Summary
Background
There is no cross-sectional comparison on therapeutic and adverse effects for treatments of metastatic castration-resistant prostate cancer (mCRPCa). We aimed to horizontally compare them for all common first-line and second-line therapies.
Methods
We conducted a network meta-analysis with a systematic review in four databases (Pubmed, Web of Science, Embase, and Cochrane Library) up to January 5th, 2025. All randomized controlled trials (RCT) related to mCRPCa treatments with a clear description in study design were included. Endpoints included the radiographic progression-free survival (rPFS), overall survival (OS), time to PSA progression (TTPP), PSA progression rate (PSARR), and adverse events. All data was extracted by two researchers and analyzed with Gemtc package in R and Stata15. This NMA protocol was registered online (ID: CRD42025633178).
Findings
After screening among 33,694 articles, 24 RCTs involving 13,059 cases were included. For first-line treatments, combination therapies with second-generation androgen receptor inhibitors (ARIs) showed superior efficacies in OS [HR of poly(ADP-ribose) polymerase inhibitors (PARPi) + ARI: 0.63 (0.32,1.25)], TTPP [HR of Lu177 + ARI: 0.07 (0.01,0.87)] and PSARR [RR of Lu177 + ARI: 33.02 (15.56,71.62)] with the highest SUCRA (Surface under the Cumulative Ranking Curve) (72%, 91% and 97% respectively). PARPi + ARI also performed best for rPFS (SUCRA: 85%, with an insignificant HR [0.12 (0.02,2.35)]. For post-docetaxel second-line treatments, ARI also emerged as the preferred option. Efficacies of post-ARI second-line treatments were not evaluated due to the lack of related RCTs. No obvious heterogeneity and publication bias was detected during the therapeutic comparison.
Interpretation
This study provided comparative evidence for first-line and post-chemotherapy second-line mCRPCa treatment options. Second-generation ARIs exhibited good efficacy, particularly when combined with other treatments. However, the safety analysis necessitated balance between benefits and adverse events, especially for combination therapies. Stronger evidence is needed through direct comparisons in future clinical trials.
Funding
The study was supported by The National Natural Science Foundation of China (No. 82172568).
Keywords: Metastatic castration-resistant prostate cancer, Network meta-analysis, Therapeutic effect, First-line treatment, Second-line treatment
Research in context.
Evidence before this study
In the treatment of metastatic castration-resistant prostate cancer (mCRPCa), five types of drugs are commonly used: second-generation antiandrogens (Abiraterone and Enzalutamide), taxane chemotherapies (Docetaxel and Cabazitaxel), poly(ADP-ribose) polymerase inhibitors (PARPi), immunotherapy (Sipuleucel-T), and radionuclides (Lu-177 and Ra-223). We performed a literature search in Pubmed until July 28th, 2024 to preliminarily summarize the efficacy of the aforementioned different treatments. The study population was restricted to mCRPCa patients and all studies with a clear description of prior treatments and grouping strategies were included. Most existing studies focus on evaluating the effectiveness of different drugs within a single class or comparing combination therapies with their respective monotherapies. A well-designed 2024 network meta-analysis recommended Talazoparib (a type of PARPi) combined with Enzalutamide as the preferred treatment, but this analysis was limited to two types of treatments: PARPi and second-generation antiandrogens (ARIs). Even though this study may reveal the good effects of the combination of different types of drugs and the necessity of comparing them, the optimal intervention still remains unclear due to the lack of cross-sectional comparisons among all treatment options for both first-line treatment and second-line treatment after docetaxel failure.
Added value of this study
To our knowledge, this is the first network meta-analysis to compare the relative effects and safety of different classes of treatments. For each efficacy endpoint, we categorized the involved randomized controlled trials (RCTs) into first-line treatment subgroups and second-line post-docetaxel groups based on prior medication history aiming to eliminate the potential influence of previous therapies on therapeutic efficacy and offers more precise guidance for clinical decision-making in specific scenarios. Additionally, eight major adverse events were also analyzed to assess the relative safety of treatments, providing valuable insights for selecting therapies that minimize adverse effects.
Implications of all the available evidence
This research provides evidence that the combination therapies of second-generation androgen receptor inhibitors (Lu-177/PARPi + ARIs) showed a promising therapeutic efficacy in all four therapeutic endpoints among first-line treatments and a relatively low incidence of adverse events. However, their effects on prolonging overall survival and radiologic progression-free survival were therapeutic but insignificant. For second-line post-docetaxel therapies, second-generation ARIs emerged as the preferred treatments rather than changing the drugs (cabazitaxel) for chemotherapy. But the effects of combination therapies were not applicable for evaluation due to a limited number of existed RCTs in this situation. The combination of docetaxel + ARIs is not recommended for its limited therapeutic effects and highest likelihood of the adverse events. Clinicians should carefully balance therapeutic benefits with potential adverse effects when treating mCRPCa. Our meta-analysis provides comprehensive comparative evidences for clinical workers on the selection of optimal treatments both for first-line treatments and post-chemotherapy second-line treatments and formation of future guidelines for mCRPCa treatments. Future clinical trials that directly compare different treatment categories are still needed to support our conclusions and contribute to clinical practice.
Introduction
Prostate cancer represents the second most common cancer among males (14.2%) with 1,466,680 new cases and 396,792 new deaths.1 Given that the prostate gland is a hormone-dependent organ, androgen deprivation therapy (ADT) has been identified as the primary treatment option for aggressive prostate cancer. However, drug resistance to androgen deprivation therapy may lead to the transformation to metastatic castration-resistant prostate cancer (mCRPCa) with a poor prognosis. This is characterized by a re-elevated PSA level and newly radiological metastasis. Certain treatments have already been recommended for the first-line and second-line management of mCRPCa according to guidelines from American Urological Association,2 European Association of Urology,3 and National Comprehensive Cancer Network,4 including taxane-based chemotherapy (docetaxel and cabazitaxel), second-generation antiandrogens (abiraterone acetate [AA] and androgen receptor inhibitors [ARIs]), poly (ADP-ribose) polymerase inhibitors (PARPi), immunotherapy (Sipuleucel-T) and PSMA-targeted radionuclide therapy [177Lu]Lu-PSMA-617 (Lu-177).
Despite the recent publications of trials focusing on the pair-wise comparisons of drug effect and safety, there remains a paucity of horizontal comparison and the optimal therapies remains unclear. In this study, a systematic review and network meta-analysis (NMA) of various systemic treatments was performed for patients with mCRPCa, aiming to directly and indirectly compare and rank the therapeutic efficacy and safety, and to provide evidence-based guidance for the selection of optimal treatment options.
Methods
Registration
This NMA was registered on the International Prospective Register of Systematic Reviews (PROSPERO; ID: CRD42025633178) website following the PRISMA statement and extension statement for network meta-analyses (Preferred Reporting Items for Systematic Reviews and Meta-Analyses).5
Ethics
Our study only involves publicly available clinical research data from published articles. The ethical statements for all published articles included in this study can be found in their respective original texts.
Search strategy and selection criteria
Two researchers (J.B.H. and L.B.H.) independently searched all available articles on four main databases (PubMed, Web of Science, Embase, and the Cochrane Library) up to December 31st, 2024. Medical Subject Headings (MeSH) and free words with all known forms of the following terms: “Castration-Resistant Prostatic Neoplasm”, “Docetaxel”, “Cabazitaxel”, “Antiandrogens”, “Immunotherapy”, “Poly(ADP-ribose) Polymerase Inhibitors”, and “Lutetium-177” were applied for literature search which was conducted on January 5th, 2025. Detailed search strategies and formulas were listed in supplementary files (Supplementary Table S1).
