Abstract
Background
Heritable pulmonary arterial hypertension (PAH) is a rare form of pre-capillary pulmonary hypertension that typically affects young patients. With increased survival and subsequent ageing of these patients, newly acquired cardiovascular conditions may influence the pulmonary haemodynamic profile and impact management.
Case summary
We report a case series of four patients with mutations in genes associated with PAH to illustrate the spectrum of pulmonary haemodynamics under the influence of superimposed acquired conditions. The first two cases involve patients with a long-standing diagnosis of heritable PAH and severe pre-capillary pulmonary hypertension, who developed overt left-sided diastolic dysfunction later in follow-up due to the acquisition of multiple cardiovascular comorbidities. The second two cases describe patients with a genetic pre-disposition to develop PAH and conditions that are risk factors for left heart disease, with mild elevation of resting pulmonary pressures, in whom exercise right heart catheterization unmasked occult left-sided diastolic dysfunction.
Discussion
Pulmonary haemodynamics are complex and dynamic over time, even in patients with or at risk of heritable PAH, when additional acquired cardiovascular conditions emerge. Correct phenotyping at diagnosis and during follow-up of patients at risk of heritable PAH, along with a clear understanding of the underlying pulmonary haemodynamic profile, is crucial for appropriate management.
Keywords: Heritable pulmonary arterial hypertension, Cardiovascular comorbidities, Pre-capillary pulmonary hypertension, Post-capillary pulmonary hypertension, Diastolic dysfunction, Exercise right heart catheterization, Case series
Learning points.
Correct haemodynamic phenotyping of patients at risk of or with long-standing pre-capillary pulmonary hypertension is critical to establish a precise diagnosis and management strategy.
Acquired cardiovascular conditions may exert a significant influence on pulmonary haemodynamics and cause left-sided diastolic disfunction in patients at risk or with diagnosed heritable pulmonary arterial hypertension (PAH).
Exercise right heart catheterization should always be considered as diagnostic tool in patients at risk for PAH or with unexplained dyspnoea and normal resting haemodynamics.
Introduction
Pulmonary arterial hypertension (PAH) is a rare and severe disease characterized by progressive remodelling of the pulmonary vasculature.1 Haemodynamically, PAH corresponds to pre-capillary pulmonary hypertension (PH), i.e. mean pulmonary artery pressure (PAp) >20 mmHg, pulmonary arterial wedge pressure (PAWP) ≤ 15 mmHg, and pulmonary vascular resistance (PVR) >2 Wood units by right heart catheterization (RHC).2,3 Among the aetiologies of PAH, heritable forms refer to patients with a pathogenic mutation in a gene associated with PAH or to genetically negative cases with familial aggregation of the disease.4,5 Recent data from PAH registries have shown an epidemiological shift in idiopathic PAH, with patients now being commonly diagnosed later in life (∼60–65 years), often in association with comorbidities, which impacts both management and prognosis.6–8 Since heritable PAH is usually diagnosed at young age,5 comorbidities are uncommon; nevertheless, with ageing acquired conditions may appear during follow-up. To date, data regarding this issue and its impact on PAH from a pathobiological, management, and prognostic perspective are lacking. This manuscript presents a case series of four patients with mutations in genes associated with PAH, illustrating the influence of superimposed acquired conditions on pulmonary haemodynamics.
Summary figure
Case 1
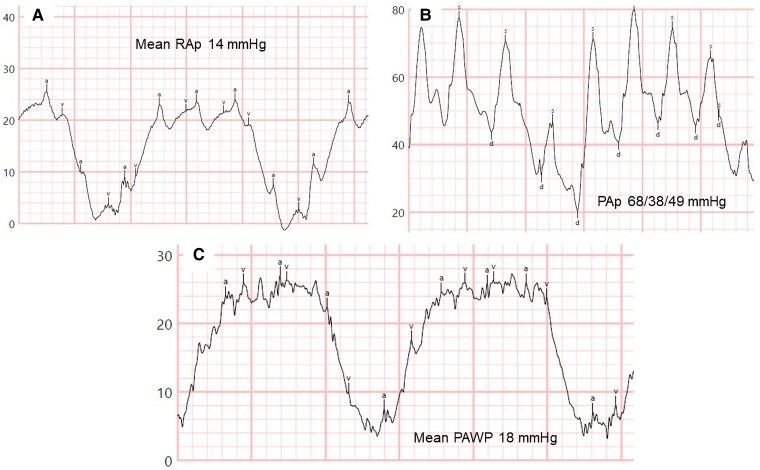
The first case involves a 72-year-old woman who was diagnosed with heritable PAH at age 48, following the identification of a pathogenic mutation in the bone morphogenetic protein receptor type 2 gene (BMPR2). Severe pre-capillary PH with low cardiac output (CO) was present at diagnosis (RHC, right atrial pressure [RAp] 18 mmHg, PAp 144/56/84 mmHg, PAWP 8 mmHg, CO 2.3 L/min, mixed venous oxygen saturation [SvO2] 52%, PVR 33 Wood units). At that time, initial dual-combination therapy with sildenafil and subcutaneous treprostinil was initiated. Treprostinil was titrated, and ambrisentan was added in 2018 due to clinical and haemodynamic deterioration. During follow-up, the patient developed systemic arterial hypertension, dyslipidaemia, Grade 3 obesity (body mass index 49 kg/m²), severe obstructive sleep apnoea, restrictive ventilatory impairment, hypoventilation syndrome with hypercapnia due to obesity, and chronic kidney disease. A repeat RHC in 2022, during hospitalization for acute heart failure, showed the following values: RAp 14 mmHg, PAp 68/38/49 mmHg, mean PAWP 18 mmHg (24 at end-expiration), CO 5.98 L/min, SvO2 71%, PVR 5.18 Wood units (Figure 1A–C). Transthoracic echocardiography revealed mild biatrial dilation, mildly increased left ventricular wall thickness, indeterminate left heart diastolic function, and mild right ventricular dilation with normal biventricular systolic function. A new haemodynamic condition, overt left heart diastolic dysfunction, developed in addition to her pre-capillary PH, 24 years after the diagnosis of heritable PAH. Due to the persistence of severe pre-capillary PH, PAH therapy was not reduced; however, diuretic therapy with furosemide and spironolactone was added to treat the elevated right- and left-sided filling pressures. To date, the patient remains clinically stable, and no additional hospitalizations have occurred.
