Abstract
A 54-year-old woman presented with an anterior neck subcutaneous tumor that had appeared one month prior. Mild tenderness was noted. As a diagnosis was difficult to make based on physical examination and ultrasonography, a magnetic resonance imaging (MRI) scan was performed. Both examinations revealed a 1-cm subcutaneous mass with well-defined margins; the MRI scan was hypointense on T1-weighted images and slightly hyperintense with low point foci on T2-weighted images. Subsequently, an excisional biopsy was performed, and the pathologic diagnosis of glomus tumor was obtained. Glomus tumors usually present as a painful subcutaneous mass beneath the nail bed but may be painless or occur in areas other than the fingers. Because glomus tumors in the neck resemble a variety of diseases, their diagnosis may be delayed. This case highlights the importance of considering glomus tumors as a potential cause of neck subcutaneous tumors.
Keywords: glomus tumor, anterior neck, subcutaneous tumor, diagnosis, tenderness
Background
A glomus tumor is a rare benign soft tissue neoplasm that arises from the glomus bodies in the skin and mucous membranes, typically affecting the distal portion of the digits and causing considerable pain and tenderness. Its occurrence in the anterior neck is extremely uncommon. Herein, we report our experience with a case of glomus tumors arising in the anterior neck, representing a rare manifestation of this tumor.
Case Report
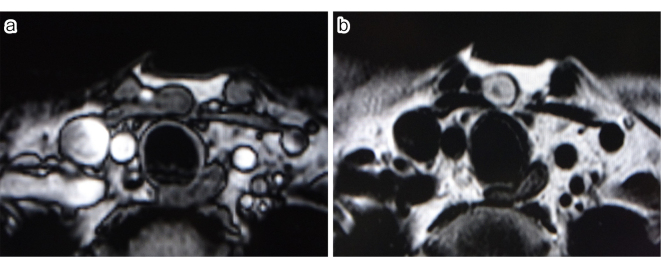
We present the case of a 54-year-old female patient who presented to our hospital with a small subcutaneous mass in the anterior neck that had been progressively enlarging for one month. The patient had no significant past medical or family history. Physical examination revealed an elastic and soft subcutaneous nodular lesion located in the inferior region of the median anterior neck, with no adhesion to the skin but with adhesion to the underlying tissues (Figure 1). The patient complained of mild point tenderness, but no spontaneous pain or cold sensitivity. Ultrasound examination revealed a well-defined solitary subcutaneous mass, measuring 10 mm × 7 mm × 11 mm, which was borderline hypoechoic and located slightly caudal to the thyroid gland. No blood flow was noted. MRI showed a 1-cm subcutaneous nodule with well-defined margins, which was hypointense on T1-weighted images and slightly hyperintense with hypointense foci on T2-weighted images (Figure 2a and b). An excisional biopsy was performed under local anesthesia because a neurogenic tumor was suspected and the patient desired treatment. The tumor was excised through a transverse incision in the neck. The tumor margins were clearly defined, allowing for easy dissection and resection from surrounding tissue. The excised specimen was a slightly erythematous solid mass measuring 15 mm × 16 mm × 7 mm with well-defined margins.
Figure 1.
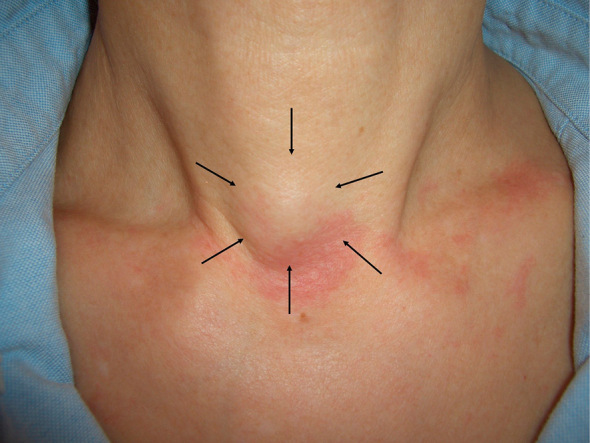
Findings at the initial examination: A nodular lesion of subcutaneous fat is seen in the lower part of the midline anterior neck.
Figure 2.
(a) T1-weighted image.
(b) T2-weighted image.
MRI findings: A 1-cm subcutaneous nodule was observed with well-defined margins in the anterior neck. It was hypointense on T1-weighted images and slightly hyperintense overall with hypointense foci on T2-weighted images.
