Abstract
Background
Muscle-invasive urothelial cancer (UC) has a high risk of recurrence after definitive treatment. Nivolumab adjuvant to radical surgery improves disease-free survival in patients with UC with a high risk of recurrence; however, its role adjuvant to chemoradiation therapy (CRT) is unknown.
Methods
The NEXT trial is a single-arm, phase-2 study evaluating the efficacy and tolerability of nivolumab adjuvant to CRT in patients with localized or locoregional UC. The primary endpoint is failure-free survival (FFS) at 2 years. Secondary endpoints include patterns of recurrence, toxicity and quality of life (QoL). Plasma cell-free DNA (cfDNA) was subjected to shallow whole-genome sequencing to correlate with outcomes.
Results
28 patients were enrolled and received 480 mg of nivolumab intravenously every 4 weeks for up to 12 cycles adjuvant to CRT. The FFS at 2 years was 33.2% (95% CI 18.5% to 59.6%). Nine (32%) patients had localized progression, and eight (29%) had distant progression. 25 (89%) had one or more high-risk features (ie, plasmacytoid differentiation, T4, N+, multiple tumors, tumors >5 cm, residual disease before CRT, carcinoma in situ, and hydronephrosis). Patients with ≤2 high-risk features had a median FFS of 45.2 months (95% CI 14.56 to not reached (NR)) compared with 8.2 months (95% CI 7.1 to NR) in those with three or more risk features (p=0.0024). Nivolumab-associated treatment-related adverse events occurred in 18 (64.3%) patients, only 3 had grade 3 TRAEs, with significant changes in QoL. Plasma cfDNA copy number instability (CNI) scores ≤25 before the first dose of adjuvant nivolumab and at cycle 4 were associated with better overall survival compared with CNI scores ≥26 (49.6 months vs 20.5 months, p=0.0024). Genome copy number changes indicated chromatin remodeling and tyrosine kinase pathways, among others, as oncogenic drivers implicated in progression.
Conclusion
Nivolumab adjuvant to CRT in localized or locally advanced UC is well tolerated. Stratification by risk factors and correlation with plasma cfDNA analyses generate hypotheses for potential patient selection and putative therapeutic targets for future study.
Trial registration number
Keywords: Bladder Cancer, Immune Checkpoint Inhibitor, Circulating tumor DNA - ctDNA
WHAT IS ALREADY KNOWN ON THIS TOPIC
Nivolumab adjuvant to radical surgery improves outcomes in patients with bladder cancer at high risk of relapse.
WHAT THIS STUDY ADDS
Nivolumab adjuvant to chemoradiation is safe and possibly effective in patients with bladder cancer who are not surgical candidates or elect bladder preservation. A limited number of high-risk disease features and low cell-free DNA (cfDNA) were prognostic of better outcomes.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study provides a rationale for conducting and stratifying patients for randomized trials to evaluate the benefit of nivolumab adjuvant to chemoradiation in patients with localized disease. Future studies are needed to evaluate the additional role of targeted therapy based on cfDNA analysis in high-risk disease management.
Background
Bladder cancer, most commonly urothelial carcinoma (UC), is the second most common genitourinary cancer and sixth most common cancer overall, accounting for approximately 83,190 new cases and 16,840 deaths predicted in the USA in 2024.1 The treatment of localized or locoregional UC primarily involves radical surgery (cystectomy for muscle-invasive tumors arising from the bladder, or nephroureterectomy for high-grade tumors arising in the upper urinary tract) with or without neoadjuvant cisplatin-based chemotherapy conferring a median overall survival (mOS) of approximately 6 years.2 However, these procedures have high perioperative morbidity and can significantly impact quality of life (QoL).3 Moreover, bladder cancer is more commonly seen in older adults, with a median age of diagnosis of approximately 73 years; this population often has significant medical comorbidities and poor performance status, which limits the eligibility for major surgical procedures.4 Considering this, organ preservation strategies have emerged as treatment options for those who are not radical surgery candidates or prefer a non-extirpative approach.
In this setting, trimodality therapy, which involves chemoradiation therapy (CRT) after maximal transurethral resection of the tumor, can have outcomes similar to radical surgery in selected patients (those with cT2N0 disease, no moderate/severe tumor-related hydronephrosis, no diffuse carcinoma in situ (CIS), solitary tumor <5–7 cm, and complete transurethral resection of the tumor).5 However, radio-sensitizing regimens may not effectively target micro-metastases, and the efficacy of a bladder-sparing approach is more appreciable in locoregional control than in the prevention of systemic disease relapse.6 7 Treatment of localized UC with 5-fluorouracil (5FU) plus mitomycin-based CRT has a disease-free survival (DFS) of only 50% at 2 years from the start of CRT.7 Radio-sensitizing chemotherapy regimens are more tolerable for older adults. Hence, CRT may be used in some patients with locally advanced disease.8
There has been significant success in enhancing the effect of standard treatments for UC with immune checkpoint inhibition (ICI).9 10 Nivolumab, an antibody against programmed death-1 receptor, has a response rate of 19.6% in previously treated locally advanced or metastatic UC11 and is effective as an adjuvant treatment for patients with localized or locoregional UC with a high risk of relapse after radical surgery, with a median metastasis-free survival of 22.9 months compared with 13.7 months in those who received placebo.12 Preclinical studies suggest that radiation-induced tumor-specific immune response is enhanced by inhibiting checkpoints in the immune system, both locally and abscopally.13 There is proven clinical benefit of adjuvant durvalumab following completion of CRT in patients with lung cancer14 and adjuvant nivolumab in patients with esophageal or gastroesophageal junction tumors, treated with neoadjuvant CRT.15 The NEXT trial was designed to assess the efficacy and safety of nivolumab adjuvant to CRT in patients with localized or locoregional UC. In this study, we chose to study nivolumab adjuvant to CRT to avoid compounding toxicities from concurrent administration with CRT.
