Abstract
Intratumoral heterogeneity is the main cause of tumor treatment failure, varying across disease sites (spatial heterogeneity) and polyclonal properties of tumors that evolve over time (temporal heterogeneity). As our understanding of intratumoral heterogeneity, the formation of which is mainly related to the genomic instability, epigenetic modifications, plastic gene expression, and different microenvironments, plays a substantial role in drug-resistant as far as tumor metastasis and recurrence. Understanding the role of intratumoral heterogeneity, it becomes clear that a single therapeutic agent or regimen may only be effective for subsets of cells with certain features, but not for others. This necessitates a shift from our current, unchanging treatment approach to one that is tailored against the killing patterns of cancer cells in different clones. In this review, we discuss recent evidence concerning global perturbations of intratumoral heterogeneity, associations of specific intratumoral heterogeneity in lung cancer, the underlying mechanisms of intratumoral heterogeneity potentially leading to formation, and how it drives drug resistance. Our findings highlight the most up-to-date progress in intratumoral heterogeneity and its role in mediating tumor drug resistance, which could support the development of future treatment strategies.
Keywords: Cancer, Heterogeneity, Drug resistance, Exosomes, Small extrachromosomal circular DNA
Introduction
Tumor cells within a tumor tissue are not uniform, they exhibit distinct molecular and biological phenotypes, forming characteristic cell subsets, which are referred to as intratumoral heterogeneity. This heterogeneity often stems from molecular and genetic changes in the daughter cells (cell subsets) during tumor growth, leading to variations in tumor growth rate, invasion ability, and response to drugs, among other characteristics [1]. Genetic sequencing results from multiple sites have revealed significant differences in the genetic makeup of malignant cells, not only between different anatomical parts and disease stages (such as primary and metastatic lesions) but also within different regions of the same tumor. This phenomenon is referred to as spatial intratumoral heterogeneity. Similarly, some studies have shown that the genetic characteristics of the same lesion can vary over time, a concept known as temporal intratumoral heterogeneity [2].
Importantly, intratumoral heterogeneity manifests not only at the genetic level but also includes epigenetic, transcriptional, phenotypic, secretory, and metabolic components [3, 4]. These substances are not identical to one another (for instance, tumors with high epigenetic variability might exhibit genetic stability) nor are they closely interconnected (for example, genetic and epigenetic alterations work together to define transcriptomic and phenotypic profiles) [5]. It has been reported that there are numerous genetic differences between primary tumors and metastatic lesions.
The presence of intratumoral heterogeneity has been confirmed through the analysis of samples from various tumors, indicating differences in terms of mutations and chromosomal imbalances between the primary tumor and the metastases [6]. Moreover, significant intratumoral heterogeneity has been observed between the primary cancer and metastasis lesions through DNA copy number assessments in various tumors, including non-small cell lung cancer (NSCLC) [7], colorectal cancer (CRC) [8], renal cell carcinoma (RCC) [9] and so on.
Intratumoral heterogeneity is the basis of drug resistance. It is necessary to design targeted drugs or drug combinations based on this heterogeneity to maximize drug effectiveness and minimize toxicity [10]. Therefore, accurate detection of intratumoral heterogeneity is clinically significant for improving therapeutic effects and prognosis [11]. Furthermore, intratumoral heterogeneity is one of the main causes of tumor recurrence and metastasis [7, 12–15]. In this review, we will provide an overview of the role and causes of intratumoral heterogeneity, intercellular communication, and how these differences influence treatment outcomes and patient survival.
Intratumoral heterogeneity in malignant cells.
Spatial heterogeneity
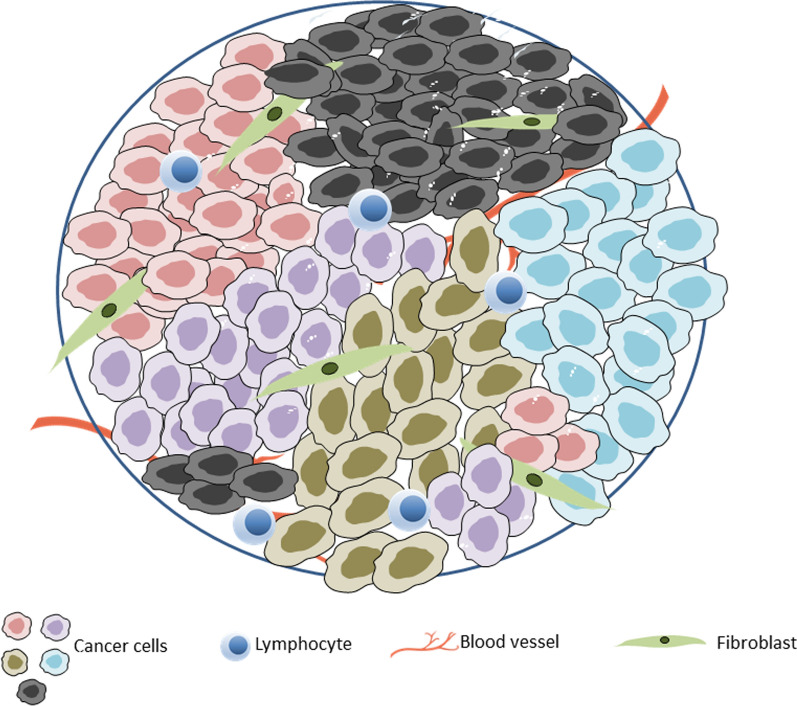
Intratumoral heterogeneity primarily encompasses spatial and temporal heterogeneity, which manifests in structural, genetic, proteomic, and functional differences (Fig. 1). Spatial heterogeneity refers to the distinctions between the primary tumor and its metastases. Characteristics such as genetic information and cell morphology can vary, with the extent of genetic discrepancies between the primary and metastatic tumors potentially indicating whether metastasis occurred late in the disease progression or originated from early dissemination. The former scenario would typically result in a lesser degree of genetic discordance. In addition to the discordance between the primary tumor and metastatic sites, comparisons of the genetic makeup often reveal substantial levels of heterogeneity among different metastases [16]. Furthermore, even when cancer cells at locoregional and distant sites originate from a common ancestor, site-specific factors (such as the interaction between cancer cells and other cells within the local tumor microenvironment) can drive genetic divergence following the initial colonization of metastatic sites. For instance, in a comprehensive study comparing the genetic profiles of primary tumors, brain metastases, and extracranial metastases, the researchers noted a branched evolutionary pattern in brain metastases, leading to genetic uniformity among distinct brain metastases despite significant genetic differences from extracranial metastases [17].
Fig. 1.
Intratumoral heterogeneity represented by cancer cells with different color in the context of tumor microenvironment resulting from different stromal cell compositions and biophysical properties such as differences in extracellular matrix composition (fibroblast), blood vessel (hypoxia and acidosis), and other factors
Nevertheless, within the same tumor tissue block, there can also be cell subsets with different genotypes at various sites, such as the potential coexistence of both EGFR mutant and EGFR wild-type cells. EGFR mutant non-small cell lung cancer (NSCLC) responds effectively to tyrosine kinase inhibitors (TKIs) targeting EGFR, whereas NSCLC cells with wild-type EGFR are resistant to these EGFR-TKIs [18, 19]. Studies by Linnoila et al. indicate that there is a significant difference in the proportion of subsets of neuroendocrine features at various sites in NSCLC, which is associated with drug resistance [20]. Studies have shown that the antigenicity types and expression levels vary among different parts of NSCLC [21]. Negative immune regulators, such as programmed cell death 1 ligand 1 (PD-L1) and tumor mutation burden, also exhibit dissimilarities in different regions of NSCLC [22–24]. It is therefore conceivable that the response to immunotherapy varies. Heterogeneity refers to the diversity of subclonal cell populations within the tumor, also known as clonal diversity.
Unlike the mutational load, the heterogeneity reflects genetic differences among cells. A tumor with a high mutation load may have low intratumoral heterogeneity, while a tumor with high heterogeneity maybe also a low mutation load. The existence of spatial heterogeneity existed in various cancer, such as CRC, NSCLC and RCC. Jain et al. reported that genetically distinct subclones within the primary tumor could lead to metastasis in different parts after dissemination, including lymph-node and distant metastases [25]. Defilippi et al. have shown that both genetic diversity and the tumor microenvironment can lead to drug resistance by influencing intratumoral heterogeneity [26]. RCC is also a highly heterogeneous tumor. Previous studies have shown that there are significant differences in genes and signaling pathway markers between primary and metastatic lesions in advanced RCC. Thus, different subclones produced by initial treatment could cause drug resistance. This issue could be addressed through personalized and combination therapies. [27]. Therefore, the future of successful treatment management for tumors hinges on accurately accounting for intratumoral heterogeneity. To achieve this goal, improved tumor specimen sampling and subclonal mutation identification are essential.
Temporal heterogeneity
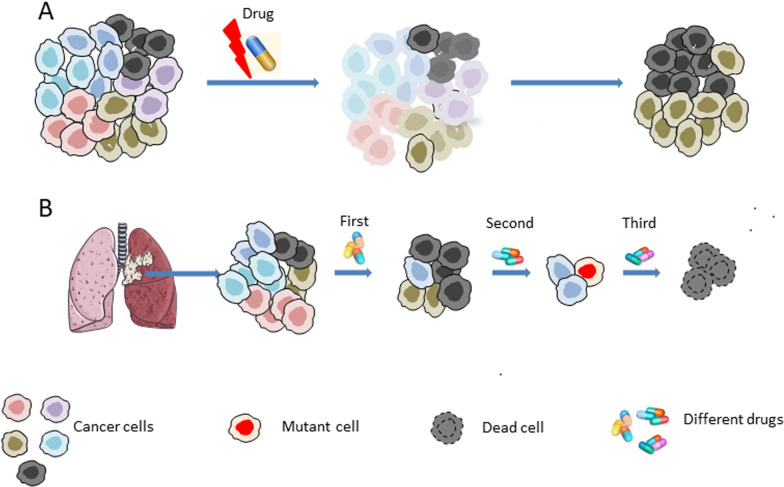
Beyond spatial heterogeneity, tumor development and treatment reveal distinct cellular, molecular, or genetic characteristics, biological phenotypes, and drug sensitivities at various clinical stages, indicating tumor temporal heterogeneity (Fig. 2). This reflects the dynamic changes in tumor gene diversity over time. Successive biopsies to study tumor evolution suggest that chemotherapy can alter the tumor mutational spectrum and induce molecular changes over time [28, 29], Especially, drugs that affect replication and cell cycle regulation. For instance, temozolomide can induce a hypermutated phenotype by enriching transitional mutations in mismatch repair (MMR) genes [1]. Compared to non-specific treatments such as cytotoxic chemotherapy, targeted therapies exert a more potent selective pressure on cancer cells carrying oncogenes. In fact, many mutations associated with tumor temporal heterogeneity occur in the context of targeted therapy. The genomic instability and the distribution of extrachromosomal DNA (eccDNA) to offspring cells result in tumor cell evolution and accumulated variation, producing different molecular, genetic characteristics, and biological phenotypes from primary cell [30–33]. During tumor treatment, anticancer drugs can drive the evolution of cancer cells and lead to new mutations, which in turn mediates cell resistance. Targeted therapy for NSCLC has achieved significant success. Currently, targeted drugs include inhibitors of EGFR, ALK, BRAF, HER2, MEK1, c-Met, and vascular endothelial growth factor (VEGF), among others.
Fig. 2.
Intratumoral heterogeneity can lead to drug resistance. A Anticancer drugs drive cancer cell evolution and new mutations, which mediates cell resistance. B Intratumoral heterogeneity pose a challenge to targeted therapies, subclonal lesions may be more effective of one targeted therapy
To date, biopsy samples have been analyzed to evaluate the temporal heterogeneity from different tumor locations in drug-resistant patients. Because this method requires a larger biopsy sample, drug resistance cannot be detected in the early or intermediate stages. This might lead to incomplete tumor responses in most patients, leaving residual tumor masses even with subsequent drug changes [34–36]. Anti-tumor therapy can lead to the emergence of new acquired resistance, which may be due to the selective proliferation of subclonal populations caused by intratumoral heterogeneity, or the continuous evolution of tumor cells leading to the emergence of new resistant cells. This provides a theoretical basis for developing treatments for minimal residual lesions. Consequently, due to the induction of drugs, the evolution of molecules and genes manifests as temporal heterogeneity in different clinical stages. However, the evolution pattern of cells under different drugs and conditions remains unclear.
