Abstract
Background
Psoriatic arthritis (PsA) is a multi-domain, inflammatory disease impacting joints, soft tissues, and skin; tumor necrosis factor inhibitors (TNFi) are typically the first biologic following inadequate response (IR) to conventional therapies. Although guidance is lacking on therapy selection after initial TNFi failure, data suggest TNFi-IR PsA patients may benefit from switching to a different mechanism of action (MOA) vs. cycling to another TNFi. Guselkumab is a fully human monoclonal antibody targeting the interleukin-23p19 subunit. Emphasizing practicality and applicability to routine clinical practice, EVOLUTION will pragmatically evaluate whether switching to guselkumab is more effective than cycling to a second TNFi (subcutaneous [SC] golimumab) in TNFi-IR PsA patients.
Methods
The multicenter, longitudinal, prospective, observational Psoriatic Arthritis Research Consortium study guided eligibility criteria, outcome measures, and sample size estimates. Adults seen in clinical practice with active PsA (≥ 1 swollen joint) while receiving TNFi treatment will be eligible. Participants will be randomized (1:1:1) to guselkumab 100 mg every 4 weeks (Q4W); guselkumab 100 mg at Week 0, Week 4, and Q8W; or SC golimumab 50 mg Q4W (no washout period). The novel primary composite endpoint is achievement of clinical Disease Activity in Psoriatic Arthritis (cDAPSA) low disease activity (≤ 13) and an Investigator’s Global Assessment (IGA) of psoriasis score of 0/1 (scale: 0–4) at Month12. Secondary endpoints include cDAPSA + IGA 0/1 at Month 6; achievement of minimal disease activity, resolution of enthesitis and dactylitis (among patients affected at baseline) at Months 6/12; and mean changes at Months 6/12 in the 12-item PsA Impact of Disease, Dermatology Life Quality Index, Patient-Reported Outcomes Measurements Information System fatigue and depression questionnaires, and Bath Ankylosing Spondylitis Disease Activity Index (patients with physician-determined axial disease). The target sample size is 150 participants (50/treatment group); all analyses are considered exploratory.
Discussion
EVOLUTION will employ a pragmatic approach, including a novel primary endpoint relevant to clinical practice, to assess whether switching to an alternate MOA biologic with guselkumab is more effective than cycling to a second TNFi among TNFi-IR PsA patients.
Trial registration
This trial was registered at ClinicalTrials.gov, NCT05669833, on 3 January 2023, https://www.clinicaltrials.gov/study/NCT05669833?term=%20NCT05669833&rank=1
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s13063-025-08777-y.
Keywords: Psoriatic arthritis, TNFi-IR, Randomized controlled trial, Subcutaneous golimumab, Guselkumab, IL-23p19-subunit inhibitor
Background
Psoriatic arthritis (PsA) is a multi-domain, chronic, inflammatory disease impacting the joints, soft tissues, and skin [1]. Owing to its diverse phenotypes and the availability of multiple advanced therapies with distinct mechanisms of action (MOAs) to treat active PsA, a targeted treatment approach is currently recommended to address the various disease domains (peripheral arthritis, axial arthritis, enthesitis, dactylitis, skin, and nail disease) affected in individual patients [2, 3]. In particular, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) recommends that therapy decisions should ideally consider active disease domains, comorbidities, previous therapies, and patient preference [3]. Tumor necrosis factor inhibitors (TNFi) were the first biologics available for patients with PsA, and given the availability of biosimilar TNFi, are typically the first advanced therapy utilized for patients with an inadequate response (IR) to conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) [4, 5]. However, many patients respond inadequately to TNFi, and require alternate therapy. Results from observational studies evaluating outcomes in patients who switched TNFi therapy have been inconsistent, with many suggesting that these patients are less likely to respond to a subsequent TNFi [4, 5]. Patients with PsA who have responded inadequately to a TNFi (TNFi-IR) comprise a difficult-to-treat population who may benefit from therapies utilizing a different MOA.
While several biologic DMARDs with alternative MOAs are now available for patients with PsA (e.g., therapies inhibiting interleukin [IL]-12/23, IL-17, and IL-23), formal evidence-based guidelines regarding therapy selection after nonresponse or loss of response to an initial TNFi are lacking. The efficacy and safety of biologics other than TNFi have been evaluated in TNFi-IR patients with PsA; however, most of these studies have been performed using a placebo control, precluding direct comparison with other biologics in this patient population [3, 6–8]. Preliminary findings from a longitudinal analysis of a real-world database suggest that patients with PsA who discontinue their initial TNFi may be more likely to achieve a clinical response by switching to an agent with a different MOA rather than by cycling to a second TNFi [9]. Prospective randomized controlled trials (RCTs) would further inform treatment decisions for this distinct population of patients with TNFi-IR PsA.
Guselkumab is a fully human monoclonal IL-23p19-subunit inhibitor that is approved for adults with active PsA at a dosing regimen of 100 mg every 8 weeks (Q8W) [10]. The efficacy and safety of guselkumab were evaluated in the phase 3, randomized, placebo-controlled DISCOVER-1 (TNFi-experienced/TNFi-naïve) [6] and DISCOVER-2 (biologic-naïve) [11] studies. In these studies, participants treated with guselkumab every 4 or 8 weeks (Q4W/Q8W) had significantly greater response rates for achieving ≥ 20%/50%/70% improvement in American College of Rheumatology response criteria (ACR20/50/70) and clear or almost clear skin vs placebo at Week 24. Guselkumab-treated participants also had significantly greater improvements in enthesitis and dactylitis than those receiving placebo. The efficacy of guselkumab Q8W in participants with TNFi-IR active PsA was evaluated and confirmed in the phase 3b COSMOS study [12].
To examine the question of cycling to a second TNFi or switching to a new MOA for patients with TNFi-IR PsA, we designed and recently initiated the EVOLUTION trial with application of an innovative, pragmatic, randomized trial approach to assess whether TNFi-IR patients seen in clinical practice will benefit more from switching to guselkumab (either Q4W or Q8W) than cycling to a second TNFi (subcutaneous [SC] golimumab). Both guselkumab and SC golimumab, a fully human monoclonal antibody that binds to both the soluble and transmembrane bioactive forms of human TNFα, are approved by the US Food and Drug Administration (FDA) to treat adults with active PsA [10, 13].
To our knowledge, EVOLUTION is the first randomized trial to compare the efficacy of switching to guselkumab vs cycling to a second TNFi and will address important knowledge gaps in the management of treatment-refractory PsA. Of note, EVOLUTION is distinct from conventional, randomized, placebo-controlled studies by incorporating pragmatic study elements [14] designed to emulate patient experience in clinical practice, including absence of a pre-defined washout period prior to initiation of the trial drug, utilization of a novel primary endpoint, and a trial population more reflective of the overall PsA patient population. This trial will assess achievement of clinical Disease Activity in Psoriatic Arthritis (cDAPSA) low disease activity (LDA; ≤ 13) [15] and an Investigator’s Global Assessment of psoriasis score of 0/1 (IGA 0/1 on 0–4 scale) [16] with direct applicability to routine care (e.g., patient population with prior TNFi exposure and assessments used in clinical practice). Additionally, this trial will assess, among guselkumab-treated patients, whether the more frequent Q4W dosing regimen may offer an incremental benefit in comparison with the Q8W regimen in this difficult-to-treat patient population.
Methods
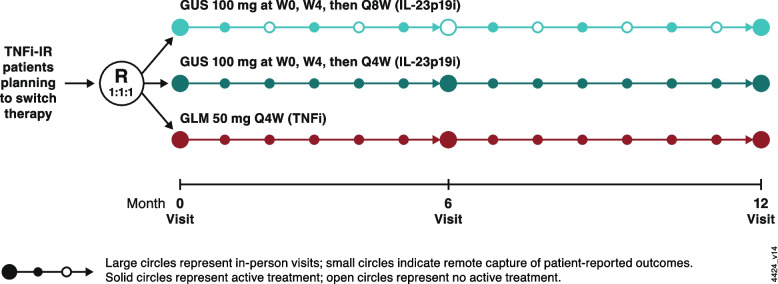
EVOLUTION is a phase 3b, pragmatic, randomized, open-label, multicenter, active-comparator study evaluating the effectiveness and safety of guselkumab 100 mg Q4W and Q8W compared with SC golimumab 50 mg Q4W in TNFi-IR patients with active PsA (Fig. 1). Eligible participants will be recruited at approximately six clinics in the United States. The study protocol follows the Standard Protocol Items Recommendation for Interventional Trials (SPIRIT) reporting guidelines [17] that can be found in Additional file 1: EVOLUTION SPIRIT checklist. Trial registration details can be found here: https://clinicaltrials.gov/study/NCT05669833.
