Abstract
Objectives:
The number and quality of endothelial progenitor cells decrease in patients with connective tissue diseases. This limits the efficacy of mononuclear cell therapy for ischemic ulcers associated with connective tissue diseases. To overcome these problems, we developed a serum-free quality and quantity control culture method that potentially improves the function of endothelial progenitor cells and expands their numbers. Here, we show the effect of quality and quantity control culture on mononuclear cells from patients with connective tissue diseases.
Methods:
Peripheral blood mononuclear cells were isolated from C57BL/6JJmsSlc-lpr/lpr mice with systemic lupus erythematosus, patients with connective tissue diseases, and healthy volunteers. Mononuclear cells were cultured using the quality and quantity control culture method, and the number of endothelial progenitor cells was analyzed using flow cytometry, an endothelial progenitor cell culture assay, and an endothelial progenitor cell colony-forming assay. Flow cytometry was also used to examine mononuclear cell subpopulations. A human umbilical vein endothelial cell tube-forming assay was used to examine the function of quality and quantity control cultured mononuclear cells.
Results:
Mice with systemic lupus erythematosus showed a significantly lower number of endothelial progenitor cells, which increased to the same levels as those of the control mice after quality and quantity control culture. In humans, the numbers of endothelial progenitor cells and M2 macrophages were significantly increased and the number of proinflammatory cells was decreased after quality and quantity control culture in both healthy volunteers and patients with connective tissue diseases. The human umbilical vein endothelial cell tube formation assay showed higher angiogenic potential in quality and quantity control cultured mononuclear cells from patients with connective tissue diseases than that in quality and quantity control cultured mononuclear cells from healthy controls.
Conclusions:
Our study suggests that the quality and quantity control culture method is effective in recovering the angiogenic ability of mononuclear cells from patients with connective tissue diseases.
Keywords: connective tissue disease, peripheral blood mononuclear cell, cell therapy, MNC-QQ cell, vasculogenesis
Introduction
Autologous mononuclear cells (MNCs) have been used for clinical vascular regenerative therapy since the discovery of endothelial progenitor cells (EPCs) by Asahara et al. in 19971,2). EPCs can differentiate into the endothelium of blood vessels. EPCs are one of the components of the MNCs in bone marrow (BM) and peripheral blood (PB), which are distinguished by the expression of CD34 and CD133. For regenerative cell therapy using EPCs, MNCs have been harvested from BM or PB treated with granulocyte-colony stimulating factor (G-CSF). Autologous BM cell therapy and G-CSF-mobilized PB CD34+ cell therapy have been shown to be safe and clinically effective in ischemic diseases2,3). However, autologous EPC therapy is limited for some diseases, such as diabetes and connective tissue diseases (CTDs), because of the lack of the number and angiogenic function of EPCs4-6).
To overcome these problems, a novel technique to promote the angiogenic function of EPCs and to expand their number, which is called serum-free quality and quantity control culture (QQc) method, was developed7). We also reported that the population of EPCs significantly increased in PBMNCs from patients with diabetes and their vasculogenic capability substantially improved through QQc. Moreover, MNC-QQ cells from patients with diabetes promoted wound healing in mice with diabetes8). Phase I and II clinical trials to investigate the safety and efficacy of autologous MNC-QQ cell therapy in patients with diabetes and chronic nonhealing ischemic extremity wounds were conducted from 2014 to 2017. PB (200 mL) was collected from the patients, and 2 × 107 MNC-QQ cells were injected into the area surrounding the ulcer. No death and other serious adverse events were observed during the 12-week follow-up after transplantation. In all 10 cases, vascular perfusion and skin perfusion pressure were increased and pain intensity was decreased9).
Autoimmune diseases and vasculitis account for approximately 20%-23% of refractory chronic wounds10). Because the number of EPCs is decreased and their angiogenic capability is impaired in patients with CTD and in patients with diabetes, QQc could be helpful as an effective cell therapy for limb salvage. Here, we compared the angiogenic potential of MNC-QQ cells from patients with CTD with that of MNC-QQ cells from healthy controls via an in vitro study.
Methods
1 Participants
This study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee and review board of Juntendo University. All participants provided informed consent. Patients with CTD were selected from the outpatient department. As the controls, healthy volunteers were recruited (Table 1).
Table 1.
