Abstract
Background
Venoarterial extracorporeal membrane oxygenation (V-A ECMO) use to support patients in cardiac failure is increasing. Despite this increased use, predicting successful weaning from ECMO can be challenging, no uniform guidelines on weaning exist. Therefore, we completed a systematic review to evaluate prognostic factors that predict successful weaning from V-A ECMO.
Methods
Following the PRIMSA guidelines, a systematic literature search of Medline, Embase, SCOPUS and CENTRAL identified original research studies of patients requiring V-A ECMO where weaning was attempted. Data was collected on demographic factors and weaning protocol, biomarkers, haemodynamic, echocardiographic factors for the successfully weaned (SW) and not successfully weaned (NSW) groups. Two investigators reviewed studies for relevance, extracted data, and assessed risk of bias using the ROBINS-I tool. The study was registered on the international prospective register of systematic reviews (PROSPERO ID# CRD42022366153).
Results
1219 records were screened, of which 20 studies were deemed sufficient to be included in the statistical analysis based on pre-specified criteria. Factors associated with successful weaning were higher left ventricular ejection fraction (LVEF) (MD 9.0, 95% CI 4.1–13.8; p < 0.001) and left ventricular outflow tract velocity time integral (LVOT VTI) at time of weaning, (MD 1.35, 95% CI 0.28–2.40 lactate at admission (MD −3.2, 95% CI −4.8 to −1.5, p < 0.001), and CK-MB at admission (MD −4.11, 95%CI −6.6 to −1.6, p = 0.001). Critical appraisal demonstrated moderate-high risk of bias owing to confounding and low sample sizes.
Conclusion
In patients on V-A ECMO support being assessed for weaning multi-parametric assessment is required. Moderate-high heterogeneity and low sample sizes warrant higher-quality studies to help guide decisions to wean patients from V-A ECMO.
Introduction
Veno-arterial (V-A) extracorporeal membrane oxygenation (ECMO) is increasingly being used [1,2] in acute cardiac failure to provide end-organ perfusion whilst definitive treatment, myocardial recover occurs or bridge to left ventricular assist device (LVAD) or heart transplant is completed.
Complications whilst on V-A ECMO support are common and effect mortality and increase with duration of support [3]. Therefore, minimising duration of V-A ECMO support, where possible, is sought. However, premature withdrawal of V-A ECMO support, may result in recurrence of cardiogenic shock and effect recovering organs. Minimizing complications associated with device support with the potential for hemodynamic deterioration if support is prematurely discontinued can be challenging.
The definition of successful V-A ECMO weaning has been proposed as when a patient survives for longer than 48 hours after ECMO explantation, with more, recent definitions as those having ECMO removed and not requiring further mechanical support because of recurring cardiogenic shock over the following 30 days [4,5]. Depending on the definition the proportion of V-A ECMO patients successfully weaned ranged between 30–75% [4–9].
A variety of clinical, haemodynamic, biochemical and echocardiographic variables have been proposed and used to guide clinical improvement and readiness to wean [10]. However, criteria and variables have not been completely reviewed to ascertain effectiveness [11] and meta-analyses as yet not completed. Therefore, we systematically reviewed a broad range of biomarkers, haemodynamic, echocardiographic and scoring systems to predict successful weaning from V-A ECMO.
Materials and methods
The study was conducted as previously outlined in our registered and published protocol (PROSPERO ID# CRD42022366153) and in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [12]. Ethics approval and patient consent were not required. The PRISMA checklist is provided in S1 Appendix.
Search strategy
The search strategy is detailed in a Material. A comprehensive search of three electronic databases (Medline, Embase, SCOPUS and CENTRAL) was conducted in October 2022, which were re-run in December 2023 and March 2024 prior to final analysis and further studies retrieved for inclusion. Appropriate Boolean operators were used to combine search terms that included V-A ECMO, ECMO, extra-corporeal life support, weaning, decannulation and ECLS. The reference lists of all included studies were also reviewed to identify any additional articles, and duplicate articles were removed. Studies that were not primarily in the English language were included if they were accompanied by an English translation. There were no limitations on the publication period. The search strategy is provided in S2 Appendix.
Study characteristics
Inclusion criteria allowed for randomized controlled trials, cohort studies, case series and conference abstracts that (1) considered adult or paediatric populations (2) involved patients who were on V-A ECMO and (3) there was an attempt to de-cannulate/wean from ECMO. Studies using ECMO as a bridge to ventricular assist device or heart transplant were excluded. Case series were included if >5 patients. Studies had to report associations between variables within the study and weaning success. Review publications, grey literature, non-English language publications, editorials, comments, letters to the editor and animal studies were excluded. Studies only assessing baseline variables with weaning success were excluded.
Study selection
Title and abstract screening were conducted by independent investigators (P.S. or H.H. or C.D.). Likewise, full-text screening was performed by two independent investigators (P.S. or H.H. or C.D.). All conflicts were resolved by a third, senior investigator (M.D.). The systematic review platform Covidence (www.covidence.org; Veritas Health Innovation, Melbourne, Australia) was used to facilitate the screening process. Publications found to fulfil eligibility criteria underwent data extraction. Literature search and decisions are provided in S1 Table.
Data extraction
Data was extracted from studies by two independent reviewers (P.S. or H.H. or C.D. or J.G.) using Microsoft Excel. Extracted variables included but not limited to patient demographics, weaning protocol, successful weaning definition, weaning success, various prognostic factors including biomarkers, haemodynamic, echocardiographic and scoring systems. The primary outcome was weaning success defined survival post removal of mechanical circulatory support and not requiring ventricular assist device or heart transplant. Meta-analysis was planned of predictors as appropriate. Missing data was reported as not reported. Authors were attempted to be contacted for further or missing data via email. Data extraction table is provided in S2 Table.
Evaluation of risk of bias
Critical appraisal of the risk of bias for individual studies was conducted using the ROBINS-I Tool (Risk of Bias in Non-Randomized Studies - of Interventions) [13]. Each included study was scored by two independent investigators (P.S. or J.G. or H.H.). Any discrepancies between the two reviewers were resolved by discussion and mutual agreement. Studies of poor-quality following risk of bias assessment were not be excluded from being included in our synthesis. Where a poor-quality study has contributed to a synthesized effect estimate, we explored the impact of study quality by performing sensitivity analysis by removing the poor-quality study to observe the impact that bias has had on the synthesized effect. Risk of bias assessment is provided in S3 Table.
Statistical analysis
Meta-analysis was completed as per the Cochrane Handbook for Systematic Reviews of Interventions when the outcomes were reported by two or more trials [14]. Statistical analysis was performed using Review Manager (version 5.3, The Cochrane Collaboration, Oxford, UK). For continuous outcomes, mean, standard deviation (SD) and sample size were extracted from each of the groups. Where studies reported median and ranges or interquartile range, derived mean and standard deviation as described by Wan et al. were calculated [15]. Mean differences with 95% confidence interval (CI) were used for continuous outcomes. An inverse variance method was applied for mean difference. Heterogeneity was assessed using I2 statistics and values between 50% and 90% were considered to represent substantial heterogeneity. A random effects meta-analysis model and exploring heterogeneity with sensitivity and subgroup analysis were applied where appropriate. Categorisation of reported risk factors of successful weaning from studies that reported multivariable adjustment was completed.
