics of viral antigen response is associated with sub-type specific glioma risk and survival. medRxiv (2021). 10.1101/2021.09.13.21263349
- 67.Chen, H. et al. Overexpression of RhoV promotes the progression and EGFR-TKI resistance of lung adenocarcinoma. Front. Oncol.11, 619013 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang, D. et al. RHOV promotes lung adenocarcinoma cell growth and metastasis through JNK/c-jun pathway. Int. J. Biol. Sci.17, 2622–2632 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shepelev, M. V., Chernoff, J. & Korobko, I. V. Rho family GTPase chp/RhoV induces PC12 apoptotic cell death via JNK activation. Small GTPases. 2, 17–26 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schoenhard, J. A., Eren, M., Johnson, C. H. & Vaughan, D. E. Alternative splicing yields novel BMAL2 variants: tissue distribution and functional characterization. Am. J. Physiol. Cell. Physiol.283, C103–C114 (2002). [DOI] [PubMed] [Google Scholar]
- 71.Brady, J. J. et al. An Arntl2-driven secretome enables lung adenocarcinoma metastatic self-sufficiency. Cancer Cell.29, 697–710 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zou, W. et al. The circadian gene ARNTL2 promotes nasopharyngeal carcinoma invasiveness and metastasis through suppressing AMOTL2-LATS-YAP pathway. Cell. Death Dis.15, 466 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ge, W. et al. Activation of the PI3K/AKT signaling pathway by ARNTL2 enhances cellular glycolysis and sensitizes pancreatic adenocarcinoma to erlotinib. Mol. Cancer. 23, 48 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qiu, B. Q. et al. ITGB1-DT/ARNTL2 axis may be a novel biomarker in lung adenocarcinoma: a bioinformatics analysis and experimental validation. Cancer Cell Int.21, 665 (2021). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The code supporting the findings of this study is available upon request from the corresponding author. The relevant data for this study are publicly accessible in the TCGA (https://portal.gdc.cancer.gov), GEO (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi? acc=GSE31210), STRING (https://cn.string-db.org/), UCSC Xena (https://xenabrowser.net/datapages/), Kaplan-Meier Plotter (https://kmplot.com/analysis/), and The Human Protein Atlas (https://www.proteinatlas.org/) databases. The KEGG pathway data used in this study are available from the KEGG database (https://www.kegg.jp/) and permission for use has been obtained from the Kanehisa laboratory.