Abstract
Introduction
Treatment mechanisms are the underlying process or pathway through which a treatment influences the body. This includes molecular, cellular and physiological processes or pathways contributing to treatment effect. Manual therapy (MT) evokes complex mechanistic responses across body systems, interacting with the individual patient and context to promote a treatment response. Challenges arise as mechanistic studies are spread across multiple professions, settings and populations. The purpose of this review is to summarize treatment mechanisms that have been reported to occur with MT application.
Methods
Four electronic databases were searched (Medline, CINAHL, Cochrane Library, and PEDro) for reviews investigating mechanistic responses which occur during/post application of MT. This review was registered a priori with PROSPERO (CRD42023444839). Methodological quality (AMSTAR-2) and risk of bias (ROBIS) were assessed for systematic and scoping reviews. Data were synthesized by mechanistic domain.
Results
Sixty-two reviews were included. Systematic reviews (n = 35), narrative reviews (n = 24), and scoping reviews (n = 4) of asymptomatic (n = 37), symptomatic (n = 43), non-specified human subjects (n = 7) and animals (n = 7) were included. Reviews of moderate quality supported neurovascular, neurological, and neurotransmitter/neuropeptide changes. Reviews of low quality supported neuroimmunce, neuromuscular, and neuroendocrine changes. Reviews of critically low quality support biomechanical changes.
Conclusions
Findings support critically low to moderate quality evidence of complex multisystem mechanistic responses occurring with the application of MT. Results support peripheral, segmental spinal, and supraspinal mechanisms occurring with the application of MT, which can be measured directly or indirectly. The clinical value of these findings has not been well established. While MT has proven to be an effective intervention to treat conditions such as pain, the current body of literature leaves uncertainty as to ‘why’ MT interventions work, and future research should look to better define which mechanisms (or combinations of mechanisms) are mediators of clinical response.
Introduction
Manual Therapy (MT) is a type of force-based manipulation which has been defined as “passive application of mechanical force to the outside of the body with therapeutic intent, often as part of pain management care (e.g., low-back pain), rehabilitation care, or general wellness and disease prevention” [1]. Techniques associated with MT include soft tissue mobilization (STM), joint mobilization (non-thrust), and manipulation (thrust). These techniques are used by healthcare professionals such as osteopaths, massage therapists, chiropractors, and physical therapists (PT). Whereas historical models of MT attributed the clinical effect to biomechanical changes within tissues directly related to the technique applied [2,3], recent evidence-based models support more complex interactive mechanistic responses across body systems, interacting with the individual patient and context to promote a treatment response [4–6].
Mechanisms are “the molecular, cellular, physiological processes or pathways contributing to a) disease development, b) treatment action, or c) pain signal sensation, transmission, perception, and modulation” [7]. Treatment mechanisms are “the underlying process or pathway through which a specific treatment produces an influence on the body” [7]. Potential treatment mechanisms associated with MT include: biomechanical (altered tissue movement, fluid loading, etc.), neurological (fMRI activation, altered neural conduction, etc.), neuroimmune (release of inflammatory and anti-inflammatory mediators, etc.), neurovascular (sympathetic response, etc.), neurotransmitter and neuropeptide (release of serotonin, beta endorphin, etc.), neuromuscular (altered neuromuscular tone, muscle recruitment), neuroendocrine (release of cortisol etc.) and other mechanisms [8].
Mechanisms are processes that can be measured in a number of different ways. Measurements can be direct or indirect. Higher level (cortical and subcortical) changes typically represent initial efferent activity (direct measure), while measurements of downstream effects represent indirect or proxy measures of the process (e.g., skin conductance as a proxy measure of ANS response, fMRI as proxy measure of cortical activation, neuroimmune markers as proxy measures of multisystem subcortical activation, somatosensory reflexes as a measure of spinal excitability, etc.) rather than direct/primary measures (e.g., EEG as a measure of cortical activity, MRI as a measure of joint position, etc.) [9]. Defined mechanistic domains and proposed direct and indirect measures within these domains are presented in S1 Appendix. Studies investigating treatment mechanisms associated with MT are of interest as recent National Institute of Health (NIH) funding has been designated to better understand the mechanisms associated with the application of force during MT [1].
MT techniques have demonstrated efficacy/effectiveness in improving range of motion, reducing disability, improving function and modulating pain [10–12]. Based on these findings, the use of selected MT techniques are commonly cited in high-level clinical practice guidelines as recommended interventions for various conditions [13–17]. Despite this endorsement, variability exists in the strength of recommendations [18,19], suggesting an inconclusive body of literature. Furthermore, reported treatment effect sizes for MT are small to moderate [20], which is likely due to individual variability in treatment response [21] when provided with a one-size-fits-all treatment approach [22]. Mechanistic-based treatment stratification represents a potential approach to match patients to treatments and improve outcomes [21,23,24]. Such an approach allows the matching of an intervention of known mechanisms to patients with underlying conditions responsive to these mechanisms [25]. Clarifying the mechanisms through which MT inhibits musculoskeletal pain could improve the effectiveness in clinical practice by better informing a mechanistic approach to identifying individuals who will respond positively to these interventions. Despite progress in this area of study the mechanisms underpinning the demonstrated effectiveness of MT remains unclear due to two notable limitations:
1) Mechanism-based studies are spread across multiple professions and settings including lab-based and clinical-based designs, animal and human models, and asymptomatic and symptomatic specimens.
2) Mechanism-based studies often lack translation of causality between treatment mechanisms and clinical outcomes (translational studies).
A recent interprofessional panel reached a consensus on current gaps that are present in mechanistic research [8]; however, the literature involves multiple physiological systems, often explored heterogeneously, making it challenging to interpret and summarize. The purpose of this review of reviews was to identify and summarize the neurological, neuroimmune, biomechanical, neurovascular, neurotransmitter/neuropeptide, neuroendocrine, and other not previously categorized treatment mechanism systems that have been reported to occur with MT application. Reviews of reviews play a crucial role in synthesizing and evaluating existing research, providing a comprehensive perspective on a specific topic or question therefore aiding both researchers and clinicians to make sense of a vast complex topic [26].
Methods
Protocol and registration
A review of systematic, scoping, and narrative reviews was performed to assess and summarize the mechanisms associated with MT application. To encompass new evidence as it becomes available, a living review building off the findings of this study will be hosted digitally at the Duke Center for Excellence in Manual and Manipulative Therapy (CEMMT). This study was registered with PROSPERO on September 03, 2023, prior to the initial literature search (CRD42023444839).
Eligibility criteria
Systematic reviews, scoping reviews, and narrative reviews with or without meta-analyses were included. Reviews including MT techniques within the scope of Physical Therapy (PT) practice were included (mobilization (non-thrust), manipulation (thrust), STM/massage, light touch). Manual techniques controlled or performed by external, non-human forces were excluded except for instrument-assisted STM in which a human provider was manually controlling a device to assist in external tissue mobilization. Internal (invasive) STM techniques such as dry needling and acupuncture were excluded. Outcomes (mechanisms) required for inclusion were neurological, neuroimmune, biomechanical, neurovascular, neurotransmitter, neuroendocrine, and other non-aforementioned mechanisms associated with MT application. In vivo models including living human and animal subjects were included. In-vitro models and cadaveric studies were excluded as the treatment mechanisms are assumed to differ in these models. Comparators included control, sham, or other MT procedures. Reviews were excluded if they did not include an outlined literature search strategy.
Information sources
Four electronic databases were searched including: Medline, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Cochrane Library, and Physiotherapy Evidence Database (PEDro). Reviews published from inception to October 3, 2023 were included in initial search. An updated search was performed September 23, 2024 to include reviews published through that date. The search strategy for each of the included databases was validated by a Health Sciences librarian and uploaded to PROSPERO prior to the search. The comprehensive search strategy is available in S2 Appendix.
Data selection
Two review authors performed title (DK, KB), abstract (DK, MF), and full text (DK, KB) screenings independently. Discrepancies between reviewers were resolved by a third review author (CC, KB). Microsoft Excel (Microsoft Corporation; version 2205-2211) was utilized to manage and organize the search results throughout the review process. Cohen’s Kappa Scores (95% CI) were calculated to assess agreement between reviewers.
Data extraction
Data were extracted using a self-developed tool to extract appropriate variables. The following items were extracted: author, year, type of review, database(s) searched, number of included studies, mechanistic domain, MT intervention, comparator, outcome and results/conclusion. Data were extracted independently by two review authors (DK, KB) with all discrepancies discussed and agreed upon by the two authors.
Methodological quality appraisal
Methodological quality for each included systematic and scoping review was performed using the AMSTAR-2 quality assessment measure [27]. Risk of Bias was assessed using the ROBIS tool [28]. Two review authors independently performed quality appraisal (KL, CC) and risk of bias assessment (SK, JB) with discrepancies resolved by a third review author (DK). Quality assessment and risk of bias was not assessed for narrative reviews.
Data synthesis
Studies were grouped by mechanistic domains (S1 Appendix) outlined in previous work [8] with mechanisms outside of those domains categorized within an ‘other’ category. No synthesized quality or GRADE was used given the focus on mechanistic outcomes rather than clinical effectiveness.
Data analysis
No analysis of data occurred. The goals of this review were to outline and summarize the status of the available literature.
Results
Selection of sources of evidence
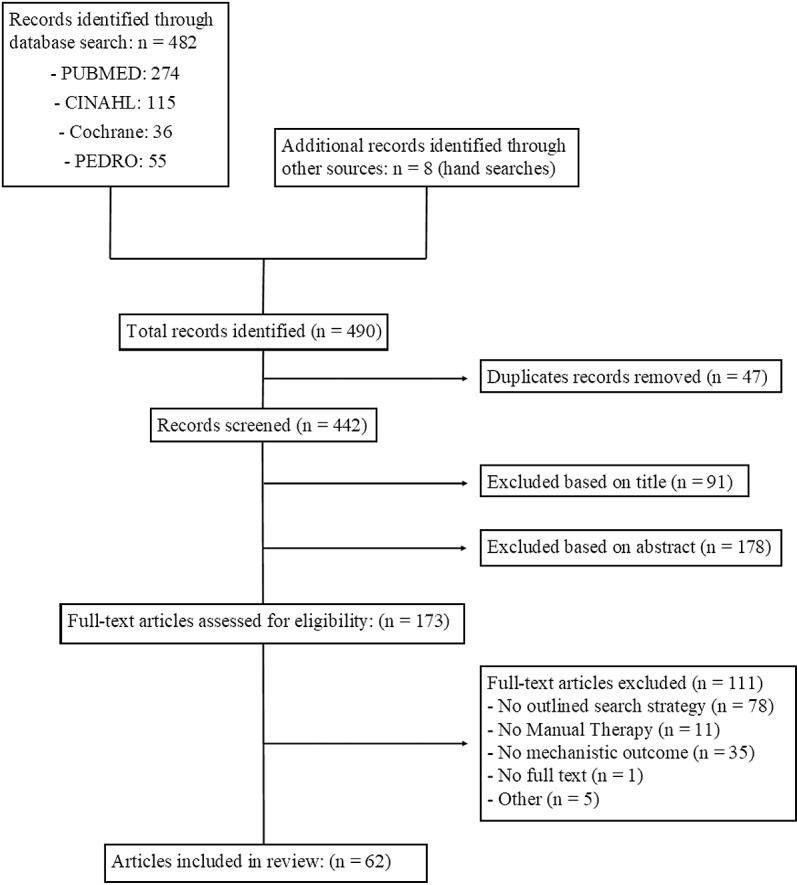
The search identified 442 reviews after duplicate removal. After title and abstract screening, 173 full-text reviews were agreed to be assessed for eligibility. Cohens Kappa coefficients to assess agreement between reviewers demonstrated moderate to strong agreement for title (k = .77; 95% CI.69 -.84) abstract (k = .83; 95% CI.78 -.89), and full text (k = .98; 95% CI.94 -.99) screening. Sixty-two reviews were agreed upon to be included in this review of reviews. A flowchart representing the process for evidence selection including rationale for full texts which were screened and excluded is presented in Fig 1. Detailed rationale for full texts which were screened and excluded are presented in S3 Appendix.
Fig 1. PRISMA Flowchart for selection of sources of evidence.
Study characteristics
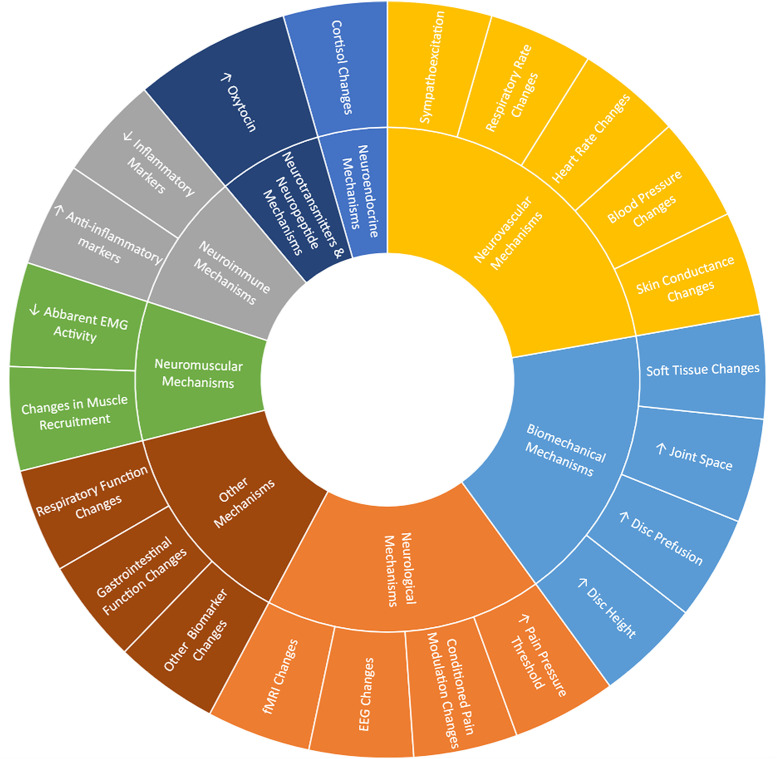
Included reviews were systematic reviews (n = 35), narrative reviews (n = 24), and scoping reviews (n = 4). Databases searched within these reviews included CINAHL (n = 35), Cochrane (n = 27), PubMed/Medline (n = 61), PEDro (n = 17), SCOPUS (n = 10), and EMBASE (n = 19). Forty-five of the included reviews also included other search strategies (e.g., searching references of included studies, consulting experts in the field) and databases (PsycINFO, AMED, MANTIS, Google Scholar, Elsevier, Science Direct, Web of Science, Index of Chiropractic Literature, SciELO, etc.). Subjects included asymptomatic humans (n = 37), symptomatic humans (n = 43), human subjects without specifications regarding status (n = 7) and animal models (n = 6). MT techniques included manipulation (n = 41), mobilization (n = 23), and STM or massage (n = 19). Reviews included studies comparing MT to sham intervention (n = 37), comparing MT to control (no intervention) (n = 45), and 14 reviews that did not specify a comparator. Treatment mechanisms from the biomechanical domain (n = 14), neurovascular domain (n = 32), neurological domain (n = 23), neurotransmitter/neuropeptide domain (n = 16), neuroimmune domain (n = 12), neuroendocrine domain (n = 11), neuromuscular domain (n = 10), and other domains (n = 7) were identified (Fig 2). Characteristics of the included reviews are presented in Table 1 with more detailed extracted data presented in S4 Appendix. Reported treatment mechanisms across the aforementioned domains are presented in Tables 2–11.
Fig 2. Treatment mechanisms occurring with Manual Therapy application.