Summary of the Profiles of the Patients with CTD and Healthy Subjects.
| Patients with CTD | Healthy subjects | |
|---|---|---|
| N | 15 | 16 |
| Age (years) | 57.3 ± 12.7 | 46.7 ± 13.5* |
| BMI | 23.0 ± 5.7 | 20.2 ± 3.1 |
| CTD duration (years) | 19.9 ± 13.6 | N/A |
| Diagnosis | ||
| Rheumatoid arthritis | 8 | N/A |
| Systemic lupus erythematosus | 3 | N/A |
| Systemic sclerosis | 3 | N/A |
| Other CTDs | 2 | N/A |
| Diabetes mellitus | 4 | N/A |
| CRP | 0.84 ± 0.93 | N/A |
Notes: Values are expressed as mean ± SD. *p < 0.05, patients with CTD vs. healthy subjects.
BMI, body mass index; CTD, connective tissue disease; N/A, not applicable
2 Collection of PBMNCs
PB samples were collected from healthy volunteers and patients with CTD, including systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and systemic sclerosis (SSc), using BD Vacutainer cell preparation tubes (CPTs; BD, Franklin Lakes, NJ, USA). PBMNCs were isolated according to the manufacturer's protocol. Briefly, whole blood in BD Vacutainer CPTs was centrifuged at 1800 g for 20 min and MNC layers were collected into 50-mL tubes. After washing with ethylenediaminetetraacetic acid-phosphate-buffered saline (EDTA-PBS), cells were treated with ammonium chloride-potassium lysis buffer (Gibco, Thermo Fisher Scientific, USA) to lyse the remaining red blood cells. After washing twice with EDTA-PBS, the cells were suspended in PBS.
3 Ex vivo expansion culture for MNC-QQ cells
PBMNCs were processed in an ex vivo serum-free expansion culture system named QQc as previously described7). PBMNCs were seeded at a density of 2 × 106 cells/2 mL/well in six-well Primaria plates (Corning Inc., NY, USA) with Stemline II medium (Sigma-Aldrich, St. Louis, MO, USA) supplemented with recombinant human (rh) Fms-related tyrosine kinase-3 ligand (rh FLT-3L, 100 ng/mL), rh vascular endothelial growth factor (rhVEGF, 50 ng/mL), rh thrombopoietin (rhTPO, 20 ng/mL), rh stem cell factor (rhSCF, 100 ng/mL), rh interleukin-6 (rhIL-6, 20 ng/mL) (all from Pepro Tech, Rocky Hill, NJ, USA), and penicillin-streptomycin (Invitrogen) and were cultured for 7 days at 37°C in a 5% carbon dioxide (CO2) atmosphere. After 7 days of not changing the medium, the MNC-QQ cells were harvested and washed with EDTA-PBS.
4 Flow cytometry
PBMNCs and MNC-QQ cells in flow cytometry staining (FACS) buffer (EDTA-PBS with 2% fetal bovine serum [FBS]) were treated with the FcR blocking reagent (Miltenyi Biotec, Auburn, CA, USA) and stained with specific antibodies as follows: Fluorescein isothiocyanate (FITC) antihuman CD19 (clone: HIB19, BioLegend, San Diego, CA, USA), PerCP/Cy5.5 antihuman CCR2 (CD192, clone: K036C2, BioLegend), BV421 antihuman CD56 (NCAM, clone: HCD56, BioLegend), PE antihuman CD34 (clone: 581, BioLegend), PE/Cy7 antihuman CD206 (MMR, clone: 15-2, BioLegend), APC antihuman CXCR4 (CD184, clone: 12G5, BioLegend), Alexa Fluor-700 antihuman CD3 (clone: UCHT1, BioLegend), APC/Cy7 antihuman CD14 (clone: HCD14, BioLegend), FITC antihuman CD4 (clone: RPA-T4, BioLegend), PerCP/Cy5.5 antihuman CD25 (clone: M-A251, BioLegend), BV421 antihuman CD127 (IL-7Rα, clone: A019D5, BioLegend), PE antihuman CD133/1 (clone: AC133, Miltenyi Biotec), PE/Cy7 antihuman CD31 (clone: WM59, BioLegend), APC/Cy7 antihuman CD8a (clone: HIT8a, BioLegend), and appropriate isotype controls. After 30 min of incubation at 4°C and washing with FACS buffer, the cells were analyzed using a BD LSRFortessa Flow Cytometer (BD Biosciences) and the FlowJo software.