Methods
Study design and participants
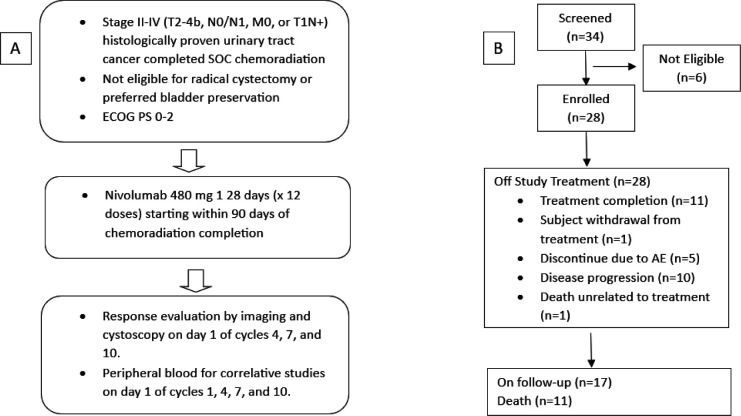
NEXT is a multi-institutional, single-arm, investigator-initiated, phase II study, evaluating the efficacy and safety of nivolumab adjuvant to CRT in patients with histologically confirmed localized or locoregional UC who were not eligible for radical surgery or elected an organ-sparing approach (Clinical trial information: NCT03171025). The study schema is summarized in figure 1A. The primary endpoint is failure-free survival (FFS) at 2 years defined as the time between the start date of CRT and the date of first recurrence or non-cancer-related death. Disease recurrence is defined as follows: (1) Local, urothelial tract: any high-grade or muscle-invasive lower or upper urothelial tract recurrence of UC including intravesical recurrences for subjects with an intact bladder that requires additional treatment other than transurethral resection. Any residual or new UC lesions requiring partial or radical cystectomy, including those thought to be a second UC primary, will be considered recurrences. (2) Local, non-urothelial tract: any recurrence in pelvic soft tissue or involving pelvic nodes below the aortic bifurcation. (3) Distant: any non-local recurrence.
Figure 1. (A) Study schema. (B) Consolidated Standards of Reporting Trials diagram. AE, adverse event; ECOG PS, Eastern Cooperative Oncology Group performance status; SOC, standard of care.
We hypothesized that targeting localized or locoregional UC and its micro-metastases with nivolumab after completion of CRT would offer an additional DFS rate of at least 20% at 2 years above the 50% DFS rate observed with CRT alone, as seen in the BC2001 study.7 The hypothesized efficacy of this organ-preserving approach matches the efficacy of the current front-line treatment approach using neoadjuvant chemotherapy followed by radical surgery. Secondary endpoints include the rate of acute and late treatment-related toxicity, patterns of recurrence, overall survival (OS) (defined as the time from day one of CRT until death), and QoL measures. Exploratory objectives include sequencing of cell-free DNA (cfDNA) collected prior to treatment at C1D1 and C4D1 to correlate with outcomes.
All patients were required to provide informed consent before enrolling in the study. The study was performed in accordance with the Declaration of Helsinki and the International Conference of Harmonization Good Clinical Practice guidelines.
Eligibility
Eligible patients were 18 years and older, had Eastern Cooperative Oncology Group performance status (ECOG PS) of 2 or less, and had received standard of care (SOC) definitive radiation therapy with radio-sensitizing chemotherapy for histologically proven stage II-IV (T2-4b, N0/N1, M0, or T1N+) UC (bladder, urethra, or lower ureter). Patients were excluded if they had non-regional metastatic disease, CIS or lymph node involvement outside of the radiation field or an absolute contraindication for immunotherapy. The presence of variant histology (adeno, sarcomatoid or squamous cell carcinoma) was not exclusionary, like the approach in BC2001. Although predominant urothelial histology was not required, neuroendocrine features were exclusionary. Non-metastatic disease was confirmed by scans prior to enrollment. Disease reassessment by cystoscopy following CRT completion was not mandated for enrollment.
Procedures and treatments
All patients received nivolumab 480 mg as a 30 min intravenous infusion on day 1 of a treatment cycle every 4 weeks (28 days) for a maximum of 12 doses or until recurrence, unacceptable toxicity, withdrawal of consent, or study completion. The first dose of nivolumab was administered within 90 days after completion of CRT. The radiation strategy and accompanying chemotherapy regimen were per SOC and based on the treatment team’s discretion. Dose interruptions were allowed to address any adverse event, laboratory abnormality, or intercurrent illness at the investigator’s judgment.
While on protocol, patients underwent physical examination with ECOG PS assessment and laboratory testing with every cycle of nivolumab. Safety and toxicity assessments were performed using the Common Terminology Criteria for Adverse Events. QoL assessment using the previously validated National Comprehensive Cancer Network/Functional Assessment of Cancer Therapy (NCCN/FACT) Bladder Symptom Index-18 (FBSI-18) questionnaire16 and Radiation Therapy Oncology Group (RTOG) toxicity evaluations were performed prior to treatment initiation and at cycles 4, 7, and 10, and at the end of treatment (EOT). Disease status was assessed with imaging studies (CT or MRI) and cystoscopy prior to treatment initiation, before cycles 4, 7, and 10, at the EOT and subsequent follow-up in patients who had completed 12 cycles of nivolumab or if patients discontinued treatment for reasons other than progression.
As part of the correlative studies, cell-free DNA (cfDNA) was extracted from plasma before cycles 1 and 4 and subjected to shallow whole-genome sequencing. Sequence data was mapped to the human reference genome (HG19) and read-count statistical analyses were performed to call somatic copy number aberrations; the copy number instability (CNI) score was derived from all statistically significant altered regions and represents a measure of circulating tumor DNA (ctDNA) level. CNI was selected for cfDNA determination given recent studies showing this biomarker to detect less than 1% tumor-derived cfDNA and to be an early predictor of response to immunotherapy.17 Circos plots were constructed to obtain z-scores of the CNI analysis for each patient. The genes located in a region that had a z-score above or below the normal threshold were then identified.
Outcomes
FFS was defined as the time from the start of CRT to the time of disease recurrence (systemic or locoregional) or death from any cause. All subjects who received at least one infusion of nivolumab during the study and had follow-up until the primary endpoint were considered evaluable for the primary endpoint of FFS at 2 years.