Results
Systematic search and study selection
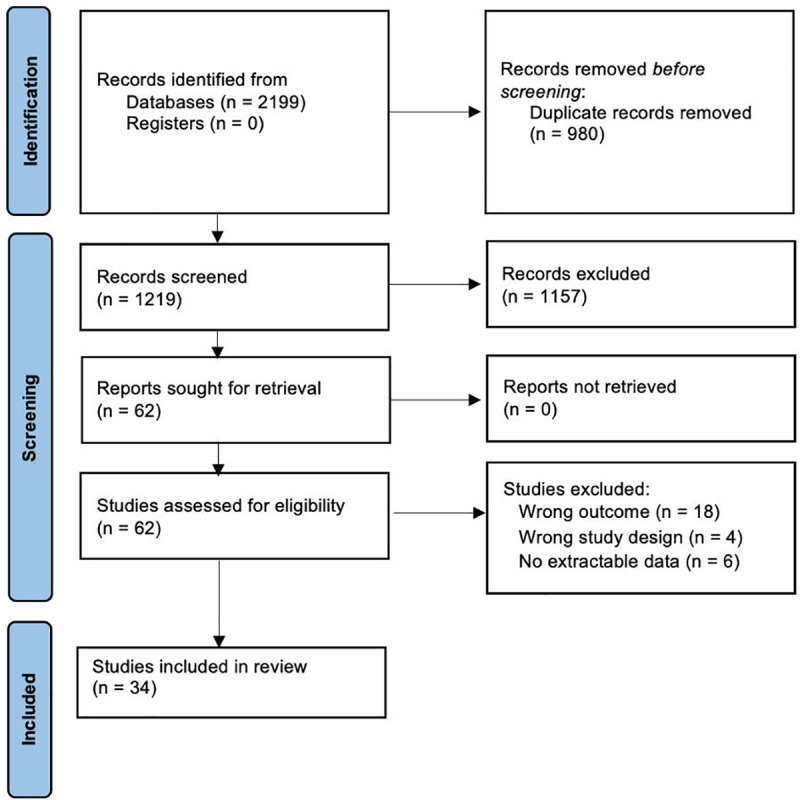
The search strategy of relevant references yielded a total of 2199 references (Fig 1). After the removal of 980 duplicates, the remaining 1219 references were screened by title and abstract. A total of 62 publications were deemed to be eligible for full-text screening, of which 28 studies were excluded with reasons. A total of 34 articles were included in the final analysis, of which 20 studies were deemed sufficient to be included in the statistical analysis. Risk of bias assessment is summarised in Fig 2. The remaining 14 studies examined differing prognostic predictors that were not able to be meta-analysed together. The sample sizes ranged from 12 to 265 patients, with a pooled sample size of 1903 patients. Twenty-six publications were retrospective cohort studies, seven were prospective cohort studies, and one not recorded. Cardiogenic shock was the primary indication for V-A ECMO in ten publications, myocarditis in two, cardiomyopathy in two, cardiac arrest in four, pulmonary embolism in one, post cardiac surgery in two, congenital heart disease in one, acute respiratory distress syndrome in one, and eleven not recorded. Geographically, fourteen publications were from Asia, eight were from North America, eleven were from Europe, and one was from the Middle East. A summary of the baseline characteristics of included papers and clinical variables is provided in Table 1.
Fig 1. PRISMA flow diagram illustrating the number of studies identified by the search and the stages in which they were chosen and eliminated.
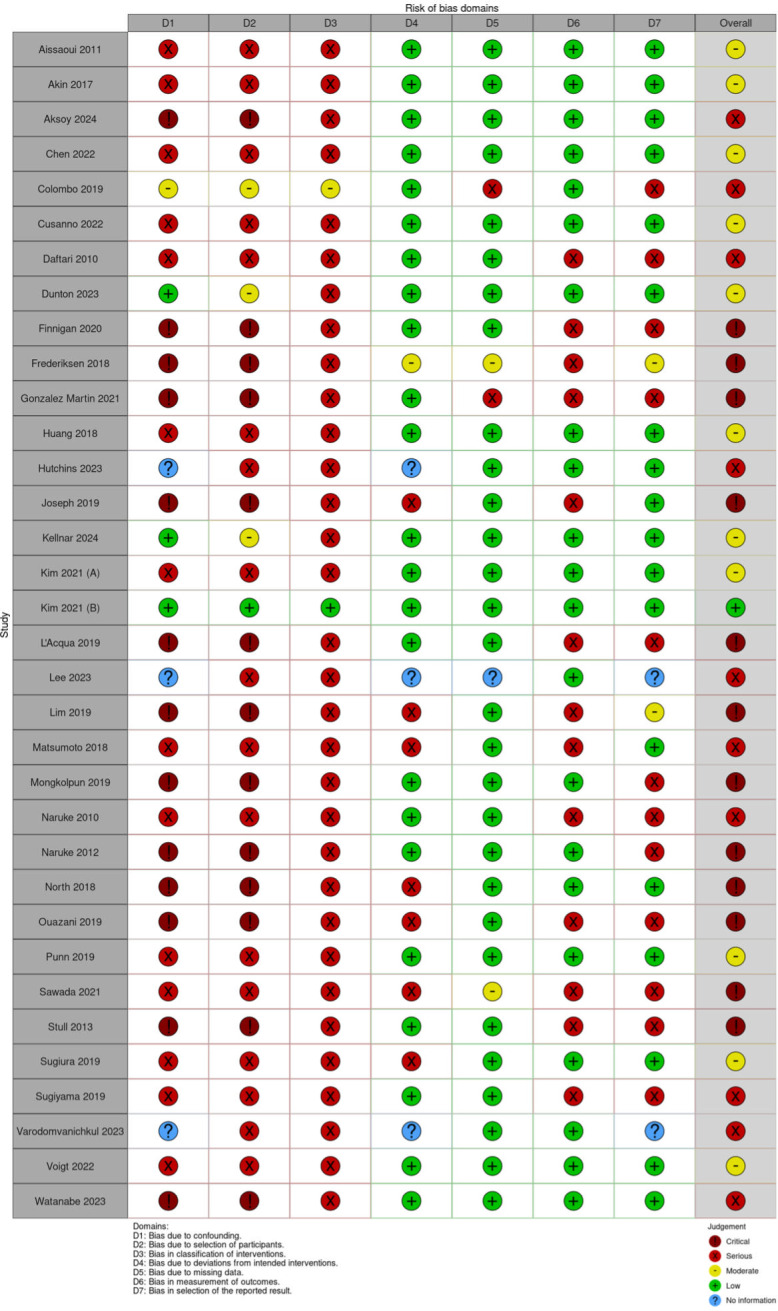
Fig 2. Risk of bias of the included studies (ROBINS-I).
