Abstract
The current study outlines a consistent and reproducible protocol for the routine clinical dose preparation of [68Ga]Ga-Pentixafor using the Eckert and Ziegler ‘Modular-Lab Standard’ non-cassette based automated module, that can be effectively used in the hospital radiopharmacy unit of a high volume nuclear medicine centre. The pre-clinical studies (including in-vitro cell line studies, in-vivo PET/CT imaging and pre-clinical dosimetry) were conducted to show the promising potential of the product for clinical use in targeting CXCR4 tumor overexpression. PET/CT image of SCID mouse bearing lymphoma xenograft tumor, at 2 h post-injection, clearly delineated the tumor. The pre-clinical dosimetry results show the suitability of the product for clinical use in patients. [68Ga]Ga-Pentixafor when administered to patients with primary aldosteronism exhibited distinct uptake in the adrenal nodules. The clinical PET/CT scan of the patients demonstrated the potential use of CXCR4 targeted imaging as a promising surgical decision-making tool for patients with primary aldosteronism.
Keywords: [68Ga]Ga-Pentixafor, Preclinical dosimetry, PET/CT, CXCR4 receptor
Subject terms: Health care, Molecular medicine
Introduction
The prominent role of chemokine (C-X-C motif) receptor 4 (CXCR4) and its endogenous ligand stromal derived factor 1 (SDF-1α / CXCL12) has been well established in a variety of tumor growth1. The preclinical studies have revealed the importance of CXCR4/CXCL12 interaction between cancer cells and tumor microenvironment (TME)2, thus demonstrating the crucial role played by overexpressed CXCR4 in the growth, progression, invasiveness and metastasis process of tumor, mainly of haematological origin3. However, the role of CXCR4 has also been reported in solid tumors of various origins namely adrenals, breast, prostate, small cell lung, melanoma etc4. Towards this, three different types of molecular probes were developed for positron emission tomography (PET) imaging of over expressed CXCR4 namely (i) analogs of bicyclams, (ii) T-140 based peptides and (iii) FC-131 based cyclic pentapeptides5. Among the three types, cyclic pentapeptides were found to exhibit high affinity for overexpressed CXCR4 and a faster renal clearance6. The suitable pharmacological and pharmacodynamic behaviour of small cyclic pentapeptide – Pentixafor, has established its potential as a promising vector for radiolabeling with 68Ga for use as a PET radiotracer for imaging overexpressed CXCR46.
The presence of macrocyclic chelator DOTA moiety in the commercially available cyclic pentapeptide molecule (Pentixafor) makes it suitable for convenient radiolabeling with Gallium-68 as compared to Fluorine-186,7. The radiolabeling of this CXCR4 binding peptide with Fluorine-18 requires a pseudo-radiometal complex namely aluminium-[18F]fluoride (Al[18F]F)8. The Al[18F]F2+ forms stable complexes with pentadentate ligands like 2,2’,2”-(1,4,7-triazacyclononane-1,4,7-triyl)triacetic acid (NOTA), hence cyclic pentapeptide (CPCR2.4 scaffold) was required to be coupled with NOTA for radiolabeling with Fluorine-18. In this case, the reaction was carried out in two steps viz. (i) coupling of CPCR2.4 scaffold with NOTA and (ii) radiolabeling NOTA-pentapetides with Fluorine-187.
The rapid and high accumulation of [68Ga]Ga-Pentixafor with low non-specific background uptake, in different lympho-proliferative diseases, offer advantages of the possibility of theranostic usage involving high contrast PET/CT imaging, for in-vivo assessment of CXCR4 expression kinetics, as well as grading of the CXCR4 overexpressed tumor for targeted therapy, while using Lutetium-177 or Yttrium-90 as the therapeutic matched pairs3,9. Apart from oncological studies, [68Ga]Ga-Pentixafor has played a pivotal role in non-invasive detection of various non-oncological diseases like myocardial infarction, atherosclerosis, urinary tract infection etc10–12.
The stringent regulatory approval necessitates the formulation and characterization of the in-house produced [68Ga]Ga-Pentixafor to be in accordance with the guidelines mentioned in current Good Manufacturing Practice (cGMP) and current Good Radiopharmacy Practice (cGRPP)13,14. The first standard operating procedures (SOP) defined in these cGMP and cGRPP guidelines were to carry out the radiopharmaceutical formulations using all GMP grade active product ingredients (APIs) in automated radiochemistry modules, with minimal or no human intervention13,14. Apart from the automated radiosynthesis and physicochemical characterization, the entire in-vitro and in-vivo pharmacological studies were to be carried out to establish the suitability of the [68Ga]Ga-Pentixafor for clinical use in human.
In the present study, we have optimized a cGMP-compliant automated radiosynthetic method for obtaining clinical grade [68Ga]Ga-Pentixafor, using a non-cassette based Eckert and Ziegler Modular-Lab Standard (EZ-ML) radiochemistry module. The quality control parameters of the produced [68Ga]Ga-Pentixafor were validated in accordance with Gallium (68Ga) Edotreotide® injection ([68Ga]Ga-DOTA-TOC) included in the European Pharmacopoeia- with respect to its suitability for clinical usage. The complete preclinical characterization performed with [68Ga]Ga-Pentixafor includes (i) in-vitro cell binding analysis of receptor affinity and specific binding; (ii) in-vivo distribution studies in tumor xenografted mice bearing lymphoma. Dosimetry studies being a mandatory requirement as part of preclinical evaluation, preclinical dosimetry was conducted in animal models. Extrapolation of animal bio-kinetics data to human reference adult male was carried out using Organ Level Internal Dose Assessment / Exponential Modeling (OLINDA/EXM) version 2.0 software, in order to assess the predictive values for, (i) organ absorbed dose coefficient (in mGy MBq−1) and (ii) whole body effective dose coefficient (in mSv MBq−1) for clinical translation to patients15.
PET/CT images of three patients with primary aldosteronism, showed distinct uptake of [68Ga]Ga-Pentixafor, in the CT described adrenal gland nodules, thereby helping in surgical decision making for such patients using the CXCR4-targeted molecular imaging tool.
Results
Automated radiosynthesis of [68Ga]Ga-Pentixafor in modular-lab standard
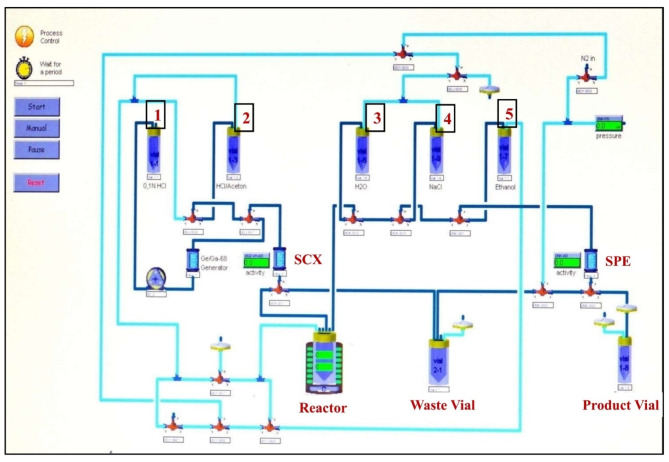
Using Modular-Lab Standard (a fixed tubing or non-cassette based fully automated radiochemistry module), radiosynthesis of pharmaceutical grade [68Ga]Ga-Pentixafor, with a total synthesis time of (15 ± 2) minutes was carried out (Fig. 1). The decay corrected RCY of all the produced batches of [68Ga]Ga-Pentixafor (n = 16) were in the range of (97.11 ± 0.60)%. The decay-corrected RCY of [68Ga]Ga-Pentixafor was calculated considering 15 min from the end of elution (EOE) of [68Ga]GaCl3 to the end of synthesis (EOS).
Fig. 1.
Eckert-Ziegler Modular Lab Standard schematic for the production of [68Ga]Ga-Pentixafor.
Quality control of [68Ga]Ga-Pentixafor
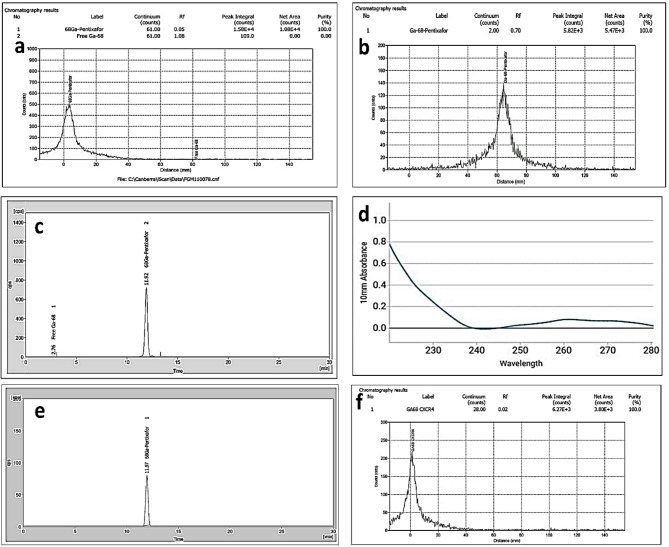
In-house produced [68Ga]Ga-Pentixafor (n = 16) was found to be clear and colourless, with pH in the range of 5.0–6.0. The radioactive concentration (RAC) was maintained at (0.122 ± 0.003) GBq/mL. The RCP of [68Ga]Ga-Pentixafor (n = 16), was estimated by radio-TLC using two different solvent systems for estimating free [68Ga]Ga3+ and [68Ga]Ga-Colloid in the final product. The RCP was found to be (99.2 ± 0.4)% in 0.1 M sodium citrate buffer (pH: 5.0) and (99.42 ± 0.47)% in 1 M CH3COONH4 (pH: 3.5)/CH3OH (1/1, v/v), with Rf: 0.05 (Fig. 2A) and 0.70 (Fig. 2B) respectively. The RCP of all the produced batches of [68Ga]Ga-Pentixafor (n = 16) ascertained by HPLC was (99.4 ± 0.3)% with Rt ~11.9 min (Fig. 2C).
Fig. 2.
