Abstract
Schlafen 11 (SLFN11), a regulator of cell fate following DNA injury, sensitizes tumor cells to DNA‐damaging agents. Patients with SLFN11‐positive tumors may benefit from DNA‐damaging chemotherapies. SLFN11 has been studied in different types of cancer including colorectal carcinomas. However, colorectal carcinomas with diffuse positivity (expression in ≥80% of tumor cells) have not been meticulously characterized. SLFN11 immunostaining of tumor microarrays (TMAs) with 3,300 primary CRCs identified 65 (~2.0%) tumors with focal staining (<10% of tumor nuclei positive), 83 (~2.5%) with patchy (≥10% and <80%) and 51 (~1.5%) with diffuse (≥80%) SLFN11 positivity. The latter was confirmed on full sections from donor blocks in 31 (~1%) cases, which were further studied including evaluation of additional immunohistochemical markers, genotyping with targeted DNA sequencing, and assessment of microsatellite instability. SLFN11‐positive carcinomas were mostly (21/31, 68%) right‐sided tumors with a female predominance (21/31, 68%) and median age of 67 years. Eighteen of 31 (58%) contained areas of mucinous differentiation. Deficiency of mismatch repair proteins was detected in 65% (20/31) of SLFN11‐positive carcinomas. Moreover, MLH1 (n = 2), MSH2, MSH6, and PMS2 germline mutations were identified in 25% (5/20) of patients with mismatch repair deficient tumors. BRAF p.V600E mutation was found in 45% (9/20) of mismatch repair deficient, but only 1 of 11 proficient tumors. Colorectal carcinomas with diffuse SLFN11 positivity were often mismatch repair deficient tumors with their typical clinical, morphological, and molecular characteristics.
Keywords: SLFN11, colorectal cancer, mismatch repair proteins, Lynch syndrome, mucinous carcinoma, DNA damaging agents
Introduction
Schlafen 11 (SLFN11) is a DNA helicase which controls cellular response to replicative stress [1, 2]. Upon DNA injury, SLFN11 permanently stalls replication forks and induces apoptosis [3]. Therefore, its expression has been increasingly recognized as a biomarker of tumor sensitivity to DNA‐targeting therapies, including platins, hydroxyurea, nucleoside analogs, topoisomerase inhibitors, and poly(ADP‐ribose) polymerase inhibitors [4, 5, 6, 7, 8]. Tumors lacking SLFN11 may be resistant to those agents and require alternative treatments [4, 7, 9, 10, 11, 12]. Thus, the analysis of SLFN11 expression is among inclusion criteria for several ongoing clinical trials (clinicaltrials.gov), and it may eventually become a part of routine clinical practice.
Colorectal carcinoma (CRC) is the third most common and second most deadly cancer worldwide with almost 2 million new cases diagnosed in 2022 [13]. Biomarker‐informed, personalized treatment may improve survival of CRC patients. Variable expression of SLFN11 was reported in primary (~5%) and metastatic (~10%) CRCs [14] and linked to sensitivity to platinum derivatives [15].
This study aimed to identify CRCs with diffuse expression of SLFN11. Such tumors have not been systematically characterized for their clinicopathological, immunophenotypic, and molecular genetic features.
Materials and methods
Tissue samples and immunohistochemical screening for SLFN11‐positive cases
This study was completed under the Office of Human Subject Research Exemption (https://www.hhs.gov) with clinically annotated de‐identified specimens. Previously characterized 3,300 primary CRCs [16] from 124 tissue microarrays were screened for SLFN11 expression using immunohistochemistry (IHC). Leica Bond Max automation platform (Leica Biosystems, Bannockburn, IL, USA) and anti‐SLFN11 D‐2 antibody (mouse monoclonal, catalog number: sc‐515,071; Santa Cruz Biotechnology, Dallas, TX, USA) was employed as previously reported [14]. Diffusely positive (≥80% of positive nuclei) tissue microarray (TMA) spots were validated with full tissue section of donor blocks. Tumors with diffuse SLFN11 expression were selected and 2nd generation TMAs (n = 2) comprising validated cases were constructed for further IHC evaluation.
Characteristics of CRCs with diffuse SLFN11 expression
Clinicopathologic data, histopathology, and IHC
pTNM categories were compliant with the 8th Edition of the American Joint Committee on Cancer staging system [17]. Tumor histopathologic classification was done following the WHO Classification of Tumours of the Digestive System, 5th edition [18].