Inclusion criteria for studies: (1) Population: Patients of any age diagnosed with mCRPCa. (2) Intervention: Patients in the intervention group received at least one of the following treatments: taxanes (Docetaxel and Cabazitaxel), AA, ARIs, PARPi, Sipuleucel-T and Lu-177. (3) Comparison: Placebo or one of the treatments above. (4) Outcome: At least one of the following outcomes was reported: overall survival (OS), radiographic progression-free survival (rPFS), PSA response rate, time to PSA progression, and the incidence of any adverse event (AE). OS was defined as the period between recruitment to death regardless of the cause. rPFS was defined as the time from randomization to a radiographic progression event or death.6 The PSA response rate was defined as the proportion of patients with a 50% decline in PSA level compared to the baseline pre-treatment level. (5) Study design: The included articles were primarily limited to randomized controlled trials (RCTs).
The exclusion criteria were as follows: (1) Patients diagnosed with other types of prostate cancer, such as castration-sensitive prostate cancer and non-metastatic CRPCa (nmCRPCa). (2) Studies without clear description of study design, diagnostic criteria, and observed outcomes. (3) Other study types: Single-arm trials, reviews, case reports et al. (4) Studies with incomplete data. (5) Studies of PARPi which restricted the research population (HRR-mutation positive/negative alone).
Study selection
According to the criteria above, the selection was conducted by two independent researchers (J.B.H. and L.B.H.). First, all potential studies were imported into the reference management software Endnote 20. Subsequently, titles and abstracts were evaluated for preliminarily exclusion. Finally, the full texts of the remaining studies were downloaded for further inclusions. Discussions were initiated to resolve disagreements with the involvement of a third researcher.
Data collection and quality assessment
The data collection was conducted following a pre-designed form, in which the following data was included: first author, publication year, study type, nation, previous treatments, baseline characteristics (average age, number of participants in each group, median follow-up time, metastatic sites and median PSA level), treatments for intervention and control group, and interested outcomes mentioned above. Since HRR gene mutation significantly impact the efficacy of PARPi, we only extracted efficacy data from studies without limitation to HRR status to maintain the comparability with other treatments. Studies focusing only on HRR(+) or HRR(−) subgroups were excluded during study selection. Detailed extracted data was displayed in Supplementary Table S4. The Risk of Bias Assessment Tool 2.0 (RoB2) was used in the quality assessment. Each RCT was evaluated in five dimensions: (1) Randomization process; (2) Deviations from intended interventions; (3) Missing outcome data; (4) Measurement of the outcome; (5) Selection of the reported result.
During this two-step process, one researcher extracted data, completed the form, and assessed the quality of included articles. Another researcher conducted a second review independently without knowledge of results from the first researcher to ensure accuracy. Disagreements were resolved through discussion.
Statistical analysis
A Bayesian-framework based statistical model was constructed. The hazard ratio (HR) with a 95% credible interval (CrI) was calculated for survival analyses (OS, rPFS, and time to PSA progression). A pooled risk ratio with a 95% CrI was calculated for categorical data (PSA response rate and adverse events). Since prior drug use may influence treatment efficacy, we categorized the included studies into different subgroups for our analysis of therapeutic effects based on the medication history since mCRPCa. For all outcomes, random-effect models were applied. Markov chains were set and underwent 50,000 iterations, and 20,000 iterations were discarded during a burn-in period.7 Then, the consistency model and inconsistency model were evaluated in comparison to the value of DIC (deviation information criterion) for each outcome. If the difference was less than five, it was deemed that the consistency was good and consistency modeling was adopted.8 The relative efficacy of different treatments was estimated using the surface under the cumulative rank curve (SUCRA).
Global and local incoherence in the network meta-analysis was illustrated by I2 statistic and forest plots.9,10 Funnel plots were applied for the preliminary assessment of publication bias. Formal statistical tests (Egger’s test and Begg’s test) were further conducted. Trim and fill sensitivity analysis were conducted in endpoints which were considered to have publication bias (P < 0.05) or may require attention to the possibility of publication bias (P < 0.1). All analyses were conducted via the Gemtc package in R software (version 4.2.2) and Stata (version 15.0).
Evaluation of the certainty of evidence
We further assessed the certainty of evidence using the GRADE approach. Puhan et al. proposed a method to extend the GRADE approach to indirect and network comparisons.11 First, direct comparisons were assessed. Evidence for direct comparisons was initially assumed to have high confidence and then rate down the confidence in stages of “High”, “Moderate”, “Low” and “Very low” based on five criteria: study limitations, indirectness, inconsistency, imprecision and publication bias. Then, assessment for indirect comparisons between was conducted by evidence loops created by connecting treatments to a common comparator. We used the lowest rating of direct comparisons involved in a loop as the result of indirect comparison. In cases of both existing direct and indirect comparison for a treatment pair, we accepted the higher rating as the final network certainty. Detailed results and reasons for downgrading were presented in Supplementary Table S3.
Roles of funding source
The study was supported by The National Natural Science Foundation of China (Grant No. 82172568). The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Article search and screening
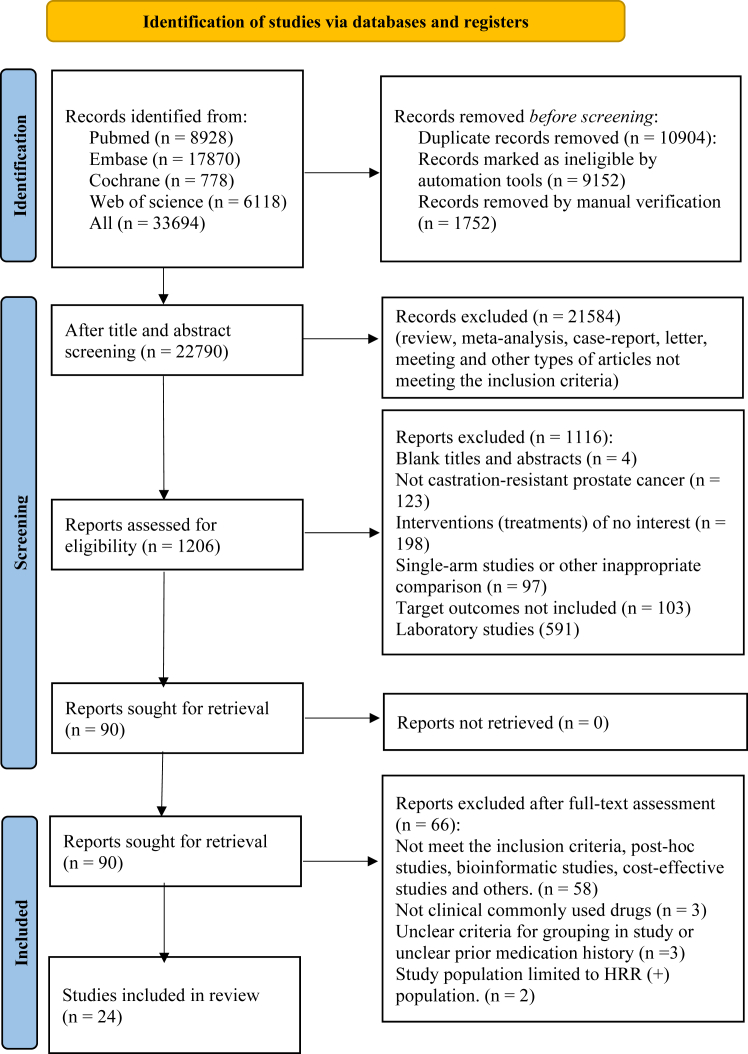
Details of the literature search are shown as a PRISMA flow chart in Fig. 1. A total of 33,694 articles were initially screened until December 31st, 2024. 90 studies were further full-text assessed. 58 articles were excluded due to uninterested topic, including post-hoc, cost-effective, and bioinformatics studies. Three articles were excluded which drugs have not been applied in clinical (Orteronel and PROSTVAC).12, 13, 14 Three RCTs were excluded for the unclear prior medication history and the control group treatments.15, 16, 17 Two RCTs involved PARPi was excluded for its limited HRR(+) population.18,19 Ultimately, 24 studies were included in this NMA.