5 Tube formation assay
Human umbilical vein endothelial cells (HUVECs; Lonza, Basel, Switzerland) were cultured in microvascular endothelial cell growth medium-2 (EGM-2 MV, Lonza) according to the manufacturer's protocol, and the medium was replaced with endothelial cell growth basal medium (EBM-2, Lonza) for 1 h. PBMNCs and MNC-QQ cells were labeled with Alexa Fluor-488 acetylated low-density lipoprotein (Ac-LDL, Invitrogen, Thermo Fisher Scientific) at 37°C for 1 h. HUVECs (2 × 104) and PBMNCs (4 × 103) or MNC-QQ cells (4 × 103) were mixed in 200 μL of PBS(−) and seeded in 24-well plates precoated with 200 μL/well Matrigel matrix (Corning Inc.). The plates were incubated for 2 h at 37°C in a 5% CO2 atmosphere before being photographed using an Olympus IX83 microscope (Tokyo, Japan). The number of closed circles was counted and normalized to that of the controls, which contained only HUVECs.
6 EPC culture assay
The EPC culture assay was performed as described previously8). PBMNCs and MNC-QQ cells were seeded at 1 × 105 cells/well in human fibronectin-coated 96-well plates with EGM-2 MV medium (Lonza) and were cultured at 37°C with 5% CO2 for 7 days. After 7 days, the attached cells were stained with Alexa Fluor-488 Ac-LDL (Invitrogen) and rhodamine-labeled Ulex europaeus agglutinin I (UEA-I, Vector Lab., Burlingame, CA, USA) for 4 h at 37°C in 5% CO2. After fixation with 4% paraformaldehyde phosphate-buffered solution (PFA; Wako, Osaka, Japan) at 4°C for 30 min, the cells were mounted using Vectashield (Vector Lab.) with 4', 6-diamidino-2-phenylindole. Three to five fields in each well were photographed using a fluorescence microscope (BZ-X710, Keyence, Osaka, Japan), and Alexa Fluor-488 Ac-LDL and rhodamine UEA-I double-positive cells were counted as early EPCs.
7 EPC colony-forming assay
An EPC-colony-forming assay (EPC-CFA) semisolid culture medium using MethoCult SF H4236 (Stem Cell Tech.) supplemented with 30% FBS (CCB, Nichirei Biosci., Tokyo, Japan), rhEGF (50 ng/mL), rhVEGF (50 ng/mL), rhSCF (100 ng/mL), rhIGF-1 (50 ng/mL), rhFGF-2 (50 ng/mL), rhIL-3 (20 ng/mL) (all chemicals obtained from Pepro Tech.), heparin (2 IU/mL) (Ajinomoto, Tokyo, Japan), and penicillin-streptomycin was diluted in 30% FBS-Iscove's Modified Dulbecco's Medium. MNCs or MNC-QQ cells were mixed with the medium and were seeded using blunt-end 18 G needles (Nipro, Osaka, Japan) in a 35-mm Primaria culture dish (2 × 105 cells/1 mL/dish). After 2 weeks of culture, the number of adherent colonies per dish was measured using a phase-contrast light microscope (Nikon, Tokyo, Japan). EPCs were classified as either definitive EPC colony-forming units (dEPC-CFUs) or primitive EPC (pEPC)-CFUs, as previously described7).
8 Isolation and culture of MNCs from mice with SLE
C57BL/6J and C57BL/6JJmsSlc-lpr/lpr male mice aged 10 weeks were purchased from Japan SLC Inc. All animal experiments were approved by the institutional guidelines of the Care and Use Committee of Juntendo University, School of Medicine, and followed the recommendations of the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. PBMNCs were isolated by density gradient centrifugation using Histopaque-1083 (Sigma-Aldrich). MNCs were cultured for 5 days at 37°C in a 5% CO2 atmosphere in Stemline II medium supplemented with 100 ng/mL mouse FLT-3L, 50 ng/mL mouse VEGF, 20 ng/mL mouse TPO, 100 ng/mL mouse SCF, 20 ng/mL mouse IL-6 (Pepro Tech), and penicillin-streptomycin. For flow cytometry analysis, the PBMNCs were incubated with anti-Sca-1 and anti-c-Kit antibodies for 20 min. Sca-1+/c-Kit+ cells, presumably representing circulating EPCs, were evaluated using LSRFortessa (BD). The vasculogenic potential of the PBMNCs was assessed using EPC-CFA5,7). A total of 2 × 105 PBMNCs per dish were seeded into a 35-mm Primaria tissue culture dish (BD Falcon). After 7 days, the EPC-CFUs were counted.
9 Statistics
The Mann-Whitney test (a nonpaired nonparametric test) was performed to compare the healthy and CTD groups. The Wilcoxon matched pairs signed-rank test (a paired nonparametric test) was performed to compare the pre- and post-QQc groups. All analyses were performed using the GraphPad Prism 8 software. Mean ± standard deviation was used to represent all data.