Expression of caudal type homeobox 2 (CDX2), cytokeratin 7 (CK7), cytokeratin 20 (CK20), beta‐catenin (β‐catenin), and tumor protein p53 (p53) was evaluated using Ventana BenchMark Ultra (Ventana Medical Systems, Tucson, AZ, USA) IHC. Staining was graded as negative, focal (<10% of tumor cells), patchy (≥10% and <80% of tumor cells) and diffuse (≥80% tumor cells). p53 immunolabeling was categorized as normal/wild type (WT) (scattered cells with variable intensity of nuclear staining), negative (complete loss of nuclear expression), or diffuse/overexpressed (strong staining in ≥80% of tumor nuclei).
DNA and RNA extraction
Genomic DNA was isolated from formalin‐fixed paraffin‐embedded (FFPE) tumor tissues of all analyzed CRCs. In five cases, non‐neoplastic DNA or RNA was obtained from peripheral blood samples or extracted from adjacent non‐neoplastic tissues in three cases.
Targeted DNA next‐generation sequencing
Tumor DNA samples were studied using AmpliSeq™ Cancer Hotspot Panel v2 Kit and Ion Torrent® next‐generation sequencing platform (Thermo‐Fisher Scientific, Waltham, MA, USA). The assay evaluated genomic status of 50 oncogenes and tumor suppressors commonly mutated in cancer.
Analysis of mismatch repair and microsatellite instability status
In all cases, immunohistochemical expression of MutS Protein Homolog 2 (MSH2), MutS Protein Homolog 2 (MSH6), MutL Protein Homolog 1 (MLH1), and Postmeiotic Segregation Increased 2 (PMS2) was evaluated with Ventana BenchMark Ultra platform.
MLH1 promoter hypermethylation, determined by the methylation‐specific PCR targeting promoter sequences following the bisulfite conversion with EZ DNA Methylation‐Gold Kit (Zymo Research, Burlington, ON, USA) [19] was analyzed in 11 cases.
An instability of mononucleotide markers, BAT‐25, BAT‐26, NR‐21, NR‐24, and MONO‐27 was assessed using Droplet Digital™ PCR (ddPCR™) MSI assay (Bio‐RAD, Hercules, CA, USA) in five cases including four tumors screened for MLH1, MSH2, MSH6, PMS2, and POLE mutations using a custom gene panel created with the Ion Ampliseq Designer for Ion Torrent NGS platform (Thermo‐Fisher Scientific).
Normal tissue
MLH1, MSH2, and MSH6 coding and exon‐intron junction sequences and PMS2 coding sequences were evaluated using the Sanger method following PCR or RT‐PCR amplification of DNA or RNA extracted from peripheral blood samples (n = 5). In addition, three DNA samples from non‐neoplastic tissues adjacent to MSH2 (n = 1), MSH6 (n = 1), and MLH1 (n = 1) mutant tumors (identified by a custom gene NGS panel) were germline tested using mutation‐specific PCR amplification and Sanger sequencing.
In five cases, the selective adaptor ligation selective amplification methylation sensitive‐multiplex ligation probe amplification (SALSA® MS‐MLPA®) kits P003, P072, P008, and ME011 (MRC‐Holland, Amsterdam, The Netherlands) were used to detect large genomic aberrations such as deletions and duplications in MLH1, MSH2, MSH6, and PMS2. The PCR amplification products were separated by capillary electrophoresis using ABI 3130XL Genetic Analyzer (Applied Biosystems, Waltham, MA, USA), and the output data were analyzed with ‘Coffalyser.net’ software (MRC‐Holland).
Details of antibodies and immunohistochemical protocols, molecular genetic methods including DNA and RNA extraction, Sanger sequencing and NGS, ddPCR™, and SALSA® MS‐MLPA® and study the deficiency of mismatch repair (dMMR) and MSI are provided in Supplementary materials and methods.
