Abstract
Anti-oxidant/Pro-oxidant oxidant imbalance leads to chronic inflammation and insulin resistance can lead to the development of metabolic syndrome (MetS). The oxidative balance score (OBS) is a tool for assessing oxidative stress associated with MetS risk. However, the association between OBS and mortality in patients with MetS remains unclear. This study analyzed 10,647 MetS patients from the 1999–2018 National Health and Nutrition Examination Survey (NHANES). OBS were calculated using a combination of 16 dietary and 4 lifestyle factors. Multivariate Cox proportional hazards regression models, Kaplan–Meier survival analysis, restricted cubic splines (RCS), and subgroup analyses were used to evaluate the potential association between OBS and the risk of all-cause and cardiovascular mortality. Sensitivity analyses confirmed the robustness of the results. This study found that OBS was inversely associated with all-cause and cardiovascular mortality in patients with MetS, a result consistent across most subgroups. Both the Kaplan–Meier curve and RCS analysis supported these findings. Sensitivity analysis was used to verify the robustness of the results. Maintaining an antioxidant-based diet and lifestyle may help reduce the risk of all-cause and cardiovascular mortality in patients with MetS. These findings underscore the significance of incorporating antioxidant-rich dietary patterns and behavioral practices in strategies aimed at preventing and managing MetS.
Keywords: Oxidative balance scores, Metabolic syndrome, Mortality, NHANES, Nutrition
Subject terms: Metabolic syndrome, Metabolic syndrome
Introduction
In recent years, the prevalence of metabolic syndrome (MetS) and related complications has become a global public health problem1. Data from 2003 to 2012 indicated that approximately 33% of the U.S. population was affected by MetS2. By 2018, it is estimated that at least one billion individuals worldwide had MetS3–5.
MetS represents a constellation of interrelated risk factors for cardiovascular disease (CVD) and diabetes. MetS is a complex of diseases, including obesity (especially central obesity), elevated triglyceride level, decreased high-density lipoprotein cholesterol (HDL-C) level, elevated blood pressure, and abnormal blood glucose. A diagnosis of MetS is made when at least three of these criteria are present6–8. Evidence suggests that free radical-induced oxidative stress plays a crucial role in the development of MetS9. Oxidative stress can induce hyperglycemia, insulin resistance, lipid metabolism disorder, and activation of inflammatory cascade by inhibiting insulin signaling, which forms the physiological basis of pre-MetS10,11.
In particular, oxidative stress promotes endothelial cell damage and dysfunction by increasing reactive oxygen species (ROS), contributing to atherosclerosis risk. ROS also impair insulin signaling, reducing cellular insulin sensitivity, and increasing the risk of diabetes and CVD11. Furthermore, oxidative imbalance activates pro-inflammatory pathways, elevates cytokine secretion, and induces chronic low-grade inflammation, which accelerates cardiovascular damage and metabolic disorders, thereby increasing mortality risk in MetS patients12. Additionally, oxidative stress induces lipid peroxidation, leading to adipocyte dysfunction, further promoting fat accumulation, insulin resistance, and metabolic disturbances, exacerbating clinical outcomes in MetS13. Despite the availability of pharmacological treatments, lifestyle and dietary interventions are often underutilized in clinical practice7,14,15.
Emerging evidence indicates that adopting a healthy lifestyle and consuming high levels of anti-oxidant nutrients can effectively delay the progression of chronic diseases16,17. Oxidative stress levels in the body are influenced by both anti-oxidant and pro-oxidant factors; the former includes specific anti-oxidant nutrients and regular physical activity, while the latter encompasses behaviors such as smoking, excessive alcohol consumption, obesity, and inadequate anti-oxidant intake18–20. However, there is still a lack of evidence on the association between lifestyle management patterns combined with anti-oxidant diets and mortality, especially in individuals with MetS.
By summarizing the factors influencing oxidative stress levels, a combination of factors including lifestyle management and an anti-oxidative/pro-oxidative diet was developed to generate an OBS21. Elevated OBS levels are indicative of increased anti-oxidant exposure22.
OBS provides a comprehensive assessment of oxidative stress levels. Monitoring OBS can identify individuals with high oxidative stress in MetS patients, allowing for early prediction of CVD, diabetes, and other metabolic disorders. Based on OBS levels, personalized interventions, such as anti-oxidant supplementation and lifestyle modifications, can help reduce oxidative damage, improve metabolic health, and ultimately enhance patient prognosis10.
The present study aimed to investigate the association between the level of OBS and all-cause and cardiovascular mortality in patients with MetS using data from the NHANES.
Materials and methodology
Study population
NHANES is a nationally representative survey of nutrition and health status in a nationally representative population of the U.S. conducted by the National Center for Health Statistics (NCHS), part of the US Centers for Disease Control and Prevention. Its primary objective is to assess the health and nutritional status of the U.S. population. Given the complex survey design and way of data collection, sample weights were provided according to oversampling, survey nonresponse, and post-stratification. The NHANES study protocol was approved by the NCHS ethics review committee, and all participants provided written informed consent. The researchers can be downloaded on the website [https://www.cdc.gov/nchs/nhanes/ind-ex.htm for free public data for analysis].
The data for this study were obtained from the NHANES database from 1999 to 2018, covering 10 survey periods. Inclusion and exclusion criteria were as follows: (1) pregnant participants (n = 1,753); (2) participants aged < 18 years (n = 42,029); (3) participants with missing mortality data (n = 1,275); (4) participants with insufficient dietary and lifestyle data to calculate OBS (n = 1,610); (5) non-MetS participants (n = 42,137); (6) participants with missing data on covariates (n = 1,838), including education, marital status, and poverty to income ratio (PIR). Finally, 10,647 participants were included in our study. The flow chart of inclusion and exclusion criteria is shown in Fig. S1.
Definition of MetS
The definition of MetS was based on previous guidelines [National Cholesterol Education Program (NCEP): National Cholesterol Education Program Adult Treatment Panel III]23. MetS was defined as meeting three or more of the following criteria: (1) Abdominal obesity (waist circumference): > 102 cm in men or > 88 cm in women; (2) Hypertriglyceridemia: plasma triglyceride (TG) ≥ 150 mg/dl (1.7 mmol/L) or current treatment with TG-lowering drugs; (3) Low HDL-C: HDL-C < 40 mg/dl in men, HDL-C < 50 mg/dl in women or receiving HDL-C lowering drugs; (4) Hypertension: systolic blood pressure (SBP) ≥ 130 mmHg or SBP ≥ 85 mmHg or being treated with antihypertensive drugs or previously diagnosed by a doctor; (5) Hyperglycemia: fasting plasma glucose (FPG) > 100 mg/dl or currently receiving hypoglycemic drugs, insulin treatment, or previously diagnosed as diabetes by a doctor.
