Abstract
Background
Cognitive training has been confirmed to significantly improve the overall cognitive function in patients. For patients with coronary heart disease, in addition to controlling common risk factors, there is a lack of effective evidence for the treatment of cognitive function in patients with coronary heart disease and its effectiveness. This randomized controlled study was designed to evaluate the effectiveness of computer-based cognitive training for improving cognitive function in such patients.
Methods
COG-T CHD is a multicenter, double-blind, parallel-designed, randomized controlled trial. The patients will be divided 1:1 into two groups by a central randomized system, a cognitive digital therapy group or a positive control group. Patients assigned to the cognitive digital therapy group will undergo computer-based cognitive training for 30 min at least five times a week for 12 weeks. At the end of the 12 weeks, the subjects were randomly divided into two groups. One group continued the 12 weeks of cognitive digital therapy training and the other group stopped the training. Patients assigned to the positive control group will undergo computer-based cognitive training with little or no difficulty changes for 30 min at least five times a week for 12 weeks. The study will last approximately 2 years, with enrollment completed in approximately 18 months, with the last enrolled patient followed for at least 24 weeks. The primary outcome is the proportion of improvement in overall cognitive function at 12 weeks, using the Basic Cognitive Ability Test (BCAT). Secondary outcomes are the proportion of improvement in the overall cognitive function from baseline at 24 weeks, the change in overall cognitive function scores at 12 and 24 weeks, and the proportion of improvement in each cognitive domain, General Self-Efficacy Scale score, EuroQol five-dimension three-level questionnaire score, and Generalized Anxiety Disorder-7 score at 12 and 24 weeks from baseline. The investigational outcome is the change in head MRI structure and function from baseline at weeks 12/24.
Discussion
COG-T CHD is the first clinical trial to evaluate the efficacy of computer-based cognitive training in patients with coronary heart disease, filling an important gap in the treatment evidence for cognitive digital therapy.
Trial registration
ClinicalTrials.gov, NCT05735041. Registered on Jan. 18, 2023.
Keywords: RCT, Mild cognitive impairment, Computerized cognitive training, Coronary heart disease
Administrative information
Note: the numbers in curly brackets in this protocol refer to SPIRIT checklist item numbers. The order of the items has been modified to group similar items (see http://www.equator-network.org/reporting-guidelines/spirit-2013-statement-defining-standard-protocol-items-for-clinical-trials/).
| Title {1} | The efficacy of computerized cognitive training in patients with coronary heart disease and cognitive impairment, no dementia: study protocol for a randomized controlled trial |
| Trial registration {2a and 2b}. | ClinicalTrials.gov: NCT05735041 |
| Protocol version {3} | (2022) Research Review No. (17–2) |
| Funding {4} | Beijing Wispirit Technology Co., LTD and Chinese Society of Cardiology's Foundation (Project code: CSC2023A03) |
| Author details {5a} |
1Department of Cardiology, Centre for Coronary Artery Disease, Beijing Anzhen Hospital, Capital Medical University, Beijing 100037, China 2 Beijing Wisdom Spirit Technology Co.,Ltd., China 3The Second Hospital of Chifeng, China 4The First Affiliated Hospital of Hebei North University, China 5Inner Mongolia Ordos Central Hospital Kangbashi Department, China 6The First Hospital of Hebei Medical University, China 7Beijing Sixth Hospital, China 8Handan Central Hospital, China 9Peking University Third Hospital, China |
| Name and contact information for the trial sponsor {5b} |
Yong Zeng, Department of Cardiology, Centre for Coronary Artery Disease, Beijing Anzhen Hospital, Capital Medical University, 2 Anzhen Road, Chaoyang District, Beijing, China 100037 Email: yzeng_anzhen@mail.ccmu.edu.cn Tel: (+ 86) 13501373114 |
| Role of sponsor {5c} | This study is a clinical study jointly initiated by the funder and the investigator. The research funder participated in the study design, but did not participate in data collection, management, analysis, interpretation, report writing, publication and other activities related to the study. Important scientific decisions and research management for this study were made by the Steering Committee. |
Introduction
Background and rationale {6a}
Cognitive impairment is a syndrome with acquired and persistent cognitive impairment as the core, which leads to the decline of patients’ daily life and work ability and behavior change [1]. Cognitive impairment is divided into mild cognitive impairment (MCI) and dementia. MCI is the impairment of memory or other cognitive functions, but the ability to live daily lives is not significantly affected and does not meet the criteria for a dementia diagnosis. Epidemiological survey shows that the prevalence of dementia in people over 60 years old is 6.0%, and the prevalence of mild cognitive impairment is 15.5% [2]. Early intervention in MCI is of great significance for delaying the course of the disease, preventing dementia and improving the quality of life.
Coronary heart disease is the most common cardiovascular disease, and its prevalence increases with age. Recent studies have shown that the severity of coronary artery disease is positively correlated with the severity of cognitive impairment [3]. The prevalence of cognitive impairment in patients with coronary heart disease is as high as 35% ~ 46% [4]. Patients with coronary heart disease combined with cognitive impairment are at increased risk of cardiovascular events, cardiovascular death, and all-cause death. Coronary heart disease is often accompanied by cerebral vascular endothelial dysfunction, which can lead to reduced cerebral blood flow, hypoperfusion, white matter injury, and cerebral infarction [5]. Reduced cardiac output in patients with coronary heart disease can aggravate cerebral hypoperfusion and neuronal injury, further aggravating cognitive function impairment [6].
There is currently no proven medication for pre-dementia, a period of cognitive impairment but no impaired ability to perform daily activities. Non-pharmacological treatment, especially cognitive training, has attracted more and more attention in recent years and is expected to become an early prevention and intervention means for patients with mild cognitive impairment. Cognitive training refers to improving cognitive function by training different cognitive domains and cognitive processing processes. Cognitive training can be carried out in one or more cognitive domains such as memory, attention, and executive processing and can be in the form of pen or computerized training. Randomized controlled trials and meta-analyses have shown that cognitive training can significantly improve overall cognitive function in patients with cognitive impairment [7, 8]. In addition, in people at high risk of cognitive impairment, such as people with diabetes, home-based multidimensional cognitive digital therapy can improve overall cognitive function and disease management [9].
For the prevention and treatment of cognitive impairment in patients with coronary heart disease, in addition to the control of common risk factors such as hypertension, diabetes, sleep apnea, and vascular factors, there is still a lack of favorable medical evidence for the treatment of cognitive function itself and its effectiveness, and there is a lack of randomized controlled studies on coronary heart disease combined with cognitive dysfunction. Therefore, the aim of this study was to evaluate the effectiveness of computer-based cognitive training in improving cognitive function in patients with coronary heart disease combined with cognitive impairment but without dementia through a randomized controlled study on cognitive training.
Objectives {7}
The primary objective of the study was to compare the proportion of cognitive digital therapy that improved overall cognitive function at 12 weeks compared with positive controls in coronary heart disease patients with cognitive impairment but without dementia.
