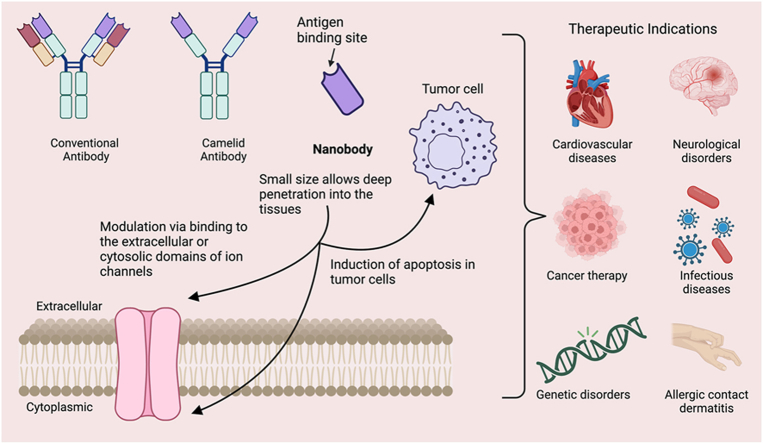
Abstract
Ion channels play instrumental roles in regulating membrane potential and cross-membrane signal transduction, thus making them attractive targets for understanding various physiological processes and associated diseases. Gaining a deeper understanding of their structural and functional properties has significant implications for developing therapeutic interventions. In recent years, nanobodies, single-domain antibody fragments derived from camelids, have emerged as powerful tools in ion channel and synthetic biology research. Their small size, high specificity, and ability to recognize difficult-to-reach epitopes offer advantages over conventional antibodies and biologics. Furthermore, their resemblance to the variable region of human IgG family III reduces immunogenicity concerns. Nanobodies have introduced new opportunities for exploring ion channel structure-function relationships and offer a promising alternative to conventional drugs, which often face challenges such as off-target effects and toxicity. This review highlights recent progress in applying nanobodies to interrogate and modulate ion channel activity, with an emphasis on their potential to overcome current technical and therapeutic limitations.
Keywords: Nanobody, Antibody engineering, Ion channels, Immunotherapy, Synthetic biology, Therapeutics
Graphical abstract
1. Introduction
Voltage- and ligand-gated ion channels constitute a diverse family of transmembrane proteins that control ion flow across membranes through gated pores. These channels are essential for regulating a wide range of biological processes, including hormone secretion, cell proliferation, cell migration, programmed cell death, neuronal excitability, muscle contraction, signal transduction, blood pressure regulation, and gene transcription [1,2]. The human genome contains hundreds of genes that encode ion channels, which can be broadly categorized as either voltage-gated or ligand-gated, based on the mechanisms regulating pore opening and closing [3]. Genetic defects in genes encoding ion channels are linked to a large number of channelopathies that affect the cardiac, neurological and immune systems [[4], [5], [6]]. For example, neuromuscular disorders such as Lambert-Eaton myasthenic syndrome and Isaacs' syndrome arise from autoantibodies that target voltage-gated calcium (Ca2+) and potassium (K+) channels, respectively [[7], [8], [9]]. Similarly, mutations in the gene encoding the Ca2+ release-activated Ca2+ (CRAC) channel, ORAI1, and its activator stromal interaction molecule 1 (STIM1), are implicated in severe combined immunodeficiency (SCID), tubular aggregate myopathy (TAM), and Stormorken syndrome [10,11]. Additionally, impaired degradation of renal epithelial Na+ channels leads to Liddle syndrome [12], which is characterized by hypertension with hypokalemic metabolic alkalosis, suppressed aldosterone secretion, and hyporeninemia. The pivotal role of ion channels in regulating a multitude of cellular functions and their involvement in a broad spectrum of diseases underscores their significance as valuable therapeutic targets.
Over the past five decades, remarkable progress has been made in the pursuit of small molecules targeting ion channels, which substantially advances the treatment of neurological and cardiovascular disorders, as well as pain management [1]. These efforts, driven by high-throughput compound screening and serendipitous discoveries, have given rise to novel classes of ion channel-targeting therapeutics [1,13]. For instance, small molecule blockers of high-voltage-activated Ca2+ channel (HVACC), such as benzothiazepines, phenylalkylamines and dihydropyridines, have become indispensable therapeutics in the clinical management of conditions like cerebral vasospasm, hypertension, cardiac arrhythmias, Parkinson's disease, and epilepsy [14,15]. More recently, emerging evidence suggests that ion channel blockers may also play a role in combating multidrug resistance in cancer, thus expanding their therapeutic potential [16,17].
Despite these advances, small-molecule modulators of ion channels still face several challenges. These include off-target effects, difficulty in achieving selectivity across ion channel subtypes or isoforms, and plasma half-lives limited to the scale of minutes to hours, which constrain their sustained efficacy and specificity. Furthermore, prolonged use of small-molecule inhibitors has been linked to the development of therapeutic resistance [18]. To address these challenges, monoclonal antibodies (mAbs) have emerged as a transformative class of therapeutics, particularly in oncology and autoimmune diseases [19]. However, mAbs present their own limitations, primarily due to their large size (∼150 kDa), which leads to reduced tissue penetration and suboptimal biodistribution [20]. Nanobodies (Nbs), derived from camelid antibodies, have garnered increasing attention as highly modular and versatile alternative ion channel modulators [21]. Owing to their small size and extraordinary binding specificity, Nbs offer several distinct advantages over mAbs and small molecules. Their compact structure allows for superior tissue penetration and the ability to access epitopes that are often inaccessible to larger mAbs. Additionally, their high specificity and tunable affinities make them particularly well-suited for targeting ion channels, thereby providing solutions to the common challenges of off-target effects and therapeutic resistance observed with small molecules.
Nanobodies are not only more modularly adaptable but can also be flexibly engineered with greater precision and tunability to modulate specific ion channel subtypes, hence addressing the issue of selectivity that has historically hindered other therapeutic approaches. These properties make nanobodies an ideal platform for developing next-generation therapies for ion channel-related diseases. This review will focus on recent advances in the use of nanobodies to modulate ion channel activity, exploring their physiological and pathophysiological roles, and highlighting their potential as next-generation therapeutics for ion channel-related diseases.
