Summary
Grass carp reovirus (GCRV) causes hemorrhagic disease in grass carp, leading to significant economic losses in China’s aquaculture. However, the cellular receptors responsible for the initiation of GCRV infection remain unclear. This study reveals that cell surface heparan sulfate (HS) acts as a crucial attachment receptor for GCRV. Removing HS with heparinase significantly reduces GCRV attachment and infection. Both HS and its homologue, heparin, inhibit the attachment of GCRV to cells. Altering HS levels in cells affects GCRV attachment and infection accordingly. GCRV outer capsid proteins VP5, VP56, and VP35, as well as purified GCRV virions, directly bind to HS. Pretreating GCRV with heparin or feeding grass carp with feed containing heparin significantly reduces mortality caused by GCRV infection. Collectively, these results highlight the crucial role of HS as an attachment receptor for GCRV and therefore provide a promising target for the prevention and control of this virus.
Subject areas: Virology, Biochemistry, Cell biology, Aquaculture, Aquaculture diseases
Graphical abstract

Highlights
-
•
Removing heparan sulfate significantly reduces GCRV attachment and infection
-
•
Heparan sulfate and its homologue inhibit the attachment of GCRV to cells
-
•
GCRV outer capsid proteins and GCRV virions directly bind to heparan sulfate
-
•
Heparan sulfate acts as a crucial attachment receptor for GCRV
Virology; Biochemistry; Cell biology; Aquaculture; Aquaculture diseases
Introduction
The grass carp (Ctenopharyngodon idellus), a significant species in Chinese aquaculture, contributes over 21% to the total freshwater aquaculture yield in the country. As of 2022, grass carp production has soared to an impressive 5.90 million tons, underscoring its considerable commercial importance.1 However, the aquaculture of grass carp has been beset by numerous diseases, notably grass carp hemorrhagic disease attributed to grass carp reovirus (GCRV). GCRV is categorized into three genotypes, with representative strains including GCRV-873 (type I), GCRV-HZ08 (type II), and GCRV-104 (type III).2 Notably, GCRV-873 (type I) was the first fish virus to be characterized and sequenced in China3 and thus served as a model in early research on disease-resistant breeding, vaccine development, and virus–host interactions. However, numerous studies have shown that most GCRVs isolated in southern China are type II, such as GCRV-HZ08, GCRV-GD108, and GCReV-109,2,4,5 indicating that type II GCRV is the predominant strain currently circulating in China. Type III GCRV is not widely distributed in China, with only one strain identified (GCRV-104).6
As obligate intracellular pathogens, viruses rely on host cells to complete their life cycle. Virus infection commences with specific interactions between viral envelope proteins or outer capsid proteins and cellular surface components, such as cellular receptors.7,8 Consequently, cellular receptors are believed to govern the tissue tropism, viral pathogenesis, and host range of viruses, rendering them pivotal targets for the prevention and control of viral diseases.9 In the case of mammalian orthoreovirus (MRV), the outer capsid proteins σ1 or σ3 facilitate viral entry by interacting with cellular receptors or co-receptors, including sialic acid,10 junctional adhesion molecule-A (JAM-A),11 GM2 Glycan,12 NgR1,13 β1 integrin,14 heparan sulfate (HS),15 PirB,16,17 and NRP1.18 However, there is no evidence suggesting that these molecules also function as receptors or co-receptors for GCRV. Recent studies have unveiled numerous host proteins (TIA1, Laminin, Fibulin-4, Ubc9, LITAF, SRB1, HSP90, and HSP70) that interact with GCRV-encoded proteins and play essential roles during GCRV infection.19,20,21,22,23,24,25,26 Nevertheless, whether these proteins serve as the functional receptors of GCRV remains elusive. Therefore, the cellular receptors for GCRV infection remain unclear.
Virus receptors exhibit considerable variation depending on the specific virus and the host species it targets. These receptors encompass proteins, carbohydrates, or lipid molecules present on the cell surface.27 HS is a linear polysaccharide bound to a core protein, forming heparan sulfate proteoglycans (HSPGs) that are widely expressed on the surface of nearly all mammalian cells and within the extracellular matrix.28 HS plays a pivotal role in orchestrating the binding of various signaling molecules to their respective receptors, thereby regulating numerous biological processes, including homeostasis, metabolism, and various pathological pathways. Owing to its ubiquitous presence and negatively charged characteristics, HS serves as a non-protein receptor utilized by numerous viruses for attachment.29 For instance, SARS-CoV-2 infection relies on cellular HS in conjunction with ACE2,30,31 while Coxsackievirus A16 utilizes cell surface HS glycosaminoglycans as its attachment receptor.32 Additionally, HS serves as a cellular receptor for enteric human adenoviruses.33 However, the precise role of HS during GCRV infection remains unresolved.
In the study, we demonstrated that cell surface HS acts as an important attachment receptor for GCRV. The removal of cell surface HS resulted in a significant decrease in GCRV attachment and subsequent infection. Moreover, both HS and its structural homolog heparin were found to impede GCRV bind to cells. Manipulating HS biosynthesis through knockdown or elevation either inhibited or facilitated GCRV attachment and infection. Furthermore, we confirmed the direct binding of purified GCRV virions and their outer capsid proteins to HS. Importantly, our findings reveal that heparin, a structural homolog of HS, reduced the mortality of grass carp caused by GCRV infection. These results highlight the crucial role of HS as an important attachment receptor for GCRV and provide a promising target for the prevention and control of this virus.
Results
Removal of cell surface heparan sulfate significantly reduced GCRV attachment and infection
HS is ubiquitously expressed across different cell types and are known as attachment or entry receptor for multiple viruses. Therefore, we speculated that GCRV may use HS as an attachment receptor. To test the role of HS in GCRV infection, we used heparinase I treatment to remove cell surface HS prior to GCRV infection. We first validated the efficacies of the heparinase I in removing HS by immunofluorescence (IF) and flow cytometry (FCM) using mouse anti-HS antibody staining following by Alexa Fluor 594-conjugated goat anti-mouse IgG. Both IF and FCM confirmed that heparinase I treatment effective removed cell surface HS (Figures S1A and S1B). Cell viability detection indicated that heparinase I treatment at 28°C for 1h had no effect on cell viability at all the examined concentrations (Figure S1C). After that, GCRV (GCRV subtype I and subtype II, hereinafter referred to as GCRV-I and GCRV-II) were incubated with heparinase I treated or untreated cells at 4°C for 1h to allow virus attachment but not the subsequent infection step, and then harvested for IF by staining with antibodies against GCRV outer capsid proteins combined with FITC-linked antibody to HSPG. As show in Figure 1, after cells treated with heparinase I, the number of both type of viral particles that attached to cell surface was significantly reduced when compared with untreated cells (Figures 1A and 1B). Interestingly, we found that red fluorescence labeled GCRV virions colocalized green fluorescence labeled HS obviously, implying the binding between GCRV virions and HS (Figures 1A and 1B). To quantify the effect of heparinase treatment on GCRV binding, we selected 15 cells from each group and analyzed the number of antibody-stained viral particles that attached to cell surface. We found that the mean number of viral particles that attached to cell surface from 12.87 (GCRV-I) and 13.33 (GCRV-II) per cell in untreated group to 5.40 (GCRV-I) and 6.00 (GCRV-I) per cell in heparinase I treated group (p < 0.01) (Figures 1C and 1D). Moreover, qPCR analysis also showed heparinase I treatment significantly reduced the attachment of both type of GCRV in a dose-dependent manner (Figures 1E and 1F). Since virus attachment is the first step to initiate infection, the effect on virus attachment should also be appeared in the subsequent infection process. It was reported that GCRV-1, but not GCRV-II, induces obvious cytopathic effects (CPE) in CIK cells.34 Therefore, we also investigated the effect of heparinase treatment on GCRV-I subsequent infection process. It could be seen that the heparinase treatment reduced the expression levels of viral proteins of GCRV-I (Figure 1G) and GCRV-I induced CPE (Figure 1H). Moreover, the supernatants from heparinase treated and untreated cells were obtained and then used for plaque assay, results showed that heparinase I treatment remarkably reduced the plaque number of GCRV-I (Figure 1I). Collectively, these results indicate that removal of cell surface HS significantly reduced GCRV attachment and infection.
Figure 1.
Removal of cell surface heparan sulfate significantly reduced GCRV attachment and infection
See also Figure S1.
(A and B) Immunofluorescence analysis of GCRV-I (A) and GCRV -II (B) attached cells that untreated or treated with heparinase I. Scale bar = 10 μm.
(C and D) Quantitative analysis the number of GCRV-I (C) and GCRV-II (D) particles that attached to heparinase I treated or untreated cells. Data are represented as mean (n = 15) ± SD. ∗∗ indicates p < 0.01.
(E and F) RT-qPCR analysis of GCRV-I (E) and GCRV -II (F) attached cells that mock treated or treated with heparinase I. Data are represented as mean (n = 3) ± SD. ∗ indicates p < 0.05, ∗∗ indicates p < 0.01.
(G–I) Western blotting (G), cytopathic effect analysis (H) and plaque assay (I) of GCRV-I infected cells that untreated or treated with heparinase I. Scale bar = 100 μm.