Figure 1.
Resting right heart catheterization tracings during follow-up of Case 1 at time of hospital admission. (A) Mean right atrial pressure 14 mmHg. (B) Pulmonary artery pressures 68/38/49 mmHg. (C) Mean pulmonary arterial wedge pressure 18 mmHg. Note the significant respiratory variations due to obesity. PAp, pulmonary artery pressures; PAWP, pulmonary arterial wedge pressure; RAp, right atrial pressure.
Case 2
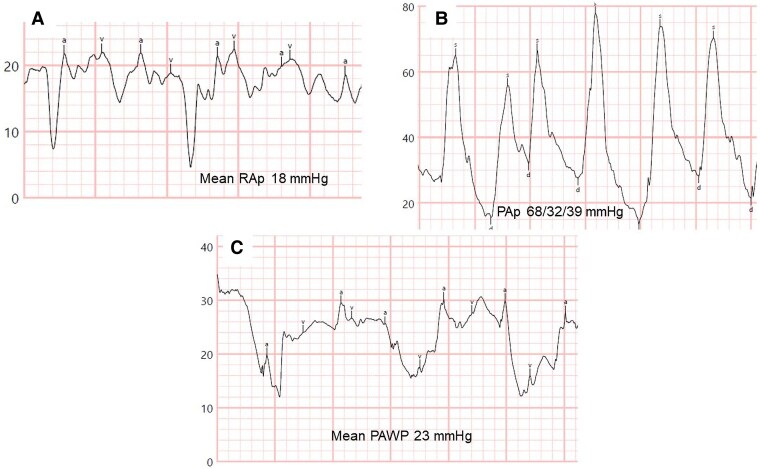
Case 2 corresponds to a woman with a late diagnosis of heritable PAH, identified at the age of 59 in 2008, with a likely pathogenic mutation in the T-Box factor 4 gene and familial aggregation (two daughters with PAH). At diagnosis, RHC showed the following haemodynamics: RAp 15 mmHg, PAp 86/11/44 mmHg, mean PAWP 8 mmHg, CO 2.8 L/min, SvO2 64%, PVR 12.9 Wood units. Dual oral combination therapy with tadalafil and ambrisentan was initiated. In 2018, due to clinical and haemodynamic deterioration, subcutaneous treprostinil was added. Over the years, the patient developed systemic arterial hypertension, dyslipidaemia, paroxysmal atrial fibrillation, severe obstructive sleep apnoea, and respiratory insufficiency, requiring chronic oxygen therapy. The development of acute pulmonary oedema in the context of paroxysmal atrial fibrillation, with invasive haemodynamic confirmation of elevated left-sided filling pressures (mean PAWP 18 mmHg), led to the discontinuation of treprostinil. In 2022, the patient was hospitalized for right and left heart failure. RHC showed the following values: RAp 18 mmHg, PAp 68/32/39 mmHg, mean PAWP 23 mmHg (26 at end-expiration), CO by Fick (due to severe tricuspid regurgitation) 5.1 L/min, PVR 3.14 Wood units (Figure 2A–C). PAWP decreased to 16 mmHg after fluid depletion. Transthoracic echocardiography, performed before diuretic treatment, revealed mildly increased left ventricular wall thickness with normal left ventricular systolic function, signs of elevated left- and right-sided filling pressures, right atrial dilation (33 cm²), severe right ventricular dilation and systolic dysfunction, moderate mitral regurgitation, and severe tricuspid regurgitation (Figure 3A–F). Due to several adverse prognostic parameters related to PAH, and clinical and haemodynamic worsening after prostacyclin withdrawal, low-dose subcutaneous treprostinil was restarted, combined with high doses of diuretics (furosemide, spironolactone, chlorthalidone). The disease progressed over the following year. Severe complications related to the subcutaneous treprostinil infusion site, along with her end-stage condition, led to the decision to switch treprostinil to oral selexipag to improve her quality of life. Six months before her death, a structured follow-up by the Palliative Care Unit was organized. The patient died in July 2023 from respiratory arrest during a brief hospitalization for right and left heart failure and acute-on-chronic respiratory failure.