Results
Identification of CRCs with diffuse SLFN11 immunopositivity
SLFN11 immunostaining of TMAs with 3,300 primary CRCs identified 65 (~2.0%) tumors with focal staining (<10% of tumor nuclei positive), 83 (~2.5%) with patchy (≥10% and <80%), and 51 (~1.5%) with diffuse (≥80%) SLFN11 positivity. The latter was confirmed on full sections from donor blocks in 31 cases, which were further studied including evaluation of additional immunohistochemical markers, genotyping with targeted DNA sequencing, and assessment of microsatellite instability. Demographic, clinicopathologic, immunohistochemical, and molecular features of CRCs with diffuse SLFN11 expression are summarized in Figure 1. Filtering and sorting of the tumors by different variables included in Figure 1 is possible in supplementary material, Table S1. Representative photomicrographs are shown in Figure 2.
Figure 1.
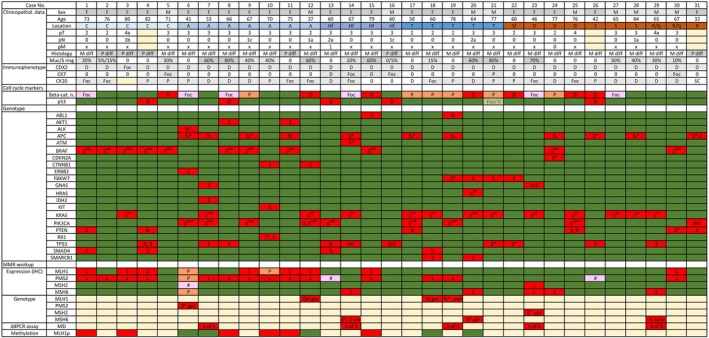
Clinicopathologic and molecular genetic characteristics of 31 CRCs with diffuse SLFN11 expression. Abbreviations (from the first to the last row left to right in consecutive order) are as follows: F, female; M, male; C, cecum; A, ascending, Hf, hepatic flexure; T, transverse; Sf, splenic flexure; D, descending; S, sigmoid; R/Sj, rectosigmoid junction; R, rectum; M diff, moderately differentiated; P diff, poorly differentiated; Muc, mucinous; S.ring, signet ring; 0, not present/negative/lack of expression; D, diffuse; Foc, focal; P, patchy; SC, scattered cells; Foc/0, indicates heterogeneity; Beta‐cat., β‐catenin; n., nuclear; S, substitution (missense mutation); fs, frameshift; *, STOP codon; superscript number, codon number; L, lost; #, intraglandular heterogeneity; Del, deletion; ger, germline; som, somatic; unk, unknown (germline versus somatic variant). Colors: blue – right colon; brown – left colon; yellow – not available; green – normal expression and WT; red – pathologic changes; orange and pink – possible pathologic changes. Dark and light gray mark female and male, respectively. Also, shades of gray are used to mark morphological differences. ddPCR assay: numbers indicate altered MSI markers.
Figure 2.

Representative images. (A–C) Adenocarcinoma of the colonic hepatic flexure (case number 14) with mucinous differentiation (A, ×100) showing diffuse SLFN11 staining (B, ×100) and MSH6 loss (C, ×100) by immunohistochemistry; this tumor had mutations in MSH6 and KRAS genes. (D–F) BRAF V600E‐mutated carcinoma of the cecum (case number 4) (D, ×100) with SLFN11 positivity (E, ×100) and MLH1 loss (F, ×100).
Demographic and clinicopathologic data
The cohort consisted of 21 females (68%) and 10 males. The patient age ranged from 32 to 84 with a median age of 67 years for females and 66 years for males. A majority (68%) of SLFN11‐positive CRCs occurred on the right side of the colon. All but one tumor with a strong SLFN11 immunoreactivity were right‐sided. The female to male ratio was 3:1 and 1:1 for right‐ and left‐sided tumors, respectively. The pTNM staging was available in 28 cases, although the status of metastatic disease was unknown in all but two patients. The most common was stage pT3 reported in 61% (17/28) of CRCs followed by pT2 (n = 8) and pT4 (n = 3). Seven (25%) of 28 patients had local metastases at the surgery.
Histopathological features, immunophenotype, and genotype
Eighteen (58%) of 31 CRCs contained areas of mucinous differentiation ranging from 5% to 80% of total tumor area. Seven cases were bona fide mucinous adenocarcinomas with extracellular mucin comprising >50% of tumor area. Two CRCs contained areas with signet ring cell morphology. Six tumors were poorly differentiated carcinomas with a solid sheet pattern.