Fig. 1.
EVOLUTION study schema. Large circles represent in-person visits (baseline, 6 months, 12 months) while small circles indicate remote capture of patient-reported outcomes. Solid circles represent active treatment, while open circles represent no active treatment. GLM golimumab, GUS guselkumab, IL-23p19i interleukin-23 p19-subunit inhibitor, Q4W/Q8W every 4/8 weeks, R randomization, TNFi-IR tumor necrosis factor inhibitor-inadequate response, W week
Objectives
The aim of EVOLUTION is to evaluate whether switching to a selective IL-23p19-subunit inhibitor (guselkumab) is more effective than cycling to a second TNFi (SC golimumab) in TNFi-IR patients with active PsA. The primary endpoint utilizes a novel composite measure of achieving both cDAPSA LDA [18] and an IGA 0/1 [16] at Month 12. At Months 6 and 12, improvements in signs and symptoms of PsA will also be assessed using additional measures of extent and severity of skin disease, enthesitis and dactylitis, and health-related quality of life (HRQoL) as described in Table 1. In addition, EVOLUTION will evaluate the relative efficacy of more frequent dosing with guselkumab 100 mg Q4W versus Q8W in TNF-IR patients with PsA. Safety will be assessed through the frequency and types of adverse events (AEs) and serious AEs (SAEs). An overview of objectives and endpoints is provided in Table 1.
Table 1.
Study objectives and endpoints
| Objectives | Endpoints |
|---|---|
| Efficacy: Compare the efficacy of guselkumab and SC golimumab in TNFi-IR patients with active PsA |
Primary Endpoint Proportion of patients achieving cDAPSA LDA (≤ 13) and IGA 0/1 at Month 12 Selected Secondary Endpoints • Month 6, proportion of patients achieving cDAPSA LDA and IGA 0/1 • Months 6 and 12, proportion of patients achieving: ◦ IGA 0/1a ◦ PSAID-12 < 4b ◦ MDA ◦ Resolution of dactylitisc ◦ Resolution of enthesitisc • Months 6 and 12, mean change from baseline in: ◦ PSAID-12 ◦ PGA ◦ DLQI ◦ PROMIS-fatigue score ◦ PROMIS-depression score ◦ BASDAId |
| Safety: Evaluate the safety of guselkumab in TNFi-IR patients with active PsA | Descriptive summary of AEs through Week 60 |
AE adverse event, BASDAI Bath Ankylosing Spondylitis Disease Activity Index, BSA body surface area, cDAPSA clinical Disease Activity Index for Psoriatic Arthritis, DLQI Dermatology Life Quality Index, IGA Investigator’s Global Assessment of psoriasis, IR inadequate responders, LDA low disease activity, MDA minimal disease activity, PGA Physician Global Assessment, PROMIS Patient Reported Outcomes Measurement Information System, PsA psoriatic arthritis, PSAID-12 Psoriatic Arthritis Impact of Disease 12-item questionnaire, SC subcutaneous, TNFi tumor necrosis factor inhibitor
a In addition to the primary endpoint, IGA 0/1 will be assessed among patients with BSA > 3% at baseline and among patients with IGA of ≥ 2 at baseline.
b Among patients with PSAID-12 ≥ 4 at baseline
c Among patients affected at baseline
dAmong patients with axial disease at baseline as defined by the treating physician
Study population
The target study population for EVOLUTION is patients who have active PsA after receiving therapy with a single TNFi and are considering switching to a new biologic therapy. Inclusion criteria for EVOLUTION were informed by findings from the longitudinal, prospective, observational cohort study, Psoriatic Arthritis Research Consortium (PARC) [19], conducted at four academic medical centers with expertise in PsA and PsA clinics (University of Pennsylvania, Cleveland Clinic, New York University, and University of Utah). Demographic and disease characteristics of patients with PsA enrolled in PARC who had previously received a biologic are shown in Table 2.
Table 2.
Baseline characteristics of biologic-experienced patients in the PARC cohort by eligibility status for EVOLUTION
| Characteristic | PARC Cohort (N = 215) | ||
|---|---|---|---|
| EVOLUTION-Eligible | EVOLUTION-Ineligible | Total | |
| Demographics | |||
| Patients, n (%) | 113 (53) | 102 (47) | 215 (100) |
| Age, years | 50.1 (13.8) | 50.8 (15.1) | 50.4 (14.4) |
| Female, n (%) | 65 (58) | 55 (54) | 120 (56) |
| White, n (%) | 87 (77) | 87 (85) | 174 (81) |
| Disease Activity | |||
| cDAPSA (0–154) | 29.0 (14.2) | 10.0 (6.3) | 20.0 (15.0) |
| cDAPSA LDA (≤ 13), n (%) | 6 (5) | 85 (83) | 91 (42) |
| % BSA (0–100) | 2.8 (7.8) | 1.1 (2.7) | 2.0 (6.0) |
| BSA ≤ 1%, n (%) | 70 (62) | 81 (79) | 151 (70) |
| MDA, n/N (%) | 1/113 (1) | 43/101 (43) | 44/214 (21) |
| PtGA | 5.6 (2.1) | 3.1 (2.0) | 4.4 (2.4) |
| PSAID-12 | 5.1 (1.8) | 2.6 (1.7) | 3.9 (2.1) |
| SJC | 6.0 (5.7) | 0.8 (1.0) | 3.5 (4.9) |
| TJC | 11.0 (8.9) | 2.4 (4.0) | 6.9 (8.2) |
Data presented as mean (standard deviation) unless otherwise noted
BSA body surface area affected by psoriasis, cDAPSA clinical Disease Activity in Psoriatic Arthritis, LDA low disease activity, MDA minimal disease activity, PARC, Psoriatic Arthritis Research Consortium, PSAID-12 Psoriatic Arthritis Impact of Disease 12-item questionnaire, PtGA patient global assessment of disease activity, SJC swollen joint count, TJC tender joint count
EVOLUTION participants are required to have a diagnosis of PsA and meet the ClASsification criteria for Psoriatic ARthritis (CASPAR). The study population will be limited to adults aged 18–80 years; patients older than 80 years may be more likely to have comorbid osteoarthritis which may confound assessments of PsA symptoms. Eligible participants must have active PsA, defined as ≥ 1 swollen joint (66-joint count). All participants must have a cDAPSA ≥ 10; those with psoriasis may have a cDAPSA score of 10–14 if they also have an IGA of ≥ 2, and those without psoriasis must have a cDAPSA > 14. In post hoc analyses from the DISCOVER-1 and -2 studies, participants with investigator-identified imaging-confirmed spondylitis had greater improvements in axial symptoms than did those receiving placebo [20, 21]. Thus, in the current study, participants identified by the investigator as having axial involvement are permitted to enroll, with the exception of those without evidence of peripheral joint disease, which is uncommon [22].
Concomitant use of oral glucocorticoids (≤ 10 mg daily), nonsteroidal anti-inflammatory drugs [NSAIDs], topical medications for psoriasis, and up two oral small molecule therapies (csDMARDs [i.e., methotrexate, leflunomide, hydroxychloroquine, sulfasalazine] or apremilast) is permitted at a stable dose (≥ 4 weeks prior to study entry and throughout study participation). Concomitant medications will be used at the discretion of the treating physician and will not be provided by the study. Prior exposure to a tyrosine kinase 2 inhibitor is permitted, but this treatment cannot be used concomitantly during the study.
