Abstract
Background
Pregnancy loss significantly affects physical and mental health. A nomogram for predicting spontaneous abortion risk was developed to improve pregnancy outcomes.
Methods
A total of 1346 pregnant women were enrolled from The Third Affiliated Hospital of Wenzhou Medical University (May 2020 - May 2022). The training set included 941 participants, and the validation set had 405. Feature selection was optimized using a random forest model, and a predictive model was constructed via multivariable logistic regression. The nomogram’s performance was assessed with receiver operator characteristic (ROC), Hosmer-Lemeshow test, calibration curve, and clinical impact curve (CIC). Discrimination and clinical utility were compared between the nomogram and its individual variables.
Results
Antithrombin III (AT-III), homocysteine (Hcy), complement component 3 (C3), protein C (PC), and anti-β2 glycoprotein I antibody (anti-β2GP1) were identified as risk factors. The nomogram demonstrated satisfactory discrimination (Training AUC: 0.813, 95% CI: 0.790–0.842; Validation AUC: 0.792, 95% CI: 0.741–0.838). The Hosmer-Lemeshow test (P = 0.331) indicated a good fit, and the CIC showed clinical net benefit. The nomogram outperformed individual variables in discrimination (AUC: 0.804, 95% CI: 0.779–0.829).
Conclusion
The developed nomogram, incorporating AT-III, Hcy, C3, PC, and anti-β2GP1, aids clinicians in identifying pregnant women at high risk for spontaneous abortion.
Keywords: Abortion, Hematological parameters, Nomogram, Receiver operator characteristic
Introduction
Pregnancy loss is a common complication of early pregnancy. The incidence of clinically confirmed miscarriage in all pregnancies is 15%, and the average prevalence of women with one miscarriage is 11% [1]. If biochemical pregnancy is included, the estimated pregnancy loss rate will be as high as 57% [2].
Miscarriage not only inflicts serious physical damage on the patient, but also profoundly impacts patient’s mental well-being, adding to the financial strain on their family, and even jeopardizes the harmony and stability of their family and society. Research shows that early pregnancy loss can cause women to suffer from high levels of post-traumatic stress, anxiety, and depression. Although their distress decreases over time, it still remains at a clinically significant level after 9 months [3].
By accurately predicting the outcome of pregnancy, we can avoid unnecessary interventions, save time and medical resources, and also provide scientific guidance for the next steps of diagnosis and treatment. Therefore, in early pregnancy, clinicians usually predict the outcome of early pregnancy by measuring the expression level and dynamic changes of specific hematological parameters, combined with ultrasound examination results and clinical manifestations of pregnant women [4, 5].
Although the exact mechanisms behind miscarriage are not fully understood, the main causes are chromosomal abnormalities, immunologic diseases, infections, endocrine diseases, thrombophilia, and inflammation [6]. At present, the hematological parameters used clinically to predict the outcome of early pregnancy include thromboelastography, lymphocyte subsets, thyroid function, antiphospholipid antibodies, antinuclear antibodies, inflammatory markers, etc. Thrombophilia is positively correlated with adverse pregnancy outcomes. Pregnant women with coagulation, anticoagulation dysfunction or disorders can develop a maternal hypercoagulable state, which worsens with the progress of pregnancy and increases the incidence of various pregnancy complications [7]. The thrombophilia workup included blood cell counts, coagulation parameters, factor levels, d-dimer, fibrinogen levels, proteins C and S, etc [8]. Autoimmune diseases can significantly increase the risk of adverse pregnancy outcomes such as spontaneous abortion [9, 10]. It has been confirmed that the incidence of adverse pregnancy outcomes such as miscarriage, stillbirth, and fetal death in patients with systemic lupus erythematosus (SLE) is significantly higher than that in the normal population. The risk of severe complications for SLE pregnant women and newborns is also significantly higher than that in the normal population [11]. Experts suggest that patients with recurrent miscarriages should undergo a comprehensive and systematic screening of immune-related indicators, such as antinuclear antibody, thyroid autoantibodies, antiphospholipid antibody, lupus anticoagulant, etc., to exclude the possibility of autoimmune factors contributing to the miscarriages [12]. Endocrine diseases such as thyroid dysfunction and abnormal glucose metabolism can increase the risk of pregnancy loss [13]. Some literature has pointed out that systemic inflammation markers such as cytokines, white blood cell count, neutrophil count, C-reactive protein, etc. are also associated with pregnancy loss [14].
This study aimed to develop a nomogram based on hematological parameters to accurately predict spontaneous abortion risk, enabling personalized care and treatment for high-risk pregnancies.
Materials and methods
Patients
The retrospective study was conducted at the Department of Gynecology and Obstetrics, The Third Affiliated Hospital of Wenzhou Medical University, Wenzhou, China, from May 2020 to May 2022. The protocol was approved by the Research Ethics Committee of The Third Affiliated Hospital of Wenzhou Medical University. All patients provided informed consent. The flow chart of the study population’s inclusion and exclusion criteria and the research process diagram are shown (Fig. 1).
Fig. 1.
The flow chart of the study population’s inclusion and exclusion criteria and the research process diagram. The research process diagram describes the steps and procedures of the study, such as the data collection, analysis, and reporting methods. AT-III: antithrombin III; Hcy: homocysteine; C3: Complement protein 3; PC: protein C; anti-β2GP1: anti-beta 2 glycoprotein 1 antibody
We enrolled 1,584 women with at least one previous pregnancy loss, confirmed pregnant by elevated serum hCG levels and menstrual history. After applying exclusion criteria—missing hematological data (n = 141), loss to follow-up (n = 50), elective abortion (n = 15), genital tract infections (n = 12), genital malformations (n = 11), and systemic comorbidities (n = 9)—1,346 women were included. Participants were split into a training set (n = 941) and a validation set (n = 405) at a 7:3 ratio.