Mechanisms of intratumoral heterogeneity
In general, internal cellular factors and the tumor microenvironment (TME) are the primary contributors to intratumoral heterogeneity. The potential mechanisms can be categorized into genomic instability, epigenetic modifications, plastic gene expression, and variations in the TME (Table 1). There are genetic and functional disparities among different tumors, leading to differences in genotype, treatment response, and prognosis. Typically, the primary tumor site dictates the treatment approach. Nonetheless, even tumors originating from the same tissue and cell type can exhibit considerable differences in genomic aberrations, invasiveness, and drug sensitivity [37–39]. Within tumors, diversity occurs at the gene, epigenetic, and protein expression levels, which can lead to sampling bias affecting the detection of relevant treatments, thereby complicating the treatment of tumors.
Table 1.
The mechanisms and strategies of intratumoral heterogeneity
| Mechanisms | Strategies | |
|---|---|---|
| Genomic instability | Elevate the mutation rate | 63 |
| Mismatch repair (MMR) | 1 | |
| EccDNA distributed to offspring cells | 40–43 | |
| Epigenetic modifications | ||
| Temporal shifts in DNA methylation patterns | 83 | |
| DNA methylation in the transcription of genes | 85 | |
| Cellular clone and cancer stem cells | ||
| Genetic variation in CSCs | 91, 92 | |
| EMT phenotype | 94 | |
| Intratumoral heterogeneity and microenvironment | ||
| Growth factors, cytokines, oxygen, and nutrients, as well as extra-cellular matrix (ECM), are different | 97, 98 | |
| The heterogeneity of infiltrating immune cells in the TME | 102 | |
| Blood supply | 106 |
Genetic heterogeneity
Genome replication and division exhibit high fidelity, resulting in a rare point mutation rate in somatic cells (0.77 × 10−9 per site per cell division) [40]. Additionally, chromosome segregation errors are relatively low, occurring approximately once every 100 cell divisions [41–43]. Most tumors display a form of genomic instability, encompassing both solid malignancies and hematopoietic tumors [44]. The tolerance for genomic instability has increased, enabling cells to evade death following DNA damage, withstand increased alterations and mutations in chromosomes, and even be stimulated by factors such as chemotherapy drugs. Therapy-induced mutations and severe genomic instability can accumulate, leading to a hypermutator phenotype and thereby increasing intratumoral heterogeneity [45, 46]. A large number of studies have shown that intratumoral heterogeneity is significantly related to prognosis [47–52].
The causes of intratumoral heterogeneity are highly complex. Heterogeneity observed in different individuals presents with distinct genetic backgrounds, including variations in chromosome number and structure, pathological types, clinical stages, differentiation levels, and cell evolution. Even tumors from the same patient can exhibit significant molecular differences, such as variations in gene expression profiles, mutation spectra, and network regulation. Genomic instability plays a crucial role in the development and progression of many cancers, ranging from single-base substitutions to whole-genome duplications [53]. The occurrence of mutations in patients with the same type of tumor also exhibits inconsistencies in the mutational gene spectrum and biological characteristics among tumor cells at different sites. This reflects the high complexity and diversity of malignant tumors during their evolution. Consequently, heterogeneity may arise from cellular pressures such as drugs and genomic instability, manifesting as differences in gene mutations or expression profiles [30, 54–57]. Genomic instability alone is insufficient to maintain intratumoral heterogeneity; other factors must also be at play to foster the development of such heterogeneity. Conversely, recent studies have indicated that heterogeneity and cellular resistance are associated with the expression of extrachromosomal DNA (eccDNA).
The etiologies mediating intratumoral heterogeneity are primarily associated with tumor cell genomic instability and the expression of eccDNA [58], which is a cancer-specific circular DNA molecule derived from chromosomes. Compared to linear chromosomes, eccDNA possesses a unique structure that can reflect the high accessibility of chromosomes and contribute to the overactivation and malignant behavior of proto-oncogenes. Additionally, the non-chromosomal inheritance and repeated mutations of eccDNA exacerbate the heterogeneity and evolution of tumors [59]. Studies have indicated that long eccDNA may encompass complete genetic information, ranging from a few hundred kilobases to several megabases, and act as amplifiers for oncogenes implicated in tumorigenesis and drug resistance [60].
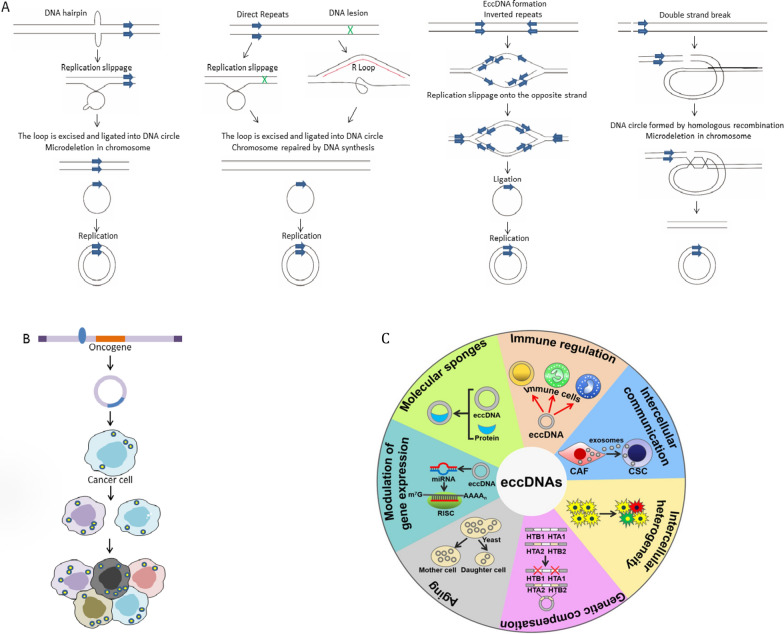
Since eccDNA is present in the cytoplasm, it cannot be evenly distributed to the two progeny cells during cell division [58, 61–63] (Fig. 3a). However, eccDNA expression remains unclear. The amplification of oncogenes on eccDNA is surprisingly common in cancer, where it can significantly increase oncogene copy number and drive genetic heterogeneity within tumors due to its lack of centromeres and susceptibility to unequal segregation. Numerous findings suggest that eccDNA substantially promotes increased transcription of the oncogenes studied, as it increases DNA copy number and is associated with enhanced chromatin accessibility.
Fig. 3.
EccDNA was randomly assigned to offspring cells to mediate intratumoral heterogeneity. A Mechanism of eccDNA formation (Ref Trends Genet. 2018 April; 34(4): 270–278). B EccDNA was randomly assigned to offspring cancer cells (Ref Int J Biol Sci. 2021; 17(4): 1010–1025) [174]. C The biological functions of eccDNAs [175]
Epigenetic modifications
Both genetic mutations and epigenetic modifications contribute to the heterogeneity of tumors. While epigenetic modifications are crucial for normal cell development and the maintenance of cell fate, cancer cells can also transmit epigenetic aberrations to other cells [64]. Epigenetic modifications can be stably inherited by future generations without altering the DNA sequence [65].
Temporal variations in DNA methylation patterns have been observed in recurrent glioblastomas and progressive lung cancers [66]. Similarly, other epigenetic processes have been shown to be related to spatial and/or temporal intra-tumor heterogeneity, such as gene regulation by noncoding RNAs, chromatin remodeling, and histone acetylation [64]. Epigenetic changes could affect the evolution and the clonal landscape of the tumor through the cell progeny [67].
Additionally, DNA methylation within tumors has been consistently observed in regulatory regions that impact the transcription of genes associated with the disease process. For instance, androgen receptor enhancers, which are ligand-dependent transcription factors driving the expression of many genes involved in cell survival and proliferation in prostate tumors, exhibit a high degree of intratumoral heterogeneity [68]. The increasing application of single-cell multi-omics analysis in tumor studies accelerates the direct analysis of genetic, epigenetic changes, and gene expression profiles. A recent single-cell RNA sequencing study of acute leukemia has shown that the RUNX1 transcription factor plays a key role in regulating intratumoral heterogeneity among patients [69].
Cellular clone and cancer stem cells
Cancer stem cells (CSCs) are a group of cells capable of self-renewal and infinite proliferation, playing a crucial role in tumor survival, proliferation, metastasis, and recurrence [9, 70], which could asymmetrically differentiate into two types of heterogeneous cells: one similar to CSCs, and the other comprising the non-oncogenic cancer cells that constitute the majority of the tumor.
Antitumor activity can be overcome by CSCs, which are capable of replicating, evading immune responses, and differentiating into transformed cells [71], and possess the ability to self-renew. The most critical mechanism driving the evolution of CSCs is treatment, where only resistant subclones survive and proliferate during therapy [72, 73]. This raises the question of whether these resistant subclones were present in the earlier tumor stages or if they were induced by drug treatment. A large number of studies have supported the existence of a small number of drug-resistant subclones in tumors, which is due to the genetic variation of CSCs [73, 74].
intratumoral heterogeneity and microenvironment
The tumor microenvironment (TME) refers to the internal environment in which tumor cells are produced and reside. This environment encompasses not only the tumor cells themselves but also various surrounding cells, such as fibroblasts, immune and inflammatory cells, and glial cells. Additionally, it includes the nearby intercellular matrix, microvessels, and bio-molecules that infiltrate them. The content of these components varies in different locations within the TME [75, 76]. Since the TME of each cancer cell is unique, cells with the same genotype may respond differently to treatment [77]. For instance, tumor cells in proximity to stromal cells exhibit significantly greater proliferation and survival, a phenomenon attributed to the paracrine effects of immune cells and fibroblasts [75, 78]. The uneven distribution of the tumor microenvironment creates numerous microenvironmental niches. Due to the varying microenvironments that tumor cells inhabit, this may result in different treatment sensitivities among genetically identical cancer cells [64]. Intratumoral heterogeneity and tumor evolution are complementary. The changes in genetic and epigenetic factors together determine the transcriptome and phenotype of tumor cells, leading to the consistent reprogramming of the TME [79].
As an important factor influencing drug resistance, the environment in which tumor cells reside has been emphasized for its significance, particularly the extrinsic compartments of tumor cells [80]. The sources and effects of TME heterogeneity are attributed to the migration of immune cells, recruitment of fibroblasts, matrix remodeling, and the development of vascular networks. Moreover, tumor heterogeneity also influences the nonmalignant compartments of the TME.
The heterogeneity of infiltrating immune cells within the TME plays a crucial role in immunotherapies. Leukocytes are typically one of the most abundant cell types in tumors, and their high mobility contributes to rapidly changing spatial heterogeneity, resulting in immunologically active or silenced niches. In addition, tumor-infiltrating lymphocytes (TILs) also contribute to this complexity [81] and cancer-associated fibroblasts (CAFs) [82, 83], as well as various myeloid cell populations, including tumor-associated macrophages (TAMs) and dendritic cells (DCs) [84] Also, the TME plays a key role in drug resistance. Consequently, the location, abundance, and functional orientation of each cell component within the TME change over time and space, thereby affecting the tumor heterogeneity.
Furthermore, differences in the tumor cell microenvironment can also cause heterogeneity, many factors work together to create this unequal microenvironment.