Local disease recurrence was defined as any high-grade lower or upper urothelial tract recurrence of cancer. Any residual or new UC lesions requiring partial or radical surgery, including those thought to be a second UC primary, were considered recurrences. Any recurrence in pelvic soft tissue or involving pelvic nodes below the aortic bifurcation was also defined as local non-urothelial recurrence. Any metastatic recurrence not meeting the above definition was defined as distant recurrence.
Secondary endpoints included safety and toxicity, QoL and FFS from nivolumab initiation. Exploratory endpoints included CNI levels before cycles 1 and 4 in relation to disease relapse and OS patterns.
Statistical analyses
Sample size determination
The sample size was chosen based on the maximum width of a two-sided 95% binomial CI for 2 year FFS. We hypothesized that the 2-year FFS would be 70% or greater with the BC2001 study as reference, which showed a 2-year DFS, both distant and metastatic, of 50% with CRT alone.7 With 28 evaluable subjects and no censored observations, the 95% exact binomial CI, estimated using the Clopper-Pearson method, extends no more than 20% from the observed FFS.
Analysis
Patients’ characteristics were summarized using descriptive statistics. All patients who received at least one infusion of nivolumab in the study and had follow-up until the primary endpoint were considered evaluable and included in the outcome analysis. The Kaplan-Meier method was used to determine time-to-event endpoints (FFS and OS). The association of predefined risk factors and CNI levels with FFS and OS was examined using log-rank tests and Cox proportional hazards models. Nivolumab-related adverse events (AEs) and RTOG AEs were summarized as the number and percentage of patients with AEs.
As the NEXT study was enrolling, it became evident that the patients receiving CRT as a bladder preservation strategy for locally advanced UC had multiple high-risk disease characteristics (further defined below), and our study population was at a higher risk of relapse than the historical reference. Because of this, we performed a post hoc stratification analysis evaluating the effect of high-risk features at baseline (plasmacytoid differentiation, T4, N+, multiple tumors, tumors >5 cm, residual disease before CRT, CIS, and hydronephrosis)5 18 19 on predefined time-to-event endpoints.
To assess the impact of the trial regimen of adjuvant nivolumab on the QoL of patients, self-reported QoL parameters from the FBSI-18 questionnaire were analyzed from the start of nivolumab on the trial. The QoL measures were tabulated chronologically as assessed on follow-up; descriptive statistics were used to assess completion rates and changes in QoL. The range and median of the QoL scores and change in QoL scores on treatment were assessed. Patients were placed in three groups: patients who dropped out because of an AE (n=5), patients who dropped out because of progression or death (n=11), and patients who either completed or decided to discontinue treatment (n=12). Because of the small sample size (n=5 for one group), it was decided to use linear trend models from baseline to cycle 10. The models assumed that the four measurement times (baseline, cycle 4, cycle 7, cycle 10) were spaced apart as indicated, at relative times 0, 4, 7, and 10. The EOT was treated as a separate category because it could occur at any time relative to the cycles.
All statistical analysis was performed using R V.4.3.2. (Copyright 2023, The R Foundation for Statistical Computing).
Results
Patients and disease characteristics
From August 2017 to January 2023, a total of 28 patients were enrolled in the study at two sites in the USA. Their outcomes and disposition are summarized in figure 1B. The median follow-up was 26.12 months (range 7.98–52.11 months) for the overall population (N=28). All patients had either completed or discontinued study treatment at the time of data cut-off. The most common reason for treatment discontinuation was disease progression, seen in 10 patients (36%), while 5 patients (18%) discontinued nivolumab because of AEs. One patient (4%) discontinued treatment due to travel burden, and one patient (4%) died due to causes unrelated to disease or treatment-related AEs (figure 1B). Patients and disease characteristics are described in table 1. The median age was 72 years (range, 54–86 years). 10 patients (36%) had greater than T2 and/or nodal involvement. Six patients received neoadjuvant systemic chemotherapy prior to CRT. Most of the patients underwent chemoradiation as they were not surgical candidates due to comorbidities or advanced disease (table 1).
Table 1. Baseline patient characteristics.
| Characteristics | Patients (N=28) |
| Sex – no (%) | |
| Male | 25 (89) |
| Female | 3 (11) |
| Age – years | |
| Median (range) | 72 (54–86) |
| Race | |
| White | 26 (93%) |
| Unknown | 2 (7%) |
| ECOG performance status | |
| 0 | 13 (46%) |
| 1 | 15 (54%) |
| Smoking status | |
| Former | 17 (61%) |
| Never smoker | 10 (36%) |
| Current | 1 (3%) |
| Site of primary disease | |
| Bladder | 27 (96%) |
| Urethra | 1 (4%) |
| Histology | |
| Urothelial | 27 (96%) |
| Adenocarcinoma | 1 (4%) |
| Urothelial with plasmacytoid or signet ring cells | |
| Yes | 3 (11%) |
| No | 24 (89%) |
| Clinical T stage | |
| T1 | 0 |
| T2 | 20 (71%) |
| T3 | 6 (21%) |
| T4 | 2 (7%) |
| Tumor greater than 5 cm | |
| Yes | 14 (50%) |
| Clinical nodal stage | |
| N0 | 25 (89%) |
| N1 | 2 (7%) |
| N2 | 1 (4%) |
| Hydronephrosis | |
| Yes | 12 (43%) |
| Multifocal disease | |
| Yes | 8 (29%) |
| CIS | |
| Yes | 7 (25%) |
| Residual disease visualized prior to chemoradiation | |
| Yes | 7 (25%) |
| No | 21 (75%) |
| Prior therapy for localized disease | |
| Yes | 9 (32%) |
| No | 19 (68%) |
| Prior systemic therapies for UC | |
| Gemcitabine and cisplatin | 4 (45%) |
| Dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin | 2 (22%) |
| Prior intravesical therapies | |
| Mitomycin C | 1 (11%) |
| BCG | 2 (22%) |
| Reason for bladder preservation approach | |
| Surgical risk due to comorbidities | 14 (50%) |
| Patient preference | 11 (39%) |
| Unresectable disease | 1 (4%) |
| Other | 2 (7%) |
One patient had prior bowel surgery and the other had a primary urethral tumor.