Results
1 Numbers of EPCs in PB from healthy mice and mice with SLE
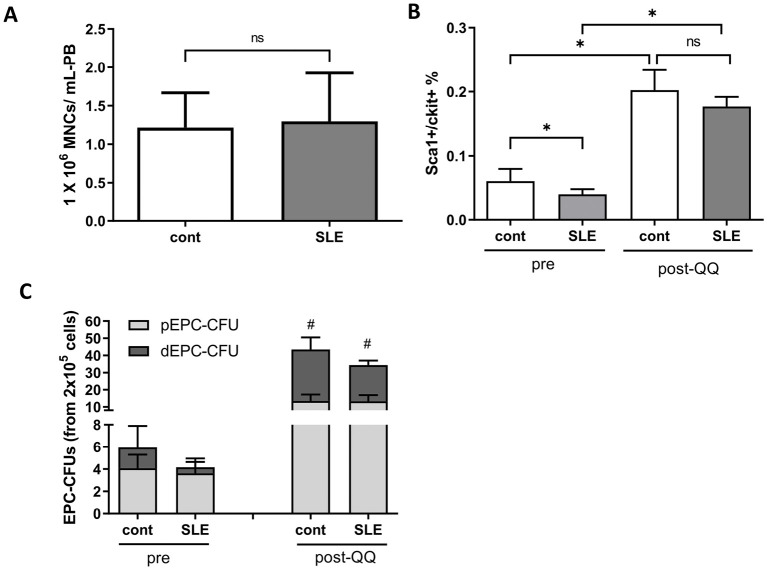
There was no difference in the number of MNCs collected from PB between healthy mice and mice with SLE (1.32 ± 0.64 × 106 cells vs. 1.37 ± 0.76 × 106 cells, p = 0.93) (Figure 1A). PBMNCs from healthy mice included more circulating EPCs (Sca-1+/c-Kit+ cells) (0.06% ± 0.019% vs. 0.04% ± 0.009%, p < 0.05) than those from mice with SLE. Following QQc, the numbers of EPCs were significantly increased and there was no difference between healthy mice and mice with SLE (0.20% ± 0.03% vs. 0.18% ± 0.01%, p = 0.20) (Figure 1B). Pre-QQc cells from healthy mice contained slightly more dEPC-CFUs than those from mice with SLE (1.87 ± 1.94 counts per well vs. 0.56 ± 0.47 counts per well, p = 0.077). After QQc, the number of dEPC-CFUs was significantly increased in both healthy mice and mice with SLE. The numbers of dEPC-CFUs derived from post-QQc cells from healthy mice and mice with SLE were not significantly different (30.0 ± 7.00 counts per well vs. 21.0 ± 2.65 counts per well, p = 0.10) (Figure 1C). These results suggest that the reduced number of EPCs in mice with SLE was recovered by QQc.
Figure 1.
Depletion of EPCs in C57BL/6JJmsSlc-lpr/lpr (SLE) mice PBMNCs was recovered by QQ culture.
(A) The number of PBMNCs in control mice and mice with SLE (n = 12). (B) Flow cytometry analysis detected EPCs that were double positive for Sca-1 and c-kit in control mice and mice with SLE pre- and post-QQc PBMNCs (pre-QQ, n = 6; post-QQ, n = 3). (C) The number of pEPC and dEPC colonies in control mice and mice with SLE pre- and post-QQc PBMNCs (pre-QQ, n = 11; post-QQ, n = 3).
*p < 0.05, #p < 0.05 versus pre-MNCs.