One of the most important factor is blood supply in tumor [85]. The nutrient supply and metabolism of tumor cells vary depending on their proximity to the vascular system, resulting in intratumoral heterogeneity. Additionally, certain stromal cells, such as inflammatory cells, fibroblasts, and pluripotent mesenchymal cells, can influence the chemotaxis and growth of tumor cells by secreting cytokines, growth factors, and extracellular matrix (ECM) components. This further promotes intratumoral heterogeneity and affects the TME [86]. It has been reported that tumors with higher intratumoral heterogeneity tend to grow faster, whereas those with lower intratumoral heterogeneity are associated with a better prognosis [87, 88]. However, can distinct cellular subgroups "communicate with one another" and "assist each other"? How do they communicate? Under cellular stress, do they exhibit "unity, mutual aid, and a search for solutions" or "isolation" and counterproductive actions, ultimately leading to their demise? These questions remain unanswered.
Single-cell transcriptome sequencing and intratumoral heterogeneity
Single-cell transcriptome sequencing (scRNA-seq) technology enables high-resolution analysis of gene expression at the single-cell level. It can identify transcriptional expression differences among various tumor cell subtypes, offering a foundation for studying strategies to address tumor heterogeneity. Additionally, it provides precise genetic information for tumor diagnosis and treatment, potentially enabling the realization of "personalized medicine."
ScRNA-seq can be utilized to analyze the mechanistic study of tumor development and evolution, assist in conducting in-depth studies of expression heterogeneity within the TME, and identify cell subsets and abnormally expressed genes that are key to tumor development. GOJO et al. [21] analyzed the molecular groups and anatomical locations of ependymomas using scRNA-seq to explore the origin and heterogeneity of the tumor. They found that ependymomas with a good prognosis primarily contained well-differentiated cells, whereas invasive ependymomas were rich in undifferentiated cell populations. This research revealed the developmental levels related to clinical behavior in ependymoma biology.
Implications of intratumoral heterogeneity on drug resistance
Clonal evolution and selection pressure
Genetically distinct subclonal cell populations emerge as a result of cell mutations, and subsequently, within a specific tumor microenvironment, such as during disease progression and drug treatment [89, 90] selective growth of clones with phenotypic advantages leads to changes in the subclonal cell population [91–93]. Clone sweeping, where a new clone supplants the ancestral clone and assumes control over the entire population, will result in a uniform cell population. However, if a new clone fails to outcompete its predecessor, some level of heterogeneity will be observed [94]. Additionally, there is branching tumor evolution, where various subclones evolve in parallel, leading to extensive subclonal diversity [73].
To gain insight into cancer evolution and intratumoral heterogeneity, single-cell sequencing or multiplex fluorescence in situ hybridization (FISH) methods have been used to reconstruct tumor subclonal structures [95]. We have observed branching tumor evolution in a range of tumors, including breast cancer [96], pancreatic cancer [91], chronic lymphoblastic leukaemia (CLL) [93], and colorectal adenomas [97]. It is conceivable that tumor stem cells play an important role in maintaining the heterogeneity of solid tumors.
The mechanisms of drug resistance
These differences in molecular characteristics between cells can occur at the genome, gene expression, and post-translational modification stages; however, most types of cancer recognize these differential molecules [9, 98]. The amplification of drug resistance genes on eccDNA may lead to drug resistance in cancer cells. The greater the degree of eccDNA quantitative variation, the greater the diversity of intratumoral heterogeneity, which also triggers cellular diversity that makes the tumor resistant to most chemotherapeutic drugs. A large quantity of eccDNA coding for dihydrofolate reductase (DHFR) was found when patients received treatment with methotrexate (MTX), and subsequently, the sensitivity to MTX decreased [99]. These studies have shown that eccDNA is related to the development of drug resistance. Reportedly, eccDNA regulates EGFR mutations to produce EGFRvIII, which leads to EGFR inhibitor resistance in glioblastoma [100–102]. Similar mechanisms are also observed in multidrug resistance mediated by MDR [103]. Collectively, cancer cells evade drug therapy through a “hide and seek” mechanism regulated by eccDNA (Fig. 3b, c). Therefore, it is important and significant to understand the molecular mechanisms behind the production and maintenance of eccDNA, and to explore the alterations in the tumor environment caused by eccDNA. During the progression and development of tumors, clonal evolution and intratumoral heterogeneity result in a series of biological and host environment changes, primarily through alterations in intercellular transcriptome expression and crosstalk. The inherent heterogeneity of the tumor, along with these changes, contributes to drug resistance, a phenomenon observed across all cancer types and treatment modalities [104].
Ample evidence suggests that intratumoral heterogeneity increases the likelihood that malignant cells survive conventional chemotherapy, radiation therapy, and especially targeted and immunotherapy [75]. Presently, conventional treatments frequently approach cancer as a uniform disease, neglecting the distinctions among tumor cells. For instance, targeted therapies that aim at the abnormal molecular characteristics of cancer cells are based on biopsies from a single tumor site and fail to represent the tumor's overall condition. Due to the heterogeneity of cancer cells, drugs may be effective against some or several cancer cell subclones, while others remain resistant. These resistant subclones can survive the destruction of most non-resistant subclones in heterogeneous tumors, eventually dominating and continuing to grow and reform the tumors. This necessitates a change in our current, static treatment approach, which should instead be tailored to target the killing patterns of different cancer cell clones.
Targeted therapy is a treatment approach that targets specific genetic or molecular markers in cancer cells, such as EGFR mutations in NSCLCs. It involves the use of agents that target these markers, which can be proteins or gene fragments, to interfere with the growth and survival of cancer cells. However, despite the initial significant response to targeted treatment, drug resistance often develops within 1–2 years. The possible reason for this is the selective expansion of drug-resistant clones during treatment, due to intratumoral heterogeneity [105–107].
All compartments within TME engage in multidirectional interactions with one another, leading to the evolution of intratumoral heterogeneity over space and time [86, 108]. The distribution and functional orientation of the immunological TME can be regulated by malignant and immune cells through the exposure or release of various immunoregulatory factors, including cell surface proteins, cytokines, growth factors, and danger signal molecules. Additionally, CAFs in the immunological TME may function in the following ways: by secreting extracellular matrix components and profibrotic cytokines, such as TGFB1; by inhibiting the function of CTLs and releasing immunosuppressive factors; and by enhancing the stemness, proliferation rate, and metabolism of cancer cells. In turn, the heterogeneity of CAFs could be exploited by cancer cells through multiple mechanisms, including a paracrine loop centered on TGFB. Importantly, various signals involved in the spatial and temporal reconfiguration of the TME originate from dying cells, which are prevalent in the TME even in the absence of therapeutic interventions [86, 108].
Developing culture techniques for primary or circulating tumor cells and conducting drug sensitivity tests are key to achieving phased, cancer cell killing patterns for different clones (Fig. 2b). This approach also offers new strategies for overcoming tumor drug resistance. The traditional monotherapy strategy is limited because the development of new drugs and the emergence of drug resistance are cyclical. There is an urgent need for a method that can identify the molecular drivers of drug resistance in tumors early, thereby better preventing and predicting the emergence of drug resistance.
Intratumoral heterogeneity and drug resistance in different cancers.
Several factors can influence drug responsiveness, including pharmacokinetics, but it is improbable that this is the primary factor affecting the relationship between intratumoral heterogeneity and drug resistance. This is because intratumoral heterogeneity seems to mainly impact drug response at the cellular level, due to variations in genotype and epigenetic modifications among cells, or disparities in the tumor microenvironment [109].
Thus far, the mortality rate for most advanced metastatic cancers remains high, even after the elimination of most tumor cells. Intratumoral heterogeneity permits a small subset of tumor cells to survive treatment-induced elimination, particularly because of the diverse phenotypes of tumor cells that enable them to adapt to drugs during the treatment process. This adaptation leads to new drug resistance and recurrence.
Moreover, the interactions and dependencies among cell subpopulations, driven by intratumoral heterogeneity, result in continuous tumor growth. There are significant variations in treatment responses across different subpopulations, leading to the belief that intratumoral heterogeneity underpins poor drug responses, acquired resistance, and disease recurrence in clinical treatment. Intratumoral heterogeneity commonly contributes to therapeutic resistance. Research has indicated that it correlates with treatment resistance and poor prognosis in various types of cancer.
Mutual communication between heterogeneous cells and tumor drug resistant cells
Vesicles released from cells are mediators to achieve inter-cell communication.
The biological phenotypes within each cancer subset possess unique advantages due to their distinct gene expression profiles. Some exhibit sensitivity to chemotherapeutic and targeted therapeutic agents, while others display drug resistance. Are the tumor cells with different biological phenotypes within the same tumor tissue interconnected? How do drug-resistant cell subpopulations impact sensitive subpopulations?
Vesicles released from cells act as mediators to achieve inter-cellular communication. Depending on their size, they can be categorized as follows: exomeres, small exosomes, large exosomes, microvesicles, exophers, migrasomes, large oncosomes, etc. (Table 2). While there is some overlap and crossover among them, their functions are distinct. Exomeres (exosome particles), small exosomes, and large exosomes are particularly significant in inducing drug resistance. CD9, CD63, CD81, Tsg101, and Alix1 are generally recognized as specific markers for exosomes. However, these markers are not unique to small exosomes and large exosomes [110–113].
Table 2.
| Exomeres | Small exosomes | Large exosomes | Microvesicles | Exophers | Migrasomes | Large oncosomes |
|---|---|---|---|---|---|---|
| − 35 nm | 60–80 nm | 90–120 nm | ≤ 1000 nm | 1–4 µm | 500–3000 nm | 1–10 µm |
Exosomes are released by multivesicular bodies (MVBs) through the fusion of cytoplasmic membranes with the extracellular membrane, forming bubbles. Their origin is from plasma membrane endocytosis, which progresses to early endosomes, then to late endosomes. Subsequently, endosomal membranes undergo inward budding to form vesicles known as Intraluminal Vesicles (ILVs). These vesicles then evolve into multivesicular bodies, which are ultimately released outside the cell. RAL-1 plays a role in the formation of MVBs and their fusion with the cytoplasmic membrane. The target protein of RAL-1 is SYX-5. In the absence of SYX-5, MVBs should accumulate under the cytoplasmic membrane and release disorders [114]. Ral GTPases are key proteins that regulate the formation of MVBs and the release of exosomes [115]. Exosome contents are typically synthesized at their naturally occurring locations and then transported to specific sites by molecular machines. This is followed by the aggregation and budding of the contents, and finally, they are cut by specific molecular machines to form a closed membrane structure. There are two primary methods of content sorting: the ESCRT (Endosomal Sorting Complex Required for Transport)-dependent pathway and the ESCRT-independent pathway. Indeed, the ESCRT complex comprises four members: ESCRT-0 and ESCRT-I, which restrict ubiquitinated transmembrane cargo subunits to specific membrane microdomains of the MVB and induce deformation of the membrane structure; ESCRT-II, which is responsible for recruiting and initiating microdomain budding; and ESCRT-III, which is involved in the cleavage that forms a closed vesicle. Independent mechanisms of the ESCRT system include neutral sphingomyelinase 2 (nSMase 2), Tetraspanin, CD63, CD81, and CD9. It has also been reported that Rabs serve as the assembly and loading tools for exosomes, with their contents being associated with Rabs. Consequently, Rab GTPases may be related to the intracellular loading speed, content, and degradation of exosomes [111, 116, 117]. The neutral sphingolipase 2 inhibitor, GW4869, is believed to suppress exosome release. Exosome release occurs through two primary pathways: SNARE (Soluble NSF Attachment Protein Receptors)-dependent or independent. SNARE proteins primarily regulate the fusion of secretory vesicles with the cell membrane. t-SNARE and v-SNARE proteins determine the interaction between vesicles and specific sites on the membranes [118–120].
How do the exosomes released from the donor cells recognize and enter the recipient cells?
