Abstract
Conservation of elephants requires physical, chemical, and biological approaches to ensure the protection of these gargantuan pachyderms. One such approach is using orange plants (as biofencing) for the repellence of elephants, which precludes catastrophic events related to the encroachment of elephants into human habitats. Elephants have sensitive olfactory discrimination of plant volatile compounds for foraging and other behavior using G-protein-coupled receptors (GPCRs). However, 2 such receptors are the A2A and A2B receptors mediating olfaction elicited by a host of ligands, including limonene, the main volatile compound in citrus plants, which is hypothesized to be the chief repelling agent. Bioinformatics at the protein and mRNA levels (BLAST/Multiple Sequence Alignments) were employed to explore the multiple expression products of A2B receptors, namely full-length and truncated proteins produced by isoform mRNAs translated from multiple methionines, while the comparison of the limonene-binding pockets of human and elephant A2B receptors and prediction servers [Netphos 3.1; Protter] was used to focus, respectively, on the contacts limonene binding entails and the post-translational modifications that are involved in cell signaling. Finally, the link between limonene and antifeedant activity was explored by considering limonene content on trees that are preferentially foraged or avoided as part of the feeding behavior by elephants. The African bush elephant (Loxodonta africana) possesses a full-length A2A receptor but unlike most mammals, expresses a highly truncated A2B receptor isoform possessing only transmembrane helices 5, 6, and 7. Truncation may lead to higher traffic and expression of the A2B receptor in olfactory interfaces/pathways and aid stronger activation. In addition, all residues in the putative limonene-binding cleft are perfectly conserved between the human and African bush elephant A2B receptors, both full length and truncated. Shallow activation sites require micromolar affinity and fewer side-chain interactions, which is speculated to be the case for the truncated A2B receptor. An N-terminal extremity N-glycosylation motif is indicative of membrane localization of the truncated A2B receptor following accurate folding. A combination of truncation, indels, substitutions, and transcript isoforms are the attributed roles in the evolution of the L. africana A2B receptor, out of which limonene receptivity may be the key. It is also inferred how limonene may act as a dietary repellent/antifeedant to a generalist herbivore, with the documented limonene content being absent in some dietary favorites including the iconic Sclerocarya birrea.
Keywords: elephants, citrus, limonene, conservation, A2A receptors, A2B receptors
Graphical abstract

Introduction
Elephants, both the African (Genus Loxodonta) and the Asian (Genus Elephas), are known for their sensitive olfactory reception of plant volatile compounds, that affect their behavior, including foraging activity. 1 Olfaction is dependent on the reception (docking) of volatile ligands to receptors at the olfactory epithelium and discrimination of smell determined by the olfactory bulb. 2 More than ~1400 olfactory receptors are attributed to olfactory reception by Asian elephants, against ~400 in humans, which demonstrates the superior olfaction (smell) of elephants compared with humans.3,4 In fact, the olfactory bulb in African elephants is a superlative in the animal world, with a resounding volume of 59.9–62.4 cm3. 3
Elephant–human conflict sacrifices hundreds of elephants every year while poaching for ivory dwindles the number of tuskers. Elephants are repelled (avoidance) by orange plants when present as a barrier, so earmarking fences made of orange trees can be a biocontrol solution for elephant conservation. The repellent/s in oranges can be of unique candidacy or an amalgam of many volatile compounds. However, limonene is the primary volatile compound found in oranges and is hypothesized here, to be a repelling agent.
Limonene has been shown in the past to be capable of serving as an agonist to A2A receptors in humans, a family of GPCRs, that binds to a ligand using transmembrane domains 3, 5, and 6 and parts of the extracellular loops, which promotes a response by G-protein coupling, generation of cAMP, mobilization of calcium, and subsequent depolarization, giving rise to a neural response.5-8
There is a second A2B receptor that is known to be a counterpart for A2A, poorly expressed on olfactory organs but expressed profusely in areas of taste reception, which has provisioned a role for A2B receptors in taste discrimination. 9 A2A and A2B belong to the wider adenosine receptors that also contain the A1 and the A3 receptors, while an auxiliary A2C receptor is found in fish, which is missing in the terrestrial counterparts.
GPCRs are known to be under the control of phosphorylation by GPCR kinases and a family of proteins called beta-arrestins.10,11 cAMP-dependent protein kinase (PKA) and Ca2+-dependent protein kinase (PKC), among others, phosphorylate serine and threonine residues for downstream signal transduction functions. 12 GPCRs are known to possess truncated splice variants from splicing phenomena, containing a smaller number of transmembrane domains, that are inactive in function, attributing a negative dominant phenotype. 13 However, GPCRs are also known to form oligomers, consequently, oligomerization using—among others—the C-terminal unstructured domains, may be able to rescue a truncated protein from its absence of independent functionality. Truncated GPCRs are also released easily from the endoplasmic reticulum (ER) to be transported to the cell membranes, again symbolizing that they may have a functional significance in the resident organism. 14 Truncated forms of olfactory receptors are thought of as being: (1) recalcitrant to protein misfolding and (2) possessing a modified trafficking pathway to the full-length GPCRs. 13
Precise specificity and stronger sensitivity in olfactory discrimination of the Asian Elephant, using a sample size of 3, was demonstrated for diverse length aliphatic hydrocarbons and enantiomers of volatile compounds, where the elephants were able to discriminate mild changes in structure—both stereochemical and stereo-optical. 3 There are hypotheses on the olfactory superiority of elephants, which entail 2 critical theories: (1) the larger size of the olfactory bulb and (2) the superior numbers of olfactory receptors. Elephants are known to contain ~3400 GPCRs, which in man, is only 800 but the expansion of the above protein superfamily cannot be attributed to the olfactory system alone. 15 Olfaction receptors can also be promiscuous or strict in relation to their binding ligands.
In this in silico study, the nuts and bolts of the relationship between limonene and limonene-responsive A2A and A2B receptors of elephants are sought to study the biochemistry impacting the physiology of smell and repellency. Limonene constitutes > 90% of the volatile compounds of the orange plant and consequently, this study is of relevance for finding a biofencing solution for the conservation of the African bush elephant and by association their Asian counterparts.
Methods
Sequence retrieval and alignment
First, protein sequences corresponding to L. africana A2A and A2B receptors were searched using the National Center for Biotechnology Information (NCBI) Protein database [https://www.ncbi.nlm.nih.gov/protein/], using word-based queries. The resulting proteins had the following identities, XP_010597015.1 (A2A Receptor);
XP_023395384.1 (Truncated A2B receptor) (Table 1).
Table 1.
The proteins and mRNA isoforms from L. africana used in this bioinformatics study.
| Protein | mRNA | Identity | Length |
|---|---|---|---|
| XP_023395384.1/XP_023395383.1 | Truncated A2B receptor | 172 aa | |
| XP_064127927.1 | Full-length A2B receptor | 333 aa | |
| XP_064128479.1/XP_010597015.1 | A2A receptor | 400 aa | |
| XM_023539615 | Transcript variant X1 (A2B) | 1541 bp | |
| XM_023539616 | Transcript variant X2 (A2B) | 1538 bp | |
| XM_064271857.1 | Full-length A2B transcript | 1885 bp |
It has come to light that the original annotation of XP_023395384.1 (Truncated A2B receptor) has been supplemented by a full-length protein (XP_064127927.1) and the original annotation suppressed. However, due to the presence of a 1541 bp mRNA (Accession XM_023539615/Transcript Variant X1) and 1538 bp mRNA (Accession XM_023539616/Transcript Variant X2) (Table 1) that when translated to the protein sequence yields the 172 amino acid region, points to a truncated protein being present perhaps on top of the full-length protein or as the major candidate.
The 1541 bp mRNA (Accession XM_023539615/Transcript Variant X1) and 1538 bp mRNA (Accession XM_023539616/Transcript Variant X2) match the start site introduced by the second methionine (first internal methionine) and this encodes the 172 amino acid length protein designated by the accession XP_023395384.1 (truncated A2B receptor) (Table 1).
A suppressed record in NCBI is defined as follows—“Suppressed: Suppressed data are data that were previously public, have been removed from the NCBI text-based search and comparative analysis results, and may be accessed only by accession number. Suppressed data often have a future date when they will return to public status.”
Sequence alignments of protein sequences were performed using the MEGA and DAMBE software (Version X).16,17 Sequence alignments indicate both conserved regions and non-conserved regions, the former informative on functional areas that are of strong relevance to many animals (thus similar in sequence) and the latter indicative of newer changes in the function of the protein in relation to the L. africana protein sequences, likely to be due to selection by nature of beneficial evolutionary outcomes manifesting at the trait level.
Phylogenetic inferences
Phylogeny elaborates the evolutionary history of a gene/protein family using a gene tree or phylogenetic tree and can be used as a tool to identify speciation and gene duplications. A maximum likelihood or neighborhood joining phylogenetic tree was constructed using MEGA software, (version X) comprising downloaded sequences [in FASTA format] derived from a search using BLASTp search tool. Relationships between adjacent nodes were based on bootstrap support from 1000 pseudo-replicates. The phylogenetic tree was constructed following the multiple sequence alignment of sequences using the default alignment tool [ClustalW] in MEGA X software.