Fig. 1.
PRISMA flow chart for search, screening and inclusion of studies.
Basic characteristics of included articles
The basic characteristics of 24 included articles are shown in Table 1 which were published between 2004 and 2024,20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43 encompassing studies conducted across the Americas, Europe, Asia, and Australia. The sample size ranged from 61 to 1717. Overall, 13,059 cases of mCRPCa were analyzed in this NMA, and the mean ages of studies ranged from 65 to 74 years old with well-balanced metastasis sites and median PSA between the intervention and control group. In terms of the prior treatment since the diagnosis of mCRPCa, 13 of the included studies focused on the first-line treatment.21, 22, 23,25,27,28,30, 31, 32, 33,37,40,42 Five studies targeted populations receiving second-line treatment after docetaxel failure.20,24,26,34,35 To minimize potential confounders introduced by treatment backgrounds, we applied strict criteria to the concept of “first-line”. In the efficacy analysis, the concept of first-line treatment should meet the following standards: 1. No treatment except continuous ADT after mCRPCa diagnosis. 2. Antiandrogens and chemotherapy during other stages of prostate cancer were acceptable with a mentioned discontinuation period or post-discontinuation response to ensure the washout of prior drug effects. Two studies were excluded from the efficacy analysis to minimize heterogeneity.38,39 Details backgrounds of included RCTs have been presented in the Supplementary Table S5. During the analysis of therapeutic effects, two studies on post-ARI second-line treatment,41,43 one study without restriction to prior treatment,29 and one study reporting both docetaxel and ARI histories36 were excluded for lack of studies to form network.
Table 1.
Detailed information of included studies in the network meta-analysis.
| Citation | Study | Country | Article type | Medication history | Number of patients | Intervention (N) | Control (N) | Age | Median Follow-up | Median PSA (ng/ml) | Metastasis Site | Interest Outcome | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 20 | de Bono 2011 | US/EURO | RCT | C | 1195 | AA (797) | placebo (398) | 69 | 20.2 | 128.8/137.7 | B/V/L | OS,rPFS, PSARR,TTPP | Low |
| 21 | Ryan 2013 | US/EURO | RCT | F | 1088 | AA (546) | placebo (542) | 70.3 | 22.2 | 22.3/21 | B/L | OS,rPFS, PSARR,TTPP | Low |
| 22 | Ye 2017 | Asia/EURO | RCT | F | 313 | AA (156) | placebo (157) | 71 | 60 | 48.57/55.73 | B/L | PSARR, TTPP | Low |
| 23 | Beer 2014 | US/EURO | RCT | F | 1717 | Enzalutamide (872) | placebo (845) | 71.3 | 22 | 54.1/44.2 | B/V/L | OS,rPFS, PSARR,TTPP | Low |
| 24 | Scher 2012 | International | RCT | C | 1199 | Enzalutamide (800) | placebo (399) | 68.7 | 14.4 | 107.7/128.3 | B/V/L | OS,rPFS, PSARR,TTPP | Low |
| 25 | Shore 2016 | US/EURO | RCT | F | 375 | Enzalutamide (184) | Bicalutamide (191) | 70.7 | 20 | 21/22 | B/L | rPFS, TTPP | Low |
| 26 | Clarke 2018 | International | RCT | C | 142 | Olaparib + AA (71) | AA (71) | 68 | 27.6 | 86/47 | B/L | OS,rPFS, PSARR | Low |
| 27 | Kantoff 2010 | US/CAN | RCT | F | 512 | Sipuleucel-T (341) | placebo (171) | 71 | 34.1 | 51.7/47.2 | B/L | OS | Low |
| 28 | Small 2006 | US | RCT | F | 127 | Sipuleucel-T (82) | placebo (45) | 71.7 | 36 | 46/47.9 | B/L | OS | Low |
| 29 | Hussain 2018 | US | RCT | M | 153 | Veliparib + AA (79) | AA (74) | 68 | 36 | 32.7/36.4 | B/V/L | OS,rPFS, PSARR | Low |
| 30 | Clarke 2022 | International | RCT | F | 796 | Olaparib + AA (399) | AA (397) | 69.1 | 19.3 | 17.9/16.8 | B/V/L | OS,rPFS | Low |
| 31 | Agarwal 2023 | International | RCT | F | 805 | Talazoparib + Enzalutamide (402) | Enzalutamide (403) | 71 | 24.8 | 18.2/16.2 | B/V/L | OS,rPFS, PSARR,TTPP | Low |
| 32 | Petrylack 2004 | International | RCT | F | 674 | Docetaxel (338) | placebo (336) | 70 | 32 | 84/90 | B/V/L | OS,PSARR | Low |
| 33 | Tannock 2004 | International | RCT | F | 1006 | Docetaxel (669) | placebo (337) | 68 | 20.8 | 114/123 | B/V/L | OS,PSARR | Low |
| 34 | de Bono 2010 | International | RCT | C | 755 | Cabazitaxel (378) | placebo (377) | 68 | 12.8 | 128/145 | B/V/L | OS,rPFS, PSARR,TTPP | Low |
| 35 | Hofman 2021 | Australia | RCT | C | 200 | Lu-177 (99) | Cabazitaxel (101) | 71.6 | 18.4 | 93.5/110 | B/V/L | rPFS, PSARR | Low |
| 36 | Sartor 2021 | US/EURO | RCT | B | 831 | Lu-177 (551) | placebo (280) | 70 | 20.9 | 77.5/74.6 | B/V/L | OS,rPFS | Low |
| 37 | Emmett 2024 | Australia | RCT | F | 162 | Lu-177 + Enzalutamide (83) | Enzalutamide (79) | 71 | 20 | – | B/V/L | rPFS, PSARR,TTPP | Low |
| 38 | Caffo 2021 | Italy | RCT | U | 246 | Docetaxel + Enzalutamide (120) | Docetaxel (126) | 70 | 24.1 | 32.7/33.2 | B/V/L | OS,rPFS, PSARR,TTPP | Low |
| 39 | Shen 2012 | China | RCT | U | 61 | Docetaxel (30) | placebo (31) | 65 | 42 | 100.86/63.45 | B/V | PSARR | Moderate |
| 40 | Zhou 2015 | China | RCT | F | 228 | Docetaxel (113) | placebo (115) | 71 | 39.1 | 70.9/100 | B/V/L | OS,PSARR | Low |
| 41 | Climen 2022 | Spain | RCT | A | 94 | Docetaxel + AA (47) | Docetaxel (47) | 69.5 | 15 | 73/34 | B/V/L | PSARR | Low |
| 42 | Fossa 2008 | Norway | RCT | F | 109 | Docetaxel (57) | placebo (52) | 71 | – | 130/163 | B/V | PSARR | Low |
| 43 | Merseburger 2022 | EURO | RCT | A | 271 | Docetaxel + Enzalutamide (136) | Docetaxel (135) | 71 | 8.1 | 36.9/28.1 | B/V | rPFS, PSARR,TTPP | Low |
Notes: ① In column “Article Type”, all the studies included were randomized controlled trials (RCT); ② In column “Medication History”, abbreviated capital letters are used to represent the treatments received by patients since the diagnosis of mCRPCa. F: First-line therapy without prior drug use; C: Second-line therapy after docetaxel failure; A: Second-line therapy after second-generation antiandrogen failure; M: The study did not set a restriction to prior drug use or without clear description; B: Second-line therapy after failure of docetaxel and second-generation antiandrogen; U: Unclear prior medication history or different limiting criteria from other studies. ③ AA: Abiraterone acetate; ④ N: Sample size of intervention and control group; ⑤ The values in the column “median follow-up” are presented in months; ⑥ In column “Median PSA”, the data is summarized in the form of “Intervention group/Control group”; ⑦ In column “Metastasis Site”, the abbreviations of the reported metastatic sites are as follows: B: Bone, V: Visceral, L: Lymph nodes; ⑧ OS: Overall survival; rPFS: Radiographic progression-free survival; PSARR: PSA response rate; TTPP: Time to PSA progression; ⑨ The risk of bias for each included article was assessed via the Risk of Bias Assessment Tool 2.0 (RoB2).