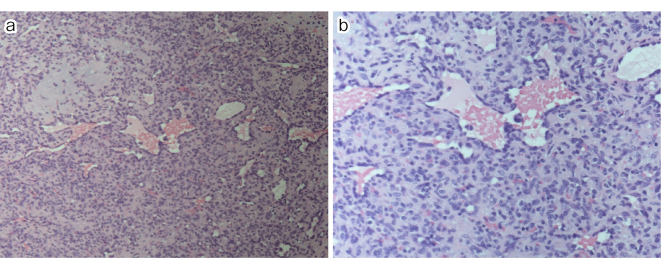
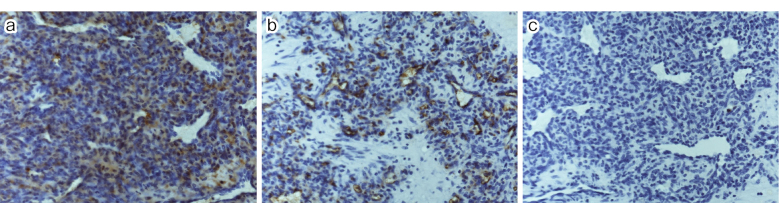
Histopathological examination revealed histologically enlarged cells and scattered vascular cavities of various sizes, which were surrounded by a layer of endothelial cells with minimal cytologic atypia. The surrounding tissue exhibited either an edematous, broad stroma with scattered spindle cells or a wide area with densely arranged spindle cells (Figure 3a and b). Immunostaining revealed perivascular spindle cells that were positive for alpha-smooth muscle actin (αSMA) but negative for CD34 and S100 (Figure 4a, b and c). Given the minimal cytologic atypia observed in these cells, the tumor was definitively diagnosed as a glomus tumor.
Figure 3.
(a) HE ×10.
(b) HE ×20.
Histopathologic findings: There were scattered vascular cavities of various sizes, hyperplasia surrounded by endothelial cell layers, and little cytologic atypia. Surrounding the hyperplasia was either a wide edematous stroma with scattered spindle-shaped cells or a large area of densely arranged spindle-shaped cells.
Figure 4.
(a) αSMA ×10.
(b) CD34 ×10.
(c) S100 ×10.
Immunostaining findings: Perivascular spindle-shaped cells were positive for αSMA and negative for CD34 and S100.
The postoperative course was uneventful, and no recurrence had been observed after one year (Figure 5).
Figure 5.
Postoperative findings at one year: No recurrence.
Discussion
Our report presents a rare case of a glomus tumor located in the anterior neck region, which is an atypical location for this type of tumor. Typically, glomus tumors arise from the glomus bodies in the fingers, where they account for 50% of cases1). The occurrence of glomus tumors in locations other than the hands or fingers is approximately 25%2). Glomus tumors have been reported in various regions of the body, such as the intestinal tract, bronchi, and penis3-5).
Glomus tumors can be classified into three categories based on their histopathological features, which include variations in the constituent glomus cells, capillaries, and smooth muscle cells6). These categories are solid glomus tumor, glomangioma, and glomangiomyoma. In our specific case, the typical histopathological findings upon hematoxylin and eosin (H&E) staining revealed a glomus tumor, and immunostaining showed positivity for the smooth muscle marker αSMA. However, markers for vascular endothelial cells (CD34) and neuroectoderm (S100 protein) were negative. Based on these findings, this case was classified as a solid glomus tumor.
To the best of our knowledge, glomus tumors originating in the neck are uncommon, and there is a limited amount of published literature on this topic7,8). Jaoude et al.8) reported 11 cases of glomus tumors, of which two were neck-onset tumors. The study highlighted the differences in diagnostic approaches required for different sites of glomus tumors. While typical glomus tumors in the fingers sometimes only required X-ray examination before surgery, neck tumors were evaluated using ultrasound, which was also the case in our patient. Al-Qattan stated that preoperative MRI is not necessary for glomus tumors that occur on the fingers if a clinical diagnosis of glomus tumor has been confirmed9). In this case, an MRI was performed because the patient had only mild tenderness and because there is a wide variety of differential diseases associated with subcutaneous masses in the neck. The most common tumor in the anterior neck, including congenital cases, is the thyroglossal duct cyst10). On ultrasound, a typical thyroglossal duct cyst appears as a smooth, well-circumscribed anechoic lesion with posterior enhancement in the anterior neck. On MRI, it consistently shows hyper-intensity on T2-weighted sequences. Signal intensity on T1-weighted images varies significantly due to differences in cystic content, with T1 hyper-intensity observed in lesions containing high proteinaceous cystic content11).