CIS, carcinoma in situ; ECOG, Eastern Cooperative Oncology Group; UC, urothelial carcinoma
Chemoradiation
The CRT characteristics are summarized in online supplemental table 1. The median number of fractions of radiation therapy (RT) patients received was 31.5 (range 20–36). The median total radiation dose was 57.2 Gy (range 46–66 Gy). 13 patients (46%) received hypo-fractionated RT. 18 patients (64%) received RT to the regional lymph nodes. Two patients (7%) could not complete the prescribed course of radiation. Radio-sensitizing regimens given concomitantly with radiation were 5-FU-based, cisplatin-based, and gemcitabine in 14 (50%), 11 (39%), and 3 (11%) patients, respectively. Three patients (11%) could not complete the prescribed chemotherapy regimen.
Clinical efficacy and survival
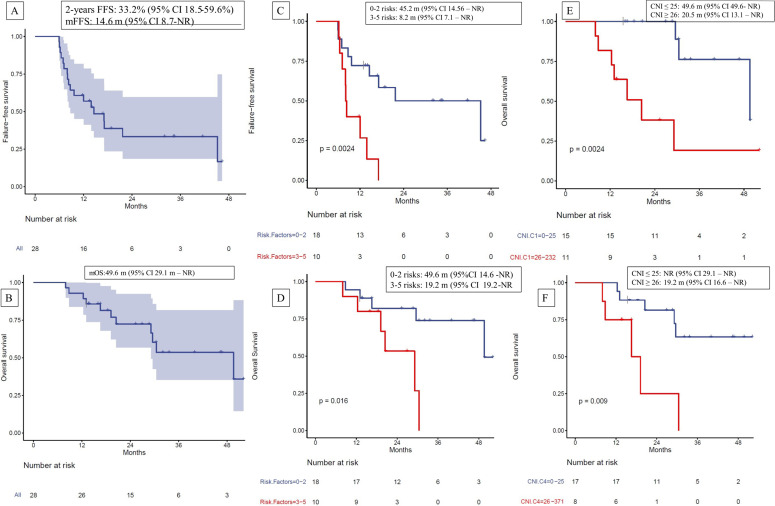
At the time of data cut-off (January 31, 2024), 17 patients (61%) had progressed, and 1 had died without any evidence of progression. Of these, 9 patients (32%) had local progression within the urothelial tract, and 8 (29%) had distant progression. The FFS at 2 years was 33.2% (95% CI 18.5% to 59.6%) (figure 2A). Five patients are still on follow-up and have not yet reached 2 years since the start of CRT. The median failure-free survival (mFFS) was 14.6 months (95% CI 8.71 months to not reached (NR)) (figure 2A). The mOS was 49.6 months (95% CI 29.1 to NR) (figure 2B). The mFFS after the start of nivolumab was 11.1 months (95% CI 5.75 months to NR) (online supplemental figure 1).
Figure 2. Kaplan-Meier curves for (A) failure-free survival (FFS) at 2 years from start of chemoradiation for the overall population; (B) overall survival (OS) for the overall population; (C) FFS stratified by risk factors; (D) mOS stratified by risk factors; (E) mOS stratified by CNI on C1D1; (F) mOS stratified by CNI on C4D1. CNI, copy number instability; mFFS, median FFS; mOS, median OS; NR, not reached.
We assessed the effect of high-risk characteristics such as plasmacytoid differentiation, T4, N+, multiple tumors, tumors >5 cm, residual disease before CRT, CIS, and hydronephrosis on mFFS. Among the 28 patients treated in the study, 25 (89%) had at least one high-risk feature. The mean number of high-risk features was 2 (range 0–5). Most patients (22, 79%) had one to three high-risk features. The mFFS of patients with three or more risk factors was significantly worse compared with those with two or fewer high-risk features (8.2 months vs 45.2 months, HR 4.66, 95% CI 1.58 to 13.70, p=0.002) (figure 2C). Similarly, the mOS of patients with three or more high-risk factors was significantly worse compared with those with two or fewer high-risk factors (OS 19.2 months vs 49.6 months, HR 4.53, 95% CI 1.20 to 17.07, p=0.016) (figure 2D).
Of the nine patients (32%) who experienced local recurrence, two had muscle-invasive disease, and seven had either CIS or Ta/T1 disease. Three patients had radical cystectomy to treat the local recurrence. None of the patients with local recurrence experienced distant recurrence. One patient died from surgical complications following radical surgery, and one from locally advanced disease.
Safety
The median number of cycles of nivolumab was 7 (range 1–12). 11 patients (39%) completed the planned 12 cycles of nivolumab therapy. The treatment-related toxicities and RTOG toxicities are listed in table 2. A total of 18 patients (64.3%) developed any grade AEs, and 3 patients (10.7%) developed grade 3 events. Of note, no toxicity greater than grade 3 was observed. The most common grade 1–2 treatment-related toxicities were pruritus (14%), diarrhea (14%), fatigue (14%), and hypothyroidism (10.7%). The observed grade 3 treatment-related toxicities were elevated liver enzymes (3.6%), diarrhea (3.6%), and polymyalgia rheumatica (3.6%) (table 2). A total of five patients (18%) required oral steroids for management of an immune-related AE. No patients required intravenous steroids.
Table 2. Treatment-related adverse events seen in two or more patients and RTOG toxicity grade 3 adverse events.
| Adverse event | Grade 1–2 n (%) | Grade 3 n (%) | Any grade n (%) |
| Total treatment-related adverse events (RTOG not included) | 18 (64.3) | 3 (10.7) | 18 (64.3) |
| Diarrhea | 4 (14) | 1 (3.6) | 5 (17.8) |
| Pruritus | 4 (14) | 0 | 4 (14) |
| Fatigue | 4 (14) | 0 | 4 (14) |
| Hypothyroidism | 3 (10.7) | 0 | 3 (10.7) |
| Liver enzyme elevation | 1 (3.6) | 1 (3.6) | 2 (7) |
| Alopecia | 2 (7) | 0 | 2 (7) |
| Arthritis | 2 (7) | 0 | 2 (7) |
| Hyperglycemia | 2 (7) | 0 | 2 (7) |
| Hyperthyroidism | 2 (7) | 0 | 2 (7) |
| Thrombocytopenia | 2 (7) | 0 | 2 (7) |
| Leukopenia | 2 (7) | 0 | 2 (7) |
| Polymyalgia rheumatica | 0 | 1 (3.6) | 1 (3.6) |
| Radiation toxicity (RTOG) toxicity | 17 (60.7) | 2 (7) | 19 (67.8) |
| RTOG+AEs (total events) | 26 (92.9) | 5 (17.8) | 27 (96.4) |
No grade 4 or higher adverse events were reported.