Table 1. Characteristics of included reviews.
| Author Year |
Type of Review | Included Database(s) | (n) Studies | MT Technique(s) | Comparator(s) | Mechanistic Domain(s) | ||
|---|---|---|---|---|---|---|---|---|
| Man | Mob | STM | ||||||
| Alanazi et al. 2024 [29] | Narr. Review | Medline | 221 | Spinal, Peripheral | ---------- | ---------- | Sham, Control | Neurovascular |
| Araujo et al. 2019 [30] | Sys. Review | Cochrane, Medline, PEDro, Embase, Other | 18 | Spinal | Spinal | ---------- | Sham, Control | Neurovascular, Neurotransmitter |
| Arribas-Romano et al. 2020 [31] | Sys. Review | Cinahl, Cochrane, Medline, PEDro, SCOPUS, Embase, Other | 17 | Lumbar | Knee | ---------- | Sham, Control | Neurological |
| Bernier Carney et al. 2020 [32] | Sys. Review | Cinahl, Medline, SCOPUS, Other | 2 | ----------- | ---------- | Gua sha | n/a | Neuroimmune, Neuroendocrine |
| Bolton et al. 2012 [33] | Narr. Review | Medline, Other | 35 | Spinal | Spinal | ---------- | Sham, Control | Neurovascular, Neuromuscular, Neuroimmune, Neuroendocrine, Neurotransmitter, Neuropeptide, Other |
| Borges et al. 2018 [34] | Sys. Review | Cochrane, Medline, PEDro, SCOPUS, Other | 9 | Spinal | ---------- | LE, UE, Lumbar, Craniofacial, Craniosacral | Sham, Control | Neurovascular |
| Chow et al. 2021 [35] | Sys. Review | Cinahl, Cochrane, Medline, Embase, Other | 8 | Spinal | ---------- | ---------- | Sham, Control | Neuroimmune, Neuroendocrine, Neurotransmitter, Neuropeptide, Neurovascular |
| Chu et al. 2014 [36] | Sys. Review | Cinahl, Cochrane, Medline, PEDro, Other | 11 | ---------- | Spinal | ---------- | Sham, Control | Neurovascular |
| Colombi et al. 2019 [37] | Narr. Review | Medline, Other | 13 | Spinal | ---------- | ---------- | Sham, Control | Neuroendocrine, Neuroimmune |
| Cook et al. 2024 [38] | Narr. Review | Medline | 13 | Spinal, Peripheral | Spinal, Peripheral | n/a | Sham, Control | Neuroimmune |
| Coronado et al. 2012[39] | Sys. Review | Cinahl, Medline, Other | 20 | Spinal | ---------- | ---------- | Sham, Control | Neurological |
| Coronado et al. 2013 [40] | Sys. Review | Cinahl, Medline, PEDro, Other | 5 | Spinal | ---------- | ---------- | Sham | Neurological, Neurotransmitter, Neuroendocrine |
| Corso et al. 2019 [41] | Sys. Review | Cinahl, Cochrane, PEDro Other |
20 | Spinal | ---------- | ---------- | Sham, Control | Neuromuscular, Neurovascular |
| Evans 2002 [42] | Narr. Review | Medline, Embase | n/a | Spinal | ---------- | ---------- | n/a | Biomechanical |
| Field 2016 [43] | Narr. Review | Medline | 65 | ---------- | ---------- | n/a | Sham | Neuroimmune, Neuroendocrine, Neuropeptide, Biomechanical, Neurovascular, Other |
| Galindez-Ibarbengoetxea et al. 2017 [44] | Sys. Review | Cinahl, Medline, PEDro, SCOPUS, Other | 11 | Cervical | ---------- | ---------- | Control | Neurovascular |
| Gay et al. 2005 [45] | Narr. Review | Cinahl, Medline, Embase, Other | Lumbar | ---------- | ---------- | n/a | Biomechanical | |
| Gay et al. 2013 [46] | Sys. Review | Cinahl, Medline | 23 | ---------- | ---------- | n/a | Sham, Control | Neurological |
| Gera et al. 2020 [47] | Sys. Review | Cochrane, Medline, Other | 18 | Spinal | Spinal | ---------- | Sham, Control | Neurovascular |
| Haavik et al. 2021 (2) [48] | Narr. Review | Cinahl, Medline, Other | 58 | Spinal | ---------- | ---------- | n/a | Neuromuscular |
| Haavik et al. 2021 [49] | Narr. Review | Cinahl, Medline, SCOPUS, Other | 23 | Spinal | ---------- | ---------- | Control | Neuroimmune, Neuropeptide, Neuroendocrine, Neurotransmitter |
| Hartnett 2021[50] | Narr. Review | Medline, Other | 57 | ---------- | ---------- | n/a | Control | Biomechanical |
| Hegedus et al. 2011 [51] | Sys. Review | Cinahl, Medline, Other | 10 | ---------- | Spinal | ---------- | Control | Neurovascular |
| Hennenhoefer et al. 2019 [52] | Narr. Review | Medline, Other | 69 | Spinal | ---------- | ---------- | n/a | Biomechanical, Neuromuscular, Neurological |
| Hillier et al. 2010 [53] | Sys. Review | Cinahl, Cochrane, Medline, Embase, Other | 4 | ---------- | ---------- | n/a | Sham | Neuroimmune |
| Holey et al. 2014 [54] | Narr. Review | Cinahl, Medline, Other | n/a | ---------- | ---------- | n/a | n/a | Neurovascular, Neurotransmitter |
| Honoré et al. 2018 [55] | Sys. Review | Cochrane, Medline, Embase, Other | 12 | Spinal | ---------- | ---------- | Sham, Control | Neurological |
| Jacobson 2011 [56] | Narr. Review | Medline, Other | 12 | ---------- | ---------- | n/a | n/a | Neurological, Biomechanical, Neuromuscular, Other |
| Jones et al. 2013 [57] | Sys. Review | Cinahl, Cochrane, Medline, Embase, Other | 12 | ---------- | ---------- | Foot | Sham., Control | Neurovascular |
| Jun et al. 2020 [58] | Scop Review | Cinahl, Medline | 10 | Lumbar | Lumbar | ---------- | Control | Neuromuscular, Biomechanical, Neurological |
| Jung et al. 2023 [59] | Sys. Review | Cinahl, Cochrane, Medline, Other | 22 | Spinal | Spinal | ---------- | Sham, Control | Neurological |
| Kingston et al. 2014 [60] | Sys. Review | Cochrane, Medline, PEDro, Embase, Other | 7 | ---------- | Spinal | ---------- | Sham, Control | Neurovascular |
| Lascurain-Aguirrebena et al. 2016 [61] | Sys. Review | Cinahl, Medline, SCOPUS, Embase, Other | 24 | ---------- | Spinal | ---------- | Sham, Control | Neurological, Neurovascular, Biomechanical |
| Lima et al. 2020 [62] | Scop Review | Cinahl, Cochrane, Medline, Embase, Other | 78 | Spinal | Spinal, LE | n/a | n/a | Neuroimmune, Neuromuscular, Neurotransmitter, Neurological, Neurovascular, Biomechanical |
| Meyer et al. 2019 [63] | Sys. Review | Medline, PEDro, Embase | 18 | Spinal | ---------- | ---------- | Sham, Control | Neurological |
| Millan et al. 2012 [64] | Sys. Review | Cochrane, Medline, Other | 43 | Spinal | ---------- | ---------- | Sham, Control | Neurological |
| Mitchell et al. 2017 [65] | Sys. Review | Cinahl, Cochrane, Medline, SCOPUS, Other | 8 | Lumbar | Lumbar | ---------- | Sham, Control | Biomechanical |
| Moyer et al. 2011 [66] | Narr. Review | Cinahl, Medline, Other | 19 | ---------- | ---------- | Full Body | Control | Neuroendocrine |
| Navarro-Santana et al. 2020 [67] | Sys. Review | Cinahl, Cochrane, Medline, PEDro, SCOPUS, Embase, Other | 17 | ---------- | Spinal, UE | ---------- | Sham, Control | Neurovascular |
| Nelson 2015 [68] | Scop Review | Cinahl, Medline | 27 | ---------- | ---------- | n/a | n/a | Neurological, Neurovascular, Neuroendocrine |
| Picchiottino et al. 2019 [69] | Sys. Review | Cochrane, Medline, PEDro, Embase, Other | 29 | Spinal | Spinal Elbow | ---------- | Sham | Neurovascular, Neurotransmitter |
| Pickar 2002 [70] | Narr. Review | Medline, Other | n/a | Spinal | ---------- | ---------- | Sham, Control | Neuromuscular, Biomechanical |
| Potter et al. 2005 [71] | Narr. Review | Cinahl, Medline, Other | 31 | Spinal, Peripheral | ---------- | ---------- | n/a | Biomechanical, Neurological, Neurovascular, Neuromuscular, Neurotransmitter |
| Riley et al. 2024 [72] | Sys. Review | Cinahl, Medline, PEDro, Other | 3 | Spinal | ---------- | ---------- | Sham | Neurological |
| Rogan et al. 2023 [73] | Scop Review | Cochrane, Medline, PEDro, Other | 33 | Spinal | Spinal | Spinal | Sham, Control | Neurovascular |
| Sampath et al. 2017 [74] | Sys. Review | Cinahl, Cochrane, Medline, PEDro, SCOPUS, Embase, Other | 8 | Spinal | ---------- | ---------- | Sham, Control | Neuroimmune, Neuroendocrine, Neurotransmitter, Neuropeptide |
| Sampath et al. 2024 [75] | Sys. Review | Cinahl, Cochrane, Medline, SCOPUS, Other | 14 | Spinal | ---------- | ---------- | Sham, Control | Neurovascular, Neuropeptide/Neurotransmitter, Neuroendocrine |
| Savva et al. 2021 [76] | Narr. Review | Cinahl, Medline, Other | 16 | Spinal, UE | ---------- | ---------- | Sham Control |
Neurological, Neurovascular |
| Schmid et al. 2008 [77] | Sys. Review | Cinahl, Cochrane, Medline | 15 | ---------- | Cervical | ---------- | Sham | Neuromuscular, Neurological, Neurovascular |
| Simmonds et al. 2012 [78] | Narr. Review | Cinahl, Medline. Other | 13 | Spinal | Spinal | ---------- | Sham, Control | Neurovascular |
| Sousa et al. 2020 [79] | Sys. Review | Medline, Other | 10 | Spinal | ---------- | Hand, Spinal | Sham, Control | Neurovascular |
| Sullivan et al. 2020 [80] | Sys. Review | Cinahl, Medline, Other | 28 | Spinal | ---------- | ---------- | Sham, Control | Neurovascular |
| Tejero-Fernandez et al. 2015 [81] | Sys. Review | Cochrane, Medline, PEDro | 5 | ---------- | ---------- | n/a | Control | Neuroimmune, Neurological, Neuroendocrine, Other |
| Vernon 2000 [82] | Narr. Review | Medline | 11 | Lumbar | ---------- | ---------- | Control | Neurological |
| Vicenzino 2007 [83] | Narr. Review | Cinahl, Cochrane, Medline, Embase, Other | 19 | ---------- | UE | ---------- | n/a | Biomechanical, Neurological, Neurovascular |
| Vigotsky et al. 2015 [84] | Narr. Review | Medline | 29 | Spinal, Knee | ---------- | ---------- | Sham, Control | Neurotransmitter, Neuropeptide |
| Voogt et al. 2015 [85] | Sys. Review | Cochrane, Medline, Embase, Other | 14 | Cervical | LE, UE, Cervical | ---------- | n/a | Neurological |
| Weerapong et al. 2005 [86] | Narr. Review | Medline, Other | 35 | ---------- | ---------- | Whole body | Control | Neurovascular |
| Xiong et al. 2015 [87] | Sys. Review | Cochrane, Medline, Embase, Other | 24 | ---------- | ---------- | n/a | Control | Neurovascular |
| Yao et al. 2014 [88] | Narr. Review | Medline | 6 | ---------- | Thorax | ---------- | Sham | Neurovascular, Neuroimmune |
| Young et al. 2024 [89] | Sys. Review | Cinahl, Cochrane, Medline, PEDro, Embase, Other | 20 | Spinal | ---------- | ---------- | Control | Biomechanical |
| Zegarra-Parodi et al. 2015 [90] | Sys. Review | Cinahl, Cochrane, Medline, PEDro | 20 | Spinal | Spinal | ---------- | Sham, Control | Neurovascular |
Definitions: LE- Lower extremity, UE- Upper Extremity, n/a- not specified.
Table 2. biomechanical treatment mechanisms associated with MT.
| Author/Year | Subjects | MT Technique | Measure | Time of Measure | Results/Conclusion | |
|---|---|---|---|---|---|---|
| Systematic Reviews |
Lascurain-Aguirrebena et al. 2016 [61] | Humans- Neurogenic UE pain, LBP, neck pain, craniofacial pain, shoulder pain, cervicogenic dizziness, whiplash, epicondylalgia | Mob- Spinal | Imaging | During and Immediately post to 6 weeks post | Lumbar: PA mobilization produced intersegmental extension at each targeted segment, greatest at L2-L3 and least at L4-L5 Cervical: PA mobilization only produced compression of the soft tissue; no intersegmental motion at any cervical joint Variability in post PA motion: 1 favoring changes in motion and 1 reporting no significant difference |
| Mitchell et al. 2017 [65] | Humans- Not specified | Man- Lumbar Mob- Lumbar |
Disc height, morphology, molecular transportation, intervertebral disc space and diffusion | n/a | All studies confirmed, either directly or indirectly, that their respective intervention influenced disc physiology primarily through water flow | |
| Young et al. 2024 [89] | Humans- Healthy, LBP, non-migraine headaches Animals- Dogs, horses |
Man- Spinal | Radiography, Ultrasound, Stiffness assessment | Immediate Post | Change in vertebral position in 2/3 included studies (0/2 supporting rated credible) ↑ Facet joint space in 4/5 included studies (all 4 supporting rated credible) Changes in spinal stiffness in 3/3 included studies (1/3 supporting rated credible) Changes in muscle stiffness in 2/6 included studies (0/2 supporting rated credible) Changes in disc pressure in 1/1 included study rated as not credible Changes in tissue characteristics in 1/1 included study rated as not credible Changes in tissue damage to artery in 0/1 included study rated as not credible |
|
| Scoping Reviews |
Lima et al. 2020 [62] | Animals- Non-Cadaveric Animal Models (Rats, Rabbits, Mice); healthy and induced pain | STM- Region not specified | Imaging | n/a | ↓ Myofibril damage → Muscle volume ↑ Cross sectional area ↑ Force production |
| Jun et al. 2020 [58] | Humans- Asymptomatic and symptomatic; Animals | Man- Lumbar Mob- Lumbar |
Dorsoventral displacement, Intervertebral disc diffusion | n/a | Six studies suggested that changes in spinal or bending stiffness are associated with an increase in mobility following SMT Insufficient fluid recovery resulting in a change in the viscoelastic properties of the soft tissues Repeated loading of the spine might cause creep and relaxation of the spinal connective tissues, which would change the resistance to the applied load and similarly the initial displacement under the applied load ↑ Intervertebral disc diffusion following SMT, but only in the participants classified as responders at follow-up |
|
| Narrative Reviews | Jacobson 2011 [56] | Humans- Asymptomatic | STM- Structural integration | n/a | None of the hypotheses about the local effects of structural integration manipulation have been assessed | |
| Hennenhoefer et al. 2019 [52] | Humans- Not specified | Man- Region not specified | Joint position | n/a | Correcting the asymmetrical movement preferences of vertebrae (or even affecting the vertebrae at all) is unlikely to be a source of therapeutic benefit | |
| Hartnett 2021 [50] | Humans- Symptomatic | STM- Region not specified | Scar tissue breakdown, tissue perfusion | n/a | Proposed mechanisms include combination of increased tissue perfusion and scar tissue breakdown- minimal strong evidence supporting the proposed physiological effects of the therapy | |
| Potter et al. 2005 [71] | Humans- Not specified | Man- Spinal and Peripheral | Joint space on MRI | Immediate Post | ↑ Joint space at MCP pre vs post ↑ Joint space at spine (facet joints) pre vs post and vs control |
|
| Field 2016 [43] | Humans- Patients with burn scar | STM- Region not specified | Imaging | n/a | ↓ Scar tissue | |
| Evans 2002 [42] | Humans- Not specified | Man- Spinal | Imaging (MRI) | Immediate post | Transient relative movements of the manipulated vertebrae associated with cavitation Gapping of zygapophyseal joints ↑ Dimensions of the intervertebral foramen |
|
| Vicenzino 2007 [83] | Humans- Post-traumatic thumb injury | Mob (MWM)- MCP | Imaging (MRI) | n/a | MRI revealed 4 deg pronated positional fault of MCP joint before treatment, which was not present with MWM | |
| Pickar 2002 [70] | Humans- Asymptomatic and symptomatic | Man- Spinal | n/a | ↑ Joint space (MCP and Lumbar Facet) | ||
| Gay et al. 2005 [45] | Humans- Not specified | Man- Lumbar | CT- Disc Height, Degree of disc protrusion, Central and lateral canal space; Intradiscal pressure | Immediate post | ↑ Disc height ↓ Disc protrusion/abnormality → Percent of the canal occupied by the disc pre vs post-treatment ↑ Intradiscal pressure in all cases except rotation with distraction, which resulted in very little change or a decrease in pressure |
Definitions: ↑- Increase, ↓- Decrease, →- No change, STM- Soft tissue mobilizations, Man- Manipulation, Mob- Mobilizations, PA- Posterior to anterior, UE- Upper extremity, LBP- Low back pain, MCP- Metacarpophalangeal, MWM- Mobilization with movement, SMT- Spinal manipulative technique.