Definitions
In China, spontaneous abortion refers to pregnancy loss that occurs before 28 weeks of gestation or with a fetal weight of less than 1000 g [17]. The European Society of Human Reproduction and Embryology (ESHRE) and the American Society for Reproductive Medicine (ASRM) define miscarriage as the termination of an intrauterine pregnancy confirmed by ultrasound or histology before 24 weeks, and use the term pregnancy loss to include miscarriage, biochemical pregnancy, and ectopic pregnancy [2, 18].
We classified pregnancy outcomes into two groups: successful pregnancy and pregnancy loss. Successful pregnancy was defined as the presence of a viable intrauterine fetus beyond 24 weeks of gestation. Pregnancy loss was defined as any spontaneous termination of pregnancy before 24 weeks of gestation, including stillbirth, embryonic demise, biochemical pregnancy.
Date collection
We collected the clinical parameters during the 4th to 8th week of pregnancy from electronic medical records, including maternal age, medical histories, obstetric histories (number of gravidity, parity, previous miscarriage, living child), body mass index (BMI), and blood pressure, and the following laboratory tests were also recorded: (1) Endocrine function examination, including thyroid function, fasting blood glucose (FBG); (2) immune system examination, including antiphospholipid antibodies (anti-cardiolipin antibodies (ACA), anti-β2 glycoprotein 1 antibodies (anti-β2GP1) and lupus anticoagulant (LAC)), anti-nuclear antibody spectrum (ANAs), complement, immunoglobulin, lymphocyte subpopulation, thyroid peroxidase antibodies and thyroglobulin antibodies (TPOAb/TGAb); (3) prethrombotic state (PTS), including routine coagulation tests, protein C (PC), protein S (PS), antithrombin III (AT-III), D-dimer, thromboela-stogram (TEG), homocysteine (Hcy), platelet count (PLT); (4) systemic inflammation markers, including white blood cell (WBC) count, neutrophil count (Neu), monocyte count, lymphocyte count, cytokine serum (interleukin-2, -4, -6, -10 (IL-2, -4, -6, -10), tumor necrosis factor-alpha (TNF-α) and interferon-gamma (IFN-γ)); (5) other blood biochemical parameters, including methylenetetrahydrofolate (MTHFR), 25-hydroxyvitamin D (25(OH)D), uric acid, creatinine, bilirubin, and blood lipid profiles (triglyceride (TG), total cholesterol (TC), high density lipoprotein cholesterol (HDL-C) and low density lipoprotein cholesterol (LDL-C)), hemoglobin (Hb), red blood cell count (RBC) and alanine aminotransferase (ALT). All measurements were conducted in strict adherence to the manufacturer’s guidelines by skilled technicians. Various laboratory tests were performed on blood samples taken from pregnant women in the 4th to 8th week of pregnancy.
Construction of the nomogram
We used a random forest classifier to train the data. A random forest model is a type of ensemble learning that combines multiple decision trees. It trains multiple decision trees simultaneously and determines the outcome by the majority vote of all trees. The random forest models were optimized by tuning hyperparameters that influence model structure and accuracy. These hyperparameters included the number of randomly selected explanatory variables (mtry), the number of trees grown (trees), and the minimum number of observations required per node (min_n). For model tuning, the hyperparameter ranges were set as follows: trees from 10 to 1000, mtry from 3 to 8, and min_n from 2 to 10. To avoid overfitting, we performed cross-validation on the training cohort to construct and evaluate the ML models. We then used the validation database to test the predictive power of the models. Next, the factors with a p value of 0.05 or less in univariate analysis will be further analyzed using multivariable logistic regression. Then, we applied multivariable logistic regression analysis with the features selected by the random forest model to identify statistically significant predictors. We established a clinical nomogram that contains independent clinical predictors, reported the features as odds ratio (OR), 95% confidence interval (95% CI), and p-value. Then the performance of the nomogram in discriminating ability, calibration ability and clinical usefulness was evaluated. Finally, we developed a nomogram that predicts the risk of spontaneous abortion in pregnancies based on the result of the multivariable logistic regression.
Validation of the nomogram
To assess the calibration of the nomogram, we plotted the calibration curve and performed the Hosmer-Lemeshow test [15]. A significant result of the test indicates that the nomogram is not well calibrated. We used the area under the curve (AUC) of the receiver operator characteristic (ROC) curve to evaluate the discrimination of the nomogram. To measure the net benefits of different threshold probabilities in the spontaneous abortion cohort, we plotted the CIC [16] to evaluate the clinical benefits of the nomogram.
Models comparison
We plotted ROC and decision curve analysis (DCA) curves to compare the discrimination and clinical utility of the nomogram and its individual variables. We used the DeLong test [17] to compare the AUC values.
Statistical analysis
Normally distributed continuous variables were described as means with standard deviations (SD), and parametric t-tests were used to test for statistical significance between the two groups; otherwise, medians with interquartile range (IQR) and non-parametric Mann–Whitney U tests were applied for variable description and group comparisons.