AEs, adverse eventsRTOG, Radiation Therapy Oncology Group
Regarding RTOG toxicity scores, all mean toxicity scores after starting nivolumab were lower than baseline, with a significant reduction in this measurement noted at cycle 4 compared with the baseline (p=0.0149), suggesting that adjuvant nivolumab did not worsen or cause long-term radiation-related toxicity during the study (table 3).
Table 3. Radiation Therapy Oncology Group toxicity analysis over time.
| Time | Estimate | SE | T value | P value |
| Baseline (reference) | 0.2356 | — | — | — |
| Cycle 4 | −0.4364 | 0.1759 | −2.4813 | 0.0149 |
| Cycle 7 | −0.0807 | 0.2026 | −0.3985 | 0.6912 |
| Cycle 10 | −0.3486 | 0.2200 | −1.5845 | 0.1165 |
| End of treatment | −0.2703 | 0.1848 | −1.4626 | 0.1470 |
| Follow-up | −0.4553 | 0.1849 | −2.4630 | 0.0157 |
Quality of life
Patients who completed or discontinued the study, not secondary to AEs, progression or death, had a mean FBSI-18 total score of 55.72 (SD=9.2) at screening and 57.10 (SD=7.17) at the EOT. In a linear model trend analysis, the change between FBSI-18 total score at screening and at the EOT was not statistically significant (p=0.94). Similar trends were observed in patients who discontinued the trial secondary to AEs (p=0.23) or disease progression (p=0.6) (table 4). However, in a combined model with strata, we looked for potential differences between the three groups, with the patients who completed treatment as the reference group. We found that the patients who eventually dropped out because of progression/death may have started with lower QoL (p=0.079) relative to the group that completed treatment. The group with AEs had a decline in QoL over time relative to the group that completed treatment (p=0.018). This group also trended towards a lower QoL at the EOT than the group that completed treatment (p=0.065) (online supplemental table 2).
Table 4. FBSI-18 score and SD of patients at the time of screening, cycles 4, 7, 10, and at the end of treatment.
| Time | All patientsScore; SD (N=5) | Patients who dropped out because of AEsScore; SD (N=5) | Patients who dropped out due to progression or deathScore; SD (N=11) | Patients who completed or dropped out for other reasonsScore; SD (N=12) |
| Screening | 52.53; 8.98 | 51.57; 15.91 | 50.18; 6.60 | 55.72; 9.20 |
| Cycle 4 | 54.32; 9.80 | 39.00; 12.73 | 51.84; 7.81 | 59.36; 7.68 |
| Cycle 7 | 54.89; 14.10 | 27.00; NA | 57.17; 2.57 | 56.81; 13.92 |
| Cycle 10 | 57.60; 8.70 | NA; NA | 51.13; NA | 58.18; 8.87 |
| EOT | 52.20; 11.43 | 30.75; NA | 48.13; 12.60 | 57.10; 7.17 |
| P value | 0.94 | 0.23 | 0.60 | 0.94 |
P- values for the model of linear trend from baseline to end of treatment.
EOT, end-of-treatment
Exploratory analysis
Patients with no documented recurrence by 9 months (n=9) had a mean CNI (mCNI) of 38.9 (SD=40.1) before initiating adjuvant immunotherapy. Similarly, those with non-muscle invasive local recurrence (n=7) had an mCNI of 31 (SD=31, p=1), and those with muscle-invasive local recurrence or distant recurrence had an mCNI of 55 (SD=69, p=0.34).
CNI levels were also assessed on C4D1. Patients with no documented recurrence by 9 months had an mCNI of 24 (SD=22.2). Those with non-muscle invasive local recurrence had an mCNI of 12.7 (SD=6.3, p=0.44), and those with muscle-invasive local recurrence or distant metastatic disease had an mCNI of 74.4 (SD=122, p=0.17). Although the difference in mean values from these groups is evident, this did not reach statistical significance as there was significant variability of CNI levels within each category.
We stratified FFS and OS based on CNI levels. We did not observe a statistically significant difference in FFS according to CNI levels. Patients with a CNI≤25 on C1D1 had an mFFS of 17.1 months (95% CI 9.7 to NR) compared with 8.7 months (95% CI 8 to NR) in those with a CNI≥26 (p=0.47) (online supplemental figure 2). Similarly, patients with a CNI≤25 on C4D1 had an mFFS of 12 months (95% CI 8.3 to NR) compared with 13.9 months (95% CI 7 to NR) in those with a CNI≥26 on C4D1 (p=0.69) (online supplemental figure 3). However, a statistically significant difference was observed in mOS of patients according to their baseline and C4D1 CNI; patients with a CNI≤25 on C1D1 had a mOS of 49.6 months (95% CI 49.6 to NR) compared with 20.5 months (95% CI 13.1 to NR) in those with a CNI≥26 (p=0.0024) (figure 2E). Similarly, in patients with a CNI≤20 on C4D1, mOS was not reached (95% CI 29.1 to NR) compared with 19.2 months (95% CI 16.6 to NR) in those with a C4D1 CNI≥26 (p=0.009) (figure 2F). Change in CNI levels between C1 and C4 was not associated with either FFS or OS in the Cox model.
The Circos plots of genes that had copy number changes suggesting deletion and amplification for all patients with documented progression, as obtained from the shallow whole genome sequencing of cfDNA of each patient are shown in online supplemental figure 4. The amplified genes were MYC, AKT2, ALK, SOX2, NKX2, and MDM4. The deleted genes were BRCA2, ARID1A, MAPK24, TSC2 and WT1. The affected genes and their pathways are listed in table 5.
