Abstract
Background
Gestational diabetes mellitus (GDM) is one of the most common complications during pregnancy and has been on a continuous increase in recent years. This study aimed to establish a combined prediction model for the risk of GDM and to provide more reliable reference information for non-invasive assessment of GDM in clinical practice.
Methods
This study retrospectively collected clinical data and ultrasound information of 122 pregnant women who underwent fetal nuchal translucency screening, which divided into 36 cases of the GDM group and 86 cases of the non-gestational diabetes mellitus(NGDM) group. The collected clinical data and ultrasound information were analyzed using Student’s t-test and Wilcoxon W test for univariate analysis. Independent risk factors for patients with GDM were screened through binary logistic regression analysis. A model was established based on the screened results, and the diagnostic performance of different models was evaluated by drawing the receiver operating characteristic curve(ROC). The optimal prediction model was selected, and the calibration curve and clinical decision curve were drawn to evaluate the goodness of fit and clinical application efficiency of the model.
Results
Univariate results showed that age, body mass index(BMI), number of abortions, gravidity, placental volume(PV), vascularization index(VI), flow index(FI), and vascularization flow index(VFI) all had statistically significant differences between the GDM and NGDM groups(p < 0.05). Binary logistic regression analysis showed that BMI, number of abortions, PV, VI, and FI were independent risk factors for the development of GDM in pregnant women (p < 0.05). Based on these results, five prediction models were established in this study. Their area under the ROC curve(AUC) were 0.67, 0.80, 0.80, 0.87, and 0.85, respectively. The model combining clinical data with 30° ultrasound data had the highest AUC, so we constructed a nomogram for this model. The results of its calibration curve showed that the model had a good fit, and the results of the clinical decision curve showed that the model had good clinical application efficiency.
Conclusion
The nomogram model combining clinical data with 30° ultrasound data has good accuracy and clinical application value for predicting the risk of GDM.
Peer review
The peer review reports can be found at 10.1186/s12911-025-02962-4.
Keywords: Ultrasound, Placenta, Gestational diabetes mellitus, Obstetrics, Nomogram
Background
Gestational Diabetes Mellitus (GDM) [1] refers to high blood sugar or carbohydrate intolerance first discovered or occurring during pregnancy, typically detected between the 24th and 28th weeks of gestation. With the rising global obesity rate and the increase in the number of women of advanced maternal age following the adjustment of China’s birth policy, the number of diabetes cases in China is growing rapidly, while gestational diabetes mellitus (GDM) has also become one of the most common complications during pregnancy [2]. Research shows that although blood sugar levels in GDM patients often return to normal after delivery, they and their offspring are at increased risk [3] of developing type 2 diabetes mellitus (T2DM) in the future. Their offspring also have a higher risk of developing obesity. Evidence suggests that early identification of high-risk groups for GDM will help clinicians to intervene timely through dietary or lifestyle adjustments [4]. This approach can prevent the risks associated with the future use of insulin or antihyperglycemic medications, thereby effectively improving perinatal outcomes. Therefore, early diagnosis and management of GDM are particularly important.
The 75 g oral glucose tolerance test (75 g OGTT) is commonly recommended for diagnosing [5] gestational diabetes mellitus (GDM) between 24 and 28 weeks of pregnancy. However, this method has several drawbacks, including its relatively late diagnostic timing, complex procedure (requiring multiple blood samples), and potential interference from the mother’s tolerance to the test (some pregnant women may find it difficult to tolerate, leading to vomiting). Therefore, there is a need to explore an early, convenient, and less interfered method to predict the risk of GDM, which could assist in early clinical intervention.
There is epidemiological evidence suggesting that factors such as advanced age, obesity, history of miscarriages, Gravida, and Parity are considered high-risk factors [6, 7] for gestational diabetes mellitus (GDM). Advanced age and obesity can lead to a decline in insulin secretion function or an increase in insulin resistance, resulting in difficulties in blood sugar control. GDM patients often present with chronic low-grade inflammation [8], and a history of miscarriages may also induce inflammatory responses in the body, thus increasing the risk of developing GDM. While estrogen and progesterone [9] help maintain metabolic balance under physiological conditions, hormonal fluctuations during pregnancy are more pronounced, which may lead to insulin dysfunction. This is particularly true for women who have experienced multiple pregnancies, as their insulin adaptability may decrease over time.
