Abstract
Background
Since the mid-20th century, knee osteoarthritis (OA) has doubled in prevalence, costing more than $27 billion annually. This study aimed to compare 6 nonoperative treatment options for knee OA (cryoneurolysis with superficial nerve block, cryoneurolysis with deep nerve block, intra-articular hyaluronic acid injections, nonsteroidal inflammatory drug injections, IA corticosteroids, and IA triamcinolone extended release [IA-TA-ER]) over 4 months, analyzing their effects on sleep disturbance, a component of health-related quality of life.
Methods
All patients with knee OA and received nonoperative interventions with at least 4 months of follow-up between 2021 and 2024 were identified from the Innovations in Genicular Outcomes Research registry, a multicenter novel real-world registry. Patient demographics were gathered/analyzed, adjusting for age, sex, study site, Kellgren-Lawrence grade, baseline score of pain severity/function, pain-catastrophizing, and analgesic use in each assessment. Sleep disturbance was assessed via least-square-means relative to the average population, with multivariate linear regressions used to assess changes pretherapy/post-therapy.
Results
Patients administered IA-TA-ER had decreased sleep disturbance relative to other cohorts (least-square-means 52.3; 95% confidence interval: 50.5-54.0; P < .03). Patients receiving IA-TA-ER or IA corticosteroids achieved achieving minimum clinically important difference for sleep disturbance improvement, 63% and 57%, respectively. Pairwise comparison revealed patients receiving IA-TA-ER were 2 times more likely to achieve minimally clinically important difference for improved sleep relative to other cohorts (P < .05).
Conclusions
Extended-release triamcinolone injections are associated with decreased sleep disturbance relative to other treatments, in both degree of improvement and proportion of patients. Further studies should examine the potential beneficial effects of IA-TA-ER on other aspects of health-related quality of life.
Keywords: Sleep disturbance, Triamcinolone acetonide extended-release, Knee osteoarthritis, Real-world registry, Cryoneurolysis
Introduction
Knee osteoarthritis (OA) is the most common form of OA, affecting more than 19% of Americans aged more than 45 years [1]. Costs related to knee OA are estimated at $27 billion US dollars annually [2,3], with a lifetime individual cost of knee-OA care between $12,400 and $16,000 [3]. Patients who have knee OA can experience pain, stiffness, and an overall decreased functional status. As the population ages, and as obesity increases, the prevalence of knee OA is expected to continue to increase [1,4].
For end-stage knee OA, total knee arthroplasty (TKA) remains the gold standard for treatment unresponsive to nonoperative therapies, with ∼800,000 performed annually in the United States [5]. There remains a wide range of nonoperative treatment options to potentially avoid or delay the need for TKA. While physical therapy and over-the-counter medications remain first-line nonoperative treatments for knee OA, there are a growing number of other potential treatment options approved by the Food and Drug Administration [6]. Such modalities can be broadly divided into local intra-articular injections with variable agents available (eg, corticosteroids, hyaluronic acid, extended-release corticosteroids, and cellular-based therapies [7,8]) and denervation therapies (cryoneurolysis and radiofrequency ablation techniques) [9]. Intra-articular injections are designed to decrease synovial inflammation and pain by downregulating pro-inflammatory mediators (eg, collagenases, aggrecan) [10]. Denervation therapies are designed to block pain signals from the knee, with cryoneurolysis involving low temperature application (−20°C to −100°C) to a targeted nerve to cause temporary Wallerian degeneration and disrupt nerve function [11,12].
The goal of such interventions is to decrease pain, including sleep disturbance, improve function, and overall delay or prevent the need for a primary TKA [13]. In general, while such nonoperative treatment options may be attractive to patients who have symptomatic knee OA, evidence of their clinical impact is limited. It is imperative to appropriately assess the safety and efficacy profiles of these interventions. While randomized controlled trials serve as one potential method [14], they are often limited by resource utilization constraints, small sample sizes, and limited generalizability given specific inclusion criteria [15]. In contrast to randomized trials, retrospective administrative and claims database studies rely on records of patients treated for knee OA [16,17]. While such studies are often easier to perform and less resource-intensive, they also frequently lack patient-reported outcomes (PROs), disease severity assessments, clinical context, and documentation of adherence to treatment.
Registries based on real-world experiences provide insights beyond what administrative records or claims-based studies can gather, revealing factors that clinical trials might miss, such as the preferences of both physicians and patients and the impact of economic and insurance considerations on treatment choices [18]. Furthermore, unlike randomized controlled trials, which are limited to specific treatment protocols, real-world registries can gather a broader scope of data that better reflect the everyday management of knee OA in actual practice settings [19]. Real-world registries can also adopt prospective methodologies, which standardize data collection and incorporate PROs and other clinical and health service utilization metrics. The Innovations in Genicular Outcomes Research (iGOR) registry, for example, follows a prospective observational study format, systematically compiling clinical data, PRO evaluations, health service utilization, and reimbursement information [20]. The iGOR registry was established to comprehensively document various treatment approaches for knee OA, assessing clinical effectiveness, safety, patient-reported health-related quality of life (HRQOL), and overall health service use proactively [19].
The aim of this study was to compare and contrast 6 nonoperative treatments for knee OA over a 4-month follow-up period, analyzing their effects on sleep disturbance, an important component of HRQOL. The 6 treatments were cryoneurolysis with superficial genicular nerve block, cryoneurolysis with deep genicular nerve block, intra-articular hyaluronic acid (IA-HA) injections, IA nonsteroidal anti-inflammatory drug (IA-NSAID) injections, IA corticosteroids (IA-CS), and IA triamcinolone extended release (IA-TA-ER) injections.