All statistical analyses were executed utilizing R statistical software, version 4.3.1 (R Foundation for Statistical Computing). Logistic regression analyses, nomogram development, and calibration plot generation were facilitated through the ‘rms’ package. ROC curves were delineated employing the ‘pROC’ package. The Hosmer-Lemeshow goodness-of-fit test was applied via the ‘resourceselection’ package. Both CIC and DCA were conducted using the ‘rmda’ function. All statistical tests were 2‐tailed, and P < 0.05 was considered statistically significant.
Results
Patients’ characteristics
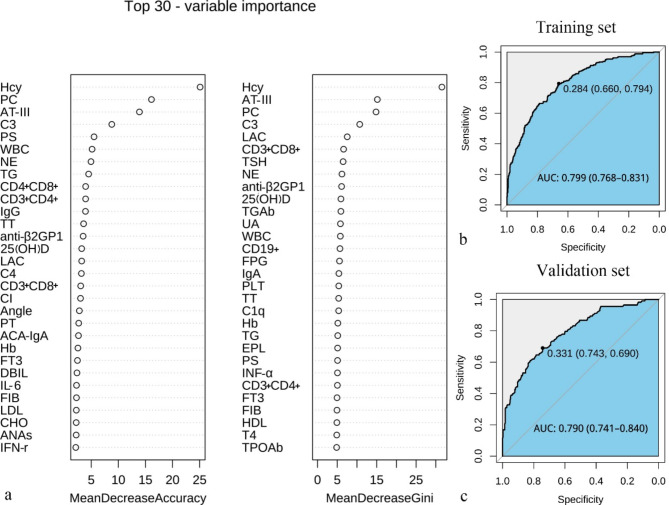
The final study cohort comprised 1346 pregnancies. Of these, 941 were assigned to the training set, which included 262 cases of pregnancy loss and 679 instances of successful pregnancy. The remaining 405 pregnancies constituted the validation set, encompassing 292 cases of pregnancy loss and 113 successful pregnancies. All patients’ baseline clinical parameters are given in Table 1. The AUC of the 70 laboratory tests in the training set is 0.799 (95% CI: 0.768 to 0.821) (Fig. 2b). Similarly, the AUC of the tests in the validation set is 0.790, with a 95% CI of 0.741 to 0.840 (Fig. 2c). This means that the test performs well on new data, demonstrating good external validity and generalizability. Of the 70 laboratory tests collected from pregnancies, 30 features were selected using a random forest classifier (Fig. 2a). These features were subsequently included in univariate and multivariable logistic regression analysis. The characteristics of laboratory tests between successful pregnancy and pregnancy loss groups in the training sets are summarized in Table 2. The risk of abortion is significantly positively correlated with Hcy, C3, C4, WBC, Neu, Th cell (CD4+), CD4+/CD8+, LAC and anti-β2GP1 (P < 0.05). On the other hand, the risk of abortion is significantly negatively correlated with AT-III, suppressor/cytotoxic T cells (CD8+) and PC (P < 0.05).
Table 1.
Baseline characteristics of the investigated patients in the training and validation sets (n = 1346)
| Characteristic | Training set (n = 941) | Validation set (n = 405) | P |
|---|---|---|---|
| Age (years) | 29.14 ± 4.50 | 28.88 ± 4.61 | 0.336 |
| BMI (kg/m2) | 21.66 ± 3.30 | 21.56 ± 3.12 | 0.611 |
| Previous abortions | 0.886 | ||
| 1 | 612/941 (65.0%) | 268/405 (66.2%) | |
| 2 | 185/941 (19.7%) | 79/405 (19.5%) | |
| ≥ 3 | 144/941 (15.3%) | 58/405 (14.3%) | |
| Previous birth | 0.524 | ||
| Nulliparous | 635/941 (67.5%) | 281/405 (69.4%) | |
| Multiparous | 306/941 (32.5%) | 124/405 (30.6%) | |
| Systolic blood pressure (mmHg) | 116.27 ± 12.30 | 115.83 ± 12.23 | 0.553 |
| Diastolic blood pressure (mmHg) | 70.68 ± 9.46 | 70.62 ± 9.60 | 0.918 |
BMI: body mass index
Fig. 2.
Top 30 important variables in the random forest model and the AUC of the 70 laboratory tests in the training and validation sets. Feature importance derived from the random forest model. The plot shows the relative importance of the variables in the random forest model (a). The AUC, or area under the curve, of the 70 laboratory tests in the training set (b) and the validation set (c) shows that the test performs well on new data and has good generalizability
Table 2.