The placenta is a crucial bridge for material exchange between the mother and fetus. In early pregnancy, a series of changes induced by gestational diabetes mellitus (GDM) may manifest in the placenta at an earlier stage. These changes primarily involve alterations in the placental morphology and micro-physiological functions [10], such as changes in placental surface area, volume, and the microvascular structure. These alterations may lead to placental insufficiency [11], exposing the fetus to a hyperglycemic environment, which increases the risk of various adverse pregnancy outcomes [12], including macrosomia, neonatal hyperglycemia, and hyperbilirubinemia [13]. Ultrasound is the preferred and most commonly used imaging method during pregnancy, offering advantages such as affordability, non-invasiveness, and absence of radiation. Compared to conventional two-dimensional Doppler ultrasound, three-dimensional power Doppler ultrasound (3D-PDU) provides a more comprehensive assessment of placental function by evaluating placental volume and microvascular perfusion [14]. It holds significant potential for detecting early changes caused by gestational diabetes mellitus (GDM), making it a valuable tool for early prediction and management [15].
Currently, most existing studies focus on using either ultrasound parameters [16] or clinical parameters [17] alone to predict GDM, with limited research combining both for early and accurate risk assessment. This gap may result in delayed detection for some patients, leading to adverse pregnancy outcomes [18]. Therefore, this study aims to address this issue by analyzing factors such as maternal age, body mass index (BMI), Miscarriage, Gravida, Parity, and ultrasound-derived parameters, including placental volume (PV) and placental microvascular flow. By integrating these clinical and ultrasound parameters, we propose a novel nomogram to provide a more comprehensive method for early GDM prediction and to bridge this critical research gap.
Methods
General information
A retrospective collection of pregnant women who underwent fetal nuchal translucency screening at the Department of Ultrasound Medicine of the First Affiliated Hospital of Shihezi University from October 2021 to October 2022 was carried out. According to the inclusion and exclusion criteria, a primary dataset of 122 patients was established. Based on the “Guidelines for the Diagnosis and Treatment of Gestational Hyperglycemia (2022)“ [19], they were divided into 36 cases of GDM group and 86 cases of non-gestational diabetes mellitus (NGDM) group. Inclusion criteria were: (1) gestational age between 11 + 0 weeks and 13 + 6 weeks, (2) singleton pregnancy, (3) no other pregnancy-related diseases, (4) complete clinical and ultrasound data of the patients. Exclusion criteria were: (1) multiple pregnancies, (2) pregestational diabetes, (3) history of hypertension, preeclampsia, thyroid disease, autoimmune diseases, or diagnosis of these diseases during this pregnancy, (4) long-term use of drugs affecting glucose or lipid metabolism, (5) incomplete clinical data (age, BMI, miscarriage, gravidity, and parity) and ultrasound data (placental thickness, PV, VI, FI, and VFI). Relevant baseline data such as maternal age, BMI, number of abortions, gravidity, parity, etc. were collected. This study was approved by the Ethics Committee of the First Affiliated Hospital of Shihezi University (Approval No: KJX-2021-018-01), and all research subjects signed informed consent forms.
Instruments and methods
(1) Instrumentation: A GE Voluson E8 color Doppler ultrasound diagnostic system (three-dimensional volume probe, frequency 4.0 ~ 8.5 MHz) equipped with Virtual Organ Computer-aided AnaLysis (VOCAL) software was used.
(2) Ultrasound examination: All research subjects, in accordance with the ALARA (As Low As Reasonably Achievable) principle, underwent a full volume scan of the placenta using a three-dimensional energy Doppler ultrasound imaging mode with a three-dimensional volume probe at 11 + 0–13 + 6 weeks of gestation. Instruct the patient to assume a supine position and adjust the instrument to ensure good visualization of low-speed blood flow (Power Doppler map, 6; frequency, low; smoothing, rise 2/fall 4; flow resolution, mild 1; line density, 7; balance, 210; ensemble, 10; line filter, 3; artifact suppression, on; quality, high 1; wall motion filter, medium 1; pulse repetition frequency, 1.3 kHz). Obtain the maximum cross-sectional image of the placenta while the pregnant woman is breathing calmly and the fetus is in a resting state. Then, stabilize the probe for 15 s to acquire the raw placental volume data.
(3) Image analysis: The VOCAL software was used to manually outline the placental contour from the A-plane measurement plane, using two different views of 15° section rotation 12 times and 30° section rotation 6 times, to reconstruct the three-dimensional image of the placenta. The placental volume (PV); vascularity index (VI), to assess the richness of vessels in the target volume; flow index (FI), to evaluate the signal strength of blood flow in the target volume; and vascularity flow index (VFI), to assess the number and richness of vessels and blood flow in the target volume, were obtained from the automatically generated three-dimensional energy Doppler histogram. Information for each patient was outlined three times by a physician having more than five years of experience in prenatal screening and being certified by the Chinese Fetal Medicine Foundation in obstetric ultrasound, and averaged to ensure data reliability. In addition, to ensure quality control, a random selection of placental images will be re-outlined by different observers, and the fluctuation range of the PV will be kept within 1cm3, ensuring consistency in image analysis. The physician analyzing the placental data was blinded to the pregnancy outcomes during the analysis.