Quality assessment
All studies were of high quality. Detailed information on the process of quality assessment is shown in Table 1 and Supplementary Fig. S1.
Results for outcomes of concern
Presentation of overall analysis results
A summary of the analysis results was presented in Tables 2 and 3 with P value and 95% credible intervals, taken placebo as the common control. More detailed results were discussed in the following sections. Supplementary Table S6 presented the SUCRA values of treatments for all the outcomes.
Table 2.
Summary of analyzing results of therapeutic effects for all 4 endpoints.
Estimated results are presented as P value and 95% credible intervals (Comparing with placebo). Different types of effects are represented by different colors: (1) Positive therapeutic effects or potential to induce adverse effects (Pink); (2) Significant positive therapeutic effects or potential to induce adverse effects (Pink with bold fonts); (3) Not reported (Grey). (4) The most influential treatment according to the SUCRA value (Deep pink).
AA: Abiraterone acetate; ARI: Second-generation androgen receptor inhibitor; CAB: Cabazitaxel; DOC: Docetaxel; FARI: First-generation androgen receptor inhibitor (Bicalutamide in this NMA); PARPi: Poly (ADP-ribose) polymerase inhibitors; Sip-T: Sipuleucel-T; OS: Overall survival; rPFS: Radiographic progression-free survival; PSARR: PSA response rate; TTPP: Time to PSA progression; SUCRA: Surface under the cumulative Ranking Curve.
Table 3.
Summary of analyzing results of therapeutic effects for 8 adverse events.
 |
Estimated results are presented as P value and 95% credible intervals (Comparing with placebo). Different types of effects are represented by different colors: (1) Positive therapeutic effects or potential to induce adverse effects (Pink); (2) Significant positive therapeutic effects or potential to induce adverse effects (Pink with bold fonts); (3) Insignificant potential to prevent adverse effects (Blue); (4) Not reported (Grey). (5) The most influential treatment according to the SUCRA value (Deep pink).
AA: Abiraterone acetate; ARI: Second-generation androgen receptor inhibitor; CAB: Cabazitaxel; DOC: Docetaxel; FARI: First-generation androgen receptor inhibitor (Bicalutamide in this NMA); PARPi: Poly (ADP-ribose) polymerase inhibitors; Sip-T: Sipuleucel-T; SUCRA: Surface under the cumulative Ranking Curve.
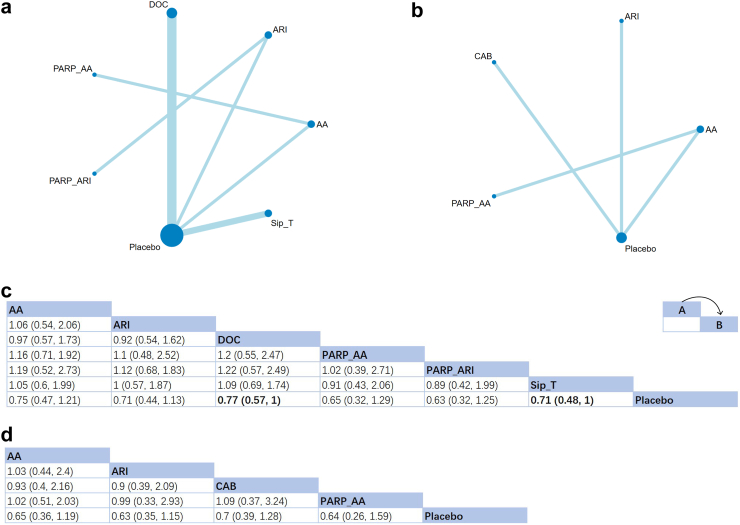
OS
13 studies were included in the assessment of the effects of seven treatment methods on OS (Fig. 2a and b). The relative effects of different treatments are shown in Fig. 2c and d. For the first-line therapy choices, all six treatments showed an active therapeutic effect on prolonging OS. Of these, the effects of docetaxel (HR: 0.77; 95% CrI [0.57, 1]) and Sipuleucel-T (HR: 0.71; 95% CrI [0.48, 1]) were of clinical significance. According to the value of SUCRA, PARPi + ARI demonstrated the most significant potential for improving OS (SUCRA = 72%), followed by PARPi + AA (SUCRA = 70%). For patients who received docetaxel as the first-line therapy but failed, all four involved treatments presented a positive therapeutic effect without statistical significance. The SUCRA rating indicated a better efficacy of second-generation antiandrogens and relative combination therapies comparing to a shift of chemotherapy drug from docetaxel to cabazitaxel (ARI: SUCRA = 66%; AA: 62%; PARPi + AA: 61%; cabazitaxel: 52%).
Fig. 2.
Network plot and results of overall survival (OS): (a) Network plot for OS (First-line therapies). (b) Network plot for OS (Second-line therapies after docetaxel failure). (c) Relative effects of different treatments (First-line therapies). (d) Relative effects of different treatments (Second-line therapies after docetaxel failure). Notes: Estimated results are presented as P value and 95% credible intervals (95% CrI). Relative effects are located at the intersections of the column and row. The arrow in the upper right corner of panel c and d shows how these two tables should be read: The P value at the intersection represents the relative effect of intervention A comparing to intervention B. Significant results are in bold. AA: Abiraterone Acetate; ARI: Second-generation androgen receptor inhibitor; CAB: Cabazitaxel; DOC: Docetaxel; PARP: poly (ADP-ribose) Polymerase Inhibitors; Sip-T: Sipuleucel-T.
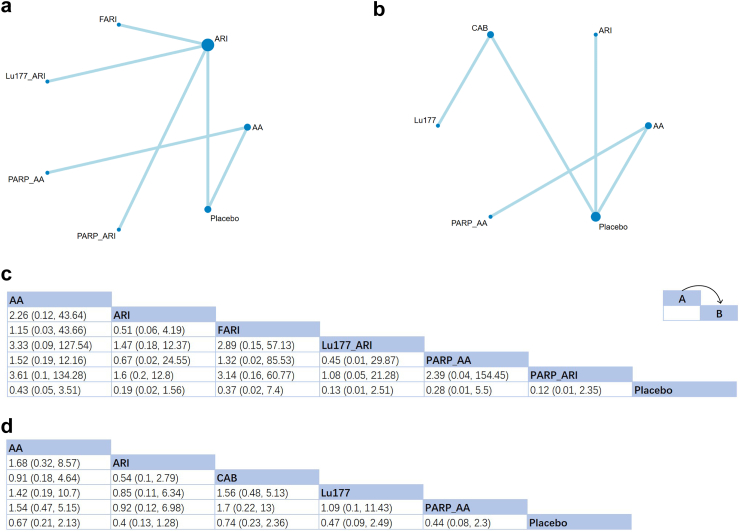
rPFS
Overall, 11 studies involving seven treatments were included in this analysis on rPFS (Fig. 3a and b). In case of first-line treatments, all of them had statistically insignificant positive effects on rPFS (Fig. 3c). The SUCRA ranking showed promising effects for improving rPFS in ARI and its related combination therapy (PARPi + ARI: SUCRA = 85%; Lu177 + ARI: 81%; ARI: 65%). For patients with a medication history of docetaxel, the therapeutic effects for all 5 treatments were positive but insignificant (Fig. 3d). Similar to the result for first-line therapies, ARI also performed as the best choice (SUCRA: 75%).