Exposure variable: oxidative balance score
The OBS is a comprehensive assessment of 4 lifestyle factors and 16 dietary nutrients, including 15 anti-oxidant factors and 5 pro-oxidative factors21,24. Data on 16 dietary nutrients were obtained from the first dietary recall interview record, including dietary fiber, carotenoids (a- and β-carotene), niacin, riboflavin, vitamin B6, vitamin B12, vitamin C (a-tocopherol equivalent), vitamin E, total folate, iron, magnesium, zinc, copper, calcium, selenium, and total fat. Data on 4 lifestyle factors were obtained from home interview records and mobile examination center tests, including body mass index (BMI), physical activity, smoking, and alcohol consumption. Smoking levels were standardized according to cotinine measurements. Total fat intake, iron intake, BMI, alcohol consumption level, and smoking level were classified as pro-oxidative factors, and the remaining 15 factors were classified as anti-oxidant factors.
Combined with previous studies21,25, we categorized alcohol into 3 groups based on sex and alcohol consumption: never drinkers, light to moderate drinkers (men 0–30 g/day, women 0–15 g/day), and heavy drinkers (men > 30 g/day, women > 15 g/day), scored as 2, 1, and 0 points. Other factor variables were scored by tertiles after stratification according to gender. For anti-oxidant factors, scores from the highest tertile to the lowest tertile were 2,1,0. In contrast, for pro-oxidant components, the highest tertile received 0 points and the lowest received 2 points. OBS was calculated by summing the 20-factor scores. Theoretical scores range from 0 to 40. Higher scores indicate greater individual exposure to anti-oxidants. The specific factor scoring scheme for the OBS is shown in Table S1.
Ascertainment of mortality
Mortality data were obtained from the National Death Index database managed by the Centers for Disease Control and can be downloaded for data analysis by researchers at the website: [https://www.cdc.gov/nchs/data-linkage/mortality-public.htm]. The most recent publicly available data include follow-up data on mortality from the date participants entered the survey through December 31, 2019. Causes of death were classified according to the International Classification of Diseases, tenth revision; in particular, I00-I09, I11, I13, and I20-I51 were used to determine cardiovascular death.
Covariates
Based on previously published studies, we considered several covariates that could confound the results21. Covariates included in this study included age, sex (male, female), race (Mexican American, non-Hispanic black, non-Hispanic white, other Hispanic, other race—including multi-racial), education (less than high school, high school or equivalent, more than high school), marital status (married or living with a partner, never married, widowed/divorced/separated), PIR (< 1.3,1.3–3.5, > 3.5), smoking history (never, former, or current)26, and drinking history (defined as drinking at least 12 alcoholic beverages per year). Hypertension was defined as SBP ≥ 140 mmHg, DBP ≥ 90 mmHg, or self-reported physician-diagnosed hypertension or current use of antihypertensive medications27. Diabetes was defined as having any of the following: HbA1c concentration ≥ 6.5% or FPG level ≥ 126 mg/dL, or self-reported physician-diagnosed diabetes or current use of glucose-lowering medications or use of insulin28. Data on each of these variables are available on the NHANES website.
Statistical analysis
To account for the effects of stratification and clustering inherent in the complex survey design, the weights from the NHANES 1999–2018 diet day one sample were applied to produce nationally representative estimates for all analyses21,29. Due to the distinct nature of the sampling weights for the periods 1999 to 2002 and 2003 to 2018, it is essential to calculate these weights separately. Specifically, the weight for the 1999 to 2002 period is derived as WTDR4YR * 2/10, while the weight for the 2003 to 2018 period is calculated as WTDRD1 * 1/10.
Baseline characteristics were represented using quartiles of the OBS. Continuous variables are presented as means ± standard deviations (SD), while categorical variables are reported as frequency counts and percentages. P-values for categorical variables were obtained using Pearson’s Chi-square test, and for continuous variables, the Kruskal–Wallis rank-sum test was employed. Differences in survival probabilities were assessed using the log-rank test in conjunction with Kaplan–Meier (K-M) survival analysis to evaluate variations in mortality risk among the four groups. Three multivariate Cox proportional hazards models were employed to assess the association between OBS levels and the risk of all-cause and cardiovascular mortality in patients with MetS. Model 1 served as an unadjusted baseline. Model 2 was adjusted for sex, age, and race, while Model 3 incorporated additional adjustments for education, marital status, PIR, smoking, alcohol consumption, history of hypertension, and history of diabetes. To elucidate the relationship between OBS levels and mortality risk, RCS with three knots were utilized for visualization based on the covariate adjustments of Model 3. The variance inflation factor (VIF) of the variables included in the models was calculated to avoid the result deviation caused by multicollinearity. We did not find evidence of collinearity in the models, given the VIF of < 5. Additionally, subgroup analyses and interaction tests were conducted using the covariate adjustments from Model 3. To avoid reverse causation and validate the stability of the current findings, sensitivity analyses were carried out by excluding participants who died within two years. To mitigate selection bias from excluding subjects with missing data, we performed a sensitivity analysis using multiple imputation.
Statistical analyses were performed using R version 4.3.1. A two-tailed p-value of less than 0.05 was considered indicative of statistical significance.
Results
Baseline characteristics of the participants
Table 1 presents the baseline characteristics of participants categorized by quartiles of the OBS. Compared to individuals in the highest quartile (Q4) of OBS, those in the lowest quartile (Q1) were more likely to be non-Hispanic Black, possess lower levels of education, be unmarried, have a lower PIR, exhibit a higher prevalence of diabetes, smoke more frequently, and higher OBS dietary/lifestyle score. Conversely, the Q1 group was relatively younger. No statistically significant differences in OBS were observed across gender, hypertension, or alcohol consumption history.
Table 1.