Table 1. Characteristics of included studies.
| Author | Country | Enrolment period | Type of study | Total number of patients | Successful weaned | Unsuccessful wean | Age | Male | BMI | Heart failure (co-morbidity) | Indication for V-A-ECMO (%) | Successful weaning definition | Weaning success (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Success wean | Unsuccess wean | Success wean | Unsuccess wean | Success wean | Unsuccess wean | |||||||||||
| Aissaoui 2011 [16] | France | 2007–2008 | Prospective cohort | 38 | 25 | 13 | 49 ± 14 | 67 ± 11 | 25 | 8 | NR | NR | 8 | CMP (47%), FM (6%), Post-cardiotomy shock (22%), Post-transplantation (10%), Other (16%) | ECMO remoV-Al and no further MCS because of recurring CS over the following 30 days | 20/38 = 53% |
| Akin 2017 [17,29] | Netherlands | 2014–2016 | Prospective cohort | 13 | 10 | 3 | 56 ± 17 | 41 ± 16 | 9 | 1 | NR | NR | 0 | PE (38%), Post-cardiotomy shock (23%), CS post-AMI (15%), Myocarditis (15%), Intoxication (8%) | Successful V-A-ECMO explantation within 48 h | 10/13 = 77% |
| Aksoy 2024 [18] | Turkey | 2010–2019 | Retrospective cohort | 55 | 27 | 28 | 2.3 | 1.6 | 19 | 20 | NR | NR | NR | Post-op complications following congenital heart surgery, including: low cardiac output syndrome, inability to wean from bypass, ECPR. | Wean trial when adequate myocardial contraction and haemodynamically stable; initiated with flow rate to 25% | 27/55 = 49% |
| Chen 2022 [19] | Taiwan | NR | Retrospective cohort | 47 | 31 | 16 | 69 ± 16 | 39 ± 18 | 15 | 9 | NR | NR | NR | NR | Weaning from ECMO and surviV-Al beyond 48 h | 31/47 = 66% |
| Colombo 2019 [20] | Italy | 2013–2017 | Retrospective cohort | 25 | 18 | 7 | NR | NR | NR | NR | NR | NR | NR | CPR (71%), CS post-AMI (17%), Myocarditis (7%), PE (4%), Takotsubo (2%), Intoxication (2%) | Device remoV-Al without requirement for re-cannulation over the following 30 days | 18/25 = 72% |
| Cusanno 2022 [9] | France | 2016–2021 | Retrospective cohort | 57 | 36 | 21 | 46 ± 19 | 57 ± 17 | NR | NR | 26 ± 6 | 25 ± 7 | 37 | Ischemic CS (35%), Refractory CA (28%), Other (37%) | NR | 36/57 = 63% |
| Daftari 2010 [21] | USA | 2000–2008 | Retrospective cohort | 27 | 16 | 11 | NR | NR | NR | NR | NR | NR | 27 | Heart failure (100%) | NR | 16/27 = 59% |
| Dunton 2023 [22] | USA | 2016–2021 | Retrospective cohort | 265 | 140 | 125 | 59.1 ± 13.6 | 59.4 ± 13.2 | 95 | 100 | 30.8 ± 8.1 | 30.4 ± 7.4 | 75 | CPR, cardiogenic shock, post cardiotomy shock | Survival to decannulation | 140/265 = 53% |
| Finnigan 2020 [23] | United Kingdom | NR | NR | 14 | NR | NR | NR | NR | NR | NR | NR | NR | NR | Post cardiac surgery support (57%), Respiratory support (43%) | NR | NR |
| Frederiksen 2018 [24] | Denmark | NR | Retrospective cohort | 29 | 15 | 14 | NR | NR | NR | NR | NR | NR | NR | NR | ECMO weaning and being alive 24 h later without hemodynamic MCS | 15/29 = 52% |
| Gonzalez Martin 2021 [25] | Spain | 2013–2020 | Retrospective cohort | 85 | 52 | 33 | NR | NR | NR | NR | NR | NR | 0 | CS (47%), ECPR (9%), Electrical storm (9%), Post-cardiotomy CS (33%), Other (1%) | Survival >24 h after explant and no mortality from cardiogenic shock/heart failure or cardiac arrest during admission | 52/85 = 61% |
| Huang 2018 [26] | Taiwan | 2014–2015 | Retrospective cohort | 46 | 28 | 18 | 59 ± 16 | 48 ± 15 | 18 | 15 | NR | NR | 8 | CS/Cardiac arrest post AMI (50%), Dilated cardiomyopathy (15%), VT/VF Arrest (11%), Myocarditis (8%), PE (4%) | ECMO removal and no mortality and/or MCS because of recurring CD over the following 48 h | 28/46 = 61% |
| Hutchins 2023 [27] | USA | 2015–2021 | Retrospective cohort | 199 | 103 | 96 | 56.2 ± 14.6 | 53.1 ± 16.2 | 67 | 71 | NR | NR | NR | Cardiogenic shock | Successful decannulation was defined as survivall without relapse to mechanical circulatory support or heart transplant within 30 days | 103/199 = 51.8% |
| Joseph 2019 [28] | USA | NR | Retrospective cohort | 30 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Kellnar 2024 [29] | Germany | 2021–2023 | Prospective cohort | 12 | 7 | 5 | 53.0 (IQR 47.0; 60.0) | 58.0 (IQR 57.5; 67.0) | NR | NR | 26.9 (IQR 25.2; 30.0) | 24.9 (IQR 23.7; 26.9) | NR | Cardiogenic shock | Not requiring further mechanical circulatory support within 30 days | 7/12 = 58% |
| Kim 2021 (A) [30] | South Korea | 2016–2018 | Prospective cohort | 92 | 64 | 28 | 60 ± 12 | 59 ± 12 | 48 | 21 | 24 ± 3 | 25 ± 4 | NR | CS post-AMI (48%) Ischemic cardiomyopathy (48%), Other (4%) | ECMO removal and not requiring further MCS over the following 30 days | 64/92 = 70% |
