Abstract
Background
Alzheimer's disease (AD) is characterized by progressive cognitive decline and synaptic dysfunction, largely driven by amyloid plaques and neurofibrillary tangles (NFTs) composed of hyperphosphorylated tau. These pathological hallmarks disrupt glutamate signaling, which is essential for synaptic plasticity and memory consolidation. This study investigates the therapeutic potential of melatonin on memory and synaptic plasticity in an AD-like mouse model, with a focus on its regulatory effects on glutamate homeostasis and metabotropic glutamate receptors (mGluRs).
Methods
The study began with an in-silico bioinformatics analysis of RNA-seq datasets from hippocampal tissues of AD patients to identify differentially expressed genes (DEGs) related to glutamate signaling and tau pathology. An AD-like model was induced via intra-hippocampal injection of cis-phospho tau in C57BL/6 mice. Memory function was assessed using behavioral tests. Synaptic plasticity was evaluated using in vitro field potential recording of hippocampal slices. Histological analyses included Nissl staining for neuronal density, Luxol Fast Blue for myelin integrity, and immunofluorescence for tau hyperphosphorylation. Molecular studies employed qPCR and Western blot to assess glutamate-related markers and tau phosphorylation. Melatonin (10 mg/kg) was administered intraperitoneally, starting either two weeks (early intervention) or four weeks (late intervention) post-induction.
Results
Key molecular targets in glutamate signaling pathways were identified using bioinformatics. AD-like mice displayed memory deficits and synaptic dysfunction. Melatonin improved cognitive function, especially with early intervention, as confirmed by behavioral tests. Histological studies revealed reduced neuronal loss, improved myelin integrity, and decreased tau hyperphosphorylation. Molecular findings showed restored mGluR expression and reduced GSK3 activity. Early intervention yielded superior outcomes, with partial restoration of synaptic plasticity observed in LTP recordings.
Conclusions
These findings underscore the neuroprotective properties of melatonin, mediated by its ability to modulate glutamate signaling and mGluR activity, offering new insights into its potential as a therapeutic agent for AD. Additionally, the results suggest that earlier administration of melatonin may significantly enhance its efficacy, highlighting the importance of timely intervention in neurodegenerative diseases.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12993-025-00271-4.
Keywords: Alzheimer's disease, Memory, Synaptic plasticity, Glutamate signaling, Melatonin, Metabotropic glutamate receptors, Cis-phospho tau
Background
Alzheimer's disease (AD) represents the most prevalent form of dementia, affecting approximately 30% of individuals aged 85 years or older. Clinically, it manifests through a spectrum of cognitive deficits and impaired neurological function. This multifaceted disease is pathologically characterized by the extracellular accumulation of amyloid-beta (Aβ) plaques and the intracellular aggregation of neurofibrillary tangles (NFTs) composed of hyperphosphorylated tau protein. Tau, a microtubule-associated protein, is crucial for neuronal stability and function.
Pathogenic post-translational modifications of tau protein, particularly hyperphosphorylation, lead to its detachment from microtubules, resulting in the formation of hyperphosphorylated tau, oligomeric tau, and misfolded tau—collectively referred to as tauopathies. These tauopathies progressively accumulate into insoluble NFTs and can be detected within the synapses of AD patients exhibiting cognitive impairments [1–3]. Tauopathies are potentially implicated in the disruption of key neurotransmitter pathways, notably glutamate, which is critical for memory and learning. Investigating the interplay between tau pathology and neurotransmitter disturbances, along with their associated signaling mechanisms, is imperative for a comprehensive understanding of the cognitive impairments underlying AD.
The significance of glutamate signaling and its receptors lies in their pivotal role in maintaining synaptic stability, plasticity, motor functions, learning, memory, and cognition. This neurotransmitter is fundamental in either sustaining cognitive health or contributing to neurodegenerative pathology. Elucidating the precise regulatory mechanisms of extracellular glutamate and its receptors is paramount to preventing excitotoxicity and neuronal loss, thereby mitigating the risk of various neurological disorders, including AD [4–6].
Glutamate mediates its effects by binding to ionotropic (iGluR) and metabotropic (mGluR) receptors—members of the G protein-coupled receptors (GPCRs) superfamily—on the cell surface. While iGluRs are primarily involved in fast synaptic transmission and have been extensively studied in the context of excitotoxicity and neurodegenerative diseases such as stroke and epilepsy, mGluRs regulate slower synaptic responses and are crucial for modulating intracellular signaling cascades, calcium homeostasis, and long-term synaptic plasticity. Recent studies have increasingly recognized mGluRs as critical regulators of neuronal and synaptic function. Unlike iGluRs, which directly mediate excitatory signals, mGluRs act through intracellular second messengers and are particularly relevant in modulating synaptic plasticity—a core aspect disrupted in AD. Dysregulation of mGluRs has been linked to abnormal signaling in the hippocampus, impaired learning, and memory, all hallmark features of AD. Furthermore, mGluRs signaling interacts closely with key pathological elements of AD, such as hyperphosphorylated tau, thereby bridging glutamate dysregulation with broader neurodegenerative mechanisms [7]. Despite their importance, the therapeutic potential of targeting mGluRs in AD remains underexplored compared to the well-documented role of iGluRs. This knowledge gap prompted our focus on mGluRs, aiming to unravel their contributions to AD pathology and assess their potential as therapeutic targets.
Despite recent advancements in developing novel therapeutic approaches for AD, currently approved treatments are predominantly "symptomatic therapies" that ameliorate cognitive and behavioral symptoms without addressing the underlying etiopathogenesis of the disease. The efficacy of these interventions varies significantly among AD patients. Enhancing cognitive function and ameliorating behavioral symptoms are primary objectives for patients with cognitive disorders, as these improvements profoundly impact their quality of life. Nonetheless, the exigent challenge in AD research is the development of interventions that delay disease onset or decelerate its progression, presenting substantial hurdles in pharmacological research [8, 9].
In this context, melatonin—a neurohormone with extensive neuroprotective and regulatory properties—has emerged as a promising candidate for addressing cognitive decline in AD. Clinical studies have consistently shown that melatonin levels are significantly reduced in AD patients, correlating with the severity of memory impairments. Despite substantial evidence supporting the beneficial effects of melatonin therapy in mitigating symptoms across a spectrum of severity in AD patients, the precise mechanisms by which melatonin exerts these effects remain to be fully elucidated [6, 7, 10]. It is postulated that melatonin, as a regulatory hormone, significantly influences the glutamatergic system, which is crucial for learning and memory [11, 12]. Dysregulation of glutamate signaling is a hallmark of AD, contributing to excitotoxicity and neuronal damage. Given its ability to directly influence critical pathways associated with cognitive decline, melatonin represents a scientifically sound and compelling therapeutic option for AD. Its selection in this study is grounded in robust evidence, highlighting its potential to prevent or slow the progression of AD-related cognitive impairments.
In this study, we investigated the therapeutic potential of melatonin in both preventing and treating an AD-like animal model in mice, with a particular focus on mGluR alterations. This focus was informed by bioinformatics analyses highlighting the critical role of the glutamatergic system in learning and memory disorders, as well as by the unique functions of mGluRs in synaptic plasticity, long-term potentiation, and intracellular signaling cascades. Unlike iGluRs, which mediate fast synaptic transmission, mGluRs regulate slower synaptic responses and are intricately linked to key AD pathologies, including tau hyperphosphorylation. By prioritizing mGluRs, this study aims to address significant gaps in understanding mGluR-related pathways in AD and their therapeutic relevance, while complementing the extensive body of research on iGluRs.
Methods
In silico study
To identify appropriate targets for investigating the molecular mechanisms underlying cognitive impairment in the AD-like model and the pathways affected by melatonin, a transcriptome analysis of brain changes in AD patients compared to control groups was first conducted. This analysis utilized RNA-seq datasets of the hippocampus from AD patients and age-matched controls, obtained from the Gene Expression Omnibus (GEO) database (GSE184942, GSE67333, and GSE236562). The selection criteria for these data sources included confirmed AD diagnosis in patients and the presence of validated plaques containing Aβ and NFTs in brain tissue samples. Additionally, genes associated with memory and learning pathways were identified using the Mouse Genome Database (MGD) and Gene Ontology (GO) Database. The analysis of raw RNA-seq data was carried out using a predefined pipeline[13] (Additional file 1).
Animal model of AD-like disease
Adult male C57BL/6 mice (25–27 g) were used. Animals were purchased from Razi Institute (Karaj, Iran) and housed at a temperature of 21°C ± 2°C, under a 12 h light/12 h dark cycle. Each mouse was housed individually in a standard plexiglas cage (60 cm in length, 40 cm in width, and 30 cm in height). Each cage was uniquely numbered, and the animals had ad libitum access to food and water. All experiments were conducted following the Guidelines for Care and Use of Laboratory Animals and approved by the “Ethics Committee of Tarbiat Modares University” (IR.MODARES.REC.1400.217).