Statistical methods
Statistical analysis was performed using SPSS 20.0 and R Studio 4.1.0 software. The normality of the data was checked by the Kolmogorov-Smirnov test. The Student’s t-test was used for normally distributed measurement data, and the Wilcoxon W test was used for non-normally distributed measurement data and count data. The significant univariate factors identified were used as independent variables, and the occurrence of gestational diabetes mellitus was used as a dependent variable in a binary logistic regression. Clinical data and ultrasound indicators were used to establish five models, and receiver operating characteristic (ROC) curves were plotted. The area under the ROC curve (AUC) was calculated to evaluate the diagnostic performance of the models. The DeLong test was used to compare whether there was a statistical significance between the models. The best model was selected to plot a nomogram. The consistency index (C-index) was calculated to evaluate the predictive performance of the nomogram, and the Youden index was used to assess the effectiveness of the predictive model in identifying GDM and NGDM. Finally, the rms package was used for internal validation of the model using Bootstrap resampling 1,000 times. Calibration curves and clinical decision curves were plotted to evaluate the clinical net benefit of the nomogram model. A P-value < 0.05 was considered statistically significant.
Results
Clinical data
A total of 206 pregnant women underwent ultrasound examinations in this study. Among them, 47 were lost to follow-up, including 31 who did not return to our hospital for subsequent prenatal check-ups and 16 with incomplete prenatal information, such as failure to undergo glucose tolerance screening at our facility. Additionally, 37 pregnant women were excluded due to other pregnancy-related complications, including preeclampsia (n = 11), hypertension (n = 8), and thyroid dysfunction (n = 18). As a result, the study ultimately included 122 participants, comprising 36 women in the GDM group (29.5%) and 86 women in the NGDM group (70.5%).
Univariate analysis
Table 1 shows the univariate analysis of the basic clinical data, placental volume, placental thickness, and placental vascular index of the gestational diabetes group and the non-gestational diabetes group for both the 15° and 30° sections. The results showed that age (p < 0.01), BMI (p = 0.03), abortion history (p < 0.01), gravidity (p < 0.01), PV30° (p < 0.01), PV15° (p < 0.01), VI30° (p < 0.01), VI15° (p < 0.01), FI30° (p < 0.01), FI15° (p < 0.01), VFI30° (p < 0.01), and VFI15° (p < 0.01) all had statistically significant group differences in predicting the risk of gestational diabetes.
Table 1.
Univariate analysis
| GDM group (n = 36) |
Non-GDM group (n = 86) |
Z/X 2 | P value | |
|---|---|---|---|---|
| Clinical | ||||
| Age | 30.86 ± 4.02 | 29.01 ± 3.34 | 3.67 | < 0.01 |
| BMI | 24.19 ± 3.85 | 22.59 ± 2.74 | 2.25 | 0.03 |
| Miscarriage | 63.89%(23/36) | 34.88%(30/86) | 2.94 | < 0.01 |
| Gravida | 83.33%(30/36) | 55.81%(48/86) | 2.88 | < 0.01 |
| Parity | 42.67%(15/36) | 25.58%(22/86) | 1.76 | 0.08 |
| Ultrasonography | ||||
| PV (cm3) | ||||
| 30° | 35.96 ± 7.24 | 44.70 ± 13.23 | 12.37 | < 0.01 |
| 15° | 36.57 ± 7.81 | 45.97 ± 13.79 | 10.88 | < 0.01 |
| VI | ||||
| 30° | 34.99 ± 7.28 | 41.16 ± 13.61 | 13.31 | < 0.01 |
| 15° | 35.44 ± 6.98 | 41.70 ± 13.72 | 17.46 | < 0.01 |
| FI | ||||
| 30° | 29.31 ± 6.01 | 33.44 ± 5.69 | 0.30 | < 0.01 |
| 15° | 29.33 ± 6.24 | 33.66 ± 5.67 | 0.35 | < 0.01 |
| VFI | ||||
| 30° | 11.22 ± 3.06 | 14.17 ± 5.73 | 12.83 | < 0.01 |
| 15° | 11.59 ± 3.74 | 14.34 ± 5.79 | 6.85 | < 0.01 |
| Placental thickness | 13.39 ± 2.74 | 13.28 ± 2.22 | 2.52 | 0.82 |
Multivariate analysis
The significant variables identified above were subjected to multivariate logistic regression analysis. The results showed that for the 30° section (Table 2), BMI (p = 0.04), number of abortions (p < 0.01), PV30° (p < 0.01), VI30° (p = 0.03), and FI30° (p < 0.01) had statistically significant differences. For the 15° section (Table 3), BMI (p = 0.03), number of abortions (p < 0.01), PV15° (p < 0.01), VI15° (p = 0.02), and FI15° (p < 0.01) had statistically significant differences. However, age (p = 0.33), gravidity (p = 0.98), VFI30° (p = 0.86), and VFI15° (p = 0.13) did not have statistical differences.