Differences between laboratory tests of successful pregnancy and pregnancy loss groups in the training sets (n = 941)
| Characteristic | successful pregnancy (n = 679) | pregnancy loss (n = 262) | P |
|---|---|---|---|
| PT (s) | 11.61 ± 0.69 | 11.56 ± 0.74 | 0.406 |
| TT (s) | 16.57 ± 1.45 | 16.73 ± 1.82 | 0.149 |
| AT-III (%) | 93.66 ± 8.98 | 89.31 ± 10.65 | < 0.001 |
| Angle (deg) | 71.05 ± 5.65 | 70.99 ± 5.68 | 0.868 |
| CI | 1.50 (0.90, 2.80) | 1.49 (0.70, 3.00) | 0.929 |
| Hcy (µmol/L) | 5.56 ± 1.16 | 6.89 ± 2.07 | < 0.001 |
| 25(OH)D (ng/mL) | 20.22 ± 7.15 | 19.32 ± 7.45 | 0.08 |
| IgG (g/L) | 12.38 ± 2.81 | 12.52 ± 3.01 | 0.527 |
| C3 (g/L) | 0.91 ± 0.21 | 1.02 ± 0.23 | < 0.001 |
| C4 (g/L) | 0.21 ± 0.07 | 0.23 ± 0.08 | 0.001 |
| TG (mmol/L) | 1.09 ± 0.63 | 1.18 ± 0.62 | 0.055 |
| WBC (*10^9/L) | 9.71 ± 5.43 | 11.13 ± 7.01 | 0.001 |
| Neu (*10^9/L) | 6.98 ± 5.03 | 8.33 ± 6.45 | 0.001 |
| Th cell (CD4+) (%) | 39.17 ± 7.21 | 40.89 ± 7.57 | 0.001 |
| suppressor/cytotoxic T cells (CD8+) (%) | 27.55 ± 5.87 | 26.07 ± 5.94 | 0.001 |
| CD4+/CD8+ | 1.53 ± 0.54 | 1.70 ± 0.67 | < 0.001 |
| PC (%) | 112.87 ± 26.85 | 99.90 ± 24.98 | < 0.001 |
| PS (%) | 66.41 ± 16.16 | 66.37 ± 15.98 | 0.971 |
| anti-β2GP1 (AU/mL) | 2.00 (2.00, 3.60) | 2.40 (2.00, 5.93) | 0.002 |
| LAC | 1.03 ± 0.09 | 1.05 ± 0.15 | 0.025 |
PT: prothrombin time; TT: thrombin time; AT-III: antithrombin III; Angle: kinetics of clot development; Hcy: homocysteine; 25(OH)D: 25-hydroxyvitamin D; IgG: Immunoglobulin G; C3: Complement protein 3; C4: Complement protein 4; TG: triglyceride; WBC: white blood cell; Neu: neutrophil; Th cell (CD4+): CD4(+) T lymphocytes; PC: protein C; PS: protein S; anti-β2GP1: anti-β2 glycoprotein 1 antibody; LAC: lupus anticoagulant
Nomogram construction and performance assessment
Univariate logistic regression analysis identified 12 variables significantly associated with spontaneous abortion in pregnancy (all P < 0.05): AT-III, Hcy, C3, C4, PC, WBC, Neu, Th cells (CD4+), suppressor/cytotoxic T cells (CD8+), CD4+/CD8+, LAC, and anti-β2GP1. These variables were further analyzed using multivariable logistic regression, which identified AT-III (OR: 0.956, 95% CI: 0.938–0.975), Hcy (OR: 1.923, 95% CI: 1.673–2.212), C3 (OR: 13.935, 95% CI: 5.258–36.926), PC (OR: 0.979, 95% CI: 0.972–0.987), and anti-β2GP1 (OR: 1.013, 95% CI: 1.003–1.022) as independent risk factors for spontaneous abortion (Table 3). Thereafter, a nomogram was developed by incorporating these five predictors (Fig. 3).
Table 3.
Univariate and multivariate logistic regression analysis of the candidate predictors in the training set
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | ||
| PT (s) | 0.917 (0.748–1.125) | 0.405 | |||
| TT (s) | 1.066 (0.977–1.163) | 0.152 | |||
| AT-III (%) | 0.953 (0.939–0.968) | < 0.001 | 0.956 (0.938–0.975) | < 0.001 | |
| Angle (deg) | 0.998 (0.973–1.023) | 0.868 | |||
| CI | 0.999 (0.940–1.061) | 0.965 | |||
| Hcy (µmol/L) | 1.917 (1.694–2.171) | < 0.001 | 1.923 (1.673–2.212) | < 0.001 | |
| 25(OH)D (ng/mL) | 0.982 (0.962–1.003) | 0.089 | |||
| IgG (g/L) | 1.016 (0.967–1.067) | 0.527 | |||
| C3 (g/L) | 9.252 (4.770-17.945) | < 0.001 | 13.935 (5.258–36.926) | < 0.001 | |
| C4 (g/L) | 24.136 (3.547-164.234) | 0.001 | |||
| TG (mmol/L) | 1.231 (0.992–1.527) | 0.059 | |||
| WBC (*10^9/L) | 1.038 (1.015–1.062) | 0.001 | |||
| Neu (*10^9/L) | 1.042 (1.017–1.068) | 0.001 | |||
| Th cell (CD4+) (%) | 1.032 (1.012–1.053) | 0.001 | |||
| suppressor/cytotoxic T cells (CD8+) (%) | 0.957 (0.934–0.981) | 0.001 | |||
| CD4+/CD8+ | 1.621 (1.278–2.055) | < 0.001 | |||
| PC (%) | 0.98 (0.974–0.986) | < 0.001 | 0.979 (0.972–0.987) | < 0.001 | |
| PS (%) | 1.000 (0.991–1.009) | 0.971 | |||
| anti-β2GP1 (AU/mL) | 1.014 (1.003–1.025) | 0.013 | 1.013 (1.003–1.022) | 0.007 | |
| LAC | 4.268 (1.106–16.473) | 0.035 | |||
PT: prothrombin time; TT: thrombin time; AT-III: antithrombin III; Angle: kinetics of clot development; Hcy: homocysteine; 25(OH)D: 25-hydroxyvitamin D; IgG: Immunoglobulin G; C3: Complement protein 3; C4: Complement protein 4; TG: triglyceride; WBC: white blood cell; Neu: neutrophil; Th cell (CD4+): CD4(+) T lymphocytes; PC: protein C; PS: protein S; anti-β2GP1: anti-β2 glycoprotein 1 antibody; LAC: lupus anticoagulant; 95% CI: 95% confidence interval; OR: odds ratio;
Fig. 3.