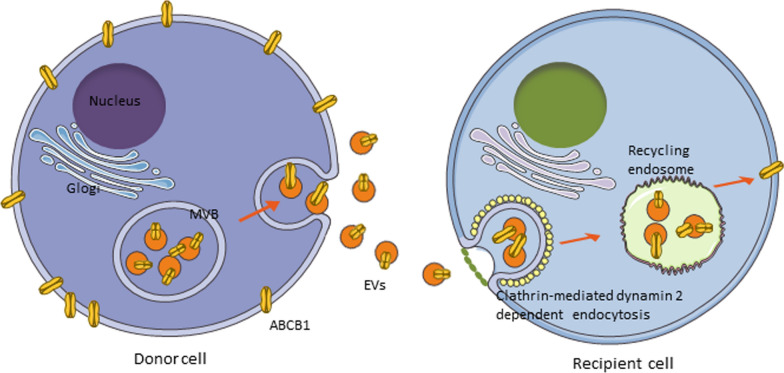
The mechanism by which exosomes are recognized by cell adhesion molecules such as integrins or receptor ligands prior to endocytic recycling, as well as through direct fusion, remains unclear. It has been reported that the ATP-binding cassette subfamily B member 1 (ABCB1) of multidrug-resistant (MDR) tumor cells can be transferred to sensitive tumor cells (where the MDR1 gene is not expressed) via exosomes when MDR cells are co-cultured with parental sensitive cells. Consequently, the sensitive tumor cells can rapidly acquire the MDR phenotype, which accounts for the transient resistance observed in tumor cells. Recently, we have discovered that anti-cancer agents can significantly increase the transfer of ABCB1 molecules. Anticancer drugs, such as vincristine (VCR), result in an elevated release of exosomal ABCB1 in MDR cells, which is linked to Rab8 expression. Moreover, receptor cells more readily accept and recycle exosomal ABCB1, a process associated with the expression levels of Rab5 and clathrin. Consequently, receptor cells transiently acquire functional ABCB1, thereby mediating cell resistance (Fig. 4). Exosomes released by tumor-associated fibroblasts can enhance stemness and EMT transformation, and mediate drug resistance [121].
Fig. 4.
Exosomes released from the donor cells recognize and enter the recipient cells, thus ransferred exosomal resistant molecules triggers drug resistance
Recently, we co-cultured EGFR wild-type cells (resistant to osimertinib) with Exon19del mutant cells (sensitive to osimertinib and transfected with GFP). We observed that the sensitive cells developed resistance to osimertinib. Further studies revealed similar effects in the co-culture of exosomes from NSCLC cells with EGFR wild-type compared to the mutant cells with Exon19del EGFR. Mechanistic studies suggest that NSCLC cells with wild-type EGFR may generate and release exosomal wild-type EGFR, which transfers to receptor cells with Exon19del mutant EGFR. This acquisition of wild-type EGFR on the receptor cell membrane can activate downstream cell survival pathways, such as the AKT and ERK pathways, thereby mediating resistance to EGFR-TKIs [122, 123]. The resistance to immune checkpoint inhibitors (ICIs) was also mediated by exosomal PD-L1 [124, 125]. Hoshino et al. [126] demonstrated that integrins α6β4 and α6β1 in exosomes were associated with lung metastasis of liver cancer. Furthermore, the transfection of Rab27b increased the content of HSP90 and vimentin in exosomes, thereby enhancing the metastatic potential of breast cancer cells [127, 128]. Similarly, exosomes isolated from highly metastatic lung cancer cells increased vimentin and N-cadherin expression and decreased the expression of E-cadherin and the tight-junction protein ZO-1, thereby enhancing motility and invasive potential [129]. In addition to proteins, mRNA, eccDNA, microRNA (miRNA), long non-coding RNA (lncRNA), circular RNA (circRNA), and other molecules can transfer to recipient cells, potentially altering their biological behavior, including cell resistance, migration, and invasion. Matsumoto et al. reported that RNAs/miRNAs are selectively loaded into exosomes [130]. Many exosomal lncRNAs and miRNAs have been associated with drug resistance, as summarized in Table 3.
Table 3.
Exosomal lncRNA/miRNA induced drug resistance in NSCLC
| lncRNA/miRNA | Drug resistance | References |
|---|---|---|
| Circ-CPA4/let-7 miRNA | PD-L1/PD-1 antibody | Hong et al. [152] |
| miR-21 | Cisplatin/gemcitabine/teniposide | Bica-Pop et al. [153] |
| miR-222-3p | Gemcitabine | Wei et al. [154] |
| miR-193a | Cisplatin | Wu et al. [155] |
| miR-130a | Zhang et al. [156] | |
| miR-425-3p | Ma et al. [157] | |
| miR-146a-5p | Yuden et al. [158] | |
| miR-103a-3p | Wang et al. [159] | |
| miR-4443 | Song et al. [160] | |
| miR-1273a | Zhao et al. [161] | |
| circ_PIP5K1A/miR-101 | Shao et al. [162] | |
| circRNA_102481/miR-30a-5p | Gefitinib/erlotinib | Yang et al. [163] |
| lncRNA UFC1 | PTEN inhibitor | Zang et al. [164] |
| lncRNA SOX2 | Zhou et al. [165] | |
| miR-214 | Gefitinib | Zhang et al. [166] |
| lncRNA UCA1/miR143 | Chen et al. [167] | |
| lncRNA RP11-838N2.4 | Erlotinib | Zhang et al. [168] |
| lncRNA H19 | Pan et al. [169] | |
| lncRNA MSTRG.292666.16 | Osimertinib | Deng et al. [170] |
| miR-184 and miR-3913-5p | Li et al. [171] | |
| miR-210 | Hisakane et al. [172] | |
| miR-136-5p | Anlotinib | Gu et al. [173] |
Emerging evidence suggests that EVs mediate communication among various cells, carrying distinct nucleotides, proteins, and metabolites from their cell of origin, particularly exosomes. Exosomes play a crucial role in cellular communication by transferring cellular proteins, nucleic acids, and lipid cargo between cells. Among these functions, exosomes contribute to multidrug resistance (MDR) by transferring resistance proteins and nucleic acids [131], but the exact mechanism is still not quite clear.
Progress has been made in targeting exosomes, including the CD47-mediated protection of exosomes from phagocytosis by monocytes and macrophages [132]. GW4869 inhibiting exosome release [133, 134]. Since exosomes can mediate the transient gain of resistance in tumor cells, inhibiting their formation and release to block cell-to-cell communication is a viable strategy. This involves “isolating” tumor cells to overcome resistance and increase anticancer efficacy.
The challenge of intratumoral heterogeneity for clinical diagnosis and treatment
Intratumoral heterogeneity affect clinical diagnosis and disease surveillance of tumor
Due to the continuity of the spatiotemporal evolution and dynamic evolution of tumors, the cell and gene variations within and between tumors are highly heterogeneous and rapidly evolving, which brings challenges to the realization of accurate tumor diagnosis [6, 135]. Real-time and dynamic monitoring of the tumor’s overall state is crucial during treatment, yet the tumor's heterogeneity poses significant challenges. For instance, while sensitive lesions may respond well to treatment, the size of insensitive lesions may remain unchanged or even increase. Although multi-regional biopsy can enhance the assessment of tumors' spatial heterogeneity, it has not been widely adopted due to its high risk [136, 137]. In recent years, technologies capable of fully reflecting intratumoral heterogeneity and monitoring tumor therapeutic efficacy have been developed. These include liquid biopsy, next-generation sequencing (NGS), and single-cell sequencing technology, which can track tumor progression and treatment response more accurately and conveniently.
Intratumoral heterogeneity affects the clinical treatment and prognosis of tumor
Intratumoral heterogeneity leads to a broader range of treatment options for patients. The question is whether the outcome can be predicted prior to treatment, thereby enabling the selection of more effective therapies. Recent studies have found that neither tumor mutation load nor copy number variations (CNV) significantly affect survival rates. Patients with lower intratumoral heterogeneity exhibit a considerably higher immune response and survival rate compared to those with higher intratumoral heterogeneity. This finding was subsequently validated in melanoma cell lines, and a possible explanation has been proposed: neoantigens on intracellular subclones with high intratumoral heterogeneity (ITH) are diluted and insufficient to induce an effective anti-tumor immune response [138].
Machine learning can help identify biomarkers associated with intratumoral heterogeneity
In recent years, transcriptome sequencing technology has made significant progress and has become the primary method for analyzing gene expression patterns and heterogeneity in malignant tumors. The development of second-generation sequencing, single-cell sequencing, and spatial transcriptomics has enabled us to integrate the gene expression of individual tumor cells with spatial information, analyzing the determinants of tumor microenvironment heterogeneity in a multifactorial and multidimensional manner. This approach helps determine the molecular basis of tumor microenvironment formation and discover potential cancer biomarkers and therapeutic targets by analyzing cell–cell interaction information. It provides new insights into the diagnosis, treatment, and prevention of cancer. Additionally, a new prognostic scoring system for tumors has been developed using machine learning methods, which can predict tumor characteristics and prognosis more accurately [139, 140].
Conclusions
The instability of several key cellular regulatory processes leads to malignant transformation. The resulting heterogeneity arises from genetic instability and is subsequently maintained through selection processes, including treatment selection pressure. This heterogeneity leads to the development of drug resistance and site-specific responses, complicating the selection of effective therapeutic agents globally. Even in the simplest scenario of an oncogene-driven cancer, heterogeneity provides the basis for the emergence of drug resistance and, ultimately, disease relapse. The manifestations of resistance are as diverse as those of cancer, ranging from a single, identical resistant subclone at the primary site to different subclones present at various metastatic sites. Clarifying the phenotype of molecular subclasses of tumor patients can better characterize tumor biology, define patient prognosis, and establish new therapeutic strategies.
Intratumoral heterogeneity is a common phenomenon in tumors and serves as the molecular basis for tumor resistance and recurrence. It has recently been demonstrated that heterogeneity and cell resistance are associated with the expression of eccDNA, which has been reported to play a key role in drug resistance through the "hide and seek" mechanism. Therefore, it is crucial to understand the molecular mechanisms behind the production and maintenance of eccDNA, and to investigate the changes in the tumor environment induced by eccDNA. Nevertheless, the novel insights from our study pertain to how heterogeneous cells communicate and assist one another. How can we achieve mutual aid, enabling sensitive cells to acquire drug resistance instantly in the face of cellular stress, such as that caused by chemotherapeutic drugs? Vesicles released from cells have been reported to mediate inter-cellular communication of eccDNA, which can facilitate a transient gain of resistance in tumor cells. Exosomes, in particular, can mediate this transient gain of resistance in tumor cells. Therefore, inhibiting exosome formation and release, and blocking cell-to-cell communication, presents a viable strategy to overcome resistance and enhance anti-cancer efficacy.
It is important to note that intratumoral heterogeneity is mediated by genetic diversity. Genetically homogeneous subclones can exhibit functionally distinct behaviors following exposure to various treatments, influenced by different patterns of proliferation, with dormant resting cells surviving cytotoxic exposure [141]. Phenotypic heterogeneity is not determined solely by genetic differences between subclones, but also by stochastic events in gene expression and protein stability, epigenetic divergence, and microenvironmental fluctuations [141–144]. Indeed, a study examining the fate of daughter cells derived from the same mother cell after antimitotic drug exposure found that the fates of the sister cells appeared to be independent of one another [145].
Observations of intratumoral heterogeneity present challenges to various therapies and raise important questions about future drug development strategies, which may benefit from considering whether clonally dominant events could be more effective targets than subclonal lesions [146]. Abundant evidence indicates that increased intratumoral heterogeneity adversely affects clinical responses to various treatments, such as targeted therapy, chemotherapy, and immunotherapy. This suggests that intratumoral heterogeneity could be a target for the development of combination treatment regimens [138]. During treatment, the complexity of drug-resistance-related gene mutations, which is due to intratumoral heterogeneity, becomes more pronounced. For instance, the ALK gene resistance mutation sites vary among different patients, including G1202R, G1269A, L1196M, I1171T, and F1174C [147]; Or the co-mutation of multiple drug resistance sites in the same patient, such as G1269A, F1174L, E1154Q and E1210K [148]; And the co-mutation of ALK and EGFR-L858R and other phenomena [149]. Based on this, combined anti-tumor strategies that target multiple signaling pathways may reduce the occurrence of tumor resistance.