Table 5. Copy number changes seen in cell-free DNA of patients with documented progression and associated affected pathways.
| Genes | Affected pathways |
| MAP2K4 Del | RTK/RAS pathway |
| ALK Amp | RTK/RAS pathway |
| ARID1A Del | Chromatin remodeling |
| TSC2 Del | PI3K pathways |
| AKT2 Amp | PI3K pathways |
| WT1 Del | WNT pathway |
| CDKN2A Del | Cell cycle progression |
| MYC Amp | Myc pathway |
| NKX2.1 Amp | Cytoskeleton remodeling |
| SOX2 Amp | Multiple pathways |
| MDM4 Amp | Cell cycle progression |
Amp, amplificationDel, deletion
Discussion
In the NEXT trial, we evaluated the efficacy and tolerability of nivolumab adjuvant to SOC CRT in patients with localized or locally advanced UC. We found a 2-year FFS of 33% since the start of CRT (figure 2A). Most of the recurrences were local and non-muscle-invasive (32% of patients), and the rate of metastatic disease progression was only 29%. The treatment was well tolerated with manageable adverse effects and there was no prolongation or worsening of radiation toxicity. Given the significant morbidity associated with definitive treatment of bladder cancer,3 QoL often motivates the decision to opt for CRT and compliance with adjuvant treatments. Importantly, we observed no significant change in QoL over time, favoring adjuvant nivolumab as a suitable option for patients from that standpoint.
The NEXT trial was designed referencing the BC2001 trial, in which the 2-year locoregional DFS with CRT was 67% with a 50% 2-year DFS (locoregional and metastatic). However, conclusive comparisons cannot be drawn as the two trials had very different patient populations. In the BC2001 study, 83% of patients had T2 disease, and none had nodal disease.7 In comparison, in the NEXT trial, 28% of enrolled patients had T3 or higher stage tumors, and 11% had at least N1 disease (table 1).
The presence of high-risk disease features in UC such as plasmacytoid differentiation, T4, N+, multiple tumors, tumors >5 cm, residual disease before CRT, CIS, and hydronephrosis is known to confer poor prognosis.518,20 The presence of one or more of these high-risk features in 89% of the enrolled patients reflects the selection bias in adjuvant trials for patients with higher risk disease. Similarly, the DUART study assessed the role of adjuvant durvalumab following concomitant durvalumab and RT in patients with T2-4, N0-2 UC who were deemed unresectable or unfit for surgical intervention. This study included 26.9% of patients with documented hydronephrosis and 27% of patients with residual disease following transurethral resection of bladder tumor (TURBT) and showed an mPFS of 21.8 months (95% CI 14.8 to NR) and mOS of 30.8 months (95% CI 22.9 to NR) with a 1-year OS of 83.3% (95% CI 70.4% to 99.7%).21 The NEXT cohort included 43% of patients with hydronephrosis and 25% with residual disease. Again, with the caveat that comparison across trials are limited by confounding factors, we note that, with a larger proportion of patients with documented hydronephrosis, a known factor associated with poor outcomes,20 CRT with adjuvant nivolumab led to a numerically worse mFFS (14.6 months vs 21.8 months) but better mOS compared with concurrent durvalumab and RT (49.6 months vs 30.8 months).21
Other recent studies have noted improved outcomes with concurrent immunotherapy and CRT. For instance, in a randomized phase II trial, concurrent nivolumab and CRT showed a 2-year locoregional/distant relapse-free survival of 59.9% (95% CI 30.7% to 80.1%) compared with 37.6% (95% CI 18% to 57.1%) with CRT alone, with similar toxicities between the two arms, further proving the potential benefit of adding immunotherapy to CRT.22 Two phase-3 clinical trials have investigated adjuvant immunotherapy following radical surgery in patients with muscle-invasive urothelial carcinoma (MIUC) showing promising results.12 23 The results of our trial add to the body of literature suggesting a clinical benefit of immunotherapy following local therapy for patients with MIUC.
The NEXT trial sheds light on an important aspect regarding appropriate choice of CRT for patients most likely to benefit from this treatment. As seen in figure 2C,D, both OS and FFS are significantly worse in patients who harbor three or more high-risk disease features. On the other hand, patients with limited risk disease can anticipate a more durable treatment benefit from CRT and adjuvant nivolumab. Enfortumab vedotin in combination with pembrolizumab has demonstrated a remarkable survival improvement for patients with locally advanced or metastatic UC with an mPFS of 12.5 months and mOS of 31.5 months24 and is the new SOC regimen in this setting.25 This regimen compares favorably to CRT with adjuvant nivolumab in locally advanced UC and was not available at the time of enrollment of patients on the NEXT trial. Given significant advances in the treatment of patients with locally advanced and metastatic disease as seen in the CheckMate 901 trial and the EV302 trial, it is reasonable to anticipate a role of “induction” systemic therapy first in patients with locally advanced and/or node-positive disease. Patients with pelvic-confined disease who have a response to systemic therapy may be considered for a potential consolidative approach afterwards.9 24 Chromosomal instability reflects the gains and losses of genomic material within cells, which are responsible for cancer genesis and progression. These genetic alterations are also important drivers of treatment resistance in patients with cancer.26 We observed that lower levels of chromosomal instability, as indicated by copy number variation in the plasma cfDNA, expressed as CNI on C1D1 and C4D1 was associated with significantly better mOS (figure 2E,F). Interestingly, the change in CNI from C1D1 to C4D1 was not prognostic for FFS or OS, this could be explained by the sample size and perhaps by the great variability observed in CNI at both time points. The amplified and deleted regions of the genome shed light on potential oncogenic drivers for future targeting (table 5). The cell cycle and receptor tyrosine kinase pathways have been identified as potential targets in UC in other studies.27,30 There are preclinical and early clinical reports of success with targeting UC with CDK 4/6 inhibitors and preclinical reports of efficacy with MEK inhibitors in UC, although these therapeutics remain experimental for this patient population.31,35 Moreover, our study adds to the cumulative evidence of the prognostic significance of ctDNA as demonstrated in the IMvigor 010, TOMBOLA and the KEYNOTE-361 trials.36,38
Our study has some important limitations. First, we performed a single-arm clinical trial, hence the actual benefit of adjuvant ICI remains to be assessed. The INTACT trial (NCT03775265) is a phase 3 clinical trial assessing the role of chemotherapy and radiation with or without atezolizumab in patients with localized UC.39 Similarly, the phase 3 KEYNOTE-992 study is assessing the role of pembrolizumab plus chemoradiotherapy versus placebo plus chemoradiotherapy in patients with MIUC.40 Additionally, the small study size and the heterogeneous nature of the patient population limit overall conclusive efficacy assessment from the NEXT trial. Nonetheless, stratification by risk factors and correlation with plasma cfDNA analyses generate hypotheses for potential patient selection and putative future therapeutic targets.
