Abstract
Background
Multidrug-resistant (MDR) bacteria are a global health threat, notably in low- and middle-income countries. The aim of this review was to estimate the prevalence of multidrug-resistant bacteria in healthcare and community settings in West Africa.
Methods
In accordance with PRISMA guidelines, we searched PubMed, CINAHL, African Index Medicus, and other databases for studies published from 2010 onward. Data on MDR bacterial prevalence, study characteristics, and infection types were extracted and analyzed via R software. Subgroup analyses were performed to explore differences in prevalence across infection settings and sample types.
Results
Out of the 5,320 articles identified, 50 studies from 13 West African countries met the inclusion criteria, with the majority from Nigeria (34%) and Ghana (22%). Among the 35,820 bacteria isolated in these studies, gram-negative bacteria (GNB), particularly Escherichia coli and Klebsiella sp., were the most frequently isolated species, accounting for 63.3% of the bacteria. The overall prevalence of MDR bacteria was 59% (95% CI: 48-69%), with significant heterogeneity between studies (I² = 98%, p < 0.001). Subgroup analysis revealed a 7% increase in MDR bacteria prevalence from the first five-year period to the last two five-year periods, and a greater prevalence of MDR bacteria in nosocomial infections (65%, 95% CI: 45-81%) than in community-acquired infections (53%, 95% CI: 31-74%). The prevalence of MDR bacteria in mixed infection settings was 58% (95% CI: 44-71%). The MDR prevalence was highest in the urine samples (72%, 95% CI: 57-84%) and superficial skin samples (69%, 95% CI: 29-92%), whereas it was lowest in the nasopharyngeal samples (26%, 95% CI: 21-33%).
Conclusion
The high prevalence of MDR bacteria in West Africa underscores the need for strengthened infection control measures, improved surveillance, and stricter antibiotic use policies. Enhanced regional collaboration is essential to mitigate the spread of AMR in both healthcare and community settings.
PROSPERO registration number
CRD42023470363.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-025-10562-w.
Keywords: Prevalence, MDR bacteria, West Africa
Introduction
Every year, the number of antimicrobial-resistant microorganisms increases. The Global Action Plan on Antimicrobial Resistance (AMR), which recognized that AMR represents a global threat to public health, was approved in May 2015 by the World Health Assembly [1]. By Jim O’Neill’s 2016 prediction, if no action is taken by 2050, there could be an additional 10 million deaths per year related to AMR. Approximately half of these deaths will occur in Africa [2]. The World Bank as well warns that without adequate measures by 2050, AMR could lead to an annual increase in healthcare costs of up to $1 trillion [3]. Among bacterial pathogens, there has been an increase in resistant strains, known as multidrug-resistant bacteria. According to the 2021 report of the Global Antimicrobial Resistance and Use Surveillance System (GLASS), 36.6% of Escherichia coli strains isolated from blood cultures were resistant to ceftriaxone, and 24.9% of Staphylococcus aureus strains were resistant to methicillin. The overall resistance rate to ceftriaxone among Klebsiella pneumoniae and Escherichia coli strains isolated from urine samples was between 40% and 50%, and the carbapenem resistance rate of Acinetobacter sp. isolated from blood samples was 65.5% [4]. The number of deaths related to antibiotic resistance was estimated to be 1.27 million in 2019. The majority of these deaths are reported in sub-Saharan Africa and Western Africa, with 27.3 deaths per 100,000 inhabitants [5].
In West Africa, the situation is particularly alarming. The lack of regulation of antibiotic use; weak bacterial infection surveillance systems; emerging armed conflicts; and health, environmental, and socioeconomic impacts play significant roles in the spread of MDR bacteria in this region.
Although some studies have attempted to measure the prevalence of MDR bacteria in specific areas of West Africa, the data remain fragmented, insufficient, and often noncomparable due to the diversity of methodologies and study populations. Furthermore, the majority of studies have focused on a single bacterial species, a specific bacterial family or a limited number of infection sources [6]. Owing to these methodological disparities and the absence of blended data across the West African region, a study providing an overall estimate of MDR bacteria prevalence is necessary. In this context, we conducted this systematic review and meta-analysis, with the primary objective of estimating the prevalence of multidrug-resistant bacteria among in and out patients in West Africa.
Study setting
When reference is made to west Africa, we imply western sub-Saharan African states. These 16 states include coastal countries that run from the Gulf of Guinea to the Senegal River, the countries covered by the Niger River lagoon and the Sahelian hinterland countries. These countries make up the Economic Community of West African States (ECOWAS), which was established by the Lagos treaty of 28th May 1975 [7] (Fig. 1). The Alliance of Sahel States including Mali, Niger, and Burkina Faso was created on 16 September 2023 following the 2023 Nigerien crisis. With an estimated population of 410 million inhabitants, an annual growth of approximately 0.9% for Cape Verde, 3.7% for Niger, and a regional average of approximately 2.4%, West Africa constitutes 30% of the African population and approximately 5% of the world population [8]. The majority of these West African countries have flawed health systems, which contrasts with the high endemicity of infectious pathologies such as respiratory infections, infectious diarrhea and meningitis. These diseases are at the root of high and irrational use of antibiotics, resulting in increased morbimortality from multidrug resistant bacterial infections both in the community and in health facilities [9]. Approximately 1.27 million of these deaths could be attributed to MDR bacteria. The mortality rate associated with this resistance is highest in the western parts of Sub-Saharan Africa, with approximately 27.3 deaths per year for 100,000 inhabitants [10].
Fig. 1.
West African region
Methods
Source of information and research strategy
This review was conducted following a pre-specified protocol registered on PROSPERO (registration number: CRD42023470363). Minor amendments were made during the process, but these did not significantly change the study’s objectives or methods. Research on the list of publications with studies about the prevalence of multiresistant bacteria in West Africa has been conducted in accordance with PRISMA guidelines (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) [11]. This research was carried out in electronic databases, including PubMed/Medline, African Index Medicus, CINAHL, African Journal Online, Google Scholar and other studies from gray literature. The keywords used were “ECOWAS”, “CEDEAO”, “Benin”, “Burkina Faso”, “Cap Vert”, “Ivory Coast”, “Gambia”, “Ghana”, “Guinea”, “Guinea-Bissau”, “Liberia”, “Mali”, “Mauritania”, “Niger”, “Nigeria”, “Sierra Leon”, “Senegal”, “Togo”, “West Africa” and “Occidental Africa”. We used the Boolean operators “AND”/“OR” for a good combination. An example of search strategy in PubMed is: (“Multidrug resistant bacteria” OR “drug resistance, multiple, bacterial“[Mesh] OR “antimicrobial resistance”[Text]) AND (“Africa, western” [MeSH] OR “West Africa*” [Text] OR “ECOWAS” OR Benin[Text] OR “Burkina Faso“[Text] OR Burkinabe*[Text] OR “Cabo Verde“[Text] OR Gambia*[Text] OR Ghan*[Text] OR Guinea*[Text] OR “Guinea-Bissau“[Text] OR “Ivory Coast“[Text] OR “Cote d’Ivoire“[Text] OR Ivorian*[Text] OR Liberia*[Text] OR Mali*[Text] OR Mauritania*[Text] OR Niger*[Text] OR Senegal*[Text] OR “Sierra Leone“[Text] OR Togo[Text]). Additionally, a manual search of the references of pertinent articles was carried out. Articles published in the last 15 years were considered.
Inclusion criteria
We included studies in our review according to the CoCoPop framework [12]:
Condition: Studies on the prevalence of multidrug-resistant bacteria without language restrictions.
Context: Conducted in one of the 16 West African countries and published within the last 15 years (from 2010 onward).
Population: Studies involving participants hospitalized or from the community.
Multidrug resistant bacteria are defined as bacteria resistant to three antibiotics belonging to three different families [13].
Before including a selected study, two coauthors independently reviewed the titles and abstracts, followed by a full-text review of potentially eligible studies. In case of disagreement between the two initial reviewers on whether to include a study, a third coauthor was consulted for arbitration. Article screening was conducted in a double-blind manner via Rayyan software [14].
Non-inclusion criteria
Studies dealing with specific bacteria such as Chlamydia, Mycoplasma, Koch’s bacillus or other mycobacteria were not included. Similarly, studies that exclusively focused on a single bacterium, a bacterial family, or a single multidrug-resistant phenotype were not included.
Assessment of the quality of the selected studies
The Joanna Briggs Institute (JBI) tool [15] was used to assess the quality of the included studies. Articles with a score above 5 were considered high quality. Articles with scores between 3 and 5 and between 0 and 2 were considered to be of medium and low quality, respectively. A supplementary file shows the table of the quality assessment via JBI tool (supplementary file 1).
Data extraction
Data were extracted by two coauthors, and the following information were obtained: the first author’s name, year of publication, country, type of study, site of the study, sociodemographic characteristics, number of patients included, types of samples, types of infection, total number of isolated bacteria, number of each species, number of gram-positive and gram-negative bacilli, number of bacteria tested with antibiograms, and number of multidrug-resistant bacteria.
Statistical analysis
The data were analyzed using R software (version 4.3.3). Descriptive statistics were performed to summarize the characteristics of the included studies, such as sample size, types of infections, and bacterial species. A meta-analysis was conducted to estimate the pooled prevalence of multidrug-resistant (MDR) bacteria, with a 95% confidence interval (CI).
To investigate potential sources of heterogeneity, subgroup analyses were performed based on infection types (nosocomial, community-acquired, or mixed), sample types, publication periods and countries. Heterogeneity between studies was assessed using the I² test. A random-effects model was used when I2 > 50%, and a fixed-effects model was used when I2 ≤ 50%. The statistical significance of differences between subgroups was evaluated using the Chi-square (χ²) test. A funnel plot was used to assess publication bias, and its symmetry was visually inspected.