2. Nanobodies as promising tools for research and theragnostic applications
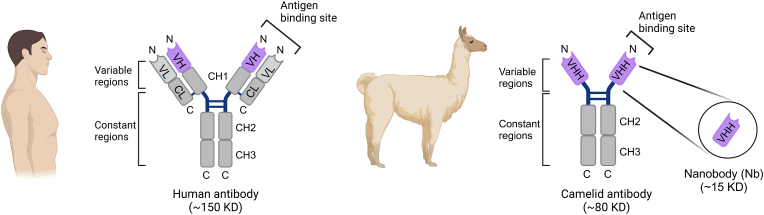
Nanobodies are a class of compact single-domain antibodies derived from a unique heavy-chain-only antibody (HCAb) naturally produced by camelid species [22]. In contrast to conventional antibodies, which contain two identical heavy-chain (CH) and light-chain (CL) polypeptides that pair to form a stably folded protein, HCAbs lack both the CL and first constant domain (CH1) (Fig. 1) [23,24]. A nanobody consists of the isolated HCAb variable domain, designated VHH, which binds to its cognate antigen and functions as the equivalent to the antigen-binding fragment (Fab) of conventional antibodies. With a molecular weight of approximately 15 kDa, nanobodies are only one-tenth the size of conventional antibodies. Similar to the variable regions of traditional antibodies, nanobodies contain three hypervariable complementarity-determining regions (CDRs) linked together by highly conserved beta-sheet framework regions [25]. The elongated third CDR, paired with its compact size, allows for the adoption of unique conformations and the ability to reach previously inaccessible target epitopes [[26], [27], [28]]. Notably, the framework regions of nanobodies exhibit high homology to human family III heavy chain domains, thereby reducing the potential risk of immunogenicity when administered to humans [25]. Additionally, nanobodies have demonstrated enhanced solubility and stability as compared to traditional antibodies and antibody fragments [29]. These favorable attributes fuel interest in the utilization of nanobodies for research, diagnostic, and therapeutic applications, including ion channel modulation (Table 1).
Fig. 1.
Schematic illustration of human and camelid antibodies and their fragments. In a typical mammalian antibody, the light chain consists of one variable (VL, light grey) and one constant (CL, dark grey) domain, while the heavy chain contains one variable (VH, purple) and three constant (CH1 to CH3, dark grey) domains. The antigen binding fragment (Fab) is formed by the paring of VL and VH domains. In contrast, the camelid antibody exists as a homodimer with heavy chains only. The isolated variable region of the camelid antibody provides a functional single-domain antibody (VHH), commonly known as a nanobody.
Table 1.
Key information on published nanobodies targeting ion channels described in this review.
| Nb | Target | Modality | Method | Immunogen | Mode of action | Therapeutic indications | KD (nM) | Ref |
|---|---|---|---|---|---|---|---|---|
| 13A7-HLE | mP2X7 | Antagonist | Llama immunization/phage display | mP2X7 transfected HEK-293 cells or with P2X7 cDNA expression vector | Blockade of ATP-induced Ca2+ influx via P2X7 | Inflammatory diseases: glomerulonephritis and allergic contact dermatitis | ND | [33] |
| 14D5-HLE | mP2X7 | Agonist | Llama immunization/phage display | mP2X7 transfected HEK-293 cells or with mP2X7 cDNA expression vector | Reducing ATP threshold required for the activation of P2X7 | Pathological conditions such as cancer or infections with intracellular parasites | ND | [33] |
| Dano1 | hP2X7 | Antagonist | Llama immunization/phage display | hP2X7 transfected HEK-293 cells or with hP2X7 cDNA expression vector | Blockade of ATP-induced IL-1β release | Glomerulonephritis, lupus nephritis, and acute dermatitis | ND | [33] |
| Nb.F3 (CaV-aβlator) | CaV1.2 β subunit | Antagonist | Llama immunization/phage display | Purified β1 and β3 proteins from HEK-293 cells overexpressing CaVβ1b and CaVβ3 | Cytosolic β-domains | Pain, hypertension, cardiac arrhythmias, epilepsy, and Parkinson's disease | 13.2 ± 7.2 | [42] |
| Nb.E8 (Chisel-1) | CaV1.2 β1 subunit | Antagonist | Llama immunization/phage display | Purified β1 and β3 proteins from HEK-293 cells over expressing CaVβ1b and CaVβ3 | Cytosolic β1-domain | Cardiovascular and neurological diseases | ND | [44] |
| Nb.C1 | hASIC1a | Antagonist | Llama immunization /phage display |
hASIC1a transfected HEK-293T cells | Extracellular domain of the channel | Potential to be used in neurological disorders | ND | [52] |
| Nb17, Nb82 | NaV 1.4 or NaV 1.5 | Antagonist | Llama immunization/phage display | Purified NaV1.4 protein fragment in complex with Calmodulin (CaM) | Cytosolic domain (CT) | Human genetic diseases caused by mutation in the CT, e.g., generalized epilepsy with febrile seizures, hypokalemic periodic paralysis, myotonia, long-QT syndrome, and Brugada syndrome | Nb17: 41.1 ± 9. 9; 60.5 ± 5.80 Nb82: 50.2 ± 8.87; 63.2 ± 6.75 |
[58] |
| VHH-D9-scTRAIL | mKV 10.1 | Antagonist | Llama immunization/phage display | mKV10.1 derived antigen | Extracellular/Induce apoptosis into the tumor cells | Human pancreatic cancer | 78 | [62] |
| A0194009G09 | KV1.3 | Antagonist | Immunoglobulin based protein engineering | Immunoglobulin recognizing the first extracellular loop of KV1.3 | Extracellular/C-type inactivation of channel | Autoimmune diseases, such as multiple sclerosis, type-1 diabetes mellitus, rheumatoid arthritis and psoriasis. | ND | [63] |
ND, not determined; P2X7, purinergic P2X receptor 7; CaV, voltage-gated Ca2+ channel; KV, voltage-gated K+ channel; ASIC, acid-sensing ion channel; IL, interleukin.