BLAST searches
Components of the BLAST toolbox, namely the BLASTp (protein-level searches) and BLASTn (nucleotide-level searches), were used for sequence searches against the respective databases. The highest matches in BLASTp results are indicative of proteins that share a high level of functionality. For BLASTn searches, > 97% sequence identity between 2 DNA sequences can be inferred as belonging to the same species, while a marginally lesser level of homology (90%–95%) is used to zoom in on the same genus.
BLASTp, that is, protein–protein BLAST searches were performed using the following parameters: standard databases were searched (non-redundant protein sequences [nr]) with no specific organism mentioned to focus/narrow down the search—and not checking any boxes to mark exclusions—allowing for a wider coverage of sequences from a spectrum of species. No algorithm parameters were modified.
BLASTn searches relied on standard databases, focusing on the core nucleotide database (Core_nt), with no entry of an organism name and no checking exclusions. The search was optimized for highly similar sequences [megablast]. No algorithm parameters were modified.
Phosphorylation sites prediction
Phosphorylation is a post-translational modification that is supplemented on the 3 amino acids—threonine, serine, and tyrosine—following translation of the corresponding mRNA. The Netphos 3.1 server was used for the prediction of phosphorylated residues in the A2B receptors. 18
The specific sequence was pasted on the sequence submission form in FASTA format, with all 3 residues—serine, threonine, and tyrosine—checked for phosphorylation site mapping. The output format was classical, while there was no requirement to modify the threshold of displayed scores.
Phosphorylated residues are functionally significant in relation to signaling, following a binding event (in this case, limonene) that is mediated by receptors, ferried across by membrane-bound proteins (via extracellular and membrane-anchored A2B receptors), and is relayed downstream to the nucleus, for gene expression.
Plotting the protein architecture
GPCRs have an N-terminal extracellular region, transmembrane regions, and a C-terminal intracellular region. The transmembrane protein architecture of the A2B receptors was plotted using the Protter server using the default parameters. 19 Protter also identifies N-glycosylations in the protein sequences that are a hallmark of membrane attachment/localization and consequently function.
Homology modeling
Homology models were built using the Swiss-Model Server https://swissmodel.expasy.org/. The target sequence (truncated A2B receptor from L. africana) was entered into the interactive workspace and searched for suitable templates. The template used for the construction of the truncated A2B receptor homology model was P29276.1.A (Adenosine receptor A2B from Rattus norvegicus), which showed the highest levels of sequence homology (89% sequence identity) to the protein of concern.
RNA secondary structure prediction
RNA secondary structure prediction was performed using the ViennaRNA Web Services toolkit. The RNA fold webpage is hosted at http://rna.tbi.univie.ac.at/cgi-bin/RNAWebSuite/RNAfold.cgi. Under fold algorithms and basic options, the default parameters—minimum free energy (MFE) and partition function—were employed for the determination of RNA structure, while avoiding isolated base pairs. No other changes were checked outside of the default options for any of the other query themes. The output included interactive RNA secondary structure plots, RNA secondary structure plots with reliability annotation (Partition function folding only), and Mountain plots.
Ballesteros–Weinstein numbering scheme
Protein IDs for sequences from L. africana that were obtained from the NCBI database were not 100% available in UniProt (https://www.uniprot.org/), and this prevented the author from aligning the GPCRs with emphasis on the Ballesteros–Weinstein numbering scheme.
It is noteworthy that the absence or deficiency of elephant A2B and A2A receptors accessible from the GPCR database—https://gproteindb.org/—prevented the usage of alignments, structures, and illustrations in the article, to represent the canonical numbering scheme.
Since the focus of the article is not solely based on the structure/sequence-numeric alignments and membrane-anchored GPCRs are illustrated using the Protter tool, 19 the author feels that although the utility of the canonical numbering scheme would be beneficial for pinpoint representation, the absence of it will not hamper the findings of the study and the overall impact of the bioinformatics exploration.
Among other parameters, the Protter server applies protein topology information and detailed protein feature annotation from UniProt, 19 the same database/repository that supplies protein-level information for the GPCR database—https://gproteindb.org/. The above factors ensure that the Protter tool is authentic and dependable for correct association and display of membrane-spanning, extracellular and intracellular regions.
To search for the human A2B protein (in https://gproteindb.org/), the “receptor page” was employed where the author clicked on the folder icon for Class A, expanded it to display receptor groups, retrieved “Nucleotide receptors,” further expanded on the term “Adenosine,” and finally selected the human A2B receptor.
Results
A2A receptor of L. africana
It has been demonstrated previously that the human A2A receptor is activated by limonene as an agonist ligand. Therefore, the homology between the human and the African bush elephant A2A receptor was sought with a BLASTp search, to ascertain their similarities and differences. The resulting output showed 87.8% sequence identity between the 2 proteins, which included a 10-residue gap at the C-terminal end (data not shown). Figure 1 showcases the phylogeny of the A2A receptor of L. africana with fellow mammals, which unveils a monophyletic clade between the African Bush elephant and the Florida manatee.
Figure 1.
Phylogeny of A2A receptor protein sequences using the Neighborhood Joining method. The sequences were first aligned using the ClustalW tool, sampled by 1000 bootstrap replications, prior to drawing the phylogenetic tree. The level of bootstrap support is indicated at the nodes of the phylogenetic tree.
Biochemistry of the A2B receptor
A2B receptor has been demonstrated in prior studies to possess parallel or overlapping functionality to the A2A receptor in functions, such as mucociliary clearance rate. 20 The A2B receptor was sought using the NCBI protein resources, and 2 candidate proteins were identified that were of 333 and 172 amino acids in length. The shorter A2B receptor only contained helices 5, 6, and 7 of the total transmembrane helical structure of any GPCR and was missing helices, 1, 2, 3, and 4.
The intracellular region between helices 5 and 6 is known to provide a number of residues to be phosphorylated by cytoplasmic GPCR kinases and therefore, the likely candidates to be phosphorylated were identified using NetPhos 3.1 server. 18 However, 3 residues within the region between helices 5 and 6 were identified as likely to phosphorylated: (1) Threonine-46, (2) Serine-61, and (3) Threonine-64 (Figure 2). Out of which, Threonine 46 is unique to the sequences from the African savanna elephant and the giant panda. Out of the above, the likely downstream pathways encompass protein kinase-C for Threonine-46 and Threonine-64, while for Serine-61, it is inferred to be protein kinase A (Figure 2).
Figure 2.
Phosphorylated residues predicted using Netphos 3.1 for the XP_023395383.1 protein from L. africana. The upper panel depicts the putative phosphorylated residues as a function of protein sequence (X-Axis) from the N-terminal end to the C-terminus, while the Y-axis showcases the probability of phosphorylation over a threshold of 0.5. Serines are highlighted in red and threonines in green in the lower panel. The Netphos 3.1 server uses serine, threonine, and tyrosine phosphorylation sites using families of neural networks, drawing from experimentally verified phosphorylation sites.
The A2B receptor too has 2 serines in the distal C-terminal region of the full-length protein, which have a higher propensity to be phosphorylated as predicated by the NetPhos 3.1 server. Serine-145 is likely to mediate downstream action via protein kinase A, while the fate of Serine-157 is unspecified (Figure 2).
Evidence for limonene receptivity of the truncated A2B receptor
In an article, the side-chain donating residues in a binding cleft for limonene was showcased in a 3-dimensional structure of the A2B receptor from humans. 5 Figure 3 shows the arrangement of helices in a classical GPCR, while Figure 4 demonstrates the side-chain architecture that contours the limonene chemistry in the human A2B receptor compared to the truncated counterpart from African bush elephants.
Figure 3.
Schematic illustration of (A) the linear and (B) the structural simplification of a GPCR. This was reproduced with permission from a specific research study where this illustration appeared. 21
Figure 4.

The side chains of the limonene binding site mapped from the human A2B receptor as provided in the work by Patel et al., 5 showcased here in analogy to the counterparts from the truncated A2B receptor where the analogous residues appear. The illustration is a modified picture of the one provided in the work by Patel et al., 5 showcasing the side chains that are relevant and their positional IDs.
In addition, Figure 4 depicts the 100% conservation of key limonene binding/contouring residues between the human A2B receptor and the counterparts (truncated and full-length A2B) from the African Bush Elephant.
Mapping the N-glycosylation sites of the A2B and A2A receptors
The author used a bioinformatics tool (Protter) to showcase the protein architecture and to identify the N-glycosylation sites in both A2A and A2B receptor proteins. Although the A2A protein showed an N-glycosylation site between helices 4 and 5, the A2B receptor manifested an N-glycosylation site on the extracellular N-terminal extremity of the truncated protein (Figure 5A and B).
Figure 5.
(A) The NES N-glycosylation site indicated in the sequence of the A2B protein from L. africana and an illustration of the receptor using Protter. (B) The N-glycosylation sites in structure of the A2A protein from L. africana illustrated using Protter.