Baseline characteristics grouped by OBS quartile.
| Variable | Total | Q1 [0–11] | Q2 (11–18] | Q3 (18–23] | Q4 (23–36] | P-value |
|---|---|---|---|---|---|---|
| N | 10,674 | 2770 | 3006 | 2286 | 2612 | |
| Age, mean ± SD (years) | 55.888(0.239) | 54.927(0.403) | 57.012(0.396) | 56.691(0.432) | 54.978(0.418) | < 0.001* |
| OBS, mean ± SD | 17.951(0.139) | 6.713(0.100) | 15.201(0.053) | 20.916(0.044) | 27.320(0.074) | < 0.001* |
| Gender (%) | 0.632 | |||||
| Female | 5807(53.448) | 1388(51.870) | 1666(54.380) | 1294(53.865) | 1459(53.521) | |
| Male | 4867(46.552) | 1382(48.130) | 1340(45.620) | 992(46.135) | 1153(46.479) | |
| Race (%) | < 0.001* | |||||
| Mexican American | 1907(7.449) | 499(8.540) | 518(6.880) | 379(6.840) | 511(7.579) | |
| Non-Hispanic Black | 2106(10.293) | 687(13.956) | 670(12.373) | 390(8.956) | 359(6.418) | |
| Non-Hispanic White | 5161(72.269) | 1193(66.048) | 1410(71.093) | 1183(74.359) | 1375(76.776) | |
| Other Hispanic | 838(4.598) | 224(5.212) | 247(4.737) | 184(4.464) | 183(4.076) | |
| Other Race—including multi-racial | 662(5.390) | 167(6.244) | 161(4.917) | 150(5.381) | 184(5.152) | |
| Education (%) | < 0.001* | |||||
| Less than High school | 3307(20.350) | 1136(29.178) | 1005(22.274) | 606(17.639) | 560(13.514) | |
| High school diploma or equivalent | 2682(27.701) | 704(29.693) | 783(29.272) | 568(26.795) | 627(25.318) | |
| More than High school | 4685(51.949) | 930(41.129) | 1218(48.454) | 1112(55.566) | 1425(61.168) | |
| Marry status (%) | < 0.001* | |||||
| Married or living with a partner | 6494(64.315) | 1571(59.039) | 1839(65.080) | 1394(65.032) | 1690(67.310) | |
| Never married | 1003(10.046) | 318(12.270) | 243(8.493) | 205(9.465) | 237(10.163) | |
| Widowed,divorced,or separated | 3177(25.638) | 881(28.691) | 924(26.427) | 687(25.504) | 685(22.527) | |
| Poverty to income ratio (%) | < 0.001* | |||||
| < 1.3 | 3536(23.594) | 1190(34.856) | 1036(25.423) | 668(19.947) | 642(15.610) | |
| 1.3 ~ 3.5 | 4254(38.139) | 1052(38.379) | 1247(40.495) | 963(39.657) | 992(34.533) | |
| > = 3.5 | 2884(38.267) | 528(26.765) | 723(34.082) | 655(40.396) | 978(49.857) | |
| Hypertension (%) | 0.085 | |||||
| No | 2792(28.129) | 726(30.308) | 751(27.382) | 579(25.557) | 736(29.088) | |
| Yes | 7882(71.871) | 2044(69.692) | 2255(72.618) | 1707(74.443) | 1876(70.912) | |
| Diabetes mellitus (%) | 0.029* | |||||
| No | 5695(60.000) | 1411(58.935) | 1541(57.845) | 1248(59.892) | 1495(62.978) | |
| Yes | 4979(40.000) | 1359(41.065) | 1465(42.155) | 1038(40.108) | 1117(37.022) | |
| Alcohol (%) | 0.913 | |||||
| No | 7967(74.369) | 2064(73.857) | 2214(74.309) | 1692(74.125) | 1997(75.032) | |
| Yes | 2707(25.631) | 706(26.143) | 792(25.691) | 594(25.875) | 615(24.968) | |
| Smoke (%) | < 0.001* | |||||
| Former | 3443(32.422) | 835(27.777) | 1002(32.911) | 764(34.834) | 842(33.829) | |
| Never | 5295(48.801) | 1213(42.096) | 1445(47.075) | 1158(49.649) | 1479(55.197) | |
| Now | 1936(18.777) | 722(30.127) | 559(20.013) | 364(15.516) | 291(10.974) | |
| OBS. dietary | 15.943(0.123) | 6.319(0.067) | 12.070(0.060) | 17.580(0.059) | 23.583(0.071) | < 0.001* |
| OBS. lifestyle | 3.189(0.027) | 2.437(0.040) | 3.131(0.038) | 3.336(0.046) | 3.738(0.042) | < 0.001* |
Q1–Q4: Grouped by quartile according to OBS. Mean ± SD for continuous variables. The P value was calculated by Kruskal–Wallis rank-sum test. % for categorical variables. P value was calculated by Chi-square test. *Significant difference.
Associations of the OBS with all-cause and cardiovascular mortality in patients with MetS
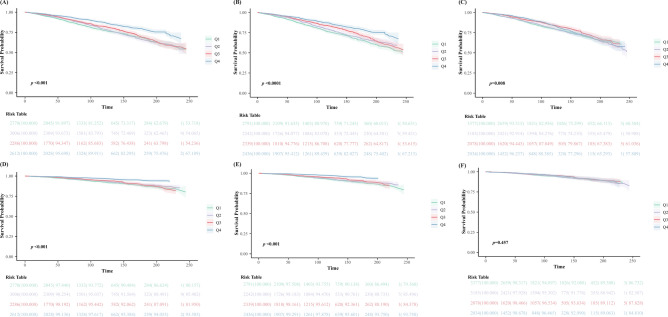
During a mean follow-up period of 105.4 months, 2505 out of 10,647 participants with MetS (23.5%) succumbed, with 725 (28.9%) of these deaths attributable to cardiovascular disease. Kaplan–Meier (K-M) survival analysis revealed significant differences in both all-cause and cardiovascular mortality rates across different quartiles of OBS. Mortality rates were lowest among participants in the highest quartile (Q4) of OBS and highest among those in the lowest quartile (Q1) (log-rank p < 0.001). The OBS dietary score showed similar results to the OBS score. However, no statistical difference was observed in the OBS lifestyle score for cardiovascular death. The detailed K-M survival curves are illustrated in Fig. 1.