Material and methods
Registry
The iGOR registry, listed on Clinicaltrials.gov with the identifier NCT05495334, is a multicenter observational database designed to track clinical and HRQOL outcomes for a period of up to 18 months following various knee OA pain treatments [20]. This registry is observational in nature, allowing treating physicians to make all clinical decisions, thereby mirroring real-life medical practice [19]. The iGOR registry captures a comprehensive array of pain management strategies ranging from oral medications such as NSAIDs and opioids to denervation techniques like radiofrequency ablation and cryo-nerve blocks, as well as intra-articular injections including corticosteroids, viscosupplements, stem cell derivatives, platelet-rich plasma, and NSAIDs. It also covers surgical interventions like arthroplasty, all targeting the management of knee OA pain. As of March 2024, participants have been recruited from 8 locations (Campbell Clinic, Cedars Sinai Medical Center, Hoag Orthopedic Institute, Ortho West, Mid State Orthopaedics, Oschner Medical Center, Sinai Hospital of Baltimore, United Rheumatology). Every participating location received approval from their respective Institutional Review Board that complies with the standards set by the International Conference on Harmonization for Good Clinical Practice, as well as the guidelines specified in Part 56 of Title 21 of the Code of Federal Regulations enforced by the US Food and Drug Administration.
Individuals who qualified for the iGOR registry encompass all patients intending to undergo treatment for knee OA, covering a range of procedures such as injections, nerve blocks, or arthroplasty, scheduled within 60 days of initial evaluation, and who are capable of providing informed consent. The exclusion criteria apply to those currently participating in a research trial that would restrict them from receiving the established standard of care, or to those anticipating a surgical procedure on a different knee. After joining the registry, patients gain access to web-based PRO tools that comply with the Health Insurance Portability and Accountability Act. They can use a mobile phone to enter their clinical and health-related outcomes, starting from the initial assessment and continuing through their follow-up visits for up to 18 months post-treatment. If patients undergo additional treatments, they are recorded in the registry for a further 18 months, starting from the new treatment episode.
Patient cohorts
Patients within the registry were identified who had symptomatic knee OA, with a 4-month follow-up, and who had undergone a nonsurgical treatment including cryoneurolysis of the deep genicular nerve, cryoneurolysis of the superficial genicular nerve, IA-HA injections, IA-NSAIDs, IA conventional corticosteroid, and IA-TA-ER. Cryoneurolysis was performed through the administration of Iovera (Pacira BioSciences, Parsippany, New Jersey), a handheld device able to apply freezing cold to peripheral nerve tissue designed to relieve pain for up to 90 days [21,22]. Specifically, cryoneurolysis is performed thorough application of a 20-gauge, 90-mm closed-end needle, enabling targeted delivery of low temperatures through Cryogen (nitrous oxide) flowing from the cartridge to the handpiece via a SmartTip (Pacira Cryotech, Inc., Fremont, California) [23].
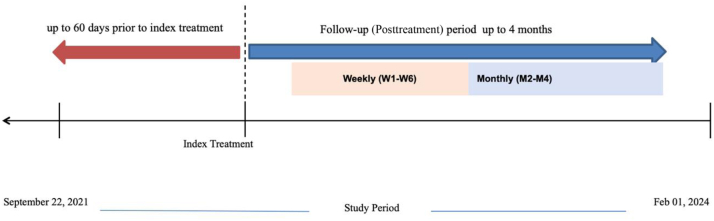
ZILRETTA (triamcinolone acetonide extended-release injectable suspension, 32 mg) was the product administered as IA-TA-ER [24]. Other formulations of IA injections (eg, hyaluronic acid, conventional corticosteroids, and ketorolac) were chosen at the providers’ discretion. A total of 481 patients between September 21, 2021 and February 1, 2024 had received an index intervention, with weekly follow-up for 6 weeks followed by monthly follow-up at 2, 3, and 4 months postintervention (Fig. 1). Baseline patient demographics were recorded, including age, sex, body mass index (BMI), medical history, surgical history, OA treatment history, employment status, physical activity level, smoking, education level, race, insurance type, baseline opioid use, baseline pain severity score, functional score, sleep disturbance score, and number of healthcare resource utilization visits.
Figure 1.
Timeline of study enrollment period.
Sleep disturbance assessment
Sleep disturbance was assessed using a Patient-Reported Outcomes Measurement Information System-Sleep Disturbance score [25]. This 8-item measurement is geared toward assessing self-reported perceptions of sleep quality, depth, and restoration associated with sleep [26]. The post-treatment Patient-Reported Outcomes Measurement Information System-Sleep Disturbance score was normalized into a T-score (range, 28.5-76) with a mean of 50 and a standard deviation (SD) of 10. A score of 50 was chosen as the average SD in the general population, with a higher overall number representing worse sleep quality.
Levels of sleep disturbance were patient-reported at weekly intervals for the first 6 weeks after index intervention, then in a monthly manner at 2, 3, and 4 months. Scores were subsequently converted to a minimally clinically important difference (MCID), a cutoff value for outcome score change, defined by iGOR patients, adjusting for age, sex, BMI, pain catastrophizing scale (PCS), Kellgren-Lawrence (KL) grade [27], and corresponding outcome measures at baseline.