| Kim 2021 (B) [31] | South Korea | 2016–2019 | Prospective cohort | 79 | 50 | 29 | 63 ± 13 | 58 ± 12 | 41 | 23 | 25 ± 3 | 25 ± 4 | NR | Post-MI CMP (52%), Idiopathic dilated CMP (18%), Fulminant myocarditis (4%), Stress-induced CMP (4%) | Successful removal of V-A-ECMO and no further mechanical circulatory support in the following 30 days | 50/79 = 63% |
| L’Acqua 2019 [32] | Italy | 2012–2018 | Retrospective cohort | 98 | 49 | 49 | NR | NR | NR | NR | NR | NR | NR | NR | Patient weaned from V-A ECMO | 49/98 = 50% |
| Lee 2023 [33] | South Korea | 2017–2019 | Retrospective cohort | 55 | 38 | 17 | NR | NR | NR | NR | NR | NR | NR | NR | NR | 38/55 = 69% |
| Lim 2019 [34] | South Korea | 2010–2018 | Retrospective cohort | 122 | 72 | 50 | 57.8 ± 15.0 | NR | NR | NR | NR | NR | NR | NR | NR | 72/122 = 59% |
| Matsumoto 2018 [35] | Japan | 1995–2014 | Retrospective cohort | 37 | 22 | 15 | 44 ± 32 | 40 ± 31 | 13 | 8 | 21 ± 3 | 22 ± 4 | NR | Myocarditis (100%) | ECMO removal | 22/37 = 59% |
| Mongkolpun 2019 [36] | Belgium | NR | Retrospective cohort | 22 | 12 | 10 | NR | NR | NR | NR | NR | NR | NR | CS post-AMI (64%), post-cardiotomy (14%), Myocarditis (14%), PE (8%) | ECMO removal and HD Stabilization without the need to increase the Vasopressor dose within 24 h | 12/22 = 55% |
| Naruke 2010 [37] | Japan | 1996–2008 | Retrospective cohort | 25 | 18 | 7 | 54 ± 14 | 49 ± 18 | 8 | 5 | NR | NR | 3 | Myocarditis (52%), CS post-AMI (36%), ACHF (12%) | ECMO weaning | 18/25 = 72% |
| Naruke 2012 [38] | Japan | NR | Retrospective cohort | 30 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | V-A-ECMO weaned of without severely deteriorated cardiac output indicated by ETCO2 < 10 mmHg or LVET < 100 ms | NR |
| North 2018 [41] | USA | 2012–2017 | Retrospective cohort | 60 | 42 | 18 | NR | NR | NR | NR | NR | NR | NR | NR | V-A-ECMO decannulation | 42/60 = 70% |
| Ouazani 2019 [42] | USA | NR | Prospective cohort | 12 | 9 | 3 | NR | NR | NR | NR | NR | NR | NR | NR | ECMO removal without requiring any further MCS | 9/12 = 75% |
| Punn 2019 [39] | USA | 2010–2018 | Prospective cohort | 63 | 25 | 38 | 58 ± 21 | 32 ± 40 | 16 | 20 | NR | NR | NR | Congenital heart defect (63%), Myocarditis (15%), Idiopathic dilated cardiomyopathy (14%), Sepsis (2%), Others (6%) | wean within 48 hours of assessment and survived without ventricular assist devices or orthotopic heart transplantation | 25/63 = 40% |
| Sawada 2021 [40] | Japan | 2013–2017 | Retrospective cohort | 50 | 24 | 26 | 76 ± 21 | 64 ± 29 | 20 | 17 | 23 ± 6 | 23 ± 6 | 5 | CS post-AMI (54%), FM (24%), CMP (10%), other heart disease (12%) | ECMO removal and survival beyond 30 days without needs for further MCS | 24/50 = 48% |
| Stull 2013 [41] | USA | 2010–2013 | Retrospective cohort | 23 | 15 | 8 | NR | NR | NR | NR | NR | NR | NR | ARDS (100%) | NR | 15/23 = 65% |
| Sugiura 2019 [42] | Japan | 2012–2016 | Retrospective cohort | 55 | 28 | 27 | 64 ± 14 | 68 ± 16 | 21 | 25 | 25 ± 4 | 25 ± 5 | NR | CS post-AMI (100%) | ECMO removal | 28/55 = 51% |
| Sugiyama 2019 [43] | Japan | 2011–2018 | Retrospective cohort | 74 | 37 | 37 | NR | NR | NR | NR | NR | NR | NR | NR | Patient survives more than 48 h after the removal of cannulas of ECMO | 37/74 = 50% |
| V-ArodomV-Anichkul 2023 [44] | Thailand | 2018–2021 | Retrospective cohort | 57 | 46 | 11 | NR | NR | NR | NR | NR | NR | NR | Cardiogenic shock | NR | 46/57 = 81% |
| Voigt 2022 [45] | Germany | 2017–2020 | Retrospective cohort | 40 | 16 | 24 | 51 ± 17 | 60 ± 15 | 10 | 18 | 28 ± 6 | 27 ± 6 | NR | Cardiac arrest (40%), Cardiogenic shock (60%) | V-A-ECMO decannulation and subsequent discharge | 16/40 = 40% |
| Watanabe 2023 [46] | Japan | 2010–2016 | Retrospective cohort | 41 | 17 | 24 | 70.6 ± 13.2 | 68.0 ± 14.5 | 12 | 17 | NR | NR | NR | NR | Survival for more than 24 hours after V-A-ECMO withdrawal without requiring reintroduction | 17/41 = 41% |
ACHF, acute on chronic heart failure; AMI, acute myocardial infarction; ARDS, acute respiratory distress syndrome; CA, cardiac arrest; CMP, cardiomyopathy; CPR, cardiopulmonary resuscitation; CS, cardiogenic shock; ECMO, extracorporeal membranous oxygenation; FM, fulminant myocarditis; MCS, mechanical cardiac support; NR, not reported; PE, pulmonary embolism; VF, ventricular fibrillation; VT, ventricular tachycardia.
Biomarkers.
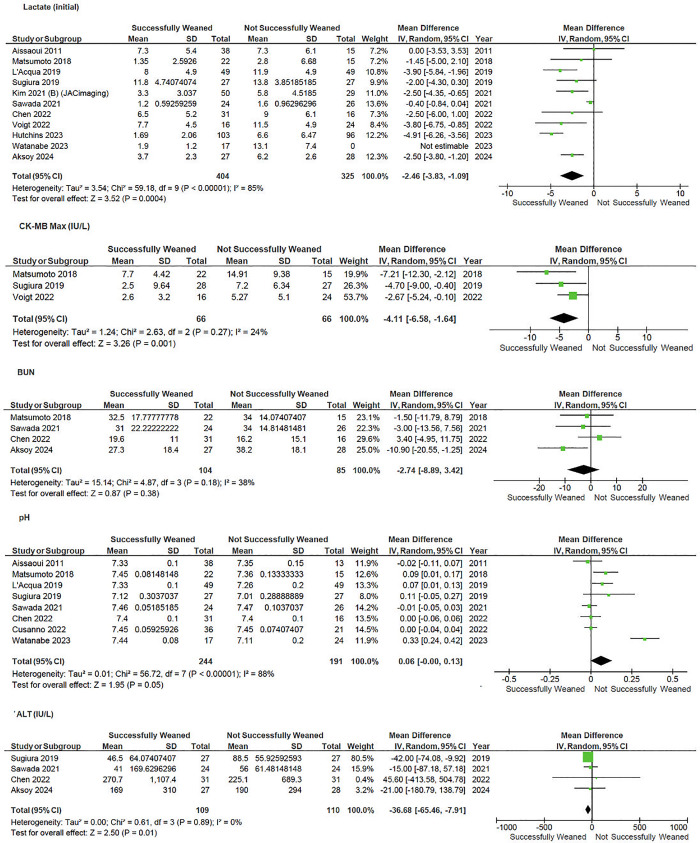
Markers of organ damage were inversely associated with weaning success—Fig 3. Lower levels of creatinine kinase (CK-MB) (MD −4.1, 95%CI −6.6 to −1.6, p = 0.001; I2 = 24%), lactate at admission (MD −3.2, 95% CI −4.8 to −1.5, p < 0.001; I2 = 90%), and lower levels of alanine aminotransferase (ALT) (MD −36.7, 95%CI −65.5 to 7.9, P = 0.01; I2 = 0%) at the time of weaning were associated with weaning success. Too few studies reported NT-ProBNP or Troponin to enable analysis.