Trial design {8}
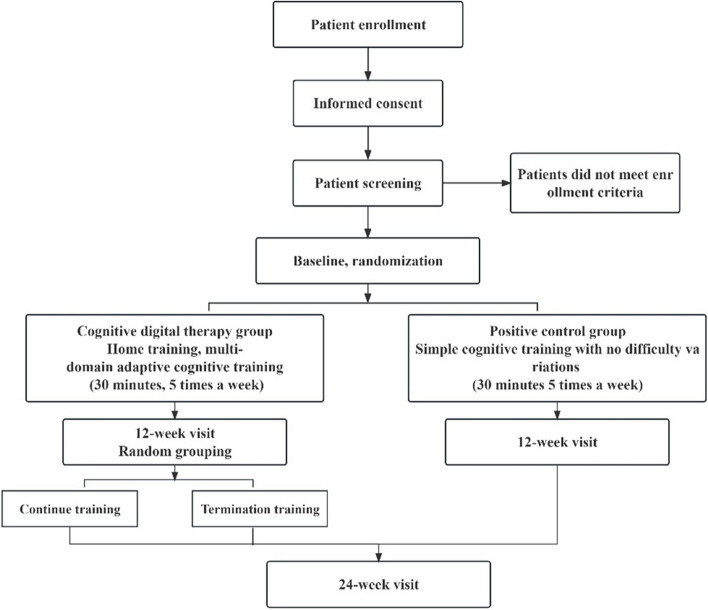
This study is a multicenter, double-blind, parallel randomized controlled study using a 1:1 parallel control design. A total of 200 patients with coronary heart disease combined with cognitive decline but no dementia were enrolled in 8 centers (Beijing Anzhen Hospital affiliated to Capital Medical University, Peking University Third Hospital, the Second Hospital of Chifeng, The First Affiliated Hospital of Hebei North University, Handan Central Hospital, The First Hospital of Hebei Medical University, Beijing Sixth Hospital, Inner Mongolia Ordos Central Hospital Kangbashi Department). All patients were stratified and randomly assigned to the cognitive digital therapy group and the positive control group according to age and educational level and were given cognitive training at least 5 times a week for at least 30 min each time for a total of 12 weeks. All subjects were interviewed at 12 weeks. After completing the 12-week visit, the researchers will unblind the subjects, the positive control group will end the intervention, and the cognitive digital therapy group will repeat the intervention 1:1 randomization: One group ended the cognitive training, and the other group continued the training until 24 weeks. Each patient will be followed up to 24 weeks. The primary outcome is the proportion of improvement in overall cognitive function at 12 weeks, defined as 0.67 standard deviation (SD) above baseline cognitive function as measured by the Basic Cognitive Ability Test (BCAT) (see Fig. 1).
Fig. 1.
Participant flow through study
Methods: participants, interventions, and outcomes
Study setting {9}
Initiated and led by Beijing Anzhen Hospital affiliated to Capital Medical University, this study was launched in January 2023 and planned to enroll 200 coronary heart disease patients with cognitive decline but no dementia from 8 centers (Beijing Anzhen Hospital affiliated to Capital Medical University, Peking University Third Hospital, The second hospital of Chifeng, The First Affiliated Hospital of Hebei North University, Handan Central Hospital, The First Hospital of Hebei Medical University, Beijing Sixth Hospital, Inner Mongolia Ordos Central Hospital Kangbashi Department) in China over 18 months. Each patient was required to sign an informed consent prior to enrollment. Each center will apply to the Ethics Committee for ethical review.
Eligibility criteria {10}
This study planned to enroll patients in the outpatient and ward of each participating center. Patients were screened and enrolled under quiet conditions in the outpatient and ward, accompanied by a family member who had lived with them for a long time. The family member could not provide help during the cognitive test.
Montreal Cognitive Assessment Scale (MoCA) assessment
The MoCA is a common tool used to assess general cognitive function and is often used to screen for mild cognitive impairment. The total score is 30 points. According to the original English results of the scale designer, a score ≥ 26 is considered normal and a score < 26 is considered as a decline in cognitive function. In this study, the MoCA examination will be completed by trained psychological testers, so patients with a score < 26 are included.
Mini-Mental State Examination Scale (MMSE) assessment
The MMSE is a common screening tool for cognitive and intelligence decline. The score on this scale is related to the educational level, and the total score is 30 points. According to the educational level, the cognitive function decline is considered as the illiteracy ≤ 17, the primary education ≤ 20, and the junior high school education ≤ 24. In this study, the MMSE examination will be completed by trained psychological testers. Since the selected patients were all with junior high school education or above, patients with MMSE score less than 24 points were excluded.
Cognitive training
After the patient’s MoCA and MMSE scores meet the enrollment criteria, each patient will receive a tablet computer specially used for cognitive training and receive one to two complete cognitive training instructions accompanied by a family member who had lived with the patient for a long time. The cognitive training guide includes the use of the cognitive training tablet, the training content that needs to be completed each week, the training process needs to pause or encounter system failures, etc., and each training should not exceed 60 min.
After the end of the first training, the patient will perform the training independently. If the patient has difficulty in completing one training independently, the researcher will conduct the training again. If the patient cannot complete one training independently after two training sessions, it is considered that the patient cannot master the cognitive training operation. During the training process, family members and patients participate in the training, but cannot remind patients when they operate independently.
Inclusion criteria
Patients must meet all of the following criteria to be included in the study:
1) Over the age of 50;
2) Completion of more than 6 years of education;
3) Patients with confirmed coronary heart disease, including chronic myocardial ischemia syndrome, acute coronary syndrome, or transcoronary CT.
Or coronary angiography showed lumen stenosis greater than 50% patients;
4) The chief complaint of cognitive decline within 1 year;
5) Montreal Cognitive Assessment Scale (MoCA) < 26 points;
6) Agree to be randomized to cognitive function tests and cognitive training and be able to receive follow-up as required.
Exclusion criteria
Patients could not be included in this study if they met any of the following criteria:
1) A definite diagnosis of dementia or a simple mental MMSE ≤ 20 points.
2) Caused by head trauma, skull tumor, stroke, Parkinson's disease, schizophrenia, Alzheimer's disease, anxiety.
Cognitive dysfunction caused by neuropsychiatric diseases such as depression;
3) Deaf and mute and other reasons cannot communicate normally;
4) Binge drinking or taking drugs that affect cognitive function (antihistamines, antipsychotics);
5) Unable to master the use of cognitive training equipment after two 1-h training instructions;
6) Patients who plan to undergo coronary intervention within 6 months or have undergone coronary intervention within 1 month;
7) Severe liver and kidney function injury or critical condition, poor prognosis, expected survival of less than 1 month;
8) Patients who had undergone general anesthesia within 3 months;
9) Prior neurosurgery or history of skull tumor;
10) Nuclear magnetic examination contraindications: such as metal implants in the body and claustrophobia;
11) Patients living alone;
12) Patients with atrial fibrillation, structural heart disease, and infective endocarditis.
Who will take informed consent? {26a}
All subjects who may be eligible for inclusion in this study were screened according to the inclusion criteria. Researchers should fully inform subjects of all relevant information of this study and ask subjects to sign two copies of informed consent. The original informed consent shall be kept in the subject’s study folder. The other copy will be kept by the subject. Subjects have the right to withdraw from the study at any time, and their decision will be recorded in the subject's study file.
Additional consent provisions for collection and use of participant data and biological specimens {26b}
N/a.
Explanation: all patient information, biological samples, and imaging data required for the exploratory endpoints of this study were included in the formal informed consent and were fully informed by the investigators at the time of enrollment.
Interventions
Explanation for the choice of comparators {6b}
A total of 200 patients were randomly divided into the following two groups according to age (≥ 75 years old, < 75 years old) and education level (completion of junior high school and below, senior high school and above): (1) cognitive digital therapy group and (2) positive control group.
Intervention description {11a}
Training program
The cognitive training software used in the experiment is an app based on cloud services, which is divided into two distinct parts: the server side and the client side.