Figure 2.
Resting right heart catheterization tracings during follow-up of Case 2 at time of hospital admission. (A) Mean right atrial pressure 18 mmHg. (B) Pulmonary artery pressures 68/32/39 mmHg. (C) Mean pulmonary arterial wedge pressure 23 mmHg. PAp, pulmonary artery pressures; PAWP, pulmonary arterial wedge pressure; RAp, right atrial pressure.
Figure 3.
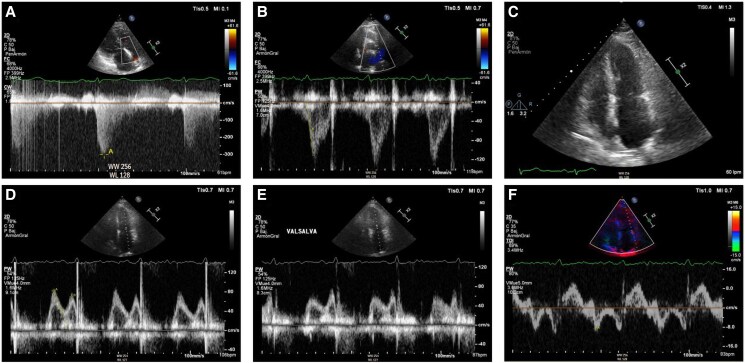
Transthoracic echocardiography during follow-up of Case 2 at time of hospital admission. (A) Apical four chamber view depicting a severely dilated right ventricle with the following dimensions: end-diastolic right ventricular basal diameter 52 mm, end-diastolic right ventricular mid diameter 55 mm, end-diastolic right ventricular longitudinal diameter 85 mm, right ventricular end-diastolic area 41.5 cm2. Right ventricular systolic dysfunction was severely reduced (fractional area change 19%, tricuspid annular plane systolic excursion 12 mm with severe tricuspid regurgitation, pulsed Doppler S wave of the tricuspid annulus 6.9 cm/s). Severe right atrial dilation (33 cm2). (B) Right ventricular-focused apical four chamber view showing severe functional tricuspid regurgitation. (C) Apical four chamber view depicting moderate mitral regurgitation due to anterior leaflet prolapse. (D) Moderate functional pulmonary regurgitation allows for estimation of a mean pulmonary artery pressure of 45 mmHg. (E) Dilated inferior vena cava of 24 mm without respiratory variation, estimated right atrial pressure of 15 mmHg. (F) Mitral inflow shows pseudonormal left ventricular filling pattern consistent with Grade 2 diastolic dysfunction (elevated left ventricular filling pressures). Mitral E-wave was 109 cm/s; mitral A-wave was 102 cm/s. Valsalva manoeuvre increased A-wave velocity. Lateral E/e′ ratio was 12.
Case 3
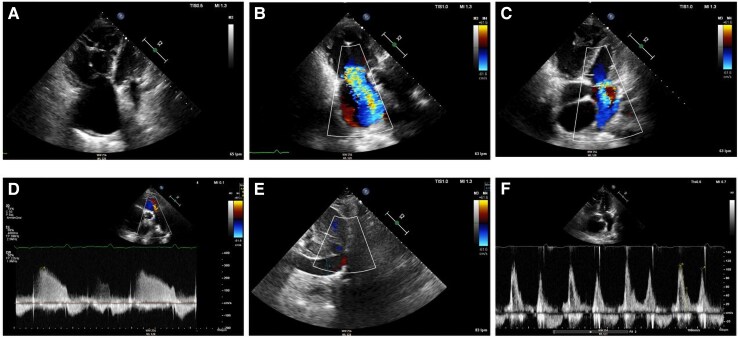
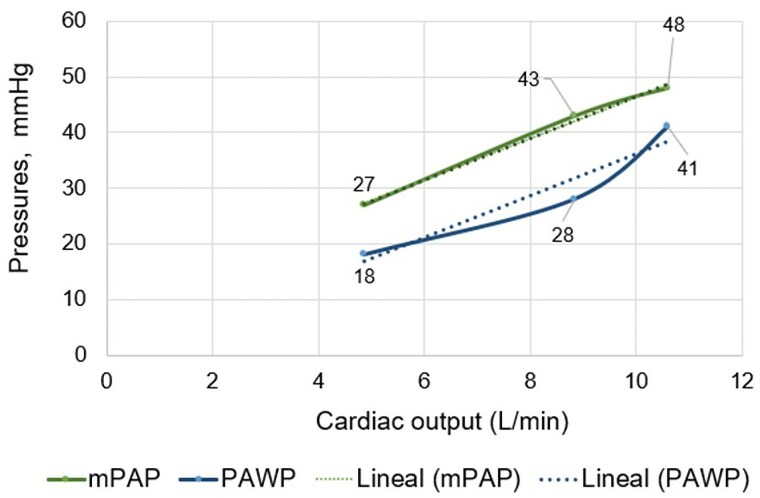
A 69-year-old woman with a pathogenic mutation in the BMPR2 gene and a family history of PAH (one sister and one daughter deceased from PAH, another sister a carrier of the same mutation) was referred to our clinic due to exertional dyspnoea for diagnostic work-up of PAH. She had obesity, systemic arterial hypertension, diabetes mellitus, dyslipidaemia, and obstructive sleep apnoea. NT-proBNP was normal at 102 pg/mL; cardiopulmonary exercise testing revealed normal functional capacity with signs of deconditioning, and no ventilatory inefficiency; electrocardiogram showed normal sinus rhythm, no signs of right-sided overload, and a left QRS axis. Mild left atrial dilation and signs of elevated left heart filling pressures with normal right heart chambers were observed in the transthoracic