Abstract
Attention deficit/hyperactivity disorder (ADHD) is a neurodevelopmental condition affecting cognitive and social functions all over childhood. Monosodium glutamate (MSG) is a common food additive associated with ADHD-like symptoms in children. Nutraceuticals, like sesamol (SE) and astaxanthin (AST), or physical activity (PHA) were reported to possess beneficial effects on human health. Meanwhile, still their neuroprotective effect against ADHD has been poorly investigated. This study aimed to investigate the impact of SE, AST and PHA either separately or combined on ADHD-like behaviors induced by MSG in rat pups. Eighty-four male Sprague Dawley rat pups were randomly allocated into seven groups; control, MSG, (PHA + MSG), (SE + MSG), (AST + MSG), (SE + AST + MSG), and (COMB [PHA + SE + AST] + MSG) and treated for eight weeks. MSG-induced ADHD-like behavior was evaluated, via assessing behavioral outcomes; neurotransmitters’ levels; five pathway biomarkers, coupled with histopathological and immunohistochemical studies. Rats exposed to PHA or treated with SE or AST either separately or combined exhibited enhanced attention, locomotor, and cognitive abilities, compared to MSG-intoxicated group. All treatments remarkably improved MSG-induced abnormalities in neurotransmitters’ levels; biochemical markers; along with histological findings, via modulating HMGB1/RAGE/JAK-2/STAT-3, PI3K/AKT/CREB/BDNF, AMPK/SIRT-1 and PERK/CHOP pathways. Nevertheless, the combination of PHA with nutraceuticals (SE and AST) elicited more favorable effects in all measured parameters and histological findings, compared to other treated groups. In conclusion, this study revealed the superiority of the combination of nutraceuticals with PHA, over other standalone treatments, in amelioration of MSG-induced ADHD-like behaviors in rat pups, via fine-tuning of HMGB1/RAGE, PI3K/AKT/CREB/BDNF, AMPK/SIRT-1 and PERK/CHOP pathways.
Graphical Abstract
Keywords: ADHD, Monosodium glutamate, Sesamol, Astaxanthin, Autophagy, Endoplasmic reticulum stress, Physical activity
Introduction
Attention deficit/hyperactivity disorder (ADHD), is a neurodevelopmental ailment, which is instigated in childhood and recognized by impaired attention, hyperactivity and impulsivity (Jerome et al. 2020). It negatively impacts the cognitive and social functions during childhood, hence extensively affecting patients’ education and social life, producing grave socio-economical burdens (Li and He 2021). Although the etiopathological mechanisms underlying ADHD are not fully understood, yet the dysregulation in catecholamine neurotransmitters, including dopamine (DA) and norepinephrine (NE) within the prefrontal cortex, hippocampus and striatum, which results in executive dysfunction, had been reported to be tightly linked with the pathogenesis of ADHD (Posner et al. 2020). Previous studies stated that disturbance in glutamatergic function throughout developmental phases is a major contributor to ADHD’s symptoms and pathophysiology. In fact, glutamate, the chief excitatory neurotransmitter in the central nervous system, controls learning and memory function and adjusts the catecholaminergic activity that had been involved in ADHD’s etiopathology (Featherstone 2010). Remarkably, excessive glutamate levels are excitotoxic, where its elevated levels with the consequent activation of its receptors are the key contributors to excitotoxicity (Pan et al. 2012), the core hallmark documented in ADHD, which is accompanied by elevated intracellular calcium level, disruption of mitochondrial function, enhanced reactive oxygen species (ROS) levels and neuroinflammation, hence ending up with neuronal apoptosis (Glaser et al. 2022).
Along with catecholaminergic dysregulation, perturbed redox status and enhanced neuroinflammation have been recognized as key players in the pathophysiology of ADHD (Leffa et al. 2018). Excessive ROS in the brain elicit a detrimental effect on the integrity of neurons, causing neuronal apoptosis (Smaga et al. 2015). Besides, elevated ROS also leads to the activation of astrocytes and microglia, along with the excessive generation of pro-inflammatory mediators (Solleiro-Villavicencio and Rivas-Arancibia 2018). One of the major contributors in the inflammatory cascades is the high mobility group box 1 protein (HMGB1), which is overexpressed during neuroinflammation. HMGB1 binds to its receptor, namely receptor for advanced glycation end products (RAGE) to trigger the downstream inflammatory response and produce various pro-inflammatory mediators, like tumour necrosis factor-alpha (TNF-α) and interleukin-1beta (IL-1β), leading to neuroinflammation and neuronal apoptosis (Lotze and Tracey 2005).
Another pathway implicated in neuroinflammation is janus kinase-2 (JAK-2)/signal transducer and transcription-3 (STAT-3) cascade that is activated by the effect of pro-inflammatory mediators. JAK-2 enhances the phosphorylation of STAT-3, leading to astroglial activation and exaggeration of inflammatory response in neurological disorders (Hou et al. 2017). Interestingly, neuroinflammation further fosters the production of ROS. Thus, neuroinflammation and oxidative stress are interrelated mechanisms in the ADHD pathophysiology (Leffa et al. 2018). Further, oxidative damage is strongly related with the occurrence of neuroinflammation, enhanced endoplasmic reticulum (ER) stress, excessive apoptosis and impaired autophagy, where these mechanisms are interconnected with the pathophysiology of neurodevelopmental disorders, such as ADHD (Coulson et al. 2022).
Besides, pro-inflammatory mediators also hamper the production of brain-derived neurotrophic factor (BDNF), thus impairing BDNF signaling. Disrupted BDNF signaling has been linked with neurological disorders, such as ADHD and mood disorders. Indeed, BDNF is essential for several aspects of brain activity, including neurological development, neuronal growth and neuronal plasticity (Rossi et al. 2006). Noteworthy, the activation of BDNF signaling is under the control of the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) pathway. Upon activation of PI3K/AKT cue, activated PI3K phosphorylates and activates AKT, with consequent activation of cyclic adenosine monophosphate (CAMP) response element-binding protein (CREB), which enhances the generation of BDNF (Luo et al. 2021). Subsequently, BDNF binds with its receptor tropomyosin receptor kinase B (TrkB), to regulate memory and neuronal survival (Mir et al. 2017). Of note, activated AKT results in halting neuroinflammation, oxidative stress and neuronal apoptosis (Luo et al. 2021). Thus, PI3K/AKT/CREB/BDNF/TrKB hub may be regarded as a striking therapeutic objective for the amelioration of neurodevelopmental disorders, like ADHD.
In addition to enhanced oxidative stress and neuroinflammation, defective autophagy is a common feature in the pathophysiology of neurodevelopmental maladies, like ADHD, where suppressed autophagy, results in neuronal loss (Abdulghani et al. 2023). Undoubtedly, autophagy is indispensable in the selective degradation of damaged organelles and misfolded proteins, thereby repressing neuronal injury, improving cognitive function and enhancing neuronal survival (Cerri and Blandini 2019). AMP-activated protein kinase (AMPK)/Sirtuin-1 (SIRT-1)/mechanistic target of rapamycin (m-TOR) cue is the chief path for regulating the autophagic processes, as the activation of both SIRT-1 and AMPK stimulates autophagy, whereas m-TOR inhibits autophagy (Garcia and Shaw 2017). AMPK, a modulator of autophagy, elicits its actions through the activation of SIRT-1 to enhance autophagy and halt apoptosis (Meijer and Codogno 2007). Moreover, activation of AMPK and SIRT-1 boosts autophagy by repression of m-TOR. Consequently, modulation of the AMPK/SIRT-1/m-TOR pathway has been proposed as an attractive approach for promoting autophagy and enhancing neuronal survival (Curry et al. 2018).
Alongside impaired autophagy, excessive ER stress possesses a pivotal part in the pathogenesis of neurological diseases including, neurodevelopmental disorders, as it enhances neuronal apoptosis (Coulson et al. 2022). ER is important for protein synthesis and appropriate protein folding, together with maintaining calcium homeostasis (Anelli and Sitia 2008). Nevertheless, stressful conditions, including exaggerated oxidative stress, inflammation, impaired autophagy and heightened glutamate level, are believed to be linked with abnormal protein misfolding and accumulation of misfolded proteins and calcium, with the resultant enhanced ER stress. Thus, instigating unfolded protein response (UPR) to relieve ER stress or otherwise induce apoptosis (Zhang et al. 2018). Among the UPR sensors accountable for the activation of ER stress pathway is protein kinase RNA-like ER kinase (PERK), where during ER stress, glucose-regulated protein 78 (GRP78) separates from PERK, thus activating PERK (Kudo et al. 2008). Upon prolonged ER stress, the UPR pathway fails to restore ER homeostasis, thereby the overactivation of UPR shifts toward the apoptotic pathway, where PERK stimulates the pro-apoptotic C/EBP homologous protein (CHOP) expression to initiate ER stress-induced apoptosis (Huang et al. 2019). Subsequently, hindering ER stress could alleviate neuronal apoptosis and promote neuronal survival.
Monosodium glutamate (MSG), a flavor enhancer, is commonly utilized to improve the palatability of many types of foods, including soups, salads and chips (Gürgen et al. 2021). Despite its extensive usage, MSG's health safety as a food chemical is still highly debatable, where previous studies had reported that MSG intake was associated with adverse outcomes on multiple organs, including cardiotoxicity, hepatotoxicity and neurotoxicity (Eid et al. 2019). In the context of neurotoxicity, MSG intake is associated with various neurological conditions, including depression and ADHD (Kazmi et al. 2017). MSG is a natural sodium salt of glutamic acid, where MSG exposure is linked with excitotoxicity through the binding of glutamate to its receptors, leading to overexcitation of the nerve cells, along with enhancing calcium influx, thereby inducing neuronal apoptosis in various brain regions, such as the hippocampus (Khafaga et al. 2021). Unquestionably, elevated level of circulating glutamate had been interconnected with the excessive production of ROS and the generation of inflammatory mediators (Gaffen and Liu 2004). Earlier reports revealed that MSG remarkably disrupted catecholamine neurotransmitters’ levels, as it lessened NE and DA levels in the brain (Liu et al. 2022). Furthermore, excitotoxicity due to the disruption of glutamatergic neurotransmission with the perturbations in the levels of catecholamine neurotransmitters resulting from MSG contributed to the appearance of various behavioral deficits including, impaired memory and cognitive function, together with anxiety-like behaviors and hyperactivity, which are core symptoms of ADHD (Rosa et al. 2015). Of note, the major contributors to MSG neurotoxicity are excitotoxicity, disruption of catecholamine neurotransmission, oxidative stress, activation of inflammatory cascades, and neuronal apoptosis (Seiva et al. 2012). Hence, MSG exposure resulted in pathophysiological events and symptoms that mimic those of ADHD. In this respect, MSG had been reported to be used in inducing ADHD-like symptoms in experimental animals to study the pathophysiological mechanisms contributing to ADHD and to investigate the effectiveness of therapeutic agents in amelioration of ADHD-like symptoms (Khaled Abd et al. 2021).
In view of the notion that ADHD’s pathophysiology is tightly linked with dysregulation in catecholamine neurotransmission. So far, dextroamphetamine and methylphenidate are the most commonly used medications for controlling ADHD symptoms, via restoring catecholamine levels (Brown et al. 2018). Nonetheless, the long-term use of these medications had been associated with the incidence of many adverse events, such as headache, dry mouth and liver injury (Ahn et al. 2016). Hence, there is an increased demand to find safer therapeutic strategies capable of ADHD amelioration. Since oxidative stress and inflammation are two main interrelated mechanisms involved in the pathological process of ADHD. Consequently, the usage of phytochemicals with antioxidant and anti-inflammatory activities is mandatory in ameliorating ADHD with its associated neuronal apoptosis (Ding et al. 2022). Amongst, sesamol (SE), a dietary natural phenolic component derived from sesame lignans, possesses antioxidant, free radical-scavenging, anti-hyperlipidemic, anti-inflammatory and anti-apoptotic properties (Kanimozhi and Prasad 2009; El‐Borai et al. 2022). Prior studies documented that SE exerted neuroprotective effects against different models of neurological diseases (Beheshtimanesh and Rajaei 2023; Wang et al. 2023). In addition, astaxanthin (AST) is a natural red–orange carotenoid pigment that is found in microalgae, crabs and shrimp. AST had been shown to possess potent antioxidant, anticancer, anti-apoptotic, anti-inflammatory and neuroprotective properties (Kishimoto et al. 2016). Previous studies reported that AST displayed a marked neuroprotective effect against different models of neurological maladies (Adiguzel et al. 2023). Based upon the documented antioxidant and anti-inflammatory properties of SE and AST, we selected to study the effects of SE and/or AST, as potential candidates, against MSG-induced ADHD-like behaviors. Apart from the usage of phytochemicals, physical activity (PHA) had been documented to exert advantageous effects against neurological disorders and cognitive impairment, where regular exercise possesses favorable actions on brain function and memory, thus guarding against neuronal damage (Valenzuela 2008; Ali et al. 2017).
Given the complex pathophysiology of ADHD, a combination regimen targeting various pathways has become an attractive therapeutic approach. Accordingly, this study was designed to inspect the neuroprotective effects of SE, AST, or PHA either alone or in combination with each other against MSG-induced ADHD-like behaviors in rat pups. Besides, this study provided insights into the mechanistic targets through which these agents exerted their beneficial actions, with highlighting the role of HMGB1/RAGE/JAK-2/STAT-3, PI3K/AKT/CREB/BDNF/TrkB, AMPK/SIRT-1/m-TOR, and PERK/CHOP/Bcl-2 cues, as possible contributors in ADHD’s pathophysiology.
Materials and Methods
Drugs and Chemicals
MSG, SE and AST were obtained from Sigma-Aldrich Chemical Co. in St. Louis, Missouri. They were freshly prepared in distilled water and were provided orally (Kaur et al. 2012; Al-Amin et al. 2015; Onyesife et al. 2023). Other chemicals and solvents used in the study were commercially available and of utmost analytical grade.
Animals
Eighty-four healthy post-weaning male Sprague–Dawley rat pups (Postnatal around 21 days' age), weighing 20–25 g, were purchased from the Nile Co. for Pharmaceuticals and Chemical Industries, Cairo, Egypt. The rats were acclimatized for a week before the experiments. They were housed at the animal house facility in the Faculty of Pharmacy, Al-Azhar University "girls," under controlled conditions (temperature of 25 ± 1°C, humidity of 50 ± 5%, with 12-h light and dark cycles) in stainless steel cages. The animals had access to a normal pellet diet and water ad libitum. All experimental techniques were accepted and supervised by the Animal Care and Use Committee of the Faculty of Pharmacy, Al-Azhar University, with ethical approval number 429/2023. The handling of animals was following the guidelines outlined in the "Guide for Care and Use of Laboratory Animals," published by the National Institutes of Health (NIH Publications No. 8023, revised 1978).
Experimental Design
Animals’ Grouping
Following the one-week acclimatization period, rats were arbitrarily assigned into seven groups comprising 12 rats per group and treated in this way:
Control group: The rats received distilled water (2.5 ml/kg/day), orally for 8 weeks.
MSG group: Rats received MSG (0.4 g/kg/day) (Mahmoud et al. 2020), orally for 8 weeks.