Immunohistochemically, all tumors that expressed CDX2 and 28/31 were CK20‐positive including 11 cases with diffuse staining. CK7 positivity was found in seven tumors, diffuse in two of them. Of 31 tumors, 25 had WT and 5 had aberrant (4 overexpressed and 1 null) pattern of p53 immunoreactivity. One tumor contained subclones with WT and null p53 phenotypes. Nuclear β‐catenin staining was present in 18/31 tumors and in 7 cases diffuse.
Gain‐of‐function substitutions in KRAS codons 12, 13, and 146 and BRAF V600E mutation were identified in 38.7% (12/31) and 32.3% (10/31) of cases. Nine tumors (29%) harbored PIK3CA mutations mostly affecting common hotspots, codon 1,047 (n = 4) and 545 (n = 3). Oncogenic PIK3CA activation was the major driver in 4 KRAS/BRAF WT tumors. Mutations in APC and TP53 tumor suppressors were detected in 35.5% (11/31) and 29% (9/31) of cases and were mutually exclusive with a few exceptions (n = 3). Detailed results of targeted NGS sequencing of tumor DNA are provided in supplementary material, Table S2.
Mismatch repair and microsatellite instability status
dMMR workup is summarized in Figure 1. IHC testing of MMR proteins identified losses of single or multiple proteins in 20/31 (65%) tumors. Hypermethylation of MLH1 promoter was documented in 6 (54.5%) of 11 dMMR cases. In four of five MLH1 non‐hypermethylated tumors germline mutations affecting MLH1 (n = 2), PMS2 and MSH6 were detected by Sanger sequencing. Subsequent NGS of four dMMR tumors not included in MLH1 hypermethylation analysis detected MSH6, MLH1 somatic, MSH2 germline, and MSH6 undetermined mutation. Moreover, MSI was identified in these four cases and MLH1 non‐hypermethylated tumor (case number 14) by ddPCR MSI assay.
A detailed description of MMR gene mutations is provided in supplementary material, Table S3.
Deficient MMR versus proficient MMR CRCs
dMMR tumors occurred predominantly in female patients (16/20, 80%), whereas distribution of mismatch proficient (pMMR) cases was balanced between females (n = 5) and males (n = 6). Also, the proportion of right‐sided location was higher in dMMR (16/20, 80%) compared with pMMR (5/11, 45%) CRCs. Median age at diagnosis was overall higher in cases with dMMR (67) than pMMR (60) tumors. This difference was even more prominent in female patients (71.5 and 58 years, respectively). pTNM data were available for 19/20 dMMR and 9/11 pMMR CRCs. In the former, 13 (68%) tumors were staged pT3 or pT4 and 4 (21%) were pN(+). Among pMMR cancers, seven (78%) were staged pT3 or pT4 and three (33%) were pN(+). Mucinous differentiation was more frequent in dMMR (14/20, including 6 mucinous carcinomas) than pMMR (4/11, including 1 mucinous carcinoma) CRCs.
BRAF p.V600E was the most common (9/20, 45%) oncogenic driver mutation in dMMR tumors, whereas KRAS (7/20, 35%) and PIK3CA (3/20, 15%) were less frequently mutated. Conversely, in pMMR tumors, mutations of the latter two genes were more common (5/11, 45% and 3/11, 27%, respectively) while BRAF p.V600E was rare (1/11, 9%).
Discussion
SLFN11 expression is emerging as a clinically valuable predictive biomarker for optimization of cancer treatment. Several clinical trials are currently investigating new treatment strategies in SLFN11‐positive tumors (clinicaltrials.gov). SLFN11 expression suppressed CRC growth in vitro and in vivo and sensitized cancer cells to cisplatin [15]. Similarly, elevated SLFN11 expression in CRC cells promoted the efficacy of a topoisomerase inhibitor irinotecan [20]. Overall, analysis of SLFN11 may become useful for clinical decision‐making in CRC patients. However, data on the SLFN11 expression in CRC are incomplete. One study reported high SLFN11 immunoreactivity in 17% (40/236) of analyzed cases, but the staining was cytoplasmic [21], a pattern deemed non‐specific [14, 22]. Another study did not detect any positivity in 39 cases of CRCs analyzed [22]. Our previous immunohistochemical screening of SLFN11 expression in various cancers identified variably positive staining in ~5% of primary CRCs [14]. The current study investigated SLFN11 expression in archival CRC specimens and found that tumors with diffuse immunoreactivity (~1%, 31/3,300) were highly enriched with dMMR tumors. These findings corroborate a previously reported trend associating SLFN11 mRNA levels and MSI status in CRC [23].