Table 11. Treatment mechanisms by domain and level of literature support.
| Domains and mechanisms | Supported by: | |||||
|---|---|---|---|---|---|---|
| Systematic Review(s) | Scoping Review(s) | Narrative Review(s) | ||||
| Moderate Quality | Low Quality | Critically low Quality | ||||
| Biomechanical | ↓ Scar tissue | [43,50] | ||||
| Joint movement during (lumbar) | [61] | [42] | ||||
| Gapping of zygapophyseal joints | [42] | |||||
| ↑ Neuroforaminal space | [89] | [71] | ||||
| ↑ Disc Height | [45] | |||||
| Joint position post (Metacarpophalangeal) | [70,71,83] | |||||
| Joint position post (spine) | [33] | [70] | ||||
| ↓ Disc protrusion | [45] | |||||
| ↑ Intradiscal pressure | [58] | [45] | ||||
| Changes in disc physiology+ | [65,89] | [58] | [45] | |||
| ↓ Myofibril damage | [62] | |||||
| No change in muscle volume | [62] | |||||
| ↑ Cross sectional area | [62] | |||||
| Change in viscoelastic properties of soft tissues+ | [89] | [58] | ||||
| Neurovascular | Sympathetic excitation | [60] | [34,61,77] | [73] | [54,71,76,78,83] | |
| Parasympathetic excitation | [34] | [73] | ||||
| ↑ Skin conductance | [30] | [60] | [36,51,67,69,77,90] | [33,71,86] | ||
| ↑ Respiratory rate | [60] | [69,77] | [33] | |||
| ↑ Blood pressure | [60] | [86] | ||||
| ↑ Heart rate | [60] | |||||
| ↓ Skin temperature | [30] | [60] | [36,67,90] | [71] | ||
| Changes in Heart rate variability+ | [57,75] | [68] | ||||
| Blood pressure changes+ | [57] | [44,51,77,80] | [43] | |||
| ↓ α -amylase | [51,69] | [88] | ||||
| ↑ Vascular endothelial growth factor -A | [62] | |||||
| Neurological | ↑ Pressure pain threshold (local) | [46,55,72] | [64] | [39,77,85] | [58] | [52,71,76,82] |
| ↑ Pressure pain threshold (remote) | [46,55] | [39,77,85] | [52] | |||
| Functional MRI (fMRI) Changes+ | [63] | |||||
| Electroencephalogram (EEG) Changes+ | [63] | [81] | [62,68] | [56] | ||
| ↓ Temporal summation | [31] | |||||
| ↑ Conditioned pain modulation | [31] | |||||
| Neurotransmitter & Neuropeptide | No change in Epinephrine | [30] | [35,74,75] | [69] | [49] | |
| No change in Norepinephrine | [30] | [35,74,75] | [69] | |||
| Changes in Substance P+ | [74] | [62] | [33,49] | |||
| Changes in Neurotensin+ | [74] | [49,84] | ||||
| Changes in β-endorphin+ | [40] | [33,54,71,84] | ||||
| Changes in Oxytocin (direction not specified)+ | [74] | [43,49,84] | ||||
| ↑ Phenethylamine | [84] | |||||
| ↑ Anandamide | [84] | |||||
| ↑ Orexin A | [84] | |||||
| ↑ Dopamine | [84] | |||||
| ↑ Serotonin | [84] | |||||
| ↑ Corticotropin releasing factor | [84] | |||||
| ↑ Na+, K+ -ATPase Activity and NGF content | [62] | |||||
| Neuroimmune | ↑ Neutrophils | [81] | ||||
| ↓ Proinflammatory cytokines | [81] | [62] | [38] | |||
| ↑ Immunoglobin A | [81] | |||||
| ↑ Natural killer cell concentration | [53] | [43,38] | ||||
| ↑ CD4 cell count | [53] | [62] | ||||
| ↓ Anti-inflammatory cytokines | [62] | [38] | ||||
| Changes in immune markers+ | [35,74] | [81] | [62] | [33,37,38,43,49] | ||
| ↑ White blood cells | [88] | |||||
| Neuro-endocrine | ↓ Cortisol | [74] | [32,40] | [43] | ||
| Changes in cortisol+ | [35,75] | [49] | ||||
| Changes in Testosterone/Cortisol ratio | [75] | |||||
| Neuromuscular | ↑ Maximal voluntary contraction | [41] | [71] | |||
| ↓ EMG activity in compensatory musculature | [77] | [52,56,71] | ||||
| ↓ Spontaneous neuronal activity | [62] | |||||
| ↓ Involuntary muscle activity | [58] | |||||
| ↑ Amplitude of muscle contraction | [56] | |||||
| ↓ Agonist–antagonist co-contraction | [56] | |||||
| ↑ Activation of stabilizing muscles during tasks | [56] | |||||
| Changes in paraspinal muscle activity+ | [70] | |||||
| Other | ↓ Creatine Kinase | [81] | ||||
| ↑ Mitochondrial biogenesis | [81] | |||||
| ↑ O2 saturation | [68] | |||||
| ↓ Central venous pressure | [68] | |||||
| ↓ Pulmonary vessel resistance | [68] | |||||
| ↓ CD11b/c and GFAP glycoproteins | [62] | |||||
| ↓ Excitability Satellitosis (glial cells) | [62] | |||||
| ↑ Transport subcutaneous nanoparticles to lymph node | [62] | |||||
| ↑ Liposome transport into bloodstream | [62] | |||||
| ↓ Gene Expression | [62] | |||||
| ↑ Intestinal function | [62] | |||||
| ↑ Serum glutamic | [56] | |||||
| ↑ Forced vital capacity | [33] | |||||
| ↑ Forced expiratory volume capacity | [33] | |||||
= Changes variable direction or not specified
Quality assessment and risk of bias
Thirty-nine reviews were appropriate for quality and risk of bias appraisal. The included reviews were primarily of critically low (n = 23) to low-quality (n = 12) with the exception of four reviews of moderate quality. Overall quality of the included reviews is presented in Table 12 with itemized scoring presented in S5 Appendix. Fourteen reviews were rated at high risk of bias and twenty-five rated as low risk of bias. Overall risk of bias of the included reviews is presented in Table 12 with itemized scoring presented in S6 Appendix.
Table 12. Risk of bias and quality of included systematic reviews.
| Author(s) | Risk of Bias (ROBIS) | Quality (AMSTAR-2) |
|---|---|---|
| Araujo et al. 2019 [30] | Low | Moderate |
| Arribas-Romano et al. 2020 [31] | Low | Low |
| Bernier Carney et al. 2020 [32] | Low | Critically Low |
| Borges et al 2018 [34] | High | Critically Low |
| Chow et al. 2021 [35] | Low | Low |
| Chu et al. 2014 [36] | Low | Critically Low |
| Coronado et al. 2013 [40] | Low | Critically Low |
| Coronado et al. 2012 [39] | Low | Critically Low |
| Corso et al. 2019 [41] | Low | Low |
| Galindez-Ibarbengoetxea et al. 2017 [44] | High | Critically Low |
| Gay et al. 2013 [46] | Low | Moderate |
| Gera et al. 2020 [47] | High | Low |
| Hegedus et al. 2011 [51] | Low | Critically Low |
| Hillier et al. 2010 [53] | Low | Low |
| Honoré et al. 2018 [55] | High | Moderate |
| Jones et al. 2013 [57] | High | Low |
| Jun et al. 2020 [58] | Low | Critically Low |
| Jung et al. 2023 [59] | Low | Low |
| Kingston et al. 2014 [60] | Low | Low |
| Lascurain-Aguirrebeña et al. 2016 [61] | High | Critically Low |
| Lima et al. 2020 [62] | High | Critically Low |
| Meyer et al.2019 [63] | Low | Low |
| Millan et al. 2012 [64] | Low | Low |
| Mitchell et al. 2017 [65] | Low | Critically Low |
| Navarro-Santana et al. 2020 [67] | Low | Critically Low |
| Nelson 2015 [68] | High | Critically Low |
| Picchiottino et al. 2019 [69] | Low | Critically Low |
| Riley et al. 2024 [72] | Low | Moderate |
| Rogan et al. 2023 [73] | High | Critically Low |
| Sampath et al. 2017 [74] | Low | Low |
| Sampath et al. 2024 [75] | Low | Low |
| Schmid et al. 2008 [77] | Low | Critically Low |
| Sousa et al. 2020 [79] | High | Critically Low |
| Sullivan et al. 2020 [80] | High | Critically Low |
| Tejero-Fernandez et al. 2015 [81] | High | Critically Low |
| Voogt et al. 2015 [85] | Low | Critically Low |
| Xiong et al. 2015 [87] | High | Critically Low |
| Young et al. 2024 [33] | Low | Critically Low |
| Zegarra-Parodi et al. 2015 [90] | High | Critically Low |
Biomechanical mechanisms
Fourteen reviews of critically low quality reported biomechanical treatment mechanisms associated with MT. (Table 2) Five reviews reported changes in joint position associated with MT techniques [42,61,70,83,89]. One of these reviews reported no correlation between joint changes and improvement in pain or impairment [83]. Two reviews questioned the concept of joint position changes with MT, most specifically at the cervical spine [52,61]. Five reviews supported physiological changes in soft tissue associated with MT (such as viscoelastic properties) [43,50,58,62,89]. Four reviews reported changes in disc characteristics following MT techniques (e.g., intradiscal pressure) [45,58,65,89]. All four reviews supported increased disc diffusion with two of these studies supporting translational association with improved clinical outcomes [45,65].
Neurovascular mechanisms
Thirty-two studies of critically low to moderate quality reported neurovascular mechanisms associated with MT. (Table 3) Twelve of the included reviews favored sympathoexcitation across outcome measures [30,34,36,54,60,61,71,73,76,77,83,86]. One review favored sympathoexcitation if the MT technique was noxious and sympathoinhibition if the technique was non-noxious [78]. A decrease in alpha-amylase levels, a proposed measure of Autonomic Nervous System (ANS) function, was reported across 3/3 reviews, indicating a sympathoinhibitory effect of MT [51,69,88]. Increased skin conductance was reported in 12 reviews following MT intervention [30,33,36,51,60,67,69,71,73,77,86,90]. No change in skin temperature was reported in 14 reviews post MT intervention [30,33,36,51,54,60,67,69,71,73,77,78,86,90]. One review, however, reported inverse responses related to both skin conductance and skin temperature in individuals with LBP [90] while other symptomatic populations did not demonstrate the same effect. Heart rate, heart rate variability and blood pressure demonstrated a change of variable direction without a clear rationale for variations.
Table 3. Neurovascular treatment mechanisms associated with MT.
| Author/Year | Subjects: | MT Technique | Measure | Time of Measure | Results/Conclusion | |
|---|---|---|---|---|---|---|
| Systematic Reviews | Corso et al. 2019 [41] | Humans- Asymptomatic | Man- Spinal | HR, HRV | Immediate post | → Resting HR or HRV → Exercising HR |
| Kingston et al. 2014 [60] | Humans- Asymptomatic and symptomatic (neck pain) | Mob- Spinal | ST, SC, RR, BP, HR | During to immediate post | Strong evidence for sympathoexcitation vs control and sham: ↑ SC ↑ RR ↑ BP ↑ HR ↓ ST |
|
| Hegedus et al. 2011 [51] | Humans- Asymptomatic and symptomatic (neck pain, lateral elbow pain) | Man- Spinal | α -amylase secretion (saliva), SC, ST | Immediate to 24 hrs post | ↑ SC in healthy subjects (lasting 10 minutes max and 5 minutes or less avg.) → ST in healthy subjects ↓ α -amylase (lasting 10 minutes) |
|
| Gera et al. 2020 [47] | Humans- Asymptomatic and symptomatic (LBP, Neck Pain, Thoracic spine pain, HTN) | Man- Spinal Mob- Spinal |
BP, HR, ECG, Pulse-ox | n/a | ↓ SBP (MD = 4:56, 95% CI = 9:20, 0.08; p = .05) ↓ DBP vs control and vs placebo group (MD = 1:96, 95% CI = 4:60, 0.69; p = 0:15) Changes in HR (direction not specified) (MD = 0:24, 95%CI = 3:59, 3.11; p = 0:89) |
|
| Zegarra-Parodi et al. 2015 [90] | Humans- Asymptomatic and symptomatic (Low Back Pain, Lateral epicondylalgia, Neck pain) | Man- Spinal Mob- Spinal, SI |
SC, ST, laser doppler flowmetry (LDF) | n/a |
Healthy populations: ↑ SC ↓ ST ↓ LDF among smokers and ↑ LDF among nonsmokers Symptomatic populations: ↑ SC in individuals with cervical and craniofacial pain ↓ SC in individuals with LBP ↓ ST in individuals with cervical pain or epicondylalgia ↑ ST in individuals with LBP ↓ LDF in individuals with epicondylalgia |
|
| Chow et al. 2021 [35] | Humans- Asymptomatic and symptomatic | Man- Spinal | HRV | n/a | → HRV | |
| Sullivan et al. 2020 [80] | Humans- Not specified | Man- Spinal | BP | Immediate | 13 studies did not report any significant changes in blood pressure and 14 studies showed significant changes in blood pressure after chiropractic intervention Cervical spine SMT more consistently demonstrate ↓ BP (Sympathetic inhibitory) |
|
| Galindez-Ibarbengoetxea et al. 2017 [44] | Humans- Asymptomatic and symptomatic (HTN) | Man- Cervical | BP, HR, ECG, Pulse-ox | Immediate to post 8-sessions | ↓ DBP → HR, SBP, ECG, Pulse-Ox |
|
| Araujo et al. 2019 [30] | Humans- Asymptomatic and symptomatic (craniofacial pain, neck pain) | Man- Spinal Mob- Spinal |
HR, RR, BP, SC, pupil | n/a |
PAIVM mobilization: ↑ SC and ↓ ST compared to no treatment in patients with neck pain (low quality evidence) ↑ SC and ↓ ST in patients with neck pain compared with placebo (low quality evidence) ↑ HR in healthy individuals (low quality evidence) ↑ SC compared to placebo in healthy individuals (low quality evidence) ↑ SC in patients with craniofacial pain (low quality evidence) ↑ HR vs placebo (low quality evidence) ↑ HR in patients with craniofacial pain (low quality evidence) → ST vs placebo or control in healthy individuals (very low quality evidence) → ST compared to placebo (low quality evidence) SNAG mobilizations: → SC in healthy individuals (low quality evidence) → ST vs placebo in healthy individuals (very low quality evidence) Manipulation: ↓ Edge light pupil cycle time in healthy participants (very low quality evidence) → Pupil diameter (low quality evidence) |
|
| Sousa et al. 2020 [79] | Humans- Asymptomatic and symptomatic (back pain, fibromyalgia) | Man- Thoracic and cervical STM- Hand, paravertebral |
Cardiac Autonomic control (heartrate variability) | Immediate to 20 weeks | 9/10 studies demonstrated improvement in cardiac autonomic control. The only study that did not observe improvement in cardiac autonomic control was performed on patients with fibromyalgia. | |
| Navarro-Santana et al. 2020 [67] | Humans- Asymptomatic and symptomatic (Elbow pain, neck pain, craniofacial pain) | Mob- Spinal | ST, SC | During to immediate post | ↑ SC (SMD 1.21, 95% CI 0.88 to 1.53, n = 269) vs control ↑ SC (SMD 0.73, 95% CI 0.51to 0.96, n = 293) vs sham ↓ ST (SMD 0.92, 95% CI − 1.47 to − 0.37, n = 128) vs control ↓ ST (SMD − 0.50, 95% CI − 0.82 to − 0.18, n = 134) vs sham |
|
| Borges et al. 2018 [34] | Humans- Asymptomatic and symptomatic (fibromyalgia) | Man- Spinal STM- LE’s, Lumbar, Craniofacial, shoulder, craniosacral |
HRV, ECG. BP, SC-Biopac System, Polar monitor | Immediately post to 1 year post | ↑ PaSNS and SNS activity | |