Fig. 3.
Network plot and results of radiographic progression-free survival (rPFS): (a) Network plot for rPFS (First-line therapies). (b) Network plot for rPFS (Second-line therapies after docetaxel failure). (c) Relative effects of different treatments (First-line therapies). (d) Relative effects of different treatments (Second-line therapies after docetaxel failure). Notes: Estimated results are presented as P value and 95% credible intervals (95% CrI). Relative effects are located at the intersections of the column and row. The arrow in the upper right corner of panel c and d shows how these two tables should be read: The P value at the intersection represents the relative effect of intervention A comparing to intervention B. Significant results are in bold. AA: Abiraterone Acetate; ARI: Second-generation androgen receptor inhibitor; CAB: Cabazitaxel; FARI: First-generation androgen receptor inhibitor, Bicalutamide in this NMA; PARP: poly (ADP-ribose) Polymerase Inhibitors; Sip-T: Sipuleucel-T.
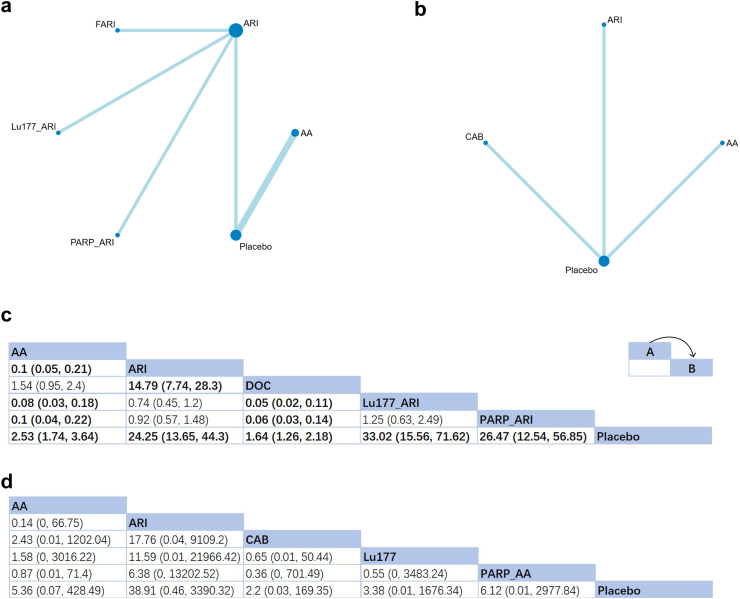
Time to PSA progression
As shown in Fig. 4a and b, the effects of six treatments on time to PSA progression were assessed in nine studies. According to the results, all the first-line drug choices showed a positive effect, while two of them remained significant (HR of ARI: 0.17, 95% CrI: [0.03, 0.94]; HR of Lu177 + ARI: 0.07, 95% CrI: [0.01, 0.87]) (Fig. 4c). The SUCRA ranking indicated that combination therapies and monotherapy of ARI might be the preferred option for controlling the time to PSA progression (Lu177 + ARI: SUCRA = 91%; PARPi + ARI: 78%; ARI: 66%). For post-docetaxel patients, positive but insignificant effects were presented for all the three treatments (ARI, AA and cabazitaxel) (Fig. 4d). The SUCRA rating suggested ARI the best treatment to control the PSA progression (ARI: 88%).
Fig. 4.
Network plot and results of time to PSA progression (TTPP): (a) Network plot for TTPP (First-line therapies). (b) Network plot for TTPP (Second-line therapies after docetaxel failure). (c) Relative effects of different treatments (First-line therapies). (d) Relative effects of different treatments (Second-line therapies after docetaxel failure). Notes: Estimated results are presented as P value and 95% credible intervals (95% CrI). Relative effects are located at the intersections of the column and row. The arrow in the upper right corner of panel c and d shows how these two tables should be read: The P value at the intersection represents the relative effect of intervention A comparing to intervention B. Significant results are in bold. AA: Abiraterone Acetate; ARI: Second-generation androgen receptor inhibitor; CAB: Cabazitaxel; FARI: First-generation androgen receptor inhibitor, Bicalutamide in this NMA; PARP: poly (ADP-ribose) Polymerase Inhibitors; Sip-T: Sipuleucel-T.
PSA response rate
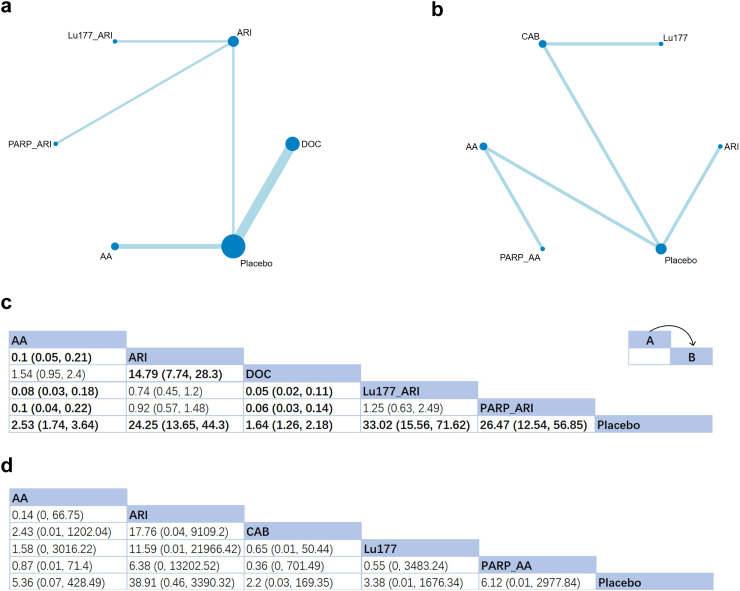
The data from 14 studies involving nine treatments were employed to ascertain their effects on PSA response rate (Fig. 5a and b). Compared with placebo, significant effects were found in all the first-line treatments (RR of AA = 2.53, 95% CrI:[1.74, 3.64]; RR of ARI = 24.25, 95% CrI: [13.65, 44.3]; RR of docetaxel = 1.64, 95% CrI: [1.26, 2.18]; RR of Lu177 + ARI = 33.02, 95% CrI: [15.56, 71.62]; RR of PARPi + ARI = 26.47, 95% CrI: [12.54, 56.85]) (Fig. 5c). According to the value of SUCRA, ARI and its related combination therapy might be optimal choices to enhance the response rate (Lu177 + ARI: SUCRA = 97%; PARPi + ARI: 82%; ARI: 71%). For patients who had undergone treatment of docetaxel, positive but insignificant therapeutic efficacies were observed for all five treatments (Fig. 5d). ARI performed the best among them with a SUCRA value of 85%.
Fig. 5.
Network plot and results of PSA response rate (PSARR): (a) Network plot for PSARR (First-line therapies). (b) Network plot for PSARR (Second-line therapies after docetaxel failure). (c) Relative effects of different treatments (First-line therapies). (d) Relative effects of different treatments (Second-line therapies after docetaxel failure). Notes: Estimated results are presented as P value and 95% credible intervals (95% CrI). Relative effects are located at the intersections of the column and row. The arrow in the upper right corner of panel c and d shows how these two tables should be read: The P value at the intersection represents the relative effect of intervention A comparing to intervention B. Significant results are in bold. AA: Abiraterone Acetate; ARI: Second-generation androgen receptor inhibitor; CAB: Cabazitaxel; DOC: Docetaxel; PARP: poly (ADP-ribose) Polymerase Inhibitors; Sip-T: Sipuleucel-T.