Intratumoral heterogeneity plays a role in tumor evolution, immune escape, and other aspects, which are closely related to tumor progression and treatment resistance. It can be utilized to guide clinical diagnostic criteria and therapeutic methods, and is expected to facilitate individualized immunotherapy, which has significant clinical transformation potential. In the future, on the one hand, the phenotype and function of immune cell subsets should be further subdivided according to various cell subtypes within the microenvironment. From the perspective of spatial and temporal heterogeneity, we should uncover the biological significance and related clinical characteristics of the co-localization of various immune cell types at a specific time and location. This involves identifying key immune parameters. The development of a biomarker network model based on the microenvironment is anticipated to enhance clinical prognosis and efficacy prediction. Regarding personalized treatment, studies indicate that the objective remission rate for monotherapy in Phase III clinical trials is less than 20.0% (15.0% in Checkmate459 [86] and 18.3% in KeyMAT-240 [87]). However, the combination of anti-angiogenesis and immunotherapy [7, 13] has significantly improved therapeutic outcomes. This clinical phenomenon suggests that administering immunotherapy to target immune cells, along with adjusting other elements in the microenvironment, can achieve immunotherapy sensitization and further benefit patients.
All these data suggest that the complexity of the tumor driving forces behind intratumoral heterogeneity, the contribution of intratumoral heterogeneity to drug resistance, the mechanisms of intratumoral heterogeneity, and, finally, understanding intratumoral heterogeneity can provide a rational therapeutic strategy for tumor patients, particularly those with identified oncogenes.
Methodological approach to literature selection and synthesis
To ensure a comprehensive and balanced review, we adopted a structured approach to select and synthesize relevant studies. First, we conducted a literature search using PubMed, Web of Science, and Google Scholar, focusing on articles published between 2010 and 2023. The search terms included “intratumoral heterogeneity,” “single-cell analysis,” and “machine learning in cancer.” We included studies that provided novel insights into the role of intratumoral heterogeneity in cancer progression and therapy resistance. Studies were excluded if they lacked experimental validation or focused solely on non-cancerous tissues. After initial screening, we synthesized the findings thematically, emphasizing recent advancements and emerging trends in the field. While this review is narrative in nature, we aimed to maintain transparency by clearly outlining our search strategy and inclusion criteria.
Acknowledgements
We would like to thank Professor Kenneth K. W. To, from School of Pharmacy, The Chinese University of Hong Kong, for editing the manuscript.
Author contributions
Shaobo Liang conceptualization; Yuechun Fu writing original draft, tables and figures preparation; Xueping Wang and Shaobo Liang revised the manuscript; all authors have read and approved the article.
Funding
This work was supported by the Foundation of Guangdong Esophageal Cancer Institute (Q202102), Guangdong Medical Science and Technology Research Fund project (A2023407), Guangdong Provincial Natural Science Foundation (2024A1515011350).
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent to publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yue-Chun Fu and Shao-Bo Liang have contributed equally to this work.
Contributor Information
Min Luo, Email: luomin2@sysucc.org.cn.
Xue-Ping Wang, Email: wangxuep@sysucc.org.cn.
References
- 1.Dagogo-Jack I, Shaw AT. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol. 2018;15(2):81–94. 10.1038/nrclinonc.2017.166. [DOI] [PubMed] [Google Scholar]
- 2.Wang J, Cazzato E, Ladewig E, Frattini V, Rosenbloom DI, Zairis S, et al. Clonal evolution of glioblastoma under therapy. Nat Genet. 2016;48(7):768–76. 10.1038/ng.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin DC, Mayakonda A, Dinh HQ, Huang P, Lin L, Liu X, et al. Genomic and epigenomic heterogeneity of hepatocellular carcinoma. Cancer Res. 2017;77(9):2255–65. 10.1158/0008-5472.CAN-16-2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raynaud F, Mina M, Tavernari D, Ciriello G. Pan-cancer inference of intra-intratumoral heterogeneity reveals associations with different forms of genomic instability. Plos Genet. 2018;14(9): e1007669. 10.1371/journal.pgen.1007669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vitale I, Shema E, Loi S, Galluzzi L. Intratumoral heterogeneity in cancer progression and response to immunotherapy. Nat Med. 2021;27(2):212–24. 10.1038/s41591-021-01233-9. [DOI] [PubMed] [Google Scholar]
- 6.Gerlinger M, Rowan AJ, Horswell S, Math M, Larkin J, Endesfelder D, et al. Intraintratumoral heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366(10):883–92. 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gregorc V, Lazzari C, Mandala M, Ippati S, Bulotta A, Cangi MG, et al. Intratumoral cellular heterogeneity: implications for drug resistance in patients with non-small cell lung cancer. Cancers (Basel). 2021. 10.3390/cancers13092023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chao S, Zhang F, Yan H, Wang L, Zhang L, Wang Z, et al. Targeting intraintratumoral heterogeneity suppresses colorectal cancer chemoresistance and metastasis. Embo Rep. 2023;24(8):e56416. 10.15252/embr.202256416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tabata M, Sato Y, Kogure Y, McClure MB, Oshikawa-Kumade Y, Saito Y, et al. Inter- and intra-intratumoral heterogeneity of genetic and immune profiles in inherited renal cell carcinoma. Cell Rep. 2023;42(7): 112736. 10.1016/j.celrep.2023.112736. [DOI] [PubMed] [Google Scholar]
- 10.Zhao B, Hemann MT, Lauffenburger DA. Intraintratumoral heterogeneity alters most effective drugs in designed combinations. Proc Natl Acad Sci USA. 2014;111(29):10773–8. 10.1073/pnas.1323934111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andor N, Maley CC, Ji HP. Genomic instability in cancer: teetering on the limit of tolerance. Cancer Res. 2017;77(9):2179–85. 10.1158/0008-5472.CAN-16-1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim ZF, Ma PC. Emerging insights of intratumoral heterogeneity and drug resistance mechanisms in lung cancer targeted therapy. J Hematol Oncol. 2019;12(1):134. 10.1186/s13045-019-0818-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goto T, Hirotsu Y, Amemiya K, Mochizuki H, Omata M. Understanding intraintratumoral heterogeneity and evolution in NSCLC and potential new therapeutic approach. Cancers (Basel). 2018. 10.3390/cancers10070212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Passaro A, Malapelle U, Del RM, Attili I, Russo A, Guerini-Rocco E, et al. Understanding EGFR heterogeneity in lung cancer. Esmo Open. 2020;5(5): e919. 10.1136/esmoopen-2020-000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghaderi N, Jung JH, Odde DJ, Peacock J. Clinically validated model predicts the effect of intratumoral heterogeneity on overall survival for non-small cell lung cancer (NSCLC) patients. Comput Methods Programs Biomed. 2021;212: 106455. 10.1016/j.cmpb.2021.106455. [DOI] [PubMed] [Google Scholar]
- 16.Almendro V, Kim HJ, Cheng Y, Gönen M, Itzkovitz S, Argani P, et al. Genetic and phenotypic diversity in breast tumor metastases. Cancer Res. 2014;74(5):1338–48. 10.1158/0008-5472.CAN-13-2357-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brastianos PK, Carter SL, Santagata S, Cahill DP, Taylor-Weiner A, Jones RT, et al. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov. 2015;5(11):1164–77. 10.1158/2159-8290.CD-15-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Chang L, Yang Y, Fang W, Guan Y, Wu A, et al. Intraintratumoral heterogeneity comparison among different subtypes of non-small-cell lung cancer through multi-region tissue and matched ctDNA sequencing. Mol Cancer. 2019;18(1):7. 10.1186/s12943-019-0939-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soucheray M, Capelletti M, Pulido I, Kuang Y, Paweletz CP, Becker JH, et al. Intratumoral heterogeneity in EGFR-Mutant NSCLC results in divergent resistance mechanisms in response to EGFR tyrosine kinase inhibition. Cancer Res. 2015;75(20):4372–83. 10.1158/0008-5472.CAN-15-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linnoila RI, Zhao B, DeMayo JL, Nelkin BD, Baylin SB, DeMayo FJ, et al. Constitutive achaete-scute homologue-1 promotes airway dysplasia and lung neuroendocrine tumors in transgenic mice. Cancer Res. 2000;60(15):4005–9. [PubMed] [Google Scholar]
- 21.Jia Q, Wu W, Wang Y, Alexander PB, Sun C, Gong Z, et al. Local mutational diversity drives intratumoral immune heterogeneity in non-small cell lung cancer. Nat Commun. 2018;9(1):5361. 10.1038/s41467-018-07767-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ben DS, Aizic A, Sabo E, Hershkovitz D. Spatial heterogeneity of PD-L1 expression and the risk for misclassification of PD-L1 immunohistochemistry in non-small cell lung cancer. Lung Cancer. 2020;147:91–8. 10.1016/j.lungcan.2020.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Naso JR, Wang G, Pender A, Wong SK, Zhu J, Ho C, et al. Intratumoral heterogeneity in programmed death-ligand 1 immunoreactivity is associated with variation in non-small cell lung carcinoma histotype. Histopathology. 2020;76(3):394–403. 10.1111/his.13983. [DOI] [PubMed] [Google Scholar]
- 24.Moutafi MK, Tao W, Huang R, Haberberger J, Alexander B, Ramkissoon S, et al. Comparison of programmed death-ligand 1 protein expression between primary and metastatic lesions in patients with lung cancer. J Immunother Cancer. 2021. 10.1136/jitc-2020-002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naxerova K, Reiter JG, Brachtel E, Lennerz JK, van de Wetering M, Rowan A, et al. Origins of lymphatic and distant metastases in human colorectal cancer. Science. 2017;357(6346):55–60. 10.1126/science.aai8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salemme V, Centonze G, Avalle L, Natalini D, Piccolantonio A, Arina P, et al. The role of tumor microenvironment in drug resistance: emerging technologies to unravel breast cancer heterogeneity. Front Oncol. 2023;13:1170264. 10.3389/fonc.2023.1170264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beksac AT, Paulucci DJ, Blum KA, Yadav SS, Sfakianos JP, Badani KK. Heterogeneity in renal cell carcinoma. Urol Oncol. 2017;35(8):507–15. 10.1016/j.urolonc.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Murugaesu N, Wilson GA, Birkbak NJ, Watkins T, McGranahan N, Kumar S, et al. Tracking the genomic evolution of esophageal adenocarcinoma through neoadjuvant chemotherapy. Cancer Discov. 2015;5(8):821–31. 10.1158/2159-8290.CD-15-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Findlay JM, Castro-Giner F, Makino S, Rayner E, Kartsonaki C, Cross W, et al. Differential clonal evolution in oesophageal cancers in response to neo-adjuvant chemotherapy. Nat Commun. 2016;7:11111. 10.1038/ncomms11111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Bruin EC, McGranahan N, Mitter R, Salm M, Wedge DC, Yates L, et al. Spatial and temporal diversity in genomic instability processes defines lung cancer evolution. Science. 2014;346(6206):251–6. 10.1126/science.1253462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Voigt W, Manegold C, Pilz L, Wu YL, Mullauer L, Pirker R, et al. Beyond tissue biopsy: a diagnostic framework to address intratumoral heterogeneity in lung cancer. Curr Opin Oncol. 2020;32(1):68–77. 10.1097/CCO.0000000000000598. [DOI] [PubMed] [Google Scholar]