| Jones et al. 2013 [57] | Humans- Asymptomatic and symptomatic (MS, Dementia, Cardiac issues, COPD) | STM- Foot | BP, HR, HRV, Blood flow (doppler sonography) | Immediately post to 20 min post | BP: Variable response: 2 studies demonstrating ↓ SBP only, 1 study showing ↓ SBP and DBP, 4 studies demonstrating no significant effect HR: Variable response: 3 studies demonstrating ↓ HR, 3 studies demonstrating no significant effect HRV: Significant changes in HRV in 3 studies (direction not specified) Blood Flow: Significant changes in blood flow locally Significant change in resistive index of the renal artery when kidney reflex point of foot was massaged (p < 0.001). Significant resistive index changes in the superior mesenteric artery (p = 0.021) when the intestinal reflex point on the foot was stimulated |
|
| Xiong et al. 2015 [87] | Humans- Symptomatic (HTN) | STM- Region not specified | BP | Post treatment plan (4-16 weeks) | → SBP vs control (MD: 3.26 (10.02, 3.49); p = 0.34) → DBP (DBP; MD: 2.41 (8.75, 3.93); p = 0.46 ↓ SBP vs antihypertensive drugs (MD: 3.47 (5.39, 1.56); p = 0.0004 → DBP vs antihypertensive drugs (MD: 0.98 (2.28, 0.32); p = 0.14) |
|
| Lascurain-Aguirrebena et al. 2016 [61] | Humans- Symptomatic | Man- Spinal | Immediately post to 6 weeks post | ↑ Sympathetic Excitation | ||
| Schmid et al. 2008 [77] | Humans- Asymptomatic and symptomatic | Mob- Cervical | BP, SC, ST, HR, RR | Immediate post | ↑ Sympathetic Excitation: ↑ SC both upper limbs ↑ HR ↑ RR ↓ DBP (During mobilization) → ST |
|
| Chu et al. 2014 [36] | Humans- Not specified | Mob- Thoracic and cervical | SC, ST | Immediate post | ||
| Picchiottino et al. 2019 [69] | Humans- Asymptomatic and symptomatic | Man- Spinal Mob- Spinal, Elbow |
SC, ST, Skin Blood flow, HR, HRV, RR, Pupil Diameter, Salivary α-amylase activity | During, Immediate post -10 min post |
Mobilization: → ST ↑ SC compared to sham in 10/10 studies → Dermal blood flow in 1/2 studies, effects in opposite directions (both increase and decrease) in the other study → HR → BP in 2/2 studies → HRV in 1/1 study ↑ RR in 3/3 studies ↓ α-amylase activity vs sham in 1/1 study Spinal SNAG: → ST → SC Mobilization with movement: ↑ SC compared to sham in 1/1 study (elbow) ↓ or ↑ ST and skin blood flow vs sham in 1/1 study ↑ HR and ↑ BP vs sham in 1/1 study Spinal Manipulation: → HR in 3/3 studies → BP in 1 study → HRV in 7/7 studies → Pupil diameter in 1/1 study |
|
| Sampath et al. 2024 [75] | Humans- Asymptomatic and symptomatic (HTN, Neck pain, Achilles tendinopathy) | Man- Spinal | HR, HRV, Edge light pupil cycle time, Root mean square of successive difference (rMSSD), Pupil diameter | Immediate post -5 min post | ↓ in HR ↓ Low-frequency/high frequency power ratio Changes in low frequency power ↓ High frequency power ↓ Edge light pupil cycle time → Pupil diameter → HRV or BP vs sham ↑ rMSSD (HRV) vs control; →rMSSD (HRV) vs sham Changes in PR interval Statistically significant within-group Change in QRS duration |
|
| Scoping Reviews |
Lima et al. 2020 [62] | Animals- (Non-Cadaveric Cats, Mice, Rabbits, Rats); healthy and induced pain | STM- Region not specified | n/a | n/a | ↑ Nerve blood flow and ↓ Intraocular pressure ↓ BP ↓ HR ↑ Vascular endothelial growth factor (VEGF)-A |
| Nelson 2015 [68] | Humans- Symptomatic (HTN) | STM- Region not specified | HRV, HR, Blood flow, Viscosity | n/a | ↓ HRV ↓ HR in 45% of studies investigating HR * Effects do not persist beyond the MT session and are not considered clinically significant |
|
| Rogan et al. 2023 [73] | Humans- Asymptomatic and symptomatic (Fascial pain, acute LBP, neck pain, HTN, elbow pain) | Man- Spinal Mob- Spinal STM- Paraspinal |
HRV, SC, ST | Immediately post to 8 weeks post | 74% demonstrated effect of single intervention on ANS ↑ SNS activity demonstrate in 51% ↑ PaSNS activity demonstrated in 24% Physiological ANS response was independent of the treatment region |
|
| Narrative Reviews | Savva et al. 2021[76] | Humans- Symptomatic | Man- Thoracic | SC, ST | n/a | ↑ Sympathetic Excitation |
| Field 2016 [43] | Humans- Symptomatic (Cardiac issues) | STM- Region not specified | ↓ SBP and DBP | |||
| Vicenzino 2007 [83] | Humans- Symptomatic (epicondylalgia) | Mob (MWM)- Elbow | HR, BP | n/a | ↑ Sympathetic Excitation | |
| Yao et al. 2014 [88] | Humans- Symptomatic (Pneumonia) | Mob- Rib and thoracic | Changes is α-amylase | Immediate post | ↓ α-amylase | |
| Potter et al. 2005 [71] | Humans- Not specified | Man- Spinal and peripheral | SC, ST, Blood flow | Immediate Post | ↑ Sympathetic outflow ↑ SC ↓ ST ↓ Blood Flow |
|
| Holey et al. 2014 [54] | Humans- Not specified | STM- Region not specified | BP, Blood Flow, ST (foot) | Immediate to two weeks post | ↓ Peripheral blood flow, followed by an increase after two weeks ↑ DBP (immediate and moderate effect size) → SBP → HR → Skin Temp ↑ Sympathetic activity, with main effect was on diastolic BP rather than systolic. → Mean arterial BP ↑ ST locally at 15 min post treatment, maintained for at least 1 hour |
|
| Weerapong et al. 2005 [86] | Humans- Asymptomatic | STM- Local and whole body | HR, BP, SC, RR | n/a | ↑ vs → HR ↑ vs → BP ↑ vs → ST ↓ RR ↑ SC |
|
| Simmonds et al. 2012 [78] | Humans- Asymptomatic | Man- Thoracic Mob- Cervical, lumbar |
HRV, ST, SC | n/a | Different ANS response to noxious and non-noxious stimuli in the spine: Noxious ↑ SNS response (excitatory); non-noxious ↓ SNS response (inhibitory) | |
| Bolton et al. 2012 [33] | Humans- Asymptomatic | Man- Spinal Mob- Cervical |
HR, ST, BP, RR, SC, Edge light pupil cycle time (ELPCT) | n/a | HR: ↓ 2 studies; ↑ 1 study; → 4 studies ST: ↓ 1 study; ↑ 1 study; → 1 study DBP: ↓ 2 studies; ↑ 1 study; → 3 studies SBP: ↓ 2 studies; ↑ 1 study; → 3 studies ↑ RR 1 study ↑ SC mobilization vs control ↑ SC HVLA vs control and vs exercise ↓ ELPCT with HVLA |
|
| Alanez et al. 2024 [29] | Humans- Asymptomatic and symptomatic | Man- Spinal, peripheral Mob- Spinal, peripheral STM- region not specified |
BP, HR, HRV, SC, ST, Pupil Diameter | n/a | → Pupil diameter with thoracic manipulation ↓ Edge Light Pupil Cycle Time with cervical manipulation Variable response on BP (→or ↓) with all included types of MT Variable response on HR with all included types of MT Variable response on LF/HF ratio with all included types of MT |
Definitions: ↑ - Increase, ↓ - Decrease, →- No change, STM- Soft tissue mobilizations, Man- Manipulation, Mob- Mobilizations, PA- Posterior to anterior, UE- Upper extremity, LBP- Low back pain, MWM- Mobilization with movement, SMT- Spinal manipulative technique, MT- Manual Therapy, ANS- Autonomic nervous system, HR- Heartrate, HRV- Heart rate variability, SC- Skin conductance, ST- Skin temperature, RR- Respiratory rate, BP- Blood pressure, SBP- Systolic blood pressure, DBP- Diastolic blood pressure, SNS- Sympathetic nervous system, PaSNS- Parasympathetic nervous system.
Neurological mechanisms
Twenty-three reviews of critically low to moderate quality reported neurological treatment mechanisms associated with MT. Twenty reviews investigated changes in pain threshold following MT application (Table 4). Increases in local pressure pain threshold (PPT) versus control and sham were demonstrated in 12 reviews [39,46,55,58,61,62,64,71,76,77,82,85]. Two reported no difference in effect between mobilization and manipulation [40,55],one reported larger PPT increase in the manipulation group [82] and one reported mixed results [72]. Two reviews reported no difference in PPT between MT and active PT management [46,55]. Several reviews reported the remote effect of MT on PPT with general support for an increase in PPT however not consistent across reviews. Four reviews reported no effect of MT on thermal pain threshold (TPT) [64,77,83]. Seven reviews reported other neurological mechanisms (Table 5), including changes in EEG activity [56,63,68,81], nerve characteristics [62], and cerebral blood flow [62,63]. Improved conditioned pain modulation (CPM) and reduced temporal summation (TS) were supported by 1 review [31].
Table 4. Neurological (pain threshold) treatment mechanisms associated with MT.
| Author/Year | Subjects: | MT Technique | Measure | Time of Measure | Results/Conclusion | |
|---|---|---|---|---|---|---|
| Systematic Reviews | Schmid et al. 2008 [77] | Humans- Asymptomatic and symptomatic | Mob- Cervical | Changes in PPT, TPT | Immediate post | ↑ PPT locally and distally → TPT |
| Voogt et al. 2015 [85] | Humans- Symptomatic (Musculoskeletal pain) | Man- Cervical Mob- Ankle, knee, elbow, cervical, shoulder, wrist |
Changes in PPT (local) Changes in PPT (Distal) |
n/a | ↑ PPT In 11/14 studies (77%) * 3/11 studies the percentage of change in PPT was less than 15% ↑ PPT ipsilateral side (epicondyle) (44.2%); contralateral side (17.7%) ↓ PPT (27.3%) on the painful side (knee) and (15.3%) on remote body part |
|
| Honoré et al. 2018 [55] | Humans- Not specified | Man- Spinal | Changes in PPT (Regional and Remote) | Immediate to 5 min post | ↑ PPT vs sham in 5/8 studies ↑ PPT vs control in 2/3 studies → PPT vs HVLAT at other regions in 2/2 studies → PPT vs mobilization in 3/3 studies → PPT vs ‘other PT’ in 2/3 studies |
|
| Coronado et al. 2013 [40] | Humans- Symptomatic (Spinal pain) | Man- Spinal | Changes in PPT | Immediate to 7 days post | → PPT vs mobilization in 1/1 study | |
| Gay et al. 2013 [46] | Humans- Asymptomatic and symptomatic (Neck Pain, Scapulocostal syndrome, low back pain, breast CA survivors, Chronic tension headaches | STM- Region not specified | Changes in PPT (local and remote), Electrical pain threshold | Immediate | ↑ PPT vs all other interventions (Hedges g = 0.254 [95% CI 0.105–0.403], P < 0.05) → PPT vs active treatment (Hedges g = 0.036 [95% CI -0.289–0.362], P = 0.83). ↑ PPT vs sham treatment (Hedges g = 0.268 [95% CI 0.078–0.457], P < 0.05) (I2 = 0.0%, P = 0.55) ↑ PPT vs no-treatment control (Hedges g = 0.471 [95% CI 0.113–0.830], P < 0.05) |
|
| Jung et al. 2023 [59] | Humans- Asymptomatic and symptomatic | Man- Spinal Mob- Spinal |
Changes in PPT (local) | Immediately to 15 minutes post | → PPT (local) in patients with chronic pain: SMD = 0.25 (95 CI = -0.02 to 0.51)- low certainty evidence → PPT (local) in health controls: SMD = 0.2595 CI = -0.02 to 0.52- moderate certainty evidence |
|
| Changes in PPT (remote) | Immediately to 15 minutes post | → PPT remote (segmental): SMD = 0.14 (95%CI = -0.09 to 0.36) in patients with chronic pain - low certainty evidence → PPT remote (non-segmental) SMD = 0.19 (95%CI = -0.05 to 0.44) in patients with chronic pain - low certainty evidence → PPT remote (segmental): SMD = 0.19 (95% CI = -0.2 to 0.58) in healthy controls- low certainty evidence ↓ PPT remote (non-segmental): SMD = 0.26 (95%CI = 0.01 to 0.51) in healthy controls- low certainty evidence |
||||
| Coronado et al. 2012 [39] | Humans- Asymptomatic and symptomatic | Man- Spinal | Changes in PPT (Cervical, Elbow, Head, Lumbar, Trap, Web space | Immediate and delayed | ↑ PPT (remote and local) vs other interventions (Hedges g = 0.315 [95% CI = 0.078; 0.552], p = 0.009) ↑ PPT (local site) (Hedges g = 0.387 [95% CI = -0.070; 0.844], p = 0.097) ↑ PPT (remote site) (Hedges g = 0.287 [95% CI = 0.073; 0.500], p = 0.008) |
|
| Millan et al. 2012 [64] | Humans- Symptomatic (clinically induced pain) | Man- Spinal | Changes in PPT, TPT | Immediate to 7 days post | ↑ PPT (19/27 studies) (4.8% to 44.2%) → TPT (6/9 studies) |
|
| Lascurain-Aguirrebena et al. 2016 [61] | Humans- Symptomatic | Mob- Spinal | During, Immediately post to 6 weeks post | ↑ PPT local and distal to site of mobilization in 5/7 studies All studies measuring thermal pain threshold reported no significant changes. |
||
| Riley et al. 2024 [72] | Humans- Symptomatic (chronic LBP, chronic neck pain) | Man- Spinal | Changes in PPT | Immediate post to 4 weeks post | ↑ PPT regionally vs other MT procedure in 1/3 studies → PPT regionally vs sham or other MT procedure in 2/3 studies |
|
| Scoping Reviews | Jun et al. 2020 [58] | Humans- Asymptomatic and symptomatic; Animals | Man- Lumbar Mob- Lumbar |
Changes in PPT (local) | n/a | ↑ PPT |
| Lima et al. 2020 [62] | Animals- (Non-Cadaveric Cats, Mice, Rabbits, Rats); healthy and induced pain | Man- Spinal | Changes in PPT | n/a | ↑ Mechanical threshold in nociceptive specific thalamic neurons → Mechanical threshold response of nociceptive specific neurons |
|
| Narrative Reviews |
Vicenzino 2007 [83] | Humans- Symptomatic (epicondylalgia, ankle sprain) | Mob (MWM)- Ankle and elbow | Changes in PPT | Immediately post to post 6-sessions | → PPT and TPT following MWM at ankle in 1 study ↓ PPT vs control for MWM at elbow (10-15%) across 3/4 studies * Magnitude of improvement in PPT was not reduced with repeated applications of over 6 successive sessions. |
| Hennenhoefer et al. 2019 [52] | Humans- Not specified | Man- Region not specified | Changes in PPT, TPT | n/a | ↑ PPT local and remote → TPT |
|
| Potter et al. 2005 [71] | Humans- Not specified | Man- Spinal and peripheral | Changes in PPT | Immediate post | ↑ PPT | |
| Savva et al. 2021 [76] | Humans- Symptomatic | Man- Spinal, elbow, wrist | Changes in PPT, TPT | n/a | ↑ PPT | |
| Vernon 2000 [82] | Humans- Asymptomatic and symptomatic (LBP) | Man- lumbar | Changes in PPT, Electrical Pain Threshold | Immediate - 2 hrs post | ↑ PPT thrust manipulation> mobilizations |
Definitions: ↑ - Increase, ↓ - Decrease, → - No change, STM- Soft tissue mobilizations, Man- Manipulation, Mob- Mobilizations, PA- Posterior to anterior, UE- Upper extremity, LBP- Low back pain, MWM- Mobilization with movement, MT- Manual Therapy, HVLAT- High velocity low amplitude thrust, PT- Physical therapy, PPT- Pressure Pain Threshold, TPT- Thermal pain threshold.