AD-like animal model was induced as explained previously [14, 15]. Briefly, the mice were anesthetized with intraperitoneal injections of ketamine/xylazine (100 mg/kg / 10 mg/kg respectively) and subjected to stereotaxic surgery. The AD-like model was induced by bilateral injection of 1μl of 1 μg/μl of cis phospho-tau (cis p-tau) into each dorsal hippocampus (total dose of 2 μg per animal). Post-operative analgesics (buprenorphine, 0.1 mg/kg) were used to reduce post-surgery pain, and tetracycline antibiotic ointment was applied to the wound site to prevent infection.
Melatonin injection
The therapeutic effect of melatonin (Goldaru Pharmacological Company, Iran) was investigated in an AD-like mouse model. Previous experiments showed that intra-hippocampal cis p-tau induced a significant impairment in working memory, but not in spatial memory, at 2 weeks after injection. Significant impairment in spatial memory is observed at 4 weeks after injection [16]. Therefore, animals showed mild memory impairment at 2 weeks and severe memory impairment at 4 weeks following cis p-tau injection. Accordingly, in the first experiment, daily melatonin injection was started at 2 weeks following cis p-tau injection (i.p., 10 mg/kg [17]), and was continued for two weeks (Fig. 2A). In the second experiment, melatonin injection (i.p., 10 mg/kg) was started from the 4th week after intra-hippocampal cis p-tau and was continued for 4 weeks later.
Fig. 2.
Improvement of working memory impairment due to melatonin treatment in AD-like animal model. A Working memory assessment pre- and post-melatonin treatment when the melatonin injection (10 mg/kg) was initiated at the 2nd week post-induction. B Working memory assessment pre- and post-melatonin treatment when the melatonin (10 mg/kg) was initiated at the 4th week post-induction. The results represent the average performance of 12 samples per group, indicating observed trends in working memory improvement. *** p < 0.001 compared to control group, ### p < 0.001 compared to AD group
The study groups included AD-like animals without treatment (AD), AD-like animals under melatonin treatment (AD-Melatonin), untreated healthy control animals (Control), and healthy control animals under melatonin treatment (Control-Melatonin) (n = 12 in all groups).
Y-maze test
To assess the biological effect of melatonin treatment on short-term memory changes in animals in different study groups, the Y-Maze behavioral test was conducted weekly. The Y-maze consists of a Y-shaped gray plexiglas box (30 cm length, 10 cm width, and 15 cm height) for the working memory task. The animal was put at the end of one area and allowed to explore freely during an 8-min session. An alternation is determined as successive entries into all three arms. Next, after recording the number of arm entries and alternations, the percentage of the alternation behavior was calculated by the below formula:
A spontaneous alternation happens when a mouse enters a different arm of the maze in each of 3 consecutive arm entries (i.e., visit from A to B or C, which are designated to the other arms, respectively). In addition, incorrect trials are considered to travel back to a previously experienced arm, such as CBC moving. For assessment, all movements were recorded using a video camera, and the data obtained were analyzed according to a standard protocol and also using EthoVision 11 software (Noldus Information Technology, Wageningen, The Netherlands).
Barnes maze test
The Barnes maze test was employed to assess spatial learning and memory in the AD-like animal model [18]. This maze was a circular platform (diameter: 92 cm, height: 50 cm) with 20 holes (diameter: 5 cm) evenly spaced around the perimeter. One of the holes was designated as the "goal hole", leading to a small escape box hidden underneath (goal box). Three distal visual cues (geometric shapes: triangle, circle, square) were placed equidistantly on the surrounding walls for spatial orientation.
Mice underwent four consecutive days of training, with three trials per day and an inter-trial interval of 15 min. Each trial lasted for a maximum of 5 min. Mice were placed at the center of the maze in a start box (12.5 × 8 × 8 cm) for 10 s before being allowed to explore. The trial ended when the mouse entered the goal box, or after 5 min had elapsed. The measured parameters included: primary latency (time to locate the goal hole for the first time), total latency (time to enter the goal box), primary errors (number of incorrect holes explored before locating the goal box), and total errors (total number of incorrect holes explored).
The probe test was conducted 24 h after the last training session. The goal box was removed, and mice were allowed to explore the maze for 90 s. The goal hole exploration frequency, non-goal hole exploration frequency, and goal sector preference (ratio of goal hole exploration to non-goal exploration) were recorded. These parameters assessed the retention of spatial memory.
All trials were recorded with a video tracking system (EthoVision XT 11, Noldus, Wageningen, Netherlands). Data were analyzed for spatial learning during training and memory retention during the probe phase.
Histological assessment
To assess the tissue changes in the brains of AD-like mouse models and the effect of melatonin treatment, histological examinations were performed. For these analyses, one hemisphere of the brain was used for each sample. Tissue preparation was carried out using a standard method [19], ultimately coronal hippocampal sections with a thickness of 8 μm were prepared. For histological studies, 5 samples per group were included.
Luxol Fast Blue (Sigma-Aldrich, Cat. No. 212171000) staining was utilized to assess myelin changes in the hippocampus of AD-like animal models and those under melatonin treatment [20]. Evaluation of the desired tissue changes was conducted using a light microscope. The images were analyzed using ImageJ v1.43 software (NIH, Bethesda, MD, USA).
Nissl staining method was employed to evaluate the extent of cellular degeneration in the brain tissue of an AD-like mouse model [21]. Evaluation of the desired tissue changes w performed using a light microscope.
To examine the changes in tauopathy levels in the study animals, immunofluorescence staining with cis pT231-tau mAb was conducted on the prepared tissue sections. The specimens were observed using a fluorescent microscope (Olympus, BX51 with Olympus DP72 digital camera), and the images were analyzed using ImageJ v1.43 software.
Molecular assessment
For assessing changes in gene expression and protein levels using qPCR and Western blot techniques, 5 samples per group were included.
Gene expression analysis
Total RNA was extracted from hippocampal tissue samples using RiboEx (GenALL, Korea, Cat. No. 301–001/301–002) according to the manufacturer's protocol. To eliminate DNA contamination from the total RNA, the extracted samples were treated with DNase I (Fermentas, Lithuania, Cat. No. EN0521). The quality and quantity of the extracted RNA were assessed by 1% agarose (Sigma-Aldrich, CAS No. 9012–36-6) gel electrophoresis, and spectrophotometry, respectively. cDNA synthesis was performed using 3 µg of total RNA with a Thermo Scientific cDNA synthesis kit (USA, Cat. No. K1622) following the manufacturer's protocol. The expression of target genes was analyzed using the qPCR technique on the StepOne ABI system (Applied Biosystems, USA) with HOT FIREPol 5X Eva Green (Solis BioDyne, Estonia, Cat. No. 08–24-0000S). The genes examined at the transcriptome level included Gls1, Glul, Grm1, Grm5, Grm2, Grm3, Grm4, Grm7, Grm8, Gsk3α, and Gsk3β, with Gapdh serving as an internal control. The primers designed for gene expression analysis are listed in Table 1. All reactions were performed at least in duplicate for each biological sample, and the specificity of PCR products was confirmed by performing a melt curve analysis and agarose gel electrophoresis. mRNA expression analysis of target genes was conducted using 2−ΔCt and Fold Change methods with 2−ΔΔCt calculation [22].
Table 1.