Genetics of the A2B receptor gene/transcript
Genetics of the A2B receptor gene/transcript was deciphered following the alignment of codon triplets to the respective amino acids of the full-length protein. The important residues around the start codon were manually assessed for their potential function which showed positional optimization. A 5’ untranslated region (UTR) region that was distinct in the transcripts XM_023539615 and XM_023539616 was shown to contain different structural elements leading to differential regulation of translation.
Discussion
A2A receptor of L. africana
The A2A receptors from 14 species were used for the compilation of a multiple sequence alignment, where only the African bush elephant and the Florida manatee A2A receptors demonstrated a 10-residue gap (Data Not Shown). Manatees and elephants are descendants of the speciation of a common ancestor named the clade Tethytheria. When the phylogeny was inferred for the A2A receptors, the African bush elephant and the Florida manatee A2A receptors formed an exclusively monophyletic cluster that was backed up by 100% bootstrap support (Figure 1).
The deleted region (10 residues) in the C-terminal extremity of both the African elephant and Florida Manatee A2A receptors was shown to eliminate a short helix region that was present in the rest of the counterparts (Data Not Shown). The elimination of a helix in the C-terminal extremity is hypothesized to improve the C-terminal “lack of structure/disorder” and to enhance structural disorder that can play a role in the oligomerization of the adenosine receptors and help in their traffic to the cell membrane. Structural disorder enhances the functional capabilities of proteins involved as chaperones, DNA- and RNA-binding proteins, and oligomers.23,24
Comparing A2A and A2B receptors (full length and truncated) of L. africana
When the truncated and full-length A2B receptors from L. africana are used in pairwise sequence alignment with the A2A receptor (Figure 6), using EMBOSS Water [https://www.ebi.ac.uk/jdispatcher/psa/emboss_water], the following features are visible from the sequence alignment. The regions immediately N-terminal and C-terminal to the initiator methionine [MNESCC] in the truncated A2B receptor is highly variable and poorly conserved between the A2A and both truncated/full-length A2B receptors (Figure 6) and can be distinguished apart from other parts of the protein sequences.
Figure 6.
Local sequence alignment of A2A [XP_064128479.1] and the full-length A2B receptor [XP_064127927.1] from L. africana to showcase similarities and divergences. In green is an insertion in the A2B receptor and in yellow is a region of weak conservation surrounding the initiator methionine of the truncated protein.
In the full-length A2B receptor [XP_064127927.1], there is an insertion of 7 residues marked by the sequence “NSQDSAT” that is missing (gapped) in the A2A receptor immediately upstream to the initiator methionine [MNESCC] of the shorter A2B receptor (Figure 6).
The region “TEPWDGAMNESC” in the full-length A2B receptor is poorly conserved between the A2A and A2B receptors (Figure 6); that is, there is very poor sequence similarity between the A2A and A2B receptors in the above area.
Sequence non-conservation is heightened in regions associated with the regulation of splicing. This feature is due to a partiality to produce custom RNA-binding sites to RNA-binding proteins mediating the synthesis of isoforms (splice variants) and to specify and regulate the production of a distinct “isoform.” It could be inferred that diverse RNA-binding proteins act as splicing silencers and activators to produce protein diversity. It is known that RNA-binding proteins recognize RNA-binding motifs specified by positional effects which points to “sequences-specific context” being an important criterion for producing protein isoforms.
Each splicing event is documented to be regulated by a multitude of RNA-binding proteins, following the guidelines of the “splicing code.” Therefore, it could be inferred that the dissimilarity (poor conservation) in the TEPWDGAMNESC sequence and the presence of the NSQDSAT sequence in A2B receptor [XP_064127927.1] compared with its counterpart A2A, maybe to accommodate distinct RNA-binding proteins at the transcript level, to fine-tune the production of a splice variant, leading to the translation of the 172 amino acid–truncated A2B receptor. This is just a hypothesis as of now, and there are further corroborating evidences—below—pointing to the above theory in the form of alternate splicing and internal translation:
The availability of 2 mRNA isoforms—XM_023539615 and XM_023539616 (Figure 7A) distinct in their 5’ UTR.
The translation of the mRNAs XM_023539615 and XM_023539616 produces the 172 amino acid protein (Figure 7) but not the 333 amino acid full-length protein XP_064127927.1.
The documented evidence of the presence of the truncated 172 amino acid protein in the NCBI database.
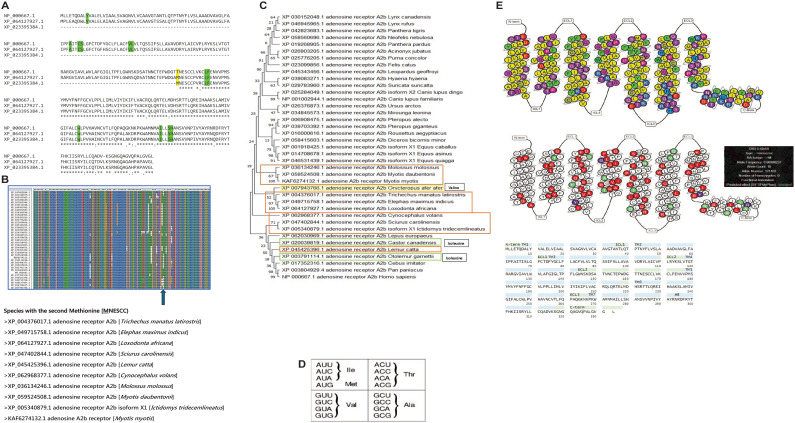
The absence of the second methionine in the human A2B receptor showcases species or lineage-specific mutations (Figure 8A). In fact, the second methionine is only found in a handful of species whose phylogeny showcases lineage-specific selection (Figure 8B and C).
There is likely internal initiation of translation from the internal methionine M NESCC resulting in the truncated protein.
The methionine M NESCC being only the second methionine in the full-length mRNA promotes internal translation from a distal site.
The initiator methionine has an open and accessible conformation in its RNA secondary structure, which is conducive to binding and being translated from the coding region for M NESCC (Figure 9A).
Threonine to methionine conversion is no wobble position mutation (Figure 8B). Threonine is coded by ACA, ACU, ACG, and ACT, which can be inferred to be the base state prior to mutation and selection. ACG (Threonine) to AUG (Methionine) is a single amino acid conversion mediated by a point mutation at the nucleotide level. However, if the threonine is encoded by ACA, ACU, or ACT, a single nucleotide mutation at the middle position only leads to an Isoleucine. Therefore, it appears, that at the specific position, there is selective pressure to transform a threonine into a methionine, either directly (one step) or via an intermediate isoleucine (2 steps) (Figure 8C and D). This is reflected in the sequences that were used for alignment and phylogeny construction (Figure 8B and C).
It appears that human A2B receptors too produce truncated isoforms containing transmembrane domains 4, 5, 6, and 7 (Supplementary Section – Table 1) that appear to be translated from an internal methionine different from that of M NESCC. This showcases the variety in truncated isoforms in A2B receptors is no rare event exclusive to elephants. Furthermore, the naturally occurring variation of the human A2B receptor demarcating an isoleucine 141 showing an affinity to being mutated into a methionine, points to a highly truncated mutant missense protein (Helices 5, 6, and 7) produced by the I → M mutation at this location, drawing similarities to the highly truncated A2B receptor from L. africana (Figure 8C).
Figure 7.
(A) 2 types of transcripts (mRNA isoforms) that are generated from the A2B receptor gene differing in their 5’ UTRs. The start codon is shown in a box while the differing 5’ UTRs are shown in a shaded blue rectangle. In addition, all 3 transcript isoforms are aligned in Supplementary Figure 1; (B) The alignment of the truncated A2B receptor mRNA with the corresponding A2B receptor protein sequence. Important conserved residues in the Kozak sequence are shown as pink residues. The start codon is colored yellow and the stop codon is colored gray.
Figure 8.
(A) Sequence alignment of the human A2B receptor [NP_000667.1] with the counterparts from L. africana. The predicted limonene-binding residues are highlighted in green. Note that there is 100% conservation of the limonene-binding residues between the 3 sequences. Furthermore, the internal methionine from where the translation is proposed to begin in the truncated A2B receptor is showcased in yellow. Of relevance, there is no second methionine in the human A2B receptor [In Yellow]; (B) Sequence alignment of A2B receptor protein sequences. The species armed with the second methionine (the translated site of the A2B truncated receptor from L. africana) are shown underneath as relevant species; (C) Phylogeny of A2B receptor protein sequences using the Maximum Likelihood method. The sequences were first aligned using the ClustalW tool, sampled by 500 bootstrap replications, prior to drawing the phylogenetic tree. However, 10 out of the 39 sequences containing the second methionine residue (Figure 8[B]) are found within the orange boxes pointing to lineage-specific evolution. Moreover, 2 isoleucines as found in 2 distinct species, which the authors infer to be a stepping stone for the conversion into an internal methionine, are shown in green. (D) Codons encoding Threonine, Methionine, Valine, Isoleucine, and Alanine; (E) [Top] The color-coded biochemical properties of amino acids in the human A2B receptor arranged by GPCR residue numbering by the Ballesteros and Weinstein system. 22 [Middle] The naturally occurring variation of the human A2B receptor showcasing the propensity of isoleucine 141 in being mutated into a methionine, hinting that a highly truncated mutant missense protein can be produced by the I → M mutation at this location (Allele frequency—0.00008237), drawing similarities to the highly truncated A2B receptor from L. africana. [Bottom] The sequence of the human A2B receptor showcases the residues classified according to structural localities. The isoleucine to methionine mutation appears to be a common adaptation to producing truncated proteins (Figure 8C).