Table 5. Neurological (other) treatment mechanisms associated with MT.
| Author/Year | Subjects: | MT Technique | Measure | Time of Measure | Results/Conclusion | |
|---|---|---|---|---|---|---|
| Systematic Reviews Sy Sy |
Tejero-Fernandez et al. 2015 [81] | Humans- Asymptomatic | STM- Region not specified | EEG | n/a | ↓ Power density in β-1 in central and frontal leads |
| Meyer et al. 2019 [63] | Humans- Asymptomatic and symptomatic (Spinal Pain) | Man- Spinal Mob- Spinal |
Blood Oxygenation level (fMRI); EEG- Somatosensory evoked potential (SEP), Cerebellar inhibition, Mental rotation reaction-time task, Motor Evoked Potentials (MEP) Cortical Silent Period |
Immediately post - 1 hr post | ↑ Activation in the insular and sensorimotor cortices ↑ Activation anterior and posterior cingulate, supplementary motor area, and precentral gyrus post-SMT vs control ↓ SEP- Statistically significant pre to post (p = .02) but not vs control (p = .4) ↓ Cerebral Inhibition- post SMT vs control ↑ Motor Evoked Potential- immediately post (10 to 120 s. post-SMT vs control Regional Metabolic Rate: ↑ Broca’s area, anterior cingulate cortex, somatosensory association cortex, Wernicke’s area, visual association cortex, cerebellar vermis, and visual cortex ↓ Inferior parietal lobule, frontal pole, inferior frontal gyrus, pars triangularis, premotor area/supplementary motor area, primary motor cortex, frontal eye field, dorsolateral prefrontal cortex, angular gyrus, fusiform gyrus, inferior temporal gyrus, and temporal pole. |
|
| Arribas-Romano et al. 2020 [31] | Humans- Symptomatic (Chronic pain) | Man- Lumbar Mob- Knee |
TS, CPM | Immediately post | ↑ CPM ↓ TS: The only therapeutic modality that resulted in significant change was MT for TS MT on Sensory Testing (all): Z = 1.95 (p = .05) |
|
| Scoping Reviews SC SC |
Nelson 2015 [68] | Humans- Symptomatic | STM- Region not specified | EEG | ↓ EEG asymmetry; Shift toward left-frontal EEG activation ↑ Blood flow to the amygdala and hypothalamus ↓ Sympathetic outflow after massage is associated with increases in structures within the brain involved in ANS regulation |
|
| Lima et al. 2020 [62] | Animals- (Non-Cadaveric Cats, Mice, Rabbits, Rats); healthy and induced pain | Man- spinal Mob- Spinal, knee, ankle |
fMRI (Spinal Cord, Cortical), Nerve Morphological Analysis | n/a |
Mobilization: ↑ (9%) vs → nerve elongation ↑ Myelin sheath thickness Capsaicin injection activate areas within spinal cord dorsal horn; No statistically significant differences in activation after knee joint mobilization Capsaicin injection activate brain pain processing areas; No statistically significant differences in activation after knee joint mobilization Manipulation: ↓ Excitability (DRG neurons) STM: Non-specific changes in mean firing rate & number of short inter-spike intervals of supraoptic neuron activity → Axonal (facial nerve) branching ↓ Motor endplate poly-innervation (across 4 articles) ↑ Brachial Plexus Nerve Conduction Velocity and Nerve action potential ↑ Dendritic arborization in pyramidal cells ↑ Dendritic branching and spine density in different brain areas ↓ Neuronal activity and conduction velocity |
|
| Jun et al. 2020 [58] | Humans- Asymptomatic and Symptomatic (low back pain); Animals | Man- Lumbar Mob- Lumbar |
n/a | n/a | Four studies hypothesized changes in the CNS or sensory and motor reflex pathways to be possible mechanisms for the changes in spinal stiffness and the decrease in pain following | |
| Narrative Review Review |
Jacobson 2011 [56] | Humans- Asymptomatic | STM- Region not specified | EEG | Immediate post |
Average evoked response (AER): ↑ Amplitude all levels of stimulus intensity ↓ Variability at maximum and minimum amplitudes of stimuli ↑ Capacity for efficient organization of sensory input ↑ Sensitivity to stimulation and selective inhibition as stimulus intensity increases |
Definitions: ↑ - Increase, ↓ - Decrease, → - No change, STM- Soft tissue mobilizations, Man- Manipulation, Mob- Mobilizations, PA- Posterior to anterior, UE- Upper extremity, LBP- Low back pain, fMRI- Functional MRI, MWM- Mobilization with movement, SMT- Spinal manipulative technique, MT- Manual Therapy, DRG- Dorsal root ganglion, CNS- Central Nervous System, EMG- Electromyogram, ECG- Electrocardiogram, EEG- Electroencephalogram, TS- Temporal summation, MVC- Mav voluntary contraction, CPM- Conditioned pain modulation.
Neurotransmitter/neuropeptide mechanisms
Sixteen reviews of critically low to moderate quality reported neurotransmitter and/or neuropeptide treatment mechanisms associated with MT. (Table 6) Increase in oxytocin levels post MT application was reported in 4 reviews [43,49,74,84] with the exception of 1 review reporting increased levels with STM and decreased levels with manipulation [84]. Substance P was included in 5 reviews with 3 reviews on spinal manipulation favoring an increase, 1 review on mobilizations favoring a decrease, and 1 review on spinal manipulation favoring no change [33,35,49,62,74]. Increased β-endorphin was reported in 5 reviews following MT application versus control, however less consistent results and less significant changes when compared with sham intervention [33,40,54,71,84]. Little to no change in Norepinephrine (NE) and Epinephrine (Epi) levels with MT were reported in 7 reviews based on low quality evidence [30,35,49,69,74,75,84].
Table 6. Neurotransmitter and neuropeptide treatment mechanisms associated with MT.
| Author/Year | Subjects: | MT Technique | Measure | Time of Measure | Results/Conclusion | |
|---|---|---|---|---|---|---|
| Systematic Reviews | Chow et al. 2021 [35] | Humans- Not specified | Man- Spinal | Plasma- Epi, NE, Substance P | 5 min to 6 hrs post | → Substance P → NE → Epi |
| Sampath et al. 2017 [74] | Humans- Asymptomatic and Symptomatic | Man- Thoracic and cervical | Substance P Neurotensin Oxytocin |
Immediately post | SMT vs control: immediately post ↑ Substance P (SMD-0.48, 95% CI - 0.87 to 0.10) - Low quality evidence ↑ Oxytocin (SMD -2.61,95%CI-3.5to-1.72) - Low quality evidence ↑ Neurotensin (SMD -1.8,95% CI-2.56to-1.04) - Low quality evidence → Substance P at short term follow-up (SMD -0.40, 95% CI e 1.2 to 0.4)- Very low quality evidence → Epi vs control (SMD 0.1,95%CI- 0.56to0.75) - Low quality evidence → NE vs control (SMD -0.06,95%CI-0.71to0.6)- Low quality evidence |
|
| Araujo et al. 2019 [30] | Humans- Asymptomatic and Symptomatic (craniofacial pain, neck pain) | Man- Spinal | Plasma- Epi and NE | n/a | → NE (low quality evidence) → Epi (low quality evidence) |
|
| Picchiottino et al. 2019 [69] | Humans- Asymptomatic and Symptomatic | Man- Spinal Mob- Spinal, elbow |
Epi and NE and NE- plasma | n/a | → Epi → NE |
|
| Coronado et al. 2013 [40] | Humans- Symptomatic (spinal pain) | Man- Spinal | β -endorphin | n/a | Variable results on β -endorphin; ↓ x1 study; ↑ x1 study | |
| Sampath et al. 2024 [75] | Humans- Asymptomatic and symptomatic (HTN, Neck pain, Achilles tendinopathy) | Man- Spinal | Plasma- Epi and NE | Immediate to 15 min post | → NE → Epi |
|
| Scoping Review | Lima et al. 2020 [62] | Animals- (Non-Cadaveric Cats, Mice, Rabbits, Rats); healthy and induced pain | Man- Spinal Mob- Spinal, knee, ankle STM- Region not specified |
n/a | n/a |
Mobilization: ↓ Substance P & TRPV1 ↑ µ -opioid receptor Adenosine A1/α-2-adrenergic receptors & Serotonin mediate pain ↓ 5-HT1/2 and α2-adrenergic receptors respectively prevented and reversed joint mobilization antihyperalgesia; No statistically significant differences elicited by GABAA and opioid blockade STM: ↑ Na + , K + -ATPase Activity and NGF content |
| Narrative Reviews | Bolton et al. 2012 [33] | Humans- Asymptomatic | Man- Thoracic and cervical | Plasma β -endorphin | Immediate to 2 hrs post | ↑ β -endorphin vs controls → β -endorphin vs sham |
| Haavik et al. 2021 [49] | Humans- Not specified | Man- Spinal | Epi | n/a | → Epi (low quality evidence from one study) | |
| Holey et al. 2014 [54] | Humans- Not specified | STM- Region not specified | Plasma β -endorphin | n/a | ↑ β-endorphin | |
| Potter et al. 2005 [71] | Humans- Not specified | Man- spinal | ↑ β-endorphin (x1); → β -endorphin (x1) | |||
| Vigotsky et al. 2015 [84] | Humans- Asymptomatic and Symptomatic (Myalgia, low back pain, stage I and II Breast Cancer, Anorexia, Fibromyalgia, Autism); Animals (Rats, mice) | Man- Spinal and Peripheral Mob- Ankle, elbow STM- Region not specified |
n/a | n/a |
Manipulation: Inconsistent changes in β -endorphin ↑ x3; → x2 ↑ NE ↑ PEA ↑ AEA ↓ Neurotensin ↑ Orexin A ↓ Oxytocin Mobilization: ↓ Glial cell activation Soft Tissue Mobilization: Inconsistent changes in β -endorphin ↑ x1 study; → x2 studies; ↓ x1 study → β -lipotropins ↑ Oxytocin ↑ Dopamine ↑ Serotonin ↓ Arginine vasopressin ↑ Corticotropin releasing factor |
|
| Field 2016 [43] | Humans- Not specified | STM- Region not specified | n/a | ↑ Oxytocin | ||
| Haavik et al. 2021 [49] | Humans- Not specified | Man- spinal | Neurotensin, Oxytocin, and Substance P | ↑ Neurotensin vs control- Moderate quality evidence from 1 study ↑ Oxytocin vs control- Moderate quality evidence from 1 study * No difference between neurotensin and oxytocin changes vs sham HVLAT ↑ Substance P (low-quality evidence) |
||
| Bolton et al. 2012 [33] | Humans- Asymptomatic | Man- Thoracic | Plasma Substance P | Immediate to 2 hrs post | ↑ Substance P versus control |
Definitions: ↑ - Increase, ↓ - Decrease, → - No change, STM- Soft tissue mobilizations, Man- Manipulation, Mob- Mobilizations, PA- Posterior to anterior, UE- Upper extremity, LBP- Low back pain, MWM- Mobilization with movement, SMT- Spinal manipulative technique, MT- Manual Therapy, NE- Norepinephrine, EPI- Epinephrine, PEA- Phenethylamine, AEA- Anandamide, HVLAT- High velocity low amplitude thrust.
Neuroimmune mechanisms
Twelve reviews of critically low to low quality reported neuroimmune treatment mechanisms associated with MT. (Table 7) General support was demonstrated for changes in cytokine levels with MT application. Trends towards a decrease in pro-inflammatory cytokines (IL-1β, TNF-α) and an increase in anti-inflammatory cytokines (IL-2, IL-10) were seen across reviews with some variability. This was supported across symptomatic and asymptomatic populations and was more significant with MT application than control and sham interventions. Other immune markers including leukocytes [62,81,88], natural killer cells [43,53], Immunoglobin (Ig)-A [81], Ig-G [33,35], and Ig-M [33,35] also demonstrated modulation with MT intervention.
Table 7. Neuroimmune treatment mechanisms associated with MT.
| Author/Year | Subjects: | MT Technique | Measure | Time of Measure | Results/Conclusion | |
|---|---|---|---|---|---|---|
| Systematic Reviews | Bernier Carney et al. 2020 [32] | Humans- Symptomatic (LBP > 3 months) | STM- Gua sha | Salivary TNF-α and Heme-oxygenase (HO-1) | First session and 8th session- immediate post, 30 min post | → TNF-α → HO-1 |
| Chow et al. 2021 [35] | Humans- Not specified | Man- Spinal | Plasma- inflammatory markers | 5 min - 12 week post | Immediate changes in the levels of selected immunological biomarkers: polymorphonuclear neutrophils, monocytes, TNF-α, IL-1β, IL-2, Ig-G, Ig-M) in asymptomatic participants vs sham manipulation or vs control groups (direction not specified) With the exception of 1 study, SMT was not associated with changes in lymphocyte levels among patients with low back pain or participants who were asymptomatic vs sham or vs control |
|
| Sampath et al. 2017 [74] | Humans- Asymptomatic and symptomatic | Man- Thoracic and cervical | TNF-α, IL-1 PBMC, Ig-G Ig-M |
Moderate quality evidence that spinal manipulation is better than control in influencing IL concentration (direction not specified) | ||
| Tejero-Fernandez et al. 2015 [81] | Humans- Asymptomatic | STM- Region not specified | Plasma (neutrophil), Saliva (Ig-A) biopsy (cytokine profile) | n/a | ↑ Neutrophils ↓ Proinflammatory cytokine production ↑ Ig-A |
|
| Hillier et al. 2010 [53] | Humans- Symptomatic (HIV) | STM- Region not specified | Plasma CD4 cell count (cells/mm3) and viral load | n/a | ↑ Natural killer cell concentration (p < 0.01) Changes in Natural Killer Cells: Z = 1.61 (p = .11); Std mean Diff.52 favoring MT ↑ CD4 cell count (p < 0.05) Changes in CD4 vs Control: Z = .96 (p = .34); Std Mean Diff:.23 favoring the control |
|
| Scoping Review | Lima et al. 2020 [62] | Animals- (Non-Cadaveric Cats, Mice, Rabbits, Rats); healthy and induced pain | Man- Spinal Mob- Spinal, knee, ankle STM- Region not specified |
n/a | n/a |
Mobilization: ↓ IL-1β & TNF-α in the nerve trunk & branches between treated/non-treated sides ↑ IL-10 → Leukocyte expression Manipulation: ↓ Satellitosis, c-Fos & PKCγ, IL-1β in DRG ↑ IL-10 in spinal cord STM: ↓ IL-1β ↓ Number and severity of adhesion in preventive group (i.e., massage right after surgery) ↓ Leukocyte infiltration ↓ Intraperitoneal protein and leukocyte levels → Inflammatory cell infiltration ↑ Number of thymocytes, CD4 + CD8 + , CD4 + and CD8 + ↓ TNF-α |
| Narrative Reviews |
Colombi et al. 2019 [37] | Humans- Asymptomatic | Man- Thoracic and cervical | Venipuncture | n/a | Inconsistent changes in TNF- α and IL-2 |
| Haavik et al. 2021 [49] | Humans- Not specified | Man- Spinal | TNF-α and IL | n/a | Significant influence on levels of immune mediators in 18/23 studies (direction not specified) Moderate quality evidence that SMT influences IL levels (direction not specified) |
|
| Field 2016 [43] | Humans- Symptomatic (Breast Cancer) | STM- Region not specified | n/a | n/a | ↑ Natural killer cell concentration ↑ Immune Function |
|
| Yao et al. 2014 [88] | Animals- Dogs and Rats | Mob- Rib, thoracic | White blood cell count | n/a | ↑ Leukocyte mobilization and flow in dogs and rats, primarily from gut-associated lymphoid tissue ↑ White blood cells (plasma) |
|
| Bolton et al. 2012 [33] | Humans- Asymptomatic | Man- Thoracic | TNF-α, IL1-β, Ig-G and Ig-M | Immediate to 2 hrs post | Variable changes in TNF- α (One study demonstrating ↓ and one demonstrating ↑ ) ↓ I-1β ↑ IL-2 induced Ig-G and Ig-M production |
|
| Cook et al. 2024 [38] | Humans and animals- not specified | Man- Spinal and Peripheral Mob- Spinal and Peripheral STM- Region not specified |
Pro-inflammatory and anti-inflammatory cytokine profiles | n/a | ↑ IL-2 ↓ IL-4 → IL-8, IL-12, IL-17, IL-23, GM-CSF Changes (variable direction) in IL-13, IL-10, TNF-α, IFN-y, IL-1 β, IL-6 Changes in TNF-α in the short term; vary depending on the technique selected and the comorbidities of the patient |
Definitions: ↑ - Increase, ↓ - Decrease, → - No change, STM- Soft tissue mobilizations, Man- Manipulation, Mob- Mobilizations, PA- Posterior to anterior, UE- Upper extremity, LBP- Low back pain, MCP- Metacarpophalangeal, MWM- Mobilization with movement, SMT- Spinal manipulative technique, MT- Manual Therapy, TNF- Tumor Necrosis Factor, IL- Interleukin, PBMC- Peripheral blood mononuclear cells, Ig- Immunoglobin, DRG- Dorsal root ganglion, PKC- Protein kinase C.
Neuroendocrine mechanisms
Twelve reviews of critically low to low quality reported neuroendocrine treatment mechanisms associated with MT. (Table 8) All reviews investigated changes in cortisol levels with general support for modulation of variable direction and effect sizes. Little to no difference from sham and control was reported in 5 reviews [32,33,66,68,81], larger response in MT groups reported in two reviews [35,74], and longer carryover of effects with MT vs control was reported in one review [32].