- 32.Guibert N, Delaunay M, Lusque A, Boubekeur N, Rouquette I, Clermont E, et al. PD-L1 expression in circulating tumor cells of advanced non-small cell lung cancer patients treated with nivolumab. Lung Cancer. 2018;120:108–12. 10.1016/j.lungcan.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Osorio JC, Arbour KC, Le DT, Durham JN, Plodkowski AJ, Halpenny DF, et al. Lesion-level response dynamics to programmed cell death protein (PD-1) blockade. J Clin Oncol. 2019;37(36):3546–55. 10.1200/JCO.19.00709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100(9):672–9. 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 35.Raha D, Wilson TR, Peng J, Peterson D, Yue P, Evangelista M, et al. The cancer stem cell marker aldehyde dehydrogenase is required to maintain a drug-tolerant tumor cell subpopulation. Cancer Res. 2014;74(13):3579–90. 10.1158/0008-5472.CAN-13-3456. [DOI] [PubMed] [Google Scholar]
- 36.Bivona TG, Doebele RC. A framework for understanding and targeting residual disease in oncogene-driven solid cancers. Nat Med. 2016;22(5):472–8. 10.1038/nm.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LJ, Kinzler KW. Cancer genome landscapes. Science. 2013;339(6127):1546–58. 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weigelt B, Geyer FC, Reis-Filho JS. Histological types of breast cancer: how special are they? Mol Oncol. 2010;4(3):192–208. 10.1016/j.molonc.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499(7457):214–8. 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lynch M. Rate, molecular spectrum, and consequences of human mutation. Proc Natl Acad Sci USA. 2010;107(3):961–8. 10.1073/pnas.0912629107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thompson SL, Compton DA. Proliferation of aneuploid human cells is limited by a p53-dependent mechanism. J Cell Biol. 2010;188(3):369–81. 10.1083/jcb.200905057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thompson SL, Compton DA. Examining the link between chromosomal instability and aneuploidy in human cells. J Cell Biol. 2008;180(4):665–72. 10.1083/jcb.200712029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Torres EM, Williams BR, Amon A. Aneuploidy: cells losing their balance. Genetics. 2008;179(2):737–46. 10.1534/genetics.108.090878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gordon DJ, Resio B, Pellman D. Causes and consequences of aneuploidy in cancer. Nat Rev Genet. 2012;13(3):189–203. 10.1038/nrg3123. [DOI] [PubMed] [Google Scholar]
- 45.McGranahan N, Swanton C. Biological and therapeutic impact of intraintratumoral heterogeneity in cancer evolution. Cancer Cell. 2015;27(1):15–26. 10.1016/j.ccell.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 46.Almendro V, Marusyk A, Polyak K. Cellular heterogeneity and molecular evolution in cancer. Annu Rev Pathol. 2013;8:277–302. 10.1146/annurev-pathol-020712-163923. [DOI] [PubMed] [Google Scholar]
- 47.Andor N, Graham TA, Jansen M, Xia LC, Aktipis CA, Petritsch C, et al. Pan-cancer analysis of the extent and consequences of intraintratumoral heterogeneity. Nat Med. 2016;22(1):105–13. 10.1038/nm.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morris LG, Riaz N, Desrichard A, Senbabaoglu Y, Hakimi AA, Makarov V, et al. Pan-cancer analysis of intraintratumoral heterogeneity as a prognostic determinant of survival. Oncotarget. 2016;7(9):10051–63. 10.18632/oncotarget.7067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peng J, Sun BF, Chen CY, Zhou JY, Chen YS, Chen H, et al. Single-cell RNA-seq highlights intra-tumoral heterogeneity and malignant progression in pancreatic ductal adenocarcinoma. Cell Res. 2019;29(9):725–38. 10.1038/s41422-019-0195-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cunnea P, Curry EW, Christie EL, Nixon K, Kwok CH, Pandey A, et al. Spatial and temporal intra-tumoral heterogeneity in advanced HGSOC: implications for surgical and clinical outcomes. Cell Rep Med. 2023;4(6): 101055. 10.1016/j.xcrm.2023.101055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng Y, Carrillo-Perez F, Pizurica M, Heiland DH, Gevaert O. Spatial cellular architecture predicts prognosis in glioblastoma. Nat Commun. 2023;14(1):4122. 10.1038/s41467-023-39933-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wan Z, Wang Y, Li A, Li C, Zheng D. Single-cell transcription analysis reveals the tumor origin and heterogeneity of human bilateral renal clear cell carcinoma. Open Life Sci. 2023;18(1):20220569. 10.1515/biol-2022-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carter SL, Eklund AC, Kohane IS, Harris LN, Szallasi Z. A signature of chromosomal instability inferred from gene expression profiles predicts clinical outcome in multiple human cancers. Nat Genet. 2006;38(9):1043–8. 10.1038/ng1861. [DOI] [PubMed] [Google Scholar]
- 54.Errico A. Lung cancer: heterogeneity in space and time. Nat Rev Clin Oncol. 2014;11(12):684. 10.1038/nrclinonc.2014.186. [DOI] [PubMed] [Google Scholar]
- 55.Alidousty C, Baar T, Martelotto LG, Heydt C, Wagener S, Fassunke J, et al. Genetic instability and recurrent MYC amplification in ALK-translocated NSCLC: a central role of TP53 mutations. J Pathol. 2018;246(1):67–76. 10.1002/path.5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakamura H, Saji H, Idiris A, Kawasaki N, Hosaka M, Ogata A, et al. Chromosomal instability detected by fluorescence in situ hybridization in surgical specimens of non-small cell lung cancer is associated with poor survival. Clin Cancer Res. 2003;9(6):2294–9. [PubMed] [Google Scholar]
- 57.Dai J, Jiang M, He K, Wang H, Chen P, Guo H, et al. DNA damage response and repair gene alterations increase tumor mutational burden and promote poor prognosis of advanced lung cancer. Front Oncol. 2021;11: 708294. 10.3389/fonc.2021.708294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ling X, Han Y, Meng J, Zhong B, Chen J, Zhang H, et al. Small extrachromosomal circular DNA (eccDNA): major functions in evolution and cancer. Mol Cancer. 2021;20(1):113. 10.1186/s12943-021-01413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kang J, Dai Y, Li J, Fan H, Zhao Z. Investigating cellular heterogeneity at the single-cell level by the flexible and mobile extrachromosomal circular DNA. Comput Struct Biotechnol J. 2023;21:1115–21. 10.1016/j.csbj.2023.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang T, Zhang H, Zhou Y, Shi J. Extrachromosomal circular DNA: a new potential role in cancer progression. J Transl Med. 2021;19(1):257. 10.1186/s12967-021-02927-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tandon I, Pal R, Pal JK, Sharma NK. Extrachromosomal circular DNAs: an extra piece of evidence to depict tumor heterogeneity. Future Sci OA. 2019;5(6):FSO390. 10.2144/fsoa-2019-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liao Z, Jiang W, Ye L, Li T, Yu X, Liu L. Classification of extrachromosomal circular DNA with a focus on the role of extrachromosomal DNA (ecDNA) in intratumoral heterogeneity and progression. Biochim Biophys Acta Rev Cancer. 2020;1874(1): 188392. 10.1016/j.bbcan.2020.188392. [DOI] [PubMed] [Google Scholar]
- 63.Kumar P, Kiran S, Saha S, Su Z, Paulsen T, Chatrath A, et al. ATAC-seq identifies thousands of extrachromosomal circular DNA in cancer and cell lines. Sci Adv. 2020;6(20):eaba2489. 10.1126/sciadv.aba2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Flavahan WA, Gaskell E, Bernstein BE. Epigenetic plasticity and the hallmarks of cancer. Science. 2017. 10.1126/science.aal2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dupont C, Armant DR, Brenner CA. Epigenetics: definition, mechanisms and clinical perspective. Semin Reprod Med. 2009;27(5):351–7. 10.1055/s-0029-1237423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Teixeira VH, Pipinikas CP, Pennycuick A, Lee-Six H, Chandrasekharan D, Beane J, et al. Deciphering the genomic, epigenomic, and transcriptomic landscapes of pre-invasive lung cancer lesions. Nat Med. 2019;25(3):517–25. 10.1038/s41591-018-0323-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Patten DK, Corleone G, Gyorffy B, Perone Y, Slaven N, Barozzi I, et al. Enhancer mapping uncovers phenotypic heterogeneity and evolution in patients with luminal breast cancer. Nat Med. 2018;24(9):1469–80. 10.1038/s41591-018-0091-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brocks D, Assenov Y, Minner S, Bogatyrova O, Simon R, Koop C, et al. Intratumor DNA methylation heterogeneity reflects clonal evolution in aggressive prostate cancer. Cell Rep. 2014;8(3):798–806. 10.1016/j.celrep.2014.06.053. [DOI] [PubMed] [Google Scholar]
- 69.Granja JM, Klemm S, McGinnis LM, Kathiria AS, Mezger A, Corces MR, et al. Single-cell multiomic analysis identifies regulatory programs in mixed-phenotype acute leukemia. Nat Biotechnol. 2019;37(12):1458–65. 10.1038/s41587-019-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sreepadmanabh M, Toley BJ. Investigations into the cancer stem cell niche using in-vitro 3-D tumor models and microfluidics. Biotechnol Adv. 2018;36(4):1094–110. 10.1016/j.biotechadv.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 71.Visvader JE. Cells of origin in cancer. Nature. 2011;469(7330):314–22. 10.1038/nature09781. [DOI] [PubMed] [Google Scholar]
- 72.Burrell RA, McGranahan N, Bartek J, Swanton C. The causes and consequences of genetic heterogeneity in cancer evolution. Nature. 2013;501(7467):338–45. 10.1038/nature12625. [DOI] [PubMed] [Google Scholar]
- 73.Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481(7381):306–13. 10.1038/nature10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Notta F, Mullighan CG, Wang JC, Poeppl A, Doulatov S, Phillips LA, et al. Evolution of human BCR-ABL1 lymphoblastic leukaemia-initiating cells. Nature. 2011;469(7330):362–7. 10.1038/nature09733. [DOI] [PubMed] [Google Scholar]
- 75.Denison TA, Bae YH. intratumoral heterogeneity and its implication for drug delivery. J Control Release. 2012;164(2):187–91. 10.1016/j.jconrel.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hjelmeland AB, Wu Q, Heddleston JM, Choudhary GS, MacSwords J, Lathia JD, et al. Acidic stress promotes a glioma stem cell phenotype. Cell Death Differ. 2011;18(5):829–40. 10.1038/cdd.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chow KH, Shin DM, Jenkins MH, Miller EE, Shih DJ, Choi S, et al. Epigenetic states of cells of origin and tumor evolution drive tumor-initiating cell phenotype and intratumoral heterogeneity. Cancer Res. 2014;74(17):4864–74. 10.1158/0008-5472.CAN-13-3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13(10):714–26. 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 79.Xing X, Yang F, Huang Q, Guo H, Li J, Qiu M, et al. Decoding the multicellular ecosystem of lung adenocarcinoma manifested as pulmonary subsolid nodules by single-cell RNA sequencing. Sci Adv. 2021. 10.1126/sciadv.abd9738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zeng T, Wang DC, Wang X, Xu F, Chen L. Prediction of dynamical drug sensitivity and resistance by module network rewiring-analysis based on transcriptional profiling. Drug Resist Updat. 2014;17(3):64–76. 10.1016/j.drup.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 81.Azizi E, Carr AJ, Plitas G, Cornish AE, Konopacki C, Prabhakaran S, et al. Single-cell map of diverse immune phenotypes in the breast tumor microenvironment. Cell. 2018;174(5):1293–308. 10.1016/j.cell.2018.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Costa A, Kieffer Y, Scholer-Dahirel A, Pelon F, Bourachot B, Cardon M, et al. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell. 2018;33(3):463–79. 10.1016/j.ccell.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 83.LeBleu VS, Neilson EG. Origin and functional heterogeneity of fibroblasts. Faseb J. 2020;34(3):3519–36. 10.1096/fj.201903188R. [DOI] [PubMed] [Google Scholar]