Exclusion criteria include prior exposure to golimumab, any biologic DMARD other than a TNFi (i.e., IL-12/23i, IL-17i, or IL-23i), or a Janus kinase inhibitor; an AE that precludes use of another TNFi (e.g., development of drug-induced SLE, allergic reaction, serious infection, heart failure symptoms, demyelination at any point during use of therapy); or any other contraindication or substantial intolerance to a TNFi. Patients will also be excluded if they are already receiving an oral glucocorticoid at a dosage > 10 mg/day, discontinue their prior TNFi for reasons of safety, tolerability, or non-efficacy related reasons (e.g., cost or copay burden), or are currently pregnant or actively trying to conceive. Participants also are not eligible if they already meet both primary endpoint criteria at screening or baseline (cDAPSA ≤ 13 and IGA 0/1). Key inclusion and exclusion criteria are provided in Table 3.
Table 3.
Key inclusion and exclusion criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
• Age 18 – 80 years • Diagnosis of PsA and meet CASPAR criteria • Active PsA: • ≥ 1 swollen joint • cDAPSA ≥ 10a • Currently receiving or previously received a TNFi and experienced inadequate response (TNFi-IR; primary or secondaryb) and planning to switch to a new biologic therapy |
• Prior exposure to golimumab (IV or SC), biologics other than TNFi (IL-12/23i, IL-17i, or IL-23i), or JAKi • An AE that precludes use of another TNFi (development of drug-induced SLE, allergic reaction, serious infection, heart failure symptoms, demyelination at any point during use of therapy) or any other contraindication or substantial intolerance to a TNFi • Receiving oral glucocorticoids > 10 mg per day • Already meet the primary endpoint criteria at screening or baseline (cDAPSA LDA and IGA 0/1)a |
AE adverse event, CASPAR Classification for Psoriatic Arthritis, cDAPSA clinical Disease Activity Index for Psoriatic Arthritis, IGA Investigators Global Assessment, IL-12i interleukin-12 inhibitor, IL-17i interleukin-17 inhibitor, IL23i interleukin-23 inhibitor, IR inadequate responders, IV intravenous, JAKi Janus kinase inhibitor, LDA low disease activity, PsA psoriatic arthritis, SC subcutaneous, SLE systemic lupus erythematosus, TNFi tumor necrosis factor inhibitor
aParticipants with psoriasis may have a cDAPSA score of 10–14 if their IGA is ≥ 2; participants without psoriasis must have a cDAPSA score > 14
bPrimary nonresponse defined as lack of response; secondary nonresponse defined as loss of response
A pragmatic approach to assessing effectiveness of therapy will involve recruiting a patient population that is representative of those seen in clinical practice, who will be enrolled in the study at the time when they switch treatment rather than first requiring a washout of their initial treatment. Recruitment methods will include referral networks, signage in waiting rooms, and other advertising efforts. Patient screening will be conducted by the study site treating physician.
Research ethics
EVOLUTION will be conducted in accordance with the principles outlined by the Declaration of Helsinki and current International Conference on Harmonisation Good Clinical Practice guidelines, in addition to applicable regulatory requirements. The protocol and any modifications will be reviewed and approved by an institutional review board (IRB) for each site. The University of Pennsylvania IRB will serve as a central IRB for some sites, while other sites will utilize a local IRB. Treating physicians at each study site will collect written informed consent from all participants prior to their participation in any study-related procedures.
Patient and public involvement
Patients and members of the public were not involved in designing the EVOLUTION study protocol.
Assessments
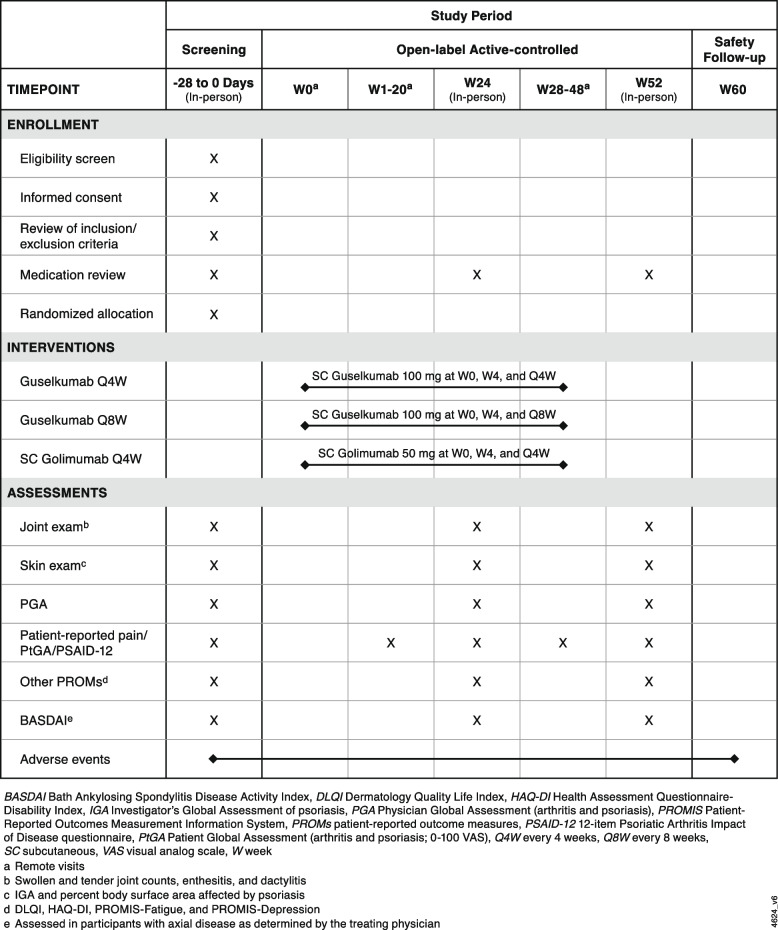
Study assessments will be collected as shown in the schedule of events (Fig. 2). The treating physician will determine the swollen joint count (SJC; 0–66) and tender joint count (TJC; 0–68) and provide a physician global assessment of arthritis and psoriasis (PGA; visual analog scale [VAS] 0–10). The physician will also assess the presence and severity of enthesitis and dactylitis utilizing the Leeds enthesitis index (LEI; enthesitis at 0–6 anatomical locations) [23] and dactylitis count (0–20), respectively. Patients will report their level of pain (VAS 0–10), global assessment of disease activity (arthritis and psoriasis; patient global assessment of disease activity [PtGA]; VAS 0–10), and physical function (using the Health Assessment Questionnaire-Disability Index [HAQ-DI]; 0–3).
Fig. 2.
EVOLUTION schedule of events
DAPSA and cDAPSA have both been validated in patients with PsA [15, 18]. The DAPSA is a continuous composite outcome capturing critical aspects of disease activity combining both provider assessments and patient-reported outcomes and is calculated using SJC (0–66), TJC (0–68), patient-reported pain, PtGA (0–10), and either erythrocyte sedimentation rate or C-reactive protein (CRP). The cDAPSA is calculated similarly with the exception of the laboratory assessment, making this a more practical instrument for use in the clinical practice setting, and the cDAPSA scale ranges from 0 to 154. Lower cDAPSA scores indicate less severe disease, with remission and LDA defined as scores ≤ 4 and ≤ 13, respectively [18]. The extent and severity of psoriatic skin involvement will be assessed using the IGA, a five-point scale ranging from 0 to 4 that averages induration, erythema, and scaling scores to categorize the severity of psoriasis (0 = cleared, 1 = minimal, 2 = mild, 3 = moderate, and 4 = severe) [16]. The combination endpoint of cDAPSA LDA and IGA 0/1 was previously assessed in post hoc analyses from the DISCOVER-1 and -2 studies, which showed that the achievement of this endpoint demonstrated a high level of agreement with achieving LDA using another composite score, the Psoriatic Arthritis Disease Activity Score (PASDAS) [24]. Together, the achievement of cDAPSA LDA and IGA 0/1 at Month 12 constitutes a novel composite primary endpoint for this study.
Patients will complete several patient-reported outcome (PRO) questionnaires to document their HRQoL. Patients will report symptoms based on the 12-item PsA Impact of Disease (PSAID-12) instrument that assesses patient perception of the following aspects of their disease and QoL: pain, fatigue, skin, work and/or leisure activities, function, discomfort, sleep, coping, anxiety, embarrassment, social life, and depression [25]. The Dermatology Life Quality Index (DLQI) is a 10-item scale that is frequently used to assess the patient’s perspective of the impact of skin disease on daily living [26]. Questionnaires from the Patient-Reported Outcomes Measurements Information System (PROMIS) will also be utilized to assess fatigue (Short Form 8a) and depression [27, 28].