Results
Procedure of study selection
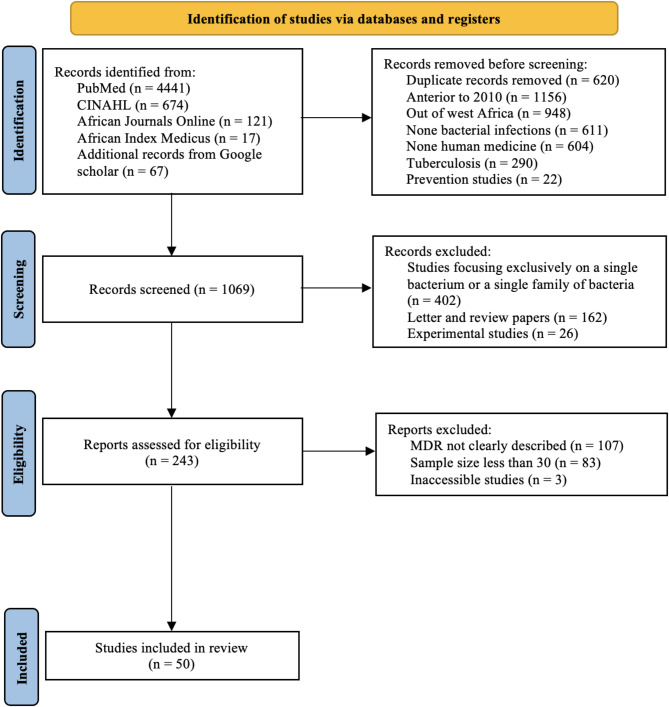
A total of 5,320 articles were found during our webliography research in PubMed, CINAHL, African Index Medicus and African Journal Online, and 67 supplementary articles were found through Google Scholar. After 620 duplicates were removed, 4,700 articles remained, 3,631 of which were removed before screening. Thus, 1,069 articles were evaluated on the basis of their titles and summaries. Among these, 826 were ruled out. Hence, 243 articles were evaluated for eligibility, 193 of which were excluded. Consequently, we ultimately included 50 articles in our systematic review (Fig. 2).
Fig. 2.
Flowchart of the studies selection
Study characteristics
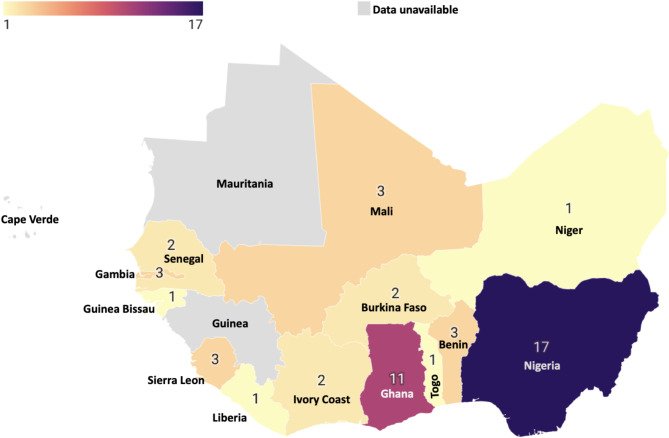
These 50 articles were issued from 13 of the 16 West African countries, predominantly Nigeria (17/50; 34%) and Ghana (11/50: 22%). Data on MDR bacteria as defined by our review were not available for Mauritania, Guinea or Cape Verde (Fig. 3).
Fig. 3.
Number of studies included per country
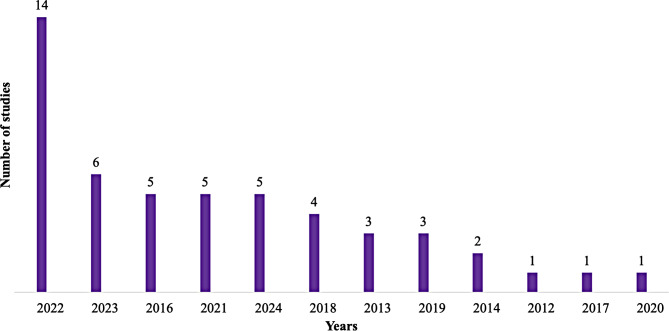
Most of the articles were published in 2022 (28%) (Fig. 4) and included 40% adults, 20% children, 40% mixed populations and 31 (62%) prospective studies. The study population was mentioned in 47 (94%) of the articles, and a total of 121,262 patients were included. the majority of the study use disc diffusion method for antimicrobial susceptibility testing, and only one was able to identify anaerobic bacteria the different characteristics of the studies included are represented in Table 1.
Fig. 4.
Number of publications per year
Table 1.
Study characteristics
| Authors | Country | Year | Study design | Population | Sex-ratio | Sample size | Type of infection | methods utilized for antimicrobial susceptibility | Anaerobic | Gram (+) | Gram (-) | Undetermined |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adegun PT et al. [16] | Nigeria | 2019 | Prospective | Adult | N/A | 154 | Nosocomial infections | modified Kirby-Bauer disc diffusion method. | N/A | 14 | 124 | N/A |
| Adeleye QA et al. [17] | Nigeria | 2024 | Retrospective | Pediatric | 1.7 | 1157 | Mixed infections | modified Kirby-Bauer disc diffusion method | N/A | 46 | 318 | N/A |
| Afum T et al. [18] | Ghana | 2022 | Retrospective | Mixed | 0.6 | 792 | Community-acquired infections | Kirby-Bauer disk diffusion and Microscan autoScan4 MIC panels | N/A | 0 | 167 | N/A |
| Aglomasa BC et al. [19] | Ghana | 2022 | Prospective | Adult | 0.4 | 60 | Nosocomial infections | Disc diffusion and MIC assay methods | N/A | 26 | 13 | N/A |
| Ahoyo TA et al. [20] | Benin | 2014 | Prospective | Mixed | 0.7 | 597 | Nosocomial infections | Agar diffusion method on Mueller Hinton agar | N/A | 420 | 507 | 30 |
| Aika IN et al. [21] | Nigeria | 2022 | Retrospective | Mixed | N/A | N/A | Mixed infections | Kirby-Bauer disk diffusion on Muller Hinton Agar | N/A | 501 | 318 | N/A |
| AKO-NAI AK et al. [22] | Nigeria | 2013 | Prospective | Adult | 1.9 | 60 | Nosocomial infections | antibiotic discs (ABTEK, Biological Limited Liverpool, UK) | N/A | 142 | 48 | N/A |
| Asafo-Adjei K et al. [23] | Ghana | 2018 | Prospective | Adult | N/A | 188 | Nosocomial infections | Kirby Bauer disc diffusion method | N/A | 7 | 149 | N/A |
| Asamoah B et al. [24] | Ghana | 2022 | Prospective | Mixed | N/A | 94,134 | Mixed infections | Kirby–Bauer disc diffusion method on Mueller–Hinton agar | N/A | 295 | 20,515 | N/A |
| Bonko MDA et al. [25] | Burkina Faso | 2021 | Prospective | Pediatric | 1.2 | 1099 | Community-acquired infections | Kirby-Bauer disc diffusion method | N/A | 6 | 135 | N/A |
| Campbell JSO et al. [26] | Sierra Leone | 2022 | Retrospective | Mixed | 0.3 | 168 | Mixed infections | Kirby–Bauer disc diffusion method | N/A | 15 | 60 | N/A |
| Chukwu EE et al. [27] | Nigeria | 2022 | Prospective | Mixed | 0.2 | 499 | Mixed infections | Kirby–Bauer disc diffusion method | N/A | 121 | 111 | N/A |
| Chukwumeze F et al. [28] | Nigeria | 2021 | Prospective | Pediatric | 0.9 | 234 | Community-acquired infections | Kirby–Bauer disk diffusion method on Mueller–Hinton agar | N/A | 14 | 18 | N/A |
| Dayyab FM et al. [29] | Nigeria | 2018 | Prospective | Adult | 1 | 100 | Nosocomial infections | N/A | N/A | 41 | 103 | N/A |
| Diarra B et al. [30] | Mali | 2024 | Prospective | Pediatric | 1.2 | 554 | Community-acquired infections | Kirby-Bauer disc diffusion method on Muller Hilton’s agar. | N/A | 0 | 192 | N/A |
| Dibbasey M et al. [31] | Gambia | 2023 | Prospective | Mixed | 1 | 159 | Mixed infections | Kirby-Bauer disc diffusion method | N/A | 8 | 3 | N/A |
| Donkor ES et al. [32] | Ghana | 2019 | Prospective | Adult | 0.3 | 307 | Community-acquired infections | Kirby Bauer disc diffusion method | N/A | 4 | 27 | N/A |
| Gnimatin JP et al. [33] | Ghana | 2022 | Retrospective | Mixed | 1.1 | 1222 | Mixed infections | disc diffusion method | N/A | 111 | 1111 | N/A |
| Iliyasu G et al. [34] | Nigeria | 2016 | Retrospective | Adult | 2.7 | 33 | Mixed infections | N/A | N/A | 7 | 17 | N/A |
| Iliyasu G et al. [35] | Nigeria | 2016 | Retrospective | Adult | 1.9 | 76 | Nosocomial infections | N/A | N/A | 36 | 48 | N/A |
| Inusah A et al. [36] | Ghana | 2021 | Retrospective | Mixed | N/A | N/A | Mixed infections | N/A | N/A | 327 | 473 | N/A |