echocardiography (Figure 4A–F). The first resting RHC depicted the following values: RAp 1 mmHg, PAp 45/15/25 mmHg, PAWP 13 mmHg, CO 5.2 L/min, SvO2 61%, PVR 2.3 Wood units. Despite the results corresponding to pre-capillary PH according to the current definition, the state of excessive volume depletion as indicated by the right heart filling pressures (RAp 1 mmHg) raised the suspicion of a falsely low value of PAWP, considering the patient’s comorbidities of Group 2 PH and the results of the non-invasive tests. Accordingly, and due to the importance of accurately phenotyping this patient (carrier of a mutation for heritable PAH), exercise RHC was performed. Resting parameters were RAp 4 mmHg, PAp 48/18/27 mmHg, PAWP 18 mmHg, CO 4.86 L/min, SvO2 66%, PVR 1.85 Wood units, i.e. a normal fluid status unmasked left-sided diastolic dysfunction. Peak exercise (45 W) values were: PAp 85/30/48 mmHg, PAWP 41 mmHg, CO 10.6 L/min, PVR 0.66 Wood units. The multipoint mean PAp/CO slope was 3.7 mmHg/L/min, while the mean PAWP/CO slope was 3.8 mmHg/L/min. In summary, exercise catheterization confirmed the clinical suspicion of post-capillary PH (PH related to left-sided diastolic dysfunction) (Figure 5). Diuretic treatment was modified, SGLT2 inhibitors were initiated, and the importance of strict control of cardiovascular risk factors was reinforced. To date, the patient remains stable with no changes in clinical status or in non-invasive follow-up tests.
Figure 4.
Transthoracic echocardiography of Case 3 at screening for pulmonary hypertension. (A) Systolic peak tricuspid regurgitation velocity 3.0 m/s. (B) Shortened right ventricular outflow tract acceleration time of pulmonary ejection of 70 ms without evident mid-systolic notch. (C) Apical four chamber view depicting normal right ventricular, right atrial, and left ventricular size and mild left atrial dilation (35 mL/m2). (D and E) Mitral inflow shows pseudonormal left ventricular filling pattern consistent with Grade 2 diastolic dysfunction (elevated left ventricular filling pressures). Mitral E-wave was 82 cm/s; mitral A-wave was 70 cm/s. Valsalva manoeuvre increased A-wave velocity. (F) Reduced lateral mitral e′-wave in tissue Doppler imaging of 8.8 cm/s. Average E/e′ ratio was 11.
Figure 5.
Mean pulmonary artery pressure and mean pulmonary arterial wedge pressure/cardiac output slopes at peak exercise of Case 3 during screening for pulmonary hypertension. The mean pulmonary artery pressure/cardiac output slope was 3.7 mmHg/L/min and the mean pulmonary arterial wedge pressure/cardiac output slope was 3.8 mmHg/L/min. CO, cardiac output; mPAP, mean pulmonary artery pressure; PAWP, mean pulmonary arterial wedge pressure.