AE
This NMA examined the eight most common adverse reactions and reported the analysis of fatigue in detail as a demonstration on interpreting the results. For fatigue, 12 treatment options were involved (Supplementary Fig. S2a). While all treatments except first-generation ARI (bicalutamide) showed the potential for increased events of fatigue, the following four treatments were statistically significant: docetaxel (RR: 1.61, 95% CrI [1.15, 2.33]), docetaxel + ARI (RR: 2.59, 95% CrI [1.44, 5.12]), Lu177 (RR: 1.64, 95% CrI [1.08, 2.61]) and PARPi + AA (RR: 1.63, 95% CrI [1.09, 3.06]) (Supplementary Fig. S2b). According to SUCRA value, docetaxel-related therapies showed significant effects on side effects (docetaxel + ARI SUCRA = 95%; docetaxel + AA = 71%; docetaxel = 68%). A brief summary of results of the remaining seven adverse reactions is presented in Table 3 and detailed results can be found in the Supplementary materials. In general, this NMA revealed significant effects of all treatments on only six out of eight AEs (fatigue, diarrhea, arthralgia, hypertension, headache, and nausea). Detailed SUCRA values are provided in Supplementary Table S6.
Heterogeneity, consistency, publication bias and certainty of evidence
The DIC results which represented good consistency for all models were reported in Supplementary Table S2, together with I2 statistics reflecting the global heterogeneity. Local heterogeneity was assessed by forest plots (Supplementary Figs. S12–S22). No global or local heterogeneity was detected in all the therapeutic endpoints. For adverse events, some local incoherences were found within certain pair-wise comparisons which may due to the small included sample, resulted in an extremely low number of adverse events in certain studies. The funnel plots (Supplementary Figs. S23 and S24), Egger’s test and Begg’s test indicated no apparent publication bias for all the endpoints, but the result of Egger’s test for PSA response rate in first-line therapies showed a relatively low P value (P = 0.06) (Supplementary Table S2). To ensure rigor, leave-one-out analyses and trim & fill sensitivity analysis were conducted to assess the potential publication bias, with no significant changes during the leave-one-out analyses (Supplementary Fig. S25). After adding three hypothetical studies, the significance of the estimated effect remained unchanged, demonstrating the stability of the result (Supplementary Fig. S26).
According to the GRADE rating, “Very low” evidence certainty was detected in three therapeutic endpoints (rPFS, PSA response rate and time to PSA progression) (Supplementary Table S3). The most common reasons for lower rating were imprecision with a 95% CrI crossing over 1 and suspected publication bias due to an insufficient number of reported RCTs in direct comparison. Importantly, due to the very low evidence certainty for PSA response rate in post-docetaxel second-line population, the reported results should be interpreted with caution.
Discussion
Due to the lack of horizontal comparisons, finding effective treatments of mCRPCa has become a challenge for clinicians. This NMA involving 24 RCTs compares the therapeutic effect and safety of all first-line and post-docetaxel second-line therapies. Ra-223 was not included in this network meta-analysis. Due to its specific effectiveness in bone metastasis, the focused populations of RCTs involving Ra-223 differed in terms of metastasis sites comparing to those in studies of other drugs, resulting in a lack of comparability. To assess the clinical efficacy, OS, rPFS, time to PSA progression, and PSA response rate were selected as the endpoints for analysis.
According to the analysis results, all the treatments involved indicated their positive therapeutic effects for both first-line and post-docetaxel second-line subgroups. For the first-line options for treatment, the ARI-related combination therapies performed well across all four endpoints. PARPi + ARI showed its best effects on OS with borderline therapeutic effects (SUCRA: 72%). For rPFS, PARPi + ARI showed its good efficacy with a SUCRA of 85%, followed by Lu177 + ARI (SUCRA: 81%), which demonstrated the efficacy in prolonging the lifespans and preventing local and distal metastases. A higher PSA response rate of ARI-based combination therapies (SUCRA of Lu177 + ARI: 97%; SUCRA of PARPi + ARI: 82%) may indicate more potential benefits. For the time to PSA progression, the combination therapy of Lu-177 + ARIs demonstrated the most effective therapeutic effect (91%), followed by PARPI + ARI (78%) and ARI monotherapy (66%). This may reflect the control on biochemical recurrence and PSA elevation in clinical practice. However, the single effect of PARPi and Lu-177 remained inconclusive due to the lack of reported RCTs. In case of second-line therapies after failure of docetaxel, ARI showed its best therapeutic efficacies in all four endpoints (SUCRA in OS: 66%; SUCRA in rPFS:75%; SUCRA in time to PSA progression: 88%; SUCRA in PSA response rate: 85%), followed by AA and AA-based combination therapy. This result may support the decision to second-generation antiandrogen therapies (especially ARIs) rather than changing chemotherapy drugs to cabazitaxel. However, SUCRA values can only indicate a potential best option. Further RCTs and clinical practice are necessary to determine the best treatment.
Numerous researchers have conducted pair-wise meta-analyses, which is consistent with our results.44, 45, 46 Two studies indicated a significant effect of abiraterone on rPFS while that of our results showed insignificant.47,48 This discrepancy may be caused by the influences of other treatment through indirect comparisons in our study. In regard to PARPi, Ditonno’s meta-analysis reported a significant efficacy of PARPi monotherapy.49 However, there is no comparability between this study and ours because it only focused on HRR (+) patients. Even though PARPi is more effective for HRR gene mutant, its synergistic effect with antiandrogens reported by several articles and the results of this NMA indicated a possible benefit for a broader range of patients.50,51 Moreover, some articles compared the combination therapies in addition to monotherapies.52,53 Francini et al. pointed out that the ARI-based combination therapies (Lu177/PARPi + ARI) were ideal choices for treatment of mCRPCa, which highly supported our findings.53
Safety of treatments is another important aspect in clinical decision. In regard to hypertension, Lacovelli et al. evaluated the efficacy of AA and second-generation ARIs. Both treatments demonstrated a significant effect (RR of abiraterone: 1.79, 95% CrI [1.45, 2.21]; RR of ARIs: 2.66, 95% CrI [1.94, 3.66]), aligning with our findings.54 According to this NMA, significant influences were seen in six AEs out of eight. Based on the SUCRA values, overall, combination therapies were more likely to cause adverse events than their respective monotherapies (except for diarrhea and hypertension). Thus, the potential to induce AEs should be carefully evaluated before implementing the combination therapy for better efficacy. Moreover, the combination therapy of docetaxel and ARI should be carefully considered for its more limited therapeutic efficacy and more possibilities to cause AEs comparing with other ARI combination therapies.
This study has the following advantages compared to all existing research. To our knowledge, this is the first NMA to comprehensively compare and rank the therapeutic effects of all systemic therapies commonly recommended by guidelines on mCRPCa. This NMA may provide valuable insight for clinical decision-making. Second, in order to eliminate the potential influence of prior medication history of mCRPCa treatment, we analyzed the therapeutic effect in subgroups of first-line therapies and second-line therapies after docetaxel failure, which results were more convincing comparing with existing meta-analyses. Furthermore, this NMA assessed the potential for triggering adverse reactions associated with all treatments, which included the most comprehensive treatments and adverse reactions.
There are still some limitations. First, the results may be affected by the limited number of studies and sample sizes, which may lead to an increase in heterogeneity and a decreased evidence certainty. Second, discrepancies in control group treatment could introduce heterogeneity into the analysis. There is a possibility of different background treatments under the same term “placebo” in different trials. Third, the impressive results related to PARPi + ARI combination therapy should still be viewed cautiously and urgently require further validation by clinical trials even though we set the strictest data-collecting criteria on HRR status. Fourth, this NMA focused on all grades of AEs. Subgroup analyses for grade 3 and 4 AEs were not performed due to the limited number of relevant reports. Lastly, some non-English studies may have been overlooked because the literature search was conducted using English-language databases.