ns, not significant; EPCs, endothelial progenitor cells; pEPC, primitive EPC; dEPC, definitive EPC; SLE, systemic lupus erythematosus; PBMNCs, peripheral blood mononuclear cells
2 Significant increase in the number of EPCs after QQc
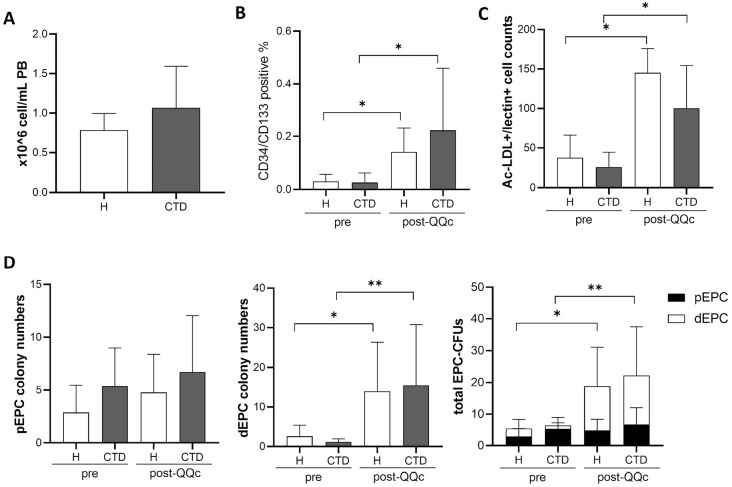
The number of MNCs in the PB did not differ significantly between healthy controls and patients with CTD (0.78 ± 0.22 × 106 cell/mL vs. 1.07 ± 0.53 × 106 cells/mL, p = 0.25) (Figure 2A). The number of EPCs was analyzed using flow cytometry. The percentages of CD34 and CD133 double-positive cells, presumably representing EPCs, were significantly increased in MNCs from both healthy controls and patients with CTD after QQc compared to before QQc (0.03% ± 0.03% vs. 0.14% ± 0.09%, p < 0.05; 0.03% ± 0.04% vs. 0.22% ± 0.24%, p < 0.05, respectively), whereas there was no difference in the percentage of those cells between healthy controls and patients with CTD both before and after QQc (Figure 2B). Concordant with the FACS results, the EPC culture assay results showed that the numbers of Ac-LDL+/lectin+ cells, representing early EPCs, derived from MNCs were significantly increased in both healthy controls and patients with CTD after QQc (37.66 ± 28.38 cells vs. 145.3 ± 30.32 cells, p < 0.05; 25.96 ± 18.59 cells vs. 100.3 ± 53.9 cells, p < 0.05, respectively), whereas there was no difference in their numbers between healthy controls and patients with CTD (Figure 2C). Moreover, QQc significantly increased the total EPC-CFUs and dEPC-CFUs that were derived from the MNCs from patients with CTD and each of those from healthy controls (Figure 2D).
Figure 2.
Number of EPCs in PBMNCs was increased by QQ culture in patients with connective tissue disease.
(A) The number of PBMNCs was counted in healthy controls and patients with CTD (healthy, n = 5; CTD, n = 12). (B) Flow cytometry analysis detected EPCs that were double positive for CD34 and CD133 in healthy and CTD pre- and post-QQc PBMNCs (healthy, n = 7; CTD, n = 12). (C) The EPCs stained with Ac-LDL and lectin were counted after the EPC culture was counted (n = 6). (D) The number of pEPC and dEPC colonies in pre- and post-QQc PBMNCs from healthy controls and patients with CTD (healthy, n = 6; CTD, n = 9).
*p < 0.05, **p < 0.01. PBMNCs, peripheral blood mononuclear cells; QQc, serum-free quality and quantity control culture; H, healthy subjects; CTD, connective tissue disease; pEPC, primitive EPC; dEPC, definitive EPC; Ac-LDL, acetylated low-density lipoprotein
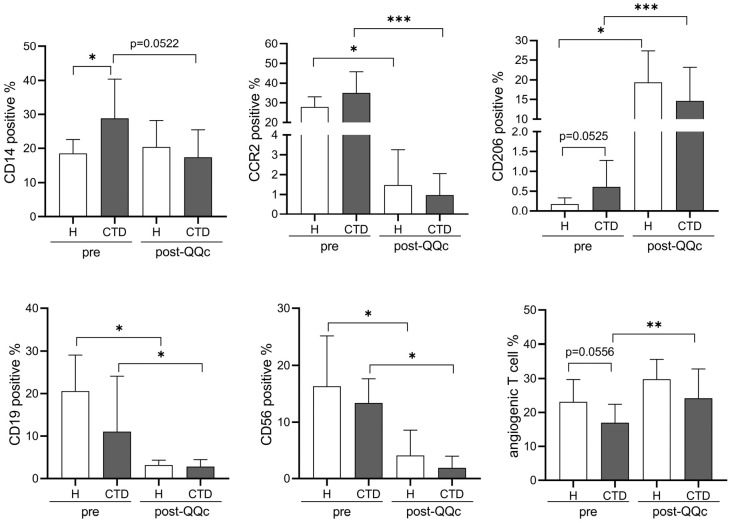
3 Cell populations change toward anti-inflammation after QQc in healthy controls and patients with CTD
We performed FACS analysis to examine the effects of QQc on cell populations involved in inflammation. The percentage of CD14-positive monocytes or macrophages was significantly higher in MNCs from patients with CTD than in those from healthy controls. This difference was lost because of the slight decrease in the percentage of CD14-positive cells in MNCs from patients with CTD after QQc, whereas there was no change in that from healthy controls. Among the macrophages, the CD206-positive M2 macrophage population was larger in MNCs from patients with CTD than in those from healthy controls, although the difference was not significant. The percentages of CD206-positive cells were significantly increased both in MNCs from healthy controls and in those from patients with CTD after QQc. The percentages of proinflammatory cells, such as CCR2-positive M1 macrophages, CD19-positive B cells, and CD56-positive natural killer (NK) cells, were significantly decreased both in MNCs from healthy controls and in those from patients with CTD after QQc, whereas there were no differences between those from healthy controls and patients with CTD. Although the percentage of angiogenic T cells in MNCs from patients with CTD was likely to be lower than that in MNCs from healthy controls, QQc significantly expanded the population of angiogenic T cells from patients with CTD (Figure 3).