PHA + MSG group: Rats were exposed to physical activity (PHA), swimming for 30 min/day and 5 days/week for 8 weeks, and received MSG (0.4 g/kg/day).
SE + MSG group: Rats received sesamol (SE) (50 mg/kg) (Singh et al. 2020), orally, daily, two hours before receiving MSG (0.4 g/kg/day), orally for 8 weeks.
AST + MSG: Rats received astaxanthin (AST) (150 mg/kg) (Baburina et al. 2023), orally, daily, two hours before receiving MSG (0.4 g/kg/day), orally for 8 weeks.
SE + AST + MSG group: Rats received SE (50 mg/kg) and AST (150 mg/kg), orally, daily, two hours before receiving MSG (0.4 g/kg/day), orally for 8 weeks.
COMB + MSG: Rats in this group received a combination of SE (50 mg/kg) and AST (150 mg/kg), orally, daily and exposed to PHA, swimming for 30 min/day and 5 days/week, along with receiving MSG (0.4 g/kg/day).
For 8 weeks, all drugs were given by oral gavage and behavior assessments were conducted one day after the last dose administration. 24 h after the termination of behavioral assessments, rats were anesthetized with an intraperitoneal injection of a ketamine (75 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.) mixture (Gowifel et al. 2020; El-Shoura et al. 2023; Khidr et al. 2023) and euthanized by cervical dislocation. Then, the brains were promptly removed for biochemical analyses and histopathological evaluations.
Physical Activity (PHA)
Physical activity (PHA) was employed as a therapeutic method to lessen the neurotoxicity induced by MSG administration. PHA was acquired by subjecting the rats to a swimming exercise protocol encompassing 30 min/day and 5 days/week for 8 weeks.
The Swimming Exercise Protocol
The followed swimming exercise protocol was modified from the methods of Viboolvorakul and Patumraj (2014) and Muhammad et al. (2024). The swimming exercise protocol involves adaptation of rats to swimming in a cylindrical tank (80 cm diameter and 90 cm depth), filled with 60 cm water at temperature of 33–36 °C. The swimming exercise protocol consisted of training once daily for 10 min for two days then the duration was increased gradually till rats swam for 30 min, on the 5th day of first week and continued until the completion of the eight weeks with a rate of 5 days/week. A rest for 24 h was permitted prior rats were subjected to the behavioral tests.
Behavioral Tests
The following behavioral tests were performed.
Y-Maze Test
Spontaneous alternation behavior is thought to assess spatial working memory (Hritcu et al. 2012). In this experiment, a black wood Y-maze having three arms (35 cm long, 25 cm high and 10 cm broad) with a symmetrical triangular middle region, was employed. The arms were designated A, B, or C. Animals were positioned at one arm’s edge and permitted to roam freely around the maze for 8 min. Once the rat's hind paws were totally within the arm, it was considered an entrance. The following equation was used to calculate the spontaneous alternation percentage (SAP) based on the number of alternations and total arm entries: SAP (%) = [number of alternations/(total arm entries 2)] × 100.
Open Field Test (OFT)
According to Cunha and Masur (1978), the experiment was conducted in an 80 × 80 × 40 cm square wooden box with red walls and a white polished floor split into 16 equal squares (4 × 4) by black lines. Each rat was placed in the center of the box and monitored for 3 min, where a video camera was located above the box to record the behavior. Numerous parameters were evaluated and analyzed statistically, comprising the latency time (time taken by the rat to move from the center of the arena), grooming frequency (number of times the rat engaged in facial scratching, licking of forelimbs, and genital grooming), and ambulation frequency (number of squares traversed by the rat).
Tissue Sampling and Preparation
After the animals’ euthanasia, their brains were detached and washed with saline. Six brains from each group were fixed in 10% neutral buffered formalin overnight for consequent histopathological and immunohistochemical examinations. Each of the residual six brains in each group was divided into two equal parts. The first part was separately homogenized in ice-cold PBS (pH = 7.4) to get 10% homogenate (w/v). Afterward, the homogenate was centrifuged at 1800 g for 10 min at 4 °C, and the resultant supernatant was utilized for various biochemical measurements, including enzyme-linked immunosorbent assay (ELISA). The other part was kept at −80°C to be used in real-time PCR analyses.
Biochemical Assessments
Colorimetric Analyses
Tissue homogenates were tested for oxidative stress biomarkers in the brain, including malondialdehyde (MDA) (Satoh 1978; Ohkawa et al. 1979), superoxide dismutase (SOD) (Nishikimi et al. 1972), and total antioxidant capacity (TAC) (Koracevic et al. 2001), which were evaluated by commercially available colorimetric assay kits (Biodiagnostic, Inc., Giza, Egypt), according to the manufacturer's instructions.
Enzyme-Linked Immunosorbent Assay (ELISA) in Brain Tissues
ELISA kit purchased from MyBioSource (Southern California, San Diego, USA) was used for the estimation of the brain content of glutamate (Cat. #: MBS756400). The brain inflammatory markers (TNF-α and IL-1β) were estimated using an ELISA Kits supplied by Cusabio Biotech Co., China (TNF-α, Cat. #: CSB-E11987r) and Ray Biotech, Inc., China (IL-1β, Cat #: IQR-IL1b). BDNF was measured using an ELISA kit (Cat. #: EK0308) from Boster Biological Technology (CA, USA). The procedures were followed precisely as instructed by the manufacturer.
Fluorometric Assays in the Brain
Brain monoamines (DA, NE and serotonin (5-HT)) were measured instantly according to Ciarlone (1978). As previously reported, monoamines were detected fluorometrically within samples at λex/λem 320/480 nm, 380/480 nm, and 355/470 nm for DA, NE, and 5-HT, respectively (Ciarlone 1978).
Analysis of Gene Expression by Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
By using Qiagen tissue extraction kit (Qiagen, Germantown, MD, USA) and following the manufacturer’s instructions, total RNA was isolated. Also, a sense-fast cDNA synthesis kit (Cat No. BIO-65053) was utilized to perform reverse transcription of the extracted mRNA. Data analyses were done by Applied Biosystem with software version 3.1 (StepOneTM, Waltham, MA, USA). Transcripts of AKT, AMPK, B-cell lymphoma protein 2 (Bcl-2)-associated X protein (BAX), B-cell lymphoma 2 (Bcl-2), Beclin-1, CHOP, SIRT-1, CREB, GRP78, HMGB1, JAK-2, m-TOR, RAGE, PERK, PI3K, STAT-3, TrkB and the housekeeping gene (β-actin), were assessed in rat brain tissue by RT-qPCR using Applied Biosystems step one plus equipment. The relative expression of target genes was calculated using the following formula: 2^(−∆∆CT) (Livak and Schmittgen 2001). The forward and reverse sequences of the primers used for the PCR amplification are shown in Table 1.
Table 1.
List of primer sequence sets used for RT-qPCR analysis in rat tissues
| Gene | Forward and backward sequences |
|---|---|
| PI3K |
F: 5′-GCCCAGGCTTACTACAGAC-3′ R: 5′-AAGTAGGGAGGCATCTCG-3′ |
| AKT |
F5’-ATGGACTTCCGGTCAGGTTCA-3’ R: 5’-GCCCTTGCCCAGTAGCTTCA −3’ |
| CREB |
F: 5′-CAGACAACCAGCAGAGTGGA-3′ R: 5′-CTGGACTGTCTGCCCATTG-3′ |
| TrkB |
F: 5′- CCTCCACGGATGTTGCTGA-3′ R: 5′-GGCTGTTGGTGATACCGAAGTA-3′ |
| AMPK |
F: 5′ -AAAGAACCCTAGCCTGAAGAGG-3′ R: 5′-ACCTTCCGAGATGAATGCTTTT-3′ |
| SIRT 1 |
F: 5′- GGCACCGATCCTCGAACAAT-3′ R: 5′-CGCTTTGGTGGTTCTGAAAGG-3′ |
| m-TOR |
F:5’TTGAGGTTGCTATGACCAGAGAGAA-3’ R:5’TTACCAGAAAGGACACCAGCCAATG-3’ |
| Beclin-1 |
F: 5′-AGCACGCCATGTATAGCAAAGA-3′ R: 5′-GGAAGAGGGAAAGGACAGCAT-3′ |
| PERK |
F: 5′-GCCGATGGGATAGTGATG-3′ R: 5′-GCAGCCTCTACAATGTCTTCT-3′ |
| CHOP |
F: 5′-TCTGCCTTTCGCCTTTGAG-3′ R: 5′-GCTTTGGGAGGTGCTTGTG-3′ |
| GRP78 |
F:5′-TAATCAGCCCACCGTAAC-3′ R:5′-GTTTCCTGTCCCTTTGTC-3′ |
| BAX |
F: 5’-CACGTCTGCGGGGAGTCA-3’ R: 5’-TAGGAAAGGAGGCCATCCCA-3’ |
| Bcl-2 |
F: 5′-GGATGACTTCTCTCGTCGCTAC-3′ R: 5′-TGACATCTCCCTGTTGACGCT-3 |
| HMGB1 |
F: 5′-CACCCTGCATATTGTGGTAGG-3′ R: 5′-CGCTGGGACTAAGGTCAACA-3′ |
| RAGE |
F: 5′-GAGTCCGAGTCTACCAGATTCC-3′ R:5′-GGTCTCCTCCTTCACAACTGTC-3′ |
| JAK-2 |
F: 5′-AGCTCCTCTCCTTGACGACT-3′ R:5′-GCACGCACTTCGGTAAGAAC-3′ |
| STAT-3 |
F: 5′-CAAAGAAAACATGGCCGGCA-3′ R:5′-GGGGGCTTTGTGCTTAGGAT-3′ |
| β-actin |
F: 5′-CCGTAAAGACCTCTATGCCA-3’ R: 5′-AAGAAAGGGTGTAAAACGCA-3’ |
Histopathological and Immunohistochemical Examinations
Histopathological Examination
Brain tissue samples in various groups were fixed in 10% formalin for 1 day then washed with tap water. By an ascending sequence of ethyl alcohol, dehydration was done. In a hot air oven at 56 ºC for 1 day, clearing and paraffin embedding was done. For routine microscopic examination with a light microscope, 5 µm sections were mounted on glass slides, deparaffinized, and stained with hematoxylin and eosin (H & E), then photomicrographs at magnification of 40X were captured (Bancroft and Gamble 2008).
Immunohistochemical Assessment
After sectioning and deparaffinization of the blocks of brain tissue using xylene, rehydration with alcohol in ascending concentrations was done. Subsequently, the sections were kept in 3% H2O2 for 10 min then 30 min in 0.1% trypsin at 37 °C, for antigen retrieval. Afterwards, an overnight incubation at 4 °C with polyclonal rabbit antibodies against glial fibrillary acidic protein (GFAP) (1:800 dilution; servicebio, USA, Cat# GB12090) was performed. Sections were treated at 37 °C for 30 min with the secondary antibody, biotinylated goat anti-rabbit (Invitrogen); stained with 3, 3-diaminobenzidine and eventually counterstained with hematoxylin. Finally, image analysis was carried out by assessing the area percent (A %) in 10 microscopic fields, via ImageJ software (version 1.48).
Statistical Analysis
Data are expressed as mean ± S.E.M. Multiple comparisons were performed using one-way ANOVA followed by Tukey Kramer as a post-hoc test. GraphPad Prism Software Ver. 5 (ISI®, USA) was used to accomplish all statistical analysis and data visualization. The level of significance was considered at p < 0.05.
Results
Regarding the current study, beside the abovementioned seven studied groups, there are another additional five groups, which include; normal control group exposed to PHA; normal control group treated with SE; normal control group treated with AST; normal control group treated with both SE and AST; and normal control group treated with a combination of SE and AST along with exposure to PHA. Of note, the results of these additional five groups in all the measured parameters and the histopathological findings were non-significant, compared to those of the normal control group. Consequently, the findings of these additional five groups were not comprised in this study to sustain simplicity of the presented data.
Combination of SE and AST with PHA Enhanced Locomotor, Attention and Cognitive Functions Assessed by Open-Field and Y-Maze Tests in MSG-Intoxicated Rats
Open-Field Test
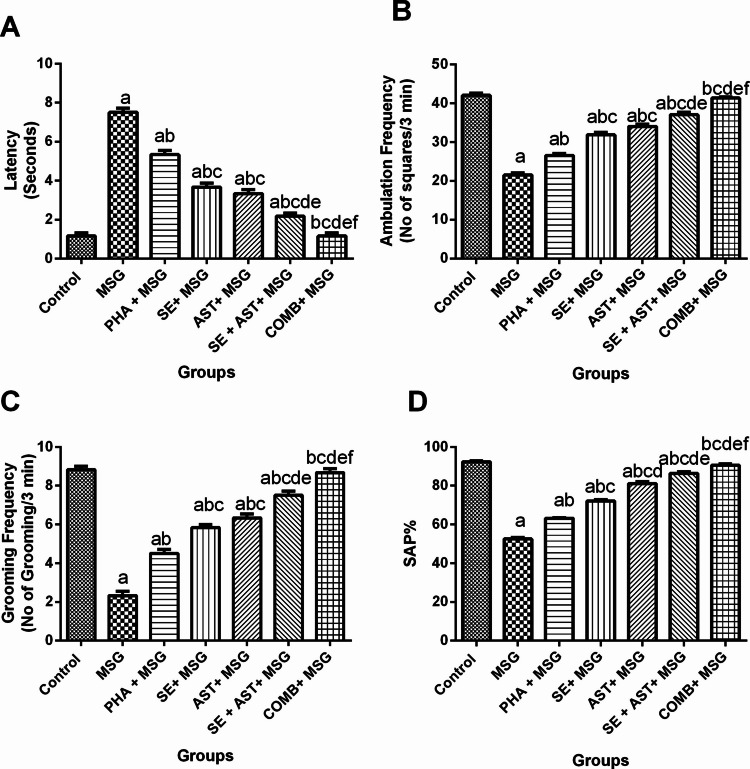
As displayed in Fig. 1, the results of the open-field test revealed that MSG affected the decision-making behavior in rats due to delayed latency (6.4-fold (F(6,35) = 140.3, p < 0.0001)) (Fig. 1A) with a substantial drop in the ambulation(Fig. 1B) and grooming (Fig. 1C) frequencies (by 48.8% (F(6,35) = 173, p < 0.0001), and 73.6% (F(6,35) = 131.9, p < 0.0001), respectively), compared to the control group. In contrast, co-treatment of MSG-intoxicated rats with PHA, SE, AST, (SE + AST), or COMB meaningfully lessened latency time (by 28.9%, 51.1%, 55.6%, 71.1% and 84.4%, respectively), compared to MSG group (Fig. 1A). Regarding ambulation frequency, it was remarkably raised in (PHA + MSG), (SE + MSG), (AST + MSG), (SE + AST + MSG), and (COMB + MSG) groups (1.2-fold, 1.5-fold, 1.6-fold, 1.7-fold, and 1.9-fold, respectively) versus MSG group (Fig. 1B). Concerning grooming frequency, it was prominently elevated in (PHA + MSG), (SE + MSG), (AST + MSG), (SE + AST + MSG), and (COMB + MSG) groups (1.2-fold, 1.4-fold, 1.5-fold, 1.6-fold, and 1.7-fold, respectively) compared to MSG group (Fig. 1C). Of note, the co-administration of a combination of PHA and SE with AST restored the latency time in addition to ambulation and grooming frequencies back to normal values.