In conclusion, among patients with localized or locally advanced UC, who either elected to forego radical surgery or were deemed ineligible for this intervention, and hence completed CRT, adjuvant nivolumab was well tolerated with no new safety signals. FFS was better in patients with fewer number of high-risk disease features. Patients with high-risk disease features may benefit from systemic therapy. The results of this study are hypothesis-generating due to the limitations previously outlined and offer direction for future studies of personalized targeted therapy tailored to tumor genomics assessed on plasma cfDNA.
supplementary material
Acknowledgements
We would like to extend our deepest gratitude to the clinical trial office at Huntsman Cancer Institute/University of Utah and the Moffitt Cancer Center for their efforts in the completion of this trial. Most importantly, we want to thank the patients and their families, without whom this work would not have been possible.
Footnotes
Funding: This clinical trial received funding from Bristol Myers Squibb Pharmaceutical and Dr. Gupta is supported by the VA merit grant # 1I01BX005765.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: This study involves human participants and was approved by University of Utah Institutional Review Board - HCI IRB #100769.
Data availability free text: The data that support the findings of this study are available on request from the corresponding author.
Data availability statement
Data are available upon reasonable request.
References
- 1.Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12–49. doi: 10.3322/caac.21820. [DOI] [PubMed] [Google Scholar]
- 2.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–66. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 3.Yang LS, Shan BL, Shan LL, et al. A systematic review and meta-analysis of quality of life outcomes after radical cystectomy for bladder cancer. Surg Oncol. 2016;25:281–97. doi: 10.1016/j.suronc.2016.05.027. [DOI] [PubMed] [Google Scholar]
- 4.Guancial EA, Roussel B, Bergsma DP, et al. Bladder cancer in the elderly patient: challenges and solutions. Clin Interv Aging. 2015;10:939–49. doi: 10.2147/CIA.S74322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zlotta AR, Ballas LK, Niemierko A, et al. Radical cystectomy versus trimodality therapy for muscle-invasive bladder cancer: a multi-institutional propensity score matched and weighted analysis. Lancet Oncol. 2023;24:669–81. doi: 10.1016/S1470-2045(23)00170-5. [DOI] [PubMed] [Google Scholar]
- 6.Mak RH, Hunt D, Shipley WU, et al. Long-term outcomes in patients with muscle-invasive bladder cancer after selective bladder-preserving combined-modality therapy: a pooled analysis of Radiation Therapy Oncology Group protocols 8802, 8903, 9506, 9706, 9906, and 0233. J Clin Oncol. 2014;32:3801–9. doi: 10.1200/JCO.2014.57.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.James ND, Hussain SA, Hall E, et al. Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med. 2012;366:1477–88. doi: 10.1056/NEJMoa1106106. [DOI] [PubMed] [Google Scholar]
- 8.Jouhadi H, Sr, Sahraoui S, Benider A. Concurrent weekly cisplatin during radiation treatment of locally advanced bladder carcinoma in elderly patients. JCO. 2008;26:16109. doi: 10.1200/jco.2008.26.15_suppl.16109. [DOI] [Google Scholar]
- 9.van der Heijden MS, Sonpavde G, Powles T, et al. Nivolumab plus Gemcitabine-Cisplatin in Advanced Urothelial Carcinoma. N Engl J Med. 2023;389:1778–89. doi: 10.1056/NEJMoa2309863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Powles T, Park SH, Voog E, et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N Engl J Med. 2020;383:1218–30. doi: 10.1056/NEJMoa2002788. [DOI] [PubMed] [Google Scholar]
- 11.Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18:312–22. doi: 10.1016/S1470-2045(17)30065-7. [DOI] [PubMed] [Google Scholar]
- 12.Bajorin DF, Witjes JA, Gschwend JE, et al. Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma. N Engl J Med. 2021;384:2102–14. doi: 10.1056/NEJMoa2034442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pilones KA, Vanpouille-Box C, Demaria S. Combination of radiotherapy and immune checkpoint inhibitors. Semin Radiat Oncol. 2015;25:28–33. doi: 10.1016/j.semradonc.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med. 2017;377:1919–29. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 15.Kelly RJ, Ajani JA, Kuzdzal J, et al. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N Engl J Med. 2021;384:1191–203. doi: 10.1056/NEJMoa2032125. [DOI] [PubMed] [Google Scholar]
- 16.Jensen SE, Beaumont JL, Jacobsen PB, et al. Measuring priority symptoms in advanced bladder cancer: development and initial validation of a brief symptom index. J Support Oncol. 2013;11:86–93. doi: 10.1016/j.suponc.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weiss GJ, Beck J, Braun DP, et al. Tumor Cell-Free DNA Copy Number Instability Predicts Therapeutic Response to Immunotherapy. Clin Cancer Res. 2017;23:5074–81. doi: 10.1158/1078-0432.CCR-17-0231. [DOI] [PubMed] [Google Scholar]