A nomogram for predicting the risk of spontaneous abortion in pregnancies. Covariates were assessed for the pregnancies and given a point in the nomogram. A higher total number of points indicated a higher likelihood of spontaneous abortion. AT-III: antithrombin III; Hcy: homocysteine; C3: Complement protein 3; PC: protein C; anti-β2GP1: anti-β2 glycoprotein 1 antibody
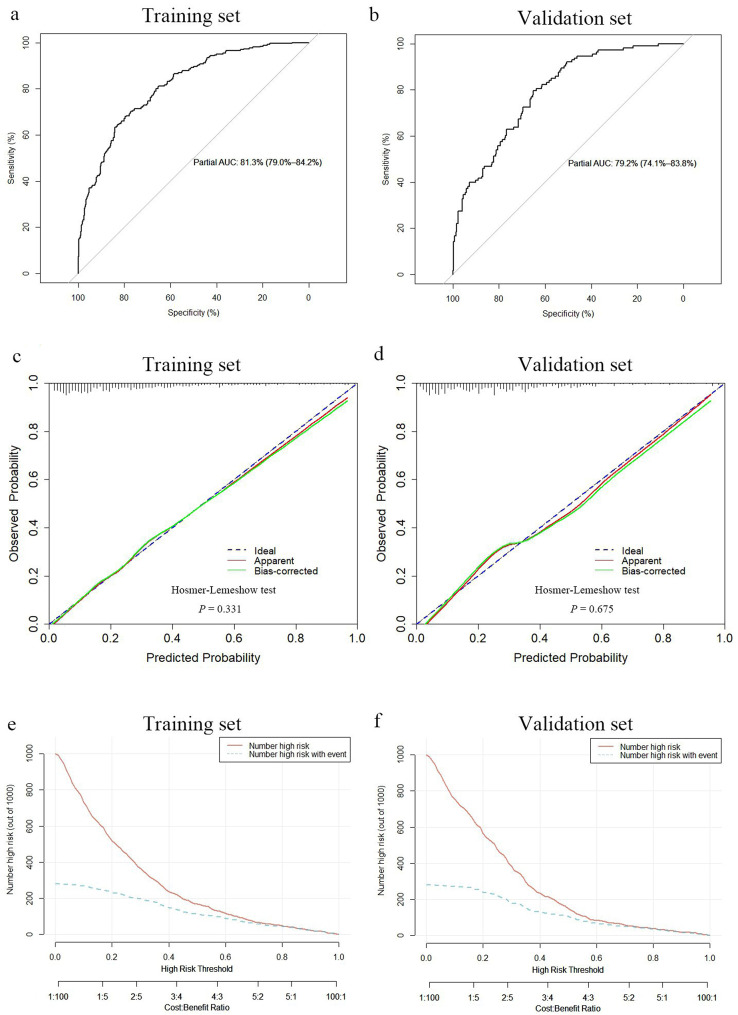
The nomogram demonstrated favorable discriminatory performance in the training set, with an AUC of 0.813 (95% CI: 0.790–0.842) (Fig. 4a). The calibration curve indicated strong agreement between predicted probabilities and observed outcomes in the training set (Fig. 4c). Additionally, the Hosmer-Lemeshow test produced a nonsignificant P value of 0.331, confirming good calibration.
Fig. 4.
Discrimination and calibration of the nomogram for predicting spontaneous abortion risk in the pregnancies and clinical impact curve depicting the clinical net benefit of the nomogram. Receiver operator characteristic curve of the nomogram in the training set (a) and validation set (b). Calibration curve of the nomogram in the training set (c) and validation set (d). Clinical impact curve for the nomogram in the training set (e) and validation set (f). For the calibration curve, the y-axis represents the actual observed spontaneous abortion probabilities, and the x‐axis represents nomogram‐predicted probabilities. The calibration curve shows how well the predicted probabilities agree with the observed probabilities. The diagonal blue dashed line represents a perfect prediction by an ideal model, and the green solid line reflects the performance of the nomogram; a closer fit to the diagonal dashed line indicates a better prediction. Clinical impact curve to predict the improved number for a population size of 1000. The red solid curve shows the predicted number of spontaneous abortions at different threshold probabilities, and the blue dashed curve represents the actual number of spontaneous abortions in the pregnancies. AUC: area under the curve
The clinical impact curve for the training set illustrated the stratification of spontaneous abortion probabilities for 1000 samples based on predicted probabilities (Fig. 4e). The predicted number of abortion cases closely matched the actual number of positive cases when the threshold probability exceeded 0.8, with a cost-to-benefit ratio of 0.6.
Validation of the nomogram
The validation set confirmed the satisfactory discriminatory performance of the nomogram, with an AUC of 0.792 (95% CI: 0.741–0.838) (Fig. 4b). Calibration was also deemed acceptable in the validation set, as evidenced by a nonsignificant Hosmer-Lemeshow test result (P = 0.675) (Fig. 4d). Furthermore, the CIC indicated that the nomogram provided a higher net benefit in the validation set when threshold probabilities exceeded 0.4 (Fig. 4f).