Fig. 1.
Effects of SE, AST and PHA on MSG-induced behavioral changes in (A) Latency Time, (B) Ambulation Frequency and (C) Grooming Frequency in open-field test and (D) Spontaneous alteration percentage in Y-maze test. The data are presented as means ± S.E.M (n = 6). Significance (a): relative to the control group, (b): relative to MSG group, (c): relative to PHA + MSG, (d): relative to SE + MSG, (e): relative to AST + MSG, and (f): relative to SE + AST + MSG. P-value < 0.05. Monosodium glutamate (MSG); Physical activity (PHA); Sesamol (SE); Astaxanthin (AST); COMB (SE + AST + PHA); Spontaneous alteration percentage (SAP)
Y-Maze Test
MSG induced a marked reduction of spontaneous alternation percentage (SAP) (by 43.1% (F(6,35) = 434.7, p < 0.0001)), compared to the control group (Fig. 1D). However, SAP was pronouncedly upsurged in (PHA + MSG), (SE + MSG), (AST + MSG), (SE + AST + MSG), and (COMB + MSG) groups (1.2-fold, 1.4-fold, 1.55-fold, 1.6-fold, and 1.7-fold, respectively) versus MSG group (Fig. 1D). Remarkably, co-administration of a combination of PHA and SE with AST elevated SAP to be as near as normal values (Fig. 1D).
Combination of SE and AST with PHA Alleviates MSG-Induced Changes in Monoamine Neurotransmitters and Glutamate Content in the Brain
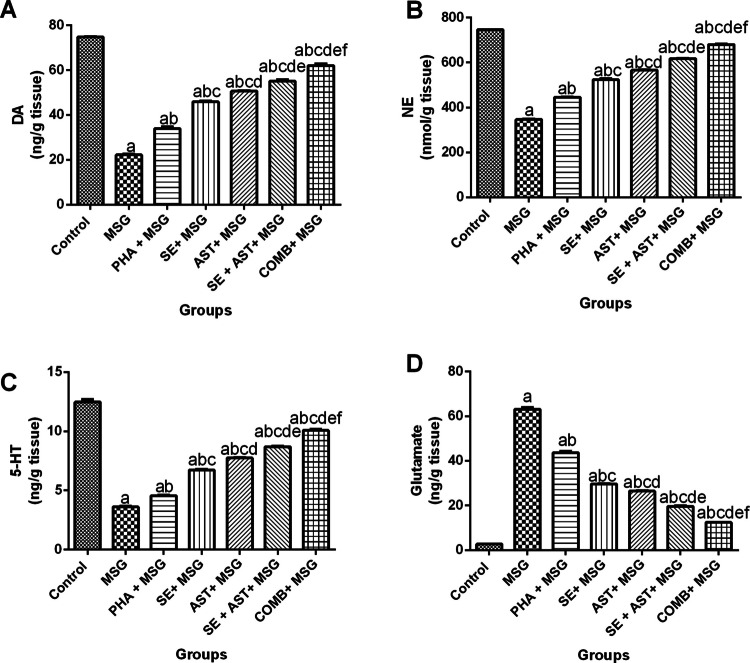
As illustrated in Fig. 2, the brain monoamine levels (DA, NE and 5-HT) were remarkably diminished upon MSG administration (by 70.3% (F(6,35) = 1025, p < 0.0001) (Fig. 2A), 53.4% (F(6,35) = 4596, p < 0.0001) (Fig. 2B), and 70.9% (F(6,35) = 917.9, p < 0.0001) (Fig. 2C), respectively), while brain glutamate content was noticeably increased (23.2-fold (F(6,35) = 2118, p < 0.0001)) (Fig. 2D), relative to the control group. Contrariwise, exposure to PHA, or administration of either SE, or AST elicited a prominent elevation in DA (1.5-fold, 2.1-fold and 2.3- fold, respectively) (Fig. 2A), NE (1.3-fold, 1.5-fold and 1.6- fold, respectively) (Fig. 2B) and 5-HT (1.3-fold, 1.9-fold, and 2.1- fold, respectively) (Fig. 2C), while glutamate levels were considerably reduced (by 30.8%, 53.2%, and 58.1%, respectively) (Fig. 2D), compared to MSG group. When SE and AST were combined, the brain monoamines’ levels (DA, NE and 5-HT) were pronouncedly amplified (2.5-fold, 1.8-fold and 2.4-fold, respectively) (Fig. 2A, 2B and 2C), in addition to marked hampering in the glutamate level (by 69%) (Fig. 2D) as compared to MSG group. Interestingly, the combination of SE and AST along with PHA showed maximum protective effects by elevation of DA (2.8-fold) (Fig. 2A), NE (1.9-fold) (Fig. 2B), and 5-HT (2.8-fold) (Fig. 2C), together with a decrement in glutamate content (by 80.3%) (Fig. 2D) compared to MSG group.
Fig. 2.
Effects of SE, AST and PHA on brain neurotransmitters in MSG-intoxicated rats: (A) DA, (B) NE, (C) 5-HT and (D) Glutamate. The data are presented as means ± S.E.M (n = 6). Significance (a): relative to the control group, (b): relative to MSG group, (c): relative to PHA + MSG, (d): relative to SE + MSG, (e): relative to AST + MSG, and (f): relative to SE + AST + MSG. P-value < 0.05. Monosodium glutamate (MSG); Physical activity (PHA); Sesamol (SE); Astaxanthin (AST); COMB (SE + AST + PHA); Dopamine (DA); Norepinephrine (NE) and Serotonin (5-HT)
Combination of SE and AST with PHA Mitigates Oxidative Stress Biomarkers in the Brain of MSG-Intoxicated Rats
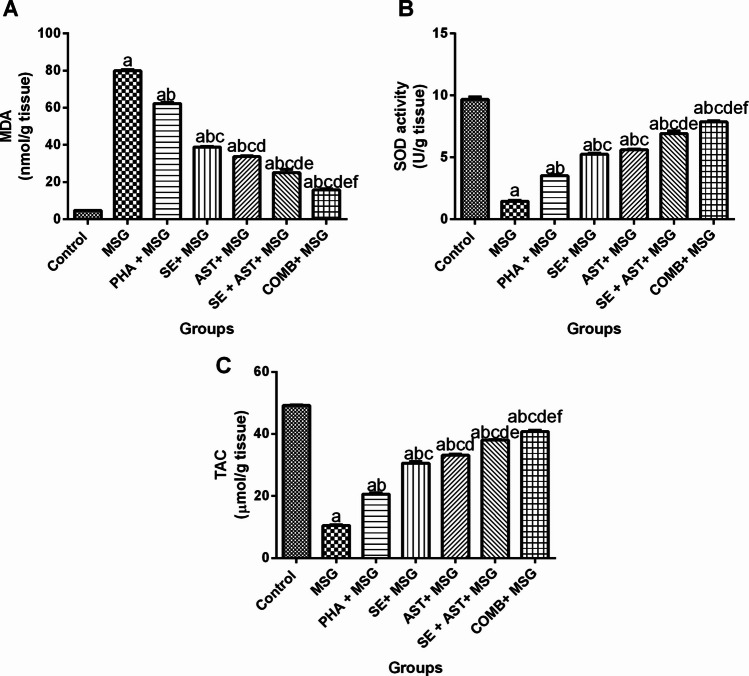
As displayed in Fig. 3, MSG-intoxicated rats exhibited profound oxidative damage as evidenced by a noticeable increase in MDA brain content (16.7-fold (F(6,35) = 1052, p < 0.0001)) (Fig. 3A), coupled with a marked diminution in SOD activity(Fig. 3B) and TAC (Fig. 3C) level (by 84.8% (F(6,35) = 501, p < 0.0001) and 78.7% (F(6,35) = 1635, p < 0.0001), respectively in the brain tissue compared to the control group. On the other hand, (PHA + MSG), (SE + MSG), (AST + MSG), (SE + AST + MSG), and (COMB + MSG) groups demonstrated a substantial reduction in the brain MDA content (by 22.1%, 51.4%, 57.8%, 68.5% and 80.3%, respectively) (Fig. 3A), along with a marked elevation in SOD activities (2.4-fold, 3.6-fold, 3.9-fold, 4.7-fold and 5.4-fold, respectively) (Fig. 3B) and TAC levels (1.9-fold, 2.9-fold, 3.2-fold, 3.6-fold and 3.9-fold, respectively) (Fig. 3C) compared to MSG group. It is worth noting that, the combination of PHA, SE and AST presented the maximum effect in curbing oxidative stress and replenishing antioxidant defense compared to other treatment groups.
Fig. 3.
Effects of SE, AST and PHA on brain oxidative stress biomarkers in MSG-intoxicated rats: (A) MDA, (B) SOD and (C) TAC. The data are presented as means ± S.E.M (n = 6). Significance (a): relative to the control group, (b): relative to MSG group, (c): relative to PHA + MSG, (d): relative to SE + MSG, (e): relative to AST + MSG, and (f): relative to SE + AST + MSG. P-value < 0.05. Monosodium glutamate (MSG); Physical activity (PHA); Sesamol (SE); Astaxanthin (AST); COMB (SE + AST + PHA); Malondialdehyde (MDA); Superoxide dismutase (SOD) and Total antioxidant capacity (TAC)
Combination of SE and AST with PHA Diminishes Neuroinflammatory Biomarkers in the Brain of MSG-Intoxicated Rats
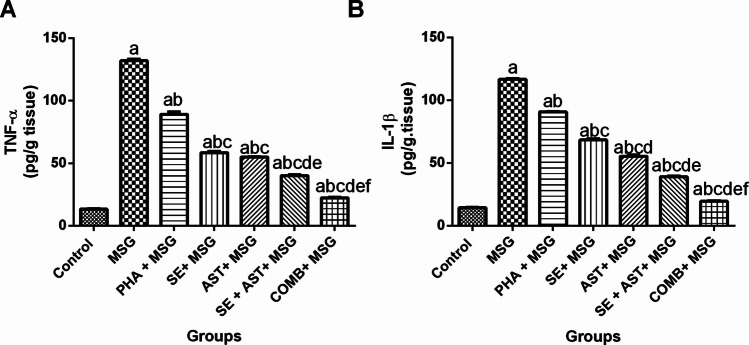
Figure 4 demonstrated that MSG administration revealed an exaggerated inflammatory response, as confirmed by striking increase of the pro-inflammatory cytokines (TNF-α, and IL-1β) levels in the brain tissues (9.9-fold (F(6,35) = 1457, p < 0.0001) (Fig. 4A) and 8.1-fold (F(6,35) = 2864, p < 0.0001) (Fig. 4B), respectively) compared to the control group. Inversely, (PHA + MSG), (SE + MSG), (AST + MSG), (SE + AST + MSG) and (COMB + MSG) groups substantially diminished TNF-α (by 32.6%, 55.7%, 58.3%, 69.5% and 83.1%, respectively) (Fig. 4A), and IL-1β (by 22.3%, 41.5%, 52.7%, 66.5% and 83.4%, respectively) (Fig. 4B) versus MSG-intoxicated rats. Remarkably, the combination of PHA, SE with AST showed the most prominent anti-inflammatory effects compared to any other group in this study.
Fig. 4.
Effects of SE, AST and PHA on brain neuroinflammatory biomarkers in MSG-intoxicated rats: (A) TNF-α and (B) IL-1β. The data are presented as means ± S.E.M (n = 6). Significance (a): relative to the control group, (b): relative to MSG group, (c): relative to PHA + MSG, (d): relative to SE + MSG, (e): relative to AST + MSG, and (f): relative to SE + AST + MSG. P-value < 0.05. Monosodium glutamate (MSG); Physical activity (PHA); Sesamol (SE); Astaxanthin (AST); COMB (SE + AST + PHA); Interleukin-1β (IL-1β) and Tumor necrosis factor-alpha (TNF-α)
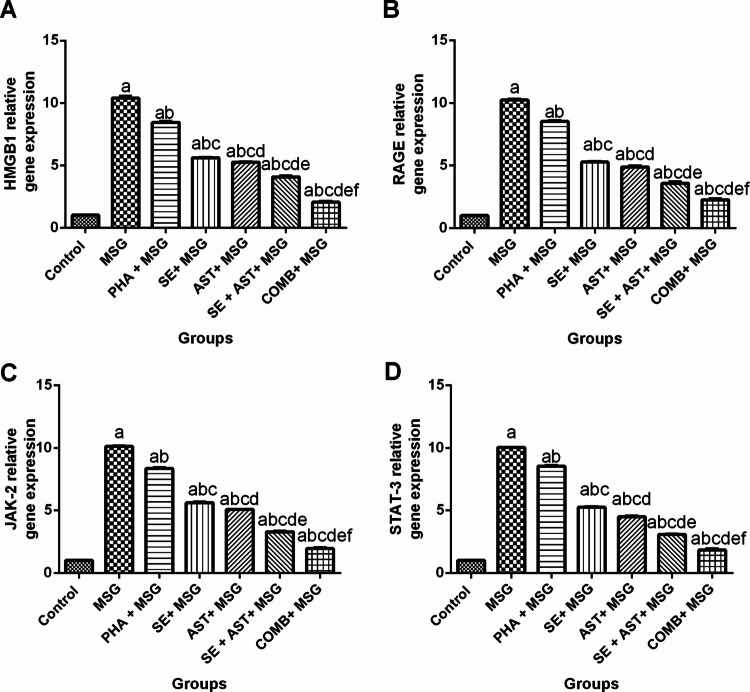
Combination of SE and AST with PHA Hinders HMGB1/RAGE and JAK-2/STAT-3 Signaling Pathways in MSG-Intoxicated Rats
As clarified in Fig. 5, MSG meaningfully upregulated HMGB1 (Fig. 5A), RAGE (Fig. 5B), JAK-2 (Fig. 5C) and STAT-3 (Fig. 5D) gene expression (10.2-fold (F(6,35) = 2038, p < 0.0001), 10.1-fold (F(6,35) = 2212, p < 0.0001), 9.9-fold (F(6,35) = 5696, p < 0.0001) and 9.8- fold (F(6,35) = 4128, p < 0.0001), respectively), compared to the control group. On the contrary, (PHA + MSG), (SE + MSG), (AST + MSG), (SE + AST + MSG) and (COMB + MSG) groups displayed extensive downregulation in the gene expression of HMGB1 (by 18.7%, 45.4%,49.5%, 60.7% and 80.2%, respectively) (Fig. 5A); RAGE (by 16.6%, 48.4%, 52.2%, 64.9% and 77.9%, respectively) (Fig. 5B); JAK-2 (by 17.5%, 44.4%, 49.7%, 67.4% and 80.7% respectively) (Fig. 5C) and STAT-3 (by 14.9%, 47.6%, 55.1%, 69.4% and 81.6% respectively) (Fig. 5D), relative to MSG-intoxicated rats. Hence, the combination of PHA, SE with AST exhibited the greatest inhibitory effect on HMGB1/RAGE and JAK-2/STAT-3 signaling pathways among other treated groups.