In this study, achievement of minimal disease activity (MDA) will be determined using the standard assessments [29] as well as a modification in which the HAQ-DI criterion is replaced by a PSAID-12 score < 4 (0–10) [30]. In EVOLUTION, MDA is defined as meeting ≥ 5/7 of the following criteria: TJC ≤ 1, SJC ≤ 1, tender entheseal points ≤ 1, body surface area (BSA) affected by psoriasis ≤ 3%, PtGA (arthritis and psoriasis) ≤ 2 (0–10), patient-reported pain ≤ 2 (0–10), and either HAQ-DI ≤ 0.5 or PSAID-12 score < 4. Patients will also rate their fatigue, spinal pain, peripheral joint pain, pain at entheseal sites, and severity and duration of morning stiffness, each on a 0–10 VAS, using the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) [31].
AEs will be reported by the patient to their physicians during a visit or between visits by text message through Research Electronic Data Capture (REDCap) for the duration of the study. In addition, patients will receive an AE survey at 4-weekly intervals to capture any other untoward effects or disease flares. All AEs reported by patients will be followed up by the coordinator and will be conveyed to the treating physician so that appropriate medical care can be determined. Other supporting documentation of the AE may be requested by the Primary Site (University of Pennsylvania), independent Data Safety Monitoring Board (DSMB), or study funder (Johnson & Johnson). All SAEs (e.g. AEs that result in death, are life threatening, or require inpatient hospitalization) should be reported to the Primary Site within 24 h of becoming aware of the event(s) using the REDCap SAE form or other secure method.
Study design
The EVOLUTION study design aims to balance feasibility and generalizability that align with clinical practice, while maintaining internal validity and minimizing systematic bias and loss to follow up. The study will comprise a screening phase (4 weeks), an open-label active treatment phase (48 weeks), and a safety follow-up phase extending 12 weeks after the last study drug administration (Week 60). Eligible patients with active PsA with current or prior use of a single TNFi who are considering a switch to a new biologic therapy will be assigned randomly (1:1:1) to one of three treatment groups: guselkumab 100 mg Q4W; guselkumab 100 mg at Weeks 0, 4, and Q8W thereafter; or SC golimumab 50 mg Q4W. These dosing regimens are consistent with prior phase 3 randomized, placebo-controlled studies of guselkumab [6, 11] and SC golimumab [32] in participants with active PsA. Of note, consistent with the pragmatic study approach, no washout period is required before study enrollment.
At the in-person Screening/Baseline visits, patients will complete the PRO assessments, and the treating physician will collect the patient’s medical history and perform the standard physical examination assessments (joint counts, enthesitis, dactylitis, skin, and physician global assessments). At Weeks 1, 2, and 4 and at 4-week intervals until study completion, patients will complete a short set of PRO assessments (PtGA, patient-reported pain, and PSAID-12) via text message link or email link (per patient preference). In-person visits to collect PROs and repeat physical examination assessments will occur at Months 6 and 12. The final safety follow-up will occur 12 weeks after the final study drug administration (AE reporting and medication review). Patients who discontinue the study intervention will be encouraged to complete any remaining trial procedures as indicated in the protocol, including the final safety visit.
Intervention
Currently, the guselkumab 100 mg Q8W dosing regimen and SC golimumab (50 mg once a month) are FDA-approved for use in adults with active PsA [10, 13]. In addition to the guselkumab Q8W dosing regimen, a Q4W regimen was also evaluated in patients with active PsA in the phase 3 DISCOVER-1 (TNF-experienced/TNFi-naive) [6] and DISCOVER-2 (biologic-naive) [11] studies. Both guselkumab dosing regimens demonstrated significant improvements in the signs and symptoms of PsA with a favorable safety profile compared with placebo. Although the majority of patients evaluated in these studies were biologic-naïve (DISCOVER-1 included a limited number of TNFi-experienced patients, some being TNFi-IR). A third phase 3 study, COSMOS, evaluated the Q8W dosing regimen and was conducted solely in TNFi-IR patients [3]. Although the COSMOS study only assessed the Q8W dosing regimen in TNFi-IR patients, it should be noted that the guselkumab Q4W dosing regimen is approved by the European Medicines Agency for patients at high risk of structural damage [33]. Findings from the TNFi-IR patients with PsA in DISCOVER-1 and COSMOS suggest that these patients may derive an incremental benefit from the more frequent guselkumab Q4W dosing regimen [3, 6, 34]. Therefore, the three interventions chosen for evaluation in EVOLUTION are guselkumab 100 mg Q4W; guselkumab 100 mg at Weeks 0, 4, and Q8W thereafter; and SC golimumab 50 mg Q4W. Concomitant use of the following medications at stable doses will be permitted: methotrexate ≤ 20 mg weekly (oral, intramuscular, or SC), apremilast ≤ 30 mg twice daily, leflunomide ≤ 20 mg/day, sulfasalazine ≤ 1500 mg twice daily, hydroxychloroquine ≤ 400 mg/day, ≤ oral corticosteroids 10 mg/day, and standard NSAIDs at approved doses.
Sample size
The initial planned sample size was a total of approximately 300 participants, with equal randomization across the three treatment groups. However, owing to recruitment challenges, the planned sample size was reduced to a total of 150 (i.e., 50 in each group), and all analyses will be considered exploratory. When applying the EVOLUTION inclusion criteria to biologic-experienced patients in the PARC cohort, approximately half would meet disease activity eligibility criteria for the current study (Table 2). Utilizing data from this EVOLUTION-eligible PARC cohort, we hypothesize that 50%, 45%, and 24% of patients in the guselkumab Q4W, guselkumab Q8W, and SC golimumab groups, respectively, will achieve the primary outcome of cDAPSA LDA + IGA 0/1 at Month 12.
Randomization and blinding
Randomization will be implemented to minimize bias in the assignment of participants to intervention groups, to increase the likelihood that known and unknown attributes are evenly balanced across intervention groups. Randomization will be stratified by site, sex, and baseline medication use (csDMARDs [methotrexate, leflunomide, hydroxychloroquine, sulfasalazine] and apremilast). The randomization schedule will be generated using the Way to Health software platform that was developed by the Penn Center for Health Incentives and Behavioral Economics (CHIBE) and is currently operated through a partnership between CHIBE and Penn Medicine Center for Health Care Innovation. REDCap (developed by Vanderbilt University; https://redcap.vanderbilt.edu/) will be used for data entry, sending and recording PROs, and monitoring patient participation and trial quality assurance. An independent DSMB will meet every 6 months to review safety findings throughout the study.
Each participant will be recruited, consented, and screened at their clinic visit by the treating physician and will be assigned a unique code that will dictate the intervention assignment. Study interventions will be provided by the study sponsor and, consistent with the open-label design, shipped directly to the study participants by the pharmacy (Pioneer Health Compounding Pharmacy). Although it is expected that most patients in this study will have had prior experience and training with self-injectable therapy, such training will be provided at the Screening/Baseline visit if needed. Self-administration will ensure consistency with real-world experience, aligning the trial with clinical practice as much as possible.
Statistical methods
Demographic and disease characteristics at baseline will be summarized by treatment group with descriptive statistics such as proportions for categorical variables and means (standard deviations [SDs]) or medians (interquartile ranges) for continuous variables. Standardized differences will be used to examine whether there were any characteristics that were sufficiently different between treatment groups at baseline. Patient disposition through Month 12 will be reported; baseline characteristics will be compared for participants who complete the study and for those who discontinue within each treatment group.
Efficacy results will be summarized by randomized treatment group using descriptive statistics (e.g., counts and percentages; means and SDs) utilizing an intention-to-treat analysis. The primary endpoint is the proportion of patients achieving both cDAPSA LDA and IGA 0/1 at Month 12. The proportion of patients achieving this composite endpoint at Month 6 will also be determined; other secondary categorical endpoints include the proportions of patients achieving IGA 0/1 (among patients with baseline IGA ≥ 2 and those with baseline BSA ≥ 3%), MDA (using both HAQ-DI and PSAID-12 criteria), PSAID-12 score < 4 (among patients with baseline score ≥ 4), and resolution of enthesitis and dactylitis (score of 0 of among those with the condition at baseline) at Months 6 and 12. Secondary continuous endpoints include mean changes at Months 6 and 12 in PGA, PtGA, PSAID-12, DLQI, PROMIS-fatigue, and PROMIS-depression. Additionally, changes in BASDAI will be assessed among all patients and also specifically in patients with axial disease (yes/no, as determined by the treating physician) at baseline.