Heparan sulfate and its homologs inhibits GCRV bind to cells
Next, we investigated whether HS could inhibit GCRV bind to cells. GCRV-I and GCRV-II suspension were preincubated with different concentrations of HS or its homolog, heparin, a structural homolog of HS that has been used as a surrogate for cell surface HS,35 at 4°C for overnight. After that, the mixtures were incubated cells at 4°C for 1h to allow virus attachment and then washed and harvested for IF analysis by staining with antibodies against GCRV outer capsid proteins combined with FITC-linked antibody to HSPG. As shown in Figure 2, IF showed that preincubated with HS or heparin significantly inhibited the attachment of both type of GCRV to cell surface (Figures 2A and 2B). Red fluorescence labeled GCRV virions also colocalized green fluorescence labeled HS obviously (Figures 2A and 2B). Quantitative analysis indicated that HS and heparin significantly reduced the number of GCRV particles that attached to cell surface (Figures 2C and 2D). qPCR analysis also indicated that preincubated with HS or heparin significantly inhibited the attachment of both type of GCRV in a dose-dependent manner (Figures 2E–2H). We also investigated the effect of preincubated with HS and heparin on the subsequent infection process of GCRV-I. Western blotting indicated that preincubated with HS or heparin significantly reduced the expression levels of viral proteins of GCRV-I (Figures 2I and 2J). Plaque assay also showed that the plaque number formed by the supernatants from HS or heparin preincubated cells was significantly reduced in a dose-dependent manner (Figures 2K and 2L). Moreover, we also found that preincubated with HS or heparin significantly inhibited GCRV-I induced CPE (Figure 2M). Taken together, these results confirm that HS and its homolog inhibits GCRV bind to cells.
Figure 2.
Heparan sulfate and its homolog inhibits GCRV bind to cells
(A and B) Immunofluorescence analysis of cells that attached with HS or heparin preincubated GCRV-I (A) or GCRV-II (B). Scale bar = 10 μm.
(C and D) Quantitative analysis the effect of HS or heparin preincubation on the number of GCRV-I (C) and GCRV-II (D) particles that attached to cell surface. Data are represented as mean (n = 15) ± SD. ∗∗ indicates p < 0.01.
(E–H) RT-qPCR analysis of cells that attached with HS or heparin preincubated GCRV-I (E, F) or GCRV-II (G, H). Data are represented as mean (n = 3) ± SD. ∗∗ indicates p < 0.01.
(I–M) Western blotting (I, J), plaque assay (K, L), and cytopathic effect analysis (M) of cells that infected with HS or heparin preincubated GCRV-I. Scale bar = 100 μm.
Heparan sulfate-knockdown cells impact GCRV attachment and infection
To further investigated the role of HS during GCRV attachment and infection, we used an optimized CRISPR-Cas13d RNA (CrRNA) system36 to knockdown two critical genes (B4GALT7 and EXT2) that essential for HS biosynthesis and then investigated the effects on GCRV attachment and infection. B4GALT7 encodes galactosyltransferase I that involved in the biosynthesis of xylose-galactose-galactose-glucuronic acid protein-glycan linkage,37 while EXT2 encodes one of two glycosyltransferases involved in the chain elongation step of HS biosynthesis.38 The effect of B4GALT7 and EXT2 knockdown on HS biosynthesis were verified by western blotting and IF. As shown in Figure S1D–S1H, all three CrRNA effectively knockdown the expression levels of two target genes (Figure S1D) and resulted in reduced HS in cell surface (Figure S1E–S1H). We therefore investigated effect of B4GALT7 and EXT2 knockdown on GCRV attachment and infection. IF showed that knockdown of two genes significantly inhibited both type of GCRV bind to cells (Figures 3A, 3B, S1I, and S1J). Quantitative analysis revealed that knockdown of two genes reduced the number of GCRV particles that attached to cell surface (Figures 3C, 3D, S1K, and S1L). qPCR analysis also indicated that knockdown of two genes inhibited the attachment of both type of GCRV in a dose-dependent manner (Figures 3E–3H). The effect of B4GALT7 and EXT2 knockdown on the subsequent GCRV infection process were also analyzed. We found that knockdown of B4GALT7 and EXT2 reduced the expression levels of viral proteins of GCRV-I (Figures 3I and 3J) and inhibited the formation of viroplasms in GCRV-I infected cells (Figures 3K and 3L). Therefore, these results indicate HS-knockdown cells impact GCRV attachment and infection.
Figure 3.
Heparan sulfate-knockdown cells impact GCRV attachment and infection
See also Figure S1.
(A and B) Immunofluorescence analysis of control or B4GALT7-knockdown cells that attached with GCRV-I (A) or GCRV-II (B). Scale bar = 10 μm.
(C and D) Quantitative analysis the number of GCRV-I (C) and GCRV-II (D) particles that attached to control or B4GALT7-knockdown cells. Data are represented as mean (n = 15) ± SD. ∗∗ indicates p < 0.01.
(E–H) RT-qPCR analysis of control or heparan sulfate-knockdown cells that attached with GCRV-I (E, F) or GCRV-II (G, H).
(I and J) Western blotting analysis of control or heparan sulfate-knockdown cells that infected with GCRV-I.
(K and L) Immunofluorescence analysis of the viroplasms in mock or heparan sulfate-knockdown cells that infected with GCRV-I. Scale bar = 10 μm.
Elevation of heparan sulfate biosynthesis facilitates GCRV attachment and infection
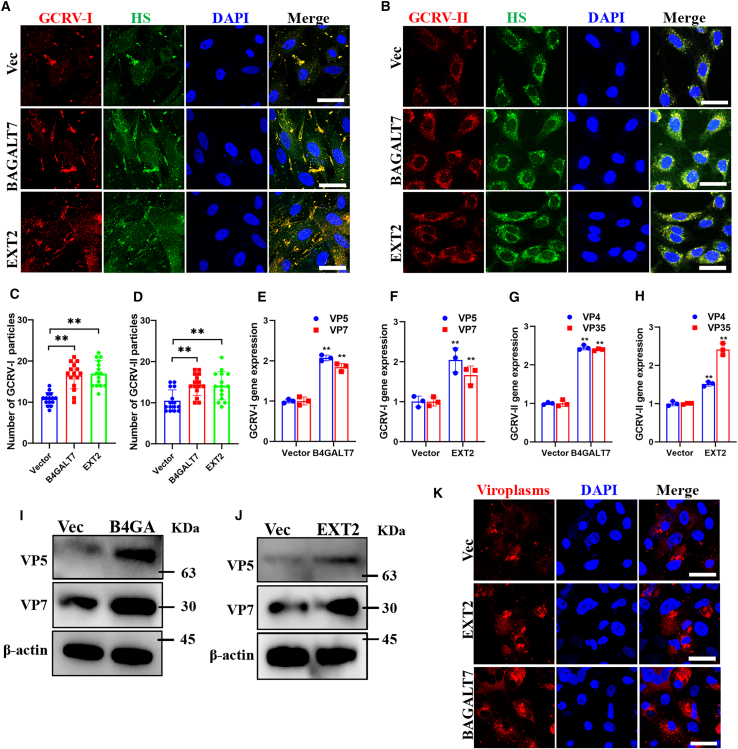
We also overexpressed the two critical genes to elevated HS biosynthesis and then investigated the effects on GCRV attachment and infection. The overexpression effects of B4GALT7 and EXT2 in transfected cells were confirmed by western blotting (Figure S1M). Moreover, IF and FCM revealed that overexpression of both genes elevated HS biosynthesis in cell surface (Figures S1N and S1O). We therefore investigated effect of B4GALT7 and EXT2 overexpression on GCRV attachment and infection. IF showed that overexpression of B4GALT7 and EXT2 facilitated both type of GCRV bind to cells (Figures 4A–4F). The colocalization between red fluorescence labeled GCRV virions and green fluorescence labeled HS were also observed (Figures 4A and 4B). Quantitative analysis confirmed the two genes increased the number of GCRV particles that attached to cell surface (Figures 4C and 4D). qPCR analysis of the overexpressed cells also showed similar results to that of IF (Figures 4E–4H). We also found that overexpression of B4GALT7 and EXT2 enhanced the expression levels of viral proteins of GCRV-I (Figures 4I and 4J) and increased the formation of GCRV-I viroplasms (Figure 4K). Thus, these results confirm that elevation of HS biosynthesis facilitates GCRV attachment and infection.
Figure 4.
Elevation of heparan sulfate biosynthesis facilitates GCRV attachment and infection
See also Figure S1.
(A and B) Immunofluorescence analysis of mock or heparan sulfate biosynthesis elevated cells that attached with GCRV-I (A) or GCRV-II (B). Scale bar = 10 μm.
(C and D) Quantitative analysis the number of GCRV-I (C) and GCRV-II (D) particles that attached to control or heparan sulfate biosynthesis elevated cells. Data are represented as mean (n = 15) ± SD. ∗∗ indicates p < 0.01.
(E–H) RT-qPCR analysis of control or heparan sulfate biosynthesis elevated cells that attached with GCRV-I (E, F) or GCRV-II (G, H).