3. Nanobodies targeting ion channels
3.1. P2X7 ion channels
P2X7 is a non-selective adenosine 5-triphosphate (ATP)-gated cation channel expressed in macrophages and regulatory T-cells that permits calcium (Ca2+) and sodium (Na+) influx and efflux of potassium ions (K+). ATP released from cells under stress during inflammation and tumor development triggers the activation of P2X7 channels. This activation initiates a proinflammatory signaling cascade and leads to the release of cytokines, including interleukin-1β (IL-1β) [30]. As such, these channels are attractive therapeutic targets for a wide range of inflammatory diseases, including multiple sclerosis, glomerulonephritis, and chronic pain [31,32].
Danquah et al. developed potent nanobodies against mouse and human P2X7 ion channels from immunized llamas (Fig. 2A). Nanobody 13A7, the most potent murine P2X7 blocker (IC50: 12 nM), could effectively inhibit ATP-induced whole-cell currents and Ca2+ influx. In contrast, nanobody 14D5 (IC50: 6 nM), strongly enhanced ATP-induced whole cell currents and Ca2+ influx in mouse P2X7-expressing cells [33]. The binding affinities and potencies were improved by the generation of a dimeric NB, involving the fusion of two single domains via a flexible peptide linker. Further, generation of a dimeric half-life extension (HLE) version through the fusion of homodimers of 13A7 or 14D5 to an anti-albumin antibody moiety (Alb8), extended both the half-life from hours to days, and the duration of P2X7 modulation (Fig. 2B). Systemic administration of dimer-HLE nanobody 13A7 ameliorated inflammation in glomerulonephritis and allergic contact dermatitis mouse models [33].
Fig. 2.
Schematic diagram showing the generation of P2X7-targeting nanobodies. A. Llama was immunized with human and mouse P2X7-transfected HEK293 cells to generate anti-P2X7 nanobodies. B. Nanobodies were isolated as monomers and re-engineered into dimer along with Alb8. C. Nbs inhibit ATP-activated P2X7 channels, which are involved in inflammatory response by inducing Ca2+ influx and ultimately causing the production of pro-inflammatory cytokines, such as IL-1β.
Similarly, the nanobody Dano1, which targets human P2X7 (hP2X7), effectively blocked ATP-induced Ca2+ influx in hP2X7-expressing cells and inhibited the release of proinflammatory cytokines with up to 1000-fold greater potency than small-molecule inhibitors. Furthermore, Dano1 demonstrated high specificity with no crosstalk between species and orthologs, positioning it as a strong candidate for translational development. Given the central role of IL-1β inhibition in managing chronic inflammatory diseases, targeting P2X7 with nanobodies presents an attractive alternative approach to suppress IL-1β (Fig. 2C). Another study used adeno-associated virus (AAV) delivery to produce an endogenous heavy-chain antibody consisting of a non-modulatory anti-P2X7 nanobody fused to murine IgG2a for in vivo cell depletion [34]. This study establishes a proof-of-concept for utilizing nanobodies as effective tools for ion channel research.
Of particular interest, anti-P2X7 nanobodies were able to cross the blood-brain barrier (BBB) and block P2X7 channels in microglia in mouse models of systemic inflammation when administered intravenously at high doses or intracerebrally at low doses [35]. Endogenous production of nanobodies following AAV administration enabled sustained channel blockade over the longer term. Furthermore, anti-P2X7 nanobody treatment reduced the risk of ischemic tissue damage in mice following induced stroke [36]. Collectively, these findings highlight the ability of nanobodies to cross the BBB and support their potential as therapeutics for brain inflammation.
3.2. Voltage-gated Ca2+ channels (VGCCs)
VGCC play a crucial role in excitable cells, mediating processes such as muscle contraction, hormonal secretion, and neurotransmitter release [15,37]. As a result, they serve as promising therapeutic targets for a myriad of cardiovascular and neurological diseases. VGCCs exist as multi-subunit complexes, with seven distinct subtypes (CaV1.1–CaV1.4 and CaV2.1–CaV2.3) comprising an α1 voltage-sensor, selectivity filter, and pore-forming subunit assembled with auxiliary proteins which include β, α2-δ, and γ [14,38]. There are four CaVβ subunit isoforms (CaVβ1–CaVβ4) encoded by distinct genes [39]. Auxiliary proteins involved in the trafficking, gating, and modulation of VGCCs are increasingly recognized as potential therapeutic targets for treating VGCC-related diseases. For example, gabapentin targets α2-δ subunits for the treatment of neuropathic pain and epilepsy [40,41]. Previous studies have shown that the association of pore-forming subunit α1 with β is essential for the formation of functional VGCC. Disrupting the α1-β interaction has been pursued as a strategy to inhibit VGCC activity [14].
Morgenstern et al. developed a llama-derived anti-CaVβ nanobody (nb.F3) fused to an E3 ubiquitin ligase to inhibit CaV1.2 channel activity [42]. Nb.F3 was shown to bind to β1 through β4 subunits in transfected cells and assemble into CaV channel complexes without disrupting channel function. To achieve inhibition, Nb.F3 was fused with the catalytic Homologous to the E6-APC Carboxyl Terminus (HECT) domain of an E3 ubiquitin ligase, neural precursor cell expressed developmentally downregulated gene 4-like (Nedd4L) (Fig. 3A). The resultant CaV-aβlator construct blocked whole-cell currents in diverse reconstituted CaV1.2 channels in HEK293 cells, as well as native mammalian cardiomyocytes, dorsal root ganglion neurons, and pancreatic β cells [42]. The CaV-aβlator approach introduced a versatile strategy for modulating protein complexes and demonstrated therapeutic potential. Subcutaneous AAV delivery of CaV-aβlator reduced hyperalgesia and Ca2+ spike recordings in mice subjected to spare nerve injury, highlighting its neuropathic therapeutic efficacy. These studies showcase the potential of the CaV-aβlator as a platform for post-translational inactivation of ion channels through the ubiquitin-proteasome system [43].
Fig. 3.
Nanobody-mediated degradation of CaV channels. A. The anti-CaVβ nanobody nb.F3 is fused with the catalytic HECT domain of the Nedd4L E3 ubiquitin ligase to generate CaV-aβlator. CaV-aβlator is capable of catalyzing the ubiquitination of the CaV1.2/CaVβ Ca2+ channel, ultimately leading to their functional inhibition. B. The anti-CaVβ1 nanobody nb.E8 is fused with the catalytic HECT domain of Nedd4L to yield Chisel-1. Chisel-1 catalyzes the ubiquitination of the CaV1.2/CaVβ1 Ca2+ channel to suppress the channel activity via proteasomal degradation.