Fig 3. Forrest plot of comparison of haemodynamic parameters on V-A ECMO Weaning.
Haemodynamics.
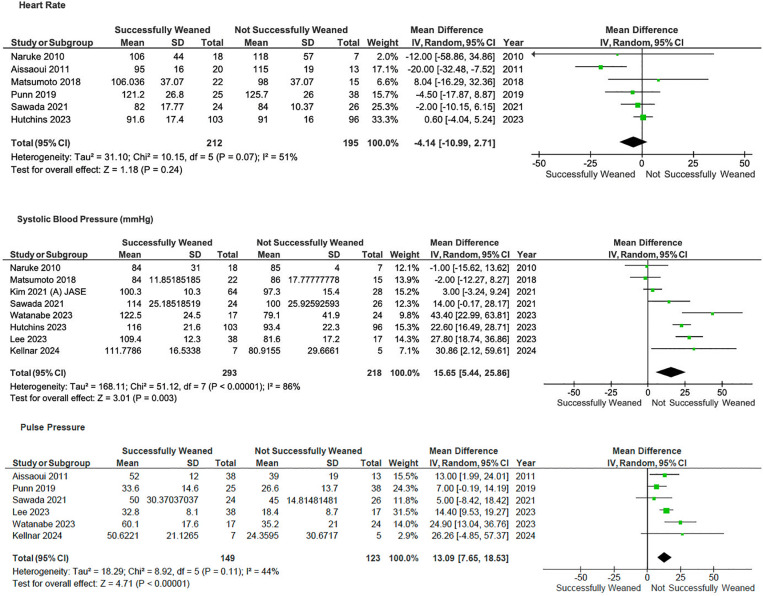
Patients with higher pulse pressure (MD 13.1, 95%CI 7.7–18.5, p < 0.001; I2 = 44%) and systolic blood pressure (MD 15.7, 95%CI 5.4–25.9, p < 0.001; I2 = 86%) were associated with weaning success—Fig 4.
Fig 4. Forrest plot of comparison of laboratory parameters on V-A ECMO.
Echocardiography.
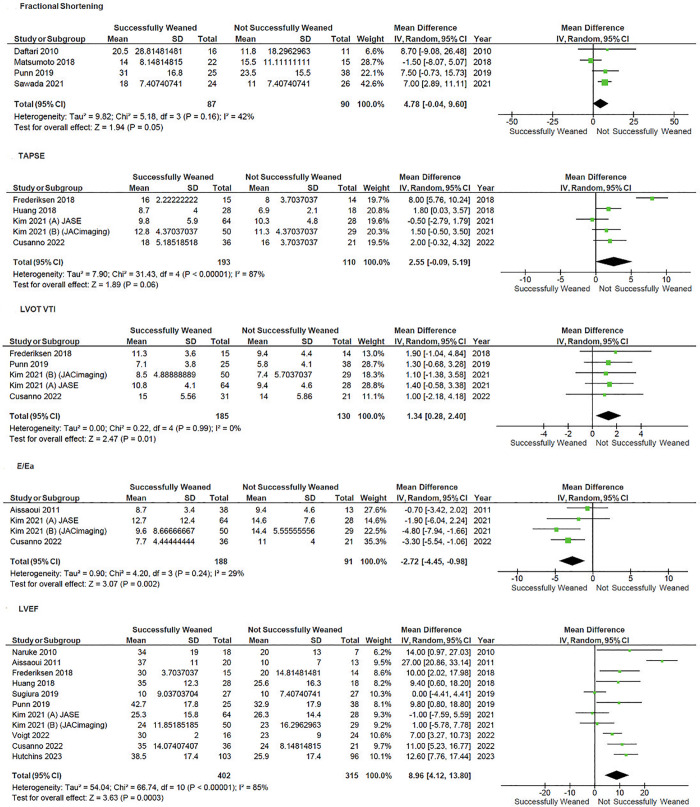
Patients with higher left ventricular ejection fraction (LVEF) at time of weaning (MD 9.0, 95% CI 4.1–13.8; p < 0.001; I2 = 85%), left ventricular outflow tract velocity time integral (LVOT VTI) (MD 1.35, 95% CI 0.28–2.40, p = 0.01; I2 = 0%), E/Ea (MD −2.72, 95% CI −4.45 to −0.98, p = 0.002; I2 = 29%) were associated with weaning success—Fig 5.
Fig 5. Forest plot of the comparison of different echocardiographic parameters on V-A ECMO weaning.
Multi-variable adjusted: Risk predictors.
Thirteen studies provided multi-variable adjusted analysis to identify predictors of successful weaning. Covariates tested varied widely between studies; only lack of renal failure or CRRT during ECMO, and post-weaning lactate, reported by more than one study as predictors of successful ECMO weaning—Table 2.
Table 2. Risk factors predictive of successful ECMO weaning from multi-variable logistic regression models.
| Baseline characteristics | Study |
|---|---|
| Normal RV function | Puerto [47] |
| No pre-existing ischemic cardiopathy | Cusanno [9] |
| Post PCI TIMI flow | Sugiura [42] |
| While on ECMO | |
| No CRRT | Kim [31] |
| No need for LV venting | Kim [31] |
| 10% improvement of tricuspid S’ during ECMO flow study | Kim [30] |
| Any improvement of lateral e’ during ECMO flow study | Kim [30] |
| ECMO duration | Punn [39] |
| Implantation-weaning test delay < 7 days | Cusanno [9] |
| Echo findings on weaning | |
| VTI | Lim [48], Punn [39] |
| LV EF | Punn [39] |
| Corrected LV ejection time/PAWP | Sawada [40] |
| Tricuspid annular S’/RSVP >0.33 | Kim [31] |
| RV EF | Huang [26] |
| Normal RV function | Puerto [47] |
| RV free wall strain | Huang [26] |
| RV FAC | Huang [26] |
| Hemodynamics | |
| HR on day of decannulation | Liu [49] |
| MAP at weaning | Lim [48] |
| MAP at 4hrs | Sugiura [42] |
| Post-test SBP >120 mmHg | Cusanno [9] |
| CVP on day of decannulation | Huang [26] |
| Post de-cannulation | |
| Lactate at 12hrs | Chen [19] |
| Lactate at 24hrs | Sugiura [42] |
| Lower vasoactive-inotropic score 24hrs post cannulation | Dunton [22] |
| Improvement of RV systolic function >24hrs after decannulation | Puerto [47] |
CRRT, continuous renal replacement therapy; CVP, central venous pressure; ECMO, extracorporeal membrane oxygenation; EF, ejection fraction; FAC, fractional area change; HR, heart rate; LV, left ventricle; MAP, mean arterial pressure; PAWP, pulmonary artery wedge pressure; PCI, percutaneous coronary intervention; RV, right ventricle; SBP, systolic blood pressure; TIMI, Thrombolysis in Myocardial Infarction score; VTI, velocity time integral.