Figure 3.
Flow cytometry analysis of the PBMNC subpopulation after QQc in healthy controls and patients with CTD.
The percentages of CD14+, CCR2+, CD206+ macrophage markers, CD19+ B cell markers, CD56+ NK cell markers, and angiogenic T cell markers in PBMNCs and MNC-QQ cells were analyzed by flow cytometry (healthy, n = 7; CTD, n = 12).
*p < 0.05, **p < 0.01, ***p < 0.001. H, healthy subjects; CTD, patients with connective tissue disease; PBMNCs, peripheral blood mononuclear cells; QQc, serum-free quality and quantity control culture; NK, natural killer
4 Significant improvement in angiogenic potential after QQc in patients with CTD
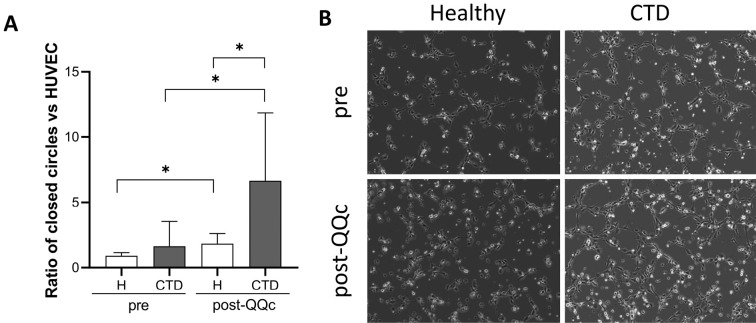
Next, we examined the angiogenic potential of MNC-QQ cells from patients with CTD and healthy controls using the HUVEC tube formation assay. There was no significant difference in tube formation between healthy MNCs and CTD MNCs cocultured with HUVECs for 2 h. QQc significantly promoted the tube formation capacity of MNCs from patients with CTD compared with that from healthy controls (1.84 ± 0.77-fold vs. 6.66 ± 5.2-fold upon HUVEC only, p < 0.05) (Figure 4).
Figure 4.
Improvement in angiogenic ability after QQc in CTD PBMNCs was analyzed using the HUVEC tube formation assay.
(A) The number of closed-circle formations on the Matrigel was counted. The graph shows the ratio of the combination of MNCs, MNC-QQ cells, and HUVECs to the HUVEC-only control group (n = 6). (B) Representative images of tube formation.
*p < 0.05. H, healthy; CTD, connective tissue disease; PBMNCs, peripheral blood mononuclear cells; QQc, serum-free quality and quantity control culture; HUVEC, human umbilical vein endothelial cell
Discussion
Chronic refractory ulcers often occur in CTDs such as RA, SSc, and SLE. Impaired wound regeneration is mainly caused by poor vasculogenesis, which partly results from the dysfunction of EPCs6,11). In this study, we showed the effect of the QQc method on PBMNCs from patients with CTD. In both mice and humans, after QQc, the percentages of the EPC population both in MNCs from healthy controls and in those from mice with SLE/patients with CTD were significantly increased. Among the total EPC population, the percentages of the definitive EPC-CFUs, which represent mature EPCs and have high vasculogenic potential, were increased after QQc. Consistent with the EPC-CFU assay, in vitro tube formation assays showed the increased angiogenic ability of both MNC-QQ cells from healthy controls and those from patients with CTD conditions compared with those of pre-QQc MNCs.