Table 2.
Multivariate analysis of clinical characteristics combined with 30-degree ultrasound data
| Variables | OR | 95%CI | P value |
|---|---|---|---|
| Age | 2.01 | 0.41–9.77 | 0.33 |
| BMI | 1.86 | 0.84–4.12 | 0.04 |
| Miscarriage | 5.08 | 1.17–22.08 | < 0.01 |
| Gravida | 1.00 | 0.16–6.02 | 0.98 |
| PV(cm3) 30° | 0.21 | 0.09–0.53 | < 0.01 |
| VI30° | 0.40 | 0.09–1.71 | 0.03 |
| FI30° | 0.24 | 0.08–0.74 | < 0.01 |
| VFI30° | 1.16 | 0.23–5.93 | 0.86 |
Table 3.
Multivariate analysis of clinical characteristics combined with 15-degree ultrasound data
| Variables | OR | 95%CI | P value |
|---|---|---|---|
| Age | 2.14 | 0.46–9.86 | 0.30 |
| BMI | 2.17 | 0.93–5.02 | 0.03 |
| Miscarriage | 4.59 | 1.51–13.94 | < 0.01 |
| Gravida | 1.15 | 0.24–5.45 | 0.66 |
| PV (cm3) 15° | 0.28 | 0.13–0.62 | < 0.01 |
| VI15° | 0.18 | 0.04–0.74 | 0.02 |
| FI15° | 0.21 | 0.07–0.57 | < 0.01 |
| VFI15° | 2.78 | 0.73–10.53 | 0.13 |
Establishment of predictive models
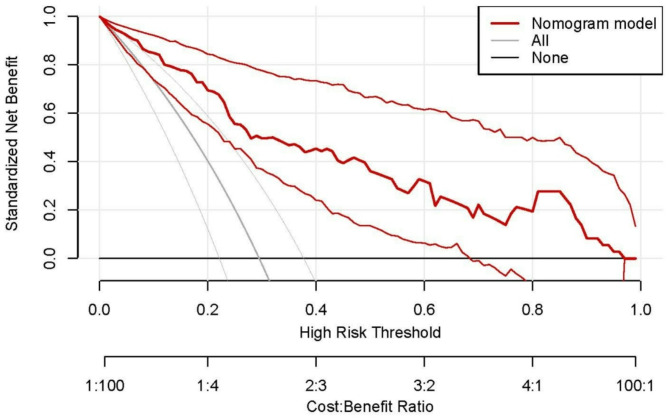
Based on the independent risk factors screened out through multifactorial analysis, we separately constructed five groups of models: a clinical data model (BMI + Miscarriage), a 30° ultrasound data model (PV 30° + VI 30°+ FI 30°), a 15° ultrasound data model (PV 15° + VI 15°+ FI 15°), a clinical data combined with 30° ultrasound data model(BMI + Miscarriage + PV 30° + VI 30°+ FI 30°), and a clinical data combined with 15° ultrasound data model (BMI + Miscarriage + PV 15° + VI 15°+ F I15°). We plotted ROC curves for each model (Fig. 1), and used the DeLong test to compare the differences in the area under the curve (AUC) of the five models (Table 4) to evaluate the predictive performance of each model for gestational diabetes. At the same time, we calculated the accuracy, sensitivity, specificity, Youden’s index, and consistency index (c-index) of each model (Table 5). ROC analysis showed that the AUCs of the five models were 0.67, 0.80, 0.80, 0.87, and 0.85, respectively. The clinical data combined with the 30° ultrasound data model had the highest predictive value for gestational diabetes. Therefore, we plotted a nomogram for this model (Fig. 2). The clinical decision curve obtained after internal validation of the model using the Bootstrap method with 1000 repetitions using the rms package (Fig. 3) indicated that this model has a high clinical application value. Meanwhile, the calibration curve results showed that this model has high consistency between the predicted probability of gestational diabetes and actual observations (Fig. 4).