Data analyses
All data analyses were performed with the assistance of Statistical Analysis Software, version 9.4 (SAS Institute, Cary, North Carolina). Descriptive statistics were performed to summarize baseline demographics between study groups of interest and measurements collected during follow-up. Multivariable generalized linear regression modeling with normal distribution was performed to assess changes in sleep disturbance preintervention and postintervention, adjusting for age, sex, BMI, PCS, KL grade, and corresponding outcome measures at baseline, and opioid use during follow-up. An MCID was generated and calculated to identify the proportion of patients achieving a clinical difference for sleep disturbance over 4 months of follow-up. Statistical significance was set at a cutoff P value of .05.
Results
Decreased sleep disturbance with use of triamcinolone extended release
All cohorts had significantly decreased sleep quality over the 4-month follow-up, defined as least-square-means (LSM) > 50, relative to the average population (P < .05) (Table 1).
Table 1.
Average follow-up adjusted sleep disturbance for 4 months.
| Treatment | Standard deviation (least squares mean) | Standard error | Lower | Upper | P value |
|---|---|---|---|---|---|
| Cryo 1 | 54.56 | 0.85 | 52.90 | 56.23 | <.0001 |
| IA-HA | 54.89 | 0.86 | 53.21 | 56.58 | <.0001 |
| IA-NSAID | 55.41 | 1.13 | 53.19 | 57.64 | <.0001 |
| IA-CS | 53.93 | 0.60 | 52.75 | 55.11 | <.0001 |
| Cryo 2 | 57.17 | 1.38 | 54.45 | 59.88 | <.0001 |
| IA-TAER | 52.26 | 0.89 | 50.52 | 54.01 | <.0001 |
Cryo_1, cryoneurolysis with superficial genicular nerve block; Cryo_2, cryoneurolysis with deep genicular nerve block; CS, corticosteroid; HA, hyaluronic acid; IA, intra-articular; NSAID, nonsteroidal anti-inflammatory drug; TAER, triamcinolone extended release.
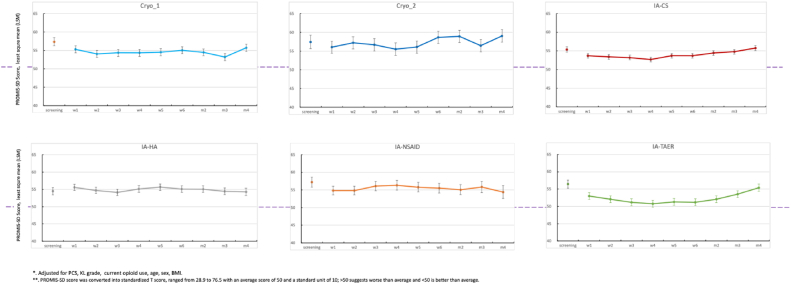
The IA-TA-ER had the least sleep disturbance (LSM = 52.3; 95% confidence interval: 50.5-54.0; P < .05) relative to all other cohorts, including IA-HA, IA-NSAID, IA-CS, and cryoneurolysis of superficial and/or deep genicular nerves (LSM > 54; 95% confidence interval: 52.8-59.9; P < .03) (Table 2). The IA-CS had significantly decreased sleep disturbance relative to cryoneurolysis with deep genicular nerve block (Fig. 2).
Table 2.
Pairwise comparison of functional scores between treatments.
| Treatment | Treatment | Standard deviation |
95% confidence interval |
P value | |
|---|---|---|---|---|---|
| Difference | Lower | Upper | |||
| Cryo 1 | IA-HA | −0.33 | −2.28 | 1.62 | .740 |
| Cryo 1 | IA-NSAID | −0.85 | −3.31 | 1.61 | .497 |
| Cryo 1 | IA-CS | 0.63 | −1.02 | 2.28 | .451 |
| Cryo 1 | Cryo 2 | −2.60 | −5.61 | 0.41 | .090 |
| Cryo 1 | IA-TAER | 2.30 | 0.31 | 4.29 | .024 |
| IA-HA | IA-NSAID | −0.52 | −2.91 | 1.87 | .669 |
| IA-HA | IA-CS | 0.96 | −0.61 | 2.53 | .229 |
| IA-HA | Cryo 2 | −2.27 | −5.26 | 0.72 | .136 |
| IA-HA | IA-TAER | 2.63 | 0.71 | 4.55 | .008 |
| IA-NSAID | IA-CS | 1.48 | −0.63 | 3.59 | .168 |
| IA-NSAID | Cryo 2 | −1.75 | −4.92 | 1.41 | .277 |
| IA-NSAID | IA-TAER | 3.15 | 0.73 | 5.57 | .011 |
| IA-CS | Cryo 2 | −3.23 | −5.97 | −0.50 | .021 |
| IA-CS | IA-TAER | 1.67 | 0.02 | 3.31 | .047 |
| Cryo 2 | IA-TAER | 4.90 | 1.88 | 7.92 | .002 |
Cryo_1, cryoneurolysis with superficial genicular nerve block; Cryo_2, cryoneurolysis with deep genicular nerve block; CS, corticosteroid; HA, hyaluronic acid; IA, intra-articular; NSAID, nonsteroidal anti-inflammatory drug; TAER, triamcinolone extended release.
Figure 2.
PROMIS sleep disturbance assessment over time following treatment. ∗Error bars represent standard error of the least square mean (LSM). Cryo_1, cryoneurolysis with superficial genicular nerve block; Cryo_2, cryoneurolysis with deep genicular nerve block; CS, corticosteroid; HA, hyaluronic acid; IA, intra-articular; NSAID, nonsteroidal anti-inflammatory drug; PROMIS, Patient-Reported Outcomes Measurement Information System; TAER, triamcinolone extended release.