Figure 9.
(A) Secondary structure prediction of the region (60 residues in total) spanning the codon triplet encoding the second methionine. The initiator codon coding for the second methionine is shown in a red arrow. A secondary structural complex with a composite hairpin-loop structure is shown in a square, which is assumed to be a regulatory structural element upstream of the start codon. The web server employed was the RNA-Fold Web Server 25 ; (B) Secondary structure prediction of the divergent regions found further upstream of the start codon in the 2 mRNA transcripts, XM_023539615 and XM_023539616. In yellow are the consecutive hairpin-loop structures that distinctively identify XM_023539616 and underlined are the deleted residues in the XM_023539616 transcript compared with XM_023539615. The divergent region is demarcated in Figure 8(B). The web server employed was the RNA-Fold Web Server 25 ; (C) 4 consecutive codons coding for a region inclusive of the 3-residue N-glycosylated motifs in the A2B (top) and A2A (bottom) receptors of L. africana. It is inferred that the NESC coding region of the A2B receptor is an insertion.
Truncated and full-length A2B receptors of L. africana
Biochemistry of the A2B receptor
There are tangible hypotheses to the truncated A2B receptor of L. africana: (1) the shorter receptor may be abolished in ligand binding activity; (2) the receptor may form a functional oligomer with another adenosine receptor subunit; (3) the receptor may function independently with either differences in ligand preference or magnitude of the signaling via phosphorylation; (4) the receptor may have a higher ability to discriminate a broader diversity of odor molecules; and (5) the receptor may have higher expression in olfactory interfaces.
Truncation of the N-terminus of GPCRs has been shown to increase the trafficking and membrane anchoring of the receptors in CB1 cannabinoid, Alpha 1D adrenergic and GPR 37 receptor proteins, all GPCRs.26,27 This is in part due to the lower complexity of the truncated N-terminal regions that assists in the proper folding and correct insertion of the receptors into the respective plasma membranes. There is a separate line of thought that the N-terminus can be employed as a negative regulatory domain that when eliminated through truncation results in a higher magnitude of signaling via the GPCRs. 28
The full-length A2B receptor is poorly expressed in most olfactory interfaces, and truncation may release the probable negative regulation of the density of the A2B receptors to increase their populations in membranes. 29 There is a suggestion that poor expression of the A2B receptor—that can be rescued by incorporating a tail region from the A2A receptor—is likely to be due to poor trafficking and prone to form an oligomer for enhanced function. 29 There may be opportunities for the truncated A2B protein to heteromerize with other receptors including A1, A2A, and A3 receptors that may improve receptor diversity. There is a theory suggesting that the A2B receptor is trafficked together with the A2A receptor for better trafficking purposes to the cell membrane, and the truncation of the A2B receptor will be a positive development for such combined trafficking efforts due to the decreased complexity of the N-terminal region in the truncated protein. 29 The empowerment of downstream signaling that may be associated with the truncated A2B protein of L. africana, and the accommodation of a diverse set of odorants as ligands, may be hallmarks of the truncated protein.
Evidence for Limonene receptivity of the truncated A2B receptor
In the human A2B receptor, limonene interacts with the S68, V85, L86, F173, W247, H280, and I276 drawing similarities to the human A2A receptor that uses the following residues S67, V84, L85, F168, E169, W246, H278, and I274, to bind using van der Waal contacts showcasing the close mimicry and analogy of the receptor-binding process between 2 closely aligned receptors. The conservation of the above residues in the A2B receptor between the human and the elephant counterparts signifies that the probable mode of action of limonene on A2B receptors is convergent in its functional capacity as an olfactory molecule. Such inferences are speculative in their current scope and will require a combination of in silico, in vivo, and in vitro experimental evidence to hone in on the biology that makes A2A and A2B receptors limonene-responsive macromolecules.
All distal residues found in helix 5 and onwards implicated in limonene-binding, are 100% conserved between the human and the African bush elephant A2B receptors (Figure 4). The residues such as Leucine-172, Phenylalanine-173, Tryptophan-247, Isoleucine-276, Histidine-279, and Serine-280 (Figure 4) in the human receptor, are conserved 100% in the truncated A2B receptor. This draws a picture that the residues that are missing due to the truncated form of the A2B receptor in the African elephant may be contoured by the presence of a separate oligomeric surface of a conjoint receptor, likely to be A2A. Such analyses will require experimental assays to prove the binding of the limonene to the truncated A2B receptor from L. africana and oligomerization using the structurally disordered C-termini. Site-directed mutagenesis (single substitution/insertion/deletion) studies on the limonene-binding pocket of the in vitro expressed receptor proteins will shed light on the key contacting residues to limonene-binding, while mutational analyses of unstructured [disordered] regions will help in demarcating the regions that are central to oligomerization. Truncated proteins such as the 172 residue A2B receptor will be less prone to misfolding/inclusion body formation and will be easier to produce using eukaryotic/prokaryotic expression systems.
Although A1 and A3 receptors are activated by nanomolar concentrations of the ligand, the A2A and A2B receptors are only activated by micromolar concentrations. Out of the 2, A2B is known as the weaker receptor, requiring a high micromolar concentration to be activated. The above “weak” nature of ligand binding in A2B receptors may be the reason for the repellence of elephants exclusively to oranges, due to oranges having the highest amounts of limonene among citrus fruits. In a study, bitter lemon was shown to contain 68%–91% limonene, while for lemons and mandarins, the values were 38%–70% and 52%–69% respectively. 30 Other studies state limonene content in oranges to be routinely > 90%. 31 Therefore, the selective nature of elephants for repellence by oranges may be due to the high limonene content emitted from orange plants.
Another theory that can be inferred is shallow receptor binding sites that are synonymous with fewer side-chain interactions and a micromolar affinity. 31 Therefore, it is inferred here that the A2B receptor due to the truncation of the first 4 transmembrane domains may be a shallow binding site that is activated by micromolar concentration of the active ingredient, limonene, making fewer interactions with side chains protruding to the binding pocket.
Even in the context of the full-length A2B protein [XP_064127927.1], there is a 100% conservation of residues that are involved in limonene-binding between human and elephant counterparts, again showcasing the high similarity between the human and the African bush elephant full-length A2B receptors (Figure 8). It should be noted here that 100% conservation of limonene-binding residues between the human and African bush elephant A2B receptors is only a theoretical measure of the ability of limonene to be a complementary ligand to the A2B receptor, which requires further experimental validation.
Evidence for membrane localization of the truncated A2B receptor
Almost all GPCRs have at least one N-glycosylation site and the N-glycosylation sites are localized to the N-terminal ectodomain of the receptor or the extracellular loops. 32 N-glycosylation occurs at the tripeptide consensus Asn-X-Ser/Thr and the above motif in an N-terminal region of a GPCR is indicative of a membrane localization of the receptor. Therefore, the Asn-X-Ser/Thr motif, indicative of an N-glycosylation site, was sought in the A2B receptor sequence of L. africana. From Residue 2 to Residue 4 of the Loxodonta A2B receptor is the sequence Asn-Glu-Ser indicative of a putative N-glycosylation site (Figure 5A). However, 70% of Asn-X-Ser/Thr motifs are N-glycosylated. It is inferred that N-glycosylation occurs at the Asn-Glu-Ser motif is a hallmark of membrane expression/localization of the A2B receptor of L. africana, and suggests that the truncated protein is functional and trafficked to the correct location. N-glycosylation is associated with protein folding, ER export, membrane insertion, and event signaling–related functions, such as ligand-binding affinity. The N-glycosylation encapsulates the addition of 3 glucoses, 9 mannoses, and 2 N-acetylglucosamines (Glc3Man9GlcNAc2) to the asparagine residue, as a post or co-translation modification. 32
It is known that N-terminal ectodomain N-glycosylation sites in GPCRs are associated with sorting of otherwise misfolded proteins to the cell surface, thereby improving cell surface presentation of misfolding-prone receptor units. 33 There is a likely functional N-glycosylation of the A2B receptor from L. africana, which proposes that the theory of unitary transport of the A2B receptor or conjoint transport of the A2B and A2A receptors from the ER to the plasma membrane, may hold true. Furthermore, the A2A receptor from L. africana has an N-glycosylation site in the extracellular linker between helices 4 and 5 (Figure 5B) that may also possess functional significance.