Radio TLC chromatogram of [68Ga]Ga-Pentixafor in (a) 0.1 M Sodium Citrate Buffer (pH ~ 5.0) Rf = 0.05 and (b) 1 M CH3COONH4 (pH:3.5) /CH3OH: 1/1 (v/v) Rf =0.70. (c) Radio-HPLC chromatogram of [68Ga]Ga-Pentixafor (Rt: 11.92 min). (d) UV/VIS spectrum of [68Ga]Ga-Pentixafor at 220 nm. (e) Radio-HPLC of [68Ga]Ga-Pentixafor (Rt: 11.97 min) at 4 h post radiolabeling, without stabilizer, on storage, at room temperature 25oC. (f) Radio-TLC of [68Ga]Ga-Pentixafor in 0.1 M Sodium Citrate Buffer (Rf: 0.02) after 1 h incubation (in healthy human serum, at 37oC) & on further storage up to 1 h at 25oC.
The concentration of peptide (Pentixafor) was found to be (2.91 ± 0.04) µg/mL (Fig. 2D) (n = 16), as estimated by spectrophotometric method using Microvolume Spectrophotometer. The specific activity of [68Ga]Ga-Pentixafor (n = 16) was found to be (41.9 ± 0.9) MBq/µg. After eluting the final product from plus C18 Sep-Pak cartridge, using 1.0 mL of 50% aqueous ethanol and further diluting with 10 mL of saline, the residual ethanol content in the final product was (4.4 ± 0.4)%, as estimated by gas chromatography. The endotoxin levels for all the produced batches of [68Ga]Ga-Pentixafor (n = 16) were < 25 EU/mL, and all of them were found to be sterile.
In-vitro saline stability of [68Ga]Ga-Pentixafor was (99.02 ± 0.50)% (n = 16) up to 4 h on storage at room temperature (25oC), with RAC ~ 0.122 GBq/mL (without use of any extra stabilizer like ascorbic acid or gentisic acid) as ascertained from the radio-HPLC chromatogram, (Rt: 12 min, Fig. 2E). Radio-TLC chromatogram (Rf: 0.02, Fig. 2F) ascertains the in-vitro serum stability of [68Ga]Ga-Pentixafor with RCP (99.01 ± 0.26)% upon 1 h incubation at 37oC and further storage upto 1 h at 25oC. The RNP of the [68Ga]Ga-Pentixafor (n = 16) was > 99% ascertained by time-decay method and the measured half-life of the final product was between 69 and 71 min. The quality of the produced [68Ga]Ga-Pentixafor with respect to its suitability for clinical usage was compared (Table 1) with the specifications stated in the European Pharmacopoeia of a similar product -Gallium (68Ga) Edotreotide Injections.
Table 1.
Comparison of quality parameters of [68Ga]Ga-Pentixafor prepared using the present method (with respect to its suitability for clinical usge) with that of [68Ga]Ga-Edotreotide as mentioned in Pharmaeuropa 23.2.
| Description | Specifications of [68Ga]Ga-Edotreotide® from Pharmaeuropa 23.2 |
QC parameters of the produced Pharmaceutical grade[68Ga]Ga-Pentixafor | |||
|---|---|---|---|---|---|
| Characteristics of the solution | Clear & colorless | Clear & colorless | |||
| pH | 4.0–8.0 | ~ 5.0–6.0 | |||
| RAC | NA | 0.122 ± 0.003 GBq/mL | |||
| RCY* | NA | (97.11 ± 0.60)%. | |||
| RNI | 62–74 min half-life | 69–71 min half-life | |||
| RNP | ≥ 98% as [68Ga]Ga3+ | > 99% | |||
| RCP | [ 68 Ga]Ga-labeled agent | ≥ 95% | Radio HPLC | (99.43 ± 0.26)% | |
| Radio TLC | (99.24 ± 0.36)% | ||||
| [ 68 Ga]Ga-Colloid | < 2% | Not observed | |||
| [68Ga]Ga 3+ | < 2% | Not observed | |||
| Absolute ethanol | < 10% v/v | (4.4 ± 0.4)% (v/v) | |||
| CP | HEPES content | < 200 µg/V | Not applicable | ||
| Tween 80 content | < 0.1% | Not applicable | |||
| Peptide content |
< 50 µg/Vmax (4.5 µg/mL) |
2.91 ± 0.04 µg/mL | |||
| Endotoxin limit (Gel Clot BET Assay Method) | < 175 EU/V | < 25 EU/mL | |||
| Sterility test (Post-facto) | Absence of any growth on 14 days of incubation | Complies | |||
| In-vitro stability | RCP | ≥ 95% | (99.02 ± 0.50)% | ||
RAC: Radioactive concentration, RCY*: Decay corrected radiochemical yield, RNI: Radionuclide identification, RNP: Radionuclide purity, RCP: Radiochemical purity, CP: Chemical purity, V: Total Volume of Injection, Vmax: Total Volume of final product (In the present setup, it is ~11.0 mL).
In-vitro cell binding studies of [68Ga]Ga-Pentixafor
In-vitro studies carried out in Daudi cells showed specific and rapid binding. The maximum cell binding of (24.5 ± 1.1)% was observed on incubating 0.2 pmol of [68Ga]Ga-Pentixafor with 3 × 106 cells (n = 3), for 60 min. The results of the % cell binding with different numbers of cells are shown in Fig. 3. Inhibition studies carried out by receptor blocking for the analysis of non-specific binding (NSB) showed that the percentage cell binding reduced to (0.3 ± 0.1)% when one million cells (1 × 106) were incubated with an addition of 0.2 nmol of non-radiolabeled DOTA-Pentixafor. This indicates the specificity of the radioconjugate for the CXCR4 receptor.
Fig. 3.
In-vitro cell binding assay result: Cell concentration (x106/mL) versus Percentage cell binding (n = 3).
In-vivo pharmacological behaviour of [68Ga]Ga-Pentixafor
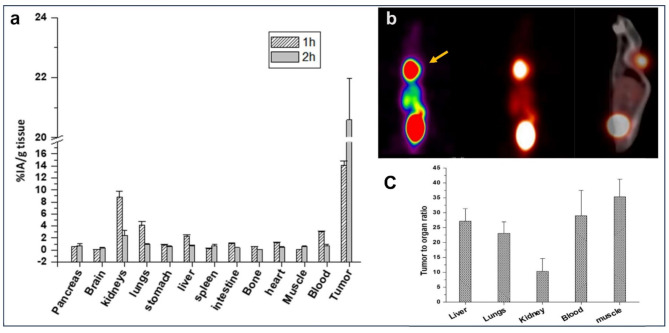
Biodistribution studies in tumor bearing SCID mice showed moderate uptake of (8.8 ± 0.9)% IA/g in the kidneys, (4.4 ± 0.6)% IA/g in the lungs and (2.3 ± 0.3)% IA/g in the liver, at 1 h p.i. However, these values decreased to (2.4 ± 0.9), (0.9 ± 0.2) and (0.8 ± 0.1)% IA/g for kidneys, lungs and liver respectively, after 2 h p.i. Other organs like stomach, intestine and heart showed low uptake of (0.9 ± 0.1), (1.1 ± 0.1), (1.2 ± 0.1)% IA/g respectively, at 1 h p.i., and cleared to negligible values within 2 h p.i. The results of biodistribution in SCID mice are given in Fig. 4A. PET/CT image of SCID mouse bearing lymphoma xenograft tumor, at 2 h p.i clearly delineated the tumor (Fig. 4B). Tumor uptake was (20.6 ± 1.4)% at 2 h post-injection (n = 3), with tumor-to-blood ratio 28.9 ± 8.0 (Fig. 4C).
Fig. 4.
(a) Biodistribution data in SCID mice bearing xenografted lymphoma tumor (n = 3). (b) PET/CT image of SCID mice bearing lymphoma tumor, injected with [68Ga]Ga-Pentixafor and imaged 2 h post-injection. (c) Tumor-to-organ ratio of [68Ga]Ga-Pentixafor in SCID mice 2 h post-injection.
Preclinical dosimetry studies
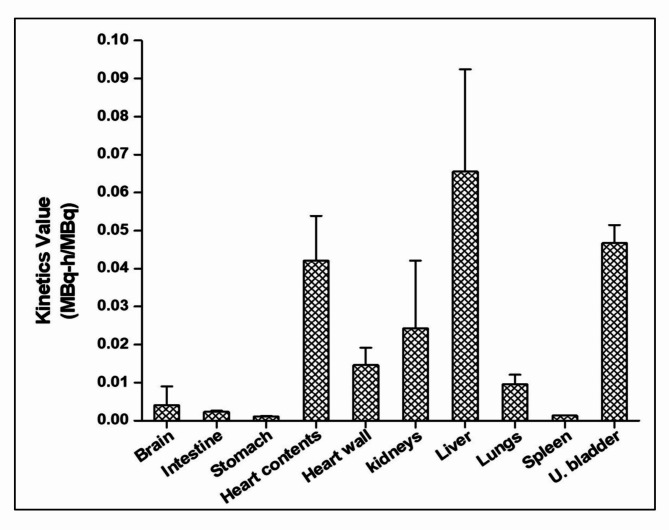
The total body effective dose coefficient for the PET tracer [68Ga]Ga-Pentixafor was estimated to be (1.10E-02 ± 4.04E-04) mSv MBq−1. The PET imaging dosage required for achieving sufficient image contrast in patients has been reported to be (150 ± 50) MBq at (60 ± 15) minutes post-administration of the tracer16. The mean whole body effective dose for a standard PET imaging protocol with administration of 150 MBq, in an adult patient, is estimated to be (1.65 ± 0.06) mSv, which is low and in the range of diagnostic medical exposure. The mean organ absorbed dose coefficient was highest for the urinary bladder wall (6.44E-02 ± 4.60E-03) mGy MBq−1, since renal excretion is the major excretory pathway observed for the radioconjugate. The absorbed doses observed (in mGy MBq−1) were lesser for the organs such as heart (3.93E-02 ± 1.34E-02), kidneys (3.85E-02 ± 2.56E-02), liver (2.12E-02 ± 7.50E-03) and other organs as per the results given in the Table 2. The dose estimates were in the comparable range as reported in the literature for 68Ga-labeled PET radiotracers17. The number of disintegrations per unit administered activity (in MBq-h/MBq) of the radioconjugate in the organs of a reference man (extrapolated from mice data) was highest for liver (0.065 ± 0.027), followed by urinary bladder contents (0.047 ± 0.005), heart contents (0.042 ± 0.012), kidneys (0.024 ± 0.018), heart wall and (0.015 ± 0.005) and lungs (0.01 ± 0.003). The results are depicted in the Fig. 5.