Table 8. Neuroendocrine treatment mechanisms associated with MT.
| Author/Year | Subjects: | MT Technique | Measure | Time of Measure | Results/Conclusion | |
|---|---|---|---|---|---|---|
| Systematic Reviews | Bernier Carney et al. 2020 [32] | Humans- Parkinson’s Disease | STM- Region not specified | Salivary cortisol (concentration and total secretion) | First session and 8th session- immediate post, 30 min post |
First session: Immediately post: ↓ Cortisol MT group (p = .002) 30 min post: ↓ Cortisol both MT (p = .0003) and control (p = .016) Eighth session: Immediately post: ↓ Cortisol MT (p = .003) and control (p = .028) 30 min post: ↓ Cortisol MT (p = .0006) and control (p = .027) Total cortisol secretion: ↓ immediately after the eighth intervention in the MT (p = .003) and control (p = .035) groups; remained ↓ in MT group (p = .004) 30-min post intervention |
| Chow et al. 2021 [35] | Humans- Asymptomatic and symptomatic | Man- Spinal | Plasma- Testosterone, T/C Ratio, O2Hb, salivary cortisol |
5min - 6 hrs post | → Testosterone → T/C ratio → Oxyhemoglobin Changes in the level of salivary cortisol in the immediate term (direction not specified) among asymptomatic participants compared with sham SMT |
|
| Sampath et al. 2017 [74] | Humans- Asymptomatic and symptomatic | Man- Thoracic and Cervical | Plasma cortisol | 5min - 2hrs post | SMT> control (SMD -0.46, 95% CI - 0.93 to 0) in reducing cortisol levels immediately after intervention. (low quality evidence) | |
| Coronado et al. 2013 [40] | Humans- Symptomatic (Spinal Pain) | Man- Spinal | Cortisol | n/a | ↓ Cortisol in 1/1 study | |
| Tejero-Fernandez et al. 2015 [81] | Humans- Asymptomatic | Man- Region not specified | Salivary and plasma cortisol | n/a | ↑ Cortisol massage and control group without significant difference | |
| Sampath et al. 2024 [75] | Humans- Asymptomatic and symptomatic (HTN, Neck pain, Achilles tendinopathy) | Man- Spinal | Plasma Cortisol, Testosterone/Cortisol ratio | Immediate to 6 hours post | Significant between-group difference of salivary cortisol 5 min post (0.35, p < 0.01) Significant between-group difference T/C ratio 6 hr post (-0.09, p < 0.01) in the intervention group |
|
| Narrative Reviews | Field 2016 [43] | Humans- Symptomatic (Cardiac issues) | STM- Region not specified | n/a | n/a | ↓ Cortisol |
| Colombi et al. 2019 [37] | Humans- Asymptomatic and symptomatic (neck pain) | Man- Thoracic and Cervical | Saliva (n = 5); Venipuncture (n = 4) | Immediate to 2 hrs post | → Cortisol in symptomatic patients (3/3 studies) | |
| Haavik et al. 2021 [49] | Humans- Not specified | Man- Spinal | Cortisol | ↑ Cortisol | ||
| Moyer et al. 2011 [66] | Humans- Asymptomatic and symptomatic | STM- Full Body, Neck and Shoulders, Feet, Back, Upper body | Blood, urinary, and salivary cortisol | Immediate to 6 weeks post | → Cortisol: Single session (first)- Massage versus control (d = 0.15, 95% CI = 0.04, 0.34) → Cortisol: Single session (last)- Massage versus control (d = 0.15, 95% CI = 0.08, 0.37) → Cortisol: Multiple doses- Massage versus control (d = 0.12, 95% CI = 0.05, 0.28) Within Group: ↓ Cortisol range 10.8% (single-dose reduction at first treatment) to 35.0% |
|
| Nelson 2015 [68] | Humans- Symptomatic | STM- Region not specified | Cortisol | n/a | → Cortisol (effect is very small or not statistically distinguishable from zero) | |
| Bolton et al. 2012 [33] | Humans- Asymptomatic | Man- Thoracic and cervical | Plasma Cortisol | Immediate to 2 hrs post | → Cortisol vs control or sham |
Definitions: ↑ - Increase, ↓ - Decrease, → - No change, STM- Soft tissue mobilizations, Man- Manipulation, Mob- Mobilizations, SMT- Spinal manipulative technique, MT- Manual Therapy.
Neuromuscular mechanisms
Ten reviews of critically low to low quality reported neuromuscular mechanisms associated with MT. (Table 9) Lima et al. assessed muscle activity during mobilization and manipulation and reported changes in muscle spindle afferent discharge, which demonstrated variability based on targeted segment and thrust velocity [62]. Post treatment responses across included reviews support increased maximum voluntary contraction [41,56,71], reduced EMG activity [62,70,71,77], and reduced muscle interference during contraction [52,56,58,70].
Table 9. Neuromuscular treatment mechanisms associated with MT.
| Author/Year | Subjects: | MT Technique | Measure | Time of Measure | Results/Conclusion | |
|---|---|---|---|---|---|---|
| Systematic Reviews | Corso et al. 2019 [41] | Humans- Asymptomatic | Man- Spinal | MVC per surface EMG, Transverse Abdominus (TA) Ultrasound | Immediate post to 60 min post | ↑ MVC vs control with PROM immediately post in plantar flexors and quad; 30 minute post in plantar flexors only; no difference at 60 min post in either group → Quad MVC after L3–4 SMT vs sham manipulation → TA thickness measured with multiple ultrasound readings at rest or during contraction vs sham |
| Schmid et al. 2008 [77] | Humans- Asymptomatic and Symptomatic | Mob- Cervical | EMG | Immediate post | ↓EMG activity of the superficial neck flexor in symptomatic patients → EMG activity in healthy controls |
|
| Scoping Reviews | Lima et al. 2020 [62] | Animals- (Non-Cadaveric Cats, Mice, Rabbits, Rats); healthy and induced pain | Man- Spinal Mob- Spinal, knee, ankle |
n/a | n/a |
Mobilization: → Muscle spindle discharge upon chemosensitization of small diameter afferents Manipulation: Trunk GTO & muscle spindle afferent discharge to impulse phase of SM ↑ Afferent discharge at 100ms ↑ Primary & secondary muscle spindle discharge over time profile ↑ EMG response with Variations in force ↑ EMG as force ↑ ; No statistically significant differences in EMG for pulse duration ↑ Compound action potentials for shorter pulse durations ↓ (25-30%) positive EMG response in the degenerative group; no statistically significant differences in spondylosis group. ↑ Resting muscle spindle discharge at 2mm either increased or decreased at 3mm ↑ Muscle spindle discharge during manipulation: ↑ position discharge & ↓ resting discharge upon longer & higher magnitude preload ↑ Spindle discharge at all contact sites with higher responsiveness at the targeted vertebra ↑ Spindle discharge with Lower thrust durations (<150ms) ↓ Spindle discharge &> 10s to return to baseline activity ↓ Spontaneous neuronal activity; attenuation of inhibitory evoked response on the contralateral hind-paw |
| Jun et al. 2020 [58] | Humans- Asymptomatic and Symptomatic (low back pain); Animals | Man- Lumbar Mob- Spinal |
LM Recruitment | n/a | Six studies suggested a link between a change in muscle activity or recruitment and the change in spinal stiffness: change in LM thickness ratio, ↑ LM recruitment, ↓ muscle activity of the erector spinae, facilitation of muscle activity, presence of muscle relaxation, correction of an altered motor function, and ↓ involuntary muscle activity | |
| Narrative Reviews | Potter et al. 2005 [71] | Humans- Not specified | Man- Spinal and peripheral | EMG reflex | During and immediately post | Reflex response elicited with latency around 4 ms. ↓ EMG activity Changes in α motor neuron activity (H-reflex) - either ↑ (2 studies) or ↓ (2 studies) ↑ Post-treatment MVC as compared to a sham manipulation and control |
| Jacobson 2011 [56] | Humans- Asymptomatic | STM- Region not specified | EMG | Immediate post | ↑ Rhythmic coherence ↑ Functional independence of muscle activation ↑ Amplitude of muscle contraction ↓ Agonist–antagonist co-contraction activation more specific to locus of primary action ↑ Differentiation between isometric and isotonic contractions movements smoother, larger, and less constrained, fewer extraneous movements; spatial excursions more dynamic and energetic ↑ Activation of stabilizing muscles during tasks ↓ Estimated energy expenditure |
|
| Haavik et al. 2021 (2) [48] | Humans- Not specified | Man- spinal | EMG, H-Reflex | n/a | Changes in neuromuscular function that occur after spinal adjustments impact the CNS and are primarily due to supraspinal excitability changes, and to a lesser degree, due to spinal cord excitability changes SMT alters supraspinal excitability SMT activates the deep muscle afferents from paravertebral tissues, particularly activating the muscle spindles and potentially Golgi tendon organs |
|
| Hennenhoefer et al. 2019 [52] | Humans- Not specified | Man- Region not specified | Muscle Tone- EMG, H-Reflex | n/a | Stabilization of motor neuron pool to correct for aberrant postural and proprioceptive behavior | |
| Bolton et al. 2012 [33] | Humans- Asymptomatic | Man- Sacral | Phasic perineal contractions (PPC) | n/a | ↑ PPC | |
| Pickar 2002 [70] | Humans- Asymptomatic and Symptomatic | Man- Spinal | EMG | ↓ or ↑ Paraspinal muscle activity |
Definitions: ↑ - Increase, ↓- Decrease, → - No change, STM- Soft tissue mobilizations, Man- Manipulation, Mob- Mobilizations, PA- Posterior to anterior, UE- Upper extremity, LBP- Low back pain, LM- Lumbar Multifidi, MCP- Metacarpophalangeal, MWM- Mobilization with movement, SMT- Spinal manipulative technique, MT- Manual Therapy, GTO- Golgi tendon organ, EMG- Electromyogram, MVC- Mav voluntary contraction.
Other mechanisms
Six reviews of critically low quality included treatment mechanisms outside of the previous domains. (Table 10) Three studies investigated cardiopulmonary responses to MT. Results suggested increased forced vital capacity, forced expiratory volume, and O2 saturation without supporting changes in VO2 Max, total lung capacity, blood lactate levels, or other reported measures of cardiopulmonary function. [33,41,68]. Other reviews reported on changes in gene expression [62], intestinal function [62], mitochondrial function [62,81] and enzyme, protein and amino acid profiles [56,62,81].
Table 10. Other mechanisms associated with MT.
| Author/Year | Subjects: | MT Technique | Measure | Time of Measure | Results/Conclusion | |
|---|---|---|---|---|---|---|
| Systematic Reviews | Tejero-Fernandez et al. 2015 [81] | Humans- Asymptomatic | STM- Region not specified | Plasma Creatine kinase measures | n/a | ↓ CK ↑ Mitochondrial biogenesis |
| Corso et al. 2019 [41] | Humans- Asymptomatic | Man- spinal | MIP, MEP, TLC, residual volume Vo2 Max Blood Lactate Concentration ECG |
Immediate post | → Rate of perceived exertion (RPE) (treadmill or ergometer) → VO2 max → Blood lactate concentration → MIP → MEP → TLC |
|
| Scoping Reviews | Nelson 2015 [68] | Humans- Symptomatic | STM- Region not specified | n/a | n/a | ↑ O2 saturation ↓ Central venous pressure ↓ Pulmonary vessel resistance |
| Lima et al. 2020 [62] | Animals- (Non-Cadaveric Cats, Mice, Rabbits, Rats); healthy and induced pain | Man- Spinal Mob- Spinal, knee, ankle STM- Region not specified |
n/a | n/a |
Mobilization: Prevention of Oxidative stress & increase in protein carbonyls and catalase → Mitochondrial function & superoxide dismutase ↑ Collapsin response mediator protein-2, galactokinase 1, tropomyosin-4, and transthyretin expressions → Gene expression observed with mobilization Pain ↓ is mediated by Cannabinoid subtype receptor 1 and 2 availability Opioid receptors mediate pain ↓ ↓ CD11b/c and GFAP glycoproteins Manipulation: ↓ Excitability Satellitosis (glial cells) Prevention of ↑ in lipid hydroperoxides and NO metabolites; ↓ of catalase activity STM: ↑ Transport of subcutaneous nanoparticles to lymph node. ↑ Liposome transport into bloodstream ↓ iNOS, COX-2, NO, PGE2, MMP-1, and proteoglycan syntheses ↓ Insulin, gastrin & somatostatin; and ↑ glucose ↓ Gene Expression: ↓TAK1 to inhibit NF-ĸB activation ↓ Matrix metalloprotease-13 & Collagen II; No differences in Collagen ↑ Gene expression in the liver ↓ Differential gene expression ↓ Intraperitoneal protein ↓ Noradrenergic innervation of lymphoid organs ↓ Tissue-type plasminogen activator and plasminogen activator inhibitor-1 levels ↑ Intestinal function: ↑ c-kit mRNA and protein levels ↑ Number, distribution, and ultrastructure of colonic interstitial cells ↓ Cohesive adhesion; delayed M2 migration intraperitoneally. ↑ Protein levels & DNA synthesis. ↑ FGF-2 and CD34 proteins Activation of MrgprB4 + neurons upon brushing stimulus |
|
| Narrative Reviews |
Field 2016 [43] | Humans- Not specified | STM- Region not specified | n/a | ↓ Bilirubin levels | |
| Jacobson 2011 [56] | Humans- Asymptomatic | STM- Region not specified | Plasma | Immediate post | ↑ Serum glutamic ↑ Oxalacetic transaminase |
|
| Bolton et al. 2012 [33] | Humans- Asymptomatic | Man- Spinal Mob- Spinal |
FVC, FEV-1, Freq. and amp. gastric contractions – electrogastrogram Plasma ACTH | n/a | ↑ FVC and FEV-1 ↑ FVC and FEV-1 with HVLA vs non-thrust ↓ Frequency and ↑ amplitude of gastric contractions post Cervical SMT → ACTH vs sham |
Definitions: ↑ - Increase, ↓ - Decrease, → - No change, STM- Soft tissue mobilizations, Man- Manipulation, Mob- Mobilizations, PA- Posterior to anterior, UE- Upper extremity, LBP- Low back pain, MCP- Metacarpophalangeal, MWM- Mobilization with movement, SMT- Spinal manipulative technique, MT- Manual Therapy, MIP- Max inspiratory pressure, MEP- Max expiratory pressure, TLC- Total lung capacity, FVC- Forced vital capacity, FEV-1- Forced expiratory volume in 1 seconds.
Discussion
Findings from this review support complex multisystem biomechanical, neurovascular, neurological, neurotransmitter/neuropeptide, neuroimmune, neuroendocrine, neuromuscular, and other mechanistic responses occurring with the application of MT. The overall quality of evidence supporting these responses was critically low to moderate, therefore these results should be interpreted with caution. Furthermore, care should be taken in assuming translation to clinical relevance as these processes are influenced by a multitude of intrinsic and extrinsic factors and are likely to demonstrate variability between individuals. Some included reviews attempted to establish clinical relevance, including Jun et al. [58], which reported correlation between being a positive responder to spinal manipulation, and improved multifidi recruitment post technique application; however, non-investigated translation to clinical outcomes should not be assumed. For example, neuroimmune treatment mechanisms favor an increase in anti-inflammatory mediators and decrease in inflammatory mediators. These changes are not unique to MT [38] and the relevance of these changes to immune system status was questioned in several of the included reviews [35,49] and furthermore by the chiropractic community in a recent statement paper [91].
Overall, the current review supports peripheral, segmental spinal, and supraspinal neurological mechanisms occurring with the application of MT, which can be measured directly or indirectly. MT has been shown to attenuate nociceptive spinal excitability [92]. This alteration in neural excitability as well as changes in tissue sensitivity as measured via PPT are theorized to occur due to facilitation of descending inhibitory mechanisms [93]. Previous animal model research [94] demonstrated that joint-based MT likely induces analgesia via non-opioidergic inhibitory pathways, that include noradrenergic and serotonergic mechanisms in the central nervous system. Changes in tissue sensitivity may also be related to changes in functional connectivity involving the PAG [95]. One included review [46] reported that 11 studies reported correlation between hypoalgesia and clinical pain reduction; however, the degree to which changes in quantitative sensory testing translate to clinical outcomes has demonstrated question [96–98], likely due to poor baseline assessment of which aberrant neurophysiological mechanisms are present within the study participant group. Descending regulatory mechanisms have also been proposed to influence neuroimmune [99,100], neuroendocrine [101], neurovascular [101], and neuromuscular responses [102]. The crosstalk between mechanisms further complicates research within this field, as discussed in a recent review on neuroimmune mechanisms associated with MT [38].
While historic models of MT promote assessment and treatment based on biomechanical principles, the overwhelming majority of mechanisms studied suggest stronger support for neurological changes than biomechanical. These findings agree with several recent reviews suggesting non-specific analgesic effects associated with MT [103,104]. Several recent publications have emphasized the need for updated training paradigms and modernized practice patterns to accommodate what is currently known and unknown regarding these complex mechanistic responses [12,105–108]. This review summarizes treatment mechanisms associated with MT application supporting complex multisystem responses; however, care must be taken in interpreting these findings due to two distinct realizations:
1) Treatment mechanisms that occur are unlikely to be unique to a specific MT intervention. It is unclear based on the results of this review which mechanisms are specific to MT versus those which are related to the fact that ‘an intervention’ was applied. This can be seen with the significant reduction in effect size across several treatment mechanisms when compared to other active controls and sham techniques. Recent work has cited the importance of better understanding the specific and shared mechanisms associated with MT [109].
2) Treatment mechanisms that occur are unlikely to be consistent across different populations. Contextual factors such as patient factors, provider factors, and environmental factors have been shown to influence mechanistic response to MT forces and therefore should be considered when investigating and interpreting mechanistic responses to MT. While contextual factors have been solidified as a critical component of mechanistic response, two recent studies obtained consensus on gaps within this field specifically related to MT treatment mechanisms [110,111].