| Isendahl J et al. [37] | Guinea-Bissau | 2014 | Prospective | Pediatric | N/A | 372 | Community-acquired infections | VITEK2 system, E-test (bioMérieux) and the disk diffusion method (Oxoid AB, Malmö, Sweden) | N/A | 33 | 15 | N/A |
| Iwalokun BA et al. [38] | Nigeria | 2012 | Prospective | Mixed | 0.9 | 103 | Community-acquired infections | disc diffusion method | N/A | 4 | 24 | N/A |
| Iwuafor AA et al. [39] | Nigeria | 2016 | Prospective | Adult | 1.2 | 71 | Nosocomial infections | Kirby-Bauer disc diffusion method on Mueller-Hinton agar | N/A | 11 | 28 | N/A |
| Karikari AB et al. [40] | Ghana | 2022 | Prospective | Mixed | N/A | 219 | Mixed infections | Kirby-Bauer disc diffusion method on Mueller-Hinton agar | N/A | 28 | 46 | N/A |
| Kebbeh A et al. [41] | Gambia | 2023 | Prospective | Adult | 0.2 | 422 | Community-acquired infections | Kirby Bauer’s disc diffusion | N/A | 0 | 54 | N/A |
| Labi AK et al. [42] | Ghana | 2016 | Retrospective | Pediatric | N/A | 8025 | Mixed infections | Kirby Bauer Disc diffusion method | N/A | 1421 | 329 | N/A |
| Lakoh S et al. [43] | Sierra Leone | 2022 | Prospective | Adult | 0.4 | 417 | Nosocomial infections | VITEK 2 compact system (bioMérieux, France) | N/A | 2 | 23 | N/A |
| Lakoh S et al. [44] | Sierra Leone | 2023 | Prospective | Adult | 0.9 | 459 | Nosocomial infections | VITEK 2 compact system (bioMérieux, France) | N/A | 8 | 51 | N/A |
| Makanjuola OB et al. [45] | Nigeria | 2018 | Retrospective | Mixed | 1.4 | 47 | Nosocomial infections | disc diffusion method | N/A | 8 | 46 | N/A |
| Ombelet S et al. [46] | Benin | 2022 | Prospective | Mixed | 1.2 | 3032 | Mixed infections | disk diffusion and E-tests | N/A | 77 | 258 | N/A |
| Onanuga A et al. [47] | Nigeria | 2018 | Prospective | Adult | N/A | 201 | Community-acquired infections | Kirby-Bauer disc diffusion method on Mueller-Hinton agar | N/A | 20 | 99 | N/A |
| Onanuga A et al. [48] | Nigeria | 2016 | Prospective | Adult | 0.9 | 200 | Community-acquired infections | Kirby-Bauer disc diffusion method on Mueller-Hinton agar | N/A | 48 | 94 | N/A |
| Otajevwo FD et al. [49] | Nigeria | 2013 | Prospective | Mixed | N/A | 100 | Community-acquired infections | Agar diffusion disc method | N/A | 5 | 27 | N/A |
| Tetteh FKM et al [50] | Ghana | 2022 | Retrospective | Pediatric | 1.1 | 471 | Mixed infections | Pheonix100 identification system (Becton Dickinson, NJ, USA) | N/A | 124 | 15 | N/A |
| Tobin EA et al. [51] | Nigeria | 2021 | Retrospective | Mixed | 1.1 | 3247 | Mixed infections | Kirby-Bauer disc diffusion method on Mueller-Hinton agar | N/A | 435 | 559 | N/A |
| Yehouenou CL et al. [52] | Benin | 2020 | Prospective | Adult | N/A | 174 | Nosocomial infections | Kirby-Bauer disk diffusion and Beckton Dickinson Phoenix automated system | N/A | 49 | 180 | N/A |
| Diedhiou M [53] | Senegal | 2023 | Retrospective | Adult | 3.2 | 243 | Nosocomial infections | N/A | N/A | 7 | 21 | N/A |
| Abdoulaye O et al. [54] | Niger | 2022 | Retrospective | Mixed | 1.6 | 77 | Mixed infections | Agar diffusion disc method | N/A | 17 | 60 | N/A |
| Moroh JLA et al. [55] | Ivory Coast | 2013 | Retrospective | Mixed | N/A | N/A | Mixed infections | disc diffusion method on Mueller Hinton agar | N/A | 534 | 2530 | N/A |
| Ky/Ba et al. [56] | Burkina Faso | 2024 | Prospective | Adult | 1.1 | 77 | Mixed infections | disc diffusion method on Mueller Hinton agar | N/A | 3 | 37 | N/A |
| Konate I et al. [57] | Mali | 2022 | Prospective | Adult | 1.8 | 222 | Community-acquired infections | N/A | N/A | 10 | 49 | N/A |
| Rahden et al. [58] | Gambia | 2024 | Retrospective | Mixed | 1.2 | 645 | Mixed infections | Kirby-Bauer disc diffusion method | N/A | 106 | 115 | 39 |
| Maiga A et al. [59] | Mali | 2023 | Prospective | Mixed | N/A | 463 | Nosocomial infections | disc diffusion method on Mueller Hinton agar | N/A | 9 | 45 | N/A |
| Arlette AS et al. [60] | Ivory Coast | 2023 | Prospective | Pediatric | N/A | N/A | Mixed infections | Agar diffusion method | N/A | 103 | 112 | N/A |
| Adeyemo AT et al. [61] | Nigeria | 2019 | Prospective | Adult | 1.4 | 90 | Mixed infections | Modified Kirby-Bauer disk diffusion method | 29 | 59 | 129 | N/A |
| Dossim S et al. [62] | Togo | 2024 | Retrospective | Mixed | N/A | N/A | Mixed infections | Kirby-Bauer disc diffusion method | N/A | 164 | 469 | N/A |
| Wembulua BS et al. [63] | Senegal | 2021 | Retrospective | Adult | 0.9 | 74 | Mixed infections | Diffusion disc method | N/A | 30 | 47 | 23 |
| Sampane-Donkor E et al. [64] | Ghana | 2017 | Prospective | Pediatric | 1.1 | N/A | Community-acquired infections | Kirby-Bauer disk diffusion method | N/A | 168 | 53 | N/A |
| Goodyer J et al. [65] | Liberia | 2022 | Retrospective | Pediatric | N/A | 100 | Mixed infections | Kirby Bauer disc diffusion method | NA | 22 | 81 | 48 |
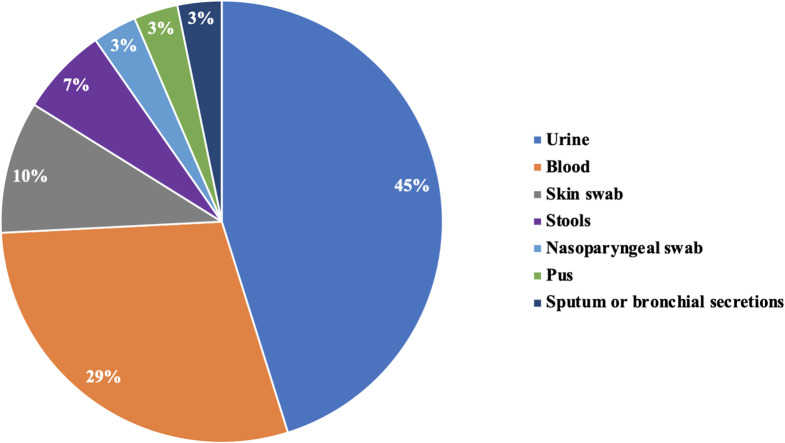
Pathogenic samples and types of infection
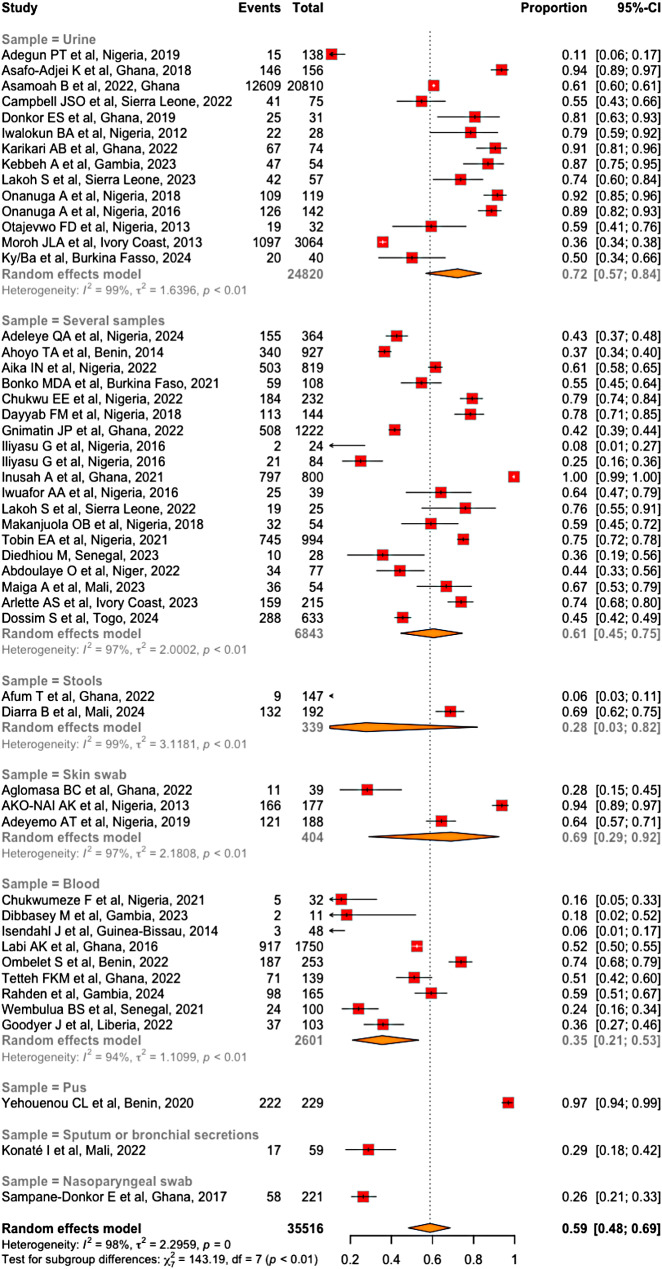
With respect to pathologic samples, 19 (38%) studies included several samples at the same time. Among the studies that had one sample, 45% and 29% were urine and blood samples, respectively (Fig. 5). The types of infection were community-acquired, nosocomial or mixed in 26%, 28% and 46% of the articles, respectively.