Table 2.
Comparison of estimated organ-absorbed dose coefficients (mGy MBq−1) and total body effective dose coefficient (mSv MBq−1) obtained in the present study (extrapolated to human from mice data) with previously reported investigations.
| Organs | Organ absorbed dose coefficients (mGy MBq−1) | ||
|---|---|---|---|
| Present study (n = 3) | Ken Hermann et al. | ||
| Mean | SD | ||
| Adrenals | 1.47E-02 | 2.07E-03 | 1.23E-02 |
| Brain | 6.82E-03 | 4.32E-03 | 1.00E-02 |
| Gall bladder wall | 1.32E-02 | 2.10E-03 | 1.46E-02 |
| Small intestine | 1.28E-02 | 6.66E-04 | 1.23E-02 |
| Stomach wall | 1.20E-02 | 6.56E-04 | 1.19E-02 |
| Heart wall | 3.93E-02 | 1.34E-02 | 2.65E-02 |
| Kidneys | 3.85E-02 | 2.56E-02 | 3.50E-02 |
| Liver | 2.12E-02 | 7.50E-03 | 1.75E-02 |
| Lungs | 7.04E-03 | 7.45E-04 | 1.10E-02 |
| Pancreas | 1.17E-02 | 7.21E-04 | 1.28E-02 |
| Red marrow | 8.67E-03 | 6.68E-04 | 1.40E-02 |
| Osteogenic cells | 8.01E-03 | 6.87E-04 | 1.84E–02 |
| Spleen | 7.46E-03 | 1.40E-04 | 5.38E-02 |
| Urinary bladder wall | 6.44E-02 | 4.60E-03 | 1.23E-02 |
| Total body effective dose (mSv/MBq) | 1.1E-02 | 4.04E-04 | 1.56E-02 |
Fig. 5.
Number of nuclear transformations in the organ per unit administered activity (Kinetics value) of the PET tracer [68Ga]Ga-Pentixafor, extrapolated to adult reference male (ICRP 89) from mice (n = 3), using OLINDA/EXM 2.0 software.
Clinical PET/CT imaging of patient
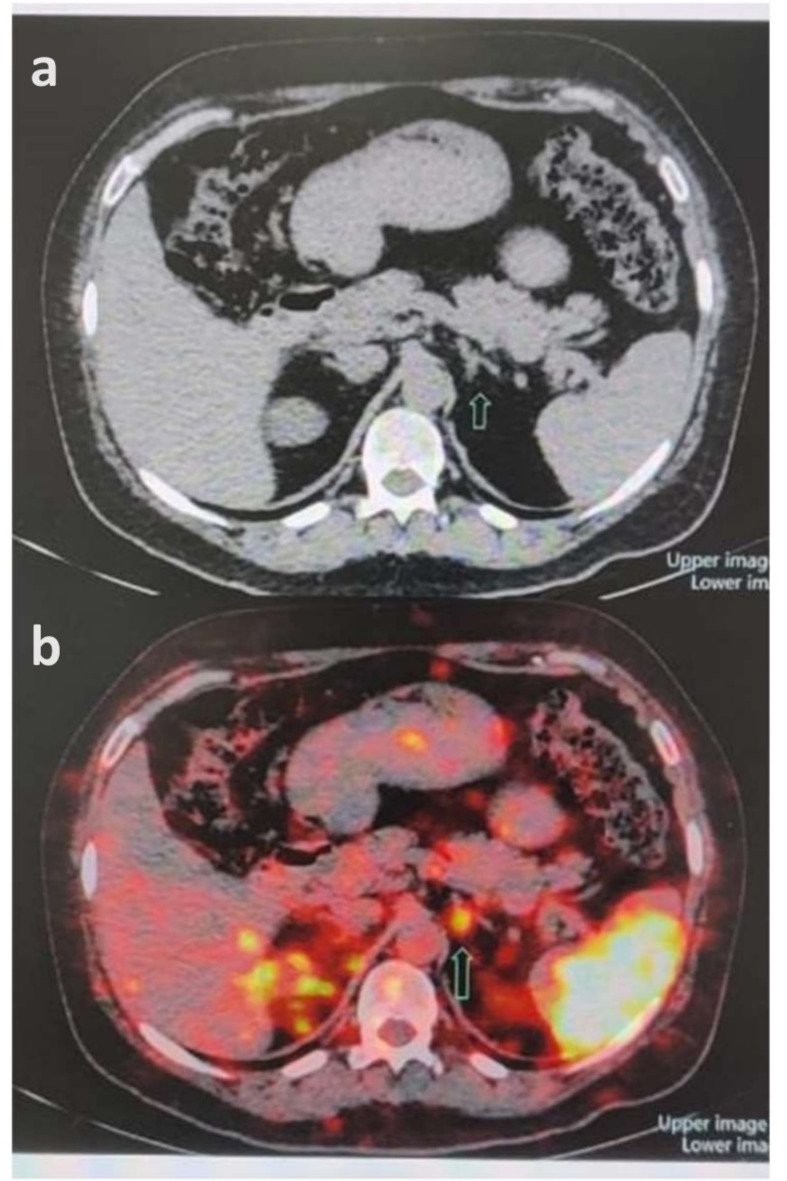
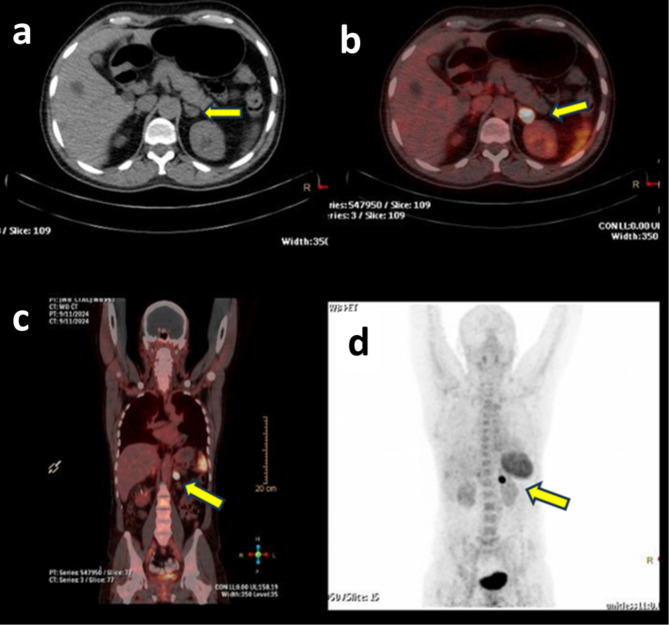
In patient 1, transaxial CT (Fig. 6A) and [68Ga]Ga-Pentixafor PET/CT (Fig. 6B) of a case of primary aldosteronism (PA) showed an intense tracer avid focus (SUVmax 9.37) corresponding to the CT-described nodule in the medial limb of left adrenal gland. PET/CT image of the patient 2, showed a focus of CXCR4 expression in the nodule arising from lateral limb of left adrenal gland 1.2 × 2.7 × 2.6 cm (SUVmax: 11.0) [Fig. 7B and D] at the CT described nodule [Fig. 7C]. Physiological uptake of the tracer was noted in the spleen, kidneys and bladder [Fig. 7A]. In patient 3, [68Ga]Ga-Pentixafor PET-CT scan showed a focus of CXCR4 expression (SUV max: 22.96) [Fig. 8B, C and D] corresponding to the CT described nodule [Fig. 8A] in the lateral limb of left adrenal gland.
Fig. 6.
(a) Transaxial CT of patient 1 and (b) [68Ga]Ga-Pentixafor PET/CT showing intense tracer avid focus (SUVmax 9.37) corresponding to the CT depicted nodule in the medial limb of left adrenal (arrows).
Fig. 7.
(a) MIP projection PET-CT image of patient 2 at one hour after i.v injection of 4.6 mCi of [68Ga]Ga-Pentixafor. (b) PET-CT coronal fused image showing intense tracer uptake in the nodule at lateral limb of left adrenal gland (arrows). (c) Transaxial CT image. (d) [68Ga]Ga-Pentixafor trans axial PET/CT fused image showing intense tracer uptake at the CT described nodule (SUVmax 11.0).
Fig. 8.
(a) Trans axial CT image of patient 3 indicating distinct nodule in the left adrenal gland. (b) [68Ga]Ga-Pentixafor trans axial PET/CT fused image showing intense tracer uptake in the left adrenal gland (arrows) (SUV max: 22.96). (c) PET/CT coronal fused image showing intense tracer uptake at the CT described nodule in the lateral limb of left adrenal gland. (d) MIP projection PET-CT image at one hour post injection of 5.0 mCi [68Ga]Ga-Pentixafor.
Discussion
In the present study, an attempt has been made to establish the feasibility of producing pharmaceutical grade [68Ga]Ga-Pentixafor, using the existing general-purpose automated radiochemistry module (Modular-Lab Standard), in high throughput nuclear medicine centres (NMCs) equipped with a dedicated hospital radiopharmacy. The nuclear medicine centres (NMCs) like in the present case, equipped with therapy wards for housing of the patients, have a high demand of various types of [68Ga] radiolabeled radiotracers, for staging of various malignancies, prior to carrying out radionuclide therapy. The hospital radio pharmacies of such high throughput NMCs demand routine production of two or three different [68Ga] radiolabeled radiotracers on a single day, for clinical applications18.