The server side is designed for researchers to use and enables the visual management of the research process. It allows operations such as protocol setting, viewing patients’ cognitive training status, and recording follow-up time. Protocol setting includes specifying the number of specific training tasks, the daily training duration, the number of training days, and the follow-up time window. The server side simultaneously presents to researchers the cognitive training status of each enrolled patient, such as the specific dates of training, the total training duration on a given day, and other information. Researchers can manage patients’ compliance with cognitive training participation by reviewing such information. According to the pre-set experimental training protocol, when a patient enters the follow-up time window, the server side will remind the researcher to conduct the follow-up work promptly, and after completion, this reminder will be marked as completed.
The client side is provided for patients to use at home. The interface of the client side displays the assigned training tasks each time. Patients click to start each training session. Five training tasks are assigned each time, with a fixed training duration of 30 min in total. After completion, a prompt will appear on the interface to inform the patient that the current cognitive training has been completed.
In the experiment, the digital therapy cognitive training used stipulates that the subjects are required to select 5 days out of a week and complete the cognitive training tasks assigned by the daily updated cognitive training software within 30 min each day.
Cognitive digital therapy group
The cognitive digital therapy group used multitask and adaptive cognitive training. These cognitive training tasks were adapted from classic psychological paradigms such as the Stroop Effect and Dual-Task Paradigm (see Fig. 2, 3). The training tasks covered cognitive domains such as sensory perception, attention, memory, thinking, and processing speed. In addition, the above cognitive domains are divided into more details. For example, the memory-related training tasks are divided into working memory, spatial memory, memory span, and other training tasks. At the same time, the digital therapy cognitive training system is equipped with an adaptive algorithm, which judges the different cognitive domains of patients according to the score of the patients’ current training tasks, and then updates the training tasks assigned to the patients with appropriate difficulty and the cognitive domains that need to be strengthened every day. There are also different levels of difficulty set within the training tasks. These settings allow patients to exercise overall cognitive function.
Fig. 2.
Cognitive training task adapted from the classic Stroop effect paradigm: the patients must pay attention to the color of the balloons and the content of the words and click on the balloons whose color does not match the written description
Fig. 3.
Cognitive training task adapted from the classic psychological paradigm size-matching task: the patients need to select the image from the three options below that have the same size as the image displayed above
Positive control group
The training content of the positive control group is cognitive training tasks with weak difficulty or no difficulty change. The patients underwent cognitive function training with a fixed program, including 15 training items, and the internal difficulty of the training items remained constant. The system presents the training content randomly according to the training scheme. The patients had the same total amount of training per day.
Criteria for discontinuing or modifying allocated interventions {11b}
This study did not involve changes in clinical routine treatment, and the routine treatment of patients during and after the study will not be affected by this study. There is also no expected harm to patients.
Strategies to improve adherence to interventions {11c}
After the training, each subsequent training will be completed in the family, once a day, at least five times a week to complete effective training; effective training is defined as completing the training task assigned by the system, and the cognitive training duration reaches 30 min.
Researchers can check the patient’s daily training records through the doctor’s side to see if the patient’s daily training is effective. A yellow star on the calendar indicates that the patient completed effective cognitive training that day; the gray stars indicate that the patient had cognitive training but did not reach an effective dose.
In addition, researchers can view patient training from the doctor’s side and check the total daily training time of the subjects, the start time of each training task, the training duration, and the score. If the training task is not completed, such as the total length of a training session is less than 30 min or the training time is often more than 60 min, it is counted as invalid training, but the training time should also be recorded in the background. The researcher should inform the patient to try to choose a fixed free time of the day for training and try to avoid the situation that there is an interruption in one training; if there is an interruption, the system stops the timing, and the interruption is completed from the beginning on the same day. If the patient did not complete effective training for two consecutive days, the researcher reminded the patient to complete the training by phone.
During the training process, the system will collect the patient’s training information and record the training duration and completion status. The abnormal training data detected in the background for two consecutive days should be verified by phone and recorded truthfully.
In order to ensure the effectiveness and applicability of cognitive training, the following methods are adopted to guide and monitor patients’ daily training: (1) assistance from doctors and trainers: if patients have questions about training tasks during the training process, they can contact doctors or trainers to solve and guide them through telephone or online communication, so as to ensure their correct and effective cognitive training; (2) family assistance: during the baseline visit, the doctor and trainer will train the patient and the patient's family. During the patient’s home training process, the patient’s family members can assist the patient in cognitive training, help the patient understand the operation of various training, and urge the patient to carry out cognitive training; (3) incentive measures: for each enrolled patient, promise to give 1 year of cognitive training software use right after the end of the study to encourage patients to actively complete the cognitive training task.
Relevant concomitant care permitted or prohibited during the trial {11d}
In this study, the optimal management of cardiovascular disease for all patients participating in the study was mainly motivated by the following two considerations. On the other hand, cardiovascular diseases such as coronary heart disease, hypertension, and diabetes are likely to affect the cognitive function of patients, so optimizing management can minimize the impact of factors other than the intergroup study intervention on the outcome event.
For all patients with coronary heart disease combined with hypertension, long-acting and combined antihypertensive drugs should be used as far as possible. For general patients, the blood pressure target should be controlled below 140/90 mmHg. Under tolerable and sustainable conditions, the blood pressure of some high-risk patients with diabetes, proteinuria, etc., can be controlled below 130/80 mmHg. For patients with coronary heart disease combined with hyperlipidemia, the blood lipids of the patients were reduced to the ideal level according to the stratification of cardiovascular disease risk. For patients with coronary heart disease combined with diabetes, the HBA1c will be below 7% if reasonable hypoglycemic treatment is adopted.
In addition, it should also include sound advice on healthy lifestyles such as limiting sodium intake, weight management, increasing physical activity, smoking cessation, and blood sugar and lipid control. Specific recommendations include (1) weight loss for overweight or obese patients; (2) diet rich in fruits, vegetables, and low-fat dairy products (DASH diet) are recommended, and the diet of patients with CKD should be adjusted appropriately; (3) reduce sodium intake to recommended levels; (4) reduce alcohol intake to recommended levels; and (5) get regular aerobic exercise. If subjects currently smoke, they are encouraged to quit.
Provisions for post-trial care {30}
The investigator should take this seriously and ensure that all patients who are harmed as a result of participating in the trial receive appropriate medical protection.
Outcomes {12}
Primary outcome
Percentage improvement in overall cognitive function at 12 weeks.
The BCAT software was introduced in 2001 by Deming Li’s research group of the Institute of Psychology of the Chinese Academy of Sciences, based on the research of the psychological and brain mechanisms of adult cognitive aging for many years, and formulated a national norm, which has been widely used [10]. At present, the software has been updated to the third edition; this set of tests in clinical, rehabilitation, drug efficacy evaluation, talent selection, education, and other aspects of a wide range of practical value is not only effective but also has strong sensitivity characteristics.
Since crystallized intelligence is greatly influenced by education, and crystallized intelligence depends on innate ability and gradually decreases in the aging process, the basic cognitive ability in this study only includes four fluid intelligence: processing speed, working memory, episodic memory, and visuospatial intelligence. Corresponding to the four tests in BCAT test software, namely symbol search, portrait memory operation span, and origami test, the subjects used the web version of the test page in the tablet browser for assessment.
1) Symbol search
This test is adapted from a subtest of the Wechsler Intelligence Scale. There is a horizontal line in the middle of the screen, two symbols above the horizontal line, and five symbols below it, and the subject’s task is to see if one of the two symbols above the horizontal line is present in the five symbols below the horizontal line. This test is limited to 120 s, and the subjects are required to complete as many tasks as possible within the prescribed time, under the premise of ensuring correctness. The total score of the test is the number of correct judgments of the subject in the specified time.