Fig. 5.
Samples used in the studies
Bacteriology
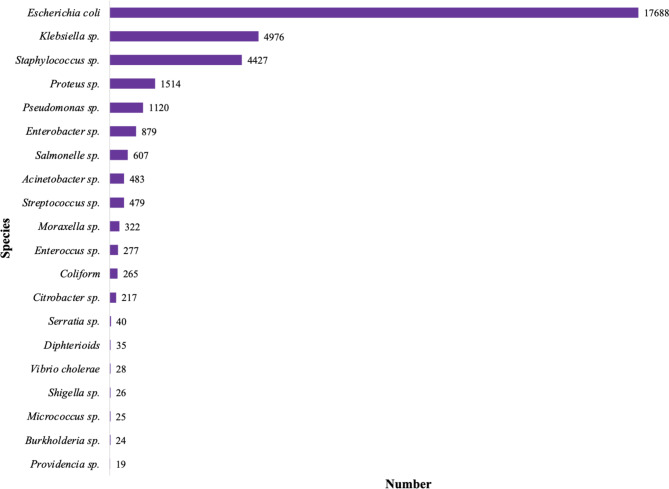
A total number of 35,820 bacteria were isolated in the study included and were divided into 69 different species. Among these bacteria, 30,053 (83.9%) were gram-negative bacteria (GNB), 5,646 (15.8%) were gram-positive bacteria (GPB), 29 (0.08%) were anaerobic, and 121 (0.3%) were undetermined. A total of 35,516 (99.3%) bacteria were tested with antibiograms. The 20 most common bacterial species are represented in Fig. 6. Escherichia coli, Klebsiella sp. and Staphylococcus sp. were the main bacteria. All the species isolated in the included studies are represented in the supplementary file 2.
Fig. 6.
Top ten of isolated species
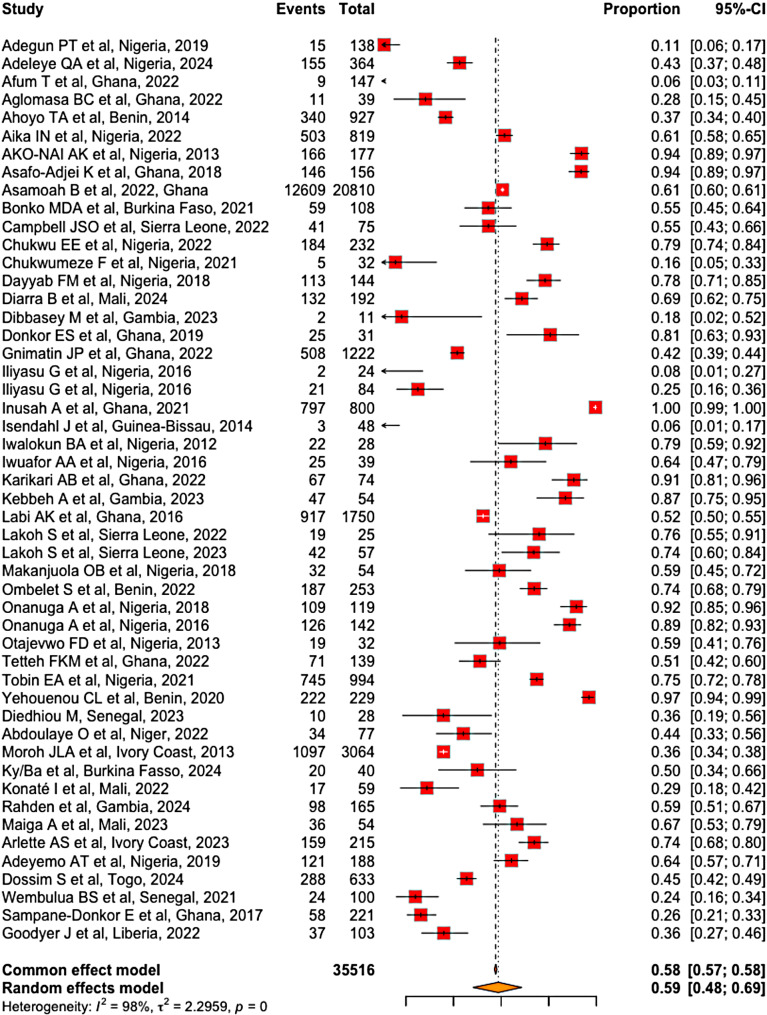
Prevalence of MDR bacteria in West Africa
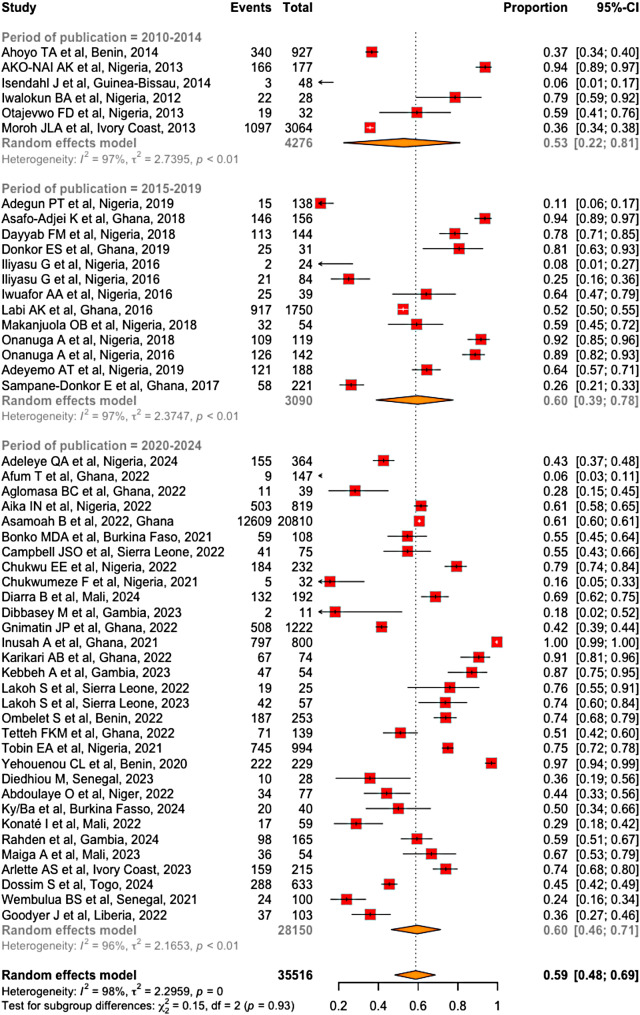
Of the 35,516 bacteria tested with antibiograms, 20,495 were multidrug resistant. Our meta-analysis revealed that the prevalence of MDR bacteria in West Africa was estimated to 59% (95% CI [48-69%]), with significant heterogeneity among the included studies (I2 = 98%, p < 0.001) (Fig. 7).
Fig. 7.
Global prevalence of MDR bacteria in West Africa
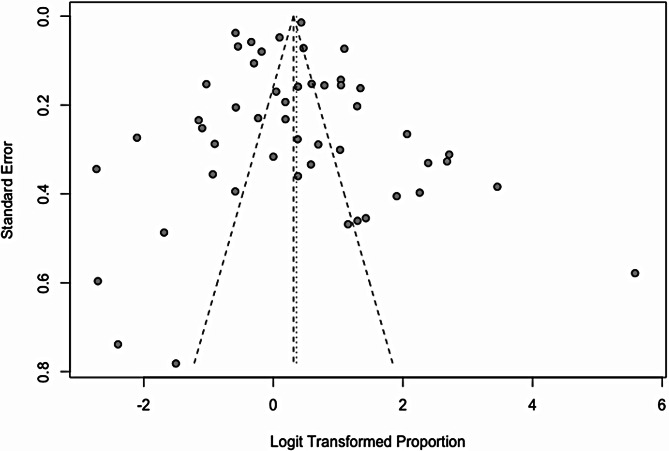
Publication bias assessment
According to the funnel plot, there was a relatively symmetrical distribution of studies around the central line. This symmetry suggests an absence of major publication bias. However, the extreme points outside the funnel shape may indicate heterogeneity among the studies, warranting subgroup analyses (Fig. 8).
Fig. 8.
Risk of publication bias assessment
Subgroup analysis
The analysis of MDR bacteria prevalence by 5-year publication periods showed a 7% increase between the 2010–2014 period (53%; 95% CI [22–81%]) compared to the 2015–2019 and 2020–2024 periods, where the reported MDR bacteria prevalence were 60% (95% CI [39–78%]) and 60% (95% CI [46-71%]), respectively (Fig. 9). this variation is not statistically significant (χ² = 0.15; df = 2; p = 0.93).
Fig. 9.