Fig. 1.
Receiver decision curve for multivariate analysis
Table 4.
Delong test for the five models
| Model cl | Model 30° | Model 15° | Model cl + 30° | |
|---|---|---|---|---|
| Model cl | ||||
| Model 30° | 0.89 | |||
| Model 15° | 0.98 | 0.61 | ||
| Model cl + 30° | < 0.01 | < 0.01 | < 0.01 | |
| Model cl + 15° | < 0.01 | < 0.01 | < 0.01 | 0.27 |
Table 5.
Comparison of prediction performance among the models
| Model | AUC | Accuracy | Sensitivity | Specificity | Youden index | c-index |
|---|---|---|---|---|---|---|
| CL | 0.67 | 0.72 | 0.75 | 0.72 | 0.47 | 0.72 |
| 15° | 0.80 | 0.75 | 0.80 | 0.75 | 0.55 | 0.80 |
| 30° | 0.80 | 0.75 | 0.73 | 0.75 | 0.48 | 0.80 |
| CL + 15° | 0.85 | 0.80 | 0.83 | 0.80 | 0.63 | 0.85 |
| CL + 30° | 0.87 | 0.80 | 0.80 | 0.80 | 0.60 | 0.88 |
Fig. 2.
Nomogram for predicting the risk of GDM
Fig. 3.
Calibration curve of the nomogram
Fig. 4.
The clinical decision curve of the nomogram
Discussion
Globally, the prevalence of gestational diabetes mellitus (GDM) is steadily increasing, posing a serious threat to the health of mothers and infants during the perinatal period. Pregnant women with GDM have enhanced placental vascular formation, accompanied by pathological responses such as endothelial cell dysfunction, increased vascular permeability, and impaired integrity of the chorionic vascular system, leading to placental dysfunction. These changes may cause various complications during pregnancy and adverse pregnancy outcomes. Therefore, early diagnosis of GDM, preferably within the first three months of pregnancy, will be beneficial for its effective long-term management and to reduce the risk of adverse maternal and infant outcomes [20].
In this study, we retrospectively analyzed 122 pregnant women who underwent routine ultrasound examinations, including 36 in the GDM group and 86 in the NGDM group. We conducted univariate and multivariate analyses of the clinical data and ultrasound data of the included population and successfully constructed a GDM prediction model combining clinical data and ultrasound data. The results showed that body mass index (BMI), number of abortions, placental volume (PV), vascular index (VI), and blood flow index (FI) are independent risk factors for gestational diabetes. These findings are generally consistent with previous research conclusions, further confirming the importance of these factors in the occurrence of GDM. However, it is important to clarify that, as this is an observational study, we can only infer correlations rather than definitive causal relationships.
Of note, a study by Wang [21] et al. showed that a higher BMI in pregnant women is an established risk factor for GDM. Higher BMI in early pregnancy is associated with abnormal lipid metabolism, which may increase the risk of GDM. This finding is highly consistent with ours. However, Xu [22] et al. believe that weight gain during pregnancy before the diagnosis of GDM increases the risk of GDM above average, but obese women gain less weight during pregnancy than average, which may benefit them from less than average weight gain during pregnancy, especially in early pregnancy. This discrepancy is currently influenced by sample size and single-center limitations. Future research should include larger samples and multiple centers to validate these results.
This study showed that multiple induced abortions are one of the independent risk factors for GDM. A cohort study by Li and colleagues observed that the risk of GDM is associated with an increased chance of abortion, which may be due to the mediating role of high-sensitivity C-reactive protein (hs-CRP) in the pathway linking abortion history and GDM [23]. Research by Matias Vaajala’s team [24] suggests that women with a history of induced abortion, miscarriage, or both have a higher chance of developing GDM during their first pregnancy and childbirth. Moreover, the risk of GDM increases with the number of induced abortions before childbirth, which is highly consistent with our research results. Therefore, clinicians should be extra vigilant when dealing with pregnant women with adverse obstetric histories to prevent adverse pregnancy outcomes.