With the constraints of space and budget, a dedicated radiochemistry module for radiosynthesis of each of the three different [68Ga] radiolabeled radiotracers {[68Ga]Ga-DOTATATE, [68Ga]Ga-PSMA-11 and [68Ga]Ga-Pentixafor} was not feasible in the hospital radiopharmacy setting of our centre19. In the present scenario, to circumvent such situation, use of cassette based automated modules has been reported for the formulation of different radiotracers on a single day20. However, such cassette based automated modules have several limitations which includes (i) adaptability (i.e. requirement for development of different fluidic labelling kit for different radiotracers) and (ii) high cost of the fluidic labelling kit, since these sterile fluidic labeling kits are for single use21. In the last few years, there have been several reports on the automated radiosynthesis of [68Ga]Ga-Pentixafor, using various commercially available modules viz. Modular Lab PharmaTracer (MLPT) and Modular-Lab EAZY (MLE) from Eckert and Ziegler, miniAiO from Trasis, Gaia/Luna from Elysia-Raytest and GRP from Scintomics. The common feature among all these automated modules is that, they are based on a single-use, disposable fluidic labeling kit22–26. These disposable fluidic labeling kits consisting of (i) reagents prefilled in colour coded vials or syringes, (ii) cassettes/hardware kits (having multiple stopcock manifolds connected to tubing with luer connector) etc., for the preparation of different radiotracers. These were mainly available from different manufactures namely Synthera®+ (IBA Radiopharma Solutions), iQS-TS (ITM Medical Isotopes), miniAIO (Trasis) etc. Especially these cassettes/hardware kits are meant for single-time use, since the kits are equipped with radiofrequency identification (RFID) chip scanner for automated recognition, of the bar-coded lot number of each of these kits27. In addition to the high cost of producing [68Ga]Ga-Pentixafor, there are operational challenges associated with synthesizing it using cassette-based automated modules. These challenges stem from the uncertainty in sourcing RFID chip-based, radiotracer-specific cassettes from commercial manufacturers28.
To obviate the issues related to sourcing of RFID chip-based radiotracer-specific cassettes, we developed a method for synthesizing [68Ga]Ga-Pentixafor using the Modular-Lab Standard, which is a fixed tubing-based automated module. This approach utilizes sterile, pyrogen-free buffers and solvents prepared in-house. As a result, we were able to synthesize multiple products—such as [68Ga]Ga-Pentixafor, [68Ga]Ga-DOTATATE, and [68Ga]Ga-PSMA-11, on the same day at every three hours interval and within the same module, without any risk of cross-contamination, while maintaining consistently high RCP for each product.
The decay-corrected RCY (97.11 ± 0.60)% and RCP (99.43 ± 0.26)% were consistent, reliable and reproducible for automated routine preparation of clinical dosages of [68Ga]Ga-Pentixafor. Consequently, the produced [68Ga]Ga-Pentixafor became available to patients at a much more affordable price. Apart from the cost-efficiency of the produced [68Ga]Ga-Pentixafor, the EZ Modular-Lab Standard (fixed tubing-based automated module) is used for routine preparation of various other [68Ga]Ga- based radiotracers namely [68Ga]Ga-DOTATATE, and [68Ga]Ga-PSMA-11 at our centre, without procuring any reagents and solvents other than peptides, thus establishing the enhanced flexibility of the use of Modular-Lab Standard as well as achieving cost-benefits.
This has provided the necessary impetus to the present work. In a hospital radiopharmacy setting like ours, it is important to meet the needs of a high-throughput nuclear medicine centre by providing cost-effective options, using in-house prepared reagents and solvents for the preparation of radiotracers and avoiding the usage of module (or radiotracer) specific sterile, disposable fluidic labeling kits. This obviates the dependence on the import of sterile, disposable fluidic labeling kits from manufacturers of automated radiochemistry modules. This helps to reduce dependency on imported kits and offers a more efficient solution for routine use.
The protocol for radiolabeling has been thoroughly optimized in a way that the temperature control, timer control, output pressure control for nitrogen air, flow rate and flow direction of solenoid valves need not be modified in the graphical user interface (GUI) based programmed time-list. This was very essential for carrying out formulation of three different [68Ga] radiolabeled radiotracers in the same EZ Modular-Lab Standard on a single day, at different time intervals in the present hospital radiopharmacy settings. A comparison of the automated module used, radiolabeling and the quality control parameters of the produced [68Ga]Ga-Pentixafor described in the earlier reports vis-à-vis the one standardized by us has been presented in Table 3.
Table 3.
The automated radiochemical synthesis of [68Ga]Ga-Pentixafor in the present method vis-a-vis earlier reports.
| Radiochemical synthesis parameters | Spreckel-meyer et al. [22] |
Nader et al. [24] |
Costes et al. [29] |
Daniel et al. [23] |
Watts et al. [26] | Sammartano et al. [25] | Martin et al. [28] | Our Method |
|---|---|---|---|---|---|---|---|---|
| Radiochemistry Module Used | Modular Lab Pharm Tracer (Eckert & Ziegler) |
Modular Lab EAZY (Eckert & Ziegler) |
miniAio (Trasis) |
Gaia/Luna (Elysia-Raytest) |
GRP™ (Scintom- ics) |
GRP™ (Scintom- ics) |
GRP™ (Scintomics) |
Modular Lab Standard (Eckert & Ziegler) |
| Process (Automated / Semi-automated) | Automated | Automated | Automated | Automated | Automated | Automated | Automated | Automated |
| Radiochemistry module type | Cassette-based | Cassette-based | Cassette-based | Cassette-based | Cassette-based | Cassette-based | Cassette-based |
Fixed Tubing (Non-Cassette based) |
| Radiolabeling temperature (o C) & Time (minutes) | 95 & 5 | 110 & 8 | 95 & 10 | 97 & 4 | 125 & 6 | 95 & 10 | 125 & 6 | 95 & 6 |
|
Ligand used for radiolabeling (µg) |
50 | 50 | 50 | 50 | 20 | 20 | 20 | 35 |
|
Total automated radiosynthesis time (minutes) |
18 | 14.5 | NR | 24 | 34 | 33 | NR | 20 |
| RCY*(%) | 80.9 ± 10.0 | 95 | 94.8 ± 2.6 | 90.1 | 73.1 ± 7.7 | 79.8 | 80 | 97.1 ± 0.6 |
| RCP (%) | 99.8 | 99 | 99.9 ± 0.01 | 98.4 | > 99 | 99.9 | > 99 | 99.4 ± 0.3 |
RCY*: Decay corrected radio chemical yield; RCP: Radiochemical purity; NR: Not reported.
An automated cleaning process of the EZ Modular-Lab Standard with 70% aqueous ethanol prior to radiolabeling of each of these [68Ga]Ga- based radiotracers {[68Ga]Ga-Pentixafor, [68Ga]Ga-DOTATATE and [68Ga]Ga-PSMA-11} was carried out to prevent any cross-contamination. Post radiolabeling of each of the [68Ga]Ga radiotracers, the Strata™ SCX (strong cation exchanger cartridges), Sep-Pak® C18 (reversed phase cartridges) and 0.22 μm membrane filter were removed and the module was purged with high purity nitrogen. Automated cleaning process of EZ Modular-Lab Standard was carried out for two times with 70% aqueous ethanol, followed by UPW. Radio-TLC of UPW was carried out in 0.1 M sodium citrate buffer at pH: 5.0 (the same solvent system used for the analysis of [68Ga]Ga-Pentixafor). The radio-TLC chromatograms did not exhibit any radioactive peak, establishing the absence of any residual [68Ga]Ga- radiotracers or free [68Ga]Ga3+, prepared in the previous batch18.
[68Ga] radiolabeled radiotracers demand high RCY due to very short physical half-life of Gallium-68 (t1/2 = 67.8 min). The incorporation of [68Ga]Ga3+ into the macrocyclic chelator (DOTA) moiety of pentixafor ligand requires (i) heating at elevated temperature albeit for a short time period and also (ii) a minimum amount of the ligand necessary for achieving maximum radiocomplexation. The present study also optimized the heating time (6 min, 95oC) for radiolabeling, in order to enhance the dc RCY (97.11 ± 0.60%) of the final product. Exploring different time points, such as 4 and 10 min, provide insights into the radio-complexation process of Pentixafor with [68Ga]GaCl3. Radio-complexation of pentixafor with [68Ga]GaCl3 at 95oC for 4 min resulted in low dc RCY (89.49%) as depicted in Fig. 9B. A review of the methods used by Spreckelmeyer et al.22 and Daniel et al.23 shows a dc RCY of {(80.9 ± 10.0)% and 87.7%}, at 95oC for 5 min heating respectively. This observation served as a reference for our studies, wherein a higher dc RCY (97.11 ± 0.60)% which is ~20.0% and ~10.0% more than the earlier reported methods22,23could be observed under similar reaction conditions. The likely reason for the improvement in the RCY could possibly be attributed to in-process measurements22and retention in 0.22 μm sterile filters23.
Fig. 9.
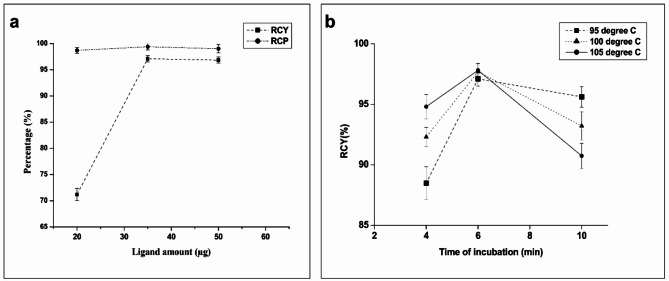
Influence of (a) Ligand amount on RCY & RCP and (b) radiocomplexation time and temperature on final RCY of [68Ga]Ga-Pentixafor.
It is notable that as per reported by Costes et al.29, the radiolabeling protocol which involved heating for 6 min at 95oC could be further improved by increasing heating time to 10 min. However, our results do not reflect a similar pattern, as the dc RCY which was (97.11 ± 0.60)% on heating at 95oC for 6 min decreased on increasing the heating time, as seen from Fig. 9B. The dc RCY (94.8 ± 2.6)% reported by Costes et al., on heating at 95oC for 10 min was low as compared to that of our dc RCY (97.11 ± 0.60)%, which could possibly be attributed to the protocol reported by Costes et al., which did not involve pre-purification or pre-concentration of the 68Ge/68Ga generator eluate29. A review of the radiolabeling parameters reported in the above studies indicates the potential factors that influence the RCY. The possible effect of carrying out radiolabeling at higher temperatures, viz. 100oC and 105oC, on the RCY were also explored. The findings, as depicted in Fig. 9B, were similar to the reports of Nader et al.24.