Prevalence of MDR bacteria per 5-year period of publication
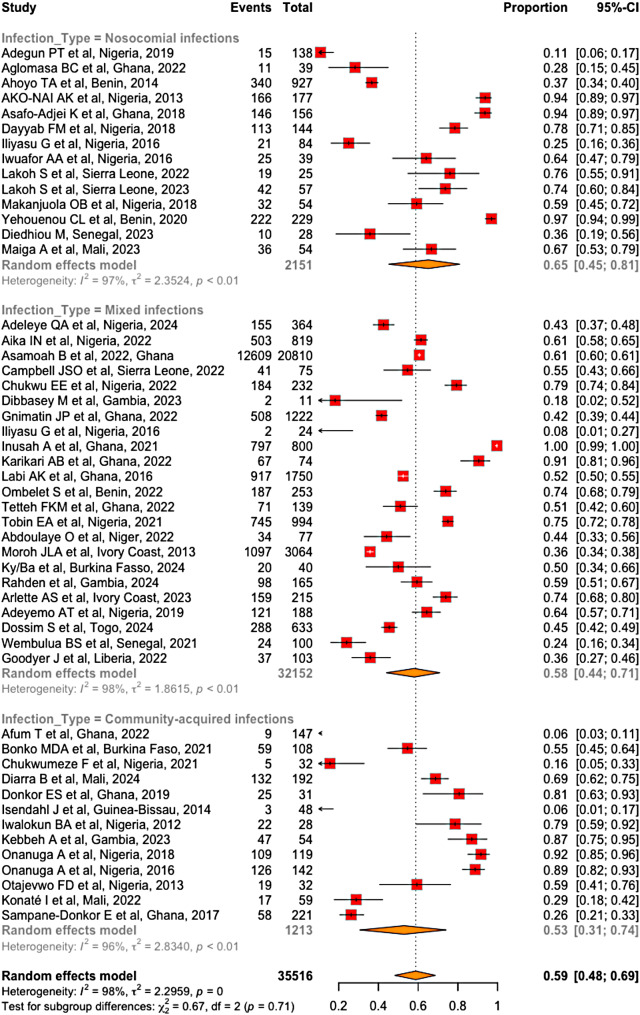
Subgroup analysis also revealed that the prevalence of MDR bacteria was greater in studies involving nosocomial infections (65%; 95% CI [45-81%]) than in studies involving both nosocomial and community-acquired infections (58% CI [44-71%]) and studies involving community-acquired infections exclusively (53%; 95% CI [31-74%]) (Fig. 10). However, these differences were not statistically significant (χ² = 0.67; df = 2; p = 0.71). Similarly, the prevalence of MDR bacteria significantly varied with respect to the type of sample (χ² = 143.19, df = 7, p < 0.01). This prevalence was greater in urine (72%; 95% CI [57-84%]) and lowest in nasopharyngeal samples (26%; 95% CI [21-33%]) (Fig. 11). it also varied significantly across countries, ranging from 6% in Guinea-Bissau to 79% in Benin (χ² = 62.40, df = 12; p < 0.01). The results of the subgroup analysis across countries are represented in Table 2.
Fig. 10.
Prevalence of MDR bacteria per infection type
Fig. 11.
Prevalence of MDR bacteria per sample type
Table 2.
Pooled prevalence of MDR bacteria across west African countries
| Country | Number of studies included | Pooled prevalence | 95%CI | i2 | p-value |
|---|---|---|---|---|---|
| Nigeria | 17 | 61% | 44 − 76% | 96% | < 0.01 |
| Ghana | 11 | 66% | 36 − 87% | 98% | < 0.01 |
| Benin | 3 | 79% | 36 − 96% | 99% | < 0.01 |
| Burkina Faso | 2 | 53% | 45 − 61% | 0% | 0.62 |
| Sierra Leone | 3 | 67% | 54 − 78% | 70% | 0.04 |
| Mali | 3 | 55% | 34 − 75% | 93% | < 0.01 |
| Gambia | 3 | 59% | 24 − 87% | 90% | < 0.01 |
| Bissau Guinea | 1 | 6% | 1 − 17% | - | - |
| Senegal | 2 | 27% | 20 − 35% | 34% | 0.22 |
| Niger | 1 | 44% | 33 − 56% | - | - |
| Ivory Coast | 2 | 55% | 29 − 79% | 99% | < 0.01 |
| Togo | 1 | 45% | 42 − 49% | - | - |
| Liberia | 1 | 36% | 27 − 46% | - | - |
Test for subgroup differences: χ² = 62.40, df = 12 (p < 0.01)
Discussion
Our systematic review included 50 studies on nonspecific bacteria published over the last 15 years from 13 West African countries. A meta-analysis of the included studies revealed a high prevalence of MDR bacteria in healthcare and community settings in this region. The stratified analysis by type of infection revealed a greater prevalence of MDR bacteria in studies focusing on nosocomial infections [16–29] than in those addressing both nosocomial and community-acquired infections [30–52] or those focusing solely on community-acquired infections [53–65]. Similarly, the prevalence of MDR bacteria varied according to the type of sample collected, being greater in urine samples [16, 20, 25, 32, 33, 39, 45, 46, 57, 59–63] and lower in nasopharyngeal samples [65].
Global prevalence
In our review, the prevalence of MDR bacteria in healthcare and community settings in West Africa was estimated at 59% (95% CI [48 − 69%]). A similar systematic review conducted in Ethiopia, including 37 studies, reported an MDR bacteria prevalence of 70.5% (95% CI [64.9 − 76.1%]) [66]. In the same country, a meta-analysis of patients living with HIV reported a MDR bacteria prevalence close to that estimated in our review (58.02%; 95% CI [46.32 − 69.73%]) [67]. Prevalence rates similar to those reported in West Africa have also been reported in other sub-Saharan African countries. For example, a Kenyan study covering three phenotypes of multidrug resistance (methicillin-resistant Staphylococcus aureus, carbapenem-resistant enterobacteria and extended-spectrum cephalosporin-resistant enterobacteria) reported an overall MDR bacteria prevalence of 61.9% [68]. Han et al. reported a prevalence of 46.8% for MDR bacteria isolated from blood cultures in South Africa. Despite some differences that may be related to the microbiological diagnostic tools, studied populations, and/or methodologies used, we noted that MDR bacteria are more prevalent in sub-Saharan African countries than in Maghreb and developed countries. In fact, MDR bacteria prevalence rates of 26.4% and 14% have been reported in Tunisia [69] and Morocco [70], respectively. Similarly, in hospitalized patients with cirrhosis in hospitals across northern, southern, and western Europe, the MDR bacteria prevalence was 29.2% [71]. These differences may be explained by several factors. In sub-Saharan Africa, healthcare and MDR bacterial surveillance systems are less effective, antibiotic stewardship policies are inadequate, healthcare and hygiene infrastructures often do not meet standard norms, and socioeconomic conditions are deficient. Different climatic contexts may also account for these discrepancies. Indubitably, scientific evidence suggests that warm climates and humidity are correlated with the spread of MDR bacteria [72]. The climate also affects the seasonality of many infectious diseases, which are often more prevalent during the rainy season. In regions where mass antibiotic prophylaxis is seasonal, an increase in bacterial resistance is sometimes observed due to the increased use of antibiotics during these periods.
Heterogeneity and subgroup analysis
In our review, the I² test revealed significant heterogeneity among the included studies (I² = 98%, p = 0.00). This prompted us to conduct subgroup analyses to identify potential factors explaining these discrepancies. We observed a significant variation in prevalence across countries, ranging from 6% in Guinea-Bissau to 79% in Benin. This variability could be attributed to the highly variable number of included studies per country, the types of infections studied (community-acquired or nosocomial infection), and the types of specimens analyzed. For instance, in Guinea-Bissau, where the prevalence of multidrug-resistant bacteria (MDR) appeared lower, only one study was included, focusing exclusively on community-acquired infections [58]. In contrast, in Benin, where the prevalence was higher, three studies were included: two focused on nosocomial infections, while the third addressed both nosocomial and community-acquired infections [18, 27, 41]. Additionally, variations in antimicrobial susceptibility testing techniques across countries may have influenced the detection capacity for multidrug-resistant bacteria.
Regarding the publication periods from 2010 to 2024, there was a 7% increase in MDR bacteria prevalence from the first five-year period to the last two five-year periods, although the difference was not statistically significant. These findings are consistent with those of Oneko M. et al. in rural Western Kenya on community-acquired, MDR invasive Nontyphoidal Salmonella from 2009 to 2013 [73]. Similarly, Shawa M. et al. reported a comparable trend of increasing bacterial resistance in a Zambian study on strains isolated from hospital setting between 2015 and 2020 [74]. Such data confirm the growing burden of MDR bacteria, as reported by many experts, which worsens each year.
In our review, the prevalence of MDR bacteria was greater in studies focused on nosocomial infections (65%; 95% CI [45 − 81%]) than in those reporting either mixed infections (58%; 95% CI [44 − 71%]) or community-acquired infections only (53%; 95% CI [31 − 74%]). A similar finding was reported in the Ethiopian review by Alemayehou [66], where the prevalence of MDR bacteria was higher in nosocomial infections (72.1%; 95% CI [61.4 − 82.7%]). Many other studies on nosocomial infections in Africa and outside the continent have reported prevalences of MDR bacteria exceeding 50% [69, 71, 75, 76], in contrast to studies on community-acquired infections where MDR bacteria are less prevalent [77–79].
This difference, which is commonly reported in the literature, is attributed to the fact that hospital-acquired bacteria are generally more resistant than community-acquired bacteria. The multidrug resistance of hospital bacteria is due to the selective pressure resulting from the frequent use of broad-spectrum antibiotics in hospitals [80]. The possibility of horizontal gene transfer of resistant elements between bacteria in the hospital environment also enhances their ability to withstand broad-spectrum antibiotics [81]. Moreover, hospitalized patients are often immunocompromised and suffer from severe infections caused by multidrug-resistant bacteria; they also undergo invasive procedures that are risk factors for infections caused by multidrug-resistant bacteria [82].
Furthermore, we found that the difference in MDR prevalence by infection type was not statistically significant (χ² = 0.67; df = 2; p = 0.71). This result highlights the increasing frequency of MDR strains in community settings. In a U.S. study conducted by Keith S. Kaye et al. among outpatients with urinary tract infections, an increasing trend in antibiotic resistance of Klebsiella pneumoniae from 2011 to 2012 was reported [79]. Oneko M et al. also reported a similar trend in a study conducted in Rural Western Kenya on Community-Acquired, MDR Invasive Nontyphoidal Salmonella from 2009 to 2013 [73]. One of the main causes of this phenomenon is the misuse of antibiotics across the human, animal, and environmental sectors which exerts selective pressure on community-acquired bacteria [83]. In fact, several surveys in West African countries have shown prevalence of antibiotic use exceeding 70% both in community [84] and healthcare settings [85–89].