Additional sensitivity analyses removing studies that included paediatric patients did not reveal any significant differences—S4 Table.
Study quality.
A total of 34 publications were eligible for quality assessment, of which 12 publications were of poor quality, i.e., ‘critical’ risk of bias (Fig 2). Many studies did not provide detailed protocols, limiting methodological assessment, appraisal of the confounding effect of intervention and bias in selection of participants into the study.
Discussion
In this systematic review of predictors of V-A ECMO weaning success 34 predominantly small observational, studies were identified. On pooled analysis, lower levels of biochemical markers of end-organ perfusion or injury (lactate, CK-MB and ALT), haemodynamic (pulse pressure and systolic blood pressure) and echocardiographic indicators of myocardial function (LVEF, LVOT VTI, E/Ea) were associated with successful weaning form V-A ECMO.
To our knowledge this review is only the second to systematically assess predictors of V-A ECMO wean success. The first, in adult patients with specifically cardiogenic shock or cardiac arrest identified similar results to our review, with lower creatine kinase and lactate levels, and higher LVEF being predictors for successful weaning from V-A-ECMO [10] Other, non-systematic, reviews have reported also reported lower creatine kinase and lactate levels, and higher LVEF and LVOT VTI being predictors for successful weaning from V-A-ECMO [50]. Several other V-variables used in clinical practice [9] Troponin, NT-ProBNP, RV to PA coupling indices were not identified owing to limited numbers of studies and patients reported with these.
Despite significant heterogeneity, small sample sizes and a significant risk of bias, there are some conclusions that can be drawn from this review and the available literature. First, determination of likely weaning success, should consider multiple variables and not be focussed on one individual predictor. Factors associated with success (or failure), were present across clinical, biochemical, haemodynamic and echocardiographic parameters and clinicians should avoid relying on one variable over the complete picture of the patient. Second, initial severity of illness (e.g., lactate), markers of end-organ perfusion, and then recovery of such are important considerations in attempting to wean [42,51,52]. Further, absolute cut offs for specific variables, e.g., LVEF or LVOT VTI to predict weaning success vary between studies, are based empirical clinical weaning protocols [50,53] and therefore cannot yet be elucidated. Overall restitution and improvement of the overall clinical state of the patient as well as cardiac function is likely key to successful weaning rather than a specific variable or level of a variable.
Formal weaning or “ramp” studies that assess haemodynamic and echocardiographic changes to alterations to ECMO flows protocols are recommended [54] but as yet no standardised protocols exist, are only variably reported in ECMO trials, and are not formally assessed in this systematic review. However, they are critical tools to assess the response of cardiac function to reduction, and then removal, of mechanical circulatory support [9,55]. Future prospective clinical trials should publish weaning strategies and protocols to enable further assessment and comparison of strategies.
Limitations
Our review is limited by the lack of large high-quality trial, with all included studies consisting of observational studies with small sample sizes and these small trials were used to investigate widely varying interventions amongst this population group, often performed without covariate adjustment. However, we completed a comprehensive review of the literature including all commonly used variables for V-A ECMO weaning. The inclusion criteria of our study were broad and included adult and paediatric patients and any aetiology leading to V-A ECMO support. It is possible that paediatric and adult patients differ in weaning factors. Additional analysis excluding studies that included paediatric patients showed no significant change in outcomes reported. Further, V-A ECMO patients by their very nature are heterogenous and a wide array of prognostic variables are utilized by clinicians in clinical practice, a wider approach to inclusion criteria increases sensitivity and generalisability of findings. Micro-circulation indices were not assessed but are not in uniform clinical practice which was our focus. The underlying aetiology leading to requirement for V-A ECMO support was not assessed and may impact timing of weaning, weaning success and need for durable ventricular support, e.g., VAD.
Conclusions
In patients requiring V-A ECMO support, multiple biochemical, haemodynamic and echocardiographic parameters of recovery, rather than a single variable should be used to guide appropriateness for weaning. Further larger studies are required to determine optimal weaning strategies.
Supporting information
(DOCX)
(DOCX)
(XLSM)
(XLSX)
(XLSX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Schrage B, Becher PM, Goßling A, Savarese G, Dabboura S, Yan I, et al. Temporal trends in incidence, causes, use of mechanical circulatory support and mortality in cardiogenic shock. ESC Heart Fail. 2021;8(2):1295–303. doi: 10.1002/ehf2.13202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mebazaa A, Combes A, van Diepen S, Hollinger A, Katz JN, Landoni G, et al. Management of cardiogenic shock complicating myocardial infarction. Intensive Care Med. 2018;44(6):760–73. doi: 10.1007/s00134-018-5214-9 [DOI] [PubMed] [Google Scholar]
- 3.Zangrillo A, Landoni G, Biondi-Zoccai G, Greco M, Greco T, Frati G, et al. A meta-analysis of complications and mortality of extracorporeal membrane oxygenation. Critical Care Resusc. 2013;15(3):172–8. doi: 10.1016/s1441-2772(23)01792-1 [DOI] [PubMed] [Google Scholar]
- 4.Chen Y-S, Chao A, Yu H-Y, Ko W-J, Wu I-H, Chen RJ-C, et al. Analysis and results of prolonged resuscitation in cardiac arrest patients rescued by extracorporeal membrane oxygenation. J Am Coll Cardiol. 2003;41(2):197–203. doi: 10.1016/s0735-1097(02)02716-x [DOI] [PubMed] [Google Scholar]