Fig. 5.
Effects of SE, AST and PHA on HMGB1/RAGE and JAK-2/STAT-3 signaling pathways in MSG- intoxicated rats: (A) HMGB1, (B) RAGE, (C) JAK-2 and (D) STAT-3. The data are presented as means ± S.E.M (n = 6). Significance (a): relative to the control group, (b): relative to MSG group, (c): relative to PHA + MSG, (d): relative to SE + MSG, (e): relative to AST + MSG, and (f): relative to SE + AST + MSG. P-value < 0.05. Monosodium glutamate (MSG); Physical activity (PHA); Sesamol (SE); Astaxanthin (AST); COMB (SE + AST + PHA); High mobility group box 1 (HMGB1); Receptor for Advanced Glycation End Products (RAGE); Janus kinase-2 (JAK-2) and Signal transducer and activator of transcription-3 (STAT-3)
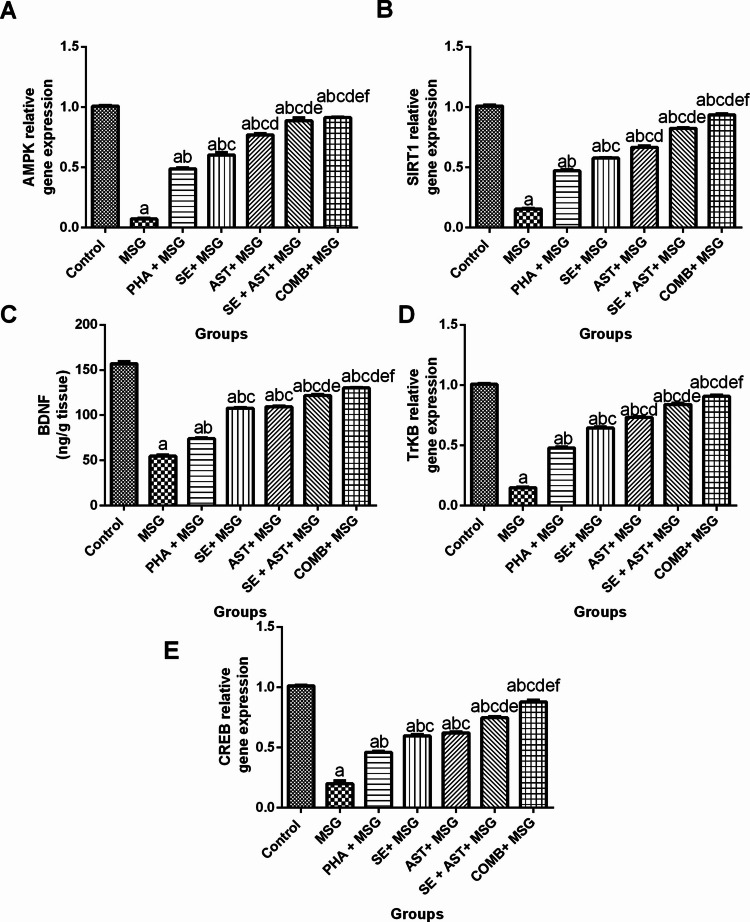
Combination of SE and AST with PHA Enhances AMPK/SIRT-1 and CREB/BDNF/TrkB Signaling Pathway in MSG-Intoxicated Rats
As illustrated in Fig. 6, MSG induced a sharp decrement in the gene expression levels of AMPK (Fig. 6A), SIRT-1(Fig. 6B), TrkB (Fig. 6D) and CREB (Fig. 6E) (by 92.9% (F(6,35) = 624.4, p < 0.0001), 84.6% (F(6,35) = 1517, p < 0.0001), 85.2% (F(6,35) = 1205, p < 0.0001) and 80.2% (F(6,35) = 435.8, p < 0.0001), respectively), in addition to BDNF level (by 65.2% (F(6,35) = 695.4, p < 0.0001)) (Fig. 6C), compared to the control group. In contrast, (PHA + MSG), (SE + MSG), (AST + MSG) (SE + AST + MSG), and (COMB + MSG) groups prominently upregulated the gene expression levels of AMPK (6.8-fold, 8.5-fold, 10.8-fold, 12.5-fold, and 12.8-fold, respectively) (Fig. 6A); SIRT-1 (threefold, 3.7-fold, 4.3-fold, 5.3-fold, and sixfold, respectively) (Fig. 6B); TrkB (3.2-fold, 4.3-fold, 4.9-fold, 5.6-fold, and 6.1-fold, respectively) (Fig. 6D) and CREB (2.3-fold, 2.9-fold, 3.1-fold, 3.7-fold, and 4.4-fold, respectively) (Fig. 6E), together with, BDNF level (1.4-fold, twofold, twofold, 2.2-fold, and 2.4-fold, respectively) (Fig. 6C), compared to MSG group. Remarkably, the combination of PHA, SE with AST demonstrated the maximal favorable effects on AMPK/SIRT-1 and CREB/BDNF/TrkB pathways among other treated groups.
Fig. 6.
Effects of SE, AST and PHA on AMPK/SIRT-1 and CREB/BDNF/TrkB signaling pathways in MSG-intoxicated rats: (A) AMPK, (B) SIRT-1, (C) BDNF, (D) TrkB and (E) CREB. The data are presented as means ± S.E.M (n = 6). Significance (a): relative to the control group, (b): relative to MSG group, (c): relative to PHA + MSG, (d): relative to SE + MSG, (e): relative to AST + MSG, and (f): relative to SE + AST + MSG. P-value < 0.05. Monosodium glutamate (MSG); Physical activity (PHA); Sesamol (SE); Astaxanthin (AST); COMB (SE + AST + PHA); AMP-enhanced protein kinase (AMPK); Sirtuin-1 (SIRT-1); Brain-derived neurotrophic factor (BDNF); Tropomyosin receptor kinase B (TrKB); Cyclic adenosine monophosphate (cAMP) response element-binding protein (CREB)
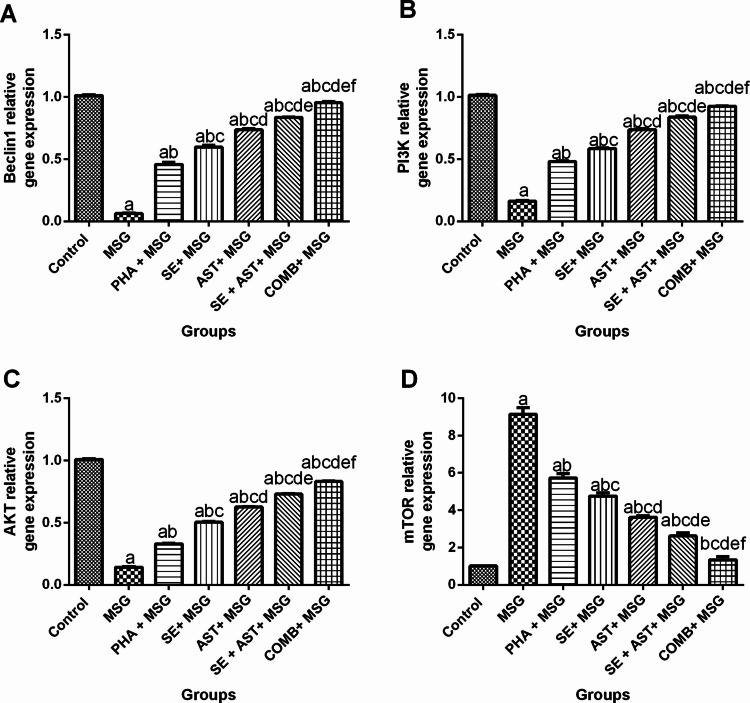
Combination of SE and AST with PHA Modulates PI3K/AKT and m-TOR/Beclin-1 Signaling Pathways in the Brain of MSG-Intoxicated Rats
As illustrated in Fig. 7, MSG induced a considerable downregulation in Beclin-1(Fig. 7A), PI3K (Fig. 7B) and AKT (Fig. 7C) gene expression (by 93.7% (F(6,35) = 1010, p < 0.0001), 84.1% (F(6,35) = 2265, p < 0.0001), and 85.8% (F(6,35) = 2381, p < 0.0001), respectively), together with upregulation of m-TOR (ninefold (F(6,35) = 205.3, p < 0.0001)) (Fig. 7D), as compared to the control group. On the other hand, (PHA + MSG), (SE + MSG), (AST + MSG), (SE + AST + MSG) and (COMB + MSG) groups both pronouncedly upregulated the gene expression levels of Beclin-1 (7.2-fold, 9.4-fold, 11.6-fold, 13.2-fold, and 15.1-fold, respectively) (Fig. 7A); PI3K (threefold, 3.6-fold, 4.6-fold, 5.2-fold, and 5.7-fold, respectively) (Fig. 7B) and AKT (3.3-fold, 3.5-fold, 4.4-fold, 5.1-fold and 5.8-fold, respectively) (Fig. 7C), and also markedly downregulated the gene expression of m-TOR (by 37.3%, 47.9%, 60.4%, 71.4% and 85.3%, respectively) (Fig. 7D), versus MSG control group. Notably, the combination of PHA, SE with AST achieved the maximum protective effect PI3K/AKT and m-TOR/Beclin-1 pathways, over any other treatments in this study, evidenced by keeping gene expression level of m-TOR as nearly as its level in the control group.
Fig. 7.
Effects of SE, AST and PHA on PI3K/AKT and m-TOR/Beclin-1 signaling pathways in MSG-intoxicated rats: (A) Beclin-1, (B) PI3K, (C) AKT and (D) m-TOR. The data are presented as means ± S.E.M (n = 6). Significance (a): relative to the control group, (b): relative to MSG group, (c): relative to PHA + MSG, (d): relative to SE + MSG, (e): relative to AST + MSG, and (f): relative to SE + AST + MSG. P-value < 0.05. Monosodium glutamate (MSG); Physical activity (PHA); Sesamol (SE); Astaxanthin (AST); COMB (SE + AST + PHA); Phosphoinositide 3-kinase (PI3K); Protein kinase B (AKT); Mechanistic target of rapamycin (m-TOR)
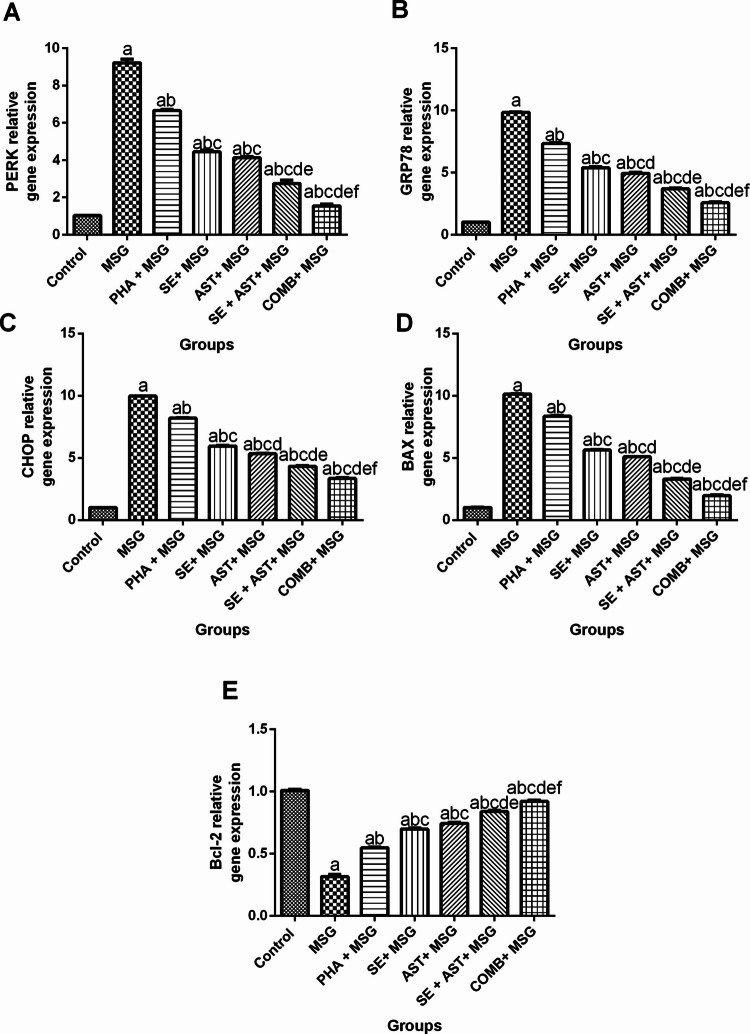
Combination of SE and AST with PHA Modulates PERK/GRP78/CHOP and BAX/Bcl-2 Signaling Pathways in the Brain of MSG-Intoxicated Rats
Figure 8 demonstrated that MSG exerted obvious upregulation in the gene expression of ER stress markers (PERK, GRP78, and CHOP), and apoptotic marker, BAX (ninefold (F(6,35) = 636.8, p < 0.0001) (Fig. 8 A), 9.6-fold (F(6,35) = 2070, p < 0.0001) (Fig. 8B), 9.8-fold (F(6,35) = 3681, p < 0.0001) (Fig. 8C), and 9.9-fold (F(6,35) = 5689, p < 0.0001) (Fig. 8D), respectively), along with marked downregulation in the gene expression of anti-apoptotic marker, Bcl-2 (by 68.6% (F(6,35) = 500.7, p < 0.0001)) (Fig. 8E), compared to the control group. Conversely, (PHA + MSG), (SE + MSG), (AST + MSG), (SE + AST + MSG) and (COMB + MSG) groups both obviously downregulated the gene expression levels of PERK (by 27.7%, 51.8%, 55.2%, 70.2% and 83.2%, respectively) (Fig. 8A); GRP78 (by 25.5%, 45.3%, 49.8%, 62.5%, and 73.9%, respectively) (Fig. 8B); CHOP (by 17.8%, 40.3%, 46.6%, 56.7%, and 66.5%, respectively) (Fig. 8C), and BAX (by 17.5%, 44.4%, 49.7%, 67.4% and 80.7%, respectively) (Fig. 8D), and also strikingly upregulated the gene expression of Bcl-2 (1.7-fold, 2.2-fold, 2.4-fold, 2.7-fold, and 2.9-fold, respectively) (Fig. 8E), versus MSG group. Of note, the combination of PHA, SE with AST employed the maximum protective effect in PERK/GRP78/CHOP and BAX/Bcl-2 axes relative to any other treatment groups.
Fig. 8.