Primers sequences were used in this study to investigate gene expression changes using the qPCR technique
| Gene | Transcript | Primer Name | Primer Sequence (5ʹ → 3ʹ) |
Amplicon length(bp) |
|---|---|---|---|---|
| Gapdh | NM_008084.4 | Forward | AACGGGAAGCTCACTGGCATGG | 304 |
| Reverse | CCACCACCCTGTTGCTGTAGCC | |||
| Gls1 | NM_001081081.2 | Forward | GCACAGACATGGTTGGGATAC | 304 |
| Reverse | AGAGGAGGAGACCAACACATC | |||
| Glul | NM_008131.5 | Forward | AGGCTTAGGTTTAGGGGATGC | 328 |
| Reverse | GCAGAGGTGACACTAGGAGAC | |||
| Grm1 | NM_016976.3 | Forward | AGCCCTGACTGCACAAGTCCCAC | 231 |
| Reverse | GGTGGAGCTTTGTAAGTTTGAGG | |||
| Grm5 | NM_001081414.2 | Forward | CATGACAGCCGATGACAACAC | 328 |
| Reverse | AGGTTGACTTTTTGGTCCCAG | |||
| Grm2 | NM_001160353.1 | Forward | ACCTCCAGTGATTATCGGGTGC | 241 |
| Reverse | CACGGCCATTGCAAACAGTAGG | |||
| Grm3 | NM_181850.3 | Forward | AGCATGTTGATCTCTCTGACC | 320 |
| Reverse | TTGAGGTGAAGTCTGTGTGTG | |||
| Grm4 | NM_001013385.2 | Forward | TGCCAGCCCATACCCATTGTC | 315 |
| Reverse | GATGCGGTTGGTCTTGGTCAG | |||
| Grm7 | NM_177328.3 | Forward | ACAGCTCCCAGACTCATAAGC | 272 |
| Reverse | TCTGGTACACCCCGAGTCTTG | |||
| Grm8 | NM_008174.4 | Forward | TGGAGAACAGCGAACACTGG | 319 |
| Reverse | TCCCAGAGACACTGAAGCAC | |||
| Gsk3α | NM_008174.4 | Forward | AAGTTCCCCCAGATCAAAGC | 274 |
| Reverse | AGTGAGGAGGGATGAGAATGG | |||
| Gsk3β | NM_001031667.1 | Forward | GACTGACCCAATGTCCATGGTG | 255 |
| Reverse | ATGTGTAGACAGGAGGTGCAGC |
Protein expression analysis
To investigate the protein changes of GLS1, Glul, GSK3, and Phospho-GSK3, the Western blotting technique was employed. Total protein was extracted from hippocampal tissues using RIPA buffer (50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 1% NP-40, 0.1% SDS, 0.5% sodium deoxycholate) supplemented with protease and phosphatase inhibitors. The protein concentrations were determined using the Bradford method, and equal amounts (20 μg) of the pooled samples from each group were loaded per lane. Proteins were separated by sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) using 12% polyacrylamide gels depending on the target protein’s molecular weight. Following electrophoresis, proteins were transferred onto poly (vinylidene fluoride) (PVDF) membranes (Millipore) using a semi-dry transfer system at 20V for 1 h. The membranes were then blocked for 1 h at room temperature with 5% non-fat dry milk in TBS-T (Tris-buffered saline containing 0.1% Tween-20) to prevent non-specific binding. Following blocking, the membranes were incubated overnight at 4°C with primary antibodies diluted in TBS-T containing 5% bovine serum albumin (BSA). After washing three times with TBS-T, the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies diluted in TBS-T for 1 h at room temperature. The membranes were washed again three times with TBS-T and then visualized using an enhanced chemiluminescence (ECL) detection system (Bio-Rad). The resulting bands were quantified using ImageJ software, and the relative expression levels of the target proteins were normalized to β-actin as the loading control. The specifications of the secondary antibodies used for evaluating the expression levels of each protein are detailed in Table 2.
Table 2.
Specifications of primary antibodies used in this study for investigating protein changes using the Western blotting technique
| Antibody | Detected Antigen | Molecular Weight | Isotype | Catalog Number | Company |
|---|---|---|---|---|---|
| β-Actin (2A3) | β-Actin | 43 KDa | monoclonal antibody | sc-517582 | Santa Cruz Biotechnology |
| GLS1(KGA/GAC) | Glutaminase (GLS1)-KGA isoform | 58 KDa, | Polyclonal antibody | 12,855–1-AP | Proteintech |
| Glutaminase (GLS1)-GAC isoform | 65 KDa | ||||
| Gl Syn (F-4) | Glutamine synthetase (GI Syn) or GLUL | 49 KDa | monoclonal antibody | sc-398034 | Santa Cruz Biotechnology |
| GSK-3α/β (0011-A) | Glycogen synthase kinase 3α(GSK-3α) | 51 KDa | monoclonal antibody | sc-7291 | Santa Cruz Biotechnology |
| Glycogen synthase kinase 3β (GSK-3β) | 47 KDa | ||||
| p-GSK-3a/b (6D3) |
Tyr 279 phosphorylated GSK-3a |
51 KDa | monoclonal antibody | sc-7292 | Santa Cruz Biotechnology |
| Tyr 216 phosphorylated GSK-3b | 47 KDa |
Field potential recording in hippocampal slices
The effect of melatonin on synaptic plasticity of the hippocampal CA1 area was investigated by in vitro field potential recording in hippocampal slices. In summary, animals were anesthetized by CO2, and their brain was removed quickly. 400-μm thick coronal slices containing the hippocampus were prepared by a vibratome and were incubated in artificial cerebrospinal fluid (aCSF) at room temperature for at least 1 h. The aCSF contained (in mM): NaCl 124, NaHCO3 26, KH2PO4 1.25, KCl 5, CaCl2 2, MgCl2 2.06, and d-glucose 10, and its pH was 7.3–7.4. The slices were transferred to an interface-type recording chamber containing aCSF solution. Field excitatory post-synaptic potential (fEPSPs) was recorded from the stratum radiatum in the dorsal hippocampus while Schaffer collaterals were stimulated. An input/output curve was plotted to calculate test pulse intensity (i.e., the intensity that produced 50% of maximum response), and evoked field potentials were recorded from the CA1 region at test pulse intensity for 20 min. To generate LTP, primed-burst stimulation (PBS) was applied, and responses were recorded for 60 min post-PBS.
Experimental groups
The experimental design included four distinct groups, each consisting of 12 animals:
Control Group (Control): Healthy mice with no treatment, serving as the baseline for comparison. Melatonin Control Group (Control-Melatonin): Healthy mice treated with melatonin (intraperitoneal injection, 10 mg/kg) for a duration-matched to the AD-melatonin groups, used to assess any effects of melatonin in non-diseased animals. AD-like Group (AD): Mice injected with 1 μg of cis-phospho tau (cis p-tau concentration; 1 μg/μl) into each dorsal hippocampus to induce an AD-like condition, with no further treatment, serving as the disease model. AD + Melatonin Group (AD-Melatonin): Mice subjected to cis p-tau injection to induce the AD-like condition, followed by daily intraperitoneal injections of melatonin (10 mg/kg). Two subgroups were included based on the timing of melatonin administration: AD-Melatonin (2 Weeks): Treatment initiated two weeks post-cis-phospho tau injection and continued for two weeks to investigate early intervention effects. AD-Melatonin (4 Weeks): Treatment initiated four weeks post-cis-phospho tau injection and continued for four weeks to assess the effects of later intervention. Each experimental group was monitored for behavioral, histological, molecular, and electrophysiological changes throughout the study.
Statistical analysis
Statistical analyses were performed using GraphPad Prism software, version 9.0 (GraphPad Software, San Diego, CA, USA). Data were presented as mean ± standard error of the mean (SEM). The normal distribution of data was tested by the Kolmogrov-Sminnov test. For comparisons among multiple groups, a one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test was applied. When comparing two groups, a Student's t-test was used. Behavioral data were analyzed using two-way ANOVA to evaluate treatment effects over time. All statistical tests were two-tailed, and a p-value of less than 0.05 (p < 0.05) was considered statistically significant.
A priori power analysis was conducted to determine the appropriate sample size. For behavioral tests, an effect size of 0.5, a significance level (α) of 0.05, and a statistical power of 0.80 were used, resulting in a minimum of 12 animals per group. For molecular, histological, and electrophysiological analyses, a subset of 5 animals per group was used, as these methods require more focused sampling.
Results
Identification of the glutamatergic system as a candidate using in silico study
In silico analyses were employed to uncover potential molecular mechanisms through which melatonin might ameliorate cognitive impairments. For RNA-seq analysis, data from the hippocampal tissues of AD patients were obtained from the datasets GSE67333, GSE184942, and GSE236562. These datasets were selected based on similar mean ages and disease stages (ABC Scoring system) (Table 3).
Table 3.
Demographic data of RNA sequencing datasets
| GEO accession | GSE67333 | GSE184942 | GSE236562 | |||
|---|---|---|---|---|---|---|
| Platforms | Illumina HiSeq 2000 | Illumina HiSeq 2000 | Illumina HiSeq 4000 | |||
| AD | Control | AD | Control | AD | Control | |
| Number of Samples | 4 | 4 | 5 | 5 | 4 | 4 |
| Average of Age | 83.5 ± 1.2 | 83.75 ± 1.5 | 84.33 ± 2.1 | 81.95 ± 3 | 82.3 ± 1.7 | 80.45 ± 2.2 |
| ABC score | ||||||
| A | 2.63≈3 | 0.673913 | 2.769231 | 1.045455 | 2.8 ± 0.5 | N/a |
| B | 2.78≈3 | 1.130435 | 2.692308 | 0.863636 | 2.5 ± 0.6 | N/a |
| C | 2.54 | 0.902174 | 2.846154 | 0.5 | 1.8 ± 1.6 | N/a |
RNA-seq analysis generated the Gene Count Table, and Batch Effect Correction was performed using the ComBat-Seq model [23]. Differential Gene Expression (DEG) analysis was conducted using the DeSeq2 package in R. Target genes were filtered from the Mouse Genome Database (MGD) and the Gene Ontology (GO) Database, focusing on learning, memory, neuroplasticity, and behavior regulation genes. Given the significant role of GPCR receptors in neurodegenerative disorders, a GPCR gene list was sourced from the HUGO Gene Nomenclature Committee (HGNC) database[24].
With criteria of −0.5 ≥ log2FC ≥ 0.5 and 0.05 ≥ p-value, a Volcano plot was generated for candidate genes (Fig. 1A) (Additional file 2). Principal Component Analysis (PCA) illustrated gene expression differences between control and AD samples (Fig. 1B). The glutamatergic system and mGluR receptors were selected for molecular investigation (Fig. 1C).
Fig. 1.