Clinical implications
Clinicians using MT interventions should appreciate that multisystem mechanisms occur beyond the local tissue to which MT is targeted. While not reasonable to assess these mechanisms clinically in real time, understanding the complex interactions across systems regulating processes such as immune function, cardiovascular function, and neuroendocrine function are important factors to consider. Many of the included reviews looked at these mechanistic responses in isolation and clinicians should appreciate the complex interaction between these systems producing the observed response which they see clinically are the sum of these mechanisms with interaction from contextual variables.
Research implications
High quality primary and secondary research within this area of study is needed to further investigate changes in the mechanistic domains outlined within this review. Researchers should address identified gaps within MT mechanisms research [110,111]. Collaboration between clinical researchers and basic scientists should be leveraged to establish translational value of these mechanisms to clinical outcomes, assess contextual factor influence on both mechanistic and clinical response, and determine if there are pain phenotypes which provide different mechanistic and clinical responses. Research should be focused on symptomatic patient populations to promote relevance of findings. Furthermore, emphasis should be placed on study designs effective in establishing causation such as those which have been proposed in previous work [6].
Limitations
Several limitations are present which must be considered in interpreting the findings of this review. Although reporting of different MT techniques occurred, this review did not attempt to differentiate between treatment mechanisms for different MT techniques (STM, manipulation, mobilization). Several of the included reviews did report on these findings in their results. Significant heterogeneity was present between the included reviews related to type of MT technique, type of mechanistic measure, and subjects. Several of the included reviews were not in English which required translation, therefore language barriers may have been a limitation during screening, quality assessment, risk of bias assessment, and data extraction. Furthermore, this review included narrative reviews which are less structured and transparent than systematic/scoping reviews allowing increased potential for bias. These reviews were included to broaden the investigated mechanisms and the results demonstrated beneficial information extracted from these reviews which would have otherwise been excluded. Inclusion criteria were used (search strategy outlined in review) in an attempt to include more transparent narrative reviews.
Summary
This review supports multisystem mechanistic responses with the application of hands-on intervention across the biomechanical, neurological, neurovascular, neuroendocrine, neurotransmitter/neuropeptide, and neuromuscular domains. The clinical value of these mechanistic responses has not been well established. Clinicians should appreciate the uncertainty related to ‘why’ these interventions work and future research should look to better define which mechanisms (or combinations of mechanisms) are mediators of clinical response.
Statement on living review
With permission from the publisher, results from this living review will be housed at the Duke Center for Excellence in Manual and Manipulative Therapy (CEMMT) website at https://sites.duke.edu/cemmt/. Results will be updated every 6 months to include newly published research. Please reach out to the corresponding author with any potential reviews which may meet the inclusion criteria to be included on the next revision.
Supporting information
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(DOCX)
Acknowledgments
We the authors would like to thank Colleen Duchon, Health Sciences Librarian at Youngstown State University for validation of search strategy. We would also like to thank Dr. Weiqing Ge and Dr. Jean-Michel Brismée for assistance with article translation.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.National Institutes of Health (NIH). In: Neural Mechanisms of Force-Based Manipulations: High Priority Research Networks [Internet]. 2 Feb 2023. Available: https://grants.nih.gov/grants/guide/rfa-files/RFA-AT-21-006.html#:~:text=Force-based%20manipulations%20refer%20to%20the%20passive%20application%20of,rehabilitation%20care%2C%20or%20general%20wellness%20and%20disease%20prevention
- 2.Cook C. The demonization of manual therapy. MSK – Muskuloskelettale Physiotherapie. 2021;25:125–32. [Google Scholar]
- 3.Pettman E. A history of manipulative therapy. J Man Manip Ther. 2007;15(3):165–74. doi: 10.1179/106698107790819873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bialosky JE, Beneciuk JM, Bishop MD, Coronado RA, Penza CW, Simon CB, et al. Unraveling the mechanisms of manual therapy: modeling an approach. J Orthop Sports Phys Ther. 2018;48(1):8–18. doi: 10.2519/jospt.2018.7476 [DOI] [PubMed] [Google Scholar]
- 5.Courtney C, Fernández de las Peñas C. Manual Therapy for Chronic Conditions: A Mechanistic Approach to Modern Manual Therapy. In: Simoneau G, editor. Mechanisms of Manual Therapy Interventions. Lacrosse, WI: Academy of Orthopaedic Physical Therapy. APTA; 2021. p. 5–18. [Google Scholar]
- 6.Damian K, Chad C, Kenneth L, David G. Time to evolve: the applicability of pain phenotyping in manual therapy. J Man Manip Ther. 2022;30(2):61–7. doi: 10.1080/10669817.2022.2052560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cook C, Abraira V, Burns J, Degenhardt B, Kawchuk G, Keter D, et al. Categorizing treatment mechanisms for complementary and integrative musculoskeletal interventions. Int J Osteopath Med. 2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keter D, Bent J, Bialosky J, Courtney C, Esteves J, Funabashi M. An international consensus on gaps in mechanisms of forced-based manipulation research; findings from a nominal group technique. 2023. [DOI] [PMC free article] [PubMed]
- 9.Lok JJ. Defining and estimating causal direct and indirect effects when setting the mediator to specific values is not feasible. Stat Med. 2016;35(22):4008–20. doi: 10.1002/sim.6990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cross KM, Kuenze C, Grindstaff TL, Hertel J. Thoracic spine thrust manipulation improves pain, range of motion, and self-reported function in patients with mechanical neck pain: a systematic review. J Orthop Sports Phys Ther. 2011;41(9):633–42. doi: 10.2519/jospt.2011.3670 [DOI] [PubMed] [Google Scholar]
- 11.Nim CG, Weber KA, Kawchuk GN, O’Neill S. Spinal manipulation and modulation of pain sensitivity in persistent low back pain: a secondary cluster analysis of a randomized trial. Chiropr Man Therap. 2021;29(1):10. doi: 10.1186/s12998-021-00367-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grenier J-P, Rothmund M. A critical review of the role of manual therapy in the treatment of individuals with low back pain. J Man Manip Ther. 2024;32(5):464–77. doi: 10.1080/10669817.2024.2316393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bier JD, Scholten-Peeters WGM, Staal JB, Pool J, van Tulder MW, Beekman E, et al. Clinical Practice Guideline for Physical Therapy Assessment and Treatment in Patients With Nonspecific Neck Pain. Phys Ther. 2018;98(3):162–71. doi: 10.1093/ptj/pzx118 [DOI] [PubMed] [Google Scholar]
- 14.George SZ, Fritz JM, Silfies SP, Schneider MJ, Beneciuk JM, Lentz TA, et al. Interventions for the management of acute and chronic low back pain: revision 2021. J Orthop Sports Phys Ther. 2021;51(11):CPG1–60. doi: 10.2519/jospt.2021.0304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pangarkar SS, Kang DG, Sandbrink F, Bevevino A, Tillisch K, Konitzer L, et al. VA/DoD clinical practice guideline: diagnosis and treatment of low back pain. J Gen Intern Med. 2019;34(11):2620–9. doi: 10.1007/s11606-019-05086-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qaseem A, Wilt TJ, McLean RM, Forciea MA, Clinical Guidelines Committee of the American College of Physicians, Denberg TD, et al. noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American college of physicians. Ann Intern Med. 2017;166(7):514–30. doi: 10.7326/M16-2367 [DOI] [PubMed] [Google Scholar]
- 17.WHO. Guideline for non-surgical management of chronic primary low back pain in adults in primary and community care settings. Geneva: World Health Organization; 2023. [PubMed] [Google Scholar]
- 18.Oliveira CB, Maher CG, Pinto RZ, Traeger AC, Lin C-WC, Chenot J-F, et al. Clinical practice guidelines for the management of non-specific low back pain in primary care: an updated overview. Eur Spine J. 2018;27(11):2791–803. doi: 10.1007/s00586-018-5673-2 [DOI] [PubMed] [Google Scholar]
- 19.Parikh P, Santaguida P, Macdermid J, Gross A, Eshtiaghi A. Comparison of CPG’s for the diagnosis, prognosis and management of non-specific neck pain: a systematic review. BMC Musculoskelet Disord. 2019;20(1):81. doi: 10.1186/s12891-019-2441-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubinstein SM, de Zoete A, van Middelkoop M, Assendelft WJJ, de Boer MR, van Tulder MW. Benefits and harms of spinal manipulative therapy for the treatment of chronic low back pain: systematic review and meta-analysis of randomised controlled trials. BMJ. 2019;364:l689. doi: 10.1136/bmj.l689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edwards RR, Dworkin RH, Turk DC, Angst MS, Dionne R, Freeman R, et al. Patient phenotyping in clinical trials of chronic pain treatments: IMMPACT recommendations. Pain. 2016;157(9):1851–71. doi: 10.1097/j.pain.0000000000000602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hancock MJ, Hill JC. Are Small Effects for Back Pain Interventions Really Surprising?. J Orthop Sports Phys Ther. 2016;46(5):317–9. doi: 10.2519/jospt.2016.0604 [DOI] [PubMed] [Google Scholar]
- 23.Cruz-Almeida Y, Fillingim RB. Can quantitative sensory testing move us closer to mechanism-based pain management?. Pain Med. 2014;15(1):61–72. doi: 10.1111/pme.12230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Granovsky Y, Yarnitsky D. Personalized pain medicine: the clinical value of psychophysical assessment of pain modulation profile. Rambam Maimonides Med J. 2013;4(4):e0024. doi: 10.5041/RMMJ.10131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clauw DJ. Diagnosing and treating chronic musculoskeletal pain based on the underlying mechanism(s). Best Pract Res Clin Rheumatol. 2015;29(1):6–19. doi: 10.1016/j.berh.2015.04.024 [DOI] [PubMed] [Google Scholar]
- 26.Shi X, Wallach JD. Umbrella reviews: a useful study design in need of standardisation. BMJ. 2022;o1740. doi: 10.1136/bmj.o1740 [DOI] [Google Scholar]
- 27.Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whiting P, Savović J, Higgins JPT, Caldwell DM, Reeves BC, Shea B, et al. ROBIS: A new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol. 2016;69:225–34. doi: 10.1016/j.jclinepi.2015.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alanazi MS, Degenhardt B, Kelley-Franklin G, Jacobson E, Fritz S, Kettner N, et al. Autonomic nervous system and viscera-related responses to manual therapy: A narrative overview. Int J Osteopath Med. 2024;54:100735. doi: 10.1016/j.ijosm.2024.100735 [DOI] [Google Scholar]
- 30.Araujo FX, Ferreira GE, Angellos RF, Stieven FF, Plentz RDM, Silva MF. Autonomic effects of spinal manipulative therapy: systematic review of randomized controlled trials. J Manipulative Physiol Ther. 2019;42(8):623–34. doi: 10.1016/j.jmpt.2018.12.005 [DOI] [PubMed] [Google Scholar]
- 31.Arribas-Romano A, Fernández-Carnero J, Molina-Rueda F, Angulo-Diaz-Parreño S, Navarro-Santana MJ. Efficacy of physical therapy on nociceptive pain processing alterations in patients with chronic musculoskeletal pain: a systematic review and meta-analysis. Pain Med. 2020;21(10):2502–17. doi: 10.1093/pm/pnz366 [DOI] [PubMed] [Google Scholar]
- 32.Bernier Carney KM, Young EE, Guite JW, Starkweather AR. A systematic review of biological mechanisms and chronic pain outcomes during stress reduction interventions. Biol Res Nurs. 2020;22(2):205–16. doi: 10.1177/1099800420907964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bolton PS, Budgell B. Visceral responses to spinal manipulation. J Electromyogr Kinesiol. 2012;22(5):777–84. doi: 10.1016/j.jelekin.2012.02.016 [DOI] [PubMed] [Google Scholar]
- 34.Amoroso Borges BL, Bortolazzo GL, Neto HP. Effects of spinal manipulation and myofascial techniques on heart rate variability: A systematic review. J Bodyw Mov Ther. 2018;22(1):203–8. doi: 10.1016/j.jbmt.2017.09.025 [DOI] [PubMed] [Google Scholar]
- 35.Chow N, Hogg-Johnson S, Mior S, Cancelliere C, Injeyan S, Teodorczyk-Injeyan J, et al. Assessment of studies evaluating spinal manipulative therapy and infectious disease and immune system outcomes: a systematic review. JAMA Netw Open. 2021;4(4):e215493. doi: 10.1001/jamanetworkopen.2021.5493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chu J, Allen DD, Pawlowsky S, Smoot B. Peripheral response to cervical or thoracic spinal manual therapy: an evidence-based review with meta analysis. J Man Manip Ther. 2014;22(4):220–9. doi: 10.1179/2042618613Y.0000000062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colombi A, Testa M. The effects induced by spinal manipulative therapy on the immune and endocrine systems. Medicina (Kaunas). 2019;55(8):448. doi: 10.3390/medicina55080448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cook CE, Keter D, Cade WT, Winkelstein BA, Reed WR. Manual therapy and exercise effects on inflammatory cytokines: a narrative overview. Front Rehabil Sci. 2024;5:1305925. doi: 10.3389/fresc.2024.1305925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coronado RA, Gay CW, Bialosky JE, Carnaby GD, Bishop MD, George SZ. Changes in pain sensitivity following spinal manipulation: a systematic review and meta-analysis. J Electromyogr Kinesiol. 2012;22(5):752–67. doi: 10.1016/j.jelekin.2011.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coronado RA, Bialosky JE, Cook CE. The temporal effects of a single session of high-velocity, low-amplitude thrust manipulation on subjects with spinal pain. Phys Ther Rev. 2010;15(1):29–35. doi: 10.1179/174328810x12647087218712 [DOI] [Google Scholar]
- 41.Corso M, Mior SA, Batley S, Tuff T, da Silva-Oolup S, Howitt S, et al. The effects of spinal manipulation on performance-related outcomes in healthy asymptomatic adult population: a systematic review of best evidence. Chiropr Man Therap. 2019;27:25. doi: 10.1186/s12998-019-0246-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Evans DW. Mechanisms and effects of spinal high-velocity, low-amplitude thrust manipulation: previous theories. J Manipulative Physiol Ther. 2002;25(4):251–62. doi: 10.1067/mmt.2002.123166 [DOI] [PubMed] [Google Scholar]
- 43.Field T. Massage therapy research review. Complement Ther Clin Pract. 2016;24:19–31. doi: 10.1016/j.ctcp.2016.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galindez-Ibarbengoetxea X, Setuain I, Andersen LL, Ramírez-Velez R, González-Izal M, Jauregi A, et al. Effects of Cervical High-Velocity Low-Amplitude Techniques on Range of Motion, Strength Performance, and Cardiovascular Outcomes: A Review. J Altern Complement Med. 2017;23(9):667–75. doi: 10.1089/acm.2017.0002 [DOI] [PubMed] [Google Scholar]
- 45.Gay RE, Bronfort G, Evans RL. Distraction manipulation of the lumbar spine: a review of the literature. J Manipulative Physiol Ther. 2005;28(4):266–73. doi: 10.1016/j.jmpt.2005.03.012 [DOI] [PubMed] [Google Scholar]
- 46.Gay CW, Alappattu MJ, Coronado RA, Horn ME, Bishop MD. Effect of a single session of muscle-biased therapy on pain sensitivity: a systematic review and meta-analysis of randomized controlled trials. J Pain Res. 2013;6:7–22. doi: 10.2147/JPR.S37272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gera C, Malik M, Kaur J, Saini M. A systematic review and meta-analysis on effect of spinal mobilization and manipulation on cardiovascular responses. Hong Kong Physiother J. 2020;40(2):75–87. doi: 10.1142/S1013702520500122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haavik H, Kumari N, Holt K, Niazi IK, Amjad I, Pujari AN, et al. The contemporary model of vertebral column joint dysfunction and impact of high-velocity, low-amplitude controlled vertebral thrusts on neuromuscular function. Eur J Appl Physiol. 2021;121(10):2675–720. doi: 10.1007/s00421-021-04727-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haavik H, Niazi IK, Kumari N, Amjad I, Duehr J, Holt K. The potential mechanisms of high-velocity, low-amplitude, controlled vertebral thrusts on neuroimmune function: a narrative review. Medicina (Kaunas). 2021;57(6):536. doi: 10.3390/medicina57060536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hartnett DA. Gua sha therapy in the management of musculoskeletal pathology: a narrative review. Physical Therapy Reviews. 2021;27(3):169–75. doi: 10.1080/10833196.2021.2011581 [DOI] [Google Scholar]