Models comparison
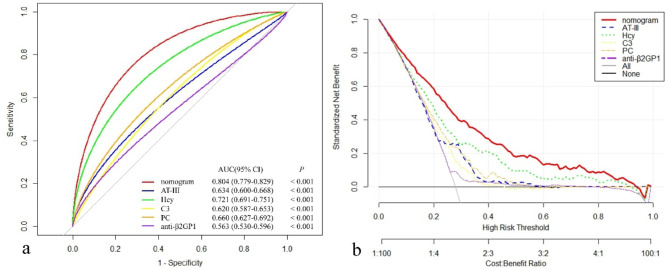
The nomogram demonstrated superior discriminatory accuracy for predicting spontaneous abortion in pregnancies, with an AUC of 0.804 (95% CI: 0.779–0.829), significantly outperforming any single variable included in the nomogram (P < 0.001) (Fig. 5a). The DCAs further revealed that the nomogram provided a higher overall net benefit compared to models containing individual risk factors across a wide range of threshold probabilities (Fig. 5b).
Fig. 5.
Models comparison in the whole study cohort. (a) Receiver operator characteristic curves of the models are presented to compare their discriminatory accuracy for predicting spontaneous abortion. P values show the difference between the AUC for the nomogram and the AUCs for other variables incorporated in the nomogram alone. (b) Decision curve analyses comparing the net benefit of the nomogram and the other variables incorporated in the nomogram alone are shown. AUC: area under the curve; CI: confidence interval; AT-III: antithrombin III; Hcy: homocysteine; C3: Complement protein 3; PC: protein C; anti-β2GP1: anti-β2 glycoprotein 1antibody
Discussion
Although numerous studies have reported the association between miscarriage and various immunological, endocrinological, systemic inflammation, thrombophilic and other blood biochemical parameters [7, 9, 10, 13], few have integrated these markers to predict the risk of miscarriage. To address this gap, we constructed a prediction nomogram based on hematological risk factors. This nomogram demonstrated favorable diagnostic accuracy, enabling clinicians to make accurate risk assessments and provide precise, evidence-based treatments.
Thrombophilia and miscarriage
Thrombophilia is a pathological condition characterized by an imbalance in the coagulation-anticoagulation system, involving platelets, the coagulation-fibrinolysis system, hemorheology. Mothers with thrombophilia may develop placental thrombosis, reducing placental perfusion and impairing maternal-fetal nutrient exchange, ultimately leading to spontaneous abortion [18]. This study identified AT-III and PC, two coagulation-related factors, are one of the key indicators for predicting miscarriage.
AT-III is a heparin-dependent serine protease inhibitor that can bind to and inactivate various serine-containing coagulation factors, thrombin, and plasmin, thus maintaining normal coagulation and preventing thrombosis [19]. PC is a protein that is activated in the presence of calcium ions and inactivates factor V and factor VIII, exerting anticoagulant effects [20]. In normal pregnancies, the synthesis of coagulation factors accelerates, increasing thrombin production while maintaining strong anticoagulant activity of AT-III. This enhances the thrombin-antithrombin complex and preserves AT-III levels. However, in abnormal pregnancies, this cascade is interrupted [21]. Studies have shown that the decrease of AT-III and PC activities increases the risk of thrombosis and miscarriage [22]. Wang et al. [21] found that AT-III levels decreased with an increasing number of prior miscarriages, with significantly lower levels in patients with four or more prior losses compared to those with normal fertility (P = 0.0111). Similarly, Sugiura et al. [23] reported that PC deficiency during pregnancy increases the risk of thrombosis by 3–10% antepartum and 7–19% postpartum, and was associated with recurrent miscarriages across all trimesters. Clinicians might consider heparin anticoagulation therapy, such as low molecular weight heparin (LMWH), to improve placental blood flow and prevent thrombosis.
Immune dysregulation and miscarriage
Immune system plays a vital role in maintaining a healthy pregnancy by regulating the maternal-fetal interface and protects the fetus from pathogens. Immune dysregulation, including autoimmune disorders, alloimmune reactions, and chronic inflammation, can lead to pregnancy loss or complications. Complement C3, the central protein of the complement cascade, is composed of α- and β-chains and plays a key role in early pregnancy and placental development [24]. Activation of C3 amplifies leukocyte inflammatory responses, enhances vascular endothelial activity, and exacerbates the procoagulant effects of APL antibodies. These processes can lead to thrombosis, placental ischemia, and hypoxia, culminating in miscarriage [25]. To confirm this hypothesis, Al Jameil et al. [26] injected Crry-IG, a C3 convertase inhibitor, into pregnant APL antibody-positive mice, blocking the classical and alternative pathways of complement C3 activation, which prevented fetal loss and growth restriction. Additionally, in patients with systemic lupus erythematosus (SLE), complement activation in the placenta generates anaphylatoxins (e.g., C3 and C1q), which mediate effector cell activation and accelerate embryonic rejection [27]. Anti-β2GP1 is a diagnostic marker of antiphospholipid syndrome and contributes to miscarriage by impairing trophoblast implantation and promoting uterine thrombosis. Previous studies [28, 29] confirmed that β2GP1 expression in the uterus inhibits platelet prothrombin activity, contributing to thrombosis and adverse pregnancy outcomes. Immunomodulatory therapies, such as corticosteroids or intravenous immunoglobulin (IVIG), may help mitigate these effects.
Hyperhomocysteinemia and miscarriage
Hcy is a sulfur-containing amino acid that is produced by the demethylation of methionine. Hyperhomocysteinemia (HHcy) occurs due to metabolic enzyme defects, or deficiencies in folic acid, vitamin B6, and B12 [30]. Elevated Hcy levels can damage endothelial cells, induce hypercoagulability, and harm placental vasculature, increasing the risk of thrombosis and miscarriage [31]. Several studies [32, 33] have consistently shown a strong correlation between HHcy and RSA. For instance, women with HHcy exhibit a higher prevalence of three or more consecutive spontaneous abortions [32]. Clinicians may recommend vitamin B12 and folic acid supplements to reduce Hcy levels, thereby lowering thrombosis and miscarriage risk.