Effects of SE, AST, and PHA on PERK/GRP78/CHOP and BAX/Bcl-2 signaling pathways in MSG-intoxicated rats: (A) PERK, (B) GRP78, (C) CHOP, (D) BAX and (E) Bcl-2. The data are presented as means ± S.E.M (n = 6). Significance (a): relative to the control group, (b): relative to MSG group, (c): relative to PHA + MSG, (d): relative to SE + MSG, (e): relative to AST + MSG, and (f): relative to SE + AST + MSG. P-value < 0.05. Monosodium glutamate (MSG); Physical activity (PHA); Sesamol (SE); Astaxanthin (AST); COMB (SE + AST + PHA); Protein kinase RNA-like ER kinase (PERK), Glucose-regulated protein 78 (GRP78), Pro-apoptotic C/EBP homologous protein (CHOP), Bcl-2-associated X protein (BAX), B-cell lymphoma 2 protein (Bcl-2)
Combination of SE and AST with PHA Improves MSG-Induced Histopathological Changes in Brain Tissues
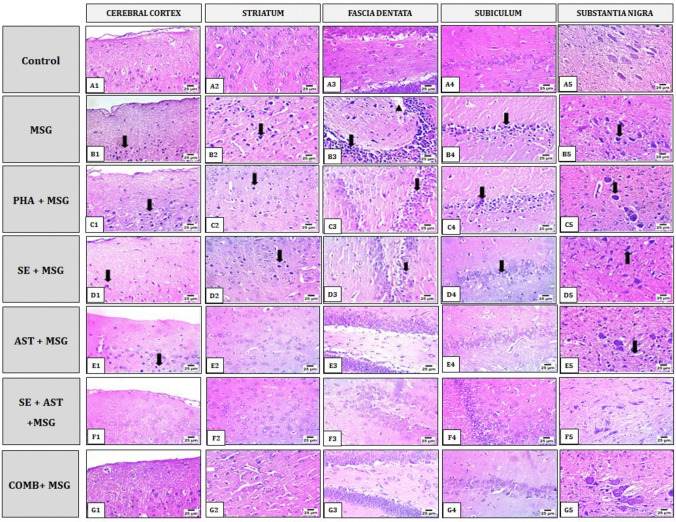
As shown in Fig. 9, histopathological examination of the brain sections from MSG-intoxicated rats showed severe nuclear pyknosis in neurons (arrows) of the cerebral cortex, striatum, subiculum and substantia nigra (Fig. 9B1, B2, B4 and B5). Severe nuclear pyknosis in neurons (arrow) of fascia dentata with hemorrhage (arrowhead) (Fig. 9B3). PHA + MSG group showed severe nuclear pyknosis in neurons (arrows) of the cerebral cortex and substantia nigra (Fig. 9C1 and C5). In the same group, mild nuclear pyknosis in neurons (arrows) of the striatum and fascia dentata (Fig. 9C2 and C3) and moderate nuclear pyknosis in neurons (arrows) of the subiculum (Fig. 9C4) were noted. In SE + MSG group, brain sections displayed mild nuclear pyknosis in neurons (arrows) of the cerebral cortex, striatum, fascia dentate and subiculum (Fig. 9D1, D2, D3 and D4). In the same group, severe nuclear pyknosis in neurons (arrows) of the substantia nigra (Fig. 9D5) was noted. The cerebral cortex and substantia nigra from AST + MSG group presented mild nuclear pyknosis in neurons (arrows) (Fig. 9E1 and E5), whereas the striatum, fascia dentata, and subiculum (arrow) in this group elicited apparent normal histological structure (Fig. 9E2, E3 and E4). In SE + AST + MSG group, brain sections of the cerebral cortex, striatum, fascia dentata, subiculum and substantia nigra (Fig. 9F1, F2, F3, F4 and F5) showed apparent normal histological picture. Furthermore, Brain sections from the COMB + MSG group showed apparent normal histological structure of the cerebral cortex, striatum, fascia dentata, subiculum, and substantia nigra (Fig. 9G1, G2, G3, G4 and G5).
Fig. 9 .
Effects of SE, AST and PHA on MSG-induced histopathological changes in brain tissues. Represented photomicrographs of brain sections stained by Hematoxylin and Eosin (magnification 40 X) of Control (A), MSG (B), PHA + MSG (C), SE + MSG (D), AST + MSG (E), SE + AST + MSG (F) and SE + AST + PHA + MSG (G) groups, showed different histopathological alteration in the cerebral cortex, striatum, hippocampus (fascia dentate and subiculum) and substantia nigra. Monosodium glutamate (MSG); Physical activity (PHA); Sesamol (SE); Astaxanthin (AST); COMB (SE + AST + PHA)
Combination of SE and AST with PHA Lessens GFAP Expression in the Brain of MSG-Intoxicated Rats
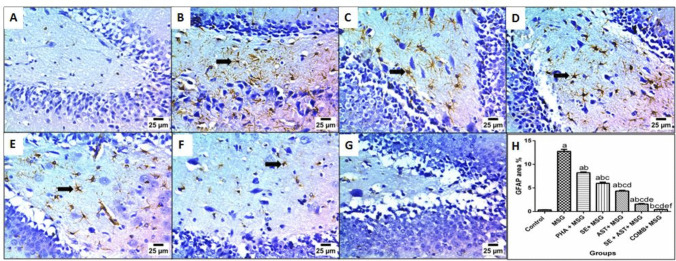
Figure 10. demonstrated that MSG administration revealed an exaggerated inflammatory response, as confirmed by an extensive upsurge in the expression of neuroinflammatory biomarker GFAP (36.4-fold (F(6,35) = 473.7, p < 0.0001)) (Fig. 10B), compared to the control group. Inversely, (PHA + MSG) (Fig. 10C), (SE + MSG) (Fig. 10D), (AST + MSG) (Fig. 10E), (SE + AST + MSG) (Fig. 10F), and (COMB + MSG) (Fig. 10G) groups noticeably diminished GFAP expression (by 35.5%, 53.1%, 65.9%, 87.7%, and 96.7%, respectively), versus MSG-intoxicated rats. Remarkably, the combination of PHA, SE with AST showed the maximum hampering of GFAP expression among treated groups in comparison to MSG-intoxicated rats by keeping the brain GFAP expression level as nearly as their levels in the control group.
Fig. 10.
Effects of SE, AST and PHA on GFAP immunoexpression in the brain of MSG-intoxicated rats. Illustrative photomicrograph of immunohistochemical staining of GFAP-positive cells in the brain (arrows). Control group (A), MSG group (B), PHA + MSG group (C), SE + MSG group (D), AST + MSG group (E) SE + AST + MSG group (F), and COMB + MSG group (G) [× 200]. Bar chart represents the area % of GFAP staining in the different groups (H). Data are presented as mean ± S.E.M (n = 6). Significance (a): relative to the control group, (b): relative to MSG group, (c): relative to PHA + MSG, (d): relative to SE + MSG, (e): relative to AST + MSG, and (f): relative to SE + AST + MSG. P < 0.05. Monosodium glutamate (MSG); Physical activity (PHA); Sesamol (SE); Astaxanthin (AST); COMB (SE + AST + PHA); Glial fibrillary acidic protein (GFAP)
Discussion
Nowadays, there is an immense interest in understanding the mechanisms involved in ADHD’s pathophysiology and investigating new therapeutic targets for amelioration of ADHD’s symptoms. ADHD is a neurodevelopmental disorder with a multifactorial etiopathology, where the pathophysiological mechanisms involved in ADHD are not fully elucidated (Song et al. 2021). MSG, a flavor enhancer, elicits an excitotoxic effect, which is associated with oxidative stress, neuroinflammation and neuronal apoptosis, eventually leading to disturbances in brain function with behavioral alterations mimicking those of ADHD in humans (Khaled Abd et al. 2021).Up till now, methylphenidate and dextroamphetamine, are the most predominant drugs designated in the management of ADHD symptoms, where they act by correcting the abnormalities in catecholaminergic functions. However, using these medications on long-term had been associated with the occurrence of numerous adverse effects, including headache, insomnia, nausea, abdominal pain, dry mouth and liver injury (Ahn et al. 2016). In addition, concerns had been raised over the long-term use of these stimulant medications due to their high likelihood for dependence and abuse, which restricted the use of these medications (Heal et al. 2009). Hence, the therapeutic usefulness and effectiveness of these conventional medications in ADHD is limited by the development of its associated adverse effects, together with their high risk of dependence. In this frame, there had been pressing need to unearth safe and effective alternatives for amelioration of ADHD. Consequently, using of effective naturally occurring agents that possess safer profiles as neuroprotective agents represents a promising therapeutic approach for alleviation of ADHD (Ahn et al. 2016; Lee et al. 2023). Among them, SE and AST possess favorable effects on health, including antioxidant, anti-inflammatory and neuroprotective potentials (Kishimoto et al. 2016; El‐Borai et al. 2022). Besides, the role of PHA had been highlighted in neurological disorders, including ADHD, as it enhances memory and cognitive function (Valenzuela 2008). During the steps of finding newer therapeutic strategies for amelioration of ADHD, integrating the use of PHA along with the intake of nutraceuticals had become an outstanding research hotspot. In this context, the aim of this study was divided into three horizons; the first one was to evaluate the neuroprotective effects of SE and AST either alone or in combination with each other against MSG-induced ADHD-like behaviors in rat pups. The second one was to investigate the role of using PHA in conjunction with the nutraceuticals (SE and AST) combination, to improve their effects. Notably the third one was to outline the possible mechanistic contributors underlying the beneficial effects of these agents, focusing on HMGB1/RAGE/JAK-2/STAT-3, PI3K/AKT/CREB/BDNF/TrkB, AMPK/SIRT-1/m-TOR and PERK/CHOP/Bcl-2 cues.
In the current study, post-weaning exposure to MSG exhibited marked depletion in the levels of brain monoamines (DA, NE and 5-HT), as well as remarkable increment in glutamate levels, which were coupled with marked alterations in spatial working memory and locomotor activity demonstrated by reduction in cognitive function in Y-maze test and prolongation in latency time in OFT, respectively, compared to normal control group. The aforementioned perturbations may be attributed to the fact that MSG dissociates in water into sodium ion and glutamate, thus augmenting glutamate levels over its normal basal levels. In turn, excessive glutamate stimulates its receptors in CNS, leading to their overactivation, thereby triggering excitotoxic neuronal damage, via provoking the generation of ROS, enhancing lipid peroxidation, disrupting calcium homeostasis and aggravating neuroinflammation, ultimately resulting in neuronal death (Mirzakhani et al. 2020). These deleterious effects with their consequent neuronal damage were corroborated by our histopathological findings that showed severe nuclear pyknosis detected in the cerebral cortex, striatum and hippocampus of MSG-intoxicated rats’ brains. Hence, the existing neuronal damage in MSG-intoxicated group may be responsible for in the observed depletion in brain neurotransmitter levels, including DA, NE and 5-HT in this group. Actually, the alterations in the neurotransmitters’ levels, along with the neurotoxic effects of MSG on hippocampus, in charge of regulating thinking, memory and learning functions, are believed to be responsible for the marked drop in learning, memory and cognitive activities, as well as the hampered attention, symptoms that are frequently noted in ADHD (Compton 2004; Rebai et al. 2017). These findings were confirmed by ours that revealed sharp drop in short-term memory detected in Y-maze test and also these findings provided justification for the depicted deterioration in memory witnessed in Y-maze results. In the context of our findings, the observed changes in neurotransmitters in MSG-intoxicated group may also account for the illustrated behavioral alterations in OFT, including prolonged latency time, reduced exploratory behavior and locomotor activity, which are usually associated with MSG-induced anxiety-related behaviors that is frequently linked with worsening in short-term memory (Onaolapo et al. 2017; van der Meer et al. 2018).
On the other hand, SE, AST, or PHA alone or in combination noticeably ameliorated the aforementioned behavioral changes, together with the disturbances in levels of neurotransmitters by variable degrees, compared to MSG-intoxicated group. These findings were further confirmed by the improvements in histopathological findings in all treated groups by various extents, compared to MSG-intoxicated group. Notably, the combination of SE with AST and PHA elicited more favorable effects compared to any other group in this study. These positive effects suggested the improvement in locomotor, memory and cognitive functions, which may be credited to the depicted abilities of the tested agents to reverse MSG-induced perturbation in glutamate levels, thereby hampering the resultant excitotoxic effects and opposing MSG-mediated alterations in neurotransmitters’ levels. Besides, these beneficial effects may be linked with the neuroprotective, antioxidant and anti-inflammatory activities of SE and AST (Kumar et al. 2011; Hernández-Marin et al. 2012), together with the positive impacts of PHA on cognitive and memory functions, with enhancing antioxidant defense machinery (Fabel et al. 2003); which acted together to enhance their favorable actions in the combination group.
It is also important to mention that myriad interrelated pathophysiological events, including excitotoxicity, oxidative stress, neuroinflammation, ER stress, as well as impaired autophagy and enhanced neuronal apoptosis may contribute in the etiopathology of ADHD (Corona 2020; Hess et al. 2021; Coulson et al. 2022). In fact, enhanced oxidative stress had been outlined as key pathological event in ADHD, where, diminished antioxidant levels had been observed in children with ADHD (Dvorakova et al. 2006; Ceylan et al. 2010; Alvarez-Arellano et al. 2020). In this point, MSG-induced elevation of glutamate level is associated with disturbance in redox balance and enhanced oxidative stress (Mirzakhani et al. 2020). These findings reinforced ours, which illustrated that MSG elicited marked perturbation in the redox status, evidenced by the diminution in antioxidant defense machinery, SOD and TAC levels, in conjunction with the sharp rise in the production of ROS, demonstrated by the elevated level of lipid peroxidation marker, MDA, in comparison to the normal control group. Undeniably, the brain is highly susceptible to ROS-induced oxidative damage, as a result of the existence of high amounts of polyunsaturated fatty acids and oxygen, due to the high oxygen consumption rate, thereby acting as a substrate for lipid peroxidation (Cobley et al. 2018). There is ample evidence suggesting that heightened production of ROS leads to oxidative damage in neurons, with consequent neuronal apoptosis (Mazon et al. 2017). Besides, excitotoxicity and oxidative stress are the two distinct pathways through which excessive glutamate induces neuroinflammation, where the elevated levels of ROS instigate the inflammatory pathways (Xu 2004; Mostafa et al. 2021). Thus excitotoxicity, oxidative damage and neuroinflammation are intimately intertwined events involved in the pathophysiology of ADHD (Yan et al. 1994; Alvarez-Arellano et al. 2020). Several lines of evidence highlighted the role of neuroinflammation and exaggerated inflammatory response in ADHD, where elevated levels of pro-inflammatory mediators, including TNF-α in children with ADHD were linked with the enhanced intensity of symptoms, such as inattention (Oades et al. 2010; Corona 2020). One of the chief pathways involved in neuroinflammation is HMGB1 cue, which plays a key role in the etiopathology of neurodevelopmental diseases, where HMGB1 is triggered by excessive ROS levels (Makris et al. 2021; Azar et al. 2022; Roustaee et al. 2024). Elevated HMGB1 promoted neuroinflammation, via activation of RAGE (Ferrero-Andrés et al. 2020), which acted concurrently with the depicted enhanced ROS levels to trigger the downstream inflammatory pathway with consequent release of pro-inflammatory mediators, such as TNF-α and IL-1β (Zádor et al. 2021); as documented in MSG-intoxicated group. Likewise, MSG-induced elevation in the levels of pro-inflammatory mediators may be in charge of the illustrated enhanced expression of JAK-2/STAT-3 pathway in this group. In this regard, the activation of JAK-2/STAT-3 cue, another crucial pathway contributing in neuroinflammation, by pro-inflammatory mediators, leads to STAT-3 phosphorylation, with resultant astroglial hyperactivation and neuronal injury (Chen et al. 2020); findings that are quite related with ours in MSG-intoxicated group. The astroglial activation was further confirmed by the depicted enhancement in GFAP immunoexpression in MSG-intoxicated group. Of note, GFAP is produced by activated astroglia that release ROS and pro-inflammatory mediators, creating a vicious cycle between oxidative stress and inflammation and hence playing a vital role in neuroinflammation with its subsequent neuronal apoptosis and hence neuronal damage (Colombo and Farina 2016). Interestingly, the presence of astrogliosis, hallmark of neuroinflammation, had been previously reported in ADHD animal model (Sanches et al. 2023). These aforementioned alterations in inflammatory markers are in line with those of Mostafa et al. (2021).