- 5.Chang W-W, Tsai F-C, Tsai T-Y, Chang C-H, Jenq C-C, Chang M-Y, et al. Predictors of mortality in patients successfully weaned from extracorporeal membrane oxygenation. PLoS One. 2012;7(8):e42687. doi: 10.1371/journal.pone.0042687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cavarocchi NC, Pitcher HT, Yang Q, Karbowski P, Miessau J, Hastings HM, et al. Weaning of extracorporeal membrane oxygenation using continuous hemodynamic transesophageal echocardiography. J Thorac Cardiovasc Surg. 2013;146(6):1474–9. doi: 10.1016/j.jtcvs.2013.06.055 [DOI] [PubMed] [Google Scholar]
- 7.Aso S, Matsui H, Fushimi K, Yasunaga H. In-hospital mortality and successful weaning from venoarterial extracorporeal membrane oxygenation: analysis of 5,263 patients using a national inpatient database in Japan. Crit Care. 2016;20:80. doi: 10.1186/s13054-016-1261-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pappalardo F, Pieri M, Arnaez Corada B, Ajello S, Melisurgo G, De Bonis M, et al. Timing and strategy for weaning from venoarterial ECMO are complex issues. J Cardiothorac Vasc Anesth. 2015;29(4):906–11. doi: 10.1053/j.jvca.2014.12.011 [DOI] [PubMed] [Google Scholar]
- 9.Cusanno A, Aissaoui N, Minville V, Porterie J, Biendel C, Volle K, et al. Predictors of weaning failure in case of VA ECMO implantation. Sci Rep. 2022;12(1):13842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charbonneau F, Chahinian K, Bebawi E, Lavigueur O, Lévesque É, Lamarche Y, et al. Parameters associated with successful weaning of veno-arterial extracorporeal membrane oxygenation: a systematic review. Crit Care. 2022;26(1):375. doi: 10.1186/s13054-022-04249-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lusebrink E, Stremmel C, Stark K, Joskowiak D, Czermak T, Born F, et al. Update on weaning from veno-arterial extracorporeal membrane oxygenation. J Clin Med. 2020;9(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906. doi: 10.1016/j.ijsu.2021.105906 [DOI] [PubMed] [Google Scholar]
- 13.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10(10):ED000142. doi: 10.1002/14651858.ED000142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aissaoui N, Luyt C-E, Leprince P, Trouillet J-L, Léger P, Pavie A, et al. Predictors of successful extracorporeal membrane oxygenation (ECMO) weaning after assistance for refractory cardiogenic shock. Intensive Care Med. 2011;37(11):1738–45. doi: 10.1007/s00134-011-2358-2 [DOI] [PubMed] [Google Scholar]
- 17.Akin S, Dos Reis Miranda D, Caliskan K, Soliman OI, Guven G, Struijs A, et al. Functional evaluation of sublingual microcirculation indicates successful weaning from VA-ECMO in cardiogenic shock. Crit Care. 2017;21(1):265. doi: 10.1186/s13054-017-1855-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aksoy T, Arslan AH, Ugur M, Ustunsoy H. Lactate and lactate clearance are predictive factors for mortality in patients with extracorporeal membrane oxygenation. Braz J Cardiovasc Surg. 2024;39(2):e20230091. doi: 10.21470/1678-9741-2023-0091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen T-Y, Chang C-H, Hsu J-Y, Sheu J-J, Kuo H-C, Hsu M-H, et al. Comparison of the predictive ability of lactate and central venous blood gas in pediatric venoarterial mode extracorporeal membrane oxygenation outcome. Pediatr Neonatol. 2022;63(5):474–83. doi: 10.1016/j.pedneo.2022.03.011 [DOI] [PubMed] [Google Scholar]
- 20.Colombo CNJ, Dammassa V, Pozzi M, et al. Echocardiographic predictors of VA ECMO weaning in patients with cardiogenic shock. Eur Heart J Acute Cardiovasc Care. 2019;8:144–5. [Google Scholar]
- 21.Daftari B. Echocardiographic predictors of successful pediatric cardiac ECMO wean. American Academy of Pediatrics National Conference and Exhibition. 2010. [Google Scholar]
- 22.Dunton K, Weeks PA, Gulbis B, Jumean M, Kumar S, Janowiak L, et al. Evaluation of vasoactive-inotropic score and survival to decannulation in adult patients on venoarterial extracorporeal life support: an observational cohort study. ASAIO J. 2023;69(9):873–8. doi: 10.1097/MAT.0000000000001982 [DOI] [PubMed] [Google Scholar]
- 23.Finnigan L, Jones H, Lotto R, Horan M, Dhannapuneni R, Guerrero R et al. Cerebral blood flow velocity during weaning of V-A ECMO in pediatrics. Perfusion. 2020;13(35):93–282. [Google Scholar]
- 24.Frederiksen CA, Nielsen R, Frederiksen AS, et al. Echocardiographic predictors of successful weaning from veno-arterial extracorporeal membrane oxygenation. Eur Heart J. 2018;39.29020337 [Google Scholar]
- 25.Gonzalez Martin J, Alonso-Fernandez-Gatta M, Merchan Gomez S, Gonzalez Cebrian M, Diego-Nieto A, Martin-Herrero F, et al. Echocardiographic recovery changes in patients supported with veno-arterial extracorporeal membrane oxygenator and weaning success. Eur Heart J. 2021;42(Supplement_1). doi: 10.1093/eurheartj/ehab724.1060 [DOI] [Google Scholar]
- 26.Huang K-C, Lin L-Y, Chen Y-S, Lai C-H, Hwang J-J, Lin L-C. Three-dimensional echocardiography-derived right ventricular ejection fraction correlates with success of decannulation and prognosis in patients stabilized by venoarterial extracorporeal life support. J Am Soc Echocardiogr. 2018;31(2):169–79. doi: 10.1016/j.echo.2017.09.004 [DOI] [PubMed] [Google Scholar]
- 27.Hutchins E, Rahrooh A, Feng J, Chandra N, Hsu JJ, Bui A. Abstract 13293: predicting successful ECMO decannulation—a novel machine learning approach. American Heart Association. 2023. [Google Scholar]
- 28.Joseph A, Venturini J, Blair JEA, Paul J, Shah A, Nathan S. Prognostic value of right ventricular function in percutaneous veno-arterial extracorporeal membrane oxygenation. BMC Cardiovasc Disord. 2019;19:A45. [Google Scholar]
- 29.Kellnar A, Naumann D, Scherer C, Lüsebrink E, Joskowiak D, Peterß S, et al. Aortic arch blood flow measurements as a predictor of successful ECMO weaning in cardiogenic shock. Heliyon. 2024;10(5):e26773. doi: 10.1016/j.heliyon.2024.e26773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim D, Jang WJ, Park TK, Cho YH, Choi JO, Jeon ES, et al. Echocardiographic predictors of successful extracorporeal membrane oxygenation weaning after refractory cardiogenic shock. J Am Soc Echocardiogr. 2021;34(4):414–22 e4. [DOI] [PubMed] [Google Scholar]
- 31.Kim D, Park Y, Choi KH, Park TK, Lee JM, Cho YH, et al. Prognostic implication of RV coupling to pulmonary circulation for successful weaning from extracorporeal membrane oxygenation. JACC Cardiovasc Imaging. 2021;14(8):1523–31. doi: 10.1016/j.jcmg.2021.02.018 [DOI] [PubMed] [Google Scholar]
- 32.L’Acqua C, Rossi F, Polvani G, Ceriani R, Bertera A, Agrifoglio M, et al. Preimplant outcome predictors in patients supported with veno-arterial extracorporeal membrane oxygenation. Does the right time exist? Minerva Anestesiol. 2019;85(8):915–6. doi: 10.23736/S0375-9393.19.13589-4 [DOI] [PubMed] [Google Scholar]