Molecular genetics of the A2B receptor gene/transcript
When the gene and the protein of the truncated L. africana A2B receptor are aligned, it can be established that the Kozak sequence of the shorter A2B receptor transcript is optimally designed to be translated efficiently. The guanosines at positions −6, −3, and + 14 and the cytosine at −1 are all arranged for optimal translation at ribosomes (Figure 7B).34,35 In the NCBI database, there are 2 separate transcripts for the A2B receptor gene, that are 100% conserved in coding sequence but are distinct in the 5’ UTR. Why there are 2 differing transcripts (XM_023539616.1 and XM_023539615.1) in the NCBI database does provide a question on the resulting biology, which the author infers to be a case of differences in transcriptional and post-transcriptional effects that may have a repercussion on quantitative dynamics of isoforms of proteins.
Low levels of structuredness near the start codon are synonymous with higher translational efficiencies. 36 Accordingly as shown in Figure 9A, the simplified structure near the AUG codon of the mRNA transcript can facilitate stronger translation. Internal Ribosome Entry Site (IRES) translation may be a strategic hallmark of the A2B receptor coding mRNA transcripts, and secondary and tertiary structures upstream of the start codon as shown in Figure 9A can be instrumental in IRES translation from the second methionine. IRES translation recruits the ribosomes for cap-independent translation from internal sites, following binding to hairpin loops and other structural elements. 36 It is documented that RNA structures have a stronger role in start codon recognition than in pre-initiation complex scanning. 36 The lack of secondary and tertiary structural elements downstream of the AUG as shown in Figure 9A can also discourage translational pausing during the elongation stage of translation of protein macromolecules. 36
When the divergent regions of the 2 mRNA molecules, XM_023539615 and XM_023539616 (In Blue—Figure 7A), were used to derive secondary structural elements using the Vienna RNA fold server, it can be observed that there are close structural similarities, such as the regions marked in orange and blue boxes, while there are 2 additional stem-loop structures in XM_023539616, which points to differential methods of scanning and regulation of the upstream region in the 2 transcripts. The shape/structure of the 2 resultant RNA molecules (Figure 9B) closely align with an RNA aptamer (Seq_04) designed in a computational study, 37 which points to distinct specificities and sensitivities of binding of regulatory molecules that may control mRNA translation from further upstream.
The extracellular N-glycosylation motif-encoding codons of the A2B and the A2A receptors appear highly divergent in relation to molecular evolution. For example, the Asparagine in the truncated A2B receptor is coded by AAU while the counterpart in the A2A receptor is AAC, while the serines are coded by AGC (A2B) and UCA (A2A), the former being a high-frequency codon (Figure 9C). Furthermore, UCA and AGC (both coding for serines) are disjointed codons found distantly in the genetic code. Therefore, this proposes that the N-terminal extremity of A2B comprising the NESC region, coded by quadruple AAU-GAA-AGC-UGC triplets, is divergent from the A2A gene (Figure 9C). The codons for the NESC region are likely to be an insertion of a DNA element that originated from a separate source, as shown by the gap in the sequence alignment. It is factual that 99% of insertions are < 50 bp and the average length of an insertion is 36 bp which is not far from the size of the inserted locus, which is 30 bp.
Limonene in the Diet of Elephants: A Defense Agent Against Mammalian Herbivore Feeding
The A2B receptor of the Asian—Indian—elephant (Elephas maximus indica) is 333 amino acids in length and is comparable to the size of other A2B receptors from a diversity of animal species. Both elephant species are repelled by high limonene in plant types, while low limonene provides a bridge to foraging.
Limonene is found in many African plant/tree species (Table 2). An example is the Baobab fruit, which has been recorded to contain as high as 6.1% limonene in total plant volatile content, while Baobab leaves have a minority presence of limonene. 38 Baobab fruit and bark are known to be part of the elephant diet. Dacryodes edulis or African Pear contains moderate levels of limonene (7.2%–27.2%). 39 The legume, Acacia nilotica, comprises 15.3% limonene in its mix of essential oils from stem barks. 40 The geographical ranges of baobabs, African pears, and Acacias overlap considerably with that of African elephants. Limonene has been branded as a defense agent/antifeedant against generalist and specialist mammalian herbivores, and the evidence from the literature points to that role. 41
Table 2.
African plant types consumed by the African Bush Elephant and their scientifically measured limonene content. The absence of monoterpenes in Sclerocarya birrea and Combretum molle is documented in a past study. 42 The relationship status between diet and elephant was determined from informal Internet accounts, and scientific literature from the continent of Africa.
| Plant type | Status | Limonene content |
|---|---|---|
| Adansonia digitata (Baobab) | Favorite | 6.1% (only in one sample) in fruit, trace levels in leaves |
| Dacryodes edulis | Moderately consumed | 7.2%–27.2% |
| Acacia nilotica | Moderately consumed | 15.3% |
| Sclerocarya birrea (Marula) | Strong favorite | Absent |
| Vachellia tortilis | Favorite | 1% |
| Colophospermum mopane | Favorite | 3.5%–5% |
| Combretum apiculatum | Favorite | Trace amounts |
| Combretum molle | Favorite | Absent |
| Dichrostachys cinerea | Only partially consumed | Trace amounts |
| Terminalia sericea | Only partially consumed | 0.11%–0.19% |
In a study performed on plant ants, low-reward plant species emitted 13 different compounds which were dominated by (S)-(-)-limonene and β-linalool. 43 Complementation of a high-reward host with (S)-(-)-limonene and β-linalool though reduced the overall attraction of ants, suggesting that the addition of (S)-(-)-limonene or β-linalool or both, can repel Pseudomyrmex ferrugineus ants from high-reward Acacia species. 43 Therefore, limonene can be suggested as a resistance-related VOC emanating from selective Acacia species. Furthermore, in Bemisia tabaci, a global pest, d-Limonene acts as an avoidance-related VOC emanated by plants. 44 It is suggested here that limonene is a resistance-related VOC for African elephants and the shortened nature of the A2B receptor is a process of adaptive evolution to express compact but stable membranous A2B receptors for stronger limonene/monoterpene signaling that promotes avoidance behavior. While repellence based on olfaction in elephants takes precedence, antifeedant activity based on distaste appears to not be a determinant of avoidance behavior. The detection of choice appears to be mediated by aromas, such as scents emanated by fruits.
S. birrea (marula), the iconic species of plants used heavily by the African elephant is documented to succumb to the destruction caused by elephant feeding habits. Of note, S. birrea has no or negligible limonene in its plant parts. Similarly, Vachellia tortilis, a species of trees favored by elephants for a diet of leaves, has only 1% limonene in leaves 45 hinting at a relationship between low limonene and increased browsing. Furthermore, parts of Colophospermum mopane, another favorite among elephants, have a low-limonene content of 3.5%–5%. 46 Dichrostachys cinerea and Terminalia sericea, 2 species damaged only minutely by elephant encroachment, have low-limonene levels, with trace amounts in the former and 0.11%–0.19% in the latter.47-49 Therefore, although low limonene appears to be a dietary attractant, it may not be universally applicable but the association points to elephant browsing behavior being promoted by low limonene in the dietary species. 50
On a different note, Asian elephants are known to have a nose for monocots over dicots, preferring palms, bamboo, other grasses, and fellow monocot species for their diet. African bush elephants are known to show a preference for tree saplings mostly, with a stronger penchant for dicots. The strongest emitters of limonene are the citrus family plants, eudicots in classification, belonging to the family Rutaceae.
Africa is known to have less species diversity in flora compared with neotropics and South East Asia, and whether the lesser choice in diet, paved the evolutionary path for foraging on widely available dicots poor in antifeedant chemicals, 51 is a valid inquiry. It may well be that olfactory limonene sensing by African bush elephants is a method of narrowing down dietary choices in dicots paying attention to chemical repellence. Elephants are known to narrow down dietary choices using a top-down selection system: landscape > habitat > available plant species; and limonene and other volatile compounds may well be a strong factor in such “foraging” decision-making. 52
Probable Signaling Mediated by Post-Translational Modifications on A2B Receptors of Elephants on Ligand Binding
It is documented that phosphorylation, palmitoylation, and disulfide bond formation regulate signaling in GPCRs.
The distal residues up to Ser-317 (the equivalent of Ser-157 in truncated receptor—Figure 10) in the human A2B receptor when removed increased the efficacy of the adenylate cyclase signaling but not phospholipase Cβ. 53 Serines in the C-terminal cytoplasmic domain have been implicated in phosphorylation and their removal/mutation results in changes in downstream signaling, especially in relation to desensitization.53,54 The Ser-157 has a high score (0.859) for phosphorylation using the NetPhos 3.1 prediction server, inferring a higher likelihood of being phosphorylated (Figure 2). It is likely that the downstream signaling initiated by ligand binding is mediated by Gs proteins and not Gq proteins (Figure 10C).
Figure 10.