2) Portrait memory
This test is adapted from the subtest of the clinical memory scale. During the test, six portraits of people were presented in turn, and the surname, job, and hobby of the person were presented on one side of the portrait at the same time, and the subjects were asked to remember the portrait and the corresponding information. After the portrait was presented, there was a memory test, in which the person was presented one by one on the screen and the subject was asked to select the person’s last name, job, and hobby. The whole test consists of two rounds of study and test process, the second round of study and test word pairs are the same as the first round, but presented in a different order. One point is given for each correct answer for a job or hobby. Two points are given for last name, up to 48 points.
3) Operation span
This test is self-designed. The test consists of two tasks, one is mental arithmetic and the other is memory. The mental arithmetic task is to complete some mixed three arithmetic problems through mental arithmetic. After the calculation formula is presented, the subjects are asked to judge whether the answer given is correct and click the corresponding option (right/wrong) with the left mouse button to answer. Then, an animal sign (such as “rat”) is displayed in the center of the screen and the subject is asked to remember the animal sign. After each formula is presented, there is an animal sign. For the formula, it is necessary to immediately judge whether it is right or wrong, while for the animal sign, it is only necessary to remember in the mind, so repeatedly, and finally ask the subject.
In accordance with the order of the animal signs presented just now, select all the animal signs that have appeared with the mouse click. The test starts with Breadth 2 (containing two sets of computations-astrological materials), and if the subject successfully passes, then Breadth 3 (containing three sets of computations-astrological materials), and so on, until the test is terminated if the subject fails twice in a row. The score of the test is divided into two parts, the calculation formula requires 80% correct rate, the score is effective, and the astrological memory part answers one point.
4) Paper-folding test
This test is adapted from the identical subtest of the intelligence test suite developed by Ekstrom et al. (1976). In the test, the subjects were asked to visualize a square of paper folded in a certain way, poke a hole with a pencil, and then unfold the paper. The paper would have several holes and their correct positions. In the presentation of each question, there is a horizontal line in the center of the screen, above which is the process of origami and piercing. Each folding process of the paper (one to three times) is clearly displayed above the horizontal line, and no other folding or moving of the paper is done. Below the horizontal line are five possible answers, and the pilot is asked to select the only correct one. There are 15 questions in the test, which are arranged in order from easy to difficult, and you get one point for each correct question, up to 15 points.
BCAT was completed by trained psychological test personnel. BCAT cognitive test included four cognitive domains, processing speed, episodic memory, working memory, and visual space, which were tested respectively for symbol search, portrait memory, operation span, and origami test. The four tests are conducted consecutively without a break, and the full assessment takes between 30 and 40 min. Before each test, there are test instructions. If the patient has questions about the instructions, the tester can explain them to ensure that the patient is clear about the operation method of the test.
After the test, the scale score of each cognitive domain adjusted based on norms will be obtained, and the Z-score will be obtained according to the corresponding relationship table between the scale score and the Z-score (Table 1). According to the cognitive conditions of different ages in the norm (Table 2), the score of this cognitive domain of the patient is determined to be lower than the norm 1 SD. Cognitive impairment is considered when the BCAT arbitrary cognitive domain score is less than 1 SD.
Table 1.
The correspondence between BCAT cognitive ability scores and Z-scores
| BCAT score | Z-score |
|---|---|
| 19 | 3.00 (2.83 ~ 3.17) |
| 18 | 2.67 (2.50 ~ 2.83) |
| 17 | 2.33 (2.17 ~ 2.50) |
| 16 | 2.00 (1.83 ~ 2.17) |
| 15 | 1.67 (1.50 ~ 1.83) |
| 14 | 1.33 (1.17 ~ 1.50) |
| 13 | 1.00 (0.83 ~ 1.17) |
| 12 | 0.67 (0.50 ~ 0.83) |
| 11 | 0.33 (0.17 ~ 0.50) |
| 10 | 0.00 (− 0.17 ~ 0.17) |
| 9 | − 0.33 (− 0.50 ~ − 0.17) |
| 8 | − 0.67 (− 0.83 ~ − 0.5) |
| 7 | − 1.00 (− 1.17 ~ − 0.83) |
| 6 | − 1.33 (− 1.50 ~ − 1.17) |
| 5 | − 1.67 (− 1.83 ~ − 1.50) |
| 4 | − 2.00 (− 2.17 ~ − 1.83) |
| 3 | − 2.33 (− 2.50 ~ − 2.17) |
| 2 | − 2.67 (− 2.83 ~ − 2.50) |
| 1 | − 3.00 (− 3.17 ~ − 2.83) |
Table 2.
Threshold for basic cognitive impairment in different age groups based on BCAT
| 18–29 years old | 30–39 years old | 40–49 years old | 50–59 years old | 60–59 years old | Over 70 years old | |
|---|---|---|---|---|---|---|
| Processing speed | − 0.16 | − 0.43 | − 0.69 | − 1.09 | − 1.39 | − 1.71 |
| Working memory | − 0.25 | − 0.45 | − 0.74 | − 1.01 | − 1.26 | − 1.43 |
| Episodic memory | − 0.34 | − 0.52 | − 0.80 | − 1.07 | − 1.20 | − 1.47 |
| Visual space | − 0.53 | − 0.69 | − 0.76 | − 0.90 | − 1.05 | − 1.22 |
Secondary outcome
1) The percentage of improvement in overall cognitive function from baseline at 24 weeks;
2) The proportion of improvement in cognitive function from baseline in each cognitive domain at 12 weeks and 24 weeks;
3) Changes in overall cognitive function scores between 12 and 24 weeks;
4) Changes in patients’ self-efficacy scores from baseline at weeks 12 and 24;
5) Changes in patients’ quality of life scores from baseline at 12 and 24 weeks;
6) Changes in patients’ anxiety and depression scores from baseline at weeks 12 and 24.
The proportion of improvement in cognitive domain function is defined as follows: based on BCAT cognitive test, four cognitive domains of processing speed, working memory, episodic memory, and visual space were evaluated. The scale score was given by the system, and Z value was obtained according to the table. Subdomain function improvement was defined as a Z-score of 0.67 SD for each subdomain above the baseline for that domain (SD being the SD of the baseline data).
General Self-Efficacy Scale: self-efficacy was measured by the Chinese version of the General Self-Efficacy Scale-Schwarzer (GSES) proposed by Schwarzer et al. [11]. GSES consisted of ten items and was scored using the 4-point Likert scale. The score ranges from 10 to 40, with the higher the score, the better the self-efficacy. Participants in this study will receive self-efficacy assessments at baseline, 12 weeks, and 24 weeks of follow-up.
EuroQol five-dimension three-level questionnaire: health status was measured using the European Five-Dimensional Health Scale (EQ-5D-3L), a self-rating scale that includes five dimensions: mobility, ability to take care of oneself, ability to perform daily activities, pain or discomfort, and depression [12]. Each dimension has three types of options (normal, moderately limited, and severely limited). Participants in this study will be evaluated for EQ-5D at baseline, 12 weeks, and 24 weeks of follow-up.
Anxiety-depression score changes: anxiety-depression status was measured by the Patient Health Questionnaire Depression Scale (PHQ-9) and the Generalized Anxiety Scale (GAD-7). PHQ-9 scale is a self-rating scale of depressive symptoms recommended by the AHA Advisory Panel on Depression and Coronary Heart Disease [13]. The scale is well accepted by subjects (nine items, 1–3 points for each item, 2–3 min for self-assessment) and has high reliability. Patients in this study will be assessed for PHQ-9 at baseline, 12 weeks, and 24 weeks. GAD-7 is a quantitative assessment tool recommended in the DSM-5 published by American Psychiatry [14]. It has simple content and strong operability. There are seven items in the scale, with each item scoring 0–3 points and taking 2–3 min. Patients in this study will be evaluated with GAD-7 at baseline, 12 weeks, and 24 weeks.