- 33.Lee J, Lee S, Joo S, Park YK, Kim KM, Jung JC, et al. Abstract: pulse pressure as a potential predictive factor for successful weaning in patients supported by venoarterial extracorporeal membrane oxygenation without left ventricular venting. ASAIO J. 2023;69:17. [Google Scholar]
- 34.Lim JY, Jung JS. Predictors of successful weaning from veno-arterial extracorporeal membrane oxygenation support. 8th EuroELSO Congress. 2019;34(1):82–251. [Google Scholar]
- 35.Matsumoto M, Asaumi Y, Nakamura Y, Nakatani T, Nagai T, Kanaya T, et al. Clinical determinants of successful weaning from extracorporeal membrane oxygenation in patients with fulminant myocarditis. ESC Heart Fail. 2018;5(4):675–84. doi: 10.1002/ehf2.12291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mongkolpun W, Bakos P, Peluso L, Annoni F, Vincent JL, Creteur J. Cutaneous blood flow as a predictor of successful weaning from VA-ECMO. Crit Care. 2019;23(P115). [Google Scholar]
- 37.Naruke T, Inomata T, Imai H, Yanagisawa T, Maekawa E, Mizutani T, et al. End-tidal carbon dioxide concentration can estimate the appropriate timing for weaning off from extracorporeal membrane oxygenation for refractory circulatory failure. Int Heart J. 2010;51(2):116–20. doi: 10.1536/ihj.51.116 [DOI] [PubMed] [Google Scholar]
- 38.Naruke T, Inomata T, Kawaguchi T, Mizutani T, Shinagawa H, Koitabashi T, et al. Prognostic impact of dramatic alteration of mixed venous oxygen saturation on the hemodynamic recovery from veno-arterial extracorporeal membrane oxygenation. Eur Heart J. 2012;33(69). [Google Scholar]
- 39.Punn R, Falkensammer CB, Blinder JJ, Fifer CG, Thorsson T, Perens G, et al. Hemodynamic and echocardiographic predictors of mortality in pediatric patients on venoarterial extracorporeal membrane oxygenation: a multicenter investigation. J Am Soc Echocardiogr. 2023;36(2):233–41. [DOI] [PubMed] [Google Scholar]
- 40.Sawada K, Kawakami S, Murata S, Nishimura K, Tahara Y, Hosoda H, et al. Predicting parameters for successful weaning from veno-arterial extracorporeal membrane oxygenation in cardiogenic shock. ESC Heart Fail. 2021;8(1):471–80. doi: 10.1002/ehf2.13097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stull C, Baram M, Pitcher H, Hirose H, Guarav K, Cavarocchi N. Predictors of a successful wean from extracorporeal membrane oxygenation (ECMO) for ARDS. Crit Care Med. 2013;41.23222263 [Google Scholar]
- 42.Sugiura A, Abe R, Nakayama T, Hattori N, Fujimoto Y, Himi T, et al. Predictors of successful weaning from veno-arterial extracorporeal membrane oxygenation after coronary revascularization for acute myocardial infarction complicated by cardiac arrest: a retrospective multicenter study. Shock. 2019;51(6):690–7. doi: 10.1097/SHK.0000000000001220 [DOI] [PubMed] [Google Scholar]
- 43.Sugiyama K, Takahashi M, Miyazaki K, Ishida T, Kobayashi M, Hamabe Y. Left ventricular wall findings in non-electrocardiography-gated contrast-enhanced computed tomography after extracorporeal cardiopulmonary resuscitation. Crit Care. 2019;23(1):357. doi: 10.1186/s13054-019-2624-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Varodomvanichkul C, Puwatnuttasit P, Ariyachaipanich A, Songmuang SB, Puwanant S. Left atrial strain for predicting successful extra-corporeal membrane oxygenator weaning from cardiogenic shock. J Am Coll Cardiol. 2023;81(8):686. doi: 10.1016/s0735-1097(23)01130-0 [DOI] [Google Scholar]
- 45.Voigt I, Mighali M, Manda D, Aurich P, Bruder O. Radiographic assessment of lung edema (RALE) score is associated with clinical outcomes in patients with refractory cardiogenic shock and refractory cardiac arrest after percutaneous implantation of extracorporeal life support. Intern Emerg Med. 2022;17:1463–70. [DOI] [PubMed] [Google Scholar]
- 46.Watanabe M, Ajiro Y, Hasegawa S, Hamada K, Tsukamoto K, Saito T, et al. Clinical factors associated with successful venoarterial extracorporeal membrane oxygenation weaning: a single-center retrospective cohort study. Critical Care Shock. 2023;26:115–24. [Google Scholar]
- 47.Puerto E, Tavazzi G, Gambaro A, Cirillo C, Pecoraro A, Martín-Asenjo R, et al. Interaction between VA-ECMO and the right ventricle. Hellenic J Cardiol. 2022;68:17–24. doi: 10.1016/j.hjc.2022.07.003 [DOI] [PubMed] [Google Scholar]
- 48.Lim JY, Jung JS. Predictors of successful weaning from veno-arterial extracorporeal membrane oxygenation support. Perfus Ger. 2019;34:88–9. [Google Scholar]
- 49.Liu S-F, Hsu CY, Lee C-K, Kao PH-L. Clinical predictors of successful weaning VA-ECMO. J Am Coll Cardiol. 2022;S29–30. [Google Scholar]
- 50.Brahmbhatt DH, Daly AL, Luk AC, Fan E, Billia F. Liberation from venoarterial extracorporeal membrane oxygenation: a review. Circ Heart Fail. 2021;14(7):e007679. [DOI] [PubMed] [Google Scholar]
- 51.Asaumi Y, Yasuda S, Morii I, Kakuchi H, Otsuka Y, Kawamura A, et al. Favourable clinical outcome in patients with cardiogenic shock due to fulminant myocarditis supported by percutaneous extracorporeal membrane oxygenation. Eur Heart J. 2005;26(20):2185–92. doi: 10.1093/eurheartj/ehi411 [DOI] [PubMed] [Google Scholar]
- 52.North M, Eckman P, Samara M, Chavez I, Schmidt C, Garberich R, et al. Peak troponin predicts successful weaning from VA ECMO in patients with acute myocardial infarction complicated by cardiogenic shock. Int J Artif Organs. 2022;45(1):68–74. doi: 10.1177/0391398821991155 [DOI] [PubMed] [Google Scholar]
- 53.Ortuno S, Delmas C, Diehl J-L, Bailleul C, Lancelot A, Naili M, et al. Weaning from veno-arterial extra-corporeal membrane oxygenation: which strategy to use? Ann Cardiothorac Surg. 2019;8(1):E1–8. doi: 10.21037/acs.2018.08.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lorusso R, Shekar K, MacLaren G, Schmidt M, Pellegrino V, Meyns B, et al. ELSO interim guidelines for venoarterial extracorporeal membrane oxygenation in adult cardiac patients. ASAIO J. 2021;67(8):827–44. doi: 10.1097/MAT.0000000000001510 [DOI] [PubMed] [Google Scholar]
- 55.Aissaoui N, Caudron J, Leprince P, Fagon J-Y, Lebreton G, Combes A, et al. Right-left ventricular interdependence: a promising predictor of successful extracorporeal membrane oxygenation (ECMO) weaning after assistance for refractory cardiogenic shock. Intensive Care Med. 2017;43(4):592–4. doi: 10.1007/s00134-016-4657-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(XLSM)
(XLSX)
(XLSX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.