(I and J) Western blotting analysis of control or heparan sulfate biosynthesis elevated cells that infected with GCRV-I.
(K) Immunofluorescence analysis of the viroplasms in mock or heparan sulfate biosynthesis elevated that infected with GCRV-I. Scale bar = 10 μm.
GCRV capsid proteins and purified GCRV virions bind to heparan sulfate
The outer capsid proteins of reovirus are speculated to crucial for virus attachment and entry.39 We therefore investigated the direct binding between GCRV capsid proteins and HS. The VP5 and VP7 are outer capsid proteins of GCRV-I,20,40 whereas the VP4, VP56, and VP35 are predicted outer capsid proteins of GCRV-II.41 These capsid proteins were purified using the HisBind purification kit and confirmed by SDS-PAGE (Figure S2). Moreover, we also purified two non-structural proteins, NS80 of GCRV-I and NS79 of GCRV-II, as the negative controls (Figure S2). Subsequently, all the purified recombinant proteins were incubated with HS-conjugated Sepharose beads or control beads lacking HS. The incubated beads were washed five times and bound proteins were eluted with binding buffer containing 2 M NaCl. As shown in Figure 5, for all the test proteins, VP5 of GCRV-I, as well as VP56 and VP35 of GCRV-II, bind to HS-conjugated Sepharose beads and were eluted by high salt buffer (elute) (Figures 5A and 5B). Nevertheless, other capsid proteins (VP7 of GCRV-I and VP4 of GCRV-II) and two non-structural proteins (NS80 of GCRV-I and NS79 of GCRV-II) not bind to the HS-conjugated Sepharose beads (Figures 5A and 5B). Importantly, all the recombinant proteins failed to bind control beads lacking HS and were present in the unbound supernatant (S) fraction (Figures 5A and 5B). Moreover, when these proteins preincubated with HS, all of them failed to bind to both of beads (Figures 5C and 5D). Collectively, these results imply the direct binding tween GCRV capsid proteins and HS.
Figure 5.
GCRV capsid proteins and purified GCRV virions bind to heparan sulfate
See also Figures S2 and S3.
(A and B) The direct binding between GCRV capsid proteins and HS-conjugated Sepharose beads.
(C and D) Heparan sulfate blocked the binding between GCRV capsid proteins and HS-conjugated Sepharose beads.
(E and F) The direct binding between purified GCRV virions and HS-conjugated Sepharose beads.
(G and H) Chondroitin sulfate (CS) did not affect the binding between GCRV capsid proteins and HS. The fractions of input (Input), supernatant after incubation (S), the fifth wash solution (W5), and the eluate solution (eluate) were detected by western blotting with the antibodies against the corresponding proteins.
To further elucidate the binding mechanisms, we predicted the binding sites of VP5, VP56, and VP35 involved in interactions with HS using molecular docking analysis. The results identified eight residues in VP5 (Arg292, Val498, Asn512, Asn515, Asn519, Asn579, and Ser581), seven residues in VP56 (Lys351, Pro352, Asn354, Gln357, Glu361, Thr365, and Arg367), and six residues in VP35 (Met64, Arg127, Asp132, Trp135, Ser160, and Arg161) as crucial for HS binding (Figure S3). To validate these findings, we constructed plasmids with these binding residues mutated to alanine and purified the corresponding mutant proteins. Our results demonstrated that all mutant proteins failed to bind to HS-conjugated Sepharose beads or control beads lacking HS, confirming the critical role of these residues in the interaction between GCRV capsid proteins and HS (Figures 5A and 5B).
We also purified both types of GCRV virions and incubated them with HS-conjugated Sepharose beads or control beads, then collected them for western blotting analysis using antibodies against VP7 from GCRV-I and VP4 from GCRV. The two capsid proteins were confirmed not to bind to HS in above. Interestingly, western blotting revealed that protein bands corresponding to VP7 or VP4 were detected in the eluate solution (Figures 5E and 5F), suggesting that both types of GCRV virions bind to the HS-conjugated Sepharose beads. Nevertheless, both types of GCRV virions failed to bind to the control beads lacking the HS moiety (Figures 5E and 5F).
Several studies have also suggested that chondroitin sulfate (CS) plays an important role during virus attachment and entry.42,43 Therefore, we also investigated the binding between GCRV capsid proteins and CS. Due to the unavailability of commercially CS-conjugated Sepharose beads, we preincubated GCRV proteins with CS. We then incubated these preincubated mixtures with HS-conjugated Sepharose beads or control beads and collected them for western blotting analysis. As shown in Figures 5G and 5H, preincubation with CS did not affect the binding between GCRV capsid proteins and HS. Therefore, these results suggest it is unlikely that GCRV capsid proteins bind to CS.
heparin reduced the mortality of grass carp caused by GCRV infection
Due to the important role of heparin and HS during GCRV attachment and infection, we therefore investigated whether heparin could alter the mortality in grass carp caused by GCRV infection. Since GCRV-II is the predominant strain currently circulating in China, and infections with GCRV-II result in over 80% mortality in yearling fish,34 we used GCRV-II rather than GCRV-I in the viral challenge experiment. About 600 grass carp with an average weigh of 5g were equally divided into four groups. Fish in these group were injected with 100 μL GCRV-II suspension (2.97 × 103 RNA copy/μL), 100 μL GCRV-II suspension that preincubated with heparin (at the final concentration of 10 μg/mL), 100 μL heparin suspension (at the final concentration of 10 μg/mL), and 100 μL PBS, respectively. The fish were monitored carefully and the number of dead individuals in each group was counted every day. As shown in Figure 6A, for the GCRV-II infected group, a final total mortality of 83.77% was reached at 15 days, with the first death recorded as early as 5 days post-infection. However, for the group infected with heparin preincubated GCRV-II suspension, the mortality was reduced to 56.00%, with the first fish dying at 6 days post-infection. Moreover, almost all of individuals survived in groups that injected with heparin or PBS. Typical hemorrhagic symptoms were found in the mouth, head, and body surface of fish from the group injected with GCRV-II suspension, whereas these symptoms were hard to detect in group injected with heparin preincubated GCRV-II suspension (Figure 6B). qPCR revealed that relative copy number of GCRV-II in group infected with heparin preincubated GCRV-II suspension was significantly lower than that of GCRV-II infected group in all examined tissues (Figures 6C, 6D, and S4A–S4C). Histological sections analysis showed that severe necrotic lesions, vacuolization, and karyorrhexis nuclei, were observed in liver and intestine samples of the GCRV-II infected group, whereas the pathological changes were less seriously in group infected heparin preincubated GCRV-II suspension (Figure 6E). TEM observation also revealed severe cell necrosis and a large number of GCRV virions were enclosed by vesicles in kidney samples of GCRV-II infected group, whereas the virions contained vesicles were hard to detected in the three remaining groups (Figures 6F and S4D). To quantify this observation, we selected 15 cells from each group and analyzed the number of virions contained vesicles. Results showed the number of virions contained vesicles in the group injected with heparin preincubated GCRV-II suspension was significantly reduced when compared with GCRV-II infected group (Figure S4E). It was reported that heparin or HS could bind to many molecules, including cytokines, to modulate the immune response and then defense against pathogens infection.44 Therefore, we investigated the expression levels of classical immune-related genes in different groups, including IRF3, IRF7, IFN1, and IFN3. The results showed that the expression levels of all these genes in the heparin-treated GCRV infected group were significantly lower than those in the GCRV-infected group (Figure S4F–S4I). These results suggest that the effects of heparin on fish pathogenesis are indeed due to its interaction with GCRV, thereby impairing the binding of GCRV to cells and the subsequent infection process, rather than modulating the immune response to defend against GCRV. Taken together, these results indicate that GCRV preincubated with HS reduced the mortality of grass carp.
Figure 6.
Pretreatment of GCRV with heparin reduce the mortality of grass carp after GCRV infection
See also Figure S4.
(A) Percent of survival in four different grass carp groups.
(B) The hemorrhagic symptoms of fish from the group injected with GCRV-II suspension or injected with GCRV-II suspension preincubated with heparin. Scale bar = 1 cm
(C and D) RT-qPCR analysis the relative copy number of GCRV-II in liver (C) and intestine (D) of different grass carp groups. Data are represented as mean (n = 3) ± SD. ∗∗ indicates p < 0.01.
(E) Histological section analysis of liver and intestine of different grass carp groups. Scale bar = 20 μm.
(F) TEM analysis of kidney samples from different grass carp groups. The red frames in the upper panes were enlarged in the bottom panes. Scale bar = 2 μm and 1μm.