Transcriptome analysis and gene expression changes in the hippocampus of AD patients. A Volcano plot depicting gene expression changes. The x-axis represents log2 fold change (log2FC), and the y-axis represents the p-value. Genes with −0.5 ≤ log2FC ≤ 0.5 and p-value ≥ 0.05 are considered significant. Blue dots on the right indicate increased expression, while dots on the left indicate decreased expression in AD samples. Red dots represent GPCR genes outside the specified range. This plot was generated using the ggplot2 package in R. B Principal Component Analysis (PCA) graph illustrating differences between control and AD samples based on gene expression. C Heatmap showing significant expression changes in genes involved in the glutamatergic system. Each row on the Y-axis represents a single gene, and dendrograms represent genes with similar expressions. The X-axis represents all samples, and the dendrogram clusters control and patient samples. The color gradient from blue to red indicates low to high expression levels. This plot was created using the p-heatmap package in R
The therapeutic effect of melatonin on working memory impairment in AD-like animals
In the first experiment, we investigated whether melatonin had a therapeutic effect in animals with mild memory impairment, AD-like model mice received melatonin at two weeks post-cis-P tau injection, and the working memory performance was assessed using the Y-Maze test. A two-way ANOVA was performed to assess the significance of differences in working memory performance across study groups and time points. The results demonstrated a statistically significant difference among the study groups (F (2,33) = 251.5, p < 0.0001) and across the evaluated time points (F (3,99) = 450, p < 0.0001). Melatonin facilitated working memory in treated AD-like mice (Fig. 2A, AD-Melatonin). As Fig. 2A shows, the spontaneous alternation was significantly increased in these animals after 2 weeks of treatment compared to the AD-like group (t = 20.88, p < 0.001). Interestingly, there was no significant difference in working memory between treated animals and the control group in 4th week (Fig. 2A). In contrast, untreated AD-like mice (Fig. 2A, AD) exhibited progressive memory impairment (t = 29.55, p < 0.0001).
In the second experiment, the therapeutic action of melatonin was evaluated after establishing a severe memory impairment in animals. Melatonin treatment was started at the fourth-week post-cis p-tau injection and continued for 4 weeks. The results of two-way ANOVA demonstrated a statistically significant difference among the study groups (F (2,33) = 679.7, p < 0.0001) and across the evaluated time points (F (7,245) = 49.79, p < 0.0001). The obtained results showed the improving effect of melatonin on working memory, so the spontaneous alternation was increased in the AD-melatonin group compared to the AD group (t = 11.45, p < 0.0001). The significant increase in spontaneous alternation was started after the 2nd week of melatonin injection (i.e., at the 6th to 8th weeks of the experiment; Fig. 2B). However, the memory impairment remained in these animals and did not reach the control values (Fig. 2B) (t = 35.38, p < 0.0001). These findings suggested that earlier melatonin intervention yielded better cognitive improvement.
The therapeutic effect of melatonin on spatial memory and learning impairment in AD-like animals
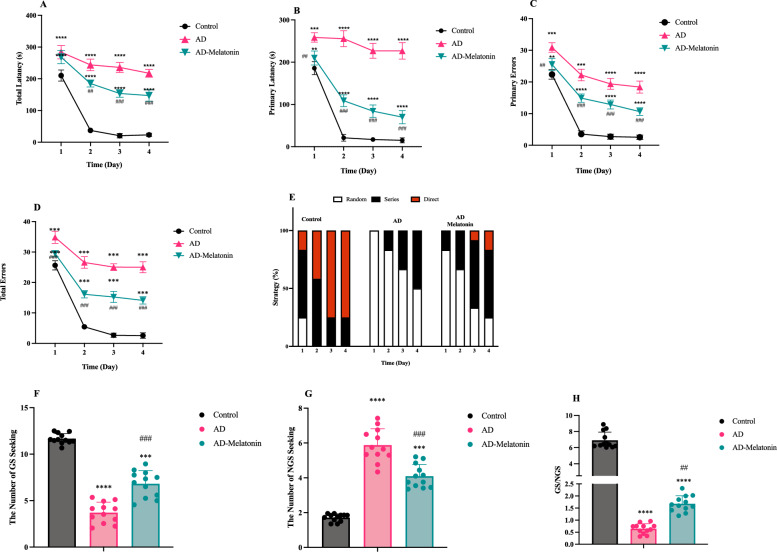
Spatial learning impairments were evident in the AD group (Figs. 3 and 4), as demonstrated by prolonged primary (Figs. 3B and 4A) (F (1, 88) = 1848, p < 0.0001 and F (1, 88) = 4195, p < 0.0001, AD 4 weeks and AD 8 weeks respectively) and total latencies (Figs. 3C and 4B) (F (1, 88) = 1499, p < 0.0001 F (1, 88) = 3645, p < 0.0001, respectively) and increased primary (Fig. 3D and 4C) F (1, 88) = 778.0, p < 0.0001 and F (1, 88) = 2612, p < 0.0001, respectively) and total errors (Figs. 3E and 4D) (F (1, 88) = 7337, p < 0.0001 and F (1, 88) = 4168, p < 0.0001, respectively) across training days. In contrast, the AD-Melatonin group showed significant improvement in all parameters (For primary latencies: F (1, 64) = 765.6, p < 0.0001 and F (1, 64) = 898.7, p < 0.0001, AD 4 weeks-Melatonin and AD 8 weeks-Melatonin respectively. For total latencies: F (1, 64) = 758.7, p < 0.0001, and F (1, 64) = 182.0, p < 0.0001, respectively. For primary error: F (1, 64) = 409.6, p < 0.0001, F (1, 64) = 264.4, and for total error: F (1, 64) = 3744, p < 0.0001 and F (1, 64) = 505.6, p < 0.0001, respectively), achieving performance comparable to controls when melatonin treatment was initiated two weeks post-Cis-Phospho Tau injection. The strategy to find the goal box was also assessed (Figs. 3F and 4E). The random strategy shows the deficiency in animal’s learning and memory. In serial strategy, the animal's learning increases, and the subject can find the goal box using its memory. Delayed treatment (initiated four weeks post-injection) improved learning metrics but did not fully restore performance to control levels.
Fig. 3.
Improvement of spatial memory and learning impairment due to melatonin treatment initiated at the 2nd-week post-induction. A Diagram illustrating the circular Barnes maze with 20 equally spaced holes, including the goal sector (GS) and non-goal sector (NGS). BPrimary latency to locate the goal hole. C: Total latency to enter the goal box. D Primary errors during exploration. E Total errors across training days. F Search strategies (direct, serial, and random) adopted by animals across training days. G Goal sector exploration frequency. H Non-goal sector exploration frequency. I Goal sector preference (goal/non-goal exploration ratio). The results represent the average performance of 12 samples per group, and data are presented as mean ± SEM. Statistical significance: *** p < 0.001 compared to the control group, ### p < 0.001 compared to the AD group
Fig. 4.
Reduced improvement in spatial memory and learning impairment due to delayed melatonin treatment initiated at the 4th-week post-induction. A Primary latency to locate the goal hole. B Total latency to enter the goal box. C: Primary errors during exploration. D Total errors across training days. E Search strategies (direct, serial, and random) adopted by animals across training days. F Goal sector exploration frequency. G Non-goal sector exploration frequency. H Goal sector preference (goal/non-goal exploration ratio). The results represent the average performance of 12 samples per group, and data are presented as mean ± SEM. Statistical significance: *** p < 0.001 compared to the control group, ### p < 0.001 compared to the AD group
The probe test revealed significant memory and learning deficits in the AD group (Figs. 3G–I, and 4F–H), as indicated by reduced goal sector (GS) preference (t = 22.28, p < 0.0001 and t = 22.04, p < 0.0001, AD 4 weeks-Melatonin and AD 8 weeks-Melatonin respectively) and increased non-goal (NGS) exploration (t = 9.225, p < 0.0001 and t = 14.90, p < 0.0001, AD 4 weeks-Melatonin and AD 8 weeks-Melatonin respectively). Melatonin treatment initiated two weeks post-injection restored these parameters (for GS: t = 10.27, p < 0.0001 and for NGS: t = 6.323, p < 0.0001, compart to AD group) to control levels. Delayed treatment resulted in partial recovery, with significant improvement compared to the AD group but not reaching control levels.
Melatonin treatment reduces myelin sheath degradation and neuronal death in the hippocampus of AD-Like model
Recent studies have indicated that cognitive impairments may reflect the degradation and deficiency of myelin in the brains of animal models [25]. Therefore, Luxol Fast Blue.
Staining was used to examine myelin changes and the effect of melatonin treatment in AD-like model animals (Fig. 5A). Results revealed significant thinning of the myelin sheath in AD-like model groups, more pronounced at the 8th week (Fig. 5B) (t = 8.586, p = 0.001, and t = 18.13, p = 0.0001, respectively). Melatonin treatment initiated in the 2nd week post-induction significantly increased Luxol Fast Blue intensity (t = 3.707, p = 0.01), although it did not restore levels to those of the control group. However, melatonin treatment initiated at the 4th-week post-induction did not improve myelin damage (t = 1.751, p > 0.05).