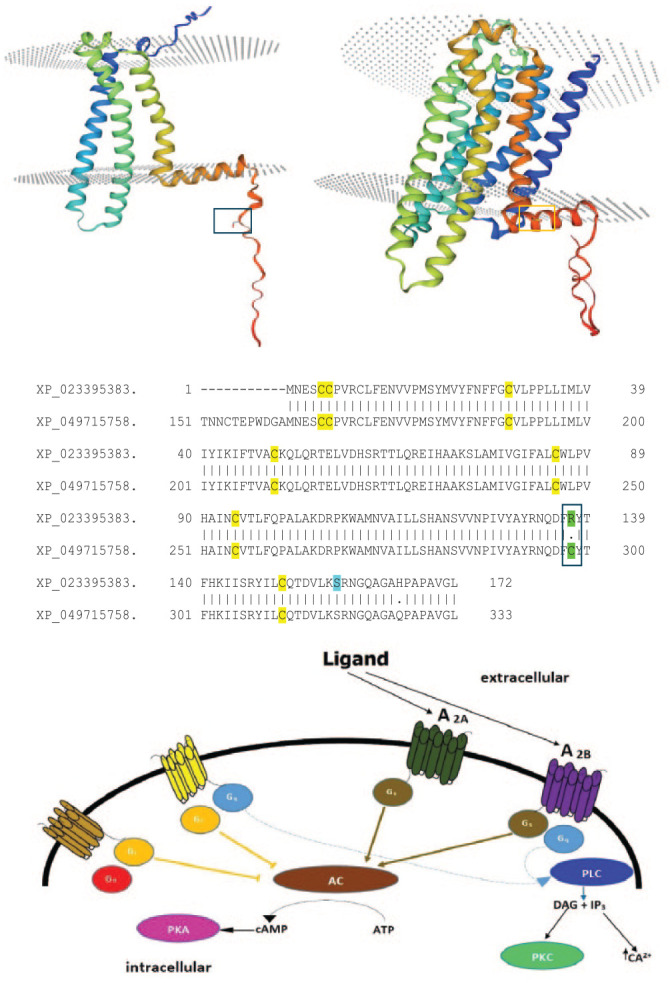
(Top) Homology models of the A2B truncated receptor from L. africana (Left) and the full-length counterpart from Elephus maximus indicus (Right). The template used for the construction of the homology models was P29276.1.A (Adenosine receptor A2B from [Rattus norvegicus]). Ser-157 in the A2B truncated receptor and Cys-298 in the full-length A2B receptor of Elephus maximus indicus are shown in boxes. The Swiss-Model server was used to build the homology models. 55 (Middle) The pairwise sequence alignment of A2B receptors of the African (XP_023395383.1) and Asian (Elephas maximum indicus—XP_049715758.1) elephants. A single (only) amino acid change between the 2 protein sequences is shown in green and Ser-157 is highlighted in cyan. (Bottom). Schematic illustration of the 4 types of adenosine receptors and their influence on downstream signaling events. This is a modified image from van Calker et al. 56 showcasing the simplified architecture and signaling interactions.
Furthermore, it has also been unveiled in past studies that the final cysteine (Cys-311) in A2B receptors is palmitoylated and depalmitoylation or release of the C-16 palmitic acid conjugate may have a role in promoting the formation of a disulfide bond with the unique cysteine (Cys-298) in the A2B receptor of the Asian elephant (Figure 10), as a mechanism of regulating downstream signaling. 53 This unique feature—a single substitution mutation from Arginine to Cysteine—distinguishes the A2B receptor from the Asian elephant against the receptor isoforms expressed in the African bush elephant (Figure 10B). Cysteine disulfide bond formation in the N-terminal extremity is known to affect the interactions of the GPCRs with G-proteins and beta-arrestins, but not ligand–receptor interactions. 57 Cys-298 and Cys-311 are only 13 residues apart and present in the flexible C-terminal tail of the A2B receptor in the Asian Elephant, and thus are conducive to disulfide bond formation, due to the structural freedom found in the above extremity in GPCRs.
In addition, a QRTEL motif found in the cytoplasmic linker between helices 5 and 6 (Figure 6A) in the truncated A2B protein and the full-length A2B receptor of L. africana, is predicted to be a PDZ-binding domain, associating with the PDZ-protein 1 scaffolding protein NHERF-2 in a multi-protein signaling complex on agonist activation and is given a role in anchoring and stabilizing the receptor in the plasma membrane. 58 The conservation of the QRTEL motif between helices 5 and 6 is again evidence for the membrane presence of the truncated A2B receptor from L. africana. However, probable signaling events unearthed here require to be given experimental evidence from site-directed mutagenesis studies, to confirm the above predictions.
It is also worth keeping in mind that tissue-specific expression, combinatorial expression, distinct signaling alone or in tandem with combinatorial receptors, distinct downstream interactions with G proteins and beta-arrestins, differences in conformational changes elicited by ligand binding and signaling are all beneficiaries of diverse protein isoforms, produced through alternate splicing and internal translation. 59 Truncated isoforms can be deemed a treasure chest of biochemical possibilities that boost the distinctiveness of responses to diverse ligands.
What Are the Options for Citrus Plants to Be Used for Biofencing in Sub-Saharan Africa?
In the continent of Africa, South Africa, Nigeria, Egypt, and Morocco are known to be growers and exporters of citrus fruits. Around 11.7% of the world’s Citrus fruit exports are from South Africa. Citrus paradisi, or grapefruit is widely grown in South Africa, with a reported limonene content of 93.33% in essential oils (Table 3).
Table 3.
Citrus fruits and their percentage of limonene in essential oils extracted mainly from the pericarp. The figures—the highest relevant to a species of Citrus—were obtained from the work by Wani et al. 60 .
| Citrus species | Reported percentage of limonene in essential oils |
|---|---|
| Citrus aurantium | 97.83 |
| Citrus sinensis | 91.14–94.95 |
| Citrus reticulata | 46.7 |
| Citrus limon | 39.74 |
| Citrus grandis | 89.96 |
| Citrus paradisi | 93.33 |
| Citrus latifolia | 47.5–48.9 |
| Citrus volkameriana | 77.27–79.36 |
| Citrus medica | 45.36 |
| Citrus deliciosa | 91.27 |
| Citrus monstruosa | 95.77 |
Citrus aurantium (Bitter Orange) of which the essential oil is measured to contain nearly 97% limonene can be classified as a good source of limonene to ward off elephants (Table 3). Bitter orange is known to be native to East Africa. Citrus aurantium is particularly suited to East Africa with a host of favorable traits: suitable temperature, altitude, rainfall, and well-drained soils which can retain moisture in full sun (International Centre for Research on Agroforestry [ICRAF] Data). It is also an evergreen that shows no invasiveness when introduced (ICRAF Data).
Citrus sinensis (Sweet Orange) of which the essential oil has a reported pinnacle percentage of 94.95% is also suited to African soils, with the rainfall, temperature, and altitude agreeing with those conditions in East Africa (ICRAF Data). Citrus sinensis grows well in well-drained, loose loam, fertile lands, but is sensitive to high salt conditions while showing no propensity to be invasive (ICRAF Data).
ICRAF data were gathered from https://apps.worldagroforestry.org/suitable-tree/
Conclusions
Here in this study, the A2A and A2B receptors of L. africana have been characterized using bioinformatics. Although the A2A receptor appears to have fewer differential hallmarks, the A2B receptor is full of unique features. An isoform of the A2B receptor is truncated to 172 residues. Second, the limonene-binding residues, as identified in the human receptor are conserved, helix 5 and onwards, in the truncated counterpart from the African elephant. Finally, the A2B receptor is hypothesized as a weaker receptor requiring a micromolar affinity of ligand while using fewer side chains for activation. There is also an N-terminal extremity N-glycosylation site in the truncated A2B receptor which is indicative of a functional presence (following export from ER) of the receptor in the plasma membrane. There are 2 transcript isoforms of the A2B gene that are likely to undergo post-transcriptional regulation. The conglomeration of the above features in the L. africana truncated A2B receptor, collectively, is predicted to have a higher disposition for upregulated expression in olfactory organs, and differences in activation properties. Limonene appears to be a volatile chemorepellent for the deterrence of consumption of plant types by the African bush elephant, a generalist herbivore in its foraging patterns.
Future Directions
This publication paves the path for in silico receptor-binding studies to be performed on A2B and A2A receptors from elephants. Furthermore, in vitro signaling assays or receptor-ligand-binding studies will be helpful in determining the function of the A2B receptors, both full-length and truncated. In addition, the membrane trafficking potential of the truncated and the full-length A2B receptors of the African bush elephant can also be conducted in a suitable cell line, using immunochemistry [Western Blots] to determine the strength of membrane saturation of the 2 types of receptors. Strong evidence for membrane localization is provided by the N-terminal N-glycosylation motif, which is a modification for folding, recruitment, and transport of the A2B receptor to the plasma membrane.