On the contrary, all these aforesaid disturbances in redox parameters and inflammatory markers were abolished by SE, AST or PHA alone or in combination by different grades, compared to MSG-intoxicated group. Remarkably, the effects of the combination of SE with AST and PHA were more pronounced compared to any other group in this study, suggesting the augmentation between SE, AST, and PHA in enhancing antioxidant defense systems and hampering inflammatory cascades. These effects could be ascribed to the antioxidant and free radical scavenging properties of both SE and AST, which is probably mediated, via donating electrons to free radicals, thus stabilizing them and changing them into inactive form, together with their ability to enhance antioxidant defense mechanisms (Gutiérrez-del-Río et al. 2021). Similarly, PHA had been shown to upregulate antioxidant systems (Fabel et al. 2003). The depicted antioxidant effects of SE, AST, or PHA alone or in combination provided a possible explanation for the observed anti-inflammatory actions of these agents, since ROS are the chief initiator for the activation of inflammatory pathways (Mostafa et al. 2021). Consequently, the observed antioxidant effects of the tested agents may be responsible for the depicted hampering in HMGB1 expression by these agents that resulted in the illustrated hindering in RAGE, which dampened the activation of inflammatory signaling, resulting in the detected suppression in the production of pro-inflammatory mediators in these groups. In this way, the represented inhibition in the release of pro-inflammatory mediators by these agents may be responsible for the observed impeding of JAK-2/STAT-3 signaling with curbed astroglial activation, evidenced by lessened GFAP immunoexpression by these groups. Noteworthy, the anti-inflammatory effects of these agents had been previously reported (El-Sayyad et al. 2023; Sharifi-Rigi et al. 2023).
It had been reported that there is an association between disruption of PI3K/AKT/CREB/BDNF pathway and the pathophysiology of neurological disorders, including ADHD (Alves et al. 2020; Yaqun et al. 2022; Zahra et al. 2022). Noteworthy, the dysregulation of BDNF/TrkB pathway had been outlined to be responsible for the impairment in learning and memory functions witnessed in ADHD children or animal models (Jichao et al. 2017). Remarkably, the activation of BDNF path is vital for neuronal survival, neuronal growth and neuronal plasticity, as well as memory and learning processes (Rossi et al. 2006), therefore, the activation of PI3K/AKT/CREB/BDNF pathway is highlighted as a striking therapeutic approach for amelioration of ADHD. In this study, MSG exhibited marked halting to the expressions of PI3K/AKT/CREB/TrkB pathway, along with the level of BDNF, compared to normal control group. Interestingly, repressed AKT signaling is usually accompanied by enhanced neuroinflammation and oxidative stress. Of note, the exaggerated levels of pro-inflammatory mediators and the suppressed AKT level are considered the key contributors in disrupting BDNF signaling, which lead to consequent enhancement in neuronal apoptosis and impairment in memory functions (Rossi et al. 2006; Luo et al. 2021); results that are closely related with ours in MSG-intoxicated group. Inversely, SE, AST or PHA alone or in combination produced elevations in PI3K,/AKT,/CREB/TrKB expressions, together with BDNF level, by variable grades, when compared to MSG-intoxicated group. Surprisingly, the actions of the combination of SE with AST and PHA on PI3K/AKT/CREB/BDNF/TrKB cue were more obvious compared to any other group in this study. These findings reflected the ability of the tested agents to abolish the abovementioned MSG-mediated hampering to PI3K/AKT pathway. In this frame, the enhanced PI3K subsequently phosphorylated AKT, which then activated CREB (Bai et al. 2019); findings that supported ours in groups exposed to SE, AST or PHA alone or in combination. Indisputably, the activated CREB promoted the transcription of BDNF that interacted with TrKB to encourage neuronal plasticity, enhance memory, suppress neuronal apoptosis and endorse neuronal survival (Palasz et al. 2020). Hence, providing a possible rationalization for the observed abilities of the tested agents to abrogate MSG-mediated memory defects and neuronal apoptosis, via activation of BDNF/TrKB cue in these groups. As a positive feedback, BDNF binding to TrKB again activates PI3K/AKT path to promote CREB activation and the transcription of BDNF another time. Since the suppressed AKT is related with exaggerated oxidative stress, neuroinflammation and neuronal apoptosis (Luo et al. 2021), thus the depicted upregulation of AKT expression by the tested agents may explain their detected abilities to abrogate MSG-mediated oxidative stress, neuroinflammation and neuronal apoptosis.
Another mechanistic event accused in the pathophysiology of neurological disorders, including ADHD, is the exaggerated ER stress, which is the main inducer of neuronal apoptosis (Mou et al. 2020). Remarkably, glutamate-induced excitotoxicity, overstated oxidative stress and neuroinflammation were outlined as the major contributors in ADHD’s pathophysiology (Vázquez-González et al. 2023). Notably, the incidence of these aforementioned three pathological events had been stated to be usually coexistent with the disruption in calcium homeostasis and induction of ER stress, which eventually leads to neuronal apoptosis (Nagar et al. 2023). These findings lend support to ours, where the depicted MSG-induced upsurge in glutamate levels, oxidative stress and neuroinflammation, provided possible explanation for the excessive ER stress noted in this group, evinced by marked upregulation in the gene expression ER stress markers, PERK/CHOP/GRP78, compared to normal control group. Upon incidence of exaggerated ER stress, overactivation of UPR occur, which stimulates PERK, via liberation of GRP78, resulting in promotion in the pro-apoptotic factor, CHOP that instigates apoptotic cascades and hampers Bcl-2 (Romine and Wiseman 2019). Along with enhanced ER stress, oxidative stress and neuroinflammation are also inducers to neuronal apoptosis (Redza-Dutordoir and Averill-Bates 2016). Given these findings, it can be realized that the depicted rise in ER stress markers, especially CHOP, acted supportively with the ongoing exaggerated ROS and inflammatory mediators to induce apoptotic pathways in MSG-intoxicated group, which is supported by the remarkable upregulation in the gene expression of pro-apoptotic BAX and downregulation in the gene expression of anti-apoptotic Bcl-2 in this group, compared to normal control group. In contrast, SE, AST or PHA alone or in combination, possibly by the virtue of their depicted antioxidant, anti-inflammatory and glutamate-hampering activities, were able to suppress MSG-induced elevation in ER stress markers, evidenced by marked lessening in the gene expression of PERK/CHOP/GRP78 pathway, by variable degrees, in these groups, compared to MSG-intoxicated group. In this context, the observed abilities of these tested agents to abate MSG-induced elevation in ER stress, oxidative stress and neuroinflammation, cooperatively resulted in hindering MSG-induced neuronal apoptosis, which is supported by anti-apoptotic Bcl-2 upregulation and prop-apoptotic BAX downregulation in these groups compared to MSG-intoxicated group. Worth mentioning, the combination of SE with AST and PHA exerted pronounced hampering in ER stress markers and apoptotic markers, compared to any other group in this study.
It is noteworthy to mention that sustained ER stress is not only linked with enhanced neuronal apoptosis, but also with diminished autophagy as well, resulting in interruption of the balance between autophagy and neuronal apoptosis, as this imbalance is a crucial pathophysiological feature in ADHD (Park et al. 2014; Chakraborty et al. 2022; Abdulghani et al. 2023). Thus, attaining the balance between autophagy and apoptosis, via enhancing autophagy and/or suppressing apoptosis, is a mainstay target for the amelioration of ADHD. Unquestionably, autophagy is capable of removing damaged organelles, hence enhancing neuronal survival and suppressing neuronal injury (Lopes da Fonseca et al. 2015). Remarkably, AMPK/SIRT-1/m-TOR cue is necessary for the regulation of autophagy, where this pathway is disrupted in neurological disorders, including ADHD, leading to neuronal death (Ibrahim et al. 2022). Our results are in line with these findings, where MSG resulted in impaired autophagy, verified by the prominent hampering in the gene expression of AMPK/SIRT-1/Beclin-1 pathway, together with upregulation in the gene expression of m-TOR, compared to the normal control group. On the contrary, SE, AST, or PHA alone or in combination exhibited noticeable enhancement in autophagy, supported by conspicuous upregulation in the gene expression of AMPK/SIRT-1/Beclin-1 pathway, along with downregulation in the gene expression of m-TOR, by different degrees, compared to MSG-intoxicated group. Of note, the combination of SE with AST and PHA produced prominent modulatory effects on AMPK/SIRT-1/m-TOR hub, compared to any other group in this study. It is important to mention that after AMPK activation, it subsequently activated SIRT-1 and hampered m-TOR, an autophagic suppressor, thus enhancing autophagy, with promoting the expression of an autophagy-related marker, beclin-1 and endorsing neuronal survival (El‐Latif et al. 2023), findings that supported ours in groups exposed to SE, AST or PHA alone or in combination. Based upon the fact that the suppressed autophagy is usually associated with enhanced ER stress (Zhang et al. 2018), hence the depicted autophagy-enhancing activities of SE, AST, or PHA may be linked with the observed hampering in ER stress, as well as the resultant repression of neuronal apoptosis and promotion of neuronal survival in these groups. Previous studies documented that SE, AST or PHA enhanced autophagy, via activation of AMPK/SIRT-1 pathway (Huang et al. 2019; El-Sayyad et al. 2023; Sharifi-Rigi et al. 2023).
Taken together, in the current study, the combination therapy of SE with AST and PHA exerted more favorable actions in all the measured behavioral outcomes, neurotransmitters’ levels, biochemical parameters, together with histopathological findings, compared to any other group in this study. These findings highlighted an augmentation between the beneficial effects of the studied treatments, SE, AST and PHA, that occurred in this combination group. Remarkably, the more pronounced actions of this combination over other studied treatments may be ascribed to the abilities of the tested agents to boost antioxidant defense, hamper neuroinflammation, suppress ER stress, hinder neuronal apoptosis, enhance neurotrophic activities and promote autophagic machineries, via their modulatory actions on HMGB1/RAGE/JAK-2/STAT-3, PERK/CHOP, PI3K/AKT/CREB/BDNF and AMPK/SIRT-1 hubs. Subsequently, these abovementioned advantageous abilities of the tested agents acted together in this combination group, thereby strengthening the favorable effects in this group to produce more prominent neuroprotective actions by this combination, compared to any other treatment in this study.
Conclusions
Our study illustrated the detrimental impact of post-weaning exposure to MSG on the brains of rat pups. Actually, MSG produced a wide range of perturbations in behavioral outcomes, neurotransmitters’ levels, neurotrophic factor, redox, neuroinflammatory, apoptotic, ER stress and autophagic markers, resembling those occurring in ADHD individuals, which was further confirmed by alterations in histopathological findings. Conversely, SE, AST, or PHA alone or in combination, by variable degrees, reversed MSG-induced alterations in all the aforementioned biochemical markers, behavioral outcomes, neurotransmitters’ levels, together with improving the histological picture. These advantageous actions of the tested agents were accounted to their antioxidant, anti-inflammatory and anti-apoptotic properties, along with their abilities to modulate HMGB1/RAGE/JAK-2/STAT-3, PI3K/AKT/CREB/BDNF/TrkB, AMPK/SIRT-1/m-TOR and PERK/CHOP/GRP78/Bcl-2 hubs, as main contributors in the underlying pathophysiological mechanisms in MSG-induced ADHD-like behaviors (Graphical abstract). Remarkably, the effects of the combination of PHA with the two nutraceuticals (AST + SE), were more favorable compared to any other group in this study, suggesting possible augmentation between the beneficial effects of PHA with those of the two tested nutraceuticals in enhancing cognitive and behavioral functions, boosting redox mechanisms and hampering inflammatory signaling. Interestingly, this study unveiled the beneficial role of combining PHA with nutraceuticals in the amelioration of ADHD-like behaviors in rat pups. Thus, shedding light on the fundamental role of using physical exercise in conjunction with nutraceuticals as a promising therapeutic avenue in the management of neurodevelopmental maladies like, ADHD.
Acknowledgements
The authors would like to thank Islam Elgohary, researcher in Pathology Department, Animal Health Research Institute (AHRI), Dokki, Giza, Egypt, for his efforts in revision of histopathological results. In addition, the authors would like to thank Faculty of Pharmacy, Al-Azhar University for providing support to the researchers so they could use the animal unit facilities.
Authors’ Contributions
Karema Abu-Elfotuh: Conceptualization, Performing in vivo experiments, Formal analysis and Writing, reviewing & editing the manuscript. Gellan A.M. Kamel: Formal analysis, writing –original draft and writing, reviewing & editing the manuscript. Mazin A.A. Najm: Formal analysis and writing, reviewing and editing the manuscript. Ahmed M.E. Hamdan: Formal analysis, data curation and writing, reviewing & editing the manuscript. Mona T. Koullah: Formal analysis and writing, reviewing & editing the manuscript. Rasha K.E. Fahmy: Data curation and writing, reviewing & editing the manuscript. Heba Abdelnaser Aboelsoud: Data curation and writing, reviewing & editing the manuscript. Manar A. Alghusn: Formal analysis and writing, reviewing and editing the manuscript. Budor R. Albalawi: Formal analysis and writing, reviewing and editing the manuscript. Ahmed M. Atwa: Data curation and Writing, reviewing & editing the manuscript. Khaled R. Abdelhakim: Data curation and writing, reviewing and editing the manuscript. Abdou M.A. Elsharkawy: Formal analysis and Writing, reviewing & editing the manuscript. Ehsan K. Mohamed: Formal analysis and writing, reviewing and editing the manuscript. Nada S. Abdou: Formal analysis and writing, reviewing and editing the manuscript. Reema Almotairi: Formal analysis and writing, reviewing and editing the manuscript. Hoda A. Salem: Formal analysis, data curation and writing, reviewing and editing the manuscript. Ayah M.H. Gowifel: Formal analysis, data curation, methodology, writing – original draft and writing, reviewing & editing the manuscript. All authors have read and agreed to the final version of the manuscript.
Funding
The authors acknowledge that the funding obtained from the Research, Development, and Innovation Authority (RDIA), Saudi Arabia, Riyadh, Reactivating & Rebuilding of Existing Labs Initiative, Number (13262-Tabuk-2023-UT-R-3–1-HW-), supporting the generation of these data and publication).
Data Availability
Data will be made available on request by the corresponding author.
Declarations
Conflict of Interest
The authors declare no competing interests.
Institutional Review Board
All experimental techniques were accepted and supervised by the Animal Care and Use Committee of the Faculty of Pharmacy, Al-Azhar University, with ethical approval number 429/2023. All animal experiments conducted in the current study complied with ARRIVE guidelines. The handling of animals was in accordance to the guidelines outlined in the "Guide for Care and Use of Laboratory Animals," published by the National Institutes of Health (NIH Publications No. 8023, revised 1978).