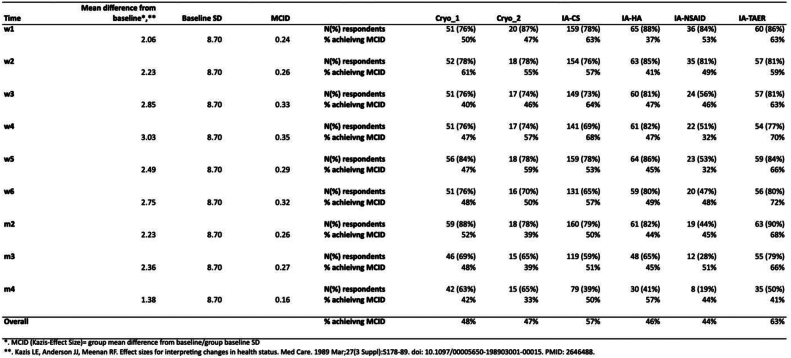
The IA-TA-ER and IA-CS were the only 2 cohorts where more than half of patients achieved MCID for sleep disturbance, 63% and 57% by the end of the study period, respectively (Table 3). Other cohorts had < 50% of patients to achieve MCID by the end of 4 months (Fig. 3). Pairwise comparisons demonstrated that patients who received IA-TA-ER were 2 times more likely to achieve MCID relative to individual cohorts (P < .05). In contrast, patients who received standard IA-CS were 1.5 times more likely than other cohorts to achieve MCID in reducing sleep disturbance (P < .05).
Table 3.
Proportion of patients achieving a minimally clinically important difference for sleep disturbance over a 4-month period.
| Time | Mean difference from baseline | Baseline SD | MCID | Number of patients | Cryo_1 | Cryo_2 | IA-CS | IA-HA | IA-NSAID | IA-TAER |
|---|---|---|---|---|---|---|---|---|---|---|
| Week 1 | N (%) respondents | 51 (76%) | 20 (87%) | 159 (78%) | 65 (88%) | 36 (84%) | 60 (86%) | |||
| 2.06 | 8.70 | 0.24 | % achieving MCID | 50% | 47% | 63% | 37% | 53% | 63% | |
| Week 2 | N (%) respondents | 52 (78%) | 18 (78%) | 154 (76%) | 63 (85%) | 35 (81%) | 57 (81%) | |||
| 2.23 | 8.70 | 0.26 | % achieving MCID | 61% | 55% | 57% | 41% | 49% | 59% | |
| Week 3 | N (%) respondents | 51 (76%) | 17 (74%) | 149 (73%) | 60 (81%) | 24 (56%) | 57 (81%) | |||
| 2.85 | 8.70 | 0.33 | % achieving MCID | 40% | 46% | 64% | 47% | 46% | 63% | |
| Week 4 | N (%) respondents | 51 (76%) | 17 (74%) | 141 (69%) | 61 (82%) | 22 (51%) | 54 (77%) | |||
| 3.03 | 8.70 | 0.35 | % achieving MCID | 47% | 57% | 68% | 47% | 32% | 70% | |
| Week 5 | N (%) respondents | 56 (84%) | 18 (78%) | 159 (78%) | 64 (86%) | 23 (53%) | 59 (84%) | |||
| 2.49 | 8.70 | 0.29 | % achieving MCID | 47% | 59% | 53% | 45% | 32% | 66% | |
| Week 6 | N (%) respondents | 51 (76%) | 16 (70%) | 131 (65%) | 59 (80%) | 20 (47%) | 56 (80%) | |||
| 2.75 | 8.70 | 0.32 | % achieving MCID | 48% | 50% | 57% | 49% | 48% | 72% | |
| Month 2 | N (%) respondents | 59 (88%) | 18 (78%) | 160 (79%) | 61 (82%) | 19 (44%) | 63 (90%) | |||
| 2.23 | 8.70 | 0.26 | % achieving MCID | 52% | 39% | 50% | 44% | 45% | 68% | |
| Month 3 | N (%) respondents | 46 (69%) | 15 (65%) | 119 (59%) | 48 (65%) | 12 (28%) | 55 (79%) | |||
| 2.36 | 8.70 | 0.27 | % achieving MCID | 48% | 39% | 51% | 45% | 51% | 66% | |
| Month 4 | N (%) respondents | 42 (63%) | 15 (65%) | 79 (39%) | 30 (41%) | 8 (19%) | 35 (50%) | |||
| 1.38 | 8.70 | 0.16 | % achieving MCID | 42% | 33% | 50% | 57% | 44% | 41% | |
| Overall | % achieving MCID | 48% | 47% | 57% | 46% | 44% | 63% |
Cryo_1, cryoneurolysis with superficial genicular nerve block; Cryo_2, cryoneurolysis with deep genicular nerve block; CS, corticosteroid; HA, hyaluronic acid; IA, intra-articular; MCID, minimally clinically important difference; NSAID, nonsteroidal anti-inflammatory drug; SD, standard deviation; TAER, triamcinolone extended release.
Figure 3.
Proportion of patients achieving a minimally clinically important difference for sleep disturbance over a 4-month period.
Discussion
This study aimed to compare 6 nonoperative treatment options for knee OA: cryoneurolysis with superficial genicular nerve block, cryoneurolysis with deep genicular nerve block, IA-HA injections, IA-NSAIDs, IA-CS, and IA-TA-ER injections over a 4-month follow-up, analyzing their effects on sleep disturbance. Overall, our data suggest use of IA-TA-ER is associated with improved sleep relative to traditional corticosteroids or other nonoperative treatment modalities for knee OA.