In our review, the prevalence of MDR bacteria was highest in studies focused on urine samples (72%; 95% CI [57 − 84%]) and superficial skin samples (69%; 95% CI [29 − 92%]). The prevalence of MDR bacteria in studies including multiple specimen types ranked third (61%; 95% CI [45 − 75%]). These findings align with those of the Ethiopian meta-analysis, in which MDR bacteria predominated in the skin, urinary tract, and multiple infection sites [66]. However, discrepant rates were reported by Assefa et al. in their review in 2024, where the highest MDR prevalence rates were observed among patients with multiple infection sites and those with pulmonary or blood infections [67]. Urinary tract infections, especially recurrent infections, are often treated with broad-spectrum antibiotics such as quinolone, third-generation cephalosporins. This repeated exposure to antibiotics favors the selection of resistant strains. Additionally, the urinary tract is often colonized by asymptomatic MDR Enterobacteriaceae. Furthermore, invasive procedures such as urinary catheterization are frequently performed on certain patients, thereby increasing the likelihood of healthcare-associated infections caused by MDR bacteria. Similarly, skin infections, particularly chronic wounds or surgical sites, often receive local antibiotic or antiseptic treatments, which also promote the proliferation of MDR bacteria through this same selection pressure mechanism.
Strengths and limitations of the study
Our study was conducted via a rigorous methodology in terms of literature searches, article screening, bias assessment, data extraction, and statistical analysis. This allowed us to include studies from several West African countries and to gain a comprehensive view of MDR bacteria in the region. However, our study has several limitations. There were three West African countries for which we did not find eligible studies. Additionally, several studies on bacterial resistance to antibiotics were identified in the West African literature, but many of them did not clearly define MDR bacteria. Most of these studies were limited to describing bacterial resistance to individual antibiotics or used definitions of MDR that differed from those used in our study. The techniques used for antimicrobial susceptibility testing were not identical across all studies, and only one study was able to identify anaerobic bacteria. This may underestimate the number of studies that could have been included. In almost all the included studies, the authors exclusively reported the overall proportion of multidrug-resistant bacteria rather than multidrug resistance by bacterial species or bacterial families. Unfortunately, this approach does not allow for identifying the most resistant bacterial species or families (i.e. Gram-negative and Gram-positive bacteria). It would be useful in future studies to classify multidrug-resistant bacteria by species or bacterial families. This would help identify the most resistant species or families.
Conclusion
This systematic review revealed a high prevalence of MDR bacteria in healthcare and community settings in West Africa. Although MDR bacteria are more frequently observed in nosocomial infections, their high prevalence at the community level is also concerning. These findings highlight the importance of strengthening infection prevention and control practices in hospitals and the need for more rigorous surveillance of MDR bacteria in West Africa. Stricter policies on the consumption and use of antibiotics are also necessary to prevent the spread of MDR bacteria. Additionally, using a standardized definition of MDR bacteria would be beneficial for a more accurate estimation of their prevalence in West Africa.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- • AMR
• Antimicrobial Resistance
- • CI
• Confidence interval
- • CINAHL
• Cumulative Index to Nursing and Allied Health Literature
- • ESBL
• Extended-spectrum beta-lactamase
- • ECOWAS
• Economic Community of West African States (CEDEAO)
- • GLASS
• Global Antimicrobial Resistance and Use Surveillance System
- • GNB
• Gram-Negative Bacteria
- • GPB
• Gram-Positive Bacteria
- • I²
• Indicator of heterogeneity in the meta-analysis
- • JBI
• Joanna Briggs Institute
- • MDR
• Multidrug resistance
- • MRSA
• Methicillin-resistant Staphylococcus aureus
- • PRISMA
• Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- • PubMed
• Public Medline
- • VRE
• Vancomycin-resistant Enterococci
Author contributions
AF formulated the research question. MD wrote the systematic review protocol. MD, OB, and AN corrected the protocol and led the submission process in PROSPERO. MD and OB performed the bibliographic research in the different databases. MD, OB, and FW performed the screening, quality assessment of the articles and data extraction. TY, SMMD, ROR, and MWG participated in the interpretation of the resistance phenotypes of the bacteria and their classification into families. MD analyzed the database with R software. OB and DN actively participated in the data analysis with R software. MD wrote the entire article. NAL, BF, PSB, and AF participated in the interpretation of the results and corrected the final version of the review. All the authors have read and approved the final version of the manuscript.
Funding
There is no specific funding for this research.
Data availability
All the data required for this research are available within the manuscript and supplementary files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.OMS. Plan d’action mondial pour combattre la résistance aux antimicrobiens [Internet]. Genève: Organization mondiale de la Santé; 2016 [cited 2024 Oct 24]. 21 p. Available from: https://iris.who.int/handle/10665/249548
- 2.O’NEILL J. Review on antimicrobial resistance: tackling drug-resistant infections globally: final report and recommendations. 2016 [Internet]. [cited 2024 Oct 24]. Available from: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf
- 3.Berthuim J, Miras M. La résistance aux antibiotiques: un enjeu de santé publique et économique. Bpi Fr Serv L’avenir; 2018.
- 4.Global antimicrobial resistance and use surveillance. System (GLASS) report 2022. Geneva: World Health Organization; 2022. License: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 5.Murray CJL, Ikuta KS, Sharara F, Swetschinski L, Aguilar GR, Gray A et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. The Lancet. 20221;399(10325):629–55. [DOI] [PMC free article] [PubMed]
- 6.Bernabé KJ, Langendorf C, Ford N, Ronat JB, Murphy RA. Antimicrobial resistance in West Africa: a systematic review and meta-analysis. Int J Antimicrob Agents. 2017;50(5):629–39. [DOI] [PubMed] [Google Scholar]
- 7.Ramdé M, (CEDEAO). Les limites de l’intégration régionale internationale: le cas de la Communauté économique des États de l’Afrique de l’Ouest. 2004 [cited 2024 Oct 31]; Available from: https://papyrus.bib.umontreal.ca/xmlui/handle/1866/15090
- 8.Tirer parti du dividende démographique en Afrique de l’Ouest. et contribuer à la réalisation des ODD| Nations Unies Commission économique pour l’Afrique [Internet]. [cited 2024 Oct 31]. Available from: https://www.uneca.org/fr/stories/tirer-parti-du-dividende-d%C3%A9mographique-en-afrique-de-l%E2%80%99ouest-et-contribuer-%C3%A0-la-r%C3%A9alisation
- 9.Ouedraogo AS, Pierre HJ, Bañuls AL, Ouédraogo R, Godreuil S. Émergence Et diffusion de la résistance aux antibiotiques en afrique de l’Ouest: facteurs favorisants et évaluation de la menace. Médecine Santé Trop. 2017;27(2):147–54. [DOI] [PubMed] [Google Scholar]
- 10.Perspectives. apport du diagnostic dans la lutte contre la résistance aux antimicrobiens en Afrique de l’Ouest - ScienceDirect [Internet]. [cited 2024 Oct 31]. Available from: https://www.sciencedirect.com/science/article/abs/pii/S2772743223000016
- 11.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hosseini MS, Jahanshahlou F, Akbarzadeh MA, Zarei M, Vaez-Gharamaleki Y. Formulating research questions for evidence-based studies. J Med Surg Public Health. 2024;2:100046. [Google Scholar]
- 13.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–81. [DOI] [PubMed] [Google Scholar]
- 14.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.JBI Manual for Evidence Synthesis. - JBI Global Wiki [Internet]. [cited 2024 Oct 25]. Available from: https://jbi-global-wiki.refined.site/space/MANUAL
- 16.Adegun PT, Odimayo MS, Olaogun JG, Emmanuel EE. Comparison of uropathogens and antibiotic susceptibility patterns in catheterized ambulant middle-aged and elderly Nigerian patients with bladder outlet obstruction. Turk J Urol. 2019;45(1):48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aglomasa BC, Adu-Asiamah CK, Asiedu SO, Kini P, Amewu EKA, Boahen KG, et al. Multidrug resistant bacteria isolates from lymphatic filariasis patients in the Ahanta West District, Ghana. BMC Microbiol. 2022;22(1):245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahoyo TA, Bankolé HS, Adéoti FM, Gbohoun AA, Assavèdo S, Amoussou-Guénou M, et al. Prevalence of nosocomial infections and anti-infective therapy in Benin: results of the first nationwide survey in 2012. Antimicrob Resist Infect Control. 2014;3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ako-nai AK, Abumere G, Akinyoola AL, Ebhodaghe BI, Attah OT, Kassim OO. Characterization of bacterial isolates from patients wounds and environmental factors predictive of postsurgical infections at the orthopedic ward in ile-ife, Nigeria. East Afr med j. 2013;90(12):380–6. [PubMed] [Google Scholar]