- 84.Chevrier S, Levine JH, Zanotelli V, Silina K, Schulz D, Bacac M, et al. An immune atlas of clear cell renal cell carcinoma. Cell. 2017;169(4):736–49. 10.1016/j.cell.2017.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fukumura D, Duda DG, Munn LL, Jain RK. Tumor microvasculature and microenvironment: novel insights through intravital imaging in pre-clinical models. Microcirculation. 2010;17(3):206–25. 10.1111/j.1549-8719.2010.00029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Junttila MR, de Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. 2013;501(7467):346–54. 10.1038/nature12626. [DOI] [PubMed] [Google Scholar]
- 87.Sharma A, Merritt E, Hu X, Cruz A, Jiang C, Sarkodie H, et al. Non-genetic intra-intratumoral heterogeneity is a major predictor of phenotypic heterogeneity and ongoing evolutionary dynamics in lung tumors. Cell Rep. 2019;29(8):2164–74. 10.1016/j.celrep.2019.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zoeller EL, Pedro B, Konen J, Dwivedi B, Rupji M, Sundararaman N, et al. Genetic heterogeneity within collective invasion packs drives leader and follower cell phenotypes. J Cell Sci. 2019. 10.1242/jcs.231514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cairns J. Mutation selection and the natural history of cancer. Nature. 1975;255(5505):197–200. 10.1038/255197a0. [DOI] [PubMed] [Google Scholar]
- 90.Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194(4260):23–8. 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- 91.Campbell PJ, Yachida S, Mudie LJ, Stephens PJ, Pleasance ED, Stebbings LA, et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature. 2010;467(7319):1109–13. 10.1038/nature09460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ding L, Ley TJ, Larson DE, Miller CA, Koboldt DC, Welch JS, et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature. 2012;481(7382):506–10. 10.1038/nature10738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Landau DA, Carter SL, Stojanov P, McKenna A, Stevenson K, Lawrence MS, et al. Evolution and impact of subclonal mutations in chronic lymphocytic leukemia. Cell. 2013;152(4):714–26. 10.1016/j.cell.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Welch JS, Ley TJ, Link DC, Miller CA, Larson DE, Koboldt DC, et al. The origin and evolution of mutations in acute myeloid leukemia. Cell. 2012;150(2):264–78. 10.1016/j.cell.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Anderson K, Lutz C, van Delft FW, Bateman CM, Guo Y, Colman SM, et al. Genetic variegation of clonal architecture and propagating cells in leukaemia. Nature. 2011;469(7330):356–61. 10.1038/nature09650. [DOI] [PubMed] [Google Scholar]
- 96.Shah SP, Roth A, Goya R, Oloumi A, Ha G, Zhao Y, et al. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature. 2012;486(7403):395–9. 10.1038/nature10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Thirlwell C, Will OC, Domingo E, Graham TA, McDonald SA, Oukrif D, et al. Clonality assessment and clonal ordering of individual neoplastic crypts shows polyclonality of colorectal adenomas. Gastroenterology. 2010;138(4):1441–54. 10.1053/j.gastro.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 98.Dianat-Moghadam H, Heidarifard M, Jahanban-Esfahlan R, Panahi Y, Hamishehkar H, Pouremamali F, et al. Cancer stem cells-emanated therapy resistance: implications for liposomal drug delivery systems. J Control Rel. 2018;288:62–83. 10.1016/j.jconrel.2018.08.043. [DOI] [PubMed] [Google Scholar]
- 99.Kapler GM, Beverley SM. Transcriptional mapping of the amplified region encoding the dihydrofolate reductase-thymidylate synthase of Leishmania major reveals a high density of transcripts, including overlapping and antisense RNAs. Mol Cell Biol. 1989;9(9):3959–72. 10.1128/mcb.9.9.3959-3972.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nozawa T, Okada M, Natsumeda M, Eda T, Abe H, Tsukamoto Y, et al. EGFRvIII is expressed in cellular areas of tumor in a subset of glioblastoma. Neurol Med Chir (Tokyo). 2019;59(3):89–97. 10.2176/nmc.oa.2018-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kielbus M, Rola R, Jarosz B, Jeleniewicz W, Cybulski M, Stenzel-Bembenek A, et al. Epidermal growth factor receptor and its oncogenic EGFRvIII variant in benign and malignant brain tumors. Anticancer Res. 2021;41(2):983–91. 10.21873/anticanres.14852. [DOI] [PubMed] [Google Scholar]
- 102.Del VC, Giacomini CP, Vogel H, Jensen KC, Florio T, Merlo A, et al. EGFRvIII gene rearrangement is an early event in glioblastoma tumorigenesis and expression defines a hierarchy modulated by epigenetic mechanisms. Oncogene. 2013;32(21):2670–81. 10.1038/onc.2012.280. [DOI] [PubMed] [Google Scholar]
- 103.Schoenlein PV, Shen DW, Barrett JT, Pastan I, Gottesman MM. Double minute chromosomes carrying the human multidrug resistance 1 and 2 genes are generated from the dimerization of submicroscopic circular DNAs in colchicine-selected KB carcinoma cells. Mol Biol Cell. 1992;3(5):507–20. 10.1091/mbc.3.5.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang A, Miao K, Sun H, Deng CX. intratumoral heterogeneity reshapes the tumor microenvironment to influence drug resistance. Int J Biol Sci. 2022;18(7):3019–33. 10.7150/ijbs.72534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Majewski IJ, Nuciforo P, Mittempergher L, Bosma AJ, Eidtmann H, Holmes E, et al. PIK3CA mutations are associated with decreased benefit to neoadjuvant human epidermal growth factor receptor 2-targeted therapies in breast cancer. J Clin Oncol. 2015;33(12):1334–9. 10.1200/JCO.2014.55.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Huang S, Benavente S, Armstrong EA, Li C, Wheeler DL, Harari PM. p53 modulates acquired resistance to EGFR inhibitors and radiation. Cancer Res. 2011;71(22):7071–9. 10.1158/0008-5472.CAN-11-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rebucci M, Michiels C. Molecular aspects of cancer cell resistance to chemotherapy. Biochem Pharmacol. 2013;85(9):1219–26. 10.1016/j.bcp.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 108.Iesato A, Nucera C. Tumor microenvironment-associated pericyte populations may impact therapeutic response in thyroid cancer. Adv Exp Med Biol. 2021;1329:253–69. 10.1007/978-3-030-73119-9_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Burrell RA, Swanton C. Tumour heterogeneity and the evolution of polyclonal drug resistance. Mol Oncol. 2014;8(6):1095–111. 10.1016/j.molonc.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zheng H, Zhan Y, Liu S, Lu J, Luo J, Feng J, et al. The roles of tumor-derived exosomes in non-small cell lung cancer and their clinical implications. J Exp Clin Cancer Res. 2018;37(1):226. 10.1186/s13046-018-0901-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu J, Ren L, Li S, Li W, Zheng X, Yang Y, et al. The biology, function, and applications of exosomes in cancer. Acta Pharm Sin B. 2021;11(9):2783–97. 10.1016/j.apsb.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang Y, Wang Y, Qin Z, Cai S, Yu L, Hu H, et al. The role of non-coding RNAs in ABC transporters regulation and their clinical implications of multidrug resistance in cancer. Expert Opin Drug Metab Toxicol. 2021;17(3):291–306. 10.1080/17425255.2021.1887139. [DOI] [PubMed] [Google Scholar]
- 113.Reclusa P, Taverna S, Pucci M, Durendez E, Calabuig S, Manca P, et al. Exosomes as diagnostic and predictive biomarkers in lung cancer. J Thorac Dis. 2017;9(Suppl 13):S1373–82. 10.21037/jtd.2017.10.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hyenne V, Apaydin A, Rodriguez D, Spiegelhalter C, Hoff-Yoessle S, Diem M, et al. RAL-1 controls multivesicular body biogenesis and exosome secretion. J Cell Biol. 2015;211(1):27–37. 10.1083/jcb.201504136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hyenne V, Labouesse M, Goetz JG. The small GTPase ral orchestrates MVB biogenesis and exosome secretion. Small Gtpases. 2018;9(6):445–51. 10.1080/21541248.2016.1251378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vietri M, Radulovic M, Stenmark H. The many functions of ESCRTs. Nat Rev Mol Cell Biol. 2020;21(1):25–42. 10.1038/s41580-019-0177-4. [DOI] [PubMed] [Google Scholar]
- 117.Wei D, Zhan W, Gao Y, Huang L, Gong R, Wang W, et al. RAB31 marks and controls an ESCRT-independent exosome pathway. Cell Res. 2021;31(2):157–77. 10.1038/s41422-020-00409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang L, Peng X, Li Y, Zhang X, Ma Y, Wu C, et al. Long non-coding RNA HOTAIR promotes exosome secretion by regulating RAB35 and SNAP23 in hepatocellular carcinoma. Mol Cancer. 2019;18(1):78. 10.1186/s12943-019-0990-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–89. 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 120.Xu K, Zhang C, Du T, Gabriel A, Wang X, Li X, et al. Progress of exosomes in the diagnosis and treatment of lung cancer. Biomed Pharmacother. 2021;134: 111111. 10.1016/j.biopha.2020.111111. [DOI] [PubMed] [Google Scholar]
- 121.Luo G, Zhang Y, Wu Z, Zhang L, Liang C, Chen X. Exosomal LINC00355 derived from cancer-associated fibroblasts promotes bladder cancer cell resistance to cisplatin by regulating miR-34b-5p/ABCB1 axis. Acta Biochim Biophys Sin (Shanghai). 2021;53(5):558–66. 10.1093/abbs/gmab023. [DOI] [PubMed] [Google Scholar]
- 122.Wu S, Luo M, To K, Zhang J, Su C, Zhang H, et al. Intercellular transfer of exosomal wild type EGFR triggers osimertinib resistance in non-small cell lung cancer. Mol Cancer. 2021;20(1):17. 10.1186/s12943-021-01307-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen R, Qian Z, Xu X, Zhang C, Niu Y, Wang Z, et al. Exosomes-transmitted miR-7 reverses gefitinib resistance by targeting YAP in non-small-cell lung cancer. Pharmacol Res. 2021;165: 105442. 10.1016/j.phrs.2021.105442. [DOI] [PubMed] [Google Scholar]
- 124.Poggio M, Hu T, Pai CC, Chu B, Belair CD, Chang A, et al. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell. 2019;177(2):414–27. 10.1016/j.cell.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xie F, Xu M, Lu J, Mao L, Wang S. The role of exosomal PD-L1 in tumor progression and immunotherapy. Mol Cancer. 2019;18(1):146. 10.1186/s12943-019-1074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic MM, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–35. 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Koh HM, Jang BG, Kim DC. Prognostic significance of Rab27 expression in solid cancer: a systematic review and meta-analysis. Sci Rep. 2020;10(1):14136. 10.1038/s41598-020-71104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ostenfeld MS, Jeppesen DK, Laurberg JR, Boysen AT, Bramsen JB, Primdal-Bengtson B, et al. Cellular disposal of miR23b by RAB27-dependent exosome release is linked to acquisition of metastatic properties. Cancer Res. 2014;74(20):5758–71. 10.1158/0008-5472.CAN-13-3512. [DOI] [PubMed] [Google Scholar]
- 129.Ghosh D, Dutta A, Kashyap A, Upmanyu N, Datta S. PLP2 drives collective cell migration via ZO-1-mediated cytoskeletal remodeling at the leading edge in human colorectal cancer cells. J Cell Sci. 2021. 10.1242/jcs.253468. [DOI] [PubMed] [Google Scholar]