HRQOL is an important measure of function in individuals who have OA. Sleep disturbance is a component of HRQOL, but few studies have assessed or quantified the effects of nonoperative interventions on sleep disturbance in patients who have symptomatic knee OA. An observational prospective study by Wilcox et al. of 429 patients who had symptomatic knee OA found that issues with sleep onset, sleep maintenance, and early morning awakenings occurred weekly among 31%, 81%, and 51% of participants, respectively [28]. Specific risk factors for sleep disturbance included White race, older age, lower education level, cardiovascular disease, more arthritic joints, poorer physical functioning, knee pain, depression, and less social support [28].
Another cross-sectional study of 517 patients who had symptomatic knee OA found that patients, on average, reported sleep disturbance several days every week, with more frequent sleep disturbance associated with higher OA symptoms (P < .001) and a higher PCS (P < .05) [29]. While there is growing evidence that the use of intra-articular injections and denervation therapies may have benefits in reducing pain and improving function in patients who have symptomatic knee OA, to our knowledge, there are no published data detailing their effects on sleep disturbances. Our data indicate that for all interventions, all groups with symptomatic knee OA had sleep disturbances relative to population controls; however, patients who received IA-TA-ER had the least number of sleep disturbances, with 63% achieving a clinically relevant improvement.
IA-TA-ER was designed as an extended-release corticosteroid injection, formulated in 75:25 (lactic to glycolic acid) poly(lactic-co-glycolic acid) microspheres ∼45 microns in diameter [30], which allow for slow and steady drug elution [31]. The mechanism of the underlying glucocorticoid, triamcinolone acetonide, is aimed at activating the glucocorticoid receptor, down-regulating pro-inflammatory cytokines, and preventing the synthesis of prostaglandins, leukotrienes, and arachidonic acid, leading to decreased pain [30]. The longer drug elution is also thought to decrease or prevent the transient elevation in blood glucose levels seen in patients given traditional IA-CS. There are several potential hypotheses to explain why triamcinolone extended release (ER) may be better than other interventions in improving sleep quality. One hypothesis is that triamcinolone ER may be better at reducing joint discomfort more effectively at night, a time when pain often feels more pronounced due to lower cortisol levels and the absence of daytime distractions. Another possibility is that sustained symptom relief provided by long-acting formulations might minimize night-time disruptions and support deeper, more restorative sleep. As mentioned before, triamcinolone ER uses microsphere technology, where the drug is encapsulated in biodegradable poly (lactic-co-glycolic acid) microspheres, potentially allowing a gradual, controlled release over ∼12 weeks. This delayed-release mechanism is hypothesized to provide prolonged symptom control, which may enhance both daytime activity and night-time rest.
The therapeutic efficacy of IA-TA-ER has been evaluated in 3 main randomized double-blinded controlled trials, 2 of which were phase II and 1 phase III [31]. A phase II, double-blind study of 228 patients who had moderate to severe knee OA randomized to IA-TA-ER or immediate-release corticosteroid injection found that patients who received IA-TA-ER had improved pain relief at 8 weeks (P < .05) and a time-weighted mean pain relief, which was significantly superior to immediate-release injections over 1 to 12 weeks (P = .04) [32]. A separate phase IIB study of 306 patients randomized to either IA-TA-ER or saline placebo injections demonstrated significant improvements in average daily pain intensity at weeks 1 to 9 in a dose-dependent fashion (32 mg more beneficial than 16 mg) [33]. Also, a phase III multicenter double-blinded study of 484 patients who had symptomatic knee OA (KL grade 2 or 3) and received either IA-TA-ER or saline placebo injections demonstrated significant week-12 improvement in pain intensity relative to control (−3.12 vs −2.13; P < .0001), indicating 50% improvement [33]. The tolerability profile of IA-TA-ER is similar to that of IA-CS and placebo, with mild adverse events. There are a number of other studies demonstrating overall safety and efficacy of TA-ER in the treatment of knee OA as well [[34], [35], [36], [37]]. Our findings add to the literature, supporting the use of IA-TA-ER in reducing sleep disturbance, potentially by decreasing knee pain and inflammation.
This study is not without potential limitations. Since the registry does not implement a standard treatment protocol, patients are not systematically organized or distributed into treatment categories through random or stratified allocation. Consequently, there may be a variation in the distribution of patients receiving different treatments, which could be attributed to the diverse treatment approaches and indications practiced across various sites and medical specialties. Inconsistent patient numbers create difficulties in preplanning for analytical comparisons across various treatments. There is a possibility that bias could influence the treatment choices that a healthcare provider makes for a certain patient demographic. This being said, it is always important to recognize that this setting offers the closest approximation to real-world conditions. The implementation of this innovative real-world registry enables the gathering of more extensive data reflective of actual clinical practice. Other forms of bias, such as the Hawthorne effect—where patients alter their behavior because they know they are being watched—pose challenges in terms of measurement [38]. The operational elements of running the registry are managed by the individual sites participating in the study, from academic institutions to private clinical practices, without the direct management of a Contract Research Organization. The availability of staff and the resources allocated for data collection might introduce a certain level of selection bias. Moreover, employing technology in data gathering facilitates wider and easier participation, but inherently biases the sample toward individuals who have greater socioeconomic status and education and who have access to and are comfortable with using technology. For disclosure purposes, the majority of authors involved in this manuscript have potential conflicts of interest with Pacira BioSciences, which developed/maintains the iGOR registry and produces several of the products listed in this study (IA-TA-ER and cryoneurolysis). Nevertheless, the implementation of the iGOR registry offers distinct advantages as a collaborative, forward-looking, real-world observational registry [19]. It excels at collecting extensive data on a range of treatments for knee OA, including clinical effectiveness and outcomes reported by patients themselves.