Fig. 1.
Association between baseline OBS and future risk of mortality. Abbreviations: K-M survival curves of OBS with mortality in patients with metabolic syndrome: (A) total OBS and all-cause mortality; (B) dietary OBS and all-cause mortality; (C) lifestyle OBS and all-cause mortality; (D) total OBS and cardiovascular mortality; (E) dietary OBS and cardiovascular mortality; (F) lifestyle OBS and cardiovascular mortality. Q1 through Q4, quartile 1 through 4.
Table 2 presents the results of the multivariate Cox regression analyses examining the relationship between OBS and all-cause mortality. In the fully adjusted model (Model 3), each unit increase in OBS correlated with a 1.1% reduction in mortality risk (HR 0.989, 95% CI 0.980–0.997, p = 0.009). Comparatively, individuals in Q4 had a 20.5% lower risk of all-cause mortality than those in Q1 (HR 0.795, 95% CI 0.663–0.952, p for trend = 0.016). OBS dietary score and OBS lifestyle score showed similar results.
Table 2.
Multivariate Cox regression analysis of OBS with all-cause and cardiovascular mortality.
| Mortality type/OBS variable forms | Model 1 | P value | Model 2 | P value | Model 3 | P value |
|---|---|---|---|---|---|---|
| HR (95%CI) | HR (95%CI) | HR (95%CI) | ||||
| OBS Total | ||||||
| All-cause death | ||||||
| Continuous | 0.978(0.971,0.985) | < 0.001* | 0.978(0.971,0.985) | < 0.001* | 0.989(0.980,0.997) | 0.009* |
| Quartile | ||||||
| Q1 [0–11] | Reference | Reference | Reference | |||
| Q2 (11–18] | 0.957(0.823,1.112) | 0.564 | 0.848(0.727,0.989) | 0.035* | 0.935(0.801,1.091) | 0.395 |
| Q3 (18–23] | 0.865(0.757,0.989) | 0.033* | 0.782(0.669,0.914) | 0.002* | 0.908(0.770,1.072) | 0.256 |
| Q4 (23–36] | 0.580(0.486,0.692) | < 0.001* | 0.628(0.528,0.746) | < 0.001* | 0.795(0.663,0.952) | 0.013* |
| P for trend | < 0.001* | < 0.001* | 0.016* | |||
| Cardiovascular death | ||||||
| Continuous | 0.967(0.955,0.978) | < 0.001* | 0.966(0.954,0.978) | < 0.001* | 0.977(0.964,0.989) | < 0.001* |
| Quartile | ||||||
| Q1 [0–11] | Reference | Reference | Reference | |||
| Q2 (11–18] | 0.836(0.640,1.092) | 0.189 | 0.741(0.565,0.972) | 0.030 | 0.812(0.619,1.066) | 0.133 |
| Q3 (18–23] | 0.841(0.662,1.068) | 0.156 | 0.765(0.596,0.982) | 0.036 | 0.889(0.678,1.166) | 0.394 |
| Q4 (23–36] | 0.410(0.290,0.581) | < 0.001* | 0.457(0.329,0.636) | < 0.001* | 0.577(0.418,0.796) | < 0.001* |
| P for trend | < 0.001* | < 0.001* | 0.002* | |||
| OBS Dietary | ||||||
| All-cause death | ||||||
| Continuous | 0.968(0.959,0.977) | < 0.001* | 0.978(0.968,0.988) | < 0.001* | 0.988(0.978,0.998) | 0.023* |
| Quartile | ||||||
| Q1 [2–10] | Reference | Reference | Reference | |||
| Q2 (10–15] | 0.863(0.724,1.028) | 0.098 | 0.861(0.721,1.028) | 0.097 | 0.950(0.794,1.137) | 0.095 |
| Q3 (15–20] | 0.763(0.669,0.870) | < 0.001* | 0.766(0.653,0.900) | 0.001* | 0.854(0.721,1.012) | 0.068 |
| Q4 (20–30] | 0.554(0.465,0.661) | < 0.001* | 0.696(0.581,0.833) | < 0.001* | 0.836(0.700,0.998) | 0.048* |
| P for trend | < 0.001* | < 0.001* | 0.029* | |||
| Cardiovascular death | ||||||
| Continuous | 0.952(0.938,0.966) | < 0.001* | 0.963(0.948,0.978) | < 0.001* | 0.972(0.973,0.988) | < 0.001* |
| Quartile | ||||||
| Q1 [2-10] | Reference | Reference | Reference | |||
| Q2 (10–15] | 0.852(0.644,1.127) | 0.261 | 0.862(0.647,1.148) | 0.309 | 0.945(0.706,1.265) | 0.705 |
| Q3 (15–20] | 0.783(0.606,1.012) | 0.062 | 0.804(0.614,1.051) | 0.111 | 0.892(0.670,1.187) | 0.433 |
| Q4 (20–30] | 0.380(0.268,0.540) | < 0.001* | 0.500(0.356,0.701) | < 0.001* | 0.600(0.429,0.841) | 0.003* |
| P for trend | < 0.001* | < 0.001* | 0.004* | |||
| OBS Lifestyle | ||||||
| All-cause death | ||||||
| Continuous | 0.971(0.935,1.008) | 0.119 | 0.855(0.824,0.889) | < 0.001* | 0.934(0.895,0.975) | 0.002* |
| Quartile | ||||||
| Q1 [0–2] | Reference | Reference | Reference | |||
| Q2 (2–3] | 1.035(0.902,1.187) | 0.622 | 0.847(0.746,0.961) | 0.010* | 1.030(0.895,1.185) | 0.680 |
| Q3 (3–4] | 0.842(0.704,1.006) | 0.058 | 0.601(0.513,0.705) | < 0.001* | 0.785(0.663,0.928) | 0.005* |
| Q4 (4–7] | 0.852(0.726,0.999) | 0.049* | 0.576(0.494,0.670) | < 0.001* | 0.776(0.655,0.918) | 0.003* |
| P for trend | 0.014* | < 0.001* | < 0.001* | |||
| Cardiovascular death | ||||||
| Continuous | 0.969(0.902,1.040) | 0.383 | 0.843(0.781,0.910) | < 0.001 | 0.925(0.858,0.996) | 0.039 |
| Quartile | ||||||
| Q1 [0–2] | Reference | Reference | Reference | |||
| Q2 (2–3] | 1.039(0.815,1.325) | 0.757 | 0.842(0.666,1.063) | 0.148 | 1.029(0.798,1.328) | 0.826 |
| Q3 (3–4] | 0.873(0.665,1.145) | 0.326 | 0.605(0.466,0.785) | < 0.001* | 0.808(0.610,1.069) | 0.136 |
| Q4 (4–7] | 0.878(0.617,1.248) | 0.467 | 0.573(0.407,0.807) | 0.001* | 0.798(0.565,1.127) | 0.201 |
| P for trend | 0.290 | < 0.001* | 0.077 | |||
HR, hazard ratio; NA, not applicable; Q1 through Q4, quartile 1 through 4. Model 1, unadjusted; Model 2, adjusted for age, gender, and race; Model 3, adjusted for age, gender, race, education, marital status, poverty-to-income ratio, hypertension history, diabetes mellitus history, drinking, and smoking. *Significant difference.