Exploratory outcome
MRI-related imaging was performed to assess changes in head MRI structure and function from baseline at weeks 12/24.
The nuclear magnetic sequence and specific parameters are as follows:
Structure image (T1 MPR): the whole brain structure image was acquired using 3D magnetization preparation rapid gradient echo (3D MPRAGE) sequence, TR (repetition time) = 2100 ms; TE (echo time) = 2.13 ms; FA (inverse angle) = 8°, FOV (field of view) = 224 mm × 224 mm, MATRIX = 320 × 320; layer thickness = 0.8 mm; number of scanning layers = 206.
T2 space: turbo spin echo sequence acquisition was adopted, TR = 5000 ms, TE = 216 ms, FA (reverse angle) = 120°, FOV (field of view) = 224 mm × 224 mm, MATRIX = 320 × 320. Slice thickness = 0.8 mm, number of slices = 206.
Resting bold: echo-planar imaging (EPI) was used, TR = 1000 ms, TE = 30 ms, FA = 55°, FOV = 192 mm × 192 mm, MATRIX = 96 × 96, layer thickness = 2 mm, scanning interval = 0 mm. Scan layers = 78.
Diffusion tensor imaging (DTI): single-shot diffusion-weighted echo planar imaging sequence (TR = 3200 ms, TE = 82 ms) was adopted. Diffusion sensitivity coefficient b value (diffusion sensitivity coefficient) is 2000, 1000, and 0 s/mm2; there are 64 diffusion sensitivity gradient directions, corresponding to b = 0 s/mm2, only a single image without diffusion tensor is collected, layer thickness = 2 mm, no layer interval, MATRIX = 110 × 110. FOV = 220 mm × 220 mm.
3D-FLAIR imaging: using Siemens equipment, 3D-FLAIR nuclear magnetic sequence parameters are as follows: TR = 5000, TE = 392, inversion time = 1800, FOV = 240, FA = 120°, slice thickness = 1 mm.
Perfusion imaging based on ASL, the sequence parameters are as follows: TR = 3500 ms, TE = 10 ms, pcasl mode, spatial resolution 3 × 3 × 3 mm3, LD = 1500 ms, TD = 2000 ms.
Participant timeline {13}
The following table outlines the timing and evaluation of the study (Table 3).
Table 3.
Research schedule
| Visit number | V1 | V2 | V3 | V4 |
|---|---|---|---|---|
| Case report form | A Screening assessment | B Baseline/randomization | C | D |
| Time to random differentiation | − 1 to 0 weeks | 0 weeks | 12 weeks | 6 months |
| Time window for evaluation | - | - | − 3 to + 7 days | ± 14 days |
| Written informed consent | × | |||
| Face-to-face visit | × | × | × | × |
| questionnaire | ||||
| Sociodemographic data | × | |||
| Lifestyle (smoking and alcohol consumption) | × | |||
| Medical history (present history, past history) | × | |||
| All medications | × | × | × | |
| Vital signs and physical examination (height, weight, blood pressure, pulse) | × | × | × | |
| Auxiliary inspection | ||||
| Blood test | × | × | × | |
| Electrocardiogram | × | × | × | |
| Echocardiogram | × | × | × | |
|
Cognitive function evaluation Cognitive Test Evaluation Based on BCAT |
× | × | × | |
| Cranial magnetic resonance scan | × | × | × | |
| scale | ||||
| Mini-mental State Examination, MMSE | × | |||
| Montreal Cognitive Assessment, MoCA | × | × | × | × |
| Functional Activities Questionnaire, FAQ | × | × | × | |
| General Self-Efficacy Scale, GSES | × | × | × | |
| EuroQol five-dimension three-level questionnaire (EQ-5D-3L) | × | × | × | |
| Patient Health Questionnaire-9, PHQ-9 | × | × | × | |
| Generalized Anxiety Disorder-7, GAD-7 | × | × | × | |
| Process Evaluation | × | × | ||
| Serious adverse events/adverse events (SAE/AE) | × | × |
Sample size {14}
The sample size was calculated based on previous studies [15] and the following assumptions (see Fig. 4):
1. After 12 weeks of intervention, 30% of patients in the cognitive digital therapy group improved their overall cognitive scores (0.67SD);
2. The overall cognitive score of the positive control group was improved by 10%;
3. Bilateral test (α = 0.05) was adopted, the minimum efficacy of the test was 90%, the 3-month loss rate was assumed to be 20%, the sample size was calculated N = 198, and 200 patients were finally included.
Fig. 4.
Sample size
Recruitment {15}
Patients were recruited from the wards of the participating centers, which were equipped with conditions suitable for cognitive training and neuropsychological scales.
Assignment of interventions: allocation
Sequence generation {16a}
Randomization
With age (≥ 75 years old, < 75 years old) and education level (completion of junior high school and below, senior high school and above) as stratified indicators, all patients meeting the study inclusion criteria were randomly divided into two groups at a 1:1 ratio according to the random table generated by the randomization server, respectively named “cognitive digital therapy group” and “positive control group”.
Concealment mechanism {16b}
Packet hiding
The clinical outcome cognitive function was assessed using an independent BCAT system. The BCAT evaluation system server is managed by the sponsor. Questionnaire evaluators and head MRI imaging analysts cannot know the grouping of patients, evaluators need to receive professional evaluation training, and staff outside the research team input the data into the computer in the form of separate data tables, so that statisticians can analyze the data without knowing the grouping.
Implementation {16c}
Central randomization
A central randomized network service system was used for randomized assignment of study patients. To ensure the confidentiality of randomization, the Network Central Randomization System (IWRS) system will not send a randomization code until patient enrollment and baseline data collection are completed.
Assignment of interventions: blinding
Who will be blinded {17a}
COG-T CHD was a double-blind trial. Randomization results are not disclosed to patients, researchers, research assistants, or data analysts.
Procedure for unblinding if needed {17b}
After completing the 12-week visit, all subjects will be unblinded, patients in the positive control group will end the intervention, and patients in the cognitive digital therapy group will be randomized 1:1 again: one group will end the cognitive training and the other group will continue the training until 24 weeks.
Data collection and management
Plans for assessment and collection of outcomes {18a}
Multiple follow-up assessments included laboratory tests, clinical tests, and questionnaires. Follow-up plans will be the same for both groups of patients who were randomized. Patients’ baseline data included sociodemographic data, anthropometric data, blood pressure, present and past history, current medication, laboratory tests, cognitive function tests, head MRI and self-efficacy, quality of life, functional activity questionnaire, and anxiety and depression scale. The research schedule is shown in Table 3.
Plans to promote participant retention and complete follow-up {18b}
Plans to promote participant retention and completion of follow-up are described in the section on improving subject compliance. Data from participants who stopped or deviated from the intervention regimen were treated in the same way as those who were lost to follow-up (mentioned in the handling of missing data section).
Data management {19}
Electronic data acquisition system (EDC)
1) Permissions: only those listed in the list are authorized to log in to the Internet-based data management system using their personal accounts and passwords.
2) Confidentiality: adopt authentication technology to meet the needs of identity authentication and integrity authentication. Sensitive data will be stored through data encryption technology, and data across regions will be encrypted and transmitted. This study will be subject to confidentiality agreements with staff who manage and operate important EDC information. EDC management staff shall not modify or delete existing information without authorization. Unauthorized persons cannot access EDC systems.