Case 4
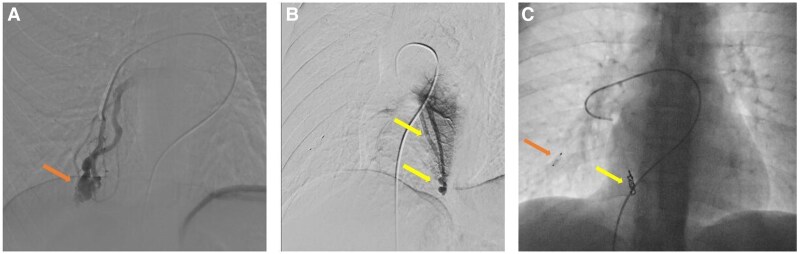
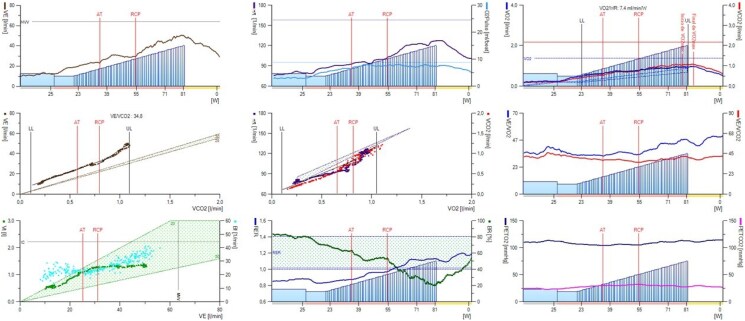
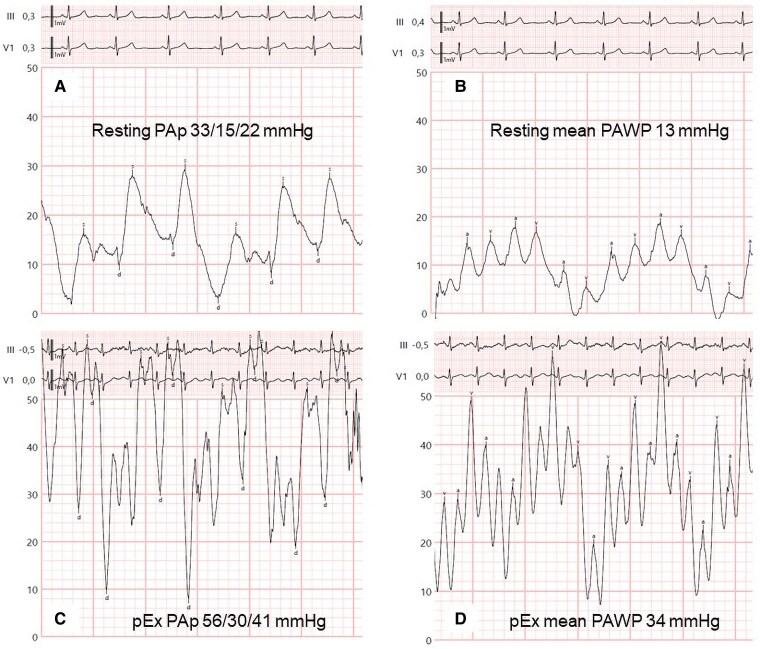
Case 4 is a 53-year-old overweight woman who is a carrier of a pathogenic mutation in the BMPR2 gene (her daughter died at age 24 from PAH) with a history of embolization of large pulmonary arteriovenous fistulae due to respiratory insufficiency and for prevention of systemic embolism (Figure 6A–C). She had a normal echocardiogram but showed signs of ventilatory inefficiency in the cardiopulmonary exercise testing (Figure 7), so she was referred for RHC. The invasive parameters were RAp 11 mmHg, PAp 33/15/22 mmHg, PAWP 13 mmHg, SvO2 65%, CO 4.35 L/min, PVR 2.07 Wood units, showing very mild pre-capillary PH (Figure 8A and B). Since there is no indication to date to start PAH treatment with these values, exercise RHC was performed to assess the response of pulmonary pressures with exercise. Invasive values at peak exercise (60 W) were PAp 56/30/41 mmHg, PAWP 34 mmHg, CO 5.9 L/min, PVR 1.19 Wood units (Figure 8C and D). The multipoint mean PAp/CO slope was 3.5 mmHg/L/min, while the mean PAWP/slope was 2.9 mmHg/L/min, indicating that exercise catheterization unmasked occult left-sided diastolic dysfunction. Accordingly, no specific therapy for PAH was initiated.
Figure 6.
Pulmonary angiography images of the pulmonary arteriovenous fistulae during embolization. (A) Pulmonary arteriovenous fistula in the basal region of de middle lobe (arrow). (B) Pulmonary arteriovenous fistula in the right lower lobe (arrows). (C) Status post-embolization of the fistula in the basal region of the middle lobe with a 6 mm large Amplatzer device (orange arrow) and of the fistula in the right lower lobe with four 5 mm coils (yellow arrow). Note that three smaller fistulae could technically not be embolized.
Figure 7.
Wasserman curves of the cardiopulmonary exercise test of Case 4 during screening for pulmonary hypertension. Exercise was performed on upright cycle ergometer. The test was maximal (respiratory exchange ratio at peak exercise 1.12, Curve 8). Functional capacity was mildly reduced [peak oxygen consumption 16.5 mL/Kg/min (69% of predicted), Curve 3]. There was moderate ventilatory inefficiency: ventilatory equivalent of carbon dioxide at first ventilatory threshold was 38 (138% of predicted) and ventilatory equivalent of oxygen at peak exercise was 46 (Curve 6). Ventilation/carbon dioxide production slope was 34.8 (Curve 4). End-tidal carbon dioxide partial pressure at rest was 24 mmHg, at first ventilatory threshold 30 mmHg, at peak exercise 28 mmHg (Curve 9). AT, first ventilatory threshold; Bf, breathing frequency; BR, breathing reserve; EqCO2, ventilatory equivalent of carbon dioxide; EqO2, ventilatory equivalent of oxygen; HR, heart rate; PETCO2, end-tidal carbon dioxide partial pressure; PETO2, end-tidal oxygen partial pressure; RCP, second ventilatory threshold; RER, respiratory exchange ratio; VE, ventilation; VE/VCO2, ventilation/carbon dioxide production; VO2, oxygen consumption; Vt, tidal volume.