In this study, we also found that GDM patients had significantly lower placental volume (PV), VI, and FI in early pregnancy. However, in Halil Gursoy Pala’s study, the mean gestational age of the included pregnant women was 33.65 ± 3.64 weeks, which differs from the gestational age in our study. The increase in PV observed in Pala’s study may be attributed to the progression of gestational age and the cumulative effect of hyperglycemia on the placenta during late pregnancy, leading to placental calcification and volume enlargement [25]. Wong and colleagues [26] pointed out that there was no significant difference in PV between the GDM group and the normal group in early pregnancy, but significantly increased in the GDM group during mid-gestation, further confirming the correlation between gestational age and PV. Han et al.‘s study showed that the reduction of PV, VI, and FI in GDM patients is associated with an increased risk of GDM [15], which is consistent with our research results. This suggests that the ultrasound parameters discussed in this study may become effective indicators for diagnosing GDM in the future. The main reasons for these inconsistent results may be as follows: First, the dysregulation of various hormones and cytokines in early pregnancy GDM patients may already indicate abnormal placental development [27]. Additionally, increased maternal insulin resistance may lead to higher glucose levels in the placenta of GDM patients [28], affecting PV. However, the duration of hyperglycemia in early pregnancy is relatively short, which limits the changes in PV. Furthermore, all of the above studies employed the GE Voluson color Doppler ultrasound diagnostic system, equipped with Virtual Organ Computer-aided AnaLysis (VOCAL) software, ensuring that there were no differences in imaging acquisition methods. In future research, our team will also pay more attention to the relationship between placental volume and different gestational weeks.
Subsequently, this study established five models to predict GDM, notably, the model combining clinical data and 30° ultrasound data showed the most significant predictive ability. This result indicates that compared to relying on a single type of data (such as clinical data or ultrasound data), combining clinical and ultrasound data can more comprehensively capture various information related to GDM, thereby improving prediction accuracy. This finding holds significant clinical implications. In future studies, we plan to expand the sample size and conduct multi-center research to more comprehensively validate the results of this study. Our goal is to assist clinicians in providing timely dietary and lifestyle interventions for pregnant women after obtaining relevant test results in early pregnancy, thereby improving pregnancy outcomes. In addition, compared to other studies, our study included a comparison between different cutting angles (including 30° and 15°) when using VOCAL to determine PV and placental vascular indices (VI, FI, and VFI). The results show that the combination of 30° ultrasound parameters and clinical data has better predictive performance, providing a new perspective for subsequent research. In this study, we constructed multiple models through multivariate analysis, including single-factor models and combined models. Compared with single-factor models, the combined model incorporating clinical data and 30° ultrasound data demonstrated significantly higher predictive accuracy, with an AUC value of 0.87, far exceeding those of models using clinical data alone (AUC 0.67) or single ultrasound data alone (AUC 0.80). This indicates that the integration of clinical and ultrasound data can effectively enhance prediction performance in GDM, with ultrasound data playing a crucial role in improving model sensitivity and specificity. The excellent performance of 30° ultrasound data in the model may be attributed to the reduced number of delineations, which minimizes errors caused by manual operations and more accurately reflects placental conditions. This, in turn, facilitates the precise identification of high-risk GDM populations. Therefore, the 30° ultrasound parameter has proven essential in improving the predictive ability of the model.
However, this study also has some limitations. First, it has inherent limitations due to its retrospective design. The exclusion of patients who were lost to follow-up or had incomplete data may have introduced selection bias. Additionally, reliance on historical data could lead to information bias due to inconsistencies in documentation. Although we implemented measures to minimize the impact of confounding factors, such as randomly selecting data for review to reduce human error, unmeasured variables may still have influenced the results. Second, the relatively small sample size limits the generalizability of the results. Third, as an observational study, it cannot establish causal relationships. Therefore, our team plans to conduct prospective studies in the future, employing standardized data collection protocols and larger sample sizes to validate these findings and address the limitations of the current study. We also look forward to exploring the applicability of the model in different populations and geographic settings. Additionally, the ultrasound technology used in this study performed well in the predictive model. However, we must acknowledge the potential challenges associated with the use of VOCAL, including the need for specialized equipment and personnel training. To address these challenges, healthcare institutions could consider exploring subsidized funding or partnerships with equipment manufacturers to reduce costs. Additionally, hospitals equipped with VOCAL technology could provide training for personnel from surrounding lower-tier hospitals. For example, our hospital regularly hosts visiting doctors from lower-tier hospitals for advanced training. This provides an opportunity to train these doctors in using VOCAL technology, enabling them to pass on the knowledge to their colleagues upon returning to their own workplaces.