BRAF p.V600E mutation was remarkably common (9/20, 45%) in dMMR SLFN11‐positive CRCs. This mutation occurs in ~10% of all CRCs but is significantly enriched in dMMR tumors [24, 25]. Only one of 11 (9%) pMMR SLFN11‐positive tumors harbored BRAF mutation. Although a functional link between oncogenic BRAF signaling and SLFN11 expression cannot be excluded, increased representation of BRAF mutants among SLFN11‐positive CRCs results from enrichment in dMMR tumors.
One study found the SLFN11 gene to be methylated in 55% of CRCs, which correlated with SLFN11 mRNA levels [15]. SLFN11 methylation was not associated with background clinicopathological features or BRAF and KRAS mutations; however, it predicted worse overall and relapse‐free survival [15].
Tumors with defective MMR machinery share a hypermutated phenotype that promotes generation of immunogenic neoantigens [26]. Therefore, dMMR/MSI‐H CRCs can be targeted by immune checkpoint blockade [27, 28]; however, ~30% of cases are unresponsive [28, 29]. Development of strategies to overcome this resistance is of paramount importance. Also, 5‐fluorouracil‐based chemotherapy, a standard of care in advanced pMMR CRCs [30], is not effective in dMMR tumors [31]. In this context, SLFN11‐positive dMMR CRCs may benefit from combining checkpoint inhibitors with DNA‐targeting drugs.
The nature of the link between MSI/dMMR and SLFN11 expression is unclear. Immune activation is a hallmark feature of MSI/dMMR CRC [32, 33, 34]. Tumor‐infiltrating lymphocytes and NK cells are robust sources of interferon (IFN)‐γ which is crucial for effective antitumor immunity [35]. Previous studies demonstrated that the SLFN11 gene is inducible by IFN signaling, which may explain the high representation of SLFN11‐positive CRCs among immunologically hot dMMR tumors [36, 37]. Interestingly, SLFN11 expression can be driven by gain‐of‐function JAK mutations [38] and activation of the JAK–STAT pathway was linked with hyperresponsiveness to IFN‐γ in an MSI‐H CRC cell line [39]. It will be of interest to analyze the relationship between SLFN11 and MMR/MSI status in other tumors. Interrelations between the expression of SLFN11 and the tumor microenvironment are more complex. Rather than a by‐effect of an IFN‐γ‐rich milieu, SLFN11 itself actively regulated the immune landscape and was associated with the efficacy of anti‐PD‐1 therapy in hepatocellular carcinoma [40].
Future research should establish a clinically relevant threshold for SLFN11 positivity in CRC. Diffuse SLFN11 expression was found in ~1% of cases in the current study. Considering the prevalence of CRC, even such a low percentage translates to a considerable number of patients who may potentially receive a more effective, biomarker‐informed therapy. This group may be much wider if non‐diffuse SLFN11 expression is found sufficient to determine improved treatment response. Tumors with any degree of SLFN11 immunopositivity constituted ~6% of all CRCs in this investigation.
In conclusion, SLFN11 is extensively expressed in a minor subset of CRCs. Among tumors with diffuse SLFN11 immunoreactivity there is a high representation of dMMR cases with their typical clinical, morphological, and molecular features.
Author contributions statement
MK and JL: study design; MK, MC, KY, TV, MS, KK: carrying out experiments; MK, OD, MiM, JL, MaM: data collection; MK, MC, AK, JL: data analysis and interpretation; MK and JL: literature search; MK and JL: generation of figures; MK and JL: drafting of the manuscript; JL and MaM: supervision. All authors approved the submitted version of the manuscript.
Supporting information
Supplementary materials and methods.
Table S1. Clinicopathological, immunophenotypic, and molecular characteristics of colorectal cancers with diffuse SLFN11 expression
Table S2. Results of targeted NGS sequencing of tumor DNA from colorectal cancers with diffuse SLFN11 expression
Table S3. Description of MMR gene mutations identified in colorectal cancers with diffuse SLFN11 expression
Acknowledgements
This study is supported as a part of NCI's intramural research program.