Confidentiality {27}
Researchers and patients are required to sign a confidentiality agreement to ensure that the use of the data is limited to the use of the project, to ensure the privacy and security of patient data, and to promise that the collected patient data will not be used for any other purpose without written permission.
The BCAT software, cognitive digital therapy software, and electronic data acquisition system (EDC) software used in the project all follow the standards of data security life cycle management and adopt a combination of management and technology to build a comprehensive data security system. According to the data security life cycle, the entire process—from data collection, transmission, processing, and storage, display, use, and destruction—includes measures such as identity authentication, permission management, access control, data encryption, data isolation, transmission security, storage security, and data destruction to protect users’ privacy, ownership, and control of their data.
All electronic data generated in this study are stored and backed up on servers owned by Anzhen Hospital affiliated to Capital Medical University and on local servers of participating research units. Data collection, processing, access, output, and other management operations are strictly in accordance with the national data security requirements of the authority management to ensure the security of medical data.
Plans for collection, laboratory evaluation, and storage of biological specimens for genetic or molecular analysis in this trial/future use {33}
All study records should be kept in a safe and secure facility for at least 15 years in accordance with clinical study regulations and regulatory requirements. All the paper materials of the original records in this study will be summarized and bound in the folder of the same specification after the completion of the project, all the electronic data will be downloaded and saved in the hard disk of the same specification, and all the data will be stored in the designated storage place according to the requirements of the regulatory authority.
Statistical methods
Statistical methods for primary and secondary outcomes {20a}
This study analyzed the proportion of overall cognitive function improvement in the cognitive digital therapy group versus the positive control group after 12 weeks of treatment. BCAT cognitive test was used to evaluate the processing speed, working memory, episodic memory, and visuospatial cognitive areas and to obtain the scale scores of each item. The Z-score for each cognitive area can be obtained from the Z-score table based on the scale score for each item in the test (Table 2.3). The overall cognitive function score will be calculated as the average of each cognitive area score. Based on the Hedges’ g of cognitive function improvement in previous studies [8], the improvement in cognitive function in the COG-T CHD study was defined as a 0.67 SD higher Z-score of the overall BCAT score after training than the baseline Z-score.
Data analysis in this study followed the principle of intention-to-treatment analysis (ITT), and patients were classified according to their initial randomization. Continuous variables are represented by means (standard deviations) and tested by P-P plots. If the distribution is skewed, the data is represented by medians (interquartile spacing) and transformed to follow an approximate normal distribution. If it cannot be converted to an approximate normal distribution, Wilcoxon test can be performed. Statistical analysis will be conducted in accordance with a pre-designed and published Complete Statistical Analysis Plan (SAP). The difference in primary outcome was detected by t test.
Interim analyses {21b}
In order to evaluate the accuracy of data entry in the EDC system, the commissioned research institution (CRO) will check the patient’s medical records during the patient’s hospitalization and periodically check the data entered in the EDC system during the patient’s follow-up. Researchers with entry error rates > 5% will be retrained. Research centers with data fraud, informed consent fraud, or missing data will be warned or patients will be terminated. The audit will be carried out by CRO staff commissioned by the sponsor. The inspectors will examine potential problems at the research center and assess whether the study implementation, data collection, and data analysis comply with protocols, standard operating procedures (Sops), and Clinical Study Quality Management Practices (GCP). All research centers should be unconditionally subject to third-party audits and government regulatory inspections when needed. During and after the study, each research unit must be able to provide CRF, source documents, and other research-related documents.
Methods for additional analyses (e.g., subgroup analyses) {20b}
The following subgroups will be analyzed in both primary and secondary outcomes to test the consistency:
Gender: female, or male;
Age: ≥ 70 years old, or < 70 years old at baseline visit;
Education: completion of junior high school and below, or senior high school and above Anticoagulant usage: reporting using anticoagulant drugs in baseline assessment or not.
Methods in analysis to handle protocol non-adherence and any statistical methods to handle missing data {20c}
In accordance with the principle of intention-to-treat analysis, we will analyze the results of the primary outcome by participant randomization, regardless of patient adherence to the intervention. For patients who were lost to follow-up, we used all available data prior to the subject’s death or loss of follow-up.
Our approach to missing values in clinical studies is consistent with that of Molenberghs and Kenward who argue that while the removal of negligible, random deletion (MAR) analyses is reasonable for primary outcome analysis and the sensitivity of the conclusions explored under the MAR hypothesis allows for the presence of models with non-random missing values. If missing values are relevant to the results analyzed (as is often the case in the analysis of health-related outcomes), the presence of missing values can bias the results of the analysis. The severity of the problem and the rate of missed visits will be predicted from the results of previous visits. To satisfy the MAR data conditions described by Little and Rubin, our prediction model includes variables that predict the loss of follow-up rate. Maximum likelihood method is used for parameter estimation. If necessary, other methods may be considered to determine whether the results are reliable and whether they provide an appropriately conservative estimate for the study.
The reliability of missing value data inference will be further explored in sensitivity analysis. These sensitivity analyses include several “worst-case” examinations, including both inverse and pooled estimation methods. These cases are of a broad type that can be parameterized into mixed model patterns and allow detection of the sensitivity to conclusions of non-random deletion (MNAR) mechanisms.
Plans to give access to the full protocol, participant-level data, and statistical code {31c}
All information about the trial has been registered on ClinicalTrials.gov with the code NCT05735041 and is updated in real time.
Oversight and monitoring
Composition of the coordinating center and trial steering committee {5d}
The sponsor or a contract clinical research management organization (CRO) commissioned by the sponsor is responsible for the project management and coordination of the operation of the study. Responsibilities include, but are not limited to: project and data management; coordination with the members of the various committees of the study; assistance in the preparation of ethics committee submissions and progress reports by the investigators; assistance in the preparation of and improvement of the study protocol; training of the personnel of the participating units of the study; research center start-up; monitoring and auditing; monitoring of data quality and data security; monitoring of the study protocol; medical guidelines, compliance and implementation of laws and regulations; and assisting with the organization and preparation of research data and articles for publication.
Composition of the data monitoring committee, its role and reporting structure {21a}
A Data Security Regulatory Board (DSMB) was set up to oversee the study. DSMB members are appointed by the Project Steering Committee to oversee the study. DSMB members of this study may include experts in cardiovascular disease, neurology, clinical trials, biostatistics, and other related fields. It is the responsibility of the DSMB to recommend to the Steering Committee whether the study should continue, whether the study protocol should be modified, or whether the study should be terminated early. The DSMB will submit its report to the Scientific Committee through the Executive Secretary. DSMB recommendations must be approved by the Scientific Committee before they can be implemented. Whether a study can continue or needs to be terminated is not only a matter of the degree of statistical significance in the interim analysis but also whether the results of the study can achieve a statistically significant difference in the original projected sample size. To assist in the latter assessment, the focal point will complement the cohort sequential analysis approach described above by calculating conditional efficacy, that is, assessing the conditional probability of a significant difference in treatment at a preset alpha level at the end of the trial, based on the data observed to date. After approximately 25% of the total events have occurred, the conditional efficacy of the primary treatment will be compared and written into an interim study report for submission to the DSMB.
Adverse event reporting and harms {22}
The local principal investigator (PI) should keep detailed records of any serious adverse events (SAE)/adverse events (AE) that occur in subjects, including description of adverse events and all associated symptoms, time of occurrence, severity, cause of adverse events, correlation with cognitive training, duration, actions taken, and final outcomes and outcomes. Researchers should use the following guidelines to grade the intensity of adverse events:
Mild: transient and may require only minimal treatment or therapeutic intervention. The event generally does not interfere with activities of daily living.