Fig. 5.
The effect of melatonin on reducing CA1 hippocampal tissue damage in the AD-like model. A Luxol Fast Blue (LFB) staining of the CA1 hippocampal region, illustrating the extent of myelin preservation and demyelination across different experimental groups. LFB is a histological stain that specifically binds to lipoproteins in the myelin sheath, allowing visualization of myelin integrity. Intact myelin appears deep blue, whereas demyelinated or damaged regions show lighter staining or loss of staining. This technique is particularly relevant in neurodegenerative conditions such as AD, where white matter integrity is progressively compromised. B Graph showing the quantitative intensity of LFB staining, representing the average percentage of myelin staining in samples from each group. This provides a comparative assessment of myelin loss and its potential restoration following melatonin treatment. C Nissl staining indicating neuronal death, with arrows pointing to degenerated or pyknotic cells in the CA1 region. C: Nissl staining indicating neuronal death, with arrows pointing to dead cells. D Quantitative analysis of the percentage of dead cells in the CA1 region, with data averaged across the samples in each group. The tissue samples displayed in the histological images include Control animals, an AD-like model with 4 weeks of disorder (AD 4W), and model under melatonin treatment (AD 4W-Melatonin), an AD-like model with 8 weeks of disorder (AD 4W), and model under melatonin treatment (AD 8W-Melatonin). The groups in the graphs are presented as Control animals, AD-like models (AD) with 4 weeks (4W) and 8 weeks (8W) of disorder, and models under melatonin treatment (AD-Melatonin) starting from the second-week post-induction (4W) and starting from the fourth-week post-induction (8W). The symbol * indicates statistically significant differences compared to the Control, and the symbol # compared to the model animals without treatment
Previous reports have shown that neuronal death in the hippocampus can lead to cognitive impairments; thus, neuronal death was assessed using Nissl staining in the CA1 region of the hippocampus across the study groups. Nissl staining showed increased neuronal death in AD-like models (Fig. 5C, D) (t = 10.46, p = 0.0005), with a greater extent of death at the 8th week (t = 9.889, p = 0.0006). Melatonin treatment significantly reduced neuronal death (t = 10.46, p = 0.01 and t = 4.331, p < 0.05, respectively), confirming that earlier intervention was more effective.
Positive impact of melatonin treatment on pathogenic changes in Gls1 and Glul protein levels in the AD-Like animal model
Considering the importance of glutamate in cognitive activities such as memory, and the findings from the in-silico study, we examined the changes in transcriptomic (Additional file 3) and protein levels of two key enzymes involved in glutamate homeostasis, Gls1 and Glul, in hippocampal samples from animal model groups treated with melatonin.
Western blot analysis revealed increased Gls1 protein levels in AD-like models (Fig. 6A–C). Descriptive analysis showed that melatonin treatment initiated in the 2nd-week post-induction reduced Gls1-65 KDa protein levels, although they did not return to control levels. The 58 KDa isoform of Gls1 remained mostly unchanged in the early stages but showed a reduction with later treatment. Similarly, Glul protein levels (Fig. 6D), which were decreased in AD-like models, demonstrated partial restoration following melatonin treatment.
Fig. 6.
The effect of melatonin treatment on Gls1, Glul, GSK3, and phosphorylated GSK3 levels in AD-like models. A Western blot for protein quantification in study animals, including Control animals, AD-like model at 4 weeks (AD 4W) and 8 weeks (AD 8W) post-induction, and under melatonin treatment initiated at two different time points. B Gls1-65 KDa protein levels across the experimental groups. C Gls1-58 KDa protein levels, illustrating the relative changes among the groups. D Glul protein levels showing trends of variation in response to melatonin treatment. E GSK3α protein levels across the groups. F GSK3β protein levels, highlighting differences between treated and untreated groups. G Phosphorylated GSK3α levels, indicating changes in kinase activity under melatonin treatment. H Phosphorylated GSK3β levels, showing the effect of treatment on kinase activity. The results were derived from pooled samples, with 5 samples pooled per group. Due to pooling, statistical analysis was not performed, and the data are presented descriptively
In summary, melatonin treatment had a positive effect in modulating pathogenic changes in Gls1 and Glul protein levels, which are critical for maintaining glutamate homeostasis and cognitive function. The effectiveness of melatonin was more pronounced when treatment started earlier, highlighting the importance of early intervention in neurodegenerative conditions.
Positive impact of melatonin treatment on gene expression changes in mGluR receptors observed in AD-Like animal models
Our literature review and bioinformatics analyses indicated that metabotropic glutamate receptors (mGluRs) play crucial roles in various CNS functions such as learning, memory, and synaptic plasticity. Therefore, we investigated these GPCRs as functional mediators of cognitive impairment in our AD-like animal models and assessed the effects of melatonin treatment on their expression patterns. mGluRs are widely distributed throughout the CNS, responsible for synaptic modulation, and are classified into three subgroups based on their signaling pathways. Group I mGluRs include mGluR1 and mGluR5 (gene names: Grm1, Grm5), Group II includes mGluR2 and mGluR3 (gene names: Grm2, Grm3), and Group III includes mGluR4, mGluR6, mGluR7, and mGluR8 (gene names: Grm4, Grm6, Grm7, Grm8). This study examined the transcriptomic changes of seven mGluRs, with the qPCR data heatmap displayed in Fig. 7 reflecting Grm expression levels in the animal model groups studied (Additional file 4).
Fig. 7.
Positive effect of melatonin treatment on mGluRs expression in the AD-like models. Heat map created from qPCR data reflecting the expression of Grm genes across the study animal groups. Columns represent the studied animal samples, including Control, AD-like models with 4 weeks of disorder (AD 4W) and those under melatonin treatment (AD 4W Melatonin), AD-like models with 8 weeks of disorder (AD 8W), and models under melatonin treatment (AD 8W-Melatonin). Rows represent different Grm genes, including Grm1 and Grm5 related to the mGluR I group, Grm2, and Grm3 related to the mGluR II group, and Grm4, Grm7, and Grm8 related to mGluR III group. The intensity of color corresponds to the relative expression for each gene calculated using 2^−∆Ct. The color gradient from red to white indicates high to low expression levels, reflecting relative gene expression among the different groups. Results are presented as the average values from independent biological samples (n = 5) within each group
qPCR analysis showed increased Grm1 and Grm5 expression in 4-week AD-like models (Fig. 7) (t = 35.63 and t = 39.76, p < 0.0001), but melatonin treatment reduced their expression significantly (t = 31.48 and t = 37.46, p < 0.0001). Conversely, 8-week models showed decreased expression (t = 8.048 and t = 12.90, p ≤ 0.001), however, melatonin treatment partially restoring levels (t = 4.63, p = 0.0001 and t = 7.92, p ≤ 0.01, respectively), suggesting progressive synaptic and cellular deterioration over time in untreated AD-like conditions.
The expression levels of Grm2 (t = 7.440, p = 0.01; t = 7.96, p = 0.01) and Grm3 (t = 5.47, p = 0.01; t = 23.22, p < 0.0001) were significantly elevated in both the 4-week and 8-week AD-like models compared to controls (Fig. 7, Additional File 4). Early melatonin treatment effectively attenuated these increases, bringing Grm2 and Grm3 expression levels closer to control values (t = 6.656, p = 0.01; t = 6.413, p = 0.01) in the 4-week models. However, in the 8-week models, late melatonin treatment exhibited only partial restorative effects on Grm3, with negligible impact on Grm2, highlighting the critical importance of early intervention. These findings suggest that the initial upregulation of mGluR2 and mGluR3 in the 4-week models may represent a compensatory response to early excitotoxicity and synaptic stress. However, as the disease progresses (8-week models), neuroinflammatory pathways and tau pathology might contribute to receptor desensitization and reduced expression, leading to further synaptic dysfunction.
Grm4 expression increased significantly in 4-week models (t = 7.166, p = 0.01), while Grm7 showed no significant change. Grm8 expression decreased slightly but significantly (t = 3.67, p < 0.05). Melatonin treatment improved Grm7 and Grm8 levels when initiated early (t = 6.46, p = 0.01 and t = 15.22, p = 0.001, respectively).
Overall, our findings demonstrate that melatonin treatment positively impacted the expression of multiple mGluR genes, which are essential for cognitive function and synaptic regulation. Importantly, the effect was more pronounced when treatment was initiated early, reinforcing the significance of timely therapeutic intervention in neurodegenerative conditions.
These results suggest that mGluR dysregulation in AD probably is dynamic and time-dependent, with early alterations likely representing a compensatory mechanism, whereas later reductions may reflect progressive neurodegeneration and receptor desensitization. The ability of melatonin to restore mGluR expression in early-stage models highlights its potential neuroprotective role in modulating glutamatergic signaling, which may contribute to its beneficial effects on cognitive impairment in AD-like conditions.