No conflicts of interest were declared.
Data availability statement
The datasets generated and/or analyzed in this study are available from the corresponding author upon reasonable request.
References
- 1. Murai J, Thomas A, Miettinen M, et al. Schlafen 11 (SLFN11), a restriction factor for replicative stress induced by DNA‐targeting anti‐cancer therapies. Pharmacol Ther 2019; 201: 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jo U, Pommier Y. Structural, molecular, and functional insights into Schlafen proteins. Exp Mol Med 2022; 54: 730–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Murai J, Tang SW, Leo E, et al. SLFN11 blocks stressed replication forks independently of ATR. Mol Cell 2018; 69: 371–384.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Winkler C, Armenia J, Jones GN, et al. SLFN11 informs on standard of care and novel treatments in a wide range of cancer models. Br J Cancer 2020; 124: 951–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Willis SE, Winkler C, Roudier MP, et al. Retrospective analysis of Schlafen11 (SLFN11) to predict the outcomes to therapies affecting the DNA damage response. Br J Cancer 2021; 125: 1666–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lok BH, Gardner EE, Schneeberger VE, et al. PARP inhibitor activity correlates with slfn11 expression and demonstrates synergy with temozolomide in small cell lung cancer. Clin Cancer Res 2017; 23: 523–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Taniyama D, Sakamoto N, Takashima T, et al. Prognostic impact of Schlafen 11 in bladder cancer patients treated with platinum‐based chemotherapy. Cancer Sci 2022; 113: 784–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hamada S, Kano S, Murai J, et al. Schlafen family member 11 indicates favorable prognosis of patients with head and neck cancer following platinum‐based chemoradiotherapy. Front Oncol 2023; 12: 978875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tang SW, Thomas A, Murai J, et al. Overcoming resistance to DNA‐targeted agents by epigenetic activation of schlafen 11 (SLFN11) expression with class I histone deacetylase inhibitors. Clin Cancer Res 2018; 24: 1944–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nogales V, Reinhold WC, Varma S, et al. Epigenetic inactivation of the putative DNA/RNA helicase SLFN11 in human cancer confers resistance to platinum drugs. Oncotarget 2015; 7: 3084–3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jo U, Murai Y, Chakka S, et al. SLFN11 promotes CDT1 degradation by CUL4 in response to replicative DNA damage, while its absence leads to synthetic lethality with ATR/CHK1 inhibitors. Proc Natl Acad Sci U S A 2021; 118: e2015654118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Murai J, Feng Y, Yu GK, et al. Resistance to PARP inhibitors by SLFN11 inactivation can be overcome by ATR inhibition. Oncotarget 2016; 7: 76534–76550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024; 74: 229–263. [DOI] [PubMed] [Google Scholar]
- 14. Kaczorowski M, Ylaya K, Chłopek M, et al. Immunohistochemical evaluation of Schlafen 11 (SLFN11) expression in cancer in the search of biomarker‐informed treatment targets: a study of 127 entities represented by 6658 tumors. Am J Surg Pathol 2024; 48: 1512–1521. [DOI] [PubMed] [Google Scholar]
- 15. He T, Zhang M, Zheng R, et al. Methylation of SLFN11 is a marker of poor prognosis and cisplatin resistance in colorectal cancer. Epigenomics 2017; 9: 849–862. [DOI] [PubMed] [Google Scholar]
- 16. Lasota J, Chłopek M, Wasąg B, et al. Colorectal adenocarcinomas harboring ALK fusion genes: a clinicopathologic and molecular genetic study of 12 cases and review of the literature. Am J Surg Pathol 2020; 44: 1224–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weiser MR. AJCC 8th edition: colorectal cancer. Ann Surg Oncol 2018; 25: 1454–1455. [DOI] [PubMed] [Google Scholar]
- 18. Nagtegaal ID, Odze RD, Klimstra D, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020; 76: 182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chan AOO, Broaddus RR, Houlihan PS, et al. CpG island methylation in aberrant crypt foci of the colorectum. Am J Pathol 2002; 160: 1823–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tian L, Song S, Liu X, et al. Schlafen‐11 sensitizes colorectal carcinoma cells to irinotecan. Anticancer Drugs 2014; 25: 1175–1181. [DOI] [PubMed] [Google Scholar]