Although the CaV-aβlator demonstrates therapeutic potential, it lacks specificity for individual CaVβ subtypes. CaVβ1–CaVβ4 share overlapping functions, such as shifting the voltage dependence of channel activation in a hyperpolarizing direction, facilitating the co-expression of the α1 subunit, and increasing channel open probability (Po). However, each β subunit subtype imparts unique properties to VGCCs, including distinct rates of inactivation and steady-state inactivation, thereby offering an opportunity to improve the specificity of CaV-aβlator. The development of Chisel-1 demonstrated potent and specific inhibition CaVβ1 subunit through targeting the unique SH3 domain and promoting targeted ubiquitination, thus reducing the Po of the channel and reducing channel surface density (N) (Fig. 3B) [44]. Chisel-1 represents the first platform capable of selectively ablating VGCC function based on the isoform identity of the auxiliary subunit. Given that many other ion channels, beyond VGCCs, also consist of pore-forming proteins paired with auxiliary subunits of various isoforms, Chisel-1 demonstrates the potential of nanobody-based tools to selectively probe and modulate the functions of distinct ion channel subunit isoforms.
The introduction of a genetically encoded enhancer of Ca2+ channels—L-type (GeeCL) facilitated the enhancement of L-type Ca2+ channel activity in a targeted manner by leveraging a fusion of a high-affinity nanobody directed at CaV complex subunits with the minimum effector domain from the CaV1.2 modulating leucine-rich repeat-containing protein 10 (Lrrc10) [45]. GeeCL selectively increased channel open probability and enabled precise modulation of neuronal excitation-transcription coupling and cardiomyocyte Ca2+ influx. Applications of this technology could include restoring impaired Ca2+ signaling in Rett syndrome neurons, demonstrating its therapeutic potential and opening new avenues for isoform-specific VGCC research [46].
3.3. Acid-sensing ion channels (ASICs)
ASICs are sodium channels activated by protons. These channels are expressed in neurons of both central and peripheral nervous systems and play roles in ischemia-induced neuronal injury, as well as the modulation of pain, fear, and addiction [46]. Four ASIC genes (ASIC1–4) encode six distinct subunits: ASIC1a, ASIC1b, ASIC2a, ASIC2b, ASIC3, and ASIC4 [47]. These subunits assemble into homo- or heterotrimers to form functional channels, with ASIC1a being the most abundant. ASIC2a deletion in mice eliminates the majority of ASIC-mediated currents [48]. While protons are the canonical ligands for ASICs, their large extracellular loops permit the binding of non-proton ligands, such as venoms from the Texas coral snake (Micrurus tener tener, MitTx) and the tarantula Psalmopoeus cambridgei (PcTx1) [[49], [50], [51]]. MitTx, a heterodimer composed of a 60-residue α-subunit and a 119-residue β-subunit, acts as a selective agonist for ASICs, whereas PcTx1, a 40-residue peptide, potently and selectively inhibits hASIC1a.
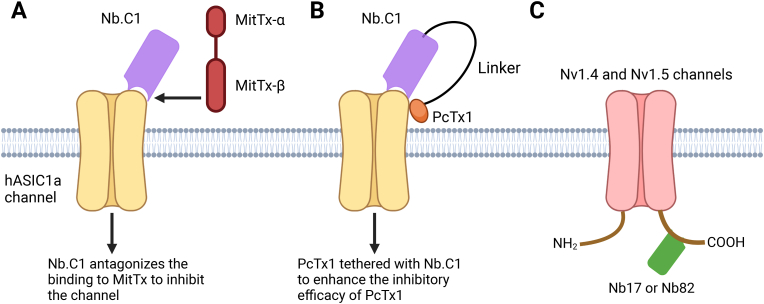
Wu et al. developed nanobody Nb.C1, which demonstrated high specificity and affinity for hASIC1a [52]. In HEK293 cells over-expressing hASIC1a, Nb.C1 prevented hASIC1a aggregation and stabilized channel expression, opening avenues for studying channel structure and function. This stabilization also improved hASIC1 sample preparation, enabling cryo-EM analysis and yielding a 2.9 Å resolution structure of the hASIC1-Nb.C1 complex in the closed conformation. This represents a proof-of-concept for using nanobody stabilization to facilitate structural analysis of ion channels [52]. Interestingly, the Nb.C1 binding site overlaps with that of MitTx, a toxin that induces severe pain by activating ASIC1a channels, but not with the ASIC1a-inhibitory PcTx1 toxin (Fig. 4A). This suggests that Nb.C1 could serve as a low-cost time-efficient alternative antidote for the pain-producing effects of snakebite venom, potentially eliminating the need for traditional antivenom derived from horses or sheep. Moreover, the distinct binding modes of PcTx1 and Nb.C1 raise the possibility that Nb.C1 can be used as a carrier to deliver PcTx1 specifically to its binding site, thereby minimizing off-target effects and enhancing PcTx1 potency [52]. This strategy could advance the therapeutic potential of PcTx1 as an analgesic or neuroprotectant (Fig. 4B). Congruently, these studies demonstrate the ability of nanobodies to be used to advance structural and functional studies of ion channels and as vehicles for enhancing drug delivery specificity.
Fig. 4.
Nanobody-mediated direct modulation of ASICs or NaV channels. A. Cartoon illustrating how Nb.C1 interferes with the binding of a venom toxin from the Texas coral snake (MitTx), thereby inhibiting MitTx -activated hASIC1a channels. B. A Nb.C1-PcTx1 fusion protein is engineered to provide more precise hASIC1a targeting with an enhanced analgesic effect. C. Nb17 and Nb82 specifically target the C-terminal regions of the 1.4 and 1.5 isoforms of NaV channels for functional tuning.