Informed Consent
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The Study Limitations
1. The use of male rat pups, where the current study evaluated behavioral, biochemical and histological changes only on male rat pups.
Recommendations
1. Further studies utilizing molecular docking for more confirmation of the actions of SE and AST on the studied molecular targets are required.
2. Future studies including both male and female should be considered to investigate possible sex differences.
3. Further clinical studies are warranted in order to confirm and compare the effects of the studied agents, sesamol and astaxanthin, with those of conventional therapies on human, especially on longer durations.
References
- Abdulghani A, Poghosyan M, Mehren A et al (2023) Neuroplasticity to autophagy cross-talk in a therapeutic effect of physical exercises and irisin in ADHD. Front Mol Neurosci 15:997054. 10.3389/fnmol.2022.997054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adiguzel E, Bozkurt NM, Unal G (2023) Independent and combined effects of astaxanthin and omega-3 on behavioral deficits and molecular changes in a prenatal valproic acid model of autism in rats. Nutr Neurosci 27(6):590–606 [DOI] [PubMed] [Google Scholar]
- Ahn J, Ahn HS, Cheong JH, Dela Peña I (2016) Natural product-derived treatments for attention-deficit/hyperactivity disorder: safety, efficacy, and therapeutic potential of combination therapy. Neural Plast 2016:1320423. 10.1155/2016/1320423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Amin MM, Rahman MM, Khan FR, Zaman F, Mahmud Reza H (2015) Astaxanthin improves behavioral disorder and oxidative stress in prenatal valproic acid-induced mice model of autism. Behav Brain Res 286:112–121. 10.1016/j.bbr.2015.02.041 [DOI] [PubMed] [Google Scholar]
- Ali AA, Khalil MG, Elariny HA, Abu-Elfotuh K (2017) The role of mental and physical activities against development of Alzheimer’s disease in socialized and isolated rats (TDR). Brain Disord Ther 6:2 [Google Scholar]
- Alvarez-Arellano L, González-García N, Salazar-García M, Corona JC (2020) Antioxidants as a potential target against inflammation and oxidative stress in attention-deficit/hyperactivity disorder. Antioxidants 9(2):176. 10.3390/antiox9020176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves CB, Almeida AS, Marques DM et al (2020) Caffeine and adenosine A2A receptors rescue neuronal development in vitro of frontal cortical neurons in a rat model of attention deficit and hyperactivity disorder. Neuropharmacol 166:107782. 10.1016/j.neuropharm.2019.107782 [DOI] [PubMed] [Google Scholar]
- Anelli T, Sitia R (2008) Protein quality control in the early secretory pathway. EMBO J 27:315–327. 10.1038/sj.emboj.7601974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azar YO, Badawi GA, Zaki HF, Ibrahim SM (2022) Agmatine-mediated inhibition of NMDA receptor expression and amelioration of dyskinesia via activation of Nrf2 and suppression of HMGB1/RAGE/TLR4/MYD88/NF-κB signaling cascade in rotenone lesioned rats. Life Sci 311:121049. 10.1016/j.lfs.2022.121049 [DOI] [PubMed] [Google Scholar]
- Baburina Y, Krestinin R, Fedorov D et al (2023) The improvement of functional state of brain mitochondria with astaxanthin in rats after heart failure. Int J Mol Sci 24. 10.3390/ijms24010031 [DOI] [PMC free article] [PubMed]
- Bai L, Zhang S, Zhou X et al (2019) Brain-derived neurotrophic factor induces thioredoxin-1 expression through TrkB/Akt/CREB pathway in SH-SY5Y cells. Biochimie 160:55–60. 10.1016/j.biochi.2019.02.011 [DOI] [PubMed] [Google Scholar]
- Bancroft JD, Gamble M (2008) Theory and practice of histological techniques. 6th edn. Churchill Livingston, Nottingham, United Kingdom, pp 161–186
- Beheshtimanesh Z, Rajaei Z (2023) Neuroprotective effects of sesamol against LPS-induced spatial learning and memory deficits are mediated via anti-inflammatory and antioxidant activities in the rat brain. Avicenna J Phytomedicine 13:213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KA, Samuel S, Patel DR (2018) Pharmacologic management of attention deficit hyperactivity disorder in children and adolescents: a review for practitioners. Transl Pediatr 7:36–47. 10.21037/tp.2017.08.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerri S, Blandini F (2019) Role of autophagy in Parkinson’s disease. Curr Med Chem 26:3702–3718. 10.2174/0929867325666180226094351 [DOI] [PubMed] [Google Scholar]
- Ceylan M, Sener S, Bayraktar AC, Kavutcu M, Bayraktar AC (2010) Oxidative imbalance in child and adolescent patients with attention-deficit/hyperactivity disorder. Prog Neuro-Psychopharmacol Boil Psychiatry 34:1491–1494 [DOI] [PubMed] [Google Scholar]
- Chakraborty J, Pakrashi S, Sarbajna A et al (2022) Quercetin attenuates copper-induced apoptotic cell death and endoplasmic reticulum stress in SH-SY5Y cells by autophagic modulation. Biol Trace Elem Res 200:5022–5041. 10.1007/s12011-022-03093-x [DOI] [PubMed] [Google Scholar]
- Chen X, Chen H, He Y et al (2020) Proteomics-guided study on Buyang Huanwu decoction for its neuroprotective and neurogenic mechanisms for transient ischemic stroke: involvements of EGFR/PI3K/Akt/Bad/14-3-3 and Jak2/Stat3/Cyclin D1 signaling cascades. Mol Neurobiol 57:4305–4321. 10.1007/s12035-020-02016-y [DOI] [PubMed] [Google Scholar]
- Ciarlone AE (1978) Further modification of a fluorometric method for analyzing brain amines. Microchem J 23:9–12. 10.1016/0026-265X(78)90034-6 [Google Scholar]
- Cobley JN, Fiorello ML, Bailey DM (2018) 13 reasons why the brain is susceptible to oxidative stress. Redox Biol 15:490–503. 10.1016/j.redox.2018.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo E, Farina C (2016) Astrocytes: key regulators of neuroinflammation. Trends Immunol 37:608–620. 10.1016/j.it.2016.06.006 [DOI] [PubMed] [Google Scholar]
- Compton DM (2004) Behavior strategy learning in rat: effects of lesions of the dorsal striatum or dorsal hippocampus. Behav Processes 67:335–342. 10.1016/j.beproc.2004.06.002 [DOI] [PubMed] [Google Scholar]
- Corona JC (2020) Role of oxidative stress and neuroinflammation in attention-deficit/hyperactivity disorder. Antioxidants 9:1039. 10.3390/antiox9111039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson RL, Mourrain P, Wang GX (2022) Sleep deficiency as a driver of cellular stress and damage in neurological disorders. Sleep Med Rev 63:101616. 10.1016/J.SMRV.2022.101616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha JM, Masur J (1978) Evaluation of psychotropic drugs with a modified open field test. Pharmacology 16:259–267 [DOI] [PubMed] [Google Scholar]
- Curry DW, Stutz B, Andrews ZB, Elsworth JD (2018) Targeting AMPK signaling as a neuroprotective strategy in Parkinson’s disease. J Parkinsons Dis 8:161–181. 10.3233/JPD-171296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Ding Y, Wu J et al (2022) “Jing-Ning Granules” can alleviate attention deficit hyperactivity disorder in rats by modulating dopaminergic D2/D1-like receptor-mediated signaling pathways. Evid Based Complement Alternat Med 2022:9139841. 10.1155/2022/9139841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorakova M, Sivoňová M, Trebatická J, Škodáček I, Waczulikova I, Muchová J, Ďuračková Z (2006) The effect of polyphenolic extract from pine bark, pycnogenol® on the level of glutathione in children suffering from attention deficit hyperactivity disorder (ADHD). Redox Rep 11:163–172 [DOI] [PubMed] [Google Scholar]
- Eid RA, Al-Shraim M, Zaki MS et al (2019) Vitamin E protects against monosodium glutamate-induced acute liver injury and hepatocyte ultrastructural alterations in rats. Ultrastruct Pathol 43:199–208. 10.1080/01913123.2019.1673860 [DOI] [PubMed] [Google Scholar]
- El-Borai NB, Elkhadrawey BA, AbuBakr HO et al (2022) Sesamol protects against aluminum oxide nanoparticles-induced hepatorenal toxicity in rats via modulation of oxidative stress, inflammation, apoptosis, and <scp>DNA</scp> damage. Environ Toxicol 37:1914–1924. 10.1002/tox.23537 [DOI] [PubMed] [Google Scholar]
- El-Latif AMA, Rabie MA, Sayed RH et al (2023) Inosine attenuates rotenone-induced Parkinson’s disease in rats by alleviating the imbalance between autophagy and apoptosis. Drug Dev Res 84:1159–1174. 10.1002/ddr.22077 [DOI] [PubMed] [Google Scholar]
- El-Sayyad SM, El-Ella DMA, Hafez MM et al (2023) Sesamol defends neuronal damage following cerebral ischemia/reperfusion: a crosstalk of autophagy and Notch1/NLRP3 inflammasome signaling. Inflammopharmacology 1:1–14. 10.1007/s10787-023-01355-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Shoura EA, Salem MA, Ahmed YH, Ahmed LK, Zaafar D (2023) Combined β-sitosterol and trimetazidine mitigate potassium dichromate-induced cardiotoxicity in rats through the interplay between NF-κB/AMPK/mTOR/TLR4 and HO-1/NADPH signaling pathways. Env Sci and Poll Res 30(25):67771–67787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabel K, Fabel K, Tam B et al (2003) VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci 18:2803–2812. 10.1111/j.1460-9568.2003.03041.x [DOI] [PubMed] [Google Scholar]
- Featherstone DE (2010) Intercellular glutamate signaling in the nervous system and beyond. ACS Chem Neurosci 1:4–12. 10.1021/CN900006N [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrero-Andrés A, Panisello-Roselló A, Roselló-Catafau J, Folch-Puy E (2020) NLRP3 inflammasome-mediated inflammation in acute pancreatitis. Int J Mol Sci 21:5386. 10.3390/ijms21155386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffen S, Liu K (2004) Overview of interleukin-2 function, production and clinical applications. Cytokine 28:109–123. 10.1016/j.cyto.2004.06.010 [DOI] [PubMed] [Google Scholar]
- Garcia D, Shaw RJ (2017) AMPK: mechanisms of cellular energy sensing and restoration of metabolic balance. Mol Cell 66:789–800. 10.1016/j.molcel.2017.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser PEA, Batten SR, Gerhardt GA (2022) The role of glutamate dysregulation in the etiology of ADHD. Glutamate Neuropsychiatr Disord Curr Emerg Treat 467–492. 10.1007/978-3-030-87480-3_16
- Gowifel AM, Khalil MG, Nada SA, Kenawy SA, Ahmed KA, Salama MM, Safar MM (2020) Combination of pomegranate extract and curcumin ameliorates thioacetamide-induced liver fibrosis in rats: impact on TGF-β/Smad3 and NF-κB signaling pathways. Toxicol Mech Meth 30(8):620–633 [DOI] [PubMed] [Google Scholar]
- Gürgen SG, Sayın O, Çeti NF et al (2021) The effect of monosodium glutamate on neuronal signaling molecules in the hippocampus and the neuroprotective effects of omega-3 fatty acids. ACS Chem Neurosci 12:3028–3037. 10.1021/acschemneuro.1c00308 [DOI] [PubMed] [Google Scholar]
- Gutiérrez-del-Río I, López-Ibáñez S, Magadán-Corpas P et al (2021) Terpenoids and polyphenols as natural antioxidant agents in food preservation. Antioxidants 10:1264. 10.3390/antiox10081264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heal DJ, Cheetham SC, Smith SL (2009) The neuropharmacology of ADHD drugs in vivo: insights on efficacy and safety. Neuropharmacol 57(7–8):608–618. 10.1016/j.neuropharm.2009.08.020 [DOI] [PubMed] [Google Scholar]
- Hernández-Marin E, Barbosa A, Martínez A (2012) The metal cation chelating capacity of astaxanthin. Does this have any influence on antiradical activity? Molecules 17:1039–1054. 10.3390/molecules17011039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess JL, Radonjić NV, Patak J et al (2021) Autophagy, apoptosis, and neurodevelopmental genes might underlie selective brain region vulnerability in attention-deficit/hyperactivity disorder. Mol Psychiatry 26:6643–6654. 10.1038/s41380-020-00974-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Zhang C, Wang K et al (2017) Paraquat and maneb co-exposure induces noradrenergic locus coeruleus neurodegeneration through NADPH oxidase-mediated microglial activation. Toxicology 380:1–10. 10.1016/j.tox.2017.02.009 [DOI] [PubMed] [Google Scholar]
- Hritcu L, Cioanca O, Hancianu M (2012) Effects of lavender oil inhalation on improving scopolamine-induced spatial memory impairment in laboratory rats. Phytomedicine 19:529–534 [DOI] [PubMed] [Google Scholar]
- Huang J, Wang X, Zhu Y et al (2019) Exercise activates lysosomal function in the brain through AMPK-SIRT1-TFEB pathway. CNS Neurosci Ther 25:796–807. 10.1111/cns.13114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim WW, Kamel AS, Wahid A, Abdelkader NF (2022) Dapagliflozin as an autophagic enhancer via LKB1/AMPK/SIRT1 pathway in ovariectomized/D-galactose Alzheimer’s rat model. Inflammopharmacology 30:2505–2520. 10.1007/s10787-022-00973-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerome D, Physician LJ-CF (2020) U (2020) Approach to diagnosis and management of childhood attention deficit hyperactivity disorder. Can Fam Physician 66:732–736 [PMC free article] [PubMed] [Google Scholar]
- Jichao S, Xinmin H, Xianguo R et al (2017) Saikosaponin A alleviates symptoms of attention deficit hyperactivity disorder through downregulation of DAT and enhancing BDNF expression in spontaneous hypertensive rats. Evid Based Complement Alternat Med 2017:2695903. 10.1155/2017/2695903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanimozhi P, Prasad NR (2009) Antioxidant potential of sesamol and its role on radiation-induced DNA damage in whole-body irradiated Swiss albino mice. Environ Toxicol Pharmacol 28:192–197. 10.1016/j.etap.2009.04.003 [DOI] [PubMed] [Google Scholar]
- Kaur A, Jindal S, Kaur IP (2012) Chopra K (2012) Effect of sesamol on the pathophysiological changes induced by surgical menopause in rodents. Climacteric 15:1–12 [DOI] [PubMed] [Google Scholar]
- Kazmi Z, Fatima I, Perveen S, Malik SS (2017) Monosodium glutamate: Review on clinical reports. Int J Food Prop 20:1–9. 10.1080/10942912.2017.1295260 [Google Scholar]