- 21. Deng Y, Cai Y, Huang Y, et al. High SLFN11 expression predicts better survival for patients with KRAS exon 2 wild type colorectal cancer after treated with adjuvant oxaliplatin‐based treatment. BMC Cancer 2015; 15: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Takashima T, Sakamoto N, Murai J, et al. Immunohistochemical analysis of SLFN11 expression uncovers potential non‐responders to DNA‐damaging agents overlooked by tissue RNA‐seq. Virchows Arch 2021; 478: 569–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ballestrero A, Bedognetti D, Ferraioli D, et al. Report on the first SLFN11 monothematic workshop: from function to role as a biomarker in cancer. J Transl Med 2017; 15: 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rajagopalan H, Bardelli A, Lengauer C, et al. Tumorigenesis: RAF/RAS oncogenes and mismatch‐repair status. Nature 2002; 418: 934. [DOI] [PubMed] [Google Scholar]
- 25. French AJ, Sargent DJ, Burgart LJ, et al. Prognostic significance of defective mismatch repair and BRAF V600E in patients with colon cancer. Clin Cancer Res 2008; 14: 3408–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Muzny DM, Bainbridge MN, Chang K, et al. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012; 487: 330–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD‐1 blockade. Science 2017; 357: 409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair‐deficient or microsatellite instability‐high colorectal cancer (CheckMate 142): an open‐label, multicentre, phase 2 study. Lancet Oncol 2017; 18: 1182–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. André T, Shiu K‐K, Kim TW, et al. Pembrolizumab in microsatellite‐instability–high advanced colorectal cancer. N Engl J Med 2020; 383: 2207–2218. [DOI] [PubMed] [Google Scholar]
- 30. Morris VK, Kennedy EB, Baxter NN, et al. Treatment of metastatic colorectal cancer: ASCO guideline. J Clin Oncol 2023; 41: 678–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sargent DJ, Marsoni S, Monges G, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil‐based adjuvant therapy in colon cancer. J Clin Oncol 2010; 28: 3219–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Llosa NJ, Cruise M, Tam A, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter‐inhibitory checkpoints. Cancer Discov 2015; 5: 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lin A, Zhang J, Luo P. Crosstalk between the MSI status and tumor microenvironment in colorectal cancer. Front Immunol 2020; 11: 552467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Phillips SM, Banerjea A, Feakins R, et al. Tumour‐infiltrating lymphocytes in colorectal cancer with microsatellite instability are activated and cytotoxic. Br J Surg 2004; 91: 469–475. [DOI] [PubMed] [Google Scholar]
- 35. Du W, Frankel TL, Green M, et al. IFNγ signaling integrity in colorectal cancer immunity and immunotherapy. Cell Mol Immunol 2021; 19: 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Borrego AR, Corona‐Ayala C, Salvador JC, et al. Gene expression regulation of the type I interferon‐induced protein Schlafen 11. FASEB J 2020; 34: 1. [Google Scholar]
- 37. Mezzadra R, De Bruijn M, Jae LT, et al. SLFN11 can sensitize tumor cells towards IFN‐γ‐mediated T cell killing. PLoS One 2019; 14: e0212053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Murai Y, Jo U, Murai J, et al. Schlafen 11 expression in human acute leukemia cells with gain‐of‐function mutations in the interferon‐JAK signaling pathway. iScience 2021; 24: 103173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yuan W, Deng D, Jiang H, et al. Hyperresponsiveness to interferon gamma exposure as a response mechanism to anti‐PD‐1 therapy in microsatellite instability colorectal cancer. Cancer Immunol Immunother 2019; 68: 257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhou C, Weng J, Liu C, et al. Disruption of SLFN11 deficiency‐induced CCL2 signaling and macrophage M2 polarization potentiates anti‐PD‐1 therapy efficacy in hepatocellular carcinoma. Gastroenterology 2023; 164: 1261–1278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials and methods.
Table S1. Clinicopathological, immunophenotypic, and molecular characteristics of colorectal cancers with diffuse SLFN11 expression
Table S2. Results of targeted NGS sequencing of tumor DNA from colorectal cancers with diffuse SLFN11 expression
Table S3. Description of MMR gene mutations identified in colorectal cancers with diffuse SLFN11 expression
Data Availability Statement
The datasets generated and/or analyzed in this study are available from the corresponding author upon reasonable request.