Table 2 also presents the results from Cox regression models evaluating the relationship between OBS and cardiovascular mortality. In the fully adjusted model (Model 3), each unit increase in OBS was associated with a 2.3% decrease in cardiovascular mortality risk (HR 0.977, 95% CI 0.964–0.989, p < 0.001). The risk of cardiovascular death was reduced by 42.3% for those in the Q4 group compared to the Q1 group (HR 0.577, 95% CI 0.418–0.796, p for trend = 0.002). OBS dietary score and OBS lifestyle score showed similar results. However, OBS lifestyle quartile scores did not show statistical differences. The weaker association may result from OBS lifestyle not accounting for factors such as sedentary behavior, sleep quality, and stress.
The population was stratified by sex. In male patients, total and dietary OBS were associated with a reduced risk of cardiovascular death, while lifestyle OBS was linked to a reduced risk of all-cause death. In female patients, OBS was associated with lower risks of both all-cause and cardiovascular mortality, except for lifestyle OBS, which was not linked to cardiovascular mortality. Thus, OBS may have greater predictive value in females. Results are provided in Tables S2 and S3.
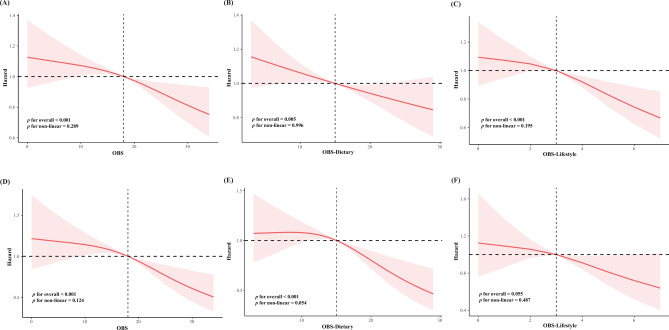
To visualize the association of OBS with the risk of death from all-cause and cardiovascular causes among patients with MetS, we constructed a model 3-based covariant-adjusted RCS with three knots. The results showed an inverse linear association between the OBS and all-cause and cardiovascular mortality in patients with MetS. The OBS dietary score showed similar results to the OBS score. However, no statistical difference was observed in the OBS lifestyle score for cardiovascular death. The detailed RCS are illustrated in Fig. 2.
Fig. 2.
Dose–response relationship between baseline OBS and future risk of mortality. Abbreviations: RCS of OBS with mortality in patients with metabolic syndrome: (A) total OBS and all-cause mortality; (B) dietary OBS and all-cause mortality; (C) lifestyle OBS and all-cause mortality; (D) total OBS and cardiovascular mortality; (E) dietary OBS and cardiovascular mortality; (F) lifestyle OBS and cardiovascular mortality. All models were adjusted for age, gender, race, education, marital status, poverty-to-income ratio, hypertension history, diabetes mellitus history, drinking, and smoking.
Subgroup analysis
To evaluate the robustness of the Cox regression results regarding OBS and mortality outcomes in patients with MetS across different subgroups, we conducted stratified analyses. These findings, detailed in Table 3, demonstrate that the inverse association between OBS and all-cause mortality was both consistent and statistically significant across most subgroups. Notably, education level was found to interact with OBS, suggesting a potential moderating effect (p = 0.012 for interaction). An inverse association was observed in participants with education above high school (HR: 0.974, 95%CI: 0.959–0.988), but not in those with lower education. These findings underscore the potential benefits of higher education, as individuals with higher education may be more likely to adopt healthier behaviors, such as improved anti-oxidant intake and lifestyle regulation, leading to better outcomes. Conversely, the relationship between OBS and cardiovascular mortality remained consistently inverse across all subgroups, reinforcing the reliability of the regression results.
Table 3.
Subgroup analysis of OBS with all-cause and cardiovascular mortality.