- Khafaga AF, El-Kazaz SE, Noreldin AE (2021) Boswellia serrata suppress fipronil-induced neuronal necrosis and neurobehavioral alterations via promoted inhibition of oxidative/inflammatory/apoptotic pathways. Sci Total Environ 785:147384. 10.1016/j.scitotenv.2021.147384 [DOI] [PubMed] [Google Scholar]
- Khaled Abd M, Kamel Moha EA, Ismail Abo HM et al (2021) Omega-3 rich oils attenuate ADHD-Like behaviour induced by dietary monosodium glutamate in rats. Pakistan J Biol Sci 24:868–880. 10.3923/pjbs.2021.868.880 [DOI] [PubMed] [Google Scholar]
- Khidr HY, Hassan NF, Abdelrahman S, El-Ansary MR, El-Yamany MF, Rabie MA (2023) Formoterol attenuated mitochondrial dysfunction in rotenone-induced Parkinson’s disease in a rat model: Role of PINK-1/PARKIN and PI3K/Akt/CREB/BDNF/TrKB axis. Int Immunopharmacol 125:111207 [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Yoshida H, Kondo K (2016) Potential anti-atherosclerotic properties of astaxanthin. Mar Drugs 14:35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koracevic D, Koracevic G, Djordjevic V et al (2001) Method for the measurement of antioxidant activity in human fluids. J Clin Pathol 54:356–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo T, Kanemoto S, Hara H et al (2008) A molecular chaperone inducer protects neurons from ER stress. Cell Death Differ 15:364–375. 10.1038/sj.cdd.4402276 [DOI] [PubMed] [Google Scholar]
- Kumar B, Kuhad A, Chopra K (2011) Neuropsychopharmacological effect of sesamol in unpredictable chronic mild stress model of depression: behavioral and biochemical evidences. Psychopharmacology 214:819–828. 10.1007/s00213-010-2094-2 [DOI] [PubMed] [Google Scholar]
- Lee JH, Jo HG, Min SY (2023) East asian herbal medicine for the treatment of children with attention deficit hyperactivity disorder: a systematic review and meta-analysis. Explore (NY) 19(3):330–355. 10.1016/j.explore.2022.11.002 [DOI] [PubMed] [Google Scholar]
- Leffa DT, Torres ILS, Rohde LA (2018) A review on the role of inflammation in attention-deficit/hyperactivity disorder. NeuroImmunoModulation 25:328–333. 10.1159/000489635 [DOI] [PubMed] [Google Scholar]
- Li JJ, He Q (2021) Polygenic scores for ADHD: a meta-analysis. Res Child Adolesc Psychopathol 49:297–310. 10.1007/s10802-021-00774-4 [DOI] [PubMed] [Google Scholar]
- Liu C, Zeng Y, Wen Y et al (2022) Natural products modulate cell apoptosis: a promising way for the treatment of ulcerative colitis. Front Pharmacol 13:806148. 10.3389/fphar.2022.806148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Lopes da Fonseca T, Villar-Piqué A, Outeiro T (2015) The Interplay between alpha-synuclein clearance and spreading. Biomolecules 5:435–471. 10.3390/biom5020435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze MT, Tracey KJ (2005) High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol 5:331–342. 10.1038/nri1594 [DOI] [PubMed] [Google Scholar]
- Luo H, Song B, Xiong G et al (2021) Cadmium inhibits neural stem/progenitor cells proliferation via MitoROS-dependent AKT/GSK-3β/β-catenin signaling pathway. J Appl Toxicol 41:1998–2010. 10.1002/jat.4179 [DOI] [PubMed] [Google Scholar]
- Mahmoud E, Mahmoud M, Hegazy E (2020) Chitosan nanoparticles suppress the oxidative stress in submandibular salivary glands and prevent the genotoxicity of monosodium glutamate in albino rats: histological, immunohistochemical and chromosomal aberrations analysis study. Egypt Dent J 64:3485–3498. 10.21608/edj.2020.91761 [Google Scholar]
- Makris G, Chouliaras G, Apostolakou F, Papageorgiou C, Chrousos GP, Papassotiriou I, Pervanidou P (2021) Increased serum concentrations of high mobility group box 1 (HMGB1) protein in children with autism spectrum disorder. Children 8(6):478. 10.3390/children8060478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazon JN, de Mello AH, Ferreira GK, Rezin GT (2017) The impact of obesity on neurodegenerative diseases. Life Sci 182:22–28. 10.1016/j.lfs.2017.06.002 [DOI] [PubMed] [Google Scholar]
- Meijer AJ, Codogno P (2007) AMP-Activated Protein Kinase and Autophagy Autophagy 3:238–240. 10.4161/auto.3710 [DOI] [PubMed] [Google Scholar]
- Mir S, Cai W, Carlson SW et al (2017) IGF-1 mediated neurogenesis involves a novel RIT1/Akt/Sox2 cascade. Sci Rep 7:3283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzakhani N, Farshid AA, Tamaddonfard E et al (2020) Comparison of the effects of hydroalcoholic extract of Capparis spinosa fruit, quercetin and vitamin E on monosodium glutamate-induced toxicity in rats. Vet Res forum an Int Q J 11:127–134. 10.30466/vrf.2018.83041.2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafa RE, Hassan A, Salama A (2021) Thymol mitigates monosodium glutamate-induced neurotoxic cerebral and hippocampal injury in rats through overexpression of nuclear erythroid 2-related factor 2 signaling pathway as well as altering nuclear factor-kappa B and glial fibrillary acidic protein expression. Open Access Maced J Med Sci 9:716–726. 10.3889/OAMJMS.2021.6170 [Google Scholar]
- Mou Z, Yuan Y, Zhang Z et al (2020) Endoplasmic reticulum stress, an important factor in the development of Parkinson’s disease. Toxicol Lett 324:20–29. 10.1016/j.toxlet.2020.01.019 [DOI] [PubMed] [Google Scholar]
- Muhammad MH, Ahmed NE, El-Shaer NO (2024) Exercise rescues cognitive deterioration in naturally aged rats via PGC1α/FNDC5/irisin/AMPK signaling pathway to restore redox, endothelial, and neuronal homeostasis. Bull EgySoci Physiol Sci 44(2):106–120 [Google Scholar]
- Nagar P, Sharma P, Dhapola R et al (2023) Endoplasmic reticulum stress in Alzheimer’s disease: molecular mechanisms and therapeutic prospects. Life Sci 330:121983. 10.1016/j.lfs.2023.121983 [DOI] [PubMed] [Google Scholar]
- Nishikimi M, Roa NA, Yogi K (1972) Measurement of superoxide dismutase. Biochem Biophys Res Commun 46:849–854 [DOI] [PubMed] [Google Scholar]
- Oades RD, Myint AM, Dauvermann MR, Schimmelmann BG, Schwarz MJ (2010) Attention-deficit hyperactivity disorder (ADHD) and glial integrity: an exploration of associations of cytokines and kynurenine metabolites with symptoms and attention. Behav Brain Funct 6:1–19. 10.1186/1744-9081-6-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa H, Ohishi W, Yagi K (1979) Malondialdeyde as lipid peroxide. Anal Biochem 95:351 [DOI] [PubMed] [Google Scholar]
- Onaolapo OJ, Aremu OS, Onaolapo AY (2017) Monosodium glutamate-associated alterations in open field, anxiety-related and conditioned place preference behaviours in mice. Naunyn Schmiedebergs Arch Pharmacol 390:677–689. 10.1007/s00210-017-1371-6 [DOI] [PubMed] [Google Scholar]
- Onyesife CO, Chukwuma IF, Okagu IU, Ndefo JC, Amujiri NA, Ogugua VN (2023) Nephroprotective effects of Piper nigrum extracts against monosodium glutamate-induced renal toxicity in rats. Sci African 19:e01453 [Google Scholar]
- Palasz E, Wysocka A, Gasiorowska A et al (2020) BDNF as a promising therapeutic agent in Parkinson’s disease. Int J Mol Sci 21:1170. 10.3390/ijms21031170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan C, Prentice H, Price AL, Wu JY (2012) Beneficial effect of taurine on hypoxia-and glutamate-induced endoplasmic reticulum stress pathways in primary neuronal culture. Amino Acids 43:845–855. 10.1007/s00726-011-1141-6 [DOI] [PubMed] [Google Scholar]
- Park E, Yu KH, Kim DK et al (2014) Protective effects of N-acetylcysteine against monosodium glutamate-induced astrocytic cell death. Food Chem Toxicol 67:1–9. 10.1016/j.fct.2014.02.015 [DOI] [PubMed] [Google Scholar]
- Posner J, Polanczyk GV, Sonuga-Barke E (2020) Attention-deficit hyperactivity disorder. Lancet 395:450–462. 10.1016/S0140-6736(19)33004-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebai O, Belkhir M, Sanchez-Gomez MV et al (2017) Differential molecular targets for neuroprotective effect of chlorogenic acid and its related compounds against glutamate induced excitotoxicity and oxidative stress in rat cortical neurons. Neurochem Res 42:3559–3572. 10.1007/s11064-017-2403-9 [DOI] [PubMed] [Google Scholar]
- Redza-Dutordoir M, Averill-Bates DA (2016) Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta 1863:2977–2992. 10.1016/j.bbamcr.2016.09.012 [DOI] [PubMed] [Google Scholar]
- Romine IC, Wiseman RL (2019) PERK signaling regulates extracellular proteostasis of an amyloidogenic protein during endoplasmic reticulum stress. Sci Rep 9:410. 10.1038/s41598-018-37207-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa SG, Quines CB, da Rocha JT et al (2015) Antinociceptive action of diphenyl diselenide in the nociception induced by neonatal administration of monosodium glutamate in rats. Eur J Pharmacol 758:64–71. 10.1016/j.ejphar.2015.03.060 [DOI] [PubMed] [Google Scholar]
- Rossi C, Angelucci A, Costantin L et al (2006) Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur J Neurosci 24:1850–1856 [DOI] [PubMed] [Google Scholar]
- Roustaee S, Sani M, Mehranpour M et al (2024) Chronic administration of lisdexamfetamine induces apoptosis and inflammation and reduces sperm quality in adult male rats. Reprod Sci 31:1278–1289. 10.1007/s43032-023-01449-9 [DOI] [PubMed] [Google Scholar]
- Sanches ES, Boia R, Leitão RA, Madeira MH, Fontes-Ribeiro CA, Ambrósio AF, Fernandes R, Silva AP (2023) Attention-deficit/hyperactivity disorder animal model presents retinal alterations and methylphenidate has a differential effect in ADHD versus control conditions. Antioxidants 12(4):937. 10.3390/antiox12040937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh K (1978) Measurement of lipid peroxide (malondialdehyde) by colorimetric method. Clin Chim Acta 90:37 [DOI] [PubMed] [Google Scholar]
- Seiva FRF, Chuffa LGA, Braga CP et al (2012) Quercetin ameliorates glucose and lipid metabolism and improves antioxidant status in postnatally monosodium glutamate-induced metabolic alterations. Food Chem Toxicol 50:3556–3561. 10.1016/j.fct.2012.07.009 [DOI] [PubMed] [Google Scholar]
- Sharifi-Rigi A, Zal F, Aarabi M-H et al (2023) The effects of astaxanthin on AMPK/autophagy axis and inflammation in type 2 diabetes patients: a randomized, double-blind, placebo-controlled trial. Gene Reports 33:101844. 10.1016/j.genrep.2023.101844 [Google Scholar]
- Singh TG, Singh HP, Kaur S, Dhiman S (2020) Protective effects of sesamol against cisplatin-induced nephrotoxicity in rats: a mechanistic approach. Obes Med 19:100269 [Google Scholar]
- Smaga I, Niedzielska E, Gawlik M et al (2015) Oxidative stress as an etiological factor and a potential treatment target of psychiatric disorders. Part 2. Depression, anxiety, schizophrenia and autism. Pharmacol Reports 67:569–580. 10.1016/j.pharep.2014.12.015 [DOI] [PubMed] [Google Scholar]
- Solleiro-Villavicencio H, Rivas-Arancibia S (2018) Effect of chronic oxidative stress on neuroinflammatory response mediated by CD4+T cells in neurodegenerative diseases. Front Cell Neurosci 12:114. 10.3389/fncel.2018.00114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Yuan H, Chen T et al (2021) An Shen Ding Zhi Ling alleviates symptoms of attention deficit hyperactivity disorder via anti-inflammatory effects in spontaneous hypertensive rats. Front Pharmacol 11:1–13. 10.3389/fphar.2020.617581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela MJ (2008) Brain reserve and the prevention of dementia. Curr Opin Psychiatry 21:296–302. 10.1097/YCO.0b013e3282f97b1f [DOI] [PubMed] [Google Scholar]
- van der Meer D, Hoekstra PJ, van Rooij D et al (2018) Anxiety modulates the relation between attention-deficit/hyperactivity disorder severity and working memory-related brain activity. World J Biol Psychiatry 19:450–460. 10.1080/15622975.2017.1287952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez-González D et al (2023) A Potential Role for Neuroinflammation in ADHD. In: Kim YK (eds) Neuroinflammation, gut-brain axis and immunity in neuropsychiatric disorders. Advances in experimental medicine and biology, vol 1411. Springer, Singapore. 10.1007/978-981-19-7376-5_15
- Viboolvorakul S, Patumraj S (2014) Exercise training could improve age-related changes in cerebral blood flow and capillary vascularity through the upregulation of VEGF and eNOS. BioMed Res Int 2014(1):230791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang Y, Wang X et al (2023) Sesamol mitigates chronic iron overload-induced cognitive impairment and systemic inflammation via IL-6 and DMT1 regulation. Mol Nutr Food Res 67(17):2300012 [DOI] [PubMed] [Google Scholar]
- Xu L (2004) Effect of glutamate on inflammatory responses of intestine and brain after focal cerebral ischemia. World J Gastroenterol 11:733. 10.3748/wjg.v11.i5.733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan QS, Jobe PC, Cheong JH et al (1994) Role of serotonin in the anticonvulsant effect of fluoxetine in genetically epilepsy-prone rats. Naunyn Schmiedebergs Arch Pharmacol 350:149–152. 10.1007/BF00241089 [DOI] [PubMed] [Google Scholar]
- Yaqun L, Haixia Y, Yuchen S et al (2022) An Shen Ding Zhi Ling ameliorates the symptoms of attention deficit hyperactivity disorder via modulating brain-derived neurotrophic factor-related signaling pathways. Evid Based Complement Alternat Med 2022:5471586. 10.1155/2022/5471586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zádor F, Joca S, Nagy-Grócz G et al (2021) Pro-inflammatory cytokines: potential links between the endocannabinoid system and the kynurenine pathway in depression. Int J Mol Sci 22:5903. 10.3390/ijms22115903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahra W, Birla H, Sen SS et al (2022) Anti-Parkinsonian effect of mucuna pruriens and ursolic acid on GSK3β/Calcium signaling in neuroprotection against rotenone-induced Parkinsonism. Phytomed Plus 2:100343. 10.1016/j.phyplu.2022.100343 [Google Scholar]
- Zhang Z, Shen Y, Luo H et al (2018) MANF protects dopamine neurons and locomotion defects from a human α-synuclein induced Parkinson’s disease model in C. elegans by regulating ER stress and autophagy pathways. Exp Neurol 308:59–71. 10.1016/j.expneurol.2018.06.016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request by the corresponding author.