3.4. Voltage-gated sodium channels
Voltage-gated sodium channels (NaV) rapidly respond to changes in membrane potential by allowing Na+ influx. These channels play an important role in the generation and propagation of action potentials in excitable tissues such as the heart, muscles, and nerves. NaV cahnnels are heteromultimeric proteins consisting of a single pore-forming α-subunit complexed with one or two small accessory β subunits. The human genome encodes nine distinct genes (SCN1A, SCN2A, SCN3A, SCN4A, SCN5A, SCN8A, SCN9A, SCN10A, and SCN11A) and four β subunit genes (SCN1B, SCN2B, SCN3B and SCN4B) [53]. While the central and peripheral nervous systems express most isoforms, skeletal and cardiac muscles exhibit a more restricted NaV repertoire [54]. Mutations in NaV-encoding genes are associated with a variety of genetic disorders affecting skeletal muscle contraction and the nervous system. These include epilepsy with febrile seizures, myotonia, long-QT syndrome, hypokalemic periodic paralysis, and Brugada syndrome [[55], [56], [57]].
The C-terminal domains of two NaV isoforms, NaV1.4 and NaV1.5, were selected as antigens for Nb production due to their regulatory roles as the binding sites for channel-interacting proteins [58]. Nb17 and Nb82 were developed to specifically recognize NaV1.4 and NaV1.5 with nM affinities, but not other isoforms. These nanobodies successfully detected NaV channel expression in mammalian cells and tissues, showcasing their potential utility in the molecular detection, visualization, and capture of NaV channels in cellular and tissue environments.
3.5. Voltage-gated potassium channels
Voltage-gated potassium channels (KV) are critical for modulating resting and action potentials as well as stabilizing membrane potentials in a variety cell types. KV channels have been implicated in cancer, autoimmune disorders, and inflammatory diseases [59]. KV1.3-specific nanobodies characterized by Ablynx exhibited valency-dependent modulatory effects [60]. Monovalent nanobodies demonstrated rapid kinetic profiles, while bivalent nanobodies not only increased avidity but also extended target residence time and blockade duration. Unsurprisingly, the composition of the bivalent nanobody resulted in varied outcomes. Homo-bivalent nanobodies amplified the effects of the monovalent component, whereas hetero-bivalent nanobodies produced mixed phenotypes. A trivalent nanobody further amplified the effects of the monomeric molecule. Among these, a homo-bivalent-HLE version demonstrated the most promise and reduced ear thickness in a delay-type hypersensitivity rat model. These findings underscore the potential of valency-optimized nanobodies for therapeutic development.
KV10.1 is frequently overexpressed in cancer cell lines [61]. An anti-KV10.1 nanobody, D9, was developed and demonstrated nanomolar affinity [62]. When fused to a single-chain tumor necrosis factor-related apoptosis-inducing ligand (VHH-D9-scTRAIL), the resulting construct induced potent tumor cell apoptosis across multiple cell lines. Remarkably, the VHH-D9-scTRAIL fusion triggered apoptosis more rapidly and effectively than either component alone, underscoring its potential as a novel strategy in cancer therapeutics.
An anti-KV1.3 nanobody, A0194009G09, was instrumental in elucidating the conformational changes required for the transition of KV1.3 channels from an open-conducting state to an inactivated conformation [63]. By stabilizing the inactivated state of both wild-type KV1.3 and two mutants, this nanobody provided valuable insights into the mechanisms of slow channel inactivation. These findings greatly enhance our understanding of KV1.3 channel dynamics and advance the development of targeted KV1.3 therapeutics.
4. Concluding remarks
Nanobodies have emerged as transformative tools for studying and modulating ion channels, providing significant advantages over traditional small molecules and monoclonal antibodies due to their small size, high specificity, and ability to target previously inaccessible epitopes. These unique properties make them highly valuable for studying the structure and function of ion channels. Further optogenetic or chemogenetic engineering of ion channel-targeting nanobody platforms, such as CaV-aβlator and Chisel-1, could offer unprecedented control over processes such as ion channel degradation, subcellular relocalization, and even post-translational modifications [[64], [65], [66], [67]]. Nanobody-based magnetic modulation of the transient receptor potential vanilloid (TRPV) ion channel is currently under investigation [68]. Continued advancements in nanobody development and screening methods are expected to expand the therapeutic and modulatory repertoire for ion channels.
The potential of nanobodies extends beyond basic research by offering innovative therapeutic opportunities for a wide range of ion channel-related diseases, including neurological, cardiovascular, and immunoinflammatory conditions. Recent advancements in nanobody engineering, such as enhanced stability, improved tissue penetration, and tunable affinity, underscore their promise as next-generation therapeutics. By addressing long-standing challenges in ion channel drug development—such as selectivity, off-target effects, and therapeutic resistance—nanobodies represent a novel and versatile platform for both research and clinical applications, with the potential to revolutionize treatments for channelopathies and other diseases involving ion channel dysfunction.
CRediT authorship contribution statement
Sher Ali: Writing – review & editing, Writing – original draft. Ashley Suris: Writing – review & editing, Writing – original draft. Yun Huang: Writing – review & editing, Funding acquisition, Conceptualization. Yubin Zhou: Writing – review & editing, Supervision, Project administration, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We gratefully acknowledge grant support from the National Institutes of Health (R01GM144986, R01CA232017, and R21AI174606 to Y.Z., as well as R35HL166557, R01DK132286, and R01CA240258 to Y.H.), the Leukemia & Lymphoma Society (to Y.Z.), and the Welch Foundation (BE-1913-20220331 to Y.Z.). Figures and the graphical abstract were created using BioRender.com.
Footnotes
Peer review under the responsibility of Editorial Board of Synthetic and Systems Biotechnology.