Achieving a high dc RCY of [68Ga]Ga-Pentixafor with a minimum amount of ligand is crucial for performing PET/CT scan requiring 142 MBq of radiotracers in a maximum volume of 7 mL30. This optimization was essential in order to minimize the peptide content per patient dose and aligns with the goal of obtaining the highest efficiency in radiolabeling for specific PET/CT imaging requirements. Towards this, experiments on different amounts of ligand (20 and 50 µg) in addition to the 35 µg used in our optimized protocol for radiosynthesis of [68Ga]Ga-Pentixafor is pertinent. The results are depicted in Fig. 9A, showing that using 50 µg of ligand does not increase the dc RCY significantly compared to when 35 µg used in our optimized protocol. The lower dc RCY reported by Daniel et al. (87.7%) and Spreckelmeyer et al. (80.9%) on using 50 µg of ligand in comparison to our study, could be attributed to factors such as not accounting the in-process measurements and retention of the final product {[68Ga]Ga-Pentixafor} in the 0.22 μm sterile filters22,23.
However, marginally lower dc RCY reported by Costes et al. (94.8%) and Nader et al. (95%), on using 50 µg of ligand, may be attributed to the experimental factors such as not pre-concentrating the 68Ge/68Ga generator eluate and not rinsing the reactor post passing the reaction mixture through the purification cartridge24,29. The considerations of these yield-limiting factors, in the present study have contributed to higher dc RCY.
On the contrary, low dc RCY (< 75%) was observed on carrying out the radiolabeling with 20 µg of pentixafor, as seen from Fig. 9A. Similar results were reported by Sammartano et al. (dc RCY: ~79.8%) and Watts et al. (dc RCY: ~73.1%), emphasizing that the peptide quantity plays a crucial role in the radiolabeling process for obtaining high RCY25,26.
The high dc RCY (97.11%) of [68Ga]Ga-Pentixafor achieved in our method, while using 35 µg of the peptide (pentixafor), is significant. Lapa et al. reported 142 MBq to be the standard injected dose of [68Ga]Ga-Pentixafor in patients with small cell lung cancer30. In our optimized protocol for radiolabeling, the single injected patient dose of [68Ga]Ga-Pentixafor will have ~ 3–4 µg of the peptide, thus keeping the total peptide content well below 20 µg which is the specified limit per patient dose, and also emphasizing the importance of achieving a high RCY of the final product31. This requirement underlines the significance of not only optimizing the protocol to achieve high RCY of the formulation but also minimizing the peptide (Pentixafor) content in the formulation in order to achieve better image quality.
Preclinical dosimetry was performed to demonstrate that there is no breach of medical exposure safety implications to an adult patient on administration of the product. In-vivo studies conducted in SCID mice bearing lymphoma tumor showed high tumor-to-organ ratios and clearly delineated the Daudi cell line induced tumor, with high imaging contrast at 2 h post-injection PET/CT imaging (Fig. 4B).
Excretion of [68Ga]Ga-Pentixafor was observed to be via the renal route through the kidneys with final collection in the bladder. The organ uptake of [68Ga]Ga-Pentixafor in C57BL/6 mice without tumor xenograft was observed to be highest in the kidneys (~ 6.3% IA/g) as compared to the other vital organs. About (72 ± 9)% injected activity (decay corrected) came out in the excreta within 3 h post-injection (n = 3). Hence urinary bladder wall is the organ receiving the highest absorbed dose. No significant uptake was observed in organs like brain, pancreas and muscle. Highest uptake was observed at 1 h post-injection in all the vital organs, with fast clearance within 3 h p.i.
The highest organ absorbed dose coefficient was observed for the urinary bladder wall (0.064 mGy MBq−1), followed by heart wall, kidneys and liver. The organ absorbed dose coefficient of urinary bladder is comparable to the value 0.08 mGy MBq−1reported by Ken Hermann et al., by PET based patient dosimetry32. For a 150 MBq PET dosage, the average organ absorbed dose to bladder was estimated to be about 9.6 mGy/150 MBq for adult, which is far below the toxicity limit of 65 Gy (TD 5/5) for the organ33. Absorbed dose coefficient for the bone marrow was 0.009 mGy MBq−1, which is close to the estimation of 0.014 mGy MBq−1by the image-based method32. The average effective whole body dose coefficient was 0.011 mSv MBq−1 as estimated in the present study. This value is slightly lower than the reported value of 0.0158 mSv MBq−1 by Ken Hermann et al. The difference can be attributed to the methodology difference of the studies, considering the fact that some patients included in the image-based study by Ken Hermann et al. had considerable uptake and retention of the radiotracer in the lesion, which have contributed to the mean value of the whole-body effective dose coefficient. In the present study, biodistribution data of normal mice without tumor xenograft was extrapolated to reference man for the dose estimation. However, the total body effective dose coefficient is observed to be in the range for other 68Ga-labeled peptides like 68Ga-NODAGA-RGDyK (0.012 mSv MBq−1 as estimated by Buchegger et al.), 68Ga-DOTANOC (0.017 mSv MBq−1 as estimated by Pettinato et al.) and 68Ga-Exendin (0.007 mSv MBq−1as estimated by Marti boss et al.)34,35.
The clinical use of [68Ga]Ga-Pentixafor for targeting CXCR4 overexpression has become increasingly promising in recent years for the diagnosing and disease staging application. The PET/CT images acquired 50–60 min post-injection of [68Ga]Ga-Pentixafor in three patients of primary aldosteronism with adrenal adenoma, demonstrates the clinical efficacy of the product prepared in the EZ Modular-Lab Standard (Figs. 6, 7 and 8).
Conclusion
An efficient, consistent, reproducible and reliable method was developed for synthesizing clinical doses of ready-to-inject [68Ga]Ga-Pentixafor, in a fully-automated process, using Modular-Lab Standard module, generally used for routine production of [68Ga]Ga-DOTATATE and [68Ga]Ga-PSMA-11. This obviates the necessity to procure a dedicated module for [68Ga]Ga-Pentixafor synthesis in those hospital radiopharmacy facilities, equipped with an old EZ Modular-Lab Standard. The QC parameters of the product with respect to its suitability for clinical usage was found to be comparable with Gallium (68Ga) Edotreotide Injections, listed in the European Pharmacopoeia. The studies document that the routine production for clinical doses of [68Ga]Ga-Pentixafor using the methodology reported herein, can be successfully deployed at the hospital radiopharmacy unit of a high volume nuclear medicine centre. This also reduces the production cost of [68Ga]Ga-Pentixafor. The pre-clinical results prove the promising potential of this [68Ga]Ga-Pentixafor for clinical use in patients with CXCR4 tumor overexpression. The estimated whole body effective dose coefficient to patient on administration of the product was estimated to be 0.011 mSv MBq−1, which is low as well as within the range of medical radiation exposure from 68Ga-labeled peptide scintigraphy. The clinical efficacy of [68Ga]Ga-Pentixafor synthesised in the module has been demonstrated in the PET/CT scan of patients with primary aldosteronism. The present study thus merits the possibility of offering, cost effective, non-invasive assessment and staging of tumors with CXCR4 overexpression, by PET/CT imaging.
Methods
Materials
[68Ga]GaCl3 was sourced from a 68Ge/68Ga generator (Matrix: SiO2, [68Ge]Ge4+ breakthrough: ≤ 0.005% of total radioactivity), which was obtained from ITM Medical Isotopes GmbH, Germany. Automated synthesizer (Modular-Lab Standard) was from Eckert & Ziegler, Germany. Pentixafor acetate (DOTA conjugated Pentixafor) was procured from Lympholucin (South Korea). HCl (30%, Suprapur® grade, trace metal basis), sodium acetate trihydrate (purity: ≥ 99.5%, BioUltra grade), glacial acetic acid (purity: ≥ 99.99%, trace metal basis), NaCl (purity: ≥ 99.999%, trace metal basis) and Ethanol (Emsure) were procured from Merck, Germany. Trace SELECT®ultrapure water (UPW) was from Honeywell Research Chemicals, USA. Disposable strong cation exchange resins (SCX), Strata™ were obtained from Phenomenex, USA. Solid phase extraction (SPE) cartridges plus C18 (360 mg) were procured from Waters Corporation, USA. Nitrogen (air purity: 99.999%) was purchased from Inox Air Products, India. Polyether sulfone (PES, pore size: 0.20 μm) membrane syringe filters were procured from Merck, Germany. Non-bleeding pH indicator strips (range: 0–14) were from Merck, India. Sterile, pyrogen-free saline was procured from Nirlife Healthcare, India. Lead shielded bio-safety cabinet (ISO Class 5) was purchased from Microfilt, India. Analytical radio-high performance liquid chromatography (radio-HPLC) was performed using HPLC system equipped with diode array detector (DAD)-UV and radioactive detectors [NaI(Tl)], connected in series, from Knauer, Germany. Radio-thin layer chromatography (radio-TLC) was performed using NaI(Tl) radioactive detector from Bioscan, USA. Gas chromatography was carried out in Chemito 7610 GC instrument, USA equipped with split/splitless injector inlet, flame ionization and thermal conductivity detector using helium as carrier gas. Dose calibrator (CRC 25R) was from Capintec, USA. Unlabeled Pentixafor, as chemical impurity in the final product was quantified by Microvolume Spectrophotometer (Denovix Inc, USA). Endotoxin limit (EL) was quantified by gel-clot BET assay method using LAL reagent from Charles River Laboratories Inc, USA, while the sterility test was performed by direct inoculation method using soyabean casein digest and fluid thioglycolate media from Himedia, India. Human B Cell Burkitt’s lymphoma cell line Daudi [Source: Cell Repository, National Centre for Cell Sciences (NCCS), Pune, India] expressing CXCR4 receptor was used for the in-vitro cell binding studies and for inducing tumor xenografts in severe combined immunodeficient (SCID) mice9,36. SCID mice and C57BL/6 mice used for this study were sourced from the Animal House Facility, Advanced Centre for Treatment, Research and Education in Cancer (ACTREC), Tata Memorial Centre, Government of India (GOI) (65/GO/ReBiBt/S/99/CPCSEA) and Animal House Facility, Bhabha Atomic Research Centre, GOI (106/GO/RBi/S/99/CPCSEA) respectively. PET/CT imaging was carried out using Gemini Digital PET/CT scanner from Philips N.V, Netherlands.