Conclusions
This manuscript aims to compare and contrast 6 nonoperative treatment options for knee OA, assessing their effects on sleep disturbance, an important component of HRQOL. The treatments examined were cryoneurolysis of the superficial genicular nerve, deep genicular nerve, IA-HA injections, nonsteroidal anti-inflammatory injections, corticosteroid injections, and extended-release corticosteroid (triamcinolone) injections. We found that the use of extended-release triamcinolone injections was associated with the greatest reduction in sleep disturbance up to 4 months following injection, with 63% of patients experiencing a meaningful clinical difference. Ultimately, our study suggests that the use of the extended-release triamcinolone injections offers significant benefit for reducing sleep disturbances relative to traditional corticosteroid injections or other nonoperative treatment modalities.
Conflicts of interest
Michael A. Mont received royalties from Microport and Stryker; is a paid consultant for DJ Orthopaedics, Johnson and Johnson, Medical Compression Systems, Merz, Orthosensor, Pacira, Sage Products, Inc., Stryker, Tissue Gene, and US Medical Innovations; received research support from DJ Orthopaedics, Johnson and Johnson, National Institutes of Health (NIAMS and NICHD), Ongoing Care Solutions, Orthosensor, Stryker, and Tissue Gene; is in the medical/orthopaedic publications editorial/governing board of Journal of Arthroplasty, Journal of Knee Surgery, Orthopedics, and Surgical Technology International; and is a board member of the American Academy of Orthopaedic Surgeons. Jennifer H. Lin is a paid employee for Pacira BioSciences, Inc. and holds stock or stock options in Pacira BioSciences, Inc. Andrew I. Spitzer received speakers bureau/paid presentations for Pacira BioSciences, Inc. and DePuy Synthes; is a paid consultant for Pacira BioSciences, Inc. and DePuy Synthes; and received research support from Pacira BioSciences, Inc. and DePuy Synthes as a Principal Investigator. Vinod Dasa received speakers bureau/paid presentations for Bioventus, Pacira, Sanofi, and Sanara; is a paid consultant for Bioventus, Pacira, Sanofi, Ferring, Medi Post, Vertex, Cartiheal, Avania, Nanochon, and Anika; holds stock or stock options in DOC SOCIAL, Goldfinch consulting, Motive, MEND, Grand Care, and Doron Therapeutics; received other financial or material support from My Medical Images, J of ortho exp and innovation, MEND, and Grand Care; and is in the medical/orthopaedic publications editorial/governing board of JOEI. Andrew L. Concoff is a paid employee for Exagen, Inc.; is a paid consultant for United Rheumatology and Pacira BioSciences, Inc.; and holds stock or stock options in Exagen, Inc. Mitchell K. Ng is a paid consultant for Ferghana Partners, Inc. and Pacira BioSciences, Inc. Mary DiGiorgi is a paid employee for Pacira BioSciences, Inc. and holds stock or stock options in Pacira BioSciences, Inc. Stan DySart is a paid employee for Pacira BioSciences, Inc. and holds stock or stock options in Pacira BioSciences, Inc. Joshua A. Urban received speakers bureau/paid presentations for Pacira BioSciences, Inc.; is a paid consultant for Pacira BioSciences, Inc. and Vertex; holds stock or stock options in Pacira BioSciences, Inc.; and received research support from Pacira BioSciences, Inc., Vertex, and SpineBiopharma as a Principal Investigator. William M. Mihalko received royalties from Aesculap Inc.: Vega Knee system and CoreHip femoral stem system; received speakers bureau/paid presentations for Pacira BioSciences, Inc.; is a paid consultant for Pacira BioSciences, Inc.; holds stock or stock options in Medtronic Inc.; received research support from Medacta Inc. as a Principal Investigator; received royalties, financial or material support from Elsevier Inc.; is in the medical/orthopaedic publications editorial/governing board of Journal of Arthroplasty, Journal of Orthopaedic Research, J Long Term Effects of Medical Implants, and Orthop Clinics N Am.; and is a board member of the Knee Society, AAHKS, Hip Society, and ASTM International. All other authors declare no potential conflicts of interest.
For full disclosure statements refer to https://doi.org/10.1016/j.artd.2025.101655.
CRediT authorship contribution statement
Michael A. Mont: Writing – review & editing, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Jennifer H. Lin: Validation, Supervision, Resources, Project administration, Methodology, Formal analysis, Data curation, Conceptualization. Andrew I. Spitzer: Writing – review & editing, Software, Resources, Project administration, Methodology, Investigation, Conceptualization. Vinod Dasa: Writing – review & editing, Validation, Software, Resources, Funding acquisition, Formal analysis. Adam Rivadeneyra: Writing – review & editing, Supervision, Resources, Project administration, Methodology, Data curation, Conceptualization. David Rogenmoser: Writing – review & editing, Validation, Project administration, Methodology, Investigation, Formal analysis, Conceptualization. Andrew L. Concoff: Writing – review & editing, Supervision, Project administration, Methodology, Formal analysis. Mitchell K. Ng: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Mary DiGiorgi: Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition. Stan DySart: Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Conceptualization. Joshua Urban: Writing – review & editing, Validation, Supervision, Software, Resources, Project administration, Methodology, Formal analysis. William M. Mihalko: Writing – review & editing, Supervision, Software, Methodology, Investigation, Funding acquisition, Formal analysis.