References
- 1.Bagal S., Brown A.D., Cox P.J., Omoto K., Owen R.M., Pryde D.C., Sidders B., Skerratt S.E., Stevens E.B., Storer R.I., et al. Ion channels as therapeutic targets: a drug discovery Perspective. J Med Chem. 2013;56:593–624. doi: 10.1021/jm3011433. [DOI] [PubMed] [Google Scholar]
- 2.Wickenden A., Priest B., Erdemli G. Ion channel drug discovery: challenges and future directions. Future Med Chem. 2012;4:661–679. doi: 10.4155/Fmc.12.4. [DOI] [PubMed] [Google Scholar]
- 3.Yu F.H., Yarov-Yarovoy V., Gutman G.A., Catterall W.A. Overview of molecular relationships in the voltage-gated ion channel superfamily. Pharmacol Rev. 2005;57:387–395. doi: 10.1124/pr.57.4.13. [DOI] [PubMed] [Google Scholar]
- 4.Pedersen S.F., Stock C. Ion channels and Transporters in cancer: Pathophysiology, regulation, and clinical potential. Cancer Res. 2013;73:1658–1661. doi: 10.1158/0008-5472.Can-12-4188. [DOI] [PubMed] [Google Scholar]
- 5.Wulff H., Castle N.A., Pardo L.A. Voltage-gated potassium channels as therapeutic targets. Nat Rev Drug Discov. 2009;8:982–1001. doi: 10.1038/nrd2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathie A. Ion channels as novel therapeutic targets in the treatment of pain. J Pharm Pharmacol. 2010;62:1089–1095. doi: 10.1111/j.2042-7158.2010.01131.x. [DOI] [PubMed] [Google Scholar]
- 7.Lambert E.H., Eaton L.M., Rooke E.D. Defect of neuromuscular conduction associated with Malignant Neoplasms. Am J Physiol. 1956;187:612–613. [Google Scholar]
- 8.Newsom-Davis J., Buckley C., Clover L., Hart I., Maddison P., Tüzüm E., Vincent A. Autoimmune disorders of neuronal potassium channels. Ann Ny Acad Sci. 2003;998:202–210. doi: 10.1196/annals.1254.022. [DOI] [PubMed] [Google Scholar]
- 9.Rana S.S., Ramanathan R.S., Small G., Adamovich B. Paraneoplastic Isaacs' syndrome: a case series and review of the literature. J Clin Neuromuscul Dis. 2012;13:228–233. doi: 10.1097/CND.0b013e318246197d. [DOI] [PubMed] [Google Scholar]
- 10.Böhm J., Chevessier F., De Paula A.M., Koch C., Attarian S., Feger C., Hantaï D., Laforêt P., Ghorab K., Vallat J.M., et al. Constitutive activation of the calcium sensor STIM1 causes tubular-aggregate myopathy. Am J Hum Genet. 2013;92:271–278. doi: 10.1016/j.ajhg.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Misceo D., Holmgren A., Louch W.E., Holme P.A., Mizobuchi M., Morales R.J., De Paula A.M., Stray-Pedersen A., Lyle R., Dalhus B., et al. A dominant STIM1 mutation causes Stormorken syndrome. Hum Mutat. 2014;35:556–564. doi: 10.1002/humu.22544. [DOI] [PubMed] [Google Scholar]
- 12.Rotin D. Role of the UPS in Liddle syndrome. BMC Biochem. 2008;9(Suppl 1):S5. doi: 10.1186/1471-2091-9-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaczorowski G.J., McManus O.B., Priest B.T., Garcia M.L. Ion channels as drug targets: the next GPCRs. J Gen Physiol. 2008;131:399–405. doi: 10.1085/jgp.200709946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zamponi G.W., Striessnig J., Koschak A., Dolphin A.C. The physiology, Pathology, and Pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol Rev. 2015;67:821–870. doi: 10.1124/pr.114.009654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zamponi G.W. Targeting voltage-gated calcium channels in neurological and psychiatric diseases. Nat Rev Drug Discov. 2016;15:19–34. doi: 10.1038/nrd.2015.5. [DOI] [PubMed] [Google Scholar]
- 16.Zarrin A., Mehdipour A.R., Miri R. Dihydropyridines and multidrug resistance: previous attempts, present state, and future trends. Chem Biol Drug Des. 2010;76:369–381. doi: 10.1111/j.1747-0285.2010.01025.x. [DOI] [PubMed] [Google Scholar]
- 17.Safa A.R. Photoaffinity labeling of the multidrug-resistance-related P-glycoprotein with photoactive analogs of verapamil. Proc Natl Acad Sci U S A. 1988;85:7187–7191. doi: 10.1073/pnas.85.19.7187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burman R.J., Rosch R.E., Wilmshurst J.M., Sen A., Ramantani G., Akerman C.J., Raimondo J.V. Why won't it stop? The dynamics of benzodiazepine resistance in status epilepticus. Nat Rev Neurol. 2022;18:428–441. doi: 10.1038/s41582-022-00664-3. [DOI] [PubMed] [Google Scholar]
- 19.Sun H., Li M. Antibody therapeutics targeting ion channels: are we there yet? Acta Pharmacol Sin. 2013;34:199–204. doi: 10.1038/aps.2012.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hutchings C.J., Colussi P., Clark T.G. Ion channels as therapeutic antibody targets. mAbs. 2019;11:265–296. doi: 10.1080/19420862.2018.1548232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wesolowski J., Alzogaray V., Reyelt J., Unger M., Juarez K., Urrutia M., Cauerhff A., Danquah W., Rissiek B., Scheuplein F., et al. Single domain antibodies: promising experimental and therapeutic tools in infection and immunity. Med Microbiol Immun. 2009;198:157–174. doi: 10.1007/s00430-009-0116-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamerscasterman C., Atarhouch T., Muyldermans S., Robinson G., Hamers C., Songa E.B., Bendahman N., Hamers R. Naturally-Occurring antibodies devoid of light-chains. Nature. 1993;363:446–448. doi: 10.1038/363446a0. [DOI] [PubMed] [Google Scholar]
- 23.Padlan E.A. Anatomy of the antibody molecule. Mol Immunol. 1994;31:169–217. doi: 10.1016/0161-5890(94)90001-9. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen V.K., Hamers R., Wyns L., Muyldermans S. Loss of splice consensus signal is responsible for the removal of the entire C(H)1 domain of the functional camel IGG2A heavy-chain antibodies. Mol Immunol. 1999;36:515–524. doi: 10.1016/s0161-5890(99)00067-x. [DOI] [PubMed] [Google Scholar]
- 25.Muyldermans S., Cambillau C., Wyns L. Recognition of antigens by single-domain antibody fragments: the superfluous luxury of paired domains. Trends Biochem Sci. 2001;26:230–235. doi: 10.1016/s0968-0004(01)01790-x. [DOI] [PubMed] [Google Scholar]