Melatonin treatment reduces pathogenic phospho-tau levels and GSK3 kinase activity in AD-like animal model
Given the well-established role of the kinase enzyme GSK3 in tau phosphorylation and the pathobiology of AD, along with its downstream targeting by the receptors studied, we examined the gene expression (Additional file 5), protein levels, and phosphorylation of the two GSK3 enzyme isoforms, GSK3α and GSK3β, in the animal model groups. The activity of these kinase isoforms is indicated by the phosphorylation of tyrosine residue 279 in GSK3α (pTyr279) and tyrosine 216 in GSK3β (pTyr216).
Western blot results indicated increased GSK3α and GSK3β levels in AD-like models. Descriptively, melatonin treatment appeared to reduce both GSK3α and GSK3β protein levels and their phosphorylation status in these models.
One of the targets of GSK3 kinase is the phosphorylation of the threonine 231 residue of the tau protein (pT231-tau). Therefore, to investigate the tissue changes in phosphorylated tau levels under melatonin treatment in the animal models, we performed immunofluorescence staining for pT231-tau (Fig. 8A). Immunofluorescence staining for pT231-tau showed significant increases in both models (Fig. 8B) (t = 15.70, p < 0.0001 and t = 25.90, p < 0.0001, respectively), which melatonin treatment reduced (t = 9.93, p = 0.001 and t = 5.52, p = 0.01, respectively), though not to baseline.
Fig. 8.
Melatonin treatment leads to a decrease in tau phosphorylation levels in the AD-Like model. A Immunofluorescence staining for pT231-tau (Green = cis pT231-tau, Blue = DAPI). B Quantitative graph of pT231-tau. Results are presented as the average values from independent biological samples (n = 5) within each group. Groups in the images are Control, AD-like model with 4 weeks of disorder (AD 4W) and under melatonin treatment from the second-week post-induction (AD 4W Melatonin), AD-like model with 8 weeks of disorder (AD 8W) and under melatonin treatment from the fourth-week post-induction (AD 8W Melatonin); and in the graphs as Control animals, AD-like models (AD) with 4 weeks (4W) and 8 weeks (8W) of disorder, and models under melatonin treatment (AD-Melatonin) starting from the second-week post-induction (4W) and starting from the fourth-week post-induction (8W). The symbol * indicates statistically significant differences compared to the Control, and the symbol # compared to model animals without treatment
These findings suggest that melatonin treatment can modulate the pathogenic activity of GSK3 and the phosphorylation of tau protein in an AD-like animal model. The results emphasize the potential of melatonin as a therapeutic agent targeting kinase pathways to mitigate the progression of AD-related pathology, though earlier interventions proved more effective.
Restoring effect of melatonin treatment on LTP generation in AD-like model
Following the observed improving effect of melatonin on working memory and changes in mGluR receptors in AD-like model animals, and considering the impact of these receptors on synaptic plasticity, the effect of melatonin was investigated on LTP generation in the AD-like model. Field potential recordings showed a significant decrease in LTP induction (t = 2.459, p < 0.05) and maintenance (t = 3.11, p < 0.01) in AD-like (Fig. 9A and B). Melatonin treatment restored LTP generation when administered in the second week after cis-P tau injection. There was a significant difference in LTP induction (t = 1.146, p < 0.05) and maintenance (t = 2.155, p < 0.01) between AD-melatonin-2nd week and AD groups (Fig. 9). However, melatonin injected at 4th week after cis-P tau injection had no significant effect on LTP (Fig. 9). These results revealed that the restoring effect of melatonin on LTP may observe only in animals with mild memory impairment.
Fig. 9.
Improvement of long-term potentiation (LTP) generation by melatonin in the AD-like animal model. A Timeline curve showing the impairment in LTP generation in the AD group that was restored when melatonin was injected in the 2nd week, but not in the 4th week, after pT231-tau injection in the AD-Like animal model. B Percentage of LTP induction and maintenance in different experimental groups animal groups. Each group consisted of 6 samples, with 3 technical replicates per sample. * p < 0.05 compared to the Control group
Discussion
In this study, an AD-like animal model exhibiting working and spatial memory impairment was employed. Behavioral manifestations showed demyelination, neuronal death, and degradation in the hippocampal region, contributing to cognitive impairments (Fig. 5). Melatonin, a neurohormone that decreases in neurodegenerative disorders[10], was used to treat these impairments. Our results indicated significant improvement in working and spatial memory (Figs. 2,3 and 4) and tissue damage (Fig. 5) in melatonin-treated AD-like model animals, consistent with recent studies on melatonin’s positive effects on spatial learning, memory, and synaptic plasticity in AD animal models [26–29]. Additionally, our findings highlighted the molecular mechanism and the importance of administration timing in melatonin’s therapeutic efficacy.
Bioinformatics analyses were conducted to compare transcriptome differences in the hippocampus of AD patients and control individuals, focusing on genes associated with learning and memory. Our analyses suggested the potential role of genes related to the glutamatergic system, especially key enzymes involved in glutamate homeostasis and its receptors (Fig. 10). Various studies have emphasized the physiological importance of glutamate in synaptic stability, plasticity, learning, and memory functions in neurological disorders, including AD, Parkinson's Disease (PD), Amyotrophic Lateral Sclerosis (ALS), schizophrenia, ischemia, and epilepsy [4–6].
Fig. 10.
Illustration of the molecular pathways involved in glutamate homeostasis and signaling through metabotropic glutamate receptors (mGluRs). The upper panel (A) depicts the synaptic interaction between presynaptic and postsynaptic neurons, highlighting the roles of glutaminase, glutamine synthetase, VGLUT, and EAAT1/2 in glutamate cycling and mGluR-mediated signaling. Group I mGluRs (mGluR1 and mGluR5) activate Gq proteins, leading to the release of intracellular Ca2+ and activation of protein kinase C (PKC). Group II mGluRs (mGluR2 and mGluR3) couple to Gi/o proteins, which inhibit adenylate cyclase, reducing cAMP levels and protein kinase A (PKA) activity, thus protecting neurons from excitotoxicity. Group III mGluRs (mGluR4, mGluR6, mGluR7, and mGluR8) also couple to Gi/o proteins, further inhibiting glutamate release. The lower panel (B) outlines the intracellular signaling cascades triggered by the activation of these mGluRs, detailing the involvement of key molecules such as PLCβ, PKC, PKA, and GSK3β, which are critical for synaptic plasticity and memory functions. Early melatonin intervention modulates these pathways, reducing glutamate toxicity and ameliorating cognitive deficits in AD models. This picture was created by Bio Render
In AD, abnormal levels of Aβ and NFTs increase glutamate levels in synaptic clefts, leading to excitotoxicity and cell death. Normally, excitotoxic glutamate levels are mitigated by the rapid conversion of glutamate- to glutamine, by glutamine synthetase (GLUL) in glial cells, maintaining normal synaptic signaling events [29, 30]. Our study showed increased Gls1 and decreased Glul levels in the hippocampus of the animal model, consistent with transcriptome analyses of AD patient brains. Dysregulation of GLS and impaired GLUL activity have been reported in various neurodegenerative diseases and are associated with synaptic dysfunction and learning deficits [31, 32]. Overall, the results from examining these two key enzymes in glutamate homeostasis indicate that toxic glutamate levels were elevated in the developed AD-like model. Early administration of melatonin showed better efficacy on Gls1 and Glul enzymes (Fig. 6), potentially leading to a more significant reduction in CNS glutamate levels in our animal model.
We hypothesized that elevated glutamate levels serve as ligands for specific receptors and channels, precipitating pathogenic changes in signaling activity, neurodegeneration, memory impairment, and synaptic plasticity dysfunction. Melatonin treatment might alter or control this pathway. Among the glutamate receptors, metabotropic glutamate receptors (mGluRs), particularly Group I (mGluR1 and mGluR5), are highly expressed in the cerebral cortex and hippocampus, playing a critical role in cognition through the Gq signaling pathway. Our AD-like model demonstrated increased expression of Group I mGluR genes, which normalized with melatonin treatment. Overexpression and hyperactivity of the mGluR I family in AD can lead to neurotoxic signals, including excessive Ca2+ release and tau hyperphosphorylation, leading to neurodegeneration [7, 33, 34].
As cognitive impairment progressed, a decrease in Group I mGluR gene expression was observed, consistent with bioinformatics data from the brains of AD patients, where reduced levels of mGluR1 and mGluR5 in the hippocampus and cerebral cortex are associated with memory impairments and neuroplasticity deficits [34–38]. In our study, melatonin induced a slight increase in Grm1 expression, but not Grm5, without fully restoring their expression to normal levels.
These changes in receptor expression can be attributed to the mechanisms of GPCR desensitization. Initially, increased ligand levels may upregulate receptor expression, but sustained exposure leads to receptor desensitization and internalization, eventually resulting in mRNA destabilization and reduced receptor levels. While mRNA destabilization as a mechanism of downregulation is well established, the exact regulatory mechanisms and their interplay are not completely understood[39]. Therefore, further studies are necessary to elucidate the precise molecular mechanisms regulating the expression of these genes. While direct evidence of melatonin’s role in mGluR5 promoter demethylation is lacking, prior studies have highlighted melatonin’s ability to modulate the activity of DNA methyltransferases (DNMTs) and histone-modifying enzymes [40, 41]. These findings provide a plausible basis for investigating whether melatonin can similarly affect the mGluR5 promoter, leading to altered receptor expression in neurodegenerative contexts.