In conclusion, the prevention and treatment of gestational diabetes is a multidisciplinary, multi-level comprehensive problem. Through early screening, timely diagnosis, and effective intervention, the risk of GDM and its related complications can be significantly reduced, ensuring maternal and infant health. This study developed a GDM prediction model that integrates clinical data and ultrasound data, demonstrating robust predictive performance and potential utility in identifying gestational diabetes during early pregnancy. The development of this model provides new ideas and methods for the early diagnosis and prevention of GDM. The next step for our team should be to further optimize and validate this model and explore its applicability in different populations. With a deeper understanding of the pathophysiology of GDM and the development of related technologies, we hope to improve the efficiency of GDM diagnosis in the future and explore more effective predictive indicators.
Conclusion
The nomogram model combining clinical data with 30° ultrasound data has good accuracy and clinical application value for predicting the risk of GDM.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- GDM
Gestational Diabetes Mellitus
- NGDM
Non-gestational diabetes mellitus
- OGTT
75 g oral glucose tolerance test
- 3D-PDU
Three-dimensional power Doppler ultrasound
- ROC
The receiver operating characteristic curve
- AUC
Area under the ROC curve
- BMI
Body mass index
- PV
Placental volume
- VI
Vascularization index
- FI
Flow index
- VFI
Vascularization flow index
- VOCAL
Virtual Organ Computer-aided AnaLysis
Author contributions
Study concept and design: TZ. Acquisition of data: TZ, LT. Analysis and interpretation of data: TZ. Drafting of the manuscript: TZ, MQ, WWW. Critical revision of the manuscript for important intellectual content: LC. Approval of the final manuscript: LC, LT. Study supervision: LC. All authors read and approved the final manuscript.
Funding
1) Supported by Shihezi University level research project (ZZZC2022061).
2) Supported by Hospital level key fund project of the First Affiliated Hospital of Shihezi University (DZ201902).
3) Supported by Xinjiang Production and Construction Corps financial science and technology plan project (2023ZD005).
The funding bodies played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Data availability
The datasets generated and analysed during the current study are not publicly available due to the data should be used for follow-up studies, but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study strictly adhered to the ethical principles of the Declaration of Helsinki and was approved by the Ethics Committee of the First Affiliated Hospital of Shihezi University (Approval No: KJX-2021-018-01). All research subjects provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tong Zhu and Lin Tang have contributed equally to this work.
References
- 1.Sweeting A, Wong J, Murphy HR, et al. A clinical update on gestational diabetes mellitus. Endocr Rev. 2022;43(5):763–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chinese Medical Association Obstetrics and Gynecology Branch. Chinese medical association perinatal medicine branch, China maternal and child health association pregnancy and diabetes professional committee. Guidelines for diagnosis and treatment of hyperglycemia in pregnancy (2022) [Part two]. Chin J Obstet Gynecol. 2022;57(1):3–12. [Google Scholar]
- 3.Wicklow B, Retnakaran R. Gestational diabetes mellitus and its implications across the life span. Diabetes Metab J. 2023;47(3):333–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buelo AK, Kirk A, Lindsay RS, et al. Exploring the effectiveness of physical activity interventions in women with previous gestational diabetes: a systematic review of quantitative and qualitative studies. Prev Med Rep. 2019;14:100877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu J, Lin X, Fang Y, et al. Lifestyle interventions to prevent adverse pregnancy outcomes in women at high risk for gestational diabetes mellitus: a randomized controlled trial. Front Immunol. 2023;14:1191184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang H, Guo X, Song Q, et al. Association between the history of abortion and gestational diabetes mellitus: a meta-analysis. Endocrine. 2023;80(1):29–39. [DOI] [PubMed] [Google Scholar]
- 7.Lee KW, Ching SM, Ramachandran V, et al. Prevalence and risk factors of gestational diabetes mellitus in Asia: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2018;18(1):494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alamolhoda SH, Yazdkhasti M, Namdari M, et al. Association between C-reactive protein and gestational diabetes: a prospective study. J Inst Obstet Gynecol. 2020;40(3):349–53. [DOI] [PubMed] [Google Scholar]