In the primary endpoint analysis, patients who discontinue study treatment or initiate/increase concomitant medications related to symptoms of PsA will be considered nonresponders. Other missing data will be imputed using multiple imputation. The difference in response rates between each guselkumab group and the SC golimumab group will be reported with a 95% confidence interval (CI); nominal p values will be determined by chi-square test first between the guselkumab Q4W and SC golimumab groups followed by guselkumab Q8W and SC golimumab groups. Subgroup analyses will also be performed to evaluate consistency of primary endpoint analysis. Response rates will be reported descriptively across subgroups defined by baseline demographics (sex, race/ethnicity, and age [above/below the median]), disease characteristics (primary [i.e., no response] versus secondary [i.e., loss of response] nonresponse to prior TNFi), and concomitant medication use (yes/no); treatment group comparisons will be assessed using standardized differences. No interim analyses are planned.
For secondary endpoints, missing data will be imputed using multiple imputation. Nominal p values will be determined by chi-square test for categorical variables and t-tests for continuous variables. We will also estimate treatment effects as odds ratios (binary variables) or mean differences (continuous variables) and respective 95% CI for categorical and continuous outcomes, respectively. No formal adjustment for multiple comparisons will be made for secondary endpoints; all p-values will be nominal. Linear regression modeling will be utilized to examine the statistical difference in the change of each continuous measure from baseline between treatment groups (guselkumab Q4W vs SC golimumab and guselkumab Q8W vs SC golimumab) adjusted for imbalanced patient characteristics. For secondary endpoints, no formal adjustments were made to control for multiplicity.
Safety
Safety analyses will include all patients who receive ≥ 1 administration of study intervention. The types and incidence of AEs, SAEs, and additional treatment burdens or side effects as reported by the patients will be summarized by treatment group. Laboratory investigations will not be performed prospectively after screening and will be monitored only as part of standard of care; however, laboratory abnormalities may be reported by the monitoring clinician as an AE.
Additional analyses
Mean changes over time in the PRO assessments collected at 4-weekly intervals (PtGA, patient-reported pain, and PSAID-12) will be analyzed descriptively. Urine pregnancy test for women of childbearing potential will be collected at baseline prior to initiating study intervention. For patients who provide additional consent, optional blood samples may be collected (maximum 50 mL) prior to the first study drug administration and stored for future analysis; the treating physician may order additional clinical tests. There will be no diagnostic genetic testing to determine predisposition to unknown conditions nor whole genome sequencing performed using the stored samples.
Study oversight
Sites will be monitored to assure that the conduct of the trial is in accordance with the approved protocol and amendments, International Conference on Harmonisation Good Clinical Practices, and applicable regulatory requirement. International HealthCare, LLC (IHC) staff (Project Manager and other regulatory support staff) will monitor the study with assistance from the Project Manager and Coordinator at each site and with direction as needed from the Principal Investigator and Project Manager from the University of Pennsylvania Office of Research Services (coordinating center). IHC will conduct virtual site audits after enrollment of the first and third participants and every 10 participants thereafter. Together, the IHC and University of Pennsylvania teams will provide oversight and day-to-day support for all study sites; these teams will meet every 2 weeks to discuss any study-wide or site-specific issues. Independent audits or compliance reviews may be conducted by the University of Pennsylvania Office of Research Services at their discretion to ensure monitoring practices are performed consistently across all participating sites.
The protocol can be modified with agreement from the coordinating center (University of Pennsylvania) and the study sponsor (Johnson & Johnson). Protocol amendments will be disseminated to each study site and approved by the relevant IRB. IHC will ensure that all sites are properly trained on the protocol changes prior to implementation of the changes at each. Any protocol deviations will be documented and reported to the IRB and study sponsor when necessary.
Discussion
TNFi are typically the first biologic therapy prescribed for patients with active PsA after treatment with csDMARDs. However, a substantial proportion of these patients do not achieve a clinical response (ACR20) within 6 months of treatment or may lose their response to treatment over time [35]. These patients represent a difficult-to-treat population for which current treatment options and formal guidelines regarding therapy selection are limited [3]. An analysis of patients enrolled in the Corrona PsA/Spondyloarthritis Registry found that among patients initiating a TNFi for PsA, 46% had received prior TNFi therapy [36]. Switching to another TNFi may not provide lasting control of disease activity, and patients often experience decreased persistence and efficacy with subsequent TNFi treatments [35]. Although guidance is lacking on therapy selection when a patient does not achieve response with a TNFi, available observational data suggest TNFi-IR PsA patients may benefit from switching to a therapy targeting a different MOA rather than cycling to another TNFi. RCTs have evaluated the efficacy and safety of biologics other than TNFi in TNFi-IR patients with PsA; however, these studies have been performed using primarily a placebo control, precluding direct comparison with other biologics in this patient population [3, 6–8].
In the phase 3 DISCOVER-1 (TNFi-experienced/TNFi-naïve) [6] and DISCOVER-2 (biologic naïve) [11] studies, compared with placebo, guselkumab was effective in improving the signs and symptoms of PsA. Improvements across PsA disease domains was generally similar regardless of dosing regimen (Q4W and Q8W). However, among TNFi-experienced DISCOVER-1 patients, including a small subset who discontinued their prior TNFi treatment due to inadequate efficacy, ACR50 and ACR70 response rates at Week 24 were numerically higher in participants receiving the guselkumab Q4W dosing regimen (29% and 18%, respectively) compared with those receiving guselkumab Q8W (13% and 7%, respectively) [6, 34]. Subsequently, the COSMOS phase 3b study demonstrated the efficacy of guselkumab in patients with active PsA who had experienced either inadequate efficacy or intolerance to ≤ 2 TNFi. However, this study only evaluated the Q8W dosing regimen [3]. Taken together, results from DISCOVER-1 and COSMOS suggest that more frequent dosing with guselkumab may provide incremental benefit for TNFi-IR patients with PsA. Of note, a separate, phase 3b study, SOLSTICE, was designed to gather additional data on the Q4W and Q8W dosing regimens to assess whether TNFi-IR patients might benefit from more frequent guselkumab administration and is currently ongoing [37].
The Th1 and Th17 pathways are known to contribute to the pathogenesis of PsA [38]. Activation of the Th1 pathway leads to upregulation of TNF, whereas IL-23 induces Th17 cell differentiation that results in upregulation of IL-17 and IL-22. Although TNF inhibition is an effective treatment approach for many patients with PsA, there remains a substantial subset who do not achieve response or lose response with TNFi. In vivo analyses using a murine model of psoriasis found that TNF blockade resulted in increased serum concentrations of IL-17 and IL-22 [39]. Recent biomarker analyses from the DISCOVER-1 and -2 studies [40] and COSMOS [41] demonstrated that treatment with guselkumab significantly reduced serum levels of IL-22 from baseline to Week 24. Additionally, pooled analyses of TNFi-IR and biologic-naïve participants from DISCOVER-1, DISCOVER-2, and COSMOS found that baseline serum levels of IL-22 were higher among those who achieved response in skin and joint assessments at Week 24 than in nonresponders [42]. Collectively, these findings suggest that for patients with TNFi-IR PsA, targeting the IL-23/Th17 pathway may be a more appropriate treatment strategy than cycling to another TNFi. EVOLUTION plans for an optional biospecimen repository to address important mechanistic studies.
To improve outcomes in patients with PsA, innovative research approaches are needed to determine optimal disease management. Accordingly, the phase 3b EVOLUTION trial has been designed as a pragmatic trial to evaluate the efficacy and safety of selectively inhibiting the IL-23p19 subunit with guselkumab in patients with active PsA after therapy with a single TNFi who are considering switching to a new biologic. Findings from EVOLUTION will provide insight into whether this patient population may derive greater benefit from switching to a biologic utilizing an alternate MOA, specifically an IL-23 p19-subunit inhibitor, rather than cycling to a second TNFi. EVOLUTION will be the first head-to-head, pragmatic, comparative trial to enroll a population of patients with PsA representative of those seen in clinical practice. Conventional RCTs in patients with PsA often prohibit participation of those who previously have received a TNFi, and when such patients are allowed to enroll, a washout period for the TNFi is typically required. Additionally, conventional RCTs often have a minimum SJC and TJC requirement for enrollment that may be higher and less representative than that of many patients with PsA seen in clinical practice. The minimal inclusion and exclusion criteria of the EVOLUTION trial were chosen to increase the pragmatic nature of the study.