Next, we also investigated whether the addition of heparin after GCRV infection could also alter the mortality of grass carp. 750 grass carp with an average weight of 5g were equally divided into five groups. Four groups were infected with GCRV-II by intraperitoneal injection as described above, whereas the remaining group were injected with equal volume of PBS as a negative control. Three days after injection, the four GCRV infected groups were fed with normal feed or fed with feed containing different concentrations of heparin (10, 100, and 200 mg/kg) for three days, whereas the negative control group were fed with normal feed all the time. The fish were monitored carefully and the number of dead individuals in each group was counted every day. Figure 7A showed that GCRV infection resulted in a final mortality of 86.67% when the infected fish fed with normal feed, however, the mortality were reduced to 57.86%, 43.57%, and 52.86% when the infected fish were fed with feed containing heparin at the concentrations of 10, 100, and 200 mg/kg, respectively. No death individual was found in the negative control group. Moreover, we found typical hemorrhagic symptoms in GCRV-II infected fish that were fed with normal feed, whereas these symptoms were not obvious when the infected fish were fed with feed containing heparin (Figure 7B). qPCR revealed that fed with feed containing heparin reduced the relative copy number of GCRV (Figures 7C, 7D and S5A–S5C). We also found that fed with feed containing heparin decreased the pathological changes in liver and intestine samples that caused by GCRV infection (Figure 7E). TEM analysis also showed a large number of GCRV virions that enclosed by vesicles were observed in GCRV infected group fed with normal feed, but hard to detect in GCRV infected groups that fed with feed containing heparin or the negative control group (Figures 7F and S5D). Quantitative analysis also indicates that the number of virions contained vesicles in GCRV infected groups that fed with feed containing heparin was remarkably reduced when compared the group that fed with normal feed (Figure S5E). Moreover, we also found the expression levels of four immune-related genes in GCRV infected groups that fed with feed containing heparin were significantly lower than those in the GCRV-infected group that fed with normal feed (Figures S5F–S5I). Collectively, these results confirm that addition of heparin after GCRV infection could also reduce the mortality of grass carp.
Figure 7.
Feeding grass carp with feed containing heparin decrease the mortality of grass carp after GCRV infection
See also Figure S5.
(A) Percent of survival in five different grass carp groups.
(B) The hemorrhagic symptoms in GCRV-infected fish that fed with normal feed or fed with feed containing heparin. Scale bar = 1 cm
(C and D) RT-qPCR analysis the relative copy number of GCRV-II in liver (C) and intestine (D) of five different grass carp groups. Data are represented as mean (n = 3) ± SD. ∗∗ indicates p < 0.01.
(E) Histological section analysis of liver and intestine of five different grass carp groups. Scale bars = 20 μm.
(F) TEM analysis of kidney samples from five different grass carp groups. The red frames in the upper panes were enlarged in the bottom panes. Scale bar = 2 μm and 1μm.
Discussion
As obligate intracellular pathogens, viruses must enter host cells to complete their life cycle. Binding to receptors on the cellular surface is the initial event crucial for successful virus infection. Furthermore, the ability to recognize and interact with specific receptors determines the host range and tissue tropism. GCRV, the most virulent pathogen in the genus Aquareovirus belonging to the family Spinareoviridae,45 induces hemorrhagic disease, posing a significant threat to the grass carp aquaculture industry in China. Despite numerous studies on GCRV,46 the cellular receptors for GCRV infection have remained unclear until now. In our study, we demonstrate that cell surface HS serves as an attachment receptor for both types of GCRV (GCRV-I and GCRV-II). These findings offer an important target for disease-resistant breeding of grass carp and hold promise for the prevention and control of this virus.
Due to its ubiquitous presence and negatively charged characteristics, HS serves as a non-protein receptor for many viruses.29 Thus, we investigated the detailed molecular events of the interaction between HS and GCRV. Viral envelope proteins or outer capsid proteins are responsible for interacting with cellular receptors. Interestingly, only the outer capsid protein VP5 of GCRV-I, and VP56 and VP35 of GCRV-II, bind to HS-conjugated Sepharose beads, while no binding was observed between other capsid proteins or non-structural proteins and HS. We also confirmed the binding between purified GCRV virions and HS. We examined the isoelectric point (pI) of each tested protein. The results showed that the pI values are 6.14 (NS80), 6.13 (VP5), 6.23 (VP7), 6.45 (NS79), 5.81 (VP4), 5.12 (VP56), and 6.93 (VP35) (Table S1), respectively. Therefore, all proteins exhibit a net negative charge when incubated with beads in the binding buffer (50 mM Tris-HCl, 10 mM sodium citrate, pH 7.4), suggesting that the interaction between HS and GCRV proteins is specific rather than due to nonspecific electrostatic adsorption. Although HS is ubiquitously expressed in most cell types, previous studies suggest that some viruses do not utilize HS as an attachment receptor due to the absence of direct interaction between HS and the virus.47 Moreover, previous studies have demonstrated that VP5, rather than VP7, of GCRV-I may be necessary for GCRV cell attachment or receptor binding.20,40 VP56 is the homologous protein of MRV σ1 outer capsid protein that responsible for cell attachment.48 Furthermore, subunit vaccines based on VP56 and VP35 proteins of GCRV-II provide protective immunity against grass carp hemorrhagic disease.49,50 Therefore, the binding of VP5, VP56, and VP35 to HS-conjugated Sepharose beads implies that these proteins are responsible for the GCRV-host receptor interaction.
Heparin, a structural homolog of HS, has been frequently utilized as a surrogate for cell surface HS in numerous research studies.35 Given the crucial role of HS in GCRV attachment and infection processes, alongside the direct binding between HS and GCRV outer capsid proteins or GCRV virions, we investigated whether heparin could serve as a drug to treat GCRV infection. Our findings revealed that pretreating GCRV with heparin reduced grass carp mortality caused by GCRV infection, along with decreased viral copy numbers and pathological changes. Nevertheless, the expression levels of immune-related genes in the heparin-treated GCRV infected group were significantly lower than those in the GCRV-infected group. Therefore, the decreased mortality and viral copy number may be attributed to heparin’s bind to GCRV virions through interaction with capsid proteins, thereby obstructing the attachment and infection of these virions, rather than modulating the immune response to defend against GCRV. Interestingly, when grass carp infected with GCRV were fed with feed containing heparin, fish mortality and viral copy numbers also significantly decreased. This suggests that heparin present in the fish feed could similarly bind to the virus, impeding its spread and subsequent infection processes. Previous studies have also supported the use of low molecular weight heparins as prophylaxis against SARS-CoV-2 infection31,51 or in inhibiting respiratory syncytial virus infection.52 Collectively, these results highlight the potential of heparin as a promising drug for the prevention and control of grass carp hemorrhagic disease.
In summary, our study elucidated the significant role of cell surface HS in GCRV attachment and conclusively demonstrated HS as an attachment receptor for GCRV. These findings offer a valuable target for disease-resistant breeding of grass carp and underscore the potential of heparin as a promising drug for the prevention and control of grass carp hemorrhagic disease.
Limitations of the study
While the aforementioned data underscore the significant role of HS during GCRV attachment and infection, it is essential to note that HS is not absolutely indispensable for GCRV attachment and infection. For instance, removing cell surface HS or preincubating GCRV with HS or heparin did not completely abolish GCRV attachment and infection. Furthermore, pretreating GCRV with heparin or feeding grass carp with feed containing heparin significantly reduced fish mortality but did not ensure the survival of all fish. These findings suggest that HS may not be the sole receptor for GCRV. Apart from HS, MRV utilizes several other molecules including sialic acid, JAM-A, GM2 Glycan, NgR1, β1 integrin, and PirB as cellular receptors or co-receptors.53 The genus Aquareovirus is closely related to the genus Orthoreovirus based on phylogenetic analysis of amino acid sequences of viral RNA polymerase.46,54 Therefore, it is plausible that GCRV may also utilize other unidentified receptors or co-receptors for attachment or entry. Moreover, it is important to note that while HS is ubiquitously expressed in most cell types, while the host range for GCRV is limited. Hence, we speculate that the entry of GCRV into host cells may depend on the cooperation of HS and other unidentified receptors or co-receptors. Further research will be conducted to explore these other receptors or co-receptors necessary for GCRV attachment and entry.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Libo He (helibowudi@ihb.ac.cn).
Materials availability
Plasmids, antibodies, and cell lines generated in this study are available upon request from the lead contact.
Data and code availability
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to re-analyze the data reported in this paper is available from the lead contact upon request.
Acknowledgments
This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB0730100), the National Natural Science Foundation of China (32073017), and the Youth Innovation Promotion Association CAS (2021338). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Author contributions
L.B.H., Y.P.W., and Z.Y.Z. conceived and coordinated the project; L.B.H. and Y.P.W. designed the experiments; Q.W., L.B.H., H.Y.W., and X.Y.W. performed the experiments; Q.W., H.Y.W., and L.B.H. analyzed the data; Y.M.L. and L.J.L. participated in viral challenge experiment; C.Y. performed bioinformatic analysis; L.B.H., Q.W., and Y.P.W. wrote the manuscript. All authors have reviewed the final version of the manuscript and approve it for publication.