| All-cause mortality | Cardiovascular mortality | |||
|---|---|---|---|---|
| HR (95% CI) | p for interaction | HR (95% CI) | p for interaction | |
| Age | 0.151 | 0.197 | ||
| ≤ 60 | 0.998(0.980,1.015) | 0.967(0.934,1.002) | ||
| > 60 | 0.980(0.968,0.991) | 0.984(0.973,0.996) | ||
| Gender | 0.953 | 0.793 | ||
| Male | 0.988(0.974,1.002) | 0.972(0.952,0.992) | ||
| Female | 0.985(0.973,0.997) | 0.981(0.966,0.997) | ||
| Race | 0.397 | 0.951 | ||
| Non-Hispanic White | 0.985(0.974,0.997) | 0.980(0.966,0.995) | ||
| Mexican American | 0.977(0.953,1.001) | 0.971(0.939, 1.005) | ||
| Non-Hispanic Black | 0.989(0.969,1.008) | 0.967(0.934,1.001) | ||
| Other Hispanic | 1.024(0.971,1.080) | 0.973(0.930, 1.016) | ||
| Other Race—Including Multi-Racial | 0.986(0.950,1.023) | 0.985(0.926, 1.047) | ||
| Education | 0.012* | 0.135 | ||
| Low High school | 0.991(0.974,1.007) | 0.985(0.966,1.005) | ||
| High school diploma or equivalent | 1.001(0.983,1.019) | 0.990(0.955,1.026) | ||
| High High school | 0.974(0.959,0.988) | 0.964(0.946, 0.981) | ||
| Marry status | 0.119 | 0.520 | ||
| Never married | 1.010(0.979,1.043) | 0.955(0.914,0.998) | ||
| Married or living with a partner | 0.983(0.971,0.995) | 0.981(0.963,0.999) | ||
| Widowed,divorced,or separated | 0.985(0.970,1.001) | 0.978(0.960,0.997) | ||
| Poverty to income ratio | 0.278 | 0.936 | ||
| < 1.3 | 0.996(0.978,1.014) | 0.977(0.962,0.993) | ||
| 1.3 ~ 3.5 | 0.981(0.968,0.994) | 0.981(0.960,1.001) | ||
| > = 3.5 | 0.986(0.967,1.006) | 0.974(0.943, 1.007) | ||
| Smoke | 0.140 | 0.530 | ||
| Never | 0.995(0.979,1.011) | 0.990(0.965,1.015) | ||
| Former | 0.973(0.957,0.989) | 0.974(0.956,0.992) | ||
| Now | 0.997(0.978,1.016) | 0.961(0.926,0.997) | ||
| Alcohol | 0.124 | 0.361 | ||
| No | 0.983(0.973,0.994) | 0.975(0.963,0.987) | ||
| Yes | 0.994(0.972,1.017) | 0.988(0.954, 1.023) | ||
| Hypertension | 0.054 | 0.935 | ||
| No | 0.982(0.972,0.993) | 0.979(0.966,0.993) | ||
| Yes | 1.004(0.982,1.026) | 0.974(0.939,1.010) | ||
| Diabetes mellitus | 0.604 | 0.794 | ||
| No | 0.984(0.969,0.998) | 0.976(0.952,1.001) | ||
| Yes | 0.990(0.977,1.003) | 0.980(0.965,0.995) | ||
Stratified analysis was constructed based on model 3. In each case, the model was not adjusted for the stratification variable itself. The P value for the interaction was calculated using the likelihood ratio test. *Significant difference.
Sensitivity analysis
Table S4 presents the results of sensitivity analyses. Exclusion of individuals who died within 2 years did not alter the main findings, supporting the robustness of the primary analysis. This approach helps mitigate potential reverse causality and further strengthens the validity of the observed results. Results from multiple imputation were consistent with the main findings, as shown in Table S5.
Discussion
This study is the first to investigate the relationship between OBS and mortality risk within a nationally representative U.S. MetS sample. Analyzing data from 10,647 participants with MetS across 10 NHANES cycles (1999–2018), we identified a significant inverse linear association between OBS and all-cause and cardiovascular mortality. This association persisted whether OBS were treated as continuous or categorical variables. Subgroup analyses and interaction tests revealed consistent inverse relationships between OBS and mortality risk, independent of all covariates except educational level. Sensitivity analyses confirmed the robustness of the results. These results underscore the importance of an antioxidant-rich diet and lifestyle as a preventive strategy for individuals with MetS.
Our research demonstrated that elevated levels of OBS, indicative of increased exposure to antioxidant factors among participants, are associated with a reduced risk of all-cause and cardiovascular mortality in patients with MetS. This finding aligns with existing literature. A cross-sectional analysis of 11,171 individuals from the 2007–2018 cycles of the NHANES revealed that higher OBS levels were linked with both a decreased risk of developing MetS and a reduction in its severity30. Similarly, a study by Xu et al., using NHANES data from the 1999–2018 cycles and involving 10,591 participants with diabetes and prediabetes, reported that high OBS levels were associated with a lower risk of all-cause and cardiovascular mortality. Considering that MetS increases the likelihood of developing diabetes and prediabetes, Xu et al.'s findings corroborate our results21.
Oxidative stress is a key factor in the onset, progression, and poor prognosis of MetS. It results from an imbalance in the ROS/NO ratio and limited anti-oxidant capacity. Factors such as increased ROS-producing enzymes, elevated ROS levels, reduced anti-oxidant enzyme activity, increased nitrite, and dysregulation of the ADMA-NO pathway all contribute to this imbalance31. Oxidative stress also impairs mitochondrial function, disrupts the electron transport chain, promotes ectopic fat deposition, and releases inflammatory factors and catecholamines, exacerbating intestinal microbiota damage and compromising intestinal barrier function13.
Our study found that all OBS were inversely associated with mortality risk, except for a weak trend between lifestyle OBS and cardiovascular mortality. This may be due to lifestyle OBS not accounting for factors such as sedentary behavior, sleep quality, and mental stress. Subgroup analysis revealed gender differences in the association between OBS levels and mortality risk reduction. Studies show that women with MetS face higher cardiovascular risk than men, possibly due to factors like central obesity, lipid profile differences, hormonal variations, and platelet and biochemical levels32. As a result, women with MetS may derive greater benefit from high anti-oxidant levels. Additionally, individuals over 60, with more than a high school education, female, stable marriages, smoking cessation, no alcohol consumption, and no history of hypertension, were more likely to have a lower risk of death due to high anti-oxidant levels.
Other potential unadjusted confounders, such as genetic susceptibility, also contribute to the poor prognosis of MetS. Studies have linked MetS to genetic factors, with specific gene variants (e.g., leptin, leptin receptor, opioid melanocortin precursor, and melanocortin 4 receptor) increasing the risk and influencing disease progression33. Additionally, GWAS studies offer greater power to identify variants associated with MetS phenotypes34.
Due to the limitations in the creation of OBS, the assignment of scores based on sex-specific tertiles may not reflect the optimal nutrient intake or ideal level of lifestyle protection. Research indicates that the relationship between essential metals and MetS prevalence is not always dose-dependent35. For example, while urinary copper and zinc were positively associated with MetS, no such association was observed for iron, manganese, or selenium. Similarly, an optimal level of lifestyle protection exists; high levels of leisure-time physical activity (LTPA) reduce the risk of MetS, but moderate LTPA has only a weak association with risk reduction36. Thus, while OBS can be a useful tool for predicting individual oxidation properties, its effectiveness depends on individual anti-oxidant intake and susceptibility to MetS.