- 20.Asafo-Adjei K, Mensah JE, Labi AK, Dayie NTKD, Donkor ES. Urinary tract infections among bladder outlet obstruction patients in Accra, Ghana: etiology, Antibiotic Resistance, and risk factors. Diseases. 2018;6(3):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dayyab FM, Iliyasu G, Aminu A, Habib ZG, Tiamiyu AB, Tambuwal SH, et al. A prospective study of hospital-acquired infections among adults in a tertiary hospital in northwestern Nigeria. Trans R Soc Trop Med Hyg. 2018;112(1):36–42. [DOI] [PubMed] [Google Scholar]
- 22.Iliyasu G, Abdu A, Dayyab FM, Tiamiyu AB, Habib ZG, Adamu B, et al. Postrenal transplant infections: single-center experience from Nigeria. Transpl Infect Dis off J Transpl Soc. 2016;18(4):566–74. [DOI] [PubMed] [Google Scholar]
- 23.Iwuafor AA, Ogunsola FT, Oladele RO, Oduyebo OO, Desalu I, Egwuatu CC, et al. Incidence, clinical outcome and risk factors of Intensive Care Unit infections in the Lagos University Teaching Hospital (LUTH), Lagos, Nigeria. PLoS ONE. 2016;11(10):e0165242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lakoh S, Yi L, Russell JBW, Zhang J, Sevalie S, Zhao Y, et al. The burden of surgical site infections and related antibiotic resistance in two geographic regions of Sierra Leone: a prospective study. Ther Adv Infect Dis. 2022;9:20499361221135128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lakoh S, Yi L, Russell JBW, Zhang J, Sevalie S, Zhao Y, et al. High incidence of catheter-associated urinary tract infections and related antibiotic resistance in two hospitals of different geographic regions of Sierra Leone: a prospective cohort study. BMC Res Notes. 2023;16(1):301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Makanjuola OB, Fayemiwo SA, Okesola AO, Gbaja A, Ogunleye VA, Kehinde AO, et al. Pattern of multidrug resistant bacteria associated with intensive care unit infections in IBADAN, NIGERIA. Ann Ib Postgrad Med. 2018;16(2):162–9. [PMC free article] [PubMed] [Google Scholar]
- 27.Yehouenou CL, Kpangon AA, Affolabi D, Rodriguez-Villalobos H, Van Bambeke F, Dalleur O, et al. Antimicrobial resistance in hospitalized surgical patients: a silently emerging public health concern in Benin. Ann Clin Microbiol Antimicrob. 2020;19(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diedhiou M, Sarr N, Dieye A, Lo S, Ba EB, Barboza D, et al. Nosocomial infections in the Intensive Care Unit of Saint Louis Regional Hospital: Status Report and prognostic factors of Mortality. EAS J Anesthesiol Crit Care. 2023;5(3):38–44. [Google Scholar]
- 29.Aminata M, Alioune BS, Yacouba C, Agaly DO, Bassirou D, Abdoulaye T, et al. Multidrug resistant bacteria isolated from nosocomial infections at University Teaching Hospital of Point-G, Bamako, Mali. Afr J Bacteriol Res JBR. 2023;15(1):39116BE70184. [PMC free article] [PubMed] [Google Scholar]
- 30.Adeleye QA, Ndubuisi EC, Isa FA. Etiologic and antimicrobial susceptibility profiles of bacterial urinary tract infection and bacterial enteritis among children at a private Multi-specialty Healthcare Facility in Abuja, Nigeria: a 5-Year separate and comparative review. Niger J Clin Pract. 2024;27(1):35–46. [DOI] [PubMed] [Google Scholar]
- 31.Aika IN, Enato E. Antibiogram of clinical isolates from primary and secondary healthcare facilities: a step toward antimicrobial stewardship. PLOS Glob Public Health. 2022;2(12):e0000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asamoah B, Labi AK, Gupte HA, Davtyan H, Peprah GM, Adu-Gyan F, et al. High resistance to Antibiotics recommended in Standard Treatment Guidelines in Ghana: a cross-sectional study of antimicrobial resistance patterns in patients with urinary tract infections between 2017–2021. Int J Environ Res Public Health. 2022;9(24):16556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campbell JSO, van Henten S, Koroma Z, Kamara IF, Kamara GN, Shewade HD, et al. Culture requests and Multi-drug Resistance among suspected urinary tract infections in two Tertiary hospitals in Freetown, Sierra Leone (2017-21): a cross-sectional study. Int J Environ Res Public Health. 2022;19(8):4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chukwu EE, Awoderu OB, Enwuru CA, Afocha EE, Lawal RG, Ahmed RA, et al. High prevalence of resistance to third-generation cephalosporins detected among clinical isolates from sentinel healthcare facilities in Lagos, Nigeria. Antimicrob Resist Infect Control. 2022;11(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dibbasey M, Dahaba M, Sarfo F, Jallow-Manneh I, Ceesay B, Umukoro S, et al. Laboratory indices of hospitalized sickle cell disease patients, prevalence and antimicrobial susceptibility of pathogenic bacterial isolates at MRCG ward in the Gambia. BMC Infect Dis. 2023;23(1):546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gnimatin JP, Weyori EW, Agossou SM, Adokiya MN. Bacterial infections epidemiology and factors associated with multidrug resistance in the northern region of Ghana. Sci Rep. 2022;12(1):22069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iliyasu G, Daiyab FM, Tiamiyu AB, Abubakar S, Habib ZG, Sarki AM, et al. Nosocomial infections and resistance pattern of common bacterial isolates in an intensive care unit of a tertiary hospital in Nigeria: a 4-year review. J Crit Care. 2016;34:116–20. [DOI] [PubMed] [Google Scholar]
- 38.Inusah A, Quansah E, Fosu K, Dadzie I. Resistance Status of Bacteria from a Health Facility in Ghana: a retrospective study. J Pathog. 2021;2021:6648247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karikari AB, Saba CK, Yamik DY. Reported cases of urinary tract infections and the susceptibility of Uropathogens from hospitals in Northern Ghana. Microbiol Insights. 2022;15:11786361221106109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Labi AK, Obeng-Nkrumah N, Bjerrum S, Enweronu-Laryea C, Newman MJ. Neonatal bloodstream infections in a Ghanaian Tertiary Hospital: are the current antibiotic recommendations adequate? BMC Infect Dis. 2016;16(1):598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ombelet S, Kpossou G, Kotchare C, Agbobli E, Sogbo F, Massou F, et al. Blood culture surveillance in a secondary care hospital in Benin: epidemiology of bloodstream infection pathogens and antimicrobial resistance. BMC Infect Dis. 2022;22(1):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tetteh FKM, Fatchu R, Ackah K, Philips TJ, Shewade HD, Fenny AP, et al. Sepsis among neonates in a Ghanaian Tertiary Military Hospital: culture results and Turnaround Times. Int J Environ Res Public Health. 2022;19(18):11659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tobin EA, Samuel SO, Inyang NJ, Adewuyi GM, Nmema EE. Bacteriological Profile and antibiotic sensitivity patterns in clinical isolates from the out-patient departments of a Tertiary Hospital in Nigeria. Niger J Clin Pract. 2021;24(8):1225–33. [DOI] [PubMed] [Google Scholar]
- 44.Abdoulaye O, Bacha BSM, Aghali NH, Abdoulaye I, Abdoulaye MB, Lo G, et al. Profile of multidrug-resistant clinical bacterial isolates at the National Hospital of Zinder (NHZ), Niger Republic in 2021: Profil Des souches bactériennes multirésistantes isolées à l’Hôpital National De Zinder (HNZ), République Du Niger en 2021. Afr J Clin Exp Microbiol. 2022;23(4):369–77. [Google Scholar]
- 45.Moroh JL, Fleury Y, Tia H, Bahi C, Lietard C, Coroller L, et al. Diversity and antibiotic resistance of uropathogenic bacteria from Abidjan. Afr J Urol. 2014;20(1):18–24. [Google Scholar]
- 46.Ky/Ba A, Tondé I, Dienderé EA, Ky AY, Tamini JR, Sanou M, et al. Bacteriological profiles of urinary tract infections in patients admitted to the nephrology-hemodialysis department of the Bogodogo University Teaching Hospital (CHU B), Ouagadougou, Burkina Faso: Profils bactériologiques des infections urinaires chez les patients admis Au service de néphrologie-hémodialyse du CHU de bogodogo (CHU B), Ouagadougou, Burkina Faso. Afr J Clin Exp Microbiol. 2024;25(3):295–302. [Google Scholar]
- 47.Rahden P, Barrow E, Bah H, Bittaye SO, Nygren D, Badjan A. Bloodstream infections at a tertiary hospital in the Gambia-A one-year retrospective study. Research Square; 2024. 10.21203/rs.3.rs-4378140/v1. [DOI] [PMC free article] [PubMed]
- 48.Arlette AS, Valérie MG, Bertin TK, Bertin KG, Anatole TA, Fernique K, et al. Multiresistant Bacteria to antibiotics in hospitals: the case of Neonatology services of the University Hospitals Centers of Abidjan, Côte d’Ivoire. South Asian J Res Microbiol. 2023;15(3):47–56. [Google Scholar]
- 49.Adeyemo AT, Kolawole BA, Rotimi VO, Aboderin AO. Multicenter Study of the Burden of Multidrug-Resistant Bacteria in the Etiology of Infected Diabetic Foot Ulcers [Internet]. bioRxiv; 2019 [cited 2024 Sep 29]. p. 625012. Available from: https://www.biorxiv.org/content/10.1101/625012v1 [DOI] [PMC free article] [PubMed]
- 50.Dossim S, Bawe LD, Dossouvi KM, Maba D, Lawani AA, Godonou AM, et al. Antibiotic resistance profiles of Bacteria isolated from patients hospitalized at the Sylvanus Olympio University Teaching Hospital in Lomé, Togo. Microbiol Res J Int. 2024;34(4):13–23. [Google Scholar]
- 51.Wembulua BS, Lakhe A, Mbaye KD, Aw NN, Diallo VC, Ka D, et al. Profils de Sensibilité aux Antibactériens des Isolats Sanguins Chez 74 patients Infectés Par Le VIH Hospitalisés à La Clinique Des maladies Infectieuses et tropicales du Chu De Fann, Dakar De 2013 à 2016. Médecine TropSanté. 2021;1(2):mtsibulletin. 10.48327/mtsibulletin.n1.2021.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goodyer J, Elbadawi H, Mayronne S, Lynch E, Michel J, Carreras I et al. Retrospective descriptive analysis of pediatric bacteraemia-Bardnesville Junction Hospital, Liberia. In: MSF Pediatric Days 2022 [Internet]. 2022 [cited 2024 Oct 29]. Available from: https://api.scienceportal.msf.org/api/v1/blobs/14243