Declaration of interests
The authors declare no competing interests.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Mouse anti-heparan sulfate | USBiological | Cat# H1890; RRID: AB_10013601 |
| Alexa Fluor® 594 conjugated goat anti-mouse IgG H&L | Abcam | Cat# ab150116; RRID: AB_2650601 |
| Alexa Fluor® 594 conjugated goat anti-rabbit IgG (H + L) | Cell Signaling Technology | Cat#8889; RRID: AB_2716249 |
| FITC-linked polyclonal antibody to heparan sulfate proteoglycan (HSPG) | Feiyue | Cat#FY-AB43649 |
| HRP-conjugated goat anti-rabbit IgG | Biosharp | Cat#BL003A; RRID: AB_2827666 |
| B4GALT7 polyclonal antibody | Proteintech | Cat#10535-1-AP, RRID: AB_2274457 |
| EXT2 polyclonal antibody | Proteintech | Cat#11348-1-AP, RRID: AB_2293839 |
| Rabbit anti-GCRV-I NS80 | This study | N/A |
| Rabbit anti-GCRV-I VP5 | This study | N/A |
| Rabbit anti-GCRV-I VP7 | This study | N/A |
| Rabbit anti-GCRV-II NS79 | This study | N/A |
| Rabbit anti-GCRV-II VP4 | This study | N/A |
| Rabbit anti-GCRV-II VP56 | This study | N/A |
| Rabbit anti-GCRV-II VP35 | This study | N/A |
| Bacterial and virus strains | ||
| BL21(DE3) Chemically Competent Cell | TransGen Biotech | Cat#CD601-02 |
| GCRV subtype I | He et al.34 | N/A |
| GCRV subtype II | He et al.34 | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| heparan sulfate | Macklin | Cat#H876459 |
| heparin | MedChemExpress | Cat#HY-17567 |
| chondroitin sulfate | Sigma | Cat#C6737 |
| heparinase I | Sigma | Cat#H2519 |
| DAPI | Beyotime | Cat#C1006 |
| M199 medium | Hyclone | Cat#SH30253.01 |
| M199 medium without phenol red | Gibco | Cat#11043023 |
| Fetal bovine serum | NEWZERUM | Cat#FBS-S500 |
| Penicillin/streptomycin (100x) | Sigma Aldrich | Cat# P4333 |
| Phosphate Buffered Saline | Biosharp | Cat#BL302A |
| BeyoGold™ His-tag Purification Resin | Beyotime | Cat# P2210 |
| Freund’s complete adjuvant | Sigma | Cat#F5881 |
| Freund’s incomplete adjuvant | Sigma | Cat#F5506 |
| heparan sulfate-sepharose beads | Solarbio | Cat#S9340 |
| Control Sepharose beads | Solarbio | Cat#S9190 |
| 0.25% Trypsin-EDTA (1X) | Biosharp | Cat#BL512A |
| Glutaraldehyde (25%) | SPI Supplies | Cat#02607-BA |
| 4% paraformaldehyde | Beyotime | Cat#P0099 |
| RIPA Lysis Buffe | Beyotime | Cat#P0013D |
| PVDF membrane | Merck millipore | Cat#ISEQ00010 |
| TBST buffer (20 X) | Solarbio | Cat#T1082 |
| Normal goat serum | Beyotime | Cat#C0265 |
| Crystal violet | Servicebio | Cat#G1014 |
| Triton X-100 | GLPBIO | Cat#GC19778 |
| Critical commercial assays | ||
| ClonExpress II One Step Cloning Kit | Vazyme | Cat#C112-01 |
| HiScript III 1st Strand cDNA Synthesis Kit | Vazyme | Cat#R312-01 |
| ChamQ SYBR qPCR Master Mix | Vazyme | Cat#Q311-02 |
| HRP-DAB Chromogenic Kit | Tiangen | Cat#PA110 |
| Cell Counting Kit-8 | Beyotime | Cat#C0038 |
| AG RNAex Pro Reagent | Accurate Biology | Cat#AG21101 |
| Lipofectamine 3000 Reagent | Thermo Fisher Scientific | Cat#L3000015 |
| Experimental models: Cell lines | ||
| CIK cells | CCTCC | Cat#GDC0081 |
| Experimental models: Organisms/strains | ||
| Grass carp: Ctenopharyngodon idellus | Guanqiao Experimental Station, Institute of Hydrobiology, CAS. | Wild type |
| Oligonucleotides | ||
| The sequences are listed in Tables S1 | This study | N/A |
| Recombinant DNA | ||
| pET-32a | Novagen | Cat#69015-3 |
| pcDNA3.1 | Invitrogen | Cat#V790-20 |
| pXR003 | Konermann et al.36 | Addgene Plasmid # 109053 |
| pcDNA3.1-Cas13d | This study | N/A |
| pET32a-VP5 | This study | N/A |
| pET32a-VP7 | This study | N/A |
| pET32a-NS80 | This study | N/A |
| pET32a-VP4 | This study | N/A |
| pET32a-VP56 | This study | N/A |
| pET32a-VP35 | This study | N/A |
| pET32a-NS79 | This study | N/A |
| pCMV-B4GALT7-flag | This study | N/A |
| pCMV-EXT2-flag | This study | N/A |
| Software and algorithms | ||
| GraphPad Prism 8 | Graphpad software | https://www.graphpad.com/scientific-software/prism/ |
| ImageJ | ImageJ software | https://imagej.nih.gov/ij/index.html |
Experimental model and study participant details
Cells and viruses
Ctenopharyngodon idella kidney (CIK) cells (China Center for Type Culture Collection (CCTCC), Cat# GDC0081) were cultured in M199 medium (HyClone, USA) supplemented with 10% fetal bovine serum (FBS), 100 IU/mL penicillin, and 100 mg/mL streptomycin under humidified conditions with 5% CO2 at 28°C. All cells used in this study were tested regularly for mycoplasma contamination by PCR. The study employed two subtypes of grass carp reovirus (GCRV), specifically GCRV-I and GCRV-II, previously isolated and identified by our laboratory,34 were used in the study for virus attachment and infection assay, as well as viral challenge experiment.
Animals
All animal experiments were conducted in strict compliance with the ARRIVE guidelines. The study protocol received approval from the Ethics Committee of the Institute of Hydrobiology, Chinese Academy of Sciences (CAS) (protocol code: IHB2023-0810). During the experiments, all surgeries were performed under MS-222 anesthesia, and utmost efforts were made to minimize any potential suffering. F Five-month-old wild-type grass carp, with an average weight of 5 g and length of 10 cm, were used in this study. These fish were sourced from a full-sib family bred at the Guanqiao Experimental Station, Institute of Hydrobiology, CAS, and the sex ratio (male: female) was approximately 1:1. Before the experiment, the fish were acclimatized in aerated fresh water at 26°C–28°C for one week before processing. To ensure that the grass carp were not previously exposed to GCRV, the fish were randomly selected for RT-PCR detection of GCRV using GCRV-specific primers (Table S2). The GCRV-free fish were fed commercial feed (Tong Wei, China) twice daily, and the water was exchanged daily. The photoperiod was 14 h:10 h light/dark, and the dissolved oxygen and pH of the water were maintained at 5–10 mg/L and 7.5–8.5, respectively. After no abnormalities were observed, viral challenge experiments were conducted.
Method details
Antibodies and reagents
Rabbit polyclonal antibodies against GCRV-I VP5 (anti-VP5), GCRV-I VP7 (anti-VP7), GCRV-I NS80 (anti-NS80), GCRV-II VP4 (anti-VP4), GCRV-II VP56 (anti-VP55), GCRV-II V35 (anti-VP35), and GCRV-II NS79 were prepared in our laboratory. Mouse anti-heparan sulfate (USBiological, USA), Alexa Fluor 594 conjugated goat anti-mouse IgG H&L (Abcam, UK), Alexa Fluor 594 conjugated goat anti-rabbit IgG (H + L) (Cell Signaling Technology, USA), FITC-linked polyclonal antibody to heparan sulfate proteoglycan (HSPG) (Feiyue, China), HRP-conjugated goat anti-rabbit IgG (Biosharp, China), B4GALT7 polyclonal antibody (Proteintech, USA), EXT2 Polyclonal antibody (Proteintech, USA), heparan sulfate (Macklin, China), heparin (MedChemExpress, China), chondroitin sulfate (Sigma, USA), heparinase I (Sigma, USA), and DAPI (Beyotime, China) were purchased from the indicated companies.
Virus attachment and infection assay
For virus attachment, CIK cells were incubated with GCRV at MOI of 100 for 1h at 4°C to allow the virus bind to the cells but not initiate the subsequent infection process, then cells were washed with M199 medium three times and harvest for qPCR and immunofluorescence (IF) analysis. For virus infection, CIK cells were infected with GCRV at MOI of 1 for 24 h at 28°C to allow the virus to complete its life cycle. The cells were then harvested and analyzed for Western blotting and IF analysis.
Heparinase treatment
CIK cells were treated with heparinase I (from Flavobacterium heparinum) at different concentrations (0, 1, 2, 5, and 10 U/ml) at 28°C for 1h, then cells were washed with M199 medium three times. The treated and untreated cells were infected with GCRV at MOI of 100 for 1h at 4°C for virus attachment assay or infected with GCRV at MOI of 1 for 24 h at 28°C for virus infection assay.
Heparan sulfate or heparin incubation assay
About 100 MOI of GCRV-I or GCRV-II suspension were preincubated with HS or its homologs, heparin, at different concentrations (0, 0.1, 1, 10, and 100 mg/mL) at 4°C for overnight. After that, the incubated mixtures were incubated with CIK cells at 4°C for 1h to allow virus bind to the cells but not initiate the subsequent infection process. Then, cells were washed with M199 medium for three times and harvested for IF and qPCR analysis.