The primary endpoint employed in the EVOLUTION trial distinguishes this study from conventional RCTs. The ACR20 response was developed to differentiate the efficacy of an active drug from placebo in clinical trials of patients with RA. It has also been the standard primary endpoint utilized in conventional RCTs in patients with PsA and facilitates comparisons of the response to treatment between relatively large patient cohorts [43]. However, because the ACR response criteria were originally developed to assess clinical response in patients with RA focusing on peripheral joint disease, they do not include the PsA disease domains of enthesitis, dactylitis, axial involvement, or skin disease, thus limiting the utility of this endpoint in patients with PsA [44]. Additionally, because the ACR response is a binary outcome, floor effects can occur in patients with lower swollen and tender joint counts at the time of treatment initiation [43]. As a result, patients with PsA may struggle to achieve significant ACR response levels of 20%, 50% or 70% reduction in tender and swollen joint counts [45]. Alternatively, the cDAPSA is a composite instrument designed specifically to evaluate PsA disease activity from the perspective of the physician and the patient and can be used to monitor disease activity over time. It includes swollen and tender joint counts and patient-reported pain and global assessment. Additionally, while achievement of clinical response using the ACR criteria requires comparison with a previous timepoint, DAPSA and cDAPSA are continuous outcome measures for which there are established cut offs that coincide with remission, low, moderate, and high disease activity, which can be useful when applying a treat-to-target strategy [18]. It should also be noted that the cDAPSA variation does not require a laboratory assessment (omitting CRP). Therefore, the cDAPSA allows for immediate interpretation by health care providers without a delay in therapeutic decision, making this a feasible choice for use in clinical practice and pragmatic trials [18]. It should be noted that the cDAPSA instrument may perform differently in patients with oligoarticular PsA than in those with polyarticular disease [46]. However, recent post hoc analyses from the phase 3 DISCOVER-1 and -2 studies found that the treatment effect of guselkumab in the achievement of cDAPSA LDA/remission was generally consistent across subgroups defined by the number of swollen and tender joints at baseline (< 10, 10 to 15, > 15) [47]. Together, the achievement of cDAPSA LDA and IGA of 0 or 1, which encompass both skin and joint assessments as well as patient-reported outcomes, constitute the novel composite primary endpoint for this study.
The pragmatic-explanatory continuum indicator summary (PRECIS) tool was designed to guide trialists when designing clinical studies [14]. A more recent, validated version (PRECIS-2) comprises nine domains: eligibility, recruitment, setting, organization, flexibility (delivery), flexibility (adherence), follow-up, primary outcome, and primary analysis [48]. When considering these domains, several elements of the EVOLUTION trial design are consistent with a pragmatic approach: participants will be representative of the patient population of interest, regardless of their anticipated response, comorbidities or past treatment adherence; the control intervention (TNFi, SC golimumab) is consistent with current clinical practice; the primary outcome will be measured objectively, without the need for adjudication, and will be meaningful to participants [49]; efficacy assessments (including remote monitoring) have been selected to minimize burden on investigators and patients; and the statistical analysis methods used will account for all randomized patients (intention to treat).
It should be noted that the EVOLUTION study population will be limited to patients with active PsA after having received only a single TNFi, who are recruited from clinical practices, and will compare only two medications: guselkumab and SC golimumab. Consequently, any conclusions from the study may not be generalizable to patients who have received more than one TNFi or other biological or targeted therapies. Existing data on the efficacy of guselkumab in treating patients with axial PsA are limited. Exploratory analyses from the DISCOVER-1 and -2 studies showed greater improvements in axial symptoms with guselkumab vs placebo among patients with investigator-identified and imaging-confirmed spondylitis. For the EVOLUTION study, patients identified by the investigator as having axial involvement are permitted to enroll; however, they must also have evidence of active peripheral joint disease. Of note, the ongoing STAR study was designed to prospectively assess the efficacy of guselkumab in treating axial symptoms and inflammation in patients with PsA [50]. EVOLUTION will assess efficacy only through 1 year, reflecting the median treatment persistence with TNFi therapy [51]. However, additional studies will be warranted to evaluate long-term outcomes in patients with TNFi-IR PsA. In addition, unlike the previous conventional RCTs of guselkumab, in which an independent joint assessor was employed, the treating physician will determine the SJC, TJC, LEI, dactylitis count, provide a PGA, and perform the standard physical examination assessments.
In summary, the EVOLUTION trial will be the first of its kind pragmatic trial evaluating this treatment paradigm and is expected to provide efficacy and safety data in TNFi-IR patients with PsA. This differs significantly from conventional RCTs [14] because the EVOLUTION trial incorporates pragmatic features, such the absence of a pre-defined washout period, and a novel primary endpoint (cDAPSA LDA and IGA 0/1) with direct applicability to informing treatment decisions for patients with TNFi-IR PsA in routine clinical care. Additionally, this study will assess, among guselkumab-treated patients, whether the more frequent Q4W dosing regimen may offer an incremental benefit to that of the Q8W regimen in this difficult-to-treat patient population.
Trial status
The trial started with recruitment on July 14, 2023, and enrollment will be completed in October 2025. Protocol version 6.0, July 09, 2024.
Supplementary Information
Additional file 1: EVOLUTION SPIRIT checklist.
Acknowledgements
Medical writing support was provided by Kristin L Leppard, MS, and Rebecca Clemente, PhD, of Johnson & Johnson under the direction of the authors in accordance with Good Publication Practice guidelines (Ann Intern Med 2022;175: 1298-1304).
Abbreviations
- ACR20
≥ 20% Improvement in American College of Rheumatology response criteria
- AE
Adverse events
- BASDAI
Bath Ankylosing Spondylitis Disease Activity Index
- BSA
Body surface area
- CASPAR
Classification criteria for psoriatic arthritis
- cDAPSA
Clinical disease activity in psoriatic arthritis
- CHIBE
Center for Health Incentives and Behavioral Economics (Penn)
- CI
Confidence interval
- CRP
C-reactive protein
- csDMARD
Conventional synthetic disease-modifying antirheumatic drug
- DLQI
Dermatology Life Quality Index
- DMARDs
Disease-modifying antirheumatic drugs
- DSMB
Data safety monitoring board
- GLM
Golimumab
- GRAPPA
Group for Research and Assessment of Psoriasis and Psoriatic Arthritis
- GUS
Guselkumab
- HAQ-DI
Health Assessment Questionnaire-Disability Index
- HRQoL
Health-related quality of life
- IGA
Investigator’s Global Assessment
- IL
Interleukin
- IR
Inadequate response
- IV
Intravenous
- LEI
Leeds Enthesitis Index
- LDA
Low disease activity
- JAKi
Janus kinase inhibitor
- MDA
Minimal disease activity
- MOA
Method of action
- NSAID
Nonsteroidal anti-inflammatory drug
- PARC
Psoriatic Arthritis Research Consortium
- PGA
Physician global assessment
- PRECIS
Pragmatic explanatory continuum indicator summary
- PRO
Patient reported outcome
- PROMIS
Patient Reported Outcomes Measurement Information System
- PsA
Psoriatic arthritis
- PSAID-12
Psoriatic arthritis impact of disease 12-item questionnaire
- PtGA
Patient global assessment of disease activity
- Q4W
Every 4 weeks
- Q8W
Every 8 weeks
- REDCap
Research Electronic Data Capture
- RCTs
Randomized controlled trials
- SAE
Serious adverse event
- SC
Subcutaneous
- SD
Standard deviation
- SJC
Swollen joint count
- SPIRIT
Standard Protocol Items: Recommendation for Interventional Trials
- TJC
Tender joint count
- TNF
Tumor necrosis factor
- TNFi
Tumor necrosis factor inhibitor
- TNF-IR
Tumor necrosis factor-inadequate response
- VAS
Visual analog scale
- W
Week
Authors’ contributions
Study/protocol design: AO, SMR, SHG, MEH, JUS, KS-E, JK, BL, JRC, AJS, SDC, CG, JAW. Data collection: AO, SMR, MEH, JUS, KS-E, JK, BL, JRC, JAW. Data analysis: AJS. Data interpretation: AO, SMR, SHG, MEH, JUS, KS-E, JK, BL, JRC, AJS, SDC, CG, JAW. Drafting/revising manuscript: AO, MEH, JK, BL, JRC, SDC, CG. Approval: AO, SMR, SHG, MEH, JUS, KS-E, JK, BL, JRC, AJS, SDC, CG, JAW. Study oversight: AO, SDC.