- 18.Lockhart K, King S, Grant A, et al. Outcomes of poorly differentiated and plasmacytoid variant bladder urothelial carcinoma. BJUI Compass . 2022;3:62–7. doi: 10.1002/bco2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matulay JT, Kamat AM. Advances in risk stratification of bladder cancer to guide personalized medicine. F1000Res. 2018;7 doi: 10.12688/f1000research.14903.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu Z, Zhao J, Li Y, et al. Prognostic value of preoperative hydronephrosis in patients with bladder cancer undergoing radical cystectomy: A meta-analysis. PLoS One. 2019;14:e0222223. doi: 10.1371/journal.pone.0222223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joshi M, Tuanquin L, Zhu J, et al. Concurrent durvalumab and radiation therapy (DUART) followed by adjuvant durvalumab in patients with localized urothelial cancer of bladder: results from phase II study, BTCRC-GU15-023. J Immunother Cancer . 2023;11:e006551. doi: 10.1136/jitc-2022-006551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kougioumtzopoulou A, Koutsoukos K, Zakopoulou R, et al. 1961O Nivolumab plus chemoradiotherapy in patients with non-metastatic muscle-invasive bladder cancer (nmMIBC), not undergoing cystectomy: A phase II, randomized study by the Hellenic GU Cancer Group. Ann Oncol. 2024;35:S1133–4. doi: 10.1016/j.annonc.2024.08.2046. [DOI] [Google Scholar]
- 23.Apolo AB, Ballman KV, Sonpavde G, et al. Adjuvant Pembrolizumab versus Observation in Muscle-Invasive Urothelial Carcinoma. N Engl J Med. 2025;392:45–55. doi: 10.1056/NEJMoa2401726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Powles T, Valderrama BP, Gupta S, et al. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N Engl J Med. 2024;390:875–88. doi: 10.1056/NEJMoa2312117. [DOI] [PubMed] [Google Scholar]
- 25.Sidaway P. A new standard of care for advanced-stage urothelial carcinoma. Nat Rev Clin Oncol. 2024;21:336. doi: 10.1038/s41571-024-00884-0. [DOI] [PubMed] [Google Scholar]
- 26.Steele CD, Abbasi A, Islam SMA, et al. Signatures of copy number alterations in human cancer. Nature New Biol. 2022;606:984–91. doi: 10.1038/s41586-022-04738-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hussain SA, Palmer DH, Syn W-K, et al. Gene expression profiling in bladder cancer identifies potential therapeutic targets. Int J Oncol. 2017;50:1147–59. doi: 10.3892/ijo.2017.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zaravinos A, Lambrou GI, Volanis D, et al. Spotlight on differentially expressed genes in urinary bladder cancer. PLoS One. 2011;6:e18255. doi: 10.1371/journal.pone.0018255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bahar ME, Kim HJ, Kim DR. Targeting the RAS/RAF/MAPK pathway for cancer therapy: from mechanism to clinical studies. Signal Transduct Target Ther. 2023;8:455. doi: 10.1038/s41392-023-01705-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang J, Nie J, Ma X, et al. Targeting PI3K in cancer: mechanisms and advances in clinical trials. Mol Cancer. 2019;18:26. doi: 10.1186/s12943-019-0954-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sathe A, Koshy N, Schmid SC, et al. CDK4/6 Inhibition Controls Proliferation of Bladder Cancer and Transcription of RB1. J Urol. 2016;195:771–9. doi: 10.1016/j.juro.2015.08.082. [DOI] [PubMed] [Google Scholar]
- 32.Sonpavde GP, Grivas P, Milowsky MI, et al. Trial in progress: A phase 2, randomized, open-label study of trilaciclib with first-line, platinum-based chemotherapy and avelumab maintenance in untreated patients with locally advanced or metastatic urothelial carcinoma (PRESERVE 3) JCO. 2022;40:TPS585. doi: 10.1200/JCO.2022.40.6_suppl.TPS585. [DOI] [Google Scholar]
- 33.G1 Therapeutics G1 therapeutics providesthird quarter 2023 financial results and operational highlights. 2023
- 34.Xue Z, Vis DJ, Bruna A, et al. MAP3K1 and MAP2K4 mutations are associated with sensitivity to MEK inhibitors in multiple cancer models. Cell Res. 2018;28:719–29. doi: 10.1038/s41422-018-0044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tate T, Plumber SA, Al-Ahmadie H. Combined mek inhibition and pparg activation eradicates muscle invasive bladder cancer in a mouse model of BBN-induced carcinogenesis. bioRxiv. 2023 doi: 10.1101/2023.08.19.553961. Preprint. [DOI]
- 36.Powles T, Assaf ZJ, Davarpanah N, et al. ctDNA guiding adjuvant immunotherapy in urothelial carcinoma. Nature New Biol. 2021;595:432–7. doi: 10.1038/s41586-021-03642-9. [DOI] [PubMed] [Google Scholar]
- 37.Powles T, Chang Y-H, Yamamoto Y, et al. Pembrolizumab for advanced urothelial carcinoma: exploratory ctDNA biomarker analyses of the KEYNOTE-361 phase 3 trial. Nat Med. 2024;30:2508–16. doi: 10.1038/s41591-024-03091-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bjerggaard Jensen J, Birkenkamp-Demtröder K, Nordentoft I, et al. 1960O Identification of bladder cancer patients that could benefit from early post-cystectomy immunotherapy based on serial circulating tumour DNA (ctDNA) testing: Preliminary results from the TOMBOLA trial. Ann Oncol. 2024;35:S1133. doi: 10.1016/j.annonc.2024.08.2045. [DOI] [Google Scholar]
- 39.Singh P, Efstathiou JA, Tangen C, et al. INTACT (S/N1806) phase III randomized trial of concurrent chemoradiotherapy with or without atezolizumab in localized muscle-invasive bladder cancer: Safety update on first 73 patients. JCO. 2021;39:428. doi: 10.1200/JCO.2021.39.6_suppl.428. [DOI] [Google Scholar]
- 40.Gupta S, Fujii Y, Van Der Heijden MS, et al. Phase 3 KEYNOTE-992 study of pembrolizumab plus chemoradiotherapy versus placebo plus chemoradiotherapy in patients with muscle-invasive bladder cancer (MIBC) JCO. 2024;42:TPS720. doi: 10.1200/JCO.2024.42.4_suppl.TPS720. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.