- 51.Hegedus EJ, Goode A, Butler RJ, Slaven E. The neurophysiological effects of a single session of spinal joint mobilization: does the effect last?. J Man Manip Ther. 2011;19(3):143–51. doi: 10.1179/2042618611Y.0000000003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hennenhoefer K, Schmidt D. Toward a theory of the mechanism of high-velocity, low-amplitude technique: a literature review. J Am Osteopath Assoc. 2019;119(10):688–95. doi: 10.7556/jaoa.2019.116 [DOI] [PubMed] [Google Scholar]
- 53.Hillier SL, Louw Q, Morris L, Uwimana J, Statham S. Massage therapy for people with HIV/AIDS. Cochrane Database Syst Rev. 2010;2010(1):CD007502. doi: 10.1002/14651858.CD007502.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holey LA, Dixon J. Connective tissue manipulation: a review of theory and clinical evidence. J Bodyw Mov Ther. 2014;18(1):112–8. doi: 10.1016/j.jbmt.2013.08.003 [DOI] [PubMed] [Google Scholar]
- 55.Honoré M, Leboeuf-Yde C, Gagey O. The regional effect of spinal manipulation on the pressure pain threshold in asymptomatic subjects: a systematic literature review. Chiropr Man Therap. 2018;2611. doi: 10.1186/s12998-018-0181-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jacobson E. Structural integration, an alternative method of manual therapy and sensorimotor education. J Altern Complement Med. 2011;17(10):891–9. doi: 10.1089/acm.2010.0258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jones J, Thomson P, Irvine K, Leslie SJ. Is there a specific hemodynamic effect in reflexology? A systematic review of randomized controlled trials. J Altern Complement Med. 2013;19(4):319–28. doi: 10.1089/acm.2011.0854 [DOI] [PubMed] [Google Scholar]
- 58.Jun P, Pagé I, Vette A, Kawchuk G. Potential mechanisms for lumbar spinal stiffness change following spinal manipulative therapy: a scoping review. Chiropr Man Therap. 2020;28(1):15. doi: 10.1186/s12998-020-00304-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jung A, Adamczyk WM, Ahmed A, van der Schalk L, Poesl M, Luedtke K, et al. No Sufficient evidence for an immediate hypoalgesic effect of spinal manual therapy on pressure pain thresholds in asymptomatic and chronic pain populations: a systematic review and meta-analysis. Phys Ther. 2023;103(3):pzad003. doi: 10.1093/ptj/pzad003 [DOI] [PubMed] [Google Scholar]
- 60.Kingston L, Claydon L, Tumilty S. The effects of spinal mobilizations on the sympathetic nervous system: a systematic review. Man Ther. 2014;19(4):281–7. doi: 10.1016/j.math.2014.04.004 [DOI] [PubMed] [Google Scholar]
- 61.Lascurain-Aguirrebeña I, Newham D, Critchley DJ. Mechanism of action of spinal mobilizations: a systematic review. Spine (Phila Pa 1976). 2016;41(2):159–72. doi: 10.1097/BRS.0000000000001151 [DOI] [PubMed] [Google Scholar]
- 62.Lima CR, Martins DF, Reed WR. Physiological responses induced by manual therapy in animal models: a scoping review. Front Neurosci. 2020;14:430. doi: 10.3389/fnins.2020.00430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meyer A-L, Amorim M-A, Schubert M, Schweinhardt P, Leboeuf-Yde C. Unravelling functional neurology: does spinal manipulation have an effect on the brain? - a systematic literature review. Chiropr Man Therap. 2019;27:60. doi: 10.1186/s12998-019-0265-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Millan M, Leboeuf-Yde C, Budgell B, Amorim M-A. The effect of spinal manipulative therapy on experimentally induced pain: a systematic literature review. Chiropr Man Therap. 2012;20(1):26. doi: 10.1186/2045-709X-20-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mitchell UH, Helgeson K, Mintken P. Physiological effects of physical therapy interventions on lumbar intervertebral discs: A systematic review. Physiother Theory Pract. 2017;33(9):695–705. doi: 10.1080/09593985.2017.1345026 [DOI] [PubMed] [Google Scholar]
- 66.Moyer CA, Seefeldt L, Mann ES, Jackley LM. Does massage therapy reduce cortisol? A comprehensive quantitative review. J Bodywork Mov Ther. 2011;15:3–14. doi: 10.1016/j.jbmt.2010.06.001 [DOI] [PubMed] [Google Scholar]
- 67.Navarro-Santana MJ, Gómez-Chiguano GF, Somkereki MD, Fernández-de-Las-Peñas C, Cleland JA, Plaza-Manzano G. Effects of joint mobilisation on clinical manifestations of sympathetic nervous system activity: a systematic review and meta-analysis. Physiotherapy. 2020;107:118–32. doi: 10.1016/j.physio.2019.07.001 [DOI] [PubMed] [Google Scholar]
- 68.Nelson NL. Massage therapy: understanding the mechanisms of action on blood pressure. A scoping review. J Am Soc Hypertens. 2015;9(10):785–93. doi: 10.1016/j.jash.2015.07.009 [DOI] [PubMed] [Google Scholar]
- 69.Picchiottino M, Leboeuf-Yde C, Gagey O, Hallman DM. The acute effects of joint manipulative techniques on markers of autonomic nervous system activity: a systematic review and meta-analysis of randomized sham-controlled trials. Chiropr Man Therap. 2019;27:17. doi: 10.1186/s12998-019-0235-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pickar JG. Neurophysiological effects of spinal manipulation. Spine J. 2002;2(5):357–71. doi: 10.1016/s1529-9430(02)00400-x [DOI] [PubMed] [Google Scholar]
- 71.Potter L, McCarthy C, Oldham J. Physiological effects of spinal manipulation: a review of proposed theories. Phys Ther Rev. 2005;10(3):163–70. doi: 10.1179/108331905x55820 [DOI] [Google Scholar]
- 72.Riley SP, Swanson BT, Shaffer SM, Flowers DW, Hofbauer MA, Liebano RE. Does manual therapy meaningfully change quantitative sensory testing and patient reported outcome measures in patients with musculoskeletal impairments related to the spine?: A “trustworthy” systematic review and meta-analysis. J Man Manip Ther. 2024;32(1):51–66. doi: 10.1080/10669817.2023.2247235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rogan S, Taeymans J, Berger I, Baur H. Manual spinal therapy techniques to stimulate the autonomic nervous system: a scoping review. Sportverletz Sportschaden. 2023;37(2):67–78. doi: 10.1055/a-1958-2730 [DOI] [PubMed] [Google Scholar]
- 74.Kovanur-Sampath K, Mani R, Cotter J, Gisselman AS, Tumilty S. Changes in biochemical markers following spinal manipulation-a systematic review and meta-analysis. Musculoskelet Sci Pract. 2017;29120–31. doi: 10.1016/j.msksp.2017.04.004 [DOI] [PubMed] [Google Scholar]
- 75.Kovanur Sampath K, Tumilty S, Wooten L, Belcher S, Farrell G, Gisselman AS. Effectiveness of spinal manipulation in influencing the autonomic nervous system - a systematic review and meta-analysis. J Man Manip Ther. 2024;32(1):10–27. doi: 10.1080/10669817.2023.2285196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Savva C, Karagiannis C, Korakakis V, Efstathiou M. The analgesic effect of joint mobilization and manipulation in tendinopathy: a narrative review. J Man Manip Ther. 2021;29(5):276–87. doi: 10.1080/10669817.2021.1904348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schmid A, Brunner F, Wright A, Bachmann LM. Paradigm shift in manual therapy? Evidence for a central nervous system component in the response to passive cervical joint mobilisation. Man Ther. 2008;13(5):387–96. doi: 10.1016/j.math.2007.12.007 [DOI] [PubMed] [Google Scholar]
- 78.Simmonds N, Miller P, Gemmell H. A theoretical framework for the role of fascia in manual therapy. J Bodyw Mov Ther. 2012;16(1):83–93. doi: 10.1016/j.jbmt.2010.08.001 [DOI] [PubMed] [Google Scholar]
- 79.Sousa KA de, Cardoso LB, Pirola FM, Auad LG, De Oliveira AS. Effect of manual therapies on cardiac autonomic control: a systematic review. mtprehabjournal. 20201–12. doi: 10.17784/mtprehabjournal.2021.19.804 [DOI] [Google Scholar]
- 80.Sullivan SG, Paolacci S, Kiani AK, Bertelli M. Chiropractic care for hypertension: Review of the literature and study of biological and genetic bases. Acta Biomed. 2020;91(13-S):e2020017. doi: 10.23750/abm.v91i13-S.10524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tejero-Fernández V, Membrilla-Mesa M, Galiano-Castillo N, Arroyo-Morales M. Immunological effects of massage after exercise: A systematic review. Phys Ther Sport. 2015;16(2):187–92. doi: 10.1016/j.ptsp.2014.07.001 [DOI] [PubMed] [Google Scholar]
- 82.Vernon H. Qualitative review of studies of manipulation-induced hypoalgesia. J Manipulative Physiol Ther. 2000;23(2):134–8. doi: 10.1016/s0161-4754(00)90084-8 [DOI] [PubMed] [Google Scholar]
- 83.Vicenzino B, Paungmali A, Teys P. Mulligan’s mobilization-with-movement, positional faults and pain relief: current concepts from a critical review of literature. Man Ther. 2007;12(2):98–108. doi: 10.1016/j.math.2006.07.012 [DOI] [PubMed] [Google Scholar]
- 84.Vigotsky AD, Bruhns RP. The role of descending modulation in manual therapy and its analgesic implications: a narrative review. Pain Res Treat. 2015;2015292805. doi: 10.1155/2015/292805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Voogt L, de Vries J, Meeus M, Struyf F, Meuffels D, Nijs J. Analgesic effects of manual therapy in patients with musculoskeletal pain: a systematic review. Man Ther. 2015;20(2):250–6. doi: 10.1016/j.math.2014.09.001 [DOI] [PubMed] [Google Scholar]
- 86.Weerapong P, Hume PA, Kolt GS. The mechanisms of massage and effects on performance, muscle recovery and injury prevention. Sports Med. 2005;35(3):235–56. doi: 10.2165/00007256-200535030-00004 [DOI] [PubMed] [Google Scholar]
- 87.Xiong XJ, Li SJ, Zhang YQ. Massage therapy for essential hypertension: a systematic review. J Hum Hypertens. 2015;29(3):143–51. doi: 10.1038/jhh.2014.52 [DOI] [PubMed] [Google Scholar]
- 88.Yao S, Hassani J, Gagne M, George G, Gilliar W. Osteopathic manipulative treatment as a useful adjunctive tool for pneumonia. J Vis Exp. 2014;(87):50687. doi: 10.3791/50687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Young KJ, Leboeuf-Yde C, Gorrell L, Bergström C, Evans DW, Axén I, et al. Mechanisms of manipulation: a systematic review of the literature on immediate anatomical structural or positional changes in response to manually delivered high-velocity, low-amplitude spinal manipulation. Chiropr Man Therap. 2024;32(1):28. doi: 10.1186/s12998-024-00549-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zegarra-Parodi R, Park PYS, Heath DM, Makin IRS, Degenhardt BF, Roustit M. Assessment of skin blood flow following spinal manual therapy: a systematic review. Man Ther. 2015;20(2):228–49. doi: 10.1016/j.math.2014.08.011 [DOI] [PubMed] [Google Scholar]
- 91.Côté P, Bussières A, Cassidy JD, Hartvigsen J, Kawchuk GN, Leboeuf-Yde C, et al. A united statement of the global chiropractic research community against the pseudoscientific claim that chiropractic care boosts immunity. Chiropr Man Therap. 2020;28(1):21. doi: 10.1186/s12998-020-00312-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Courtney CA, Witte PO, Chmell SJ, Hornby TG. Heightened flexor withdrawal response in individuals with knee osteoarthritis is modulated by joint compression and joint mobilization. J Pain. 2010;11(2):179–85. doi: 10.1016/j.jpain.2009.07.005 [DOI] [PubMed] [Google Scholar]
- 93.Courtney CA, Steffen AD, Fernández-de-Las-Peñas C, Kim J, Chmell SJ. Joint Mobilization Enhances Mechanisms of Conditioned Pain Modulation in Individuals With Osteoarthritis of the Knee. J Orthop Sports Phys Ther. 2016;46(3):168–76. doi: 10.2519/jospt.2016.6259 [DOI] [PubMed] [Google Scholar]
- 94.Skyba DA, Radhakrishnan R, Rohlwing JJ, Wright A, Sluka KA. Joint manipulation reduces hyperalgesia by activation of monoamine receptors but not opioid or GABA receptors in the spinal cord. Pain. 2003;106(1–2):159–68. doi: 10.1016/s0304-3959(03)00320-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gay CW, Robinson ME, George SZ, Perlstein WM, Bishop MD. Immediate changes after manual therapy in resting-state functional connectivity as measured by functional magnetic resonance imaging in participants with induced low back pain. J Manipulative Physiol Ther. 2014;37(9):614–27. doi: 10.1016/j.jmpt.2014.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wan DWL, Arendt-Nielsen L, Wang K, Xue CC, Wang Y, Zheng Z. Pain adaptability in individuals with chronic musculoskeletal pain is not associated with conditioned pain modulation. J Pain. 2018;19(8):897–909. doi: 10.1016/j.jpain.2018.03.002 [DOI] [PubMed] [Google Scholar]
- 97.Zheng Z, Wang K, Yao D, Xue CCL, Arendt-Nielsen L. Adaptability to pain is associated with potency of local pain inhibition, but not conditioned pain modulation: a healthy human study. Pain. 2014;155(5):968–76. doi: 10.1016/j.pain.2014.01.024 [DOI] [PubMed] [Google Scholar]
- 98.Gierthmühlen J, Binder A, Förster M, Baron R. Do we measure what patients feel?: an analysis of correspondence between somatosensory modalities upon quantitative sensory testing and self-reported pain experience. Clin J Pain. 2018;34(7):610–7. doi: 10.1097/AJP.0000000000000582 [DOI] [PubMed] [Google Scholar]
- 99.Gonçalves Dos Santos G, Delay L, Yaksh TL, Corr M. Neuraxial cytokines in pain states. Front Immunol. 2020;103061. doi: 10.3389/fimmu.2019.03061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wrona D. Neural-immune interactions: an integrative view of the bidirectional relationship between the brain and immune systems. J Neuroimmunol. 2006;172(1–2):38–58. doi: 10.1016/j.jneuroim.2005.10.017 [DOI] [PubMed] [Google Scholar]
- 101.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10(6):397–409. doi: 10.1038/nrn2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gritsenko V, Kalaska JF, Cisek P. Descending corticospinal control of intersegmental dynamics. J Neurosci. 2011;31(33):11968–79. doi: 10.1523/JNEUROSCI.0132-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nim CG, Downie A, O’Neill S, Kawchuk GN, Perle SM, Leboeuf-Yde C. The importance of selecting the correct site to apply spinal manipulation when treating spinal pain: Myth or reality? A systematic review. Sci Rep. 2021;11(1):23415. doi: 10.1038/s41598-021-02882-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sørensen PW, Nim CG, Poulsen E, Juhl CB. Spinal manipulative therapy for nonspecific low back pain: does targeting a specific vertebral level make a difference?: a systematic review with meta-analysis. J Orthop Sports Phys Ther. 2023;53(9):529–39. doi: 10.2519/jospt.2023.11962 [DOI] [PubMed] [Google Scholar]
- 105.Keter D, Griswold D, Learman K, Cook C. Priorities in updating training paradigms in orthopedic manual therapy: an international Delphi study. J Educ Eval Health Prof. 2023;20:4. doi: 10.3352/jeehp.2023.20.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Keter D, Griswold D, Learman K, Cook C. Modernizing patient-centered manual therapy: Findings from a Delphi study on orthopaedic manual therapy application. Musculoskelet Sci Pract. 2023;65102777. doi: 10.1016/j.msksp.2023.102777 [DOI] [PubMed] [Google Scholar]
- 107.Keter D, Hutting N, Vogsland R, Cook CE. Integrating person-centered concepts and modern manual therapy. JOSPT Open. 2024;2(1):60–70. doi: 10.2519/josptopen.2023.0812 [DOI] [Google Scholar]
- 108.Kerry R, Young KJ, Evans DW, Lee E, Georgopoulos V, Meakins A, et al. A modern way to teach and practice manual therapy. Chiropr Man Therap. 2024;32(1):17. doi: 10.1186/s12998-024-00537-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McDevitt AW, O’Halloran B, Cook CE. Cracking the code: unveiling the specific and shared mechanisms behind musculoskeletal interventions. Arch Physiother. 2023;13(1):14. doi: 10.1186/s40945-023-00168-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Keter DL, Bent JA, Bialosky JE, Courtney CA, Esteves JE, Funabashi M, et al. An international consensus on gaps in mechanisms of forced-based manipulation research: findings from a nominal group technique. J Man Manip Ther. 2024;32(1):111–7. doi: 10.1080/10669817.2023.2262336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Griswold D, Learman K, Rossettini G, Palese A, Ickert E, Wilhelm M, et al. Identifying priority gaps in contextual factors research and force-based manipulation. An international and interdisciplinary Delphi study. J Man Manip Ther. 2024;32(1):118–26. doi: 10.1080/10669817.2023.2255820 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.