- 130.Matsumoto Y, Kano M, Akutsu Y, Hanari N, Hoshino I, Murakami K, et al. Quantification of plasma exosome is a potential prognostic marker for esophageal squamous cell carcinoma. Oncol Rep. 2016;36(5):2535–43. 10.3892/or.2016.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Krishnan SR, Bebawy M. Circulating biosignatures in multiple myeloma and their role in multidrug resistance. Mol Cancer. 2023;22(1):79. 10.1186/s12943-022-01683-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546(7659):498–503. 10.1038/nature22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Catalano M, O’Driscoll L. Inhibiting extracellular vesicles formation and release: a review of EV inhibitors. J Extracell Vesicles. 2020;9(1):1703244. 10.1080/20013078.2019.1703244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Essandoh K, Yang L, Wang X, Huang W, Qin D, Hao J, et al. Blockade of exosome generation with GW4869 dampens the sepsis-induced inflammation and cardiac dysfunction. Biochim Biophys Acta. 2015;1852(11):2362–71. 10.1016/j.bbadis.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cai W, Lin D, Wu C, Li X, Zhao C, Zheng L, et al. Intratumoral heterogeneity of ALK-rearranged and ALK/EGFR coaltered lung adenocarcinoma. J Clin Oncol. 2015;33(32):3701–9. 10.1200/JCO.2014.58.8293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yan T, Cui H, Zhou Y, Yang B, Kong P, Zhang Y, et al. Multi-region sequencing unveils novel actionable targets and spatial heterogeneity in esophageal squamous cell carcinoma. Nat Commun. 2019;10(1):1670. 10.1038/s41467-019-09255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li X, Guo X, Li D, Du X, Yin C, Chen C, et al. Multi-regional sequencing reveals intraintratumoral heterogeneity and positive selection of somatic mtDNA mutations in hepatocellular carcinoma and colorectal cancer. Int J Cancer. 2018;143(5):1143–52. 10.1002/ijc.31395. [DOI] [PubMed] [Google Scholar]
- 138.Wolf Y, Bartok O, Patkar S, Eli GB, Cohen S, Litchfield K, et al. UVB-induced intratumoral heterogeneity diminishes immune response in melanoma. Cell. 2019;179(1):219–35. 10.1016/j.cell.2019.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hamidi H, Senbabaoglu Y, Beig N, Roels J, Manuel C, Guan X, et al. Molecular heterogeneity in urothelial carcinoma and determinants of clinical benefit to PD-L1 blockade. Cancer Cell. 2024;42(12):2098–112. 10.1016/j.ccell.2024.10.016. [DOI] [PubMed] [Google Scholar]
- 140.Zhou QQ, Guo J, Wang Z, Li J, Chen M, Xu Q, et al. Rapid visualization of PD-L1 expression level in glioblastoma immune microenvironment via machine learning cascade-based Raman histopathology. J Adv Res. 2024;65:257–71. 10.1016/j.jare.2023.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kreso A, O’Brien CA, van Galen P, Gan OI, Notta F, Brown AM, et al. Variable clonal repopulation dynamics influence chemotherapy response in colorectal cancer. Science. 2013;339(6119):543–8. 10.1126/science.1227670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Meads MB, Gatenby RA, Dalton WS. Environment-mediated drug resistance: a major contributor to minimal residual disease. Nat Rev Cancer. 2009;9(9):665–74. 10.1038/nrc2714. [DOI] [PubMed] [Google Scholar]
- 143.Varley KE, Mutch DG, Edmonston TB, Goodfellow PJ, Mitra RD. Intra-intratumoral heterogeneity of MLH1 promoter methylation revealed by deep single molecule bisulfite sequencing. Nucl Acids Res. 2009;37(14):4603–12. 10.1093/nar/gkp457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Marusyk A, Almendro V, Polyak K. Intra-tumour heterogeneity: A looking glass for cancer? Nat Rev Cancer. 2012;12(5):323–34. 10.1038/nrc3261. [DOI] [PubMed] [Google Scholar]
- 145.Gascoigne KE, Taylor SS. Cancer cells display profound intra- and interline variation following prolonged exposure to antimitotic drugs. Cancer Cell. 2008;14(2):111–22. 10.1016/j.ccr.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 146.Swanton C. Intraintratumoral heterogeneity: evolution through space and time. Cancer Res. 2012;72(19):4875–82. 10.1158/0008-5472.CAN-12-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Katayama R. Drug resistance in anaplastic lymphoma kinase-rearranged lung cancer. Cancer Sci. 2018;109(3):572–80. 10.1111/cas.13504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kang J, Chen HJ, Zhang XC, Su J, Zhou Q, Tu HY, et al. Heterogeneous responses and resistant mechanisms to crizotinib in ALK-positive advanced non-small cell lung cancer. Thorac Cancer. 2018;9(9):1093–103. 10.1111/1759-7714.12791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kim S, Kim TM, Kim DW, Go H, Keam B, Lee SH, et al. Heterogeneity of genetic changes associated with acquired crizotinib resistance in ALK-rearranged lung cancer. J Thorac Oncol. 2013;8(4):415–22. 10.1097/JTO.0b013e318283dcc0. [DOI] [PubMed] [Google Scholar]
- 150.Tan X, He S, Wang F, Li L, Wang W. Migrasome, a novel organelle, differs from exosomes. Biochem Biophys Rep. 2023;35: 101500. 10.1016/j.bbrep.2023.101500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang H, Freitas D, Kim HS, Fabijanic K, Li Z, Chen H, et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat Cell Biol. 2018;20(3):332–43. 10.1038/s41556-018-0040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hong W, Xue M, Jiang J, Zhang Y, Gao X. Circular RNA circ-CPA4/let-7 miRNA/PD-L1 axis regulates cell growth, stemness, drug resistance and immune evasion in non-small cell lung cancer (NSCLC). J Exp Clin Cancer Res. 2020;39(1):149. 10.1186/s13046-020-01648-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 153.Bica-Pop C, Cojocneanu-Petric R, Magdo L, Raduly L, Gulei D, Berindan-Neagoe I. Overview upon miR-21 in lung cancer: focus on NSCLC. Cell Mol Life Sci. 2018;75(19):3539–51. 10.1007/s00018-018-2877-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wei F, Ma C, Zhou T, Dong X, Luo Q, Geng L, et al. Exosomes derived from gemcitabine-resistant cells transfer malignant phenotypic traits via delivery of miRNA-222-3p. Mol Cancer. 2017;16(1):132. 10.1186/s12943-017-0694-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wu H, Mu X, Liu L, Wu H, Hu X, Chen L, et al. Bone marrow mesenchymal stem cells-derived exosomal microRNA-193a reduces cisplatin resistance of non-small cell lung cancer cells via targeting LRRC1. Cell Death Dis. 2020;11(9):801. 10.1038/s41419-020-02962-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang T, Zhang P, Li HX. CAFs-derived exosomal miRNA-130a confers cisplatin resistance of NSCLC cells through PUM2-dependent packaging. Int J Nanomed. 2021;16:561–77. 10.2147/IJN.S271976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ma Y, Yuwen D, Chen J, Zheng B, Gao J, Fan M, et al. Exosomal transfer of cisplatin-induced miR-425-3p confers cisplatin resistance in NSCLC through activating autophagy. Int J Nanomed. 2019;14:8121–32. 10.2147/IJN.S221383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yuwen DL, Sheng BB, Liu J, Wenyu W, Shu YQ. MiR-146a-5p level in serum exosomes predicts therapeutic effect of cisplatin in non-small cell lung cancer. Eur Rev Med Pharmacol Sci. 2017;21(11):2650–8. [PubMed] [Google Scholar]
- 159.Wang H, Huang H, Wang L, Liu Y, Wang M, Zhao S, et al. Cancer-associated fibroblasts secreted miR-103a-3p suppresses apoptosis and promotes cisplatin resistance in non-small cell lung cancer. Aging (Albany). 2021;13(10):14456–68. 10.18632/aging.103556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Song Z, Jia G, Ma P, Cang S. Exosomal miR-4443 promotes cisplatin resistance in non-small cell lung carcinoma by regulating FSP1 m6A modification-mediated ferroptosis. Life Sci. 2021;276: 119399. 10.1016/j.lfs.2021.119399. [DOI] [PubMed] [Google Scholar]
- 161.Zhao X, Li M, Dai X, Yang Y, Peng Y, Xu C, et al. Downregulation of exosomal miR1273a increases cisplatin resistance of nonsmall cell lung cancer by upregulating the expression of syndecan binding protein. Oncol Rep. 2020;44(5):2165–73. 10.3892/or.2020.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shao N, Song L, Sun X. Exosomal circ_PIP5K1A regulates the progression of non-small cell lung cancer and cisplatin sensitivity by miR-101/ABCC1 axis. Mol Cell Biochem. 2021;476(6):2253–67. 10.1007/s11010-021-04083-8. [DOI] [PubMed] [Google Scholar]
- 163.Yang B, Teng F, Chang L, Wang J, Liu DL, Cui YS, et al. Tumor-derived exosomal circRNA_102481 contributes to EGFR-TKIs resistance via the miR-30a-5p/ROR1 axis in non-small cell lung cancer. Aging (Albany). 2021;13(9):13264–86. 10.18632/aging.203011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zang X, Gu J, Zhang J, Shi H, Hou S, Xu X, et al. Exosome-transmitted lncRNA UFC1 promotes non-small-cell lung cancer progression by EZH2-mediated epigenetic silencing of PTEN expression. Cell Death Dis. 2020;11(4):215. 10.1038/s41419-020-2409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhou D, Xia Z, Xie M, Gao Y, Yu Q, He B. Exosomal long non-coding RNA SOX2 overlapping transcript enhances the resistance to EGFR-TKIs in non-small cell lung cancer cell line H1975. Hum Cell. 2021;34(5):1478–89. 10.1007/s13577-021-00572-6. [DOI] [PubMed] [Google Scholar]
- 166.Zhang Y, Li M, Hu C. Exosomal transfer of miR-214 mediates gefitinib resistance in non-small cell lung cancer. Biochem Biophys Res Commun. 2018;507(1–4):457–64. 10.1016/j.bbrc.2018.11.061. [DOI] [PubMed] [Google Scholar]
- 167.Chen X, Wang Z, Tong F, Dong X, Wu G, Zhang R. lncRNA UCA1 promotes gefitinib resistance as a ceRNA to target FOSL2 by sponging miR-143 in non-small cell lung cancer. Mol Ther Nucl Acids. 2020;19:643–53. 10.1016/j.omtn.2019.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 168.Zhang W, Cai X, Yu J, Lu X, Qian Q, Qian W. Exosome-mediated transfer of lncRNA RP11838N2.4 promotes erlotinib resistance in non-small cell lung cancer. Int J Oncol. 2018;53(2):527–38. 10.3892/ijo.2018.4412. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 169.Pan R, Zhou H. Exosomal transfer of lncRNA H19 promotes erlotinib resistance in non-small cell lung cancer via miR-615-3p/ATG7 axis. Cancer Manag Res. 2020;12:4283–97. 10.2147/CMAR.S241095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Deng Q, Fang Q, Xie B, Sun H, Bao Y, Zhou S. Exosomal long non-coding RNA MSTRG.292666.16 is associated with osimertinib (AZD9291) resistance in non-small cell lung cancer. Aging (Albany). 2020;12(9):8001–15. 10.18632/aging.103119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Li X, Chen C, Wang Z, Liu J, Sun W, Shen K, et al. Elevated exosome-derived miRNAs predict osimertinib resistance in non-small cell lung cancer. Cancer Cell Int. 2021;21(1):428. 10.1186/s12935-021-02075-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hisakane K, Seike M, Sugano T, Yoshikawa A, Matsuda K, Takano N, et al. Exosome-derived miR-210 involved in resistance to osimertinib and epithelial-mesenchymal transition in EGFR mutant non-small cell lung cancer cells. Thorac Cancer. 2021;12(11):1690–8. 10.1111/1759-7714.13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gu G, Hu C, Hui K, Zhang H, Chen T, Zhang X, et al. Exosomal miR-136-5p derived from anlotinib-resistant NSCLC cells confers anlotinib resistance in non-small cell lung cancer through targeting PPP2R2A. Int J Nanomedicine. 2021;16:6329–43. 10.2147/IJN.S321720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Paulsen T, Kumar P, Koseoglu MM, Dutta A. Discoveries of extrachromosomal circles of DNA in normal and tumor cells. Trends Genet. 2018;34(4):270–8. 10.1016/j.tig.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang M, Chen X, Yu F, Ding H, Zhang Y, Wang K. Extrachromosomal circular DNAs: origin, formation and emerging function in cancer. Int J Biol Sci. 2021;17(4):1010–25. 10.7150/ijbs.54614. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.