Moderate: remission with additional specific treatment interventions. The event will interfere with activities of daily living and cause discomfort, but will not pose a significant or permanent risk of injury to the subject.
Severe: interruption of activities of daily living, or significant impact on clinical status, or may require intensive treatment.
Frequency and plans for auditing trial conduct {23}
The Ethics Committee of Anzhen Hospital reviewed the trial every 12 months. The review process is independent from investigators and the sponsor.
As the cognitive training utilized in this trial was an intervention with an extremely low-risk level, no data monitoring committee was established for this trial. A project management team was constituted when the trial was initiated. Every Friday, the specific implementation details of each participating hospital were reported and final solutions were discussed and formulated.
Furthermore, the trial hired personnel from CRO and SMO companies to provide corresponding services for the specific execution of the trial, ensuring adequate quality guarantee.
The composition of the project management team included the researcher team of the sponsor, the project manager, and the CRA of each trial sub-center. The project team conducted detailed reviews on aspects such as the specific enrollment progress of the trial, the verification of original data, and the collection and judgment of adverse events.
Plans for communicating important protocol amendments to relevant parties (e.g., trial participants, ethical committees) {25}
Protocol revision is a written description or formal statement of a study protocol change that may affect the conduct, potential benefit, or subject safety of a clinical study, including study purpose, study design, study participants, sample size, study procedures, or significant study administration protocols. Changes in study protocol management information do not have a significant impact on the way clinical studies are conducted, do not affect the safety of subjects, and are minor corrections or clarifications (e.g., changes in telephone numbers, changes in organizational arrangements). Study protocol amendments must be approved by the CRO, the regulatory authority (if necessary), and the Ethics Committee. If for the purpose of ensuring the safety of the subjects, the study protocol revision may be implemented prior to approval by the Ethics Committee. Although changes to the study protocol are subject to a formal approval process, the investigator may take urgent measures to ensure the safety of the study subjects, regardless of whether such measures are contrary to the original protocol. In such cases, the CRO and IRB/EC of the research Centre should be notified in a timely manner.
Dissemination plans {31a}
The main results of this study will be published under the title of this study. Article writing is done by an authoring committee approved by the Steering Committee (SC). The preparation committee will consist of committee members, statisticians, and researchers. They will prepare the main report of the study under the name of this study. The results of the study will be published in the journal and in the field of cardiovascular and neurology and report at national and international conferences.
Discussion
Coronary heart disease and cognitive impairment are common diseases in the elderly. Cognitive impairment in patients with coronary heart disease will accelerate the course of coronary heart disease due to memory loss, executive ability decline, and other problems. Secondary prevention in patients with coronary heart disease requires a lot of drug support. For patients with coronary heart disease with cognitive impairment, non-drug treatment or better choice can be provided by training in one or more cognitive domains such as memory, attention, and executive processing. This multicenter, double-blind, parallel-designed, randomized controlled study was designed to investigate the effectiveness of cognitive digital therapy in improving overall cognitive function in patients with coronary heart disease combined with cognitive impairment.
The highlight of this study is the design of electronic targeted cognitive function training for patients with coronary heart disease, including attention, memory, executive function, thinking, processing speed, perception, and other multidimensional cognitive functions. The training system will adaptively adjust the training difficulty and training plan according to the current training time and training results of patients. Patients in the positive control group received cognitive function training with a fixed regimen of low or no change in difficulty. Researchers can guide and monitor the daily training of patients through the physician side of the device to ensure the effectiveness and applicability of the training. Considering the impact of underlying diseases such as hypertension and diabetes on the cognitive function of enrolled patients, all participants were optimally managed for cardiovascular disease in order to minimize the impact of factors other than the intergroup study intervention on the outcome event.
Secondary outcomes will compare changes in processing speed, working memory, episodic memory, visuospatial equigraded cognitive domains, patient self-efficacy score, patient quality of life score, and patient anxiety and depression score after cognitive training from baseline to more finely assist in assessing the effectiveness of digital drugs in patients with coronary heart disease. Exploratory outcomes will study the effects of cognitive training on brain structure and function as measured by MRI to explore the anatomical mechanisms by which cognitive training improves cognitive function.
To improve the accuracy and reliability of the trial, patients were randomly grouped by age and education level. After patient enrollment and baseline data collection, the IWRS system was used to ensure the confidentiality of the grouping. The assessment of clinical outcome cognitive function was performed using an independent BCAT system, allowing statisticians to analyze the data without knowing the grouping. In order to ensure the smooth conduct of the research, all participating centers must complete the training of researchers in data collection and reporting. Throughout the clinical study process, the CRO will conduct regular inspections of all study participating centers.
To the authors’ knowledge, this is the first study to examine the effects of computer-based cognitive training for improving cognitive function in patients with coronary heart disease. The results of this study can provide a new treatment idea for patients with coronary heart disease complicated with cognitive impairment and help fill the evidence gap on the role of digital therapy in such patients. The results may be informative to future clinical guidelines.
Trial status
At present, the study has completed the enrollment of all patients and entered the follow-up and data collection stage. The study completed the first patient enrollment on January 18, 2023, and the final patient enrollment on November 17, 2023, a total of 224 patients. The last follow-up of the last patient is expected to be on May 19, 2024.
The actual sample size is larger than the calculated sample size, which is specifically explained here. During the initial implementation of the scheme, there were 32 subjects whose MRI data were not collected in the baseline period, which belonged to the scheme deviation. In order to ensure the integrity of the exploratory outcome indicators, 24 subjects were added to the original sample size during the inclusion process. The confidence in the original sample size was calculated using the primary outcome measure, so the primary endpoint was not missing and was not affected in any way. This study was a randomized double-blind trial, and all subjects were blinded in the study, so the randomness of the scheme was not affected.
Reasons for not submitting before the completion of patient enrollment: In order to balance the number of nuclear magnetic indicators collected in the regions where each center is located, 24 more exploratory indicators were included in the Beijing area, and in order to obtain amended ethical records, the submission was not submitted before the completion of patient enrollment.
Acknowledgements
We thank the patients, the staff, Beijing Wispirit Technology Co. and Chinese Society of Cardiology who have supported this study.
Patient and public involvement
Patients and/or the public were not involved in the design, conduct, reporting, or dissemination plans of this research.
Patient consent for publication
Not applicable.