- 26.Vincke C., Muyldermans S. Introduction to heavy chain antibodies and derived Nanobodies. Methods Mol Biol. 2012;911:15–26. doi: 10.1007/978-1-61779-968-6_2. [DOI] [PubMed] [Google Scholar]
- 27.Muyldermans S., Atarhouch T., Saldanha J., Barbosa J.A., Hamers R. Sequence and structure of VH domain from naturally occurring camel heavy chain immunoglobulins lacking light chains. Protein Eng. 1994;7:1129–1135. doi: 10.1093/protein/7.9.1129. [DOI] [PubMed] [Google Scholar]
- 28.Desmyter A., Transue T.R., Ghahroudi M.A., Thi M.H., Poortmans F., Hamers R., Muyldermans S., Wyns L. Crystal structure of a camel single-domain VH antibody fragment in complex with lysozyme. Nat Struct Biol. 1996;3:803–811. doi: 10.1038/nsb0996-803. [DOI] [PubMed] [Google Scholar]
- 29.Kunz P., Zinner K., Mucke N., Bartoschik T., Muyldermans S., Hoheisel J.D. The structural basis of nanobody unfolding reversibility and thermoresistance. Sci Rep. 2018;8:7934. doi: 10.1038/s41598-018-26338-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrari D., Pizzirani C., Adinolfi E., Lemoli R.M., Curti A., Idzko M., Panther E., Di Virgilio F. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol. 2006;176:3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- 31.Scheuplein F., Schwarz N., Adriouch S., Krebs C., Bannas P., Rissiek B., Seman M., Haag F., Koch-Nolte F. NAD+ and ATP released from injured cells induce P2X7-dependent shedding of CD62L and externalization of phosphatidylserine by murine T cells. J Immunol. 2009;182:2898–2908. doi: 10.4049/jimmunol.0801711. [DOI] [PubMed] [Google Scholar]
- 32.Idzko M., Ferrari D., Eltzschig H.K. Nucleotide signalling during inflammation. Nature. 2014;509:310–317. doi: 10.1038/nature13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Danquah W., Meyer-Schwesinger C., Rissiek B., Pinto C., Serracant-Prat A., Amadi M., Iacenda D., Knop J.H., Hammel A., Bergmann P., et al. Nanobodies that block gating of the P2X7 ion channel ameliorate inflammation. Sci Transl Med. 2016;8 doi: 10.1126/scitranslmed.aaf8463. [DOI] [PubMed] [Google Scholar]
- 34.Gonde H., Demeules M., Hardet R., Scarpitta A., Junge M., Pinto-Espinoza C., Varin R., Koch-Nolte F., Boyer O., Adriouch S. A methodological approach using rAAV Vectors encoding nanobody-based biologics to Evaluate ARTC2.2 and P2X7 in vivo. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.704408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinto-Espinoza C., Guillou C., Rissiek B., Wilmes M., Javidi E., Schwarz N., Junge M., Haag F., Liaukouskaya N., Wanner N., et al. Effective targeting of microglial P2X7 following intracerebroventricular delivery of nanobodies and nanobody-encoding AAVs. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.1029236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilmes M., Pinto Espinoza C., Ludewig P., Stabernack J., Liesz A., Nicke A., Gelderblom M., Gerloff C., Falzoni S., Tolosa E., et al. Blocking P2X7 by intracerebroventricular injection of P2X7-specific nanobodies reduces stroke lesions. J Neuroinflammation. 2022;19:256. doi: 10.1186/s12974-022-02601-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Catterall W.A., Lenaeus M.J., Gamal El-Din T.M. Structure and Pharmacology of voltage-gated sodium and calcium channels. Annu Rev Pharmacol Toxicol. 2020;60:133–154. doi: 10.1146/annurev-pharmtox-010818-021757. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y., Yamada Y., Fan M., Bangaru S.D., Lin B., Yang J. The beta subunit of voltage-gated Ca2+ channels interacts with and regulates the activity of a novel isoform of Pax6. J Biol Chem. 2010;285:2527–2536. doi: 10.1074/jbc.M109.022236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buraei Z., Yang J. The ss subunit of voltage-gated Ca2+ channels. Physiol Rev. 2010;90:1461–1506. doi: 10.1152/physrev.00057.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dolphin A.C. Calcium channel auxiliary alpha2delta and beta subunits: trafficking and one step beyond. Nat Rev Neurosci. 2012;13:542–555. doi: 10.1038/nrn3311. [DOI] [PubMed] [Google Scholar]
- 41.Gee N.S., Brown J.P., Dissanayake V.U., Offord J., Thurlow R., Woodruff G.N. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the alpha2delta subunit of a calcium channel. J Biol Chem. 1996;271:5768–5776. doi: 10.1074/jbc.271.10.5768. [DOI] [PubMed] [Google Scholar]
- 42.Morgenstern T.J., Park J., Fan Q.R., Colecraft H.M. A potent voltage-gated calcium channel inhibitor engineered from a nanobody targeted to auxiliary Ca(V)beta subunits. Elife. 2019;8 doi: 10.7554/eLife.49253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun L., Tong C.K., Morgenstern T.J., Zhou H., Yang G., Colecraft H.M. Targeted ubiquitination of sensory neuron calcium channels reduces the development of neuropathic pain. Proc Natl Acad Sci U S A. 2022;119 doi: 10.1073/pnas.2118129119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morgenstern T.J., Nirwan N., Hernandez-Ochoa E.O., Bibollet H., Choudhury P., Laloudakis Y.D., Ben Johny M., Bannister R.A., Schneider M.F., Minor D.L., Jr., et al. Selective posttranslational inhibition of Ca(V)beta(1)-associated voltage-dependent calcium channels with a functionalized nanobody. Nat Commun. 2022;13:7556. doi: 10.1038/s41467-022-35025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Del Rivero Morfin P.J., Chavez D.S., Jayaraman S., Yang L., Geisler S.M., Kochiss A.L., Tuluc P., Colecraft H.M., Marx S.O., Liu X.S., et al. A genetically encoded actuator boosts L-type calcium channel function in diverse physiological settings. Sci Adv. 2024;10 doi: 10.1126/sciadv.adq3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waldmann R., Champigny G., Bassilana F., Heurteaux C., Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997;386:173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- 47.Kellenberger S., Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev. 2002;82:735–767. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- 48.Wemmie J.A., Chen J., Askwith C.C., Hruska-Hageman A.M., Price M.P., Nolan B.C., Yoder P.G., Lamani E., Hoshi T., Freeman J.H., Jr., et al. The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron. 2002;34:463–477. doi: 10.1016/s0896-6273(02)00661-x. [DOI] [PubMed] [Google Scholar]