- 9.Tao Z, Cheng Z. Hormonal regulation of metabolism-recent lessons learned from insulin and estrogen. Clin Sci (London, England: 1979). 2023;137(6):415–434. [DOI] [PMC free article] [PubMed]
- 10.Carrasco-Wong I, Moller A, Giachini FR, et al. Placental structure in gestational diabetes mellitus. Biochim Et Biophys Acta Mol Basis Dis. 2020;1866(2):165535. [DOI] [PubMed] [Google Scholar]
- 11.Diniz MS, Hiden U, Falcão-Pires I, et al. Fetoplacental endothelial dysfunction in gestational diabetes mellitus and maternal obesity: a potential threat for programming cardiovascular disease. Biochim Et Biophys Acta Mol Basis Dis. 2023;1869(8):166834. [DOI] [PubMed] [Google Scholar]
- 12.Venkatesh KK, Khan SS, Powe CE. Gestational diabetes and long-term cardiometabolic health. JAMA. 2023;330(9):870–1. [DOI] [PubMed] [Google Scholar]
- 13.Bedell S, Hutson J, de Vrijer B, et al. Effects of maternal obesity and gestational diabetes mellitus on the placenta: current knowledge and targets for therapeutic interventions. Curr Vasc Pharmacol. 2021;19(2):176–92. [DOI] [PubMed] [Google Scholar]
- 14.Reijnders IF, Mulders A, Koster M, et al. First-trimester maternal haemodynamic adaptation to pregnancy and placental, embryonic and fetal development: the prospective observational Rotterdam periconception cohort. Bjog-an Int J Obstet Gynecol. 2022;129(5):785–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han Z, Zhang Y, Li X, et al. Investigation into the predictive potential of three-dimensional ultrasonographic placental volume and vascular indices in gestational diabetes mellitus. Front Endocrinol. 2021;12:689888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ehlers E, Talton OO, Schust DJ, et al. Placental structural abnormalities in gestational diabetes and when they develop: a scoping review. Placenta. 2021;116:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu YT, Zhang CJ, Mol BW, et al. Early prediction of gestational diabetes mellitus in the Chinese population via advanced machine learning. J Clin Endocrinol Metab. 2021;106(3):e1191–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye W, Luo C, Huang J, et al. Gestational diabetes mellitus and adverse pregnancy outcomes: systematic review and meta-analysis. BMJ (Clinical research ed.). 2022;377:e067946. [DOI] [PMC free article] [PubMed]
- 19.Chinese Medical Association Obstetrics and Gynecology Branch. Chinese medical association perinatal medicine branch, China maternal and child health association pregnancy and diabetes professional committee. Guidelines for diagnosis and treatment of hyperglycemia in pregnancy (2022) [Part two]. Chin J Obstet Gynecol. 2022;57(2):81–90. [Google Scholar]
- 20.Sharma AK, Singh S, Singh H, et al. Deep insight of the pathophysiology of gestational diabetes mellitus. Cells. 2022;11(17):2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Wu P, Huang Y, et al. BMI and lipidomic biomarkers with risk of gestational diabetes in pregnant women. Obes (Silver Spring Md). 2022;30(10):2044–54. [DOI] [PubMed] [Google Scholar]
- 22.Xu H, Hutcheon JA, Liu X, et al. Risk of gestational diabetes mellitus in relation to early pregnancy and gestational weight gain before diagnosis: a population-based cohort study. Acta Obstet Gynecol Scand. 2022;101(11):1253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Q, Wang H, Sun L, et al. The mediating role of inflammation in the association between pregnancy loss history and gestational diabetes mellitus. Diabetol Metab Syndrome. 2023;15(1):132. [DOI] [PMC free article] [PubMed]
- 24.Vaajala M, Liukkonen R, Ponkilainen V, et al. Previous induced abortion or miscarriage is associated with increased odds for gestational diabetes: a nationwide register-based cohort study in Finland. Acta Diabetol. 2023;60(6):845–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Three-dimensional ultrasonographic placental volume in gestational diabetes mellitus - PubMed [EB/OL] [2024-12-19]. https://pubmed.ncbi.nlm.nih.gov/25731652/ [DOI] [PubMed]
- 26.Wong CH, Chen CP, Sun FJ, et al. Comparison of placental three-dimensional power doppler indices and volume in the first and the second trimesters of pregnancy complicated by gestational diabetes mellitus. J Maternal-Fetal Neonatal Med Off J Eur Assoc Perinatal Med Fed Asia Oceania Perinatal Soc. 2019;32(22):3784–91. [DOI] [PubMed]
- 27.Nanda S, Savvidou M, Syngelaki A, et al. Prediction of gestational diabetes mellitus by maternal factors and biomarkers at 11 to 13 weeks. Prenat Diagn. 2011;31(2):135–41. [DOI] [PubMed] [Google Scholar]
- 28.Thaweethai T, Soetan Z, James K, et al. Distinct insulin physiology trajectories in euglycemic pregnancy and gestational diabetes mellitus. Diabetes Care. 2023;46(12):2137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analysed during the current study are not publicly available due to the data should be used for follow-up studies, but are available from the corresponding author on reasonable request.