Heparan sulfate-sepharose binding assay
To investigate the binding between GCRV proteins and heparan sulfate, the outer capsid proteins of GCRV-I (VP5 and VP7) and GCRV-II (VP4, VP56, and VP35), as well as two non-structural proteins (NS80 of GCRV-I and NS79 of GCRV-II), were purified. Briefly, the complete ORF sequences of the corresponding proteins were amplified and ligated into the pET-32a expression vector. The resulting plasmids were transformed into E. coli BL21 and then the bacteria were induced with 1 mM IPTG for 10 h at 20°C to express the fusion protein. The fusion proteins were purified using BeyoGold His-tag Purification Resin (Beyotime, China). Moreover, we also purified GCRV virions from GCRV-I infected cells and GCRV-II infected grass carp as described previously.3,5 Purified GCRV proteins or virions were incubated with heparan sulfate-sepharose beads (Solarbio, China) or control Sepharose beads (Solarbio, China) at 4°C for 1h. Then, the incubated beads were washed with binding buffer (50 mM Tris-HCl, 10 mM sodium citrate, pH 7.4) five times, and bound protein was eluted with binding buffer containing 2 M NaCl. The samples were analyzed by western blotting by using antibodies against the corresponding proteins.
Moreover, to further investigate the binding between GCRV proteins and heparan sulfate, the purified GCRV proteins were pre-incubated with heparan sulfate or chondroitin sulfate at 4°C for 1h, then the incubated mixtures with incubated with heparan sulfate-sepharose beads or control Sepharose beads at 4°C for another 1h. The beads were washed, eluted, and analyzed as described above.
Plasmid construction and transfection
To investigate the role of heparan sulfate during GCRV attachment and infection, two endogenous genes (B4GALT7 and EXT2) that critical for heparan sulfate biosynthesis were cloned for overexpression. The complete ORF sequences of the two genes were amplified from grass carp cDNA and then inserted into pCMV-flag vector. All resulting plasmids were validated through DNA sequencing. A list of primers utilized for plasmid construction can be found in Table S2.
Transfection was performed as previously described, but with some modifications.55 CIK cells grown in six-well plates were transfected with plasmids using the Lipofectamine 3000 transfection reagent (Thermo Fisher Scientific, USA) according to the manufacturer’s instructions. After 24 h post transfection, the transfected cells were used for virus attachment or infection assay as described above.
Knockdown of heparan sulfate biosynthesis by CRISPR-Cas13d
To further explore the role of heparan sulfate during GCRV attachment and infection, we employed an optimized CRISPR-Cas13d RNA (CrRNA) system to knock down the two endogenous genes that critical for heparan sulfate biosynthesis.36 The codon sequences of Cas13d were optimized and then fused with the nuclear localization sequence (NLS) of the simian virus 40 large T antigen. Subsequently, they were inserted into the pcDNA3.1 vector for expression. The pXR003 vector was utilized to drive the expression of CrRNA, where the human U6 promoter was replaced by the zebrafish U6 promoter to enhance CrRNA expression. Specific CrRNAs targeting each gene were designed using the web-based platform cas13design.56 Three CrRNAs (#1, #2, and #3) were designed for each gene (Table S2), and a CrRNA targeting luciferase served as a negative control. Plasmids encoding Cas13d and each CrRNA were transfected into CIK cells. After 24 h post transfection, the transfected cells were used for virus attachment or infection assay as described above.
RT-qPCR
RT-qPCR was used to investigate the effects of heparan sulfate on GCRV attachment. CIK cells were infected with GCRV at MOI of 100 for 1h at 4°C to allow the virus bind to the cells but not the subsequent infection process, then cells were washed with M199 medium three times and harvested. Total RNA was isolated using the AG RNAex Pro Reagent (Accurate Biology, China), and first-strand cDNA was obtained using a HiScript III 1st Strand cDNA Synthesis Kit (Vazyme, China). RT-qPCR was performed using a fluorescence quantitative PCR instrument (Bio-Rad, USA). Each reaction mixture contained 0.8 μL forward and reverse primers (Table S2), 1 μL cDNA template, 10 μL 2× ChamQ SYBR qPCR Master Mix (Vazyme, China), and 7.4 μL ddH2O. Three replicates were performed for each sample, and β-actin was used as an internal control for the normalization of gene expression. The program was as follows: 95°C for 10 s; 40 cycles of 95°C for 15 s, 56°C for 30 s, and 72°C for 30 s; and melt curve construction. The relative expression levels were calculated using the 2-ΔΔCt method.57 Data are presented as mean (n ≥ 3) ± standard deviation (SD) of three replicates.
Western blotting
Western blotting was employed to examine the effects of heparan sulfate on GCRV infection. CIK cells were infected with GCRV at MOI of 1 for 24 h at 28°C to allow virus complete its life cycle. Cells were harvested and lysed in RIPA lysis buffer (Beyotime, China) on ice for 30 min and collected by centrifuge at 12,000×g at 4°C for 10 min. Proteins were separated by 15% SDS-PAGE and transferred to a PVDF membrane (Millipore, China). The membranes were washed with Tris-Buffered Saline Tween 20 (TBST) buffer (Solarbio, China) and then incubated with 5% nonfat milk powder, followed by incubation with primary antibodies at a 1:1000 dilution overnight. After washing in TBST, PVDF membranes were incubated with HRP-conjugated goat anti-rabbit IgG (Biosharp, China) at room temperature for 1 h. Finally, the immunoblot signals were detected using an HRP-DAB Chromogenic Kit (Tiangen, China).
Plaque assay
Plaque assay was performed to investigate the effects of heparan sulfate on progeny virus production. CIK cells were infected with GCRV at MOI of 1 for 24 h at 28°C. Supernatants were collected from these cells and then used to infect fresh CIK cells in 12 well plates. The cells were overlaid with a medium containing 0.7% melted soft agar. After 24–48 hpi, plaques formed, and the medium was removed. The cells were then fixed with 4% paraformaldehyde and stained with 1% crystal violet (Servicebio, China).
Immunofluorescence microscopy
CIK cells grown in glass-bottomed cell culture dishes were infected with GCRV at MOI of 100 for 1h at 4°C for virus attachment or at MOI of 1 for 24 h at 28°C for virus infection. Then, immunofluorescence was then performed on virus-attached cells to stain virions on the cell surface using antibodies against viral outer capsid proteins (VP5 and VP7 for GCRV-I, as well as VP4, VP56, and VP35 for GCRV-II), or on virus-infected cells to stain viroplasms in the cytoplasm using an antibody against NS80. For the virus attached cells, cells were fixed with 4% paraformaldehyde but not permeabilized with 0.2% Triton X-100. For the virus infected cells, cells were fixed with 4% paraformaldehyde and then permeabilized with 0.2% Triton X-100. The permeabilized or not permeabilized cells were blocked in 10% normal goat serum (Beyotime, China) at room temperature for 1 h. The cells were incubated with primary antibodies diluted in 1% normal goat serum for 2 h, rinsed three times for 10 min each with PBS containing 1% normal goat serum, and then incubated with secondary antibodies. DAPI staining was used to visualize the nuclei. Finally, the cells were rinsed with PBS, mounted with 50% glycerol, and visualized using a confocal microscope (Leica, Germany).
Viral challenge experiment and sample collection
About 600 grass carp were equally divided into four groups. Fish in these group were injected with 100 μL GCRV-II suspension (2.97 × 103 RNA copies/μL), 100μL GCRV-II suspension that preincubated with heparin (at the final concentration of 10 μg/mL), 100 μL heparin suspension (at the final concentration of 10 μg/mL), and 100 μL PBS, respectively. At 5 days post infection, five fish from each group were collected and the liver, intestine, spleen, kidney, gill samples were removed for analysis. The remaining fish were monitored carefully and the number of dead individuals in each group was counted every day. The experiment was concluded and the total mortality was calculated when no mortality was recorded for seven consecutive days.
To further investigated whether the addition of heparin after GCRV infection could also alter the mortality of grass carp. 750 grass carp were equally divided into five groups. Four groups were infected with GCRV-II by intraperitoneal injection as described above, whereas the remaining group were injected with equal volume of PBS as a negative control. Three days after injection, the four GCRV infected groups were fed with normal feed or fed with feed containing different concentrations of heparin (10, 100, and 200 mg/kg) for three days, whereas the negative control group were fed with normal feed all the time. At 5 days post infection, five fish from each group were collected and the liver, intestine, spleen, kidney, gill samples were removed for analysis. The remaining fish were monitored as described above.
Hematoxylin and eosin staining
Hematoxylin and eosin staining were prepared as described previously.5 Briefly, liver and intestine samples of grass carp from different groups were fixed in Bouin’s fixative overnight at 4°C. Following dehydration, the samples were embedded in HistoResin (Leica, Germany). Serial sections of 4 mm thickness were cut using a microtome (Leica, Germany), dried on slides at 42°C overnight, stained with hematoxylin and eosin (H&E), mounted in Permount (Thermo Fisher Scientific, USA), and imaged with phase contrast with a 63× oil immersion objective lens.