In conclusion, this NMA demonstrated that all included first-line and second-line treatments were efficacious. For first-line treatments, ARI and its relative combination therapies (PARPi/Lu177 + ARI) may be the optimal choices for prolonging the survival time, preventing the development of local and distal metastases and regulating the serum PSA level. For second-line therapy after docetaxel failure, ARI was also considered the best among all treatments. The combination therapies were identified to have the greatest tendency to induce most AEs (nausea for PARPi + AA, headache for PARPi + ARI and fatigue/anaemia/arthralgia for docetaxel + ARI). It is therefore essential to weigh up the balance of therapeutic and adverse effects, particularly in the case of combination therapies (especially docetaxel + ARI, with a limited therapeutic efficacy and potential to induce adverse effect). Further clinical trials are required to provide direct comparisons and to reveal the efficacy and safety of different treatments, with a view to guiding the clinical decision-making on the management of mCRPCa.
Contributors
All authors contributed to the study conception and design and had access to the study data through pre-established data-management processes. Writing - original draft preparation: Bohao Jiang, Benqiao Wang, Yiming Chen, Yaang Chen, Bohan Li, Jianbin Bi; Writing - review and editing: Bohao Jiang, Benqiao Wang; Data extraction and verification: Bohao Jiang, Bohan Li, Benqiao Wang; Conceptualization: Bohan Li; Methodology: Benqiao Wang, Yiming Chen; Formal analysis and investigation: Bohao Jiang, Yaang Chen; Funding acquisition: Jianbin Bi; Resources: Jianbin Bi; Supervision: Jianbin Bi, and all authors commented on previous versions of the manuscript. All authors read and approved the final version of the manuscript.
Data sharing statement
In this study, all data analyses were conducted using the Gemtc package in R (version 4.2.2) and STATA 15. The raw data needed for the analyses are presented in the Supplementary table, and the code used for the analyses has been uploaded to GitHub (https://github.com/NelsonJiang1999/codes-for-nma.git).
Declaration of interests
We declare no competing interests.
Acknowledgements
The research was funded by the National Natural Science Foundation of China (Grant No. 82172568). The authors acknowledge the support received from the foundation. We are grateful to Dr. Gejun Zhang for sharing his clinical experience in the treatment of metastatic castration-resistant prostate cancer. We appreciate the constructive suggestions provided by all the reviewers, which have significantly improved the quality of our paper.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2025.103129.
Contributor Information
Bohan Li, Email: li18698881347@163.com.
Jianbin Bi, Email: jianbinbi@cmu.edu.cn.
Appendix ASupplementary data
References
- 1.Bray F., Laversanne M., Sung H., et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–263. doi: 10.3322/caac.21834. [DOI] [PubMed] [Google Scholar]
- 2.Lowrance W., Dreicer R., Jarrard D.F., et al. Updates to advanced prostate cancer: AUA/SUO guideline (2023) J Urol. 2023;209(6):1082–1090. doi: 10.1097/JU.0000000000003452. [DOI] [PubMed] [Google Scholar]
- 3.Tilki D., van den Bergh R.C.N., Briers E., et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG guidelines on prostate cancer. Part II-2024 update: treatment of relapsing and metastatic prostate cancer. Eur Urol. 2024;86(2):164–182. doi: 10.1016/j.eururo.2024.04.010. [DOI] [PubMed] [Google Scholar]
- 4.Schaeffer E.M., Srinivas S., Adra N., et al. NCCN Guidelines® insights: prostate cancer, version 3.2024. J Natl Compr Canc Netw. 2024;22(3):140–150. doi: 10.6004/jnccn.2024.0019. [DOI] [PubMed] [Google Scholar]
- 5.Hutton B., Salanti G., Caldwell D.M., et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 6.Eisenhauer E.A., Therasse P., Bogaerts J., et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 7.Hajihashemi M., Aghababaei Samani K. Multi-strategy evolutionary games: a Markov chain approach. PLoS One. 2022;17(2) doi: 10.1371/journal.pone.0263979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dempster A.P. The direct use of likelihood for significance testing. Stat Comput. 1997;7(4):247–252. [Google Scholar]
- 9.Krahn U., Binder H., König J. A graphical tool for locating inconsistency in network meta-analyses. BMC Med Res Methodol. 2013;13:35. doi: 10.1186/1471-2288-13-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salanti G., Marinho V., Higgins J.P. A case study of multiple-treatments meta-analysis demonstrates that covariates should be considered. J Clin Epidemiol. 2009;62(8):857–864. doi: 10.1016/j.jclinepi.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Dumville J.C., Soares M.O., O’Meara S., Cullum N. Systematic review and mixed treatment comparison: dressings to heal diabetic foot ulcers. Diabetologia. 2012;55(7):1902–1910. doi: 10.1007/s00125-012-2558-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saad F., Fizazi K., Jinga V., et al. Orteronel plus prednisone in patients with chemotherapy-naive metastatic castration-resistant prostate cancer (ELM-PC 4): a double-blind, multicentre, phase 3, randomised, placebo-controlled trial. Lancet Oncol. 2015;16(3):338–348. doi: 10.1016/S1470-2045(15)70027-6. [DOI] [PubMed] [Google Scholar]
- 13.Kantoff P.W., Schuetz T.J., Blumenstein B.A., et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28(7):1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gulley J.L., Borre M., Vogelzang N.J., et al. Phase III trial of PROSTVAC in asymptomatic or minimally symptomatic metastatic castration-resistant prostate cancer. J Clin Oncol. 2019;37(13):1051–1061. doi: 10.1200/JCO.18.02031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Penson D.F., Armstrong A.J., Concepcion R., et al. Enzalutamide versus bicalutamide in castration-resistant prostate cancer: the STRIVE trial. J Clin Oncol. 2016;34(18):2098–2106. doi: 10.1200/JCO.2015.64.9285. [DOI] [PubMed] [Google Scholar]
- 16.de Bono J., Mateo J., Fizazi K., et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091–2102. doi: 10.1056/NEJMoa1911440. [DOI] [PubMed] [Google Scholar]
- 17.de Wit R., de Bono J., Sternberg C.N., et al. Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer. N Engl J Med. 2019;381(26):2506–2518. doi: 10.1056/NEJMoa1911206. [DOI] [PubMed] [Google Scholar]
- 18.Fizazi K., Piulats J.M., Reaume M.N., et al. Rucaparib or physician’s choice in metastatic prostate cancer. N Engl J Med. 2023;388(8):719–732. doi: 10.1056/NEJMoa2214676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chi K.N., Rathkopf D., Smith M.R., et al. Niraparib and abiraterone acetate for metastatic castration-resistant prostate cancer. J Clin Oncol. 2023;41(18):3339–3351. doi: 10.1200/JCO.22.01649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Bono J.S., Logothetis C.J., Molina A., et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryan C.J., Smith M.R., de Bono J.S., et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ye D., Huang Y., Zhou F., et al. A phase 3, double-blind, randomized placebo-controlled efficacy and safety study of abiraterone acetate in chemotherapy-naive patients with mCRPC in China, Malaysia, Thailand and Russia. Asian J Urol. 2017;4(2):75–85. doi: 10.1016/j.ajur.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beer T.M., Armstrong A.J., Rathkopf D.E., et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424–433. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scher H.I., Fizazi K., Saad F., et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 25.Shore N.D., Chowdhury S., Villers A., et al. Efficacy and safety of enzalutamide versus bicalutamide for patients with metastatic prostate cancer (TERRAIN): a randomised, double-blind, phase 2 study. Lancet Oncol. 2016;17(2):153–163. doi: 10.1016/S1470-2045(15)00518-5. [DOI] [PubMed] [Google Scholar]