Emerging technologies are elucidating the causal link between MetS and poor prognosis. Untargeted metabolomics identified novel markers associated with MetS, while Mendelian analysis suggested a potential causal role of abnormal LysoPC metabolism in metabolic risk37. Additionally, genome-wide association studies and Mendelian randomization have established a causal relationship between short telomere length and metabolic syndrome traits38. Our study highlights OBS, a marker of oxidative stress, as a predictor of mortality risk in MetS patients. However, the observational nature of the study limits causal inference.
Our findings can be interpreted through three primary mechanisms. First, a higher OBS may reflect a robust antioxidant defense system that mitigates oxidative stress, thereby lowering the risk of all-cause and cardiovascular mortality in patients with MetS. Adherence to a Mediterranean diet, along with management of waist circumference, weight, alcohol intake, and physical activity, can diminish oxidative stress, reduce MetS incidence, prevent cardiovascular disease and type 2 diabetes, and potentially extend lifespan39. Second, persistent low-grade systemic inflammation can accelerate the progression of MetS and worsen prognosis by exacerbating dyslipidemia and insulin resistance. Adopting a plant-based diet, maintaining weight control, ceasing smoking, and limiting alcohol consumption are effective strategies to alleviate chronic systemic inflammation40. Third, an elevated OBS may enhance insulin sensitivity, facilitate glucose uptake, and improve insulin utilization, thereby reducing the risk of insulin resistance—a crucial factor in MetS pathogenesis. Engaging in moderate physical activity can increase peripheral tissue sensitivity to insulin, whereas chronic smoking impairs insulin uptake in these tissues41.
Despite its limitations, OBS has significant clinical applications. By integrating diet and lifestyle factors, OBS offers a comprehensive method for assessing redox status. It allows early identification of individuals at high risk for MetS and poor clinical outcomes. Furthermore, OBS-based strategies, such as consuming antioxidant-rich foods, exercising regularly, and quitting smoking or limiting alcohol, provide valuable treatment guidance for individuals with MetS.
Our study exhibits several notable strengths. Firstly, the utilization of the extensive NHANES database, coupled with long-term follow-up data on mortality, enhances the robustness and reliability of our findings. Secondly, by incorporating 16 dietary factors and 4 lifestyle factors, the OBS offers a more comprehensive assessment of systemic oxidative stress, surpassing the limitations inherent in single-index measures.
However, there are several limitations that must be addressed. Firstly, as an observational study based on participants with MetS in the US, our research is limited in its ability to establish causal relationships. Future prospective studies are required to elucidate the causal links and underlying mechanisms. Secondly, the reliance on the NHANES database, which uses 24-h dietary recalls and household interviews to collect data on nutrient intake and lifestyle factors, presents challenges in accurately calculating the OBS. Thirdly, while we controlled for potential confounding variables in our multivariate regression models, other unaccounted factors may still influence the OBS. Fourth, the criteria for MetS are not uniform, possibly limiting the comparability between studies. Future research should focus on comparing different criteria to identify the most accurate and efficient definition. Finally, the exclusion of individuals with missing data may have introduced selection bias, potentially impacting the generalizability of our findings.
Conclusion
This study demonstrates an inverse relationship between the OBS and both all-cause and cardiovascular mortality among patients with MetS. Strategies to improve OBS, such as physical exercise, smoking cessation, alcohol restriction, weight management, and anti-oxidant intake, may reduce MetS risk. Further randomized controlled trials are needed to confirm the effectiveness of these dietary and lifestyle interventions in MetS prevention.
Supplementary Information
Acknowledgements
The data used in this study were from the NHANES. We thank all the staff of and participants in the NHANES for their contribution.
Author contributions
Concept and design: Jing, Gao. Acquisition, analysis, or interpretation of data: Yuqing Li. Drafting of the manuscript: Yuqing Li. Critical review of the manuscript for important intellectual content: All authors. Statistical analysis: Ping Li, Ran Chu, Weiwei Tian. Obtained funding: Jing Gao, Yin Liu. Administrative, technical, or material support: Jiaxin Wang. Supervision: Jing Gao, Yin Liu. All authors reviewed the manuscript.
Funding
This work was supported by the Tianjin Health Research Project [No. TJWJ2022XK032] and the Key Project of Scientific and Technological Support Plan of Tianjin in 2020 [No. 20YFZCSY00820].
Data availability
Data will be made available on request. Any queries or requirements of data can be obtained by contacting corresponding author Jing Gao or first author Yuqing Li.
Ethics approval and consent to participate
The National Health and Nutrition Examination Survey (NHANES) is a publicly available dataset sanctioned by the Institutional Review Board (IRB) of the National Center for Health Statistics (NCHS). All participants consented in writing during their involvement in the survey. Since no further IRB approval was necessary for the secondary analysis, this study was exempt from additional ethical review.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-90640-w.