Group II mGluRs (mGluR2 and mGluR3) stimulate Gi/o signaling and are predominantly expressed pre-synaptically, reducing glutamate release and protecting neurons from excitotoxicity. Increased expression of Group II mGluR genes was observed in the AD-like model, consistent with reports in AD patients and animal models [35]. Early melatonin intervention reduced these gene expressions. Abnormal activation of Group II mGluRs could suppress synaptic plasticity and increase tau phosphorylation. Therefore, regulating the activity of mGluR IIs might also contribute to ameliorating tauopathy.
Group III mGluRs (mGluR 4, mGluR 7, and mGluR 8), signaling through Gi/o proteins, are crucial in nervous system disorders. The AD-like model exhibited reduced expression of Group III mGluR genes, partially modified by melatonin treatment. Activation of these receptors is believed to exert neuroprotective effects by regulating the N-methyl-D-aspartate receptor (NMDAR) and Phosphoinositide 3-kinase (PI3K) pathways and reducing neuroinflammation [7].
Our findings suggest that melatonin may positively regulate mGluRs to mitigate AD-like damage, as evidenced by the alterations in mGluR expression levels observed in this study. However, we acknowledge that these changes might result from indirect mechanisms, such as melatonin’s well-documented antioxidant and anti-inflammatory properties, rather than direct interactions with mGluRs. While our research focused on exploring the therapeutic effects of melatonin on glutamate signaling and cognitive impairment, we did not perform direct experimental assays, such as receptor-binding studies or functional tests, to confirm a direct regulatory effect of melatonin on mGluRs. We recognize this as a limitation of the current study.
Increased levels and activity of GSK3 kinase isoforms, key downstream signaling targets of Group I and II mGluRs, were observed in the AD-like model. Histological studies showed increased pT231-tau levels, supporting the hypothesis that tauopathies act in a prion-like manner by activating GSK3, leading to further tauopathies [42]. Melatonin reduced GSK3 activation and pT231-tau levels in the animal models. The findings of our study highlight the dual regulatory effects of melatonin on both GSK3α and GSK3β protein levels and their phosphorylation status, emphasizing its broad neuroprotective potential in molecular pathways associated with synaptic plasticity and cognitive function. While our primary focus was on GSK3β, given its well-established role in tau hyperphosphorylation and AD pathology, recent evidence suggests that GSK3α also plays a critical role in modulating synaptic plasticity. For instance, a recent study [43] demonstrated that conditional knockdown of GSK3α, but not GSK3β, significantly enhances long-term potentiation (LTP) in the CA1 region of the hippocampus. This finding aligns with the hypothesis that GSK3α may contribute to distinct signaling pathways essential for synaptic maintenance, which complement the effects of GSK3β in neurodegenerative processes. Our observation that melatonin reduces both GSK3α and GSK3β levels and phosphorylation status supports the idea that its therapeutic effects may extend beyond GSK3β modulation, potentially influencing broader mechanisms of synaptic restoration and cognitive improvement. More interestingly, our electrophysiological studies showed a decrease in LTP induction in the AD-like model, in which melatonin treatment initiated two weeks post-induction ameliorated, whereas delayed treatment did not.
Given the overlapping yet distinct roles of GSK3α and GSK3β in AD pathology, future investigations should aim to dissect their contributions to synaptic function, tau pathology, and downstream neurodegenerative mechanisms. This perspective reinforces the necessity for a comprehensive approach to studying AD-related molecular pathways, recognizing the interplay between distinct kinases and signaling networks.
This study provides significant insights into the neuroprotective effects of melatonin treatment in an AD-like model, particularly its role in improving cognitive function and synaptic plasticity. However, some limitations must be acknowledged. First, the study exclusively used male animals to reduce variability and focus on the primary research objectives. While this approach enhanced consistency, it limits the generalizability of our findings and neglects potential sex-specific effects of melatonin on cognitive and synaptic outcomes. Future studies should include both male and female animals to comprehensively evaluate sex-based differences in melatonin’s therapeutic effects. Second, while the current study highlights melatonin's ability to modulate mGluRs and downstream signaling pathways, the lack of direct functional tests or binding studies to confirm specific receptor interactions is a limitation. Further studies are needed to validate the disruptions in proposed signaling pathways and the effect of melatonin on cognitive improvement. Third, behavioral and molecular analyses in this study focused on a limited range of parameters. However, the inclusion of additional experimental groups, such as the control + melatonin group, and further analyses could have provided a more comprehensive understanding. Finally, Detailed mechanistic insights into how melatonin modulates synaptic plasticity at the molecular level remain needed. These limitations underscore the need for broader and more integrative approaches in subsequent studies to fully elucidate the therapeutic potential of melatonin in AD-like conditions. By addressing these limitations, we aim to strengthen the translational impact of our findings and provide a more holistic understanding of melatonin’s neuroprotective effects.
Conclusions
Our study demonstrates that melatonin treatment significantly enhances memory performance and synaptic plasticity in an AD-like mouse model. The neuroprotective effects of melatonin are primarily mediated through the modulation of glutamate homeostasis and metabotropic glutamate receptors (mGluRs). Electrophysiological studies underscore the critical role of timely intervention, with earlier melatonin treatment yielding more pronounced improvements in cognitive function and synaptic plasticity. The timing of melatonin administration is crucial for its efficacy. Early treatment initiates more robust neuroprotective responses, suggesting melatonin's potential as a preventive therapeutic agent. Future clinical trials should focus on optimizing melatonin's timing and dosage for AD patients.
Supplementary Information
Acknowledgements
We acknowledge the support from “Goldaru Pharmaceutical Company”, which generously provided the effective substance of melatonin utilized in this research. Their contributions were purely academic and did not influence the study’s outcomes. Additionally, we extend our appreciation to Dr. Golalizadeh Lahi for their valuable guidance and expertise in the statistical analysis, which significantly contributed to the rigor and accuracy of our findings.
Abbreviations
- Aβ
Amyloid-beta
- aCSF
Artificial cerebrospinal fluid
- AD
Alzheimer's disease
- Cis P-Tau
Cis-phospho tau
- CNS
Central nervous system
- fEPSP
Excitatory postsynaptic field potentials
- GLUL
Glutamine synthetase
- GLS1
Glutaminase 1
- GPCRs
G protein-coupled receptors
- GSK3
Glycogen synthase kinase 3
- iGluR
Ionotropic glutamate receptor
- i.p.
Intraperitoneal (i.p.) injection
- mGluR
Metabotropic glutamate receptor
- NFTs
Neurofibrillary tangles
- qPCR
Quantitative polymerase chain reaction
- RNA-seq
RNA sequencing
Author contributions
N. K. Sh. S: Conceptualization, Investigation, Formal analysis, Writing—Original Draft, Histological Studies, Bioinformatics Analysis, Molecular Studies, Visualization. F. B: Investigation, Animal Model Development, Behavioral Studies, Electrophysiological Studies, Data Curation. J. M-Z: Supervision, Project Administration, Review & Editing, Experimental Design, Critical Insights. K. Sha: Resources, Preparation of Phospho-Tau and Antibodies, Methodology. M. B: Conceptualization, Supervision, Funding Acquisition, Bioinformatics Analysis, Writing—Review & Editing. All authors reviewed the manuscript.
Funding
This work was supported by the “Cognitive Science and Technologies Council” [grant number 11316] and the Research Affairs Department of Tarbiat Modares University.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request. RNA-seq data of hippocampal tissues from AD patients and control individuals used in this study are publicly accessible in the Gene Expression Omnibus (GEO) database under the accession numbers GSE67333, GSE184942, and GSE236562.
Declarations
Ethics approval and consent to participate
All experiments were conducted following the Guidelines for the care and use of laboratory animals and approved by the “Ethics Committee of Tarbiat Modares University” (IR.MODARES.REC.1400.217).