Funding
Johnson & Johnson
Name and contact information for the trial sponsor: Dr. Alexis Ogdie, University of Pennsylvania School of Medicine.
Study responsible physician: Alexis Ogdie.
Role of sponsor: Authors who are employees of the study sponsor participated in the study design and protocol development. A medical writer employed by the funding agency provided writing and editorial support. Authors who are employees of the study sponsor and the funding agency will participate in analysis and interpretation of data resulting from this study and will serve as authors on future publications based on results of this study. All authors will satisfy ICMJE authorship criteria (https://www.icmje.org/recommendations/browse/roles-and-responsibilities/defining-the-role-of-authors-and-contributors.html) and will participate in the decision to approve and submit future reports for publication.
Data availability
Data will be available according to the data sharing policy of Johnson & Johnson (https://innovativemedicine.jnj.com/our-innovation/clinical-trials/transparency). Trial results will be posted online as required by ClinicalTrials.gov, and results will be shared with patients as required by local regulations.
Declarations
Ethics approval and consent to participate
The study is being conducted in compliance with the Declaration of Helsinki and International Council for Harmonization Guidelines for Good Clinical Practice. The protocol will be approved by each site’s institutional review board. Each study patient must have given written informed consent prior to conducting any study-related procedures.
Consent for publication
Not applicable.
Competing interests
AO: grant/research support to the University of Pennsylvania from AbbVie, Janssen, Novartis, and Pfizer and to Forward from Amgen and Bristol Myers Squibb; consultant fees from AbbVie, Amgen, Bristol Myers Squibb, Celgene, CorEvitas, Eli Lilly, Gilead, Happify, Janssen, Novartis, Pfizer, and UCB; her husband has received royalties from Novartis. SMR: consultant for, AbbVie, Amgen, Fresnius Kabi, Janssen, Novartis, UCB. SHG: employee University of Pennsylvania School of Medicine). MEH: received consulting fees and/or honoraria from AbbVie, Amgen, Bristol Myers Squibb, Eli Lilly, Janssen, Novartis, Sanofi Genzyme/Regeneron and UCB (less than $10,000 each). JUS: consultant for AbbVie, Janssen, Kaleido, Novartis, Pfizer, Sanofi, and UCB; received funding for investigator-initiated studies from Janssen, Novartis, and Pfizer. KSE: None. JK: None. BAL: None. AJSS: receives compensation from the American College of Physicians for editorial work, serves on the External Advisory board for the Florida Atlantic University Summer Institute in Biostatistics, received speaker’s honorarium from NYU in 2023, received consultant fees from WilmerHale on behalf of Gilead Sciences in 2022. SDC: employee of and owns stock in Johnson & Johnson. CG: employee of and owns stock in Johnson & Johnson. JAW: received research funding from AbbVie, Merck, and Pfizer; consulting for AbbVie, Eli Lilly, Janssen, Novartis, and UCB. JRC: received grant/research support from AbbVie, Amgen, Bristol Myers Squibb, CorEvitas, Eli Lilly, Janssen, Myriad, Novartis, Pfizer, Sanofi, UCB, and NIAMS P30AR072583; received consulting fees from AbbVie, Amgen, Bristol Myers Squibb, CorEvitas, Eli Lilly, Janssen, Myriad, Novartis, Pfizer, Sanofi, and UCB.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ritchlin CT, Colbert RA, Gladman DD. Psoriatic Arthritis. N Engl J Med. 2017;376:957–70. [DOI] [PubMed] [Google Scholar]
- 2.Gossec L, Baraliakos X, Kerschbaumer A, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis. 2020;79:700–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coates LC, Soriano ER, Corp N, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): updated treatment recommendations for psoriatic arthritis 2021. Nat Rev Rheumatol. 2022;18:465–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merola JF, Lockshin B, Mody EA. Switching biologics in the treatment of psoriatic arthritis. Semin Arthritis Rheum. 2017;47:29–37. [DOI] [PubMed] [Google Scholar]
- 5.Mease P. A short history of biological therapy for psoriatic arthritis. Clin Exp Rheumatol. 2015;33:S104–8. [PubMed] [Google Scholar]
- 6.Deodhar A, Helliwell PS, Boehncke WH, et al. Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNFα inhibitor treatment (DISCOVER-1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395:1115–25. [DOI] [PubMed] [Google Scholar]
- 7.Orbai AM, Husni ME, Gladman DD, et al. Secukinumab efficacy on psoriatic arthritis GRAPPA-OMERACT core domains in patients with or without prior tumor necrosis factor inhibitor use: Pooled analysis of four Phase 3 studies. Rheumatol Ther. 2021;8:1223–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orbai AM, Gratacos J, Turkiewicz A, et al. Efficacy and safety of ixekizumab in patients with psoriatic arthritis and inadequate response to TNF inhibitors: 3-Year follow-up (SPIRIT-P2). Rheumatol Ther. 2021;8:199–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogdie A, McLean R, Blachley T, et al. The impact of second-line therapeutic on disease control after discontinuation of first line TNF inhibitor in patients with PsA: analysis from the CorEvitas Psoriatic Arthritis/Spondyloarthritis Registry [abstract]. Arthritis Rheumatol. 2022;74 (suppl 9). https://acrabstracts.org/abstract/the-impact-of-second-line-therapeutic-on-disease-control-after-discontinuation-of-first-line-tnf-inhibitor-in-patients-with-psa-analysis-from-the-corevitas-psoriatic-arthritis-spondyloarthritis-regis/. Accessed 9 Mar 2023.