Appendix A. Supplementary data
References
- 1.Katz J.N., Arant K.R., Loeser R.F. Diagnosis and treatment of Hip and knee osteoarthritis: a review. JAMA. 2021;325:568–578. doi: 10.1001/JAMA.2020.22171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ong K.L., Runa M., Lau E., Altman R.D. Cost-of-illness of knee osteoarthritis: potential cost savings by not undergoing arthroplasty within the first 2 years. Clin Outcomes Res. 2019;11:245–255. doi: 10.2147/CEOR.S170119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Losina E., Paltiel A.D., Weinstein A.M., Yelin E., Hunter D.J., Chen S.P., et al. Lifetime medical costs of knee osteoarthritis management in the United States: impact of extending indications for total knee arthroplasty. Arthritis Care Res. 2015;67:203. doi: 10.1002/ACR.22412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jain N.B., Higgins L.D., Ozumba D., Guller U., Cronin M., Pietrobon R., et al. Trends in epidemiology of knee arthroplasty in the United States, 1990–2000. Arthritis Rheum. 2005;52:3928–3933. doi: 10.1002/ART.21420. [DOI] [PubMed] [Google Scholar]
- 5.Lespasio M., Piuzzi N.S., Husni M.E., Muschler G.F., Guarino A., Mont M.A. Knee osteoarthritis: a primer. Perm J. 2017;21 doi: 10.7812/TPP/16-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jevsevar D.S. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;21:571–576. doi: 10.5435/JAAOS-21-09-571. [DOI] [PubMed] [Google Scholar]
- 7.Piuzzi N.S., Chughtai M., Khlopas A., Harwin S.F., Miniaci A., Mont M.A., et al. Platelet-rich plasma for the treatment of knee osteoarthritis: a review. J Knee Surg. 2017;30:627–633. doi: 10.1055/s-0037-1603795. [DOI] [PubMed] [Google Scholar]
- 8.Rossi L.A., Murray I.R., Chu C.R., Muschler G.F., Rodeo S.A., Piuzzi N.S. Classification systems for platelet-rich plasma. Bone Joint J. 2019;101-B:891–896. doi: 10.1302/0301-620X.101B8.BJJ-2019-0037.R1. [DOI] [PubMed] [Google Scholar]
- 9.Cherian J.J., Jauregui J.J., Leichliter A.K., Elmallah R.K., Bhave A., Mont M.A. The effects of various physical non-operative modalities on the pain in osteoarthritis of the knee. Bone Joint J. 2016;98-B:89–94. doi: 10.1302/0301-620X.98B1.36353. [DOI] [PubMed] [Google Scholar]
- 10.Ayhan E., Kesmezacar H., Akgun I. Intraarticular injections (corticosteroid, hyaluronic acid, platelet rich plasma) for the knee osteoarthritis. World J Orthop. 2014;5:351. doi: 10.5312/WJO.V5.I3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang Y., Deng Q., Yang L., Ma J., Wang Z., Huang D., et al. Efficacy and safety of ultrasound-guided radiofrequency treatment for chronic pain in patients with knee osteoarthritis: a systematic review and meta-analysis. Pain Res Manag. 2020;2020 doi: 10.1155/2020/2537075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buelt A., Narducci D.M. Osteoarthritis management: updated guidelines from the American college of Rheumatology and arthritis foundation. Am Fam Physician. 2021;103:120–121. [PubMed] [Google Scholar]
- 13.Najm A., Alunno A., Gwinnutt J.M., Weill C., Berenbaum F. Efficacy of intra-articular corticosteroid injections in knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Jt Bone Spine. 2021;88 doi: 10.1016/J.JBSPIN.2021.105198. [DOI] [PubMed] [Google Scholar]
- 14.Smith C.S., Mollon B., Vannabouathong C., Fu J.M., Sales B., Bhandari M., et al. An assessment of randomized controlled trial quality in the journal of bone & joint Surgery: update from 2001 to 2013. J Bone Joint Surg Am. 2020;102:E116. doi: 10.2106/JBJS.18.00653. [DOI] [PubMed] [Google Scholar]
- 15.Puzzitiello R.N., Lachance A.D., Michalowski A., Menendez M.E., Salzler M.J. Comparing orthopaedic randomized control trials published in high-impact medical and orthopaedic journals. J Am Acad Orthop Surg. 2023;31:E974–E983. doi: 10.5435/JAAOS-D-22-00604. [DOI] [PubMed] [Google Scholar]
- 16.Ng M.K., Piuzzi N.S., Jason Wong C.H., Delanois R.E., Bozic K.J., Browne J.A., et al. How to create an orthopaedic arthroplasty administrative database Project: a step-by-step guide Part I: study design. J Arthroplasty. 2023;38:407–413. doi: 10.1016/J.ARTH.2022.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Ng M.K., Piuzzi N.S., Wong C.H.J., Delanois R.E., Bozic K.J., Browne J.A., et al. How to create an orthopaedic arthroplasty database Project: a step-by-step guide Part II: study execution. J Arthroplasty. 2023;38:414–418. doi: 10.1016/j.arth.2022.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Willke R.J., Burke L.B., Erickson P. Measuring treatment impact: a review of patient-reported outcomes and other efficacy endpoints in approved product labels. Control Clin Trials. 2004;25:535–552. doi: 10.1016/j.cct.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Concoff A., Dasa V., DiGiorgi M., Eten K., Lin J., Mihalko W., et al. Innovations in genicular outcomes registry (iGOR): protocol for a real-world registry study of treatments for knee osteoarthritis. Ther Adv Musculoskelet Dis. 2024;16 doi: 10.1177/1759720X241304193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Innovations in genicular outcomes registry - full text view - ClinicalTrials.gov n.d. https://classic.clinicaltrials.gov/ct2/show/NCT05495334