Transmission electron microscopy
Transmission electron microscopy (TEM) experiments were performed as previously described, with some modifications.55 Kidney samples of grass carp that collected from different groups were pre-fixed with 2.5% glutaraldehyde for 24 h at 4°C, then followed by post-fixation with 1% osmium tetroxide (OsO4) for 2 h at 4°C. The samples were dehydrated stepwise in a graded series of ethanol and embedded in the epoxy resin Epon-812 overnight. The specimens were cut using a Leica DMIRB ultrathin microtome at 70 nm thickness, double stained with uranyl acetate and lead citrate, and observed with an HC-1 80.0 kV Hitachi TEM system (Hitachi, Japan).
Quantification and statistical analysis
All experiments were performed at least three times, one-way analysis of variance (ANOVA) and unpaired two-tailed Student’s t test were used to analyze statistical significance. Data are represented as mean (n ≥ 3) ± standard deviation (SD). Statistical significance is depicted with stars (∗ indicates p < 0.05, ∗∗ indicates p < 0.01).
Published: February 15, 2025
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2025.112033.
Supplemental information
References
- 1.Fisheries Bureau of Ministry of Agriculture and Rural Affairs in China . China Agriculture Press; 2023. China Fishery Statistical Yearbook of 2022. [Google Scholar]
- 2.Wang Q., Zeng W., Liu C., Zhang C., Wang Y., Shi C., Wu S. Complete genome sequence of a reovirus isolated from grass carp, indicating different genotypes of GCRV in China. J. Virol. 2012;86 doi: 10.1128/JVI.02333-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fang Q., Attoui H., Cantaloube J.F., Biagini P., Zhu Z., de Micco P., de Lamballerie X. Sequence of genome segments 1, 2, and 3 of the grass carp reovirus (Genus Aquareovirus, family Reoviridae) Biochem. Biophys. Res. Commun. 2000;274:762–766. doi: 10.1006/bbrc.2000.3215. [DOI] [PubMed] [Google Scholar]
- 4.Ye X., Tian Y.Y., Deng G.C., Chi Y.Y., Jiang X.Y. Complete genomic sequence of a reovirus isolated from grass carp in China. Virus Res. 2012;163:275–283. doi: 10.1016/j.virusres.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 5.Pei C., Ke F., Chen Z.Y., Zhang Q.Y. Complete genome sequence and comparative analysis of grass carp reovirus strain 109 (GCReV-109) with other grass carp reovirus strains reveals no significant correlation with regional distribution. Arch. Virol. 2014;159:2435–2440. doi: 10.1007/s00705-014-2007-5. [DOI] [PubMed] [Google Scholar]
- 6.Fan Y., Rao S., Zeng L., Ma J., Zhou Y., Xu J., Zhang H. Identification and genomic characterization of a novel fish reovirus, Hubei grass carp disease reovirus, isolated in 2009 in China. J. Gen. Virol. 2013;94:2266–2277. doi: 10.1099/vir.0.054767-0. [DOI] [PubMed] [Google Scholar]
- 7.Casasnovas J.M. Virus-receptor interactions and receptor-mediated virus entry into host cells. Subcell. Biochem. 2013;68:441–466. doi: 10.1007/978-94-007-6552-8_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anwar M.N., Akhtar R., Abid M., Khan S.A., Rehman Z.U., Tayyub M., Malik M.I., Shahzad M.K., Mubeen H., Qadir M.S., et al. The interactions of flaviviruses with cellular receptors: Implications for virus entry. Virology. 2022;568:77–85. doi: 10.1016/j.virol.2022.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Maginnis M.S. Virus-Receptor Interactions: The Key to Cellular Invasion. J. Mol. Biol. 2018;430:2590–2611. doi: 10.1016/j.jmb.2018.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barton E.S., Youree B.E., Ebert D.H., Forrest J.C., Connolly J.L., Valyi-Nagy T., Washington K., Wetzel J.D., Dermody T.S. Utilization of sialic acid as a coreceptor is required for reovirus-induced biliary disease. J. Clin. Investig. 2003;111:1823–1833. doi: 10.1172/JCI16303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell J.A., Schelling P., Wetzel J.D., Johnson E.M., Forrest J.C., Wilson G.A.R., Aurrand-Lions M., Imhof B.A., Stehle T., Dermody T.S. Junctional adhesion molecule a serves as a receptor for prototype and field-isolate strains of mammalian reovirus. J. Virol. 2005;79:7967–7978. doi: 10.1128/JVI.79.13.7967-7978.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reiss K., Stencel J.E., Liu Y., Blaum B.S., Reiter D.M., Feizi T., Dermody T.S., Stehle T. The GM2 glycan serves as a functional coreceptor for serotype 1 reovirus. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1003078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konopka-Anstadt J.L., Mainou B.A., Sutherland D.M., Sekine Y., Strittmatter S.M., Dermody T.S. The Nogo receptor NgR1 mediates infection by mammalian reovirus. Cell Host Microbe. 2014;15:681–691. doi: 10.1016/j.chom.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koehler M., Petitjean S.J.L., Yang J., Aravamudhan P., Somoulay X., Lo Giudice C., Poncin M.A., Dumitru A.C., Dermody T.S., Alsteens D. Reovirus directly engages integrin to recruit clathrin for entry into host cells. Nat. Commun. 2021;12:2149. doi: 10.1038/s41467-021-22380-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan C.W., Gamage A.M., Yap W.C., Wei Tang L.J., Sun Y., Yang X.L., Pyke A., Chua K.B., Wang L.F. Pteropine orthoreoviruses use cell surface heparan sulphate as an attachment receptor. Emerg. Microbes Infect. 2023;12 doi: 10.1080/22221751.2023.2208683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shang P., Simpson J.D., Taylor G.M., Sutherland D.M., Welsh O.L., Aravamudhan P., Natividade R.D.S., Schwab K., Michel J.J., Poholek A.C., et al. Paired immunoglobulin-like receptor B is an entry receptor for mammalian orthoreovirus. Nat. Commun. 2023;14:2615. doi: 10.1038/s41467-023-38327-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sutherland D.M., Strebl M., Koehler M., Welsh O.L., Yu X., Hu L., Dos Santos Natividade R., Knowlton J.J., Taylor G.M., Moreno R.A., et al. NgR1 binding to reovirus reveals an unusual bivalent interaction and a new viral attachment protein. Proc. Natl. Acad. Sci. USA. 2023;120 doi: 10.1073/pnas.2219404120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shang P., Dos Santos Natividade R., Taylor G.M., Ray A., Welsh O.L., Fiske K.L., Sutherland D.M., Alsteens D., Dermody T.S. NRP1 is a receptor for mammalian orthoreovirus engaged by distinct capsid subunits. Cell Host Microbe. 2024;32:980–995.e9. doi: 10.1016/j.chom.2024.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song L., Wang H., Wang T., Lu L. Sequestration of RNA by grass carp Ctenopharyngodon idella TIA1 is associated with its positive role in facilitating grass carp reovirus infection. Fish Shellfish Immunol. 2015;46:442–448. doi: 10.1016/j.fsi.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang H., Yu F., Li J., Lu L. Laminin receptor is an interacting partner for viral outer capsid protein VP5 in grass carp reovirus infection. Virology. 2016;490:59–68. doi: 10.1016/j.virol.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Yu F., Wang H., Liu W., Lu L. Grass carp Ctenopharyngodon idella Fibulin-4 as a potential interacting partner for grass carp reovirus outer capsid proteins. Fish Shellfish Immunol. 2016;48:169–174. doi: 10.1016/j.fsi.2015.11.029. [DOI] [PubMed] [Google Scholar]
- 22.Yu F., Wang H., Wang L., Lu L. Orthoreovirus outer-fiber proteins are substrates for SUMO-conjugating enzyme Ubc9. Oncotarget. 2016;7:79814–79827. doi: 10.18632/oncotarget.12973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu J., Wang H., Zhang Y., Li Y., Lu L. Grass carp reovirus NS26 interacts with cellular lipopolysaccharide-induced tumor necrosis factor-alpha factor, LITAF. Virus Genes. 2016;52:789–796. doi: 10.1007/s11262-016-1370-6. [DOI] [PubMed] [Google Scholar]
- 24.Ou M., Huang R., Luo Q., Xiong L., Chen K., Wang Y. Characterisation of scavenger receptor class B type 1 in rare minnow (Gobiocypris rarus) Fish Shellfish Immunol. 2019;89:614–622. doi: 10.1016/j.fsi.2019.04.031. [DOI] [PubMed] [Google Scholar]