- 10.Tremfya. Package insert. Horsham, PA: Janssen Biotech, Inc.; 2024. [Google Scholar]
- 11.Mease PJ, Rahman P, Gottlieb AB, et al. Guselkumab in biologic-naive patients with active psoriatic arthritis (DISCOVER-2): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395:1126–36. [DOI] [PubMed] [Google Scholar]
- 12.Coates LC, Gossec L, Theander E, et al. Efficacy and safety of guselkumab in patients with active psoriatic arthritis who are inadequate responders to tumour necrosis factor inhibitors: results through one year of a phase IIIb, randomised, controlled study (COSMOS). Ann Rheum Dis. 2022;81:359–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simponi. Package Insert. Horsham, PA: Janssen Biotech, Inc.; 2023. [Google Scholar]
- 14.Thorpe KE, Zwarenstein M, Oxman AD, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. CMAJ. 2009;180:E47-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schoels M, Aletaha D, Funovits J, Kavanaugh A, Baker D, Smolen JS. Application of the DAREA/DAPSA score for assessment of disease activity in psoriatic arthritis. Ann Rheum Dis. 2010;69:1441–7. [DOI] [PubMed] [Google Scholar]
- 16.Langley RG, Feldman SR, Nyirady J, van de Kerkhof P, Papavassilis C. The 5-point Investigator’s Global Assessment (IGA) scale: a modified tool for evaluating plaque psoriasis severity in clinical trials. J Dermatolog Treat. 2015;26:23–31. [DOI] [PubMed] [Google Scholar]
- 17.Chan AW, Tetzlaff JM, Gøtzsche PC, et al. SPIRIT 2013 explanation and elaboration: Guidance for protocols of clinical trials. BMJ. 2013;346:e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schoels MM, Aletaha D, Alasti F, Smolen JS. Disease activity in psoriatic arthritis (PsA): defining remission and treatment success using the DAPSA score. Ann Rheum Dis. 2016;75:811–8. [DOI] [PubMed] [Google Scholar]
- 19.Walsh JA, Wan MT, Willinger C, et al. Measuring outcomes in psoriatic arthritis: Comparing routine assessment of patient index data and psoriatic arthritis impact of disease. J Rheumatol. 2020;47:1496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mease PJ, Gladman DD, Poddubnyy D, et al. Efficacy of guselkumab on axial-related symptoms through up to 2 years in adults with active psoriatic arthritis in the phase 3, randomized, placebo-controlled DISCOVER-2 study. Rheumatol Ther. 2023;10:1637–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mease PJ, Helliwell PS, Gladman DD, et al. Efficacy of guselkumab on axial involvement in patients with active psoriatic arthritis and sacroiliitis: a post-hoc analysis of the phase 3 DISCOVER-1 and DISCOVER-2 studies. Lancet Rheumatol. 2021;3:E715–23. [DOI] [PubMed] [Google Scholar]
- 22.Ogdie A, Hur P, Liu M, et al. Effect of multidomain disease presentations on patients with psoriatic arthritis in the Corrona Psoriatic Arthritis/Spondyloarthritis Registry. J Rheumatol. 2021;48:698–706. [DOI] [PubMed] [Google Scholar]
- 23.Healy PJ, Helliwell PS. Measuring clinical enthesitis in psoriatic arthritis: assessment of existing measures and development of an instrument specific to psoriatic arthritis. Arthritis Rheum. 2008;59:686–91. [DOI] [PubMed] [Google Scholar]
- 24.Aletaha D, Merola JF, Sharaf M, et al. A novel composite endpoint including low peripheral joint disease activity state and clear/almost clear skin in patients with active psoriatic arthritis: post hoc analyses of 2 phase 3 randomized, double-blind, placebo-controlled studies. Ann Rheum Dis. 2024;83:373. [Google Scholar]
- 25.Gossec L, de Wit M, Kiltz U, et al. A patient-derived and patient-reported outcome measure for assessing psoriatic arthritis: elaboration and preliminary validation of the Psoriatic Arthritis Impact of Disease (PsAID) questionnaire, a 13-country EULAR initiative. Ann Rheum Dis. 2014;73:1012–9. [DOI] [PubMed] [Google Scholar]
- 26.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210–6. [DOI] [PubMed] [Google Scholar]
- 27.Cella D, Yount S, Rothrock N, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007;45:S3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63:1179–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coates LC, Fransen J, Helliwell PS. Defining minimal disease activity in psoriatic arthritis: a proposed objective target for treatment. Ann Rheum Dis. 2010;69:48–53. [DOI] [PubMed] [Google Scholar]
- 30.Johnson K, Ye JY, Chandran V, Gladman DD. A novel role for the psoriatic arthritis impact of disease (PsAID) questionnaire. Semin Arthritis Rheum. 2019;49:241–5. [DOI] [PubMed] [Google Scholar]
- 31.Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol. 1994;21:2286–91. [PubMed] [Google Scholar]
- 32.Kavanaugh A, McInnes I, Mease P, et al. Golimumab, a new human tumor necrosis factor α antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: Twenty-four-week efficacy and safety results of a randomized, placebo-controlled study. Arthritis Rheum. 2009;60:976–86. [DOI] [PubMed] [Google Scholar]
- 33.Tremfya (EMA). Package Insert. Leiden, The Netherlands: Janssen B.V; 2022. [Google Scholar]
- 34.Ritchlin CT, Deodhar A, Boehncke WH, et al. Multidomain efficacy and safety of guselkumab through 1 year in patients with active psoriatic arthritis with and without prior tumor necrosis factor inhibitor experience: analysis of the phase 3, randomized, placebo-controlled DISCOVER-1 study. ACR Open Rheumatol. 2023;5:149–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glintborg B, Ostergaard M, Krogh NS, et al. Clinical response, drug survival, and predictors thereof among 548 patients with psoriatic arthritis who switched tumor necrosis factor α inhibitor therapy: results from the Danish Nationwide DANBIO Registry. Arthritis Rheum. 2013;65:1213–23. [DOI] [PubMed] [Google Scholar]
- 36.Mease PJ, Karki C, Liu M, et al. Discontinuation and switching patterns of tumour necrosis factor inhibitors (TNFis) in TNFi-naive and TNFi-experienced patients with psoriatic arthritis: an observational study from the US-based Corrona registry. RMD Open. 2019;5:e000880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogdie A, Merola JF, Mease PJ, et al. Efficacy and safety of guselkumab in patients with active psoriatic arthritis who had inadequate efficacy and/or intolerance to one prior tumor necrosis factor inhibitor: study protocol for SOLSTICE, a phase 3B, multicenter, randomized, double-blind, placebo-controlled study. BMC Rheumatol. 2023. [DOI] [PMC free article] [PubMed]
- 38.Mantravadi S, Ogdie A, Kraft WK. Tumor necrosis factor inhibitors in psoriatic arthritis. Expert Rev Clin Pharmacol. 2017;10:899–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma HL, Napierata L, Stedman N, et al. Tumor necrosis factor alpha blockade exacerbates murine psoriasis-like disease by enhancing Th17 function and decreasing expansion of Treg cells. Arthritis Rheum. 2010;62:430–40. [DOI] [PubMed] [Google Scholar]
- 40.Sweet K, Song Q, Loza MJ, et al. Guselkumab induces robust reduction in acute phase proteins and type 17 effector cytokines in active psoriatic arthritis: results from phase 3 trials. RMD Open. 2021;7:e001679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schett G, Chen W, Gao S, et al. Effect of guselkumab on serum biomarkers in patients with active psoriatic arthritis and inadequate response to tumor necrosis factor inhibitors: results from the COSMOS phase 3b study. Arthritis Res Ther. 2023;25:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siebert S, Coates LC, Schett G, et al. Immunological differences between PsA patients who are tumor necrosis factor inhibitor-naïve and who have inadequate response to tumor necrosis factor inhibitors. Ann Rheum Dis. 2022;81:254–5. [Google Scholar]
- 43.McGagh D, Coates LC. Assessment of the many faces of PsA: single and composite measures in PsA clinical trials. Rheumatology (Oxford). 2020;59:i29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Felson DT, Anderson JJ, Boers M, et al. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum. 1995;38:727–35. [DOI] [PubMed] [Google Scholar]
- 45.Coates LC, FitzGerald O, Gladman DD, et al. Reduced joint counts misclassify patients with oligoarticular psoriatic arthritis and miss significant numbers of patients with active disease. Arthritis Rheum. 2013;65:1504–9. [DOI] [PubMed] [Google Scholar]
- 46.Karmacharya P, Stull C, Stephens-Shields A, et al. Responsiveness and minimal clinically important difference in patient-reported outcome measures among patients with psoriatic arthritis. Arthritis Care Res (Hoboken). 2023;75:2182–9. [DOI] [PMC free article] [PubMed]
- 47.Mease PJ, Gottlieb AB, Mcinnes IB, et al. Achievement of low disease activity/remission in guselkumab-treated patients with moderately-highly active psoriatic arthritis regardless of baseline characteristics: pooled post-hoc analysis of two phase 3/randomized studies [abstract]. Congress of Clinical Rheumatology-East Destin, FL. 2024.
- 48.Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ. 2015;350:h2147. [DOI] [PubMed] [Google Scholar]
- 49.Ogdie A, Husni ME, Bush K, et al. Patient identified treatment goals in psoriatic arthritis: decreasing pain and increasing activity level are high priorities. EULAR 2019 abstract, AB0769 2019;78:1853–4.
- 50.Gladman DD, Mease PJ, Bird P, et al. Efficacy and safety of guselkumab in biologic-naive patients with active axial psoriatic arthritis: study protocol for STAR, a phase 4, randomized, double-blinded, placebo-controlled trial. Trials. 2022;23:743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.George MD, Baker JF, Ogdie A. Comparative persistence of methotrexate and tumor necrosis factor inhibitors in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol. 2020;47:826–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: EVOLUTION SPIRIT checklist.
Data Availability Statement
Data will be available according to the data sharing policy of Johnson & Johnson (https://innovativemedicine.jnj.com/our-innovation/clinical-trials/transparency). Trial results will be posted online as required by ClinicalTrials.gov, and results will be shared with patients as required by local regulations.