Figure 8.
Resting and exercise right heart catheterization tracings of Case 4 during screening for pulmonary hypertension. (A) Resting pulmonary artery pressures 33/15/22 mmHg. (B) Resting mean pulmonary arterial wedge pressure 13 mmHg. (C) Pulmonary artery pressures 56/30/41 mmHg at peak exercise. (D) Mean pulmonary arterial wedge pressure at peak exercise 34 mmHg. PAp, pulmonary artery pressures; PAWP, pulmonary arterial wedge pressure; pEx, peak exercise.
Discussion
Heritable PAH represents the paradigm of pre-capillary PH. The characteristic picture of a patient with heritable PAH is that of a young woman without comorbidities and advanced pulmonary arterial remodelling with severely increased PAp and PVR and a normal PAWP. Nevertheless, with improvements in the diagnosis and management of these patients and increased survival, patients may develop superimposed acquired conditions that introduce additional pathobiological mechanisms, influencing the development of pathological changes in the pulmonary vasculature. Furthermore, occult left heart diastolic dysfunction may be the aetiology of the mild elevation of pulmonary pressures in patients at risk for heritable PAH, either due to cardiovascular comorbidities and/or to an alteration related to the heritable condition (BMPR2 mutation) leading to left-sided overload, such as pulmonary arteriovenous fistulae.9,10
We present herein two women (Cases 1 and 2) with a long-standing diagnosis of heritable PAH and very severely increased PAp and PVR, requiring triple-combination therapy. Due to the high number of cardiopulmonary conditions acquired during follow-up, these patients developed, 22 and 14 years after the diagnosis of PAH, overt left-sided diastolic dysfunction, leading to resting elevation of PAWP. From a clinical point of view, the presence of left heart failure with preserved ejection fraction (HFpEF) in a patient with severe pre-capillary PH warrants careful assessment, as PAH therapy may be detrimental for left-sided pathology and require dose adjustment. In these two patients, it was decided to maintain PAH therapy unchanged due to the presence of several parameters of adverse prognosis of PAH. However, it became mandatory to prescribe combined treatment with different diuretics to decrease left- and right-sided filling pressures and treat systemic and pulmonary congestion. If the patient had had a less severe profile of PAH, with no high-risk features, it might have been decided to decrease the dose or withdraw one of the PAH medications, alongside combined diuretic treatment. In this regard, endothelin receptor antagonists are associated with the highest risk of left-sided decompensation and should therefore be considered for withdrawal first. Alternatively, the dose of prostacyclin infusion could be reduced. Careful follow-up is essential, as PAH may worsen with therapy reduction. It can be particularly challenging to achieve a balance between treatment strategies that favour right- vs. left-sided disease, and this may impact prognosis. Regarding pathobiological mechanisms, pre-capillary PH results from vasoconstriction, abnormal cell proliferation, fibrosis, in situ thrombosis, and inflammation of mainly small muscular-type pulmonary arteries. Contrarily, post-capillary PH originates from passive backward transmission of elevated left-sided filling pressures secondary to systolic or diastolic left ventricular dysfunction into the pulmonary vasculature. Interstitial fibrosis causing left atrial stiffness and reduced left atrial compliance, as well as impaired left atrial contractility, contributes to pathogenic alterations in the pulmonary circuit and right heart.3,11 As illustrated here and as previously reported in other settings of PH, the presence of severe long-standing pre-capillary PH, with advanced remodelling of the pulmonary arteries and significantly increased arterial resistance does not protect the pulmonary venous system from developing elevated pressures and venous congestion.12,13
The last two cases depict two patients with a genetic pre-disposition to develop pre-capillary PH, albeit with the incomplete penetrance of the BMPR2 mutation. At rest they had a very mild increase of PAp and PVR, which strictly corresponded to the definition of pre-capillary PH. Exercise RHC unmasked that the mild elevation of resting PAp was at the expense of occult left-sided diastolic dysfunction, i.e. HFpEF. Exercise RHC is recommended in the current European Guidelines in patients at risk for PAH or with unexplained dyspnoea and normal resting haemodynamics. Fluid challenge or passive leg raise during RHC may also help unmask occult HFpEF.2,14,15 Correct phenotyping of patients at risk for PAH is of utmost importance to ensure proper management. While the information provided by the resting haemodynamics could have led the clinician to start PAH therapy early in this specific setting of heritable PAH, aiming to delay disease progression, the results of the exercise RHC, conversely, advised against its initiation. Consequently, in these two patients, recommendations for strict control of cardiovascular risk factors to avoid progression of diastolic dysfunction should be provided, and, depending on the presence of exertional symptoms and congestion, therapy with SGLT2 inhibitors and diuretics should be initiated. While there was no indication for PAH therapy in these two patients, the presence of a pathological mutation for heritable PAH requires periodic monitoring to assess for clinical, functional, or imaging deterioration suggestive of PAH, which will need invasive haemodynamic confirmation to then start PAH therapy as indicated by the European Guidelines.2
A pathophysiological continuum ranging from classical idiopathic PAH to combined pre-and post-capillary PH from HFpEF with intermediate phenotypes of patients with idiopathic PAH and comorbidities has been proposed,11 the latter group currently being the most prevalent in registries.6–8 Whether a similar pattern might occur in the specific setting of heritable PAH remains to be elucidated. In any case, maintaining a critical approach both at diagnosis and during follow-up of patients at risk of heritable PAH, and paying special attention to all clinical elements, diagnostic tests, and prognostic indicators is imperative to ensure correct management of the patient, given the dynamic and changing nature of pulmonary haemodynamics.