In mice with SLE, the number and angiogenic function of EPCs in PB were decreased, in agreement with a previous report11). However, there was no significant difference in the number of EPCs in human healthy controls and patients with CTD, although many studies have reported a decrease in the number of EPCs in patients with CTD6,11,12). One reason for this inconsistency would be that we included patients with CTD with a variety of disease levels and medications in this study. Grisar et al.13) reported the effects of medication and activity on circulating angiogenic cells in patients with RA. They found that the number of EPCs was much lower in patients with active RA than in those with inactive RA or those receiving antitumor necrosis factor therapy13,14). Thus, the EPC-expanding effects of QQc on patients in the active stage of CTD remain to be seen in the future.
Our results showed a tendency for a reduction in angiogenic T cells in CTD MNCs compared with healthy MNCs, whereas no significant difference in EPC percentage was found between them. Similar to EPCs, the number of angiogenic T cells is also known to be decreased in patients with RA15). Angiogenic T cells are a subset of T cells that enhance endothelial function by cooperating with EPCs. The decrease in the number of angiogenic T cells and higher levels of IFN alpha in patients with CTD were reported to be associated with the occurrence of cardiovascular events15), and this association might be mediated by an impaired endothelial repair mechanism. QQc increased the percentage of angiogenic T cells in MNCs from patients with CTD to a level similar to that observed in those from healthy controls. These results also show the promising applicability of potentially angiogenic MNC-QQ cells harvested from patients with CTD.
M2 macrophages are anti-inflammatory macrophages that are important in wound healing. We showed that the number of CD206-positive cells, which represent M2 macrophages, was slightly increased in PBMNCs from patients with CTD than in those from healthy controls. Some reports have demonstrated an increase in the number of CD14-positive monocytes/macrophages and M2 macrophages in patients with CTD16-18). The number of M2 macrophages was significantly increased after QQc, whereas the numbers of immune system cells, CD56-positive NK cells, CD19-positive B cells, and proinflammatory CCR2-positive M1 macrophages were decreased. These results suggest that QQc could also contribute to the creation of an anti-inflammatory environment in MNC transplantation therapy.
In this study, we only performed an in vitro analysis of MNC-QQ cells obtained from patients with CTD. The wound healing and angiogenic effects of MNC-QQ cells from healthy mice and mice with diabetes mellitus in vitro and in vivo were previously reported7,8,19). Recently, phase I and IIa clinical trials were conducted to investigate the safety and efficacy of autologous MNC-QQ cell therapy in patients with chronic nonhealing ischemic extremity wounds9). In these trials, two patients with SSc were included and MNC-QQ cell transplantation improved vascular perfusion and pain in patients with SSc as effectively as it did in other patients with diabetes mellitus. Thus, we believe that MNC-QQ cell therapy serves as a noninvasive and effective therapy for collagen tissue diseases with refractory chronic ulcers.
This study had some limitations. First, the number of participants with CTD was small and the study included patients with various diseases, such as RA and SLE. As far as we could obtain information, most patients were well controlled with medication. Thus, further analysis with a larger number of participants is required to determine the effects of QQc methods on each type and pathological stage of CTDs. Second, we only performed in vitro analyses. Although we confirmed that there was no difference in the cell population and the angiogenic potential of MNC-QQ cells between healthy controls and patients with CTD, the in vivo function of MNC-QQ cells in CTD model mice should be examined in more detail in the future.
Author Contributions: R.T. and S. Furukawa designed the study; S. Furukawa, R.H., and A.S. performed the experiments and analyzed the data; R.T. and S. Fujimura supervised the experiment; S. Furukawa wrote the manuscript.
Conflicts of Interest: Rica Tanaka was the Chief Executive Officer of ReEir, Inc.
Ethical Approval: This study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee and review board of Juntendo University (approval number: M12-0902). All animal experiments followed the recommendations of the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and followed the institutional guidelines of the Care and Use Committee of Juntendo University, School of Medicine (approval number: 1052).
Consent to Participate: All participants provided their written informed consent to participate in this study.
Consent for Publication: Written informed consent for the publication of the information regarding the participants was obtained.
Acknowledgments
We would like to thank the Laboratory of Cell Biology, Biomedical Research Core Facilities, Juntendo University Graduate School of Medicine, for the technical assistance. We would like to thank Editage (www.editage.com) and Caroline Fedor for English language editing.
Funding Statement
Funding: This study was supported by the National Institutes of Biomedical Innovation, Health and Nutrition Regenerative Medicine Development Support Program. This study was also supported by JSPS KAKENHI (Grant Number 19H03816 and 15K20326).