- 25.Jiang L., Liu A.Q., Zhang C., Zhang Y.A., Tu J. Hsp90 Regulates GCRV-II Proliferation by Interacting with VP35 as Its Receptor and Chaperone. J. Virol. 2022;96 doi: 10.1128/jvi.01175-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hou G., Zhang Q., Li C., Ding G., Hu L., Chen X., Lv Z., Fan Y., Zou J., Xiao T., et al. An Aquareovirus Exploits Membrane-Anchored HSP70 To Promote Viral Entry. Microbiol. Spectr. 2023;11 doi: 10.1128/spectrum.04055-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valero-Rello A., Baeza-Delgado C., Andreu-Moreno I., Sanjuán R. Cellular receptors for mammalian viruses. PLoS Pathog. 2024;20 doi: 10.1371/journal.ppat.1012021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agelidis A., Shukla D. Heparanase, Heparan Sulfate and Viral Infection. Adv. Exp. Med. Biol. 2020;1221:759–770. doi: 10.1007/978-3-030-34521-1_32. [DOI] [PubMed] [Google Scholar]
- 29.Cagno V., Tseligka E.D., Jones S.T., Tapparel C. Heparan Sulfate Proteoglycans and Viral Attachment: True Receptors or Adaptation Bias? Viruses. 2019;11:596. doi: 10.3390/v11070596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clausen T.M., Sandoval D.R., Spliid C.B., Pihl J., Perrett H.R., Painter C.D., Narayanan A., Majowicz S.A., Kwong E.M., McVicar R.N., et al. SARS-CoV-2 Infection Depends on Cellular Heparan Sulfate and ACE2. Cell. 2020;183:1043–1057.e15. doi: 10.1016/j.cell.2020.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bermejo-Jambrina M., Eder J., Kaptein T.M., van Hamme J.L., Helgers L.C., Vlaming K.E., Brouwer P.J.M., van Nuenen A.C., Spaargaren M., de Bree G.J., et al. Infection and transmission of SARS-CoV-2 depend on heparan sulfate proteoglycans. EMBO J. 2021;40 doi: 10.15252/embj.2020106765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X., Shi J., Ye X., Ku Z., Zhang C., Liu Q., Huang Z. Coxsackievirus A16 utilizes cell surface heparan sulfate glycosaminoglycans as its attachment receptor. Emerg. Microbes Infect. 2017;6:e65. doi: 10.1038/emi.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rajan A., Palm E., Trulsson F., Mundigl S., Becker M., Persson B.D., Frängsmyr L., Lenman A. Heparan Sulfate Is a Cellular Receptor for Enteric Human Adenoviruses. Viruses. 2021;13:298. doi: 10.3390/v13020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He L., Zhang A., Pei Y., Chu P., Li Y., Huang R., Liao L., Zhu Z., Wang Y. Differences in responses of grass carp to different types of grass carp reovirus (GCRV) and the mechanism of hemorrhage revealed by transcriptome sequencing. BMC Genom. 2017;18:452. doi: 10.1186/s12864-017-3824-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rabenstein D.L. Heparin and heparan sulfate: structure and function. Nat. Prod. Rep. 2002;19:312–331. doi: 10.1039/b100916h. [DOI] [PubMed] [Google Scholar]
- 36.Konermann S., Lotfy P., Brideau N.J., Oki J., Shokhirev M.N., Hsu P.D. Transcriptome Engineering with RNA-Targeting Type VI-D CRISPR Effectors. Cell. 2018;173:665–676.e14. doi: 10.1016/j.cell.2018.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sandler-Wilson C., Wambach J.A., Marshall B.A., Wegner D.J., McAlister W., Cole F.S., Shinawi M. Phenotype and response to growth hormone therapy in siblings with B4GALT7 deficiency. Bone. 2019;124:14–21. doi: 10.1016/j.bone.2019.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang W., Yang M., Shen Y., Chen K., Wu D., Yang C., Bai J., He D., Gao J. Clinical survey of a pedigree with hereditary multiple exostoses and identification of EXT-2 gene deletion mutation. Mol. Med. Rep. 2022;25:141. doi: 10.3892/mmr.2022.12657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mainou B.A. The Orchestra of Reovirus Cell Entry. Curr. Clin. Microbiol. Rep. 2017;4:142–149. doi: 10.1007/s40588-017-0067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fang Q., Seng E.K., Ding Q.Q., Zhang L.L. Characterization of infectious particles of grass carp reovirus by treatment with proteases. Arch. Virol. 2008;153:675–682. doi: 10.1007/s00705-008-0048-3. [DOI] [PubMed] [Google Scholar]
- 41.Tian Q., Huo X., Liu Q., Yang C., Zhang Y., Su J. VP4/VP56/VP35 Virus-like Particles Effectively Protect Grass Carp (Ctenopharyngodon idella) against GCRV-II Infection. Vaccines (Basel) 2023;11:1373. doi: 10.3390/vaccines11081373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Misinzo G., Delputte P.L., Meerts P., Lefebvre D.J., Nauwynck H.J. Porcine circovirus 2 uses heparan sulfate and chondroitin sulfate B glycosaminoglycans as receptors for its attachment to host cells. J. Virol. 2006;80:3487–3494. doi: 10.1128/JVI.80.7.3487-3494.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fons N.R., Kines R.C., Thompson C.D., Day P.M., Lowy D.R., Schiller J.T. Chondroitin Sulfate Proteoglycans Are De Facto Cellular Receptors for Human Papillomavirus 16 under High Serum Conditions. J. Virol. 2022;96 doi: 10.1128/jvi.01857-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Collins L.E., Troeberg L. Heparan sulfate as a regulator of inflammation and immunity. J. Leukoc. Biol. 2019;105:81–92. doi: 10.1002/JLB.3RU0618-246R. [DOI] [PubMed] [Google Scholar]
- 45.Matthijnssens J., Attoui H., Bányai K., Brussaard C.P.D., Danthi P., Del Vas M., Dermody T.S., Duncan R., Fāng Q., Johne R., et al. ICTV Virus Taxonomy Profile: Spinareoviridae 2022. J. Gen. Virol. 2022;103 doi: 10.1099/jgv.0.001781. [DOI] [PubMed] [Google Scholar]
- 46.Li P.W., Zhang J., Chang M.X. Structure, function and immune evasion strategies of aquareoviruses, with focus on grass carp reovirus. Rev. Aquac. 2023;16:410–432. [Google Scholar]
- 47.Tan C.W., Sam I.C., Chong W.L., Lee V.S., Chan Y.F. Polysulfonate suramin inhibits Zika virus infection. Antiviral Res. 2017;143:186–194. doi: 10.1016/j.antiviral.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 48.Tian Y., Jiao Z., Dong J., Sun C., Jiang X., Ye X. Grass carp reovirus-GD108 fiber protein is involved in cell attachment. Virus Genes. 2017;53:613–622. doi: 10.1007/s11262-017-1467-6. [DOI] [PubMed] [Google Scholar]
- 49.Gao Y., Pei C., Sun X., Zhang C., Li L., Kong X. Novel subunit vaccine based on grass carp reovirus VP35 protein provides protective immunity against grass carp hemorrhagic disease. Fish Shellfish Immunol. 2018;75:91–98. doi: 10.1016/j.fsi.2018.01.050. [DOI] [PubMed] [Google Scholar]
- 50.Pei C., Gao Y., Sun X., Li L., Kong X. A developed subunit vaccine based on fiber protein VP56 of grass carp reovirus providing immune protection against grass carp hemorrhagic disease. Fish Shellfish Immunol. 2019;90:12–19. doi: 10.1016/j.fsi.2019.04.055. [DOI] [PubMed] [Google Scholar]
- 51.Eder J., Bermejo-Jambrina M., Vlaming K.E., Kaptein T.M., Zaderer V., Kemper E.M., Wilflingseder D., Reitsma S., de Bree G.J., Cohn D.M., Geijtenbeek T.B.H. Inhalation of Low Molecular Weight Heparins as Prophylaxis against SARS-CoV-2. mBio. 2022;13 doi: 10.1128/mbio.02558-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guo Y., Wang Z., Dong L., Wu J., Zhai S., Liu D. Ability of low-molecular-weight heparin to alleviate proteinuria by inhibiting respiratory syncytial virus infection. Nephrology. 2008;13:545–553. doi: 10.1111/j.1440-1797.2008.01012.x. [DOI] [PubMed] [Google Scholar]
- 53.Sutherland D.M., Aravamudhan P., Dermody T.S. An Orchestra of Reovirus Receptors: Still Searching for the Conductor. Adv. Virus Res. 2018;100:223–246. doi: 10.1016/bs.aivir.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 54.Mohd Jaafar F., Goodwin A.E., Belhouchet M., Merry G., Fang Q., Cantaloube J.F., Biagini P., de Micco P., Mertens P.P.C., Attoui H. Complete characterisation of the American grass carp reovirus genome (genus Aquareovirus: family Reoviridae) reveals an evolutionary link between aquareoviruses and coltiviruses. Virology. 2008;373:310–321. doi: 10.1016/j.virol.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 55.He L., Wang Q., Liang X., Wang H., Chu P., Yang C., Li Y., Liao L., Zhu Z., Wang Y. Grass Carp Reovirus Induces Formation of Lipid Droplets as Sites for Its Replication and Assembly. mBio. 2022;13 doi: 10.1128/mbio.02297-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guo X., Rahman J.A., Wessels H.H., Méndez-Mancilla A., Haro D., Chen X., Sanjana N.E. Transcriptome-wide Cas13 guide RNA design for model organisms and viral RNA pathogens. Cell Genom. 2021;1 doi: 10.1016/j.xgen.2021.100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to re-analyze the data reported in this paper is available from the lead contact upon request.