Contributor Information
Irene Martín de Miguel, Cardiology Department, Pulmonary Hypertension Multidisciplinary Unit, Hospital Universitario 12 de Octubre, Av. de Córdoba s/n, 28041 Madrid, Spain; Servicio Madrileño de Salud, Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), C. de Melchor Fernández Almagro 3, 28029 Madrid, Spain; Instituto de Investigación Sanitaria Hospital 12 de Octubre (imas12), Av. de Córdoba s/n, 28041 Madrid, Spain.
Alejandro Cruz Utrilla, Cardiology Department, Pulmonary Hypertension Multidisciplinary Unit, Hospital Universitario 12 de Octubre, Av. de Córdoba s/n, 28041 Madrid, Spain; Servicio Madrileño de Salud, Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), C. de Melchor Fernández Almagro 3, 28029 Madrid, Spain; Instituto de Investigación Sanitaria Hospital 12 de Octubre (imas12), Av. de Córdoba s/n, 28041 Madrid, Spain.
Teresa Segura de La Cal, Cardiology Department, Pulmonary Hypertension Multidisciplinary Unit, Hospital Universitario 12 de Octubre, Av. de Córdoba s/n, 28041 Madrid, Spain; Servicio Madrileño de Salud, Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), C. de Melchor Fernández Almagro 3, 28029 Madrid, Spain; Instituto de Investigación Sanitaria Hospital 12 de Octubre (imas12), Av. de Córdoba s/n, 28041 Madrid, Spain.
Fernando Sarnago Cebada, Servicio Madrileño de Salud, Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), C. de Melchor Fernández Almagro 3, 28029 Madrid, Spain; Instituto de Investigación Sanitaria Hospital 12 de Octubre (imas12), Av. de Córdoba s/n, 28041 Madrid, Spain; Interventional Cardiology Unit, Cardiology Department, Hospital Universitario 12 de Octubre, Av. de Córdoba s/n, 28041 Madrid, Spain.
Maite Velázquez Martín, Servicio Madrileño de Salud, Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), C. de Melchor Fernández Almagro 3, 28029 Madrid, Spain; Instituto de Investigación Sanitaria Hospital 12 de Octubre (imas12), Av. de Córdoba s/n, 28041 Madrid, Spain; Interventional Cardiology Unit, Cardiology Department, Hospital Universitario 12 de Octubre, Av. de Córdoba s/n, 28041 Madrid, Spain.
Carmen Jiménez López-Guarch, Servicio Madrileño de Salud, Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), C. de Melchor Fernández Almagro 3, 28029 Madrid, Spain; Instituto de Investigación Sanitaria Hospital 12 de Octubre (imas12), Av. de Córdoba s/n, 28041 Madrid, Spain; Cardiac Imaging Unit, Cardiology Department, Hospital Universitario 12 de Octubre, Av. de Córdoba s/n, 28041 Madrid, Spain.
Fernando Arribas Ynsaurriaga, Servicio Madrileño de Salud, Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), C. de Melchor Fernández Almagro 3, 28029 Madrid, Spain; Instituto de Investigación Sanitaria Hospital 12 de Octubre (imas12), Av. de Córdoba s/n, 28041 Madrid, Spain; Cardiology Department, Hospital Universitario 12 de Octubre, Av. de Córdoba s/n, 28041 Madrid, Spain.
Pilar Escribano Subías, Cardiology Department, Pulmonary Hypertension Multidisciplinary Unit, Hospital Universitario 12 de Octubre, Av. de Córdoba s/n, 28041 Madrid, Spain; Servicio Madrileño de Salud, Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), C. de Melchor Fernández Almagro 3, 28029 Madrid, Spain; Instituto de Investigación Sanitaria Hospital 12 de Octubre (imas12), Av. de Córdoba s/n, 28041 Madrid, Spain; ERN-LUNG (European Reference Network on rare respiratory diseases), Theodor-Stern-Kai 7, 60596 Frankfurt am Main, Germany.