Preparation of reagents and preconditioning of plus C18 SPE cartridges
The reagents for the radiosynthesis of [68Ga]Ga-Pentixafor were prepared under aseptic conditions in a bio-safety cabinet (ISO Class 5 grade).
-
i.
A solution of 0.05 M HCl was prepared by mixing 530 µL of 30% HCl (Suprapur® grade, trace metal basis) with 99.47 mL of ultrapure water (Trace SELECT®). This solution was used for eluting [68Ga]Ga3+ from the 68Ge/68Ga generator.
-
ii.
To prepare the acidified NaCl solution, mixed 250 µL of 30% HCl (Suprapur® grade, trace metal basis) with 10 mL of 5 M NaCl (purity: ≥ 99.999%, trace metal basis). This solution was used for pre-concentration and elution of [68Ga]GaCl3 from the Strata™ SCX cartridge.
-
iii.
1 M CH3COONa buffer was prepared by aseptically mixing 3.6 mL of 1 M CH3COONa solution (purity: ≥ 99.5%, BioUltra grade) with 16.4 mL of 1 M CH3COOH (purity: ≥ 99.99%, trace metal basis). The pH of the resulting 1 M CH3COONa buffer was maintained at ~ 4.0.
-
iv.
For the preparation of the stock solution of Pentixafor, 1 mg of lyophilized ligand/ peptide in the powdered form was dissolved in 1 mL of ultrapure water (Trace SELECT®) and then aliquoted in volumes of 50 µL. The aliquots were stored at −20oC.
-
v.
For the preconditioning of Plus C18 SPE cartridges, 5 mL of ethanol (Emsure grade) was used. After 10 min of ethanol pre-conditioning, we dried the cartridge with 15 mL of air. Finally, we passed 5 mL of ultrapure water (Trace SELECT®) through the cartridge and dried the cartridge with 25 mL of air.
Automated radiochemical synthesis of [68Ga]Ga-Pentixafor
In the present radiopharmacy settings of our facility, the Modular-Lab Standard is being routinely used for the automated production of clinical dosages of [68Ga]Ga-DOTATATE and [68Ga]Ga-PSMA-11. The same fixed tubing (or non-cassette based) Modular-Lab Standard automated radiochemistry module, without any modification, used for the radiochemical synthesis of [68Ga]Ga-Pentixafor is shown in the Fig. 1. Prior to automated radiochemical synthesis of [68Ga]Ga-Pentixafor in Modular-Lab Standard, an automated cleaning and sanitization process of the module with 70% aqueous ethanol and ultrapure water was mandatory.
It is noteworthy that neither of the steps in the existing time lists for radiolabeling sequence programmed with graphical user interface (GUI) software was modified for clinical dosage automated production of [68Ga]Ga-Pentixafor. The following work-flow was applied in the EZ Modular-Lab Standard for the automated production of [68Ga]Ga-Pentixafor.
• step 1- pre-concentration of [68Ga]GaCl3
-
(i)
[68Ga]Ga3+ (~1.61 GBq) in the form of [68Ga]GaCl3 was eluted from the 68Ge/68Ga generator using 4–5 mL of 0.05 N HCl. This solution was then passed through the Strata SCX column. In the SCX column, [68Ga]Ga3+ remained trapped, while any [68Ge]Ge3+ and other non-radioactive metallic impurities were washed out from the column and collected in the waste vial.
-
(ii)
The trapped [68Ga]Ga3+ in the SCX column was pre-concentrated with 512 µL of acidified NaCl (0.2 N HCl in 5 M NaCl) and then eluted into the reaction vessel.
• step 2- radio complexation
512 µL of pre-concentrated [68Ga]GaCl3 (~ 1.61 GBq) was mixed with 2.0 mL of 1 M CH3COONa buffer containing 35 µL of Pentixafor (35 µg, 28.66 nmoles). The reaction mixture was then incubated at 95oC for 6 min at pH ~ 4.0 and cooled to room temperature (25oC) thereafter by adding 2.0 mL of ultrapure water.
• step 3- purification
-
(i)
The reaction mixture was loaded onto preconditioned plus C18 column and the eluate was collected in a waste vial.
-
(ii)
The plus C18 column, loaded with [68Ga]Ga-Pentixafor reaction mixture, was rinsed with 2 mL of sterile pyrogen-free saline to remove unlabeled free [68Ga]Ga3+.
-
(iii)
The purified [68Ga]Ga-Pentixafor (~ 1.34 GBq) was eluted from plus C18 column using 1.0 mL of 50% aqueous ethanol. It was then passed through 0.20 μm PES membrane syringe filter into the final product vial.
-
(iv)
Sterile pyrogen-free saline (10–10.5 mL) was then passed through the plus C18 column and 0.20 μm PES membrane filter to dilute the final product, ensuring that the residual ethanol content of the final product is < 10% v/v.
Quality control of [68Ga]Ga-Pentixafor
The pH of the product was determined by spotting 1–2 µL of the final product and observing the color change of a pH strip with a narrow range. The radiochemical purity (RCP) was evaluated by radio-TLC on a silica gel (60Ao) using two different solvent systems: (i) 0.1 M sodium citrate buffer at pH: 5.0, and (ii) 1 M CH3COONH4 (pH: 3.5)/CH3OH (1/1, v/v). RCP was also determined by radio-HPLC using a gradient mode (solvent: 0.1% TFA in H2O and CH3CN) with the following gradient method: 0–20 min, 90–30% water; 20–21 min, 30–0% water; 21–30 min, 0% water; and 30–40 min, 0–100% water. For HPLC, we used Eurosphere C-18 reversed-phase column (dimension: 300 mm x 4 mm, Particle size: 5 μm) coupled with NaI(Tl) detector and DAD-UV detector (wavelength set at 220 nm) at a flow rate of 1.0 mL/min. The ethanol levels in the final product were detected by gas chromatography using a standardized procedure, with helium as carrier gas and polyethylene glycol capillary column {Dimension: 25 m (L) x 0.32 mm (ID), Thickness: 0.5 μm}. The column was kept at a temperature of 70oC during the operation. The endotoxin limit (EL) was measured using gel-clot BET assay method with lysate sensitivity 0.125 EU/mL at a maximum valid dilution (MVD) of 200. Sterility test was conducted using direct inoculation method.
The in-vitro stability of [68Ga]Ga-Pentixafor in saline was assessed by radio-HPLC on incubating [68Ga]Ga-Pentixafor (RAC: ~0.122 GBq/mL) at room temperature (25oC) upto 4 h. Also, in-vitro serum stability was evaluated by radio-TLC. For this analysis, 100 µL of [68Ga]Ga-Pentixafor (RAC: ~0.122 GBq/mL) was incubated with 300 µL human serum (obtained from healthy volunteers) at 37oC, for 1 h. Post incubation, serum proteins were precipitated using equal volume of acetonitrile, centrifugation at 8000 rpm for 5 min, and the supernatant from the centrifugate were stored further at 25oC (room temperature) upto 1 h. Radio-TLC was performed with the serum samples post 1 h of storage.
The concentration of peptide (Pentixafor) content in the final product was quantified by Bradford Assay at 220 nm, using Microvolume Spectrophotometer with in-built Absorbance Colorometrics App. Pentixafor solutions of known concentrations (6, 5, 4, 3, 2 and 1 µg/mL) were prepared in a volume of 1.0 mL. The standard Pentixafor solutions were prepared in a matrix containing 50% aqueous ethanol and NaCl (0.9%). Each of the standard Pentixafor solutions (50 µL of each standard) were incubated with Bradford reagent (200 µL) at room temperature (25oC) for 5 min. Absorbance was measured for each of the concentrations at 220 nm wavelength. The known concentrations were plotted against the corresponding absorbance resulting in a standard curve. The linearity of the data (r2: 0.996–0.997) for Pentixafor against the standard curve demonstrates that Beer’s law is obeyed over the concentration ranging from 1.0 to 6.0 µg/mL. The concentration of Pentixafor in the samples of [68Ga]Ga-Pentixafor was estimated from the standard curve.
In-vitro cell binding assay and receptor blocking assay
In-vitro analysis of receptor affinity and selectivity of [68Ga]Ga-Pentixafor radioconjugate was carried out by cell binding assays, using Human B Cell Burkitt’s lymphoma cell line Daudi. Daudi cell line was demonstrated as a positive control for CXCR4 expression in the studies conducted by Peter Herhaus et al., and high contrast visualization of tumor induced using Daudi cell line has been demonstrated in the previous studies by Andreas et al.36,37. Hence, for the in-vitro cell binding studies, Daudi cells were used to analyse CXCR4 receptor specificity of the product [68Ga]Ga-Pentixafor.
Cells were grown in a tissue culture dish using Iscove’s Modified Dulbecco’s Medium (IMDM) supplemented by 10% fetal bovine serum (FBS) at 37oC, in 5% CO2. Different concentrations of cells viz. 0.1, 0.25, 0.5, 1, 2 and 3 million cells in 1mL cell binding buffer [RPMI, 0.2% Bovine serum albumin (BSA)], were incubated with 0.2 pmol of radiolabeled agent for 60 min, followed by washing the cells twice with cold phosphate buffered saline (PBS). Receptor blocking analysis was carried out for the estimation of non-specific binding. For this study, non-specific binding assay was carried out by incubating a fixed number of cells (1 × 106 cells) with 0.2 pmol of radiolabeled agent with an addition of 0.2 nmol (1000 times higher concentration) of non-radiolabeled conjugate DOTA-Pentixafor. After washing in cold PBS, the cells were centrifuged and pellets were counted in a well-type NaI(Tl) gamma counter to determine the percentage cell binding.