- 26.Clarke N., Wiechno P., Alekseev B., et al. Olaparib combined with abiraterone in patients with metastatic castration-resistant prostate cancer: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2018;19(7):975–986. doi: 10.1016/S1470-2045(18)30365-6. [DOI] [PubMed] [Google Scholar]
- 27.Kantoff P.W., Higano C.S., Shore N.D., et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 28.Small E.J., Schellhammer P.F., Higano C.S., et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24(19):3089–3094. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 29.Hussain M., Daignault-Newton S., Twardowski P.W., et al. Targeting androgen receptor and DNA repair in metastatic castration-resistant prostate cancer: results from NCI 9012. J Clin Oncol. 2018;36(10):991–999. doi: 10.1200/JCO.2017.75.7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clarke N.W., Armstrong A.J., Thiery-Vuillemin A., et al. Abiraterone and olaparib for metastatic castration-resistant prostate cancer. NEJM Evid. 2022;1(9) doi: 10.1056/EVIDoa2200043. [DOI] [PubMed] [Google Scholar]
- 31.Agarwal N., Azad A.A., Carles J., et al. Talazoparib plus enzalutamide in men with first-line metastatic castration-resistant prostate cancer (TALAPRO-2): a randomised, placebo-controlled, phase 3 trial. Lancet. 2023;402(10398):291–303. doi: 10.1016/S0140-6736(23)01055-3. [DOI] [PubMed] [Google Scholar]
- 32.Petrylak D.P., Tangen C.M., Hussain M.H., et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 33.Tannock I.F., de Wit R., Berry W.R., et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 34.de Bono J.S., Oudard S., Ozguroglu M., et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 35.Hofman M.S., Emmett L., Sandhu S., et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021;397(10276):797–804. doi: 10.1016/S0140-6736(21)00237-3. [DOI] [PubMed] [Google Scholar]
- 36.Sartor O., de Bono J., Chi K.N., et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med. 2021;385(12):1091–1103. doi: 10.1056/NEJMoa2107322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Emmett L., Subramaniam S., Crumbaker M., et al. [(177)Lu]Lu-PSMA-617 plus enzalutamide in patients with metastatic castration-resistant prostate cancer (ENZA-p): an open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2024;25(5):563–571. doi: 10.1016/S1470-2045(24)00135-9. [DOI] [PubMed] [Google Scholar]
- 38.Caffo O., Ortega C., Nolè F., et al. Docetaxel and prednisone with or without enzalutamide as first-line treatment in patients with metastatic castration-resistant prostate cancer: CHEIRON, a randomised phase II trial. Eur J Cancer. 2021;155:56–63. doi: 10.1016/j.ejca.2021.06.016. [DOI] [PubMed] [Google Scholar]
- 39.Shen Y.J., Bian X.J., Xie H.Y., et al. [Docetaxel plus prednisone versus mitoxantrone plus prednisone as first-line chemotherapy for metastatic hormone-refractory prostate cancer: long-term effects and safety] Zhonghua Wai Ke Za Zhi. 2012;50(6):539–542. [PubMed] [Google Scholar]
- 40.Zhou T., Zeng S.X., Ye D.W., et al. A multicenter, randomized clinical trial comparing the three-weekly docetaxel regimen plus prednisone versus mitoxantone plus prednisone for Chinese patients with metastatic castration refractory prostate cancer. PLoS One. 2015;10(1) doi: 10.1371/journal.pone.0117002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Climent M.A., Font A., Durán I., et al. A phase II randomised trial of abiraterone acetate plus prednisone in combination with docetaxel or docetaxel plus prednisone after disease progression to abiraterone acetate plus prednisone in patients with metastatic castration-resistant prostate cancer: the ABIDO-SOGUG trial. Eur J Cancer. 2022;175:110–119. doi: 10.1016/j.ejca.2022.08.002. [DOI] [PubMed] [Google Scholar]
- 42.Fosså S.D. A randomized phase II trial comparing weekly taxotere plus prednisolone versus prednisolone alone in androgen-independent prostate cancer. Front Radiat Ther Oncol. 2008;41:108–116. doi: 10.1159/000139885. [DOI] [PubMed] [Google Scholar]
- 43.Merseburger A.S., Attard G., Åström L., et al. Continuous enzalutamide after progression of metastatic castration-resistant prostate cancer treated with docetaxel (PRESIDE): an international, randomised, phase 3b study. Lancet Oncol. 2022;23(11):1398–1408. doi: 10.1016/S1470-2045(22)00560-5. [DOI] [PubMed] [Google Scholar]
- 44.Roviello G., Sigala S., Sandhu S., et al. Role of the novel generation of androgen receptor pathway targeted agents in the management of castration-resistant prostate cancer: a literature based meta-analysis of randomized trials. Eur J Cancer. 2016;61:111–121. doi: 10.1016/j.ejca.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 45.Tiwari A., Alcover K., Carpenter E., et al. Utility of cell-based vaccines as cancer therapy: systematic review and meta-analysis. Hum Vaccin Immunother. 2024;20(1) doi: 10.1080/21645515.2024.2323256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sadaghiani M.S., Sheikhbahaei S., Werner R.A., et al. (177) Lu-PSMA radioligand therapy effectiveness in metastatic castration-resistant prostate cancer: an updated systematic review and meta-analysis. Prostate. 2022;82(7):826–835. doi: 10.1002/pros.24325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cao Q., Bai P., Shi D., et al. CYP17 inhibitors improve the prognosis of metastatic castration-resistant prostate cancer patients: a meta-analysis of published trials. J Cancer Res Ther. 2020;16(5):990–1001. doi: 10.4103/jcrt.JCRT_295_18. [DOI] [PubMed] [Google Scholar]
- 48.Zhou Z.R., Liu S.X., Zhang T.S., Xia J., Li B. Abiraterone for treatment of metastatic castration-resistant prostate cancer: a systematic review and meta-analysis. Asian Pac J Cancer Prev. 2014;15(3):1313–1320. doi: 10.7314/apjcp.2014.15.3.1313. [DOI] [PubMed] [Google Scholar]
- 49.Ditonno F., Bianchi A., Malandra S., et al. PARP inhibitors in metastatic prostate cancer: a comprehensive systematic review and meta-analysis of existing evidence. Clin Genitourin Cancer. 2024;22(2):402–412.e17. doi: 10.1016/j.clgc.2023.12.011. [DOI] [PubMed] [Google Scholar]
- 50.Asim M., Tarish F., Zecchini H.I., et al. Synthetic lethality between androgen receptor signalling and the PARP pathway in prostate cancer. Nat Commun. 2017;8(1):374. doi: 10.1038/s41467-017-00393-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schiewer M.J., Goodwin J.F., Han S., et al. Dual roles of PARP-1 promote cancer growth and progression. Cancer Discov. 2012;2(12):1134–1149. doi: 10.1158/2159-8290.CD-12-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ai J., Jian L., Wen X., et al. Comparative effectiveness of first-line systemic treatments for metastatic castration-resistant prostate cancer: a systematic review and network meta-analysis. Clin Transl Oncol. 2024;26(10):2559–2571. doi: 10.1007/s12094-024-03506-4. [DOI] [PubMed] [Google Scholar]
- 53.Francini E., Agarwal N., Castro E., et al. Intensification approaches and treatment sequencing in metastatic castration-resistant prostate cancer: a systematic review. Eur Urol. 2025;87(1):29–46. doi: 10.1016/j.eururo.2024.09.008. [DOI] [PubMed] [Google Scholar]
- 54.Iacovelli R., Ciccarese C., Bria E., et al. The cardiovascular toxicity of abiraterone and enzalutamide in prostate cancer. Clin Genitourin Cancer. 2018;16(3):e645–e653. doi: 10.1016/j.clgc.2017.12.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.