Although there are just more than 400,000 elephants in Africa, Asian elephants add up to < 50,000. In Sri Lanka, in 2020 and 2019, a staggering 409 and 361 elephant deaths (respectively) occurred at the hands of humans, transforming the landscape of elephant conservation into a burning issue and a timely one. Endeavoring to study the repellence of elephants by orange plants can lead to a watershed moment for the conservation of these endangered pachyderms. It is hoped that the probable receptor interfaces and pathways identified by computational biology in this article will have ripple effects in conservation biology. Already, a project titled Project Orange Elephant has provided proof of concept data on the repellence induced by citrus family plants on Asian elephants. 61
In a recent publication by Bester et al., 2023 [1], there is emerging evidence that elephants avoid limonene including other monoterpenes, basing their selection on olfactory cues for foraging, which supports the insights drawn from this bioinformatics study. Elephant conservation efforts require studies to limit the encroachment of elephants on farms, and the building of biofences with orange plants, to deter the crop raiding by elephants at night, appears promising. Also, synthetic limonene sprays could be applied on the perimeter to deter elephant encroachment, which could be performed supplementary to “biofencing” studies. Other limonene-rich citrus family members—especially wild relatives of citrus crops—can also be used to ascertain their effectiveness in elephant conservation. Of note, the aromatic rice variety Lanka-Samurdhi (At405) developed in Sri Lanka has ~10 times more limonene than elite varieties of rice. Such cultivars can act as a buffering zone for irrigated paddy cultivation to keep the elephants at bay.
Supplemental Material
Supplemental material, sj-docx-1-bbi-10.1177_11779322251315922 for A “Dock-Work” Orange: A Dual-Receptor Biochemical Theory on the Deterrence Induced by Citrusy Aroma on Elephant Traffic Central to a Conservation Effort by Dilantha Gunawardana in Bioinformatics and Biology Insights
Acknowledgments
The author thanks Michelle Alexander for drawing the elephant picture and the Limonene illustration, and LK Graphics for illustrating the G-protein-coupled receptor.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Dilantha Gunawardana  https://orcid.org/0000-0002-5086-0215
https://orcid.org/0000-0002-5086-0215
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Bester T, Schmitt MH, Shrader AM. The deterrent effects of individual monoterpene odours on the dietary decisions of African elephants. Anim Cogn 2023;26:1049–1063. 10.1007/s10071-023-01755-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nevo O, Schmitt MH, Ayasse M, Valenta K. Sweet tooth: elephants detect fruit sugar levels based on scent alone. Ecol Evol. 2020;10:11399-11407. doi: 10.1002/ece3.6811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rizvanovic A, Amundin M, Laska M. Olfactory discrimination ability of Asian elephants (Elephas maximus) for structurally related odorants. Chem Senses. 2013;38:107-118. doi: 10.1093/chemse/bjs106 [DOI] [PubMed] [Google Scholar]
- 4. Niimura Y, Matsui A, Touhara K. Extreme expansion of the olfactory receptor gene repertoire in African elephants and evolutionary dynamics of orthologous gene groups in 13 placental mammals. Genome Res. 2014;24:1485-1496. doi: 10.1101/gr.170828.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patel M, Narke D, Kurade M, et al. Limonene-induced activation of A2A adenosine receptors reduces airway inflammation and reactivity in a mouse model of asthma. Purinergic Signal. 2020;16:415-426. doi: 10.1007/s11302-020-09734-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gessi S, Poloni TE, Negro G, et al. A2A adenosine receptor as a potential biomarker and a possible therapeutic target in Alzheimer’s disease. Cells. 2021;10:2345. doi: 10.3390/cells10092345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fredholm BB, Altiok N. Adenosine A2B receptor signalling is altered by stimulation of bradykinin or interleukin receptors in astroglioma cells. Neurochem Int. 1994;25:99-102. doi: 10.1016/0197-0186(94)90052-3 [DOI] [PubMed] [Google Scholar]
- 8. Fleming KM, Mogul DJ. Adenosine A3 receptors potentiate hippocampal calcium current by a PKA-dependent/PKC-independent pathway. Neuropharmacology. 1997;36:353-362. doi: 10.1016/s0028-3908(96)00131-4 [DOI] [PubMed] [Google Scholar]
- 9. Kataoka S, Baquero A, Yang D, et al. A2BR adenosine receptor modulates sweet taste in circumvallate taste buds. PLoS ONE. 2012;7:e30032. doi: 10.1371/journal.pone.0030032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nash CA, Nelson CP, Mistry R, et al. Differential regulation of beta2-adrenoceptor and adenosine A2B receptor signalling by GRK and arrestin proteins in arterial smooth muscle. Cell Signal. 2018;51:86-98. doi: 10.1016/j.cellsig.2018.09.007 [DOI] [PubMed] [Google Scholar]
- 11. Navarro G, Gonzalez A, Campanacci S, et al. Experimental and computational analysis of biased agonism on full-length and a C-terminally truncated adenosine A2A receptor. Comput Struct Biotechnol J. 2020;18:2723-2732. doi: 10.1016/j.csbj.2020.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yan K, Gao LN, Cui YL, Zhang Y, Zhou X. The cyclic AMP signaling pathway: exploring targets for successful drug discovery (Review). Mol Med Rep. 2016;13:3715-3723. doi: 10.3892/mmr.2016.5006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wise H. The roles played by highly truncated splice variants of G protein-coupled receptors. J Mol Signal. 2012;7:13. doi: 10.1186/1750-2187-7-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gimelbrant AA, Stoss TD, Landers TM, McClintock TS. Truncation releases olfactory receptors from the endoplasmic reticulum of heterologous cells. J Neurochem. 1999;72:2301-2311. doi: 10.1046/j.1471-4159.1999.0722301.x [DOI] [PubMed] [Google Scholar]
- 15. Gurevich VV, Gurevich EV. Molecular mechanisms of GPCR signaling: a structural perspective. Int J Mol Sci. 2017;18:2517. doi: 10.3390/ijms18122517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xia X, Xie Z. DAMBE: software package for data analysis in molecular biology and evolution. J Hered. 2001;92:371-373. doi: 10.1093/jhered/92.4.371 [DOI] [PubMed] [Google Scholar]
- 17. Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol Biol Evol. 2018;35:1547-1549. doi: 10.1093/molbev/msy096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Blom N, Gammeltoft S, Brunak S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol. 1999;294:1351-1362. doi: 10.1006/jmbi.1999.3239 [DOI] [PubMed] [Google Scholar]
- 19. Omasits U, Ahrens CH, Müller S, Wollscheid B. Protter: interactive protein feature visualization and integration with experimental proteomic data. Bioinformatics. 2014;30:884-886. doi: 10.1093/bioinformatics/btt607 [DOI] [PubMed] [Google Scholar]
- 20. Hua X, Naselsky WC, Bennett WD, Ledent C, Senior BA, Tilley SL. Adenosine increases nasal mucociliary clearance rate in mice through A2A and A2B adenosine receptors. Laryngoscope. 2013;123:306-310. doi: 10.1002/lary.23658 [DOI] [PubMed] [Google Scholar]
- 21. Latorraca NR, Venkatakrishnan AJ, Dror RO. GPCR dynamics: structures in motion. Chem Rev. 2017;117:139-155. doi: 10.1021/acs.chemrev.6b00177 [DOI] [PubMed] [Google Scholar]
- 22. Pándy-Szekeres G, Caroli J, Mamyrbekov A, et al. GPCRdb in 2023: state-specific structure models using AlphaFold2 and expansion of ligand resources. Nucleic Acids Res. 2023;51:D395-D402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tompa P, Kovacs D. Intrinsically disordered chaperones in plants and animals. Biochem Cell Biol. 2010;88:167-174. doi: 10.1139/Y10-015 [DOI] [PubMed] [Google Scholar]
- 24. Tompa P, Csermely P. The role of structural disorder in the function of RNA and protein chaperones. FASEB J. 2004;18:1169-1175. doi: 10.1096/fj.04-1671fje [DOI] [PubMed] [Google Scholar]
- 25. Gruber AR, Lorenz R, Bernhart SH, Neuböck R, Hofacker IL. The Vienna RNA websuite. Nucleic Acids Res. 2008;36:W70-W74. doi: 10.1093/nar/gkn188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Uddin MS, Naider F, Becker JM. Dynamic roles for the N-terminus of the yeast G protein-coupled receptor Ste2p. Biochim Biophys Acta Biomembr. 2017;1859:2058-2067. doi: 10.1016/j.bbamem.2017.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Uddin MS, Hauser M, Naider F, Becker JM. The N-terminus of the yeast G protein-coupled receptor Ste2p plays critical roles in surface expression, signaling, and negative regulation. Biochim Biophys Acta. 2016;1858:715-724. doi: 10.1016/j.bbamem.2016.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Uddin MS, Kim H, Deyo A, Naider F, Becker JM. Identification of residues involved in homodimer formation located within a beta-strand region of the N-terminus of a yeast G protein-coupled receptor. J Recept Signal Transduct Res. 2012;32:65-75. doi: 10.3109/10799893.2011.644461 [DOI] [PubMed] [Google Scholar]