Lead author biography

Irene Martin de Miguel is a cardiologist at the University Hospital 12 de Octubre in Madrid with specialities in pulmonary hypertension, adult congenital heart disease and cardio-obstetrics. She was trained at University Hospital Gregorio Marañon in Madrid and completed a 1-year research and clinical fellowship at the Adult Congenital Heart Disease Clinic of the Mayo Clinic, Rochester, USA. She is a member of the European Society of Cardiology (ESC) and the Working Groups on Pulmonary Circulation & Right Ventricular Function and Adult Congenital Heart Disease from the ESC, and a member of the Spanish Society of Cardiology (SEC).
Consent: Informed written consent was obtained from the three living patients and from a relative of the deceased patient, following the guidelines set out by the Committee on Publication Ethics.
Funding: I.M.d.M. is supported by a National Río Hortega Grant from Instituto de Salud Carlos III (ISCIII) (CM23/00235). A.C.U. is supported by a National Juan Rodés Grant from ISCIII (JR23/00071).
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Hassoun PM. Pulmonary arterial hypertension. N Engl J Med 2021;385:2361–2376. [DOI] [PubMed] [Google Scholar]
- 2. Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: developed by the task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Endorsed by the International Society for heart and lung transplantation (ISHLT) and the European reference network on rare respiratory diseases (ERN-LUNG). Eur Heart J 2022;43:3618–3731.36017548 [Google Scholar]
- 3. Martin de Miguel I, Cruz-Utrilla A, Oliver E, Escribano-Subias P. Novel molecular mechanisms involved in the medical treatment of pulmonary arterial hypertension. Int J Mol Sci 2023;24:4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Morrell NW, Aldred MA, Chung WK, Elliott CG, Nichols WC, Soubrier F, et al. Genetics and genomics of pulmonary arterial hypertension. Eur Respir J 2019;53:1801899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sztrymf B, Coulet F, Girerd B, Yaici A, Jais X, Sitbon O, et al. Clinical outcomes of pulmonary arterial hypertension in carriers of BMPR2 mutation. Am J Respir Crit Care Med 2008;177:1377–1383. [DOI] [PubMed] [Google Scholar]
- 6. Hoeper MM, Pausch C, Grünig E, Klose H, Staehler G, Huscher D, et al. Idiopathic pulmonary arterial hypertension phenotypes determined by cluster analysis from the COMPERA registry. J Heart Lung Transplant 2020;39:1435–1444. [DOI] [PubMed] [Google Scholar]
- 7. Stolfo D, Barbisan D, Ameri P, Lombardi CM, Monti S, Driussi M, et al. Performance of risk stratification scores and role of comorbidities in older vs younger patients with pulmonary arterial hypertension. J Heart Lung Transplant 2023;42:1082–1092. [DOI] [PubMed] [Google Scholar]
- 8. Rosenkranz S, Pausch C, Coghlan JG, Huscher D, Pittrow D, Grünig E, et al. Risk stratification and response to therapy in patients with pulmonary arterial hypertension and comorbidities: a COMPERA analysis. J Heart Lung Transplant 2023;42:102–114. [DOI] [PubMed] [Google Scholar]
- 9. Kularatne M, Eyries M, Savale L, Humbert M, Montani D. Isolated pulmonary arteriovenous malformations associated with BMPR2 pathogenic variants. Chest 2023;164:e23–e26. [DOI] [PubMed] [Google Scholar]
- 10. Soon E, Southwood M, Sheares K, Pepke-Zaba J, Morrell NW. Better off blue: BMPR-2 mutation, arteriovenous malformation, and pulmonary arterial hypertension. Am J Respir Crit Care Med 2014;189:1435–1436. [DOI] [PubMed] [Google Scholar]
- 11. Opitz CF, Hoeper MM, Gibbs JS, Kaemmerer H, Pepke-Zaba J, Coghlan JG, et al. Pre-capillary, combined, and post-capillary pulmonary hypertension: a pathophysiological continuum. J Am Coll Cardiol 2016;68:368–378. [DOI] [PubMed] [Google Scholar]
- 12. Hoeper MM, Rosenkranz S. Was Paul Wood wrong about pre-capillary pulmonary hypertension protecting against pulmonary congestion in left heart disease? Eur Heart J 2022;43:3432–3434. [DOI] [PubMed] [Google Scholar]
- 13. Omote K, Sorimachi H, Obokata M, Reddy YNV, Verbrugge FH, Omar M, et al. Pulmonary vascular disease in pulmonary hypertension due to left heart disease: pathophysiologic implications. Eur Heart J 2022;43:3417–3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. D’Alto M, Romeo E, Argiento P, Motoji Y, Correra A, Di Marco GM, et al. Clinical relevance of fluid challenge in patients evaluated for pulmonary hypertension. Chest 2017;151:119–126. [DOI] [PubMed] [Google Scholar]
- 15. Montani D, Girerd B, Jaïs X, Laveneziana P, Lau EMT, Bouchachi A, et al. Screening for pulmonary arterial hypertension in adults carrying a BMPR2 mutation. Eur Respir J 2021;58:2004229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.