Therefore, while such predictions can guide clinical decision-making, it is essential to involve the patient in a shared decision-making process, providing full transparency about the limitations and uncertainties of the model. Clinicians should ensure that patients understand the nature of the prediction and are supported emotionally, so they can make informed decisions that align with their values and reproductive goals.
Our study identified five independent risk factors for spontaneous abortion and developed a predictive model with a relatively high AUC, good calibration, and significant clinical utility. The nomogram and CIC further highlight its potential for guiding risk assessment in clinical practice. However, this study has several limitations. First, its retrospective design and single-center setting may limit the generalizability of the findings. Second, although we adjusted for baseline variables and covariates, unmeasured confounders, including treatments such as progesterone and heparin, may still have influenced the results. Finally, the analysis focused exclusively on hematological parameters to assess miscarriage risk. Future studies should include clinical indicators like maternal age, BMI, medical history, and symptoms to improve the model’s predictive accuracy.
Conclusion
We have developed and validated a novel tool to assess the risk of spontaneous abortion in pregnancies. This tool is a nomogram that incorporates several biomarkers and antibodies, such as AT-III, Hcy, C3, PC, and anti-β2GP1. It shows reliable accuracy and the ability to differentiate pregnancy outcomes. This allows clinicians and patients to adopt more targeted and effective treatment strategies. However, large multicenter studies are needed to further validate its utility before widespread clinical adoption.
Acknowledgements
The authors thank the Department of Gynecology of The Third Affiliated Hospital of Wenzhou Medical University, for their continuous support during the study.
Abbreviations
- AT
III Antithrombin III
- Hcy
Homocysteine
- C3
Complement component 3
- PC
Protein C
- Anti-β2GP1
Anti-β2GP1 Anti-β2 glycoprotein I antibody
Author contributions
Junmiao Xiang: Conceptualization, Methodology, Software, Investigation, Formal Analysis, Funding Acquisition, Writing - Original Draft, Writing - Review & Editing; Lin Liu: Data Curation, Writing - Original Draft; Ruru Bao: Visualization, Validation, Writing - Original Draft; Zhuhua Cai: Conceptualization, Resources, Supervision, Writing - Review & Editing.
Funding
This work was supported by Foundation of Wenzhou Municipal Health Commission (2020041).
Data availability
Data available on request from the authors.
Declarations
Ethics approval and consent to participate
This study was reviewed and approved by the Ethics Committee of The Third Affiliated Hospital of Wenzhou Medical University. The patients/participants provided written informed consent to participate in this study.
Consent for publication
Before participating in the study, all participants signed up with informed permission.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Quenby S, Gallos ID, Dhillon-Smith RK, Podesek M, Stephenson MD, Fisher J, Brosens JJ, Brewin J, Ramhorst R, Lucas ES. Miscarriage matters: the epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet. 2021;397(10285):1658–67. [DOI] [PubMed] [Google Scholar]
- 2.No RG-tG. The investigation and treatment of couples with recurrent first-trimester and second-trimester miscarriage. RCOG: London, UK; 2011.
- 3.Farren J, Jalmbrant M, Falconieri N, Mitchell-Jones N, Bobdiwala S, Al-Memar M, Tapp S, Van Calster B, Wynants L, Timmerman D, et al. Posttraumatic stress, anxiety and depression following miscarriage and ectopic pregnancy: a multicenter, prospective, cohort study. Am J Obstet Gynecol. 2020;222(4):367. e361-367 e322. [DOI] [PubMed] [Google Scholar]
- 4.Li C, Xiao L, Tang L, Zeng D, Huang S. Application of QF-PCR technology combined with early pregnancy ultrasound in prenatal screening for fetal chromosomal aneuploidy. Altern Ther Health Med. 2023;29(7):198–203. [PubMed] [Google Scholar]
- 5.Parisi F, Coco C, Cetin I, group Ss. Prospective multicentre Italian pregnancy cohort study (SIMPLE) on the associations of maternal first trimester SIMPLE nutritional score with early placental function markers and pregnancy outcomes. BMJ Open. 2022;12(10):e062940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao QY, Li QH, Fu YY, Ren CE, Jiang AF, Meng YH. Decidual macrophages in recurrent spontaneous abortion. Front Immunol. 2022;13:994888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kupferminc MJ. Thrombophilia and pregnancy. Reprod Biol Endocrinol. 2003;1:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hazra D, Sen I, Stephen E, Agarwal S, Nair SC, Mammen J. Evaluation of Factor VIII as a Risk Factor in Indian Patients with DVT. Surg Res Pract 2015, 2015:307879. [DOI] [PMC free article] [PubMed]
- 9.Marder W, Littlejohn EA, Somers EC. Pregnancy and autoimmune connective tissue diseases. Best Pract Res Clin Rheumatol. 2016;30(1):63–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radin M, Schreiber K, Cuadrado MJ, Cecchi I, Andreoli L, Franceschini F, Caleiro T, Andrade D, Gibbone E, Khamashta M, et al. Pregnancy outcomes in mixed connective tissue disease: a multicentre study. Rheumatology (Oxford). 2019;58(11):2000–8. [DOI] [PubMed] [Google Scholar]