The present case involves a glomus tumor arising on the anterior neck with a pain complaint similar to those reported previously. While glomus tumors on fingertips typically cause severe pain in response to temperature or tactile stimuli12), this case is unique in that the level of pain experienced was relatively mild. There are reports that glomus tumors in the oral cavity do not cause pain, suggesting that the degree of pain may differ depending on the site of the tumor13). Glomus tumors misdiagnosed as complex regional pain syndrome have also been reported14). Considering all the above stated, the degree and nature of pain associated with glomus tumors can vary significantly between cases. Still, it is worth noting that most glomus tumors are diagnosed from the presence of local pain.
In this case, the tumor showed progressive enlarging for one month. There are many reports on the long-term course of glomus tumors, and their growth rate is generally considered to be slow. However, this case showed a rapid progression. There may be two reasons for this. One possibility is that it followed a specific and rapid growth pattern, while the other is that cases with a high growth rate may have been missed. However, these considerations cannot be fully addressed in this case alone. Further investigation and clarification will be required in future cases.
Author Contributions: N.M., performed the surgery; K.Y., and N.M., wrote the manuscript.
Conflicts of Interest: There are no conflicts of interest.
Ethical Approval: As this is a case report, publication in a paper does not require approval from the institution's ethics committee based on Japanese Guidelines.
Consent to Participate: The authors have not obtained consent to participate because a case report does not require it.
Consent for Publication: The authors have not obtained written consent because the person is not identifiable.
Acknowledgments
We thank all the medical staff involved in the diagnosis and treatment of this case.
References
- 1.Mravic M, LaChaud G, Nguyen A, et al. Clinical and histopathological diagnosis of glomus tumor: an institutional experience of 138 cases. Int J Surg Pathol. 2015 May;23(3):181-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rimington TR, Lefton CS. Simultaneous solitary glomus tumors in nonadjacent digits. Am J Orthop. 2009 May;38:E82-4. [PubMed] [Google Scholar]
- 3.Chen JH, Lin L, Liu KL, et al. Malignant glomus tumor of the intestinal ileum with multiorgan metastases: a case report and review of literature. World J Gastroenterol. 2020 Feb;26(7):770-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Zhang QP, Ji YQ, et al. Bronchial glomus tumor with calcification: a case report. World J Clin Cases. 2021 May;9(14):3320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dagur G, Warren K, Miao Y, et al. Unusual glomus tumor of the penis. Curr Urol. 2016 Oct;9(3):113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calduch L, Monteagudo C, Martinez-Ruiz E, et al. Familial generalized multiple glomangiomyoma: report of a new family, with immunohistochemical and ultrastructural studies and review of the literature. Pediatr Dermatol. 2002 Sep;19(5):402-8. [DOI] [PubMed] [Google Scholar]
- 7.Morales Olavarria C, Carnevale C, Sarria-Echegaray P, et al. Neck glomangiomyoma: a case report and literature review. Mol Clin Oncol. 2022 Nov;17(5):154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abou Jaoude JF, Roula Farah A, Sargi Z, et al. Glomus tumors: report on eleven cases and a review of the literature. Chir Main. 2000 Sep;19(4):243-52. [DOI] [PubMed] [Google Scholar]
- 9.Al-Qattan MM, Al-Namla A, Al-Thunayan A, et al. Magnetic resonance imaging in the diagnosis of glomus tumours of the hand. J Hand Surg Br. 2005 Oct;30(5):535-40. [DOI] [PubMed] [Google Scholar]
- 10.Allard R. The thyroglossal cyst. Head Neck Surg. 1982 Nov;5(2):134-46. [DOI] [PubMed] [Google Scholar]
- 11.Mittal MK, Malik A, Sureka B, et al. Cystic masses of neck: a pictorial review. Indian J Radiol Imaging. 2012 Oct;22(4):334-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamble P, Ariwala D, Mohanty SS. The glomus tumor of finger - a case series. J Orthop Case Rep. 2023 Mar;13(3):17-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanchez-Romero C, Oliveira MEP, Castro JFL, et al. Glomus tumor of the oral cavity: report of a rare case and literature review. Braz Dent J. 2019 Apr;30:185-90. [DOI] [PubMed] [Google Scholar]
- 14.Ajala RT, Lyon KA, Lyon PR, et al. Extradigital glomus tumor mimics an intrinsic nerve tumor in a trauma patient: case report and literature review. Cureus. 2021 Nov;13(11):e19256. [DOI] [PMC free article] [PubMed] [Google Scholar]