- 49.Bohlen C.J., Chesler A.T., Sharif-Naeini R., Medzihradszky K.F., Zhou S., King D., Sanchez E.E., Burlingame A.L., Basbaum A.I., Julius D. A heteromeric Texas coral snake toxin targets acid-sensing ion channels to produce pain. Nature. 2011;479:410–414. doi: 10.1038/nature10607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu Y., Chen Z., Li W.G., Cao H., Feng E.G., Yu F., Liu H., Jiang H., Xu T.L. A nonproton ligand sensor in the acid-sensing ion channel. Neuron. 2010;68:61–72. doi: 10.1016/j.neuron.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 51.Escoubas P., De Weille J.R., Lecoq A., Diochot S., Waldmann R., Champigny G., Moinier D., Menez A., Lazdunski M. Isolation of a tarantula toxin specific for a class of proton-gated Na+ channels. J Biol Chem. 2000;275:25116–25121. doi: 10.1074/jbc.M003643200. [DOI] [PubMed] [Google Scholar]
- 52.Wu Y., Chen Z., Sigworth F.J., Canessa C.M. Structure and analysis of nanobody binding to the human ASIC1a ion channel. Elife. 2021;10 doi: 10.7554/eLife.67115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fozzard H.A., Hanck D.A. Structure and function of voltage-dependent sodium channels: comparison of brain II and cardiac isoforms. Physiol Rev. 1996;76:887–926. doi: 10.1152/physrev.1996.76.3.887. [DOI] [PubMed] [Google Scholar]
- 54.Whitaker W.R., Clare J.J., Powell A.J., Chen Y.H., Faull R.L., Emson P.C. Distribution of voltage-gated sodium channel alpha-subunit and beta-subunit mRNAs in human hippocampal formation, cortex, and cerebellum. J Comp Neurol. 2000;422:123–139. doi: 10.1002/(sici)1096-9861(20000619)422:1<123::aid-cne8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 55.George A.L., Jr. Inherited disorders of voltage-gated sodium channels. J Clin Investig. 2005;115:1990–1999. doi: 10.1172/JCI25505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Escayg A., MacDonald B.T., Meisler M.H., Baulac S., Huberfeld G., An-Gourfinkel I., Brice A., LeGuern E., Moulard B., Chaigne D., et al. Mutations of SCN1A, encoding a neuronal sodium channel, in two families with GEFS+2. Nat Genet. 2000;24:343–345. doi: 10.1038/74159. [DOI] [PubMed] [Google Scholar]
- 57.Wulff H., Christophersen P., Colussi P., Chandy K.G., Yarov-Yarovoy V. Antibodies and venom peptides: new modalities for ion channels. Nat Rev Drug Discov. 2019;18:339–357. doi: 10.1038/s41573-019-0013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Srinivasan L., Alzogaray V., Selvakumar D., Nathan S., Yoder J.B., Wright K.M., Klinke S., Nwafor J.N., Labanda M.S., Goldbaum F.A., et al. Development of high-affinity nanobodies specific for Na(V)1.4 and Na(V)1.5 voltage-gated sodium channel isoforms. J Biol Chem. 2022;298 doi: 10.1016/j.jbc.2022.101763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hernandez-Resendiz I., Hartung F., Pardo L.A. Antibodies targeting K(V) potassium channels: a promising treatment for cancer. Bioelectricity. 2019;1:180–187. doi: 10.1089/bioe.2019.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stortelers C., Pinto-Espinoza C., Van Hoorick D., Koch-Nolte F. Modulating ion channel function with antibodies and nanobodies. Curr Opin Immunol. 2018;52:18–26. doi: 10.1016/j.coi.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 61.Pardo L.A., Stuhmer W. The roles of K(+) channels in cancer. Nat Rev Cancer. 2014;14:39–48. doi: 10.1038/nrc3635. [DOI] [PubMed] [Google Scholar]
- 62.Hartung F., Kruwel T., Shi X., Pfizenmaier K., Kontermann R., Chames P., Alves F., Pardo L.A. A novel anti-Kv10.1 nanobody fused to single-chain TRAIL enhances apoptosis Induction in cancer cells. Front Pharmacol. 2020;11:686. doi: 10.3389/fphar.2020.00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chandy K.G., Sanches K., Norton R.S. Structure of the voltage-gated potassium channel K(V)1.3: insights into the inactivated conformation and binding to therapeutic leads. Channels. 2023;17 doi: 10.1080/19336950.2023.2253104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tan P., He L., Huang Y., Zhou Y. Optophysiology: Illuminating cell physiology with optogenetics. Physiol Rev. 2022;102:1263–1325. doi: 10.1152/physrev.00021.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tan P., He L., Zhou Y. Engineering Supramolecular Organizing Centers for optogenetic control of innate immune responses. Adv Biol (Weinh) 2021;5 doi: 10.1002/adbi.202000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gil A.A., Carrasco-Lopez C., Zhu L., Zhao E.M., Ravindran P.T., Wilson M.Z., Goglia A.G., Avalos J.L., Toettcher J.E. Optogenetic control of protein binding using light-switchable nanobodies. Nat Commun. 2020;11:4044. doi: 10.1038/s41467-020-17836-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu D., Lee H., Hong J., Jung H., Jo Y., Oh B.H., Park B.O., Heo W.D. Optogenetic activation of intracellular antibodies for direct modulation of endogenous proteins. Nat Methods. 2019;16:1095–1100. doi: 10.1038/s41592-019-0592-7. [DOI] [PubMed] [Google Scholar]
- 68.Unda S.R., Pomeranz L.E., Marongiu R., Yu X., Kelly L., Hassanzadeh G., Molina H., Vaisey G., Wang P., Dyke J.P., et al. Bidirectional regulation of motor circuits using magnetogenetic gene therapy. Sci Adv. 2024;10:eadp9150. doi: 10.1126/sciadv.adp9150. [DOI] [PMC free article] [PubMed] [Google Scholar]