- 11.Zucchi D, Fischer-Betz R, Tani C. Pregnancy in systemic lupus erythematosus. Best Pract Res Clin Rheumatol 2023:101860. [DOI] [PubMed]
- 12.Kuon RJ, Strowitzki T, Sohn C, Daniel V, Toth B. Immune profiling in patients with recurrent miscarriage. J Reprod Immunol. 2015;108:136–41. [DOI] [PubMed] [Google Scholar]
- 13.Vella K, Vella S, Savona-Ventura C, Vassallo J. Thyroid dysfunction in pregnancy - a retrospective observational analysis of a Maltese cohort. BMC Pregnancy Childbirth. 2022;22(1):941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salmon JE. A noninflammatory pathway for pregnancy loss: innate immune activation? J Clin Invest. 2004;114(1):15–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kramer AA, Zimmerman JE. Assessing the calibration of mortality benchmarks in critical care: the Hosmer-Lemeshow test revisited. Crit Care Med. 2007;35(9):2052–6. [DOI] [PubMed] [Google Scholar]
- 16.Kerr KF, Brown MD, Zhu K, Janes H. Assessing the clinical impact of risk prediction models with decision curves: guidance for correct interpretation and appropriate use. J Clin Oncol. 2016;34(21):2534–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demler OV, Pencina MJ, D’Agostino Sr RB. Misuse of DeLong test to compare AUCs for nested models. Stat Med. 2012;31(23):2577–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McNamee K, Dawood F, Farquharson RG. Thrombophilia and early pregnancy loss. Best Pract Res Clin Obstet Gynaecol. 2012;26(1):91–102. [DOI] [PubMed] [Google Scholar]
- 19.Stansfield BK, Wise L, Ham PB 3rd, Patel P, Parman M, Jin C, Mathur S, Harshfield G, Bhatia J. Outcomes following routine antithrombin III replacement during neonatal extracorporeal membrane oxygenation. J Pediatr Surg. 2017;52(4):609–13. [DOI] [PubMed] [Google Scholar]
- 20.Gardiner JE, Griffin JH. Studies on human protein C inhibitor in normal and factor V/VIII deficient plasmas. Thromb Res. 1984;36(3):197–203. [DOI] [PubMed] [Google Scholar]
- 21.Wang P, Yang H, Wang G, Tian J. Predictive value of thromboelastography parameters combined with antithrombin III and D-Dimer in patients with recurrent spontaneous abortion. Am J Reproductive Immunol (New York NY: 1989). 2019;82(4):e13165. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Lin X, Wu Q, Zhao M, Xian S, Lin D, Sun L, He J, Bao Y, Duan C. Thrombophilia markers in patients with recurrent early miscarriage. Clin Lab. 2015;61(11):1787–94. [DOI] [PubMed] [Google Scholar]
- 23.Sugiura M. Pregnancy and delivery in protein C-deficiency. Curr Drug Targets. 2005;6(5):577–83. [DOI] [PubMed] [Google Scholar]
- 24.Mohlin FC, Gros P, Mercier E, Gris JR, Blom AM. Analysis of C3 gene variants in patients with idiopathic recurrent spontaneous pregnancy loss. Front Immunol. 2018;9:1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Carolis S, Botta A, Santucci S, Salvi S, Moresi S, Di Pasquo E, Del Sordo G, Martino C. Complementemia and obstetric outcome in pregnancy with antiphospholipid syndrome. Lupus. 2012;21(7):776–8. [DOI] [PubMed] [Google Scholar]
- 26.Al Jameil N, Tyagi P, Al Shenefy A. Incidence of anticardiolipin antibodies and lupus anticoagulant factor among women experiencing unexplained recurrent abortion and intrauterine fetal death. Int J Clin Exp Pathol. 2015;8(3):3204–9. [PMC free article] [PubMed] [Google Scholar]
- 27.Lood C, Tydén H, Gullstrand B, Sturfelt G, Jönsen A, Truedsson L, Bengtsson AA. Platelet activation and anti-phospholipid antibodies collaborate in the activation of the complement system on platelets in systemic lupus erythematosus. PLoS ONE. 2014;9(6):e99386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franklin RD, Hollier N, Kutteh WH. beta2-Glycoprotein 1 as a marker of antiphospholipid syndrome in women with recurrent pregnancy loss. Fertil Steril. 2000;73(3):531–5. [DOI] [PubMed] [Google Scholar]
- 29.Chopra A, Radhakrishnan R, Sharma M. Porphyromonas gingivalis and adverse pregnancy outcomes: a review on its intricate pathogenic mechanisms. Crit Rev Microbiol. 2020;46(2):213–36. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Feng D, He S, Wu Q, Su Z, Ye H. Meta-analysis: association of homocysteine with recurrent spontaneous abortion. Women Health. 2021;61(7):713–20. [DOI] [PubMed] [Google Scholar]
- 31.Freyburger G, Labrouche S, Sassoust G, Rouanet F, Javorschi S, Parrot F. Mild hyperhomocysteinemia and hemostatic factors in patients with arterial vascular diseases. Thromb Haemost. 1997;77(3):466–71. [PubMed] [Google Scholar]
- 32.Nelen WL, Blom HJ, Steegers EA, den Heijer M, Thomas CM, Eskes TK. Homocysteine and folate levels as risk factors for recurrent early pregnancy loss. Obstet Gynecol. 2000;95(4):519–24. [DOI] [PubMed] [Google Scholar]
- 33.Raziel A, Kornberg Y, Friedler S, Schachter M, Sela BA, Ron-El R. Hypercoagulable thrombophilic defects and hyperhomocysteinemia in patients with recurrent pregnancy loss. Am J Reproductive Immunol (New York NY: 1989). 2001;45(2):65–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request from the authors.