- 21.Iovera home | patient n.d. https://www.iovera.com/
- 22.Roth Z.A., Sutton K., Wenende J., Pecka S. Preoperative cryoneurolysis for total knee arthroplasty: a case series. J PeriAnesthesia Nurs. 2023;38:33–38. doi: 10.1016/J.JOPAN.2022.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Nygaard N.P.B., Koch-Jensen C., Vægter H.B., Wedderkopp N., Blichfeldt-Eckhardt M., Gram B. Cryoneurolysis for the management of chronic pain in patients with knee osteoarthritis; a double-blinded randomized controlled sham trial. BMC Musculoskelet Disord. 2021;22:1–10. doi: 10.1186/S12891-021-04102-1/TABLES/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Treatment for Osteoarthritis Knee Pain | ZILRETTA® (triamcinolone acetonide extended-release injectable suspension) n.d. https://zilretta.com/
- 25.Yu L., Buysse D.J., Germain A., Moul D.E., Stover A., Dodds N.E., et al. Development of short forms from the PROMISTM sleep disturbance and sleep-related impairment item banks. Behav Sleep Med. 2012;10:6–24. doi: 10.1080/15402002.2012.636266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.George S.Z., Bolognesi M.P., Ryan S.P., Horn M.E. Sleep disturbance, dyspnea, and anxiety following total joint arthroplasty: an observational study. J Orthop Surg Res. 2022;17:1–9. doi: 10.1186/S13018-022-03288-X/TABLES/4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Felson D.T., Niu J., Guermazi A., Sack B., Aliabadi P. Defining radiographic incidence and progression of knee osteoarthritis: suggested modifications of the Kellgren and Lawrence scale. Ann Rheum Dis. 2011;70:1884–1886. doi: 10.1136/ARD.2011.155119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilcox S., Brenes G.A., Levine D., Sevick M.A., Shumaker S.A., Craven T. Factors related to sleep disturbance in older adults experiencing knee pain or knee pain with radiographic evidence of knee osteoarthritis. J Am Geriatr Soc. 2000;48:1241–1251. doi: 10.1111/J.1532-5415.2000.TB02597.X. [DOI] [PubMed] [Google Scholar]
- 29.Tighe C.A., Youk A., Ibrahim S.A., Weiner D.K., Vina E.R., Kwoh C.K., et al. Pain catastrophizing and arthritis self-efficacy as mediators of sleep disturbance and osteoarthritis symptom severity. Pain Med. 2020;21:501–510. doi: 10.1093/PM/PNZ187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson J.M., Shive M.S. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv Drug Deliv Rev. 1997;28:5–24. doi: 10.1016/S0169-409X(97)00048-3. [DOI] [PubMed] [Google Scholar]
- 31.Paik J., Duggan S.T., Keam S.J. Triamcinolone acetonide extended-release: a review in osteoarthritis pain of the. Knee. 2019;79:455–462. doi: 10.1007/s40265-019-01083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bodick N., Lufkin J., Willwerth C., Kumar A., Ballal R., Clayman M., et al. An intra-articular, extended-release formulation of triamcinolone acetonide prolongs and amplifies analgesic effect in patients with osteoarthritis of the knee: a randomized clinical trial. J Bone Joint Surg Am. 2015;97:877–888. doi: 10.2106/JBJS.N.00918. [DOI] [PubMed] [Google Scholar]
- 33.Conaghan P.G., Hunter D.J., Cohen S.B., Kraus V.B., Berenbaum F., Lieberman J.R., et al. Effects of a single intra-articular injection of a microsphere formulation of triamcinolone acetonide on knee osteoarthritis pain: a double-blinded, randomized, placebo-controlled, multinational study. J Bone Joint Surg Am. 2018;100:666–677. doi: 10.2106/JBJS.17.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Householder N.A., Raghuram A., Agyare K., Thipaphay S., Zumwalt M. A review of recent Innovations in cartilage regeneration strategies for the treatment of primary osteoarthritis of the knee: intra-articular injections. Orthop J Sports Med. 2023;11 doi: 10.1177/23259671231155950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ross E., Katz N.P., Conaghan P.G., Kivitz A., Turk D.C., Spitzer A.I., et al. Improved treatment effect of triamcinolone acetonide extended-release in patients with concordant baseline pain scores on the average daily pain and western ontario and McMaster universities osteoarthritis index pain scales. Pain Ther. 2022;11:289–302. doi: 10.1007/S40122-021-00335-Z/TABLES/4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spitzer A.I., Richmond J.C., Kraus V.B., Gomoll A., Jones D.G., Huffman K.M., et al. Safety and efficacy of repeat administration of triamcinolone acetonide extended-release in osteoarthritis of the knee: a phase 3b, open-label study. Rheumatol Ther. 2019;6:109–124. doi: 10.1007/S40744-019-0140-Z/FIGURES/5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Langworthy M.J., Conaghan P.G., Ruane J.J., Kivitz A.J., Lufkin J., Cinar A., et al. Efficacy of triamcinolone acetonide extended-release in participants with unilateral knee osteoarthritis: a post hoc analysis. Adv Ther. 2019;36:1398–1411. doi: 10.1007/S12325-019-00944-3/METRICS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akgülle A.H., Haidar M., Baştürk D.K., Gündoğdu M., Coşkun Ö.K. Hawthorne effect in gait analysis of children with in-toeing caused by increased femoral anteversion. Indian J Orthop. 2022;56:1789–1794. doi: 10.1007/S43465-022-00729-X/FIGURES/5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.