- 53.Afum T, Asandem DA, Asare P, Asante-Poku A, Mensah GI, Musah AB, et al. Diarrhea-causing Bacteria and their antibiotic resistance patterns among Diarrhea patients from Ghana. Front Microbiol. 2022;13:894319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bonko MDA, Tahita MC, Kiemde F, Lompo P, Yougbaré S, Some AM, et al. Antibiotic susceptibility profile of bacterial isolates from febrile children under 5 years of age in Nanoro, Burkina Faso. Trop Med Int Health TM IH. 2021;26(10):1220–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chukwumeze F, Lenglet A, Olubiyo R, Lawal AM, Oluyide B, Oloruntuyi G, et al. Multidrug resistance and high mortality associated with community-acquired bloodstream infections in children in conflict-affected northwest Nigeria. Sci Rep. 2021;11(1):20814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Diarra B, Guindo I, Koné B, Dembélé M, Cissé I, Thiam S, et al. High frequency of antimicrobial resistance in Salmonella and Escherichia coli causing diarrheal diseases at the Yirimadio community health facility, Mali. BMC Microbiol. 2024;24(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Donkor ES, Horlortu PZ, Dayie NT, Obeng-Nkrumah N, Labi AK. Community acquired urinary tract infections among adults in Accra, Ghana. Infect Drug Resist. 2019;12:2059–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Isendahl J, Manjuba C, Rodrigues A, Xu W, Henriques-Normark B, Giske CG, et al. Prevalence of community-acquired bacteraemia in Guinea-Bissau: an observational study. BMC Infect Dis. 2014;14:3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iwalokun BA, Iwalokun SO, Hodonu SO, Aina OA, Agomo PU. Evaluation of microalbuminuria in relation to asymptomatic bacteruria in Nigerian patients with sickle cell anemia. Saudi J Kidney Dis Transpl off Publ Saudi Cent Organ Transpl Saudi Arab. 2012;23(6):1320–30. [DOI] [PubMed] [Google Scholar]
- 60.Kebbeh A, Dsane-Aidoo P, Sanyang K, Darboe SMK, Fofana N, Ameme D, et al. Antibiotics susceptibility patterns of uropathogenic bacteria: a cross-sectional analytic study at Kanifing General Hospital, the Gambia. BMC Infect Dis. 2023;23(1):723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Onanuga A, Omeje MC, Eboh DD. Carriage of multi-drug-resistant urobacteria by asymptomatic pregnant women in YENAGOA, BAYELSA state, NIGERIA. Afr J Infect Dis. 2018;12(2):14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Onanuga A, Selekere TL. Virulence and antimicrobial resistance of common urinary bacteria from asymptomatic students of Niger Delta University, Amassoma, Bayelsa State, Nigeria. J Pharm Bioallied Sci. 2016;8(1):29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Otajevwo FD. Urinary tract infection among symptomatic outpatients visiting a tertiary hospital based in midwestern Nigeria. Glob J Health Sci. 2013;5(2):187–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Konate I. Profil bactériologique et pronostique des pneumonies bactériennes non tuberculeuses chez les patients VIH Au Mali. Rev Malienne Infect Microbiol. 2022;17(1):46–53. [Google Scholar]
- 65.Sampane-Donkor E, Badoe EV, Annan JA, Nii-Trebi N. Colonization of antibiotic resistant bacteria in a cohort of HIV infected children in Ghana. Pan Afr Med J. 2017;26:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alemayehu T. Prevalence of multidrug-resistant bacteria in Ethiopia: a systematic review and meta-analysis. J Glob Antimicrob Resist. 2021;26:133–9. [DOI] [PubMed] [Google Scholar]
- 67.Assefa M, Amare A, Tigabie M, Girmay G, Setegn A, Wondmagegn YM, et al. Burden of multidrug-resistant bacteria among HIV-positive individuals in Ethiopia: a systematic review and meta-analysis. PLoS ONE. 2024;19(8):e0309418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ita T, Luvsansharav UO, Smith RM, Mugoh R, Ayodo C, Oduor B, et al. Prevalence of colonization with multidrug-resistant bacteria in communities and hospitals in Kenya. Sci Rep. 2022;12(1):22290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rhim H, Trad RB, Haddad O, Kadri Y, Mastouri M. Comparative study of multidrug-resistant bacterial infections in hospitals and community settings in the region of Monastir– Tunisia. Tunis Médicale. 2022;100(5):390. [PMC free article] [PubMed] [Google Scholar]
- 70.Kamouni E, Zouhair S, Arsalane L. The multidrug-resistant bacteria: frequency and antibiotic resistance levels in the region of Marrakesh, Morocco. IJSAR. 2022;9(4):14–21. [Google Scholar]
- 71.Fernández J, Prado V, Trebicka J, Amoros A, Gustot T, Wiest R, et al. Multidrug-resistant bacterial infections in patients with decompensated cirrhosis and with acute-on-chronic liver failure in Europe. J Hepatol. 2019;70(3):398–411. [DOI] [PubMed] [Google Scholar]
- 72.MacFadden DR, McGough SF, Fisman D, Santillana M, Brownstein JS. Antibiotic resistance increases with local temperature. Nat Clim Change. 2018;8(6):510–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oneko M, Kariuki S, Muturi-Kioi V, Otieno K, Otieno VO, Williamson JM et al. Emergence of Community-Acquired, Multidrug-Resistant Invasive Nontyphoidal Salmonella Disease in Rural Western Kenya, 2009–2013. Clin Infect Dis. 2015;61 Suppl 4:S310-6. 10.1093/cid/civ674. PMID: 26449946. [DOI] [PubMed]
- 74.Shawa M, Paudel A, Chambaro H, Kamboyi H, Nakazwe R, Alutuli L, et al. Trends, patterns and relationship of antimicrobial use and resistance in bacterial isolates tested between 2015–2020 in a national referral hospital of Zambia. PLoS ONE. 2024;19(4):e0302053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Poletajew S, Pawlik K, Bonder-Nowicka A, Pakuszewski A, Nyk Ł, Kryst P. Multi-drug-resistant Bacteria as Etiological Factors of Infections in a Tertiary Multidisciplinary Hospital in Poland. Antibiotics. 2021;10(10):1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shaker M, Zaki A, Asser SL, Sayed IE. Trends and predictors of antimicrobial resistance among patients with urinary tract infections at a tertiary hospital facility in Alexandria, Egypt: a retrospective record-based classification and regression tree analysis. BMC Infect Dis. 2024;24(1):246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abu-Gharbia MA, Al-Arabi G, Salem JM. Community-acquired urinary tract infections: epidemiology, etiology, and β-Lactam resistance. Sohag J Sci. 2024;9(1):47–55. [Google Scholar]
- 78.Klingeberg A, Willrich N, Schneider M, Schmiemann G, Gágyor I, Richter D, et al. The percentage of Antibiotic Resistance in Uncomplicated Community-acquired urinary tract infections. Dtsch Arztebl Int. 2024;121(6):175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kaye KS, Gupta V, Mulgirigama A, Joshi AV, Ye G, Scangarella-Oman NE, et al. Prevalence, regional distribution, and trends of antimicrobial resistance among female outpatients with urine Klebsiella spp. isolates: a multicenter evaluation in the United States between 2011 and 2019. Antimicrob Resist Infect Control. 2024;13(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chan MC, Chiu SK, Hsueh PR, Wang NC, Wang CC, Fang CT. Risk factors for healthcare-associated extensively drug-resistant Acinetobacter baumannii infections: a case-control study. PLoS ONE. 2014;9(1):e85973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sher AA, VanAllen ME, Ahmed H, Whitehead-Tillery C, Rafique S, Bell JA, et al. Conjugative RP4 plasmid-mediated transfer of Antibiotic Resistance genes to commensal and multidrug-resistant enteric Bacteria in Vitro. Microorganisms. 2023;11(1):193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang Y, Ren J, Yao Z, Wang W, Wang S, Duan J, et al. Clinical impact and risk factors of Intensive Care Unit-acquired nosocomial infection: a propensity score-matching study from 2018 to 2020 in a Teaching Hospital in China. Infect Drug Resist. 2023;16:569–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morehead MS, Scarbrough C. Emergence of global antibiotic resistance. Prim Care. 2018;45(3):467–84. [DOI] [PubMed] [Google Scholar]
- 84.Bassoum O, Lèye MMM, Sougou NM, Diongue M, Niang K, Tine JAD, et al. Practices about antibiotic use among urban residents: a cross-sectional survey in Rufisque, Senegal. Cent Afr J Public Health. 2019;5(1):1–12. [Google Scholar]
- 85.Abubakar U. Antibiotic use among hospitalized patients in northern Nigeria: a multicenter point-prevalence survey. BMC Infect Dis. 2020;20(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tapha O, Degbey CC, Yacouba A, Mahouna Tchioundjro E, Nadakou NT, Alkassoum Salifou I, et al. Antimicrobial use in hospitalized patients: a point prevalence survey across four tertiary hospitals in Niger. JAC Antimicrob Resist. 2024;6(5):dlae175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Umeokonkwo CD, Onah CK, Adeke AS, Igwe-Okomiso DO, Umeokonkwo AA, Madubueze UC, et al. Antimicrobial use among paediatric inpatients in a Nigerian tertiary hospital: a three-year point prevalence survey. J Infect Prev. 2023;24(2):71–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kamara IF, Kanu J, Maruta A, Fofanah BD, Kamara KN, Sheriff B, et al. Antibiotic use among hospitalised patients in Sierra Leone: a national point prevalence survey using the WHO survey methodology. BMJ Open. 2023;13(12):e078367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gnimavo MS, Boya B, Mudenda S, Allabi AC. Antibiotic use at the Centre Hospitalier Universitaire De Zone d’Abomey Calavi/Sô-Ava (CHUZ/AS) in Benin: a point prevalence survey. JAC Antimicrob Resist. 2025;7(1):dlae220. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data required for this research are available within the manuscript and supplementary files.