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Colom-Cadena M, Davies C, Sirisi S, Lee J-E, Simzer EM, Tzioras M, et al. Synaptic oligomeric tau in Alzheimer’s disease — A potential culprit in the spread of tau pathology through the brain. Neuron. 2023; https://linkinghub.elsevier.com/retrieve/pii/S0896627323003057 [DOI] [PubMed]
- 2.Young-Pearse TL, Lee H, Hsieh Y-C, Chou V, Selkoe DJ. Moving beyond amyloid and tau to capture the biological heterogeneity of Alzheimer’s disease. Trends Neurosci. 2023;46:426–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simons M, Levin J, Dichgans M. Tipping points in neurodegeneration. Neuron. 2023. [DOI] [PubMed]
- 4.Cox MF, Hascup ER, Bartke A, Hascup KN. Friend or Foe? Defining the Role of Glutamate in Aging and Alzheimer’s Disease. Front Aging. 2022. 10.3389/fragi.2022.929474/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang R, Reddy PH. Role of Glutamate and NMDA Receptors in Alzheimer’s Disease. J Alzheimer’s Dis. 2017;57:1041–8. 10.3233/JAD-160763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bukke VN, Archana M, Villani R, Romano AD, Wawrzyniak A, Balawender K, et al. The dual role of glutamatergic neurotransmission in alzheimer’s disease: from pathophysiology to pharmacotherapy. Int J Mol Sci. 2020;21:7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srivastava A, Das B, Yao AY, Yan R. Metabotropic glutamate receptors in alzheimer’s disease synaptic dysfunction: therapeutic opportunities and hope for the future. J Alzheimer’s Dis. 2020;78:1345–61. 10.3233/JAD-201146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cummings J. New approaches to symptomatic treatments for Alzheimer’s disease. Mol Neurodegener. 2021;16:2. 10.1186/s13024-021-00424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cummings J, Zhou Y, Lee G, Zhong K, Fonseca J, Cheng F. Alzheimer’s disease drug development pipeline: 2023. Alzheimer’s & Dementia. 2023;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nous A, Engelborghs S, Smolders I. Melatonin levels in the Alzheimer’s disease continuum: a systematic review. Alzheimers Res Ther. 2021;13:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhangar RR, Kale PP, Kadu PK, Prabhavalkar K. Possible benefits of considering glutamate with melatonin or orexin or oxytocin as a combination approach in the treatment of anxiety. Curr Pharmacol Rep. 2020;6:1–7. [Google Scholar]
- 12.Wang C, An Y, Xia Z, Zhou X, Li H, Song S, et al. The neuroprotective effect of melatonin in glutamate excitotoxicity of R28 cells and mouse retinal ganglion cells. Front Endocrinol (Lausanne). 2022;13:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corchete LA, Rojas EA, Alonso-López D, De Las RJ, Gutiérrez NC, Burguillo FJ. Systematic comparison and assessment of RNA-seq procedures for gene expression quantitative analysis. Sci Rep. 2020;10:19737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fatemeh B, Koorosh S, Amir S, Yaghoub F, Javad M-Z. Intra-hippocampal cis-P tau microinjection induces long-term changes in behavior and synaptic plasticity in mice. Behav Brain Func. 2023;19:9. 10.1186/s12993-023-00211-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pourhamzeh M, Joghataei MT, Mehrabi S, Ahadi R, Hojjati SMM, Fazli N, et al. The interplay of tau protein and β-amyloid: while tauopathy spreads more profoundly than amyloidopathy, both processes are almost equally pathogenic. Cell Mol Neurobiol. 2021;41:1339–54. 10.1007/s10571-020-00906-2. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Fatemeh B, Koorosh S, Amir S, Yaghoub F, Javad M-Z. Intra-hippocampal cis-P tau microinjection induces long-term changes in behavior and synaptic plasticity in mice. Behav Brain Funct. 2023;19:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hajmirzaeyian A, Chamanara M, Rashidian A, Shakyba S, Nassireslami E, Akhavan-Sigari R. Melatonin attenuated the behavioral despair induced by acute neurogenic stress through blockade of N-methyl D-aspartate receptors in mice. Heliyon. 2021;7: e05900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gawel K, Gibula E, Marszalek-Grabska M, Filarowska J, Kotlinska JH. Assessment of spatial learning and memory in the Barnes maze task in rodents—methodological consideration. Naunyn Schmiedebergs Arch Pharmacol. 2019;392:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sampedro-Carrillo EA. Sample Preparation and Fixation for Histology and Pathology. 2022;33–45. [DOI] [PubMed]
- 20.Carriel V, Campos A, Alaminos M, Raimondo S, Geuna S. Staining Methods for Normal and Regenerative Myelin in the Nervous System. 2017. p. 207–18. [DOI] [PubMed]
- 21.Kang J, Watanabe H, Shen J. Protocols for assessing neurodegenerative phenotypes in Alzheimer’s mouse models. STAR Protoc. 2021;2: 100654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25:402–8. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Parmigiani G, Johnson WE. ComBat-seq: batch effect adjustment for RNA-seq count data. NAR Genom Bioinform. 2020;2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thathiah A, De Strooper B. The role of G protein-coupled receptors in the pathology of Alzheimer’s disease. Nat Rev Neurosci. 2011;12:73–87. [DOI] [PubMed] [Google Scholar]
- 25.Depp C, Sun T, Sasmita AO, Spieth L, Berghoff SA, Nazarenko T, et al. Myelin dysfunction drives amyloid-β deposition in models of Alzheimer’s disease. Nature. 2023;618:349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang P, Sui H-J, Li X-J, Bai L-N, Bi J, Lai H. Melatonin ameliorates microvessel abnormalities in the cerebral cortex and hippocampus in a rat model of Alzheimer’s disease. Neural Regen Res. 2021;16:757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamsrijai U, Wongchitrat P, Nopparat C, Satayavivad J, Govitrapong P. Melatonin attenuates streptozotocin-induced Alzheimer-like features in hyperglycemic rats. Neurochem Int. 2020;132:104601. [DOI] [PubMed] [Google Scholar]
- 28.Luengo E, Buendia I, Fernández-Mendívil C, Trigo-Alonso P, Negredo P, Michalska P, et al. Pharmacological doses of melatonin impede cognitive decline in tau-related Alzheimer models, once tauopathy is initiated, by restoring the autophagic flux. J Pineal Res. 2019;67:e12578. [DOI] [PubMed] [Google Scholar]
- 29.Frieg B, Görg B, Homeyer N, Keitel V, Häussinger D, Gohlke H. Molecular mechanisms of glutamine synthetase mutations that lead to clinically relevant pathologies. PLoS Comput Biol. 2016;12:e1004693. 10.1371/journal.pcbi.1004693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merlo SA, Belluscio MA, Pedreira ME, Merlo E. Memory persistence: from fundamental mechanisms to translational opportunities. Transl Psychiatry. 2024;14:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding L, Xu X, Li C, Wang Y, Xia X, Zheng JC. Glutaminase in microglia: A novel regulator of neuroinflammation. Brain Behav Immun. 2021;92:139–56. [DOI] [PubMed] [Google Scholar]
- 32.Gao G, Li C, Zhu J, Wang Y, Huang Y, Zhao S, et al. Glutaminase 1 regulates neuroinflammation after cerebral ischemia through enhancing microglial activation and pro-inflammatory exosome release. Front Immunol. 2020;11:1. 10.3389/fimmu.2020.00161/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ménard C, Quirion R. Group 1 metabotropic glutamate receptor function and its regulation of learning and memory in the aging brain. Front Pharmacol. 2012. 10.3389/fphar.2012.00182/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang J, He Y, Chen X, Huang L, Li J, You Z, et al. Metabotropic glutamate receptor 5 (mGluR5) is associated with neurodegeneration and amyloid deposition in Alzheimer’s disease: A [18F]PSS232 PET/MRI study. Alzheimers Res Ther. 2024;16:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eng AG, Kelver DA, Hedrick TP, Swanson GT. Transduction of group I mGluR-mediated synaptic plasticity by β-arrestin2 signalling. Nat Commun. 2016;7:13571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Surdin T, Preissing B, Rohr L, Grömmke M, Böke H, Barcik M, et al. Optogenetic activation of mGluR1 signaling in the cerebellum induces synaptic plasticity. Science. 2023;26:105828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mango D, Ledonne A. Updates on the Physiopathology of Group I Metabotropic Glutamate Receptors (mGluRI)-Dependent Long-Term Depression. Cells. 2023;12:1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mecca AP, McDonald JW, Michalak HR, Godek TA, Harris JE, Pugh EA, et al. PET imaging of mGluR5 in Alzheimer’s disease. Alzheimers Res Ther. 2020;12:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flores-Espinoza E, Thomsen ARB. Beneath the surface: endosomal GPCR signaling. Trends Biochem Sci. 2024;49:520–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Linowiecka K, Slominski AT, Reiter RJ, Böhm M, Steinbrink K, Paus R, et al. Melatonin: a potential regulator of DNA methylation. Antioxidants. 2023;12:1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monayo SM, Liu X. The prospective application of melatonin in treating epigenetic dysfunctional diseases. Front Pharmacol. 2022;13:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frost B. Alzheimer’s disease and related tauopathies: disorders of disrupted neuronal identity. Trends Neurosci. 2023. [DOI] [PMC free article] [PubMed]
- 43.Ebrahim Amini A, Miyata T, Lei G, Jin F, Rubie E, Bradley CA, et al. Specific Role for GSK3α in limiting long-term potentiation in CA1 pyramidal neurons of adult mouse hippocampus. Front Mol Neurosci. 2022;15:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request. RNA-seq data of hippocampal tissues from AD patients and control individuals used in this study are publicly accessible in the Gene Expression Omnibus (GEO) database under the accession numbers GSE67333, GSE184942, and GSE236562.