References
- 1.Hirode, G. & Wong, R. J. Trends in the prevalence of metabolic syndrome in the United States, 2011–2016. Jama.323(24), 2526–2528 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguilar, M., Bhuket, T., Torres, S., Liu, B. & Wong, R. J. Prevalence of the metabolic syndrome in the United States, 2003–2012. Jama.313(19), 1973–1974 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Saklayen, M. G. The global epidemic of the metabolic syndrome. Current Hypertens. Rep.20(2), 12 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu, J., Ma, J., Orekoya, O., Vangeepuram, N. & Liu, J. Trends in metabolic syndrome among US Youth, from 1999 to 2018. JAMA Pediatrics.176(10), 1043–1045 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ndumele, C. E. et al. A Synopsis of the evidence for the science and clinical management of Cardiovascular-Kidney-Metabolic (CKM) syndrome: A scientific statement from the American heart association. Circulation.148(20), 1636–1664 (2023). [DOI] [PubMed] [Google Scholar]
- 6.Alberti, K. G. et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation.120(16), 1640–1645 (2009). [DOI] [PubMed] [Google Scholar]
- 7.Nilsson, P. M., Tuomilehto, J. & Rydén, L. The metabolic syndrome—What is it and how should it be managed?. Eur. J. Prevent. Cardiol.26(2_suppl), 33–46 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Prasun, P. Mitochondrial dysfunction in metabolic syndrome. Biochimica et biophysica acta Molecular basis of disease.1866(10), 165838 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Guo, Q. et al. Oxidative stress, nutritional antioxidants and beyond. Sci. China Life Sci.63(6), 866–874 (2020). [DOI] [PubMed] [Google Scholar]
- 10.Rani, V., Deep, G., Singh, R. K., Palle, K. & Yadav, U. C. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci.148, 183–193 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Grandl, G. & Wolfrum, C. Hemostasis, endothelial stress, inflammation, and the metabolic syndrome. Semin. Immunopathol.40(2), 215–224 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carrier, A. Metabolic syndrome and oxidative stress: A complex relationship. Antioxid. Redox Signal.26(9), 429–431 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Spahis, S., Borys, J. M. & Levy, E. Metabolic syndrome as a multifaceted risk factor for oxidative stress. Antioxid. Redox Signal.26(9), 445–461 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Zujko, M. E., Rożniata, M. & Zujko, K. Individual diet modification reduces the metabolic syndrome in patients before pharmacological treatment. Nutrients.13(6), 2102 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamaoka, K. & Tango, T. Effects of lifestyle modification on metabolic syndrome: A systematic review and meta-analysis. BMC Med.10, 138 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doughty, K. N., Del Pilar, N. X., Audette, A. & Katz, D. L. Lifestyle medicine and the management of cardiovascular disease. Current Cardiol. Rep.19(11), 116 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Bonekamp, N. E. et al. Diet in secondary prevention: The effect of dietary patterns on cardiovascular risk factors in patients with cardiovascular disease: A systematic review and network meta-analysis. Nutr. J.23(1), 18 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernández-Ruiz, Á. et al. Oxidative Balance Scores (OBSs) integrating nutrient, food and lifestyle dimensions: Development of the NutrientL-OBS and FoodL-OBS. Antioxidants11(2), 300 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li, Y. et al. Healthy lifestyle and life expectancy free of cancer, cardiovascular disease, and type 2 diabetes: Prospective cohort study. BMJ368, l6669 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nyberg, S. T. et al. Association of healthy lifestyle with years lived without major chronic diseases. JAMA Internal Med.180(5), 760–768 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu, Z. et al. Association between the oxidative balance score and all-cause and cardiovascular mortality in patients with diabetes and prediabetes. Redox Biol.76, 103327 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu, Y. et al. The association between oxidative balance score and frailty in adults across a wide age spectrum: NHANES 2007–2018. Food Funct.15(9), 5041–5049 (2024). [DOI] [PubMed] [Google Scholar]
- 23.Wei, X., Min, Y., Song, G., Ye, X. & Liu, L. Association between triglyceride-glucose related indices with the all-cause and cause-specific mortality among the population with metabolic syndrome. Cardiovasc. Diabetol.23(1), 134 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan, Z. et al. Trends in oxidative balance score and prevalence of metabolic dysfunction-associated steatotic liver disease in the united states: National health and nutrition examination survey 2001 to 2018. Nutrients.15(23), 4931 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang, W. et al. Association between the oxidative balance score and telomere length from the national health and nutrition examination survey 1999–2002. Oxidat. Med. Cell. Longev.2022, 1345071 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu, B. et al. Is systemic inflammation a missing link between cardiometabolic index with mortality? Evidence from a large population-based study. Cardiovasc. Diabetol.23(1), 212 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang, X. et al. The neutrophil-to-lymphocyte ratio is associated with all-cause and cardiovascular mortality among individuals with hypertension. Cardiovasc. Diabetol.23(1), 117 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding, L. et al. The prognostic value of the stress hyperglycemia ratio for all-cause and cardiovascular mortality in patients with diabetes or prediabetes: Insights from NHANES 2005–2018. Cardiovasc. Diabetol.23(1), 84 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen, R. & Zou, T. The association between cardiovascular health and depression: Results from the 2007–2020 NHANES. Psychiatry Res.331, 115663 (2024). [DOI] [PubMed] [Google Scholar]
- 30.Xu, Z. et al. Oxidative balance score was negatively associated with the risk of metabolic syndrome, metabolic syndrome severity, and all-cause mortality of patients with metabolic syndrome. Front. Endocrinol.14, 1233145 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tain, Y. L. & Hsu, C. N. Metabolic syndrome programming and reprogramming: mechanistic aspects of oxidative stress. Antioxidants11(11), 2108 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santilli, F., D’Ardes, D., Guagnano, M. T. & Davi, G. Metabolic syndrome: Sex-related cardiovascular risk and therapeutic approach. Current Med. Chem.24(24), 2602–2627 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Joy, T., Lahiry, P., Pollex, R. L. & Hegele, R. A. Genetics of metabolic syndrome. Current Diabetes Rep.8(2), 141–148 (2008). [DOI] [PubMed] [Google Scholar]
- 34.Phillips, C. M. Nutrigenetics and metabolic disease: Current status and implications for personalised nutrition. Nutrients.5(1), 32–57 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma, J. et al. Associations between essential metals exposure and metabolic syndrome (MetS): Exploring the mediating role of systemic inflammation in a general Chinese population. Environ. Int.140, 105802 (2020). [DOI] [PubMed] [Google Scholar]
- 36.He, D. et al. Association between leisure time physical activity and metabolic syndrome: A meta-analysis of prospective cohort studies. Endocrine.46(2), 231–240 (2014). [DOI] [PubMed] [Google Scholar]
- 37.Wu, Q. et al. Multi-stage metabolomics and genetic analyses identified metabolite biomarkers of metabolic syndrome and their genetic determinants. EBioMedicine.74, 103707 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loh, N. Y., Noordam, R. & Christodoulides, C. Telomere length and metabolic syndrome traits: A Mendelian randomisation study. Aging cell.20(8), e13445 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiśniewska, K., Okręglicka, K. M., Nitsch-Osuch, A. & Oczkowski, M. Plant-based diets and metabolic syndrome components: The questions that still need to be answered—A narrative review. Nutrients.16(1), 165 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas, M. S., Calle, M. & Fernandez, M. L. Healthy plant-based diets improve dyslipidemias, insulin resistance, and inflammation in metabolic syndrome. A narrative review. Adv. Nutr.14(1), 44–54 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yeh, H. C., Duncan, B. B., Schmidt, M. I., Wang, N. Y. & Brancati, F. L. Smoking, smoking cessation, and risk for type 2 diabetes mellitus: a cohort study. Ann. Internal Med.152(1), 10–17 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request. Any queries or requirements of data can be obtained by contacting corresponding author Jing Gao or first author Yuqing Li.