In-vivo pharmacological behaviour of [68Ga]Ga-Pentixafor
(I) PET/CT imaging of [68Ga]Ga-Pentixafor in tumor-bearing SCID mice
Receptor specific uptake of [68Ga]Ga-Pentixafor was analysed by PET/CT imaging in severe combined immunodeficient (SCID) mice bearing xenografted lymphoma tumor. Towards this, Daudi cell line expressing CXCR4 receptor was used for inducing lymphoma tumor in the mice. Mice were injected subcutaneously with Daudi cells (1 x 106 cells per mouse) at the proximal flank area for tumor induction. Tumor was allowed to grow to about 1cm3 volume. The radiolabeled agent [68Ga]Ga-Pentixafor was injected (~7.4MBq) through the tail vein. PET/CT imaging of mice was carried out, 2h post-injection (p.i.), for the evaluation of the tumor uptake. PET/CT imaging studies were carried out in SCID mice bearing xenografted lymphoma tumor under Isoflurane-oxygen anesthesia, following the ethics guidelines for animal experimentation.
(II) In-vivo biodistribution studies in SCID mice
In-vivo localization of [68Ga]Ga-Pentixafor was analysed in SCID mice bearing lymphoma tumor induced by Daudi cell line. Six mice, divided in to three groups, were injected with [68Ga]Ga-Pentixafor through the tail vein and sacrificed the mice at 60 min and 120 min p.i. time points. The method used for euthanasia of mice involved inducing hypoxia by exposing them to high concentrations of carbon dioxide (CO2). A cylinder of compressed CO2 was used to control the gas inflow into the CO2 chamber. The animals were placed in the chamber and exposed to 100% CO2 at a fill rate of 30% displacement of the chamber volume per minute, with CO2 added to the existing air in the chamber. This asphyxiation method led to rapid unconsciousness in the mice with minimal distress within 3 min. An additional exposure time of 3 min was used for euthanasia. This was immediately followed by bilateral thoracotomy for biodistribution analysis. Subsequently, vital organs were excised and weighed. Quantification of radioactivity associated with each organ and blood was carried out using a flat type Na(I)Tl single channel analyzer. In the in vitro studies and animal studies, three replicates per time point have been used. This is the sample size which is frequently adopted in preliminary radiopharmaceutical evaluations, in order to ensure adequate statistical robustness, while balancing feasibility and ethical considerations for usage of animals. The results are expressed as percentage of injected activity per gram of tissue [% IA/g, mean ± standard deviation of replicates (SD)].
Preclinical dosimetry studies of [68Ga]Ga-Pentixafor
The Medical Internal Radiation Dose (MIRD) schema of internal dosimetry, as implemented in the OLINDA EXM 2.0 software, was used to perform the preclinical dosimetry study38. For this analysis, C57BL/6 mice without tumor xenografts were used. Nine mice, divided into three groups, were used. Biodistribution data of mice at 1 h-, 2 h- and 3 h- p.i. of [68Ga]Ga-Pentixafor, was extrapolated to adult reference man for the estimation of dose. As per the MIRD pamphlet no. 23 notation, the mean absorbed dose D (rT, TD) to the target tissue (rT), from the radioactivity distributed uniformly in the region of source tissue (rS), over a time-integration period (TD), can be written as, D (rT, TD) = ∑rs à (rS, TD)S(rT ← rS), where ‘à (rS, TD)’ is the time-integrated activity (total number of nuclear transformations, otherwise known as cumulated activity) in rS over TD, while ‘S(rT ← rS)’ is absorbed dose in rT per nuclear transformation in rS39.
Organ distribution data of [68Ga]Ga-Pentixafor (non-corrected for the physical decay of radionuclide) in non-tumor bearing C57BL/6 mice were used for the estimation of percentage injected activity (%IA) in each organ at three time points 1 h-, 2 h- and 3 h-. Time-activity curves were plotted by exponential fitting of time post-injection versus percentage injected activity (%IA) in the organ. Activity in the organs/ tissue were integrated from time zero to infinity for the estimation of cumulated activity (MBq-h) of the radiotracer in the organs. The number of disintegrations per unit activity administered (kinetics value or residence time in MBq-h/MBq) was estimated for each organ by dividing cumulated activity by administered activity. To estimate the biological half-life of the radioconjugate, we subtracted the activity eliminated from the animal body through excreta from the injected activity. This allowed to estimate the percentage of injected activity that remained inside the animal body at specific time points. The mouse organ distribution data was extrapolated to human using relative mass scaling method, by applying correction factor for mass differences between mice and human species. The non-decay corrected values of percentages of injected activity (%IA) in the mouse organs were extrapolated to human organs by the equation,
%IAhuman = %IAanimal x [weightbody/weightorgan]animal x[weightorgan/weightbody]human40.
These %IAhumanorgan uptake values were plotted for three time points post injection, for obtaining time-activity curves (TAC) by a mono-exponential fitting, to determine the cumulated activity in each source organ. Activity of the blood pool in the heart cavity, was assigned to ‘heart content’. Activity in the heart after removing the blood inside, was assigned to ‘heart wall’41. The difference in the activity in the body and that in the organs was considered as ‘remainder’40. The number of disintegrations per unit administered activity in each source organ was determined by dividing the cumulated activity in the source organ by the total injected activity. Voiding bladder model of the OLINDA/EXM 2.0 software was used to estimate the number of nuclear transformations in the excretory organ urinary bladder. Organ absorbed doses per unit activity administered (mGy MBq−1) and effective whole-body dose per unit activity administered (mSv MBq−1) to adult human were estimated using OLINDA/EXM 2.0 software, applying S-values (mGy MBq−1 h−1) for adult reference male model (ICRP-89)42. Data have been expressed as mean ± standard deviation (SD).
PET/CT imaging of [68Ga]Ga-Pentixafor in patients
Three patients with Primary Aldosteronism, underwent [68Ga]Ga-Pentixafor PET/CT scan as per the institution protocol. An information sheet explaining the details of the imaging procedure was provided to patients and relatives on the day of consultation. Informed consent was obtained from each patient and relative on the day of the scan. In order to study the [68Ga]Ga-Pentixafor uptake in the adrenal nodules, clinical grade [68Ga]Ga-Pentixafor was formulated using EZ Modular-Lab Standard, and a diagnostic dose was administered to each of the patients and clinical PET/CT imaging after 50–60 min of injection was performed as per the institution protocol.
Patient 1 (male, age 40 years) was suffering from aldosteronism, hypertension, nausea and giddiness with imbalance. CT abdomen showed well defined heterogeneously enhancing nodule at the medial limb of the left adrenal gland. In order to study the [68Ga]Ga-Pentixafor uptake in the nodule, the patient was administered with 52 MBq [68Ga]Ga-Pentixafor. The clinical PET/CT imaging was performed after 60 min of injection. Patient 2 (52 year, male) was suffering from primary hyperaldosteronism. CT abdomen showed left adrenal hyperplasia. Aldosterone level was 158.6 ng/dl. Adrenal venous sampling indicated bilateral adrenal hyperplasia. Patient was referred to our centre for [68Ga]Ga-Pentixafor scan. Patient was administered with 170 MBq of [68Ga]Ga-Pentixafor. The clinical PET/CT imaging was performed after 60 min of injection. Patient 3 (57 year, Male) was suffering from hypertension and hypokalemic tetany. CT scan showed distinct nodule in the left adrenal gland. This patient was administered with 185 MBq of [68Ga]Ga-Pentixafor and underwent PET/CT imaging 50 min post injection.
Regulatory clearance aspects
All animal experiments were initiated with the approval (Approval number: BAEC/04/21) of Institutional Animal Ethics Committee (IAEC), Bhabha Atomic Research Centre (BARC), Department of Atomic Energy (DAE), Government of India, following the principal of good laboratory practices (GLP). Also, all the animal experiments were carried out in accordance with the guidelines laid down by Animal Research: Reporting In Vivo Experiments (ARRIVE).
In India, DAE-Radiopharmaceuticals Committee (RPC), Government of India, is the regulatory committee granting approval for experimental protocol and clinical usage of radiopharmaceuticals in human patients. Accordingly the experimental protocol of the reported [68Ga]Ga-Pentixafor and its clinical usage in patients was approved by the DAE-RPC, in their 60th Meeting (Approved unique product ID: 24034). The study was conducted in accordance with the declaration of Helsinki and good clinical practice.
Acknowledgements
The authors wish to thank the staff of the Hospital Radiopharmacy Section, Radiopharmaceutical Evaluation Section, Radiation Hazard Control Unit, Scintigraphy Section and the Animal House Facility of RMC, BARC, for providing the facilities to carry out the work. The authors also thank the Director, Health Safety and Environment Group, BARC, for his support and encouragement in carrying out the work.
Author contributions
S.B: Conceptualization; S.R.M, S.S, A.M, A.C, M.T, S.L: Methodology, investigation, validation; S.R, T.U: Image acquisition and analysis; G.M, S.B, S.R.M: Clinical study; A.M, S.R.M, S.S, A.C: Writing original draft; S.B, A.M, A.C, G.M, K, M.K.R; Results interpretation, supervision; S.B: Writing- review and editing, project administration. All authors contributed to and approved the final manuscript.
Data availability
The raw data supporting the conclusions of this manuscript will be available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that they have no conflict of interest with regard to this study.
Footnotes
The original online version of this Article was revised: Modifications have been made to table 3 and section ‘In-vivo pharmacological behaviour of [68Ga]Ga-Pentixafor'. Full information regarding the corrections made can be found in the correction for this Article.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sreeja Raj Menon and Sudeep Sahu contributed equally.