Abbreviations
- CHD
Coronary heart disease
- FAQ
Function activity questionnaire
- BCAT
Basic cognitive ability test
- MMSE
Simple Mental State Examination Scale
- MoCA
Montreal Cognitive Assessment Scale General Self-Efficacy Scale
- GSES
General Self-Efficacy Scale
- EQ-5D
EuroQol Five Dimensions Questionnaire
- PHQ-9
Patient Health Questionnaire-9
- GAD-7
Generalized Anxiety Disorder-7
- CCC
Central Coordination Centre
- CI
Confidence interval
- SD
Standard deviation
- CRF/eCRF
Case Report Form/Electronic Case Report Form
- DSMB
Data and Security Monitoring Board
- EC
Ethics Committee
- EDC
Electronic data acquisition system
- GCP
Clinical trial quality management practice
- CRO
Contract Research Organization
- IC
Informed consent
- ICMJE
International Medical Journal Editorial Board
- ITT
Intentional therapy
- PI
Principal investigator
- SC
Steering Committee
Authors’ contributions {31b}
Principal investigator: Professor Ma Changsheng, National Clinical Research Centre for Cardiovascular Diseases, Beijing Anzhen Hospital, Capital Medical University. Professor Zeng Yong, National Clinical Research Centre for Cardiovascular Diseases, Beijing Anzhen Hospital, Capital Medical University. 2. Responsibilities of each party: This study is a clinical study jointly initiated by the funder and the investigator. The research funder participated in the study design, but did not participate in data collection, management, analysis, interpretation, report writing, publication, and other activities related to the study. Important scientific decisions and research management for this study were made by the Steering Committee. Contract Research Organization (CRO): a contract clinical research management organization commissioned by the sponsor or sponsor is responsible for the project management and coordination of the operation of the study. Responsibilities include, but are not limited to: project and data management; coordination with the members of the various committees of the study; assistance in the preparation of ethics committee submissions and progress reports by the investigators; assistance in the preparation of and improvement of the study protocol; training of the personnel of the participating units of the study; research center start-up; monitoring and auditing; monitoring of data quality and data security; monitoring of the study protocol, medical guidelines, compliance and implementation of laws and regulations; and assisting with the organization and preparation of research data and articles for publication. The Steering Committee (SC), composed of experts in neurology, cardiology, and clinical research, is responsible for approving the final study protocol and making decisions on important issues during the conduct, analysis, and reporting of clinical studies. The Steering Committee has the authority to add new members to enhance the integrity of research execution and research analysis. The Steering Committee also includes funded principal investigators. Data Management team: responsible for maintaining research IT systems and data entry and validation. In this study, the Electronic Data Capture (EDC) system will be used for data collection and management, and the Interactive Web Response System (IWRS) will be used for central randomization. The data management team will design electronic CRF forms, design and maintain electronic medical records, and monitor data quality during project implementation.
Funding {4}
Funding for this study was supported by Beijing Wispirit Technology Co., Ltd. and Chinese Society of Cardiology’s Foundation (Project code: CSC2023A03).
Data availability {29}
The DSMB will monitor the data transfer and sharing process during the study and receive input from the Data Management Board. The principal investigator will have access to the cleansed data set. The data sets for this study are stored on a data server and/or file transfer protocol center created specifically for this study, and all data sets will be password protected. Principal investigators at each research center have access to their own data sets and can request access to data sets at other centers. To ensure confidentiality, data shared with project team members will not contain any identifiable participant information.
Declarations
Ethics approval and consent to participate {24}
The research protocol has been reviewed and approved by the Ethics Committee of Beijing Anzhen Hospital affiliated to Capital Medical University (Ethics code: (2022) Research Review No. (17–2)). All eight centers (Beijing Anzhen Hospital affiliated to Capital Medical University, Peking University Third Hospital, The Second Hospital of Chifeng, The First Affiliated Hospital of Hebei North University, Handan Central Hospital, The First Hospital of Hebei Medical University, Beijing Sixth Hospital, Inner Mongolia Ordos Central Hospital Kangbashi Department) involved in the study have received ethical approval. The design, implementation, and reporting of this study protocol shall comply with ICH-GCP, applicable local regulations and the ethical provisions of the World Medical Association (WMA) Declaration of Helsinki.
Prior to subject registration, the Ethics Committee overseeing the study will review and approve the study protocol, information about the subjects expected to be enrolled, informed consent, and any subsequent modifications. Prior to the start of the study, the principal investigator must sign the Study Protocol Signature page confirming that he/she agrees to conduct the study in accordance with the study documentation and the instructions and procedures in this study protocol, and to provide all relevant data and records to the auditors, inspectors, IRB/ECs, and regulatory authorities as required. If regulators require an inspection of a participating clinical center, the investigator must notify the CRO immediately.
Consent for publication {32}
Not applicable.
Competing interests {28}
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yi Ye and Qing Chen contributed equally to this work.
References
- 1.Sachdev P, Andrews G, Hobbs MJ, Sunderland M, Anderson TM. Neurocognitive disorders: cluster 1 of the proposed meta-structure for DSM-V and ICD-11. Psychol Med. 2009;39(12):2001–12. [DOI] [PubMed] [Google Scholar]
- 2.Jia L, Du Y, Chu L, Zhang Z, Li F, Lyu D, et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: a cross-sectional study. Lancet Public Health. 2020;5(12):e661–71. [DOI] [PubMed] [Google Scholar]
- 3.Song R, Xu H, Dintica CS, Pan KY, Qi X, Buchman AS, et al. Associations Between cardiovascular risk, structural brain changes, and cognitive decline. J Am Coll Cardiol. 2020;75(20):2525–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosengart TK, Sweet J, Finnin EB, Wolfe P, Cashy J, Hahn E, et al. Neurocognitive functioning in patients undergoing coronary artery bypass graft surgery or percutaneous coronary intervention: evidence of impairment before intervention compared with normal controls. Ann Thorac Surg. 2005;80(4):1327–34 discussion 1334-1335. [DOI] [PubMed] [Google Scholar]
- 5.Saleem M, Herrmann N, Dinoff A, Mazereeuw G, Oh PI, Goldstein BI, et al. Association between endothelial function and cognitive performance in patients with coronary artery disease during cardiac rehabilitation. Psychosom Med. 2019;81(2):184–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tublin JM, Adelstein JM, Del Monte F, Combs CK, Wold LE. Getting to the heart of Alzheimer disease. Circ Res. 2019;124(1):142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hill NTM, Mowszowski L, Naismith SL, Chadwick VL, Valenzuela M, Lampit A. Computerized cognitive training in older adults with mild cognitive impairment or dementia: a systematic review and meta-analysis. Am J Psychiatry. 2017;174(4):329–40. [DOI] [PubMed] [Google Scholar]
- 8.Sherman DS, Mauser J, Nuno M, Sherzai D. The efficacy of cognitive intervention in mild cognitive impairment (MCI): a meta-analysis of outcomes on neuropsychological measures. Neuropsychol Rev. 2017;27(4):440–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bahar-Fuchs A, Barendse MEA, Bloom R, Ravona-Springer R, Heymann A, Dabush H, et al. Computerized cognitive training for older adults at higher dementia risk due to diabetes: findings from a randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2020;75(4):747–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kong HY, Cheng DM, Pang W, Sun SD, Liu J, Huang CY, et al. Homocysteine levels and cognitive function scores measured with MMSE and BCAT of middle-aged and elderly subjects in Tianjin City. J Nutr Health Aging. 2013;17(6):527–32. [DOI] [PubMed] [Google Scholar]
- 11.Caruso R, Pittella F, Zaghini F, Fida R, Sili A. Development and validation of the Nursing Profession Self-Efficacy Scale. Int Nurs Rev. 2016;63(3):455–64. [DOI] [PubMed] [Google Scholar]
- 12.Balestroni G, Bertolotti G. EuroQol-5D (EQ-5D): an instrument for measuring quality of life. Monaldi Arch Chest Dis. 2012;78(3):155–9. [DOI] [PubMed] [Google Scholar]
- 13.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–7. [DOI] [PubMed] [Google Scholar]
- 15.Tang Y, Xing Y, Zhu Z, He Y, Li F, Yang J, et al. The effects of 7-week cognitive training in patients with vascular cognitive impairment, no dementia (the Cog-VACCINE study): a randomized controlled trial. Alzheimers Dement. 2019;15(5):605–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The DSMB will monitor the data transfer and sharing process during the study and receive input from the Data Management Board. The principal investigator will have access to the cleansed data set. The data sets for this study are stored on a data server and/or file transfer protocol center created specifically for this study, and all data sets will be password protected. Principal investigators at each research center have access to their own data sets and can request access to data sets at other centers. To ensure confidentiality, data shared with project team members will not contain any identifiable participant information.