- 29. Moriyama K, Sitkovsky MV. Adenosine A2A receptor is involved in cell surface expression of A2B receptor. J Biol Chem. 2010;285:39271-39288. doi: 10.1074/jbc.M110.167640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bourgou S, Rahali FZ, Ourghemmi I, Saïdani Tounsi M. Changes of peel essential oil composition of four Tunisian citrus during fruit maturation. Scientificworldjournal. 2012;2012:528593. doi: 10.1100/2012/528593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yatawara AK, Hodoscek M, Mierke DF. Ligand binding site identification by higher dimension molecular dynamics. J Chem Inf Model. 2013;53:674-680. doi: 10.1021/ci400038k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goth CK, Petaja-Repo UE, Rosenkilde MM. G protein-coupled receptors in the sweet spot: glycosylation and other post-translational modifications. ACS Pharmacol Transl Sci. 2020;3:237-245. doi: 10.1021/acsptsci.9b00063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mentesana PE, Konopka JB. Mutational analysis of the role of N-glycosylation in alpha-factor receptor function. Biochemistry. 2001;40:9685-9694. doi: 10.1021/bi010396j [DOI] [PubMed] [Google Scholar]
- 34. De Angioletti M, Lacerra G, Sabato V, Carestia C. Beta+45 G → C: a novel silent beta-thalassaemia mutation, the first in the Kozak sequence. Br J Haematol. 2004;124:224-231. doi: 10.1111/j.1365-2141.2004.04952.x [DOI] [PubMed] [Google Scholar]
- 35. Kozak M. Point mutations close to the AUG initiator codon affect the efficiency of translation of rat preproinsulin in vivo. Nature. 1984;308:241-246. doi: 10.1038/308241a0 [DOI] [PubMed] [Google Scholar]
- 36. Cao X, Zhang Y, Ding Y, Wan Y. Identification of RNA structures and their roles in RNA functions. Nat Rev Mol Cell Biol. 2024;25:784-801. doi: 10.1038/s41580-024-00748-6 [DOI] [PubMed] [Google Scholar]
- 37. Muhammad AM, Zari A, Alsubhi NH, Al-Zahrani MH, Alghamdi RA, Labib MM. Novel design of RNA aptamers as cancer inhibitors and diagnosis targeting the tyrosine kinase domain of the NT-3 growth factor receptor using a computational sequence-based approach. Molecules. 2022;27:4518. doi: 10.3390/molecules27144518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Braca A, Sinisgalli C, De Leo M, et al. Phytochemical profile, antioxidant and antidiabetic activities of adansonia digitata L. (Baobab) from Mali, as a source of health-promoting compounds. Molecules. 2018;23:3078. doi: 10.3390/molecules23123078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jirovetz L, Buchbauer G, Ngassoum MB, Parmentier M. Chemical composition and olfactory characterization of essential oils of fruits and seeds of African pear (Dacryodes edulis (G. Don) H. J. Lam) from Cameroon. Flavour Fragr J. 2005;20:215-218. doi: 10.1002/ffj.1512 [DOI] [Google Scholar]
- 40. Ogunbinu A, Okeniyi S, Flamini G, Cioni P, Ogunwande I, Babalola I. Essential oil composition of Acacia nilotica Linn., and Acacia albida Delile (Leguminosae) from Nigeria. J Essent Oil Res. 2010;22:540-542. doi: 10.1080/10412905.2010.9712352 [DOI] [Google Scholar]
- 41. Langenheim JH. Higher plant terpenoids: a phytocentric overview of their ecological roles. J Chem Ecol. 1994;20:1223-1280. doi: 10.1007/BF02059809 [DOI] [PubMed] [Google Scholar]
- 42. Zunckel M, Chiloane K, Sowden M, Otter L. Biogenic volatile organic compounds: the state of knowledge in southern Africa and the challenges for air quality management. S Afr J Sci. 2007;103:107-112. [Google Scholar]
- 43. Razo-Belman R, Molina-Torres J, Martínez O, Heil M. Plant-ants use resistance-related plant odours to assess host quality before colony founding. J Ecol. 2018;106:379-390. doi: 10.1111/1365-2745.12843 [DOI] [Google Scholar]
- 44. Johnston N, Paris T, Paret ML, Freeman J, Martini X. Repelling whitefly (Bemisia tabaci) using limonene-scented kaolin: a novel pest management strategy. Crop Prot. 2022;154:105905. doi: 10.1016/j.cropro.2021.105905 [DOI] [Google Scholar]
- 45. Muhaisen MH. A review on chemical constituents of Acacia tortilis (Leguminosae). IOSR J Pharm. 2021;11:10-21. [Google Scholar]
- 46. O’Connor T, Ferguson A, Clegg BW, Pallett N, Midgley JJ, Shimbani J. Emergent trees in Colophospermum mopane woodland: influence of elephant density on persistence versus attrition. PeerJ. 2024;12:e16961. doi: 10.7717/peerj.16961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Abraham JO, Goldberg ER, Botha J, Staver AC. Heterogeneity in African savanna elephant distributions and their impacts on trees in Kruger National Park, South Africa. Ecol Evol. 2021;11:5624-5634. doi: 10.1002/ece3.7465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nel AL, Murhekar S, Matthews B, White A, Cock IE. The interactive antimicrobial activity of Terminalia sericea Burch ex DC. Leaf extracts and conventional antibiotics against bacterial triggers of selected autoimmune inflammatory diseases. South Afr J Bot. 2020;133:17-29. doi: 10.1016/j.sajb.2020.06.013 [DOI] [Google Scholar]
- 49. Kamte SLN, Ranjbarian F, Campagnaro GD, et al. Trypanosoma brucei inhibition by essential oils from medicinal and aromatic plants traditionally used in Cameroon (Azadirachta indica, Aframomum melegueta, Aframomum daniellii, Clausena anisata, Dichrostachys cinerea, and Echinops giganteus). Int J Environ Res Public Health. 2017;14:737. doi: 10.3390/ijerph14070737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shrader AM, Bell C, Bertolli L, Ward D. Forest or the trees: at what scale do elephants make foraging decisions? Acta Oecologica. 2012;42:3-10. [Google Scholar]
- 51. Qian H, Kessler M, Zhang J, et al. Angiosperm phylogenetic diversity is lower in Africa than South America. Sci Adv. 2023;9:eadj1022. doi: 10.1126/sciadv.adj1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Terborgh J, Davenport LC, Ong L, Campos-Arceiz A. Foraging impacts of Asian megafauna on tropical rain forest structure and biodiversity. Biotropica. 2017;50:84-89. doi: 10.1111/btp.12488 [DOI] [Google Scholar]
- 53. Ryzhov S, Zaynagetdinov R, Goldstein AE, Matafonov A, Biaggioni I, Feoktistov I. Differential role of the carboxy-terminus of the A(2B) adenosine receptor in stimulation of adenylate cyclase, phospholipase Cbeta, and interleukin-8. Purinergic Signal. 2009;5:289-298. doi: 10.1007/s11302-008-9129-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Matharu AL, Mundell SJ, Benovic JL, Kelly E. Rapid agonist-induced desensitization and internalization of the A(2B) adenosine receptor is mediated by a serine residue close to the COOH terminus. J Biol Chem. 2001;276:30199-30207. doi: 10.1074/jbc.M010650200 [DOI] [PubMed] [Google Scholar]
- 55. Waterhouse A, Bertoni M, Bienert S, et al. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 2018;46:W296-W303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. van Calker D, Biber K, Domschke K, Serchov T. The role of adenosine receptors in mood and anxiety disorders. J Neurochem. 2019;151:11-27. doi: 10.1111/jnc.14841 [DOI] [PubMed] [Google Scholar]
- 57. Schihada H, Klompstra TM, Humphrys LJ, et al. Isoforms of GPR35 have distinct extracellular N-termini that allosterically modify receptor-transducer coupling and mediate intracellular pathway bias. J Biol Chem. 2022;298:102328. doi: 10.1016/j.jbc.2022.102328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sitaraman SV, Wang L, Wong M, et al. The adenosine 2b receptor is recruited to the plasma membrane and associates with E3KARP and Ezrin upon agonist stimulation. J Biol Chem. 2002;277:33188-33195. doi: 10.1074/jbc.M202522200 [DOI] [PubMed] [Google Scholar]
- 59. Marti-Solano M, Crilly SE, Malinverni D, et al. Author correction: combinatorial expression of GPCR isoforms affects signalling and drug responses. Nature. 2020;588:E24. doi: 10.1038/s41586-020-2999-9. Erratum for: Nature. 2020;587(7835):650-656. doi: 10.1038/s41586-020-2888-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wani A, Akhtar N, Mir TUG, Singh R. Extraction designs and therapeutic attributes associated with limonene: a review. Plant Arch. 2021;21:254. [Google Scholar]
- 61. Dharmarathne C, Fernando C, Weerasinghe C, et al. Project orange elephant is a conflict-specific holistic approach to mitigating human-elephant conflict in Sri Lanka. Commun Biol. 2020;3:43. doi: 10.1038/s42003-020-0760-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-bbi-10.1177_11779322251315922 for A “Dock-Work” Orange: A Dual-Receptor Biochemical Theory on the Deterrence Induced by Citrusy Aroma on Elephant Traffic Central to a Conservation Effort by Dilantha Gunawardana in Bioinformatics and Biology Insights