References
- 1.Asahara T, Murohara T, Sullivan, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997 Feb;275(5302):964-7. [DOI] [PubMed] [Google Scholar]
- 2.Qadura M, Terenzi DC, Verma S, et al. Concise review: cell therapy for critical limb ischemia: an integrated review of preclinical and clinical studies. Stem Cells. 2018 Feb;36(2):161-71. [DOI] [PubMed] [Google Scholar]
- 3.Wahid FSA, Ismail NA, Wan Jamaludin WF, et al. Efficacy and safety of autologous cell-based therapy in patients with no-option critical limb ischaemia: a meta-analysis. Curr Stem Cell Res Ther. 2018;13(4):265-83. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka R, Wada M, Kwon SM, et al. The effects of flap ischemia on normal and diabetic progenitor cell function. Plast Reconstr Surg. 2008 Jun;121(6):1929-42. [DOI] [PubMed] [Google Scholar]
- 5.Tsukada S, Masuda H, Jung SY, et al. Impaired development and dysfunction of endothelial progenitor cells in type 2 diabetic mice. Diabetes Metab. 2017 Apr;43(2):154-62. [DOI] [PubMed] [Google Scholar]
- 6.Del Papa N, Pignataro F. The role of endothelial progenitors in the repair of vascular damage in systemic sclerosis. Front Immunol. 2018 Jun;9:1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masuda H, Tanaka R, Fujimura S, et al. Vasculogenic conditioning of peripheral blood mononuclear cells promotes endothelial progenitor cell expansion and phenotype transition of anti-inflammatory macrophage and T lymphocyte to cells with regenerative potential. J Am Heart Assoc. 2014 Jun;3(3):e000743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka R, Ito-Hirano R, Fujimura S, et al. Ex vivo conditioning of peripheral blood mononuclear cells of diabetic patients promotes vasculogenic wound healing. Stem Cells Transl Med. 2021 Jun;10(6):895-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanaka R, Fujimura S, Kado M, et al. Phase I/IIa feasibility trial of autologous quality- and quantity-cultured peripheral blood mononuclear cell therapy for non-healing extremity ulcers. Stem Cells Transl Med. 2022 Mar;11(2):146-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shanmugam VK, Angra D, Rahimi H, et al. Vasculitic and autoimmune wounds. J Vasc Surg Venous Lymphat Disord. 2017 Mar;5(2):280-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mak A, Chan JKY. Endothelial function and endothelial progenitor cells in systemic lupus erythematosus. Nat Rev Rheumatol. 2022 May;18(5):286-300. [DOI] [PubMed] [Google Scholar]
- 12.Yoshimi R, Nakajima H. Current state and issues of regenerative medicine for rheumatic diseases. Front Med (Lausanne). 2022 Jan;9:813952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grisar J, Aletaha D, Steiner CW, et al. Depletion of endothelial progenitor cells in the peripheral blood of patients with rheumatoid arthritis. Circulation. 2005 Jan;111(2):204-11. [DOI] [PubMed] [Google Scholar]
- 14.Grisar J, Aletaha D, Steiner CW, et al. Endothelial progenitor cells in active rheumatoid arthritis: effects of tumour necrosis factor and glucocorticoid therapy. Ann Rheum Dis. 2007 Oct;66(10):1284-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez-Carrio J, Alperi-Lopez M, Lopez P, et al. Angiogenic T cells are decreased in rheumatoid arthritis patients. Ann Rheum Dis. 2015 May;74(5):921-7. [DOI] [PubMed] [Google Scholar]
- 16.Higashi-Kuwata N, Jinnin M, Makino T, et al. Characterization of monocyte/macrophage subsets in the skin and peripheral blood derived from patients with systemic sclerosis. Arthritis Res Ther. 2010;12(4):R128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohamed ME, Gamal RM, El-Mokhtar MA, et al. Peripheral cells from patients with systemic sclerosis disease co-expressing M1 and M2 monocyte/macrophage surface markers: relation to the degree of skin involvement. Hum Immunol. 2021 Sep;82(9):634-9. [DOI] [PubMed] [Google Scholar]
- 18.McGarry T, Hanlon MM, Marzaioli V, et al. Rheumatoid arthritis CD14+ monocytes display metabolic and inflammatory dysfunction, a phenotype that precedes clinical manifestation of disease. Clin Transl Immunology. 2021;10(1):e1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen TN, Kado M, Hagiwara H, et al. MMP9 secreted from mononuclear cell quality and quantity culture mediates STAT3 phosphorylation and fibroblast migration in wounds. Regen Ther. 2021 Nov;18:464-71. [DOI] [PMC free article] [PubMed] [Google Scholar]