Change history
4/23/2025
A Correction to this paper has been published: 10.1038/s41598-025-98171-0
References
- 1.Jacobson, O. & Weiss, I. D. CXCR4 chemokine receptor overview: Biology, Pathology and Applications in Imaging and Therapy. Theranostics3, 1–2. 10.7150/thno.5760 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo, F. et al. CXCL12/CXCR4: a symbiotic bridge linking cancer cells and their stromal neighbors in oncogenic communication networks. Oncogene35, 816–826. 10.1038/onc.2015.139 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Lapa, C. et al. [68Ga]Pentixafor-PET/CT for imaging of chemokine receptor CXCR4 expression in multiple myeloma - comparison to [18F]FDG and laboratory values. Theranostics7, 205–212. 10.7150/thno.16576 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hadebe, B., Sathekge, M. M., Aldous, C. & Vorster, M. Current Status of 68Ga-Pentixafor in Solid Tumours. Diagnostics 12, 2135, (2022). 10.3390/diagnostics12092135 [DOI] [PMC free article] [PubMed]
- 5.Luker, G. D. et al. At the Bench: pre-clinical evidence for multiple functions of CXCR4 in cancer. J. Leukoc. Biol.109, 969–989. 10.1002/JLB.2BT1018-715RR (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osl, T., Schmidt, A., Schwaiger, M., Schottelius, M. & Wester, H. J. A new class of pentixa for and pentixa ther-based theranostic agents with enhanced CXCR4-targeting efficiency. Theranostics10, 8264–8280. 10.7150/thno.45537 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poschenrieder, A. et al. First 18F-Labeled Pentixafor-Based Imaging Agent for PET Imaging of CXCR4 Expression In Vivo. Tomography 2, 85–93, (2016). 10.18383/j.tom.2016.00130 [DOI] [PMC free article] [PubMed]
- 8.Archibald, S. J. & Allott, L. The aluminium-[18F]fluoride revolution: simple radiochemistry with a big impact for radiolabelled biomolecules. EJNMMI Radiopharmacy Chem.610.1186/s41181-021-00141-0 (2021). [DOI] [PMC free article] [PubMed]
- 9.Schottelius, M. et al. [177Lu]pentixather: Comprehensive preclinical characterization of a first CXCR4-directed endoradiotherapeutic agent. Theranostics7, 2350–2362. 10.7150/thno.19119 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thackeray, J. T. et al. Molecular imaging of the chemokine receptor CXCR4 after Acute myocardial infarction. JACC Cardiovasc. Imaging. 8, 1417–1426. 10.1016/j.jcmg.2015.09.008 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Hyafil, F. et al. Imaging the cytokine receptor CXCR4 in atherosclerotic plaques with the radiotracer 68Ga-Pentixafor for PET. J. Nucl. Med.58, 499–506. 10.2967/jnumed.116.179663 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Derlin, T., Wester, H. J., Bengel, F. M. & Hueper, K. Visualization of posttraumatic splenosis on chemokine receptor CXCR4-Targeted PET/CT. Clin. Nucl. Med.42, e317–e318. 10.1097/RLU.0000000000001590 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Todde, S. et al. Guidance on validation and qualification of processes and operations involving radiopharmaceuticals. EJNMMI Radiopharm Chem.210.1186/s41181-017-0025-9 (2017). [DOI] [PMC free article] [PubMed]
- 14.Elsinga, P. et al. Guidance on current good radiopharmacy practice (cGRPP) for the small-scale preparation of radiopharmaceuticals. Eur. J. Nucl. Med. Mol. Imaging. 37, 1049–1062. 10.1007/s00259-010-1407-3 (2010). [DOI] [PMC free article] [PubMed]
- 15.Stabin, M. G., Sparks, R. B. & Crowe, E. O. L. I. N. D. A. E. X. M. The second-generation Personal Computer Software for Internal Dose Assessment in Nuclear Medicine. J. Nucl. Med.46, 1023–1027 (2005). [PubMed] [Google Scholar]
- 16.Lindenberg, L., Ahlman, M., Lin, F., Mena, E. & Choyke, P. Advances in PET imaging of the CXCR4 receptor: [68Ga]Ga-PentixaFor. Semin Nucl. Med.54, 163–170. 10.1053/j.semnuclmed.2023.09.002 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herrmann, K. et al. First-in-human experience of CXCR4-directed endoradiotherapy with 177Lu-and 90Y-labeled pentixather in advanced-stage multiple myeloma with extensive intra-and extramedullary disease. J. Nucl. Med.57, 248–251. 10.2967/jnumed.115.167361 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Menon, S. R. et al. On the optimization of the protocol for Automated Radiosyntheses of [68Ga]Ga-Pentixafor, [68Ga]Ga-FAPI-4 and [68Ga]Ga-DOTATATE in a modular-lab Standard. Asia Ocean. J. Nucl. Med. Biol.12, 149–160. 10.22038/AOJNMB.2024.77059.1545 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sudeep Sahu. Automated multidoses production of [68Ga] PentixaFor in general purpose commercial module and its preclinical evaluation in lymphoma model. Nucl. Med. Biol.108, S211 (2022). [Google Scholar]
- 20.Vis, R., Lavalaye, J. & van de Garde, E. M. W. GMP-compliant 68Ga radiolabelling in a conventional small-scale radiopharmacy: a feasible approach for routine clinical use. EJNMMI Res.5, 1–7. 10.1186/s13550-015-0105-3 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boschi, S., Lodi, F., Malizia, C., Cicoria, G. & Marengo, M. Automation synthesis modules review. Appl. Radiat. Isot.76, 38–45. 10.1016/j.apradiso.2012.09.010 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Spreckelmeyer, S., Schulze, O. & Brenner, W. Fully-automated production of [68Ga]Ga-PentixaFor on the module modular Lab-PharmTracer. EJNMMI Radiopharm Chem.510.1186/s41181-020-0091-2 (2020). [DOI] [PMC free article] [PubMed]
- 23.Daniel, T., Balouzet Ravinet, C., Clerc, J., Batista, R. & Mouraeff, Y. Automated synthesis and quality control of [68Ga]Ga-PentixaFor using the Gaia/Luna Elysia-Raytest module for CXCR4 PET imaging. EJNMMI Radiopharm Chem.810.1186/s41181-023-00187-2 (2023). [DOI] [PMC free article] [PubMed]
- 24.Nader, M. et al. Improved production of 68Ga-Pentixafor using cartridge mediated cation exchange purification. Appl. Radiat. Isot.18910.1016/j.apradiso.2022.110447 (2022). [DOI] [PubMed]
- 25.Sammartano, A., Migliari, S., Scarlattei, M., Baldari, G. & Ruffin, L. Synthesis, validation and quality controls of [68Ga]-dotapentixafor for pet imaging of chemokine receptor cxcr4 expression. Acta Biomed.91, 1–10. 10.23750/abm.v91i4.9106 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watts, A. et al. Automated radiosynthesis, quality control, and biodistribution of Ga-68 pentixafor: first Indian experience. Indian J. Nuclear Med.36, 237–244. 10.4103/ijnm.ijnm_216_20 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menon, S. R. et al. Automated radiosynthesis of Pharmaceutical Grade [68Ga]Ga-NODAGA-Lys40-Exendin-4 and demonstration of its efficacy for use in patients. J. Radioanal. Nucl. Chem.333, 3873–3891. 10.1007/s10967-024-09535-1 (2024). [Google Scholar]
- 28.Martin, R., Jüttler, S. & Müller, M. and H. J. Wester Cationic eluate pretreatment for automated synthesis of [68Ga]CPCR4.2. Nucl. Med. Biol.41: 84–89. https://doi.org/10.1016/j.nucmedbio.2013.09.002 (2014) [DOI] [PubMed]
- 29.Costes, J. et al. [68Ga]Ga-PentixaFor: development of a fully automated in hospital production on the Trasis miniAllinOne synthesizer. J. Label. Comp. Radiopharm. 66, 400–410. 10.1002/jlcr.4061 (2023). [DOI] [PubMed] [Google Scholar]
- 30.Lapa, C. et al. [68Ga]Pentixafor-PET/CT for Imaging of Chemokine Receptor 4 expression in small cell lung Cancer-initial experience. Oncotarget7, 9288 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wester, H. J. et al. Disclosing the CXCR4 expression in lymphoproliferative diseases by targeted molecular imaging. Theranostics5, 618–630. 10.7150/thno.11251 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herrmann, K. et al. Biodistribution and radiation dosimetry for the chemokine receptor CXCR4-targeting probe 68Ga-pentixafor. J. Nucl. Med.56, 410–416. 10.2967/jnumed.114.151647 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Emami, B. et al. Tolerance of normal tissue to therapeutic irradiation. Int. J. Radiation Oncol. Biol. Phys.21, 109–122 (1991). [DOI] [PubMed] [Google Scholar]
- 34.Boss, M. et al. PET-Based human dosimetry of 68Ga-NODAGA-Exendin-4, a Tracer for β-Cell imaging. J. Nucl. Med.61, 112–116. 10.2967/jnumed.119.228627 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eberlein, U. & Lassmann, M. Dosimetry of [68Ga]-labeled compounds. Appl. Radiat. Isot.76, 70–74. 10.1016/j.apradiso.2012.06.033 (2013). [DOI] [PubMed] [Google Scholar]
- 36.Poschenrieder, A., Schottelius, M., Schwaiger, M. & Wester, H. J. Preclinical evaluation of [68Ga]NOTA-pentixafor for PET imaging of CXCR4 expression in vivo — a comparison to [68Ga]pentixafor. EJNMMI Res.610.1186/s13550-016-0227-2 (2016). [DOI] [PMC free article] [PubMed]
- 37.Herhaus, P. et al. Targeted positron emission tomography imaging of CXCR4 expression in patients with acute myeloid leukemia. Haematologica101, 932–940. 10.3324/haematol.2016.142976 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Howell, R. W. et al. The MIRD Perspective 1999. J. Nucl. Med.40, 3s–10s (1999). [PubMed] [Google Scholar]
- 39.Dewaraja, Y. K. et al. MIRD pamphlet 23: quantitative SPECT for patient-specific 3-dimensional dosimetry in internal radionuclide therapy. J. Nucl. Med.53, 1310–1325. 10.2967/jnumed.111.100123 (2012). [DOI] [PMC free article] [PubMed]
- 40.Cona, M. M. et al. Biodistribution and radiation dosimetry of radioiodinated hypericin as a cancer therapeutic. Int. J. Oncol.44, 819–829. 10.3892/ijo.2013.2217 (2014). [DOI] [PubMed] [Google Scholar]
- 41.Senthamizhchelvan, S. et al. Radiation dosimetry of 82Rb in humans under pharmacologic stress. J. Nucl. Med.52, 485–491. 10.2967/jnumed.110.083477 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.ICRP Publication 103. The 2007 Recommendations of the International Commission on Radiological Protection. (2007). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this manuscript will be available from the corresponding author upon reasonable request.